Abstract
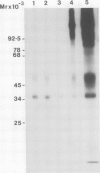
The interaction of phytohaemagglutinin (PHA) with the human T lymphocyte antigen receptor (Ti) was explored. Nonidet-P40 lysates of surface-labelled HPB-ALL cells were immunoprecipitated with PHA, using a rabbit anti-(PHA)-serum, as well as clonotypic monoclonal antibodies (H1-2D4 and T40/25) and a rabbit antiserum (R-43) against Ti. One- and two-dimensional SDS-polyacrylamide electrophoresis under reducing and non-reducing conditions showed that both the clonotypic antibodies and PHA precipitated a disulphide cross-linked heterodimer having a mol. wt. of approximately 79 000 (unreduced) and a comprising subunits of mol. wts. approximately 50 000 and 39 000 (reduced). Further evidence that PHA binds Ti was obtained by (i) cross-immunodepletion with H1-2D4 and PHA; (ii) immunoprecipitation with H1-2D4 of a glycoprotein fraction specifically eluted from a PHA immunoprecipitate; (iii) immunoprecipitation with PHA of a solubilised H1-2D4 immunoprecipitate; (iv) 2-D (non-equilibrium pH gradient electrophoresis/SDS) analyses of H1-2D4 and PHA immunoprecipitates, indicated that H1-2D4 and PHA recognise coincident beta polypeptides. PHA also binds a Ti-like disulphide cross-linked heterodimer on tonsil lymphocytes and two other T-cell leukaemias (HUT-78 and J6). The data further suggest that PHA and R-43 recognise a subpopulation of Ti molecules on HPB-ALL cells that are not bound by H1-2D4, suggesting that there may be at least two forms of Ti. Similar experiments indicate that Concanavalin A (Con A) and wheat germ agglutinin (WGA) also probably bind Ti, whereas Helix pomatia agglutinin (HPA) does not.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acuto O., Meuer S. C., Hodgdon J. C., Schlossman S. F., Reinherz E. L. Peptide variability exists within alpha and beta subunits of the T cell receptor for antigen. J Exp Med. 1983 Oct 1;158(4):1368–1373. doi: 10.1084/jem.158.4.1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan D., Crumpton M. J. Preparation and characterization of the plasma membrane of pig lymphocytes. Biochem J. 1970 Nov;120(1):133–143. doi: 10.1042/bj1200133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnstable C. J., Bodmer W. F., Brown G., Galfre G., Milstein C., Williams A. F., Ziegler A. Production of monoclonal antibodies to group A erythrocytes, HLA and other human cell surface antigens-new tools for genetic analysis. Cell. 1978 May;14(1):9–20. doi: 10.1016/0092-8674(78)90296-9. [DOI] [PubMed] [Google Scholar]
- Beverley P. C., Callard R. E. Distinctive functional characteristics of human "T" lymphocytes defined by E rosetting or a monoclonal anti-T cell antibody. Eur J Immunol. 1981 Apr;11(4):329–334. doi: 10.1002/eji.1830110412. [DOI] [PubMed] [Google Scholar]
- Boylston A. W., Goldin R. D., Moore C. S. A human T cell tumor which expresses the putative T cell antigen receptor. Eur J Immunol. 1984 Mar;14(3):273–275. doi: 10.1002/eji.1830140313. [DOI] [PubMed] [Google Scholar]
- Brown J. M., Leon M. A., Lightbody J. J. Isolation of a human lymphocyte mitogen from wheat germ with N-acetyl-D-glucosamine specificity. J Immunol. 1976 Nov;117(5 PT2):1976–1980. [PubMed] [Google Scholar]
- Burns G. F., Boyd A. W., Beverley P. C. Two monoclonal anti-human T lymphocyte antibodies have similar biologic effects and recognize the same cell surface antigen. J Immunol. 1982 Oct;129(4):1451–1457. [PubMed] [Google Scholar]
- Chang T. W., Kung P. C., Gingras S. P., Goldstein G. Does OKT3 monoclonal antibody react with an antigen-recognition structure on human T cells? Proc Natl Acad Sci U S A. 1981 Mar;78(3):1805–1808. doi: 10.1073/pnas.78.3.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ey P. L., Prowse S. J., Jenkin C. R. Isolation of pure IgG1, IgG2a and IgG2b immunoglobulins from mouse serum using protein A-sepharose. Immunochemistry. 1978 Jul;15(7):429–436. doi: 10.1016/0161-5890(78)90070-6. [DOI] [PubMed] [Google Scholar]
- Goding J. W., Harris A. W. Subunit structure of cell surface proteins: disulfide bonding in antigen receptors, Ly-2/3 antigens, and transferrin receptors of murine T and B lymphocytes. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4530–4534. doi: 10.1073/pnas.78.7.4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene W. C., Parker C. M., Parker C. W. Opposing effects of mitogenic and nonmitogenic lectins on lymphocyte activation. Evidence that wheat germ agglutinin produces a negative signal. J Biol Chem. 1976 Jul 10;251(13):4017–4025. [PubMed] [Google Scholar]
- Kahn C. R., Baird K. L., Jarrett D. B., Flier J. S. Direct demonstration that receptor crosslinking or aggregation is important in insulin action. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4209–4213. doi: 10.1073/pnas.75.9.4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammer G. M., Kurrasch R., Scillian J. J. Capping of the surface OKT3 binding molecule prevents the T-cell proliferative response to antigens: evidence that this molecule conveys the activation signal. Cell Immunol. 1984 Aug;87(1):284–294. doi: 10.1016/0008-8749(84)90152-7. [DOI] [PubMed] [Google Scholar]
- Kanellopoulos J. M., Wigglesworth N. M., Owen M. J., Crumpton M. J. Biosynthesis and molecular nature of the T3 antigen of human T lymphocytes. EMBO J. 1983;2(10):1807–1814. doi: 10.1002/j.1460-2075.1983.tb01662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappler J., Kubo R., Haskins K., Hannum C., Marrack P., Pigeon M., McIntyre B., Allison J., Trowbridge I. The major histocompatibility complex-restricted antigen receptor on T cells in mouse and man: identification of constant and variable peptides. Cell. 1983 Nov;35(1):295–302. doi: 10.1016/0092-8674(83)90232-5. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Meuer S. C., Acuto O., Hussey R. E., Hodgdon J. C., Fitzgerald K. A., Schlossman S. F., Reinherz E. L. Evidence for the T3-associated 90K heterodimer as the T-cell antigen receptor. Nature. 1983 Jun 30;303(5920):808–810. doi: 10.1038/303808a0. [DOI] [PubMed] [Google Scholar]
- Meuer S. C., Hodgdon J. C., Hussey R. E., Protentis J. P., Schlossman S. F., Reinherz E. L. Antigen-like effects of monoclonal antibodies directed at receptors on human T cell clones. J Exp Med. 1983 Sep 1;158(3):988–993. doi: 10.1084/jem.158.3.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuer S. C., Hussey R. E., Fabbi M., Fox D., Acuto O., Fitzgerald K. A., Hodgdon J. C., Protentis J. P., Schlossman S. F., Reinherz E. L. An alternative pathway of T-cell activation: a functional role for the 50 kd T11 sheep erythrocyte receptor protein. Cell. 1984 Apr;36(4):897–906. doi: 10.1016/0092-8674(84)90039-4. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Oppermann H., Levinson A. D., Varmus H. E., Levintow L., Bishop J. M. Uninfected vertebrate cells contain a protein that is closely related to the product of the avian sarcoma virus transforming gene (src). Proc Natl Acad Sci U S A. 1979 Apr;76(4):1804–1808. doi: 10.1073/pnas.76.4.1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen M. J., Kissonerghis A. M., Lodish H. F. Biosynthesis of HLA-A and HLA-B antigens in vivo. J Biol Chem. 1980 Oct 25;255(20):9678–9684. [PubMed] [Google Scholar]
- Palacios R. Mechanism of T cell activation: role and functional relationship of HLA-DR antigens and interleukins. Immunol Rev. 1982;63:73–110. doi: 10.1111/j.1600-065x.1982.tb00412.x. [DOI] [PubMed] [Google Scholar]
- Parker C. W., Dankner R. E., Falkenhein S. F., Greene W. C. Suggestive evidence for both stimulatory and inhibitory domains on human lymphocytes, as indicated by phospholipid turnover studies with wheat germ agglutinin and other lectins. Immunol Commun. 1976;5(1-2):13–25. doi: 10.3109/08820137609020609. [DOI] [PubMed] [Google Scholar]
- Van Wauwe J. P., De Mey J. R., Goossens J. G. OKT3: a monoclonal anti-human T lymphocyte antibody with potent mitogenic properties. J Immunol. 1980 Jun;124(6):2708–2713. [PubMed] [Google Scholar]
- Walsh F. S., Crumpton M. J. Orientation of cell-surface antigens in the lipid bilayer of lymphocyte plasma membrane. Nature. 1977 Sep 22;269(5626):307–311. doi: 10.1038/269307a0. [DOI] [PubMed] [Google Scholar]
- de Kretser T. A., Crumpton M. J., Bodmer J. G., Bodmer W. F. Demonstration of two distinct light chains in HLA-DR-associated antigens by two-dimensional gel electrophoresis. Eur J Immunol. 1982 Mar;12(3):214–221. doi: 10.1002/eji.1830120309. [DOI] [PubMed] [Google Scholar]