Abstract
Alpha7 nicotinic acetylcholine receptors (α7 nAChRs) are important drug targets in neurological disorders and inflammation, making their detection and localization by validated antibodies highly desirable. However, tests in knockout animals raised questions about specificity of antibodies to mouse α7 nAChRs. To date, methods for validating antibodies for rat or human α7 nAChR have not been reported. We developed a gel-shift assay for western blots using GH4C1 cells expressing either native rat receptors or α7 nAChR-green fluorescent protein (GFP) chimeras to evaluate seven commercially available α7 nAChR antibodies. Blots with anti-GFP antibody detected GFP or α7 nAChR-GFP expressed in GH4C1 cells, and 125I-α-bungarotoxin binding and RNA analysis demonstrated α7 nAChR expression. Validated samples were used to evaluate α7 nAChR antibodies by western blot and immunofluorescence studies. These methods confirmed that two of seven α7 nAChR antibodies identify gel-shifts for α7 nAChR/nAChR-GFP but only one antibody demonstrated low background and significant immunofluorescence differences between wild-type and α7 nAChR expressing GH4C1 cells. However, that polyclonal antibody displayed lot-to-lot variability. Our findings suggest that careful validation methods are required for all α7 nAChR receptor species and antibody lots and that the gel-shift assay may allow for relatively rapid antibody screening.
Keywords: antibody validation, Chrna7, MAb 306, MAb 319, monoclonal, NBP1-79948, protein detection
Introduction
Pentameric alpha7 nicotinic receptors (α7 nAChRs) are emerging as a possible therapeutic drug target in a variety of disorders.1 These receptors are implicated in treatments for Alzheimer’s,2 Parkinson’s,3 schizophrenia,4 bipolar disorder,5 cystic fibrosis,6 and chronic inflammation.7 Besides being located on neurons in both the central nervous system and in autonomic ganglia, α7 nAChRs are reportedly present in a variety of other cell types, including macrophages, microglia, and lymphocytes.7–9 Therefore, methods for localizing these receptors in cells and tissues are both useful and necessary.
The snake neurotoxin, α-bungarotoxin (αBGT), not only binds α7 nAChRs with high affinity and blocks acetylcholine (ACh) binding but also recognizes α9 and α10 nAChRs, and some subtypes of class A gamma-aminobutyric acid (GABAa) receptors.10,11 αBGT has been used to detect and quantify α7 nAChR densities (e.g., Loring et al.12) but is not necessarily specific for α7 nAChRs. α7 nAChR selective antibodies would be a powerful tool to localize α7 nAChRs, and many antibodies are commercially available that claim α7 nAChR specificity. However, a variety of reports indicate that most, if not all, antibodies do not specifically detect α7 nAChRs. In these studies,13–17 western blots and immunohistochemical staining by these commercial antibodies do not show significant differences between wild-type (WT) and α7 nAChR knockout mouse tissue samples. Although mouse knockouts are useful for validating antibodies against mouse receptors, equivalent tests for rat or human receptors are not currently available, and additional methods are needed to test whether antibodies cross-react with other species.
Antibody validation is a general problem in many fields of biological research.18 To overcome the problems of poor reproducibility using antibody reagents, Uhlen et al.19 suggest that all publications include at least one type of antibody validation out of five possible “pillars”: (1) genetic (knockdown the target protein expression), (2) orthogonal testing (expression of target protein detected by antibody compared with an alternate antibody-independent method), (3) independent antibody verification (target protein is detected by two antibodies with non-overlapping epitopes), (4) tagged protein expression (the target protein is expressed with a tag that can be detected separately), and (5) immunocapture followed by mass spectrometry. These authors emphasize that the “five pillars” should be used in a manner that is specific to the application at hand.
In this work, we investigated two tests for validating antibodies against rat α7 nAChRs in western blotting and fluorescent immunocytochemistry applications. For immunofluorescence, we compared antibody staining between native rat GH4C1 cells and cells transfected with rat α7 DNA. This is genetic validation with a twist (Pillar 1), as instead of knocking down expression of the target protein, the procedure allows overexpression of transfected gene products in cells that do not normally express the antibody target. Furthermore, this includes an orthogonal test (Pillar 2), as surface receptor expression in GH4C1 cells can be measured independently with 125I-αBGT binding.20,21 For western blots, we used tagged protein expression (Pillar 4): We compared expression of WT α7 nAChR in GH4C1 cells with a chimeric receptor that includes green fluorescent protein (GFP) attached at the C-terminal.22 Glycosylated subunits for the major rat α7 nAChR splice variant have 502 amino acids and are nominally 56.5 kDa without carbohydrates. However, attached carbohydrates alter the electrophoretic mobility of proteins,23 causing difficulty in predicting apparent sizes on western blots. The added GFP in the chimera increases the molecular weight by 25 kDa, and we used a gel-shift assay in western blots to screen for antibodies that detect appropriate size differences between native and chimeric receptors. We then used both these methods to screen seven commercially available antibodies for α7 nAChRs and checked their ability to locate rat α7 nAChR by immunofluorescence in transfected GH4C1 cells. In principal, the same methods can be adapted for human nAChRs or receptors of any other species.
Materials and Methods
Plasmids and RNA
Total GH4C1 RNA was extracted with a TRIzol Plus RNA purification kit (Invitrogen; Carlsbad, CA). Purified RNA was quantified with a NanoDrop ND-1000 UV-Vis spectrophotometer. Complementary DNA (cDNA) synthesis from 1 µg of total RNA for each reaction was carried out using AffinityScript QPCR cDNA synthesis kit (Agilent Technologies; Santa Clara CA). α7 nAChR primers (5′: ACATGTCTGAGTACCCCGGA, and 3′: AGGACCACCCTCCATAGGAC) were designed using Pubmed primer BLAST (NCBI; Bethesda, MD) and obtained from Integrated DNA Technologies (Coralville, IA). The primers were designed to amplify both mouse and rat α7 nAChR cDNA using 32 cycles of 57.5C annealing (30 sec) and 68C extension steps (1 min) to get an expected 264-bp amplicon. Approximately 100 ng of cDNA (equivalent to one tenth of the starting RNA amount) was used to perform polymerase chain reaction (PCR) analysis using Platinum Taq polymerase (Invitrogen). PCR products were analyzed by gel electrophoresis on a 1.5% agarose gel using a 100-bp DNA ladder (New England Biolabs; Ipswich, MA). Purified PCR products were sequenced by Genewiz (Cambridge, MA). The full-length rat α7 nAChR and α7 nAChR-GFP sequences cloned into Invitrogen pRep4 plasmids have been previously described.22 The GFP plasmid (eGFPN1) was obtained from Clontech (Mountain View, CA).
Reagents and Antibodies
αBGT was purchased from Biotoxins, Inc. (St. Cloud, FL). αBGT was radioiodinated with iodogen (Pierce Chemical; Rockford, IL) as previously described24 and typically had specific activities of 250 to 400 Ci/mMole. Alexa Fluor 488-labeled αBGT (catalog B13422) and rabbit anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH; catalog PA1988) were obtained from Thermo Fisher Scientific (Waltham, MA). The details of the seven different antibodies to α7 nAChR are listed in Table 1; these include ab23832 rabbit polyclonal antibody from Abcam (Cambridge, MA); M220 (MAb 306) mouse monoclonal antibody from Sigma-Aldrich (St. Louis, MO); sc-58607 (MAb 319) rat monoclonal and sc-5544 (H-302) rabbit polyclonal antibodies from Santa Cruz Biotechnology (Santa Cruz, CA); MABN529, mouse monoclonal and AB15332 rabbit polyclonal antibodies from EMD Millipore (Billerica, MA); and NBP1-79948 (lot QC0391-42133 and QC42753-42341) rabbit polyclonal from Novus Biologicals (Littleton, CO). The first four antibodies (ab23832, M220, sc-58607, and sc-5544) have been used in the literature to identify rat α7 nAChRs (see CiteAb.com), whereas the last three antibodies (MABN529, AB15332, and NBP1-79948) have never been cited, but differ in species reactivity according to the suppliers. NBP1-79948 has a reported reactivity to human, mouse, and rat α7 nAChRs, whereas MABN592 has reported reactivity to human and mouse, and AB15332 has reported activity against only mouse α7 nAChRs. However, the major human α7 nAChR splice variant differs from the major rat splice variant by 30 amino acids out of 502, whereas the mouse α7 nAChR differs from rat by only 2. Therefore theoretically, most antibodies with reactivity to mouse α7 nAChRs will very likely cross-react with rat α7 nAChRs. Anti-GFP was from Abcam, ab290 (lot GR158277-1). Secondary horseradish peroxidase (HRP)–conjugated anti-rabbit (catalog 7074S) and anti-mouse (catalog 7076S) antibodies were purchased from Cell Signaling Technology (Danvers, MA) whereas HRP-conjugated anti-rat antibody (catalog sc-2006) was from Santa Cruz Biotechnology. Alexa Fluor 488-conjugated secondary rabbit antibody (catalog 4412S) was from Cell Signaling Technology; Alexa Fluor 488-conjugated ab150117 anti-mouse and ab150157 anti-rat secondary antibodies were purchased from Abcam. HRP- and Alexa Fluor-conjugated secondary antibodies were used at 1:1000 dilutions for immunodetection procedures. Primary antibodies were normally used at the dilutions indicated by the suppliers, with additional dilutions as deemed necessary.
Table 1.
Summary of α7 nAChR (α7) Antibodies Used.
| Company (Alphabetical Order) | Catalog No. | Lot No. | Reported Species Reactivity | Reported Epitope Target on α7 nAChR Sequence | Western Blot/Immunofluorescence Data | Monoclonal or Polyclonal | Secondary Antibody Target | # Citations (CiteAb) | # Rat Citations (CiteAb) |
|---|---|---|---|---|---|---|---|---|---|
| Abcam | ab23832a | GR89867-1 | h, r, m | 1–100 α7 Amino acid sequence | −/− | Polyclonal | Rabbit | 17 | 4 |
| EMD Millipore | AB15332 | 2630253 | m | 300–400 α7 Amino acid sequence | +/+b | Polyclonal | Rabbit | 0 | 0 |
| EMD Millipore | MABN529 | Q2459834 | h, m | Cytoplasmic domain | −/− | Monoclonal | Mouse | 0 | 0 |
| Novus Biologicals | NBP1-79948 | QC0391-42133 | h, r, m | N-terminal domain | +/++ | Polyclonal | Rabbit | 0 | 0 |
| Novus Biologicals | NBP1-79948 | QC42753-42341 | h, r, m | N-terminal domain | −/NA | Polyclonal | Rabbit | 0 | 0 |
| Santa Cruz Biotech. | sc-5544a (H-302) | D2214 | h, r, m | 367–502 α7 Amino acid sequence | −/− | Polyclonal | Rabbit | 31 | 6 |
| Santa Cruz Biotech. | sc-58607 (MAb 319)a | L0312 | h, r, m, c | Cytoplasmic domain | −/− | Monoclonal | Rat | 5 Sigma, 3 Santa Cruz | 1 Santa Cruz |
| Sigma-Aldrich | M220 (MAb 306)a | 040K4705 | h, r, c | 380–400 α7 Amino acid sequence | +/+ | Monoclonal | MouseTotal citations | 1066 | 415 |
Reported species reactivity and epitope locations are taken from the manufacturer’s literature. Key to western blot/immunofluorescence data: +/+ indicates positive western blot data showing antibody recognition of both α7 and α7-GFP chimeras and a significant difference in immunofluorescence between transfected and untransfected cells but with significant background; +/++ indicates positive western blot data showing antibody recognition of both α7 and α7-GFP chimeras and a significant difference in immunofluorescence between transfected and untransfected cells but with low background; +/– indicates positive western blot result but negative immunofluorescence staining; –/– indicates negative immunodetection using both applications; –/NA indicates negative western blot result and unavailability of immunofluorescence data. Abbreviations: h, human; r, rat; m, mouse; c, chicken; nAChR, nicotinic acetylcholine receptor; GFP, green fluorescent protein.
Previously evaluated in the literature using mouse knockouts.
Detected a band appropriate for rat α7nAChR on blots, but did not detect rat α7nAChR-GFP.
Cell Culture
GH4C1-rat pituitary cells were obtained from American Type Culture Collection (Manassas, VA) and grown at 37C in 5% CO2 in F-10 complete growth medium: Ham’s F-10 basic medium containing 1% penicillin-streptomycin (both from Thermo Fisher Scientific) supplemented with 10% fetal bovine serum (Premium Select from Atlanta Biologicals, Lawrenceville, GA).
Transfection
Cells were plated at 500,000 cells/well in a BD falcon six-well plate in F-10 complete growth medium 24 hr before transfection with lipofectamine LTX (Invitrogen). Cells were serum starved (1 ml F-10 medium only) for 1 hr before adding transfection reagents consisting of 3 µg plasmid DNA, 9 µl lipofectamine LTX, and 3 µl Plus reagent per well in 300 µl reduced serum Opti-MEM (Invitrogen). Four hr post-transfection, 1 ml complete growth medium was added to make the final volume of 2 ml/well. Next day, the supernatant medium was replaced by complete growth medium to maintain good cell viability. The transfection efficiency was monitored under the fluorescent microscope 48 to 72 hr post-transfection. Three days later, cells transfected with receptor were selected with hygromycin (100 μg/ml; Invivogen, San Diego, CA). Transfected cells were cultured for immunodetection experiments. Transfections using eGFPN1 were performed as a positive control for each experiment.
Western Blot
Cells were grown in a six-well BD Falcon plate and washed with ice-cold phosphate buffered saline (PBS) on the experimental day. After washing, cells were immediately scraped off the surface and centrifuged at 12,000 × g at 4C for 10 min. Supernatant was discarded and cells were resuspended in a radioimmunoprecipitation assay lysis buffer [150 mM NaCl, 20 mM Tris, 1% NP-40 (Tergitol-type NP-40 [CAS 9016-45-9]; Sigma-Aldrich, St. Louis, MO), 0.1% sodium dodecyl sulfate (SDS), 1 mM EDTA, pH 8.0] containing the Halt protease inhibitor cocktail (Thermo Fisher Scientific). Cell suspensions were sonicated for 10 to 15 sec to improve cell lysis, and then sat on ice for 30 to 40 min and during this time vortexed every 5 to 10 min, followed by centrifuging at 9600 × g at 4C for 10 min. Supernatants were collected in fresh 1.5-ml centrifuge tubes and total proteins were quantified using the bicinchoninic acid (BCA) assay (Thermo Fisher Scientific). Cell lysates were reduced using the Bolt 1× lithium dodecyl sulfate (LDS) loading buffer containing 0.1 M dithiothreitol (Thermo Fisher Scientific), followed by heating the samples at 70C for 10 min. Samples were kept on ice for 5 min before 40 μg total sample protein was loaded in individual wells on a Bolt 4–12% Bis-Tris protein gel for protein separation (45 min run at 150 volts). Manufacturer’s recommended running and transfer buffers were used with the iBlot 2 dry blotting system to transfer the protein onto nitrocellulose. Blocking was done with 5% bovine serum albumin (BSA) in Tris-buffered saline with 0.1% Tween-20 (TBS-T); the membrane was rocked overnight at 4C in a primary antibody solution (in 5% BSA at recommended dilution). On the following day, the blot was washed with TBS-T three to five times and incubated with secondary antibody conjugated to HRP at a 1:1000 dilution for 1 hr. Protein–antibody complexes were visualized using the SuperSignal West Pico Chemiluminescent substrate (Thermo Fisher Scientific) using the ChemiDoc XRS Imager from Bio-Rad (Hercules, CA). GAPDH immunostaining was performed on each blot to confirm equivalent protein loading across the wells, and molecular weights were confirmed using SuperSignal molecular weight ladder from Thermo Fisher Scientific.
Immunofluorescence
Cells were plated on 10 µg/cm2 poly-l-lysine pre-coated sterile coverslips that were kept in 24-multiwell plates. Next day, cells were washed with 1 ml PBS/well, fixed for 15 min in 4% formaldehyde (made in PBS), and permeabilized in 0.4% Triton X-100. Cells were washed with ice-cold PBS three to five times before incubating the cells with 2% BSA for 1 hr at room temperature to minimize nonspecific binding. Cells were incubated in α7 nAChR primary antibody (1:100 to 1:1000 in the 2% BSA blocking buffer) overnight at 4C. The following day, samples were washed and exposed to Alexa Fluor 488 fluorochrome conjugated secondary antibody (1:1000) in the dark for 1 hr. Samples were washed three times with 1 ml ice-cold PBS. Coverslips were mounted on glass slides using mounting medium (Sigma-Aldrich Fluoroshield, catalog F6057) and left overnight at 4C in the dark. Next day, the samples were analyzed under the fluorescent microscope. Alexa Fluor 488-αBGT staining was performed in the same manner as α7 nAChR antibody treatment.
[125I]-Labeled αBGT Binding Assay
Radioactive binding assay was performed as described by Koperniak et al.25 to detect surface α7 nAChR. Cells were plated at 200,000 density per well in a 24-well plate on Day 1. 125I-αBGT binding was determined in quadruplicate when cells were 80% confluent. Cells were washed three times with sodium bicarbonate and 0.1% BSA containing Hank’s balanced salt solution (HBSS). Cells were incubated with 10 nM 125I-αBGT for 3 hr at 4C to measure total surface binding. Nonspecific binding was determined by the addition of 1 µM αBGT. After washing the cells three times in HBSS + BSA, cells were lysed for 10 to 15 min on ice by the addition of 100 μl extraction buffer (0.5 M NaOH + 1% Triton X-100). Lysates were transferred into polypropylene tubes and counted for 1 min with a Packard gamma counter.
Results
Rat α7 nAChR Expression in GH4C1 Cells
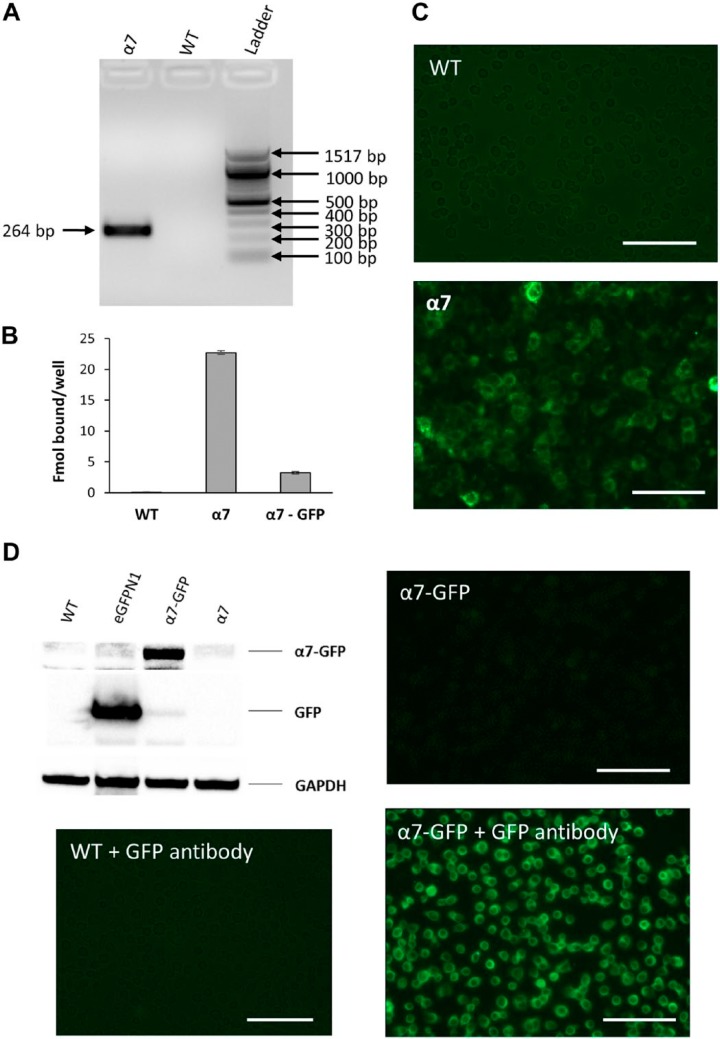
PCR results confirmed the presence of α7 nAChR RNA signal in GH4C1 cells transfected with rat α7 nAChR gene whereas no signal was observed in GH4C1-WT cells (Fig. 1A). 125I-αBGT binding experiments revealed surface rat α7 nAChR expression on transfected GH4C1 cells. As reported by Lee et al.,22 rat α7 nAChR-transfected GH4C1 cells showed approximately five times more αBGT binding as obtained with rat α7 nAChR-GFP-transfected GH4C1 cells (Fig. 1B). Similarly, Alexa Fluor 488-αBGT staining showed substantial labeling in GH4C1-rat α7 nAChR cells whereas GH4C1-WT cells showed no labeling under similar conditions (Fig. 1C). Moreover, 5 mM nicotine blocked Alexa Fluor 488 staining in α7 nAChR-transfected GH4C1 cells, confirming the presence of rat α7 nAChR on the surface of GH4C1 cells (data not shown). As observed by Lee et al.,22 fluorescence imaging of GH4C1-rat α7 nAChR-GFP cells demonstrated significantly lower GFP fluorescence (Fig. 1D), as compared with eGFPN1-transfected GH4C1 cells.
Figure 1.
Establishing rat α7 nAChR expression in GH4C1 cells. Expression of α7 nAChR RNA and protein levels in GH4C1 cells. (A) PCR analysis showing Chrna7 RNA expression in GH4C1-WT and α7 nAChR-transfected (α7) cells. α7 nAChR cDNA replicon band observed in the α7 cells at 264 bp, as expected, whereas no Chrna7 signal was observed in GH4C1-WT cells. (B) 125I-αBGT binding data comparing GH4C1-WT cells transfected with α7 or α7-GFP chimeras. α7- and α7-GFP-transfected GH4C1 cells showed significant binding whereas no binding was observed in untransfected cells. Bars in the figure represent the mean of specific binding and the error bars are the square root of the sum of the standard deviations squared for total and nonspecific binding. (C) Alexa Fluor 488-labeled αBGT binding showing fluorescence in α7-transfected GH4C1 cells compared with WT counterparts. (D) Western blot and immunofluorescence data to validate and compare WT with α7- and α7-GFP-transfected GH4C1 cells using a 1:2500 dilution of ab290 GFP antibody. A 25-kDa GFP band was observed only in the eGFPN1-transfected cells lysates (3-sec exposure). Similarly, an 80-kDa α7-GFP band was observed only in the α7-GFP transfected GH4C1 cell lysates (88-sec exposure). Forty μg of total protein was loaded in each lane and GAPDH was used as the loading control. WT GH4C1 cells do not show significant immunostaining with ab290 GFP antibody and Alexa Fluor conjugated rabbit secondary antibody. Immunofluorescence data showing only mild fluorescence in the α7-GFP-transfected cells that was increased significantly with GFP antibody staining. Scale bar, 40 μm. Abbreviations: nAChR, nicotinic acetylcholine receptors; WT, wild-type; GFP, green fluorescent protein.
Rat α7 nAChR-GFP Chimera
As Lee et al.22 reported, GFP tagged on the C-terminus of α7 nAChR decreased surface expression leading to lower αBGT binding compared with native receptor. 125I-αBGT binding confirmed the presence of α7 nAChR on transfected GH4C1 cells, thereby orthogonally validating the presence of both native rat α7 nAChR and the GFP chimera. No binding was observed in GH4C1-WT cells. Furthermore, a western blot with Abcam ab290 GFP antibody showed a GFP protein band shift in SDS–polyacrylamide gel electrophoresis from 25 to 80 kDa confirming the presence of GFP in GFP and α7 nAChR-GFP-transfected GH4C1 cells (Fig. 1D). GFP antibody data also validated the cell lysates for their use in assessing the effectiveness of α7 nAChR antibodies. Full-length GFP has a molecular weight of approximately 25 kDa that was detected by Abcam ab290 GFP antibody in GFP-transfected GH4C1 cell lysates. As GFP was tagged with α7 nAChR (molecular weight 55 kDa), the α7 nAChR–GFP chimera band appears around 80 kDa. GFP antibody immunofluorescence detected no significant staining in WT cells, though GH4C1-rat α7 nAChR-GFP cells showed intense staining (Fig. 1D). However, it was difficult to interpret GFP antibody fluorescence data for GH4C1 cells transfected with eGFPN1, as GFP-transfected cells are naturally fluorescent.
α7 nAChR Antibodies Comparing WT Versus α7 nAChR-Transfected GH4C1 Cells
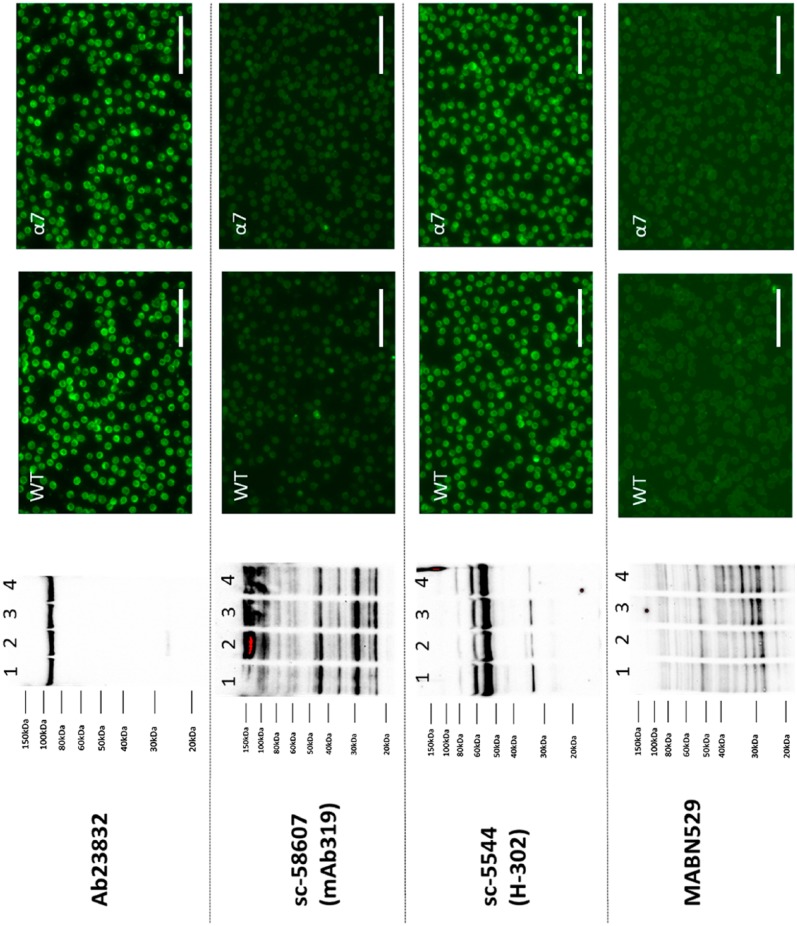
After validating the gel-shift assay for α7 nAChR and α7 nAChR-GFP cell lysates, we then tested seven commercially available α7 nAChR antibodies (Table 1) for their suitability to detect rat α7 nAChR. As stated on their respective company websites, most of the α7 nAChR antibodies are supposed to detect three species: rat, mouse, and human receptors. We included EMD Millipore AB15332 and MABN529, even though the company lists them as suitable for mouse receptors as they had not been tested for rat receptors, which are highly similar. Standard western blot and immunocytochemistry protocols were performed on these validated lysates and cells to compare the staining pattern between WT and rat α7 nAChR-transfected GH4C1 cells. Western blot results from Abcam rabbit polyclonal ab23832, rabbit polyclonal sc-5544, rat monoclonal sc-58607 (MAb 319), and EMD Millipore MABN529 α7 nAChR antibodies were inconclusive as the staining pattern did not show a significant difference between GH4C1-WT and GH4C1-rat α7 nAChR cells (Fig. 2). In fact, ab23832 antibody could not detect a 55-kDa band, representing full-length α7 nAChR protein. Ab23832 detected a single nonspecific band just below 100 kDa that was present in both untransfected and transfected cell lysates with similar intensity (Fig. 2). The remaining three antibodies could all detect bands in the 55-kDa region on the blot irrespective of the rat α7 nAChR transfection. Both MAb 319 and MABN529 showed several nonspecific bands, making the interpretation of the data very difficult. All four above-mentioned antibodies demonstrated a similar staining pattern between GH4C1-WT and GH4C1-rat α7 nAChR cells by immunofluorescence. In our experimental conditions, none of these antibodies was able to detect rat α7 nAChR protein to any substantial amount when comparing GH4C1-WT and GH4C1-rat α7 nAChR cell lysates.
Figure 2.
Antibodies that did not recognize rat α7 nAChR expression in GH4C1 cells. Representative images of the western blot and immunofluorescence results for four different α7 nAChR (α7) antibodies are shown. Left panel: Western blot data showed no ostensible difference between WT and α7 or α7-GFP transfected cells (Lane 1: GH4C1-WT, Lane 2: GH4C1 eGFPN1, Lane 3: GH4C1 α7-GFP, Lane 4: GH4C1 α7). Right panel: Immunofluorescence results demonstrate identical staining pattern between untransfected and α7 transfected GH4C1 cells. Strong nonspecific binding can be seen with ab23832 and sc-5544 α7 nAChR antibodies. Abcam ab23832 (lot GR89867-1) antibody used at a 1:1000 dilution for both immunoblotting and immunofluorescence showing no significant difference between WT and α7-transfected cells. 1:1000 dilution of sc-58607 (lot L0312) does not detect any significant bands in GH4C1 cells but a 1:100 dilution of the same antibody detects multiple bands throughout the blot in different molecular weight region irrespective of α7 presence. Only mild immunofluorescence staining was seen in the GH4C1 cells with sc-58607 antibody at a 1:1000 dilution. Sc-5544 (lot D2214) showing intense immunoblot and immunofluorescence staining at a 1:1000 dilution in both WT and α7-transfected GH4C1 cells. EMD Millipore MABN529 (lot Q2459834) antibody showing no immunofluorescence staining at a 1:1000 dilution but showing nonspecific binding on the western blot. Scale bar, 40 μm. Abbreviations: nAChR, nicotinic acetylcholine receptor; WT, wild-type; GFP, green fluorescent protein.
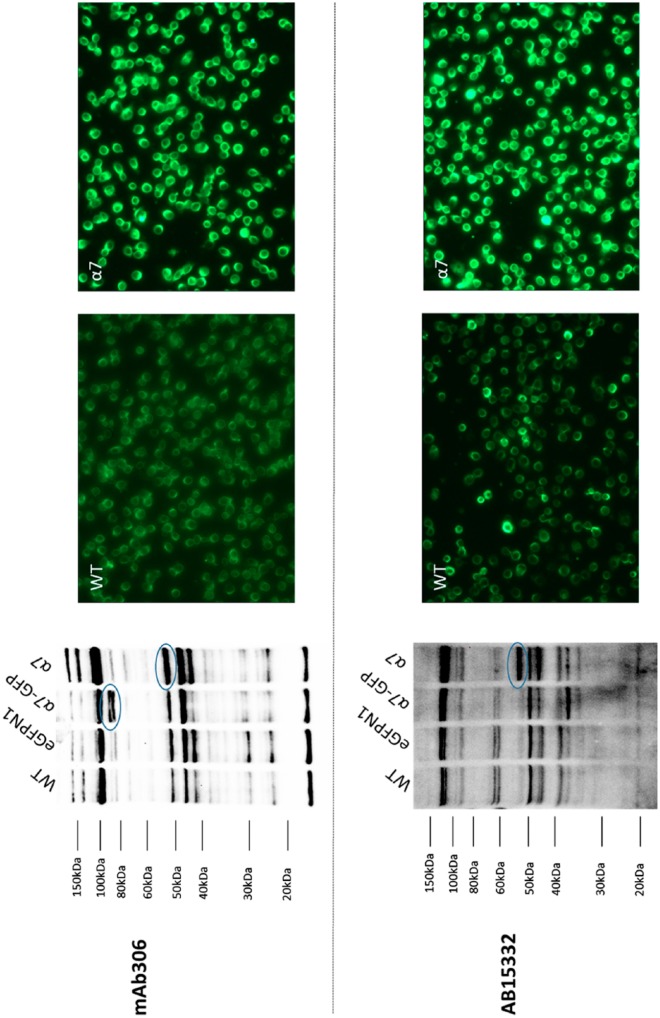
MAb 306 mouse monoclonal Sigma-Aldrich α7 nAChR antibody detected both the rat α7 nAChR band at 55 kDa in GH4C1-rat α7 nAChR cell lysates and GFP-tagged rat α7 nAChR band at 80 kDa in GH4C1-rat α7 nAChR-GFP cell lysates (Fig. 3). However, the antibody detects an unexpected band in the same molecular weight region as rat α7 nAChR, that is, 55 kDa in the GH4C1-WT cell lysates. Also, MAb 306 binds nonspecific bands starting from the 20-kDa region to the 120-kDa region on the blot. Furthermore, MAb 306 staining showed no difference in the staining intensity between GH4C1-WT and GH4C1-rat α7 nAChR cells by immunofluorescence at a 1:100 antibody dilution (data not shown). However, at a 1:750 dilution, MAb 306 antibody showed significantly more staining in GH4C1-rat α7 nAChR cells as compared with GH4C1-WT cells but with a high background. AB15332 rabbit polyclonal antibody showed α7 nAChR band at 55 kDa on the western blot at a 1:1000 dilution. However, AB15332 failed to detect the 80-kDa α7 nAChR–GFP chimera band (55-kDa α7 nAChR plus 25-kDa GFP; Fig. 3). AB15332 (diluted 1:750) showed differential staining similar to those observed with MAb 306 (diluted 1:750). Novus Biologicals NBP1-79948 rabbit polyclonal α7 nAChR antibody (lot QC0391-42133) revealed an expected 55-kDa band for GH4C1-α7 nAChR and an 80-kDa band for GH4C1-α7 nAChR-GFP (Fig. 4A), but also binds to nonspecific bands. In addition, NBP1-79948 showed some difference in immunofluorescence with low background between WT and rat a7 transfected cells at 1:1000, but not at lower dilutions such as 1:100. We tested another lot (lot QC42753-42341) of NBP1-79948 antibody and observed no significant difference on the western blot between GH4C1-WT and rat α7 nAChR-transfected GH4C1 cells indicating significant lot-to-lot variability (Fig. 4B). Unfortunately, polyclonal lot QC0391-42133 is no longer available. Thus, even though two commercially available antibodies passed the gel-shift assay and showed signs of differential fluorescent staining between cells expressing receptors and those that do not, we were unable to find blocking conditions that had satisfactorily low background staining for either western blots or immunofluorescence. We conclude that, in our hands, none of the seven commercially available antibodies can successfully be used for routine immunodetection procedures.
Figure 3.
Antibodies that may recognize rat α7 nAChR expression in GH4C1 cells. Representative images of the western blot and immunofluorescence results for MAb 306, Sigma-Aldrich M220 (lot 040K4705) mouse monoclonal α7 nAChR (α7) antibody and AB15332 (lot 2630253), EMD Millipore rabbit polyclonal α7 nAChR antibody. Left panel: Western blot data showing bands (circled) for α7 or α7-GFP with MAb 306 when used at a 1:1000 dilution. Similarly, a 1:1000 dilution of Ab15332 binds α7 but was unable to detect the α7-GFP band at approximately 80 kDa. Both antibodies also bind many nonspecific bands in both untransfected and transfected GH4C1 cells. Right panel: Immunofluorescence results demonstrate staining pattern between untransfected and α7 transfected GH4C1 cells. Mild nonspecific binding can be seen in GH4C1-WT cells with both MAb 306 and AB15332 at a 1:750 dilution. α7 nAChR-transfected GH4C1 cells showed intense staining with both MAb 306 and Ab15332. Scale bar, 40 μm. Abbreviations: nAChR, nicotinic acetylcholine receptors; GFP, green fluorescent protein; WT, wild-type.
Figure 4.
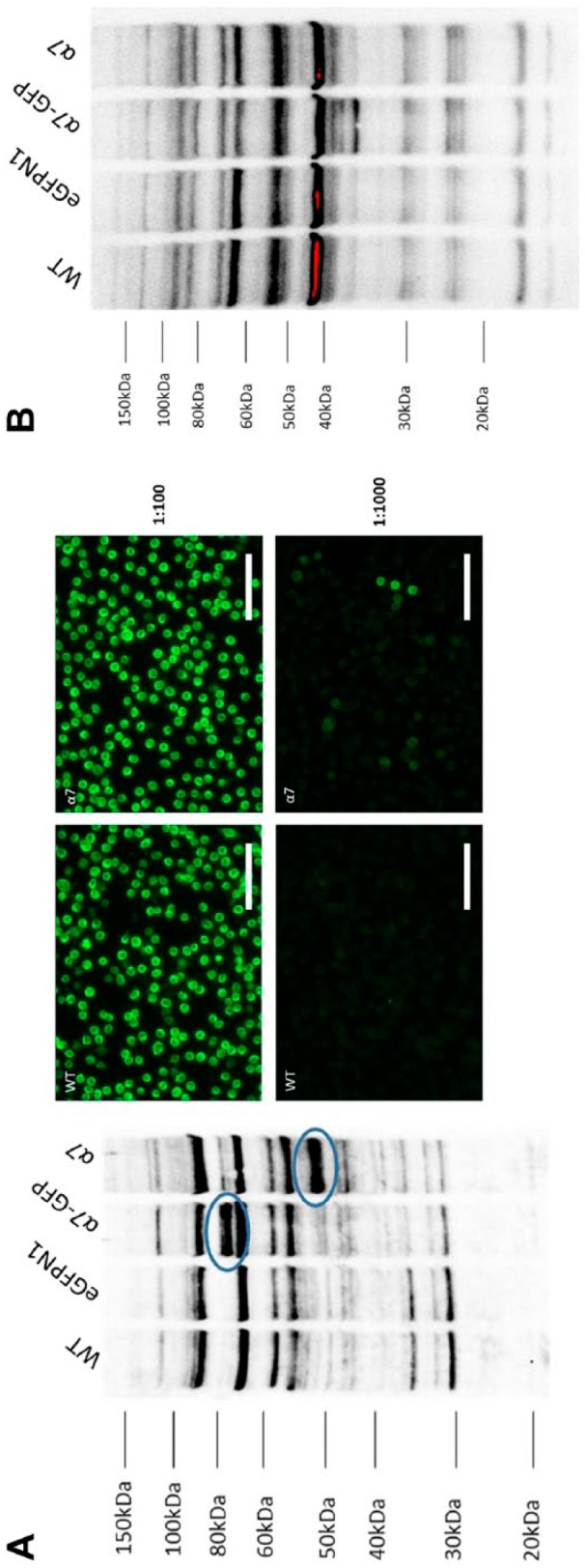
α7 nAChR detection using two different lots of NBP1-79948 antibody. (A) Representative images of the western blot and immunofluorescence results for Novus NBP1-79948, rabbit polyclonal α7 nAChR antibody (lot QC0391-42133) at a 1:1000 dilution. Western blot showing immunoreactivity toward both α7- and α7-GFP-transfected GH4C1 cells. Multiple cross-reactive nonspecific bands were found across each lane. Labeling pattern between untransfected and α7 nAChR-transfected GH4C1 cells looks similar at a 1:100 dilution. 1:1000 dilution of the same antibody from the same lot showed significant differences between WT GH4C1 cells and cells transfected with α7 nAChR. (B) Immunoblotting results obtained with lot QC42753-42341 of NBP1-79948 α7 nAChR antibody did not show significant differences between untransfected and α7 nAChR-transfected GH4C1 cell extracts. Scale bar, 40 μm. Abbreviations: nAChR, nicotinic acetylcholine receptors; GFP, green fluorescent protein; WT, wild-type.
Discussion
Antibodies are often blamed as one source of the current lack of reproducibility in biological research.18,26,27 The ideal antibody will both recognize its target antigen and avoid binding to any other antigens with little lot-to-lot variability across all detection applications. However, there are no universally accepted guidelines for how to validate whether individual antibodies meet these goals.28 Uhlen et al.19 recently proposed five possible “pillars” for validating antibodies, and suggest that all scientific publications should use at least one pillar. However, which pillars are used depends on the proposed application of the antibody. We investigated three of the possible five “pillars” for seven commercially available α7 nAChR antibodies used in the applications of western blotting and fluorescent immunocytochemistry: (1) genetic, (2) orthogonal testing, and (3) tagged protein expression. The two other “pillars” are not appropriate as Pillar 3 Independent antibody verification (the target protein is detected by two antibodies with non-overlapping epitopes) requires at least one validated antibody for the target to validate another (no validated antibody currently exists for rat α7 nAChR), and Pillar 5 Immunocapture followed by mass spectrometry is relatively specific for immunoprecipitation applications.
Transfection with rat α7 nAChR DNA (Fig. 1A) resulted in surface 125I-αBGT binding (Fig. 1B) and fluorescent toxin binding (Fig. 1C) indicating successful target protein expression in GH4C1 cells. These cells are suitable for genetic antibody validation due to the specificity of toxin binding (orthogonal testing) to show target expression. Chimeric rat α7 nAChR-GFP acts as a second genetic test with the advantage of having a tag that can both be detected by anti-GFP antibodies and should cause a shift in size for western blots. Both fluorescent αBGT binding and anti-GFP staining suggest that the density of transfected receptors is not uniform among transfected cells. However, this pattern is not observed in the cases where antibodies show apparent differential staining between transfected and non-transfected cells making it difficult to distinguish specific labeling from background (e.g., Fig. 3).
We grouped the antibodies by results. Figure 2 shows four antibodies that fail to recognize rat α7 nAChRs on western blots: Santa Cruz sc-5544 (H-302) and sc-58607 (MAb 319), EMD Millipore MABN529, and Abcam ab23832. Different dilutions of the α7 nAChR antibodies were tried unsuccessfully to specifically target α7 nAChR protein in western blots, and immunofluorescence is indistinguishable between GH4C1 cells with and without α7 nAChR expression. The result with MAb 319 is somewhat surprising as we had previously shown that MAb 319 (from a different supplier) immunoprecipitates solubilized rat α7 125I-αBGT binding sites in transfected GH4C1 cells,21 although Moser et al.16 reported finding an identical single 48-kDa band in western blots from both WT and α7 nAChR knockout mouse brain, which is consistent with our results.
Rommel et al.17 used Abcam ab23832 rabbit polyclonal α7 nAChR antibody (raised against exons 1-4, N-terminus of α7 nAChR) to detect the possible presence of α7 nAChR on mouse keratinocytes. However, the study revealed no staining differences between WT and α7 nAChR knockout mouse tissues. Blocking peptide did not prevent significant staining in the WT samples, confirming that the staining was nonspecific. Ab23832 immunoblotting gave two bands: one at approximately 45 kDa and the other around 56 kDa. Analysis on 2D gel electrophoresis, followed by matrix-assisted laser desorption/ionization-time of flight (MALDI-TOF) mass spectrometry, confirmed that the 56-kDa band that was originally thought to represent α7 nAChR in fact corresponds to β-enolase, a protein very similar to α7 nAChR in both sequence and molecular weight parameters, whereas the 45-kDa band corresponds to β-actin. These results are similar to our own findings and the results of Herber et al.13 and Moser et al.16 Since publication by Rommel et al.,17 Abcam discontinued sales of ab23832, which is to be commended.
Sigma-Aldrich MAb 306 (Fig. 3) shows the gel-shift between rat α7 nAChR and α7 nAChR–GFP, suggesting that it does recognize rat α7, but also shows the same problems with nonspecific binding reported by others. MAb 306 has been shown by western blots to bind to αBGT-affinity purified mouse α7 nAChR16 and to rat α7 nAChRs, overexpressed in cell lines.21 Furthermore, Fabian-Fine et al.29 showed that MAb 306 binds to a 56-kDa band that may represent rat α7 nAChR in the hippocampus, but also binds to a 44-kDa protein localized in mitochondria. In our western blots, MAb 306 cross-reacts with several unidentified nonspecific proteins which are also present in GH4C1-WT cells in addition to bands corresponding with native and chimeric α7 nAChR. Our data with MAb 306 showed bands close to 55 kDa in all lanes, whether transfected with α7 nAChR or not, but the labeling in transfected cells was much stronger. Because MAb 306 cross-reacts with nonspecific proteins in the untransfected GH4C1 cells, immunofluorescence was difficult to interpret, but we do see enhanced immunofluorescence in α7 nAChR-transfected GH4C1 cells compared with untransfected cells. Previously, both Herber et al.13 and Moser et al.16 found that MAb 306 nonspecifically cross-reacts with 30- and 50-kDa proteins in mouse tissues, consistent with our high-immunofluorescence backgrounds and western blotting results. This nonspecific binding of MAb 306 to shared target epitopes or structurally similar endogenous proteins could explain why these previous studies failed to observe significant immunohistochemical staining differences between tissues from α7 nAChR knockout and WT mice.
Millipore AB15332 (Fig. 3) fails the gel-shift western blot assay, but does show enhanced labeling of a 52-kDa band in α7 nAChR-transfected relative to WT cells. This is a potential problem for tagged protein expression (Pillar 4 of Uhlen et al.19), that the tag may interfere with some antibodies that bind to epitopes near the tagging site. Polyclonal AB15332 is targeted to a synthetic mouse peptide near the large cytoplasmic domain at some distance from the C terminal where GFP is located. The peptide antigen (not disclosed) is for mouse α7 nAChR, but rat α7 differs from mouse by only one amino acid in the cytoplasmic loop region. Therefore, the reason that this antibody apparently recognizes native rat α7 nAChRs but not chimeric receptors in western blots is unclear. However, if this result can happen for a chimeric receptor, it begs the question of how different splice variants in receptor regions not involving antibody epitopes might affect antibody reactivity. In this case, AB15332 shows significant and non-uniform background immunofluorescence in native cells, but the intensity is clearly much higher in α7 nAChR-transfected cells, suggesting that the antibody does recognize the receptor. However, the variability of the background in untransfected cells in culture renders using this antibody problematic for localizing α7 nAChRs in rat tissue, similar to those problems observed with MAb 306.
Novus NBP1-79948 rabbit polyclonal anti-α7 nAChR (Fig. 4) had different effects depending on lots. One lot showed bands for both α7 nAChR and α7 nAChR–GFP chimera, whereas the second detected none. Although this shows the advantage of the gel-shift assay for quickly assessing antibodies, it also points out the necessity for individual antibody lot validation. In our hands, Novus NBP1-79948 (lot QC0391-42133) did show significant difference in immunofluorescence staining between WT and α7 nAChR-transfected GH4C1 cells at a 1:1000 dilution. However, because of lot-to-lot variability, different batches of the same antibody may not demonstrate similar immunofluorescence results.
For western blots, the gel-shift assay is useful to rapidly screen different lots of antibodies to see if they possibly recognize denatured α7 nAChRs, with the caveat that tagging the target may cause some false negatives (e.g., EMD Millipore AB15332). Out of seven antibodies tested on western blots, four failed to recognize the targets, and in the one case we tested two lots of the same polyclonal antibody, the results failed to replicate. In terms of “pillars” for validating antibodies, this is an example where target expression seems equally useful as knockout animals when cell lines are available that lack the antibody target. In terms of immunofluorescence, no antibody was able to replicate the pattern of fluorescent αBGT binding to the cells in culture, although the same three antibodies that detected bands on western blots showed strong differences between transfected and untransfected cells. These are uniform cell populations when compared with tissues from animals, in which case, cells will be expected to vary widely in the types of epitopes they express, making the problem of sorting out specific from nonspecific binding even more difficult.
At the time of this writing, 27 companies offer a total of 225 α7 nAChR antibodies for sale according to Antibodypedia (www.antibodypedia.com). Apparently none of these are validated by genetic strategies, orthogonal strategies, or tagged target proteins. However, articles are being published using these antibodies, sometimes as the sole evidence that α7 nAChRs are present in tissue. Extreme caution must be exercised when antibody data are the primary evidence for the presence or localization of α7 nAChRs. Data should be corroborated using other methods such as αBGT binding, novel conotoxin binding,30 PCR methods, in situ hybridization, or electrophysiology. The problem of antibody validation is not unique to nicotinic receptors and is also a serious issue for antibodies against G-protein coupled receptors.18,31–34 Non-reproducible results in biomedical research due to poorly characterized antibodies can be addressed only through a coordinated effort by scientists, antibody suppliers, funding agencies, and scientific journals.19,27
Footnotes
Authors’ Note: This work was performed in partial fulfillment of the requirements of a PhD degree of Brijesh K. Garg, a PhD candidate in the Department of Pharmaceutical Sciences, Northeastern University, Boston, MA.
Competing Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: RHL and BKG designed the experiments, BKG performed the experiments, and BKG and RHL drafted the manuscript. Both authors have read and approved the final manuscript.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by unrelated consulting by R.H.L.
Contributor Information
Brijesh K. Garg, Department of Pharmaceutical Science, Northeastern University, Boston, Massachusetts
Ralph H. Loring, Department of Pharmaceutical Science, Northeastern University, Boston, Massachusetts.
Literature Cited
- 1. Quik M, Zhang D, McGregor M, Bordia T. Alpha7 nicotinic receptors as therapeutic targets for Parkinson’s disease. Biochem Pharmacol. 2015;97:399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wevers A, Burghaus L, Moser N, Witter B, Steinlein OK, Schütz U, Schütz U, Achnitz B, Krempel U, Nowacki S, Pilz K, Stoodt J, Lindstrom J, De Vos RA, Jansen Steur EN, Schröder H. Expression of nicotinic acetylcholine receptors in Alzheimer’s disease: postmortem investigations and experimental approaches. Behav Brain Res. 2000;113:207–15. [DOI] [PubMed] [Google Scholar]
- 3. Banerjee C, Nyengaard JR, Wevers A, de Vos RA, Jansen Steur EN, Lindstrom J, Pilz K, Nowacki S, Bloch W, Schröder H. Cellular expression of alpha7 nicotinic acetylcholine receptor protein in the temporal cortex in Alzheimer’s and Parkinson’s disease—a stereological approach. Neurobiol Dis. 2000;7:666–72. [DOI] [PubMed] [Google Scholar]
- 4. Leonard S, Adams C, Breese CR, Adler LE, Bickford P, Byerley W, Coon H, Griffith JM, Miller C, Myles-Worsley M, Nagamoto HT, Rollins Y, Stevens KE, Waldo M, Freedman R. Nicotinic receptor function in schizophrenia. Schizophr Bull. 1996;22:431–45. [DOI] [PubMed] [Google Scholar]
- 5. Hong CJ, Lai IC, Liou LL, Tsai SJ. Association study of the human partially duplicated alpha7 nicotinic acetylcholine receptor genetic variant with bipolar disorder. Neurosci Lett. 2004;355:69–72. [DOI] [PubMed] [Google Scholar]
- 6. Maouche K, Medjber K, Zahm JM, Delavoie F, Terryn C, Coraux C, Pons S, Cloëz-Tayarani I, Maskos U, Birembaut P, Tournier JM. Contribution of α7 nicotinic receptor to airway epithelium dysfunction under nicotine exposure. Proc Natl Acad Sci U S A. 2013;110:4099–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, Li JH, Wang H, Yang H, Ulloa L, Al-Abed Y, Czura CJ, Tracey KJ. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421:384–8. [DOI] [PubMed] [Google Scholar]
- 8. Downs AM, Bond CE, Hoover DB. Localization of α7 nicotinic acetylcholine receptor mRNA and protein within the cholinergic anti-inflammatory pathway. Neuroscience. 2014;266:178–85. [DOI] [PubMed] [Google Scholar]
- 9. Egea J, Buendia I, Parada E, Navarro E, Leon R, Lopez MG. Anti-inflammatory role of microglial alpha7 nAChRs and its role in neuroprotection. Biochem Pharmacol. 2015;97:463–72. [DOI] [PubMed] [Google Scholar]
- 10. McCann CM, Bracamontes J, Steinbach JH, Sanes JR. The cholinergic antagonist alpha-bungarotoxin also binds and blocks a subset of GABA receptors. Proc Natl Acad Sci U S A. 2006;103:5149–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hannan S, Mortensen M, Smart TG. Snake neurotoxin α-bungarotoxin is an antagonist at native GABA(A) receptors. Neuropharmacology. 2015;93:28–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Loring RH, Dahm LM, Zigmond RE. Localization of alpha-bungarotoxin binding sites in the ciliary ganglion of the embryonic chick: an autoradiographic study at the light and electron microscopic level. Neuroscience. 1985;14:645–60. [DOI] [PubMed] [Google Scholar]
- 13. Herber DL, Severance EG, Cuevas J, Morgan D, Gordon MN. Biochemical and histochemical evidence of nonspecific binding of alpha7nAChR antibodies to mouse brain tissue. J Histochem Cytochem. 2004;52:1367–76. [DOI] [PubMed] [Google Scholar]
- 14. Jones IW, Wonnacott S. Precise localization of alpha7 nicotinic acetylcholine receptors on glutamatergic axon terminals in the rat ventral tegmental area. J Neurosci. 2004;24:11244–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jones IW, Wonnacott S. Why doesn’t nicotinic ACh receptor immunoreactivity knock out? Trends Neurosci. 2005;28:343–5. [DOI] [PubMed] [Google Scholar]
- 16. Moser N, Mechawar N, Jones I, Gochberg-Sarver A, Orr-Urtreger A, Plomann M, Salas R, Molles B, Marubio L, Roth U, Maskos U, Winzer-Serhan U, Bourgeois JP, Le Sourd AM, De Biasi M, Schröder H, Lindstrom J, Maelicke A, Changeux JP, Wevers A. Evaluating the suitability of nicotinic acetylcholine receptor antibodies for standard immunodetection procedures. J Neurochem. 2007;102:479–92. [DOI] [PubMed] [Google Scholar]
- 17. Rommel FR, Raghavan B, Paddenberg R, Kummer W, Tumala S, Lochnit G, Gieler U, Peters EM. Suitability of nicotinic acetylcholine receptor α7 and muscarinic acetylcholine receptor 3 antibodies for immune detection: evaluation in murine skin. J Histochem Cytochem. 2015;63:329–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Baker M. Reproducibility crisis: blame it on the antibodies. Nature. 2015;521:274–6. [DOI] [PubMed] [Google Scholar]
- 19. Uhlen M, Bandrowski A, Carr S, Edwards A, Ellenberg J, Lundberg E, Rimm DL, Rodriguez H, Hiltke T, Snyder M, Yamamoto T. A proposal for validation of antibodies. Nat Methods. 2016;13:823–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Quik M, Choremis J, Komourian J, Lukas RJ, Puchacz E. Similarity between rat brain nicotinic alpha-bungarotoxin receptors and stably expressed alpha-bungarotoxin binding sites. J Neurochem. 1996;67:145–54. [DOI] [PubMed] [Google Scholar]
- 21. Sweileh W, Wenberg K, Xu J, Forsayeth J, Hardy S, Loring RH. Multistep expression and assembly of neuronal nicotinic receptors is both host-cell- and receptor-subtype-dependent. Brain Res. 2000;75:293–302. [DOI] [PubMed] [Google Scholar]
- 22. Lee HK, Gwalani L, Mishra V, Anandjiwala P, Sala F, Sala S, Ballesta JJ, O’Malley D, Criado M, Loring RH. Investigating the role of protein folding and assembly in cell-type dependent expression of alpha7 nicotinic receptors using a green fluorescent protein chimera. Brain Res. 2009;1259:7–16. [DOI] [PubMed] [Google Scholar]
- 23. Unal ES, Zhao R, Qiu A, Goldman ID. N-linked glycosylation and its impact on the electrophoretic mobility and function of the human proton-coupled folate transporter (HsPCFT). Biochim Biophys Acta. 2008;1778:1407–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schulz DW, Loring RH, Aizenman E, Zigmond RE. Autoradiographic localization of putative nicotinic receptors in the rat brain using 125I-neuronal bungarotoxin. J Neurosci. 1991;11:287–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Koperniak TM, Garg BK, Boltax J, Loring RH. Cell-specific effects on surface α7 nicotinic receptor expression revealed by over-expression and knockdown of rat RIC3 protein. J Neurochem. 2013;124:300–9. [DOI] [PubMed] [Google Scholar]
- 26. Bradbury AR, Pluckthun A. Getting to reproducible antibodies: the rationale for sequenced recombinant characterized reagents. Protein Eng Des Sel. 2015;28:303–5. [DOI] [PubMed] [Google Scholar]
- 27. Weller MG. Quality issues of research antibodies. Anal Chem Insights. 2016;11:21–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bordeaux J, Welsh A, Agarwal S, Killiam E, Baquero M, Hanna J, Anagnostou V, Rimm D. Antibody validation. Biotechniques. 2010;48:197–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fabian-Fine R, Skehel P, Errington ML, Davies HA, Sher E, Stewart MG, Fine A. Ultrastructural distribution of the alpha7 nicotinic acetylcholine receptor subunit in rat hippocampus. J Neurosci. 2001;21:7993–8003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hone AJ, Whiteaker P, Mohn JL, Jacob MH, McIntosh JM. Alexa Fluor 546-ArIB[V11L;V16A] is a potent ligand for selectively labeling alpha 7 nicotinic acetylcholine receptors. J Neurochem. 2010;114:994–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cécyre B, Thomas S, Ptito M, Casanova C, Bouchard JF. Evaluation of the specificity of antibodies raised against cannabinoid receptor type 2 in the mouse retina. Naunyn Schmiedebergs Arch Pharmacol. 2014;387:175–84. [DOI] [PubMed] [Google Scholar]
- 32. Jositsch G, Papadakis T, Haberberger RV, Wolff M, Wess J, Kummer W. Suitability of muscarinic acetylcholine receptor antibodies for immunohistochemistry evaluated on tissue sections of receptor gene-deficient mice. Naunyn Schmiedebergs Arch Pharmacol. 2009;379:389–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Michel MC, Wieland T, Tsujimoto G. How reliable are G-protein-coupled receptor antibodies? Naunyn Schmiedebergs Arch Pharmacol. 2009;379:385–8. [DOI] [PubMed] [Google Scholar]
- 34. Pradidarcheep W, Stallen J, Labruyere WT, Dabhoiwala NF, Michel MC, Lamers WH. Lack of specificity of commercially available antisera against muscarinergic and adrenergic receptors. Naunyn Schmiedebergs Arch Pharmacol. 2009;379:397–402. [DOI] [PubMed] [Google Scholar]