Abstract
Menin is a scaffold protein that interacts with several epigenetic mediators to regulate gene transcription, and suppresses pancreatic β-cell proliferation. Tamoxifen-inducible deletion of multiple endocrine neoplasia type 1 (MEN1) gene, which encodes the protein menin, increases β-cell mass in multiple murine models of diabetes and ameliorates diabetes. Glucagon-like-peptide-1 (GLP1) is another key physiological modulator of β-cell mass and glucose homeostasis. However, it is not clearly understood whether menin crosstalks with GLP1 signaling. Here, we show that menin and protein arginine methyltransferase 5 (PRMT5) suppress GLP1 receptor (GLP1R) transcript levels. Notably, a GLP1R agonist induces phosphorylation of forkhead box protein O1 (FOXO1) at S253, and the phosphorylation is mediated by PKA. Interestingly, menin suppresses GLP1-induced and PKA-mediated phosphorylation of both FOXO1 and cAMP response element binding protein (CREB), likely through a protein arginine methyltransferase. Menin-mediated suppression of FOXO1 and CREB phosphorylation increases FOXO1 levels and suppresses CREB target genes, respectively. A small-molecule menin inhibitor reverses menin-mediated suppression of both FOXO1 and CREB phosphorylation. In addition, ex vivo treatment of both mouse and human pancreatic islets with a menin inhibitor increases levels of proliferation marker Ki67. In conclusion, our results suggest that menin and PRMT5 suppress GLP1R transcript levels and PKA-mediated phosphorylation of FOXO1 and CREB, and a menin inhibitor may reverse this suppression to induce β-cell proliferation.
Keywords: diabetes, islets, glucagon-like-peptide-1 receptor, menin, protein arginine methyltransferase 5
the mass of insulin-producing pancreatic β-cells is reduced in both Type 1 and late-stage Type 2 diabetes (12, 42, 84). There is an urgent need to understand cellular mechanisms and develop new therapeutics that can help increase the β-cell mass to ameliorate diabetes (8, 16, 31, 51). In this context, we have previously reported that excision of the multiple endocrine neoplasia type 1 (MEN1) gene, which encodes the protein menin, ameliorates diabetes in murine models of diabetes (84, 85). Menin knockout reverses preexisting hyperglycemia in high-fat diet-induced diabetes and obese (db/db) mice and prevented hyperglycemia in streptozotocin-induced diabetes. This amelioration of diabetes is explained, in part, by increased proliferation of insulin-positive β-cells in pancreatic islets and increased plasma insulin levels (84, 85). Similarly, others have reported that menin suppresses pregnancy-associated and glucose-induced increases in pancreatic β-cell proliferation, insulin production, and secretion (34, 40, 64, 87). In addition, key stimuli involved in glucose homeostasis, like glucose, insulin, and somatostatin, regulate the expression of menin (40, 79, 87). Taken together, these reports point to a major physiological role of menin in regulating pancreatic β-cell proliferation and glucose homeostasis. However, the cellular mechanisms by which menin suppresses pancreatic β-cell proliferation remain to be delineated. Therefore, we wanted to explore whether and how menin regulates signaling by hormonal mediators that are key regulators of β-cell proliferation.
Both insulin and IGF have been shown to promote β-cell proliferation (1, 5, 14, 17, 19, 22, 41, 45, 75, 80, 81, 88). We reported recently that menin downregulates the insulin receptor substrate 2 (IRS2), which plays a key role in signaling downstream of the insulin and IGF receptors (IR and IGFR, respectively), as well as in glucose-induced β-cell proliferation (50, 70, 71, 78). Reduction of β-cell mass observed upon IRS2 knockout is reversed by haploinsufficiency of forkhead box protein O1 (FOXO1), highlighting FOXO1 as a key regulator of β-cell mass via the phosphatidylinositol 3-kinase/protein kinase B (PI3K/Akt) pathway (9, 10, 23, 24, 36, 37, 47). Interestingly, it has been reported that FOXO1 increases menin transcript and protein levels (87). However, it is not known whether menin regulates FOXO1 levels or function.
Glucagon-like peptide 1 (GLP1) is another key incretin hormone involved in regulating β-cell mass and glucose homeostasis (6, 7, 17, 18, 44, 53, 55, 56, 59, 80). GLP1 acts via the glucagon-like peptide-1 receptor (GLP1R) to activate PKA and phosphorylation of cAMP response element binding protein (CREB) at S133 (32). Phosphorylated CREB then recruits a histone acetyltransferase called CREB-binding protein, leading to transcriptional activation of several proproliferative genes, including cyclin A2 and IRS2 (32, 35, 69). Our previous observations indicate that several proproliferative genes upregulated by GLP1R agonists, including cyclin A2 and IRS2, are also upregulated upon menin excision, suggesting a potential cross talk between menin and GLP1R signaling (28, 84). GLP1R agonists also regulate FOXO1 phosphorylation at S256 for human FOXO1 and S253 for mouse FOXO1 via a PI3K/Akt-dependent mechanism, and this phosphorylation leads to its cytosolic localization, ubiquitination, and, ultimately, proteasomal degradation (11, 23, 24, 55, 76). However, it is not known whether and how menin regulates signaling downstream of GLP1R to regulate β-cell proliferation. Therefore, we hypothesized that menin suppresses GLP1R signaling to regulate β-cell proliferation. Specifically, we sought to determine whether and how menin suppresses GLP1R-mediated activation of CREB and inactivation of FOXO1 to regulate β-cell proliferation.
Recent research has identified several interacting partners of menin that enable menin to exert its actions; for example, the transcription factor JunD, mixed lineage leukemia protein 1 (MLL1, a histone H3 lysine 4 methyltransferase), and protein arginine methyltransferase 5 (PRMT5, a transcriptional repressor) (26, 27, 30, 43), prompting us to explore the role of these interactions in modulating GLP1R signaling. Moreover, elucidation of menin structure in conjunction with its binding partner MLL1 has enabled the development of targeted therapeutics to inhibit menin function (15, 30, 67). Therefore, we also investigated whether a menin inhibitor can reverse the effects of menin to promote GLP1R signaling and β-cell proliferation.
MATERIALS AND METHODS
Mice and excision of floxed Men1 locus.
The Institutional Animal Care and Use Committee at the University of Pennsylvania approved all experimental protocols involving the use of mice. Menl/l and littermate Menl/l-Ubc9-CreER mice generated for this study have been described in a previous study from our laboratory (84). The mice were maintained at University Laboratory Animal Resources (ULAR) facility of University of Pennsylvania under a 12:12-h light-dark cycle. Mice were genotyped as previously described using tail DNA (66). Four-week-old, male, Menl/l and littermate Menl/l-Ubc9-CreER mice were treated with tamoxifen (200 mg·kg body wt−1·day−1 ip) for 2 days followed by 1 day off and finally then another 2 days to achieve menin excision. Sixteen weeks after tamoxifen treatment, mice were euthanized, and organs were harvested at 20 wk of age. Seven-week-old C57BL/6J mice obtained from Jackson Laboratory (Bar Harbor, ME) were acclimatized for 1 wk at the University of Pennsylvania ULAR facility. At 8 wk of age, pancreatic islets were isolated and used for in vitro culture and treatment with menin inhibitor, as described below.
Isolation and culture of mouse islets.
Mouse islets used for measurement of GLP1R levels were isolated individually from Menl/l and littermate Menl/l-Ubc9-CreER mice described above at 20 wk of age using a collagenase digestion and Ficoll gradient separation protocol described previously (27). The islets were dissolved in TRIzol (Life Technologies, Waltham, MA), stored at −80°C until they were used for extraction of RNA. Islets used for in vitro culture were isolated from eight 8-wk-old C57BL/6J mice, pooled, and cultured in RPMI-1640 medium (Sigma-Aldrich, St. Louis, MO) supplemented with 10% FBS without antibiotics. The islets were then divided into four groups for treatment with drugs for 48 h, as indicated in the figure legends.
Human islet culture.
Human islets were obtained from Center for Islet Transplantation, University of Pennsylvania, and cultured in CMRL-1066 medium (cat. no. 98–304-CV, University of Miami Diabetes Research Institute) supplemented with 10% FBS, but without antibiotics. The islets were then divided into treatment groups and treated with drugs for the duration indicated in the figure legends. At the end of drug treatment, islets were processed for RNA extraction as described above for mouse islets.
Cell culture.
Cells were cultured in high glucose (4.5 g/l) DMEM (cat. no. 11965, Life Technologies), supplemented with 10% FBS and 1% penicillin-streptomycin (cat. no. 15140, Life Technologies). Menin-expressing pancreatic islet-derived menin excisable (PIME-Menl/l) and menin-null PIME cells (PIME-MenΔ/Δ) were generated in our laboratory, as previously described (84). Menin-null mouse embryonic fibroblasts (MEFs) that were complemented with either vector control (MEF-Men1Δ/Δ) or menin (MEF-Men1Δ/Δ+Menin) were generated, as previously described (66). Wild-type MIN6 cells used in this study were a gift from the laboratory of Dr. Mehboob Hussain (69). For generating menin-overexpressing MIN6 cells, FLAG-tagged menin or vector control, and green fluorescent protein plasmids were used to generate recombinant retroviruses, as previously described (66). The recombinant retroviruses were used to transduce wild-type MIN6 cells on day 0 and day 1, followed by selection with puromycin (2 μg/ml) starting at day 2 and continuing for 5 more days. Cells were then propagated in puromycin-free, high-glucose DMEM, 10% FBS, and 1% penicillin-streptomycin. PRMT5 knockdown or vector control MEFs were obtained as described earlier (27).
Drug treatment, preparation of samples for SDS-PAGE, and immunoblotting.
Cells were starved in serum-free, low-glucose (1 g/l) DMEM (cat. no. 11885; Life Technologies) before treatment with exendin-4 (cat. no. E7144; Sigma-Aldrich, or cat. no. 120214; Abcam, Cambridge, MA), forskolin (cat. no. F3917; Sigma-Aldrich), MI-2-2 (67) (Chemzon Scientific, Montreal, Canada), Akt inhibitor (MK-2206, cat. no. S1078; Selleckchem, Houston, TX) or PKA inhibitor (H-89, cat. no. B1427; Sigma-Aldrich), as detailed in the figure captions. Treatment was stopped by washing with ice-cold DPBS (cat. no. 14190235; Life Technologies). Cells were harvested in DPBS with cell scraper and pelleted by centrifugation at 3,000 g for 15 min and resuspended in either TRIzol for RNA extraction (see below) or for cell lysate preparation using lysis buffer (pH 7.5) containing 20 mM Tris(hydroxymethyl)aminomethane hydrochloride (Tris·HCl), 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 2.5 mM sodium pyrophosphate, 1 mM 2-glycerophosphate, 1 mM sodium orthovanadate, 1 mM PMSF, 1 mM DTT, and 1 mM NaF, protease inhibitor 1×, and Triton X-100 at 1%. The resulting suspension was sonicated and centrifuged at ~20,000 g for 15 min to collect supernatant. NuPAGE LDS Sample Buffer (cat. no. NP0007; Thermo Fisher Scientific, Walkersville, MD) was added to the supernatants to make the SDS-PAGE samples. Immunoblotting was performed using standard techniques. Antibodies used for immunoblotting are as follows, anti-FOXO1 (cat. no. 2880; Cell Signaling, Danvers, MA), anti-pFOXO1 S253 (cat. no. 9461; Cell Signaling), CREB (350900; Life Technologies), anti-pCREB S133 (cat. no. 4276; Cell Signaling), anti-Akt (cat. no. 9272; Cell Signaling), anti-pAkt S473 (cat. no. 4060; Cell Signaling), anti-Menin (cat. no. A300-105A; Bethyl, Montgomery, TX), and anti-PKACα (cat. no. 4782; Cell Signaling).
RNA extraction and qPCR.
RNA was isolated from cells or tissues dissolved in TRIzol using a phenol-chloroform extraction protocol and RNeasy extraction kit (Qiagen, Valencia, CA). RNA was reverse transcribed to cDNA using SSIIIRT (Life Technologies), according to the kit manufacturer’s instructions followed by quantitative PCR (qPCR) of the cDNA product using a QuantiTect SYBR Green (Qiagen) protocol on an AB7500 Fast real-time PCR system (Life Technologies). Primers used for amplification are listed in Table 1.
Table 1.
Primer sequences used for qPCR
| Target Gene | Primer Sequence |
|---|---|
| Mouse primers | |
| GLP1R primer pair 1 | Forward 5′ ACG GTG TCC CTC TCA GAG AC 3′ |
| Reverse 5′ ATC AAA GGT CCG GTT GCA GAA 3′ | |
| GLP1R primer pair 2 | Forward 5′ CCC AGG TTC CTT CGT GAA TGT 3′ |
| Reverse 5′ GTT GTC CTT ATG TAG CCA GAG AC 3′ | |
| Menin primer pair 1 | Forward 5′ GGA CCC AGA ATG CTT TGC C 3′ |
| Reverse 5′ GAC TGC ACA AGG AAA GTT GCC 3′ | |
| Menin primer pair 2 | Forward 5′ GAA AGA TAC CCC ATG GCG CTA 3′ |
| Reverse 5′ TTT CGG TTG CGA CAG TGG TAG 3′ | |
| IRS2 primer pair 1 | Forward 5′ GTG GGT TTC CAG AAC GGC CT 3′ |
| Reverse 5′ ATG GGG CTG GTA GCG CTT CA 3′ | |
| IRS2 primer pair 2 | Forward 5′ AGT AAA CGG AGG TGG CTA CA 3′ |
| Reverse 5′ AAG CTG CTG AGA AGT CAG GT 3′ | |
| NR4A2 | Forward 5′ GCA TAC AGG TCC AAC CCA GT 3′ |
| Reverse 5′ AAT GCA GGA GAA GGC AGA AA 3′ | |
| Cyclin A2 | Forward 5′ GCG CTG TGC ACG CTC TG 3′ |
| Reverse 5′ TCC TTA AGA GGA GCA ACC CGT 3′ | |
| HPRT1 | Forward 5′ TGC TCG AGA TGT CAT GAA GG 3′ |
| Reverse 5′ TAT GTC CCC CGT TGA CTG AT 3′ | |
| FOXO1 | Forward 5′ CAG CCG CGC AAG ACC AGC TC 3′ |
| Reverse 5′ TTG AAT TCT TCC AGC CCG CCG AG 3′ | |
| Human primers | |
| GLP1R (25) | Forward 5′ TTG GGG TGA ACT TCC TCA TC 3′ |
| Reverse 5′ CTT GGC AAG TCT GCA TTT GA 3′ | |
| Ki67 primer pair 1 | Forward 5′ TAC GTG AAC AGG AGC CAG CA 3′ |
| Reverse 5′ GTT CCC TGA GCA ACA CTG TC 3′ | |
| Ki67 primer pair 2 | Forward 5′ TCT GAC CCT GAT GAG AAA GCT C 3′ |
| Reverse 5′ AGT TGT TCC CTG AGC AAC AC 3′ | |
| Primers used to amplify chromatin immunoprecipitation DNA product | |
| Amplicon 1 | Forward 5′ GGT GGC ACC CAA CTA GTC CGA 3′ |
| Reverse 5′ GCC CCC ACC GAG TCA GTG CT 3′ | |
| Amplicon 2 | Forward 5′ AGG AGG AGA TCG GGG TGC GG 3′ |
| Reverse 5′ ATG TTC GGC GCT GTG CTC CC 3′ |
Chromatin immunoprecipitation.
Chromatin immunoprecipitation (ChIP) was performed using the QuickChIP kit (cat. no. 30101K; Imgenex, Littleton, CO), according to the manufacturer’s instructions. Anti-menin (A300-105A; Bethyl) and anti-PRMT5 (Ab31751; Abcam) were used for ChIP followed by qPCR. Primers used for ChIP DNA product amplification are listed in Table 1.
cAMP accumulation assay.
cAMP was measured using the cAMP EIA kit (cat. no. 581001; Cayman Chemicals, Ann Arbor, MI) based on the kit manufacturer’s instructions. Briefly, MIN6 cells were plated at a density of 1.6 × 106 cells/well in six-well plates and cultured in high glucose (4.5 g/l) DMEM for 24 h. After this time, cells were starved in serum-free, low-glucose (1 g/l) DMEM and treated with drugs for time durations specified in figure legends. Drug treatment was stopped by washing the cells with DPBS followed by extraction of cAMP using 0.1 M HCl (hydrochloric acid) for quantitation using the kit manufacturer’s protocol.
Proximity ligation assay.
Proximity ligation assay described by Soderberg et al. (68) was performed using the Duolink mouse/rabbit starter kit (cat. no. DUO92101; Sigma-Aldrich). Briefly, 1 × 104 cells were plated in eight-well Nunc Laboratory-Tek Chamber Slide System (cat. no. 177402; Thermo Fisher Scientific). One day after being plated, cells were starved and treated with drugs, as indicated in figure legends. Drug treatment was stopped by washing with DPBS followed by fixation using 4% paraformaldehyde in DPBS. After fixation, cells were either stored at 4°C until further use or used immediately to perform proximity ligation assay using the protocol described by Thymiakou and Episkopou (72). Antibodies used for the assay are as follows: anti-PKA (rabbit, cat. no. SC-903; Santa Cruz Biotechnology, Dallas, TX), anti-FOXO1 (mouse, cat. no. 05-1075; Millipore, Billerica, MA), anti-CREB (mouse, cat. no. 350900; Life Technologies), and anti-PRMT5 (rabbit, cat. no. Ab31751; Abcam).
Immunohistochemistry.
Immunohistochemistry was performed as previously described (84). Briefly, mouse islets were fixed in 4% paraformaldehyde followed by agarose and paraffin embedding and sectioning. Tissue sections were costained using anti-insulin (guinea pig serum, cat. no. Ab7842; Abcam) and Ki67 (Rabbit pAb, cat. no. Ab15580; Abcam) followed by secondary antibodies; FITC-conjugated goat anti-guinea pig (Ab6904; Abcam) and Alexa Fluor 546-conjugated goat anti-rabbit IgGs. DAPI (4′,6-diamidino-2-phenylindole) was used to stain for nuclei, followed by microscopic analysis using an Olympus IX81 microscope (Center Valley, PA) and slidebook software (Intelligent Imaging Innovations, Denver, CO) to capture images. Ki67-positive cells were counted manually and normalized to islet area (arbitrary units), measured using ImageJ software (NIH, Bethesda, MD).
Immunoprecipitation.
After being harvested, cells were lysed by incubating in immunoprecipitation (IP) lysis buffer (lysis buffer + 10% glycerol) for 1 h at 4°C followed by centrifugation at 20,000 g for 15 min to collect supernatant. The supernatant (1 mg protein at a concentration of 3 μg/μl) was incubated overnight at 4°C with 0.1 μg/μl of mouse anti-FOXO1 (cat. no. 05–1075; Millipore), mouse anti-CREB (cat. no. 350900; Life Technologies), or mouse IgG control (cat. no. N103; Calbiochem) antibodies. The antibody-antigen complexes were precipitated by incubation with protein A agarose (cat. no. 15918014; Thermo Fisher Scientific) for 4 h at 4°C, washed 2× with IP lysis buffer, and eluted with 3× loading buffer (NuPAGE LDS Sample Buffer, cat. no. NP0007; Thermo Fisher Scientific). The eluates were separated by SD-PAGE followed by immunoblotting with anti-PRMT5 (rabbit, cat. no. Ab31751; Abcam,) and Clean-Blot IP detection reagent (cat. no. 21230; Thermo Fisher Scientific), and visualized using ECL Western blotting detection reagents (cat. no. RPN2106; GE-Amersham Biosciences, Pittsburgh, PA).
In vitro kinase assay.
NH2-terminal biotin-linked FOXO1, CREB, methyl-FOXO1, and methyl-CREB peptides used for the assay were synthesized at GenScript (Piscataway, NJ). The peptide sequences are listed in Fig. 6D. The kinase reaction was performed using PKA assay kit (cat. no. 17–134; Millipore) and recombinant PKA catalytic subunit (cat. no. V5161; Promega, Madison, WI), according to the manufacturer’s instructions with slight modifications. Briefly, 2.5 nmol of peptides were incubated with 100 ng recombinant PKA catalytic subunit in assay dilution buffer, and Mg/ATP cocktail containing 10 μCi ATP ([γ-32P], 3000 Ci/mmol, cat. no. BLU502A001MC, Perkin Elmer, Waltham, MA) to a total volume of 45 μl, for 10 min at room temperature. The reaction was stopped by applying the reaction mixture to phosphocellulose membranes (cat. no. MSPHN6B10; Millipore), followed by washing 5× with aqueous 0.75% phosphoric acid and 1× with acetone. The membranes were then suspended in scintillation fluid (cat. no. 1205-440; Betaplate Scint, Perkin Elmer) and counted on a LS6500 scintillation counter (Beckman Coulter, Brea, CA) to quantify the amount of phosphate incorporated. Procedure for in vitro kinase assay using immunoprecipitated full-length FOXO1 is described in the figure legend.
Fig. 6.
PRMT5 interacts with FOXO1 and CREB. Coimmunoprecipitation of PRMT5 from MIN6 cells using antibodies against CREB and FOXO1 for the immunoprecipitation followed by Western blot analyses with antibodies against PRMT5, FOXO1, or CREB. Representative blots (A) and quantitation of PRMT5 pulldown compared with IgG controls (B; results expressed as means ± SE from three similar experiments; *P < 0.05), showing PRMT5 is enriched in both anti-FOXO1 (lane 2) and anti-CREB (lane 3) antibody groups compared with IgG control (lanes 4). C: proximity ligation assay in vector-transfected menin-null (Men1Δ/Δ) MEF cells and menin-complemented (Men1Δ/Δ+Menin) MEF cells using anti-PRMT5 and anti-CREB antibodies, shows colocalization of PRMT5 with CREB, and menin complementation increases the frequency of this association (two similar experiments).
Statistical analysis.
Statistical analysis was performed using GraphPad Prism V6 software (GraphPad Software, La Jolla, CA) and Student’s t-test or ANOVA, as specified within the figure legends.
RESULTS
Menin downregulates GLP1 receptor expression through recruitment of the transcriptional repressor PRMT5.
Menin suppresses pancreatic β-cell proliferation and is increasingly being appreciated as a regulator of glucose homeostasis (66, 84). However, it is not known whether menin regulates GLP1, an incretin hormone, which maintains glucose homeostasis by regulating pancreatic β-cell mass and insulin secretion. To investigate whether menin suppresses GLP1 receptor (GLP1R) signaling, first, we determined whether menin regulates expression of GLP1R. Men1f/f and littermate Men1f/f-Ubc9-CreER mice were treated with tamoxifen (200 mg·kg body wt−1·day−1 ip) to excise menin (Fig. 1A). Menin excision led to increased GLP1R mRNA levels in pancreatic islets, as well as in the liver (Fig. 1, B and C, respectively). Similarly, menin excision in PIME cells (PIME-Men1Δ/Δ), a cell line derived from islets of mice with floxed Men1 alleles (84), resulted in a pronounced increase in levels of GLP1R mRNA levels compared with menin-expressing controls (PIME Menl/l) (Fig. 1D). Consistently, complementation of menin-null MEFs with menin (MEF-Men1Δ/Δ+Menin) decreased GLP1R mRNA levels compared with menin-null cells (MEF-Men1Δ/Δ) (Fig. 1, E and F). Unlike homogenous systems like liver cells and multiple cell lines tested, there was substantial variability in terms of the impact of the Men1 deletion on the expression of GLP1R in islets. It is possible that the effect appears to be blunted because of contribution from other endocrine cell types and/or traces of exocrine contamination.
Fig. 1.
Menin suppresses GLP1R transcript levels by binding to the GLP1R promoter and recruiting PRMT5. At 4 wk of age, Menl/l and littermates Menl/l-Ubc9-CreER mice were treated with tamoxifen (200 mg·kg body wt−1·day−1 ip) for 2 days followed by 1 day off and finally another 2 days to achieve menin excision. Sixteen weeks after tamoxifen treatment, mice were euthanized, and organs were harvested at 20 wk of age. Menin excision upon tamoxifen treatment (A) in Menf/f-Ubc9-CreER mice leads to increased GLP1R transcript in pancreatic islets (n = 4) (B) and liver (n = 3 for Menl/l and n = 6 for MenΔ/Δ) (C). D: menin-null pancreatic islet-derived menin excisable (PIME-MenΔ/Δ) cells express higher GLP1R transcript levels compared with menin expressing PIME-Menf/f cells (n = 5). Menin complemented MEF cells (MEF-Men1Δ/Δ+Menin; E) when compared with vector complemented menin-null (MEF-Men1Δ/Δ) MEF cells (triplicates), which expressed lower levels of GLP1R mRNA (F). G and H: two different amplicons were used to amplify DNA obtained by chromatin-immunoprecipitation (ChIP) using MIN6 cells. Alignment showing GLP1R promoter sequence homology (black) vs. nonhomologous region (white) between different eutherian species, including humans and primates, with Mouse-Ch17:30901346-30901495 (target region for amplicon pair 1) (G) and Mouse-Ch17:30901523-30901674 (target region for amplicon pair 2) (H) upstream from the GLP1R transcription start site (TSS, Mouse-Ch17:30901877). Chromatin-immunoprecipitation (ChIP) using MIN6 cells and quantitative RT-PCR shows greater enrichment for anti-menin (I), and anti-PRMT5 (J) antibodies compared with IgG controls (representative plots of two similar experiments). PRMT5 knockdown in MEFs (K) increases GLP1R transcript levels (L) compared with vector controls (plots of three similar experiments). Data sets are expressed as means ± SE and were analyzed using unpaired t-test. #P < 0.0001. $P = 0.0546. *P < 0.002. & P < 0.001. ¶P < 0.01.
We further investigated how menin represses expression of GLP1R. Because we have previously shown that menin recruits PRMT5 to target genes and represses gene transcription (26, 27), we determined whether both menin and PRMT5 are recruited to the GLP1R promoter to repress its transcription. We tested whether menin and PRMT5 bind to the GLP1R promoter at regions between 382 to 531 bp and 203 to 354 bp upstream of the GLP1R transcription start site (Fig. 1, G and H). Sequence alignment of these DNA regions using Ensembl 87 (86) shows that these regions are conserved between rodent and human promoters and various other eutherian species (Fig. 1, G and H). ChIP in MIN6 cells followed by qPCR using two different amplicons described above indicated that both menin and PRMT5 bind to the GLP1R promoter (Fig. 1, I and J, respectively). PRMT5 knockdown using shRNA (Fig. 1K) led to an increase in GLP1R transcript by severalfold (~15-fold) in PRMT5 knockdown MEFs compared with vector controls (Fig. 1L). Taken together, these results are consistent with the notion that both menin and PRMT5 are recruited to the GLP1R locus to repress its transcription.
Menin inhibits GLP1R agonist and forskolin-induced and PKA-mediated phosphorylation of FOXO1 and CREB.
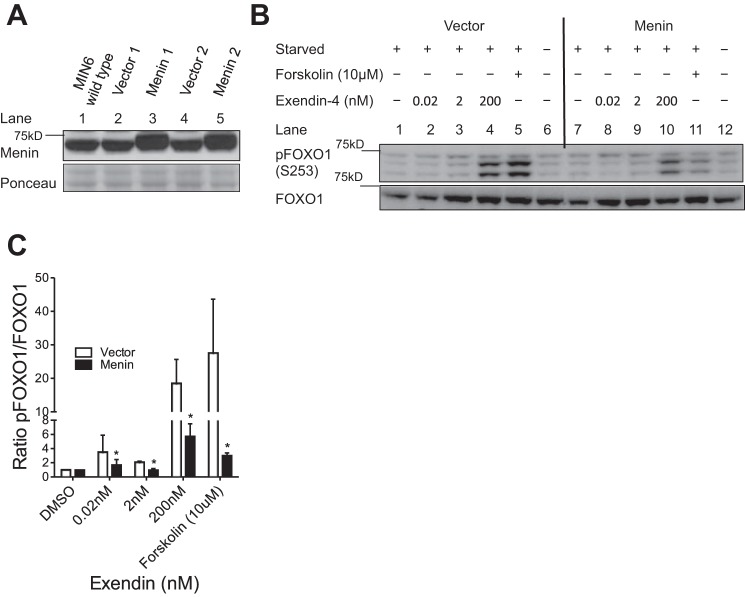
Lack of access to a commercial antibody specific for GLP1R limited our ability to explore the effect of menin on GLP1R protein levels and distribution within the islets (4, 57). However, since menin represses GLP1R expression, we determined whether menin could inhibit GLP1R-mediated phosphorylation of FOXO1. First, using retroviral transduction, we generated two independent pairs of MIN6 cell lines overexpressing either menin or vector controls (Fig. 2A). The cells were serum starved for 48 h followed by treatment with either a GLP1R agonist (exendin-4) or forskolin for 30 min. Treatment of vector control cells with either a GLP1R agonist or forskolin induced FOXO1 (S253) phosphorylation in a concentration-dependent manner (Fig. 2B, lanes 1–5). Notably, overexpression of menin markedly diminished exendin-4 or forskolin-induced FOXO1 phosphorylation (Fig. 2B, lanes 8–11 vs. lanes 2–5). Total FOXO1 levels were similar across the GLP1R agonist/forskolin treatment and menin expression groups (Fig. 2B). Quantification of S253 phosphorylated FOXO1 and total FOXO1 bands from Western blots using ImageJ shows that ectopic expression of menin substantially reduced the amount of phosphorylated FOXO1 (Fig. 2C).
Fig. 2.
Menin reduces GLP1R agonist and forskolin-induced phosphorylation of FOXO1. A: two different clones of either menin-overexpressing or vector-transfected MIN6 cells were generated. MIN6 cells were starved in serum-free, low-glucose medium for 48 h and then treated with the drugs indicated for 30 min. Representative Western blots (B) and quantitation (means ± SE) (C) of Western blots from three similar experiments demonstrating that menin overexpression blunted phosphorylation of FOXO1 (pFOXO1-S253) in response to treatment with either increasing concentrations of exendin-4 (0, 0.02, 2, and 200 nM) or forskolin (10 μM) (lanes 7 to 12) compared with vector-transfected controls (lanes 1 to 6). Data sets were analyzed using two-way repeated-measures ANOVA, *P < 0.05.
To further extend these findings to additional cell lines, MEF cells were first serum starved (24 h) and then treated with forskolin for 30 min followed by Western blot analysis to detect the impact of menin on FOXO1 phosphorylation. The results indicated that forskolin induced a concentration-dependent increase in phosphorylation of FOXO1 (S253) in MEFs and menin complementation (MEF-Men1Δ/Δ+Menin) suppressed this phosphorylation (Supplemental Fig. S1A, even numbered lanes vs. odd numbered lanes), while total FOXO1 levels were similar across the forskolin treatment and menin expression groups (Supplemental Fig. S1A). Similarly, menin complementation also suppressed CREB phosphorylation (S133) in MEFs, while total CREB levels were similar in forskolin-treated or menin-overexpressing cells (Supplemental Fig. S1A). These results indicate that menin inhibits phosphorylation of FOXO1 at S253 induced by either GLP1R agonist or forskolin.
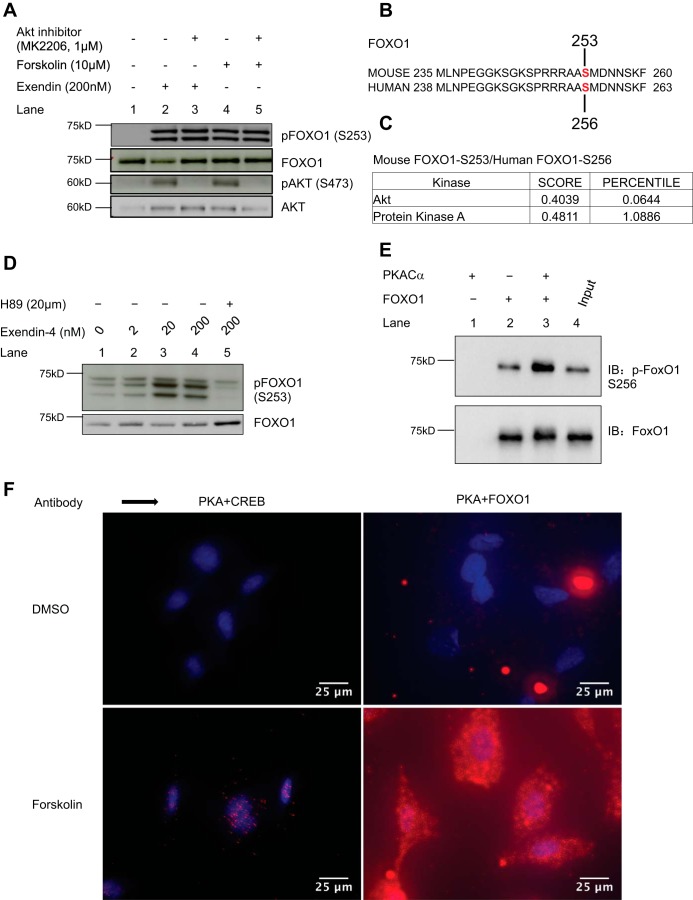
It has been reported that GLP1R agonists mediate FOXO1 phosphorylation via activation of PI3K/Akt pathway (24). Unexpectedly, however, an Akt inhibitor (MK2206) did not inhibit exendin-4 and forskolin-induced S253 phosphorylation of FOXO1 (Fig. 3A, lanes 2 vs. 3 and 4 vs. 5). This was despite the successful inhibition of S473 phosphorylation of Akt, which is essential for PI3K-mediated activation of Akt (2, 62) (Fig. 3A, lanes 2 vs. 3, and 4 vs. 5), suggesting that kinases other than Akt could be involved in inducing this GLP1R agonist or forskolin-induced phosphorylation of FOXO1.
Fig. 3.
GLP1R agonist and forskolin-induced phosphorylation of FOXO1 is PKA-mediated. A: FOXO1 phosphorylation in MIN6 cells induced by either exendin-4 (lane 2, 200 nM) or forskolin (lane 4, 10 μM) is not inhibited by an AKT inhibitor (MK2206, 1 μM, lanes 3 and 5), while phosphorylation of AKT (pAKT-S473) is inhibited as expected (lanes 3 and 5). B: alignment of mouse and human FOXO1 sequences, indicating the S253 and S256 positions, respectively. C: Scansite prediction results showing that both mouse and human FOXO1 can be phosphorylated by PKA at S253 or S256, respectively, with a score of 0.4811 compared with a score of 0.4039 for Akt. D: pretreatment of MIN6 cells with a PKA inhibitor (lane 5, H89, 20 μM) for 30 min inhibits exendin-4 induced phosphorylation of FOXO1 (lanes 1 to 4) (two similar experiments). E: PKA phosphorylates FOXO1 in vitro. Cells (HEK293T cells) transduced with FOXO1 plasmid were collected after 24 h for immunoprecipitation with anti-FOXO1 antibody. The FOXO1 immunoprecipitates were incubated with or without 0.1 μg of purified recombinant active PKA catalytic subunit (PKACα) in kinase buffer (35 mM Tris·HCl pH 7.4, 10 mM MgCl2, 0.5 mM EGTA, 0.1 mM CaCl2) containing 100 μM ATP for 30 min at 30°C in a final volume of 40 μl. Reactions were terminated by the addition of concentrated sample buffer and separated by SDS-PAGE followed by Western blot analysis. Increased S256-FOXO1 phosphorylation was observed upon coincubation with PKACα (top panel, lane 3) compared with vehicle controls (top panel, lane 2). F: proximity ligation assay in MEF cells starved for 24 h in serum-free, low-glucose medium shows increased nuclear colocalization of PKA and CREB upon forskolin (10 μM) stimulation, as expected (compare top left and bottom left panels). Similarly, forskolin treatment increased colocalization of PKA and FOXO1, which was more diffuse and not restricted to the nuclear compartment (two similar experiments).
Since cAMP/PKA activation is the major canonical pathway activated by GLP1R agonists and because of known cross-reactivity of kinases, we determined whether the S253 site in FOXO1 could be phosphorylated by PKA (55, 74). The Scansite3 kinase prediction program predicted that both mouse FOXO1 (S253) and human FOXO1 (S256) could be phosphorylated by PKA, with a score of 0.481 (1.089 percentile) compared with a score of 0.404 (0.064 percentile) for Akt at the same sites (49) (Fig. 3, B and C). Indeed, treatment of MIN6 cells with a PKA inhibitor, H-89, blocked exendin-4-induced phosphorylation of FOXO1 (Fig. 3D, lane 5). To further determine whether PKA can directly phosphorylate FOXO1, we performed an in vitro kinase assay using recombinant catalytic PKA subunit (PKACα). Recombinant PKACα phosphorylated S256 of human FOXO1 immunoprecipitated from human embryonic kidney (HEK)-293T cells overexpressing human FOXO1 (Fig. 3E). Consistently, PKACα also phosphorylated a FOXO1 peptide sequence (14 amino acids long) containing the serine corresponding to S253 of mouse FOXO1 (Fig. 7, B and C).
Fig. 7.
PRMT5 suppresses FOXO1 and CREB phosphorylation by PKA and CREB target genes. A: alignment of FOXO1 and CREB sequences showing conserved arginine residues around the target serine residues and the PKA consensus sequence. B: peptide sequences showing the arginines surrounding the serine phosphorylation sites S133 and S253 for unmodified peptides CREB and FOXO1, respectively. Unmodified peptides and methylated peptides, methyl-CREB (symmetrically dimethylated at R130 and R131), and methyl-FOXO1 (symmetrically dimethylated at R248 and R250) were used for in vitro kinase assay using recombinant PKACα. C: in vitro kinase assay using recombinant PKACα and [γ-32P]adenosine 5′-triphosphate ([γ-32P]ATP) shows PKA-mediated phosphate incorporation in kemptide (positive control) and unmodified CREB and FOXO1 peptides but not in methyl-CREB and methyl-FOXO1 peptides (results are expressed as means ± SE of two similar experiments analyzed using Student’s t-test; *P < 0.05 and **P < 0.01). Representative Western blots (D) and quantitation (E) showing PRMT5 knockdown in MEFs increased FOXO1 phosphorylation upon forskolin (10 μM) treatment (F) and increased CREB phosphorylation in MEFs (G) Values are expressed as means ± SE of two similar experiments. Data were analyzed using Student’s t-test, #P < 0.001 and §P < 0.05. H and I: PRMT5 knockdown in MEFs leads to increased CREB target gene, IRS2 and NR4A2 transcription (three similar experiments, §P < 0.05 and ¶P < 0.01).
We further determined whether the catalytic subunit of PKA (PKACα) interacts with FOXO1 within the cells using the proximity ligation assay. The proximity ligation assay allows for sensitive detection of protein-protein interactions and subcellular compartmentation using antibodies attached to DNA strands (68). The assay showed no or minimal signal for interaction of PKACα with either CREB or FOXO1 under basal (DMSO) conditions or in single antibody controls (Fig. 3F and Supplemental Fig. S2D). However, incubation with forskolin elicited a predominantly nuclear signal for the PKACα-CREB interaction as expected, while the signal was more diffuse for the PKACα-FOXO1 interaction (Fig. 3F). Taken together, these results indicate that exendin-4 induces S253 phosphorylation of FOXO1 via PKA, and menin suppresses PKA-mediated FOXO1 phosphorylation.
Menin increases FOXO1 levels and a menin inhibitor reverses menin-mediated elevation of FOXO1 levels and suppression of FOXO1 and CREB phosphorylation.
GLP1R agonists have been shown to modulate FOXO1 phosphorylation, which is a key event leading to its cytosolic localization, inactivation of transcriptional activity, and eventual degradation (11, 23, 24, 55, 76). Because menin suppressed phosphorylation of FOXO1 and we did not notice an effect of menin on FOXO1 levels under serum-starved conditions (Fig. 2B and Supplemental Fig. S2A), we asked whether menin altered FOXO1 levels in MEFs cultured with serum-supplemented culture medium. Menin-complemented MEFs (MEF-Men1Δ/Δ+Menin) showed increased levels of both FOXO1 mRNA and protein (Fig. 4, A and B) compared with vector controls. We then determined whether a menin inhibitor (MI-2-2) could reverse the effect of menin on FOXO1 levels, as well as the exendin-4-induced phosphorylation of FOXO1 and CREB in MIN6 cells (67). Treatment of MIN6 cells with MI-2-2, which blocks the interaction between menin and its partners, such as MLL (67) for 48 h, decreased FOXO1 mRNA levels, and exendin-4 treatment for 30 min did not further decrease the FOXO1 mRNA levels (Fig. 4C). PKA inhibitor (H-89) treatment for 30 min before exendin-4 treatment or 1 h before harvesting reversed the reduction of FOXO1 mRNA observed upon either exendin-4 or MI-2-2 treatment (Fig. 4C).
Fig. 4.
Menin increases FOXO1 levels and a menin inhibitor increases GLP1R agonist-induced and PKA-mediated phosphorylation of FOXO1 and CREB and decreases FOXO1 levels. Menin-complemented MEF cells (MEF-Men1Δ/Δ+Menin) express higher levels of FOXO1 mRNA (n = 2) (A) and FOXO1 protein (B), compared with vector-transfected menin-null (MEF-Men1Δ/Δ) MEF cells (representative blots of three similar experiments). C: MIN6 cells were starved in low-glucose, serum-free DMEM for 3 h, at which time, the cells were treated with either vehicle or MI-2-2 (500 nM) for a total of 48 h before treatment with exendin-4 (2 nM) for an additional 30 min. PKA inhibitor (H-89) pretreatment was initiated 30 min before exendin-4 treatment. Menin inhibitor (MI-2-2) treatment of MIN6 cells for 48 h decreases FOXO1 transcript levels in a concentration-dependent manner, both in the presence and absence of exendin-4 (2 nM, 30 min) (two experiments). Values are expressed as means ± SE. Data were analyzed using Student’s t-test (*P < 0.05 and $P = 0.08) or two-way ANOVA for effect of MI-2-2 on FOXO1 mRNA (P < 0.001, MI-2-2 vs. vehicle). D: menin inhibitor (MI-2-2, 500 nM) increases both basal and exendin-4 (2 nM)-induced FOXO1 and CREB phosphorylation levels (pFOXO1-S253 and pCREB-S133, respectively) and decreases FOXO1 protein levels (lanes 5 and 6) compared with vehicle alone and exendin-4 alone treatment groups (lanes 1 and 2, respectively). The effect of exendin-4 and/or menin inhibitor is reversed by PKA inhibitor, H-89 (lanes 3, 4, 7, and 8) (representative plots). Quantitation of pFOXO1/FOXO1 (E) and pCREB/CREB ratios (F) from three similar experiments for lanes 1, 2, 5, and 6. Values are expressed as means ± SE. Data were analyzed using one-way ANOVA followed by Tukey’s multiple-comparisons test (*P < 0.05 vs. DMSO, §P = 0.07 vs. Exendin, and &P = 0.09 vs. DMSO).
Furthermore, exendin-4 treatment induced phosphorylation of FOXO1 and CREB (S253 and S133, respectively) as expected, and the induced phosphorylation was blocked by treatment with a PKA inhibitor (Fig. 4D, lane 2 vs. 4). We also explored whether menin inhibitor affects exendin-4-induced phosphorylation of FOXO1. Notably, treatment with the menin inhibitor alone increased phosphorylation of FOXO1 and CREB to a similar extent as with exendin-4 treatment (Fig. 4D, lane 2 vs. 5, and Fig. 4, E and F). PKA inhibitor (H-89) inhibited this phosphorylation in both groups (Fig. 4D, lanes 5 and 6 vs. 7 and 8, respectively). Moreover, treatment of the cells with either exendin-4 alone, MI-2-2 alone, or the combination was able to reduce FOXO1 levels, and this effect was blocked by a PKA inhibitor (Fig. 4D, lanes 2, 5, and 6 vs. lanes 4, 7, and 8, respectively). Together, these results indicated that MI-2-2 increased phosphorylation of FOXO1 and CREB, and decreased FOXO1 levels in a PKA-dependent manner. However, this PKA-mediated effect was not associated with an increase in the PKACα levels (Supplemental Fig. S1B).
Menin decreases GLP1R signaling downstream of adenylyl cyclase without affecting total cellular cAMP accumulation.
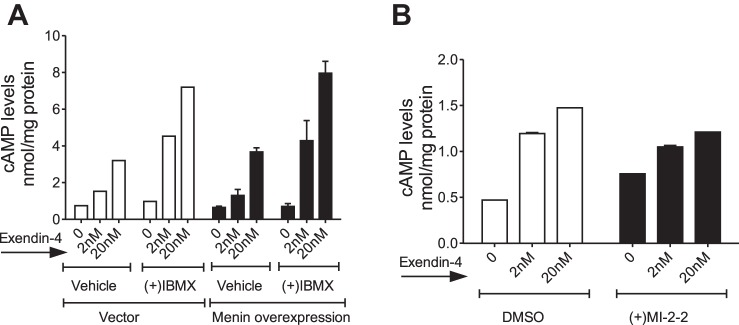
Our data indicated that apart from suppressing GLP1R transcription, menin could also act downstream of the GLP1R. First, menin suppresses phosphorylation of FOXO1 (S253) and CREB (S133), not only in response to GLP1R agonists, but also in response to forskolin in MIN6 and MEF cells (Fig. 2B, lanes 5 and 11, Fig. 2C, and Fig. 4D; and Supplemental Fig. S1A, compare even numbered lanes with odd numbered lanes (Supplemental Fig. S1A). In addition, menin suppresses CREB target genes cyclin A2 and nuclear receptor subfamily 4, group A, member 2 (NR4A2) (Supplemental Fig. S1, C and D), consistent with our previous report, wherein menin reduced the levels of IRS2 and cyclin A2 (28, 84). Furthermore, both menin and PKA inhibitor suppress forskolin-induced increases in NR4A2 mRNA in PIME cells (Supplemental Fig. S1E), indicating that menin modulates transcription of CREB target genes downstream of adenylyl cyclase activation. Considering the above and the fact that menin inhibitor did not increase PKACα levels (Fig. 4E), we asked whether this effect was mediated by increased cAMP accumulation or increased PKA activity. First, we measured cAMP accumulation in response to exendin-4 in MIN6 cells overexpressing menin or vector controls in the presence and absence of phosphodiesterase (PDE) inhibitor, 3-isobutyl-1-methylxanthine (IBMX), which prevents PDE-mediated degradation of cAMP (Fig. 5A). Exendin-4 induced cAMP accumulation in a concentration-dependent manner, and IBMX treatment further increased the cAMP levels, as expected. However, menin overexpression did not reduce cAMP accumulation compared with vector controls, both in the presence and absence of IBMX (Fig. 5A). Moreover, menin inhibitor treatment of MIN6 cells also did not increase cAMP accumulation compared with vehicle controls (Fig. 5B). Also, menin excision in PIME cells did not increase PKA activity (Supplemental Fig. S3B). Together, these data indicate that menin suppresses PKA-mediated phosphorylation, likely via mechanisms downstream of cAMP accumulation or altered PKA activity.
Fig. 5.
Menin decreases GLP1R signaling downstream of adenylyl cyclase without affecting cAMP accumulation. A: menin does not affect cAMP levels upon GLP1R agonist stimulation. MIN6 cells overexpressing menin or vector controls were starved for 48 h followed by pretreatment with the phosphodiesterase inhibitor, 3-isobutyl-1-methylxanthine (IBMX; 45 μM) for 5 min and then treated with different concentrations of exendin-4 (2 and 20 nM) for another 30 min, and cells were collected for cAMP accumulation assay. While both exendin-4 and IBMX induced cAMP accumulation as expected, menin overexpression did not differentially affect cAMP accumulation compared with vector controls, both in the presence and absence of IBMX (two experiments). B: MIN6 cells were starved in low-glucose, serum-free DMEM for 3 h, at which time, the cells were treated with either vehicle or MI-2-2 (500 nM) for a total of 48 h before treatment with varying concentrations of exendin (2 nM or 20 nM) for an additional 30 min. MI22 treatment did not affect cAMP accumulation compared with vehicle (DMSO) controls (representative plot of two similar experiments).
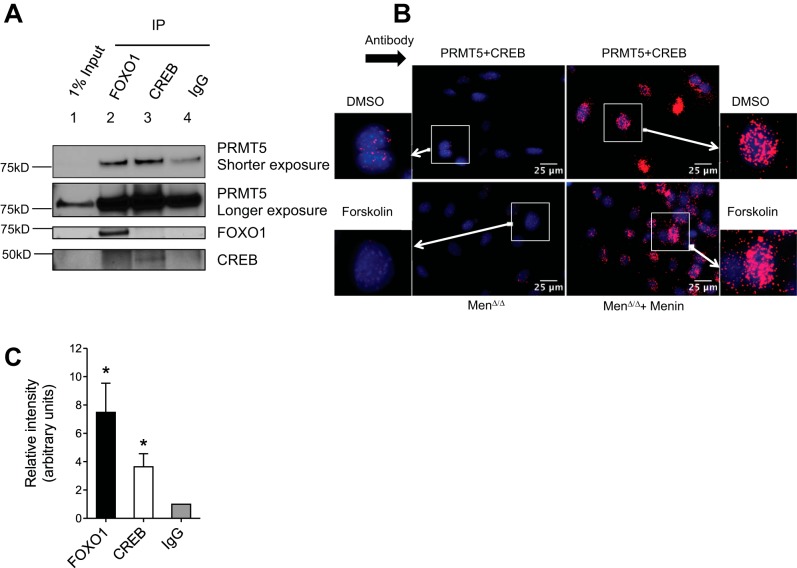
Menin recruits PRMT5 to FOXO1 and CREB to suppress their phosphorylation by PKA.
Since menin did not affect cAMP accumulation upon exendin-4 stimulation, we explored whether menin inhibits phosphorylation of CREB and FOXO1 by binding to either the substrates (CREB and FOXO1) or the enzyme (PKA), by performing coimmunoprecipitation in HEK-293T cells upon overexpression of FOXO1 and/or menin, and detected an interaction of menin via its COOH-terminal end with FOXO1 (Supplemental Fig. S2, A and B). However, we were unable to detect any direct endogenous interaction of menin with either the substrates or PKA using both coimmunoprecipitation and the proximity ligation assay (Supplemental Fig. S2C, coimmunoprecipitation data not shown). Therefore, we asked whether other mechanisms could be responsible for suppression of the phosphorylation event.
Posttranslational modifications of proteins have been shown to interfere with the ability of kinases to phosphorylate substrates (61). For example, protein arginine methyltransferase-1 (PRMT1)-mediated asymmetric methylation of arginine residues R248 and R250 suppresses Akt-mediated phosphorylation of FOXO1 (S253, mouse FOXO1) (82). In addition, the arginine residues surrounding the kinase target serine residues are conserved across species in both FOXO1 and CREB, and they are a part of the consensus sequence for PKA activity (Fig. 7A) (52, 61). Therefore, we determined whether PRMT1 interacts with either menin or FOXO1. Using proximity ligation assay, we failed to detect PRMT1 interaction with menin but detected a modest interaction of PRMT1 with FOXO1 in our system (data not shown). Because we have previously reported that menin interacts with another protein arginine methyltransferase, PRMT5, which symmetrically methylates the arginine residues, we examined whether PRMT5 interacts with FOXO1 and CREB (26, 27). To this end, we performed coimmunoprecipitation of endogenous FOXO1 and CREB in MIN6 cells and were able to coimmunoprecipitate PRMT5 with both FOXO1 (Fig. 6A, lane 2, and Fig. 6B) and CREB (Fig. 6A, lane 3, and Fig. 6B) (Supplemental Fig. S3A). We confirmed the interaction of PRMT5 with CREB using the proximity ligation assay (Fig. 6C). Interestingly, complementation of menin-null MEF cells with menin (Men1Δ/Δ+Menin) markedly increased the PRMT5-CREB interaction as compared with the vector transfected menin-null (Men1Δ/Δ) MEF cells. These data suggest that menin recruits PRMT5 to both FOXO1 and CREB.
Since PRMT5 symmetrically dimethylates arginine residues, we then asked whether symmetric dimethylation of arginine residues surrounding the target serine residues could suppress phosphorylation by PKA. To this end, we synthesized cognate peptides of CREB and FOXO1 around the serine residues phosphorylated by PKA, either with or without the symmetrically dimethylated arginine residues (Fig. 7, A and B). Arginine residues R130 and R131 were symmetrically methylated in the CREB peptide, and arginine residues R248 and R250 were symmetrically methylated in the FOXO1 peptide (Fig. 7B). The symmetrically methylated or unmodified CREB and FOXO1 peptides were then used for an in vitro kinase assay using recombinant PKACα to measure the amount of phosphorylation. We noticed a drastic decrease in the amount of phosphate incorporation upon methylation (Fig. 7C). Multiple attempts to determine whether purified PRMT5 was able to methylate peptides derived from FOXO1 and CREB, or the recombinant proteins of FOXO1 and CREB, failed to detect their methylation in the presence of tritiated SAM, even though PRMT5 was able to methylate histone H4 as a positive control (data not shown). It is possible that additional cofactors are necessary for PRMT5 to methylate FOXO1 or CREB. Alternatively, it is also possible that other types of PRMTs can mediate menin-dependent methylation. Nevertheless, these results suggest that symmetric methylation of arginines surrounding the serine residues S133 and S256, respectively in CREB and FOXO1, suppresses the ability of PKA to phosphorylate these serine residues.
To determine the functional relevance of PRMT5 in regulating FOXO1 and CREB phosphorylation, we knocked down PRMT5 in MEFs using shRNA. PRMT5 knockdown (Fig. 7D, lanes 1–3 vs. lanes 4–6, and Fig. 7E for quantitation) did not affect basal S253 pFOXO1 (Fig. 7D, lane 1 vs. lane 4, and Fig. 7F for quantitation) but resulted in remarkable increase in basal levels of S133 pCREB (Fig. 7D, lanes 1 vs. 4 and Fig. 7G for quantitation). Forskolin-mediated induction of phosphorylation of both pFOXO1 and pCREB was increased upon PRMT5 knockdown (Fig. 7D, lanes 1–3 vs. lanes 4–6, and Fig. 7, F and G for quantitation), indicating that PRMT5 suppresses PKA-mediated phosphorylation of FOXO1 and CREB. Further, PRMT5 knockdown also resulted in increased mRNA levels of CREB target genes IRS2 and NR4A2 (Fig. 7, H and I, respectively). Taken together, these results suggest that PRMT5 suppresses FOXO1 and CREB phosphorylation and could play an important role in regulating PKA-mediated gene transcription.
Menin inhibition increases proliferation in MEFs and mouse islets in a menin-dependent manner and increases GLP1R and CREB target gene transcript levels.
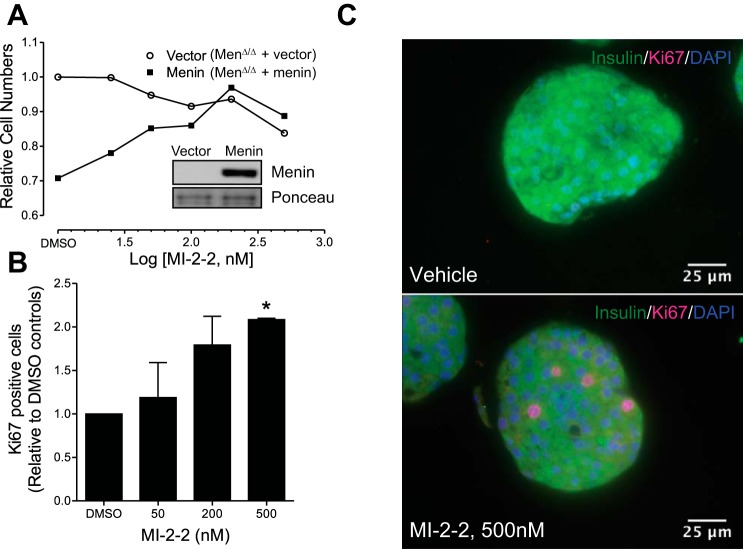
On the basis of the above data, which indicates that menin plays a crucial role in modulating GLP1R signaling, we asked whether inhibiting menin would affect proliferation in pancreatic islets. First, using the MEF cells in which proliferation is suppressed by menin complementation (Men1Δ/Δ+Menin) (Fig. 8A, compare cell numbers in DMSO controls), we demonstrated that menin inhibitor (MI-2-2) increases proliferation in a concentration-dependent manner (Fig. 8A, solid squares). However, the menin inhibitor (MI-2-2) did not affect proliferation of vector complemented menin null cells (Fig. 8A, open circles). These data indicate that the menin inhibitor-induced cell proliferation depends on the expression of target menin because the menin inhibitor failed to induce proliferation in menin-null cells (Fig. 8A).
Fig. 8.
Menin inhibitor increases proliferation in MEF cells and mouse islets in a menin-dependent manner and increases GLP1R and CREB target gene transcript levels. A: menin-null MEF cells were transfected with vector or menin (inset) and treated with varying concentrations of menin inhibitor (MI-2-2) for 6 days. Menin-expressing MEFs proliferate at a lower rate compared with menin-null MEFs under vehicle (DMSO) treatment conditions. MI-2-2 increases proliferation in MEF cells complemented with menin, in a concentration-dependent manner, but not in vector-complemented menin-null MEF (Vector) cells (representative plots of three similar experiments). B: graph showing quantification of cells expressing the proliferation marker Ki67 in mouse islets treated with vehicle (DMSO) or MI-2-2 (50, 200, and 500 nM) in a dose-dependent manner (means ± SE of two independent experiments, each using pooled islets from eight mice). C: representative immunofluorescence microscopy images showing increased number of Ki67 expressing cells in mouse islets treated with MI22 (500 nM, bottom) compared with vehicle (DMSO)-treated islets (top). *Significantly different (P < 0.05) compared with vehicle-treated controls using unpaired t-test.
To further determine whether the menin inhibitor can also affect proliferation of primary mouse islets, we isolated and treated murine pancreatic islets with the menin inhibitor for 48 h followed by immunohistochemistry analysis for the proliferation marker Ki67. The results indicate that menin inhibitor increased proliferation in a concentration-dependent manner, as evidenced by increased incidence of Ki67-positive cells in the mouse islets (Fig. 8, B and C). We extended our studies using MI-2-2 to human islets. Treatment of human islets with different concentrations of MI-2-2 (50 nM, 200 nM, and 500 nM) for 48 h increased GLP1R mRNA levels approximately twofold when compared with vehicle controls (Fig. 9A). To explore the effect of this increase in GLP1R mRNA with MI-2-2 treatment on proliferation, we treated islets from two other donors for a longer duration of 7 days and measured both GLP1R and Ki67 mRNA. Treatment with either MI-2-2 alone (250 nM and 500 nM) or exendin-4 alone (100 nM) increased both GLP1R mRNA and Ki67 mRNA twofold in islets from an 11-yr-old donor (donor 2) (Fig. 9, B and C). Treatment with a combination of MI-2-2 (500 nM) and exendin-4 (100 nM) did not appear to increase the levels of either GLP1R or Ki67 mRNAs, beyond that achieved by single treatment alone (Fig. 9, B and C). Treatment of islets from a 37-yr-old donor (donor 3) increased GLP1R mRNA marginally (13–50%) when compared with vehicle treatment (Fig. 9D). MI-2-2 treatment alone did not affect Ki67 mRNA levels, while treatment with exendin-4 alone (100 nM) or the combination of MI-2-2 (500 nM) and exendin-4 (100 nM) increased the Ki67 mRNA levels by ~30% (Fig. 9E). We speculate that this difference in response to treatments between the two donors might be related to differential plasticity for stimulating proliferation owing to the age difference (3, 63). Another key observation from these experiments is that the magnitude of increase in Ki67 mRNA corresponds to the magnitude of increase in GLP1R mRNA (Fig. 9, B–E), further supporting our hypothesis that menin suppresses proliferation in pancreatic islets, at least partly, by inhibiting GLP1R signaling.
Fig. 9.
Menin inhibitor increases transcript levels of GLP1R and proliferation marker Ki67 in human islets. A: menin inhibitor treatment (50, 200, and 500 nM) of human islets from donor 1 (50 yr old) for 48 h increases GLP1R mRNA levels compared with vehicle controls. Treatment of human islets for 7 days with either menin inhibitor alone (MI-2-2, 250 nM or 500 nM), exendin-4 alone (100 nM), or a combination of MI22 (500 nM) and exendin-4 (100 nM) increases GLP1R mRNA levels (B and D) and Ki67 mRNA levels (C and E) compared with vehicle (DMSO)-treated controls. F: schematic depicting proposed role of menin and PRMT5 in regulating GLP1 signaling: 1, Menin and PRMT5 downregulate GLP1R transcript; 2, GLP1R agonists and forskolin induce phosphorylation of FOXO1 via a PKA-mediated mechanism; 3, Menin suppresses GLP1R agonist and forskolin-induced and PKA-mediated phosphorylation of FOXO1 and CREB; 4, Menin increases both transcript and protein levels of FOXO1; 5, PRMT5 interacts with CREB and FOXO1, and symmetric dimethylation of conserved arginines surrounding the target serines blocks PKA-mediated phosphorylation of these serine residues.
DISCUSSION
In this study, we found that menin suppresses pancreatic β-cell proliferation, at least partly, by suppressing GLP1 signaling (Fig. 9F). Menin achieves this suppression by transcriptional downregulation of the GLP1R, and via suppression of PKA-mediated phosphorylation of CREB (S133) and FOXO1 (human S256, mouse S253), both involving a role for PRMT5. In addition, we demonstrate the utility of a menin inhibitor to reverse the menin-mediated suppression and to increase proliferation in mouse and human islets. This is the first study to identify that 1) menin and PRMT5 downregulate GLP1R transcript; 2) GLP1R agonists and forskolin induce phosphorylation of FOXO1 via a PKA-mediated mechanism; 3) menin suppresses GLP1R agonist and forskolin-induced, and PKA-mediated phosphorylation of FOXO1 and CREB; 4) menin increases both transcript and protein levels of FOXO1; and 5) PRMT5 interacts with CREB and FOXO1, and symmetric dimethylation of conserved arginines surrounding the target serines blocks PKA-mediated phosphorylation of these serine residues. Although our current study lacks direct evidence of PRMT5-mediated methylation of either CREB or FOXO1, we show that loss of PRMT5 leads to increased phosphorylation of both FOXO1 and CREB.
Menin suppresses GLP1R transcript, possibly via recruitment of PRMT5.
We demonstrate here that menin suppresses GLP1R transcript levels via recruitment to the GLP1R promoter of the menin binding partner PRMT5, which is a known transcriptional repressor (26, 27). In addition, treatment with a menin inhibitor increased the GLP1R mRNA levels in human islets. Waser et al. (77) demonstrated recently that GLP1R expression within the human pancreas is primarily restricted to insulin and somatostatin-producing cells, with high levels of the receptor being expressed in benign insulinomas. Furthermore, they demonstrated using normal and neoplastic human pancreatic tissues that GLP1R is unlikely to be related to neoplastic transformation (77). The increase in GLP1R levels observed in the liver upon menin excision not only offers corroborative evidence that menin regulates GLP1R transcript levels but also suggests a broader role for menin in maintaining glucose homeostasis. GLP1 receptors in the liver have been reported to be involved in hepatoportal glucose sensing and the suppression of hepatic glucose production, and they are required for antidiabetic actions of oligofructose (6, 13, 59). In light of our findings and the findings of previous studies discussed above, the increase in GLP1R mRNA levels observed with loss of menin function and PRMT5 could have important physiological and therapeutic implications to increase β-cell mass and treat diabetes, which we explored further in our study.
GLP1R and forskolin-induced phosphorylation of FOXO1 is PKA mediated.
We demonstrate here using Akt and PKA inhibitors, in vitro kinase assay, and proximity ligation assay that PKA associates with and mediates phosphorylation of FOXO1 induced by GLP1R agonist and forskolin. In agreement with our results, Lee et al. (38) have previously demonstrated that prostaglandin E2-mediated phosphorylation of FOXO1 in vascular endothelial cells is a PKA-mediated process. However, to date, this is the first report indicating that GLP1R-induced phosphorylation of FOXO1 in β-cells is a PKA-mediated process. Although we demonstrate here that GLP1 agonists induce FOXO1 phosphorylation directly via PKA, it is possible that the insulinotropic effect of GLP1 agonists also contributes to the physiological prolongation of this effect via autocrine stimulation of insulin signaling. A consequence of FOXO1 phosphorylation is that it marks FOXO1 for degradation leading to a decrease in total FOXO1 levels (23). Reports that both MIN6 cells and human islets secrete GLP1 (48, 73), coupled with our observations that a short-term (30–60 min) PKA inhibitor treatment inhibits basal, GLP1 agonist, and menin inhibitor-induced phosphorylation of FOXO1, and increases FOXO1 levels further emphasize the role of GLP1 signaling via PKA in maintaining FOXO1 levels.
Menin and PRMT5 suppress GLP1R agonist and forskolin-induced, PKA-mediated phosphorylation of FOXO1 and CREB to affect FOXO1 levels and CREB target genes, respectively.
We demonstrate here that menin and PRMT5 suppress the GLP1R- and forskolin-induced phosphorylation of both CREB and FOXO1, which, in turn, could affect CREB target gene transcription and FOXO1 levels. The observations in this study with regard to the effect of menin and PRMT5 on CREB target genes, cyclin A2, NR4A2, and IRS2 are consistent with our previous report, wherein menin reduced the levels of IRS2 and cyclin A2 (28, 84).
With respect to FOXO1, we demonstrate that menin mediates its effect on phosphorylation of FOXO1 and FOXO1 levels via suppression of PKA signaling. Although the apparent lack of increase in FOXO1 levels upon menin overexpression under serum-starved and low-glucose conditions might appear contradictory (Fig. 2B and Supplemental Fig. S1A), this observation further points toward the key role of the phosphorylation event in maintaining FOXO1 levels. The lack of a sustained kinase-activating stimulus, either serum growth factors or GLP1R agonists, leads to a decrease in pFOXO1 levels and buildup of FOXO1, and this cannot be reversed by a short-term (30 min) treatment with GLP1R agonists or forskolin (Fig. 2, B and D). However, upon exposure to a prolonged kinase-activating stimulus like serum or high glucose, the role of menin in increasing FOXO1 levels becomes apparent (Fig. 4B). The fact that the menin inhibitor alone increases pFOXO1 levels and decreases total FOXO1 levels in MIN6 cells despite the serum-depleted and low-glucose culture conditions, and without the need for GLP1R agonist stimulation, might be related to the secretory capacity of these cells. Since menin has been shown to decrease insulin synthesis and secretion (64), it is possible that the menin inhibitor effect observed here is mediated by increased autocrine stimulation, resulting from increased insulin secretion upon inhibiting menin. In addition, GLP1 secreted by MIN6 cells could play a role in increasing pFOXO1 and reducing FOXO1 levels upon menin inhibitor treatment (48).
Apart from regulating the degradation of FOXO1, PKA-mediated phosphorylation could further regulate FOXO1 levels by modulating transcription. As it has been reported that FOXO1 can bind to its own promoter to promote transcription (20), it is conceivable that the modulation of FOXO1 transcript levels by menin or its inhibitor (Fig. 4, A and C, respectively) are mediated, at least partially, by an autoregulatory feedback loop involving regulation of FOXO1 levels by a phosphorylation event.
Menin does not decrease whole cell cAMP accumulation.
Neither menin nor the menin inhibitor altered whole cell cAMP levels. In addition, deletion of menin in PIME cells did not alter PKA activity (Supplemental Fig. S3B). Although these observations helped us to hypothesize other mechanisms leading to menin-mediated suppression of GLP1R-induced phosphorylation of FOXO1 and CREB, they do not definitively eliminate the possibility that menin regulates adenylyl cyclase or PKA activities in specific subcellular compartments. The cAMP levels and PKA activity are regulated by means of compartmentalization within specific subcellular locations, through mechanisms involving PDEs, A-kinase anchoring proteins (AKAPs), and cellular and temporal barriers (21, 29, 33, 39, 54, 58, 60, 83, 89). It is possible that menin modulates these compartmentalized cAMP levels or PKA activity, which we have not tested in our study.
PRMT5 interacts with both CREB and FOXO1 and might play a role in suppressing PKA-mediated phosphorylation of these substrates.
To our knowledge, this is the first report of PRMT5 interaction with FOXO1 and CREB to suppress their phosphorylation, role of menin in modulating this interaction, and symmetric dimethylation suppressing PKA-mediated phosphorylation of its substrates. Despite confirming the role of PRMT5 in suppressing PKA-mediated phosphorylation of FOXO1 and CREB, our study is limited by the lack of direct evidence supporting PRMT5-mediated methylation of FOXO1 and CREB. We attempted to test whether PRMT5 can methylate full-length FOXO1 and CREB by an in vitro methylation assay using 1) recombinant PRMT5-MEP50, FOXO1, and CREB, and 2) PRMT5, FOXO1, and CREB immunoprecipitated from HEK-293T cells transduced with the respective plasmids for overexpression (data not shown). However, despite detecting methylation of histone H4 used as a positive control, we were not able to detect any considerable methylation of either FOXO1 or CREB in our experiments (data not shown). The presence of other protein regulators is sometimes required to observe PRMT activity; for example, MEP50 has been shown to alter PRMT5 activity and affinity to substrates like histone H4 (46, 65). It is possible that we failed to detect in vitro PRMT5 activity with FOXO1 and CREB because of the absence of an essential, but currently unidentified, regulator in our experiments.
Menin inhibitor increases proliferation in islets.
The menin inhibitor (MI-2-2) used in this study was able to increase proliferation in menin-expressing MEFs in a menin-dependent manner. Treatment with MI-2-2 also increased proliferation in mouse islets in a dose-dependent manner, confirming the utility of menin inhibitors to increase proliferation in islets. However, in human islets, the limited number of samples and inability to culture these samples for longer durations (beyond 7 days) limits our studies. Increasing the in vitro treatment duration beyond 7 days led to an overall decrease in islet mRNA quality and yield, indicating a loss of islets due to prolonged culture (data not shown). Despite the mixed results, those islets showing the highest increases in GLP1R mRNA also showed higher increases in the proliferation marker Ki67 mRNA. Investigating further, we recognized that islets from younger donors showed the highest increases in both GLP1R and Ki67 mRNA, indicating that age might be a consideration to be made, while employing these inhibitors. In addition, donor 3 was overweight with a body mass index (BMI) of 27.8 kg/m2, compared with a BMI of 24.3 kg/m2 for the 50-yr-old donor 1 and 16.8 kg/m2 for the 11-yr-old donor 2, raising the possibility that comorbidities associated with overweight contributed to this differential effect. The limitation of in vitro culture could have also affected the ability to observe an additive effect of exendin-4 on menin inhibitor-mediated increase in proliferation marker. Although the majority of cells from islets are β-cells and the increase of Ki67 mRNA likely, at least in part, reflected the increase of β-cell replication, at this time, we cannot rule out that the menin inhibitor may also increase replication of other types of cells in human islets. Similarly, our study is also limited by lack of evidence concerning the effect of menin or PRMT5 on GLP1R protein levels. Further work is needed to demonstrate a physiological impact of menin loss/gain on GLP1R-mediated glucose homeostasis. Therefore, additional studies are needed to examine the effect of the menin inhibitor. Regardless of the temporal limitations of in vitro culture, our study demonstrates that menin inhibitor can increase proliferation in islets, possibly via increasing GLP1R levels and signaling. This increase in GLP1R levels could further be harnessed for combination therapy with a GLP1R agonist to increase proliferation and β-cell mass.
In conclusion, our study demonstrates the following novel findings: menin and PRMT5 downregulate GLP1R transcript; GLP1R-mediated phosphorylation of FOXO1 is PKA mediated, unlike previous reports identifying it as an Akt-mediated process; and menin increases FOXO1 levels. Menin suppresses GLP1R-mediated phosphorylation of both CREB and FOXO1, involving mechanisms beyond the downregulation of GLP1R. PRMT5 interacts with CREB and FOXO1 and suppresses their phosphorylation, and symmetric dimethylation of arginine residues surrounding target serines in substrates inhibits the ability of PKA to phosphorylate these substrates. Finally, menin inhibition increases GLP1R levels, GLP1 agonist-mediated phosphorylation of FOXO1 and CREB, and cell proliferation in islets. These findings offer a rationale to further study menin function and its inhibitors to increase β-cell mass and to treat diabetes.
GRANTS
We acknowledge the services of Islet Cell Biology Core at the University of Pennsylvania’s Institute of Diabetes Obesity and Metabolism, which is funded by the Diabetes Research Center Grant (P30DK19525). This work was funded, in part, by a Juvenile Diabetes Research Foundation (JDRF) postdoctoral fellowship (3-PDF-2014-201-A-N) awarded to AB. Muhammad, a JDRF innovative grant, and NIH Grant R01DK097555 (to X. Hua).
DISCLOSURES
X. Hua holds equity in Novapeutics, LLC.
AUTHOR CONTRIBUTIONS
A.B.M. and X.H. conceived and designed research; A.B.M., B.X., and C.L. performed experiments; A.B.M., C.L., A.N., X.M., R.A.S., and X.H. analyzed data; A.B.M., C.L., A.N., X.M., R.A.S., and X.H. interpreted results of experiments; A.B.M. and B.X. prepared figures; A.B.M. drafted manuscript; A.B.M., A.N., X.M., R.A.S., and X.H. edited and revised manuscript; R.A.S. and X.H. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge the Islet Cell Biology Core at the Institute of Diabetes Obesity and Metabolism, of the University of Pennsylvania, for isolating murine islets used in experiments to study the in vitro effect of MI-2-2.
REFERENCES
- 1.Agudo J, Ayuso E, Jimenez V, Salavert A, Casellas A, Tafuro S, Haurigot V, Ruberte J, Segovia JC, Bueren J, Bosch F. IGF-I mediates regeneration of endocrine pancreas by increasing beta cell replication through cell cycle protein modulation in mice. Diabetologia 51: 1862–1872, 2008. doi: 10.1007/s00125-008-1087-8. [DOI] [PubMed] [Google Scholar]
- 2.Alessi DR, Cohen P. Mechanism of activation and function of protein kinase B. Curr Opin Genet Dev 8: 55–62, 1998. doi: 10.1016/S0959-437X(98)80062-2. [DOI] [PubMed] [Google Scholar]
- 3.Arda HE, Li L, Tsai J, Torre EA, Rosli Y, Peiris H, Spitale RC, Dai C, Gu X, Qu K, Wang P, Wang J, Grompe M, Scharfmann R, Snyder MS, Bottino R, Powers AC, Chang HY, Kim SK. Age-dependent pancreatic gene regulation reveals mechanisms governing human β cell function. Cell Metab 23: 909–920, 2016. doi: 10.1016/j.cmet.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aroor A, Nistala R. Tissue-specific expression of GLP1R in mice: is the problem of antibody nonspecificity solved? Diabetes 63: 1182–1184, 2014. doi: 10.2337/db13-1937. [DOI] [PubMed] [Google Scholar]
- 5.Assmann A, Ueki K, Winnay JN, Kadowaki T, Kulkarni RN. Glucose effects on beta-cell growth and survival require activation of insulin receptors and insulin receptor substrate 2. Mol Cell Biol 29: 3219–3228, 2009. doi: 10.1128/MCB.01489-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ayala JE, Bracy DP, James FD, Julien BM, Wasserman DH, Drucker DJ. The glucagon-like peptide-1 receptor regulates endogenous glucose production and muscle glucose uptake independent of its incretin action. Endocrinology 150: 1155–1164, 2009. doi: 10.1210/en.2008-0945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bastien-Dionne PO, Valenti L, Kon N, Gu W, Buteau J. Glucagon-like peptide 1 inhibits the sirtuin deacetylase SirT1 to stimulate pancreatic β-cell mass expansion. Diabetes 60: 3217–3222, 2011. doi: 10.2337/db11-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borowiak M, Melton DA. How to make beta cells? Curr Opin Cell Biol 21: 727–732, 2009. doi: 10.1016/j.ceb.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bouchi R, Foo KS, Hua H, Tsuchiya K, Ohmura Y, Sandoval PR, Ratner LE, Egli D, Leibel RL, Accili D. FOXO1 inhibition yields functional insulin-producing cells in human gut organoid cultures. Nat Commun 5: 4242, 2014. doi: 10.1038/ncomms5242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buteau J, Accili D. Regulation of pancreatic beta-cell function by the forkhead protein FoxO1. Diabetes Obes Metab 9, Suppl 2: 140–146, 2007. doi: 10.1111/j.1463-1326.2007.00782.x. [DOI] [PubMed] [Google Scholar]
- 11.Buteau J, Spatz ML, Accili D. Transcription factor FoxO1 mediates glucagon-like peptide-1 effects on pancreatic beta-cell mass. Diabetes 55: 1190–1196, 2006. doi: 10.2337/db05-0825. [DOI] [PubMed] [Google Scholar]
- 12.Butler PC, Meier JJ, Butler AE, Bhushan A. The replication of beta cells in normal physiology, in disease and for therapy. Nat Clin Pract Endocrinol Metab 3: 758–768, 2007. doi: 10.1038/ncpendmet0647. [DOI] [PubMed] [Google Scholar]
- 13.Cani PD, Knauf C, Iglesias MA, Drucker DJ, Delzenne NM, Burcelin R. Improvement of glucose tolerance and hepatic insulin sensitivity by oligofructose requires a functional glucagon-like peptide 1 receptor. Diabetes 55: 1484–1490, 2006. doi: 10.2337/db05-1360. [DOI] [PubMed] [Google Scholar]
- 14.Chowdhury S, Wang X, Srikant CB, Li Q, Fu M, Gong YJ, Ning G, Liu JL. IGF-I stimulates CCN5/WISP2 gene expression in pancreatic β-cells, which promotes cell proliferation and survival against streptozotocin. Endocrinology 155: 1629–1642, 2014. doi: 10.1210/en.2013-1735. [DOI] [PubMed] [Google Scholar]
- 15.Cierpicki T, Grembecka J. Challenges and opportunities in targeting the menin-MLL interaction. Future Med Chem 6: 447–462, 2014. doi: 10.4155/fmc.13.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Claiborn KC, Stoffers DA. Toward a cell-based cure for diabetes: advances in production and transplant of beta cells. Mt Sinai J Med 75: 362–371, 2008. doi: 10.1002/msj.20058. [DOI] [PubMed] [Google Scholar]
- 17.Cornu M, Modi H, Kawamori D, Kulkarni RN, Joffraud M, Thorens B. Glucagon-like peptide-1 increases beta-cell glucose competence and proliferation by translational induction of insulin-like growth factor-1 receptor expression. J Biol Chem 285: 10538–10545, 2010. doi: 10.1074/jbc.M109.091116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D’Alessio D, Vahl T, Prigeon R. Effects of glucagon-like peptide 1 on the hepatic glucose metabolism. Horm Metab Res 36: 837–841, 2004. doi: 10.1055/s-2004-826172. [DOI] [PubMed] [Google Scholar]
- 19.Escribano O, Gómez-Hernández A, Díaz-Castroverde S, Nevado C, García G, Otero YF, Perdomo L, Beneit N, Benito M. Insulin receptor isoform A confers a higher proliferative capability to pancreatic beta cells enabling glucose availability and IGF-I signaling. Mol Cell Endocrinol 409: 82–91, 2015. doi: 10.1016/j.mce.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 20.Essaghir A, Dif N, Marbehant CY, Coffer PJ, Demoulin JB. The transcription of FOXO genes is stimulated by FOXO3 and repressed by growth factors. J Biol Chem 284: 10,334–10,342, 2009. doi: 10.1074/jbc.M808848200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Faruque OM, Le-Nguyen D, Lajoix AD, Vives E, Petit P, Bataille D, Hani EH. Cell-permeable peptide-based disruption of endogenous PKA-AKAP complexes: a tool for studying the molecular roles of AKAP-mediated PKA subcellular anchoring. Am J Physiol Cell Physiol 296: C306–C316, 2009. doi: 10.1152/ajpcell.00216.2008. [DOI] [PubMed] [Google Scholar]
- 22.George M, Ayuso E, Casellas A, Costa C, Devedjian JC, Bosch F. Beta cell expression of IGF-I leads to recovery from type 1 diabetes. J Clin Invest 109: 1153–1163, 2002. doi: 10.1172/JCI0212969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Glauser DA, Schlegel W. The emerging role of FOXO transcription factors in pancreatic beta cells. J Endocrinol 193: 195–207, 2007. doi: 10.1677/JOE-06-0191. [DOI] [PubMed] [Google Scholar]
- 24.Gross DN, van den Heuvel AP, Birnbaum MJ. The role of FoxO in the regulation of metabolism. Oncogene 27: 2320–2336, 2008. doi: 10.1038/onc.2008.25. [DOI] [PubMed] [Google Scholar]
- 25.Gupta NA, Mells J, Dunham RM, Grakoui A, Handy J, Saxena NK, Anania FA. Glucagon-like peptide-1 receptor is present on human hepatocytes and has a direct role in decreasing hepatic steatosis in vitro by modulating elements of the insulin signaling pathway. Hepatology 51: 1584–1592, 2010. doi: 10.1002/hep.23569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gurung B, Feng Z, Hua X. Menin directly represses Gli1 expression independent of canonical Hedgehog signaling. Mol Cancer Res 11: 1215–1222, 2013. doi: 10.1158/1541-7786.MCR-13-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gurung B, Feng Z, Iwamoto DV, Thiel A, Jin G, Fan CM, Ng JM, Curran T, Hua X. Menin epigenetically represses Hedgehog signaling in MEN1 tumor syndrome. Cancer Res 73: 2650–2658, 2013. doi: 10.1158/0008-5472.CAN-12-3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gurung B, Muhammad AB, Hua X. Menin is required for optimal processing of the microRNA let-7a. J Biol Chem 289: 9902–9908, 2014. doi: 10.1074/jbc.M113.520692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Horvat SJ, Deshpande DA, Yan H, Panettieri RA, Codina J, DuBose TD Jr, Xin W, Rich TC, Penn RB. A-kinase anchoring proteins regulate compartmentalized cAMP signaling in airway smooth muscle. FASEB J 26: 3670–3679, 2012. doi: 10.1096/fj.11-201020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang J, Gurung B, Wan B, Matkar S, Veniaminova NA, Wan K, Merchant JL, Hua X, Lei M. The same pocket in menin binds both MLL and JUND but has opposite effects on transcription. Nature 482: 542–546, 2012. doi: 10.1038/nature10806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jamiolkowski RM, Guo LY, Li YR, Shaffer SM, Naji A. Islet transplantation in type I diabetes mellitus. Yale J Biol Med 85: 37–43, 2012. [PMC free article] [PubMed] [Google Scholar]
- 32.Jhala US, Canettieri G, Screaton RA, Kulkarni RN, Krajewski S, Reed J, Walker J, Lin X, White M, Montminy M. cAMP promotes pancreatic beta-cell survival via CREB-mediated induction of IRS2. Genes Dev 17: 1575–1580, 2003. doi: 10.1101/gad.1097103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Josefsen K, Lee YC, Thams P, Efendic S, Nielsen JH. AKAP 18 alpha and gamma have opposing effects on insulin release in INS-1E cells. FEBS Lett 584: 81–85, 2010. doi: 10.1016/j.febslet.2009.10.086. [DOI] [PubMed] [Google Scholar]
- 34.Karnik SK, Chen H, McLean GW, Heit JJ, Gu X, Zhang AY, Fontaine M, Yen MH, Kim SK. Menin controls growth of pancreatic beta-cells in pregnant mice and promotes gestational diabetes mellitus. Science 318: 806–809, 2007. doi: 10.1126/science.1146812. [DOI] [PubMed] [Google Scholar]
- 35.Kim SJ, Nian C, McIntosh CH. Glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1 modulate beta-cell chromatin structure. J Biol Chem 284: 12896–12904, 2009. doi: 10.1074/jbc.M809046200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kitamura T, Ido Kitamura Y. Role of FoxO proteins in pancreatic beta cells. Endocr J 54: 507–515, 2007. doi: 10.1507/endocrj.KR-109. [DOI] [PubMed] [Google Scholar]
- 37.Kitamura T, Nakae J, Kitamura Y, Kido Y, Biggs WH III, Wright CV, White MF, Arden KC, Accili D. The forkhead transcription factor Foxo1 links insulin signaling to Pdx1 regulation of pancreatic beta cell growth. J Clin Invest 110: 1839–1847, 2002. doi: 10.1172/JCI200216857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee JW, Chen H, Pullikotil P, Quon MJ. Protein kinase A-alpha directly phosphorylates FoxO1 in vascular endothelial cells to regulate expression of vascular cellular adhesion molecule-1 mRNA. J Biol Chem 286: 6423–6432, 2011. doi: 10.1074/jbc.M110.180661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lester LB, Langeberg LK, Scott JD. Anchoring of protein kinase A facilitates hormone-mediated insulin secretion. Proc Natl Acad Sci USA 94: 14,942–14,947, 1997. doi: 10.1073/pnas.94.26.14942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lu Y, Fei XQ, Yang SF, Xu BK, Li YY. Glucose-induced microRNA-17 promotes pancreatic beta cell proliferation through down-regulation of menin. Eur Rev Med Pharmacol Sci 19: 624–629, 2015. [PubMed] [Google Scholar]
- 41.Ma F, Wei Z, Shi C, Gan Y, Lu J, Frank SJ, Balducci J, Huang Y. Signaling cross talk between growth hormone (GH) and insulin-like growth factor-I (IGF-I) in pancreatic islet β-cells. Mol Endocrinol 25: 2119–2133, 2011. doi: 10.1210/me.2011-1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marrif HI, Al-Sunousi SI. Pancreatic β cell mass death. Front Pharmacol 7: 83, 2016. doi: 10.3389/fphar.2016.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matkar S, Thiel A, Hua X. Menin: a scaffold protein that controls gene expression and cell signaling. Trends Biochem Sci 38: 394–402, 2013. doi: 10.1016/j.tibs.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miao XY, Gu ZY, Liu P, Hu Y, Li L, Gong YP, Shu H, Liu Y, Li CL. The human glucagon-like peptide-1 analogue liraglutide regulates pancreatic beta-cell proliferation and apoptosis via an AMPK/mTOR/P70S6K signaling pathway. Peptides 39: 71–79, 2013. doi: 10.1016/j.peptides.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 45.Modi H, Jacovetti C, Tarussio D, Metref S, Madsen OD, Zhang FP, Rantakari P, Poutanen M, Nef S, Gorman T, Regazzi R, Thorens B. Autocrine action of IGF2 regulates adult β-cell mass and function. Diabetes 64: 4148–4157, 2015. doi: 10.2337/db14-1735. [DOI] [PubMed] [Google Scholar]
- 46.Morales Y, Cáceres T, May K, Hevel JM. Biochemistry and regulation of the protein arginine methyltransferases (PRMTs). Arch Biochem Biophys 590: 138–152, 2016. doi: 10.1016/j.abb.2015.11.030. [DOI] [PubMed] [Google Scholar]
- 47.Nakae J, Biggs WH III, Kitamura T, Cavenee WK, Wright CV, Arden KC, Accili D. Regulation of insulin action and pancreatic beta-cell function by mutated alleles of the gene encoding forkhead transcription factor Foxo1. Nat Genet 32: 245–253, 2002. doi: 10.1038/ng890. [DOI] [PubMed] [Google Scholar]
- 48.Nakashima K, Shimoda M, Hamamoto S, Tatsumi F, Hirukawa H, Tawaramoto K, Kanda Y, Kaku K. Self-inducible secretion of glucagon-like peptide-1 (GLP-1) that allows MIN6 cells to maintain insulin secretion and insure cell survival. Mol Cell Endocrinol 349: 281–288, 2012. doi: 10.1016/j.mce.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 49.Obenauer JC, Cantley LC, Yaffe MB. Scansite 2.0: Proteome-wide prediction of cell signaling interactions using short sequence motifs. Nucleic Acids Res 31: 3635–3641, 2003. doi: 10.1093/nar/gkg584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oliveira JM, Rebuffat SA, Gasa R, Gomis R. Targeting type 2 diabetes: lessons from a knockout model of insulin receptor substrate 2. Can J Physiol Pharmacol 92: 613–620, 2014. doi: 10.1139/cjpp-2014-0114. [DOI] [PubMed] [Google Scholar]
- 51.Pagliuca FW, Melton DA. How to make a functional β-cell. Development 140: 2472–2483, 2013. doi: 10.1242/dev.093187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pearce LR, Komander D, Alessi DR. The nuts and bolts of AGC protein kinases. Nat Rev Mol Cell Biol 11: 9–22, 2010. doi: 10.1038/nrm2822. [DOI] [PubMed] [Google Scholar]
- 53.Pedersen J, Holst JJ. Glucagon like-peptide 1 receptor and the liver. Liver Int 31: 1243–1245, 2011. doi: 10.1111/j.1478-3231.2011.02626.x. [DOI] [PubMed] [Google Scholar]
- 54.Peercy BE, Sherman AS. How pancreatic beta-cells discriminate long and short timescale cAMP signals. Biophys J 99: 398–406, 2010. doi: 10.1016/j.bpj.2010.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Portha B, Tourrel-Cuzin C, Movassat J. Activation of the GLP-1 receptor signalling pathway: a relevant strategy to repair a deficient beta-cell mass. Exp Diabetes Res 2011: 376509, 2011. doi: 10.1155/2011/376509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Prigeon RL, Quddusi S, Paty B, D’Alessio DA. Suppression of glucose production by GLP-1 independent of islet hormones: a novel extrapancreatic effect. Am J Physiol Endocrinol Metab 285: E701–E707, 2003. doi: 10.1152/ajpendo.00024.2003. [DOI] [PubMed] [Google Scholar]
- 57.Pyke C, Knudsen LB. The glucagon-like peptide-1 receptor—or not? Endocrinology 154: 4–8, 2013. doi: 10.1210/en.2012-2124. [DOI] [PubMed] [Google Scholar]
- 58.Rababa’h A, Singh S, Suryavanshi SV, Altarabsheh SE, Deo SV, McConnell BK. Compartmentalization role of A-kinase anchoring proteins (AKAPs) in mediating protein kinase A (PKA) signaling and cardiomyocyte hypertrophy. Int J Mol Sci 16: 218–229, 2014. doi: 10.3390/ijms16010218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Redondo A, Trigo MV, Acitores A, Valverde I, Villanueva-Peñacarrillo ML. Cell signalling of the GLP-1 action in rat liver. Mol Cell Endocrinol 204: 43–50, 2003. doi: 10.1016/S0303-7207(03)00146-1. [DOI] [PubMed] [Google Scholar]
- 60.Rogne M, Taskén K. Compartmentalization of cAMP signaling in adipogenesis, lipogenesis, and lipolysis. Horm Metab Res 46: 833–840, 2014. doi: 10.1055/s-0034-1389955. [DOI] [PubMed] [Google Scholar]
- 61.Rust HL, Thompson PR. Kinase consensus sequences: a breeding ground for crosstalk. ACS Chem Biol 6: 881–892, 2011. doi: 10.1021/cb200171d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sable CL, Filippa N, Hemmings B, Van Obberghen E. cAMP stimulates protein kinase B in a Wortmannin-insensitive manner. FEBS Lett 409: 253–257, 1997. doi: 10.1016/S0014-5793(97)00518-8. [DOI] [PubMed] [Google Scholar]
- 63.Saunders D, Powers AC. Replicative capacity of β-cells and type 1 diabetes. J Autoimmun 71: 59–68, 2016. doi: 10.1016/j.jaut.2016.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sayo Y, Murao K, Imachi H, Cao WM, Sato M, Dobashi H, Wong NC, Ishida T. The multiple endocrine neoplasia type 1 gene product, menin, inhibits insulin production in rat insulinoma cells. Endocrinology 143: 2437–2440, 2002. doi: 10.1210/endo.143.6.8950. [DOI] [PubMed] [Google Scholar]
- 65.Schapira M, Ferreira de Freitas R. Structural biology and chemistry of protein arginine methyltransferases. MedChemComm 5: 1779–1788, 2014. doi: 10.1039/C4MD00269E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schnepp RW, Chen YX, Wang H, Cash T, Silva A, Diehl JA, Brown E, Hua X. Mutation of tumor suppressor gene Men1 acutely enhances proliferation of pancreatic islet cells. Cancer Res 66: 5707–5715, 2006. doi: 10.1158/0008-5472.CAN-05-4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shi A, Murai MJ, He S, Lund G, Hartley T, Purohit T, Reddy G, Chruszcz M, Grembecka J, Cierpicki T. Structural insights into inhibition of the bivalent menin-MLL interaction by small molecules in leukemia. Blood 120: 4461–4469, 2012. doi: 10.1182/blood-2012-05-429274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Söderberg O, Leuchowius KJ, Gullberg M, Jarvius M, Weibrecht I, Larsson LG, Landegren U. Characterizing proteins and their interactions in cells and tissues using the in situ proximity ligation assay. Methods 45: 227–232, 2008. doi: 10.1016/j.ymeth.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 69.Song WJ, Schreiber WE, Zhong E, Liu FF, Kornfeld BD, Wondisford FE, Hussain MA. Exendin-4 stimulation of cyclin A2 in beta-cell proliferation. Diabetes 57: 2371–2381, 2008. doi: 10.2337/db07-1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stamateris RE, Sharma RB, Kong Y, Ebrahimpour P, Panday D, Ranganath P, Zou B, Levitt H, Parambil NA, O’Donnell CP, García-Ocaña A, Alonso LC. Glucose induces mouse beta cell proliferation via IRS2, mTOR and cyclin D2 but not the insulin receptor. Diabetes 65: 981–995, 2016. doi: 10.2337/db15-0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tao H, Wang MM, Zhang M, Zhang SP, Wang CH, Yuan WJ, Sun T, He LJ, Hu QK. MiR-126 suppresses the glucose-stimulated proliferation via IRS-2 in INS-1 β cells. PLoS One 11: e0149954, 2016. doi: 10.1371/journal.pone.0149954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Thymiakou E, Episkopou V. Detection of signaling effector-complexes downstream of bmp4 using PLA, a proximity ligation assay. J Vis Exp 49: 2631, 2011. doi: 10.3791/2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Timper K, Dalmas E, Dror E, Rütti S, Thienel C, Sauter NS, Bouzakri K, Bédat B, Pattou F, Kerr-Conte J, Böni-Schnetzler M, Donath MY. Glucose-dependent insulinotropic peptide stimulates glucagon-like peptide 1 production by pancreatic islets via interleukin 6, produced by α cells. Gastroenterology 151: 165–179, 2016. doi: 10.1053/j.gastro.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 74.Ubersax JA, Ferrell JE Jr. Mechanisms of specificity in protein phosphorylation. Nat Rev Mol Cell Biol 8: 530–541, 2007. doi: 10.1038/nrm2203. [DOI] [PubMed] [Google Scholar]
- 75.Ueki K, Okada T, Hu J, Liew CW, Assmann A, Dahlgren GM, Peters JL, Shackman JG, Zhang M, Artner I, Satin LS, Stein R, Holzenberger M, Kennedy RT, Kahn CR, Kulkarni RN. Total insulin and IGF-I resistance in pancreatic beta cells causes overt diabetes. Nat Genet 38: 583–588, 2006. doi: 10.1038/ng1787. [DOI] [PubMed] [Google Scholar]
- 76.Wang HW, Mizuta M, Saitoh Y, Noma K, Ueno H, Nakazato M. Glucagon-like peptide-1 and candesartan additively improve glucolipotoxicity in pancreatic β-cells. Metabolism 60: 1081–1089, 2011. doi: 10.1016/j.metabol.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 77.Waser B, Blank A, Karamitopoulou E, Perren A, Reubi JC. Glucagon-like-peptide-1 receptor expression in normal and diseased human thyroid and pancreas. Mod Pathol 28: 391–402, 2015. doi: 10.1038/modpathol.2014.113. [DOI] [PubMed] [Google Scholar]
- 78.Withers DJ, Burks DJ, Towery HH, Altamuro SL, Flint CL, White MF. Irs-2 coordinates IGF-1 receptor-mediated beta-cell development and peripheral insulin signalling. Nat Genet 23: 32–40, 1999. doi: 10.1038/12631. [DOI] [PubMed] [Google Scholar]
- 79.Wuescher L, Angevine K, Hinds T, Ramakrishnan S, Najjar SM, Mensah-Osman EJ. Insulin regulates menin expression, cytoplasmic localization, and interaction with FOXO1. Am J Physiol Endocrinol Metab 301: E474–E483, 2011. doi: 10.1152/ajpendo.00022.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xie J, El Sayed NM, Qi C, Zhao X, Moore CE, Herbert TP. Exendin-4 stimulates islet cell replication via the IGF1 receptor activation of mTORC1/S6K1. J Mol Endocrinol 53: 105–115, 2014. doi: 10.1530/JME-13-0200. [DOI] [PubMed] [Google Scholar]
- 81.Xuan S, Szabolcs M, Cinti F, Perincheri S, Accili D, Efstratiadis A. Genetic analysis of type-1 insulin-like growth factor receptor signaling through insulin receptor substrate-1 and -2 in pancreatic beta cells. J Biol Chem 285: 41,044–41,050, 2010. doi: 10.1074/jbc.M110.144790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yamagata K, Daitoku H, Takahashi Y, Namiki K, Hisatake K, Kako K, Mukai H, Kasuya Y, Fukamizu A. Arginine methylation of FOXO transcription factors inhibits their phosphorylation by Akt. Mol Cell 32: 221–231, 2008. doi: 10.1016/j.molcel.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 83.Yang JH, Polanowska-Grabowska RK, Smith JS, Shields CW IV, Saucerman JJ. PKA catalytic subunit compartmentation regulates contractile and hypertrophic responses to β-adrenergic signaling. J Mol Cell Cardiol 66: 83–93, 2014. doi: 10.1016/j.yjmcc.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yang Y, Gurung B, Wu T, Wang H, Stoffers DA, Hua X. Reversal of preexisting hyperglycemia in diabetic mice by acute deletion of the Men1 gene. Proc Natl Acad Sci USA 107: 20358–20363, 2010. doi: 10.1073/pnas.1012257107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yang Y, Wang H, Hua X. Deletion of the Men1 gene prevents streptozotocin-induced hyperglycemia in mice. Exp Diabetes Res 2010: 876701, 2010. doi: 10.1155/2010/876701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yates A, Akanni W, Amode MR, Barrell D, Billis K, Carvalho-Silva D, Cummins C, Clapham P, Fitzgerald S, Gil L, Girón CG, Gordon L, Hourlier T, Hunt SE, Janacek SH, Johnson N, Juettemann T, Keenan S, Lavidas I, Martin FJ, Maurel T, McLaren W, Murphy DN, Nag R, Nuhn M, Parker A, Patricio M, Pignatelli M, Rahtz M, Riat HS, Sheppard D, Taylor K, Thormann A, Vullo A, Wilder SP, Zadissa A, Birney E, Harrow J, Muffato M, Perry E, Ruffier M, Spudich G, Trevanion SJ, Cunningham F, Aken BL, Zerbino DR, Flicek P. Ensembl 2016. Nucleic Acids Res 44, D1: D710–D716, 2016. doi: 10.1093/nar/gkv1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang H, Li W, Wang Q, Wang X, Li F, Zhang C, Wu L, Long H, Liu Y, Li X, Luo M, Li G, Ning G. Glucose-mediated repression of menin promotes pancreatic β-cell proliferation. Endocrinology 153: 602–611, 2012. doi: 10.1210/en.2011-1460. [DOI] [PubMed] [Google Scholar]
- 88.Zhou L, Pelengaris S, Abouna S, Young J, Epstein D, Herold J, Nattkemper TW, Nakhai H, Khan M. Re-expression of IGF-II is important for beta cell regeneration in adult mice. PLoS One 7: e43623, 2012. doi: 10.1371/journal.pone.0043623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhou YY, Cheng H, Bogdanov KY, Hohl C, Altschuld R, Lakatta EG, Xiao RP. Localized cAMP-dependent signaling mediates β2-adrenergic modulation of cardiac excitation-contraction coupling. Am J Physiol Heart Circ Physiol 273: H1611–H1618, 1997. [DOI] [PubMed] [Google Scholar]