Abstract
Background
Ayahuasca is a plant tea containing the psychedelic 5-HT2A agonist N,N-dimethyltryptamine and harmala monoamine-oxidase inhibitors. Acute administration leads to neurophysiological modifications in brain regions of the default mode network, purportedly through a glutamatergic mechanism. Post-acutely, ayahuasca potentiates mindfulness capacities in volunteers and induces rapid and sustained antidepressant effects in treatment-resistant patients. However, the mechanisms underlying these fast and maintained effects are poorly understood. Here, we investigated in an open-label uncontrolled study in 16 healthy volunteers ayahuasca-induced post-acute neurometabolic and connectivity modifications and their association with mindfulness measures.
Methods
Using 1H-magnetic resonance spectroscopy and functional connectivity, we compared baseline and post-acute neurometabolites and seed-to-voxel connectivity in the posterior and anterior cingulate cortex after a single ayahuasca dose.
Results
Magnetic resonance spectroscopy showed post-acute reductions in glutamate+glutamine, creatine, and N-acetylaspartate+N-acetylaspartylglutamate in the posterior cingulate cortex. Connectivity was increased between the posterior cingulate cortex and the anterior cingulate cortex, and between the anterior cingulate cortex and limbic structures in the right medial temporal lobe. Glutamate+glutamine reductions correlated with increases in the “nonjudging” subscale of the Five Facets Mindfulness Questionnaire. Increased anterior cingulate cortex-medial temporal lobe connectivity correlated with increased scores on the self-compassion questionnaire. Post-acute neural changes predicted sustained elevations in nonjudging 2 months later.
Conclusions
These results support the involvement of glutamate neurotransmission in the effects of psychedelics in humans. They further suggest that neurometabolic changes in the posterior cingulate cortex, a key region within the default mode network, and increased connectivity between the anterior cingulate cortex and medial temporal lobe structures involved in emotion and memory potentially underlie the post-acute psychological effects of ayahuasca.
Keywords: ayahuasca, psychedelic after-effects, magnetic resonance imaging, mindfulness, human
Significance Statement
Psychedelics are intriguing drugs that induce transient but intense modifications in perception, emotion, and cognition. Despite human use dating back millennia, their mechanism of action is still poorly understood. Recent research in patients has shown that ayahuasca, a plant psychedelic used traditionally in the Amazon for religious and medicinal purposes, exerts rapid and potent antidepressant effects in treatment-resistant patients. These beneficial effects are observed after a single dose and intriguingly persist for weeks, long after the immediate acute effects have disappeared. Here we demonstrate using 2 neuroimaging techniques that during the post-acute phase, that is within 24 hours after intake, ayahuasca leads to metabolic and connectivity changes in the brain. These changes are associated with enhanced psychological capacities that are beneficial in the therapeutic context. These findings provide a biological basis for the post-acute or “after-glow” stage of psychedelic effects, and contribute to elucidate the therapeutic mechanisms of these substances.
Introduction
Psychedelics are the object of renewed interest as potential therapeutic tools in psychiatry (Sessa, 2005; Vollenweider and Kometer, 2010; Grob et al., 2011). Among these substances is ayahuasca, an Amazonian psychoactive beverage typically obtained from the plants Banisteriopsis caapi and Psychotria viridis. The ayahuasca tea contains a combination of the psychedelic indole N,N-dimethyltryptamine (DMT) from P. viridis, and β-carboline (harmala) alkaloids with monoamine-oxidase-inhibiting properties from B. caapi (McKenna et al., 1984). DMT is a classical serotonergic psychedelic that stimulates the 5-HT2A receptor (Carbonaro et al., 2015). Agonism at this level by DMT and other psychedelics recruits glutamatergic neurotransmission (Carbonaro et al., 2015), induces neuronal excitatory effects (Kłodzinska et al., 2002), and increases glutamate release (Scruggs et al., 2003; Muschamp et al., 2004). However, unlike psilocybin or LSD, DMT is orally inactive due to extensive degradation by monoamine-oxidase (Riba et al., 2015). Interestingly, the presence of β-carbolines in the ayahuasca tea inhibits DMT metabolism, allowing for psychoactive effects after oral intake (Riba et al., 2003). Additionally, DMT shows neuroprotective effects mediated through interaction with the sigma-1 receptor (Szabo et al., 2016).
Interest in the general pharmacology and therapeutic properties of ayahuasca has greatly increased in recent years (Domínguez-Clavé et al., 2016). Acute administration to healthy volunteers induces a transient introspective state characterized by dream-like visions with eyes closed, recollection of personal memories, and intense emotion (Riba et al., 2001, 2003). Neurophysiological studies show a suppression of the inhibitory alpha rhythm in the occipital and parietal cortex, including the posterior cingulate cortex (PCC) (Schenberg et al., 2015; Valle et al., 2016), a key node of the default mode network (DMN) with a prominent role in self-reflection and consciousness (Vogt and Laureys, 2005). Furthermore, decreased activity for the most part of the DMN and reduced connectivity of the PCC have been observed during the acute effects of ayahuasca (Palhano-Fontes et al., 2015). On the other hand, radiotracer data shows increased blood flow in the anterior cingulate cortex (ACC) and in the insula, amygdala, and hippocampus, brain areas involved in cognitive control, emotion, and memory (Riba et al., 2006). Recently, ayahuasca was found to induce post-acute increases in “decentering” ability, that is, the capacity to observe one’s thoughts and emotions in a detached manner, and to reduce automatic negative judgmental attitudes and inner reactivity (Soler et al., 2016). It thus enhanced a series of “mindfulness” capacities traditionally cultivated by meditation schools and that are known to be impaired in many forms of psychopathology (Soler et al., 2014b). In a subsequent review, the authors postulated that the mindfulness-enhancing properties of ayahuasca could be used in a therapeutic context to facilitate emotional reprocessing in patients with depression, addiction, and personality disorders (Domínguez-Clavé et al., 2016).
In line with the above, 2 recent open-label uncontrolled clinical studies have found rapid reductions in psychopathology after administration of single ayahuasca doses to depressed patients. Remarkably, antidepressant effects appeared within hours after dosing and were maintained for 3 weeks (Osório et al., 2015; Sanches et al., 2016). Analogous improvements have also been observed after psilocybin (Carhart-Harris et al., 2016b). In all cases, sustained antidepressant effects were in sharp contrast with the much shorter duration of the acute psychedelic state. This disparity of time courses suggests that 5-HT2A stimulation leads to persistent effects beyond the time frame of the acute inebriation. In fact, early researchers coined the term “after-glow” to designate a positive post-acute phase of psychedelic drug effects characterized by elevated mood and openness (Pahnke et al., 1970). This post-acute phase has been reported to extend between 6 and 8 weeks after the acute psychedelic effects and characterized as a window of opportunity for therapeutic intervention (Halpern, 1996; Winkelman, 2014). Recent clinical studies have described persisting positive after-effects following psilocybin and LSD (Griffiths et al., 2008; Lebedev et al., 2016).
Here, we investigated the neural correlates of the psychedelic “after-glow” in healthy volunteers to improve our understanding of the neural mechanisms potentially involved in the rapid and sustained therapeutic effects observed in patients. Using 2 different neuroimaging techniques, we assessed post-acute neurometabolic and connectivity changes induced by ayahuasca in healthy volunteers. Our study focused on the PCC and the ACC, 2 brain regions that have consistently been identified as targets of psychedelics (Carhart-Harris et al., 2012, 2016a; Palhano-Fontes et al., 2015; Valle et al., 2016). We postulated 3 hypotheses. First, that excitatory effects in PCC and ACC during acute ayahuasca would lead to measurable decreases of glutamate-glutamine and energy metabolites in the post-acute phase. Second, that neurometabolic changes would parallel enhanced connectivity between the PCC and the ACC by decreasing the normal pattern of anticorrelated brain activity existing between these brain regions. Third, that the measured neurometabolic and connectivity changes would correlate with increased mindfulness capacities in the immediate post-acute phase and at follow-up 2 months later.
Materials and Methods
Ethics
The study was approved by the Ethics Committee at Hospital de Sant Pau (Barcelona, Spain). All participants provided written informed consent to participate in the study.
Participants
The study included 16 healthy volunteers (6 females) with prior experience with ayahuasca. Exclusion criteria included a current or past history of psychiatric disorders, alcohol or other substance dependence, evidence of significant illness, and pregnancy. Detailed information on the recruitment procedure and the characteristics of study participants is provided in the supplementary file.
Drug
A single ayahuasca batch was used in the study. All participants ingested ayahuasca from this single batch, so alkaloid concentrations were the same for all subjects. The ayahuasca used was analyzed and the total volume ingested by each participant was recorded. Alkaloid concentrations were determined using a previously described method implementing liquid chromatography-electrospray ionization-tandem mass spectrometry (McIlhenny et al., 2009). Based on the analysis, the ayahuasca used in the session contained 0.3 mg/mL DMT, 0.86 mg/mL harmine, 0.17 mg/mL tetrahydroharmine, and 0.04 mg/mL harmaline. Participants took a mean ± SD volume of ayahuasca during the session of 148 ± 29 mL. Thus, the mean ± SD alkaloid amounts ingested were: 45 ± 9 mg DMT, 126 ± 25 mg harmine, 26 ± 5 mg tetrahydroharmine, and 5 ± 1 mg harmaline. For a 70-kg person, the dose would be equivalent to 0.64 mg DMT/kg, within the range found to be psychoactive in laboratory studies by our group (Riba et al., 2001, 2003). In the present study, we conducted post- vs. pre-ayahuasca comparisons of the dependent variables. No placebo was used in the present investigation.
Study Design
Participants were assessed twice in an MRI scanner. The first measurement was conducted in the 24 h prior to an ayahuasca session. The baseline assessment included the acquisition of anatomical high-resolution T1 images, 1H-MR spectroscopy data, and resting-state BOLD activity. Participants also completed 3 mindfulness questionnaires before the first scan. The post-acute assessment was conducted in the 24 hours after the ayahuasca session. It involved again obtaining structural T1 images, 1H-MR spectroscopy, resting-state BOLD data, and the same mindfulness questionnaires. The second MRI assessment was conducted for each participant at approximately the same time of the day as the first assessment, with a variation of about 30 minutes. The meals ingested by participants were equivalent on each assessment day. Participants were asked to fill out the mindfulness questionnaires indicating how they felt during the post-acute stage. Additionally, participants were asked to fill out the Hallucinogen Rating Scale, a subjective effects questionnaire, to indicate retrospectively how they had felt during the acute psychedelic phase. Two months later, a follow-up was conducted. Participants were requested to fill out again the mindfulness questionnaires they had been administered at baseline and in the post-acute phase.
Image Acquisition and Analysis
Images were acquired on a 3T Siemens Magneto TIM Trio scanner using a 32-channel phased-array head coil. The assessment protocol involved obtaining: (1) high resolution T1 structural images; (2) spectroscopy data in 3 volumes of interest (VOIs); and (3) resting state BOLD data for functional connectivity analysis. Each measurement is described briefly below and in depth in the supplementary information.
MR Spectroscopy: Neurometabolite Assessment
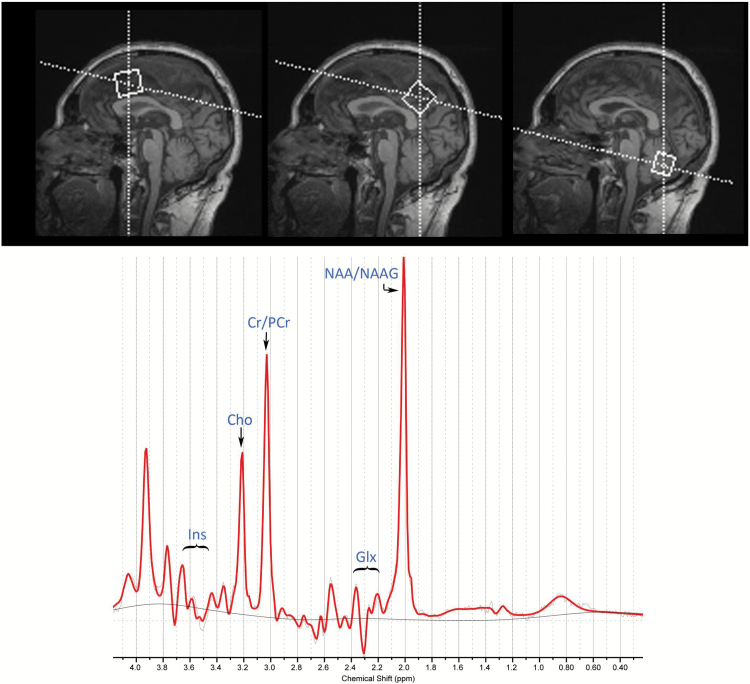
Single voxel spectra were obtained from 3 VOIs placed in the PCC, ACC, and cerebellum, as shown in Figure 1 (top panel) for one of the participants.
Figure 1.
Location of the 1H-MRS voxels (top) and a representative spectrum (bottom). The top panel shows sagittal views of voxel placement over the anterior cingulate (left), posterior cingulate (center), and cerebellum (right). The dimensions of the respective voxels were: 25 x 25 x 25 mm3 in the anterior cingulate cortex (ACC) and posterior cingulate cortex (PCC) and 20 x 40 x 20 mm3 in the cerebellum.
Metabolite concentrations were quantified for total creatine plus phosphocreatine (Cr), total choline containing compounds (glycerophosphocholine plus phosphocholine; Cho), inositol (Ins), combined glutamate plus glutamine (Glx), and total N-acetylaspartate plus N-acetylaspartylglutamate (NAA-NAAG). We used absolute metabolite levels, since ratios with creatine have been found to be a potential confounding factor and the source of increased variability (Li et al., 2003). See the supplementary materials for further details on VOI placement, quality assurance of measurements, and tissue corrections.
The obtained millimolar (mM) metabolite concentrations were compared at each VOI using repeated-measures ANOVA with ayahuasca intake (post- vs. pre-ayahuasca) and metabolite type as factors (Cr, Cho, Ins, Glx, and NAA-NAAG). When a significant effect of ayahuasca intake or the interaction ayahuasca intake by metabolite type was found, pair-wise comparisons were conducted using Student’s t tests. Results were considered significant for P < .05. Statistical results are presented in tabulated form showing uncorrected P values and values corrected for multiple comparisons using the false discovery rate (FDR). Effect size is provided using Cohen’s d (see supplementary file for details).
Resting State BOLD Signal Acquisition and Functional Connectivity Analysis
Resting state functional images were collected using a 7-minute sequence during which participants stayed awake with eyes closed. The functional connectivity analysis was conducted using the CONN software (Whitfield-Gabrieli and Nieto-Castanon, 2012). The analysis included several preprocessing steps (see supplementary file), followed by a first-level statistical analysis calculating seed-to-voxel connectivity maps for each subject and condition (pre-ayahuasca and post-acute). Individual z-maps were used in the subsequent random-effects second-level analysis to assess drug-induced changes in connectivity (post- vs. pre-ayahuasca intake) using paired-samples Student’s t tests.
Post-acute ayahuasca-induced changes in seed-to-voxel connectivity were assessed using 3 spherical seeds with 10-mm radius. The first was placed in the PCC at MNI coordinates x = 0, y = -56, z = 28, based on previously published data (Whitfield-Gabrieli and Nieto-Castanon, 2012). The 2 additional seeds were placed in the ACC. The first seed was placed in the dorsal ACC (dACC) at MNI coordinates x = 5, y = 14, z = 42, over BA 32, a subregion of the ACC involved in executive top-down control, and positive task activation (Kelly et al., 2009). A second more rostral seed (here denoted as “superior rostral ACC” or srACC) was placed at MNI coordinates x = 0, y = 15, z = 30) in BA 24 (Dixon et al., 2014). This subregion of the ACC has extensive connectivity with limbic structure in the medial temporal lobe (Vogt, BA, 2009) and has been found to be hypoactive in depressed patients (Fales et al., 2008).
The significance maps obtained in the second-level analysis were corrected for multiple comparisons at the cluster level using the family wise error correction (FWE). Significance level was set at FWE < 0.05 for a spatial extension of at least 20 continuous voxels. Further details on image acquisition and analysis can be found in the supplementary file.
Mindfulness Measures
Assessment Instruments and Statistical Analysis
Scores on mindfulness facets were assessed before and after ayahuasca intake using the Spanish versions of the following instruments: (1) the Five Facet Mindfulness Questionnaire (FFMQ) (Cebolla et al., 2012); (2) the Experiences Questionnaire (EQ) (Soler et al., 2014b); and (3) the short version of the Self-Compassion (SC) questionnaire (Garcia-Campayo et al., 2014). Additionally, the Mindsens Composite Index was calculated with items from the FFMQ and EQ. This index is most sensitive to meditation practice and accurately discriminates between meditators and nonmeditators (Soler et al., 2014a). The Mindsens index was used to assess to what extent the changes induced by ayahuasca were comparable with those induced by meditation practice.
Scores on mindfulness facets (FFMQ, EQ, and SC) were obtained before and after ayahuasca intake. Baseline assessment was conducted in the 24 hours prior to ayahuasca intake, and the second was conducted within the 24 hours after intake, before going into the MRI scanner. Participants were contacted by email 2 months after participation to conduct a follow-up assessment. Fourteen of the 16 participants responded and returned the completed questionnaires. This follow-up analysis had consequently less power to detect changes and should be thus considered exploratory. In all instances, participants were asked to fill out the questionnaires indicating how they felt at the moment of completion. Further details on the instruments used are provided in the supplementary file.
Ayahuasca-induced changes in mindfulness measures were assessed using paired-samples t tests. Scores at follow-up were compared with pre-ayahuasca values. Results were considered significant for P < .05. Statistical results are presented visually as bar graphs and in tabulated form (supplementary material). The supplementary table shows uncorrected P values and values corrected for multiple comparisons using the FDR.
Acute Subjective Effects
Assessment Instruments and Statistical Analysis
To establish that participants had experienced psychoactive effects during the acute phase, the intensity of the psychedelic effects during the acute inebriation were retrospectively assessed using a Spanish version of the Hallucinogen Rating Scale (HRS). The HRS was administered only once, in the course of the post-ayahuasca assessment, before the MRI scan. Participants were requested to fill out the questionnaire indicating how they had felt during the acute effects of ayahuasca.
To statistically verify that the dose ingested was indeed psychoactive, scores obtained in the 6 HRS subscales (see supplementary materials) were compared with scores obtained previously in a laboratory study from a sample of 12 healthy volunteers who received a placebo and an ayahuasca dose equivalent to 0.75 mg DMT/kg body weight (Valle et al., 2016). The statistical comparison was carried out using independent-samples Student’s t tests. Results were considered significant for P < .05. Results are shown in a figure with significance symbols denoting P values after multiple comparisons correction using the FDR.
Exploratory Correlation Analysis
Correlation analyses were conducted to explore potential associations between neurometabolic and functional connectivity changes on the one hand and scores on subjective effects measures and mindfulness questionnaires on the other. In all calculations, difference values (post- minus pre-ayahuasca) were used. For connectivity data, the difference in z-values (post- minus pre-ayahuasca) was calculated for clusters showing ayahuasca-induced significant changes in connectivity in the paired-samples comparison between normalized statistical maps (post- vs. pre-ayahuasca). Correlations with mindfulness measures at follow-up were calculated using the difference values between follow-up scores and pre-ayahuasca values. Correlations were considered significant for P < .05. Given the exploratory nature of this analysis, we did not implement corrections for multiple comparisons, and results should be interpreted with caution.
Results
All 16 participants completed the baseline and post-acute assessments. There were no drop-outs in the study.
MR Spectroscopy: Neurometabolite Assessment
Average metabolite concentrations in the baseline and post-acute assessments in the 3 VOIs examined are shown in Table 1. Data from several participants were below the signal-to-noise threshold either in the pre- or post-ayahuasca assessment and had to be excluded from further analysis. Usable data for Glx assessment in the PCC were obtained from 12 participants, 13 for the ACC, and 11 for the cerebellum. Inositol in the PCC could be adequately measured in 13 participants. All other metabolites could be quantified in the PCC, ACC, and cerebellum in 14 participants.
Table 1.
Neurometabolite Levels at Baseline and in the 24 Hours after Ayahuasca Intake for the 3 Examined Regions of Interest
| Brain Region and Metabolite | Baseline | Post-Ayahuasca | n | df | t Value | P Value | P FDR | Cohen’ d |
|---|---|---|---|---|---|---|---|---|
| PCC | ||||||||
| Inositol | 8.26 (1.06) | 8.32 (0.95) | 13 | 12 | -0.24 | 0.817 | 0.817 | – |
| Glx | 6.60 (0.64) | 6.25 (0.57) | 12 | 11 | 2.31 | 0.041** | 0.068* | 0.67 |
| Cr | 8.88 (0.50) | 8.61 (0.46) | 14 | 13 | 3.03 | 0.010** | 0.043** | 0.81 |
| Cho | 1.79 (0.19) | 1.77 (0.18) | 14 | 13 | 0.83 | 0.421 | 0.526 | – |
| NAA-NAAG | 12.83 (0.70) | 12.03 (0.93) | 14 | 13 | 2.74 | 0.017** | 0.043** | 0.73 |
| ACC | ||||||||
| Inositol | 8.75 (0.82) | 8.57 (0.79) | 14 | – | – | – | – | – |
| Glx | 5.38 (0.39) | 5.51 (0.50) | 13 | – | – | – | – | – |
| Cr | 8.39 (0.40) | 8.40 (0.44) | 14 | – | – | – | – | – |
| Cho | 2.22 (0.20) | 2.20 (0.20) | 14 | – | – | – | – | – |
| NAA-NAAG | 11.20 (0.41) | 11.22 (0.36) | 14 | – | – | – | – | – |
| Cerebellum | ||||||||
| Inositol | 10.67 (2.19) | 10.05 (1.51) | 14 | – | – | – | – | – |
| Glx | 6.79 (0.65) | 6.76 (0.75) | 12 | – | – | – | – | – |
| Cr | 13.58 (1.73) | 13.19 (1.28) | 14 | – | – | – | – | – |
| Cho | 3.38 (0.53) | 3.32 (0.36) | 14 | – | – | – | – | – |
| NAA-NAAG | 13.02 (1.80) | 12.98 (0.94) | 14 | – | – | – | – | – |
Abbreviations: ACC, anterior cingulate cortex; Cho, glycerophosphocholine+phosphocholine; Cr, creatine+phosphocreatine; Glx, glutamate+glutamine; NAA-NAAG, N-acetylaspartate+N-acetylaspartylglutamate; PCC, posterior cingulate cortex.
Pair-wise comparisons between baseline and post-acute values were carried out for the PCC only, based on results from the ANOVA, which were significant only for this region. Comparisons were carried out using paired-samples Student’s t tests. Values are expressed as mean (SD) in millimolar concentration units. *P < .01; **P < .05; P FDR, FDR corrected P value.
In the PCC VOI, the 2-factor repeated-measures ANOVA showed a significant effect of metabolite type [F(4,44)= 761, P<.001], a trend for ayahuasca intake [F(1,12)=3.88, P=.075], and significant interaction between ayahuasca intake and metabolite type [F(4,44)=4.11, P<.023]. As shown in Table 1, the pair-wise comparisons showed significant decreases in Cr and NAA-NAAG levels in the post-intake assessment. Glx levels were significantly decreased in the initial t test (P=.041), but this effect was marginally significant following the FDR correction (P=.068). Ins and Cho concentrations remained unchanged.
In the ACC VOI, only an effect of metabolite type was observed [F(4,48)=997, P<.001], but not effect of ayahuasca intake [F(1,12)=0.32, P=.860] or the interaction [F(4,48)=0.55, P=.606].
In the cerebellum VOI, the ANOVA yielded a significant effect of metabolite type [F(4,44)=321, P<.001], but no effects of ayahuasca intake [F(1,11)=1.44, P=.255] or the interaction between intake and metabolite type [F(4,44)=0.54, P=.632]. Mean metabolite concentrations at baseline assessment and in the post-acute phase for these 3 VOIs are also shown in Table 1.
Functional Connectivity Assessment
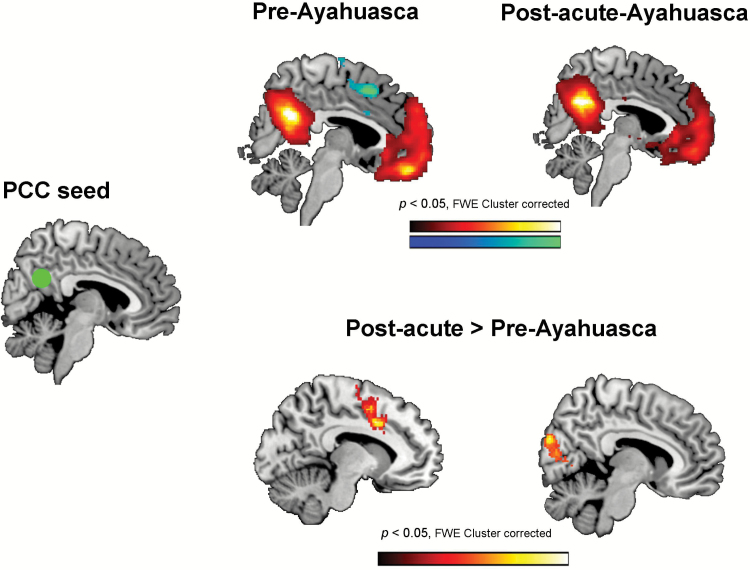
Usable pre-ayahuasca and post-acute resting-state functional connectivity scans were obtained from all 16 participants. No subjects had to be eliminated due to excessive movement (see the supplementary information file for further details). Figure 2 shows brain maps corresponding to the analysis of functional connectivity between the PCC seed (0, -56, 28) and the rest of the brain. The top panels display the results of the first-level statistical analysis separately for the pre-ayahuasca and the post-acute assessments. The bottom panels display the results of the second-level comparison between post-acute vs. pre-ayahuasca correlation maps. The statistical maps are shown corrected for multiple comparisons at the cluster level (FWE P<.05). Uncorrected maps can be found in the supplementary information file (supplementary Figure 1)
Figure 2.
Seed-to-voxel connectivity maps between the posterior cingulate cortex (PCC) seed (green circle) at MNI coordinates x = 0, y = -56, z = 28, and the rest of the brain. The top panels show statistically significant positive (hot colors) and negative (cold colors) correlations in the pre-ayahuasca and post-acute assessments. The bottom panels show the results of the second-level random effects analysis (post-acute vs. pre-intake). Only significant increases in connectivity were found, located in 2 main clusters: the anterior cingulate cortex (ACC) (left brain map) and the visual cortex (right brain map). In all statistical maps results are shown corrected for multiple comparisons at the cluster level (FWE < 0.05, z > 2.5, 20 contiguous voxels).
As shown in the upper section of Figure 2 and the supplementary file, prior to ayahuasca intake the BOLD time series in the PCC seed showed a pattern of positive correlations with brain regions known to participate in the DMN (Raichle et al., 2001). On the other hand, the PCC seed showed negative correlations with the ACC and other regions participating in the task-positive networks (TPNs) (Fox et al., 2005). In the post-acute phase, the negative correlation with the ACC was strongly reduced, suggesting a decrease in the orthogonality of BOLD activity between the PCC and the ACC.
The second-level random effects analysis confirmed the orthogonality decreases seen in the post-acute assessment maps. Brain areas demonstrating statistically significant changes in PCC seed-to-voxel connectivity are shown in the lower section of Figure 2 and are listed in Table 2. A cluster of significantly increased connectivity (reduced anticorrelation) was found in the ACC. A large significant cluster was also found over visual areas in the occipital lobe, where connectivity changed from negative to positive (see supplementary Figure 1). Additional clusters of increased connectivity were found in the pre- and post-central gyri and in the superior temporal gyrus and the insula. No areas of functional connectivity decrease were found in the second-level analysis.
Table 2.
Brain Areas Showing Post-Ayahuasca Statistically Significant Changes in Seed-to-Voxel PCC Resting-State Functional Connectivity
| Brain area | BA | MNI (x, y, z) | Number of voxels | Maximum t value |
|---|---|---|---|---|
| Areas showing increased connectivity | ||||
| Cuneus (occipital lobe) | 18/19 | (2, -88, 24) | 3824 | 6.80 |
| Anterior cingulate gyrus | 24/32 | (-10, 8, 38) | 605 | 6.80 |
| Precentral gyrus/ postcentral gyrus | 6/4 | (-54, -2, 32) | 1301 | 5.23 |
| Superior temporal gyrus/ insula | 22/13 | (-32, -8, -2) | 1401 | 5.10 |
Data shows results for the pair-wise comparison (post- vs. pre-intake) corrected for multiple comparisons at the cluster level (FWE < 0.05, z > 2.5, 20 contiguous voxels). The MNI coordinates indicate the location of the voxel with the maximum t value.
The PCC seed was located at (0, -56, 28) MNI coordinates. Data shows results of the pair-wise comparison (post- vs. pre-intake) corrected for multiple comparisons at the cluster level (FWE < 0.05, z > 2.5, 20 contiguous voxels). The MNI coordinates indicate the location of the voxel with the maximum t value. No areas were found showing significant decreases in connectivity with the PCC seed.
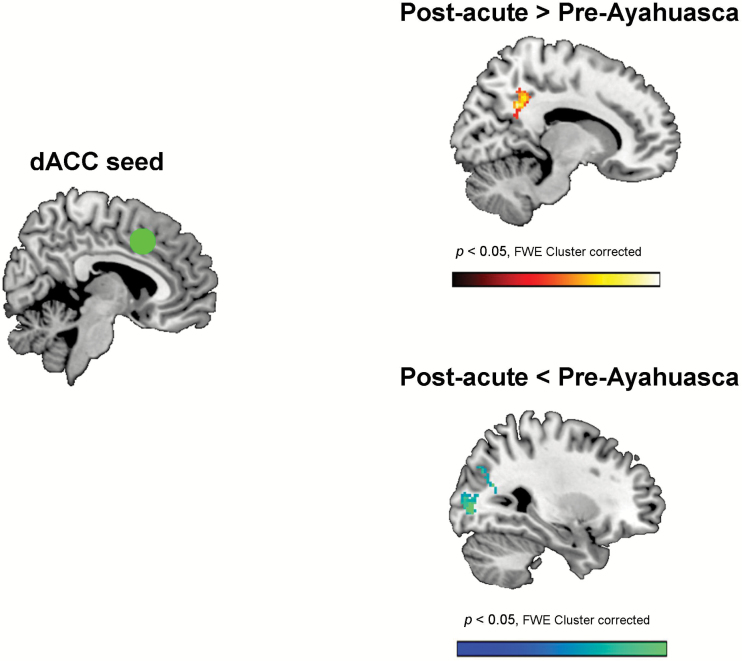
Figure 3 shows the results of the second-level random effects statistical comparison between post-acute vs. pre-intake seed-to-voxel connectivity for the dACC seed (dorsal ACC, x = 5, y = 14, z = 42). Uncorrected maps for the first-level and second-level analyses can be found in supplementary Figure 2.
Figure 3.
Statistical map showing the results of the second-level analysis (post- vs. pre-intake) of changes in connectivity between the dorsal anterior cingulate (dACC) seed (green circle) at MNI coordinates x = 5, y = 14, z = 42, and the rest of the brain. As shown in the top panel a significant increase in connectivity was found with voxels in the precuneus/posterior cingulate cortex. As shown in the bottom panel, significant decreases (cold colors) were found with voxels located in the cuneus (visual association cortex: BA 18 and 19). Results are shown corrected for multiple comparisons at the cluster level (FWE < 0.05, z > 2.5, 20 contiguous voxels).
Significant connectivity increases were seen between the seed and voxels in the medial parietal cortex corresponding to the precuneus and posterior cingulate cortex. On the other hand, functional connectivity was decreased between the dACC seed and visual association areas (BA 18 and 19) in the occipital lobes. Cluster details are provided in Table 3.
Table 3.
Brain Areas Showing Ayahuasca-Induced Statistically Significant Changes in Seed-to-Voxel Resting-State Functional Connectivity for the dACC Seed at MNI Coordinates (5, 14, 42) and the srACC Seed at (0, 15, 30)
| Brain Area | BA | MNI (x, y, z) | Number of voxels | Maximum t value |
|---|---|---|---|---|
| dAAC seed (5, 14, 42) | ||||
| Areas showing increased connectivity | ||||
| Precuneus, posterior cingulate cortex | 31 | (-6, -42, 40) | 864 | 3.76 |
| Areas showing decreased connectivity | ||||
| Cuneus (occipital lobe) | 18/19 | (28, -82, 0) | 739 | 5.56 |
| srACC seed (0, 15, 30) | ||||
| Areas showing increased connectivity | ||||
| Parahippocampal gyrus, hippocampus, amygdala (R) | 35/28 | (24, -18, -24) | 660 | 6.04 |
| Angular gyrus, inferior parietal lobule (L) | 7/40/39 | (-32, -74, 54) | 633 | 6.02 |
| Precuneus, posterior cingulate cortex (L&R) | 31/7 | (14, -54, 28) | 1436 | 5.02 |
| Areas showing decreased connectivity | ||||
| Cuneus (occipital lobe) | 18/19 | (-2, -94, 6) | 790 | 4.76 |
Abbreviations: dACC, dorsal anterior cingulate cortex; srACC, superior rostral anterior cingulate cortex.
Data shows results for the pair-wise comparison (post- vs. pre-intake) corrected for multiple comparisons at the cluster level (FWE < 0.05, z > 2.5, 20 contiguous voxels). The MNI coordinates indicate the location of the voxel with the maximum t value.
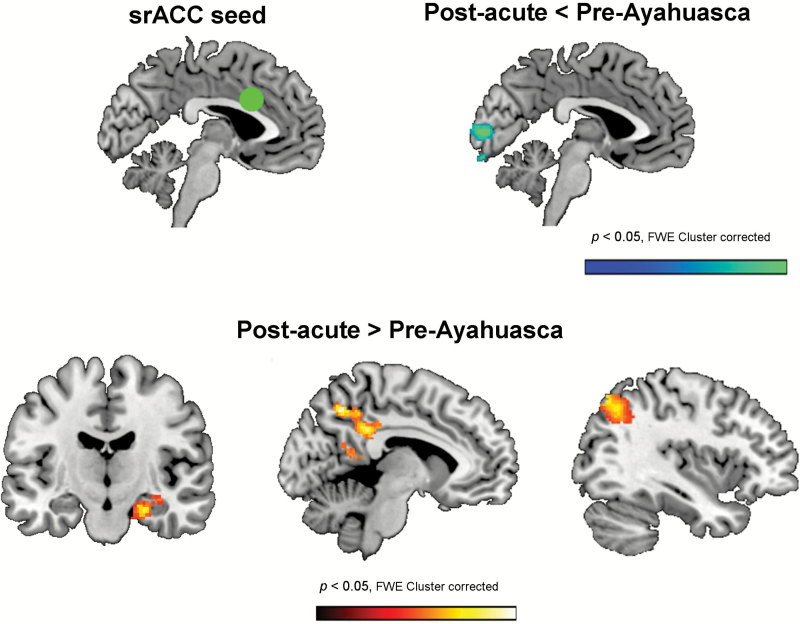
Finally, Figure 4 shows the statistical maps corresponding to the second-level random effects statistical comparison of seed-to-voxel changes between post-acute and pre-intake connectivity for the srACC seed (superior rostral ACC, x = 0, y = 15, z = 30). Cluster data are presented in Table 3, and uncorrected maps for the first-level and second-level analyses can be found in supplementary Figure 3.
Figure 4.
Statistical maps showing the results of the second-level analysis (post- vs. pre-intake) of changes in connectivity between the superior rostral anterior cingulate (srACC) seed (green circle) at MNI coordinates x = 0, y = 15, z = 30, and the rest of the brain. As shown in the top panel, decreases in connectivity (cold colors) were found with voxels located in the cuneus (visual cortex: BA 18 and 19). Increases in connectivity were found with 3 separate clusters (bottom panel): (a) the right medial temporal lobe (left brain map); (b) the precuneus and posterior cingulate cortex (center brain map); and (c) the left angular gyrus and left parietal lobule (right brain map). Results are shown corrected for multiple comparisons at the cluster level (FWE < 0.05, z > 2.5, 20 contiguous voxels).
As shown therein, in the post-acute stage, this subregion of the ACC increased its connectivity with limbic structures within the right MTL, including areas of the parahippocampal gyrus, the hippocampus, and the amygdala. Increased connectivity was also found in the left parietal lobe and in the medial parietal lobe, over the posterior cingulate cortex. As shown in the first-level maps (supplementary Figure 3), this increase reflected a reduction of the anticorrelation between the ACC and the PCC. Finally, connectivity between the srACC and the visual cortex was significantly reduced in the post-acute stage. The inspection of the first-level maps shows that BOLD activity in this area changed from being positively to negatively coupled with that in the srACC.
Mindfulness Measures
Figure 5 shows bar graphs of scores on those subscales of the FFMQ, EQ, and SC questionnaires that showed statistically significant differences from pre-intake values in the post-acute assessment or at follow-up. Tabulated data for all subscales can be found in supplementary Tables 1 and 2. As shown in supplementary Table 1, participants already scored higher than the general population in several facets and higher than meditators in the 3 subscales of the SC questionnaire.
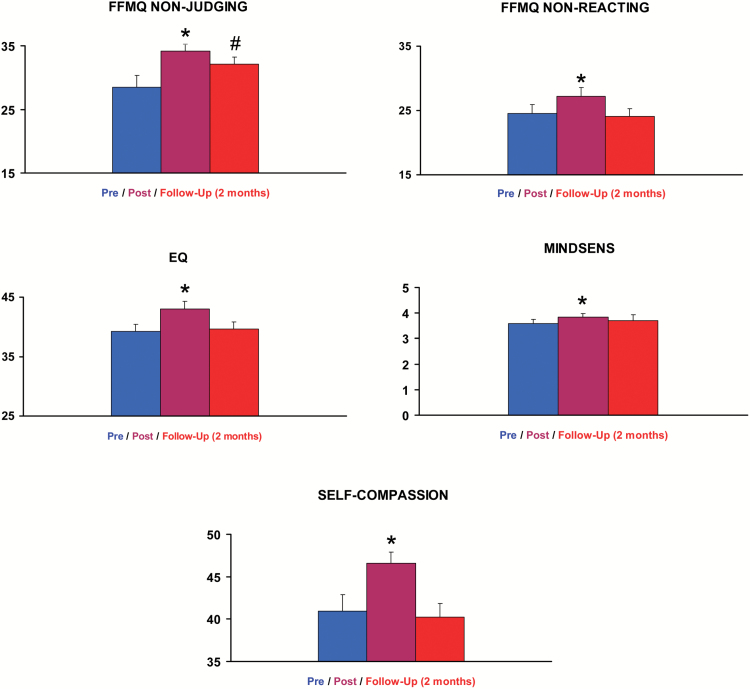
Figure 5.
The graph bars shows mean scores on mindfulness measures that showed statistically significant post- vs. pre-ayahuasca intake changes. Data from n = 16 participants at the pre- and post-acute assessments, and from n = 14 participants at follow-up 2 months later. FFMQ, Five Facets Mindfulness Questionnaire; EQ, Experiences Questionnaire; MINDSENS, Mindsens composite index; SC, Self-Compassion Questionnaire. The error bars denote 1 standard error of mean. Significant differences in the statistical comparison (post-acute or follow-up vs. pre-intake) are denoted as *P<.05 after FDR correction. #Significance lost after FDR correction. Scores on nonsignificant subscales are provided in the supplementary information file.
Despite high baseline values, further increases were observed in the post-acute assessment. The paired-samples comparison (post-acute vs. pre-ayahuasca) of the 5 subscales of the FFMQ showed statistically significant increases in scores on: nonjudging: [t(15) = -2.92, P=.011, P (FDR) = 0.021]; and nonreacting: [t(15) = -2.61, P=.020, P (FDR) = 0.031]. The other 3 subscales were not significantly modified. At follow-up 2 months later, the nonjudging score remained significantly higher than baseline: [t(13) = -2.22, P=.045], but this effect did not survive multiple comparisons correction using FDR (P=.495). Scores on the other 4 scales were not different from baseline values.
The EQ questionnaire also showed significant effects of ayahuasca, with higher scores in the post-acute stage [t(15) = -3.58, P=.003, P (FDR)=.011]. At follow-up, scores were not different from baseline values.
The Mindsens composite score was significantly increased relative to baseline [t(15) = -3.63, P = .002, P (FDR) = .011]. Again, at follow-up, scores were not significantly different from those at baseline.
Analysis of scores on the SC questionnaire showed statistically significant increases [t(15) = -3.00, P=.009, P (FDR)=.020]. At follow-up 2 months later, values were not different from baseline.
Acute Subjective Effects
The analysis of scores on the different HRS subscales showed that ayahuasca intake led to significant psychoactive effects during the acute stage (supplementary Figure 4). The comparative analysis with data from a previous laboratory study by our group (using independent samples Student’s t tests) showed significant differences from placebo for all subscales, except for volition. Scores in the present study on somaesthesia, affect, perception, volition, and intensity were not different from those we had obtained after a medium 0.75-mg DMT/kg ayahuasca dose (Valle et al., 2016). Only cognition was significantly different, with higher scores obtained here: t(26) = 4.25, P (FDR) <.01.
Exploratory Correlation Analyses
We examined potential association between neurometabolic and functional connectivity changes, and scores on mindfulness measures and subjective effects. In all correlations, difference values (post-acute minus pre-ayahuasca) were used. For follow-up measures, the difference values from pre-ayahuasca scores were used. Scatter plots for the nonjudging subscale are shown in Figure 6. Additional scatter plots are included in supplementary Figure 5.
Figure 6.
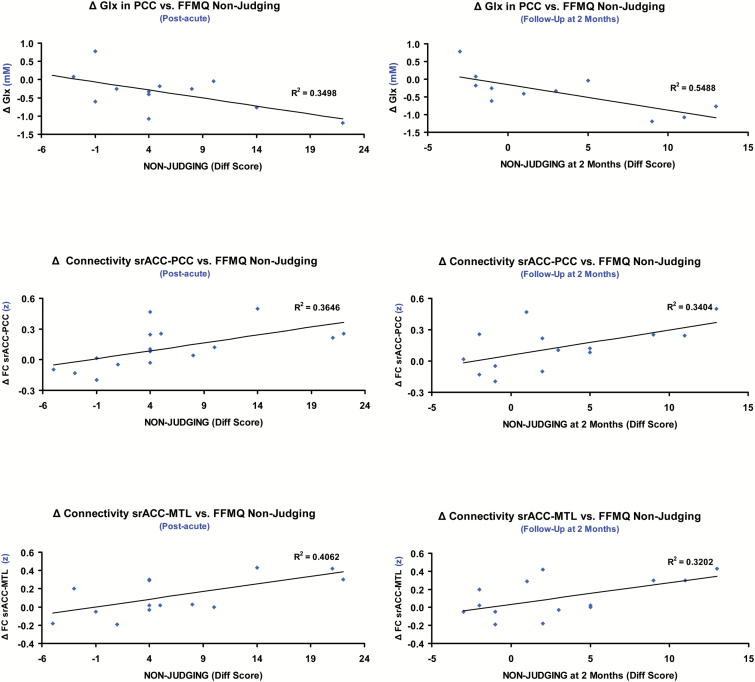
Scatter plots showing significant correlations between difference scores at the post-acute and follow-up assessments (relative to baseline values) for the FFMQ nonjudging subscale and changes in neuroimaging measures (post-acute minus pre-intake). The top panels show the correlations with changes (Δ) in Glx (glutamate+glutamine) concentrations (millimolar) in the posterior cingulate cortex (PCC) voxel as measured using MRS. The middle panels show the correlations with changes in functional connectivity (Δ in z values) between the superior rostral anterior cingulate (srACC) seed and the precuneus/PCC cluster. The lower panels show the correlations with changes in functional connectivity (Δ in z values) between the srACC seed and the right medial temporal lobe (MTL) cluster. The left column shows correlations with FFMQ nonjudging difference scores in the post-acute assessment and the right column shows correlations with difference scores at follow-up. R and P values are reported in the main text.
Associations between neurometabolic and connectivity measures, and acute psychedelic effects
Scores on the cognition subscale of the HRS correlated with Cr decreases (n=14; r=–0.735; r2=0.540; P=.003) and NAA-NAAG decreases in the PCC (n=14; r=–0.622; r2=0.387; P=.018). No other significant correlations were found.
Associations between neurometabolic and connectivity measures, and post-acute mindfulness effects
Glx decreases in the PCC correlated with increases in the nonjudging FFMQ subscale (n=12; r=–0.589; r2=0.506; P=.044). Increased srACC-PCC connectivity correlated with increases in the nonjudging (n=16; r=0.604; r2=0.365; P=.013) and Non-Reacting (n=16; r=0.522; r2=0.272; P=.038) subscales of the FFMQ. Increased srACC-MTL connectivity similarly correlated with increases in the nonjudging (n=16; r=0.637; r2=0.406; P=.008) and nonreacting (n=16; r=0.656; r2=0.430; P=.006) subscales of the FFMQ, and with the SC score (n=16; r=0.514; r2=0.264; P=.042).
Association between neurometabolic and connectivity measures, and mindfulness effects at follow-up
Glx decreases in the PCC correlated with difference in the nonjudging subscale 2 months after baseline assessment (n=11; r=–0.740; r2=0.548; P=.009). Positive correlations with sustained effects on nonjudging were also found for post-acute increases in srACC-PCC (n=14; r=0.584; r2=0.341; P=.028) and ACC-MTL (n=14; r=0.566; r2=0.320; P=.035) connectivity.
Discussion
Here we investigated the neural correlates of the psychedelic “after-glow” induced by ayahuasca in healthy volunteers. Using 2 different MRI techniques, we evidenced significant neurometabolic and functional connectivity changes hours after the acute effects of ayahuasca had disappeared. These modifications were associated with immediate changes in the psychological sphere that were marginally maintained 2 months later. Our results replicate previous findings of enhanced mindfulness capacities, including increased “decentering,” and decreased judgmental and reactive attitudes during the post-acute phase of ayahuasca (Soler et al., 2016). Ayahuasca had the power to increase FFMQ, EQ, and Mindsens scores in individuals with already high baseline scores (Soler et al., 2014a). We also found increases in self-compassion, a previously unexplored facet in this context.
MRS showed neurometabolic changes in the PCC, a region rich in 5-HT2A receptors (Carhart-Harris et al., 2012; Beliveau et al., 2016) and a target region of psychedelics (Palhano-Fontes et al., 2015; Carhart-Harris et al., 2016a; Valle et al., 2016). Glx levels in the PCC were lower in the post-acute assessment compared with baseline values, an effect that was however only marginally significant when corrected for multiple comparisons. We thus obtained partial evidence for the previously postulated involvement of glutamate neurotransmission in the effects of psychedelics (Kłodzinska et al., 2002; Moreno et al., 2011).
While we did not measure Glx levels during the acute psychedelic phase, our MRS findings are compatible with increased glutamate levels during the acute stage. Cortical glutamate levels increase in periods of external perceptual stimulation or during active cognitive tasks, while they fall below baseline levels during stimulation- or task-free periods (Mangia et al., 2007; Huang et al., 2015; Terhune et al., 2015). Also, acute psilocybin decreases brain aspartate (Preller et al., 2016), a neurotransmitter whose levels vary in an anticorrelated fashion with those of glutamate (Mangia et al., 2007). The post-acute Glx decreases in the PCC may result from increased excitatory activity during the acute phase. EEG and MEG studies in humans have shown a decrease of alpha oscillations, an inhibitory rhythm, in the parieto-occipital cortex during the acute effects of psychedelics (Kometer et al., 2013; Carhart-Harris et al., 2016a; Valle et al., 2016).
The post-acute Glx reductions in the PCC are also consistent with the observed reductions in Cr, NAA, and NAAG. Cr and N-acetyl compounds have been associated with metabolic activity, and NAAG has been directly linked to glutamatergic pathways (Rae, 2014). Additionally, the inverse correlation found between Cr and NAA-NAAG variations and scores on the HRS-Cognition subscale suggest a relationship between the intensity of acute effects and subsequent neurometabolic reductions. Neurometabolic changes may have contributed to the antidepressant effects reported for ayahuasca (Osório et al., 2015; Sanches et al., 2016). Depressed patients show abnormally high glutamate levels in the parieto-occipital cortex (Bhagwagar et al., 2007). Glutamate reductions in these areas correlate with clinical improvement in depression (Abdallah et al., 2014).
The functional connectivity analysis also evidenced post-acute changes. Activity in the PCC and associated areas within the DMN (Raichle et al., 2001) has been associated with the personal sense of “self.” Psychedelics acutely loosen the boundaries of the “self” and increase the cross-talk between networks (Carhart-Harris et al., 2012, 2016a). Here, we found a post-acute increase in coupling between the PCC and a subregion of the ACC pertaining to the TPNs.
While DMN and TPN activity are typically anticorrelated (Fox et al., 2005), psilocybin and LSD acutely increase DMN-TPN connectivity (Carhart-Harris et al., 2013), and general inter-network connectivity (Roseman et al., 2014; Tagliazucchi et al., 2016). Our results suggest that cross-talk lingers beyond the acute stage and contributes to the “after-glow,” reflected as enhanced mindfulness capacities. Increased DMN-TPN connectivity correlated with reduced judgmental processing, inner reactivity, and increased self-kindness, providing a neurobiological basis for these modifications. Conventional mindfulness training also increases DMN-TPN connectivity (Doll et al., 2015).
Visual areas showed increased coupling with the PCC but reduced with the ACC. This pattern suggests a greater interplay between internally generated visual information and spontaneous mind-wandering, and a reduction in cognitive control. These effects could explain increased phosphenes or “entoptic activity” persisting days after ayahuasca use (Frecska et al., 2012). A previous neuroimaging study found increased activity in the visual cortex under ayahuasca (de Araujo et al., 2012).
The superior rostral ACC (srACC) seed also demonstrated increased functional coupling with parahippocampal, hippocampal, and amygdalar areas of the MTL. Prior studies had identified these areas as targets of acute ayahuasca (Riba et al., 2004, 2006; de Araujo et al., 2012). LSD acutely decreases fear recognition (Dolder et al., 2016), an effect mediated by the amygdala, and psilocybin increases synchronization between the hippocampus and the ACC (Tagliazucchi et al., 2014).
Our data suggest that during the “after-glow” there is an enhanced interplay between the ACC, participating in executive tasks and in the binding of cognitive and emotional information, with limbic structures with key roles in emotion and memory processes. This finding is particularly relevant in the interpretation of the antidepressant effects of ayahuasca. Other researchers have found abnormal interactions between the srACC and the amygdala in depressed patients, possibly indicating decreased cognitive control over negative emotions (Fales et al., 2008).
Our data show for the first time that the modification induced by psychedelics on brain dynamics leads to changes in its neurometabolic, functional, and psychological balance beyond the acute stage. As previously reported (Griffiths et al., 2011; Lebedev et al., 2016), the post-acute phase in our study showed positive psychological effects, highlighting the paradoxical nature of psychedelics (Carhart-Harris et al., 2016c). While the acute inebriation shares features with psychosis (Schmid et al., 2015; Carhart-Harris et al., 2016c), psychedelics may lead to mid-term increases in psychological well-being. Increasing mindfulness capacities is clearly a desirable effect, especially in a psychotherapeutic context. Here post-acute scores were above values reported for meditators (Soler et al., 2014a), a population that also shows a pattern of decreased DMN-TPN anticorrelation (Brewer et al., 2011; Froeliger et al., 2012). Considering that maladaptive ruminations in depression have been associated with greater DMN “dominance” over TPN activity (Hamilton et al., 2011), our results provide another interesting link between psychedelic-induced neural modifications and the therapeutic potential of ayahuasca.
The post-acute phase of the psychedelic experience had received little attention from modern neuroscience. Although investigators had postulated the capacity of psychedelics to modulate brain plasticity (Vollenweider and Kometer, 2010), most research had assessed mid- and long-term effects only from a psychological perspective. The changes in personality and life attitudes reported in the 1960s (Savage et al., 1966; Pahnke, 1969) have recently been replicated as increases in trait openness (Griffiths et al., 2006; Carhart-Harris et al., 2016c). Also, in a structural neuroimaging study of regular ayahuasca users, we found a cortical thinning of the PCC, the area showing neurometabolic decreases in the present study. PCC thinning was inversely correlated with increased self-transcendence, a personality trait closely related to openness (Bouso et al., 2015).
Our MRS and connectivity data provide a biological basis for the therapeutic effects of ayahuasca (Osório et al., 2015; Sanches et al., 2016). Its potential to influence brain dynamics at multiple levels suggests its usefulness to treat disorders that are highly refractory to therapeutic intervention. Its combined effect on the psychological and neural spheres may be particularly well suited to treat addiction disorders (Fernández and Fábregas, 2014), where high impulsivity and self-centeredness coexist with alterations in brain function and structure (Vaquero et al., 2016).
Our study has several limitations that need to be mentioned. We assessed a small sample of individuals before and after ayahuasca intake, with no control for placebo or time effects. The difficulties associated with Glx quantification allowed measurement in an even smaller sample. All participants had previous experience with ayahuasca, which may have biased our sample to individuals who usually experience positive effects after intake. Additionally, participants showed high baseline scores on several mindfulness facets. While this could be considered a limitation, it is also true that these capacities show “ceiling” effects and are difficult to increase in high scorers (Montero-Marin et al., 2016). The correlation analysis should be considered exploratory and interpreted with caution. Finally, our study investigated only the sub-acute stage of ayahuasca effects. The observed connectivity modifications cannot be interpreted as indicating persistent network changes. Future studies should consider using larger samples and double-blind, placebo-controlled designs. Also, the role of prior exposure to ayahuasca could be better established by recruiting less experienced or even ayahuasca-naive individuals
To conclude, the present results indicate that ayahuasca and potentially other psychedelics induce neural modifications beyond the time frame of the acute inebriation. Neurometabolic decreases in the PCC and the increased inter-network connectivity were associated with enhanced mindfulness facets. These associations provide hints to a potential biological basis for the therapeutic effects of ayahuasca.
Statement of Interest
None.
Supplementary Material
Acknowledgments
The authors thank Alexander Lebedev for performing the Frame Displacement analysis of the fMRI data. We are also grateful to Anna Ermakova for her critical reading of the manuscript; Núria Bargalló, Anna Calvo, and Cesar Garrido for technical assistance; and the study volunteers for their participation.
This study was funded by the Beckley Foundation. Marta Valle was supported by FIS through a grant (CP04/00121) from the Spanish Health Ministry in collaboration with Institut de Recerca de l’Hospital de la Santa Creu i Sant Pau, Barcelona. José Alexandre S. Crippa and Jaime E. C. Hallak are recipients of CNPq Research fellowship awards.
References
- Abdallah CG, Niciu MJ, Fenton LR, Fasula MK, Jiang L, Black A, Rothman DL, Mason GF, Sanacora G (2014) Decreased occipital cortical glutamate levels in response to successful cognitive-behavioral therapy and pharmacotherapy for major depressive disorder. Psychother Psychosom 83:298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beliveau V, Ganz M, Feng L, Ozenne B, Højgaard L, Fisher PM, Svarer C, Greve DN, Knudsen GM (2016) A high-resolution in vivo atlas of the human brain’s serotonin system. J Neurosci 37:120–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhagwagar Z, Wylezinska M, Jezzard P, Evans J, Ashworth F, Sule A, Matthews PM, Cowen PJ (2007) Reduction in occipital cortex gamma-aminobutyric acid concentrations in medication-free recovered unipolar depressed and bipolar subjects. Biol Psychiatry 61:806–812. [DOI] [PubMed] [Google Scholar]
- Bouso JC, Palhano-Fontes F, Rodríguez-Fornells A, Ribeiro S, Sanches R, Crippa JAS, Hallak JEC, de Araujo DB, Riba J (2015) Long-term use of psychedelic drugs is associated with differences in brain structure and personality in humans. Eur Neuropsychopharmacol 25:483–492. [DOI] [PubMed] [Google Scholar]
- Brewer JA, Worhunsky PD, Gray JR, Tang Y- Y, Weber J, Kober H (2011) Meditation experience is associated with differences in default mode network activity and connectivity. Proc Natl Acad Sci U S A 108:20254–20259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbonaro TM, Eshleman AJ, Forster MJ, Cheng K, Rice KC, Gatch MB (2015) The role of 5-HT2A, 5-HT 2C and mGlu2 receptors in the behavioral effects of tryptamine hallucinogens N,N-dimethyltryptamine and N,N-diisopropyltryptamine in rats and mice. Psychopharmacology (Berl) 232:275–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris RL, Erritzoe D, Williams T, Stone JM, Reed LJ, Colasanti A, Tyacke RJ, Leech R, Malizia AL, Murphy K, Hobden P, Evans J, Feilding A, Wise RG, Nutt DJ (2012) Neural correlates of the psychedelic state as determined by fMRI studies with psilocybin. Proc Natl Acad Sci U S A 109:2138–2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris RL, Leech R, Erritzoe D, Williams TM, Stone JM, Evans J, Sharp DJ, Feilding A, Wise RG, Nutt DJ (2013) Functional connectivity measures after psilocybin inform a novel hypothesis of early psychosis. Schizophr Bull 39:1343–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris RL, et al. (2016a) Neural correlates of the LSD experience revealed by multimodal neuroimaging. Proc Natl Acad Sci U S A 113:4853–4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris RL, Bolstridge M, Rucker J, Day CMJ, Erritzoe D, Kaelen M, Bloomfield M, Rickard JA, Forbes B, Feilding A, Taylor D, Pilling S, Curran VH, Nutt DJ (2016b) Psilocybin with psychological support for treatment-resistant depression: an open-label feasibility study. Lancet Psychiatry 3:619–627. [DOI] [PubMed] [Google Scholar]
- Carhart-Harris RL, Kaelen M, Bolstridge M, Williams TM, Williams LT, Underwood R, Feilding A, Nutt DJ (2016c) The paradoxical psychological effects of lysergic acid diethylamide (LSD). Psychol Med 46:1379–1390. [DOI] [PubMed] [Google Scholar]
- Cebolla A, Garcia Palacios A, Soler J, Guillén V, Baños R, Botella C (2012) Psychometric properties of the Spanish validation of the Five Facets of Mindfulness Questionnaire (FFMQ). Eur J Psychiatry 26:118–126. [Google Scholar]
- de Araujo DB, Ribeiro S, Cecchi GA, Carvalho FM, Sanchez TA, Pinto JP, de Martinis BS, Crippa JA, Hallak JEC, Santos AC (2012) Seeing with the eyes shut: neural basis of enhanced imagery following Ayahuasca ingestion. Hum Brain Mapp 33:2550–2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon ML, Fox KCR, Christoff K (2014) Evidence for rostro-caudal functional organization in multiple brain areas related to goal-directed behavior. Brain Res 1572:26–39. [DOI] [PubMed] [Google Scholar]
- Dolder PC, Schmid Y, Müller F, Borgwardt S, Liechti ME (2016) LSD acutely impairs fear recognition and enhances emotional empathy and sociality. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol 41:2638–2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doll A, Hölzel BK, Boucard CC, Wohlschläger AM, Sorg C (2015) Mindfulness is associated with intrinsic functional connectivity between default mode and salience networks. Front Hum Neurosci 9:461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domínguez-Clavé E, Soler J, Elices M, Pascual JC, Álvarez E, de la Fuente Revenga M, Friedlander P, Feilding A, Riba J (2016) Ayahuasca: pharmacology, neuroscience and therapeutic potential. Brain Res Bull 126:89–101. [DOI] [PubMed] [Google Scholar]
- Fales CL, Barch DM, Rundle MM, Mintun MA, Snyder AZ, Cohen JD, Mathews J, Sheline YI (2008) Altered emotional interference processing in affective and cognitive-control brain circuitry in major depression. Biol Psychiatry 63:377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández X, Fábregas JM (2014) Experience of treatment with ayahuasca for drug addiction in the Brazilian Amazon. In: The therapeutic use of ayahuasca (Labate BC, Cavnar C, eds), pp161–182. Berlin, Heidelberg: Springer. [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME (2005) The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A 102:9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frecska E, Móré CE, Vargha A, Luna LE (2012) Enhancement of creative expression and entoptic phenomena as after-effects of repeated ayahuasca ceremonies. J Psychoactive Drugs 44:191–199. [DOI] [PubMed] [Google Scholar]
- Froeliger B, Garland EL, Kozink RV, Modlin LA, Chen N-K, McClernon FJ, Greeson JM, Sobin P (2012) Meditation-state functional connectivity (msFC): strengthening of the dorsal attention network and beyond. Evid Based Complement Alternat Med 2012:680407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Campayo J, Navarro-Gil M, Andrés E, Montero-Marin J, López-Artal L, Demarzo MMP (2014) Validation of the Spanish versions of the long (26 items) and short (12 items) forms of the Self-Compassion Scale (SCS). Health Qual Life Outcomes 12:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths R, Richards W, Johnson M, McCann U, Jesse R (2008) Mystical-type experiences occasioned by psilocybin mediate the attribution of personal meaning and spiritual significance 14 months later. J Psychopharmacol (Oxf) 22:621–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths RR, Richards WA, McCann U, Jesse R (2006) Psilocybin can occasion mystical-type experiences having substantial and sustained personal meaning and spiritual significance. Psychopharmacology (Berl) 187:268–283. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Johnson MW, Richards WA, Richards BD, McCann U, Jesse R (2011) Psilocybin occasioned mystical-type experiences: immediate and persisting dose-related effects. Psychopharmacology (Berl) 218:649–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grob CS, Danforth AL, Chopra GS, Hagerty M, McKay CR, Halberstadt AL, Greer GR (2011) Pilot study of psilocybin treatment for anxiety in patients with advanced-stage cancer. Arch Gen Psychiatry 68:71–78. [DOI] [PubMed] [Google Scholar]
- Halpern JH. (1996) The use of hallucinogens in the treatment of addiction. Addict Res 4:177–189. [Google Scholar]
- Hamilton JP, Furman DJ, Chang C, Thomason ME, Dennis E, Gotlib IH (2011) Default-mode and task-positive network activity in major depressive disorder: implications for adaptive and maladaptive rumination. Biol Psychiatry 70:327–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z, Davis Iv HH, Yue Q, Wiebking C, Duncan NW, Zhang J, Wagner N-F, Wolff A, Northoff G (2015) Increase in glutamate/glutamine concentration in the medial prefrontal cortex during mental imagery: a combined functional mrs and fMRI study. Hum Brain Mapp 36:3204–3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly AMC, Di Martino A, Uddin LQ, Shehzad Z, Gee DG, Reiss PT, Margulies DS, Castellanos FX, Milham MP (2009) Development of anterior cingulate functional connectivity from late childhood to early adulthood. Cereb Cortex 19:640–657. [DOI] [PubMed] [Google Scholar]
- Kłodzinska A, Bijak M, Tokarski K, Pilc A (2002) Group II mGlu receptor agonists inhibit behavioural and electrophysiological effects of DOI in mice. Pharmacol Biochem Behav 73:327–332. [DOI] [PubMed] [Google Scholar]
- Kometer M, Schmidt A, Jäncke L, Vollenweider FX (2013) Activation of serotonin 2A receptors underlies the psilocybin-induced effects on α oscillations, N170 visual-evoked potentials, and visual hallucinations. J Neurosci 33:10544–10551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebedev AV, Kaelen M, Lövdén M, Nilsson J, Feilding A, Nutt DJ, Carhart-Harris RL (2016) LSD-induced entropic brain activity predicts subsequent personality change. Hum Brain Mapp 37:3203–3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li BSY, Wang H, Gonen O (2003) Metabolite ratios to assumed stable creatine level may confound the quantification of proton brain MR spectroscopy. Magn Reson Imaging 21:923–928. [DOI] [PubMed] [Google Scholar]
- Mangia S, Tkác I, Gruetter R, Van de Moortele P-F, Maraviglia B, Uğurbil K (2007) Sustained neuronal activation raises oxidative metabolism to a new steady-state level: evidence from 1H NMR spectroscopy in the human visual cortex. J Cereb Blood Flow Metab 27:1055–1063. [DOI] [PubMed] [Google Scholar]
- McIlhenny EH, Pipkin KE, Standish LJ, Wechkin HA, Strassman R, Barker SA (2009) Direct analysis of psychoactive tryptamine and harmala alkaloids in the Amazonian botanical medicine ayahuasca by liquid chromatography-electrospray ionization-tandem mass spectrometry. J Chromatogr A 1216:8960–8968. [DOI] [PubMed] [Google Scholar]
- McKenna DJ, Towers GH, Abbott F (1984) Monoamine oxidase inhibitors in South American hallucinogenic plants: tryptamine and beta-carboline constituents of ayahuasca. J Ethnopharmacol 10:195–223. [DOI] [PubMed] [Google Scholar]
- Montero-Marin J, Puebla-Guedea M, Herrera-Mercadal P, Cebolla A, Soler J, Demarzo M, Vazquez C, Rodríguez-Bornaetxea F, García-Campayo J (2016) Psychological effects of a 1-month meditation retreat on experienced meditators: the role of non-attachment. Front Psychol 7:1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno JL, Holloway T, Albizu L, Sealfon SC, González-Maeso J (2011) Metabotropic glutamate mGlu2 receptor is necessary for the pharmacological and behavioral effects induced by hallucinogenic 5-HT2A receptor agonists. Neurosci Lett 493:76–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muschamp JW, Regina MJ, Hull EM, Winter JC, Rabin RA (2004) Lysergic acid diethylamide and [-]-2,5-dimethoxy-4-methylamphetamine increase extracellular glutamate in rat prefrontal cortex. Brain Res 1023:134–140. [DOI] [PubMed] [Google Scholar]
- Osório F de L, Sanches RF, Macedo LR, dos Santos RG, Maia-de-Oliveira JP, Wichert-Ana L, de Araujo DB, Riba J, Crippa JA, Hallak JE (2015) Antidepressant effects of a single dose of ayahuasca in patients with recurrent depression: a preliminary report. Rev Bras Psiquiatr (Sao Paulo) 37:13–20. [DOI] [PubMed] [Google Scholar]
- Pahnke WN. (1969) Psychedelic drugs and mystical experience. Int Psychiatry Clin 5:149–162. [PubMed] [Google Scholar]
- Pahnke WN, Kurland AA, Unger S, Savage C, Grof S (1970) The experimental use of psychedelic (LSD) psychotherapy. JAMA 212:1856–1863. [PubMed] [Google Scholar]
- Palhano-Fontes F, Andrade KC, Tofoli LF, Santos AC, Crippa JAS, Hallak JEC, Ribeiro S, de Araujo DB (2015) The psychedelic state induced by ayahuasca modulates the activity and connectivity of the default mode network. PloS One 10:e0118143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preller KH, Pokorny T, Hock A, Kraehenmann R, Stämpfli P, Seifritz E, Scheidegger M, Vollenweider FX (2016) Effects of serotonin 2A/1A receptor stimulation on social exclusion processing. Proc Natl Acad Sci U S A 113:5119–5124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae CD. (2014) A guide to the metabolic pathways and function of metabolites observed in human brain 1H magnetic resonance spectra. Neurochem Res 39:1–36. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL (2001) A default mode of brain function. Proc Natl Acad Sci U S A 98:676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riba J, Rodríguez-Fornells A, Urbano G, Morte A, Antonijoan R, Montero M, Callaway JC, Barbanoj MJ (2001) Subjective effects and tolerability of the South American psychoactive beverage Ayahuasca in healthy volunteers. Psychopharmacology (Berl) 154:85–95. [DOI] [PubMed] [Google Scholar]
- Riba J, Valle M, Urbano G, Yritia M, Morte A, Barbanoj MJ (2003) Human pharmacology of ayahuasca: subjective and cardiovascular effects, monoamine metabolite excretion, and pharmacokinetics. J Pharmacol Exp Ther 306:73–83. [DOI] [PubMed] [Google Scholar]
- Riba J, Anderer P, Jané F, Saletu B, Barbanoj MJ (2004) Effects of the South American psychoactive beverage ayahuasca on regional brain electrical activity in humans: a functional neuroimaging study using low-resolution electromagnetic tomography. Neuropsychobiology 50:89–101. [DOI] [PubMed] [Google Scholar]
- Riba J, Romero S, Grasa E, Mena E, Carrió I, Barbanoj MJ (2006) Increased frontal and paralimbic activation following ayahuasca, the pan-Amazonian inebriant. Psychopharmacology (Berl) 186:93–98. [DOI] [PubMed] [Google Scholar]
- Riba J, McIlhenny EH, Bouso JC, Barker SA (2015) Metabolism and urinary disposition of N,N-dimethyltryptamine after oral and smoked administration: a comparative study. Drug Test Anal 7:401–406. [DOI] [PubMed] [Google Scholar]
- Roseman L, Leech R, Feilding A, Nutt DJ, Carhart-Harris RL (2014) The effects of psilocybin and MDMA on between-network resting state functional connectivity in healthy volunteers. Front Hum Neurosci 8:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanches RF, de Lima Osório F, Dos Santos RG, Macedo LRH, Maia-de-Oliveira JP, Wichert-Ana L, de Araujo DB, Riba J, Crippa JAS, Hallak JEC (2016) Antidepressant effects of a single dose of ayahuasca in patients with recurrent depression: a SPECT study. J Clin Psychopharmacol 36:77–81. [DOI] [PubMed] [Google Scholar]
- Savage C, Fadiman J, Mogar R, Allen MH (1966) The effects of psychedelic (LSD) therapy on values, personality, and behavior. Int J Neuropsychiatry 2:241–254. [PubMed] [Google Scholar]
- Schenberg EE, Alexandre JFM, Filev R, Cravo AM, Sato JR, Muthukumaraswamy SD, Yonamine M, Waguespack M, Lomnicka I, Barker SA, da Silveira DX (2015) Acute biphasic effects of ayahuasca. PloS One 10:e0137202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid Y, Enzler F, Gasser P, Grouzmann E, Preller KH, Vollenweider FX, Brenneisen R, Müller F, Borgwardt S, Liechti ME (2015) Acute effects of lysergic acid diethylamide in healthy subjects. Biol Psychiatry 78:544–553. [DOI] [PubMed] [Google Scholar]
- Scruggs JL, Schmidt D, Deutch AY (2003) The hallucinogen 1-[2,5-dimethoxy-4-iodophenyl]-2-aminopropane (DOI) incr eas es cortical extracellular glutamate levels in rats. Neurosci Lett 346:137–140. [DOI] [PubMed] [Google Scholar]
- Sessa B. (2005) Can psychedelics have a role in psychiatry once again? Br J Psychiatry J Ment Sci 186:457–458. [DOI] [PubMed] [Google Scholar]
- Soler J, Cebolla A, Feliu-Soler A, Demarzo MP, Pascual JC, Baños R, García-Campayo J (2014a) Relationship between meditative practice and self-reported mindfulness: the MINDSENS Composite Index. PLoS ONE 9:e86622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soler J, Franquesa A, Feliu-Soler A, Cebolla A, García-Campayo J, Tejedor R, Demarzo M, Baños R, Pascual JC, Portella MJ (2014b) Assessing decentering: validation, psychometric properties, and clinical usefulness of the Experiences Questionnaire in a Spanish sample. Behav Ther 45:863–871. [DOI] [PubMed] [Google Scholar]
- Soler J, Franquesa A, Feliu-Soler A, Cebolla A, Garcia-Campayo J, Tejedor R, Demarzo MMP, Baños R, Pascual JC, Portella MJ (2014c) Assessing decentering: validation, psychometric properties and clinical usefulness of the Experiences Questionnaire in a Spanish sample. Behav Ther 45:863–871. [DOI] [PubMed] [Google Scholar]
- Soler J, Elices M, Franquesa A, Barker S, Friedlander P, Feilding A, Pascual JC, Riba J (2016) Exploring the therapeutic potential of Ayahuasca: acute intake increases mindfulness-related capacities. Psychopharmacology (Berl) 233:823–829. [DOI] [PubMed] [Google Scholar]
- Szabo A, Kovacs A, Riba J, Djurovic S, Rajnavolgyi E, Frecska E (2016) The endogenous hallucinogen and trace amine N,N-dimethyltryptamine (DMT) displays potent protective effects against hypoxia via sigma-1 receptor activation in human primary iPSC-derived cortical neurons and microglia-like immune cells. Front Neurosci 10:423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagliazucchi E, Carhart-Harris R, Leech R, Nutt D, Chialvo DR (2014) Enhanced repertoire of brain dynamical states during the psychedelic experience. Hum Brain Mapp 35:5442–5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagliazucchi E, Roseman L, Kaelen M, Orban C, Muthukumaraswamy SD, Murphy K, Laufs H, Leech R, McGonigle J, Crossley N, Bullmore E, Williams T, Bolstridge M, Feilding A, Nutt DJ, Carhart-Harris R (2016) Increased global functional connectivity correlates with LSD-induced ego dissolution. Curr Biol CB 26:1043–1050. [DOI] [PubMed] [Google Scholar]
- Terhune DB, Murray E, Near J, Stagg CJ, Cowey A, Cohen Kadosh R (2015) Phosphene perception relates to visual cortex glutamate levels and covaries with atypical visuospatial awareness. Cereb Cortex 25:4341–4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valle M, Maqueda AE, Rabella M, Rodríguez-Pujadas A, Antonijoan RM, Romero S, Alonso JF, Mañanas MÀ, Barker S, Friedlander P, Feilding A, Riba J (2016) Inhibition of alpha oscillations through serotonin-2A receptor activation underlies the visual effects of ayahuasca in humans. Eur Neuropsychopharmacol 26:1161–1175. [DOI] [PubMed] [Google Scholar]
- Vaquero L, Cámara E, Sampedro F, Pérez de Los Cobos J, Batlle F, Fabregas JM, Sales JA, Cervantes M, Ferrer X, Lazcano G, Rodríguez-Fornells A, Riba J (2016) Cocaine addiction is associated with abnormal prefrontal function, increased striatal connectivity and sensitivity to monetary incentives, and decreased connectivity outside the human reward circuit. Addict Biol 22:844–856. [DOI] [PubMed] [Google Scholar]
- Vogt BA. (2009) Regions and subregions of the cingulate cortex. In: Cingulate neurobiology and disease (Vogt BA, ed), pp 3–30. Oxford, New York: Oxford University Press. [Google Scholar]
- Vogt BA, Laureys S (2005) Posterior cingulate, precuneal and retrosplenial cortices: cytology and components of the neural network correlates of consciousness. Prog Brain Res 150:205–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollenweider FX, Kometer M (2010) The neurobiology of psychedelic drugs: implications for the treatment of mood disorders. Nat Rev Neurosci 11:642–651. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Nieto-Castanon A (2012) Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect 2:125–141. [DOI] [PubMed] [Google Scholar]
- Winkelman M. (2014) Psychedelics as medicines for substance abuse rehabilitation: evaluating treatments with LSD, peyote, ibogaine and ayahuasca. Curr Drug Abuse Rev 7:101–116. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.