Abstract
It is well established that NADH/NAD+ redox balance is heavily perturbed in diabetes, and the NADH/NAD+ redox imbalance is a major source of oxidative stress in diabetic tissues. In mitochondria, complex I is the only site for NADH oxidation and NAD+ regeneration and is also a major site for production of mitochondrial reactive oxygen species (ROS). Yet how complex I responds to the NADH/NAD+ redox imbalance and any potential consequences of such response in diabetic pancreas have not been investigated. We report here that pancreatic mitochondrial complex I showed aberrant hyperactivity in either type 1 or type 2 diabetes. Further studies focusing on streptozotocin (STZ)-induced diabetes indicate that complex I hyperactivity could be attenuated by metformin. Moreover, complex I hyperactivity was accompanied by increased activities of complexes II to IV, but not complex V, suggesting that overflow of NADH via complex I in diabetes could be diverted to ROS production. Indeed in diabetic pancreas, ROS production and oxidative stress increased and mitochondrial ATP production decreased, which can be attributed to impaired pancreatic mitochondrial membrane potential that is responsible for increased cell death. Additionally, cellular defense systems such as glucose 6-phosphate dehydrogenase, sirtuin 3, and NQO1 were found to be compromised in diabetic pancreas. Our findings point to the direction that complex I aberrant hyperactivity in pancreas could be a major source of oxidative stress and β cell failure in diabetes. Therefore, inhibiting pancreatic complex I hyperactivity and attenuating its ROS production by various means in diabetes might serve as a promising approach for anti-diabetic therapies.
Keywords: Diabetes, Pancreas, Complex I, Hyperactivity, Mitochondria, Redox imbalance, Streptozotocin
Graphical abstract
Highlights
-
•
Pancreatic mitochondrial complex I shows hyperactivity in diabetes.
-
•
Complex I hyperactivity is associated with increased NADH/NAD+ redox imbalance.
-
•
Complex I hyperactivity is associated with increased oxidative stress and cell death.
-
•
Complex I hyperactivity is linked with compromised cellular anti-oxidative stress capacity such as decreased sirt3 and NQO1 expressions.
1. Introduction
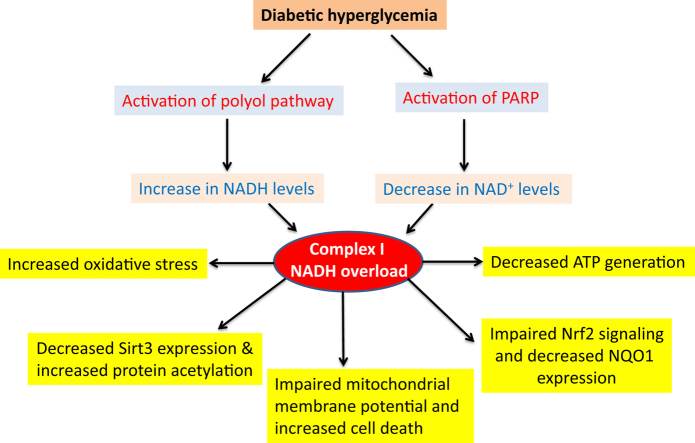
The detrimental effects of diabetes mellitus and its complications are due to persistent hyperglycemic glucotoxicity resulting from impairment of glucose metabolism [1], [2], [3]. As glucose provides electrons stored in NADH and FADH2 that are delivered to mitochondrial electron transport chain, persistent hyperglycemia would over-supply NADH, leading to NADH/NAD+ redox imbalance. Moreover, in addition to the conventional metabolic pathways that can over-produce NADH in the presence of high blood glucose [2], [4], persistent hyperglycemia-triggered activation of the polyol pathway converting NADPH to NADH [5], [6] and of the poly ADP ribose polymerase (PARP) pathway depleting NAD+ [7], [8] are also well known to contribute to the NADH/NAD+ redox imbalance in diabetes [2], [4], [9], [10]. While lactate dehydrogenase can regenerate NAD+ by converting glycolysis-generated pyruvate to lactate, the level of lactate dehydrogenase in β cells is very low [11]. Therefore, excess NADH would overload or overwhelm complex I that is a major site in mitochondria for NAD+ recycling. Moreover, NADH/NAD+ redox imbalance is known to induce cellular oxidative stress [12], probably originated from complex I because complex I is also one of the major sites of ROS production [13]. Indeed, redox imbalance between NADH and NAD+ and oxidative stress have been recognized as the major mechanisms by which high level of blood glucose (hyperglycemia) causes β cell failure [12], [14]. In spite of these well-known establishments, how pancreatic mitochondrial complex I handles NADH overload or NADH/NAD+ redox imbalance and any potential consequences of this handling in diabetes remain unknown.
The present study was designed to test our hypothesis that complex I activity is up-regulated in diabetes, and consequently ROS production and oxidative stress would be elevated given that the more NADH provided to complex I, the more ROS generated by complex I [15], [16], [17], [18]. In this article, we first presented our findings that NADH/NAD+ redox imbalance also occurred in streptozotocin (STZ) diabetic pancreas and this redox imbalance was associated with activation of the polyol pathway and of the poly ADP-ribose polymerase (PARP). We then demonstrated that pancreatic mitochondrial complex I activity indeed exhibited up-regulation or hyperactivity in type 1 streptozotocin (STZ) diabetic mice and rats, in type2 diabetic rats and mice, and in cultured β cells. Further experiments focusing on STZ-induced diabetes in rats revealed that complex I's hyperactivity could be attenuated by metformin. We also found that as NAD+ was low in diabetes, so was sirt3, which is an NAD+-dependent deacetylase. Consequently, mitochondrial protein acetylation was increased. Moreover, activity and protein level of NQO1, a cellular antioxidant, were also found to be attenuated. All these abnormalities contribute to impaired mitochondrial membrane potential, decreased mitochondrial ATP production, and increased oxidative stress and cell death that lead to progression and accentuation of diabetes.
2. Materials and methods
2.1. Chemicals and reagents
Streptozotocin (STZ), NADH, NAD+, ATP, cytochrome c, antimycin A, sodium cyanide, decylubiquinol, n-dodecyl-beta-D-maltoside, sodium succinate, nitro blue tetrazolium (NBT) tablets, EDTA, dimethyl sulfoxide, dichlorophenolindophenol (DCPIP), NADPH, glucose-6-phosphate, and metformin hydrochloride were purchased form Sigma (St. Louis, MO). Lead nitrate, tricine, amino- N- caproic acid, Tris base, and Bis-tris were obtained from VWR International. Serva blue G-250 was purchased from Serva (Heidelberg, Germany). Coomassie brilliant blue G-250, SDS-PAGE protein standard markers, ECL Western blot detection kit, and immunoblotting membrane were purchased from Bio-Rad (Richmond, CA).
2.2. Animals and STZ diabetes induction
All animal procedures were approved by Institutional Care and Use Committee of University of North Texas Health Science Center and the use of animals was in accordance with NIH guidelines for the care and use of laboratory animals. Zucker diabetic rats and Sprague-Dawley rats were obtained from Charles River. Tallyho mouse and db/db mouse were purchased from Jackson laboratories. For STZ induction of diabetes in rats, STZ was freshly prepared in 1 ml of 100 mM citrate buffer (pH 4.5) and injected (IP) to overnight fasted rats at a dosage of 60 mg/kg body weight [19]. For STZ induction of diabetes in mouse, an STZ dosage of 50 mg/kg body weight was used and mice received one injection per day for 5 days [20]. Blood glucose levels were determined using blood collected via tail-pricking by FreeStyle blood glucose test strips made by Abbot (Alameda, CA), and animals with blood levels exceeding 300 mg/dl were considered diabetic. Control mice and rats received 1 ml citrate buffer only. Four weeks (unless otherwise indicated) after STZ treatments, animals were sacrificed and tissues were collected. For metformin treatment of STZ diabetic rats, metformin (150 mg/kg body weight [21]) was administered once per day for 4 weeks via IP injection starting on day 3 after STZ injection.
2.3. INS-1 cell culture and isolation of β cells from pancreas
INS-1 cells were purchased from AddexBio (San Diego, CA) and were cultured with medium supplemented with either 5 mM (normaglycemia) or 20 mM (hyperglycemia) glucose. Basic medium used was RPMI-1640 containing 2 mM glutamate, 10 mM HEPES, 1.5 g/L sodium bicarbonate, 1 mM sodium pyruvate. Confluent cells were collected followed by mitochondrial isolation. For β cell mitochondrial preparation, pancreatic tissues from control and diabetic rats were dissected following sacrifice of the animals. Pancreatic duct was perfused with collagenase P (0.3 mg/ml) and the pancreata were digested in the same collagenase solution for about 20 min with gentle shaking at 37 °C [22], [23]. This is followed by islet collection by handpicking. The obtained islets were used for β cell mitochondrial isolation by the method described below.
2.4. Pancreatic mitochondria isolation
Pancreatic mitochondria preparation from either mouse or rat was achieved using a gradient centrifugation method adapted from previously published work [24]. Briefly, pancreas was rapidly dissected after animal euthanization and rinsed with mitochondrial isolation buffer containing 70 mM sucrose, 230 mM mannitol, 15 mM MOPS (pH 7.2), and 1 mM K2EDTA. Tissues were then homogenized in the mitochondrial isolation buffer (1 g/10 ml) followed by centrifugation of the homogenate at 4 °C at 800 g for 10 min. The resulting supernatant was further centrifuged at 8000 g for 10 min at the same temperature. The obtained pellet containing mitochondria was washed once with mitochondrial isolation buffer and centrifuged again at 8000 g for another 10 min. The final mitochondrial pellet was either used immediately or frozen at − 80 °C until use. β cell mitochondria were isolated the same way.
2.5. Enzyme activity assays
In-gel mitochondrial complex I activity was determined by non-gradient BN-PAGE as previously described [25], [26], [27]. Briefly, after resolution of mitochondrial complexes by BN-PAGE, complex I activity was stained in a solution containing 50 mM potassium phosphate buffer (pH 7.0), NADH (0.1 mg/ml), and NBT (0.2 mg/ml). Complex IV was also analyzed using BN-PAGE in-gel staining method as previously described [26]. The staining solution for complex IV activity contained 50 mM sodium phosphate (pH 7.2), 20 mM 3,3′-diaminobenzidine tetrachloride (DAB), and 50 mg of cytochrome c. Complex II activity was determined spectrophotometrically using succinate as the substrate and DCPIP as the electron acceptor [28]. The assay solution in 1 ml of 100 mM potassium phosphate (pH 7.4) contained mitochondrial protein (30 µg), DCPIP (150 µM), decylubiquinone (100 µM), antimycin A (1.2 µM), MgCl2 (200 mM), KCN (2 mM), and succinate (8 mM). The reaction was monitored at 600 nm. Complex III activity was also determined spectrophotometrically [28] with an assay solution (1 ml) containing 100 mM potassium phosphate (pH 7.4), mitochondrial extract (30 µMg), rotenone (6 µM), antimycin A (2 µM), KCN (2 mM), and decylubiquinol (60 µM). The reaction was monitored at 550 nm. Complex V activity was determined as outlined previously [26]. NQO1 activity and aldose reductase activity were measured, respectively, according to methods as previously described [29], [30].
2.6. Other methods
NAD+/NADH ratio was measured using a kit purchased from BioVision (Milpitas, CA) according to the manufacturer's instructions. ATP content and NADP/NADP+ ratio were measured using kits that were also from BioVision (Milpitas, CA). SDS-PAGE and Western blot assays were conducted according to standard protocols as previously reported [26], [31]. Protein carbonyls were measured by labeling carbonyl groups with biotin-hydrazide followed by Western blot detection and densitometric quantification of the signal intensity [32], [33]. Lipid peroxidation was determined as thiobarbituric acid reactive substances (TBARS) [34] and hydrogen peroxide levels were measured by the Amplex Red method [35] using a kit purchased from Invitrogen. Cell death marker caspase 3 activities and mitochondrial membrane potential were measured, respectively, by kits from BioAssay Systems (Hayward, CA). Nrf2 nuclear content was determined by electrophoretic mobility shift assay using a kit from Signosis Inc. (Santa Clara, CA). Mass spectrometric peptide sequencing and protein identification were conducted as previously described [36].
2.7. Data analysis
Gel images were documented using a digital scanner (Epson Perfection 1670). Data were presented as mean ± SEM. Statistical data analysis was carried out using GraphPad's 2-tailed unpaired t-test (GraphPad, San Diego, CA). A p value less than 0.05 (p < 0.05) was considered statistically significant.
3. Results
3.1. Pancreatic NADH/NAD+ redox balance is perturbed in streptozotocin-induced diabetes
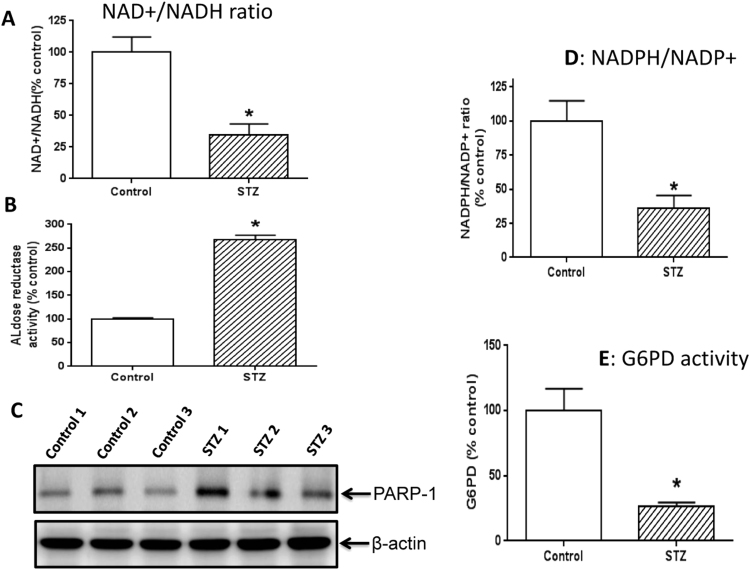
It is well established that NADH/NAD+ redox balance is perturbed in many diabetic tissues with NADH being in excess [9], [10], [37], [38], [39], [40], [41], [42]. We confirmed that this redox imbalance also occurred in STZ diabetic pancreas as the ratio between NAD+ and NADH was much less in diabetes than in controls (Fig. 1A). This redox imbalance in numerous diabetic tissues has been reported to be driven by at least two pathways that are up-regulated in diabetes [42], [43], [44]. One is the polyol pathway [45], [46], [47], [48], [49], [50] and the other is the poly ADP ribose polymerase (PARP) pathway [51], [52], [53]. To confirm that this is also the case in STZ-diabetic pancreas, we measured the rate-limiting enzyme (aldose reductase) in the polyol pathway and the protein content of PARP-1. Results show that the activity of aldose reductase indeed increased (Fig. 1B) and PARP-1 protein content also increased (Fig. 1C). Additionally, we also found that the NADP+/NADPH ratio was lower in diabetes than in controls (Fig. 1D); and given that G6PD is responsible for converting NADP+ to NADPH [54], the process of NADPH regeneration from NADP+ also appeared to be further impaired as G6PD activity was decreased (Fig. 1E). Nonetheless, it is likely that the change in NADPH/NADP+ ratio reflects an overall increase in oxidative stress rather than a sole decrease in G6PD activity. These results indicate that the redox imbalance between NADP+ and NADPH also occurs in STZ diabetic pancreas.
Fig. 1.
Measurement of redox imbalance and relevant parameters. (A) NAD+/NADH ratio was lower in STZ diabetes than in control. (B) Aldose reductase activity was much higher in STZ diabetes than in control. (C) PARP-1 expression was increased in STZ diabetes. (D) NADPH/NADP+ ratio was lower in STZ diabetes than in control. (E) G6PD activity was highly suppressed in STZ diabetes. N=3 for each assay.
3.2. Pancreatic mitochondrial complex I exhibits aberrant hyperactivity in diabetes
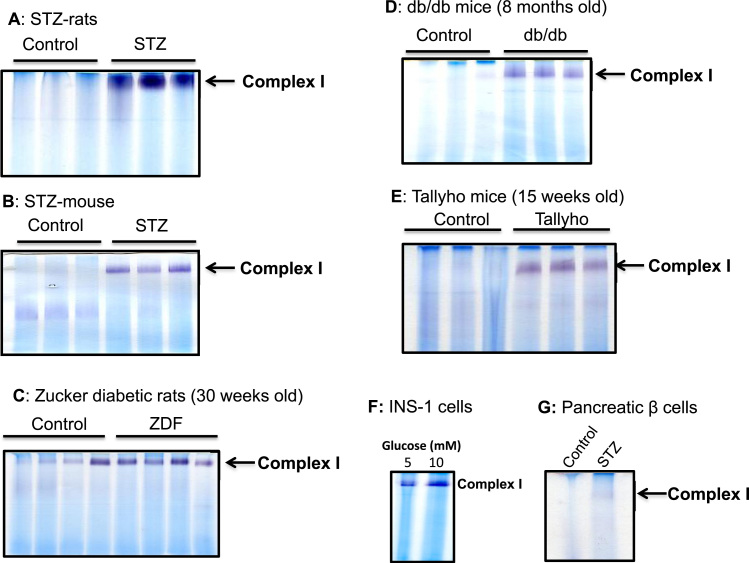
To investigate how pancreatic mitochondrial complex I activity would respond to diabetic NADH/NAD+ redox imbalance, we first measured pancreatic mitochondrial complex I activity using in-gel based BN-PAGE analysis. To this end, we used both STZ-diabetic rats and mice. Results in Fig. 2 A and B demonstrate that in these type I diabetic rats and mice, complex I activity was tremendously up-regulated. We then tested type II diabetic animal models that include Zucker diabetic rats, db/db mouse, and Tallyho mouse. Results in Fig. 2 C to E demonstrate that among all the tested type II diabetic animal models, complex I activity was also enormously up-regulated. These results indicate that complex I activity was upregulated regardless of the types of diabetes. Furthermore, when β cells (INS-1) were cultured in the presence of high level glucose (20 mM), complex I activity was also found to be higher than that in the presence of normal level glucose (5 mM) (Fig. 2F). We also measured complex I activity in pancreatic β cells isolated from STZ diabetic rats. Results in Fig. 2G demonstrate that β cell complex I activity was also up-regulated, indicating that the observed complex I hyperactivity in Fig. 2A could be partially contributed by diabetic β cells.
Fig. 2.
Increased pancreatic mitochondria complex I activity in diabetes. (A) Comparison of complex I activity between control and STZ rat. (B) Comparison of complex I activity between control and STZ mouse. (C) Complex I activity between control and Zucker diabetic rat. (D) Complex I activity between control and db/db mouse. (E) Complex I activity between control and Tallyho mouse, a type 2 diabetes model. (F) Comparison of complex I activity from INS-1 cells cultured in the presence of normal and high glucose levels. (G) Comparison of complex I activity in β cell mitochondria isolated from control and STZ-pancreas. For A to E, each lane represented an independent animal. All activities were measured by BN-PAGE.
Based on the above findings that complex I hyperactivity could be detected in both type 1 and type 2 diabetic pancreas, we then decided to carry out all the following studies using STZ-diabetic rats as induction of this model of diabetes is less time-consuming.
3.3. Pancreatic complex I hyperactivity in STZ diabetic rat is caused by diabetic hyperglycemia but not by acute STZ toxicity and is attenuated by metformin
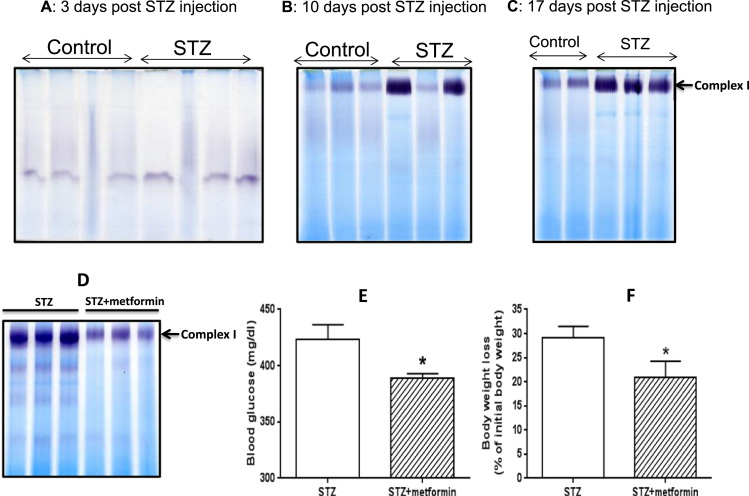
With the STZ diabetes model, the first question we asked was whether complex I hyperactivity observed in Fig. 2A was due to STZ acute toxicity. To this end, we did a time-dependent analysis of complex I activity following STZ injection. Results show that by day 3 after STZ injection, no up-regulation in complex I activity in the STZ diabetic pancreas could be detected (Fig. 3A). By day 10, 2 out of 3 STZ treated rats showed increased pancreatic mitochondrial complex I activities (Fig. 3B). By day 17, all the three STZ treated rats showed pancreatic complex I hyperactivity (Fig. 3C). These results demonstrate that pancreatic complex I hyperactivity was induced by persistent levels of hyperglycemia, but not by the acute toxicity of STZ, which is known to be rapidly eliminated out of the body within 24 h of injection [55], [56]. Moreover, when the diabetic rats were treated by metformin for 4 weeks (150 mg/kg, one IP injection per day), complex I hyperactivity was remarkably decreased (Fig. 3D). We also found that, in addition to lowering blood glucose significantly (Fig. 3E), metformin also attenuated body weight loss significantly (Fig. 3F), which agrees with data reported in the literature (for example, references [57], [58], [59]). These results further demonstrate that complex I's aberrant hyperactivity was induced by persistent high blood glucose.
Fig. 3.
Time-dependent in-gel complex I activity measurement post STZ injection and effect of metformin on complex I hyperactivity, body weight change, and blood glucose. (A) 3 days post STZ injection; (B) 10 days post STZ injection; (C) 17 days post STZ injection. (D) Metformin attenuated complex I hyperactivity induced by diabetic hyperglycemia. (E) Metformin significantly decreased blood glucose in STZ diabetic rats. (F) Metformin attenuated diabetic rat's body weight loss significantly. For all the gel analyses, each lane represents mitochondrial sample prepared from one single animal sacrificed for tissue collection and mitochondrial isolation.
3.4. Activities of complexes II to IV but not complex V are also higher in diabetic pancreas
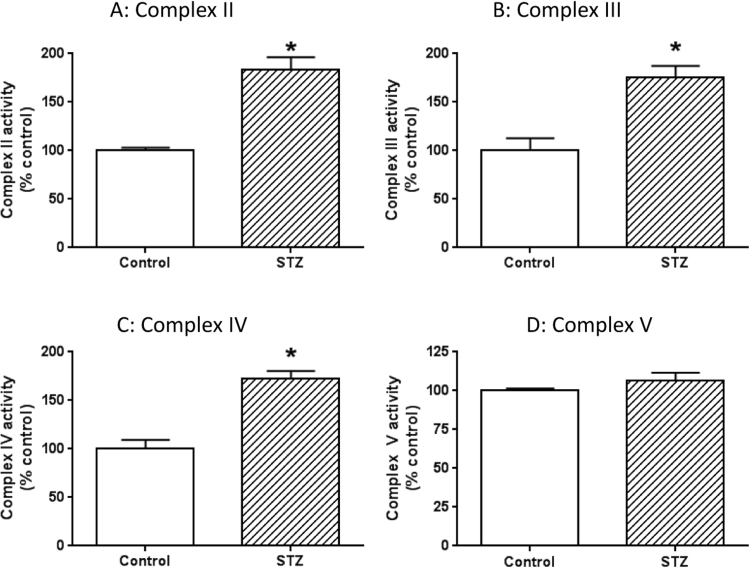
As complex I activity was greatly elevated in all the diabetic conditions (Fig. 2), we then wondered whether the activities of other mitochondrial oxidative phosphorylation components were also elevated. To address this question, we measured the activities of complex II to complex V using STZ diabetic rats. Results in Fig. 4 show that the activities of complexes II to IV were also up-regulated in diabetic pancreas. Interestingly, complex V activity showed no detectable changes between diabetes and healthy controls.
Fig. 4.
Measurements of activities of complexes II to V between control and STZ- diabetes. Shown are (A) Complex II activity, (B) Complex III activity, (C) Complex IV activity, (D) Complex V activity. N = 3 for each assay.
3.5. Decreased ATP production and increased oxidative stress in diabetic pancreas
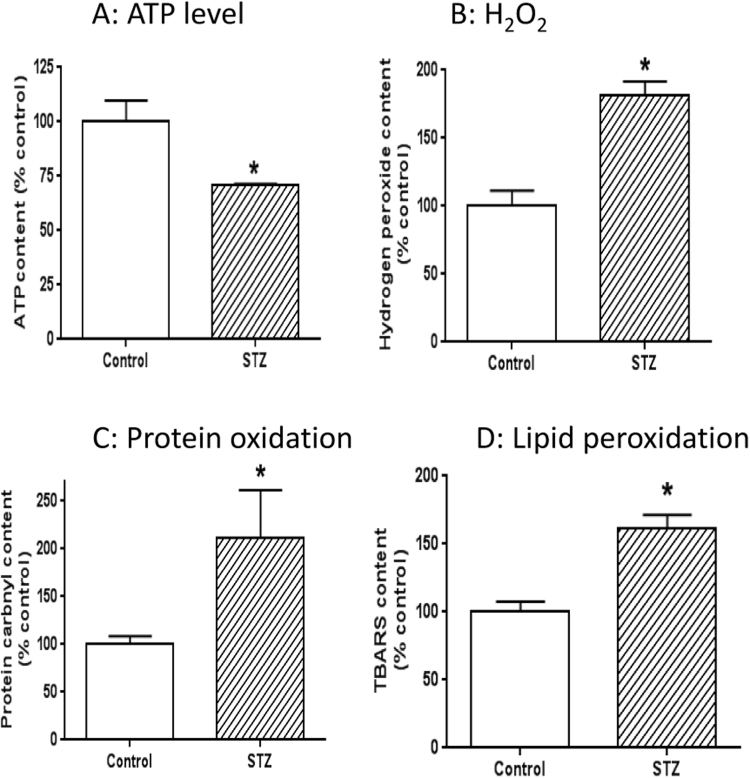
Our findings that complexes I to IV activities were higher while complex V activity showed no detectable change suggest that the elevated activities of the mitochondrial electron transport chain is not used for ATP production. Rather, these elevated activities could be used for ROS production given that higher electron transport chain activity can also increase mitochondrial ROS production [18], a phenomenon that is well known as leak respiration [60], [61]. To test this hypothesis, we measured cellular ATP content, mitochondrial protein carbonyls, H2O2 content, and lipid peroxidation via TBARS content. Results indicate that ATP output by mitochondria was decreased in diabetes (Fig. 5A), and that H2O2 content (Fig. 5B), protein carbonyl content (Fig. 5C), and TBARS levels (Fig. 5D) were all increased in diabetes. Taken together, these results indicate that overflow of NADH via complex I is partially used for leak respiration that increases ROS production and oxidative stress [60], [61].
Fig. 5.
Measurement of cellular ATP content, H2O2 content, protein carbonyl content, and lipid peroxidation magnitude. (A) ATP level was lower in STZ diabetes than in control. (B) H2O2 content was higher in STZ diabetes than in control. (C) Mitochondrial protein carbonyl content was higher in STZ-diabetes than in control. (D) TBARS content, reflecting the level of lipid peroxidation, was higher in STZ diabetic rats than in control rats. N = 3 for each assay.
3.6. Complex I hyperactivity is partly contributed by NDUFS3 up-regulation
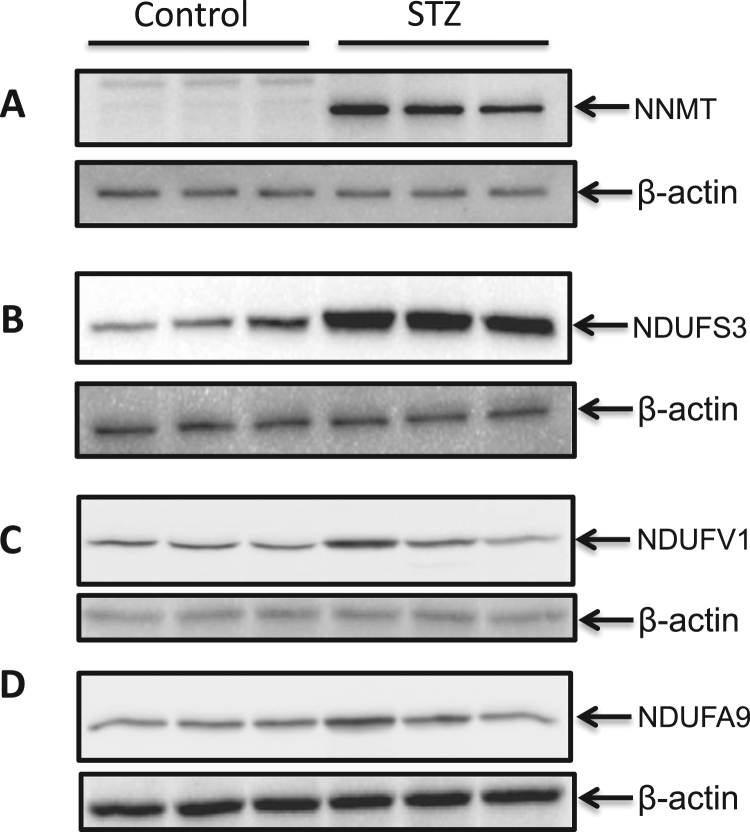
It is known that in diabetes and under overnutrition conditions, the enzyme nicotinamide N-methyl transferase (NNMT) is up-regulated [62], which in turn can up-regulate NDUFS3 [63], [64] that is a complex I subunit involved in complex I assembly and function [65]. Therefore, complex I can exhibit an elevated activity due to NDUFS3 up-regulation by NNMT. To test whether this is the case in our system, we measured protein content of both NNMT and NDUFS3 by Western blot assays using respective antibodies. Results in Fig. 6 demonstrate that the expression of both NNMT and NDUFS3 were increased in diabetic pancreas, indicating that upregulation of NDUFS3 cloud be partly responsible for complex I hyperactivity observed in this study. We also measured the content of another two complex I subunits NDUFV1 and NDUFA9, but did not detect any significant difference between control and diabetes. This Result indicates that not all complex I subunits are upregulated in STZ diabetes.
Fig. 6.
Western blot analysis of NNMT and complex I subunits NDUFS3, NDUFV1, and NDUFA9. Both NNMT expression and NDUFS3 expression were found to be upregulated (A and B). In contrast, no differences were observed for NDUFV 1 and NDUFA9 between control and STZ-diabetes. Three animals per group were used for all the measurements.
3.7. Decreased Sirt3 expression and increased protein acetylation in pancreatic mitochondria
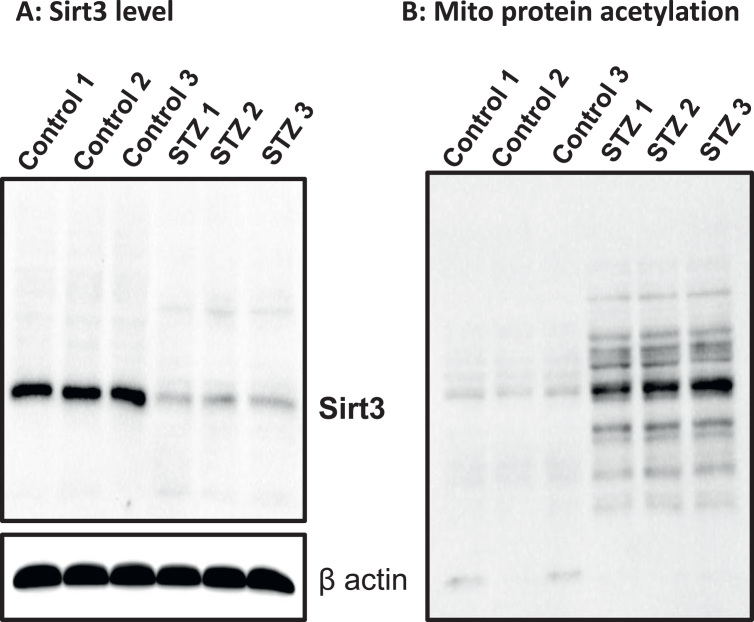
Sirtuin 3 (Sirt3) is an NAD+-dependent deacetylase in the mitochondria and is known to be down regulated in other tissues when the level of NAD+ is low [66], [67]. We focused our studies on mitochondrial sirt3 instead of other sirtuins as our investigation was centered on mitochondria. To test whether this was also the case in diabetic pancreas, we measured Sirt3 levels by Western blot assay. Results in Fig. 7A show that the content of Sirt3 was heavily decreased in diabetic pancreas. Moreover, when total mitochondrial protein acetylation profile was assessed by anti-acetylation Western blot analysis, an increased acetylation on numerous proteins could be detected in diabetes (Fig. 7B), suggesting that mitochondrial function could be impaired by acetylation, albeit that complex I activity is aberrantly higher in diabetes than in healthy controls.
Fig. 7.
Measurements of sirt3 levels and mitochondrial protein acetylation. (A) Western blot measurement of sirt3. (B) Western blot measurement of protein acetylation.
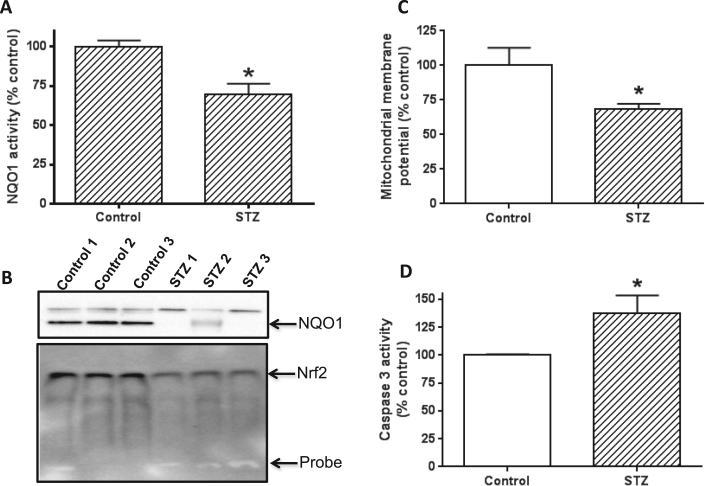
3.8. NQO1 activity and expression are decreased in diabetes
NQO1 is an antioxidant enzyme [68] and is known to be down regulated in diabetes due to redox imbalance and oxidative stress [69], [70]. To test whether this was also the case in our system, we measured NQO1 enzymatic activity and protein content. Results show that NQO1 activity was significantly lower in the diabetic animals than in the controls (Fig. 8A). This decreased activity was apparently contributed by a decreased NQO1 protein content as shown in Fig. 8B (upper panel). Moreover, decreased NQO1 content was found to be driven by a decreased level of nuclear Nrf2, a transcription factor that regulates NQO1 expression [71], [72]. These results agree with the data in Fig. 1D and E that pancreatic antioxidant capacity was compromised in diabetes. The data also agree with the findings in Fig. 5B–D that oxidative stress was increased in diabetes.
Fig. 8.
Measurements of NQO1 activity and content, and assessment of mitochondrial membrane potential and cell death. (A) Comparison of NQO1 activity between control and STZ diabetes. (B) Upper panel: Comparison of NQO1 content between control and STZ diabetes; lower panel: decreased nuclear Nrf2 content detected by electrophoretic gel shift assay. (C) Comparison of mitochondrial membrane potential between control and STZ diabetes. (D) Comparison of caspase 3 activity between control and STZ diabetes. Increased caspase 3 activity indicates increased cell death. Three animals per group were used for each measurement. N = 3 for each panel.
3.9. Mitochondrial membrane potential is impaired and cell death is increased in STZ diabetic pancreas
Our findings that mitochondrial electron transport chain activities were increased under hyperglycemic conditions while mitochondrial ATP output was decreased suggest that there is an uncoupling effect on electron transport chain activity and oxidative phosphorylation (ATP production). To test this hypothesis, we measured mitochondrial membrane potential. Results in Fig. 8C indeed shows that in STZ treated rats, pancreatic mitochondrial membrane potential was significantly impaired, indicating that leak mitochondrial respiration had occurred in the pancreas of the diabetic rats. Consequently, increased pancreatic cell death in the STZ diabetic rats was detected as caspase 3 activity, a marker for cell apoptosis [73], [74], was higher in the STZ diabetic rats than in the control rats (Fig. 8D).
4. Discussion
The major findings of the present study are that pancreatic mitochondrial complex I exhibits increased enzyme activity in both type 1 and type 2 diabetes. This increase could be attenuated by metformin and was in response to NADH/NAD+ redox imbalance and NADH overload of complex I, which also increases the activities of complexes II to IV, but not complex V. The observed mitochondrial uncoupling effect, i.e., more NADH supplies but less ATP output, demonstrates increased oxidative stress in the diabetic pancreas. Our data indicate that there is an overall redox balance perturbation that not only down-regulates mitochondrial sirts3, but also the antioxidant enzyme NQO1. Therefore, our findings provide a link between complex I ROS production and diabetic pathogenesis and reveal a widespread oxidative stress caused by complex I hyperactivity and a compromised anti-oxidation capacity in diabetic pancreas.
We initially tested several tissues for complex I activity change in STZ-diabetes. Among those tissues tested, no changes in complex I activity was detected in the brain, the liver, and the heart. The reason why complex I activity in the brain, the liver, and the heart did not exhibit detectable increases is not known at the present time. In contrast, an increased complex I activity in kidney [19], lung [39], and pancreas was observed by BN-PAGE, and the magnitude of increase in these tissues appeared to be kidney < lung < pancreas. Therefore, pancreas would be a very good organ for studying mitochondrial complex I hyperactivity in diabetes. It should be noted that we did not observe changes in complex I activity in skeletal muscle. In fact it has been reported that complex I activity in skeletal muscle is decreased in diabetes [75], [76], [77]. This makes sense because skeletal muscle cells require insulin for glucose entry for further metabolism, but in diabetes skeletal muscle cells cannot obtain glucose even though glucose is in excess due to insulin resistance or insulin deficiency [78]. We did not analyze complex I activity in diabetic adipose tissues, but it is conceivable that complex I activity change in this tissue should be similar to that in the skeletal muscle as adipocyte is also dependent on insulin for glucose uptake [79]. Additionally, it would also be of interest to evaluate pancreatic complex I activity change in gestational diabetes.
In diabetes, persistent hyperglycemia overloads metabolic pathways with excess NADH [4]. Given that pancreatic β cells employ a supply-driven mechanism for glucose metabolism [80], [81], β cells could be particularly under NADH pressure. Therefore, it is reasonable for complex I to adapt to this pressure by increasing its activity to attempt to alleviate the pressure. Nonetheless, this activity increase is not enough to consume all the NADH pool under diabetic conditions, similar to the fact that excess glucose is eliminated via the urinary system but blood glucose level will never go low by this means of elimination.
The downside problem of pancreatic complex I hyperactivity in diabetes should be severe. This is because elevated complex I activities, while attempting to oxidize more NADH and regenerating more NAD+, will also produce more superoxide anion [82], [83], a precursor of nearly all the other forms of ROS in the body [84]. Indeed, complex I is known to be the major site of ROS production in disease state and has been implicated in a variety of diseases [85], [86]. Therefore, it is imaginable that complex I hyperactivity in pancreas could lead to accentuated oxidative stress to β cells, which can impair β cell function and insulin release, and worsens diabetes situation.
In diabetes, many pathways are actually upregulated. There are two well-studied pathways that warrant discussions. One pathway is the polyol pathway that comprises two reactions catalyzed by aldose reductase and sorbitol dehydrogenase [87], respectively. The overall products of this pathway are NADH and fructose that are produced at the consumption of NAD+ and NADPH. Therefore, this pathway has been considered as the major source of NADH/NAD+ redox imbalance in diabetes [88]. In fact, inhibition of the first reaction catalyzed by aldose reductase has been investigated by numerous investigators as potential approaches for preventing or treating diabetes and its complications [46], [89], [90], [91]. It should be noted that the reason that a lowered NADPH level is capable of driving the increase in NADH level is because the cellular pool of NADPH is usually many folds greater than that of NADH [92], [93].
The second well-studied pathway is the poly ADP ribose polymerase (PARP), in particular, PARP-1. This pathway is activated by DNA oxidative damage [94] but is often over-activated in diabetes [53], [95]. As PARP uses NAD+ as its substrate, its over activation can actually deplete cellular NAD+ pool and leads to cell death [8], [96]. Therefore, this pathway also contributes significantly to diabetic NADH/NAD+ redox imbalance [42] and has also been considered as a therapeutic target for preventing or treating diabetes and its complications [97]. Indeed, it has been reported that PARP-1 knockout mouse is resistant to STZ diabetes induction [98], [99] and inhibition of PARP-1 can prevent diabetes occurrence [7], [100]. Based on findings of these two well-studied pathways, it is conceivable that inhibiting complex I hyperactivity could provide another promising approach for diabetes therapy, which should be the focus of future studies.
In the present study, we also measured two NAD+-dependent enzymes sirt3 and NQO1. With respect to sirt3, we found that its expression is much suppressed in diabetic pancreas, which agrees with previous reports that sirtuin proteins tend to be down regulated in diabetes due to attenuated level of NAD+ [101], [102]. Our further experiments demonstrate that in the presence of lowered sirt3 content, mitochondrial protein acetylation was increased (Fig. 7B). We attempted to analyze acetylation to complex I subunits following BN-PAGE separation of complex I band, but couldn't get enough proteins for mass spectrometry peptide sequencing to reach a conclusive result. Nonetheless, it is known that protein acetylation can also increase the function of the target proteins [103], [104], [105]. Regardless, pancreatic complex I acetylation will need to be further studied not only on the relationship between acetylation and complex I function, but also on identification of more subunits that undergo increased acetylation in diabetes.
With respect to NQO1, we also found that its activity was decreased due to decreased expression of the protein (Fig. 8B). NQO1 is one of the second phase antioxidants that are upregulated by the Nrf2 signaling pathway [68]. Our study indicates that NQO1's decreased expression (Fig. 8B, the upper panel) in diabetic pancreas is indeed controlled by an impaired Nrf2 signaling pathway as less nuclear Nrf2 content was detected by electrophoretic mobility shift assay (Fig. 8B, the lower panel).
One caveat of the present study is that we did not distinguish complex I activity among different cell populations in the pancreas, such as glucagon-secreting α cells, somatostatin-secreting γ cells, and pancreatic polypeptide (pp)-secretion cells. Nonetheless, given that β cells are predominant cell types (nearly 70%) in the islet [22], and that β cell complex I was found to be hyperactive in diabetic pancreas (Fig. 2G), our findings in the whole pancreas tissues may well-represent what would occur in diabetic β cells. A practical problem in studying only β cells is that the mitochondrial yield is very low when pancreas from a single rat is used for β cell mitochondria isolation. This is the main reason that we used the whole pancreas as the source of mitochondria in this study.
Our findings that complex I hyperactivity also imposed pressure on complexes II to IV, but not complex V (Fig. 4D) suggest that the processes of the mitochondrial oxidative phase is partially disconnected from the mitochondrial phosphorylation phase, thereby leading to low mitochondrial ATP output (Fig. 5A) even though NADH is over supplied. This is actually supported by the observation that mitochondrial membrane potential is impaired in diabetic pancreas (Fig. 8C), which indicates that mitochondrial permeability transition pore is aberrant. Therefore, more electrons are diverted to or leaked for ROS production that can induce oxidative stress (Fig. 5 B to D) and eventually leads to increased cell death (Fig. 8D). It is conceivable that relieving complex I NADH overload pressure by restoring NADH/NAD+ redox balance may serve as a novel approach for treating diabetes and its complications.
5. Conclusion
In summary, the present study present convincing evidence that complex I activity in pancreas is aberrantly upregulated by diabetic hyperglycemia. This upregulation or hyperactivity is in response to NADH/NAD+ redox imbalance caused by overproduction of NADH by the conventional metabolic pathways and by activation of the polyol pathway and the PARP pathway. Furthermore, due to NADH/NAD+ redox imbalance and complex I hyperactivity, cellular oxidative stress is increased and mitochondrial ATP production is decreased. These findings, together with the evidence of decreased expression of sirt3 and NQO1, provide insights into the mechanisms of pancreatic mitochondrial dysfunction and possibly β cell failure in diabetes. Finally, while mechanisms underlying complex I hyperactivity in diabetes are multifactorial, our study points to the direction that complex I could be a promising therapeutic target for diabetes, particularly, from the angle of restoring NADH/NAD+ redox balance and attenuating oxidative stress.
Acknowledgements
This work was supported in part by UNTHSC seed Grants RI10015 and RI10039 to LJY who was also supported in part by NIH (Grant#: R01NS079792). SJ was supported in part by the 2016 Innovation Talents of International Cooperation Projects of China Scholarship Council (Grant no. [2015]7642).
Footnotes
This work was partially presented on the 25th Research Appreciation Day of the University of North Texas Health Science Center in April 2017.
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.bbrep.2017.07.007.
Appendix A. Transparency document
Supplementary material
References
- 1.Newsholme P., Cruzat V.F., Keane K.N., Carlessi R., de Bittencourt P.I., Jr. Molecular mechanisms of ROS production and oxidative stress in diabetes. Biochem. J. 2016;473:4527–4550. doi: 10.1042/BCJ20160503C. [DOI] [PubMed] [Google Scholar]
- 2.Luo X., Wu J., Jing S., Yan L.J. Hyperglycemic stress and carbon stress in diabetic glucotoxicity. Aging Dis. 2016;7:90–110. doi: 10.14336/AD.2015.0702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zheng H., Wu J., Jin Z., Yan L.J. Protein modifications as manifestations of hyperglycemic glucotoxicity in diabetes and its complications. Biochem. Insights. 2016;9:1–9. doi: 10.4137/BCI.S36141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luo X., Li R., Yan L.J. Roles of Pyruvate, NADH, and Mitochondrial Complex I in Redox Balance and Imbalance in β Cell Function and Dysfunction. J. Diabetes Res. 2015;2015 doi: 10.1155/2015/512618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dunlop M. Aldose reductase and the role of the polyol pathway in diabetic nephropathy. Kidney Int. 2000;(Suppl 77):S3–S12. doi: 10.1046/j.1523-1755.2000.07702.x. [DOI] [PubMed] [Google Scholar]
- 6.Chung S.S., Ho E.C., Lam K.S., Chung S.K. Contribution of polyol pathway to diabetes-induced oxidative stress. J. Am. Soc. Nephrol. 2003;14:S233–S236. doi: 10.1097/01.asn.0000077408.15865.06. [DOI] [PubMed] [Google Scholar]
- 7.Long C.A., Boulom V., Albadawi H., Tsai S., Yoo H.J., Oklu R., Goldman M.H., Watkins M.T. Poly-ADP-ribose-polymerase inhibition ameliorates hind limb ischemia reperfusion injury in a murine model of type 2 diabetes. Ann. Surg. 2013;258:1087–1095. doi: 10.1097/SLA.0b013e31828cced3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chiu J., Xu B.Y., Chen S., Feng B., Chakrabarti S. Oxidative stress-induced, poly(ADP-ribose) polymerase-dependent upregulation of ET-1 expression in chronic diabetic complications. Can. J. Physiol. Pharmacol. 2008;86:365–372. doi: 10.1139/Y08-033. [DOI] [PubMed] [Google Scholar]
- 9.Ido Y., Williamson J.R. Hyperglycemic cytosolic reductive stress 'pseudohypoxia': implications for diabetic retinopathy. Invest Ophthalmol. Vis. Sci. 1997;38:1467–1470. [PubMed] [Google Scholar]
- 10.Williamson J.R., Chang K., Frangos M., Hasan K.S., Ido Y., Kawamura T., Nyengaard J.R., van den Enden M., Kilo C., Tilton R.G. Hyperglycemic pseudohypoxia and diabetic complications. Diabetes. 1993;42:801–813. doi: 10.2337/diab.42.6.801. [DOI] [PubMed] [Google Scholar]
- 11.Sekine N., Cirulli V., Regazzi R., Brown L.J., Gine E., Tamarit-Rodriguez J., Girotti M., Marie S., MacDonald M.J., Wollheim C.B. Low lactate dehydrogenase and high mitochondrial glycerol phosphate dehydrogenase in pancreatic beta-cells. Potential Role Nutr. Sens. J. Biol. Chem. 1994;269:4895–4902. [PubMed] [Google Scholar]
- 12.Yan L.J. Pathogenesis of chronic hyperglycemia: from reductive stress to oxidative stress. J. Diabetes Res. 2014;2014:137919. doi: 10.1155/2014/137919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murphy M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Watson J.D. Type 2 diabetes as a redox disease. Lancet. 2014;383:841–843. doi: 10.1016/S0140-6736(13)62365-X. [DOI] [PubMed] [Google Scholar]
- 15.Vinogradov A.D., Grivennikova V.G. Oxidation of NADH and ROS production by respiratory complex I. Biochim. Biophys. Acta. 1857;2016:863–871. doi: 10.1016/j.bbabio.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 16.Treberg J.R., Quinlan C.L., Brand M.D. Evidence for two sites of superoxide production by mitochondrial NADH-ubiquinone oxidoreductase (complex I) J. Biol. Chem. 2011;286:27103–27110. doi: 10.1074/jbc.M111.252502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cooper J.M., Mann V.M., Krige D., Schapira A.H. Human mitochondrial complex I dysfunction. Biochim. Biophys. Acta. 1992;1101:198–203. doi: 10.1016/s0005-2728(05)80019-2. [DOI] [PubMed] [Google Scholar]
- 18.Skrha J. Caloric restriction and oxidative stress. In: EFarooqui T., Farooqui A.A., editors. Oxidative stress in Vertebrates and Invertebrates. John Wiley & Sons, Inc; Hoboken, New Jersy: 2012. pp. 83–102. [Google Scholar]
- 19.Wu J., Luo X., Yan L.J. Two dimensional blue native/SDS-PAGE to identify mitochondrial complex I subunits modified by 4-hydroxynonenal (HNE) Front. Physiol. 2015;6 doi: 10.3389/fphys.2015.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tesch G.H., Allen T.J. Rodent models of streptozotocin-induced diabetic nephropathy. Nephrology. 2007;12:261–266. doi: 10.1111/j.1440-1797.2007.00796.x. [DOI] [PubMed] [Google Scholar]
- 21.Zhai L., Gu J., Yang D., Hu W., Wang W., Ye S. Metformin ameliorates podocyte damage by restoring renal tissue nephrin expression in type 2 diabetic rats. J. Diabetes. 2016 doi: 10.1111/1753-0407.12437. [DOI] [PubMed] [Google Scholar]
- 22.Kelly C.B., Blair L.A., Corbett J.A., Scarim A.L. Isolation of islets of langerhans from rodent pancreas. In: Ozcan S., editor. Diabetes Mellitus. Humana Press; Totowa, New Jersy: 2003. pp. 3–14. [Google Scholar]
- 23.Stange G., Van De Casteele M., Heimberg H. Purification of rat pancreatic B-cells by fluorescence-activated cell sorting. Methods Mol. Med. 2003;83:15–22. doi: 10.1385/1-59259-377-1:015. [DOI] [PubMed] [Google Scholar]
- 24.Yan L.J., Thangthaeng N., Forster M.J. Changes in dihydrolipoamide dehydrogenase expression and activity during postnatal development and aging in the rat brain. Mech. Ageing Dev. 2008;129:282–290. doi: 10.1016/j.mad.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luo X., Wu J., Jin Z., Yan L.J. Non-gradient blue native polyacrylamide gel electrophoresis. Curr. Protoc. Protein Sci. 2017;87 doi: 10.1002/cpps.21. (19 29 11-19 29 12) [DOI] [PubMed] [Google Scholar]
- 26.Yan L.J., Forster M.J. Resolving mitochondrial protein complexes using nongradient blue native polyacrylamide gel electrophoresis. Anal. Biochem. 2009;389:143–149. doi: 10.1016/j.ab.2009.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yan L.J., Yang S.H., Shu H., Prokai L., Forster M.J. Histochemical staining and quantification of dihydrolipoamide dehydrogenase diaphorase activity using blue native PAGE. Electrophoresis. 2007;28:1036–1045. doi: 10.1002/elps.200600574. [DOI] [PubMed] [Google Scholar]
- 28.Trounce I.A., Kim Y.L., Jun A.S., Wallace D.C. Assessment of mitochondrial oxidative phosphorylation in patient muscle biopsies, lymphoblasts, and transmitochondrial cell lines. Methods Enzymol. 1996;264:484–509. doi: 10.1016/s0076-6879(96)64044-0. [DOI] [PubMed] [Google Scholar]
- 29.Lind C., Cadenas E., Hochstein P., Ernster L. DT-diaphorase: purification, properties, and function. Methods Enzymol. 1990;186:287–301. doi: 10.1016/0076-6879(90)86122-c. [DOI] [PubMed] [Google Scholar]
- 30.Bagnasco S.M., Uchida S., Balaban R.S., Kador P.F., Burg M.B. Induction of aldose reductase and sorbitol in renal inner medullary cells by elevated extracellular NaCl. Proc. Natl. Acad. Sci. USA. 1987;84:1718–1720. doi: 10.1073/pnas.84.6.1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yan L.J., Orr W.C., Sohal R.S. Identification of oxidized proteins based on sodium dodecyl sulfate-polyacrylamide gel electrophoresis, immunochemical detection, isoelectric focusing, and microsequencing. Anal. Biochem. 1998;263:67–71. doi: 10.1006/abio.1998.2799. [DOI] [PubMed] [Google Scholar]
- 32.Yan L.J. Analysis of oxidative modification of proteins. Curr. Protoc. Protein Sci. 2009;(Chapter 14 ():14. doi: 10.1002/0471140864.ps1404s55. (Unit14) [DOI] [PubMed] [Google Scholar]
- 33.Wu J., Luo X., Jing S., Yan L.J. Two-dimensional gel electrophoretic detection of protein carbonyls derivatized with biotin-hydrazide. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2016;1019:128–131. doi: 10.1016/j.jchromb.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yan L.J., Lodge J.K., Traber M.G., Packer L. Apolipoprotein B carbonyl formation is enhanced by lipid peroxidation during copper-mediated oxidation of human low-density lipoproteins. Arch. Biochem. Biophys. 1997;339:165–171. doi: 10.1006/abbi.1996.9867. [DOI] [PubMed] [Google Scholar]
- 35.Zhou M., Diwu Z., Panchuk-Voloshina N., Haugland R.P. A stable nonfluorescent derivative of resorufin for the fluorometric determination of trace hydrogen peroxide: applications in detecting the activity of phagocyte NADPH oxidase and other oxidases. Anal. Biochem. 1997;253:162–168. doi: 10.1006/abio.1997.2391. [DOI] [PubMed] [Google Scholar]
- 36.Yan L.J., Thangthaeng N., Sumien N., Forster M.J. Serum dihydrolipoamide dehydrogenase is a labile enzyme. J. Biochem. Pharmacol. Res. 2013;1:30–42. [PMC free article] [PubMed] [Google Scholar]
- 37.Forbes J.M., Coughlan M.T., Cooper M.E. Oxidative stress as a major culprit in kidney disease in diabetes. Diabetes. 2008;57:1446–1454. doi: 10.2337/db08-0057. [DOI] [PubMed] [Google Scholar]
- 38.Maleki S., Sepehr R., Staniszewski K., Sheibani N., Sorenson C.M., Ranji M. Mitochondrial redox studies of oxidative stress in kidneys from diabetic mice. Biomed. Opt. Express. 2012;3:273–281. doi: 10.1364/BOE.3.000273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu J., Jin Z., Yan L.J. Redox imbalance and mitochondrial abnormalities in the diabetic lung. Redox Biol. 2017;11:51–59. doi: 10.1016/j.redox.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Teodoro J.S., Rolo A.P., Palmeira C.M. The NAD ratio redox paradox: why does too much reductive power cause oxidative stress? Toxicol. Mech. Methods. 2013;23:297–302. doi: 10.3109/15376516.2012.759305. [DOI] [PubMed] [Google Scholar]
- 41.Choudhuri S., Mandal L.K., Paine S.K., Sen A., Dutta D., Chowdhury I.H., Mukherjee A., Saha A., Bhadhuri G., Bhattacharya B. Role of hyperglycemia-mediated erythrocyte redox state alteration in the development of diabetic retinopathy. Retina. 2013;33:207–216. doi: 10.1097/IAE.0b013e318256202e. [DOI] [PubMed] [Google Scholar]
- 42.Manna P., Sil P.C. Impaired redox signaling and mitochondrial uncoupling contributes vascular inflammation and cardiac dysfunction in type 1 diabetes: protective role of arjunolic acid. Biochimie. 2012;94:786–797. doi: 10.1016/j.biochi.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 43.Trueblood N., Ramasamy R. Aldose reductase inhibition improves altered glucose metabolism of isolated diabetic rat hearts. Am. J. Physiol. 1998;275:H75–H83. doi: 10.1152/ajpheart.1998.275.1.H75. [DOI] [PubMed] [Google Scholar]
- 44.Drel V.R., Pacher P., Stevens M.J., Obrosova I.G. Aldose reductase inhibition counteracts nitrosative stress and poly(ADP-ribose) polymerase activation in diabetic rat kidney and high-glucose-exposed human mesangial cells. Free Radic. Biol. Med. 2006;40:1454–1465. doi: 10.1016/j.freeradbiomed.2005.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Forbes J.M., Cooper M.E. Mechanisms of diabetic complications. Physiol. Rev. 2013;93:137–188. doi: 10.1152/physrev.00045.2011. [DOI] [PubMed] [Google Scholar]
- 46.Tang W.H., Martin K.A., Hwa J. Aldose reductase, oxidative stress, and diabetic mellitus. Front Pharmacol. 2012;3:87. doi: 10.3389/fphar.2012.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee A.Y., Chung S.S. Contributions of polyol pathway to oxidative stress in diabetic cataract. FASEB J. 1999;13:23–30. doi: 10.1096/fasebj.13.1.23. [DOI] [PubMed] [Google Scholar]
- 48.Lo A.C., Cheung A.K., Hung V.K., Yeung C.M., He Q.Y., Chiu J.F., Chung S.S., Chung S.K. Deletion of aldose reductase leads to protection against cerebral ischemic injury. J. Cereb. Blood Flow Metab. 2007;27:1496–1509. doi: 10.1038/sj.jcbfm.9600452. [DOI] [PubMed] [Google Scholar]
- 49.Ng T.F., Lee F.K., Song Z.T., Calcutt N.A., Lee A.Y., Chung S.S., Chung S.K. Effects of sorbitol dehydrogenase deficiency on nerve conduction in experimental diabetic mice. Diabetes. 1998;47:961–966. doi: 10.2337/diabetes.47.6.961. [DOI] [PubMed] [Google Scholar]
- 50.Tang W.H., Wu S., Wong T.M., Chung S.K., Chung S.S. Polyol pathway mediates iron-induced oxidative injury in ischemic-reperfused rat heart. Free Radic. Biol. Med. 2008;45:602–610. doi: 10.1016/j.freeradbiomed.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 51.Mouchiroud L., Houtkooper R.H., Auwerx J. NAD(+) metabolism: a therapeutic target for age-related metabolic disease. Crit. Rev. Biochem. Mol. Biol. 2013;48:397–408. doi: 10.3109/10409238.2013.789479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Du X., Matsumura T., Edelstein D., Rossetti L., Zsengeller Z., Szabo C., Brownlee M. Inhibition of GAPDH activity by poly(ADP-ribose) polymerase activates three major pathways of hyperglycemic damage in endothelial cells. J. Clin. Investig. 2003;112:1049–1057. doi: 10.1172/JCI18127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dolle C., Rack J.G., Ziegler M. NAD and ADP-ribose metabolism in mitochondria. FEBS J. 2013;280:3530–3541. doi: 10.1111/febs.12304. [DOI] [PubMed] [Google Scholar]
- 54.Yan L.J., Christians E.S., Liu L., Xiao X., Sohal R.S., Benjamin I.J. Mouse heat shock transcription factor 1 deficiency alters cardiac redox homeostasis and increases mitochondrial oxidative damage. EMBO J. 2002;21:5164–5172. doi: 10.1093/emboj/cdf528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu J., Yan L.J. Streptozotocin-induced type 1 diabetes in rodents as a model for studying mitochondrial mechanisms of diabetic beta cell glucotoxicity. Diabetes Metab. Syndr. Obes. 2015;8:181–188. doi: 10.2147/DMSO.S82272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Karunanyake E.H., Hearse D.J., Mellows G. The synthesis of C14Streptozotocin and its distribution and excretion in the rat. Biochem. J. 1974;142:673–683. doi: 10.1042/bj1420673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rath D., Kar D.M., Panigrahi S.K., Maharana L. Antidiabetic effects of Cuscuta reflexa Roxb. in streptozotocin induced diabetic rats. J. Ethnopharmacol. 2016;192:442–449. doi: 10.1016/j.jep.2016.09.026. [DOI] [PubMed] [Google Scholar]
- 58.Saleh D.O., Bayoumi A.R., El-Eraky W.I., El-Khatib A.S. Streptozotocin-induced vascular and biochemical changes in rats: effects of rosiglitazone vs. metformin. Bull. Fac. Pharm. Cairo Univ. 2013;51:131–138. [Google Scholar]
- 59.Kurup S.B., Mini S. Protective potential of Averrhoa bilimbi fruits in ameliorating the hepatic key enzymes in streptozotocin-induced diabetic rats. Biomed. Pharmacother. 2017;85:725–732. doi: 10.1016/j.biopha.2016.11.088. [DOI] [PubMed] [Google Scholar]
- 60.Turrens J.F. Superoxide production by the mitochondrial respiratory chain. Biosci. Rep. 1997;17:3–8. doi: 10.1023/a:1027374931887. [DOI] [PubMed] [Google Scholar]
- 61.Laustsen C., Nielsen P.M., Norlinger T.S., Qi H., Pedersen U.K., Bertelsen L.B., Ostergaard J.A., Flyvbjerg A., Ardenkjaer-Larsen J.H., Palm F., Stodkilde-Jorgensen H. Antioxidant treatment attenuates lactate production in diabetic nephropathy. Am. J. Physiol. Ren. Physiol. 2017;312:F192–F199. doi: 10.1152/ajprenal.00148.2016. [DOI] [PubMed] [Google Scholar]
- 62.Kraus D., Yang Q., Kong D., Banks A.S., Zhang L., Rodgers J.T., Pirinen E., Pulinilkunnil T.C., Gong F., Wang Y.C., Cen Y., Sauve A.A., Asara J.M., Peroni O.D., Monia B.P., Bhanot S., Alhonen L., Puigserver P., Kahn B.B. Nicotinamide N-methyltransferase knockdown protects against diet-induced obesity. Nature. 2014;508:258–262. doi: 10.1038/nature13198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Parsons R.B., Aravindan S., Kadampeswaran A., Evans E.A., Sandhu K.K., Levy E.R., Thomas M.G., Austen B.M., Ramsden D.B. The expression of nicotinamide N-methyltransferase increases ATP synthesis and protects SH-SY5Y neuroblastoma cells against the toxicity of Complex I inhibitors. Biochem. J. 2011;436:145–155. doi: 10.1042/BJ20101685. [DOI] [PubMed] [Google Scholar]
- 64.Milani Z.H., Ramsden D.B., Parsons R.B. Neuroprotective effects of nicotinamide N-methyltransferase and its metabolite 1-methylnicotinamide. J. Biochem. Mol. Toxicol. 2013;27:451–456. doi: 10.1002/jbt.21508. [DOI] [PubMed] [Google Scholar]
- 65.Jaokar T.M., Patil D.P., Shouche Y.S., Gaikwad S.M., Suresh C.G. Human mitochondrial NDUFS3 protein bearing Leigh syndrome mutation is more prone to aggregation than its wild-type. Biochimie. 2013;95:2392–2403. doi: 10.1016/j.biochi.2013.08.032. [DOI] [PubMed] [Google Scholar]
- 66.Cerutti R., Pirinen E., Lamperti C., Marchet S., Sauve A.A., Li W., Leoni V., Schon E.A., Dantzer F., Auwerx J., Viscomi C., Zeviani M. NAD(+)-dependent activation of Sirt1 corrects the phenotype in a mouse model of mitochondrial disease. Cell Metab. 2014;19:1042–1049. doi: 10.1016/j.cmet.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vedantham S., Thiagarajan D., Ananthakrishnan R., Wang L., Rosario R., Zou Y.S., Goldberg I., Yan S.F., Schmidt A.M., Ramasamy R. Aldose reductase drives hyperacetylation of Egr-1 in hyperglycemia and consequent upregulation of proinflammatory and prothrombotic signals. Diabetes. 2014;63:761–774. doi: 10.2337/db13-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dinkova-Kostova A.T., Talalay P. NAD(P)H: quinone acceptor oxidoreductase 1 (NQO1), a multifunctional antioxidant enzyme and exceptionally versatile cytoprotector. Arch. Biochem. Biophys. 2010;501:116–123. doi: 10.1016/j.abb.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Alhaider A.A., Korashy H.M., Sayed-Ahmed M.M., Mobark M., Kfoury H., Mansour M.A. Metformin attenuates streptozotocin-induced diabetic nephropathy in rats through modulation of oxidative stress genes expression. Chem. Biol. Interact. 2011;192:233–242. doi: 10.1016/j.cbi.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 70.Guo Z., Yan X., Wang L., Wu J., Jing X., Liu J. Effect of telmisartan or insulin on the expression of adiponectin and its receptors in the testis of streptozotocin-induced diabetic rats. Horm. Metab. Res. 2016;48:404–412. doi: 10.1055/s-0042-101549. [DOI] [PubMed] [Google Scholar]
- 71.Jaiswal A.K. Nrf2 signaling in coordinated activation of antioxidant gene expression. Free Radic. Biol. Med. 2004;36:1199–1207. doi: 10.1016/j.freeradbiomed.2004.02.074. [DOI] [PubMed] [Google Scholar]
- 72.Huang X.S., Chen H.P., Yu H.H., Yan Y.F., Liao Z.P., Huang Q.R. Nrf2-dependent upregulation of antioxidative enzymes: a novel pathway for hypoxic preconditioning-mediated delayed cardioprotection. Mol. Cell Biochem. 2014;385:33–41. doi: 10.1007/s11010-013-1812-6. [DOI] [PubMed] [Google Scholar]
- 73.Ouyang Y.B., Tan Y., Comb M., Liu C.L., Martone M.E., Siesjo B.K., Hu B.R. Survival- and death-promoting events after transient cerebral ischemia: phosphorylation of Akt, release of cytochrome C and Activation of caspase-like proteases. J. Cereb. Blood Flow Metab. 1999;19:1126–1135. doi: 10.1097/00004647-199910000-00009. [DOI] [PubMed] [Google Scholar]
- 74.Elsherbiny N.M., Al-Gayyar M.M., Abd El Galil K.H. Nephroprotective role of dipyridamole in diabetic nephropathy: effect on inflammation and apoptosis. Life Sci. 2015;143:8–17. doi: 10.1016/j.lfs.2015.10.026. [DOI] [PubMed] [Google Scholar]
- 75.van Tienen F.H., Praet S.F., de Feyter H.M., van den Broek N.M., Lindsey P.J., Schoonderwoerd K.G., de Coo I.F., Nicolay K., Prompers J.J., Smeets H.J., van Loon L.J. Physical activity is the key determinant of skeletal muscle mitochondrial function in type 2 diabetes. J. Clin. Endocrinol. Metab. 2012;97:3261–3269. doi: 10.1210/jc.2011-3454. [DOI] [PubMed] [Google Scholar]
- 76.Warren B.E., Lou P.H., Lucchinetti E., Zhang L., Clanachan A.S., Affolter A., Hersberger M., Zaugg M., Lemieux H. Early mitochondrial dysfunction in glycolytic muscle, but not oxidative muscle, of the fructose-fed insulin-resistant rat. Am. J. Physiol. Endocrinol. Metab. 2014;306:E658–E667. doi: 10.1152/ajpendo.00511.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ritov V.B., Menshikova E.V., Azuma K., Wood R., Toledo F.G., Goodpaster B.H., Ruderman N.B., Kelley D.E. Deficiency of electron transport chain in human skeletal muscle mitochondria in type 2 diabetes mellitus and obesity. Am. J. Physiol. Endocrinol. Metab. 2010;298:E49–E58. doi: 10.1152/ajpendo.00317.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yki-Jarvinen H. Insulin resistance in patients with IDDM. In: Chrousos G.P., Olefsky J.M., Samols E., editors. Hormone Resistance and Hypersensitivity States. Lippincott William & Wilkins; Baltimore: 2002. pp. 175–185. [Google Scholar]
- 79.Barnett A.H. Second ed. Oxford University Press; Oxford, United Kingdom: 2012. Type 2 Diabetes. [Google Scholar]
- 80.Efrat S., Tal M., Lodish H.F. The pancreatic beta-cell glucose sensor. Trends Biochem. Sci. 1994;19:535–538. doi: 10.1016/0968-0004(94)90056-6. [DOI] [PubMed] [Google Scholar]
- 81.Reyes A., Cardenas M.L. All hexokinase isoenzymes coexist in rat hepatocytes. Biochem. J. 1984;221:303–309. doi: 10.1042/bj2210303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hirst J. Mitochondrial complex I. Annu. Rev. Biochem. 2013;82:551–575. doi: 10.1146/annurev-biochem-070511-103700. [DOI] [PubMed] [Google Scholar]
- 83.Hirst J., King M.S., Pryde K.R. The production of reactive oxygen species by complex I. Biochem. Soc. Trans. 2008;36:976–980. doi: 10.1042/BST0360976. [DOI] [PubMed] [Google Scholar]
- 84.Ames B.N., Shigenaga M.K. Oxidants are a major contributor to aging. Ann. N.Y. Acad. Sci. 1992;663:85–96. doi: 10.1111/j.1749-6632.1992.tb38652.x. [DOI] [PubMed] [Google Scholar]
- 85.Schapira A.H. Human complex I defects in neurodegenerative diseases. Biochim. Biophys. Acta. 1998;1364:261–270. doi: 10.1016/s0005-2728(98)00032-2. [DOI] [PubMed] [Google Scholar]
- 86.Fassone E., Rahman S. Complex I deficiency: clinical features, biochemistry and molecular genetics. J. Med. Genet. 2012;49:578–590. doi: 10.1136/jmedgenet-2012-101159. [DOI] [PubMed] [Google Scholar]
- 87.Chung S.S., Chung S.K. Aldose reductase in diabetic microvascular complications. Curr. Drug Targets. 2005;6:475–486. doi: 10.2174/1389450054021891. [DOI] [PubMed] [Google Scholar]
- 88.Ying W. NAD+/NADH and NADP+/NADPH in cellular functions and cell death: regulation and biological consequences. Antioxid. Redox Signal. 2008;10:179–206. doi: 10.1089/ars.2007.1672. [DOI] [PubMed] [Google Scholar]
- 89.Reddy A.B., Ramana K.V. Aldose reductase inhibition: emerging drug target for the treatment of cardiovascular complications. Recent Pat. Cardiovasc. Drug Discov. 2010;5:25–32. doi: 10.2174/157489010790192683. [DOI] [PubMed] [Google Scholar]
- 90.Ohmura C., Watada H., Azuma K., Shimizu T., Kanazawa A., Ikeda F., Yoshihara T., Fujitani Y., Hirose T., Tanaka Y., Kawamori R. Aldose reductase inhibitor, epalrestat, reduces lipid hydroperoxides in type 2 diabetes. Endocr. J. 2009;56:149–156. doi: 10.1507/endocrj.k08e-237. [DOI] [PubMed] [Google Scholar]
- 91.Tang J., Du Y., Petrash J.M., Sheibani N., Kern T.S. Deletion of aldose reductase from mice inhibits diabetes-induced retinal capillary degeneration and superoxide generation. PLoS One. 2013;8:e62081. doi: 10.1371/journal.pone.0062081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Veech R.L., Eggleston L.V., Krebs H.A. The redox state of free nicotinamide-adenine dinucleotide phosphate in the cytoplasm of rat liver. Biochem. J. 1969;115:609–619. doi: 10.1042/bj1150609a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sander B.J., Oelshlegel F.J., Jr., Brewer G.J. Quantitative analysis of pyridine nucleotides in red blood cells: a single-step extraction procedure. Anal. Biochem. 1976;71:29–36. doi: 10.1016/0003-2697(76)90006-3. [DOI] [PubMed] [Google Scholar]
- 94.Mueller-Dieckmann C., Kernstock S., Lisurek M., von Kries J.P., Haag F., Weiss M.S., Koch-Nolte F. The structure of human ADP-ribosylhydrolase 3 (ARH3) provides insights into the reversibility of protein ADP-ribosylation. Proc. Natl. Acad. Sci. USA. 2006;103:15026–15031. doi: 10.1073/pnas.0606762103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Horvath E.M., Magenheim R., Kugler E., Vacz G., Szigethy A., Levardi F., Kollai M., Szabo C., Lacza Z. Nitrative stress and poly(ADP-ribose) polymerase activation in healthy and gestational diabetic pregnancies. Diabetologia. 2009;52:1935–1943. doi: 10.1007/s00125-009-1435-3. [DOI] [PubMed] [Google Scholar]
- 96.Obrosova I.G., Drel V.R., Pacher P., Ilnytska O., Wang Z.Q., Stevens M.J., Yorek M.A. Oxidative-nitrosative stress and poly(ADP-ribose) polymerase (PARP) activation in experimental diabetic neuropathy: the relation is revisited. Diabetes. 2005;54:3435–3441. doi: 10.2337/diabetes.54.12.3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pacher P., Liaudet L., Soriano F.G., Mabley J.G., Szabo E., Szabo C. The role of poly(ADP-ribose) polymerase activation in the development of myocardial and endothelial dysfunction in diabetes. Diabetes. 2002;51:514–521. doi: 10.2337/diabetes.51.2.514. [DOI] [PubMed] [Google Scholar]
- 98.Pieper A.A., Brat D.J., Krug D.K., Watkins C.C., Gupta A., Blackshaw S., Verma A., Wang Z.Q., Snyder S.H. Poly(ADP-ribose) polymerase-deficient mice are protected from streptozotocin-induced diabetes. Proc. Natl. Acad. Sci. USA. 1999;96:3059–3064. doi: 10.1073/pnas.96.6.3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Masutani M., Suzuki H., Kamada N., Watanabe M., Ueda O., Nozaki T., Jishage K., Watanabe T., Sugimoto T., Nakagama H., Ochiya T., Sugimura T. Poly(ADP-ribose) polymerase gene disruption conferred mice resistant to streptozotocin-induced diabetes. Proc. Natl. Acad. Sci. USA. 1999;96:2301–2304. doi: 10.1073/pnas.96.5.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sarras M.P., Jr., Mason S., McAllister G., Intine R.V. Inhibition of poly-ADP ribose polymerase enzyme activity prevents hyperglycemia-induced impairment of angiogenesis during wound healing. Wound Repair Regen. 2014;22:666–670. doi: 10.1111/wrr.12216. [DOI] [PubMed] [Google Scholar]
- 101.Turkmen K., Karagoz A., Kucuk A. Sirtuins as novel players in the pathogenesis of diabetes mellitus. World J. Diabetes. 2014;5:894–900. doi: 10.4239/wjd.v5.i6.894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jing E., Emanuelli B., Hirschey M.D., Boucher J., Lee K.Y., Lombard D., Verdin E.M., Kahn C.R. Sirtuin-3 (Sirt3) regulates skeletal muscle metabolism and insulin signaling via altered mitochondrial oxidation and reactive oxygen species production. Proc. Natl. Acad. Sci. USA. 2011;108:14608–14613. doi: 10.1073/pnas.1111308108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Alrob O.A., Sankaralingam S., Ma C., Wagg C.S., Fillmore N., Jaswal J.S., Sack M.N., Lehner R., Gupta M.P., Michelakis E.D., Padwal R.S., Johnstone D.E., Sharma A.M., Lopaschuk G.D. Obesity-induced lysine acetylation increases cardiac fatty acid oxidation and impairs insulin signalling. Cardiovasc. Res. 2014;103:485–497. doi: 10.1093/cvr/cvu156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Palmieri E.M., Spera I., Menga A., Infantino V., Porcelli V., Iacobazzi V., Pierri C.L., Hooper D.C., Palmieri F., Castegna A. Acetylation of human mitochondrial citrate carrier modulates mitochondrial citrate/malate exchange activity to sustain NADPH production during macrophage activation. Biochim. Biophys. Acta. 1847;2015:729–738. doi: 10.1016/j.bbabio.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 105.Fernandes J., Weddle A., Kinter C.S., Humphries K.M., Mather T., Szweda L.I., Kinter M. Lysine acetylation activates mitochondrial aconitase in the heart. Biochemistry. 2015;54:4008–4018. doi: 10.1021/acs.biochem.5b00375. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material