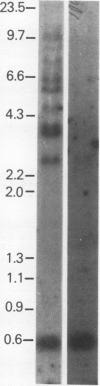
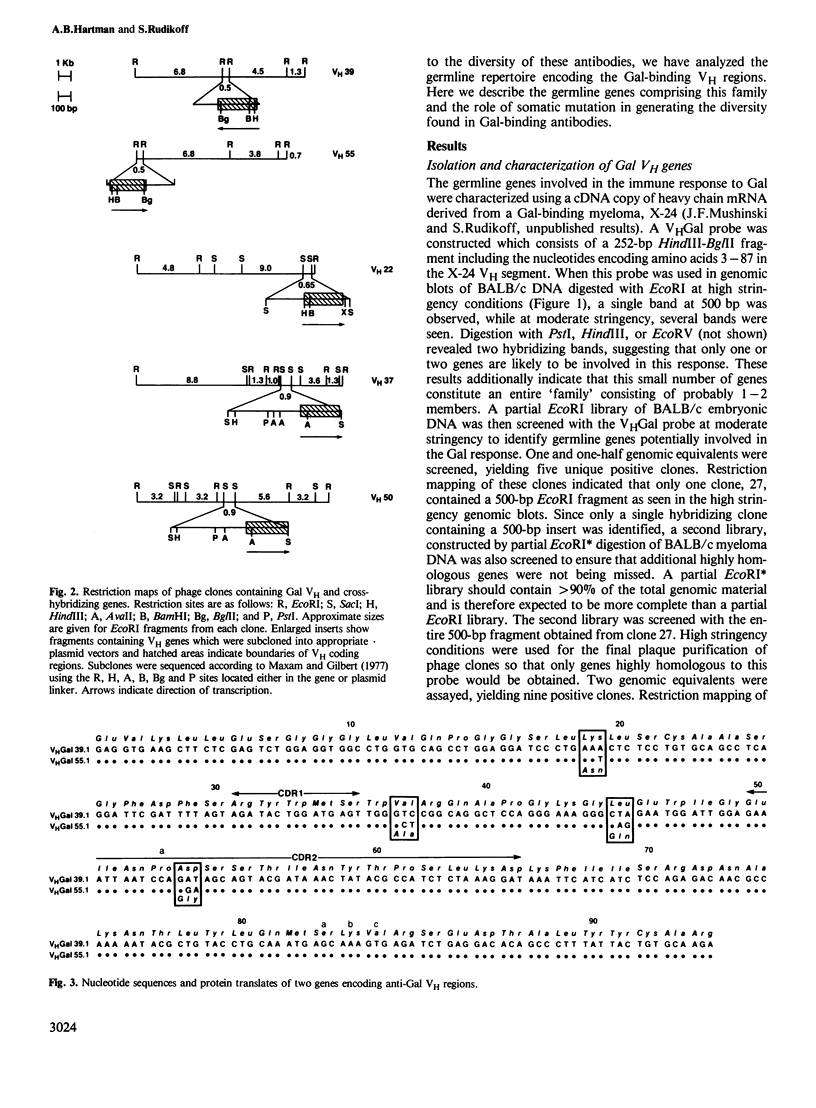
Abstract
The immune response to beta-(1,6)-galactan in the BALB/c mouse has been well characterized and includes the amino acid sequence determination of 13 monoclonal antibodies. The genetic potential encoding the VH regions of these antibodies has been determined by isolation and sequencing of homologous germline genes. The germline repertoire encoding these proteins was found to consist of two closely related genes. One of these directly encodes the VH segments of seven Gal-binding proteins, and the second directly encodes one additional protein sequence. Sequence variations found in the VH regions of five other Gal-binding proteins can be explained by somatic mutations leading to single base substitutions in the more frequently used gene. Since four of the hybridoma proteins exhibiting somatic mutations are of the IgM class, these results indicate that somatic mutation, in this system, is not associated with class switching and can apparently be initiated early in B-cell development. The two Gal genes are the only members of a very restricted multigene family and probably result from a gene duplication estimated to occur 1.4-2.8 million years ago. Three other genes hybridizing at moderate stringency to a VHGal probe were also sequenced and were found to be members of two additional VHIII families. Studies of the silent to replacement substitution ratios of these and other VH genes indicate that the number of silent substitutions found in immunoglobulin VH genes is lower than expected when compared with proteins such as preproinsulin and globin. Analysis of base composition reflected in these sequences indicates a marked increase of A-T% in the first and second codon positions of complementarity determining regions (CDR) which may be important in facilitating point mutations.
Full text
PDF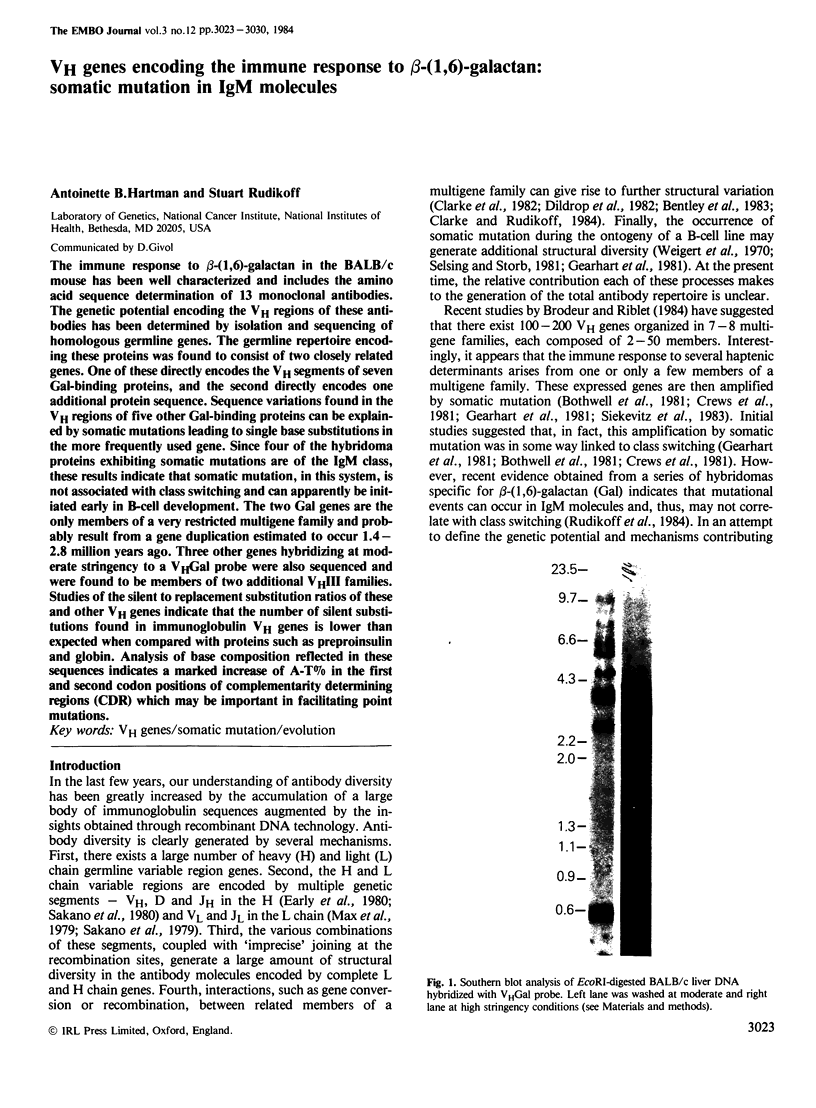



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bentley D. L., Rabbitts T. H. Evolution of immunoglobulin V genes: evidence indicating that recently duplicated human V kappa sequences have diverged by gene conversion. Cell. 1983 Jan;32(1):181–189. doi: 10.1016/0092-8674(83)90508-1. [DOI] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Bothwell A. L., Paskind M., Reth M., Imanishi-Kari T., Rajewsky K., Baltimore D. Heavy chain variable region contribution to the NPb family of antibodies: somatic mutation evident in a gamma 2a variable region. Cell. 1981 Jun;24(3):625–637. doi: 10.1016/0092-8674(81)90089-1. [DOI] [PubMed] [Google Scholar]
- Clarke S. H., Rudikoff S. Evidence for gene conversion among immunoglobulin heavy chain variable region genes. J Exp Med. 1984 Mar 1;159(3):773–782. doi: 10.1084/jem.159.3.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook W. D., Rudikoff S., Giusti A. M., Scharff M. D. Somatic mutation in a cultured mouse myeloma cell affects antigen binding. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1240–1244. doi: 10.1073/pnas.79.4.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews S., Griffin J., Huang H., Calame K., Hood L. A single VH gene segment encodes the immune response to phosphorylcholine: somatic mutation is correlated with the class of the antibody. Cell. 1981 Jul;25(1):59–66. doi: 10.1016/0092-8674(81)90231-2. [DOI] [PubMed] [Google Scholar]
- Dildrop R., Brüggemann M., Radbruch A., Rajewsky K., Beyreuther K. Immunoglobulin V region variants in hybridoma cells. II. Recombination between V genes. EMBO J. 1982;1(5):635–640. doi: 10.1002/j.1460-2075.1982.tb01220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Early P., Huang H., Davis M., Calame K., Hood L. An immunoglobulin heavy chain variable region gene is generated from three segments of DNA: VH, D and JH. Cell. 1980 Apr;19(4):981–992. doi: 10.1016/0092-8674(80)90089-6. [DOI] [PubMed] [Google Scholar]
- Gearhart P. J., Johnson N. D., Douglas R., Hood L. IgG antibodies to phosphorylcholine exhibit more diversity than their IgM counterparts. Nature. 1981 May 7;291(5810):29–34. doi: 10.1038/291029a0. [DOI] [PubMed] [Google Scholar]
- Givol D., Zakut R., Effron K., Rechavi G., Ram D., Cohen J. B. Diversity of germ-line immunoglobulin VH genes. Nature. 1981 Jul 30;292(5822):426–430. doi: 10.1038/292426a0. [DOI] [PubMed] [Google Scholar]
- Jolley M. E., Glaudemans C. P., Rudikoff S., Potter M. Structural requirements for the binding of derivatives of D-galactose to two homogeneous murine immunoglobulins. Biochemistry. 1974 Jul 16;13(15):3179–3184. doi: 10.1021/bi00712a028. [DOI] [PubMed] [Google Scholar]
- Jolley M. E., Rudikoff S., Potter M., Glaudemans C. P. Spectral changes on binding of oligosaccharides to murine immunoglobulin A myeloma proteins. Biochemistry. 1973 Jul 31;12(16):3039–3044. doi: 10.1021/bi00740a015. [DOI] [PubMed] [Google Scholar]
- Kocher H. P., Berek C., Jaton J. C. The immune response of BALB/c mice to phosphorylcholine is restricted to a limited number of VH- and VL-isotypes. Mol Immunol. 1981 Dec;18(12):1027–1033. doi: 10.1016/0161-5890(81)90018-3. [DOI] [PubMed] [Google Scholar]
- Loh D. Y., Bothwell A. L., White-Scharf M. E., Imanishi-Kari T., Baltimore D. Molecular basis of a mouse strain-specific anti-hapten response. Cell. 1983 May;33(1):85–93. doi: 10.1016/0092-8674(83)90337-9. [DOI] [PubMed] [Google Scholar]
- Max E. E., Seidman J. G., Leder P. Sequences of five potential recombination sites encoded close to an immunoglobulin kappa constant region gene. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3450–3454. doi: 10.1073/pnas.76.7.3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ollo R., Sikorav J. L., Rougeon F. Structural relationships among mouse and human immunoglobulin VH genes in the subgroup III. Nucleic Acids Res. 1983 Nov 25;11(22):7887–7897. doi: 10.1093/nar/11.22.7887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin P. Coding strategy differences between constant and variable segments of immunoglobulin genes. Nucleic Acids Res. 1984 Jul 11;12(13):5515–5527. doi: 10.1093/nar/12.13.5515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rechavi G., Bienz B., Ram D., Ben-Neriah Y., Cohen J. B., Zakut R., Givol D. Organization and evolution of immunoglobulin VH gene subgroups. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4405–4409. doi: 10.1073/pnas.79.14.4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudikoff S., Pawlita M., Pumphrey J., Heller M. Somatic diversification of immunoglobulins. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2162–2166. doi: 10.1073/pnas.81.7.2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudikoff S., Pawlita M., Pumphrey J., Mushinski E., Potter M. Galactan-binding antibodies. Diversity and structure of idiotypes. J Exp Med. 1983 Nov 1;158(5):1385–1400. doi: 10.1084/jem.158.5.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakano H., Hüppi K., Heinrich G., Tonegawa S. Sequences at the somatic recombination sites of immunoglobulin light-chain genes. Nature. 1979 Jul 26;280(5720):288–294. doi: 10.1038/280288a0. [DOI] [PubMed] [Google Scholar]
- Sakano H., Maki R., Kurosawa Y., Roeder W., Tonegawa S. Two types of somatic recombination are necessary for the generation of complete immunoglobulin heavy-chain genes. Nature. 1980 Aug 14;286(5774):676–683. doi: 10.1038/286676a0. [DOI] [PubMed] [Google Scholar]
- Selsing E., Storb U. Somatic mutation of immunoglobulin light-chain variable-region genes. Cell. 1981 Jul;25(1):47–58. doi: 10.1016/0092-8674(81)90230-0. [DOI] [PubMed] [Google Scholar]
- Sheppard H. W., Gutman G. A. Allelic forms of rat kappa chain genes: evidence for strong selection at the level of nucleotide sequence. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7064–7068. doi: 10.1073/pnas.78.11.7064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siekevitz M., Huang S. Y., Gefter M. L. The genetic basis of antibody production: a single heavy chain variable region gene encodes all molecules bearing the dominant anti-arsonate idiotype in the strain A mouse. Eur J Immunol. 1983 Feb;13(2):123–132. doi: 10.1002/eji.1830130207. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Weigert M. G., Cesari I. M., Yonkovich S. J., Cohn M. Variability in the lambda light chain sequences of mouse antibody. Nature. 1970 Dec 12;228(5276):1045–1047. doi: 10.1038/2281045a0. [DOI] [PubMed] [Google Scholar]
- Wilson A. C., Carlson S. S., White T. J. Biochemical evolution. Annu Rev Biochem. 1977;46:573–639. doi: 10.1146/annurev.bi.46.070177.003041. [DOI] [PubMed] [Google Scholar]