Abstract
A potent growth factor, PC13 embryonal carcinoma-derived growth factor (ECDGF), has been isolated from serum-free medium conditioned by PC13 murine embryonal carcinoma cells. ECDGF is a single chain, cationic hydrophobic molecule of 17 500 daltons. ECDGF will induce DNA synthesis in established fibroblast cell lines and the immediate differentiated progeny of PC13 EC cells in vitro, and consequently appears to differ from other well characterised growth factors both in structure and action.
Full text
PDF
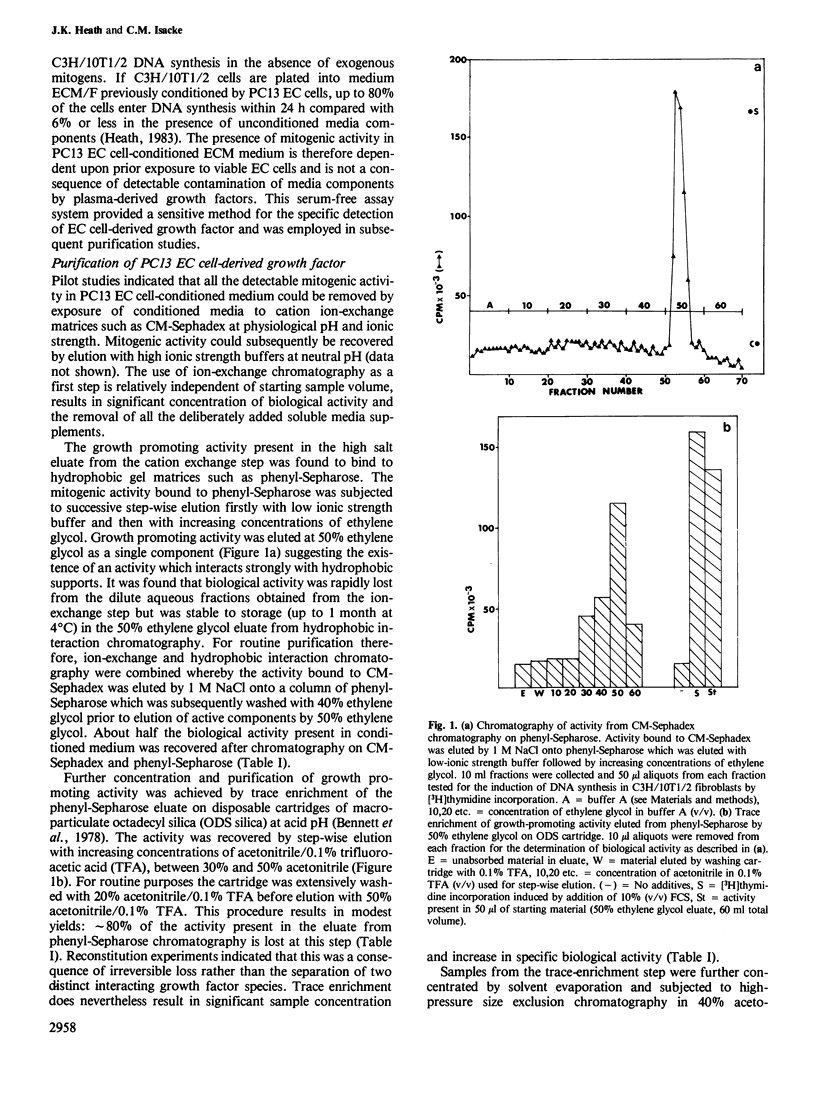




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Assoian R. K., Komoriya A., Meyers C. A., Miller D. M., Sporn M. B. Transforming growth factor-beta in human platelets. Identification of a major storage site, purification, and characterization. J Biol Chem. 1983 Jun 10;258(11):7155–7160. [PubMed] [Google Scholar]
- Bennett H. P., Hudson A. M., Kelly L., McMartin C., Purdon G. E. A rapid method, using octadecasilyl-silica, for the extraction of certain peptides from tissues. Biochem J. 1978 Dec 1;175(3):1139–1141. doi: 10.1042/bj1751139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie D. L., Gagnon J. Isolation, characterization and N-terminal sequences of the CNBr-cleavage peptides from human complement Factor B. Localization of a free thiol group and a sequence defining the site cleaved by factor D. Biochem J. 1982 Mar 1;201(3):555–567. doi: 10.1042/bj2010555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochran B. H., Reffel A. C., Stiles C. D. Molecular cloning of gene sequences regulated by platelet-derived growth factor. Cell. 1983 Jul;33(3):939–947. doi: 10.1016/0092-8674(83)90037-5. [DOI] [PubMed] [Google Scholar]
- Diwan S. B., Stevens L. C. Development of teratomas from the ectoderm of mouse egg cylinders. J Natl Cancer Inst. 1976 Oct;57(4):937–942. doi: 10.1093/jnci/57.4.937. [DOI] [PubMed] [Google Scholar]
- Heath J. K., Deller M. J. Serum-free culture of PC13 murine embryonal carcinoma cells. J Cell Physiol. 1983 Jun;115(3):225–230. doi: 10.1002/jcp.1041150302. [DOI] [PubMed] [Google Scholar]
- Heath J., Bell S., Rees A. R. Appearance of functional insulin receptors during the differentiation of embryonal carcinoma cells. J Cell Biol. 1981 Oct;91(1):293–297. doi: 10.1083/jcb.91.1.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldin C. H., Westermark B., Wasteson A. Platelet-derived growth factor. Isolation by a large-scale procedure and analysis of subunit composition. Biochem J. 1981 Mar 1;193(3):907–913. doi: 10.1042/bj1930907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isacke C. M., Deller M. J. Teratocarcinoma cells exhibit growth cooperativity in vitro. J Cell Physiol. 1983 Dec;117(3):407–414. doi: 10.1002/jcp.1041170316. [DOI] [PubMed] [Google Scholar]
- Jansen M., van Schaik F. M., Ricker A. T., Bullock B., Woods D. E., Gabbay K. H., Nussbaum A. L., Sussenbach J. S., Van den Brande J. L. Sequence of cDNA encoding human insulin-like growth factor I precursor. Nature. 1983 Dec 8;306(5943):609–611. doi: 10.1038/306609a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maciag T., Hoover G. A., Weinstein R. High and low molecular weight forms of endothelial cell growth factor. J Biol Chem. 1982 May 25;257(10):5333–5336. [PubMed] [Google Scholar]
- Marquardt H., Todaro G. J. Human transforming growth factor. Production by a melanoma cell line, purification, and initial characterization. J Biol Chem. 1982 May 10;257(9):5220–5225. [PubMed] [Google Scholar]
- Marquardt H., Wilson G. L., Todaro G. J. Isolation and characterization of a multiplication-stimulating activity (MSA)-like polypeptide produced by a human fibrosarcoma cell line. J Biol Chem. 1980 Oct 10;255(19):9177–9181. [PubMed] [Google Scholar]
- Massagué J. Epidermal growth factor-like transforming growth factor. I. Isolation, chemical characterization, and potentiation by other transforming factors from feline sarcoma virus-transformed rat cells. J Biol Chem. 1983 Nov 25;258(22):13606–13613. [PubMed] [Google Scholar]
- McKeehan W. L. The effect of temperature during trypsin treatment on viability and multiplication potential of single normal human and chicken fibroblasts. Cell Biol Int Rep. 1977 Jul;1(4):335–343. doi: 10.1016/0309-1651(77)90063-7. [DOI] [PubMed] [Google Scholar]
- Nagarajan L., Anderson W. B. Insulin promotes the growth of F9 embryonal carcinoma cells apparently by acting through its own receptor. Biochem Biophys Res Commun. 1982 Jun 15;106(3):974–980. doi: 10.1016/0006-291x(82)91806-x. [DOI] [PubMed] [Google Scholar]
- Pruss R. M., Herschman H. R. Variants of 3T3 cells lacking mitogenic response to epidermal growth factor. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3918–3921. doi: 10.1073/pnas.74.9.3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayner M. J., Graham C. F. Clonal analysis of the change in growth phenotype during embryonal carcinoma cell differentiation. J Cell Sci. 1982 Dec;58:331–344. doi: 10.1242/jcs.58.1.331. [DOI] [PubMed] [Google Scholar]
- Read S. M., Northcote D. H. Minimization of variation in the response to different proteins of the Coomassie blue G dye-binding assay for protein. Anal Biochem. 1981 Sep 1;116(1):53–64. doi: 10.1016/0003-2697(81)90321-3. [DOI] [PubMed] [Google Scholar]
- Rees A. R., Adamson E. D., Graham C. F. Epidermal growth factor receptors increase during the differentiation of embryonal carcinoma cells. Nature. 1979 Sep 27;281(5729):309–311. doi: 10.1038/281309a0. [DOI] [PubMed] [Google Scholar]
- Reznikoff C. A., Bertram J. S., Brankow D. W., Heidelberger C. Quantitative and qualitative studies of chemical transformation of cloned C3H mouse embryo cells sensitive to postconfluence inhibition of cell division. Cancer Res. 1973 Dec;33(12):3239–3249. [PubMed] [Google Scholar]
- Rizzino A., Orme L. S., De Larco J. E. Embryonal carcinoma cell growth and differentiation. Production of and response to molecules with transforming growth factor activity. Exp Cell Res. 1983 Jan;143(1):143–152. doi: 10.1016/0014-4827(83)90116-7. [DOI] [PubMed] [Google Scholar]
- Rozengurt E., Brown K. D., Pettican P. Vasopressin inhibition of epidermal growth factor binding to cultured mouse cells. J Biol Chem. 1981 Jan 25;256(2):716–722. [PubMed] [Google Scholar]
- TODARO G. J., GREEN H. Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J Cell Biol. 1963 May;17:299–313. doi: 10.1083/jcb.17.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterfield M. D., Scrace G. T., Whittle N., Stroobant P., Johnsson A., Wasteson A., Westermark B., Heldin C. H., Huang J. S., Deuel T. F. Platelet-derived growth factor is structurally related to the putative transforming protein p28sis of simian sarcoma virus. Nature. 1983 Jul 7;304(5921):35–39. doi: 10.1038/304035a0. [DOI] [PubMed] [Google Scholar]



