Abstract
Chronic pain with comorbid emotional disorders is a prevalent neurological disease in patients under various pathological conditions, yet patients show considerable difference in their vulnerability to developing chronic pain. Understanding the neurobiological basis underlying this pain vulnerability is essential to develop targeted therapies of higher efficiency in pain treatment of precision medicine. However, this pain vulnerability has not been addressed in preclinical pain research in animals to date. In this study, we investigated individual variance in both sensory and affective/emotional dimensions of pain behaviors in response to chronic neuropathic pain condition in a mouse model of chronic pain. We found that mice displayed considerably diverse sensitivities in the chronic pain-induced anxiety- and depression-like behaviors of affective pain. Importantly, the mouse group that was more vulnerable to developing anxiety was also more vulnerable to developing depressive behavior under the chronic pain condition. In contrast, there was relatively much less variance in individual responses in the sensory dimension of pain sensitization. Molecular analysis revealed that those mice vulnerable to developing the emotional disorders showed a significant reduction in the protein level of DNA methyltransferase 3a in the emotion-processing central nucleus of the amygdala. In addition, social stress also revealed significant individual variance in anxiety behavior in mice. These findings suggest that individual pain vulnerability may be inherent mostly in the emotional/affective component of chronic pain and remain consistent in different aspects of negative emotion, in which adaptive changes in the function of DNA methyltransferase 3a for DNA methylation in central amygdala may play an important role. This may open a new avenue of basic research into the neurobiological mechanisms underlying pain vulnerability.
Keywords: Pain vulnerability, DNA methyltransferase 3a, negative emotion, central amygdala, affective pain, chronic pain
Background
Chronic pain is one of the most prevalent neurological diseases; however, clinical reports have clearly shown that individuals vary substantially in their pain responses and vulnerability to developing chronic pain.1 This individual pain vulnerability can be manifested as large variability in developing chronic pain under a similar pathological condition, in variable pain magnitude and sensitivity under acute and chronic pain conditions, and in differential responses to analgesic drugs.2,3 In many pain-causing injuries or diseases, not all patients actually develop chronic pain.1,2,4,5 Besides the intrinsic features such as gender and age, there are many known risk factors that can predispose individuals to the development of chronic pain, including genetic and epigenetic changes, biological adaptations, neuropsychological disorders, and prior stressful life events including pain experience. Understanding of these risk factors is critical in order to develop targeted therapies with much increased analgesic efficiency for treatment of chronic pain. However, while some studies have addressed genetics-based group variance in pain experience and responses,1,2,6 preclinical research is absent in addressing individual vulnerability for developing chronic pain and underlying neurobiological mechanisms in animals.
Pain as a subjective and adverse experience has two dimensions: sensory and affective.7–9 Clinically, affective dimension of pain includes sensory pain-induced emotional disorders such as stress, anxiety, and depression, which are often comorbid with chronic sensitization of sensory pain and further exacerbate pain sensitivity, chronicity, and experience with substantial variance in individual vulnerability and drug response.10–13 In preclinical research, numerous studies have shown that chronic pain induces anxiety- and depression-like behaviors of affective pain in animal models of pain.14–22 As affective pain is processed and modulated by the corticolimbic circuits including the amygdala that integrate many brain functions including emotion, reward, and memory,23–25 it may greatly influence individual variability and susceptibility in pain experience and substantially contribute to the development of chronic pain. In fact, the central nucleus of the amygdala (CeA) is known as a key brain structure that regulates emotion-related behaviors and integrates responses to chronic pain and resultant emotion disorders in both humans and animals.24,26–29
Mechanistic studies on individual pain vulnerability and associated risk factors require valid animal pain models, which are currently lacking. Among the potential risk factors, a prominent one is likely epigenetic changes, as adaptive chromatin modifications are induced by life events and experience that are individual based.1 Recently, epigenetic mechanisms have been critically implicated in the pathogenesis of chronic pain in rapidly growing numbers of studies including ours.30–37 Particularly, DNA methylation, a more persistent chromatin modification for gene repression,38–42 attracts increased attention for the mechanisms of chronic pain in the most recent studies.43–45 Therefore, in this study, we analyzed individual variance and vulnerability in development of chronic pain-induced behaviors of affective/emotion disorders as well as sensory pain sensitization, and explored potential involvement of DNA methyltransferase 3a (Dnmt3a), a key epigenetic regulator for DNA methylation, in pain vulnerability.
Materials and methods
Animals
Adult male C57BL/6J mice and CD-1 strain mice were used in this study. Mice were housed in constant room temperature with free access to food and water. All procedures involving the use of animals conformed to the guidelines by the University of Texas MD Anderson Cancer Center Animal Care and Use Committee.
Neuropathic pain model
A mouse model of chronic neuropathic pain was induced by partial sciatic nerve ligation (PNL) adopted from a previous report.46 Surgical procedures were performed under isoflurane anesthesia. After skin shaving and cleaning on the right thigh, an incision of 1 cm long was made below the pelvis. The muscles and tendons in the area were then bluntly dissected to expose the sciatic nerve at the mid-thigh level. The partial nerve injury was produced by a ligature with 9-0 silk suture by approximately 1/3 to 1/2 of the diameter of the sciatic nerve. In sham-operated control mice, the sciatic nerve was exposed but left intact without ligation. The wound was then closed with one muscle suture and two stainless steel wound clips. After the surgery, the animals were carefully monitored until fully ambulatory before being returned to their home cages.
Measurement of sensory pain
Thresholds of sensory pain were measured by the paw-withdrawal test with von Frey filaments for mechanical allodynia and with the Hargreaves analgesia apparatus (Stoelting, Wood dale, IL) for thermal hyperalgesia on a freely moving mouse. Pain thresholds were measured one day before the PNL or sham surgery for baseline and once every three days after the surgery for up to 30 days.
Open field test
Open field test (OFT) is a well-established classic test for anxiety-like behavior and general locomotor activity in rodents.47–49 After habituation, a mouse was placed in one corner of an open arena (length, width, and height = 42 cm) and was allowed to freely explore and move in the arena for 15 min in a test session. A central zone was defined by a 21 × 21 cm area in the center of the arena. Locomotor activity was recorded automatically by an automated video-tracking system (EthoVision 8.0 software, Noldus, Attleboro, MA). Time the mouse spent in the central zone (central time) and total distance traveled during the 15 min session were recorded and analyzed for each mouse. A decrease in the central time was considered as an index of anxiety-like behavior. For analysis of individual variance, those PNL mice with central times less than the averaged central time of sham control mice were assigned to vulnerable group and those with central times more than the control average assigned to resistant group. This group assignment remained the same for analyses of these two mouse groups in other behavioral tests of emotion and sensory pain.
Elevated plus maze test
The elevated plus maze test (EPMT) is another widely used behavioral test for anxiety and the first-choice test for screening anxiolytic drugs in rodents.48–50 The EPMT apparatus was consisted of two opposite open arms and two closed arms (31 × 6.5 × 15 cm) elevated 38 cm above the floor. In an EPMT, a mouse was placed in the junction of the four arms facing an open arm and was allowed 5 min of free exploration. Time spent in open and closed arms (expressed as % of time spent in the open arms vs. the time spent in all arms) and total distance traveled during the 5 min test period were recorded and analyzed by the video-tracking system. A decrease in the time spent in the open arms was considered as an index of anxiety-like behavior. EPMT results of the vulnerable and resistant mouse groups assigned by OFT were compared, and each mouse was matched to its performance in OFT.
Forced swim test
Forced swim test (FST) is a main behavioral test for depression-like behaviors in rodents and for screening antidepressant drugs.51 A mouse was placed in a clear plastic cylinder filled with water (20–30 cm deep, 25 ± 1℃) for 6 min and was recorded with the video-tracking system. Time the animal spent immobile during the test was considered as a measure of despair-like behavior. Immobility was defined as cessation of all active swimming and escaping activities. The same vulnerable and resistant mouse groups as assigned by OFT were compared for their performance in FST.
Model of social defeat stress
Mouse model of social defeat stress (SDS) was induced according to the procedures in previous reports.52,53 CD1 mice were screened and selected for use as aggressors. For SDS, a C57BL/6J mouse was exposed to a novel CD1 aggressor for 5 min daily in a home cage and then separated from the aggressor by a perforated barrier for the remainder of the day. This procedure was repeated for 10 consecutive days. Control mice were subjected to the same procedures but without the presence of an aggressor. After the last social defeat episode, the stressed and control mice were housed individually. Behavioral test of social interaction (SI) was performed 24 h after the last defeat episode in an open arena that included a small wire-mesh enclosure centered against one wall of the arena. An interaction zone was defined by an area projecting 8 cm around the wire-mesh enclosure. In an SI test, a stressed or control mouse was placed in a corner of the arena, and locomotor activity was recorded for 250 s without an aggressor, followed immediately by an additional 250 s recording with a novel CD1 aggressor in the wire-mesh enclosure. Locomotor activity, including time spent in the interaction zone (interaction time) and total distance traveled, was automatically recorded with the video-tracking system. An SI ratio was calculated for each mouse by: SI ratio = interaction time with the aggressor/interaction time without the aggressor. In the stressed group, mice with an SI ratio <1 was assigned to susceptible group and those with an SI ratio >1 to resilient group.53
Western blots
After the behavior experiment, the mouse was deeply anesthetized by isoflurane and decapitated within 1 h. The brain was cut in a vibratome in cold (4℃) artificial cerebrospinal fluid to obtain brain slices (0.5 mm thick). Both sides of CeA from mice of vulnerable and resistant groups were punched out from the slices with a blunt-end syringe needle (0.8 mm inner diameter). CeA tissues were frozen in liquid nitrogen and stored in a −80℃ freezer. Tissue homogenates were prepared with radioimmunoprecipitation assay buffer (Cell signaling), and protein concentration was determined by bicinchoninic acid analysis (Thermo). Equal protein amount (10 µg) of samples was used for immunoreactivity of Dnmt3a and β-actin (Abcam Biotechnology, Cambridge, MA). The immunopositive signals were quantified by Quantity One software (Bio-Rad), and protein levels were normalized to β-actin.
Statistics analysis
Unpaired, two-tailed student’s t test was used to compare simple averages of two groups. One-way analysis of variance (ANOVA) with post hoc analysis was used to compare and analyze experimental data of multiple groups for multiple comparisons. Behavioral data with multiple measurements were analyzed by two-way ANOVA for repeated measures. The Bonferronic method was used for post hoc tests in both one-way and two-way ANOVA. Data are presented as mean ± SEM, and p < 0.05 was considered statistically significant. All statistical analyses were performed with the Prism software version 6 (GraphPad Software, La Jolla, CA).
Results
Nerve injury induces chronic sensitization of sensory pain with little individual variance
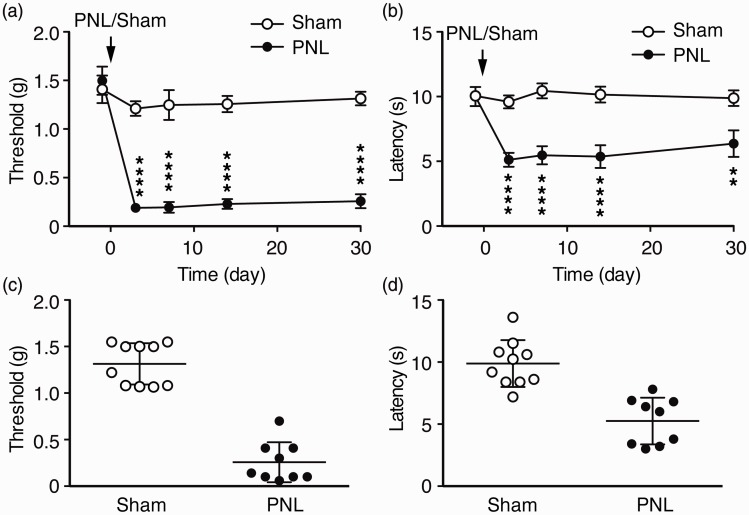
We used the mouse model of chronic pain induced by PNL46–49 to analyze the sensory pain-associated negative emotion behaviors. Compared to control mice that received sham surgery, PNL mice displayed significant and long-lasting sensitized behavior of sensory pain as mechanical allodynia and thermal hyperalgesia. The allodynia reached maximum three days after PNL surgery and remained for more than 30 days without recovery (Time: F(4, 68) = 20.0, p < 0.0001; PNL: F(1, 17) = 207.8, p < 0.0001; Interaction, F(4, 68) = 12.3, p < 0.0001, Figure 1(a)). The hyperalgesia had a similar time course, lasting more than 30 days with little recovery (Time: F(4, 68) = 4.7, p < 0.001; PNL: F(1, 17) = 87.6, p < 0.0001; Interaction, F(4, 68) = 4.5, p < 0.001, Figure 1(b)). We then examined the data of individual mice in both groups 30 days after the surgery to determine individual variance in the behavior of sensory pain. As shown in Figure 1(c) and (d), the sham mice and PNL mice were clearly separated into two groups with little overlap in their distribution of pain behaviors of both allodynia and hyperalgesia. This suggests that nerve injury induces strong behavioral sensitization of sensory pain in all mice with minimal individual variance.
Figure 1.
Nerve injury induces chronic neuropathic pain. Paw-withdrawal thresholds for mechanical allodynia (a) and paw-withdrawal latencies for thermal hyperalgesia (b) were measured over a 30 days period in sham-operated mice (n = 10) and in mice operated with partial sciatic nerve ligation (PNL, n = 9). Baseline thresholds were measured 1 day before the surgery operated on day 0 (arrows). (c) and (d) Data distribution of individual mice for the sensory pain behaviors in the same sham and PNL mice at 30 days after the surgery. **p < 0.01, ****p < 0.0001.
PNL: partial sciatic nerve ligation.
Individual variance and pain vulnerability are inherent in anxiety behavior
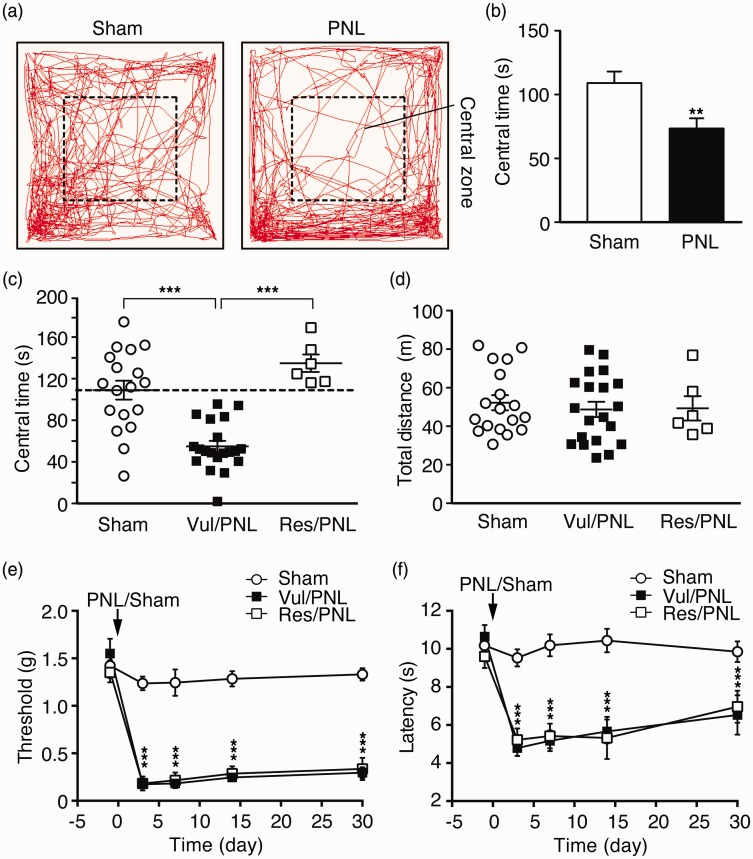
We determined affective behaviors of negative emotion in these control mice and in PNL mice with chronic neuropathic pain, using established behavioral tests for anxiety and depression in rodents. OFT is a well-established behavioral test for anxiety and locomotor activity in rodents.47–49 In OFT, we found that, 30 days after the surgery, the PNL mice showed significant anxiety-like behavior with significantly reduced time spent in the central zone (central time) when compared to the sham mice (sham, 109.0 ± 9.1 s; PNL, 73.5 ± 8.0 s, t = 2.89, p < 0.01, Figure 2(a) and (b)). Further analyzing the data of individual mice, we found a surprisingly wide distribution of central times as the index of anxiety among individual mice in both sham and PNL groups. In the sham group, these control mice displayed large variance in central times (Figure 2(c)), indicating that mice under normal conditions are quite variable in this anxiety-related behavior as measured by OFT. The PNL mice with chronic pain for 30 days also displayed a wide range of central times. Although the distribution data of sham and PNL mice appeared continuous, more PNL mice, compared to sham mice, were clustered to a range of less central times (Figure 2(c)), resulting in the significantly less central times (more anxiety) in the group average of PNL mice as shown in Figure 2(b).
Figure 2.
Chronic pain-induced anxiety-like behavior displays large individual variance. (a) Traces of locomotor activity in a sham and a PNL mouse in an open field test (OFT). (b) Averaged group data of the time spent in the central zone (central time) in sham (n = 18) and PNL (n = 26) mice. (c) Individual data of the same mice groups as in (b), with the PNL mice divided into vulnerable (vul/PNL) group (mice with central times below the group average of central time in sham mice (109.0 s, n = 18) indicated by the dashed line, n = 20) and resistant (res/PNL) group (mice with central times above the average, n = 6). (d) Individual data of total distance traveled in the three mouse groups. Behaviors of mechanical (e) and thermal (f) pain responses in the three mouse groups. *p < 0.05. ***p < 0.001.
PNL: partial sciatic nerve ligation.
To facilitate the analysis and comparison of the difference in the anxiety level among the PNL mice, we used the group average of the sham mice (central time = 109.0 s) as a cutoff line to approximately assign each PNL mouse into two groups: vulnerable group with central time < 109.0 and resistant group with central time >109.0, with a separation margin of more than ±7% from the sham average (Figure 2(c)). This arbitrary group assignment provided an initial means for further analysis of anxiety levels and levels of other emotion behaviors among individual mice although the anxiety levels within each group were recognizably variable. A similar analytic method has been used in previous studies of stress in mice, which also display large individual variance in stress-induced social avoidance behaviors in both control and stressed group.53–55 In our results, one-way ANOVA showed that there was a significant difference among the sham, vulnerable PNL (vul/PNL), and resistant PNL (res/PNL) groups, F(2, 41) = 23.23, p < 0.0001, and post hoc analysis revealed that the central time of vulnerable PNL group was significantly less than that of sham mice (sham, 109.0 ± 9.1 s; vul/PNL, 55.0 ± 5.2 s, p < 0.001) and than that of resistant PNL mice (res/PNL, 135.0 ± 8.4 s, p < 0.001), but no difference between sham and resistant PNL groups (Figure 2(c)). In contrast, there was no significant difference in the total distance traveled by mice among the three mouse groups (Figure 2(d)), which is consistent with a previous report under similar experimental conditions,56 suggesting little influence of locomotor activity on the results of OFT 30 days after the surgery. There was also no significant difference in the PNL-induced sensitization of sensory pain between vulnerable and resistant PNL mice (Figure 2(e) and (f)). Taken together, these results suggest that mice display considerable and inherent individual variance and differential vulnerabilities to sensory pain-induced anxiety-like behavior of affective pain, but little variance in behaviors of sensory pain.
Pain vulnerability is consistent in different anxiety tests
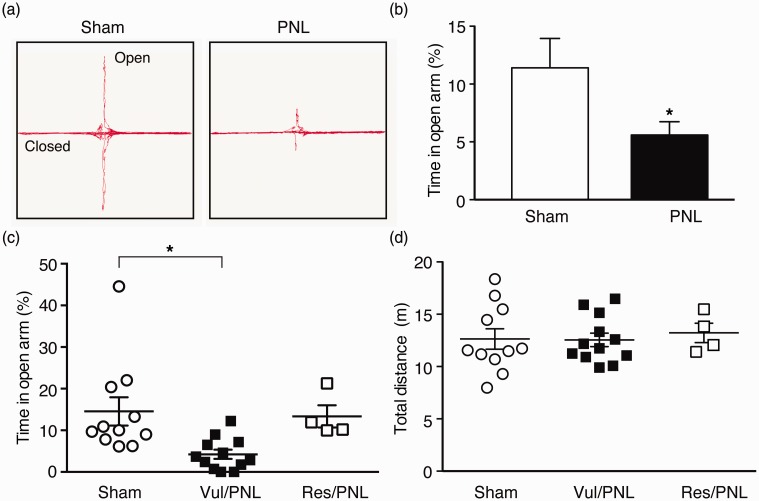
We next examined the anxiety-like behavior of the same mice with the EPMT, another commonly used test for anxiety behavior and for screening anxiolytic drugs in rodents.48–50 When group averages of sham mice and all PNL mice were compared, PNL mice spent significantly less time in open arms (Sham, 11.4 ± 2.5%; PNL, 5.6 ± 1.1%, t = 2.25, p < 0.05, Figure 3(a) and (b)), suggesting again anxiety-like behavior as measured in EPMT in the pain mice 30 days after PNL. Using the same group assignment obtained in OFT above, we analyzed individual data of the vulnerable and resistant PNL mice groups as well as the sham group in EPMT. We found that, while all mouse groups displayed a range of variance in open-arm time, there was a significant difference among sham, vul/PNL, and res/PNL groups as revealed by one-way ANOVA, F(2, 24) = 5.31, p = 0.01. More importantly, the vulnerable PNL mice displayed a higher level of anxiety with significantly reduced open-arm time when compared to sham mice (Sham, 14.6 ± 3.4%; vul/PNL, 4.2 ± 1.1%, p < 0.05), whereas the resistant PNL mice showed no difference from sham mice in open-arm time (sham, 14.6 ± 3.4%; res/PNL, 13.4 ± 2.7%, p > 0.05, Figure 3(c)). There was no significant difference in total distance traveled during the EPMT among the three groups (Figure 3(d)), indicating minimal effect of locomotor activity in the results of EPMT. These results indicate that vulnerable mice defined in OFT were also mostly vulnerable in EPMT for chronic pain-induced anxiety-like behavior although some overlap was present between the two PNL groups. Thus, it appears that the individual variance or vulnerability to developing chronic pain-induced anxiety behavior of affective pain in mice is largely consistent across the two behavioral tests that measure differential aspects of anxiety,48,49 further supporting individual pain vulnerability inherent and manifested in the variable degrees of anxiety behavior in chronic pain development.
Figure 3.
Individual pain vulnerability to anxiety is consistent in a different test of anxiety. (a) Traces of locomotor activity in a sham and PNL mouse in an elevated plus maze test (EPMT). (b) Averaged group data of % time spent in open arms (open-arm time) from the sham mice (n = 11) and PNL mice (n = 16) in EPMT. Individual data of open-arm times (c) and total distance traveled (d) in sham mice (n = 11) and in the vulnerable (n = 12) and resistant (n = 4) PNL mice, which were divided by the OFT above.
PNL: partial sciatic nerve ligation.
Stress vulnerability is also manifested in anxiety behavior
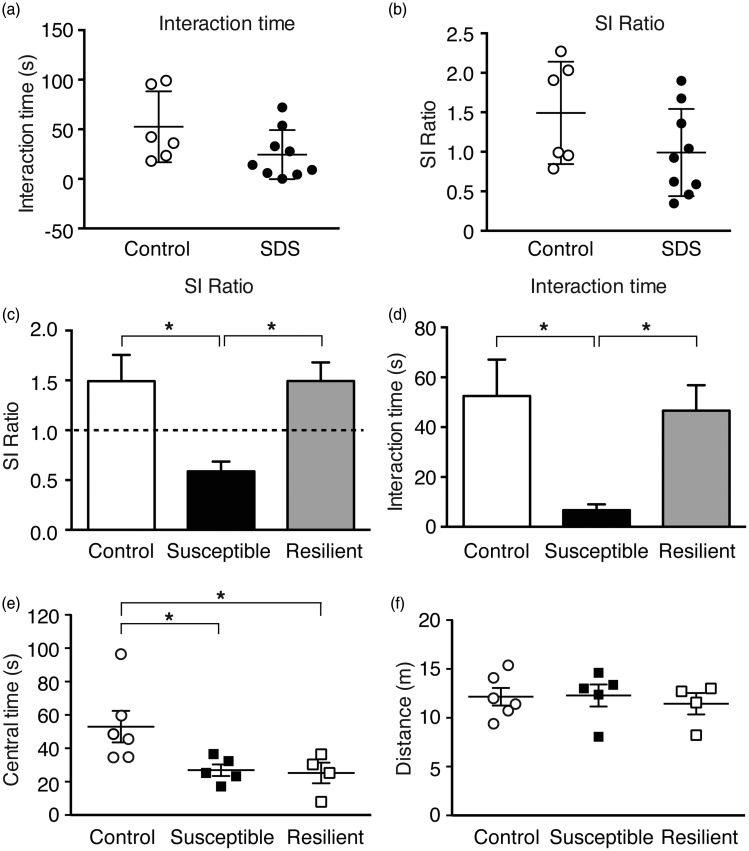
To further assess the pain vulnerability as observed in anxiety behavior induced by sensory pain, an aversive stressor, we examined the effect of stress, another neuropsychological disorder that shows great individual susceptibility in humans, using the mouse model of SDS, which has been demonstrated to display overlapping behaviors of anxiety and depression with large individual variability.53,55 We found that control mice showed a range of variances in the time spent interacting with the aggressor mouse (interaction time); and stressed mice also displayed a similar range of variances in interaction times with a continuous distribution (Figure 4(a)). These individual variances were also reflected in the SI ratio (defined by interaction time with the aggressor/interaction time without the aggressor) in both control and stressed groups (Figure 4(b)). According to the criteria used in previous studies53–55 the SI ratio of 1 (the SI time is equal with and without the aggressor) was used as the threshold to divide the stressed mice into susceptible (SI ratio < 1) and resilient (SI ratio > 1) groups. As shown in Figure 4(c), there was a main difference in the SI ratio of control, susceptible, and resilient mouse groups, F(2, 12) = 6.04, p = 0.01. Post hoc analysis revealed significant difference between control and susceptible groups (p < 0.01) and between susceptible and resilient groups (p < 0.05), but not between control and resilient groups (p > 0.05). Similar results were obtained in the interaction time among the three mouse groups (main: F(2, 12) = 4.96, p < 0.05; control vs. susceptible: p < 0.05; susceptible vs. resilient: p < 0.05; control vs. resilient: p > 0.05, Figure 4(d)). These results of group differences and data distributions are consistent with those reported in the original studies on the SDS model.54,55
Figure 4.
Social stress induces anxiety with individual variability. Individual data of the time spent in the interaction zone (interaction time, (a)) and social interaction (SI) ratios (b) in control mice (n = 6) and in mice treated with social defeat stress (SDS, n = 9). Averaged data of SI ratios (c) and interaction time (d) in control mice (n = 6) and in stressed mice of susceptible group (SI ratio < 1, n = 5) and of resilient group (SI ratio > 1, n = 4). Individual data of central times (e) and total distance traveled (f) in the control mice (n = 6) and in the susceptible (n = 5) and resilient (n = 4) groups of stressed mice defined above in OFT.
SDS: social defeat stress; SI: social interaction.
We then determined the anxiety-like behavior in the SDS model with OFT. We found that the stress treatment had a significant effect on anxiety behavior among the three mouse groups, F(2, 12) = 4.6, p < 0.05. Compared to the control group, both susceptible and resilient groups of stressed mice showed significantly increased anxiety reflected in decreased central time (control, 53.0 ± 9.4 s; sus/stress, 26.9 ± 3.4 s, p < 0.05; res/stress, 25.3 ± 6.3 s, p < 0.05, Figure 4(e)), an observation in line with the previous study.55 There was no difference in total distance traveled among the three mouse groups (Figure 4(f)). This result of social stress-induced anxiety in both stress-susceptible and stress-resistant mice is in strong contrast to that of chronic pain-induced anxiety varying considerably among individual mice, indicating that individual vulnerability in anxiety is dependent on and impacted by different types of stressors such as chronic pain and social stress. These results also further support behavioral validity of the pain-induced individual vulnerability measure by the behavioral tests for anxiety in mice.
Pain vulnerability is consistent in depression behavior
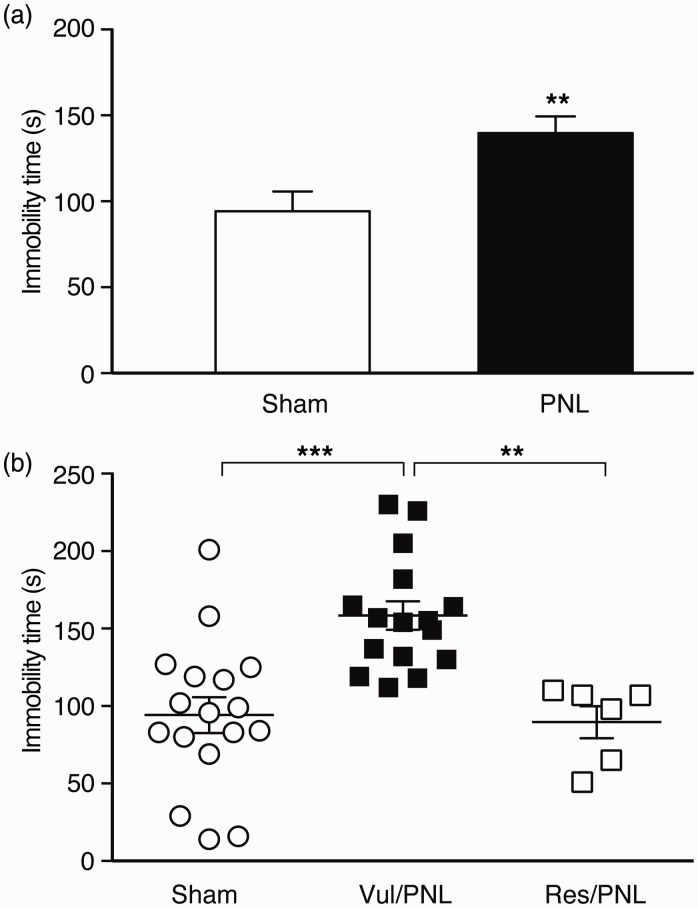
Next, we determined another behavior of negative emotion, depression-like behavior, in the vulnerable and resistant PNL mice as assigned in OFT, using the FST 30 days after the surgery. When averages of the sham mouse group and the pain group of pooled PNL mice were compared, the pain group spent significantly more time in immobility (sham, 94.2 ± 11.6 s; PNL, 139.7 ± 9.7 s, t = 3.02, p < 0.01, Figure 5(a)), indicating chronic pain-induced depressive behavior. When the vulnerable and resistant PNL mice as defined in OFT were compared with the sham group, the pain had a significant effect on the animal behavior of depression, F(2, 36) = 12.2, p < 0.0001. Interestingly, the anxiety-vulnerable mice displayed a significantly higher level of the depression index when compared to either the sham mice (vul/PNL, 158.4 ± 9.1 s; sham, 94.2 ± 11.6 s, p < 0.001) or the anxiety-resistant mice (vul/PNL, 158.4 ± 9.1 s; res/PNL, 89.7 ± 10.3 s, p < 0.01) with no difference between the sham and res/PNL groups (p > 0.05, Figure 5(b)). These findings support an intriguing notion that individual pain vulnerability is inherent in the chronic pain-induced negative emotion that is consistently manifested at least in mouse behaviors of anxiety and depression.
Figure 5.
Individual pain vulnerability is consistent in depression-like behavior. (a) Group data of immobility time in a forced swim test in sham (n = 17) and PNL (n = 22) mice. (b) Individual data of immobility times in the sham mice (n = 17) and PNL mice divided into vulnerable (n = 16) and resistant (n = 6) mice by the OFT above.
PNL: partial sciatic nerve ligation.
Dnmt3a in central amygdala is involved in pain vulnerability
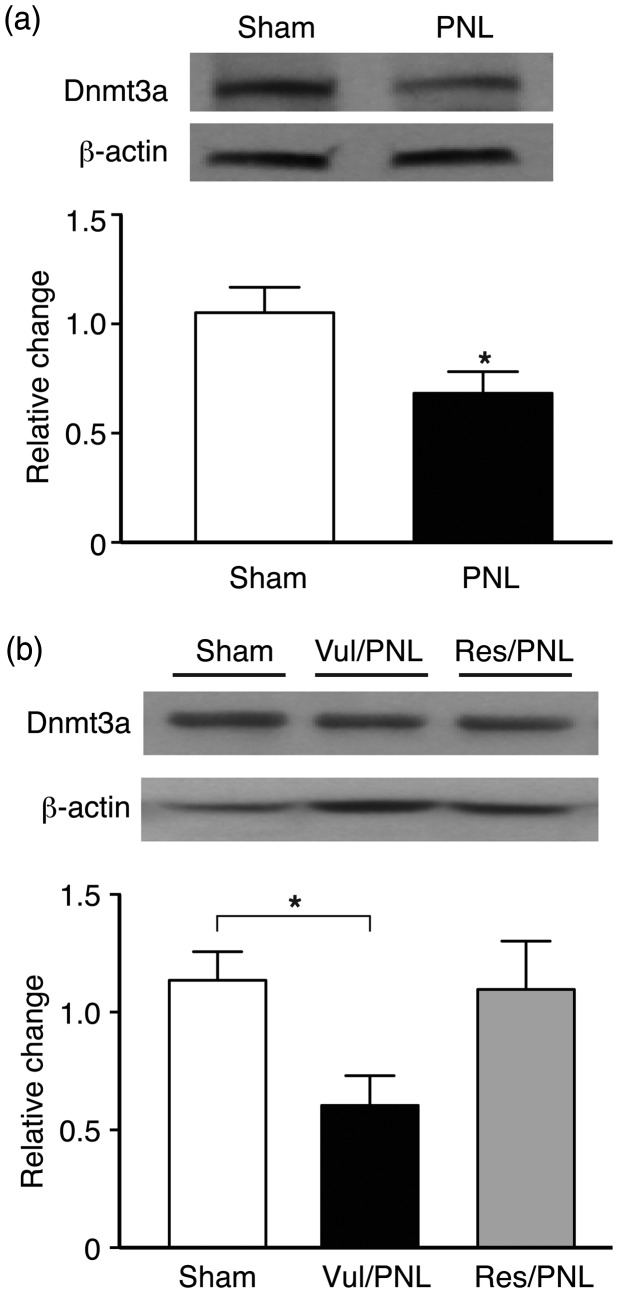
Finally, to explore a potential epigenetic mechanism that underlies the pain vulnerability in negative emotion behaviors described above, we targeted CeA, a brain site critical in processing and integrating emotion-related behaviors including pain, and determined the CeA protein level of the Dnmt3a, which catalyzes de novo DNA methylation in response to environmental and pathological conditions38,39,41,42,57 and has recently been implicated in pain mechanism in peripheral nociceptive neurons.45,58 In CeA tissues collected from different groups mice after the behavioral experiments above, we found that the CeA Dnmt3a protein was dramatically decreased in the mice with chronic pain 30 days after the PNL surgery (t = 2.38, p < 0.05, Figure 6(a)). When the two PNL groups of vulnerable and resistant mice with differential pain vulnerability were considered in data analysis, pain also had a significant overall effect on the mouse groups, F(2, 21) = 4.9, p < 0.05. More interestingly, we found that the anxiety-vulnerable group of pain mice displayed a significant decrease in CeA Dnmt3a protein (p < 0.05), but not in the anxiety-resistant group of pain mice (Figures 6(b)). These findings indicate that Dnmt3a in CeA may play an important role in mediating pain vulnerability reflected in the various degrees of negative emotion behaviors under chronic pain.
Figure 6.
Chronic pain decreases Dnmt3a in central amygdala of only vulnerable mice. (a) Western blots (top) and group data (bottom) of Dnmt3a protein levels in the central nucleus of the amygdala (CeA) from sham mice (n = 13) and PNL mice (n = 11) 30 days after the surgery. (b) Western blots (top) and group data (bottom) of CeA Dnmt3a protein in sham mice (n = 12) and in vulnerable (n = 9) and resistant (n = 3) groups of PNL mice 30 days after the surgery. All Dnmt3a proteins were normalized to β-actin and expressed as levels relative to the average of sham mice.
PNL: partial sciatic nerve ligation; Dnmt3a: DNA methyltransferase 3a.
Discussion
In this study, we have presented original evidence to demonstrate that (1) mice display considerable individual variance in and vulnerability to chronic pain-induced negative emotion of affective pain, but relatively much less in nociceptive response of sensory pain, (2) the pain vulnerability remains largely consistent in different aspects of negative emotion (anxiety and depression), and (3) Dnmt3a in CeA as a key regulator of DNA methylation may play an important role in the pain vulnerability.
Individual variability is a key factor for the development of precision medicine with individual-based, targeted therapies for treatment of various diseases including chronic pain. Identifying vulnerable individuals and underlying risk factors and mechanisms are pivotal process to achieve the goals. However, despite the prevalent clinical evidence for the individual variance in pain responses and vulnerability to developing chronic pain, this issue has not been addressed in current animal studies of pain either in study designs or in data analysis. A close area of pain studies that has well developed is research of pain responses based on sub-populations of humans and animals, i.e., on populations of different genders or differential genetic background.59 Based on our findings in this study, it appears that individual pain vulnerability is likely inherent in the negative emotion responses of affective pain. The affective component of chronic pain is processed by the corticolimbic circuits of the brain that integrate sensory pain information, current emotional and cognitive state, and memory of prior pain and other psychophysiological experience,7,23,60 which are highly individual dependent. Thus, the affective processing and evaluation of pain conditions and resultant behavioral responses is likely a very important factor that largely accounts for the pain vulnerability and plays a key role in the exacerbation and chronification of pain development. The current study presents original evidence supporting this notion and provides an animal model for studies on individual pain vulnerability and underlying mechanisms.
It is highly intriguing to find in this study that the individual pain vulnerability holds true across different aspects of negative emotion under the chronic pain conditions, at least in anxiety- and depression-like behaviors in mice. In other words, mice that are vulnerable to developing anxiety are generally also vulnerable to developing depression under the pain conditions. Given the multidimensional nature of negative emotion, one behavioral test can only evaluate certain aspects of the complex behaviors, and different tests may assess differential as well as overlapping features of the emotion behaviors in rodents.48,49 Our findings with different behavioral measurements provide general and fundamental, though not comprehensive, evaluation of the animals’ emotional status under the chronic pain conditions. Yet, the consistency in this individual pain vulnerability of the animals’ emotional status suggests that some common factors or adaptive responses contribute to the pain vulnerability, which are less prominent in resistant individuals.
DNA methylation as a longer lasting chromatin modification that represses gene expression has been implicated in the pathogenesis of many chronic neurological diseases and recently in the mechanisms of chronic pain.39–42,44 Specifically, most recent reports demonstrate Dnmt3a as an important regulator of DNA methylation in the maladaptive responses of peripheral nociceptive neurons.45,58 Our current results suggest that sensory pain-induced adaptive response in the gene-regulating functions of CeA Dnmt3a could be a crucial process in the development of pain vulnerability to negative emotion. Apparently, our finding of the Dnmt3a involvement is just an important first step to identify the function of CeA Dnmt3a in the pain vulnerability, and extensive further studies are warranted to determine the causal role of CeA Dnmt3a and Dnmt3a-mediated DNA methylation and gene repression in the pain vulnerability. As DNA methylation is a rather general gene-repressing process, and Dnmt3a targets many genes, much more studies are needed to identify the target genes in CeA that are responsible for adaptive changes in CeA circuits and for the consequent emotion behaviors of pain vulnerability.
Our results show that individual mice in sham control group had a wide range of distribution in the indices for anxiety and depression behaviors, indicating that individuals are different in dealing with stressful stimulation (e.g., pain) and environment under normal conditions. This brings an interesting question of whether mice with higher sensitivity in the behavioral indices of negative emotion in normal conditions would also be more sensitive and vulnerable to developing negative emotion under chronic pain. Answering this question would require repeating all the behavioral tests on the same animal before and 30 days after the surgery. The potential issues with this experiment approach include a possible effect of memory with repeated behavioral tests that would confound the test results and a possible impact of surgery itself on the emotion behavior. It is worth pointing out that it is somewhat arbitrary to use the average of sham group to divide the pain group into vulnerable and resistant groups, but it provides an initial step to address the relative vulnerability of pain mice. Further studies with more assigned groups of pain animals and with same-animal comparisons could better address the individual pain vulnerability that appears to have a continuous distribution among individual animals.
It is interesting to note that it seems that a prolonged period (weeks to one month) of pain condition was required for the animals to develop significant affective behaviors of negative emotion. It may reflect a slow process in which constant pain stimulation induces molecular and cellular adaptations in the corticolimbic network that integrates prior and current neuropsychological and environmental factors, and ultimately mediates the emotion behaviors. The individual variance itself would also delay the behavioral measurements to reach a statistically significant level when group averages are compared. Based on the processing nature of affective pain, it is likely that the longer the pain condition is present, the more individuals there are that would develop behavioral disorders of affective pain and the more severe the emotion disorders become, an intriguing topic to be explored in future studies.
In summary, the current study provides some key characteristics of individual variance and pain vulnerability in mice and presents important initial evidence for CeA Dnmt3a as an epigenetic regulator in pain vulnerability. This study may open a new avenue of basic research addressing the issues of individual pain vulnerability in animals. Such future studies will reveal further psychophysiological characteristics of pain vulnerability and identify underlying cellular and molecular mechanisms. Characterization and understanding of individual variability and vulnerability to chronic pain-induced affective disorders in animals will ultimately provide important insights that are critical for the development of individually targeted strategies in clinical treatment and prevention of chronic pain.
Author Contributions
Wei Wang and Zhizhong Z Pan designed the study and experiments. Wei Wang, Caiyue Li, and Youqing Cai performed the behavioral experiments in animals and protein analysis of amygdala tissues. Wei Wang, Caiyue Li, and Zhizhong Z Pan conducted data analysis. Wei Wang and Zhizhong Z Pan wrote the manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interests with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by NIH National Institute of Dental and Craniofacial Research grant DE025943.
References
- 1.Denk F, McMahon SB, Tracey I. Pain vulnerability: a neurobiological perspective. Nat Neurosci 2014; 17: 192–200. [DOI] [PubMed] [Google Scholar]
- 2.Mogil JS. Pain genetics: past, present and future. Trends Genet 2012; 28: 258–266. [DOI] [PubMed] [Google Scholar]
- 3.Twycross R, Ross J, Kotlinska-Lemieszek A, et al. Variability in response to drugs. J Pain Symptom Manage 2015; 49: 293–306. [DOI] [PubMed] [Google Scholar]
- 4.Coghill RC, McHaffie JG, Yen YF. Neural correlates of interindividual differences in the subjective experience of pain. Proc Natl Acad Sci U S A 2003; 100: 8538–8542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet 2006; 367: 1618–1625. [DOI] [PubMed] [Google Scholar]
- 6.Zorina-Lichtenwalter K, Meloto CB, Khoury S, et al. Genetic predictors of human chronic pain conditions. Neuroscience 2016; 338: 36–62. [DOI] [PubMed] [Google Scholar]
- 7.Fields H. State-dependent opioid control of pain. Nat Rev Neurosci 2004; 5: 565–575. [DOI] [PubMed] [Google Scholar]
- 8.Johansen JP, Fields HL, Manning BH. The affective component of pain in rodents: direct evidence for a contribution of the anterior cingulate cortex. Proc Natl Acad Sci U S A 2001; 98: 8077–8082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neugebauer V, Galhardo V, Maione S, et al. Forebrain pain mechanisms. Brain Res Rev 2009; 60: 226–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Altindag O, Gur A, Altindag A. The relationship between clinical parameters and depression level in patients with myofascial pain syndrome. Pain Med 2008; 9: 161–165. [DOI] [PubMed] [Google Scholar]
- 11.Miller LR, Cano A. Comorbid chronic pain and depression: who is at risk? J Pain 2009; 10: 619–627. [DOI] [PubMed] [Google Scholar]
- 12.Wiech K, Tracey I. The influence of negative emotions on pain: behavioral effects and neural mechanisms. Neuroimage 2009; 47: 987–994. [DOI] [PubMed] [Google Scholar]
- 13.Descalzi G, Ikegami D, Ushijima T, et al. Epigenetic mechanisms of chronic pain. Trends Neurosci 2015; 38: 237–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alba-Delgado C, Llorca-Torralba M, Horrillo I, et al. Chronic pain leads to concomitant noradrenergic impairment and mood disorders. Biol Psychiatry 2013; 73: 54–62. [DOI] [PubMed] [Google Scholar]
- 15.Goffer Y, Xu D, Eberle SE, et al. Calcium-permeable AMPA receptors in the nucleus accumbens regulate depression-like behaviors in the chronic neuropathic pain state. J Neurosci 2013; 33: 19034–19044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ji G, Fu Y, Ruppert KA, et al. Pain-related anxiety-like behavior requires CRF1 receptors in the amygdala. Mol Pain 2007; 3: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Narita M, Kaneko C, Miyoshi K, et al. Chronic pain induces anxiety with concomitant changes in opioidergic function in the amygdala. Neuropsychopharmacology 2006; 31: 739–750. [DOI] [PubMed] [Google Scholar]
- 18.Parent AJ, Beaudet N, Beaudry H, et al. Increased anxiety-like behaviors in rats experiencing chronic inflammatory pain. Behav Brain Res 2012; 229: 160–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roeska K, Doods H, Arndt K, et al. Anxiety-like behaviour in rats with mononeuropathy is reduced by the analgesic drugs morphine and gabapentin. Pain 2008; 139: 349–357. [DOI] [PubMed] [Google Scholar]
- 20.Terzi D, Gaspari S, Manouras L, et al. RGS9-2 modulates sensory and mood related symptoms of neuropathic pain. Neurobiol Learn Mem 2014; 115: 43–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu MG, Chen J. Preclinical research on pain comorbidity with affective disorders and cognitive deficits: challenges and perspectives. Prog Neurobiol 2014; 116: 13–32. [DOI] [PubMed] [Google Scholar]
- 22.Norman GJ, Karelina K, Zhang N, et al. Stress and IL-1beta contribute to the development of depressive-like behavior following peripheral nerve injury. Mol Psychiatry 2010; 15: 404–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Apkarian AV, Baliki MN, Geha PY. Towards a theory of chronic pain. Prog Neurobiol 2009; 87: 81–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baliki MN, Apkarian AV. Nociception, pain, negative moods, and behavior selection. Neuron 2015; 87: 474–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fields HL, Margolis EB. Understanding opioid reward. Trends Neurosci 2015; 38: 217–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neugebauer V. Amygdala pain mechanisms. Handb Exp Pharmacol 2015; 227: 261–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shackman AJ, Salomons TV, Slagter HA, et al. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat Rev Neurosci 2011; 12: 154–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vogt BA. Pain and emotion interactions in subregions of the cingulate gyrus. Nat Rev Neurosci 2005; 6: 533–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zald DH. The human amygdala and the emotional evaluation of sensory stimuli. Brain Res Brain Res Rev 2003; 41: 88–123. [DOI] [PubMed] [Google Scholar]
- 30.Denk F, McMahon SB. Chronic pain: emerging evidence for the involvement of epigenetics. Neuron 2012; 73: 435–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Descalzi G, Ikegami D, Ushijima T, et al. Epigenetic mechanisms of chronic pain. Trends Neurosci 2015; 38: 237–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Doehring A, Geisslinger G, Lotsch J. Epigenetics in pain and analgesia: an imminent research field. Eur J Pain 2011; 15: 11–16. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Z, Cai YQ, Zou F, et al. Epigenetic suppression of GAD65 expression mediates persistent pain. Nat Med 2011; 17: 1448–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chiechio S, Zammataro M, Morales ME, et al. Epigenetic modulation of mGlu2 receptors by histone deacetylase inhibitors in the treatment of inflammatory pain. Mol Pharmacol 2009; 75: 1014–1020. [DOI] [PubMed] [Google Scholar]
- 35.Zhao X, Tang Z, Zhang H, et al. A long noncoding RNA contributes to neuropathic pain by silencing Kcna2 in primary afferent neurons. Nat Neurosci 2013; 16: 1024–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hou YY, Cai YQ, Pan ZZ. Persistent pain maintains morphine-seeking behavior after morphine withdrawal through reduced MeCP2 repression of GluA1 in rat central amygdala. J Neurosci 2015; 35: 3689–3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou CH, Zhang MX, Zhou SS, et al. SIRT1 attenuates neuropathic pain by epigenetic regulation of mGluR1/5 expressions in type 2 diabetic rats. Pain 2017; 158: 130–139. [DOI] [PubMed] [Google Scholar]
- 38.Bestor TH. The DNA methyltransferases of mammals. Hum Mol Genet 2000; 9: 2395–2402. [DOI] [PubMed] [Google Scholar]
- 39.Bird A. DNA methylation patterns and epigenetic memory. Genes Dev 2002; 16: 6–21. [DOI] [PubMed] [Google Scholar]
- 40.Jones PA, Baylin SB. The fundamental role of epigenetic events in cancer. Nat Rev Genet 2002; 3: 415–428. [DOI] [PubMed] [Google Scholar]
- 41.Dulac C. Brain function and chromatin plasticity. Nature 2010; 465: 728–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schubeler D. Function and information content of DNA methylation. Nature 2015; 517: 321–326. [DOI] [PubMed] [Google Scholar]
- 43.Jiang BC, He LN, Wu XB, et al. Promoted interaction of C/EBPalpha with demethylated Cxcr3 gene promoter contributes to neuropathic pain in mice. J Neurosci 2017; 37: 685–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Livshits G, Malkin I, Freidin MB, et al. Genome-wide methylation analysis of a large population sample shows neurological pathways involvement in chronic widespread musculoskeletal pain (CWP). Pain 2017; 158: 1053–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhao JY, Liang L, Gu X, et al. DNA methyltransferase DNMT3a contributes to neuropathic pain by repressing Kcna2 in primary afferent neurons. Nat Commun 2017; 8: 14712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Malmberg AB, Basbaum AI. Partial sciatic nerve injury in the mouse as a model of neuropathic pain: behavioral and neuroanatomical correlates. Pain 1998; 76: 215–222. [DOI] [PubMed] [Google Scholar]
- 47.Fernando AB, Robbins TW. Animal models of neuropsychiatric disorders. Annu Rev Clin Psychol 2011; 7: 39–61. [DOI] [PubMed] [Google Scholar]
- 48.Haller J, Alicki M. Current animal models of anxiety, anxiety disorders, and anxiolytic drugs. Curr Opin Psychiatry 2012; 25: 59–64. [DOI] [PubMed] [Google Scholar]
- 49.Ramos A. Animal models of anxiety: do I need multiple tests? Trends Pharmacol Sci 2008; 29: 493–498. [DOI] [PubMed] [Google Scholar]
- 50.Walf AA, Frye CA. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc 2007; 2: 322–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fernando AB, Robbins TW. Animal models of neuropsychiatric disorders. Annu Rev Clin Psychol 2011; 7: 39–61. [DOI] [PubMed] [Google Scholar]
- 52.Covington HE, 3rd, Maze I, Sun H, et al. A role for repressive histone methylation in cocaine-induced vulnerability to stress. Neuron 2011; 71: 656–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Golden SA, Covington HE, 3rd, Berton O, et al. A standardized protocol for repeated social defeat stress in mice. Nat Protoc 2011; 6: 1183–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Berton O, McClung CA, Dileone RJ, et al. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science 2006; 311: 864–868. [DOI] [PubMed] [Google Scholar]
- 55.Krishnan V, Han MH, Graham DL, et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell 2007; 131: 391–404. [DOI] [PubMed] [Google Scholar]
- 56.Urban R, Scherrer G, Goulding EH, et al. Behavioral indices of ongoing pain are largely unchanged in male mice with tissue or nerve injury-induced mechanical hypersensitivity. Pain 2011; 152: 990–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Martinowich K, Hattori D, Wu H, et al. DNA methylation-related chromatin remodeling in activity-dependent BDNF gene regulation. Science 2003; 302: 890–893. [DOI] [PubMed] [Google Scholar]
- 58.Sun L, Zhao JY, Gu X, et al. Nerve injury-induced epigenetic silencing of opioid receptors controlled by DNMT3a in primary afferent neurons. Pain 2017; 158: 1153–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mogil JS, Bailey AL. Sex and gender differences in pain and analgesia. Prog Brain Res 2010; 186: 141–157. [DOI] [PubMed] [Google Scholar]
- 60.Price DD. Psychological and neural mechanisms of the affective dimension of pain. Science 2000; 288: 1769–1772. [DOI] [PubMed] [Google Scholar]