Abstract
Background
Ethanol-evoked oxidative stress, which contributes to myocardial dysfunction in proestrus rats, is mediated by increases in NADPH oxidase activity, malondialdehyde (MDA) and ERK1/2 phosphorylation. Whether these biochemical responses, which are triggered by alcohol-derived acetaldehyde in non-cardiac tissues, occur in proestrus rats’ hearts remains unknown. Therefore, we elucidated the roles of alcohol dehydrogenase (ADH), cytochrome P4502E1 (CYP2E1) and catalase, which catalyze alcohol oxidation to acetaldehyde, in these alcohol-evoked biochemical and hemodynamic responses in proestrus rats.
Method
Conscious proestrus rats prepared for measurements of left ventricular (LV) function and blood pressure (BP) received ethanol (1.5 g/kg, i.v infusion over 30 min) or saline 30 min after an ADH and CYP2E1 inhibitor, 4-MP (82 mg/kg, i.p), a catalase inhibitor, 3-AT (0.5 g/kg, i.v), their combination or vehicle. LV function and BP were monitored for additional 60 min after ethanol or saline infusion before collecting the hearts for ex vivo measurements of LV reactive oxygen species (ROS), NADPH oxidase activity, MDA, and ERK1/2 phosphorylation.
Results
Ethanol reduced LV function (dP/dtmax and LVDP) and BP, and increased cardiac NADPH oxidase activity, ROS and MDA levels, and ERK1/2 phosphorylation. Either inhibitor partially, and their combination significantly, attenuated these responses despite the substantially higher blood ethanol level, and the increased cardiac oxidative stress and reduced BP caused by 3-AT alone or with 4-MP. The inhibitors reduced cardiac MDA level and reversed ethanol effect on cardiac and plasma MDA.
Conclusions
Ethanol oxidative metabolism plays a pivotal role in the ethanol-evoked LV oxidative stress and dysfunction in proestrous rats. Notably, catalase inhibition (3-AT) caused cardiac oxidative stress and hypotension.
Keywords: Ethanol, Myocardial Dysfunction, Alcohol Dehydrogenase, Cytochrome P4502E1, Catalase, Oxidative Stress
INTRODUCTION
Alcohol metabolism plays an important role in organ injury, particularly through the generation of reactive oxygen species (ROS) (Zakhari, 2006). Hepatic alcohol dehydrogenase (ADH) along with cytochrome P4502E1 (CYP2E1) and catalase, in other tissues such as brain (Zakhari, 2006), are major enzymes that catalyze ethanol oxidation to a highly reactive and toxic metabolite, acetaldehyde. While ethanol-evoked oxidative stress is implicated in heart injury (Matyas et al., 2016, Piano and Phillips, 2014, Piano, 2002), this evidence is based on chronic exposure to ethanol. It is noteworthy that our studies unraveled oxidative stress-related myocardial dysfunction caused by acute ethanol only in the presence of endogenous estrogen (E2) (Ibrahim et al., 2014, El-Mas and Abdel-Rahman, 2014, Yao and Abdel-Rahman, 2016) or exogenous E2 administration to OVX (ovariectomized) (El-Mas and Abdel-Rahman, 2000) or male (El-Mas and Abdel-Rahman, 2015) rats. However, the role of these metabolizing enzymes in the alcohol-evoked myocardial oxidative stress/dysfunction in proestrus rats remains unknown.
Ethanol-evoked increase in ADH catalytic activity is associated with increases in malondialdehyde (MDA) and ROS levels (Kalaz et al., 2012, El-Mas and Abdel-Rahman, 2014, Hoek et al., 2002, Haorah et al., 2005). Notably, ROS is a product of oxidation of polyunsaturated fatty acids, and MDA level reflects lipid peroxidation (Grotto et al., 2009). ADH inhibition abrogated ethanol-evoked oxidative stresses (Nishitani and Matsumoto, 2006), and protected hepatocytes from ethanol-induced apoptosis (Bailey and Cunningham, 1998). Whether ADH inhibition protects against the acute ethanol-evoked myocardial dysfunction in female rats remains unknown. Besides ADH, the CYP2E1 plays an important role in ethanol oxidation in liver or other organs (Zimatkin et al., 2006, Zimatkin and Deitrich, 1997). CYP2E1 activity is induced by chronic (Takahashi et al., 1993) or acute (Haorah et al., 2005) alcohol administration. Further, CYP2E1 inhibition, knockdown or knockout attenuated oxidative stress (Jin et al., 2013, Lu et al., 2008, Bardag-Gorce et al., 2000), and myocardial contractile dysfunction (Zhang et al., 2013) caused by alcohol. Nonetheless, dual pharmacological inhibition of ADH and CYP2E1 by 4-MP (Plumlee et al., 2005) may not be sufficient to fully inhibit ethanol oxidative metabolism in the presence of E2.
Catalase, the third enzyme that catalyzes ethanol oxidation to acetaldehyde (Zimatkin and Deitrich, 1997), also serves an antioxidant role in many tissues including heart (Chelikani et al., 2004). The latter role is consistent with the ability of catalase overexpression to protect against ethanol-evoked dysfunction of myocytes isolated from male mice (Zhang et al., 2003). By marked contrast, we showed that acute ethanol-evoked increase in cardiac catalase activity was associated with myocardia oxidative stress/dysfunction in E2 replete rats (Ibrahim et al., 2014, El-Mas and Abdel-Rahman, 2014, Yao and Abdel-Rahman, 2016). Based on these contradictory findings it remains unknown whether catalase protects against or contributes to ethanol-evoked myocardial dysfunction in our model system. While sex difference and approaches (isolated myocytes vs. intact animal LV function studies) must be considered, it is notable that pharmacological catalase inhibition reduced oxidation of alcohol (Thurman and Handler, 1989), and attenuated ethanol-induced behavioral effects (Koechling and Amit, 1994, Pastor and Aragon, 2008). Whether a similar pharmacological approach exacerbates or attenuates the E2-dependent myocardial oxidative stress/dysfunction in female rats has not been investigated.
The aim of this study was to elucidate the mechanistic roles of the ethanol metabolizing enzymes, ADH, CYP2E1 and catalase, in the E2-dependent myocardial oxidative stress and dysfunction caused by acute ethanol in female rats.
MATERIAL AND METHODS
Animals
Female SD rats (200–250 g, Charles River, Raleigh, NC) were kept in the university animal care center at temperature of 23 ± 1°C, humidity of 50 ± 10%, and a 12-h light/dark cycle and with access free to food (Prolab Rodent Chow, Granville Milling, Creedmoor, NC) and water. The surgical and animal care procedures were approved by the East Carolina University Institutional Animal Care and Use Committee and were consistent with the Guide for the Care and Use of Laboratory Animals (2011).
Catheterization
The animals were anesthetized by the mixture of ketamine (90 mg/kg) and xylazine (10 mg/kg i.p). Vascular and left ventricular (LV) catheterizations were performed as detailed in our recent study (Yao and Abdel-Rahman, 2016, Yao and Abdel-Rahman, 2017) under sterile conditions. Briefly, heparinized arterial/venous catheters (Konigsberg Instruments Inc., Pasadena, CA) were placed into the abdominal aorta/vena cava via the femoral artery/vein for monitoring blood pressure (BP) and administering the pharmacological intervention (ethanol metabolizing enzyme inhibitors, ethanol or saline), respectively. The rats received buprenorphine (0.03 mg/kg; s.c) for postoperative analgesia.
Hemodynamic recording
LV function and BP measurements were conducted in conscious freely moving rats as detailed in our previous studies (Yao and Abdel-Rahman, 2016, Ibrahim et al., 2014, Yao and Abdel-Rahman, 2017). BP and LV indices were simultaneously recorded by ML870 (PowerLab 8/30; Colorado Springs, CO) via Gould-Statham pressure transducers (Oxnard-CA).
Plasma ethanol concentration
The whole blood was centrifuged and the plasma was collected for measurement of ethanol concentration as descripted in our previous studies (Yao and Abdel-Rahman, 2016, El-Mas et al., 2008, Yao and Abdel-Rahman, 2017). The reaction between ethanol and ADH took place in a 96-well plate. An Infinite® 200 PRO multimode microplate reader (Tencan Group Ltd., Männedorf, Schweiz) was used to detect the absorbance at 340 nm.
ROS level
We followed the methods in our previous studies (Yao and Abdel-Rahman, 2016, McGee and Abdel-Rahman, 2012, Yao and Abdel-Rahman, 2017). The supernatant of the homogenized myocardial tissue was used to measure the ROS formation by a fluorometric assay using DCFH-DA (Molecular Probes-Thermo Fisher Scientific Inc., Raleigh, NC). The fluorescence intensities were read at 485/530 nm (excitation/emission) with Infinite® 200 PRO multimode microplate readers (Tencan Group Ltd., Männedorf, Schweiz) at 37°C.
NADPH oxidase (Nox) activity
As described in a previous study (La Favor et al., 2013), 20 µl supernatant of the homogenized LV tissue (100 µg protein)/standard was incubated with 10 µM Amplex Red (Molecular Probes, OR), 2.0 U/ml horseradish peroxidase, 30 U/ml superoxide dismutase and 100 µM NADPH (Sigma Aldrich, St. Louis, MO) for 90 min at 37°C. Fluorescence intensity (530 nM ex/590 nM em) was measured.
MDA level
As described in our previous studies (Yao and Abdel-Rahman, 2016, Yao and Abdel-Rahman, 2017), TBARS Assay Kit (Cayman Chemical, Ann Arbor, MI, USA) was used for measuring MDA levels. The MDA-TBA adduct was measured colorimetrically at 530–540 nm.
Western blot
We followed the protocols described in our studies (Yao and Abdel-Rahman, 2016, Ibrahim et al., 2014, Yao and Abdel-Rahman, 2017). The supernatant (40 µg of protein) of the homogenized LV tissue was separated in a 4–12% gel electrophoresis (Novex Tis-Glycine gel, Life Technologies, CA), and transferred to nitrocellulose membrane, and then incubated with a primary antibody (mouse anti-p-ERK1/2 and rabbit anti ERK1/2; 1:500, Cell Signaling, Danvers, MA). Thereafter, the membrane was incubated with a secondary antibody (IRDye680-conjugated goat anti-mouse and IRDye800-conjugated goat anti-rabbit, 1:15000, LI-COR Biosciences, Lincoln, NE), and the protein bands were detected and quantified by Odyssey Infrared Imager (LI-COR Biosciences, Lincoln, NE).
Experimental protocols
Experiment 1. Effect of inhibiting ADH, CYP2E1 or catalase on ethanol-evoked myocardial dysfunction and hypotension in proestrus rats
Our previous findings documented that acute ethanol administration causes myocardial dysfunction (lower dP/dtmax and LVDP), at least partly, via oxidative stress (higher ROS and MDA levels) (Ibrahim et al., 2014, El-Mas and Abdel-Rahman, 2014, Yao and Abdel-Rahman, 2016). Here, we investigated the effect of the ADH and CYP2E1 inhibitor 4-methylpyrazole (4-MP) (Knoester et al., 2002), the catalase inhibitor 3-Amino-1,2,4-triazole (3-AT) (Correa et al., 2008) or their combination on the ethanol-evoked myocardial dysfunction in proestrus rats. The proestrus phase, determined by vaginal smear (Marcondes et al., 2001), coincides with the highest endogenous plasma E2 levels (El-Mas and Abdel-Rahman, 2014). Fifty-two female SD rats, divided into 8 groups (5–8 rats/group), were used in this experiment. The LV function and BP were measured in conscious rats 2 days after LV and femoral vascular catheterization. At least 30 min was allowed for stabilization of the measured variables. The 8 groups were divided into 4 pairs with each pair receiving 4-MP (82 mg/kg, i.p), 3-AT (0.5 g/kg, i.v), their combination or saline 30 min before i.v infusion of ethanol (1.5 g/kg), delivered as 0.39 ml of 50% ethanol per 100 g body weight over 30 min, or equal volume of saline. The dose of 4-MP (Waller et al., 1982, Jamal et al., 2007) and 3-AT (Correa et al., 2008) was based on reported studies. The ethanol regimen was based on our studies (El-Mas and Abdel-Rahman, 2014, El-Mas and Abdel-Rahman, 2015). Mean arterial pressure (MAP), LV developed pressure (LVDP) and maximum rate of LV pressure rise (dP/dtmax) were recorded for 90 min. Blood samples were collected before any treatment and at 30, 60 and 90 min after ethanol or saline infusion. At the end of the hemodynamic measurements, all rats were euthanized, and the hearts were collected for conducting the ex vivo biochemical studies.
Experiment 2. Effect of ADH, CYP2E1 or catalase inhibition on blood ethanol level and mediators of myocardial oxidative stress caused by alcohol
In these ex vivo biochemical studies, we measured the time-course changes in blood ethanol concentration in ethanol-treated rats in the absence or presence of the enzyme inhibitors, 4-MP, 3-AT, or their combination. Further, we measured ROS, MDA, Nox activity, ERK1/2 phosphorylation level in the hearts of the treatment and control groups used in the study under experiment 1, above.
Drugs
Ethanol was purchased from Midwest Grain Products Co. (Weston, MO) and diluted in saline. 3-AT and 4-MP (Sigma Aldrich, St. Louis, MO) were dissolved in saline.
Data analysis and statistics
The hemodynamic responses caused by pretreatment with the inhibitor(s) of ethanol metabolizing enzymes were analyzed by two-tailed t-test vs. their vehicle, saline (Table 1). The areas under the curve for hemodynamic indices and the biochemical data were analyzed by one-way ANOVA followed by post-hoc Tukey’s t-test comparison and Bonferroni correction when the pairwise t-tests were performed. The statistical analysis was conducted with Prism version 5 (GraphPad Software, Inc. La Jolla, CA). Values are presented as mean ± SEM. p < 0.05 was considered significant.
Table 1.
Mean arterial pressure (MAP), left ventricular developed pressure (LVDP) and maximum rate of left ventricular pressure rise (dP/dtmax) values before (baseline) and 30 min after pretreatment with 4-MP, 3-AT, their combination or vehicle (saline). These rat groups subsequently received ethanol or its vehicle (saline). Values are mean± SEM.
| Pretreatment | Group | N | Hemodynamic variable |
Baseline | After pretreatment |
|---|---|---|---|---|---|
| Saline | Saline | 5 | MAP (mmHg) | 99.7±1.7 | 103.0±2.7 |
| LVDP (mmHg) | 161.7±5.1 | 162.3±5.8 | |||
| dP/dtmax (mmHg/s) | 6870±1032 | 6647±1066 | |||
|
| |||||
| Ethanol | 6 | MAP (mmHg) | 101.4±8.1 | 99.4±5.9 | |
| LVDP (mmHg) | 160.3±11.1 | 160.5±11.2 | |||
| dP/dtmax (mmHg/s) | 7227±1236 | 6952±1300 | |||
|
| |||||
| 4-MP | Saline | 7 | MAP (mmHg) | 100.1±5.2 | 95.4±6.1 |
| LVDP (mmHg) | 165.3±7.4 | 163.2±7.2 | |||
| dP/dtmax (mmHg/s) | 7182±619 | 6859±572 | |||
|
| |||||
| Ethanol | 8 | MAP (mmHg) | 103.4±5.5 | 100.0±6.3 | |
| LVDP (mmHg) | 170.5±8.8 | 169.45±8.3 | |||
| dP/dtmax (mmHg/s) | 7051±682 | 6589±644 | |||
|
| |||||
| 3-AT | Saline | 6 | MAP (mmHg) | 102.7±4.6 | 93.7±6.4 |
| LVDP (mmHg) | 164.9±15.5 | 147.9±14.3 | |||
| dP/dtmax (mmHg/s) | 7468±1465 | 6492±1388 | |||
|
| |||||
| Ethanol | 7 | MAP (mmHg) | 102.2±3.8 | 90.9±3.5*# | |
| LVDP (mmHg) | 166.3±10.3 | 156.6±10.2 | |||
| dP/dtmax (mmHg/s) | 7331±393 | 6540±355 | |||
|
| |||||
| 4-MPAT | Saline | 6 | MAP (mmHg) | 98.6±2.8 | 84.6±4.4*# |
| LVDP (mmHg) | 167.9±7.1 | 153.2±8.4 | |||
| dP/dtmax (mmHg/s) | 7488±515 | 6735±422 | |||
|
| |||||
| Ethanol | 7 | MAP (mmHg) | 97.6±4.5 | 88.2±3.9* | |
| LVDP (mmHg) | 159.9±7.4 | 149.7±9.5 | |||
| dP/dtmax (mmHg/s) | 6908±735 | 5961±880 | |||
p < 0.05 vs. corresponding value in saline-pretreated group;
p < 0.05 vs. corresponding pretreatment (baseline) value.
RESULTS
Inhibition of ADH and CYP2E1 (4-MP), catalase (3-AT) or their combination mitigated ethanol-evoked myocardial dysfunction in proestrus rats
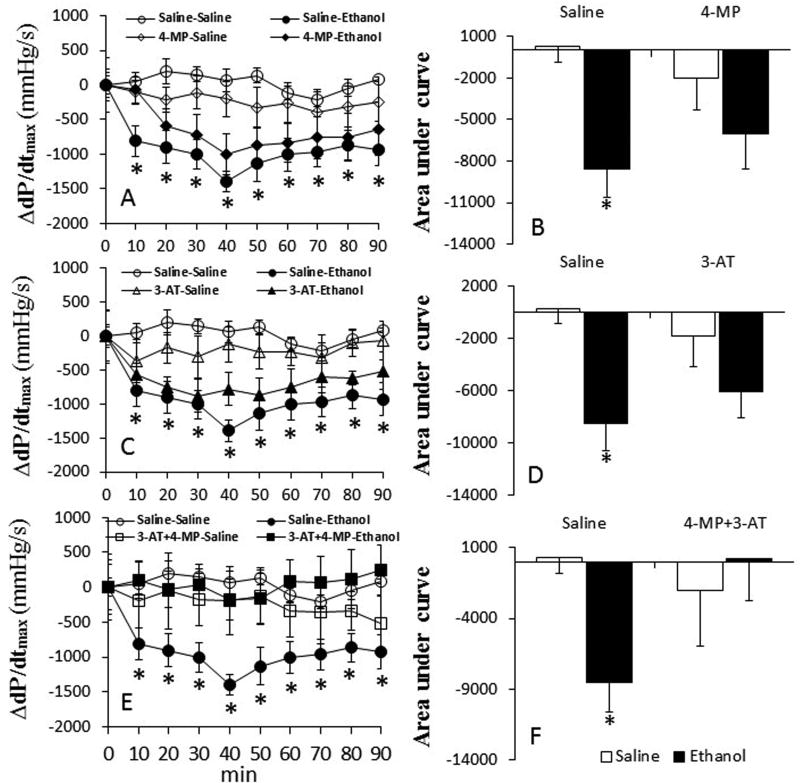
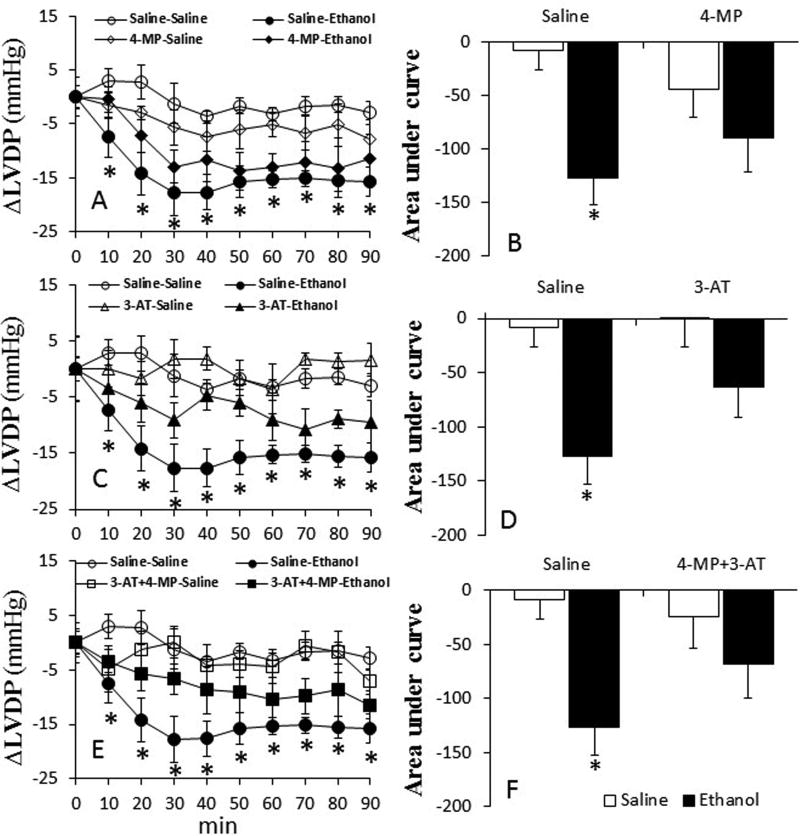
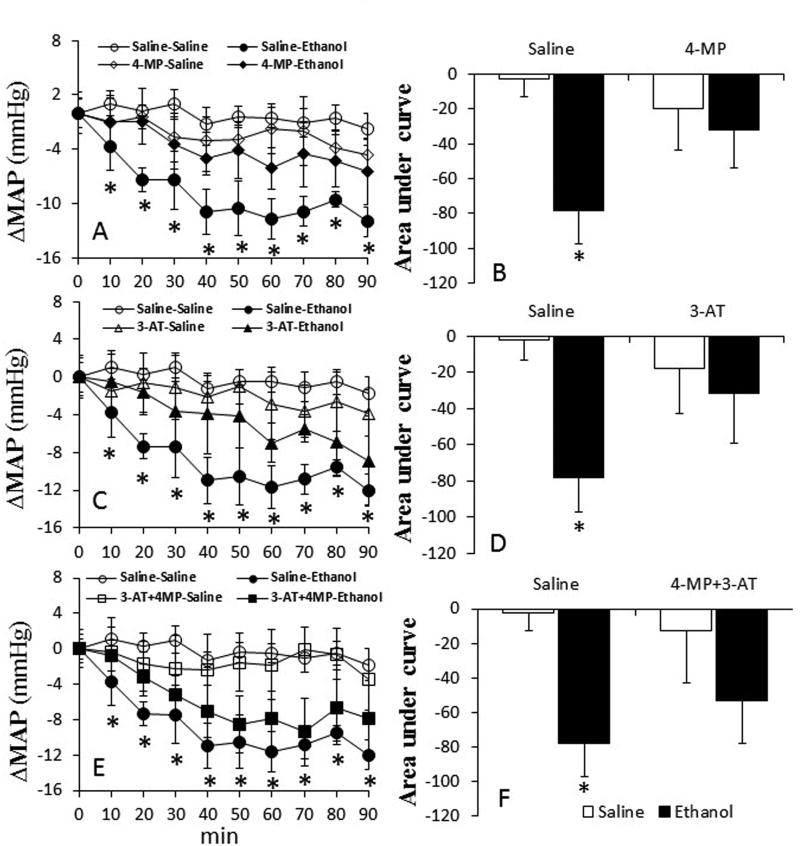
Inhibition of ADH and CYP2E1 by 4-MP alone had no effect on all hemodynamic indices. However, catalase inhibition alone (3-AT) or in combination with 4-MP, reduced (p < 0.05) myocardial function (dP/dtmax, LVDP) and MAP (Table 1). In saline (vehicle for enzyme inhibitors) pretreated rats, ethanol (1.5 g/kg) reduced (p < 0.05) dP/dtmax, LVDP and MAP (Figs. 1–3). Pretreatment with 4-MP or 3-AT partially, while their combination significantly (p < 0.05), attenuated these adverse hemodynamic effects of ethanol (Fig. 1E and F).
Fig. 1.
Prior inhibition of ADH, CYP2E1 and catalase by 4-MP, 3-AT or their combination (30 min) ameliorates ethanol-evoked myocardial dysfunction in conscious female rats. The line graphs show the time course of the changes in the maximum rate of rise of left ventricular pressure (dP/dtmax) caused by ethanol in the absence or presence of 4-MP (A), 3-AT (C) or their combination (E). The bar graphs (B, D and F) represent the areas under the curves in these groups, respectively. Values are mean ± SEM. Data were analyzed by one-way ANOVA followed by post-hoc Tukey’s t-test comparison. *p < 0.05, versus saline + saline. #p < 0.05, versus saline-ethanol.
Fig. 2.
Prior inhibition of ADH, CYP2E1 and catalase by 4-MP, 3-AT or their combination (30 min) ameliorates ethanol-evoked myocardial dysfunction in conscious female rats. The line graphs show the time course of the changes in left ventricular developed pressure (LVDP) caused by ethanol in the absence or presence of 4-MP (A), 3-AT (C) or their combination (E). The bar graphs (B, D and F) represent the areas under curves in these groups, respectively. Values are mean ± SEM. Data were analyzed by one-way ANOVA followed by post-hoc Tukey’s t-test comparison. *p < 0.05, versus saline-saline.
Fig. 3.
Prior inhibition of ADH, CYP2E1 and catalase by 4-MP, 3-AT or their combination (30 min) ameliorates ethanol-evoked myocardial dysfunction in conscious female rats. The line graphs show the time course of the changes in mean arterial pressure (MAP) caused by ethanol in the absence or presence of 4-MP (A), 3-AT (C) or their combination (E). The bar graphs (B, D and F) represent the areas under curves in these groups, respectively. Values are mean ± SEM. Data were analyzed by one-way ANOVA followed by post-hoc Tukey’s t-test comparison. *p < 0.05, versus saline-saline.
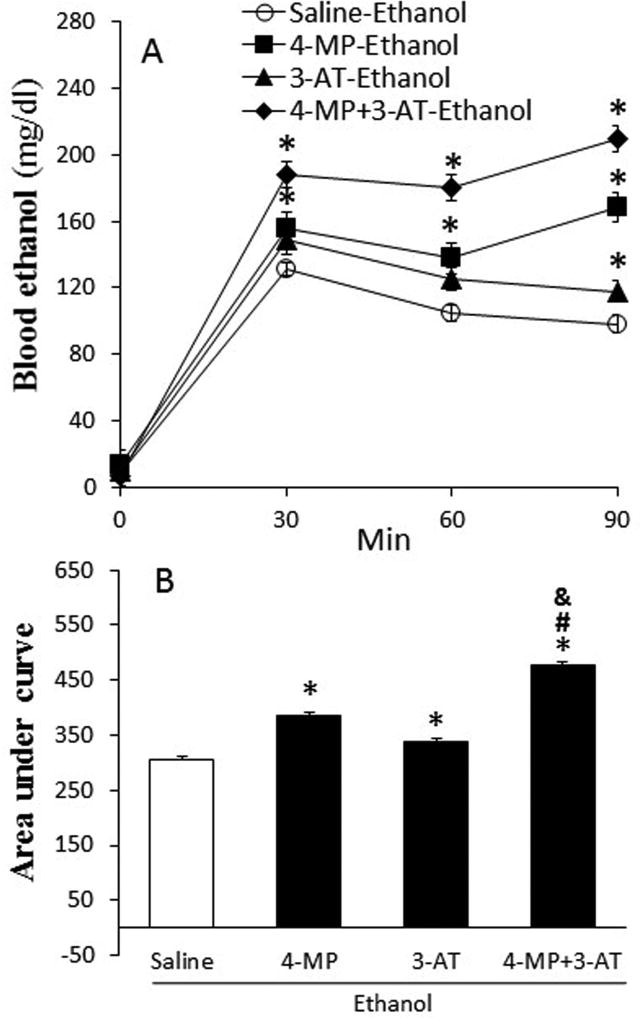
The effect of 4-MP, 3-AT or their combination on blood ethanol
Blood ethanol concentration (BAC) reached 131 ± 8.2 mg/dl at 30 min after ethanol (1.5 g/kg) infusion, and then dropped to 104.8 ± 8.7 and 98.3 ± 5.9 mg/dl at 60 and 90 min, respectively (Fig. 4A). However, BAC was higher (p < 0.05) in rats pretreated with 4-MP or 3-AT and synergistically increased by their combination (Fig. 4).
Fig. 4.
Ethanol metabolism was suppressed by inhibition of ADH, CYP2E1 and catalase by 4-MP, 3-AT or their combination. Panel A shows the time curse of blood ethanol concentration at 30, 60, and 90 min after ethanol infusion (1.5 g/kg; i.v). The bar graph (B) represents the areas under curves in these groups. Values are mean ± SEM. Data were analyzed by one-way ANOVA followed by Tukey's Multiple Comparison Test. *p < 0.05, versus saline. #p < 0.05, versus 4-MP. &p < 0.05, versus 4-MP+3-AT.
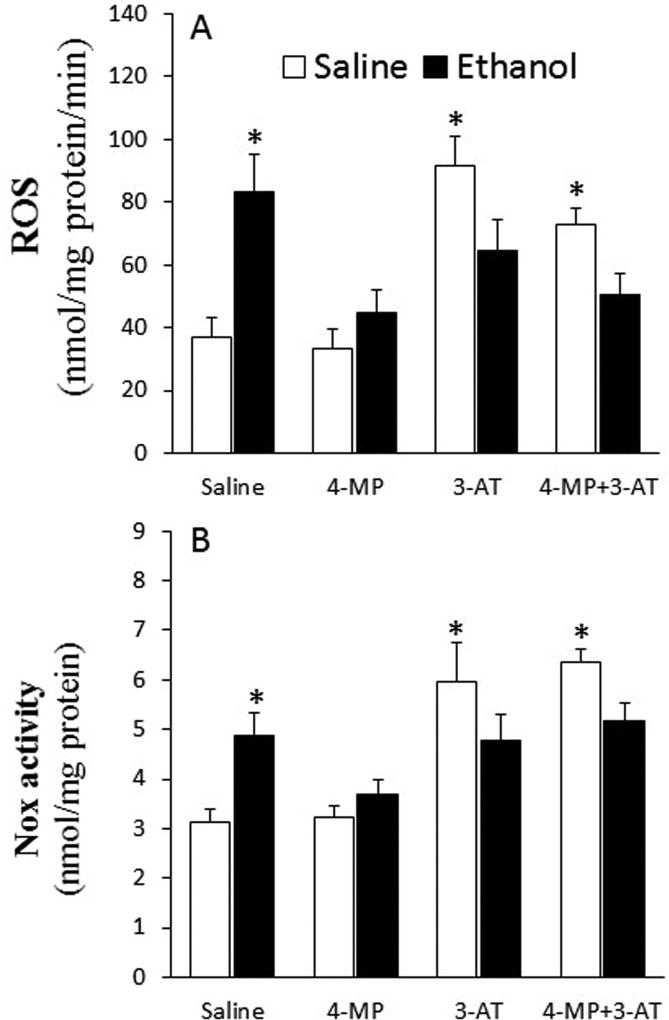
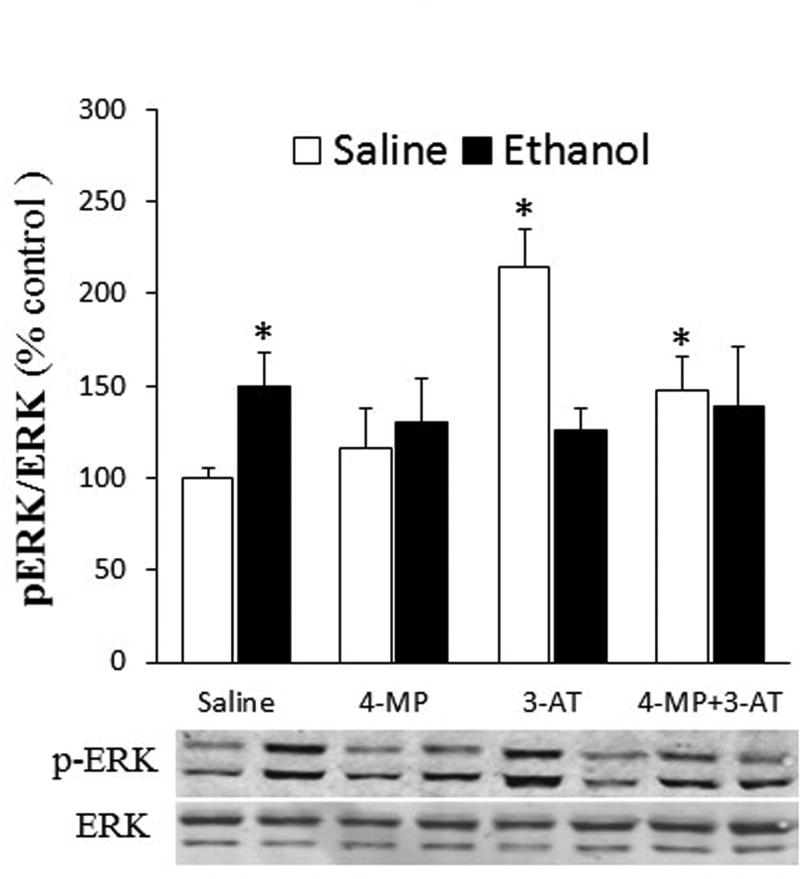
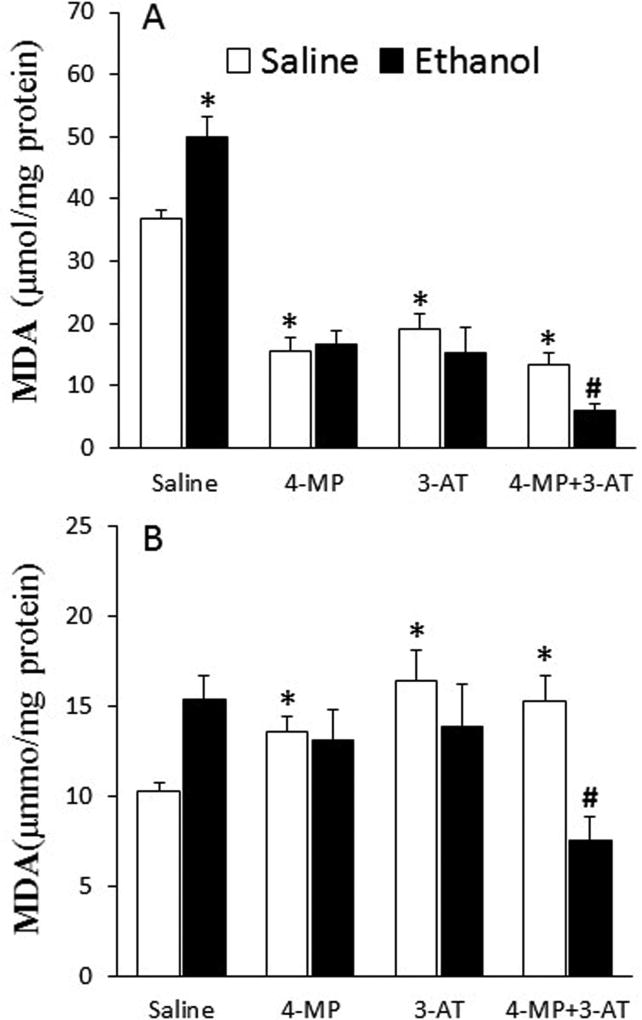
Inhibition of ADH and CYP2E1 (4-MP), catalase (3-AT) or their combination abrogated ethanol-evoked myocardial oxidative stress in proestrus rats
Ethanol increased (p < 0.05) ROS level (Fig. 5A), Nox activity (Fig. 5B), ERK1/2 phosphorylation (Fig. 6), and MDA level (Fig. 7A) in the hearts as well as MDA level in plasma (Fig. 7B) of proestrus rats. These ethanol-evoked biochemical responses, which reflect oxidative stress, were abrogated in rats pretreated with 4-MP, 3-AT or their combination (Figs. 5 and 7). Notably, catalase inhibition (3-AT) increased (p < 0.05) myocardial ROS level, Nox activity, and ERK1/2 phosphorylation as well as plasma MDA levels (Figs. 5–7). However, 4-MP, 3-AT or their combination reduced (p < 0.05) myocardial MDA levels (Fig. 7A). Despite this significant (p < 0.05) reduction in cardiac or elevation in plasma MDA levels, caused by combining 4-MP and 3-AT, subsequent ethanol administration reduced (p < 0.05) cardiac (Fig. 7A) and plasma (Fig. 7B) MDA levels. Collectively, the attenuation of the ethanol-evoked increases in mediators of oxidative stress by 4-MP, 3-AT or their combination (Figs. 5–7) paralleled the abrogation of reductions in myocardial function in the same rats (Figs. 1 and 2).
Fig. 5.
Effect of the inhibition of ADH, CYP2E1 and catalase by 4-MP, 3-AT or their combination on myocardial ROS formation (A) and NADPH oxidase (Nox, B) activity. Data were analyzed by one-way ANOVA followed by Tukey's Multiple Comparison Test. *p < 0.05, versus saline-saline.
Fig. 6.
Effect of the inhibition of ADH, CYP2E1 and catalase by 4-MP, 3-AT or their combination on myocardial ERK1/2 phosphorylation. Data were analyzed by one-way ANOVA followed by Tukey's Multiple Comparison Test. *p < 0.05, versus saline-saline. #p < 0.05, versus 3-AT-saline.
Fig. 7.
Effect of the inhibition of ADH, CYP2E1 and catalase by 4-MP, 3-AT or their combination on myocardial (A) and plasma (B) malondialdehyde (MDA) levels. Data were analyzed by one-way ANOVA followed by Tukey's Multiple Comparison Test. *p < 0.05, versus saline-saline. #p < 0.05, versus 4-MP+3-AT-saline.
DISCUSSION
The present study generated important data on the roles of three ethanol metabolizing enzymes, ADH, CYP2E1 and catalase, in myocardial oxidative stress/dysfunction caused by acute ethanol in E2 replete female rats. We show that ADH and CYP2E1 (4-MP) or catalase (3-AT) inhibition partially, while their combination drastically, attenuated the ethanol-evoked deleterious cardiac effects. The results of our ex vivo biochemical studies suggest an important role for the first oxidative metabolite of ethanol in triggering mediators of myocardial oxidative stress. The latter is a major contributor to the E2-dependent myocardial dysfunction caused by alcohol. It is imperative to be cognizant of the induction of cardiac oxidative stress and the hypotension caused by 3-AT when interpreting the present findings.
We documented a pivotal role for myocardial oxidative stress in the sex/E2 dependent myocardial dysfunction caused by acute ethanol administration in proestrus rats (Ibrahim et al., 2014, El-Mas and Abdel-Rahman, 2014, Yao and Abdel-Rahman, 2016). Realizing that acetaldehyde, the first ethanol oxidative metabolite, causes MAPK-dependent oxidative stress in many tissues (Yan et al., 2016, Ku et al., 2007), it was important to elucidate the role of this metabolic pathway in ethanol-evoked myocardial dysfunction. However, we needed to consider the possibility that this metabolic pathway is not involved in this deleterious cardiac effect of ethanol because acetaldehyde generation does not explain some effects of ethanol in other tissues (Haber et al., 2004, Manautou et al., 1992, Czech and Hartleb, 2002), and catalase overexpression protects against ethanol evoked dysfunction of isolated cardiac myocytes (Yao et al., 2015). Therefore, it was important to study, for the first time, the consequences of inhibiting ADH, CYP2E1 and catalase, which catalyze ethanol metabolism to acetaldehyde (Zakhari, 2006) in our model system. We administered 4-MP, an inhibitor of both ADH and CYP2E1 (Knoester et al., 2002), 3-AT, an inhibitor of catalase (Correa et al., 2008), alone or in combination. This approach permitted evaluation of the contribution of the three ethanol metabolizing enzymes to the ethanol-evoked myocardial dysfunction.
In support of our hypothesis, ethanol-evoked myocardial dysfunction was attenuated by prior inhibition of ADH and CYP2E (4-MP) or catalase (3-AT) and virtually abolished by their combination (Figs. 1–3). The parallel attenuation of ethanol-evoked increases in oxidative stress (ROS) and related molecular events (MDA; ERK1/2 phosphorylation) (Figs. 5–7) suggest mechanistic roles for these biochemical effects in ethanol-evoked myocardial dysfunction and its rescue following the inhibition of ethanol oxidative metabolism. This protective effect resembles protection against ethanol-evoked apoptosis in hepatocytes (Bailey and Cunningham, 1998) by ADH inhibition (Nishitani and Matsumoto, 2006) via reducing ethanol-elicited oxidative stresses (Bailey and Cunningham, 1998). Further, genetic and pharmacological CYP2E1 inhibition attenuated chronic ethanol-induced myocardial contractile dysfunction in mice (Zhang et al., 2013), but these studies were conducted in vitro on myocytes isolated from male mice. Finally, pharmacological inhibition of catalase depressed oxidation of alcohol (Thurman and Handler, 1989), prevented ethanol-induced behavioral stimulation (Pastor and Aragon, 2008) and motivation for alcohol consumption (Koechling and Amit, 1994). However, the effects of catalase inhibition on the E2-dependent myocardial oxidative stress and dysfunction have not been investigated.
The present study further supports the role of catalase in our model system because reported studies, including ours, show that E2 enhances catalase catalytic activity in the heart of female rats (Ibrahim et al., 2014, El-Mas and Abdel-Rahman, 2014, Yao and Abdel-Rahman, 2016, Campos et al., 2014). Further, acute ethanol causes additional increase in cardiac catalase activity in the presence of E2 receptor-alpha activation along with myocardial oxidative stress and dysfunction (Yao and Abdel-Rahman, 2017). The ability of catalase inhibition (3-AT) to attenuate ethanol-evoked LV dysfunction (Figs. 1–2) and oxidative stress (Figs. 5 and 7) supports a contributory role for catalase in these adverse effects. The partial attenuation of the ethanol-evoked myocardial dysfunction (dP/dtmax and LVDP) by 3-AT or 4-MP (Figs. 1–2), might be explained by: (i) the ability of the available enzyme(s) to compensate for the inhibited enzyme(s) or (ii) the higher blood alcohol concentration achieved (Fig. 4) because alcohol itself exerts direct cardiotoxic effect (Awtry and Philippides, 2010). Notably, the accumulation of non-oxidative ethanol metabolites such as fatty acid ethyl ester in blood (Doyle et al., 1994) and heart (Laposata and Lange, 1986) is increased following inhibition of ethanol oxidative metabolism (Werner et al., 2002). The present findings argue against the contribution of these alternative mechanisms because combined enzyme inhibition (3-AT plus 4-MP) conferred much better hemodynamic protection (Fig. 2). Interestingly, in these rats, the synergistically higher BAC (Fig. 5) correlated with substantial reductions in cardiac and plasma MDA levels (Fig. 7). While MDA reduction might explain the improved cardiac function, it remains to be determined how ethanol-evoked increase in MDA observed here and in our previous studies (Ibrahim et al., 2014) is reversed in the presence of combined ADH, CYP2E1 and catalase inhibition (Fig. 7).
The present study provides physiologically relevant data with catalase inhibition in the absence of ethanol. Consistent with its antioxidant role (Chelikani et al., 2004), and the ability of catalase inhibition to increase ROS production in isolated ventricular myocytes (Boles et al., 2014), 3-AT caused myocardial oxidative stress (higher ROS) level (Fig. 5A), at least partly, via enhanced Nox activity (Fig. 5B) and ERK1/2 phosphorylation (Fig. 6). Further, a central mechanism might play a role in the reduced BP because injection of 3-AT into the fourth ventricle (Valenti et al., 2010) or nucleus tractus solitarius (Cardoso et al., 2009) decreases BP in conscious rats. We also showed that 3-AT significantly increased plasma MDA level (Fig. 7B), which is consistent with higher plasma MDA in patients with lower catalase activity (Begenik et al., 2013). Notably, the inconsistency between lower myocardial and higher plasma MDA levels following 4-MP, 3-AT or their combination (Fig. 7A) also exists between lower liver and higher plasma MDA levels in reported studies (El-Bassiouni et al., 1998).
It is imperative to consider the impact of the biochemical and hemodynamic effects of 3-AT, given alone or with 4-MP, on data interpretation. First, while statistically nonsignificant, the reductions in LVDP and dP/dtmax caused by 3-AT might limit, at least partly, the ethanol-evoked LV dysfunction. This possibility is supported by the inability of ethanol to reduce BP when BP was significantly reduced in rats pretreated with 3-AT alone or in combination with 4-MP. Second, the 3-AT evoked increases in ROS and MDA levels, Nox activity and ERK1/2 phosphorylation might have limited the ethanol-evoked increases in these mediators of oxidative stress. Notably, the abrogation of the ethanol-evoked increases in ROS level and Nox activity despite a lack of change in their basal levels, in 4-MP pretreated rats, argues against this possibility. Further, we observed unexplained reductions in cardiac MDA caused by 4-MP, 3-AT and their combination and an additional reduction in rats treated with both inhibitors and ethanol (Fig. 7). Further studies are needed to elucidate the mechanisms of these biochemical effects caused by these enzyme inhibitors and ethanol.
The current study yields a novel insight into the role of ethanol oxidative metabolism in myocardial dysfunction caused by alcohol via accumulation of cardiotoxic molecules (ROS and MDA). While inhibiting ADH and CYP2E1 (4-MP), catalase (3-AT) or their combination substantially increased BAC (Fig. 4), this pharmacological approach abrogated the ethanol-evoked increases in cardiac Nox activity, ROS and MDA levels, as well as ERK1/2 phosphorylation. The findings support the contribution of these molecules, generated via oxidative metabolism of ethanol, to ethanol-evoked myocardial dysfunction in female rats. Nonetheless, at the doses used in behavioral studies, the enzyme inhibitors (3-AT and 4-MP) exerted biochemical and cardiovascular effects that might have influenced data interpretation.
Acknowledgments
This work was supported by Grant 2R01 AA014441-9 from the National Institute on Alcohol Abuse and Alcoholism. The authors thank Ms. Kui Sun for her technical assistance.
References
- Awtry EH, Philippides GJ. Alcoholic and cocaine-associated cardiomyopathies. Prog Cardiovasc Dis. 2010;52:289–299. doi: 10.1016/j.pcad.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Bailey SM, Cunningham CC. Acute and chronic ethanol increases reactive oxygen species generation and decreases viability in fresh, isolated rat hepatocytes. Hepatology. 1998;28:1318–1326. doi: 10.1002/hep.510280521. [DOI] [PubMed] [Google Scholar]
- Bardag-Gorce F, Yuan QX, Li J, French BA, Fang C, Ingelman-Sundberg M, French SW. The effect of ethanol-induced cytochrome p4502E1 on the inhibition of proteasome activity by alcohol. Biochem Biophys Res Commun. 2000;279:23–29. doi: 10.1006/bbrc.2000.3889. [DOI] [PubMed] [Google Scholar]
- Begenik H, Soyoral YU, Erkoc R, Emre H, Taskin A, Tasdemir M, Aslan M. Serum malondialdehyde levels, myeloperoxidase and catalase activities in patients with nephrotic syndrome. Redox Rep. 2013;18:107–112. doi: 10.1179/1351000213Y.0000000048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boles S, Polak A, Littlejohns B, Lin H, Suleiman MS. Catalase Inhibition Augments Reactive Oxygen Species Production in Isolated Ventricular Myocytes. Heart. 2014;100:A3. [Google Scholar]
- Campos C, Casali KR, Baraldi D, Conzatti A, Araujo AS, Khaper N, Llesuy S, Rigatto K, Bello-Klein A. Efficacy of a low dose of estrogen on antioxidant defenses and heart rate variability. Oxid Med Cell Longev. 2014;2014:218749. doi: 10.1155/2014/218749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso LM, Colombari DS, Menani JV, Toney GM, Chianca DA, Jr, Colombari E. Cardiovascular responses to hydrogen peroxide into the nucleus tractus solitarius. Am J Physiol Regul Integr Comp Physiol. 2009;297:R462–469. doi: 10.1152/ajpregu.90796.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelikani P, Fita I, Loewen PC. Diversity of structures and properties among catalases. Cell Mol Life Sci. 2004;61:192–208. doi: 10.1007/s00018-003-3206-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa M, Manrique HM, Font L, Escrig MA, Aragon CM. Reduction in the anxiolytic effects of ethanol by centrally formed acetaldehyde: the role of catalase inhibitors and acetaldehyde-sequestering agents. Psychopharmacology (Berl) 2008;200:455–464. doi: 10.1007/s00213-008-1219-3. [DOI] [PubMed] [Google Scholar]
- Czech E, Hartleb M. Non-oxidative metabolism of ethanol and its influence on the metabolic pethaway of serotonin and tranferrin. Problems Forensic Sci LII. 2002:37–51. [Google Scholar]
- Doyle KM, Bird DA, al-Salihi S, Hallaq Y, Cluette-Brown JE, Goss KA, Laposata M. Fatty acid ethyl esters are present in human serum after ethanol ingestion. J Lipid Res. 1994;35:428–437. [PubMed] [Google Scholar]
- El-Bassiouni EA, Abo-Ollo MM, Helmy MH, Ismail S, Ramadan MI. Changes in the defense against free radicals in the liver and plasma of the dog during hypoxia and/or halothane anaesthesia. Toxicology. 1998;128:25–34. doi: 10.1016/s0300-483x(98)00045-6. [DOI] [PubMed] [Google Scholar]
- El-Mas MM, Abdel-Rahman AA. Ovariectomy alters the chronic hemodynamic and sympathetic effects of ethanol in radiotelemetered female rats. Clin Exp Hypertens. 2000;22:109–126. doi: 10.1081/ceh-100100066. [DOI] [PubMed] [Google Scholar]
- El-Mas MM, Abdel-Rahman AA. Nongenomic effects of estrogen mediate the dose-related myocardial oxidative stress and dysfunction caused by acute ethanol in female rats. Am J Physiol Endocrinol Metab. 2014;306:E740–747. doi: 10.1152/ajpendo.00465.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Mas MM, Abdel-Rahman AA. Estrogen modulation of the ethanol-evoked myocardial oxidative stress and dysfunction via DAPK3/Akt/ERK activation in male rats. Toxicol Appl Pharmacol. 2015;287:284–292. doi: 10.1016/j.taap.2015.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Mas MM, Fan M, Abdel-Rahman AA. Endotoxemia-mediated induction of cardiac inducible nitric-oxide synthase expression accounts for the hypotensive effect of ethanol in female rats. J Pharmacol Exp Ther. 2008;324:368–375. doi: 10.1124/jpet.107.127498. [DOI] [PubMed] [Google Scholar]
- Grotto D, Maria LS, Valentini J, Paniz C, Schmitt G, Garcia SC, Pomblum VJ, Rocha JBT, Farina M. Importance of the lipid peroxidation biomarkers and methodological aspects FOR malondialdehyde quantification. Quím. Nova. 2009;32:169–174. [Google Scholar]
- Haber PS, Apte MV, Moran C, Applegate TL, Pirola RC, Korsten MA, McCaughan GW, Wilson JS. Non-oxidative metabolism of ethanol by rat pancreatic acini. Pancreatology. 2004;4:82–89. doi: 10.1159/000077608. [DOI] [PubMed] [Google Scholar]
- Haorah J, Knipe B, Leibhart J, Ghorpade A, Persidsky Y. Alcohol-induced oxidative stress in brain endothelial cells causes blood-brain barrier dysfunction. J Leukoc Biol. 2005;78:1223–1232. doi: 10.1189/jlb.0605340. [DOI] [PubMed] [Google Scholar]
- Hoek JB, Cahill A, Pastorino JG. Alcohol and mitochondria: a dysfunctional relationship. Gastroenterology. 2002;122:2049–2063. doi: 10.1053/gast.2002.33613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim BM, Fan M, Abdel-Rahman AA. Oxidative stress and autonomic dysregulation contribute to the acute time-dependent myocardial depressant effect of ethanol in conscious female rats. Alcohol Clin Exp Res. 2014;38:1205–1215. doi: 10.1111/acer.12363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamal M, Ameno K, Uekita I, Kumihashi M, Wang W, Ijiri I. Catalase mediates acetaldehyde formation in the striatum of free-moving rats. Neurotoxicology. 2007;28:1245–1248. doi: 10.1016/j.neuro.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Jin M, Ande A, Kumar A, Kumar S. Regulation of cytochrome P450 2e1 expression by ethanol: role of oxidative stress-mediated pkc/jnk/sp1 pathway. Cell Death Dis. 2013;4:e554. doi: 10.1038/cddis.2013.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalaz EB, Evran B, Develi S, Erata GO, Uysal M, Kocak-Toker N. Effect of binge ethanol treatment on prooxidant-antioxidant balance in rat heart tissue. Pathophysiology. 2012;19:49–53. doi: 10.1016/j.pathophys.2012.01.003. [DOI] [PubMed] [Google Scholar]
- Knoester PD, Jonker DM, Van Der Hoeven RT, Vermeij TA, Edelbroek PM, Brekelmans GJ, de Haan GJ. Pharmacokinetics and pharmacodynamics of midazolam administered as a concentrated intranasal spray. A study in healthy volunteers. Br J Clin Pharmacol. 2002;53:501–507. doi: 10.1046/j.1365-2125.2002.01588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koechling UM, Amit Z. Effects of 3-amino-1,2,4-triazole on brain catalase in the mediation of ethanol consumption in mice. Alcohol. 1994;11:235–239. doi: 10.1016/0741-8329(94)90036-1. [DOI] [PubMed] [Google Scholar]
- Ku BM, Lee YK, Jeong JY, Mun J, Han JY, Roh GS, Kim HJ, Cho GJ, Choi WS, Yi GS, Kang SS. Ethanol-induced oxidative stress is mediated by p38 MAPK pathway in mouse hippocampal cells. Neurosci Lett. 2007;419:64–67. doi: 10.1016/j.neulet.2007.03.049. [DOI] [PubMed] [Google Scholar]
- La Favor JD, Anderson EJ, Dawkins JT, Hickner RC, Wingard CJ. Exercise prevents Western diet-associated erectile dysfunction and coronary artery endothelial dysfunction: response to acute apocynin and sepiapterin treatment. Am J Physiol Regul Integr Comp Physiol. 2013;305:R423–434. doi: 10.1152/ajpregu.00049.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laposata EA, Lange LG. Presence of nonoxidative ethanol metabolism in human organs commonly damaged by ethanol abuse. Science. 1986;231:497–499. doi: 10.1126/science.3941913. [DOI] [PubMed] [Google Scholar]
- Lu Y, Zhuge J, Wang X, Bai J, Cederbaum AI. Cytochrome P450 2E1 contributes to ethanol-induced fatty liver in mice. Hepatology. 2008;47:1483–1494. doi: 10.1002/hep.22222. [DOI] [PubMed] [Google Scholar]
- Manautou JE, Buss NJ, Carlson GP. Oxidative and non-oxidative metabolism of ethanol by the rabbit lung. Toxicol Lett. 1992;62:93–99. doi: 10.1016/0378-4274(92)90082-u. [DOI] [PubMed] [Google Scholar]
- Marcondes FK, Miguel KJ, Melo LL, Spadari-Bratfisch RC. Estrous cycle influences the response of female rats in the elevated plus-maze test. Physiology & behavior. 2001;74:435–440. doi: 10.1016/s0031-9384(01)00593-5. [DOI] [PubMed] [Google Scholar]
- Matyas C, Varga ZV, Mukhopadhyay P, Paloczi J, Lajtos T, Erdelyi K, Nemeth BT, Nan M, Hasko G, Gao B, Pacher P. Chronic plus binge ethanol feeding induces myocardial oxidative stress, mitochondrial and cardiovascular dysfunction, and steatosis. Am J Physiol Heart Circ Physiol. 2016;310:H1658–1670. doi: 10.1152/ajpheart.00214.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee MA, Abdel-Rahman AA. Enhanced vascular neuronal nitric-oxide synthase-derived nitric-oxide production underlies the pressor response caused by peripheral N-methyl-D-aspartate receptor activation in conscious rats. J Pharmacol Exp Ther. 2012;342:461–471. doi: 10.1124/jpet.112.194464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council. Guide for the Care and Use of Laboratory Animals. 8. National Academies Press; Washington, DC: 2011. [Google Scholar]
- Nishitani Y, Matsumoto H. Ethanol rapidly causes activation of JNK associated with ER stress under inhibition of ADH. FEBS letters. 2006;580:9–14. doi: 10.1016/j.febslet.2005.11.030. [DOI] [PubMed] [Google Scholar]
- Pastor R, Aragon CM. Ethanol injected into the hypothalamic arcuate nucleus induces behavioral stimulation in rats: an effect prevented by catalase inhibition and naltrexone. Behav Pharmacol. 2008;19:698–705. doi: 10.1097/FBP.0b013e328315ecd7. [DOI] [PubMed] [Google Scholar]
- Piano MR. Alcoholic cardiomyopathy: incidence, clinical characteristics, and pathophysiology. Chest. 2002;121:1638–1650. doi: 10.1378/chest.121.5.1638. [DOI] [PubMed] [Google Scholar]
- Piano MR, Phillips SA. Alcoholic cardiomyopathy: pathophysiologic insights. Cardiovasc Toxicol. 2014;14:291–308. doi: 10.1007/s12012-014-9252-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plumlee CR, Lazaro CA, Fausto N, Polyak SJ. Effect of ethanol on innate antiviral pathways and HCV replication in human liver cells. Virol J. 2005;2:89. doi: 10.1186/1743-422X-2-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Lasker JM, Rosman AS, Lieber CS. Induction of cytochrome P-4502E1 in the human liver by ethanol is caused by a corresponding increase in encoding messenger RNA. Hepatology. 1993;17:236–245. [PubMed] [Google Scholar]
- Thurman RG, Handler JA. New perspectives in catalase-dependent ethanol metabolism. Drug Metab Rev. 1989;20:679–688. doi: 10.3109/03602538909103570. [DOI] [PubMed] [Google Scholar]
- Valenti VE, Abreu LC, Sato MA, Ferreira C. ATZ (3-amino-1,2,4-triazole) injected into the fourth cerebral ventricle influences the Bezold-Jarisch reflex in conscious rats. Clinics (Sao Paulo) 2010;65:1339–1343. doi: 10.1590/S1807-59322010001200018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waller MB, McBride WJ, Lumeng L, Li TK. Effects of intravenous ethanol and of 4-methylpyrazole on alcohol drinking in alcohol-preferring rats. Pharmacol Biochem Behav. 1982;17:763–768. doi: 10.1016/0091-3057(82)90359-8. [DOI] [PubMed] [Google Scholar]
- Werner J, Saghir M, Warshaw AL, Lewandrowski KB, Laposata M, Iozzo RV, Carter EA, Schatz RJ, Fernandez-Del Castillo C. Alcoholic pancreatitis in rats: injury from nonoxidative metabolites of ethanol. Am J Physiol Gastrointest Liver Physiol. 2002;283:G65–73. doi: 10.1152/ajpgi.00419.2001. [DOI] [PubMed] [Google Scholar]
- Yan T, Zhao Y, Zhang X. Acetaldehyde Induces Cytotoxicity of SH-SY5Y Cells via Inhibition of Akt Activation and Induction of Oxidative Stress. Oxid Med Cell Longev. 2016;2016:4512309. doi: 10.1155/2016/4512309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao C, Behring JB, Shao D, Sverdlov AL, Whelan SA, Elezaby A, Yin X, Siwik DA, Seta F, Costello CE, Cohen RA, Matsui R, Colucci WS, McComb ME, Bachschmid MM. Overexpression of Catalase Diminishes Oxidative Cysteine Modifications of Cardiac Proteins. PLoS ne. 2015;10:e0144025. doi: 10.1371/journal.pone.0144025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao F, Abdel-Rahman AA. Estrogen receptor ERalpha plays a major role in ethanol-evoked myocardial oxidative stress and dysfunction in conscious female rats. Alcohol. 2016;50:27–35. doi: 10.1016/j.alcohol.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao F, Abdel-Rahman AA. Estrogen Receptors alpha and beta Play Major Roles in Ethanol-Evoked Myocardial Oxidative Stress and Dysfunction in Conscious Ovariectomized Rats. Alcohol Clin Exp Res. 2017;41:279–290. doi: 10.1111/acer.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakhari S. Overview: how is alcohol metabolized by the body? Alcohol Res Health. 2006;29:245–254. [PMC free article] [PubMed] [Google Scholar]
- Zhang RH, Gao JY, Guo HT, Scott GI, Eason AR, Wang XM, Ren J. Inhibition of CYP2E1 attenuates chronic alcohol intake-induced myocardial contractile dysfunction and apoptosis. Biochim Biophys Acta. 2013;1832:128–141. doi: 10.1016/j.bbadis.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Klein AL, Alberle NS, Norby FL, Ren BH, Duan J, Ren J. Cardiac-specific overexpression of catalase rescues ventricular myocytes from ethanol-induced cardiac contractile defect. Journal of molecular and cellular cardiology. 2003;35:645–652. doi: 10.1016/s0022-2828(03)00080-4. [DOI] [PubMed] [Google Scholar]
- Zimatkin SM, Deitrich RA. Ethanol metabolism in the brain. Addict Biol. 1997;2:387–400. doi: 10.1080/13556219772444. [DOI] [PubMed] [Google Scholar]
- Zimatkin SM, Pronko SP, Vasiliou V, Gonzalez FJ, Deitrich RA. Enzymatic mechanisms of ethanol oxidation in the brain. Alcohol Clin Exp Res. 2006;30:1500–1505. doi: 10.1111/j.1530-0277.2006.00181.x. [DOI] [PubMed] [Google Scholar]