Abstract
Objective
Prenatal exposures are known to alter fetal neurodevelopment and autonomic control. We aimed to explore the correlation between fetal autonomic activity, measured by fetal heart rate variability, and 18-month developmental outcome in subjects with congenital heart disease.
Study Design
From 2010–2013, 5 fetuses with hypoplastic left heart syndrome, 9 with transposition of the great arteries, and 9 with tetralogy of Fallot were included in this prospective cohort study. A maternal abdominal fetal electrocardiogram monitor recorded fetal heart rate at 34–38 weeks gestational age. We assessed associations between fetal heart rate parameters including interquartile range and standard deviation of the fetal RR intervals and 18-month Bayley Scales of Infant Development III scores using Pearson’s correlation coefficient. Multivariable regression modeling identified predictors of neurodevelopmental scores.
Results
Fetal heart rate variability parameters at 34–38 weeks gestational age correlated with 18-month Cognition (r=0.47, p=0.03) and Motor scores (r=0.66, p=0.001). The interquartile range of the fetal RR intervals predicted Cognition (β=0.462, p=0.028, R2=0.282) and Motor (β=0.637, p<0.001, R2=0.542) scores.
Conclusions
In fetuses with congenital heart disease, low heart rate variability at 34–38 weeks gestational age predicts diminished 18-month cognitive and motor performance. Prenatal autonomic activity may serve as a marker of early childhood development in these high-risk patients.
Introduction
The autonomic nervous system drives the cardiovascular response to physiologic challenges in the form of changes in heart rate and blood pressure. Markers of autonomic regulation, including heart rate (HR) and HR variability, have been associated with impaired neurologic outcomes in fetuses and newborns exposed to stressors including prenatal alcohol and maternal depression (1,2, 3). Early profiles of autonomic function have been associated with postnatal outcomes in fetuses with medical conditions such as low birth weight, neurological lesions, as well as healthy subjects (4, 5, 6). Maeda et al measured fetal HR and movement in 12 fetuses with central nervous system lesions and 14 normal fetuses at 28 to 38 weeks gestational age. Actocardiographic indices predicted the severity of fetal central nervous system lesions (6). Bornstein et al found that 30 and 36 week gestation healthy fetuses with higher HR variability achieved higher levels of symbolic play at 27 months (7). DiPietro et al measured fetal HR variability between 20 and 38 weeks gestational age in healthy subjects. The study found that HR variability beginning at 28 weeks gestation measured using a Doppler ultrasound cardiotocography monitor, correlated with 2-year infant mental and psychomotor development and 2.5-year infant language development (8).
In our study, we used an innovative technique to measure fetal HR: a maternal abdominal fetal electrocardiogram (fECG). This monitor uses an increased sampling frequency compared with Doppler which leads to more precise and accurate measurements of RR intervals, providing HR variability measurements not attainable in prior studies (9).
Our research population is also unique as we focused on fetuses with congenital heart disease (CHD). We previously reported, using this same cohort, fetuses with CHD demonstrate altered autonomic regulation as measured by HR variability (10). We hypothesized that structural heart disease and the hemodynamic changes that accompany it serve as a form of antenatal stressor. Infants with CHD also have been found to have abnormal HR variability and decreased HR response likely resulting from a combination of fetal, preoperative, and surgical factors (11). In this study, we specifically assessed fetal markers. The utility of autonomic markers as predictors of neurodevelopment in the CHD population has not yet been reported. We aimed to investigate the association between autonomic regulation in fetuses with CHD and 18-month neurodevelopment. We hypothesized that CHD fetuses with decreased HR variability would demonstrate lower 18-month neurodevelopmental scores.
Materials/subjects and Methods
Participants
Fetuses with hypoplastic left heart syndrome (HLHS), transposition of the great arteries (TGA), or tetralogy of Fallot (TOF) diagnosed on fetal echocardiogram were recruited in this prospective observational cohort study performed at Morgan Stanley Children’s Hospital of New York Presbyterian in New York City. Enrollment of pregnant mothers took place between 11/2010 and 3/2013.
Participants were less than 24-weeks gestational age at enrollment. Exclusion criteria included multiple gestation, evidence of chromosomal abnormalities, structural brain malformations, placental insufficiency, intrauterine growth retardation or sustained cardiac arrhythmia.
Neurodevelopmental assessments were conducted at 18 months using the Bayley Scales of Infant Development-III (BSID-III). The Columbia University Medical Center Institutional Review Board approved this study. Written informed consent was obtained from pregnant mothers.
Measures
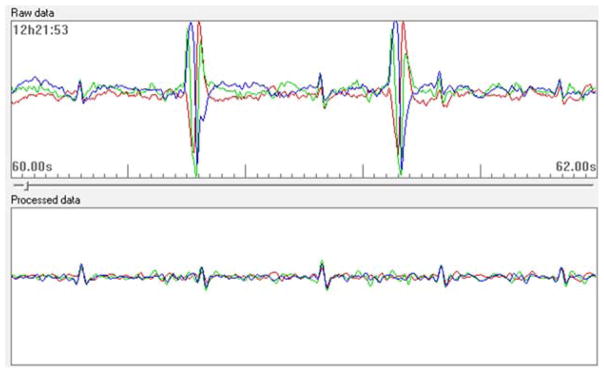
Fetal HR and HR variability were recorded non-invasively using the Monica AN24, a maternal abdominal fECG monitor. The Monica AN24 has been validated in multiple studies in its ability to extract fetal ECG from maternal abdominal recordings and thereby assess fetal heart rate parameters (12, 13, 14). This technology derives heart rate from R-R intervals of the fetal HR collected at a sampling rate of 300Hz with 13 bit precision (Figure 1). By comparison, traditional Doppler recordings provide fetal HR and HR variability based on averaged fetal HR derived from ultrasound detection of heart movements rather than R-R intervals computed from the raw ECG signals. Recordings were carried out in a quiet room with participants lying in the left lateral position at approximately 15 degrees, as recommended by Bamber et al to minimize the risk of aortocaval compression (15). Four electrodes were placed in a diamond-shaped pattern on the maternal abdomen: two placed in a straight line at the height of the umbilicus, one 3 cm above the umbilicus and one approximately 5 cm above the symphysis pubis. A fifth electrode was placed laterally to this arrangement and used for electrical reference. In order to reduce electrical impedance, the skin was lightly abraded to remove superficial dry squamous cells. The 5 electrodes were then connected to the Monica AN24 monitor that was then secured to the maternal abdominal wall with an elastic band to standardize placement of the device across studies. Fetal ECG recordings were scheduled to be 50 minutes in duration in an attempt to capture sufficient representation of the quiet and active sleep states. In addition to R-R intervals, two robust time domain measures of fetal autonomic variability were calculated from fECG data: 1) interquartile range of the fetal RR intervals (IQR) and 2) standard deviation of the fetal RR intervals (SD).
Figure 1.
Sample of Monica AN24 abdominal monitor raw signal. This sample demonstrates the raw fetal ECG data obtained from the monitor with each color representing output from a different electrode.
We recorded fetal ECG at 34–38 weeks gestational age. Fetal state strongly influences fetal HR in late gestation. Fetal sleep states emerge in the third trimester as an early indicator of neural maturation (16, 17). There are four fetal state assignments: quiet awake, quiet asleep, active awake, active asleep (17). We used established methods to measure sleep states using HR variability patterns and focused solely on the most frequent fetal state, active sleep in our analysis (17, 18). These methods require a minimum period of 3 continuous minutes of the same HR variability pattern to be assigned a state classification.
A certified psychologist assessed neurodevelopment at 18 months of age using the BSID-III. This assessment provides three summary scores: Cognitive, Language, and Motor.
Statistics
Pearson’s correlation coefficient tested associations between fetal HR variability and 18-month BSID-III scores. Univariate and multivariable regression modeling identified fetal predictors of neurodevelopmental score and included the potential covariates sex and gestational age at time of fetal testing. Potential predictor variables were entered simultaneously into the models. CHD subgroup analysis was conducted using dummy variables, whereby the TGA and TOF groups were compared with the reference group of HLHS.
Results
We enrolled 32 CHD fetuses: 11 with HLHS, 11 with d-TGA, and 12 with TOF. One patient did not survive the newborn period. After data quality analysis, a total of 23 fetuses were included in the analysis. The subject attrition resulted from recording sessions with poor signal quality or insufficient data defined by less than 30% continuous epochs of fetal active sleep (n=8). The final sample of fetuses with high data quality included 5 HLHS fetuses, 9 d-TGA fetuses, and 9 TOF fetuses. There were 12 male subjects and 11 female subjects. Mothers self-identified as White Non-Hispanic (N=12), Black Non-Hispanic (N=3), White Hispanic (N=4), Other Hispanic (N=2). Two patients declined to respond. The average gestational age of the CHD fetuses was 36.3 weeks (range 34–38.7) when the fetal heart rate measures were obtained. The average gestational age at birth was 38.6 weeks (range 35.6–40.1).
Our intention was for the recordings to be 50 minutes in length in order to allow for the capture of all fetal states. However, the recordings could not always reach this duration as a result of the time constraints on the pregnant subjects who also had other clinical appointments. The average recording was 39.3 minutes in duration with a range of 22 to 49 minutes with one study lasting 115 minutes.
Correlation of HR Variability Parameters with 18-month Bayley Scores
Results of IQR and SD as well as 18-month Bayley scores were assessed by diagnostic group (Table 1). The IQR of the fetal RR intervals at 34–38 weeks GA correlated with 18-month Cognition and Motor scores (Figure 2 and 3). Gestational age at time of testing was included as a covariate in the analysis (Table 2). The multivariate regression analysis found no association between gestational age at birth and the 18-month Bayley scores for cognition, motor, or language.
Table 1.
Heart rate variability parameters and 18-month Bayley Scores by congenital heart disease subgroup at 34–38 weeks gestational age. ANOVA of the CHD diagnostic subgroups found no significant correlation between subgroup and 18-month cognition, language, or motor scores.
| Diagnosis | Mean HR (beats per min) | SD RRi (sec) | IQR RRi (sec) | Cognition Score | Language Score | Motor Score |
|---|---|---|---|---|---|---|
| HLHS | 137.03 | 0.0168 | 0.0176 | 30.2 | 22.2 | 27.6 |
| TGA | 139.10 | 0.0174 | 0.025 | 52.6 | 39.3 | 44.3 |
| TOF | 138.61 | 0.0165 | 0.0229 | 43.4 | 37.3 | 44.2 |
| ANOVA p value | 0.127 | 0.187 | 0.083 |
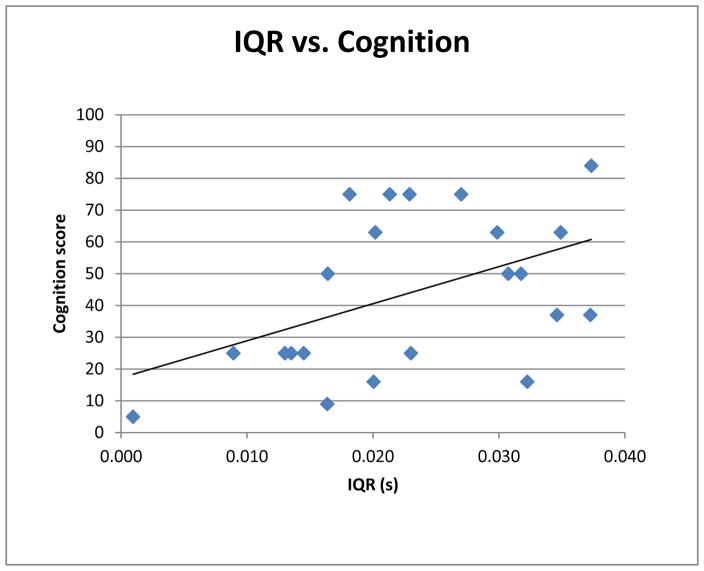
Figure 2.
Correlation between fetal heart rate variability at 34–38 weeks gestational age and 18-month Bayley Scales of Infant Development-III Cognition Scores. The interquartile range of the fetal RR intervals correlated significantly with 18-month cognition scores (p=0.03, r=0.47).
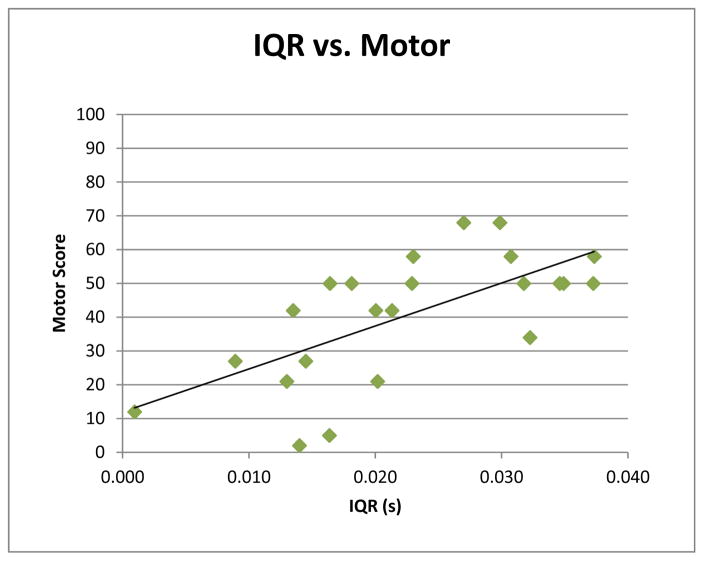
Figure 3.
Correlation between fetal heart rate variability at 34–38 weeks gestational age and 18-month Bayley Scales of Infant Development-III Motor Scores. The interquartile range of the fetal RR intervals significantly correlated with 18-month motor scores (p=0.001, r=0.66).
Table 2.
Regression models are presented for 18-month Bayley Score of Infant Development-III cognition and motor scores and 1) IQR, 2) IQR and gestational age at fetal testing, and 3) IQR and CHD subgroups (HLHS compared with TOF, and TGA compared with TOF). The regression models show that IQR predicts infant cognition and motor scores. In the multivariable model including IQR and dummy variables for CHD subgroups, IQR continued to predict infant motor scores.
| Cognition | Motor | |||||
|---|---|---|---|---|---|---|
| R square | Beta | p-value | R square | Beta | p-value | |
| IQR | 0.217 | 0.466 | 0.029 | 0.441 | 0.664 | 0.001 |
| IQR and GA at fetal testing | 0.282 | 0.462 | 0.028 | 0.542 | 0.637 | <0.001 |
| Gestation | 0.256 | 0.20 | 0.319 | 0.05 | ||
| IQR using dummy variables for CHD subgroups | 0.299 | 0.412 | 0.08 | 0.567 | 0.608 | 0.001 |
| HLHS | −0.094 | 0.69 | −0.180 | 0.32 | ||
| TGA | 0.082 | 0.72 | −0.084 | 0.63 | ||
IQR predicted Cognition (β=0.462, p=0.028, R2=0.282) and Motor (β=0.637, p<0.001, R2=0.542) scores. Sex was not a significant covariate in the multivariate regression analysis, however gestational age at test was. Of note, mean fetal HR and SD of the fetal RR intervals did not significantly correlate with 18-month Bayley scores. No significant correlation between fetal measures and 18-month language scores was found.
CHD Subgroup Analysis
Despite the small sample sizes of the CHD subgroups, we evaluated the effect of CHD diagnosis on the relationship between IQR at 34–38 weeks gestational age and 18-month BSID-III scores using dummy variables. When comparing TGA and TOF with HLHS, the association between IQR and Cognition was no longer significant, indicating that the diagnostic group assignment impacts the association between fetal HR variability and 18-month outcome. In contrast, the relationship between IQR and 18-month Motor scores remained significant. However, the strength of the association between IQR and Motor (β =0.608, p=0.001, R2 =0.567) decreased.
Discussion
Our study found that in CHD fetuses, low HR variability at 34–38 weeks of pregnancy predicts poorer 18-month cognitive and motor performance. These results support prior findings that trajectories in fetal HR variability are correlated with developmental outcomes of fetuses exposed to antenatal stressors (8, 19, 20). This is the first study to show that measures of autonomic regulation late in gestation predict neurodevelopmental outcomes in fetuses with CHD. There are undoubtedly a multitude of pre- and postnatal variables that impact neurodevelopmental outcomes. Despite these factors, we were able to elucidate a late gestation prenatal marker for short-term CHD neurodevelopmental outcomes. Furthermore, these fetal heart rate variability measures account for a greater amount of explained variance in neurodevelopment than previously reported in other high-risk groups.
These findings suggest that the anatomic defects associated with CHD may impact HR variability during late gestation as well as post-gestational neurodevelopment. In a separate cohort, Williams et al. demonstrated fetuses with CHD experience altered cerebral blood flow as measured by middle cerebral artery resistance, and that this altered resistance correlates with lower 18-month developmental scores (21, 22). Alterations in cerebral blood flow caused by the anatomic cardiac defects may affect brain development. Alternatively, changes in fetal cerebrovascular resistance may be secondary to an underlying process that also affects brain development. Similarly, in the case of altered HR variability, the heart defect can either directly alter autonomic variability through changes in hemodynamics, or, the mechanism that causes the cardiac anomaly may also affect autonomic nervous system development. Our working hypothesis is that diminished fetal HR variability is the consequence of abnormal brainstem development, the primary brain region responsible for autonomic regulation. This may be related to the alterations in blood flow to the brain, perhaps mediated by placental function, seen in the setting of CHD. Alternatively, there could be some common genetic dysregulation of heart and brain development that could also explain some of the variance in neurodevelopmental outcomes.
Our findings documented a significant correlation between HR variability at 34–38 weeks gestational age and 18-month developmental outcomes in CHD fetuses. In another study by our team comparing HR variability parameters in control (non-CHD) and CHD fetuses, SD and IQR were found to be significantly decreased in CHD fetuses only after 34 weeks gestation. These findings suggest the normal trend of increased autonomic control with increasing gestational age is altered and may be related to the pathway that results in delayed neurodevelopmental outcomes in infancy. Studies have noted a five-fold increase in white-matter between 35 and 41 weeks gestation as well as increased synaptic formation during this period (23). Therefore, prenatal stressors, such as CHD, or a shared genetic influence on heart and brain defects may be most likely to demonstrate a significant effect in this late gestational window of rapid cerebral growth.
Our findings show that fetuses with HLHS may demonstrate the strongest relationship between HR variability and neurodevelopmental outcomes. Among the CHD subgroups studied, children with HLHS evidence the most severe forms of neurodevelopmental impairment (20). In comparison with TGA and TOF fetuses, HLHS fetuses have only a single cardiac ventricle and atresia or hypoplasia of the ascending aorta that normally delivers oxygenated blood to the fetal brain. The limitation of cerebral oxygen delivery has been proposed as a reason for impaired neurodevelopmental outcomes in this CHD subgroup. Further studies with larger sample sizes of CHD fetuses are needed to elucidate these findings.
The effect size of the association between fetal IQR and 18-month cognition and motor scores is larger than that reported in prior studies (8). Reasons for this difference could be related to the improved fetal HR recording precision with the non-invasive fECG monitor providing superior measurement of beat-to-beat variability. We utilized the commercially available Monica AN24 fECG monitor that allowed for precise measurement of cardiac intervals. This device has been found to be an alternative to other fetal electrographic monitors (13). With further validation, non-invasive maternal abdominal fECG monitors could be used to identify fetuses at higher risk for poor neurodevelopmental outcomes. Identifying at-risk CHD fetuses could facilitate the early initiation of developmental therapies as well as inform education of families, which have both been found to improve developmental outcomes (24).
There were limitations in this study. The sample size of CHD fetuses was modest and therefore CHD subgroup analysis was under powered. Further investigation aims to assess differences between CHD subgroups in a larger multi-center sample. Moreover, the data quality initially obtained was variable and some early collections provided inadequate data. In our study, all the recordings were in active sleep. Lengthier recording times that included multiple active and quiet fetal states would help address data loss issues and increase the power to detect differences in HR variability patterns. Since the culmination of this study, we are finding that overnight fetal studies provide more stable estimates in multiple fetal states as well as improve the quality of recordings. Repeated and longer duration recordings would also allow consideration of additional linear and non-linear assessments of fetal HR variability patterns. Similarly, measurement over time of recurrent fetal activity patterns coupled to changes in HR derived from non-invasive abdominal recordings could provide another tool for uncovering fetal antecedents of neurodevelopmental outcomes (25).
Conclusion
HR variability in late gestation CHD fetuses predicts 18-month neurodevelopmental scores. Non-invasive measurement of fetal HR using a maternal abdominal fECG monitor can be used as an early biomarker for risk of adverse neurodevelopmental outcomes in this and perhaps other high-risk populations.
Acknowledgments
We thank Christina Wiess BA (Columbia University Medical Center Department of Pediatric Cardiology) for her assistance with data collection and management. We are grateful to Natalie H. Brito PhD (NYS Psychiatric Institute) for her guidance and technical assistance with data analysis.
Statement of Financial Support: IA Williams received support from Grant #1K23HD061601 from the NICHD/NIH as well as from Grant # KL2 RR024157 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. These sources contributed to the design and conduct of this study as well as data collection. WP Fifer received support from NIH grant R37 HD032774 which contributed to data analysis, management, and interpretation.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest relevant to this article to disclose.
References
- 1.Fifer WP, Fingers ST, Youngman M, Gomez-Gribben E, Myers MM. Effects of alcohol and smoking during pregnancy on infant autonomic control. Dev Psychobiol. 2009;51(3):234–242. doi: 10.1002/dev.20366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoyer D, Heinicke E, Jaekel S, Tetschke F, Paolo DD, Haueisen J, et al. Indices of fetal development derived from heart rate patterns. Early Hum Dev. 2009;85:379–386. doi: 10.1016/j.earlhumdev.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 3.Sandman CA, Cordova CJ, Davis EP, Glynn LM, Buss C. Patterns of fetal heart rate response at ~30 weeks gestation predict size at birth. J Dev Orig Health Dis. 2011;2(4):212–217. doi: 10.1017/S2040174411000250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.May LE. Effect on postnatal health and beyond. Physiology of prenatal exercise and fetal development. SpringerBriefs in Physiology. 2012:19–23. [Google Scholar]
- 5.Nijhuis JG. Development of fetal heart rate and behavior: Indirect measures to assess the fetal nervous system. Eur J of Obstet, Gynecol, and Reprod Biol. 1999;87:1–2. doi: 10.1016/s0301-2115(99)00143-8. [DOI] [PubMed] [Google Scholar]
- 6.Maeda K, Morokuma S, Yoshida S, Ito T, Pooh R, Serizawa M. Fetal behavior analyzed by ultrasonic actocardiogram in cases with central nervous system lesions. J Perinat Med. 2006;34:398–403. doi: 10.1515/JPM.2006.079. [DOI] [PubMed] [Google Scholar]
- 7.Bornstein MH, DiPietro JA, Hahn CS, Painter K, Haynes OM, Costigan KA. Prenatal cardiac function and postnatal cognitive development: An exploratory study. Infancy. 2002;3:475–494. [Google Scholar]
- 8.DiPietro JA, Bornstein MH, Hahn CS, Costigan K, Achy-Brou A. Fetal heart rate and variability: stability and prediction to developmental outcomes in early childhood. Child Dev. 2007;78(6):1788–98. doi: 10.1111/j.1467-8624.2007.01099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen W, Ommani S, Hassan S, Mirza FG, Solomon M, Brown R, et al. Accuracy and reliability of fetal heart rate monitoring using maternal abdominal surface electrodes. Acta Obstet Gynecol Scand. 2012;91:1306–1313. doi: 10.1111/j.1600-0412.2012.01533.x. [DOI] [PubMed] [Google Scholar]
- 10.Siddiqui S, Wilpers A, Myers M, Nugent JD, Fifer WP, Williams IA. Autonomic regulation in fetuses with congenital heart disease. Early Hum Dev. 2015;91(3):195–8. doi: 10.1016/j.earlhumdev.2014.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaltman JR, Hanna BD, Gallagher PR, Gaynor JW, Godinez RI, Tanel RE, et al. Heart rate variability following neonatal heart surgery for complex congenital heart disease. Pacing and Clin Electrophysiol. 2006;29:471–478. doi: 10.1111/j.1540-8159.2006.00378.x. [DOI] [PubMed] [Google Scholar]
- 12.Reinhard J, Hayes-Gill B, Yi Q, Hatzmann H, Schiermeier S. Comparison of non-invasive fetal electrocardiogram to Doppler cardiotocogram during the 1st stage of labor. J Perinat Med. 2010;38:179–185. doi: 10.1515/jpm.2010.025. [DOI] [PubMed] [Google Scholar]
- 13.Graatsma E, Jacod B, Van Egmond L, Mulder E, Visser G. Fetal electrocardiography: feasibility of long-term fetal heart rate recordings. BJOG. 2009;116:334–338. doi: 10.1111/j.1471-0528.2008.01951.x. [DOI] [PubMed] [Google Scholar]
- 14.Seliger G, Stenzel A, Kowalski EM, Hoyer D, Nowack S, Seeger S, et al. Evaluation of standardized, computerized Dawes/Redman heart-rate analysis based on different recording methods and in relation to fetal beat-to-beat heart rate variability. J Perinat Med. 2015 doi: 10.1515/jpm-2015-0169. [DOI] [PubMed] [Google Scholar]
- 15.Bamber JH, Dresner M. Aortocaval Compression in Pregnancy: The Effect of Changing the Degree and Direction of Lateral Tilt on Maternal Cardiac Output. Anesthesia & Analgesia. 2003;97(1):256–258. doi: 10.1213/01.ane.0000067400.79654.30. [DOI] [PubMed] [Google Scholar]
- 16.Peirano P, Algarín C, Uauy R. Sleep-wake states and their regulatory mechanisms throughout early human development. J Pediatr. 2003;143:S70–9. doi: 10.1067/s0022-3476(03)00404-9. [DOI] [PubMed] [Google Scholar]
- 17.Nijhuis JG. Behavioural states: Concomitants, clinical implications, and the assessment of the condition of the nervous system. Eur J of Obstet & Gynecol and Reprod Biol. 1986;21:301–308. doi: 10.1016/0028-2243(86)90008-0. [DOI] [PubMed] [Google Scholar]
- 18.Grieve PG, Myers MM, Stark RI. Behavioral states in the fetal baboon. Early Hum Dev. 1994;39(3):159–75. doi: 10.1016/0378-3782(94)90195-3. [DOI] [PubMed] [Google Scholar]
- 19.Robertson CM, Sauve RS, Joffe AR, Alton GY, Moddemann DM, Blakley PM, et al. The western Canadian complex pediatric therapies project follow-up group: Outcomes from an interprovincial program of newborn open heart surgery. J Pediatr. 2004;144(1):86–92. doi: 10.1016/j.jpeds.2003.09.048. [DOI] [PubMed] [Google Scholar]
- 20.Mahle WT, Clancy RR, Moss EM, Gerdes M, Jobes DR, Wernovsky G. Neurodevelopmental outcome and lifestyle assessment in school-aged and adolescent children with hypoplastic left heart syndrome. Pediatrics. 2000;105(5):1082–1089. doi: 10.1542/peds.105.5.1082. [DOI] [PubMed] [Google Scholar]
- 21.Williams IA, Tarullo AR, Grieve PG, Wilpers A, Vignola EF, Myers MM, et al. Fetal cerebrovascular resistance and neonatal EEG predict 18-month neurodevelopmental outcome in infants with congenital heart disease. Ultrasound Obstet Gynecol. 2012;40(3):304–9. doi: 10.1002/uog.11144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Williams IA, Fifer C, Jaeggi E, Levine JC, Michelfelder EC, Szwast AL. The association of fetal cerebrovascular resistance with early neurodevelopment in single ventricle congenital heart disease. Am Heart J. 2013;165(4):544–550. doi: 10.1016/j.ahj.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kinney HC. The near-term (late preterm) human brain and risk for periventricular leukomalacia: a review. Semin Perinatol. 2006;30(2):81–88. doi: 10.1053/j.semperi.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 24.Moeller MP. Early intervention and language development in children who are deaf and hard of hearing. Pediatrics. 2000;106(e43) doi: 10.1542/peds.106.3.e43. [DOI] [PubMed] [Google Scholar]
- 25.DiPietro JA, Kivlighan KT, Costigan KA, Rubin SE, Shiffler DE, Henderson JL, et al. Prenatal antecedents of newborn neurological maturation. Child Dev. 2010;81(1):115–30. doi: 10.1111/j.1467-8624.2009.01384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]