Abstract
Background: High phenylalanine levels in phenylketonuria (PKU) have been associated with brain oxidative stress and amino acid imbalance. Exercise has been shown to improve brain function in hyperphenylalaninemia and neurodegenerative diseases. This study aimed to verify the effects of exercise on coordination and balance, plasma and brain amino acid levels, and brain oxidative stress markers in PKU mice.
Methods: Twenty wild-type (WT) and 20 PAHenu2 (PKU) C57BL/6 mice were placed in cages with (exercise, Exe) or without (sedentary, Sed) running wheels during 53 days. At day 43, a balance beam test was performed. Plasma and brain were collected for analyses of amino acid levels and the oxidative stress parameters superoxide dismutase (SOD) activity, sulfhydryl and reduced glutathione (GSH) contents, total radical-trapping antioxidant potential (TRAP), and total antioxidant reactivity (TAR).
Results: SedPKU showed poor coordination (p < 0.001) and balance (p < 0.001), higher plasma and brain phenylalanine (p < 0.001), and increased brain oxidative stress (p < 0.05) in comparison to SedWT. ExePKU animals ran less than ExeWT (p = 0.018). Although no improvement was seen in motor coordination and balance, exercise in PKU restored SOD, sulfhydryl content, and TRAP levels to controls. TAR levels were increased in ExePKU in comparison to SedPKU (p = 0.012). Exercise decreased plasma and brain glucogenic amino acids in ExePKU, but did not change plasma and brain phenylalanine in both WT and PKU.
Conclusions: Exercise prevents oxidative stress in the brain of PKU mice without modifying phenylalanine levels. Hence, exercise positively affects the brain, demonstrating its value as an intervention to improve brain quality in PKU.
Electronic supplementary material
The online version of this chapter (doi:10.1007/8904_2015_498) contains supplementary material, which is available to authorized users.
Introduction
Phenylketonuria (PKU, MIM 261600) is characterized by accumulation of phenylalanine (Phe) to toxic levels due to absent activity of Phe hydroxylase (PAH, EC 1.14.16.1). High Phe concentration can impair brain function even in early-treated PKU patients, who have shown poor cognitive function (Gonzalez et al. 2011; Weglage et al. 2013; Jahja et al. 2014). Although the mechanisms are not yet fully understood, oxidative stress and brain amino acid imbalance caused by high Phe levels are speculated to underlie the impaired clinical outcomes. At the biochemical level, high blood Phe disturbs the concentration of other large neutral amino acids in the brain (de Groot et al. 2010; Martynyuk et al. 2010). Moreover, high Phe levels have been related to oxidative stress in blood from patients (Sierra et al. 1998; van Bakel et al. 2000; Schulpis et al. 2005; Sitta et al. 2009a, b; Sanayama et al. 2011), in the brain of animal models of the disease (Ercal et al. 2002; Moraes et al. 2014) and in in vitro experiments (Hagen et al. 2002; Sitta et al. 2009b; Fernandes et al. 2010; Moraes et al. 2010).
PKU treatment is based on a Phe-restricted diet, which aims to prevent high Phe concentrations in blood and tissues (Surtees and Blau 2000). Although efficient in lowering Phe levels, this diet is extremely hard to follow (Vilaseca et al. 2010). Therefore, other treatment strategies are still needed for PKU in order to improve patients’ clinical and biochemical outcomes. In this way, exercise could be a concomitant treatment in PKU. Exercising regularly can lead to peripheral and central adaptations such as strengthening brain antioxidant capacity (Elokda and Nielsen 2007; Radak et al. 2007; Tsou et al. 2015) and improving dopaminergic and serotoninergic systems (Stroth et al. 2010; Wipfli et al. 2011; Chang et al. 2012; Lin and Kuo 2013). Additionally, aerobic exercise has improved cognition in elderly individuals (Kirk-Sanchez and McGough 2014) and in patients with neurodegenerative diseases (Petzinger et al. 2013; Radak et al. 2010). Moreover, in rats chemically subjected to hyperphenylalaninemia, regular exercise improved the brain antioxidant system (Mazzola et al. 2011). However, little is known about the effects of exercise in PKU and whether it can be beneficial for patients. Therefore, this study aimed to determine the effects of voluntary exercise on behavioral and biochemical parameters in a genetic mouse model of PKU, by the means of motor coordination and balance performance, plasma and brain amino acid concentrations, and brain oxidative stress parameters.
Methods
Animals
All the experimental procedures were approved by the Animal Welfare Committee of the University of Groningen, the Netherlands. A total of 40 adult (4 months old) C57Bl/6 homozygous (−/−) PAHenu2 (PKU) and (+/+) PAHenu2 (wild type, WT) female mice were used in this experiment. WT and PKU animals were individually housed and randomly assigned to sedentary (Sed) or exercise (Exe) groups. Mice were given water and regular chow ad libitum, while kept in a 12:12-h light–dark regime and weighed weekly.
Voluntary Exercise
Mice from ExeWT and ExePKU had free access to a running wheel placed in their home cage throughout the experiment, i.e., 4 days of acclimatization plus 53 days of voluntary training. Daily running wheel activity was calculated as described before (Mulder et al. 2014).
Balance Beam Test
The balance beam is a sensorimotor integration test, which focuses on hind limb functioning (Carter et al. 1999; Soderling et al. 2003). The apparatus consisted of a 50-mm wide beam with a “safe cage” placed at the end of it. Animals performed nonconsecutive four trials (5, 10, 40, and 100 cm) on the beam, which were recorded. The number of hind limb steps and slips was counted in the 100-cm trial using the video files.
Tissue Preparation
Animals were sacrificed by cervical dislocation. Blood was centrifuged at 1,500 × g for 10 min and then plasma was harvested and stored at −80°C. The total brain was immediately frozen in liquid nitrogen. Shortly before analysis, brain tissue was grinded in liquid nitrogen and then divided into weighed aliquots. Later, the aliquots were homogenized in specific buffers as required for each technique and sonified (30 s per sample at 11–12 W). The brain homogenates were then centrifuged at 1,000 × g for 10 min at 4°C, and the supernatant was used for the biochemical measurements.
Plasma and Brain Amino Acid Levels
Brain homogenates were prepared using phosphate-buffered saline (pH 7.4) at a 1:4 weight to volume ratio (mg/μL). Plasma and brain amino acid concentrations were determined using HPLC coupled to derivatization with ninhydrin, according to the manufacturer’s protocol (Pharmacia Biotech, Cambridge, UK).
Oxidative Stress Parameters
Cerebral tissue was homogenized in 50 mM Tris–HCl buffer containing 1 mM EDTA (pH 8.2) at a 1:10 (w/v) ratio. All measurements were normalized by protein concentration using albumin as standard (Lowry et al. 1951).
Superoxide Dismutase (SOD) Activity Assay
This assay is based on the capacity of pyrogallol to autoxidize and on the ability of SOD to inhibit this reaction (Marklund 1985). Therefore, SOD activity can be indirectly assayed spectrophotometrically at 420 nm by comparing the samples’ values with a standard curve. These data are expressed as percentage of control (%SedWT).
Sulfhydryl Content
5,5′dithiobis(2-nitrobenzoic acid) (DTNB) color reagent is reduced by thiols, thus generating a yellow derivative (TNB) which can be spectrophotometrically read at 412 nm (Aksenov and Markesbery 2001). Oxidation of free thiol groups in proteins leads to the formation of disulfide bonds, which will not react with DTNB. Therefore, the sulfhydryl content is inversely correlated to oxidative damage to proteins. The results are expressed as nmol TNB/mg protein.
Reduced Glutathione (GSH) Content
This method is based on the reaction of GSH with the fluorophore ortho-phthalaldehyde (Browne and Armstrong 1998). Briefly, metaphosphoric acid was used to deproteinize samples, which were then centrifuged at 1,000 × g for 10 min. Then, sodium phosphate buffer at pH 8.0 and ortho-phthalaldehyde 1 mg/mL solution were added to the samples’ supernatants. After standing in the dark for 15 min, the fluorescence of this mixture was measured at excitation 350 nm and emission 420 nm. A calibration curve was made with a commercial GSH solution, and the results were expressed as μmol GSH/mg protein.
Total Radical-Trapping Antioxidant Potential (TRAP) and Total Antioxidant Reactivity (TAR)
TRAP and TAR were determined by measuring the chemiluminescence intensity of luminol induced by ABAP thermolysis (free radical source) in a scintillation counter (Evelson et al. 2001). After adding 3 mL of 10 mM ABAP and 10 μL of 5.6 mM luminol to scintillation vials, the initial light intensity was obtained. Ten microliters of 160 μM Trolox or 30 μL of sample was added to assess antioxidant content. At this point, the luminescence intensity is practically abolished. The consumption of active antioxidants present in samples results in the return of the luminescence (TRAP). For each sample, the time required to return of luminescence (TAR) was compared to that obtained by employing Trolox under identical experimental conditions. Values were calculated as Trolox equivalents and were represented as nmol Trolox/mg protein.
Statistical Analysis
The statistical analyses were performed with the Pearson’s correlation coefficient, independent Student’s t-test, or two-way ANOVA followed by the Tukey post hoc test for multiple comparisons and repeated measures ANOVA for longitudinal analyses. The SPSS was used and p < 0.05 was considered to be statistically significant. Number of animals per group varied due to technical sampling problems or due to exclusion of outliers (values that were two or more SDs away from the group mean).
Results
In order to evaluate the effects of voluntary exercise in PKU mice, we performed a study in which WT and PKU animals had free access to running wheels (Exe groups) and compared these to animals that did not have the apparatus in their home cages (Sed groups). As shown in Fig. 1, ExePKU group ran significantly less than did the ExeWT group (6,064 ± 1,937 m/day and 10,627 ± 4,868 m/day, respectively, p = 0.018), and the effect of the genotype was significant (p = 0.027). Exercise did not modify body weight (p = 0.758), and both PKU groups were lighter than WT groups throughout the experiment (p = 0.001) (Fig. 1).
Fig. 1.
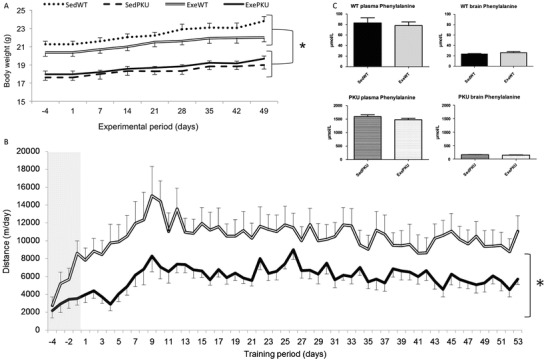
(a) Body weight from sedentary (Sed) and exercise (Exe) wild-type (WT) and PAHenu2 (PKU) mice, (b) daily running wheel activity ExeWT and ExePKU mice during the 4-day acclimatization period (gray area) followed by 53 days of training and (c) phenylalanine levels in the plasma and brain of Sed and Exe WT and PKU. Data are shown as mean ± SEM (n = 10/group, except for plasma phenylalanine in SedPKU where n = 8). *p < 0.05, repeated measures ANOVA
PKU animals from both Sed and Exe groups showed poor performance in the balance beam test as compared to WT mice, as shown in Table 1. When crossing the beam, both PKU groups had a higher number of steps (p < 0.001) and slips (p < 0.001) in comparison to SedWT, representing deficits in motor coordination and balance, respectively. Neither Exe group differed from the Sed groups, therefore showing no exercise effect for this task.
Table 1.
Balance beam test outcomes
| SedWT | SedPKU | ExeWT | ExePKU | |
|---|---|---|---|---|
| Time (s) | 19 ± 9 | 22 ± 9 | 11 ± 5 | 17 ± 5 |
| Number of steps | 40 ± 3 | 54 ± 7* | 39 ± 6 | 50 ± 7* |
| Number of slips | 8 ± 5 | 29 ± 10* | 9 ± 8 | 27 ± 11* |
Results are expressed as mean ± SD (n = 10/group)
*p < 0.001, compared to SedWT (Tukey post hoc)
As shown before (Ney et al. 2008; Solverson et al. 2012; Sawin et al. 2014), Phe levels in plasma and brain of SedPKU mice were higher in comparison to respective levels in SedWT group (p < 0.001). Exercise did not modify Phe levels when comparing each Exe group to its Sed control (Fig. 1). A comparison of plasma values between PKU groups showed that exercise decreased levels of alanine (p = 0.038), citrulline (p = 0.002), glutamine (p = 0.040), glycine (p = 0.006), ornithine (p = 0.008), and proline (p = 0.028) (Fig. 2). Furthermore, exercise reduced brain amino acid levels in ExePKU compared to SedPKU for histidine (p = 0.036), isoleucine (p = 0.011), methionine (p = 0.005), proline (p = 0.005), and valine (p = 0.010), and a positive correlation (r = 0.817; p = 0.004) between brain proline levels and distance run was found only for ExePKU (Fig. 2). No effects of exercise were found on plasma and brain amino acid levels for WT mice (Supplemental Table 1).
Fig. 2.
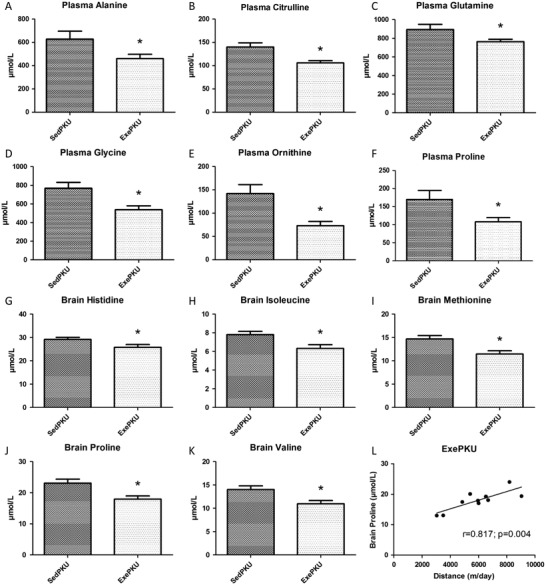
Plasma amino acid levels of (a) alanine, (b) citrulline, (c) glutamine, (d) glycine, (e) ornithine, and (f) proline and brain amino acid levels of (g) histidine, (h) isoleucine, (i) methionine, (j) proline, (k) valine, and (l) correlation between brain proline levels and distance ran in sedentary (Sed) and exercise (Exe) PAHenu2 (PKU) mice. Results are expressed as mean ± SEM (n = 10/group, except for plasma levels in SedPKU where n = 8). *p < 0.05
Regarding oxidative stress in the brain, the SedPKU group showed lower superoxide dismutase activity (p = 0.029), sulfhydryl (p = 0.043), GSH (p = 0.007), and TRAP (p = 0.003) in comparison to the SedWT group (Fig. 3). Exercise prevented those changes, so the ExePKU group reached levels similar to SedWT for SOD, sulfhydryl, TRAP and also tended to restore GSH levels (p = 0.049). Furthermore, exercise led to higher levels of TAR only for the ExePKU group in comparison to the SedPKU group (p = 0.012), although TAR levels were not lower in SedPKU in comparison to SedWT. No exercise effect was found for WT animals (ExeWT group).
Fig. 3.
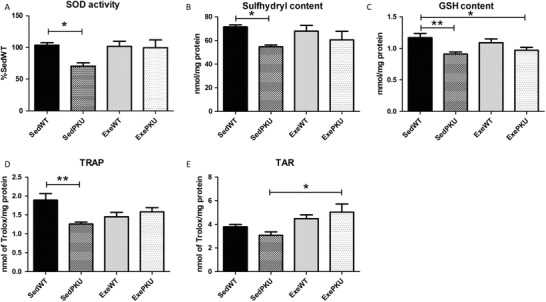
Oxidative stress parameters (a) superoxide dismutase (SOD) activity, (b) sulfhydryl and (c) reduced glutathione (GSH) contents, (d) total radical-trapping antioxidant potential (TRAP), and (e) total antioxidant reactivity (TAR) in the brain of sedentary (Sed) and exercise (Exe) wild-type (WT) and PAHenu2 (PKU) mice. Results are expressed as mean ± SEM (n = 8–10/group). *p < 0.05 and **p < 0.01
Discussion
To the best of our knowledge, this study was the first to evaluate the long-term effects of voluntary exercise in a genetic mouse model of PKU. The protocol used was voluntary exercise by animals having free access to running wheels in their home cages. Therefore, Exe animals could run whenever and for as long as they desired. PKU mice, although running less than controls, improved brain oxidative stress markers, while no changes in plasma and blood Phe levels were found.
The lower running wheel activity of the ExePKU group might be caused by their specific motor problems and/or early fatigue. Both SedPKU and ExePKU groups showed worse performance in the balance beam task than WT mice. As various brain regions are affected by Phe toxicity (Qin and Smith 2007; Fernandes et al. 2010), the balance beam findings indicate possible motor cortex and cerebellum impairments in this PKU mouse model. Voluntary training has been effective in improving motor performance in the rotarod test in healthy mice (Clark et al. 2008). However, the exercise used in the present study did not improve balance beam outcomes in both PKU and WT groups. In this way, perhaps this exercise protocol was not able to address those skills specifically necessary for crossing a narrow beam. On the other hand, the exercise used in the present study decreased plasma glucogenic amino acid levels only in the PKU mice. Skeletal muscles produce alanine to get rid of nitrogen groups during amino acid catabolism, thus preventing accumulation of ammonia to toxic levels (Graham and MacLean 1998). The lower plasma levels of alanine, glutamate precursors (glutamine and proline), and urea cycle intermediates (ornithine and citrulline) in ExePKU may indicate that trained PKU mice did not have an efficient nitrogen buffering mechanism. Supporting this hypothesis, supplementations of ornithine, citrulline, and glutamine have shown to postpone fatigue by decreasing blood ammonia in rodents (Meneguello et al. 2003; Takeda et al. 2011; Kim and Kim 2013). In this way, the PKU mice in the present study might have run less than WT due to fatigue, besides motor problems.
Exercise decreased brain levels of glucogenic amino acids, including branched-chain amino acids (BCAA) that were not changed in plasma. Lower availability of brain large neutral amino acids in PKU has been related to the clinical problems caused by high Phe levels, and this can be explained by the a critical imbalance in the competition to cross the blood–brain barrier when Phe is relatively more concentrated (de Groot et al. 2010). However, in the present study, among the amino acids that were decreased in plasma of ExePKU mice, proline was the only amino acid also decreased in the brain in comparison to SedPKU. Moreover, as concentrations of Phe and other large neutral amino acids did not change in the brain due to exercise, competition between aromatic amino acids and BCAA might not have hampered BCAA uptake in ExePKU in comparison to SedPKU. This observation could indicate an increased amino acid metabolism to yield energy, as the brain has high activity of the key enzymes especially for BCAA catabolism (Piscopo et al. 2011). Furthermore, brain proline levels show a positive correlation with the daily distance run only for PKU mice (r = 0.817; p = 0.004); therefore, the amount of running, and hence the amount of training, might have influenced this result.
The PKU mouse model used in this study showed oxidative stress in the brain by lower levels of SOD activity, sulfhydryl, and GSH contents and TRAP levels, which was mostly prevented by the voluntary exercise. Solverson et al. (2012) have found metabolic stress in the same strain of PKU mice (C57BL/6). As the brain is the most affected organ in PKU, our results corroborate the already stated hypothesis that oxidative stress is involved in the pathophysiology of the disease (Ribas et al. 2011). While exercise did not change any oxidative stress parameter in the control (WT) mice, the PKU group benefitted from exercising. ExePKU had SOD, sulfhydryl content and TRAP restored to control levels, and increased TAR in comparison to SedPKU. In this study, PKU animals that voluntarily exercised showed similar oxidative stress parameters to those of controls, thus preventing the impairments caused by PKU without changing brain Phe levels. Previous research on voluntary wheel running has shown enhancement of antioxidant enzymatic activity in arteries of old mice thus preventing age-related oxidative stress (Durrant et al. 2009). Furthermore, exercise has been shown to prevent oxidative stress in the brain of animal models of neurodegenerative diseases (Ang et al. 2010; Souza et al. 2013) as well as in hyperphenylalaninemia (Mazzola et al. 2011). In the same way, exercise improved brain oxidative stress parameters in PKU animals in the present study.
The focus of PKU treatment strategies should not only be on reducing Phe but also on enhancing central parameters that are impaired by high Phe levels (van Spronsen et al. 2009; van Vliet et al. 2015). In this way, the intermittent stress caused by exercise might be able to overcome high Phe issues and improve PKU outcomes. Moreover, even though PKU animals showed less physical activity than WT mice, only the PKU group showed changes in amino acid and oxidative stress levels. Therefore, PKU mice were more responsive to exercise effects. Future studies might evaluate the effects of exercise when introduced at early ages as well as in male mice, therefore shedding light in the importance of physical activity in this population. Although therapeutic strategies in PKU primarily address high Phe-related problems, the beneficial effects of exercise on other PKU-related problems as shown here should open new avenues in combined treatment strategies.
Conclusions
Exercise decreased levels of glucogenic amino acids in plasma and brain of PKU mice, but did not improve motor coordination or balance. Voluntary exercise training prevented oxidative stress in the brain of PKU mice without changing Phe levels in the plasma or brain. Therefore, exercise may be a concomitant strategy for PKU patients to improve brain redox status and hence brain biochemistry and function.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Acknowledgments
This research project has been made possible thanks to a fellowship from PKU Academy under the auspices of EXCEMED, Excellence in Medical Education, the Abel Tasman Talent Program from the University Medical Center Groningen and the University of Groningen. We thank Pim de Blaauw for the amino acid analyses and Wanda Douwenga and Jan Keijser for their technical support.
Abbreviations
- BCAA
Branched-chain amino acid
- Exe
Exercise
- GSH
Reduced glutathione
- PAH
Phenylalanine hydroxylase
- Phe
Phenylalanine
- PKU
Phenylketonuria
- Sed
Sedentary
- SOD
Superoxide dismutase
- TAR
Total antioxidant reactivity
- TRAP
Total radical-trapping antioxidant potential
- WT
Wild type
Concise 1: Sentence Take-Home Message
Voluntary training improved brain oxidative stress and reduced brain and plasma glucogenic amino acids in phenylketonuria mice without changing phenylalanine levels.
Compliance with Ethics Guidelines
Conflict of Interest
Priscila Nicolao Mazzola, Vibeke Bruinenberg, Karen Anjema, Danique van Vliet, Carlos Severo Dutra-Filho, Francjan J. van Spronsen, and Eddy A. van der Zee declare that they have no conflict of interest.
Animal Rights
All institutional and national guidelines for the care and use of laboratory animals were followed.
Details of the Contribution of Individual Authors
Priscila Nicolao Mazzola, Vibeke Bruinenberg, Karen Anjema, and Danique van Vliet collected the data. Priscila Nicolao Mazzola performed the statistical analyses and drafted the manuscript. All authors participated in the study design, contributed to the interpretation of the results, and revised the manuscript.
Footnotes
Electronic supplementary material
The online version of this chapter (doi:10.1007/8904_2015_498) contains supplementary material, which is available to authorized users.
Competing interests: None declared
Contributor Information
Priscila Nicolao Mazzola, Email: pku@priscilamazzola.com.
Collaborators: Matthias Baumgartner, Marc Patterson, Shamima Rahman, Verena Peters, Eva Morava, and Johannes Zschocke
References
- Aksenov MY, Markesbery WR. Changes in thiol content and expression of glutathione redox system genes in the hippocampus and cerebellum in Alzheimer’s disease. Neurosci Lett. 2001;302:141–145. doi: 10.1016/S0304-3940(01)01636-6. [DOI] [PubMed] [Google Scholar]
- Ang ET, Tai YK, Lo SQ, Seet R, Soong TW. Neurodegenerative diseases: exercising toward neurogenesis and neuroregeneration. Front Aging Neurosci. 2010;2:25. doi: 10.3389/fnagi.2010.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne RW, Armstrong D. Reduced glutathione and glutathione disulfide. Methods Mol Biol. 1998;108:347–352. doi: 10.1385/0-89603-472-0:347. [DOI] [PubMed] [Google Scholar]
- Carter RJ, Lione LA, Humby T, et al. Characterization of progressive motor deficits in mice transgenic for the human Huntington’s disease mutation. J Neurosci. 1999;19:3248–3257. doi: 10.1523/JNEUROSCI.19-08-03248.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YK, Liu S, Yu HH, Lee YH. Effect of acute exercise on executive function in children with attention deficit hyperactivity disorder. Arch Clin Neuropsychol. 2012;27:225–237. doi: 10.1093/arclin/acr094. [DOI] [PubMed] [Google Scholar]
- Clark PJ, Brzezinska WJ, Thomas MW, Ryzhenko NA, Toshkov SA, Rhodes JS. Intact neurogenesis is required for benefits of exercise on spatial memory but not motor performance or contextual fear conditioning in C57BL/6J mice. Neuroscience. 2008;155:1048–1058. doi: 10.1016/j.neuroscience.2008.06.051. [DOI] [PubMed] [Google Scholar]
- de Groot MJ, Hoeksma M, Blau N, Reijngoud DJ, van Spronsen FJ. Pathogenesis of cognitive dysfunction in phenylketonuria: review of hypotheses. Mol Genet Metab. 2010;99(Suppl 1):S86–S89. doi: 10.1016/j.ymgme.2009.10.016. [DOI] [PubMed] [Google Scholar]
- Durrant JR, Seals DR, Connell ML, et al. Voluntary wheel running restores endothelial function in conduit arteries of old mice: direct evidence for reduced oxidative stress, increased superoxide dismutase activity and down-regulation of NADPH oxidase. J Physiol. 2009;587:3271–3285. doi: 10.1113/jphysiol.2009.169771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elokda AS, Nielsen DH. Effects of exercise training on the glutathione antioxidant system. Eur J Cardiovasc Prev Rehabil. 2007;14:630–637. doi: 10.1097/HJR.0b013e32828622d7. [DOI] [PubMed] [Google Scholar]
- Ercal N, Aykin-Burns N, Gurer-Orhan H, McDonald JD. Oxidative stress in a phenylketonuria animal model. Free Radic Biol Med. 2002;32:906–911. doi: 10.1016/S0891-5849(02)00781-5. [DOI] [PubMed] [Google Scholar]
- Evelson P, Travacio M, Repetto M, Escobar J, Llesuy S, Lissi EA. Evaluation of total reactive antioxidant potential (TRAP) of tissue homogenates and their cytosols. Arch Biochem Biophys. 2001;388:261–266. doi: 10.1006/abbi.2001.2292. [DOI] [PubMed] [Google Scholar]
- Fernandes CG, Leipnitz G, Seminotti B, et al. Experimental evidence that phenylalanine provokes oxidative stress in hippocampus and cerebral cortex of developing rats. Cell Mol Neurobiol. 2010;30:317–326. doi: 10.1007/s10571-009-9455-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez MJ, Gutierrez AP, Gassio R, Fuste ME, Vilaseca MA, Campistol J. Neurological complications and behavioral problems in patients with phenylketonuria in a follow-up unit. Mol Genet Metab. 2011;104(Suppl):S73–S79. doi: 10.1016/j.ymgme.2011.07.015. [DOI] [PubMed] [Google Scholar]
- Graham TE, MacLean DA. Ammonia and amino acid metabolism in skeletal muscle: human, rodent and canine models. Med Sci Sports Exerc. 1998;30:34–46. doi: 10.1097/00005768-199801000-00006. [DOI] [PubMed] [Google Scholar]
- Hagen MEK, Pederzolli CD, Sgaravatti AM, et al. Experimental hyperphenylalaninemia provokes oxidative stress in rat brain. Biochim Biophys Acta. 2002;1586:344–352. doi: 10.1016/S0925-4439(01)00112-0. [DOI] [PubMed] [Google Scholar]
- Jahja R, Huijbregts SC, de Sonneville LM, van der Meere JJ, van Spronsen FJ. Neurocognitive evidence for revision of treatment targets and guidelines for phenylketonuria. J Pediatr. 2014;164:895.e2–899.e2. doi: 10.1016/j.jpeds.2013.12.015. [DOI] [PubMed] [Google Scholar]
- Kim DI, Kim KS. Walnut extract exhibits anti-fatigue action via improvement of exercise tolerance in mice. Lab Anim Res. 2013;29:190–195. doi: 10.5625/lar.2013.29.4.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk-Sanchez NJ, McGough EL. Physical exercise and cognitive performance in the elderly: current perspectives. Clin Interv Aging. 2014;9:51–62. doi: 10.2147/CIA.S39506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin TW, Kuo YM. Exercise benefits brain function: the monoamine connection. Brain Sci. 2013;3:39–53. doi: 10.3390/brainsci3010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Marklund SL. Pyrogallol autoxidation. In: Greenwald RA, editor. Handbook of methods for oxygen radical research. Boca Raton: CRC; 1985. pp. 243–247. [Google Scholar]
- Martynyuk AE, van Spronsen FJ, Van der Zee EA. Animal models of brain dysfunction in phenylketonuria. Mol Genet Metab. 2010;99(Suppl 1):S100–S105. doi: 10.1016/j.ymgme.2009.10.181. [DOI] [PubMed] [Google Scholar]
- Mazzola PN, Terra M, Rosa AP, et al. Regular exercise prevents oxidative stress in the brain of hyperphenylalaninemic rats. Metab Brain Dis. 2011;26:291–297. doi: 10.1007/s11011-011-9264-8. [DOI] [PubMed] [Google Scholar]
- Meneguello MO, Mendonca JR, Lancha AH, Jr, Costa Rosa LF. Effect of arginine, ornithine and citrulline supplementation upon performance and metabolism of trained rats. Cell Biochem Funct. 2003;21:85–91. doi: 10.1002/cbf.1000. [DOI] [PubMed] [Google Scholar]
- Moraes TB, Zanin F, da Rosa A, et al. Lipoic acid prevents oxidative stress in vitro and in vivo by an acute hyperphenylalaninemia chemically-induced in rat brain. J Neurol Sci. 2010;292:89–95. doi: 10.1016/j.jns.2010.01.016. [DOI] [PubMed] [Google Scholar]
- Moraes TB, Dalazen GR, Jacques CE, de Freitas RS, Rosa AP, Dutra-Filho CS. Glutathione metabolism enzymes in brain and liver of hyperphenylalaninemic rats and the effect of lipoic acid treatment. Metab Brain Dis. 2014;29:609–615. doi: 10.1007/s11011-014-9491-x. [DOI] [PubMed] [Google Scholar]
- Mulder CK, Papantoniou C, Gerkema MP, Van Der Zee EA. Neither the SCN nor the adrenals are required for circadian time-place learning in mice. Chronobiol Int. 2014;31:1075–1092. doi: 10.3109/07420528.2014.944975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ney DM, Hull AK, van Calcar SC, Liu X, Etzel MR. Dietary glycomacropeptide supports growth and reduces the concentrations of phenylalanine in plasma and brain in a murine model of phenylketonuria. J Nutr. 2008;138:316–322. doi: 10.1093/jn/138.2.316. [DOI] [PubMed] [Google Scholar]
- Petzinger GM, Fisher BE, McEwen S, Beeler JA, Walsh JP, Jakowec MW. Exercise-enhanced neuroplasticity targeting motor and cognitive circuitry in Parkinson’s disease. Lancet Neurol. 2013;12:716–726. doi: 10.1016/S1474-4422(13)70123-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piscopo P, Crestini A, Adduci A, et al. Altered oxidative stress profile in the cortex of mice fed an enriched branched-chain amino acids diet: possible link with amyotrophic lateral sclerosis? J Neurosci Res. 2011;89:1276–1283. doi: 10.1002/jnr.22655. [DOI] [PubMed] [Google Scholar]
- Qin M, Smith CB. Regionally selective decreases in cerebral glucose metabolism in a mouse model of phenylketonuria. J Inherit Metab Dis. 2007;30:318–325. doi: 10.1007/s10545-007-0583-1. [DOI] [PubMed] [Google Scholar]
- Radak Z, Kumagai S, Taylor AW, Naito H, Goto S. Effects of exercise on brain function: role of free radicals. Appl Physiol Nutr Metab. 2007;32:942–946. doi: 10.1139/H07-081. [DOI] [PubMed] [Google Scholar]
- Radak Z, Hart N, Sarga L, et al. Exercise plays a preventive role against Alzheimer’s disease. J Alzheimers Dis. 2010;20:777–783. doi: 10.3233/JAD-2010-091531. [DOI] [PubMed] [Google Scholar]
- Ribas GS, Sitta A, Wajner M, Vargas CR. Oxidative stress in phenylketonuria: what is the evidence? Cell Mol Neurobiol. 2011;31:653–662. doi: 10.1007/s10571-011-9693-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanayama Y, Nagasaka H, Takayanagi M, et al. Experimental evidence that phenylalanine is strongly associated to oxidative stress in adolescents and adults with phenylketonuria. Mol Genet Metab. 2011;103:220–225. doi: 10.1016/j.ymgme.2011.03.019. [DOI] [PubMed] [Google Scholar]
- Sawin EA, Murali SG, Ney DM. Differential effects of low-phenylalanine protein sources on brain neurotransmitters and behavior in C57Bl/6-Pah(enu2) mice. Mol Genet Metab. 2014;111:452–461. doi: 10.1016/j.ymgme.2014.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulpis KH, Tsakiris S, Traeger-Synodinos J, Papassotiriou I. Low total antioxidant status is implicated with high 8-hydroxy-2-deoxyguanosine serum concentrations in phenylketonuria. Clin Biochem. 2005;38:239–242. doi: 10.1016/j.clinbiochem.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Sierra C, Vilaseca MA, Moyano D, et al. Antioxidant status in hyperphenylalaninemia. Clin Chim Acta. 1998;276:1–9. doi: 10.1016/S0009-8981(98)00091-6. [DOI] [PubMed] [Google Scholar]
- Sitta A, Barschak AG, Deon M, et al. L-carnitine blood levels and oxidative stress in treated phenylketonuric patients. Cell Mol Neurobiol. 2009;29:211–218. doi: 10.1007/s10571-008-9313-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitta A, Manfredini V, Biasi L, et al. Evidence that DNA damage is associated to phenylalanine blood levels in leukocytes from phenylketonuric patients. Mutat Res. 2009;679:13–16. doi: 10.1016/j.mrgentox.2009.07.013. [DOI] [PubMed] [Google Scholar]
- Soderling SH, Langeberg LK, Soderling JA, et al. Loss of WAVE-1 causes sensorimotor retardation and reduced learning and memory in mice. Proc Natl Acad Sci U S A. 2003;100:1723–1728. doi: 10.1073/pnas.0438033100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solverson P, Murali SG, Brinkman AS, et al. Glycomacropeptide, a low-phenylalanine protein isolated from cheese whey, supports growth and attenuates metabolic stress in the murine model of phenylketonuria. Am J Physiol Endocrinol Metab. 2012;302:E885–E895. doi: 10.1152/ajpendo.00647.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza LC, Filho CB, Goes AT, et al. Neuroprotective effect of physical exercise in a mouse model of Alzheimer’s disease induced by beta-amyloid(1)(-)(4)(0) peptide. Neurotox Res. 2013;24:148–163. doi: 10.1007/s12640-012-9373-0. [DOI] [PubMed] [Google Scholar]
- Stroth S, Reinhardt RK, Thone J, et al. Impact of aerobic exercise training on cognitive functions and affect associated to the COMT polymorphism in young adults. Neurobiol Learn Mem. 2010;94:364–372. doi: 10.1016/j.nlm.2010.08.003. [DOI] [PubMed] [Google Scholar]
- Surtees R, Blau N. The neurochemistry of phenylketonuria. Eur J Pediatr. 2000;159(Suppl 2):S109–S113. doi: 10.1007/PL00014370. [DOI] [PubMed] [Google Scholar]
- Takeda K, Machida M, Kohara A, Omi N, Takemasa T. Effects of citrulline supplementation on fatigue and exercise performance in mice. J Nutr Sci Vitaminol. 2011;57:246–250. doi: 10.3177/jnsv.57.246. [DOI] [PubMed] [Google Scholar]
- Tsou YH, Shih CT, Ching CH, et al. Treadmill exercise activates Nrf2 antioxidant system to protect the nigrostriatal dopaminergic neurons from MPP+ toxicity. Exp Neurol. 2015;263:50–62. doi: 10.1016/j.expneurol.2014.09.021. [DOI] [PubMed] [Google Scholar]
- van Bakel MM, Printzen G, Wermuth B, Wiesmann UN. Antioxidant and thyroid hormone status in selenium-deficient phenylketonuric and hyperphenylalaninemic patients. Am J Clin Nutr. 2000;72:976–981. doi: 10.1093/ajcn/72.4.976. [DOI] [PubMed] [Google Scholar]
- van Spronsen FJ, Hoeksma M, Reijngoud DJ. Brain dysfunction in phenylketonuria: is phenylalanine toxicity the only possible cause? J Inherit Metab Dis. 2009;32:46–51. doi: 10.1007/s10545-008-0946-2. [DOI] [PubMed] [Google Scholar]
- van Vliet D, Anjema K, Jahja R, et al. BH4 treatment in BH4-responsive PKU patients: preliminary data on blood prolactin concentrations suggest increased cerebral dopamine concentrations. Mol Genet Metab. 2015;114:29–33. doi: 10.1016/j.ymgme.2014.11.009. [DOI] [PubMed] [Google Scholar]
- Vilaseca MA, Lambruschini N, Gomez-Lopez L, et al. Quality of dietary control in phenylketonuric patients and its relationship with general intelligence. Nutr Hosp. 2010;25:60–66. [PubMed] [Google Scholar]
- Weglage J, Fromm J, van Teeffelen-Heithoff A, et al. Neurocognitive functioning in adults with phenylketonuria: results of a long term study. Mol Genet Metab. 2013;110(Suppl):S44–S48. doi: 10.1016/j.ymgme.2013.08.013. [DOI] [PubMed] [Google Scholar]
- Wipfli B, Landers D, Nagoshi C, Ringenbach S. An examination of serotonin and psychological variables in the relationship between exercise and mental health. Scand J Med Sci Sports. 2011;21:474–481. doi: 10.1111/j.1600-0838.2009.01049.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


