Abstract
It is with great pleasure that I write this foreword and introduction to this Special Issue dedicated to the protective actions of the pro-resolving mediators and edited by my colleague Dr. Jesmond Dalli. Many of my collaborators and colleagues that helped to uncover the actions and clinical potential of the resolvins and other specialized proresolving mediators (SPM), namely, the superfamily of pro-resolving mediators that includes the resolvin (E-series, D-series and DPA-derived), protectin and maresin families, as well as the arachidonic acid-derived lipoxins, join me in this special issue. They have given contributions that present exciting new results on the remarkable actions and potency of these unique molecules, the SPM moving forward the importance of their mediators and pathways in human biology. Each contribution to this issue is presented by world authorities in their respective fields covering discoveries that demonstrate the importance and impact of resolution mediators in biology, medicine and surgery. While some of the authors were students and/or fellows with me and others, they are today the founding “resolutionists” of a new era of appreciation of autacoid biosynthesis and metabolomics in human health and disease with their rigorous attention to experimental detail and discovery. The chapters of this issue are filled with exciting new discoveries demonstrating the dynamics and potential of resolution mediators.
Keywords: resolvin, protectin, maresin, inflammation, omega-3, lipoxin
Introduction
Claude Bernard wrote “Physiology, pathology and therapeutics developed as distinct sciences. That was the wrong road. Only today can we begin to see the conception of an experimental, scientific medicine in the fusion of these three in a single point of view” in his Introduction to the Study of Experimental Medicine that appeared in France in 1865. These words remain profound today, as they teach us of the importance of a multidisciplinary approach, as we have taken, to study the molecules, mediators and mechanisms involved in the resolution phase of the acute inflammatory response. The resolution of inflammation is central to human health and its potential failure lies at the heart of many diseases where uncontrolled inflammation amplifies as well as creates illness.
Why study resolution of inflammation mechanisms and novel mediators?
In the post-genomic era, the forefront in medicine and health is controlling unwanted inflammation and infection. Hence, elucidating resolution mechanisms for inflammation can harness more effective treatments. Inflammation-associated diseases are a significant public health concern, placing considerable financial burden that impacts millions in the USA and worldwide (www.cdc.gov). It is well appreciated that human phagocytes (neutrophils & macrophages) play pivotal roles in host defense, the acute inflammatory response and its timely resolution (Colgan et al., 2013; Nathan and Ding, 2010; Serhan, 2010, 2014; Tabas and Glass, 2013). Uncontrolled inflammation is now widely appreciated as a unifying component in many chronic diseases including vascular diseases, metabolic syndrome, neurological diseases, and many others (Pomponi et al., 2010; Serhan et al., 2007; Tracey, 2002; Zhang and Spite, 2012). Since the acute inflammatory response is protective, evolved to permit repair of injured tissues and eliminate invading organisms (Cotran et al., 1999b), it is ideally self-limited and leads to complete resolution of leukocyte infiltrates and clearance of cellular debris enabling return to homeostasis (Figure 1). Although resolution of disease is appreciated by clinicians, resolution was considered a passive process (Cotran et al., 1999b), until our contributions (Serhan, 2004; Serhan et al., 2002) and now many others worldwide (Buckley et al., 2013; Chan and Moore, 2010; Miki et al., 2013; Rossi and Sawatzky, 2008; Wu et al., 2009) to obtain new evidence demonstrating that resolution of self-limited inflammation is an active process. With our strategy employing unbiased LM-lipidomics, genetically engineered animals (rabbits and mice), exudates and human cell systems, we obtained the first evidence that resolution is actively “turned on” and not simply a passive process (Serhan, 2007, 2009; Serhan et al., 2002; Serhan et al., 2003).
Figure 1. Function of Resolvins and Superfamily of SPM in acute inflammation resolution.
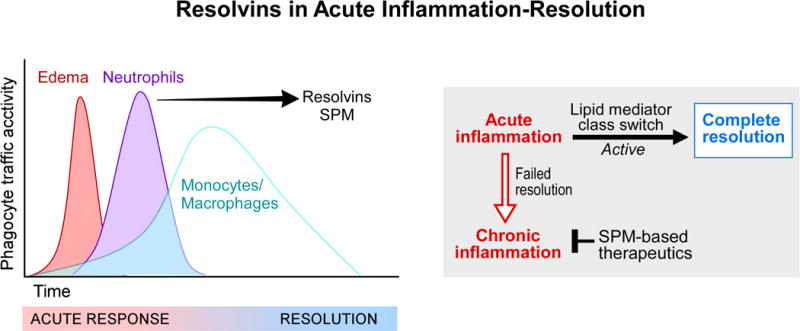
(Left) In self-limited inflammation, acute vascular and leukocyte trafficking are rapid and protective to rid the host of invaders, resolving with time to return to homeostasis, namely the loss of pus and inflammatory exudate cells from the site to return to function of the tissue. Edema and neutrophil infiltration are governed by chemoattractants, both exogenous (microbial derived) and endogenous, such as leukotriene B4 and chemokines. Resolvins and other SPM are temporally biosynthesized when neutrophils reach maximal numbers and begin to reduce in number from the inflamed site in tissues. Monocytes and resolution-phase macrophages (Stables et al., 2011) enter in a nonphlogistic fashion to help repair and remodel tissues as needed for complete resolution of the site.
(Right) Lipid mediator class switching is the process we introduced (Levy et al., 2001) to describe the temporal change in lipid mediators from initiation of inflammation to resolutionphase mediators as a biosynthetically active process, switching from PG and LT production to translational regulation of the leukocyte enzymes required to biosynthesize lipoxins, protectins and D-series resolvins. Failed resolution mechanisms may be responsible for persistent recurring bursts of acute infiltrates of leukocytes that can lead to chronic inflammation and the amplification of tissue injury. The potential of SPM-based therapeutics exemplifies a new approach, namely “one-to-many” counter-regulating pro-inflammatory targets to stop the progression (Business Wire, 2009) to chronic is depicted.
Local Mediators in Resolution
Key to our discoveries and resulting paradigm change was our identification of a novel genus of pro-resolving mediators that include resolvins, protectins, their aspirin-triggered forms (Buckley et al., 2013; Serhan et al., 2007) as well as the maresins (Serhan et al., 2009), which provided evidence that the resolution phase is orchestrated by local mediators and their biosynthesis from n-3 precursors EPA and DHA (Figures 2 and 3). From this, it’s now become evident that resolution programs of acute inflammation hold promise and remain at the forefront of medicine and biology (Buckley et al., 2013; Haeggström and Hamberg, 2013). Challenges ahead are whether we can harness these novel mediators that stimulate resolution (i.e. we’ve coined agonists of resolution as pharmacologic agents immunoresolvents (Dalli et al., 2013b; Serhan, 2011; Serhan et al., 2012)), as disease treatments. Dietary n-3 supplements are widely used but <25% are directed by health care providers (Bailey et al., 2013). Clinical trials with n-3 fatty acids have shown mixed results (De Caterina, 2011; Ramaswami et al., 2016; Ramsden, 2016; Roncaglioni et al., 2013). It is clear that fatty acids themselves are not suitable drugs. Hence, it was deemed critical for public health to establish the mechanisms that underlie their essential health requirements.
Figure 2. Resolvins, protectins and maresins in the frame of resolution of inflammation.
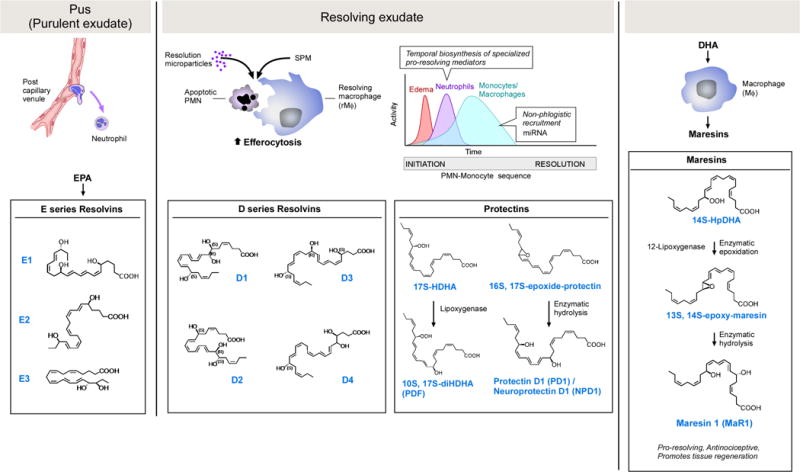
(Left) Pus production from the first step of PMN-endothelial interactions is the nidus of E-series resolvin formation and actions, with three separate potent bioactive molecules produced. Microparticles, macrophages and apoptotic PMN produce resolvins and SPM (Dalli and Serhan, 2012), which enhance phagocytosis and limit further PMN infiltration. D-series resolvins are biosynthesized from DHA; four are depicted, six are currently elucidated and functionally defined. Maresins and protectins are also produced in inflammatory exudates from DHA. The potent PD1/neuroprotectin D1 is biosynthesized via an epoxide intermediate that is synthesized and confirmed via total organic synthesis, as is the eMaresin 13(14)-epoxide (see text for details). The 17S-hydroperoxy precursor to 10S,17S-diDHA, a.k.a. PDx, gives the trans-cis-trans conjugation (Serhan et al., 2015; Serhan et al., 2006). The complete stereochemistry of each resolvin, protectin and maresin is established, and their potent stereoselective actions are confirmed.
Figure 3. Biosynthesis routes for resolvins and SPM.
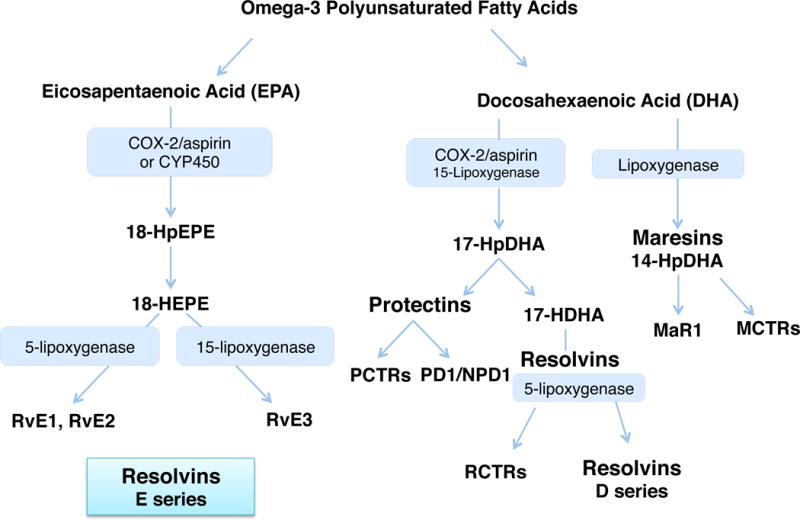
The main biosynthesis routes are depicted. Each was confirmed via label tracking of precursors and intermediates as well as trapping of proposed intermediates. In addition to lipoxygenase-initiated pathways that produce mediators with alcohols, for example in PD1 or D-series resolvins in predominantly the 17S configuration, aspirin acetylation of cyclooxygenase-2 (COX-2) produces intermediates predominantly in the R configuration at the 17-carbon position producing the 17R epimers of 17R-PD1 and D-series resolvins coined the aspirin-triggered resolvin and protectin mediators. Statins can also lead to S-nitrosylation of COX-2 that, like aspirin acetylation, changes the enzyme’s catalytic site or produce R epimer-containing intermediates (see text for details).
Using a systems approach with resolving exudates, we elucidated novel n-3 essential fatty acid-derived SPM pathways (Serhan, 2009; Serhan and Savill, 2005) that carry specific antiphlogistic pro-resolving actions (see Table 1); each stimulated at pico-nanogram potencies. Their biosynthesis and complete stereochemistry of each major resolvin (RvE1, RvD1, RvD2, RvD3, RvD4, RvD5 and RvD6) conferring their potent biologic actions reviewed in (Serhan, 2017; Serhan and Petasis, 2011) are the focus of our investigations (Figures 2 and 3) first established from the C.N. Serhan laboratories and now independently confirmed by many worldwide. For example, following our publications, the structures and potent pico to nanogram actions of Rv are extended to many organs including vascular (Miyahara et al., 2013), airway (Seki et al., 2010), dermal (reviewed in (Lee, 2012; Serhan et al., 2008)), ocular (Li et al., 2010), pain (Feng et al., 2012; Huang et al., 2011; Lima-Garcia et al., 2011; Xu et al., 2010; Xu et al., 2013), fibrosis, wound healing (Campbell et al., 2010; de Paiva et al., 2012; El Kebir et al., 2012; Hisada et al., 2009; Ishida et al., 2010; Jin et al., 2009; Keyes et al., 2010; Kim et al., 2012; Lund et al., 2010; Qu et al., 2012; Rajasagi et al., 2011; Vassiliou et al., 2008; Wan et al., 2011). This is also the case for D series resolvins (RvD1 (Bang et al., 2010; Hellmann et al., 2011; Li et al., 2013; Liao et al., 2012; Liu et al., 2012; Palmer et al., 2011; Rogerio et al., 2012; Settimio et al., 2012; Tang et al., 2013; Terrando et al., 2013), RvD2 (Bohr et al., 2013; Pope et al., 2016)) and neuroprotectins/protectins (Bazan et al., 2010; Isobe et al., 2012; Kenchegowda et al., 2013; Park et al., 2011; Schwab et al., 2007; Sheets et al., 2010); each has actions in organs throughout the body and experimental animal system. Protectin D1 also stops viral replication (Morita et al., 2013), and PDx, its isomer, is also bioactive (Serhan et al., 2006; White et al., 2014), demonstrating action of host-derived mediators directly on microbes of interest in host defense.
Table 1.
Specialized Pro-resolving Mediators (SPM): Anti-Phlogistic Pro-Resolving Actions
| SPM defining bioactions |
|---|
|
To pinpoint SPM in vivo actions, we also defined the first resolution indices that permit identification of SPM and drugs that shorten resolution intervals (Bannenberg et al., 2005; Chiang et al., 2008; Schwab et al., 2007). These indices are now used worldwide to monitor resolution in many systems (Hilberath et al., 2011; Morris et al., 2010; Navarro-Xavier et al., 2010; Pruss et al., 2011). Focusing on human phagocyte-directed actions (i.e. phagocytosis of apoptotic PMN and bacteria) as key characteristics for SPM structural elucidation proved that SPM impact many diverse preclinical disease models (Serhan, 2010), as well as pain (Xu and Ji, 2011), wound healing, cancer (Janakiram and Rao, 2009), and tissue regeneration (Serhan et al., 2012). Several other groups have reported on total organic syntheses that confirmed our original SPM structures and stereochemical assignments (reviewed in (Serhan, 2010; Serhan and Petasis, 2011)), including PD1 (Ogawa and Kobayashi, 2011), RvD1, RvD2 (Rodriguez and Spur, 2004), RvE1 (Ogawa and Kobayashi, 2009) and RvD5 (Rodriguez and Spur, 2012). Importantly, availability of commercial Rv has help to confirm and extend their many actions in controlling inflammation-resolution (Serhan and Chiang, 2013) and even viral influenza infection (Morita et al., 2013). SPM standards and protocols for targeted LC-MS-MS that originated from my NIH-supported research permitted identification of Rv and SPM in human tissues of healthy individuals and in human diseases (Mas et al., 2012; Psychogios et al., 2011), human adipose (Claria et al., 2013) and reduced levels in those with multiple sclerosis (Pruss et al., 2013), trout (Hong et al., 2005), and even salmon (Raatz et al., 2011); see Figure 4 and references within, and for a recent review (Serhan, 2017). Hence, these structures are conserved in evolution from fish to humans and govern potent bioactions in all major organ systems. This is because phagocytes travel throughout the body to protect each organ from invaders or to clear debris from within, a programmed response (Schwab et al., 2007). The ability of SPM to control phagocyte function is at the heart of their versatile proresolving mechanism of action throughout the organs of the body. Each resolvin and SPM has its own receptors that are GPCR (Serhan et al., 2008), which evoke rapid intracellular signaling and long-term actions via regulating specific miR involved in resolution of inflammation (Krishnamoorthy et al., 2012; Recchiuti et al., 2011).
Figure 4. Identification of resolvins and SPM in vivo.
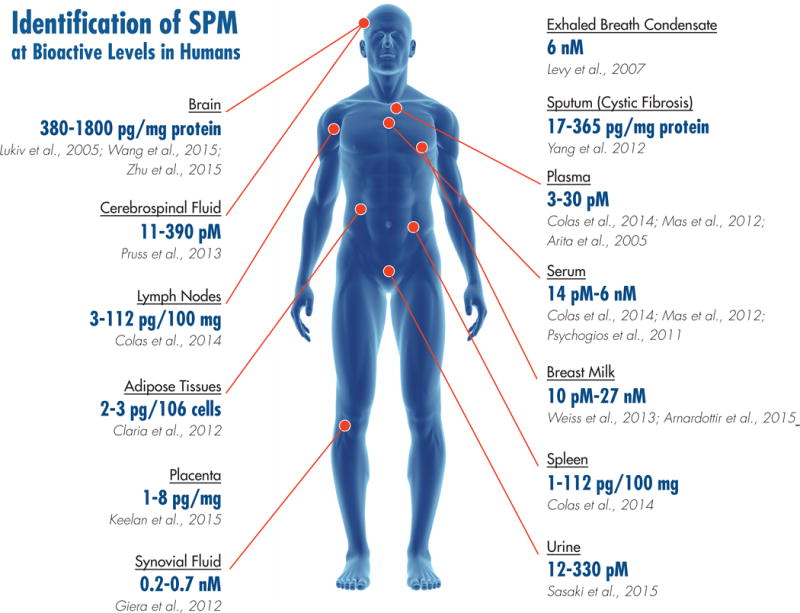
Illustration depicts the location of SPM identified by rigorous LC-MS-MS-based methods that are present in concentrations that are bioactive in experimental model systems in vivo. See accompanying references in Figure 4.
SPM Circuits: Alpha Signals Omega
Identification of SPM and their temporal biosynthesis in vivo taught us that a) resolution is active process, not passive, b) mediators of inflammation-resolution are temporally produced to control phagocyte functions and tissue numbers (Serhan, 2011; Serhan et al., 2008; Serhan and Savill, 2005), c) SPM also control pain (Ji et al., 2011), d) help clear infections (Chiang et al., 2012) and e) SPM regulate pro-inflammatory pathways and mediators (Chiang et al., 2012; Spite et al., 2009). Most important, f) SPM are not immunosuppressive, (Chiang et al., 2012; Spite et al., 2009; Spite and Serhan, 2010) unlike many current clinically used anti-inflammatories (Dinarello, 2010). SPM enhance the killing and clearance of bacteria (Chiang et al., 2012; Oh et al., 2011), an unexpected discovery that is confirmed in other laboratories and provided a new view on drugs that can function together with pro-resolving agonists to help lower unwanted side effects and/or emergence of bacterial resistance; see Table 2. Key to the cell-cell signaling actions of SPM is their ability to activate specific G-protein coupled receptors (GPCR)(Dalli et al., 2013a). RvE1 activates two separate receptors (Serhan et al., 2011), for example, to control PMN and macrophages (Arita et al., 2007; Ohira et al., 2010). RvD1 activates GPR32, a human orphan receptor (Krishnamoorthy et al., 2010; Recchiuti et al., 2011) that regulates PMN, MΦ phagocytosis as well as resolution phase miRNA that are each regulated in GPR32-dependent MΦ responses (Krishnamoorthy et al., 2010; Recchiuti et al., 2011). Hence, it is now clear that anti-inflammation and pro-resolving actions are not equivalent. The ability of SPM to clear dead cells, bacteria and infection is unique to resolvins and other SPM, and most importantly the beginning to the inflammatory challenge signals its termination in self-limited responses (vide infra).
Table 2.
SPM Shorten Resolution of Infections, Increase Survival and Outcomes in Murine Infection & Disease Models
| SPM | Increase Survival | Disease | Shorten resolution intervals Ri | Reference |
|---|---|---|---|---|
| LXA4 | + | Bacterial infections | (Walker et al., 2011) | |
| 15-epi-LXA4 | + | Lung injury | (El Kebir et al., 2009) | |
| RvE1 | + | Colitis | + | (Arita et al., 2005) |
| + | Candida yeast | + | (Haas-Stapleton et al., 2007) | |
| + | Acid-induced lung injury | + | (Levy and Serhan, 2014) | |
| RvD5, PD1 | + | E. coli infection | + | (Chiang et al., 2012) |
| RvD1, RvD5, PD1 | + | Acute lung injury | + | (Wang et al., 2011) |
| RvD2 | + | Cecal ligation and puncture sepsis | + | (Chiang et al., 2017; Spite et al., 2009) |
| RvD2 | + | Burn wound sepsis | + | (Bohr et al., 2013) |
|
RvD2 RvD3 RvD4 MCTRs |
+ + |
Burn wound sepsis Aging mice, peritonitis Skin Inflammation peritonitis, organ protection |
+ + + + |
(Bohr et al., 2013) (Arnardottir et al., 2014) (Winkler et al., 2016) (Dalli et al., 2014) |
Neural Signals for Resolution and Novel Pro-Resolving Circuits?
During inflammation-resolution, human PMN and MΦ biosynthesize specific functionally distinct profiles (Dalli and Serhan, 2012) of lipid–derived mediators (LM). Those that are pro-inflammatory include classic prostaglandins (PG) and leukotrienes (LT) (Samuelsson, 2012), whereas the new SPM profiles (Figure 2) are generated by phagocytes during resolution from omega-3 essential fatty acids (EFA), e.g. EPA, n-3 DPA and DHA. During natural resolution, apoptotic PMN are lost from inflammatory sites and tissues return to homeostasis (Serhan and Savill, 2005). In contained exudates, LM production is temporally dissociated. PG and LT appear first with PMN entry, followed by LX and Rv in resolution. Apoptotic PMN switch from producing LT to SPM, revealed with targeted LC-MS-MS-based metabolomics as a key source of SPM during resolution (Dalli and Serhan, 2012). Our results demonstrated for the first time that the type of LM produced during inflammation–resolution is both spatial and temporal in mice (Schwab et al., 2007; Serhan et al., 2002) and with isolated human PMN and MΦ (Dalli and Serhan, 2012). Also, LM in resolution of Lyme disease (Blaho et al., 2009), infections (Chiang et al., 2012) and the temporal role of leukocyte lipoxygenase in these are now being appreciated (Blaho et al., 2011). Hence, endogenous circuits that control resolution of acute inflammation are of general interest and operative throughout the whole organism and human body. Communications between brain and innate immune functions such as vagus nerve are of particular interest given its ability to signal anti-inflammatory pathways (Tracey, 2002). We found that vagus nerve controls resolution of acute inflammation via activating a novel resolvin circuit (Mirakaj et al., 2014).
Each of the SPM, given their unique structures and roles, opens new opportunity for treating human disease. For an example of this possibility, let’s take the stereochemical assignment of SPM, i.e. RvD2 (7S,16R,17S-trihydroxy-4Z,8E,10Z,12E,14E,19Z-docosahexaenoic acid); this enabled us to uncover that RvD2 regulates both PMN and MΦ to limit tissue damage and enhance killing and clearance of bacteria (Spite et al., 2009). RvD2 controls murine survival in sepsis from cecal puncture (Spite et al., 2009), prolongs survival in severe burn and sepsis, (Jones et al., 2012; Kurihara et al., 2013) reduces colitis (Bento et al., 2011) and is temporally elevated during transition from initiation to resolution phase (Dalli et al., 2013b). These new findings point to specific functions of RvD2 and SPM (Table 2) in activating resolution (Chiang et al., 2015) and are responses activated by specific GPCR (Chiang et al., 2015; Chiang et al., 2017). We have approached the work up and elucidation of each SPM family member in a similar fashion, giving them more than factor status.
Neural Control of Resolution SPM: A New Link?
Vagus nerve exerts local anti-inflammatory effects (Tracey, 2002) that we found to directly govern resolution. Hence, it is important to establish the molecules and mechanism(s) involved. Vagotomy disrupts resolution and human MΦ incubated with acetylcholine (Ach) produce RvD1 and RvD2 (Mirakaj et al., 2014). Thus, we determined the impact of vagus control of resolution on LM/SPM biosynthesis with unbiased LM-lipidomic/metabolomic profiles, and establish the human RvD2 circuit and its human disease expression (Mirakaj et al., 2014), a new circuit that is also operative in PCTR production (Dalli et al., 2017).
Relationship of SPM in the World of Eicosanoids: Birth of the Resolvins & SPM
The history of the prostaglandins and their roles in physiologic processes date back to the 1930’s, with relaxation of myometrium (Weissmann, 1980). While von Euler coined the name prostaglandins for these active substances, it soon became clear that they were not produced by the prostate gland. Bergström and colleagues at the Karolinska determined the structures of the main prostaglandins in the late 1950’s, and in the early 1960’s Samuelsson established that arachidonic acid was the precursor, and biosynthesis of intermediates are rapidly converted from arachidonic acid to prostaglandins and other potent mediators.
Prostaglandins act on smooth muscle, gut, airway and blood vessels. Sir John Vane and his colleagues demonstrated that aspirin and other NSAIDs of the time blocked cyclooxygenase (Vane, 1982). Thromboxane A2 and prostacyclin were identified next, and their roles in platelet aggregation and vasodilation elucidated (Samuelsson, 1983). Each of the cardinal signs of inflammation, known to physicians of ancient societies, rubor, tumor, dolor and functio laesa, are evoked by these potent mediators from arachidonic acid, thromboxanes, prostaglandins and prostacyclin (Vane, 1982; Weissmann, 1980). Next to arrive on the world map of autacoid biosynthesis and metabolomics from the arachidonic acid cascade was the structural elucidation of the leukotrienes, which are the slow-reacting substances of anaphylaxis (SRS-A). These leukocyte-derived conjugated trienes LTC4, LTD4 and LTE4 are potent mediators in anaphylaxis, and leukotriene B4 is a potent chemoattractant of human neutrophils (Samuelsson, 1983).
Confusion on Omega-3 PUFA in Human Health: Novel Role in Resolution of Inflammation
Numerous reports of the past ~40 years suggest that supplementation of dietary omega-3 polyunsaturated fatty acids (ω-3 PUFA) has beneficial effects in human diseases and laboratory animals (De Caterina et al., 1993; Lands, 1987). These include antithrombotic, immunoregulatory and anti-inflammatory responses relevant in arteriosclerosis, arthritis and asthma (De Caterina et al., 1993) as well as antitumor and antimetastatic effects (Iigo et al., 1997). Their potential for preventative actions in cardiovascular diseases was bolstered with the finding that major dietary ω-3 PUFAs, eicosapentaenoic acid (C20:5 ω-3; EPA) and docosahexaenoic acid (C22:6 ω-3; DHA) have a dramatic effect on ischemia-induced ventricular fibrillation and can protect against sudden cardiac death (Billman et al., 1999). Emergence of such preventative and/or therapeutic actions of ω-3 PUFA supplementation in infant nutrition, cardiovascular diseases and mental health called for recommended dietary intakes by an international workshop (Simopoulos et al., 1999). However, the molecular mechanisms(s) for dietary ω-3 protective actions remain unexplained at the cellular and molecular levels and still a subject of wide interest.
At the time, it was widely believed that the actions of the major lipid of fish oil are caused by i) preventing conversion of arachidonic acid (C20:4 ω-6, AA) to proinflammatory eicosanoids (i.e., PG and leukotrienes (LT)); ii) serving as an alternate substrate producing 5-series LT that are less potent; and/or iii) conversion by cyclooxygenase (COX) to 3-series prostanoids (i.e., PGI3) with potencies equivalent to their 4-series PG counterparts to maintain antithrombotic actions (Billman et al., 1999; De Caterina et al., 1993; Iigo et al., 1997). These and other explanations offered (Billman et al., 1999; De Caterina et al., 1993; Iigo et al., 1997; Simopoulos et al., 1999) are not embraced by us because of the lack of molecular evidence and the high concentrations of ω-3 PUFA required to evoke putative beneficial actions. Although the proinflammatory roles of LT and PG are well appreciated (Marcus, 1999; Weissmann, 1991), new evidence emerged regarding other eicosanoids derived from arachidonate, namely lipoxins (LXs) and their endogenous analogs, the aspirin-triggered 15-epimer lipoxins (ATL), potent counterregulators of PMN-mediated injury and acute inflammation (Chiang et al., 1999; Clària and Serhan, 1995; Serhan et al., 1995). Acetylation of COX-2 by aspirin (ASA) prevents the formation of prostanoids (Herschman, 1998), but the acetylated enzyme remains active in situ, generating 15R-hydroeicosatetraenoic acid (15R-HETE) from C20:4 (Chiang et al., 1998; Xiao et al., 1997), which is converted by inflammatory cells to 15-epimeric lipoxins (ATL, a.k.a. aspirin-triggered lipoxins). Synthetic analogs of these natural local mediators with prolonged bio-half-life display potent anti-inflammatory properties (Chiang et al., 1999; Clish et al., 1999; Serhan, 1999), providing evidence that cell-cell “cross-talk” can convert arachidonic acid to mediators with anti-inflammatory properties (Serhan et al., 2000b), thus changing our view of the mechanism of action of this drug. Importantly, LX and ATL stimulate monocytes in a non-phlogistic fashion (Maddox et al., 1997; Maddox and Serhan, 1996), suggesting their role in monocyte/macrophage-mediating processes, i.e. tissue repair, healing and resolution. Hence, aspirin enables the endogenous resolution mechanisms and programs unlike other NSAIDs.
Since PMN-vessel interactions are pivotal to recruitment and PMN-dependent tissue injury (Cotran et al., 1999a), the local signals involved in their “cross-talk dialog” were our interest and starting point in this line of investigation. Our finding that aspirin-acetylated COX-2 remains active in vivo (Chiang et al., 1998) to generate specific ATL that can be effectors of well-established anti-inflammatory therapy offers a mechanism for ASA beneficial impact that cannot be attributed to prostanoids (Herschman, 1998; Marcus, 1999). Because new therapeutic uses for aspirin and related NSAIDs continue to be uncovered that require molecular definition, including prophylaxis against colorectal cancer and lower risk of myocardial infarction (Levy, 1997), and in view of overlapping beneficial profiles assigned to dietary ω-3 PUFA in human disease (Billman et al., 1999; De Caterina et al., 1993; Iigo et al., 1997; Simopoulos et al., 1999), we first sought evidence for novel mechanisms involved in the biosynthesis and production of lipid-derived signals that would provide a basis as well to explain some of the beneficial actions of ω-3 PUFA (Serhan et al., 2000a). Aspirin jump-starts resolution by triggering novel lipid mediators.
The resolution of inflammation has origins in the Canon of Medicine, which was brought to Europe in the 11th Century (Avicenna (Abu cAli Sina) adapted by Laleh Bakhtiar, 1999; Serhan, 2011). The connection of omega-3 PUFA to novel mediators and resolution was elucidated with identification of novel structures of the SPM and new (Table 1) proresolving actions (Serhan and Savill, 2005). Earlier studies suggested that the omega-3 PUFA simply become prostaglandins, thromboxanes, or leukotriene-like structures that were without potent bioactivities. The discovery of novel structures that were previously unknown which stimulate resolution of inflammation and infection by evoking new biological functions has opened this area of investigation and the biology of resolution and resolution pharmacology. Thus, the SPM super-family members are each both tool compounds as well as physiologic mediators.
Temporal lipid mediator class (and family) switching (Figure 1) is central to this biosynthesis for active resolution (Levy et al., 2001). In addition to the resolvins, protectins and maresins (Hong et al., 2003; Serhan et al., 2002), conjugates of the epoxide intermediates in each of the SPM pathways were uncovered in recent studies; these novel SPM-peptido conjugates enhance tissue regeneration and bacterial killing and clearance; namely, these series are coined respectively MCTR, RCTR and PCTR, the maresin, resolvin and protectin conjugates in tissue regeneration CTR (Dalli et al., 2014; Dalli et al., 2015b). Their complex structures and potent actions underscore the critical role and importance of the specific epoxide-containing intermediates in SPM biosynthesis, stereochemistry and function (Aursnes et al., 2015; Serhan et al., 2015). Hence, resolution-phase mediators help control inflammation, infection and tissue regeneration, major essential processes that protect the host. Each member of the structurally distinct families evokes the cardinal signs of resolution of inflammation (Dalli et al., 2015a) to bring about resolution via counter regulation of pro-inflammatory mediators, giving them their unprecedented dual anti-inflammatory, proresolving status (Table 3).
Table 3.
Counter-Regulate & Reduce Cardinal Signs of Inflammation
|
The major hubs of PUFA-derived signaling families of molecules depicted in Figure 5 are involved in the initiation and resolution of inflammation. These molecules can also be used as biotemplates for designer drugs and new therapeutics that can control inflammation by stimulating resolution (Lee and Zeldin, 2015; Leslie, 2015; Serhan, 2014), namely resolution agonists. Now that the resolvin and lipoxin structures have opened the path to resolution of inflammation, many other resolution-phase mediators have been discovered including hydrogen sulfide gas, annexin peptides and carbon monoxide, to name a few (Perretti et al., 2015). There will likely be many more discoveries in the years ahead that will demonstrate the importance of resolution-phase mediators such as the resolvins, as well as their impact in human biology and potential in clinical development of novel therapeutics for a wide range of diseases. The contributions in this special issue can thus serve as a guide by experts to navigate the sea of resolution signals and the impact of the SPM pathways and superfamily of SPM mediators in human health, personalized medicine and nutrition as well as disease, hence marking the dawn of resolution physiology and pharmacology.
Figure 5. Hubs of conserved bioactive lipid mediator metabolomes.
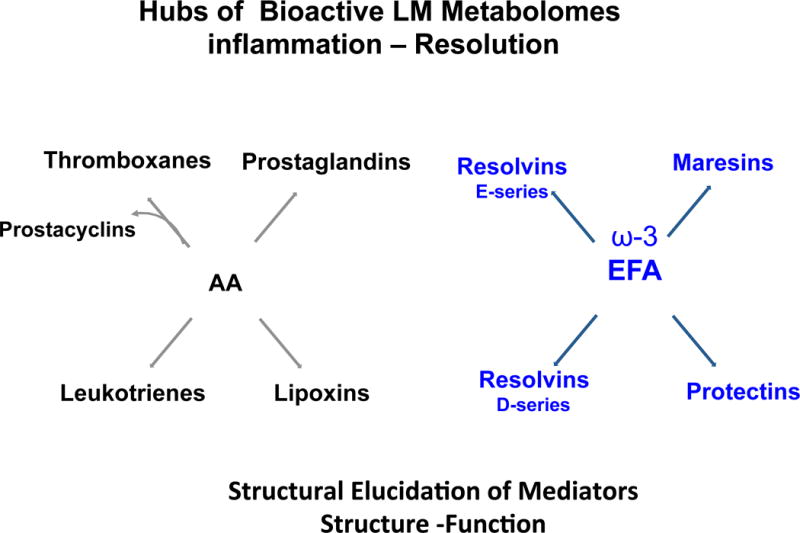
(Left) The arachidonic acid-derived mediators.
(Right) The ω-3 essential fatty acid-derived mediators.
These families and their function illustrate the importance of structural elucidation in metabolomics based on structure-function in biologic and human systems.
Acknowledgments
The author thanks Mary H. Small for expert help in manuscript preparation. I also gratefully thank my students, post-doctoral fellows, colleagues and many collaborators cited herein that have enthusiastically worked tirelessly along with me to bring forward the field of resolution and resolution pharmacology.
Funding: Studies in the Serhan Lab were supported by the National Institutes of Health [grant numbers P01GM095467, R01GM038765 and R01DE025020].
Abbreviations
- DHA
docosahexaenoic acid
- EPA
eicosapentaenoic acid
- LC-MS-MS
liquid chromatography tandem mass spectrometry
- LM
lipid-derived mediators
- LOX
lipoxygenase
- LT
leukotriene
- LX
lipoxin
- PG
prostaglandins
- MΦ
macrophage
- Maresins
macrophage mediators in resolving inflammation
- PMN
polymorphonuclear leukocyte
- PD protectin PD1/NPD1
protectin D1/neuroprotectin D1
- SPM
specialized pro-resolving mediators
- Rv, Resolvins
bioactive omega-3 derived resolution phase interaction products
- E series Rv
resolvins from EPA
- D series Rv
resolvins from DHA
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arita M, Ohira T, Sun YP, Elangovan S, Chiang N, Serhan CN. Resolvin E1 selectively interacts with leukotriene B4 receptor BLT1 and ChemR23 to regulate inflammation. J Immunol. 2007;178:3912–3917. doi: 10.4049/jimmunol.178.6.3912. [DOI] [PubMed] [Google Scholar]
- Arita M, Yoshida M, Hong S, Tjonahen E, Glickman JN, Petasis NA, Blumberg RS, Serhan CN. Resolvin E1, an endogenous lipid mediator derived from omega-3 eicosapentaenoic acid, protects against 2,4,6-trinitrobenzene sulfonic acid-induced colitis. Proc Natl Acad Sci USA. 2005;102:7671–7676. doi: 10.1073/pnas.0409271102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnardottir H, Orr SK, Dalli J, Serhan CN. Human milk proresolving mediators stimulate resolution of acute inflammation. Mucosal Immunol. 2016;9:757–766. doi: 10.1038/mi.2015.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnardottir HH, Dalli J, Colas RA, Shinohara M, Serhan CN. Aging delays resolution of acute inflammation in mice: reprogramming the host response with novel nano-proresolving medicines. J Immunol. 2014;193(8):4235–4244. doi: 10.4049/jimmunol.1401313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aursnes M, Tungen JE, Colas RA, Vlasakov I, Dalli J, Serhan CN, Hansen TV. Synthesis of the 16S,17S-Epoxyprotectin Intermediate in the Biosynthesis of Protectins by Human Macrophages. J Nat Prod. 2015;78:2924–2931. doi: 10.1021/acs.jnatprod.5b00574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avicenna (Abu cAli Sina) adapted by Laleh Bakhtiar. The Canon of Medicine (al-Qanun fi’l-tibb) Great Books of the Islamic World, Inc.; Chicago: 1999. [Google Scholar]
- Bailey RL, Gahche JJ, Miller PE, Thomas PR, Dwyer JT. Why US Adults Use Dietary Supplements. JAMA Intern Med. 2013;173(5):355–361. doi: 10.1001/jamainternmed.2013.2299. [DOI] [PubMed] [Google Scholar]
- Bang S, Yoo S, Yang TJ, Cho H, Kim YG, Hwang SW. Resolvin D1 attenuates activation of sensory transient receptor potential channels leading to multiple anti-nociception. Br J Pharmacol. 2010;161:707–720. doi: 10.1111/j.1476-5381.2010.00909.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannenberg GL, Chiang N, Ariel A, Arita M, Tjonahen E, Gotlinger KH, Hong S, Serhan CN. Molecular circuits of resolution: Formation and actions of resolvins and protectins. J Immunol. 2005;174:4345–4355. doi: 10.4049/jimmunol.174.7.4345. [DOI] [PubMed] [Google Scholar]
- Barden AE, Moghaddami M, Mas E, Phillips M, Cleland LG, Mori TA. Specialised pro-resolving mediators of inflammation in inflammatory arthritis. Prostaglandins Leukot Essent Fatty Acids. 2016;107:24–29. doi: 10.1016/j.plefa.2016.03.004. [DOI] [PubMed] [Google Scholar]
- Bazan NG, Calandria JM, Serhan CN. Rescue and repair during photoreceptor cell renewal mediated by docosahexaenoic acid-derived neuroprotectin D1. J Lipid Res. 2010;51:2018–2031. doi: 10.1194/jlr.R001131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bento AF, Claudino RF, Dutra RC, Marcon R, Calixto JB. Omega-3 fatty acid-derived mediators 17(R)-hydroxy docosahexaenoic acid, aspirin-triggered resolvin D1 and resolvin D2 prevent experimental colitis in mice. J Immunol. 2011;187:1957–1969. doi: 10.4049/jimmunol.1101305. [DOI] [PubMed] [Google Scholar]
- Billman GE, Kang JX, Leaf A. Prevention of sudden cardiac death by dietary pure w-3 polyunsaturated fatty acids in dogs. Circulation. 1999;99:2452–2457. doi: 10.1161/01.cir.99.18.2452. [DOI] [PubMed] [Google Scholar]
- Blaho VA, Buczynski MW, Brown CR, Dennis EA. Lipidomic analysis of dynamic eicosanoid responses during the induction and resolution of Lyme arthritis. J Biol Chem. 2009;284:21599–21612. doi: 10.1074/jbc.M109.003822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaho VA, Zhang Y, Hughes-Hanks JM, Brown CR. 5-Lipoxygenase-deficient mice infected with Borrelia burgdorferi develop persistent arthritis. J Immunol. 2011;186(5):3076–3084. doi: 10.4049/jimmunol.1003473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohr S, Patel SJ, Sarin D, Irimia D, Yarmush ML, Berthiaume F. Resolvin D2 prevents secondary thrombosis and necrosis in a mouse burn wound model. Wound Repair Regen. 2013;21:35–43. doi: 10.1111/j.1524-475X.2012.00853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley CD, Gilroy DW, Serhan CN, Stockinger B, Tak PP. The resolution of inflammation. Nat Rev Immunol. 2013;13:59–66. doi: 10.1038/nri3362. [DOI] [PubMed] [Google Scholar]
- Business Wire. Resolvyx announces positive data from Phase 2 clinical trial of the reesolvin RX-10045 in patients with dry eye syndrome. Business Wire. 2009 http://www.businesswire.com/news/home/20090824005320/en/Resolvyx-Announces-Positive-Data-Phase-20090824005322-Clinical.
- Campbell EL, MacManus CF, Kominsky DJ, Keely S, Glover LE, Bowers BE, Scully M, Bruyninckx WJ, Colgan SP. Resolvin E1-induced intestinal alkaline phosphatase promotes resolution of inflammation through LPS detoxification. Proc Natl Acad Sci U S A. 2010;107(32):14298–14303. doi: 10.1073/pnas.0914730107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan MMY, Moore AR. Resolution of inflammation in murine autoimmune arthritis is disrupted by cyclooxygenase-2 inhibition and restored by prostaglandin E2-mediated lipoxin A4 production. J Immunol. 2010;184:6418–6426. doi: 10.4049/jimmunol.0903816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang N, Dalli J, Colas RA, Serhan CN. Identification of resolvin D2 receptor mediating resolution of infections and organ protection. J Exp Med. 2015;212(8):1203–1217. doi: 10.1084/jem.20150225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang N, de la Rosa X, Libreros S, Serhan CN. Novel Resolvin D2 Receptor Axis in Infectious Inflammation. J Immunol. 2017;198(2):842–851. doi: 10.4049/jimmunol.1601650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang N, Fredman G, Bäckhed F, Oh SF, Vickery TW, Schmidt BA, Serhan CN. Infection regulates pro-resolving mediators that lower antibiotic requirements. Nature. 2012;484:524–528. doi: 10.1038/nature11042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang N, Gronert K, Clish CB, O’Brien JA, Freeman MW, Serhan CN. Leukotriene B4 receptor transgenic mice reveal novel protective roles for lipoxins and aspirin-triggered lipoxins in reperfusion. J Clin Invest. 1999;104:309–316. doi: 10.1172/JCI7016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang N, Schwab JM, Fredman G, Kasuga K, Gelman S, Serhan CN. Anesthetics impact the resolution of inflammation. PLoS ONE. 2008;3:e1879. doi: 10.1371/journal.pone.0001879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang N, Takano T, Clish CB, Petasis NA, Tai HH, Serhan CN. Aspirin-triggered 15-epi-lipoxin A4 (ATL) generation by human leukocytes and murine peritonitis exudates: Development of a specific 15-epi-LXA4 ELISA. J Pharmacol Exp Ther. 1998;287:779–790. [PubMed] [Google Scholar]
- Clària J, Dalli J, Yacoubian S, Gao F, Serhan CN. Resolvin D1 and resolvin D2 govern local inflammatory tone in obese fat. J Immunol. 2012;189:2597–2605. doi: 10.4049/jimmunol.1201272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claria J, Nguyen BT, Madenci A, Ozaki CK, Serhan CN. Diversity of lipid mediators in human adipose tissue depots. Am J Physiol Cell Physiol. 2013;304:C1141–C1149. doi: 10.1152/ajpcell.00351.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clària J, Serhan CN. Aspirin triggers previously undescribed bioactive eicosanoids by human endothelial cell-leukocyte interactions. Proc Natl Acad Sci USA. 1995;92:9475–9479. doi: 10.1073/pnas.92.21.9475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clish CB, O’Brien JA, Gronert K, Stahl GL, Petasis NA, Serhan CN. Local and systemic delivery of a stable aspirin-triggered lipoxin prevents neutrophil recruitment in vivo. Proc Natl Acad Sci USA. 1999;96:8247–8252. doi: 10.1073/pnas.96.14.8247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colas RA, Shinohara M, Dalli J, Chiang N, Serhan CN. Identification and signature profiles for pro-resolving and inflammatory lipid mediators in human tissue. Am J Physiol Cell Physiol. 2014;307:C39–54. doi: 10.1152/ajpcell.00024.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colgan SP, Ehrentraut SF, Glover LE, Kominsky DJ, Campbell EL. Contributions of neutrophils to resolution of mucosal inflammation. Immunol Res. 2013;55:1–3. 75–82. doi: 10.1007/s12026-012-8350-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotran RS, Kumar V, Collins T. Robbins Pathologic Basis of Disease. 6th. W.B. Saunders Co.; Philadelphia: 1999a. p. 78. Chap. 3. [Google Scholar]
- Cotran RS, Kumar V, Collins T. Robbins Pathologic Basis of Disease. 6th. W.B. Saunders Co.; Philadelphia: 1999b. p. 1425. [Google Scholar]
- Dalli J, Chiang N, Serhan CN. Identification of sulfido-conjugated mediators that promote resolution of infection and organ protection. Proc Natl Acad Sci USA. 2014;111:E4753–4761. doi: 10.1073/pnas.1415006111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalli J, Chiang N, Serhan CN. Elucidation of novel 13-series resolvins that increase with atorvastatin and clear infections. Nat Med. 2015a;21:1071–1075. doi: 10.1038/nm.3911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalli J, Colas RA, Arnardottir H, Serhan CN. Vagal Regulation of Group 3 Innate Lymphoid Cells and the Immunoresolvent PCTR1 Controls Infection Resolution. Immunity. 2017;46(1):92–105. doi: 10.1016/j.immuni.2016.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalli J, Consalvo AP, Ray V, Di Filippo C, D’Amico M, Mehta N, Perretti M. Proresolving and Tissue-Protective Actions of Annexin A1-Based Cleavage-Resistant Peptides Are Mediated by Formyl Peptide Receptor 2/Lipoxin A4 Receptor. J Immunol. 2013a;190:6478–6487. doi: 10.4049/jimmunol.1203000. [DOI] [PubMed] [Google Scholar]
- Dalli J, Ramon S, Norris PC, Colas RA, Serhan CN. Novel proresolving and tissue regenerative resolvin and protectin sulfido-conjugated pathways. FASEB J. 2015b;29:2120–2136. doi: 10.1096/fj.14-268441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalli J, Serhan CN. Specific lipid mediator signatures of human phagocytes: microparticles stimulate macrophage efferocytosis and pro-resolving mediators. Blood. 2012;120:e60–72. doi: 10.1182/blood-2012-04-423525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalli J, Winkler JW, Colas RA, Arnardottir H, Cheng CYC, Chiang N, Petasis NA, Serhan CN. Resolvin D3 and aspirin-triggered resolvin D3 are potent immunoresolvents. Chem Biol. 2013b;20:188–201. doi: 10.1016/j.chembiol.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Caterina R. n-3 fatty acids in cardiovascular disease. N Engl J Med. 2011;364:2439–2450. doi: 10.1056/NEJMra1008153. [DOI] [PubMed] [Google Scholar]
- De Caterina R, Endres S, Kristensen SD, Schmidt EB. n-3 Fatty Acids and Vascular Disease. Springer-Verlag; London: 1993. [Google Scholar]
- de Paiva CS, Schwartz CE, Gjorstrup P, Pflugfelder SC. Resolvin E1 (RX-10001) Reduces Corneal Epithelial Barrier Disruption and Protects Against Goblet Cell Loss in a Murine Model of Dry Eye. Cornea. 2012;31:1299–1303. doi: 10.1097/ICO.0b013e31823f789e. [DOI] [PubMed] [Google Scholar]
- Dinarello CA. Anti-inflammatory agents: present and future. Cell. 2010;140:935–950. doi: 10.1016/j.cell.2010.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Kebir D, Gjorstrup P, Filep JG. Resolvin E1 promotes phagocytosis-induced neutrophil apoptosis and accelerates resolution of pulmonary inflammation. Proc Natl Acad Sci U S A. 2012;109(37):14983–14988. doi: 10.1073/pnas.1206641109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Kebir D, József L, Pan W, Wang L, Petasis NA, Serhan CN, Filep JG. 15-epi-lipoxin A4 inhibits myeloperoxidase signaling and enhances resolution of acute lung injury. Am J Respir Crit Care Med. 2009;180:311–319. doi: 10.1164/rccm.200810-1601OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Q, Feng F, Feng X, Li S, Wang S, Liu Z, Zhang X, Zhao Q, Wang W. Resolvin D1 reverses chronic pancreatitis-induced mechanical allodynia, phosphorylation of NMDA receptors,and cytokines expression in the thoracic spinal dorsal horn. BMC Gastroenterol. 2012;12:148. doi: 10.1186/1471-230X-12-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giera M, Ioan-Facsinay A, Toes R, Gao F, Dalli J, Deelder AM, Serhan CN, Mayboroda OA. Lipid and lipid mediator profiling of human synovial fluid in rheumatoid arthritis patients by means of LC-MS/MS. Biochim Biophys Acta. 2012;1821(11):1415–1424. doi: 10.1016/j.bbalip.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas-Stapleton EH, Lu Y, Hong S, Arita M, Favoreto S, Nigam S, Serhan CN, Agabian N. Candida albicans modulates host defense by biosynthesizing the pro-resolving mediator Resolvin E1. PLoS ONE. 2007;2:e1316. doi: 10.1371/journal.pone.0001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeggström JZ, Hamberg M. Resolving resolvins. Chem Biol. 2013;20:138–140. doi: 10.1016/j.chembiol.2013.02.003. [DOI] [PubMed] [Google Scholar]
- Hellmann J, Tang Y, Kosuri M, Bhatnagar A, Spite M. Resolvin D1 decreases adipose tissue macrophage accumulation and improves insulin sensitivity in obese-diabetic mice. FASEB J. 2011;25:2399–2407. doi: 10.1096/fj.10-178657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herschman HR. Recent progress in the cellular and molecular biology of prostaglandin synthesis. Trends Cardiovasc Med. 1998;8:145–150. doi: 10.1016/S1050-1738(98)00004-8. [DOI] [PubMed] [Google Scholar]
- Hilberath JN, Carlo T, Pfeffer MA, Croze RH, Hastrup F, Levy BD. Resolution of Toll-like receptor 4-mediated acute lung injury is linked to eicosanoids and suppressor of cytokine signaling 3. FASEB J. 2011;25(6):1827–1835. doi: 10.1096/fj.10-169896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisada T, Ishizuka T, Aoki H, Mori M. Resolvin E1 as a novel agent for the treatment of asthma. Expert Opin Ther Targets. 2009;13(5):513–522. doi: 10.1517/14728220902865622. [DOI] [PubMed] [Google Scholar]
- Hong S, Gronert K, Devchand P, Moussignac RL, Serhan CN. Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood and glial cells: autacoids in anti-inflammation. J Biol Chem. 2003;278:14677–14687. doi: 10.1074/jbc.M300218200. [DOI] [PubMed] [Google Scholar]
- Hong S, Tjonahen E, Morgan EL, Yu L, Serhan CN, Rowley AF. Rainbow trout (Oncorhynchus mykiss) brain cells biosynthesize novel docosahexaenoic acid-derived resolvins and protectins – mediator lipidomic analysis. Prostaglandins Other Lipid Mediat. 2005;78:107–116. doi: 10.1016/j.prostaglandins.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Huang L, Wang CF, Serhan CN, Strichartz G. Enduring prevention and transient reduction of post-operative pain by intrathecal resolvin D1. Pain. 2011;152:557–565. doi: 10.1016/j.pain.2010.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iigo M, Nakagawa T, Ishikawa C, Iwahori Y, Asamoto M, Yazawa K, Araki E, Tsuda H. Inhibitory effects of docosahexaenoic acid on colon carcinoma 26 metastasis to the lung. Br J Cancer. 1997;75:650–655. doi: 10.1038/bjc.1997.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida T, Yoshida M, Arita M, Nishitani Y, Nishiumi S, Masuda A, Mizuno S, Takagawa T, Morita Y, Kutsumi H, Inokuchi H, Serhan CN, Blumberg RS, Azuma T. Resolvin E1, an endogenous lipid derived from eicosapentaenoic acid, prevents dextran sulfate sodium-induced colitis. Inflamm Bowel Dis. 2010;16:87–95. doi: 10.1002/ibd.21029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isobe Y, Kato T, Arita M. Emerging roles of eosinophils and eosinophil-derived lipid mediators in the resolution of inflammation. Front Immunol. 2012;3:270. doi: 10.3389/fimmu.2012.00270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janakiram NB, Rao CV. Role of lipoxins and resolvins as anti-inflammatory and proresolving mediators in colon cancer. Curr Mol Med. 2009;9(5):565–579. doi: 10.2174/156652409788488748. [DOI] [PubMed] [Google Scholar]
- Ji RR, Xu ZZ, Strichartz G, Serhan CN. Emerging roles of resolvins in the resolution of inflammation and pain. Trends Neurosci. 2011;34(11):599–609. doi: 10.1016/j.tins.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y, Arita M, Zhang Q, Saban DR, Chauhan SK, Chiang N, Serhan CN, Dana MR. Anti-angiogenesis effect of the novel anti-inflammatory and pro-resolving lipid mediators. Invest. Ophthalmol Vis Sci. 2009;50:4743–4752. doi: 10.1167/iovs.08-2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CN, Dalli J, Dimisko L, Wong E, Serhan CN, Irimia D. Microfluidic chambers for monitoring leukocyte trafficking and humanized nano-proresolving medicines interactions. Proc Natl Acad Sci U S A. 2012;109:20560–20565. doi: 10.1073/pnas.1210269109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keelan JA, Mas E, D’Vaz N, Dunstan JA, Li S, Barden AE, Mark PJ, Waddell BJ, Prescott SL, Mori TA. Effects of maternal n-3 fatty acid supplementation on placental cytokines, pro-resolving lipid mediators and their precursors. Reproduction. 2015;149(2):171–178. doi: 10.1530/REP-14-0549. [DOI] [PubMed] [Google Scholar]
- Kenchegowda S, He J, Bazan HE. Involvement of pigment epithelium-derived factor, docosahexaenoic acid and neuroprotectin D1 in corneal inflammation and nerve integrity after refractive surgery. Prostaglandins Leukot Essent Fatty Acids. 2013;88(1):27–31. doi: 10.1016/j.plefa.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyes KT, Ye Y, Lin Y, Zhang C, Perez-Polo JR, Gjorstrup P, Birnbaum Y. Resolvin E1 protects the rat heart against reperfusion injury. Am J Physiol Heart Circ Physiol. 2010;299:H153–H164. doi: 10.1152/ajpheart.01057.2009. [DOI] [PubMed] [Google Scholar]
- Kim TH, Kim GD, Jin YH, Park YS, Park CS. Omega-3 fatty acid-derived mediator, Resolvin E1, ameliorates 2,4-dinitrofluorobenzene-induced atopic dermatitis in NC/Nga mice. Int Immunopharmacol. 2012;14(4):384–391. doi: 10.1016/j.intimp.2012.08.005. [DOI] [PubMed] [Google Scholar]
- Krishnamoorthy S, Recchiuti A, Chiang N, Fredman G, Serhan CN. Resolvin D1 receptor stereoselectivity and regulation of inflammation and pro-resolving microRNAs. Am J Pathol. 2012;180:2018–2027. doi: 10.1016/j.ajpath.2012.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamoorthy S, Recchiuti A, Chiang N, Yacoubian S, Lee CH, Yang R, Petasis NA, Serhan CN. Resolvin D1 binds human phagocytes with evidence for pro-resolving receptors. Proc Natl Acad Sci USA. 2010;107:1660–1665. doi: 10.1073/pnas.0907342107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurihara T, Jones CN, Yu YM, Fischman AJ, Watada S, Tompkins RG, Fagan SP, Irimia D. Resolvin D2 restores neutrophil directionality and improves survival after burns. FASEB J. 2013;27:2270–2281. doi: 10.1096/fj.12-219519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lands WEM. Proceedings of the AOCS Short Course on Polyunsaturated Fatty Acids and Eicosanoids. American Oil Chemists’ Society; Champaign, IL: 1987. [Google Scholar]
- Lee CH. Resolvins as new fascinating drug candidates for inflammatory diseases. Arch Pharm Res. 2012;35(1):3–7. doi: 10.1007/s12272-012-0121-z. [DOI] [PubMed] [Google Scholar]
- Lee CR, Zeldin DC. Resolvin Infectious Inflammation by Targeting the Host Response. N Engl J Med. 2015;373(22):2183–2185. doi: 10.1056/NEJMcibr1511280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie M. Inflammation’s stop signals. Science. 2015;347(6217):18–21. doi: 10.1126/science.347.6217.18. [DOI] [PubMed] [Google Scholar]
- Levy BD, Clish CB, Schmidt B, Gronert K, Serhan CN. Lipid mediator class switching during acute inflammation: signals in resolution. Nat Immunol. 2001;2:612–619. doi: 10.1038/89759. [DOI] [PubMed] [Google Scholar]
- Levy BD, Kohli P, Gotlinger K, Haworth O, Hong S, Kazani S, Israel E, Haley KJ, Serhan CN. Protectin D1 is generated in asthma and dampens airway inflammation and hyper-responsiveness. J Immunol. 2007;178:496–502. doi: 10.4049/jimmunol.178.1.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy BD, Serhan CN. Resolution of acute inflammation in the lung. Annu Rev Physiol. 2014;76:27.21–27.26. doi: 10.1146/annurev-physiol-021113-170408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy GN. Prostaglandin H synthases, nonsteroidal anti-inflammatory drugs, and colon cancer. FASEB J. 1997;11:234–247. [PubMed] [Google Scholar]
- Li D, Hodges RR, Jiao J, Carozza RB, Shatos MA, Chiang N, Serhan CN, Dartt DA. Resolvin D1 and aspirin-triggered resolvin D1 regulate histamine-stimulated conjunctival goblet cell secretion. Mucosal Immunol. 2013;6:1119–1130. doi: 10.1038/mi.2013.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, He J, Schwartz CE, Gjorstrup P, Bazan HEP. Resolvin E1 improves tear production and decreases inflammation in a dry eye mouse model. J Ocul Pharmacol Ther. 2010;26:431–439. doi: 10.1089/jop.2010.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Z, Dong J, Wu W, Yang T, Wang T, Guo L, Chen L, Xu D, Wen F. Resolvin D1 attenuates inflammation in lipopolysaccharide-induced acute lung injury through a process involving the PPARgamma/NF-kappaB pathway. Respir Res. 2012;13:110. doi: 10.1186/1465-9921-13-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima-Garcia J, Dutra R, da Silva K, Motta E, Campos M, Calixto J. The precursor of resolvin D series and aspirin-triggered resolvin D1 display anti-hyperalgesic properties in adjuvant-induced arthritis in rats. Br J Pharmacol. 2011;164:278–293. doi: 10.1111/j.1476-5381.2011.01345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Fiala M, Mizwicki MT, Sayre J, Magpantay L, Siani A, Mahanian M, Chattopadhyay M, La Cava A, Wiedau-Pazos M. Neuronal phagocytosis by inflammatory macrophages in ALS spinal cord: inhibition of inflammation by resolvin D1. Am J Neurodegener Dis. 2012;1(1):60–74. [PMC free article] [PubMed] [Google Scholar]
- Lukiw WJ, Cui JG, Marcheselli VL, Bodker M, Botkjaer A, Gotlinger K, Serhan CN, Bazan NG. A role for docosahexaenoic acid-derived neuroprotectin D1 in neural cell survival and Alzheimer disease. J Clin Invest. 2005;115:2774–2783. doi: 10.1172/JCI25420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund T, Mangsbo SM, Scholz H, Gjorstrup P, Totterman TH, Korsgren O, Foss A. Resolvin E1 reduces proinflammatory markers in human pancreatic islets in vitro. ExpClin Endocrinol Diabetes. 2010;118(4):237–244. doi: 10.1055/s-0029-1241825. [DOI] [PubMed] [Google Scholar]
- Maddox JF, Hachicha M, Takano T, Petasis NA, Fokin VV, Serhan CN. Lipoxin A4 stable analogs are potent mimetics that stimulate human monocytes and THP-1 cells via a G-protein linked lipoxin A4 receptor. J Biol Chem. 1997;272:6972–6978. doi: 10.1074/jbc.272.11.6972. [DOI] [PubMed] [Google Scholar]
- Maddox JF, Serhan CN. Lipoxin A4 and B4 are potent stimuli for human monocyte migration and adhesion: selective inactivation by dehydrogenation and reduction. J Exp Med. 1996;183:137–146. doi: 10.1084/jem.183.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus AJ. Platelets: their role in hemostasis, thrombosis, and inflammation. In: Gallin JI, Snyderman R, editors. Inflammation: Basic Principles and Clinical Correlates. Lippincott Williams & Wilkins; Philadelphia: 1999. pp. 77–95. [Google Scholar]
- Mas E, Croft KD, Zahra P, Barden A, Mori TA. Resolvins D1, D2, and other mediators of self-limited resolution of inflammation in human blood following n-3 fatty acid supplementation. Clin Chem. 2012;58:1476–1484. doi: 10.1373/clinchem.2012.190199. [DOI] [PubMed] [Google Scholar]
- Miki Y, Yamamoto K, Taketomi Y, Sato H, Shimo K, Kobayashi T, Ishikawa Y, Ishii T, Nakanishi H, Ikeda K, Taguchi R, Kabashima K, Arita M, Arai H, Lambeau G, Bollinger JM, Hara S, Gelb MH, Murakami M. Lymphoid tissue phospholipase A2 group IID resolves contact hypersensitivity by driving antiinflammatory lipid mediators. J Exp Med. 2013;210(6):1217–1234. doi: 10.1084/jem.20121887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirakaj V, Dalli J, Granja T, Rosenberger P, Serhan CN. Vagus nerve controls resolution and pro-resolving mediators of inflammation. J Exp Med. 2014;211(6):1037–1048. doi: 10.1084/jem.20132103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyahara T, Runge S, Chatterjee A, Chen M, Mottola G, Fitzgerald JM, Serhan CN, Conte MS. D-series resolvins attenuate vascular smooth muscle cell activation and neointimal hyperplasia following vascular injury. FASEB J. 2013;27:2220–2232. doi: 10.1096/fj.12-225615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita M, Kuba K, Ichikawa A, Nakayama M, Katahira J, Iwamoto R, Watanebe T, Sakabe S, Daidoji T, Nakamura S, Kadowaki A, Ohto T, Nakanishi H, Taguchi R, Nakaya T, Murakami M, Yoneda Y, Arai H, Kawaoka Y, Penninger JM, Arita M, Imai Y. The lipid mediator protectin D1 inhibits influenza virus replication and improves severe influenza. Cell. 2013;153:112–125. doi: 10.1016/j.cell.2013.02.027. [DOI] [PubMed] [Google Scholar]
- Morris T, Stables M, Colville-Nash P, Newson J, Bellingan G, de Souza PM, Gilroy DW. Dichotomy in duration and severity of acute inflammatory responses in humans arising from differentially expressed proresolution pathways. Proc Natl Acad Sci USA. 2010;107:8842–8847. doi: 10.1073/pnas.1000373107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C, Ding A. Nonresolving inflammation. Cell. 2010;140:871–882. doi: 10.1016/j.cell.2010.02.029. [DOI] [PubMed] [Google Scholar]
- Navarro-Xavier RA, Newson J, Silveira VLF, Farrow SN, Gilroy DW, Bystrom J. A new strategy for the identification of novel molecules with targeted proresolution of inflammation properties. J Immunol. 2010;184:1516–1525. doi: 10.4049/jimmunol.0902866. [DOI] [PubMed] [Google Scholar]
- Norling LV, Headland SE, Dalli J, Arnardottir HH, Haworth O, Jones HR, Irimia D, Serhan CN, Perretti M. Proresolving and cartilage-protective actions of resolvin D1 in inflammatory arthritis. JCI Insight. 2016;1(5):e85922. doi: 10.1172/jci.insight.85922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa N, Kobayashi Y. Total synthesis of resolvin E1. Tetrahedron Lett. 2009;50:6079–6082. [Google Scholar]
- Ogawa N, Kobayashi Y. Total synthesis of the antiinflammatory and proresolving protectin D1. Tetrahedron Lett. 2011;52:3001–3004. [Google Scholar]
- Oh SF, Pillai PS, Recchiuti A, Yang R, Serhan CN. Pro-resolving actions and stereoselective biosynthesis of 18S E-series resolvins in human leukocytes and murine inflammation. J Clin Invest. 2011;121:569–581. doi: 10.1172/JCI42545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohira T, Arita M, Omori K, Recchiuti A, Van Dyke TE, Serhan CN. Resolvin E1 receptor activation signals phosphorylation and phagocytosis. J Biol Chem. 2010;285:3451–3461. doi: 10.1074/jbc.M109.044131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer CD, Mancuso CJ, Weiss JP, Serhan CN, Guinan EC, Levy O. 17(R)-Resolvin D1 differentially regulates TLR4-mediated responses of primary human macrophages to purified LPS and live E. coli. J Leukoc Biol. 2011;90:459–470. doi: 10.1189/jlb.0311145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CK, Lü N, Xu ZZ, Liu T, Serhan CN, Ji RR. Resolving TRPV1 and TNF-α-mediated spinal cord synaptic plasticity and inflammatory pain with neuroprotectin D1. J Neurosci. 2011;31:15072–15085. doi: 10.1523/JNEUROSCI.2443-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perretti M, Leroy X, Bland EJ, Montero-Melendez T. Resolution Pharmacology: Opportunities for Therapeutic Innovation in Inflammation. Trends Pharmacol Sci. 2015;36(11):737–755. doi: 10.1016/j.tips.2015.07.007. [DOI] [PubMed] [Google Scholar]
- Pomponi MF, Gambassi G, Pomponi M, Masullo C. Alzheimer’s disease: Fatty acids we eat may be linked to a specific protection via low-dose aspirin. Aging Dis. 2010;1(1):37–59. [PMC free article] [PubMed] [Google Scholar]
- Pope NH, Salmon M, Davis JP, Chatterjee A, Su G, Conte MS, Ailawadi G, Upchurch GR., Jr D-series resolvins inhibit murine abdominal aortic aneurysm formation and increase M2 macrophage polarization. FASEB J. 2016;30(12):4192–4201. doi: 10.1096/fj.201600144RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruss H, Kopp MA, Brommer B, Gatzemeier N, Laginha I, Dirnagl U, Schwab JM. Non-resolving aspects of acute inflammation after spinal cord injury (SCI): indices and resolution plateau. Brain Pathol. 2011;21(6):652–660. doi: 10.1111/j.1750-3639.2011.00488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruss H, Rosche B, Sullivan AB, Brommer B, Wengert O, Gronert K, Schwab JM. Proresolution lipid mediators in multiple sclerosis - differential, disease severity-dependent synthesis - a clinical pilot trial. PLoS One. 2013;8(2):e55859. doi: 10.1371/journal.pone.0055859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psychogios N, Hau DD, Peng J, Guo AC, Mandal R, Bouatra S, Sinelnikov I, Krishnamurthy R, Eisner R, Gautam B, Young N, Xia J, Knox C, Dong E, Huang P, Hollander Z, Pedersen TL, Smith SR, Bamforth F, Greiner R, McManus B, Newman JW, Goodfriend T, Wishart DS. The human serum metabolome. PLoS One. 2011;6(2):e16957. doi: 10.1371/journal.pone.0016957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu X, Zhang X, Yao J, Song J, Nikolic-Paterson DJ, Li J. Resolvins E1 and D1 inhibit interstitial fibrosis in the obstructed kidney via inhibition of local fibroblast proliferation. J Pathol. 2012;228:506–519. doi: 10.1002/path.4050. [DOI] [PubMed] [Google Scholar]
- Raatz SK, Golovko MY, Brose SA, Rosenberger TA, Burr GS, Wolters WR, Picklo MJ., Sr Baking reduces prostaglandin, resolvin, and hydroxy-fatty acid content of farm-raised Atlantic salmon (Salmo salar) J Agric Food Chem. 2011;59:11278–11286. doi: 10.1021/jf202576k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajasagi NK, Reddy PBJ, Suryawanshi A, Mulik S, Gjorstrup P, Rouse BT. Controlling herpes simplex virus-induced ocular inflammatory lesions with the lipid-derived mediator resolvin E1. J Immunol. 2011;186:1735–1746. doi: 10.4049/jimmunol.1003456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaswami R, Serhan CN, Levy BD, Makrides M. Fish Oil Supplementation in Pregnancy. N Engl J Med. 2016;375(26):2599–2601. doi: 10.1056/NEJMclde1614333. [DOI] [PubMed] [Google Scholar]
- Ramsden CE. Breathing Easier with Fish Oil - A New Approach to Preventing Asthma? N Engl J Med. 2016;375(26):2596–2598. doi: 10.1056/NEJMe1611723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recchiuti A, Krishnamoorthy S, Fredman G, Chiang N, Serhan CN. MicroRNAs in resolution of acute inflammation: identification of novel resolvin D1-miRNA circuits. FASEB J. 2011;25:544–560. doi: 10.1096/fj.10-169599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez AR, Spur BW. First total synthesis of 7(S),16(R),17(S)-Resolvin D2, a potent anti-inflammatory lipid mediator. Tetrahedron Lett. 2004;45:8717–8720. [Google Scholar]
- Rodriguez AR, Spur BW. First total synthesis of the anti-inflammatory lipid mediator Resolvin D6. Tetrahedron Lett. 2012;53:86–89. doi: 10.1016/j.tetlet.2020.151857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogerio AP, Haworth O, Croze R, Oh SF, Uddin M, Carlo T, Pfeffer MA, Priluck R, Serhan CN, Levy BD. Resolvin D1 and aspirin-triggered resolvin D1 promote resolution of allergic airways responses. J Immunol. 2012;189(4):1983–1991. doi: 10.4049/jimmunol.1101665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roncaglioni MC, Tombesi M, Avanzini F, Barlera S, Caimi V, Longoni P, Marzona I, Milani V, Silletta MG, Tognoni G, Marchioli R. n-3 fatty acids in patients with multiple cardiovascular risk factors. N Engl J Med. 2013;368(19):1800–1808. doi: 10.1056/NEJMoa1205409. [DOI] [PubMed] [Google Scholar]
- Rossi AG, Sawatzky DA. The Resolution of Inflammation. In: Parnham MJ, editor. Progress in Inflammation Research. Birkhäuser Verlag AG; Basel: 2008. p. 238. [Google Scholar]
- Samuelsson B. Leukotrienes: mediators of immediate hypersensitivity reactions and inflammation. Science. 1983;220:568–575. doi: 10.1126/science.6301011. [DOI] [PubMed] [Google Scholar]
- Samuelsson B. Role of basic science in the development of new medicines: examples from the eicosanoid field. J Biol Chem. 2012;287:10070–10080. doi: 10.1074/jbc.X112.351437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki A, Fukuda H, Shiida N, Tanaka N, Furugen A, Ogura J, Shuto S, Mano N, Yamaguchi H. Determination of omega-6 and omega-3 PUFA metabolites in human urine samples using UPLC/MS/MS. Anal Bioanal Chem. 2015;407:1625–1639. doi: 10.1007/s00216-014-8412-5. [DOI] [PubMed] [Google Scholar]
- Schwab JM, Chiang N, Arita M, Serhan CN. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature. 2007;447:869–874. doi: 10.1038/nature05877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki H, Fukunaga K, Arita M, Arai H, Nakanishi H, Taguchi R, Miyasho T, Takamiya R, Asano K, Ishizaka A, Takeda J, Levy BD. The anti-inflammatory and proresolving mediator resolvin E1 protects mice from bacterial pneumonia and acute lung injury. J Immunol. 2010;184:836–843. doi: 10.4049/jimmunol.0901809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN. Lipoxins and aspirin-triggered 15-epi-lipoxins. In: Gallin JI, Snyderman R, editors. Inflammation: Basic Principles and Clinical Correlates. Lippincott Williams & Wilkins; Philadelphia: 1999. pp. 373–385. [Google Scholar]
- Serhan CN. A search for endogenous mechanisms of anti-inflammation uncovers novel chemical mediators: missing links to resolution. Histochem. Cell Biol. 2004;122(4):305–321. doi: 10.1007/s00418-004-0695-8. [DOI] [PubMed] [Google Scholar]
- Serhan CN. Resolution phases of inflammation: novel endogenous anti-inflammatory and pro-resolving lipid mediators and pathways. Annu Rev Immunol. 2007;25:101–137. doi: 10.1146/annurev.immunol.25.022106.141647. [DOI] [PubMed] [Google Scholar]
- Serhan CN. Systems approach to inflammation-resolution: identification of novel anti-inflammatory and pro-resolving mediators. J Thromb Haemost. 2009;7(Suppl 1):44–48. doi: 10.1111/j.1538-7836.2009.03396.x. [DOI] [PubMed] [Google Scholar]
- Serhan CN. Novel resolution mechanisms in acute inflammation: to resolve or not? Am J Pathol. 2010;177:1576–1591. doi: 10.2353/ajpath.2010.100322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN. The resolution of inflammation: the devil in the flask and in the details. FASEB J. 2011;25:1441–1448. doi: 10.1096/fj.11-0502ufm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510(7503):92–101. doi: 10.1038/nature13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN. Treating inflammation and infection in the 21st century: new hints from decoding resolution mediators and mechanisms. FASEB J. 2017 doi: 10.1096/fj.201601222R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Brain SD, Buckley CD, Gilroy DW, Haslett C, O’Neill LAJ, Perretti M, Rossi AG, Wallace JL. Resolution of inflammation: state of the art, definitions and terms. FASEB J. 2007;21:325–332. doi: 10.1096/fj.06-7227rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Chiang N. Resolution phase lipid mediators of inflammation: agonists of resolution. Curr Opin Pharmacol. 2013 doi: 10.1016/j.coph.2013.1005.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8:349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Clish CB, Brannon J, Colgan SP, Chiang N, Gronert K. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J Exp Med. 2000a;192:1197–1204. doi: 10.1084/jem.192.8.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Dalli J, Colas RA, Winkler JW, Chiang N. Protectins and maresins: New pro-resolving families of mediators in acute inflammation and resolution bioactive metabolome. Biochim Biophys Acta. 2015;1851:397–413. doi: 10.1016/j.bbalip.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Dalli J, Karamnov S, Choi A, Park CK, Xu ZZ, Ji RR, Zhu M, Petasis NA. Macrophage pro-resolving mediator maresin 1 stimulates tissue regeneration and controls pain. FASEB J. 2012;26:1755–1765. doi: 10.1096/fj.11-201442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Gotlinger K, Hong S, Lu Y, Siegelman J, Baer T, Yang R, Colgan SP, Petasis NA. Anti-inflammatory actions of neuroprotectin D1/protectin D1 and its natural stereoisomers: assignments of dihydroxy-containing docosatrienes. J Immunol. 2006;176:1848–1859. doi: 10.4049/jimmunol.176.3.1848. [DOI] [PubMed] [Google Scholar]
- Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, Moussignac RL. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter pro-inflammation signals. J Exp Med. 2002;196:1025–1037. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Jain A, Marleau S, Clish C, Kantarci A, Behbehani B, Colgan SP, Stahl GL, Merched A, Petasis NA, Chan L, Van Dyke TE. Reduced inflammation and tissue damage in transgenic rabbits overexpressing 15-lipoxygenase and endogenous anti-inflammatory lipid mediators. J Immunol. 2003;171:6856–6865. doi: 10.4049/jimmunol.171.12.6856. [DOI] [PubMed] [Google Scholar]
- Serhan CN, Krishnamoorthy S, Recchiuti A, Chiang N. Novel anti-inflammatory -pro-resolving mediators and their receptors. Curr Top Med Chem. 2011;11:629–647. doi: 10.2174/1568026611109060629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Maddox JF, Petasis NA, Akritopoulou-Zanze I, Papayianni A, Brady HR, Colgan SP, Madara JL. Design of lipoxin A4 stable analogs that block transmigration and adhesion of human neutrophils. Biochemistry. 1995;34:14609–14615. doi: 10.1021/bi00044a041. [DOI] [PubMed] [Google Scholar]
- Serhan CN, Petasis NA. Resolvins and protectins in inflammation-resolution. Chem Rev. 2011;111:5922–5943. doi: 10.1021/cr100396c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol. 2005;6:1191–1197. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- Serhan CN, Takano T, Chiang N, Gronert K, Clish CB. Formation of endogenous “antiinflammatory” lipid mediators by transcellular biosynthesis: Lipoxins and aspirin-triggered lipoxins inhibit neutrophil recruitment and vascular permeability. Am J Respir Crit Care Med. 2000b;161:S95–S101. doi: 10.1164/ajrccm.161.supplement_1.ltta-19. [DOI] [PubMed] [Google Scholar]
- Serhan CN, Yang R, Martinod K, Kasuga K, Pillai PS, Porter TF, Oh SF, Spite M. Maresins: novel macrophage mediators with potent anti-inflammatory and pro-resolving actions. J Exp Med. 2009;206:15–23. doi: 10.1084/jem.20081880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Settimio R, Clara DF, Franca F, Francesca S, Michele D. Resolvin D1 reduces the immunoinflammatory response of the rat eye following uveitis. Mediators Inflamm. 2012;2012:318621. doi: 10.1155/2012/318621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheets KG, Zhou Y, Ertel MK, Knott EJ, Regan CE, Jr, Elison JR, Gordon WC, Gjorstrup P, Bazan NG. Neuroprotectin D1 attenuates laser-induced choroidal neovascularization in mouse. Mol Vis. 2010;16:320–329. [PMC free article] [PubMed] [Google Scholar]
- Simopoulos AP, Leaf A, Salem N., Jr Workshop on the essentiality of and recommended dietary intakes for omega-6 and omega-3 fatty acids. J Am Coll Nutr. 1999;18:487–489. doi: 10.1080/07315724.1999.10718888. [DOI] [PubMed] [Google Scholar]
- Spite M, Norling LV, Summers L, Yang R, Cooper D, Petasis NA, Flower RJ, Perretti M, Serhan CN. Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature. 2009;461:1287–1291. doi: 10.1038/nature08541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spite M, Serhan CN. Novel lipid mediators promote resolution of acute inflammation: impact of aspirin and statins. Circulation Res. 2010;107:1170–1184. doi: 10.1161/CIRCRESAHA.110.223883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stables MJ, Shah S, Camon EB, Lovering RC, Newson J, Bystrom J, Farrow S, Gilroy DW. Transcriptomic analyses of murine resolution-phase macrophages. Blood. 2011;118:e192–e208. doi: 10.1182/blood-2011-04-345330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabas I, Glass CK. Anti-inflammatory therapy in chronic disease: challenges and opportunities. Science. 2013;339(6116):166–172. doi: 10.1126/science.1230720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Zhang MJ, Hellmann J, Kosuri M, Bhatnagar A, Spite M. Proresolution Therapy for the Treatment of Delayed Healing of Diabetic Wounds. Diabetes. 2013;62:618–627. doi: 10.2337/db12-0684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrando N, Gomez-Galan M, Yang T, Carlstrom M, Gustavsson D, Harding RE, Lindskog M, Eriksson LI. Aspirin-triggered resolvin D1 prevents surgery-induced cognitive decline. FASEB J. 2013;27:3564–3571. doi: 10.1096/fj.13-230276. [DOI] [PubMed] [Google Scholar]
- Tracey KJ. The inflammatory reflex. Nature. 2002;420(6917):853–859. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- Vane JR. Adventures and excursions in bioassay: the stepping stones to prostacyclin, Les Prix Nobel: Nobel Prizes, Presentations, Biographies and Lectures. Almqvist & Wiksell, Stockholm. 1982:181–206. [Google Scholar]
- Vassiliou EK, Kesler OM, Tadros JH, Ganea D. Bone marrow-derived dendritic cells generated in the presence of resolvin E1 induce apoptosis of activated CD4+ T cells. J Immunol. 2008;181:4534–4544. doi: 10.4049/jimmunol.181.7.4534. [DOI] [PubMed] [Google Scholar]
- Walker J, Dichter E, Lacorte G, Kerner D, Spur B, Rodriguez A, Yin K. Lipoxin A4 increases survival by decreasing systemic inflammation and bacterial load in sepsis. Shock. 2011;36(4):410–416. doi: 10.1097/SHK.0b013e31822798c1. [DOI] [PubMed] [Google Scholar]
- Wan M, Godson C, Guiry PJ, Agerberth B, Haeggström JZ. Leukotriene B4/antimicrobial peptide LL-37 proinflammatory circuits are mediated by BLT1 and FPR2/ALX and are counter-regulated by lipoxin A4 and resolvin E1. FASEB J. 2011;25:1697–1705. doi: 10.1096/fj.10-175687. [DOI] [PubMed] [Google Scholar]
- Wang B, Gong X, Wan JY, Zhang L, Zhang Z, Li HZ, Min S. Resolvin D1 protects mice from LPS-induced acute lung injury. Pulm Pharmacol Ther. 2011;24(4):434–441. doi: 10.1016/j.pupt.2011.04.001. [DOI] [PubMed] [Google Scholar]
- Wang X, Zhu M, Hjorth E, Cortés-Toro V, Eyjolfsdottir H, Graff C, Nennesmo I, Palmblad J, Eriksdotter M, Sambamurti K, Fitzgerald JM, Serhan CN, Granholm AC, Schultzberg M. Resolution of inflammation is altered in Alzheimer’s disease. Alzheimers Dement. 2015;11:40–50.e42. doi: 10.1016/j.jalz.2013.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss GA, Troxler H, Klinke G, Rogler D, Braegger C, Hersberger M. High levels of anti-inflammatory and pro-resolving lipid mediators lipoxins and resolvins and declining docosahexaenoic acid levels in human milk during the first month of lactation. Lipids Health Dis. 2013;12:89. doi: 10.1186/1476-511X-12-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissmann G. Current concepts: Prostaglandins in acute inflammation. Upjohn. 1980:1–32. [Google Scholar]
- Weissmann G. Aspirin Sci Am. 1991;264:84–90. doi: 10.1038/scientificamerican0191-84. [DOI] [PubMed] [Google Scholar]
- White PJ, St-Pierre P, Charbonneau A, Mitchell PL, St-Amand E, Marcotte B, Marette A. Protectin DX alleviates insulin resistance by activating a myokine-liver glucoregulatory axis. Nat Med. 2014;20(6):664–669. doi: 10.1038/nm.3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler JW, Orr SK, Dalli J, Cheng CY, Sanger JM, Chiang N, Petasis NA, Serhan CN. Resolvin D4 stereoassignment and its novel actions in host protection and bacterial clearance. Sci Rep. 2016;6:18972. doi: 10.1038/srep18972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SH, Liao PY, Yin PL, Zhang YM, Dong L. Elevated expressions of 15-lipoxygenase and lipoxin A4 in children with acute poststreptococcal glomerulonephritis. Am J Pathol. 2009;174:115–122. doi: 10.2353/ajpath.2009.080671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao G, Tsai AL, Palmer G, Boyar WC, Marshall PJ, Kulmacz RJ. Analysis of hydroperoxide-induced tyrosyl radicals and lipoxygenase activity in aspirin-treated human prostaglandin H synthase-2. Biochemistry. 1997;36:1836–1845. doi: 10.1021/bi962476u. [DOI] [PubMed] [Google Scholar]
- Xu ZZ, Zhang L, Liu T, Park JY, Berta T, Yang R, Serhan CN, Ji RR. Resolvins RvE1 and RvD1 attenuate inflammatory pain via central and peripheral actions. Nat Med. 2010;16:592–597. doi: 10.1038/nm.2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu ZZ, Berta T, Ji RR. Resolvin E1 Inhibits Neuropathic Pain and Spinal Cord Microglial Activation Following Peripheral Nerve Injury. J Neuroimmune Pharmacol. 2013;8:37–41. doi: 10.1007/s11481-012-9394-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu ZZ, Ji RR. Resolvins are potent analgesics for arthritic pain. Br J Pharmacol. 2011;164:274–277. doi: 10.1111/j.1476-5381.2011.01348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Eiserich JP, Cross CE, Morrissey BM, Hammock BD. Metabolomic profiling of regulatory lipid mediators in sputum from adult cystic fibrosis patients. Free Radic Biol Med. 2012;53(1):160–171. doi: 10.1016/j.freeradbiomed.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang MJ, Spite M. Resolvins: anti-inflammatory and proresolving mediators derived from omega-3 polyunsaturated fatty acids. Annu Rev Nutr. 2012;32:203–227. doi: 10.1146/annurev-nutr-071811-150726. [DOI] [PubMed] [Google Scholar]
- Zhu M, Wang X, Schultzberg M, Hjorth E. Differential regulation of resolution in inflammation induced by amyloid-beta42 and lipopolysaccharides in human microglia. J Alzheimers Dis. 2015;43(4):1237–1250. doi: 10.3233/JAD-141233. [DOI] [PubMed] [Google Scholar]


