Abstract
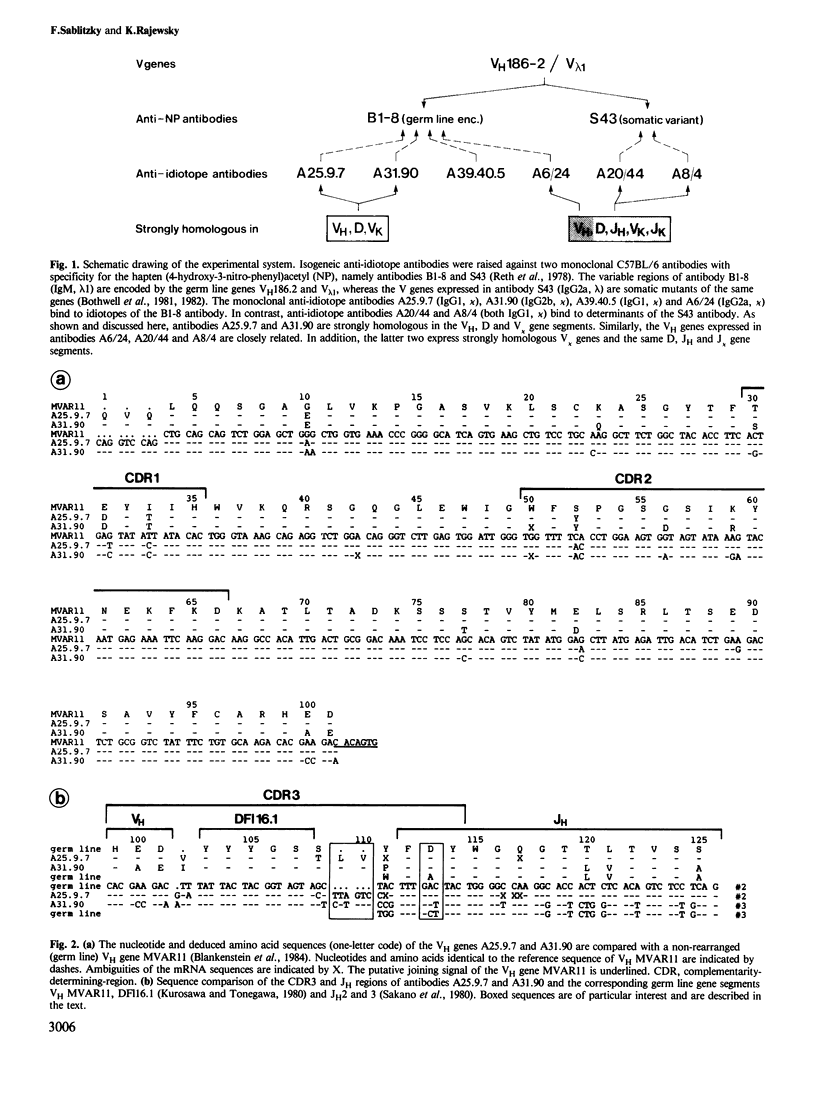
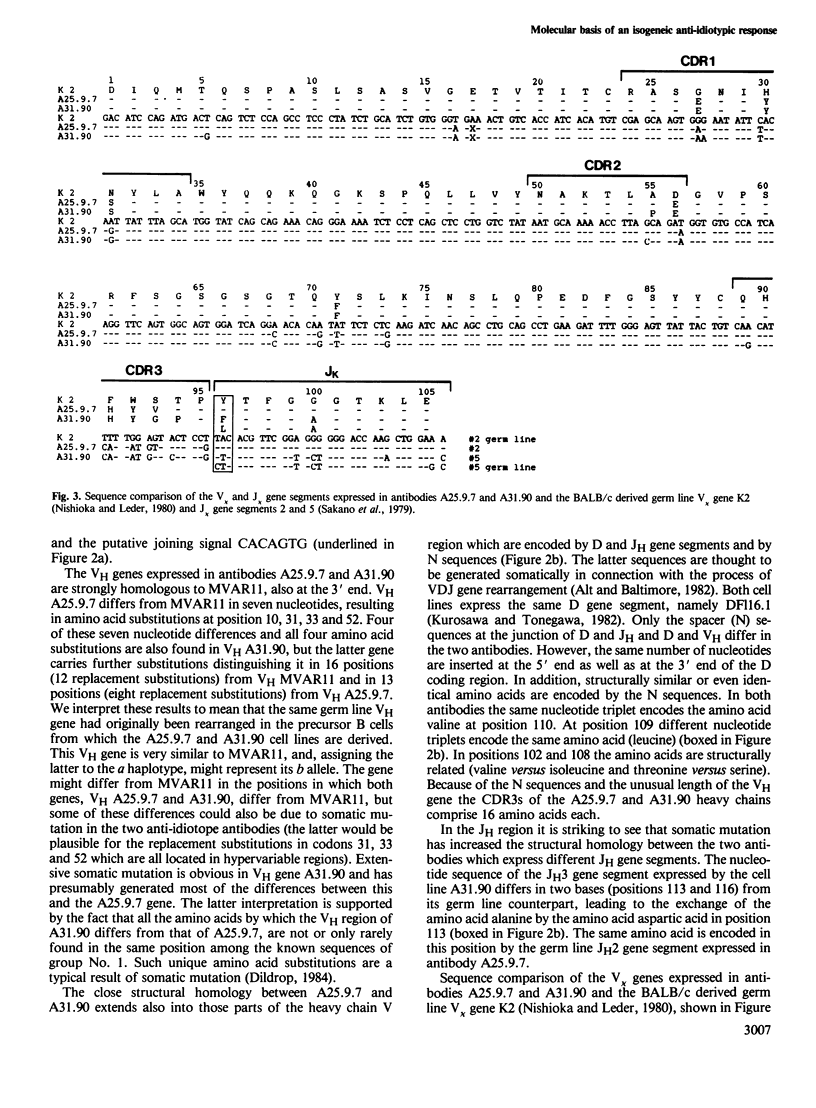
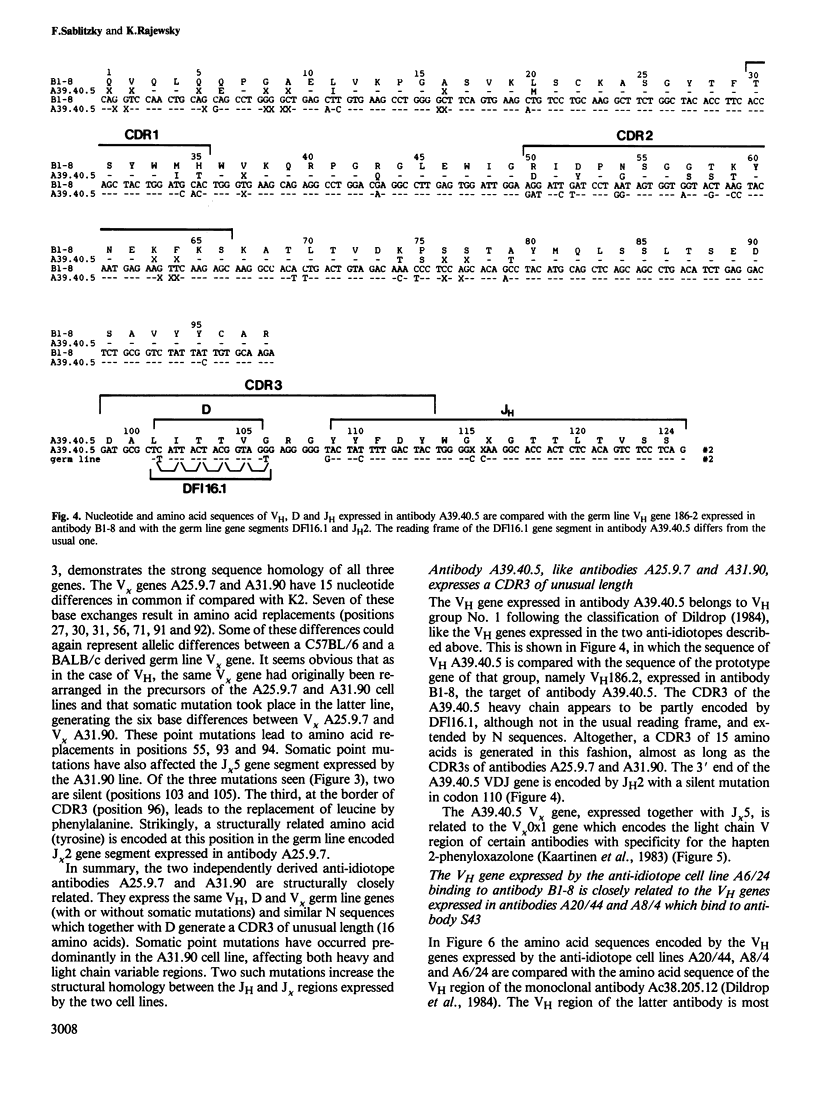
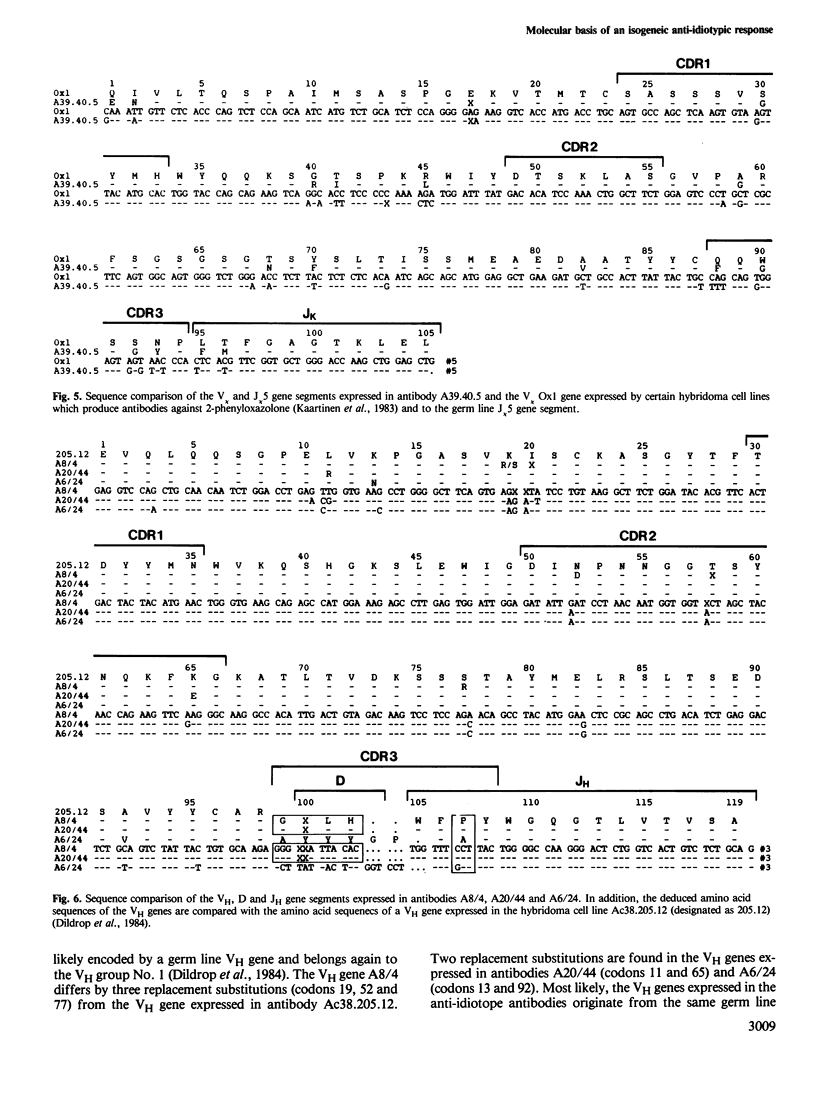
The nucleotide sequences of the variable region genes expressed in the heavy and light chains of six isogeneic anti-idiotope antibodies recognizing idiotopes on two closely related antibodies with specificity for the hapten (4-hydroxy-3-nitrophenyl)acetyl (NP) were determined. In two independently derived anti-idiotope cell lines the same or strongly homologous V kappa, VH and D region genes had originally been rearranged. The two lines express long and partly homologous N sequences (presumed to be not of germ line origin) at the border of D, resulting in CDR3s of unusual length. An unusually long CDR3, partly encoded by N sequences, is also present in the heavy chain of a third anti-idiotope antibody. The VH regions of the three remaining anti-idiotope antibodies originate from a single VH gene which belongs to the same VH group as the VH genes expressed in the other anti-idiotopes. Two of these antibodies, expressing similar V, D and J elements, had been isolated from the same mouse and appear to have diverged from the same B cell precursor by at least two rounds of somatic mutation. Somatic point mutations have occurred in most, if not all anti-idiotope V region sequences. In two instances somatic mutations in J increase the structural homology between anti-idiotopes. The anti-idiotypic response in this system is thus genetically restricted and may depend upon the selection of non-germ line sequences, suggesting an explanation for the low frequency at which anti-idiotope antibodies are expressed in this system.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alt F. W., Baltimore D. Joining of immunoglobulin heavy chain gene segments: implications from a chromosome with evidence of three D-JH fusions. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4118–4122. doi: 10.1073/pnas.79.13.4118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyreuther K., Bovens J., Brüggemann M., Dildrop R., Kelsoe G., Krawinkel U., Müller C., Nishikawa S., Radbruch A., Reth M. Idiotypic determinants used in the analysis of antibody diversification and as regulatory targets. Ann N Y Acad Sci. 1983;418:121–129. doi: 10.1111/j.1749-6632.1983.tb18060.x. [DOI] [PubMed] [Google Scholar]
- Bothwell A. L., Paskind M., Reth M., Imanishi-Kari T., Rajewsky K., Baltimore D. Heavy chain variable region contribution to the NPb family of antibodies: somatic mutation evident in a gamma 2a variable region. Cell. 1981 Jun;24(3):625–637. doi: 10.1016/0092-8674(81)90089-1. [DOI] [PubMed] [Google Scholar]
- Bothwell A. L., Paskind M., Reth M., Imanishi-Kari T., Rajewsky K., Baltimore D. Somatic variants of murine immunoglobulin lambda light chains. Nature. 1982 Jul 22;298(5872):380–382. doi: 10.1038/298380a0. [DOI] [PubMed] [Google Scholar]
- Brüggemann M., Radbruch A., Rajewsky K. Immunoglobulin V region variants in hybridoma cells. I. Isolation of a variant with altered idiotypic and antigen binding specificity. EMBO J. 1982;1(5):629–634. doi: 10.1002/j.1460-2075.1982.tb01219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dildrop R., Bovens J., Siekevitz M., Beyreuther K., Rajewsky K. A V region determinant (idiotope) expressed at high frequency in B lymphocytes is encoded by a large set of antibody structural genes. EMBO J. 1984 Mar;3(3):517–523. doi: 10.1002/j.1460-2075.1984.tb01840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmonds M., Vaughan M. H., Jr, Nakazato H. Polyadenylic acid sequences in the heterogeneous nuclear RNA and rapidly-labeled polyribosomal RNA of HeLa cells: possible evidence for a precursor relationship. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1336–1340. doi: 10.1073/pnas.68.6.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamlyn P. H., Browniee G. G., Cheng C. C., Gait M. J., Milstein C. Complete sequence of constant and 3' noncoding regions of an immunoglobulin mRNA using the dideoxynucleotide method of RNA sequencing. Cell. 1978 Nov;15(3):1067–1075. doi: 10.1016/0092-8674(78)90290-8. [DOI] [PubMed] [Google Scholar]
- Hohn B., Murray K. Packaging recombinant DNA molecules into bacteriophage particles in vitro. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3259–3263. doi: 10.1073/pnas.74.8.3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber R., Deisenhofer J., Colman P. M., Matsushima M., Palm W. Crystallographic structure studies of an IgG molecule and an Fc fragment. Nature. 1976 Dec 2;264(5585):415–420. doi: 10.1038/264415a0. [DOI] [PubMed] [Google Scholar]
- Jerne N. K. Towards a network theory of the immune system. Ann Immunol (Paris) 1974 Jan;125C(1-2):373–389. [PubMed] [Google Scholar]
- Kaartinen M., Griffiths G. M., Markham A. F., Milstein C. mRNA sequences define an unusually restricted IgG response to 2-phenyloxazolone and its early diversification. 1983 Jul 28-Aug 3Nature. 304(5924):320–324. doi: 10.1038/304320a0. [DOI] [PubMed] [Google Scholar]
- Kearney J. F., Radbruch A., Liesegang B., Rajewsky K. A new mouse myeloma cell line that has lost immunoglobulin expression but permits the construction of antibody-secreting hybrid cell lines. J Immunol. 1979 Oct;123(4):1548–1550. [PubMed] [Google Scholar]
- Kurosawa Y., Tonegawa S. Organization, structure, and assembly of immunoglobulin heavy chain diversity DNA segments. J Exp Med. 1982 Jan 1;155(1):201–218. doi: 10.1084/jem.155.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leder P., Tiemeier D., Enquist L. EK2 derivatives of bacteriophage lambda useful in the cloning of DNA from higher organisms: the lambdagtWES system. Science. 1977 Apr 8;196(4286):175–177. doi: 10.1126/science.322278. [DOI] [PubMed] [Google Scholar]
- Max E. E., Maizel J. V., Jr, Leder P. The nucleotide sequence of a 5.5-kilobase DNA segment containing the mouse kappa immunoglobulin J and C region genes. J Biol Chem. 1981 May 25;256(10):5116–5120. [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKean D., Huppi K., Bell M., Staudt L., Gerhard W., Weigert M. Generation of antibody diversity in the immune response of BALB/c mice to influenza virus hemagglutinin. Proc Natl Acad Sci U S A. 1984 May;81(10):3180–3184. doi: 10.1073/pnas.81.10.3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishioka Y., Leder P. Organization and complete sequence of identical embryonic and plasmacytoma kappa V-region genes. J Biol Chem. 1980 Apr 25;255(8):3691–3694. [PubMed] [Google Scholar]
- Rajewsky K. Symmetry and asymmetry in idiotypic interactions. Ann Immunol (Paris) 1983 Jul-Aug;134D(1):133–141. doi: 10.1016/s0769-2625(83)80064-6. [DOI] [PubMed] [Google Scholar]
- Rajewsky K., Takemori T. Genetics, expression, and function of idiotypes. Annu Rev Immunol. 1983;1:569–607. doi: 10.1146/annurev.iy.01.040183.003033. [DOI] [PubMed] [Google Scholar]
- Reth M., Hämmerling G. J., Rajewsky K. Analysis of the repertoire of anti-NP antibodies in C57BL/6 mice by cell fusion. I. Characterization of antibody families in the primary and hyperimmune response. Eur J Immunol. 1978 Jun;8(6):393–400. doi: 10.1002/eji.1830080605. [DOI] [PubMed] [Google Scholar]
- Reth M., Kelsoe G., Rajewsky K. Idiotypic regulation by isologous monoclonal anti-idiotope antibodies. Nature. 1981 Mar 19;290(5803):257–259. doi: 10.1038/290257a0. [DOI] [PubMed] [Google Scholar]
- Rudikoff S., Pawlita M., Pumphrey J., Heller M. Somatic diversification of immunoglobulins. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2162–2166. doi: 10.1073/pnas.81.7.2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sablitzky F., Radbruch A., Rajewsky K. Spontaneous immunoglobulin class switching in myeloma and hybridoma cell lines differs from physiological class switching. Immunol Rev. 1982;67:59–72. doi: 10.1111/j.1600-065x.1982.tb01055.x. [DOI] [PubMed] [Google Scholar]
- Sakano H., Hüppi K., Heinrich G., Tonegawa S. Sequences at the somatic recombination sites of immunoglobulin light-chain genes. Nature. 1979 Jul 26;280(5720):288–294. doi: 10.1038/280288a0. [DOI] [PubMed] [Google Scholar]
- Sakano H., Maki R., Kurosawa Y., Roeder W., Tonegawa S. Two types of somatic recombination are necessary for the generation of complete immunoglobulin heavy-chain genes. Nature. 1980 Aug 14;286(5774):676–683. doi: 10.1038/286676a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Schmidt W. E., Jung H. D., Palm W., Hilschmann N. Beitrag zur dreidimensionalen Strukturaufklärung der Antikörper. Die Primärstruktur des kristallisierbaren monoklonalen Immunoglobulins IgG1 KOL, I. Hoppe Seylers Z Physiol Chem. 1983 Jun;364(6):713–747. [PubMed] [Google Scholar]
- Schuler W., Weiler E., Kolb H. Characterization of syngeneic anti-idiotypic antibody against the idiotype fo BALB/c myeloma protein J558. Eur J Immunol. 1977 Sep;7(9):649–654. doi: 10.1002/eji.1830070914. [DOI] [PubMed] [Google Scholar]
- Seppälä I. J., Eichmann K. Induction and characterization of isogeneic anti-idiotypic antibodies to BALB/c myeloma S117: lack of reactivity with major idiotypic determinants. Eur J Immunol. 1979 Mar;9(3):243–250. doi: 10.1002/eji.1830090314. [DOI] [PubMed] [Google Scholar]
- Siekevitz M., Huang S. Y., Gefter M. L. The genetic basis of antibody production: a single heavy chain variable region gene encodes all molecules bearing the dominant anti-arsonate idiotype in the strain A mouse. Eur J Immunol. 1983 Feb;13(2):123–132. doi: 10.1002/eji.1830130207. [DOI] [PubMed] [Google Scholar]


