Abstract
Rapid access to enantioenriched spirocycles possessing a 1,4-dicarbonyl moiety spanning an all-carbon quaternary stereogenic spirocenter was achieved using a masked bromomethyl vinyl ketone reagent. The developed protocol entails an enantioselective palladium-catalyzed allylic alkylation reaction followed by a one-pot unmasking/RCM sequence that provides access to the spirocyclic compounds in good yields and selectivities.
Keywords: Allylic alkylation, Spirocycle, Asymmetric catalysis, Palladium catalysis, Masked bromomethyl vinyl ketone
Graphical Abstract
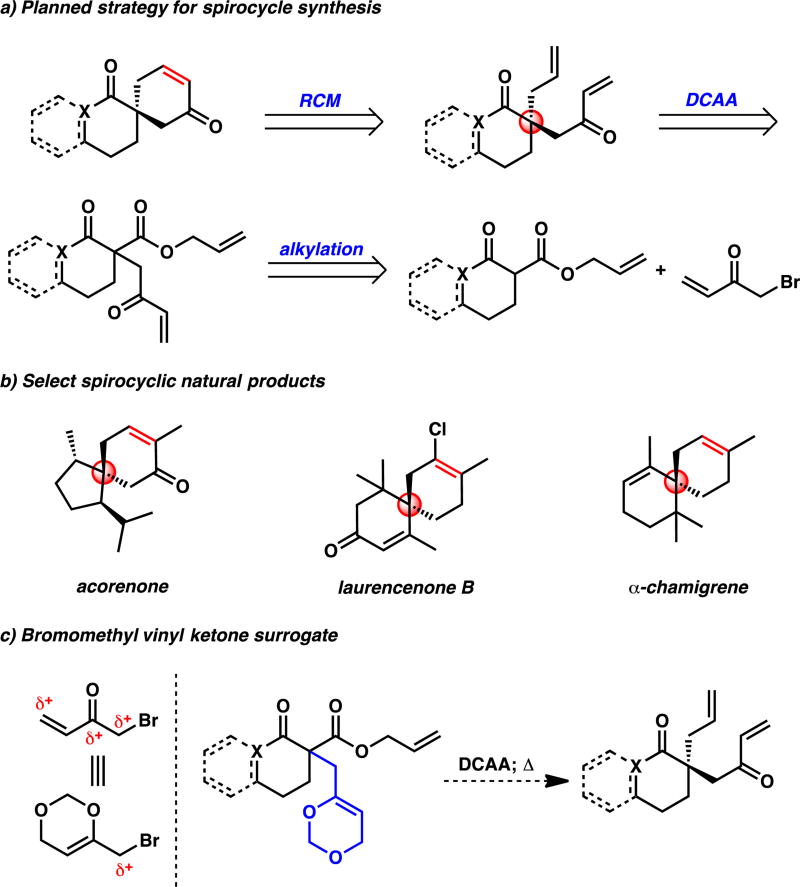
The widespread prevalence of spirocycles in biologically active molecules has inspired the development of many methods for the synthesis1 and, more recently, the enantioselective synthesis2 of this motif. During the course of our ongoing efforts in natural product synthesis, the preparation of an enantioenriched spirocyclic cyclohexenone derivative bearing both an all-carbon quaternary stereogenic spirocenter as well as a 1,4-dicarbonyl moiety spanning the spirocenter was required. This goal was challenging not only due to the difficulties in constructing 1,4-dicarbonyls,3 but also due to the inherent challenges of enantioselectively synthesizing an all-carbon quaternary stereocenter.4 As the enantioselective synthesis of all-carbon quaternary stereocenters via palladium-catalyzed allylic alkylation has been developed extensively by our group,5 we envisioned that rapid entry to the spirocyclic cyclohexenone framework could be achieved if the olefin was disconnected via a ring-closing metathesis reaction (RCM) and the resultant α-quaternary carbonyl derivative could be synthesized asymmetrically via our allylic alkylation methodology (Figure 1a). In addition to the application to our own synthetic endeavor, we imagined that this strategy would be amenable to the synthesis of a wide array of all-carbon quaternary spirocyclic compounds, such as acorenone, laurencenone B, and α-chamigrene (Figure 1b).6 However, this plan hinged on the challenging use of bromomethyl vinyl ketone as an alkylating reagent.
Figure 1.
Strategy and inspiration for the catalytic enantioselective synthesis of all-carbon quaternary spirocycles.
Nucleophilic addition to bromomethyl vinyl ketone can be problematic due to the three electrophilic positions on the molecule, which include positions for Michael addition, 1,2-addition, and SN2 displacement (Figure 1c, left). As a solution to this issue, Funk has developed the use of 6-(bromomethyl)-4H-1,3-dioxin as a bromomethyl vinyl ketone surrogate (Figure 1c, left).7 Following alkylation, the dioxin functionality of this reagent can be unmasked under thermal conditions to release formaldehyde and reveal the latent enone. Therefore, we envisioned that we could obviate the challenges of using bromomethyl vinyl ketone by utilizing Funk’s dioxin reagent in our planned strategy (Figure 1c, right). However, the use of a substrate bearing such a bulky substituent with Lewis basic oxygens had not yet been explored in our palladium-catalyzed allylic alkylation chemistry.
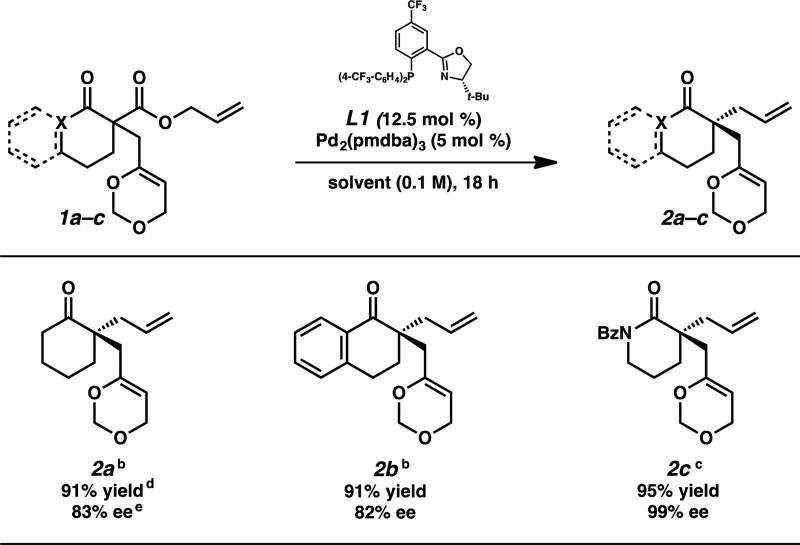
Fortuitously, we rapidly discovered that the standard conditions developed by our group for palladium-catalyzed allylic alkylation reactions were adaptable to this new substrate class (Table 1). The use of a catalyst prepared from Pd2(pmdba)3 and (S)-(CF3)3-t-Bu-PHOX (L1) provided access to a variety of dioxin-substituted allylic alkylation products in consistently high yields and enantioselectivities. Cyclohexanone 2a was obtained in 91% yield and 83% ee. Moreover, tetralone 2b was afforded in similarly high yield and selectivity, and we were pleased to find that lactam 2c could be accessed in an excellent 95% yield and 99% ee. Based on these results in combination with the previously established trends in our palladium-catalyzed allylic alkylation methodology,5 we infer that the masked methyl vinyl ketone substituent should be broadly applicable to all of the ring systems tolerated in this chemistry.
Table 1.
Enantioselective Palladium-Catalyzed Allylic Alkylations with Substrates Bearing a Masked Methyl Vinyl Ketone.a
Reactions performed on 0.2 mmol scale.
Performed using THF at 23 °C.
Performed using toluene at 40 °C.
Isolated yield.
Determined by chiral HPLC or SFC.
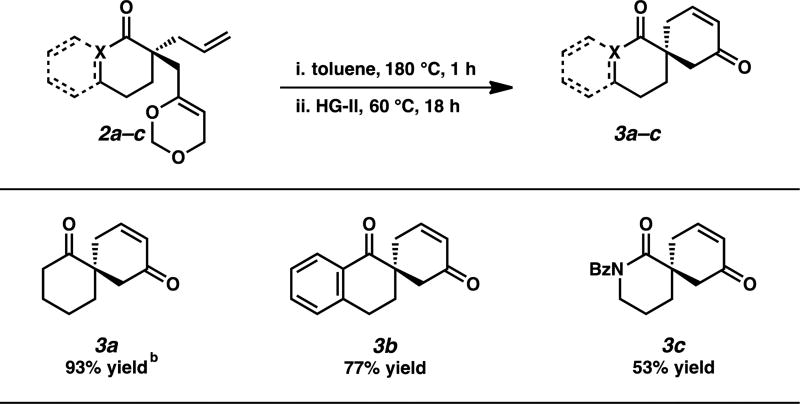
With the feasibility of utilizing substrates bearing a masked methyl vinyl ketone functionality in our allylic alkylation chemistry established, we moved to demonstrate the ease with which this strategy can provide access to the desired spirocyclic compounds. Though the masked methyl vinyl ketone synthon has been shown to provide access to bridged and fused bicycle systems,7 to the best of our knowledge, the utility of this reagent for the synthesis of spirocycles has yet to be demonstrated. We were pleased to find that the planned thermal unmasking/RCM sequence proceeded smoothly in a single reaction vessel. In this procedure, dioxin 2 is unmasked via heating in toluene at 180 °C for one hour, whereupon the reaction is cooled to 60 °C and a solution of Hoveyda-Grubbs second-generation catalyst is introduced to complete the annulation. Using this newly developed protocol, spirocyclic cyclohexenones 3a, 3b, and 3c were obtained in good to excellent yields, thus demonstrating the viability of this strategy for the synthesis of enantioenriched spirocycles.
In summary, we have demonstrated that substrates bearing a bulky, highly oxygenated methyl vinyl ketone surrogate can be utilized in an enantioselective palladium-catalyzed allylic alkylation reaction. The resulting allylic alkylation products are obtained in high yields and selectivities with neither the increased sterics nor the added Lewis basic oxygen atoms adversely affecting reactivity. Furthermore, we developed a one-pot unmasking/RCM procedure showcasing that these allylic alkylation products can be easily advanced to enantioenriched spirocycles bearing both an all-carbon quaternary stereogenic spirocenter as well as a 1,4-dicarbonyl functionality spanning the spirocenter. This simple two-step strategy is amenable to the synthesis of a range of enantioenriched spirocyclic natural products; further results in this area will be reported in due course.
Supplementary Material
Table 2.
One-Pot Synthesis of Spirocyclic Compounds.a
Reactions performed on 0.1 mmol scale.
Isolated yield.
Highlights.
Rapid access to enantioenriched spirocycles possessing a 1,4-dicarbonyl moiety spanning an all-carbon quaternary stereogenic spirocenter was achieved using a masked bromomethyl vinyl ketone reagent. The developed protocol entails an enantioselective palladium-catalyzed allylic alkylation reaction followed by a one-pot unmasking/RCM sequence that provides access to the spirocyclic compounds in good yields and selectivities.
Acknowledgments
The NIH-NIGMS (R01GM080269) and Caltech are thanked for support of our research program. J.C.H. thanks the Camille and Henry Dreyfus postdoctoral program, and S.E.S. thanks the NIH-NIGMS for a predoctoral fellowship (F31GM120804). Dr. Mona Shahgholi and Naseem Torian (Caltech) are thanked for mass spectrometry assistance. Dr. Scott Virgil (Caltech) is thanked for assistance with instrumentation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.For reviews on the synthesis of spirocycles, see: Krapcho AP. Synthesis. 1974:383–419.Sannigrahi M. Tetrahedron. 1999;55:9007–9071.Pradhan R, Patra M, Behera AK, Mishra BK, Behera RK. Tetrahedron. 2006;62:779–828.Kotha S, Deb AC, Lahiri K, Manivannan E. Synthesis. 2009:165–193.Zhuo C-X, Zheng C, You S-L. Acc. Chem. Res. 2014;47:2558–2573. doi: 10.1021/ar500167f.D’yakonov VA, Trapeznikova OA, de Meijere A, Dzhemilev UM. Chem. Rev. 2014;114:5775–5814. doi: 10.1021/cr400291c.
- 2.For reviews on the enantioselective synthesis of spirocycles, see: Rios R. Chem. Soc. Rev. 2012;41:1060–1074. doi: 10.1039/c1cs15156h.Franz AK, Hanhan NV, Ball-Jones NR. ACS Catal. 2013;3:540–553.Ball-Jones NR, Badillo JJ, Franz AK. Org. Biomol. Chem. 2012;10:5165–5181. doi: 10.1039/c2ob25184a.Trost BM, Brennan MK. Synthesis. 2009:3003–3025.Cheng D, Ishihara Y, Tan B, Barbas CF., III ACS Catal. 2014;4:743–762.
- 3.(a) Arason KM, Bergmeier SC. Org. Prep. Proced. Int. 2002;34:337–366. [Google Scholar]; (b) DeMartino MP, Chen K, Baran PS. J. Am. Chem. Soc. 2008;130:11546–11560. doi: 10.1021/ja804159y. [DOI] [PubMed] [Google Scholar]
- 4.For reviews on the synthesis of quaternary stereocenters, see: Douglas CJ, Overman LE. Proc. Natl. Acad. Sci. USA. 2004;101:5363–5367. doi: 10.1073/pnas.0307113101.Das JP, Marek I. Chem. Commun. 2011;47:4593–4623. doi: 10.1039/c0cc05222a.Quasdorf KW, Overman LE. Nature. 2014;516:181–191. doi: 10.1038/nature14007.Corey EJ, Guzman-Perez A. Angew. Chem. Int. Ed. 1998;37:388–401. doi: 10.1002/(SICI)1521-3773(19980302)37:4<388::AID-ANIE388>3.0.CO;2-V.Christoffers J, Mann A. Angew. Chem. Int. Ed. 2001;40:4591–4597. doi: 10.1002/1521-3773(20011217)40:24<4591::aid-anie4591>3.0.co;2-v.Trost BM, Jiang C. Synthesis. 2006:369–396.Liu Y, Han S-J, Liu W-B, Stoltz BM. Acc. Chem. Res. 2015;48:740–751. doi: 10.1021/ar5004658.
- 5.(a) Craig RA, II, Loskot SA, Mohr JT, Behenna DC, Harned AM, Stoltz BM. Org. Lett. 2015;17:5160–5163. doi: 10.1021/acs.orglett.5b02376. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Numajiri Y, Jiménez-Osés G, Wang B, Houk KN, Stoltz BM. Org. Lett. 2015;17:1082–1085. doi: 10.1021/ol503425t. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Reeves CM, Eidamshaus C, Kim J, Stoltz BM. Angew. Chem. Int. Ed. 2013;52:6718–6721. doi: 10.1002/anie.201301815. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Bennett NB, Duquette DC, Kim J, Liu W-B, Marziale AN, Behenna DC, Virgil SC, Stoltz BM. Chem. Eur. J. 2013;19:4414–4418. doi: 10.1002/chem.201300030. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Behenna DC, Liu Y, Yurino T, Kim J, White DE, Virgil SC, Stoltz BM. Nat. Chem. 2012;4:130–133. doi: 10.1038/nchem.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Behenna DC, Mohr JT, Sherden NH, Marinescu SC, Harned AM, Tani K, Seto M, Ma S, Novák Z, Krout MR, McFadden RM, Roizen JL, Enquist JA, White DE, Levine SR, Petrova KV, Iwashita A, Virgil SC, Stoltz BM. Chem. Eur. J. 2011;17:14199–14223. doi: 10.1002/chem.201003383. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Behenna DC, Stoltz BM. J. Am. Chem. Soc. 2004;126:15044–15045. doi: 10.1021/ja044812x. [DOI] [PubMed] [Google Scholar]
- 6.Smith LK, Baxendale IR. Org. Biomol. Chem. 2015;13:9907–9933. doi: 10.1039/c5ob01524c. [DOI] [PubMed] [Google Scholar]
- 7.Greshock TJ, Funk RL. J. Am. Chem. Soc. 2002;124:754–755. doi: 10.1021/ja0123554. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.