Abstract
Background
Prevalence of Opisthorchis viverrini, Schistosoma mekongi and soil-transmitted helminths (STH) remains high in Lao People’s Democratic Republic (Lao PDR), despite control efforts including mass-drug administration, education and communication campaigns. New approaches are required to advance helminth control.
Methods
An ecohealth study was conducted on two Mekong islands in Southern Laos. Demographic and behavioural data were collected by questionnaire. Human and animal reservoir stools were examined. Bithynia spp. and Neotricula aperta snails were examined using shedding. Fresh water fish were examined using digestion technique. Multivariate random-effects analysis was used to find risk factors associated with helminth infections.
Results
Human infection rates with O. viverrini, hookworm, S. mekongi, Trichuris trichiura, Ascaris lumbricoides and Taenia spp. were 60.7%, 44.1%, 22.2%, 4.1%, 0.6% and 0.1%, respectively. Heavy intensity infections were 4.2%, 3.6% and 1.8% for O. viverrini, S. mekongi and hookworm, respectively. O. viverrini and S. mekongi infection rates among dogs and cats were 25.0% and 14.7%, respectively. Of the cats tested, 53.1% were infected with O. viverrini. Prevalence of O. viverrini and S. mekongi in snails was 0.3% and 0.01%, respectively. Overall prevalence of O. viverrini infection in fresh water fish was 26.9%, with the highest infection rates occurring in Hampala dispa (87.1%), Cyclocheilichthys apogon (85.7%) and Puntius brevis (40.0%). Illiteracy and lower socioeconomic status increased the risk of O. viverrini infection, while those aged 10–16 years and possessing latrines at home were less likely to be infected. Household dogs and cats that consumed raw fish were significantly and positively associated with O. viverrini infection of the household members. For S. mekongi, children under 9 years old were exposed significantly to this infection, compared to older age groups.
Conclusions
There is a pressing need to design and implement an integrated helminth control intervention on the Mekong Islands in southern Lao PDR. Given the highly dynamic transmission of O. viverrini, S. mekongi, STH and extended multiparasitism, annual mass-drug administration is warranted along with environmental modifications, health education and improved access to clean water and adequate sanitation to consolidate morbidity control and move towards elimination.
Trail registration number
Our findings presented here are from a cross-sectional study, therefore, it has not been registered.
Electronic supplementary material
The online version of this article (doi:10.1186/s40249-017-0343-x) contains supplementary material, which is available to authorized users.
Keywords: Opisthorchis viverrini, Schistosoma mekongi, Animal hosts, Bithynia species., Neotricula aperta, Cyprinidae fish, Southern Lao People's Democratic Republic, Laos
Multilingual abstract
Please see Additional file 1 for translations of the abstract into the five official working languages of the United Nations.
Background
Helminthiases are neglected tropical diseases (NTDs) of major public health concern in many low- and middle-income countries (LMIC) in the tropics and sub-tropics, including in Lao People’s Democratic Republic (Lao PDR) [1–4]. Liver flukes (Opisthorchis viverrini), blood flukes (Schistosoma mekongi) and soil-transmitted helminths (STH) such as round worm (Ascaris lumbricoides), whipworm (Trichuris trichiura) and two-hookworm species (Ancylostoma duodenale, Necator americanus) are among the most prevalent infections in Lao PDR. O. viverrini is endemic nationwide but is most prevalent in the central and southern parts of the country. It occurs in the lowlands, along the Mekong River, where fish are abundant and local inhabitants prefer to consume traditional dishes prepared with raw fish [1, 4–6]. S. mekongi is only endemic in two districts of the most southern province, Champasack, bordering Cambodia [7–10]. STH are highly prevalent in the northern part of the country and in the mountainous areas along Lao-Vietnamese border [4, 11].
Infections with these helminths negatively affect human health and wellbeing. For example, untreated or chronic infection with O. viverrini may lead to severe hepatobiliary morbidity including cholangiocarcinoma (CCA), a fatal bile duct cancer [12, 13]. Chronic infection with S. mekongi may result in portal hypertension and is associated with peri-portal liver fibrosis [14–17]. In Champasack Province, O. viverrini and S. mekongi are co-endemic [5, 7, 18], further increasing the risk of hepatobiliary morbidity. Finally, anaemia and undernourishment are associated with long-lasting STH infections [19, 20].
Helminths have complex life cycles; O. viverrini, for example, involves two aquatic intermediate hosts, namely freshwater snails (of the genus Bithynia) and freshwater fish (of the Cyprinidae family). Humans and other mammals are infected by eating raw or undercooked fish [21]. The life cycle of S. mekongi involves humans and other mammals (such as dogs, pigs and possibly rats) [22, 23]. The Neotricula aperta snail, which lives in the crevices of submerged rocks in the Mekong River, serves as intermediate host. The cercariae emerge from the infected snails during the daytime and lie under the water surface [9, 24]. Humans and animals are infected with this parasite via skin penetration when they come into contact with infested waters [8]. Lao PDR adheres to the preventive chemotherapy control strategy promoted by WHO [3, 25, 26]. Over the last decade, considerable efforts were employed to implement this strategy through deworming programmes targeting school-children [27] and through mass-drug administration (MDA) alongside information, education and communication (IEC) campaigns in high risk provinces of the country [28]. Despite these efforts, the prevalence of helminth infections, including multiple infections, remains high in many places [4, 18, 26, 29–31]. Given the complexity of the transmission cycle of helminth infections and the risky behaviour of humans in endemic communities, it may be necessary to adapt the control strategy to improve the effectiveness of interventions.
Ecohealth research is an emerging field of research studying human health in close connectivity with the ecosystem [32]. It is increasingly conducted to strengthen the sustainability of infectious disease control programmes [33–35] and was widely introduced in Southeast Asia (SEA) by the Canadian International Development Research Centre (IDRC) in the late 2000s [36, 37]. Ecohealth has been defined as follows: i) “EcoHealth involves research and practice to promote sustainability of individuals, animals and biodiversity by linking complex interaction of ecosystem, socio-cultural and economic factors” and ii) “Ecohealth is a comprehensive approach to understanding health at its human, animal and environmental interface in a socio-ecological systems context”. Here, we employ an ecohealth approach to determine the prevalence and risk factors of O. viverrini, S. mekongi and STH infections in humans in the ecological environment of Khong district, where potential animal reservoir and intermediate hosts, like molluscs and fish, live in close connectivity.
Methods
Study area
Khong district is an island district located at the Southern border of Champasack Province, Lao PDR (Fig. 1a). It has an estimated population of 100,000 people and comprises a few dozen islands in the Mekong River (geographical coordinates: 13.57°-14.14°N latitude and 105.44°-106.08°E longitude). The district is a known endemic area for O. viverrini, S. mekongi and STH. Done Khon and Done Som are among the biggest islands and are popular tourist destinations. Done Khon has about 260 households with a total population of 1560 people, while Done Som has some 378 households with a total population of 2344 people.
Fig. 1.
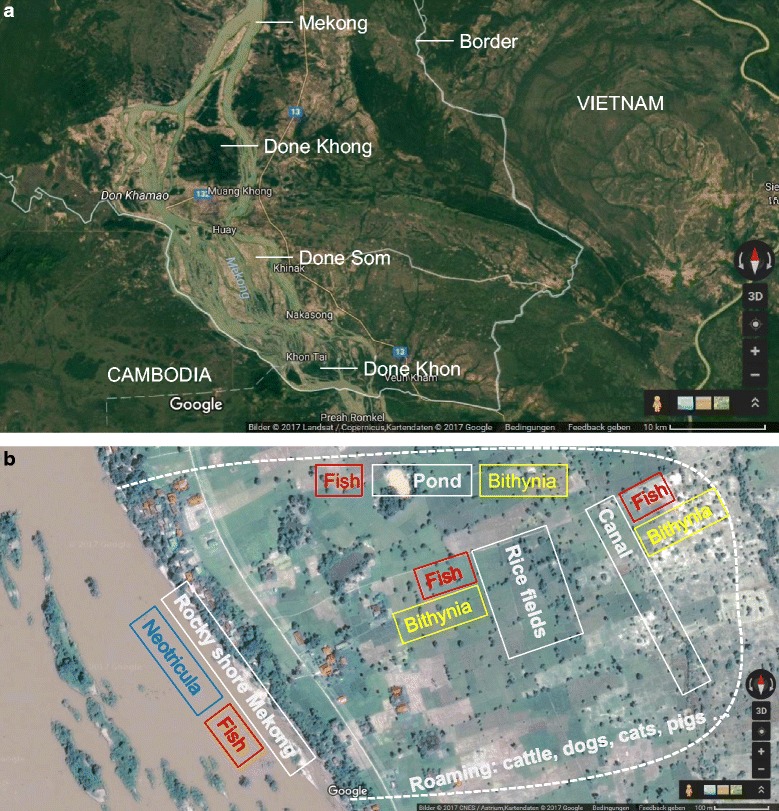
Study map: a Khong District with main Mekong islands; b Selected western shore of Done Som with human settlements and ecological features. (Source: Google Map)
Study design and population surveyed
Our cross-sectional study was carried out between October 2011 and August 2012 on Done Khon and Done Som islands. These study sites were selected based on a three-stage random sampling. First, we randomly selected two islands out of 10 known endemic islands for the targeted diseases. For each island, 323 study participants were required based on our sample calculation using a formula of simple random sampling, e.g., Z1- α/2 2 × p(1-p)/d2 with a 30% proportion and 5% precision. Based on previous experiences, about 40% of all study participants (129 persons) failed to submit complete stool samples when they were asked to submit multiple stool samples (i.e., at least two). With this in mind, at least 904 study participants from both islands were required for this study. Second, two villages were selected on each island. Finally, about 30 households in each village were randomly selected to meet the required sample size. All members of the selected households, aged 2 years and older and available on the survey day, were invited to participate in the study.
Potential animal reservoir hosts, i.e., dogs, cats, pigs and buffaloes, from selected households were also enrolled and examined for helminth infections. Due to the small numbers of these animals in the study villages (0.4 animals per household; from village record), we examined all of those present during the survey. Village health volunteers helped to identify the domestic animals and conduct follow-up examinations.
We collected intermediate hosts for O. viverrini (Bithynia spp. snails and Cyprinoid fish) and for S. mekongi (Neotricula aperta snails) from selected sites in the study villages and examined them for infection (Fig. 1b).
Snails of the genus Bithynia spp. were collected with a scoop [38] from water bodies near the study villages (e.g., ponds, canals, and rice fields). From each water body, 5–10 sites with an area of 1 × 1 m were identified as collecting points. All Bithynia snails collected from each site were counted, recorded and examined separately. Cyprinoid freshwater fish were captured from the same selected water bodies as well as from the Mekong using a fishing net. Each captured fish was measured for length and weight and were examined at the field station for the presence of O. viverrini metacercariae.
N. aperta snails [39] live in the rocky area of the Mekong River. We identified 10 sites along the Mekong River, where water was frequently used by study villagers for their daily needs. Submerged stones were dredged and snails were hand-picked from them [38]. At each site, N. aperta snails were collected for 20 min by five malacologists. All collected snails were counted, placed in a plastic bag and carried to the field station for examination.
Field procedures and laboratory examinations
In each village, a house, school or temple was identified as a field study station. Two questionnaires were administered to all participating households. A household questionnaire was administered to the heads of households for collecting data on household characteristics (e.g., building type, toilette and water supply), asset ownership (e.g., farm engine, boat, car, motorbike, electricity, television, bicycle, telephone and agriculture land) and animal ownership (e.g., buffalo, cow, goat and pig). An individual questionnaire was used to interview all household members to collect demographic data (e.g., age, sex, educational attainment and professional activities and behavioural risks (e.g., food consumption habits, water contact, animal raising and personal hygiene). Parents or legal guardians answered for children under 10 years of age.
Eligible study participants were invited to submit two stool samples over consecutive days for parasitological analysis. The first stool container (pre-labelled with participant’s name, unique identity number, age and date of collection) was handed to the study participants on the registration day, along with a detailed explanation of stool collection. The second empty container was handed out after study participants returned the first filled container.
Two Kato-Katz (KK) thick smears [40] were prepared from each stool sample (i.e. four smears per person) and examined under light microscopes by an experienced technician within 1 h of sample preparation. Eggs were counted and recorded for each helminth species separately.
We collected faecal samples from potential domestic reservoir animals owed by study households, namely cats, dogs, pigs and water buffaloes. To collect fresh faecal samples [41] from small animals (cats, dogs and pigs), rectal enemas were performed using Sodium Chloride (NaCl) solution and petroleum jelly lubricant. Faecal samples from water buffaloes were collected by rectal swab. All faecal samples were immediately preserved in a 10% formalin solution and transported to the National Institute of Public Health (NIOPH), Vientiane, for processing using the formalin ether concentration technique (FECT) [42].
Bithynia spp. and N. aperta snails were examined for the presence of cercariae infection using the shedding test, previously described by Sri-Aroon and colleagues [43]. In summary, the fresh water snails were put into a transparent plastic container filled with Mekong water and exposed to artificial light. After 2 h, the container was examined under a stereoscope for the presence of cercariae. The infected snails were identified, counted and recorded separately.
The species identification of captured Cyprinoid fish was performed based on guidelines available at FishBase website [44, 45]. Fish digestion was performed using the pepsin enzyme digestion technique [25]. The residue was examined for the presence of O. viverrini metacercariae. The metacercariae were counted and recorded for each infected fish.
Data management and analysis
Information from questionnaires and data forms were double entered into EpiData, version 3.1 (EpiData Association; Odense, Denmark) and validated for their correctness and completeness. Statistical analyses were performed with STATA, version 13.1 (StataCorp., College Station, USA). Only study participants with at least two KK thick smear examinations and with complete questionnaires were retained in the final analysis. Participants were stratified into five age groups: (i) ≤ 9 years, (ii) 10–16 years, (iii) 17–36 years, (iv) 37–50 years, and (v) ≥ 51 years. Socioeconomic status (SES) of the household was calculated using an asset-based method. Indicator data were defined by principal component analysis (PCA). The procedure is widely used and details can be found elsewhere [5, 46, 47]. SES conditions in the household were categorized into one of five wealth quintiles, namely (i) most poor, (ii) very poor, (iii) poor, (iv) less poor, and (v) least poor according to their cumulative standardized asset scores. Details of this widely used approach have been presented elsewhere [5].
The intensity of helminth egg counts was expressed as eggs per gram of stool (EPG) obtained from Kato-Katz examination. Based on WHO recommendations, infection intensity was classified as light (O. viverrini: 1-999 EPG; S. mekongi: 1-100 EPG; hookworm: 1-1999 EPG; T. trichiura: 1-999 EPG; A. lumbricoides: 1-4999 EPG), moderate (O. viverrini: 1000-9999 EPG; S. mekongi: 101-400 EPG; hookworm: 2000-3999 EPG; T. trichiura: 1000-9999 EPG; A. lumbricoides: 5000-49,999 EPG), and heavy (O. viverrini: 1-999 EPG; S. mekongi: 1-100 EPG; hookworm: 1-1999 EPG; T. trichiura: 1-999 EPG; A. lumbricoides: 1-4999 EPG), respectively [25, 31, 48].
Prevalence of parasitic infections was determined and stratified by age, sex and study area (Done Khon versus Done Som). Chi-square test was used to examine the association among categorical variables. The geometric mean for helminth egg counts was calculated for infected individuals. Univariate random-effects logistic regression analysis was used to associate O. viverrini and S. mekongi infections (outcome) with potential risk factors (predictors). The crude odds ratio (cOR), 95% confidence interval (95% CI) and P-value were calculated. Explanatory variables with a P-value of <15% were included in the stepwise multivariate random-effects logistic regression model. Adjusted odds ratio (aOR) was calculated. Smoothed age distribution of O. viverrini, S. mekongi, hookworm and T. trichiura infections by gender was established. Statistical significance was defined as yielding a P-value smaller than 0.05.
Results
Characteristics of the study participants
A total of 994 study participants were included in this final analysis (Fig. 2). Of these, 475 (47.8%) were from Done Khon and 519 (52.2%) from Done Som. There were slightly more female than male participants (51.8% vs 48.2%). Age ranged from 2 to 88 years (median age 29.8 years). The schooling rates did not differ between the two study islands. Subsistent rice farming and fishing were the main professional activities (60.0%). Less than half of the study participants reported having access to a latrine at home (Done Khon 49.7%, Done Som 38.9%). People living in Done Som had a lower socioeconomic status than in Done Khon (Most poor, 25.8% vs 16.4%, respectively). The sociodemographic characteristics of study participants are summarized in Table 1.
Fig. 2.
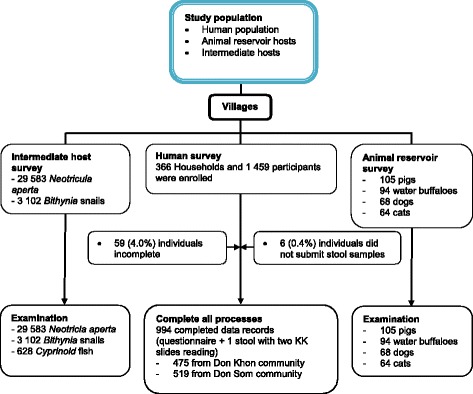
Study diagram
Table 1.
Socio-demographic characteristics of study participants from two study islands (Done Khon and Done Som, Khong District (n = 994)
| Characteristics | Overall n (%) | Study area | x 2 | P-valuea | |
|---|---|---|---|---|---|
| Done Khon, n (%) | Done Som, n (%) | ||||
| Age (years) | |||||
| Mean (range) | 29.8 (2–88) | 30.0 (2–87) | 29.6 (2–88) | ||
| Age group | |||||
| ≤ 9 | 216 (21.7) | 99 (20.8) | 117 (22.5) | ||
| 10–16 | 185 (18.6) | 91 (19.2) | 94 (18.1) | ||
| 17–36 | 204 (20.5) | 102 (21.5) | 102 (19.7) | ||
| 37–50 | 203 (20.4) | 88 (18.5) | 115 (22.2) | ||
| ≥ 51 | 186 (18.7) | 95 (20.0) | 91 (17.5) | 3.3 | 0.511 |
| Sex | |||||
| Male | 479 (48.2) | 212 (44.6) | 267 (51.5) | ||
| Female | 515 (51.8) | 263 (55.4) | 252 (48.6) | 4.6 | 0.032 |
| Educational level | |||||
| Pre-schooler | 108 (10.9) | 52 (10.9) | 56 (10.8) | ||
| Illiteracy | 97 (9.8) | 59 (12.4) | 38 (7.3) | ||
| Primary school | 538 (54.1) | 237 (49.9) | 301 (58.0) | ||
| High school/above | 251 (25.3) | 127 (26.7) | 124 (23.9) | 10.4 | 0.015 |
| Occupation | |||||
| Preschool child | 108 (10.9) | 52 (11.0) | 56 (10.8) | ||
| Student | 290 (29.1) | 137 (28.8) | 153 (29.5) | ||
| Farmer and fisher | 596 (60.0) | 286 (60.2) | 310 (59.7) | 0.05 | 0.975 |
| Socioeconomic status | |||||
| Least poor | 195 (19.6) | 126 (26.5) | 69 (13.3) | ||
| Less poor | 203 (20.4) | 73 (15.4) | 130 (25.1) | ||
| Poor | 192 (19.3) | 107 (22.5) | 85 (16.4) | ||
| Very poor | 192 (19.3) | 91 (19.2) | 101 (19.5) | ||
| Most poor | 212 (21.3) | 78 (16.4) | 134 (25.8) | 48.6 | <0.001 |
| Latrine available | |||||
| No | 556 (55.9) | 239 (50.3) | 317 (61.1) | ||
| Yes | 438 (44.1) | 236 (49.7) | 202 (38.9) | 11.7 | 0.001 |
| Opened defecation this year | |||||
| No | 484 (48.7) | 256 (53.9) | 228 (43.9) | ||
| Yes | 510 (51.3) | 219 (46.1) | 291 (56.1) | 9.9 | 0.002 |
P-valuea: the comparison between Done Khone and Done Som Island
Helminth infections in humans
Helminth infections were very frequent on the two islands. O. viverrini, hookworm, S. mekongi, and T. trichiura were found in 60.7%, 44.1%, 22.2% and 4.1% of the participants, respectively. Very few participants were infected with A. lumbricoides (0.6%) and Taenia spp. (0.1%). The prevalence of O. viverrini was almost two-times higher in Done Som compared to Done Khon (77.3% vs. 42.5%, P < 0.001). S. mekongi prevalence was similar on both islands (P = 0.329). Multi-parasitism was diagnosed in 40.5% of the study participants. Details of the helminth infections are given in Table 2.
Table 2.
Prevalence of Opisthorchis viverrini, Schistosoma mekongi, soil-transmitted helminth and other intestinal helminth infections among study participants from two islands (Done Khon and Done Som) of Khong District (n = 994)
| Parasites | Positive, n (%) (n = 994) |
Done Khon, n (%) (n = 475) |
Done Som, n (%) (n = 519) |
x 2 | P-valuea |
|---|---|---|---|---|---|
| Trematodes | |||||
| Opisthorchis viverrini | 603 (60.7) | 202 (42.5) | 401 (77.3) | 125.4 | <0.001 |
| Schistosoma mekongi | 221 (22.2) | 112 (23.6) | 109 (21.0) | 0.9 | 0.329 |
| Soil-transmitted helminth | |||||
| Hookworm | 438 (44.1) | 196 (41.3) | 242 (46.6) | 2.9 | 0.090 |
| Trichuris trichiura | 41 (4.1) | 21 (4.4) | 20 (3.9) | 0.2 | 0.653 |
| Ascaris lumbricoides | 6 (0.6) | 6 (1.3) | 0 | 6.6 | 0.010 |
| Cestodes | |||||
| Taeniaspp. | 1 (0.1) | 1 (0.2) | 0 | 1.1 | 0.296 |
| Multiparasitism | |||||
| No infection | 202 (20.3) | 127 (26.7) | 75 (14.5) | ||
| Single species | 379 (38.1) | 197 (41.5) | 182 (35.1) | ||
| Multiple species | 413 (40.5) | 151 (31.8) | 261 (40.5) | 43.9 | <0.001 |
P-valuea: the comparison between Done Khone and Done Som Island
Figure 3 displays the smoothed age prevalence of helminth infections by gender. O. viverrini infection appears to be acquired at a young age, with prevalence increasing gradually (Fig. 3a). Hookworm infection is acquired at a very young age. For males, the prevalence peaked among adolescents aged 10–20 years and plateaued among older age groups. For females, prevalence peaked between 10 and 20 years old and again after 50 years old (Fig. 3b). For males, two prevalence peaks were observed; the first among children under 10 years old and the second among adults between 40 and 50 years old. For females, only one peak was seen among children under 10 years old. T. trichiura prevalence was distributed similarly among males and females independent of age (Fig. 3c). S. mekongi prevalence was differently distributed among males and females (Fig. 3d).
Fig. 3.
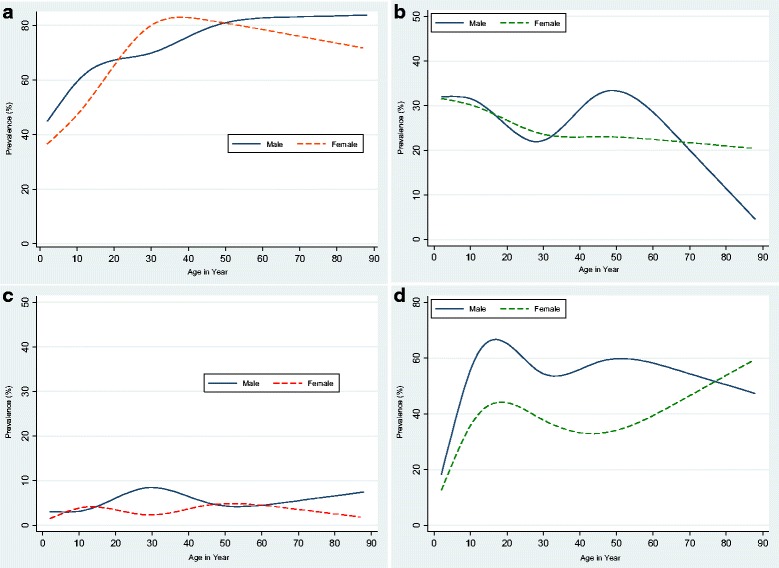
Age distribution of major helminth infections by gender on Done Khon and Done Som islands. The figures represent the smoothed age distribution of male (solid line) and female (dotted line) study participants for an infection with (a): Opisthorchis viverrini, (b): hookworm, (c): Trichuris trichiura and (d): Schistosoma mekongi
Human helminth infection intensities are summarized in Table 3. Most helminth infections were categorized as light infections. Nevertheless, O. viverrini, S. mekongi and hookworm accounted for infections of heavy intensity in some cases (4.2%, 3.6% and 1.8%, respectively).
Table 3.
Infection intensity of Opisthorchis viverrini, Schistosoma mekongi and soil-transmitted helminths among study participants from two islands (Done Khon and Done Som) of Khong District (n = 994)
| Infections | Light | Moderate | Heavy | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Overall | Done Khon | Done Som | Overall | Done Khon | Done Som | Overall | Done Khon | Done Som | |
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |
| Opisthorchis viverrini | 409 (67.8) | 174 (86.1) | 235 (58.6) | 169 (28.0) | 27 (13.4) | 142 (35.4) | 25 (4.2) | 1 (0.5) | 24 (6.0) |
| Schistosoma mekongi | 187 (84.6) | 100 (89.3) | 87 (79.8) | 26 (11.8) | 10 (8.9) | 16 (14.7) | 8 (3.6) | 2 (1.8) | 6 (5.5) |
| Hookworm | 420 (95.9) | 191 (97.5) | 229 (94.6) | 10 (2.3) | 2 (1.0) | 8 (3.3) | 8 (1.8) | 3 (1.5) | 5 (2.1) |
| Trichuris trichiura | 41 (97.6) | 22 (100.0) | 19 (95.0) | 1 (2.4) | 0 | 1 (5.0) | 0 | 0 | 0 |
| Ascaris lumbricoides | 5 (83.3) | 5 (83.3) | 0 | 1 (16.7) | 1 (16.7) | 0 | 0 | 0 | 0 |
Prevalence of helminth infections in animal reservoirs and intermediate hosts
Table 4 summarizes the results of infections in animals, snails and Cyprinoid fish. Analysis of animal faeces showed that overall prevalence of O. viverrini infection in cats, dogs and pigs was 53.1%, 25.0% and 0.9%, respectively, while only dogs (14.7%) were found to be infected with S. mekongi. Examination of intermediate host snails for O. viverrini (Bithynia spp.,) and for S. mekongi (N. aperta) detected infection rates of 0.3% and 0.01%, respectively (Table 4). A similar rate of O. viverrini infection was found in Bithynia spp. from Done Khon and Done Som (0.1% vs. 0.5%, P = 0.045), while only the N. aperta snails from Done Khon (0.02%) were found to be infected with S. mekongi.
Table 4.
Prevalence of Opisthorchis viverrini and Schistosoma mekongi infections in animals on Done Khon and Done Som islands
| Infections | No. exam | Overall, n (%) |
Done Khon, n (%) | Done Som, n (%) | x 2 | P-valuea | ||
|---|---|---|---|---|---|---|---|---|
| No. exam | No. positive | No. exam | No. positive | |||||
| Opisthorchis viverrini | ||||||||
| Dog | 68 | 17 (25.0) | 44 | 10 (22.7) | 24 | 7 (29.2) | 0.34 | 0.558 |
| Cat | 64 | 34 (53.1) | 25 | 15 (60.0) | 39 | 19 (48.7) | 0.78 | 0.378 |
| Pig | 105 | 1 (0.9) | 43 | 0 | 62 | 1 (1.6) | 0.70 | 0.403 |
| Water buffalo | 94 | 0 | 32 | 0 | 62 | 0 | na | na |
| Intermediate snails | ||||||||
| Bithynia spp. | 3102 | 9 (0.3) | 1719 | 2 (0.1) | 1383 | 7 (0.5) | 4.03 | 0.045 |
| Minute intestinal fluke (MIF) | ||||||||
| Dog | 68 | 3 (4.4) | 44 | 3 (6.8) | 24 | 0 | 1.71 | 0.191 |
| Cat | 64 | 18 (28.1) | 25 | 5 (20.0) | 39 | 13 (33.3) | 1.33 | 0.247 |
| Large trematode eggs | ||||||||
| Water buffaloes | 94 | 18 (19.1) | 32 | 9 (28.1) | 62 | 9 (14.5) | 2.52 | 0.112 |
| Pig | 105 | 4 (3.8) | 43 | 2 (4.6) | 62 | 2 (3.2) | 0.14 | 0.708 |
| Schistosoma mekongi | ||||||||
| Dog | 68 | 10 (14.7) | 44 | 7 (16.0) | 24 | 3 (13.0) | 0.14 | 0.704 |
| Cat | 64 | 0 | 25 | 0 | 39 | 0 | na | |
| Pig | 105 | 0 | 43 | 0 | 62 | 0 | na | |
| Water buffalo | 94 | 0 | 32 | 0 | 62 | 0 | na | |
| Intermediate snails | ||||||||
| Neotricula aperta | 29,583 | 4 (0.01) | 16,342 | 4 (0.02) | 13,241 | 0 | 3.24 | 0.072 |
P-valuea: the comparison between Done Khone and Done Som Island
na Not applicable
Table 5 displays the prevalence of O. viverrini infection in the Cyprinoid fish collected from habitats in Done Khon and Done Som islands. In total, 628 fish representing 21 species were digested and examined. Of these, 622 represented 19 species of Cyprinoid fish, five fish were from the Osphronemidiae family and one fish from the Anabantidae family. Only Cyprinoidae fish species were infected with O. viverrini, with an overall prevalence of 26.9% and an average of 228.7 metacercariae per fish. The highest infection intensity was seen in Cyclocheilichthys apogon, with an average of 168.7 metacercariae per infected fish. Only one fish of the Anabas testudineus from Anabantidae family was examined. It was found positive for minute intestinal fluke metacercariae.
Table 5.
Prevalence of Opisthorchis viverrini and minute intestinal flukes (MIF) metacercariae in cyprinoid fish from Done Khon and Done Som islands
| Scientific name Species |
Lao name | No. exam.\ | No. of fish infected O. viverrini positive (%) |
No. of O. viverrini metacercariae Mean, SD (range) |
No. of fish infected MIF positive (%) |
No. of MIF metacercariae Mean, SD (range) |
Weight (gram) Mean, SD (range) |
|---|---|---|---|---|---|---|---|
| Morulius chrysophekadion | Pa phea | 1 | 1 (100.0) | 2.0, na | 0 | 0 | 138 (na) |
| Hampala dispa | Pa soud | 101 | 88 (87.1) | 112.1, ± 188.0 (3–1468) | 9 (8.9) | 8.6, ± 10.6 (1–48) | 11.4, ± 11.1 (1.9–66.4) |
| Cyclocheilichthys apogon | Pa dok-ngew | 21 | 18 (85.7) | 168.7, ± 283.9 (2–984) | 5 (23.8) | 6.4, ± 4.4 (2–12) | 7.1, ± 4.6 (1.5–20.1) |
| Puntius brevis | Pa khao-mon | 100 | 40 (40.0) | 120.2, ± 322.2 (1–1940) | 22 (22.2) | 10.7, ± 10.9 (1–48) | 8.5, ± 9.3 (1.1–39.1) |
| Henicorhynchus lineatus | Pa soi | 14 | 3 (21.4) | 31, ± 37.3 (7–74) | 0 | 0 | 11.8, ± 5.3 (3.7–23) |
| Barbonymus gonionotus | Pa pak-khao | 16 | 2 (13.0) | 10, ± 7.1 (5–15) | 0 | 0 | 38.2, ± 25.5 (3.9–84.9) |
| Barbonymus altus | Pa wien-fai | 17 | 2 (11.8) | 2.5, ± 0.7 (2–3) | 0 | 0 | 21.5, ± 7.5 (2.9–36.1) |
| Poropuntius deauratus | Pa chad | 163 | 10 (6.1) | 21.6, ± 43.6 (1–142) | 16 (9.8) | 9.3, ± 12.6 (1–42) | 14.3, ± 23.4 (1.4–148.6) |
| Puntioplites falcifer | Pa sa-khang | 34 | 2 (6.0) | 6.0, ± 5.7 (2–10) | 1 (2.9) | 2.0, na | 19.2, ± 8.8 (3.3–38.7) |
| Scaphognathops bandanensis | Pa pieng | 40 | 2 (5.0) | 1.5, ± 0.7 (1–2) | 2 (5.0) | 7.5, ± 7.8 (2–13) | 33.1, ± 14.9 (6.2–68.7) |
| Albulichthys albuloides | Pa ta-sai | 69 | 1 (1.5) | 1, na | 22 (31.9) | 19.7, ± 34.9 (1–132) | 14.4, ± 5.3 (4.9–27.9) |
| Opsarius koratensis | Pa sew-oua | 16 | 0 | 0 | 1 (6.3) | 2.0, na | 6.3, ± 5.3 (1.9–14.6) |
| Paralaubuca typus | Pa tab | 5 | 0 | 0 | 1 (20.0) | 2.0, na | 8.5, ± 5.1 (5.1–17.3) |
| Mystacoleucus atridorsalis | Pa lang-khon | 9 | 0 | 0 | 0 | 0 | 9.1, ± 14.1 (1.4–36.9) |
| Cyclocheilichthys enoplus | Pa choox | 5 | 0 | 0 | 0 | 0 | 30.7, ± 11.9 (18.6–48.6) |
| Luciosoma bleekeri | Pa mak-vai | 4 | 0 | 0 | 0 | 0 | 26.1, ± 3.9 (23.7–31.9) |
| Osteochilus melanopleurus | Pa nok-khao | 3 | 0 | 0 | 0 | 0 | 8.8, ± 9.7 (2.8–20) |
| Raiamas guttatus | Pa sa-nak | 3 | 0 | 0 | 0 | 0 | 38.6, ± 29.8 (10–69.4) |
| Probarbus labeamajor | Pa oearn | 1 | 0 | 0 | 0 | 0 | 45.9 (na) |
| Trichogaster trichopterus a | Pa ka-deuth | 5 | 0 | 0 | 0 | 0 | 4.6, ± 2.4 (2.3–7.8) |
| Anabas testudineus b | Pa kheng | 1 | 0 | 0 | 1 (100) | 3.0, na | 9.7 (na) |
| Total | 628 | 169 (26.9) | 106.9 ± 228.7 (1–1940) | 12.7 | 11.9, ±20.7 (1–132) | 15.0, ±17.4 (1.1–148.6) |
Belongs to the
aOsphronemidae and
bAnabantidae family, NA Not appropriate, SD standard deviation, No Numb
Risk factor analysis for O. viverrini and S. mekongi infections in human
Table 6 shows the association between risk factors of O. viverrini and S. mekongi infections. The stepwise multivariate analysis showed that illiteracy (illiteracy vs. preschool children: aOR = 6.0, 95% CI: 3.3–11.0), P = 0.028) and lower socioeconomic status were associated with an increased risk of being infected with O. viverrini (less poor vs least poor: aOR = 3.1, 95% CI: 1.7–7.5, P = 0.013), while school children in the age group 10–16 years (aOR = 0.1, 95% CI: < 0.1–0.4, P = 0.003) and those with a latrine at home (aOR = 0.2, 95% CI: 0.1–0.4), P = 0.001) were more likely to be protected against the infection. Furthermore, having household dogs and cats that eat raw fish was significantly and positively associated with O. viverrini infection of the household members (aOR = 1.9, 95% CI: 1.2–3.1, P = 0.007). The age group was the only factor significantly associated with S. mekongi infection. Children in the age group ≤9 years old were significantly exposed to this infection compared to older age groups (age group 10–16: aOR = 0.5, 95% CI: 0.2–0.9, P = 0.047, age group 17–36: aOR = 0.2, 95% CI: < 0.1–0.8, P = 0.022; age group 37–50: aOR = 0.2, 95% CI: < 0.1–0.8, P = 0.021 and age group ≥51: aOR = 0.2, 95% CI: < 0.1–0.8, P = 0.024). The model revealed that age group (10–16 year: aOR = 1.7, 95% CI: 1.1–2.7, P = 0.015), educational level (illiteracy: aOR = 7.4, 95% CI: 3.2–17.3, P < 0.001, and primary school: aOR = 4.8, 95% CI: 2.0–11.3, P < 0.001) and raising pigs at home (aOR = 1.3, 95% CI: 1.1–1.7, P = 0.047) were significant risk factors for STH infection, while being a women (aOR = 0.4, 95% CI: 0.3–0.6, P < 0.001) or having a latrine at home (aOR = 0.6, 95% CI: 0.4–0.8, P < 0.001) were protective factors.
Table 6.
Stepwise multivariate logistic regression (backward elimination) analyses the association between underlying risk factors and O. viverrini, S. mekongi and STH infections among study participants on both islands (Done Khon and Done Som islands (n = 994)
| Characteristics | O. viverrini | S. mekongi | Soil-transmitted helminth | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crude OR
(95% CI) |
P-value | Adjusted OR (95% CI) | P-value | Crude OR
(95% CI) |
P-value | Adjusted OR (95% CI) | P-value | Crude OR
(95% CI) |
P-value | Adjusted OR (95% CI) | P-value | |
| ≤ 9 | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| 10–16 | 1.6 (1.1–2.4) | 0.022 | 0.1 (< 0.1–0.4) | 0.003 | 0.6 (0.4–1.1) | 0.075 | 0.5 (0.2–0.9) | 0.047 | 2.7 (1.8–4.0) | < 0.001 | 1.7 (1.1–2.7) | 0.015 |
| 17–36 | 3.3 (2.2–4.9) | < 0.001 | NS | NS | 0.5 (0.3–0.8) | 0.005 | 0.2 (< 0.1–0.8) | 0.022 | 1.9 (1.3–2.9) | 0.001 | NS | NS |
| 37–50 | 4.3 (2.8–6.4) | < 0.001 | NS | NS | 0.5 (0.4–0.9) | 0.006 | 0.2 (< 0.1–0.8) | 0.021 | 2.2 (1.5–3.2) | < 0.001 | NS | NS |
| ≥ 51 | 4.2 (2.7–6.4) | < 0.001 | NS | NS | 0.5 (0.4–0.9) | 0.011 | 0.2 (< 0.1–0.8) | 0.024 | 2.4 (1.6–3.6) | < 0.001 | NS | NS |
| Sex | ||||||||||||
| Male/Female | 1/1.1 (0.8–1.5) | 0.579 | NA | NA | 1/0.8 (0.6–1.2) | 0.401 | NA | NA | 1/0.5 (0.4–0.6) | < 0.001 | 1/0.4 (0.3–0.6) | < 0.001 |
| Educational level | ||||||||||||
| Preschooler | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| Illiteracy | 6.0 (3.3–11.0) | < 0.001 | 9.4 (1.3–68.9) | 0.028 | 0.6 (0.3–1.2) | 0.131 | NS | NS | 4.0 (2.2–7.2) | < 0.001 | 7.4 (3.2–17.3) | < 0.001 |
| Primary school | 4.0 (2.5–6.2) | < 0.001 | NS | NS | 0.7 (0.3–1.2) | 0.140 | NS | NS | 2.9 (1.8–4.6) | < 0.001 | 4.8 (2.0–11.3) | < 0.001 |
| High school/above | 2.7 (1.7–4.3) | < 0.001 | NS | NS | 0.6 (0.4–1.1) | 0.101 | NS | NS | 2.8 (1.7–4.6) | < 0.001 | NS | NS |
| Occupation | ||||||||||||
| Preschool child | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| Student | 1.2 (0.8–1.8) | 0.377 | NA | NA | 1.5 (1.0–1.9) | 0.034 | NS | NS | 1.9 (1.2–2.8) | 0.003 | NS | NS |
| Farmer | 3.1 (2.1–4.6) | < 0.001 | NS | NS | 2.0 (1.0–2.6) | 0.017 | NS | NS | 1.8 (1.3–2.7) | 0.002 | NS | NS |
| Socio - economic status | ||||||||||||
| Least poor | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| Less poor | 2.4 (1.3–4.7) | 0.007 | 6.5 (1.2–37.5) | 0.037 | 1.5 (0.9–2.3) | 0.321 | NA | NA | 1.5 (1.0–2.2) | 0.041 | NS | NS |
| Poor | 2.2 (1.1–4.2) | 0.018 | NS | NS | 1.9 (1.1–2.9) | 0.082 | NS | NS | 1.2 (0.8–1.8) | 0.340 | NA | NA |
| Very poor | 1.5 (0.8–2.9) | 0.213 | NS | NS | 0.9 (0.5–1.6) | 0.816 | NA | NA | 1.2 (0.8–1.8) | 0.395 | NA | NA |
| Most poor | 3.7 (2.0–7.0) | < 0.001 | NS | NS | 0.9 (0.6–1.6) | 0.753 | NA | NA | 1.3 (0.9–1.9) | 0.209 | NA | NA |
| Latrine available | ||||||||||||
| No/Yes | 1/0.4 (0.3–0.7) | < 0.001 | 1/0.2 (0.1–0.4) | 0.001 | 1/0.8 (0.6–1.1) | 0.148 | NA | NA | 1/0.6 (0.5–0.8) | < 0.001 | 1/0.6 (0.4–0.8) | < 0.001 |
| Has ever heard about diseases | ||||||||||||
| No/Yes | 1/1.5 (0.9–2.3) | 0.090 | NS | NS | 1/0.8 (0.6–1.1) | 0.154 | NA | NA | NA | NA | NA | NA |
| Known about transmission route | NA | NA | NA | NA | ||||||||
| No/Yes | 1/1.8 (1.0–3.2) | 0.041 | NS | NS | 1/0.8 (0.5–1.5) | 0.660 | NA | NA | NA | NA | NA | NA |
| Open defecation this year | ||||||||||||
| No/Yes | 1/1.8 (1.4–2.3) | < 0.001 | NS | NS | 1/1.1 (0.8–1.5) | 0.482 | NA | NA | 1/1.6 (1.2–2.1) | < 0.001 | NS | NS |
| Water contact for fishing/farming | ||||||||||||
| No/Yes | 1/1.5 (1.0–2.1) | 0.038 | NS | NS | 1/0.9 (0.6–1.4) | 0.730 | NA | NA | 1/1.6 (1.2–2.3) | < 0.005 | NS | NS |
| Eating raw/undercooked fish | ||||||||||||
| No/Yes | 1/4.3 (2.6–6.9) | < 0.001 | NS | NS | 1/0.6 (0.4–0.8) | 0.004 | NS | NS | 1/0.8(0.5–3.2) | 0.872 | NS | NS |
| Raising cats at home | ||||||||||||
| No/Yes | 1/1.0 (0.8–1.3) | 0.959 | NA | NA | 1/0.8 (0.6–1.2) | 0.542 | NA | NA | 1/1.2 (0.9–1.6) | 0.094 | NS | NS |
| Raising dogs at home | ||||||||||||
| No/Yes | 1/0.9 (0.7–1.2) | 0.397 | NA | NA | 1/0.7 (0.4–1.4) | 0.343 | NA | NA | 1/1.2 (0.9–1.5) | 0.132 | NS | NS |
| Raising pigs at home | ||||||||||||
| No/Yes | 1.1 (0.9–1.5) | 0.398 | NA | NA | 1.2 (0.9–1.6) | 0.336 | NA | NA | 1/1.2 (0.9–1.6) | 0.132 | 1/1.3 (1.1–1.7) | 0.047 |
| Raising buffaloes at home | ||||||||||||
| No/Yes | 1/1.1 (0.9–1.5) | 0.394 | NA | NA | 1/1.3 (0.9–1.8) | 0.133 | NS | NS | 1/1.1 (0.8–1.2) | 0.845 | NA | NA |
| Observed dog/cat eat raw/undercooked fish | ||||||||||||
| No/Yes | 1/1.3 (0.9–1.6) | 0.059 | 1/1.9 (1.2–3.1) | 0.007 | NA | NA | NA | NA | 1/1.3 (0.8–1.7) | 0.169 | NA | NA |
NA Not appropriate for analysis (all variables with P-value ≥15% and are removed by model), NS Not significant (all variables with P-value <15%, but are not significant after adjusted analysis
Discussion
The Khong District, with its dozens of islands in the Mekong, has a distinct ecological setting (Fig. 1). Human settlements line the island shores, while the rest of the island is used for agricultural activities, particularly rice farming. The Mekong River as well as the diverse water bodies on the islands represent a rich ecosystem for fish and mollusc populations. On two Mekong islands, highly endemic for multiple species of helminth infections, we studied the transmission of O. viverrini, S. mekongi and STH using an ecohealth approach [32, 37] to better assess the relation of human infection status to environmentally present reservoir and intermediate hosts. Heavy infections and multi-parasitism were prevalent among the human population and age-gender distributions revealed parasite-specific patterns. Examination of potential animal reservoir hosts from the study participants’ households (cats, dogs, pigs and buffaloes) yielded ten different helminth species, with many of them having zoonotic capacity. Infection rates of intermediate snail hosts Bithynia sp. and N. aperta were low but reflect on-going transmission. In addition, infection rates of locally caught cyprinoid fish with O. viverrini and minute intestinal fluke (MIF) metacercariae were very high, pointing to a high risk of infection when they are consumed raw or undercooked.
In this study, we document high infection rates of O. viverrini, S. mekongi and selected species of STH, namely hookworm infections. The high infection rates are a surprise given that MDA campaigns were conducted annually between 2008 and 2013 [26], in which praziquantel (40 mg/kg BW single dose) and albendazole (400 mg single dose) were provided to the entire population (older than 4 years). In addition, biannual deworming (with mebendazole) takes place in all Lao primary schools [27]. Local health authorities confirmed that all Mekong islands were targeted, but we could not find coherent information on the number of treatment rounds conducted on our study islands. Nevertheless, our results indicate that the impact of the intervention is insufficient.
The Ministry of Health’s objective is to eliminate S. mekongi as a public health problem in Lao PDR by 2016. On our study islands, S. mekongi cannot be considered eliminated given the high infection rates. Our data indicate that S. mekongi infection in dogs may fuel the transmission by constantly infecting Neotricula populations in the Mekong. Of similar importance are cats and dogs for the transmission of O. viverrini. Hence, animal reservoirs in households should also be a target of integrated parasite control on the Mekong islands, and throughout Lao PDR.
Several factors might account for the persisting high O. viverrini infection rates among humans on the Mekong islands. One such factor is the high infection prevalence among cyprinoid fish. More than 80 species of the Cyprinidae family and at least 13 species of other families can serve as a secondary intermediate host [25]. In our study, O. viverrini metacercariae were identified in 11 cyprinoid fish species, while some had particularly high O. viverrini metacercariae infection rates, e.g. in 87.1% of Hampala dispa. All the cyprinoid species in which we detected an infection are known to be good O. viverrini transmitting species [49–52]. They were identified in all water bodies examined in this study. Fish are mostly likely infected while small and living in rice fields, canals and ponds. The metacercariae remain alive as the fish grow and move into the Mekong.
Cyprinoid fish accumulate the metacercariae over a long time. Low infection rates in Bithynia snails may be sufficient for transmission [53]. We found a low infection rate of 0.3% in Bithynia sp. snails. Other studies have detected infection rates between 0.3–8.3% [54]. But infection rates may vary considerably, depending on sampling locality and season [54, 55]. It is important to note that even low infection prevalence rates are sufficient for maintaining transmission.
We observed low S. mekongi infection rates in N. aperta (0.02%) compared to other reports. The presence of infected molluscs gives evidence that S. mekongi transmission is currently on-going. Therefore, abandoning control activities would inevitably lead to an increase in infection rates among humans. There are many more S. mekongi endemic Mekong islands, which might display a different N. aperta population distribution and infection pattern [9, 10].
A major finding from our study is the dramatically high helminth infection rates among domestic cats, dogs, pigs and buffaloes. Ten different parasite species were detected in these animal hosts residing in the households of our study participants. By using FECT, we could distinguish O. viverrini eggs in dogs and cats from other small trematode eggs. Our results showed higher rates than Aunpromma et al. (2012) found in neighbouring Thailand, where 0.37% and 35.5% of the dogs and cats were infected, respectively [56]. The infection rate among dogs, in particular, was 20 times higher than that found in the study of Aunpromma et al. (2012). Through observation and from interviewing animal owners in both communities, it appears that most of the dogs and cats were free-roaming and usually accompanied their owners to the rice field where they caught and ate fish directly from the canals or rice fields. Moreover, raw and undercooked fish were often fed to these animals. These phenomena, in combination with the high infection rates of dogs and cats, likely maintain the transmission of O. viverrini and other fish-borne trematode infections in the communities.
Only dogs were diagnosed with S. mekongi in this study, which is consistent with other study findings [9, 22]. We did not find any S. mekongi eggs in pigs or water buffaloes, though both animals were found to be infected in earlier investigations [57]. However, they are not of importance for transmission on our study islands. On other Mekong islands where these animals are more free-roaming, their infection status could be higher and, thus, their contribution to transmission of greater importance.
The results of our risk factor analysis for O. viverrini infection differed from many previous studies [5, 30, 49]. More than half of our risk factors dropped out after multivariate analysis, whereas the initial univariate analysis showed significant associations between infection and age group, occupation, socioeconomic status, latrine availability, history of open defecation this year, and eating raw and/or undercooked fish (Table 6). The association between O. viverrini and socioeconomic status was not clear for our study population. The study area was geographically very small. Therefore, the variation in socioeconomic status and living conditions might not have varied enough to results in risk differentiation. Furthermore, control activities such as the annual treatments between 2008 and 2013, have had an impact on infection status, which in turn might have blurred important associations. For example, eating raw/undercooked fish was not significantly associated with O. viverrini infection, although deeply rooted habits of eating raw or improperly cooked fish is a well-known factor in sustaining helminth infections in humans and difficult to control [30, 53, 58].
In our multivariable analysis, we did not find any association between S. mekongi infection and risk factors, except for age. Children under 9 years old had a higher risk of infection than older study participants. This result is likely due to MDA over the years having reduced infection rates among older villagers. Therefore, controls targeting lower age groups could further contribute to eliminating S. mekongi on the Mekong islands.
Our study suffers from some limitations. Our diagnostic procedure most likely underestimated the true infection burden. Although examining a duplicate Kato-Katz thick smear per faecal sample has a considerably higher sensitivity than a single smear, the egg detection rate remains far below that of a multiple stool sample diagnostic procedure [5, 18]. Furthermore, the Kato-Katz technique cannot differentiate small trematode eggs [59]. It is therefore possible that some of the infections in humans were counted as O. viverrini infections instead of MIF.
Conclusions
We conclude that human intestinal helminth infections, namely O. viverrini, S. mekongi and hookworms are still highly endemic on the Mekong islands in Khong District. The low prevalence of O. viverrini and S. mekongi infection in intermediate snail hosts point at on-going transmission. Animal reservoir hosts, particularly cats and dogs, have high O. viverrini infection rates, while only dogs are infected with S. mekongi. An appropriate integrated control approach involving interventions targeting human behaviour, animal reservoirs, and environmental modification might improve the effectiveness of interventions and lead to the elimination of infections.
Acknowledgements
We sincerely thank the population of the study villages and the authorities at the village, district and provincial departments for their active participation and their interest in the study. Furthermore, the support of the Centre of Malariology, Parasitology, and Entomology in Vientiane and in the province and districts is highly appreciated. We thank Mrs. Amena Briet for her efficient English editing.
Funding
We are grateful to the International Development Research Centre; Foreign Affairs, Trade and Development Canada (through the Global Health Research Initiative); and the Australian Agency for International Development for funding support.
Availability of data and materials
All datasets analysed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- 95% CI
95% confidence interval
- A. duodenale
Ancylostoma duodenale
- A. lumbricoides
Ascaris lumbricoides
- aOR
Adjusted odds ratio
- BW
Body weight
- CCA
Cholangiocarcinoma
- cOR
Crude odds ratio
- EPG
Eggs per gram of stool
- FECT
Formalin ether concentration technique
- IEC
Information, education and communication
- KAPP
Knowledge, attitude, practice and perception
- Lao PDR
Lao People’s Democratic Republic
- LMIC
Low and middle income countries
- MDA
Mass drug administration
- MIF
Minute intestinal flukes
- N. americanus
Necator americanus
- N. aperta
Neotricula aperta
- NaCl
Sodium chloride
- NIOPH
National Institute of Public Health
- NTDs
Neglected tropical diseases
- O. viverrini
Opisthorchis viverrini
- PCA
Principle Component Analysis
- S. mekongi
Schistosoma mekongi
- S. stercoralis
Strongyloides stercoralis
- STH
Soil transmitted helminth
- T. trichiura
Trichuris trichiura
- WHO
World Health Organization
Additional file
Multilingual abstract in the five official working languages of the United Nations. (PDF 975 kb)
Authors’ contributions
YV, PO, KA and SS designed the study; SS, YV, SP, KT implemented the study; YV, PO and SS analyzed and interpreted the data; YV wrote the first draft of the manuscript; PO and SS revised the manuscript. All authors read and approved the final version of the manuscript.
Ethics approval and consent to participate
This study was approved by the National Ethics Committee for Health Research, Ministry of Health (MOH), Vientiane, Lao PDR (reference no.043/NECR). Field activities were approved by the MOH steering committee. An inception meeting was organized at the study site prior to starting field work. Village and local health authorities and villagers were invited to participate in this meeting. Study participants were informed of the study aims and procedures, the benefits and risks of participating, as well as of their right to withdraw from the study at any time. Before enrolment, written informed consent was obtained from all study participants. For study participants under the age of 18, written informed consent was obtained from the parents or legal guardians. In addition, oral consent was obtained from each child participant between 12 and 17 years old. Permission to collect faecal samples from animals was obtained from their owners. All diagnosed infections were treated free of charge according to the Lao National guidelines [60]. Soil-transmitted helminths (STH) and/or trematode infections were treated with a single oral dose of 400 mg albendazole and praziquantel (40 mg/kg BW), respectively [60–63].
Consent for publication
A written, informed consent to share and disseminate data was obtained from all study participants before enrolment. For children aged below 18 years, the consent was obtained from their parent or legal guardian.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1186/s40249-017-0343-x) contains supplementary material, which is available to authorized users.
Contributor Information
Youthanavanh Vonghachack, Email: vonghachack.y@gmail.com.
Peter Odermatt, Email: peter.odermatt@swisstph.ch.
Keoka Taisayyavong, Email: vkeomisy@yahoo.com.
Souphanh Phounsavath, Email: souphanh_psv@hotmail.com.
Kongsap Akkhavong, Email: kongsapa@gmail.com.
Somphou Sayasone, Phone: +856 21 250 670, Email: somphou.sayasone@yahoo.com.
References
- 1.Sripa B, Kaewkes S, Intapan PM, Maleewong W, Brindley PJ. Food-borne trematodiases in Southeast Asia epidemiology, pathology, clinical manifestation and control. Adv Parasitol 2010;72:305-50. [DOI] [PubMed]
- 2.Utzinger J, Bergquist R, Olveda R, Zhou XN. Important helminth infections in Southeast Asia diversity, potential for control and prospects for elimination. Adv Parasitol. 2010;72:1–30. doi: 10.1016/S0065-308X(10)72001-7. [DOI] [PubMed] [Google Scholar]
- 3.WHO . Prevention and control of schistosomiasis and soil-transmitted helminthiasis: first report of the joint WHO expert committees. Geneva: World Health Organization Technical Report Series No 912; 2002. p. 912. [PubMed] [Google Scholar]
- 4.Rim HJ, Chai JY, Min DY, Cho SY, Eom KS, Hong SJ, Sohn WM, Yong TS, Deodato G, Standgaard H, et al. Prevalence of intestinal parasite infections on a national scale among primary schoolchildren in Laos. Parasitol Res. 2003;91(4):267–272. doi: 10.1007/s00436-003-0963-x. [DOI] [PubMed] [Google Scholar]
- 5.Sayasone S, Mak TK, Vanmany M, Rasphone O, Vounatsou P, Utzinger J, Akkhavong K, Odermatt P. Helminth and intestinal protozoa infections, multiparasitism and risk factors in Champasack province, Lao People's Democratic Republic. PLoS Negl Trop Dis. 2011;5(4):e1037. doi: 10.1371/journal.pntd.0001037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xayaseng V, Phongluxa K, van Eeuwijk P, Akkhavong K, Odermatt P. Raw fish consumption in liver fluke endemic areas in rural southern Laos. Acta Trop. 2013;127(2):105–111. doi: 10.1016/j.actatropica.2013.03.016. [DOI] [PubMed] [Google Scholar]
- 7.Sayasone S, Rasphone O, Vanmany M, Vounatsou P, Utzinger J, Tanner M, Akkhavong K, Hatz C, Odermatt P. Severe morbidity due to Opisthorchis Viverrini and Schistosoma Mekongi infection in Lao People's Democratic Republic. Clin Infect Dis. 2012;55(6):e54–e57. doi: 10.1093/cid/cis528. [DOI] [PubMed] [Google Scholar]
- 8.WHO The control of schistosomiasis. Second report of the WHO expert committee. World Health Organ Tech Rep Ser. 1993;830:1–86. [PubMed] [Google Scholar]
- 9.Urbani C, Sinoun M, Socheat D, Pholsena K, Strandgaard H, Odermatt P, Hatz C. Epidemiology and control of mekongi schistosomiasis. Acta Trop. 2002;82(2):157–168. doi: 10.1016/S0001-706X(02)00047-5. [DOI] [PubMed] [Google Scholar]
- 10.Muth S, Sayasone S, Odermatt-Biays S, Phompida S, Duong S, Odermatt P. Schistosoma Mekongi in Cambodia and Lao People's Democratic Republic. Adv Parasitol. 2010;72:179–203. doi: 10.1016/S0065-308X(10)72007-8. [DOI] [PubMed] [Google Scholar]
- 11.Laymanivong S, Hangvanthong B, Keokhamphavanh B, Phommasansak M, Phinmaland B, Sanpool O, Maleewong W, Intapan PM. Current status of human hookworm infections, ascariasis, trichuriasis, schistosomiasis mekongi and other trematodiases in Lao People's Democratic Republic. Am J Trop Med Hyg. 2014;90(4):667–669. doi: 10.4269/ajtmh.13-0636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sripa B, Bethony JM, Sithithaworn P, Kaewkes S, Mairiang E, Loukas A, Mulvenna J, Laha T, Hotez PJ, Brindley PJ. Opisthorchiasis and Opisthorchis-associated cholangiocarcinoma in Thailand and Laos. Acta Trop. 2011;120(Suppl 1):S158–S168. doi: 10.1016/j.actatropica.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sripa B. Pathobiology of opisthorchiasis: an update. Acta Trop. 2003;88(3):209–220. doi: 10.1016/j.actatropica.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 14.Richter J, Azoulay D, Dong Y, Holtfreter MC, Akpata R, Calderaro J, El-Scheich T, Breuer M, Neumayr A, Hatz C, et al. Ultrasonography of gallbladder abnormalities due to schistosomiasis. Parasitol Res. 2016;115(8):2917–2924. doi: 10.1007/s00436-016-5116-0. [DOI] [PubMed] [Google Scholar]
- 15.Keang H, Odermatt P, Odermatt-Biays S, Cheam S, Degremont A, Hatz C. Liver morbidity due to Schistosoma Mekongi in Cambodia after seven rounds of mass drug administration. Trans R Soc Trop Med Hyg. 2007;101(8):759–765. doi: 10.1016/j.trstmh.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 16.Dumurgier C, Tay KH, Surith TN, Rathat C, Buisson Y, Monchy D, Sinuon M, Socheat D, Urbani C, Chaem S, et al. Place of surgery in the prevention of recurrences of digestive haemorrhages at the patients presenting a portal hypertension due to Schistosoma Mekongi. Bulletin de la Societe de pathologie exotique (1990) 2006;99(5):365–371. [PubMed] [Google Scholar]
- 17.Monchy D, Dumurgier C, Heng TK, Hong K, Khun H, Hou SV, Sok KE, Huerre MR. Histology of liver lesions due to Schistosoma Mekongi. About six cases with severe portal hypertension operated in Cambodia. Bulletin de la Societe de pathologie exotique (1990) 2006;99(5):359–364. [PubMed] [Google Scholar]
- 18.Vonghachack Y, Sayasone S, Bouakhasith D, Taisayavong K, Akkavong K, Odermatt P. Epidemiology of Strongyloides stercoralis on Mekong islands in southern Laos. Acta Trop. 2015;141PB:289–294. doi: 10.1016/j.actatropica.2014.09.016. [DOI] [PubMed] [Google Scholar]
- 19.Matangila JR, Doua JY, Linsuke S, Madinga J. Inocencio da Luz R, van Geertruyden JP, Lutumba P: malaria, schistosomiasis and soil transmitted helminth burden and their correlation with anemia in children attending primary schools in Kinshasa. Democratic Republic of Congo PLoS One. 2014;9(11):e110789. doi: 10.1371/journal.pone.0110789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soares Magalhaes RJ, Langa A, Pedro JM, Sousa-Figueiredo JC, Clements AC, Vaz Nery S. Role of malnutrition and parasite infections in the spatial variation in children's anaemia risk in northern Angola. Geospat Health. 2013;7(2):341–354. doi: 10.4081/gh.2013.91. [DOI] [PubMed] [Google Scholar]
- 21.Kaewkes S. Taxonomy and biology of liver flukes. Acta Trop. 2003;88(3):177–186. doi: 10.1016/j.actatropica.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 22.Strandgaard H, Johansen MV, Pholsena K, Teixayavong K, Christensen NO. The pig as a host for Schistosoma mekongi in Laos. J Parasitol. 2001;87(3):708–709. doi: 10.1645/0022-3395(2001)087[0708:TPAAHF]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 23.Kitikoon V, Schneider CR, Sornmani S, Harinasuta C, Lanza GR. Mekong schistosomiasis. 2. Evidence of the natural transmission of Schistosoma japonicum, Mekong strain, at Khong Island, Laos. Southeast Asian J Trop Med Public Health. 1973;4(3):350–358. [PubMed] [Google Scholar]
- 24.Shimada M, Kato-Hayashi N, Chigusa Y, Nakamura S, Ohmae H, Sinuon M, Socheat D, Kitikoon V, Matsuda H. High susceptibility of Neotricula aperta gamma-strain from Krakor and Sdau in Cambodia to Schistosoma Mekongi from Khong Island in Laos. Parasitol Int. 2007;56(2):157–160. doi: 10.1016/j.parint.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 25.WHO Control of foodborne trematode infections. Report of a WHO study group. World Health Organ Tech Rep Ser. 1995;849:1–157. [PubMed] [Google Scholar]
- 26.WHO . Report of the WHO expert consultation on Foodborne Trematode infections and Taeniasis/Cysticercosis. 2011. [Google Scholar]
- 27.Phommasack B, Saklokham K, Chanthavisouk C, Nakhonesid-Fish V, Strandgaard H, Montresor A, Shuey DA, Ehrenberg J. Coverage and costs of a school deworming programme in 2007 targeting all primary schools in Lao PDR. Trans R Soc Trop Med Hyg. 2008;102(12):1201–1206. doi: 10.1016/j.trstmh.2008.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Phongluxa K, van Eeuwijk P, Soukhathammavong PA, Akkhavong K, Odermatt P. Perceived illness drives participation in mass deworming campaigns in Laos. Acta Trop. 2015;141(Pt B):281–288. doi: 10.1016/j.actatropica.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 29.Aye Soukhathammavong P, Rajpho V, Phongluxa K, Vonghachack Y, Hattendorf J, Hongvanthong B, Rasaphon O, Sripa B, Akkhavong K, Hatz C, et al. Subtle to severe hepatobiliary morbidity in Opisthorchis Viverrini endemic settings in southern Laos. Acta Trop. 2015;141(Pt B):303–309. doi: 10.1016/j.actatropica.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 30.Forrer A, Sayasone S, Vounatsou P, Vonghachack Y, Bouakhasith D, Vogt S, Glaser R, Utzinger J, Akkhavong K, Odermatt P. Spatial distribution of, and risk factors for, Opisthorchis Viverrini infection in southern Lao PDR. PLoS Negl Trop Dis. 2012;6(2):e1481. doi: 10.1371/journal.pntd.0001481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sayasone S, Vonghajack Y, Vanmany M, Rasphone O, Tesana S, Utzinger J, Akkhavong K, Odermatt P. Diversity of human intestinal helminthiasis in Lao PDR. Trans R Soc Trop Med Hyg. 2009;103(3):247–254. doi: 10.1016/j.trstmh.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 32.Leung Z, Middleton D, Morrison K. One health and EcoHealth in Ontario: a qualitative study exploring how holistic and integrative approaches are shaping public health practice in Ontario. BMC Public Health. 2012;12:358. doi: 10.1186/1471-2458-12-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Asakura T, Mallee H, Tomokawa S, Moji K, Kobayashi J. The ecosystem approach to health is a promising strategy in international development: lessons from Japan and Laos. Glob Health. 2015;11:3. doi: 10.1186/s12992-015-0093-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Furie GL, Balbus J. Global environmental health and sustainable development: the role at Rio+20. Cien Saude Colet. 2012;17(6):1427–1432. doi: 10.1590/S1413-81232012000600007. [DOI] [PubMed] [Google Scholar]
- 35.Nguyen V, Nguyen-Viet H, Pham-Duc P, Stephen C, McEwen SA. Identifying the impediments and enablers of ecohealth for a case study on health and environmental sanitation in Ha Nam. Vietnam Infect Dis Poverty. 2014;3(1):36. doi: 10.1186/2049-9957-3-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nguyen-Viet H, Doria S, Tung DX, Mallee H, Wilcox BA, Grace D. Ecohealth research in Southeast Asia: past, present and the way forward. Infect Dis Poverty. 2015;4:5. doi: 10.1186/2049-9957-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kingsley J, Patrick R, Horwitz P, Parkes M, Jenkins A, Massy C, Henderson-Wilson C, Arabena K. Exploring ecosystems and health by shifting to a regional focus: perspectives from the Oceania EcoHealth chapter. Int J Environ Res Public Health. 2015;12(10):12706–12722. doi: 10.3390/ijerph121012706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kitikoon V, Sornmani S, Schneider CR. Studies on Tricula aperta and related taxa, the snail intermediate hosts of Schistosoma Mekongi. I. Geographical distribution and habitats. Malacol Rev. 1981;14(1):1. [Google Scholar]
- 39.Davis GM, Kitikoon V, Temcharoen P. Monograph on "Lithoglyphopsis" aperta, the snail host of Mekong River schistosomiasis. Malacologia. 1976;15(2):241–287. [PubMed] [Google Scholar]
- 40.Katz N, Chaves A, Pellegrino J. A simple device for quantitative stool thick-smear technique in Schistosomiasis mansoni. Rev Inst Med Trop Sao Paulo. 1972;14(6):397–400. [PubMed] [Google Scholar]
- 41.Enes JE, Wages AJ, Malone JB, Tesana S. Prevalence of Opisthorchis viverrini infection in the canine and feline hosts in three villages, Khon Kaen Province, northeastern Thailand. Southeast Asian J Trop Med Public Health. 2010;41(1):36–42. [PMC free article] [PubMed] [Google Scholar]
- 42.Ebrahim A, El-Morshedy H, Omer E, El-Daly S, Barakat R. Evaluation of the Kato-Katz thick smear and formol ether sedimentation techniques for quantitative diagnosis of Schistosoma mansoni infection. Am J Trop Med Hyg. 1997;57(6):706–708. doi: 10.4269/ajtmh.1997.57.706. [DOI] [PubMed] [Google Scholar]
- 43.Sri-Aroon P, Butraporn P, Limsoomboon J, Kaewpoolsri M, Chusongsang Y, Charoenjai P, Chusongsang P, Numnuan S, Kiatsiri S. Freshwater mollusks at designated areas in eleven provinces of Thailand according to the water resource development projects. Southeast Asian J Trop Med Public Health. 2007;38(2):294–301. [PubMed] [Google Scholar]
- 44.Search FishBase: http://www.fishbase.org/search.php.
- 45.http://fish.mongabay.com/data/Laos.htm.
- 46.Steinmann P, Zhou XN, Li YL, Li HJ, Chen SR, Yang Z, Fan W, Jia TW, Li LH, Vounatsou P, et al. Helminth infections and risk factor analysis among residents in Eryuan county, Yunnan province. China Acta Trop. 2007;104(1):38–51. doi: 10.1016/j.actatropica.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 47.Raso G, Utzinger J, Silue KD, Ouattara M, Yapi A, Toty A, Matthys B, Vounatsou P, Tanner M, N'Goran EK. Disparities in parasitic infections, perceived ill health and access to health care among poorer and less poor schoolchildren of rural cote d'Ivoire. Tropical Med Int Health. 2005;10(1):42–57. doi: 10.1111/j.1365-3156.2004.01352.x. [DOI] [PubMed] [Google Scholar]
- 48.Maleewong W, Intapan P, Wongwajana S, Sitthithaworn P, Pipitgool V, Wongkham C, Daenseegaew W. Prevalence and intensity of Opisthorchis Viverrini in rural community near the Mekong River on the Thai-Laos border in northeast Thailand. J Med Assoc Thail. 1992;75(4):231–235. [PubMed] [Google Scholar]
- 49.Sayasone S, Odermatt P, Phoumindr N, Vongsaravane X, Sensombath V, Phetsouvanh R, Choulamany X, Strobel M. Epidemiology of Opisthorchis viverrini in a rural district of southern Lao PDR. Trans R Soc Trop Med Hyg. 2007;101(1):40–47. doi: 10.1016/j.trstmh.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 50.Rim HJ, Sohn WM, Yong TS, Eom KS, Chai JY, Min DY, Lee SH, Hoang EH, Phommasack B, Insisengmay S: Fishborne trematode metacercariae detected in freshwater fish from Vientiane municipality and Savannakhet Province, Lao PDR. Korean J Parasitol 2008, 46(4):253-260. doi: 210.3347/kjp.2008.3346.3344.3253. Epub 2008 Dec 3320. [DOI] [PMC free article] [PubMed]
- 51.Manivong K, Komalamisra C, Waikagul J, Radomyos P: Opisthorchis viverrini Metacercariae in Cyprinoid fish from three rivers in Khammouane Province, Lao PDR. J Trop Med Parasitol 2009, 32 (1):23-29.
- 52.Rim HJ, Sohn WM, Yong TS, Eom KS, Chai JY, Min DY, Lee SH, Hoang EH, Phommasack B, Insisiengmay S. Fishborne trematode metacercariae in Luang Prabang, Khammouane, and Saravane Province, Lao PDR. Korean J Parasitol. 2013;51(1):107–114. doi: 10.3347/kjp.2013.51.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chai JY, Darwin Murrell K, Lymbery AJ. Fish-borne parasitic zoonoses: status and issues. Int J Parasitol. 2005;35(11–12):1233–1254. doi: 10.1016/j.ijpara.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 54.Kiatsopit N, Sithithaworn P, Saijuntha W, Boonmars T, Tesana S, Sithithaworn J, Petney TN, Andrews RH. Exceptionally high prevalence of infection of Bithynia Siamensis goniomphalos with Opisthorchis viverrini cercariae in different wetlands in Thailand and Lao PDR. Am J Trop Med Hyg. 2012;86(3):464–469. doi: 10.4269/ajtmh.2012.11-0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kiatsopit N, Sithithaworn P, Kopolrat K, Andrews RH, Petney TN. Seasonal cercarial emergence patterns of Opisthorchis viverrini infecting Bithynia Siamensis goniomphalos from Vientiane Province. Lao PDR Parasit Vectors. 2014;7(1):551. doi: 10.1186/s13071-014-0551-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aunpromma S, Tangkawattana P, Papirom P, Kanjampa P, Tesana S, Sripa B, Tangkawattana S. High prevalence of Opisthorchis viverrini infection in reservoir hosts in four districts of Khon Kaen Province, an opisthorchiasis endemic area of Thailand. Parasitol Int. 2012;61(1):60–64. doi: 10.1016/j.parint.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 57.Kitikoon V, Schneider CR, Bhaibulaya M, Sittilerd S, Thirachantra S. Mekong schistosomiasis. 4. A parasitological survey of wild rodents, domestic pigs and cattle on Khong Island, Laos. Southeast Asian J Trop Med Public Health. 1975;6(2):223–229. [PubMed] [Google Scholar]
- 58.Phongluxa K, Xayaseng V, Vonghachack Y, Akkhavong K, van Eeuwijk P, Odermatt P. Helminth infection in southern Laos: high prevalence and low awareness. Parasit Vectors. 2013;6(1):328. doi: 10.1186/1756-3305-6-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lovis L, Mak TK, Phongluxa K, Aye Soukhathammavong P, Vonghachack Y, Keiser J, Vounatsou P, Tanner M, Hatz C, Utzinger J, et al. Efficacy of praziquantel against Schistosoma Mekongi and Opisthorchis Viverrini: a randomized, single-blinded dose-comparison trial. PLoS Negl Trop Dis. 2012;6(7):e1726. doi: 10.1371/journal.pntd.0001726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.MoH . L: infectious and parasitic diseases. 2. 2004. pp. 109–181. [Google Scholar]
- 61.Satoh M, Kokaze A. Treatment strategies in controlling strongyloidiasis. Expert Opin Pharmacother. 2004;5(11):2293–2301. doi: 10.1517/14656566.5.11.2293. [DOI] [PubMed] [Google Scholar]
- 62.Bisoffi Z, Buonfrate D, Angheben A, Boscolo M, Anselmi M, Marocco S, Monteiro G, Gobbo M, Bisoffi G, Gobbi F. Randomized clinical trial on Ivermectin versus Thiabendazole for the treatment of Strongyloidiasis. PLoS Negl Trop Dis. 2011;5(7):e1e254. doi: 10.1371/journal.pntd.0001254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Suputtamongkol Y, Premasathian N, Bhumimuang K, Waywa D, Nilganuwong S, Karuphong E, Anekthananon T, Wanachiwanawin D, Silpasakorn S. Efficacy and safety of single and double doses of ivermectin versus 7-day high dose albendazole for chronic strongyloidiasis. PLoS Negl Trop Dis. 2011;5(5):e1044. doi: 10.1371/journal.pntd.0001044. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All datasets analysed during the current study are available from the corresponding author upon reasonable request.


