Abstract
Aquaporin 2 (AQP2) trafficking is regulated by phosphorylation and dephosphorylation of serine residues in the AQP2 COOH terminus. Vasopressin (VP) binding to its receptor (V2R) leads to a cascade of events that result in phosphorylation of serine 256 (S256), S264, and S269, but dephosphorylation of S261. To identify which phosphatase is responsible for VP-induced S261 dephosphorylation, we pretreated cells with different phosphatase inhibitors before VP stimulation. Sanguinarine, a specific protein phosphatase (PP) 2C inhibitor, but not inhibitors of PP1, PP2A (okadaic acid), or PP2B (cyclosporine), abolished VP-induced S261 dephosphorylation. However, sanguinarine and VP significantly increased phosphorylation of ERK, a kinase that can phosphorylate S261; inhibition of ERK by PD98059 partially decreased baseline S261 phosphorylation. These data support a role of ERK in S261 phosphorylation but suggest that, upon VP treatment, increased phosphatase activity overcomes the increase in ERK activity, resulting in overall dephosphorylation of S261. We also found that sanguinarine abolished VP-induced S261 dephosphorylation in cells expressing mutated AQP2 S256A, suggesting that the phosphorylation state of S261 is independent of S256. Sanguinarine alone did not induce AQP2 membrane trafficking, nor did it inhibit VP-induced AQP2 membrane accumulation in cells and kidney tissues, suggesting that S261 does not play an observable role in acute AQP2 membrane accumulation. In conclusion, PP2C activity is required for S261 AQP2 dephosphorylation upon VP stimulation, which occurs independently of S256 phosphorylation. Understanding the pathways involved in modulating PP2C will help elucidate the role of S261 in cellular events involving AQP2.
Keywords: aquaporin, ERK, phosphatase, PP2C, vasopressin receptor
the ability to concentrate urine is essential for adaptation to terrestrial life. In response to vasopressin (VP) stimulation, aquaporin 2 (AQP2) water channels accumulate on the apical membrane of kidney principal cells and increase water reabsorption from the collecting duct (7, 12, 29). AQP2 trafficking is regulated by phosphorylation and dephosphorylation of its COOH terminus on four different serine residues: S256, S261, S264, and S269 (5, 18). Under physiological conditions, binding of VP to vasopressin receptor type 2 (V2R) increases cAMP levels (21), which leads to activation of protein kinase A (PKA) (19) and, subsequently, phosphorylation of the key S256 residue (12, 16, 40). S264 and S269 are also phosphorylated in response to VP stimulation or hypertonicity (5, 8, 16, 18). On the contrary, S261 is dephosphorylated in response to VP stimulation (5, 17, 30, 32, 40). It is well known that protein phosphorylation and signaling represent a balance between the actions of kinases and phosphatases. However, while many studies have yielded a wealth of knowledge on the kinases that can phosphorylate some of these essential serine residues on AQP2 (5, 33), there has been less exploration of the other side of the balance, the phosphatases.
There are three major groups in the serine/threonine phosphatase family: phosphoprotein phosphatases (PPPs), metal-dependent protein phosphatases (PPMs), and the aspartate-based phosphatases targeting RNA polymerase II. Representative members of the PPP family include protein phosphatase (PP) 1 (PPP1CA gene), PP2A (PPP2CA gene), PP2B (PPP3CB gene, commonly known as calcineurin), PP4 (PPP4C gene), PP5 (PPP5C gene), PP6 (PPP6C gene), and PP7 (2). The PPM family includes manganese/magnesium-dependent PPs, such as PP2C (PPM1A gene), while FCP and SCP families represent the aspartate-based phosphatases (6, 34).
In this study we use in vitro and in situ models to focus on the effect of phosphatase inhibition on AQP2 phosphorylation and trafficking. We aimed to identify the phosphatase that is responsible for VP-induced dephosphorylation of S261, the only serine residue that is dephosphorylated when VP binds to V2R. Using a specific antibody against phosphorylated S261 AQP2, we showed that VP-induced S261 dephosphorylation is dependent on PP2C activity and that this dephosphorylation of S261 occurs independently of S256.
MATERIALS AND METHODS
Reagents.
All cell culture media were purchased from Invitrogen (Grand Island, NY), and fetal bovine serum (FBS) was purchased from Atlanta Biologicals (Laurenceville, GA). All chemical reagents were purchased from Sigma-Aldrich (St. Louis, MO).
Cell culture.
LLC-PK1 cells stably expressing c-myc-tagged AQP2 (LLC-AQP2) or an AQP2 S256A mutant (LLC-AQP2 S256A) were grown in Dulbecco’s modified Eagle’s medium (DMEM), 10% FBS, and additional l-glutamine (15). Cells were passaged twice a week, and 4′,6-diamidino-2-phenylindole (DAPI) staining was used weekly to test for mycoplasma contamination.
Cell treatment and immunocytochemistry.
Cells were plated onto glass coverslips (Ted Pella, Redding, CA) 3 days before the experiment at a density of 70,000 cells/ml. Cells were starved for ≥1 h in DMEM without FBS before treatment. Cells were treated with okadaic acid (OA, 1 μM; Calbiochem, Billerica, MA), cyclosporine A (CsA, 70 nM; Selleckchem, Houston, TX), or sanguinarine (15 μM; Tocris, Minneapolis, MN) diluted in dimethyl sulfoxide (DMSO) for 40 min. Cells were treated with PD98059 (30 μM; Tocris) for 30 min. Ten minutes before the end of treatments with various phosphatase inhibitors or PD98059, lysine-vasopressin (10 nM) was added. Cells treated with VP alone for 10 min served as a positive control, and cells treated with DMSO served as a negative control.
After treatment, cells were fixed in 4% paraformaldehyde containing 5% sucrose and then washed three times with PBS. Cells were permeabilized in 0.1% Triton X-100 for 4 min. After three washes with PBS (5 min each), nonspecific binding was blocked by 1% bovine serum albumin (BSA) in PBS. Cells were incubated with mouse anti-c-myc IgG for 1 h at room temperature to detect c-myc-tagged AQP2 in LLC-AQP2 cells. After the cells were washed three times (5 min each) in PBS, the secondary antibody donkey anti-mouse Alexa 488 (15 μg/ml; Jackson ImmunoResearch, West Grove, PA) was applied for 1 h at room temperature. LLC-AQP2 cells were costained with rhodamine-conjugated wheat germ agglutinin (WGA, 2 μg/ml; Lectin Kit, Vector Laboratories, Burlingame, CA) for 10 min as a plasma membrane marker for subsequent quantification of specific AQP2 membrane labeling. Finally, the cells were mounted in Vectashield (Vector Laboratories) with DAPI diluted 2:1 in 0.1 M Tris·HCl, pH 8.0. Images of LLC-AQP2 cells were acquired with a Nikon 90i microscope.
Kidney tissue preparation and immunohistochemistry.
The kidney tissue slices were prepared as previously described (3, 4). All animal experiments were approved by the Massachusetts General Hospital Institutional Committee on Research Animal Care in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Briefly, adult Sprague-Dawley rats were anesthetized with 2% isoflurane inhalation. The left ventricle was punctured, and kidneys were perfused with PBS (37°C) until clear PBS was leaking out of an incision in the inferior vena cava (~1 min). Kidneys were removed, and ~0.5-mm slices were cut with a Stadie-Riggs microtome. Before treatment, all kidney slices were equilibrated in CO2-saturated Hanks’ balanced salt solution (in mM: 110 NaCl, 5 KCl, 1.2 MgSO4, 1.8 CaCl2, 4 NaOAc, 1 C6H7NaO7, 6 d-glucose, 6 l-alanine, 1 NaH2PO4, 3 Na2HPO4, and 25 NaHCO3) for 15 min. Drugs were added directly into the Hanks’ balanced salt solution to obtain their final concentration. When the experiment ended, slices were fixed overnight in 4% paraformaldehyde-lysine-periodate fixative. The slices were then washed five times with PBS, and the tissues were cryoprotected overnight with PBS containing 30% sucrose and then embedded in OCT compound 4583 (Tissue-Tek, Miles, Elkhart, IN). Cryosections (5 μm) were cut using a microtome (model CM3050S, Leica Microsystems, Deerfield, IL). Sections were placed onto positive-charged slides (Denville Scientific, Metuchen, NJ) and stored at −20°C until use. Before they were stained, sections were rehydrated in PBS for 20 min and then permeabilized with 0.1% Triton X-100 for 10 min. After the sections were washed three times, nonspecific binding was blocked with 1% BSA in PBS for 15 min, and the tissues were incubated overnight at 4°C with goat anti-AQP2 (0.2 μg/ml; C17, Santa Cruz Biotechnology, Dallas, TX). Sections were then incubated with a secondary antibody, donkey anti-goat conjugated to Alexa 488 or Cy3 (15 or 1.9 μg/ml, respectively), for 1 h at room temperature. After three washes in PBS, sections were mounted in Vectashield-DAPI diluted 2:1 in 0.1 M Tris·HCl, pH 8.0. Images were acquired using a Nikon 90i epifluorescence microscope or a Nikon A1R confocal laser-scanning microscope.
Western blot analysis.
LLC-AQP2 cells were grown on 35-mm tissue culture dishes (Corning, Corning, NY) for 3 days. Before treatment, cells were starved in DMEM without serum for 1 h. After treatment, cells were washed with cold PBS and lysed in RIPA buffer (Boston Bioproducts, Ashland, MA) supplemented with a protease inhibitor cocktail (Complete mini, Roche Diagnostics, Mannheim, Germany) and phosphatase inhibitors (1 mM NaF, 5 mM EDTA, and 1 mM sodium orthovanadate). Cell lysates were mixed for 30 min at 4°C and centrifuged for 15 min at 17,000 g. Protein concentration was determined using the bicinchoninic acid (BCA) protein assay kit according to the manufacturer’s instructions (Pierce, Rockford, IL). Samples were mixed with NuPage SDS sample buffer and NuPage sample reducing buffer and then incubated at 70°C for 10 min. After samples were centrifuged for 15 min at 17,000 g, 20 μg of protein were loaded in each well of NuPage 4–12% Bis-Tris gels (Invitrogen). Then protein was transferred onto a Sequi-Blot polyvinylidene difluoride membrane (Bio-Rad, Hercules, CA). Membranes were blocked with 0.1% PBS-Tween 20 (PBS-T) containing 5% nonfat milk or 1% BSA for 1 h at room temperature. Membranes were incubated overnight at 4°C with the primary antibodies: anti-AQP2 (C-17) and anti-phosphorylated AQP2 (pSer261, Symansism, Temecula, CA), anti-phosphorylated AQP2 (pSer256, Abcam, Cambridge, MA), and anti-p44/42 MAPK and anti-phosphorylated p44/42 MAPK [Erk 1/2 (Thr202/Tyr204) Cell Signaling Technology, Danvers, MA]. After incubation, the membranes were washed five times (5 min each) with PBS-T before secondary anti-rabbit IgG antibodies conjugated to horseradish peroxidase (Jackson ImmunoResearch) were applied (0.16 μg/ml). Membranes were incubated in enhanced chemiluminescence substrate (Western Lightning ECL, Amersham, Arlington Heights, MA) and exposed to Hyblot ES film (Denville Scientific). Then membranes were stripped with Western blot stripping buffer (Thermo Scientific, Rockford, IL) for ≥15 min. Band intensities in the different experimental conditions were quantified using ImageJ (National Institutes of Health, Bethesda, MD). Band intensity of the phosphoserine antibodies was corrected according to the band intensity of total AQP2 on the same membrane.
Quantification of AQP2 at the cell membrane.
Acquired images were imported into Volocity software (PerkinElmer, Waltham, MA). Quantification of AQP2 membrane accumulation was performed under blinded conditions in which the region of interest for quantification was determined using WGA membrane staining while the Alexa 488 channel (containing the AQP2 signal) was shut down. First, DAPI was used to stain the nucleus. Then the plasma membrane was outlined by the WGA staining. Quantification was performed on cells that were not adjacent to each other. Using this approach, we then quantified the fluorescence of Alexa 488 (AQP2) in the three regions of interest: cell membrane, cytoplasm, and nucleus, respectively. Mean fluorescence in the cell membrane was corrected using the fluorescence in the nucleus as a measure of nonspecific background labeling. Each treatment was performed in triplicate in each experiment; for image quantifications, 30 cells from ≥10 different images were used. These analyses were repeated in three independent experiments.
PKA activity assay.
The effect of CsA and sanguinarine on PKA activity was tested by using a PKA kinase activity ELISA kit according to the manufacturer’s instructions (Enzo Life Sciences, Farmingdale, NY). Briefly, after treatment, cells were lysed in buffer prepared according to the manufacturer’s protocol. Protein content was determined using the BCA method, and samples were diluted to 2 μg of crude protein per reaction sample. The reaction was initiated by addition of ATP onto the plate. ELISA results were measured at 450 nm using a multimode plate reader (model DTX880, Beckman Coulter, Fullerton, CA). Each condition was performed in duplicate, and results were averaged from three independent experiments.
Data analysis.
Images of immunostained cells and tissues were analyzed using Volocity software (PerkinElmer). Western blot results were quantified using ImageJ. Values are means ± SE. Statistical significance was determined as appropriate by one-way ANOVA with Tukey’s test using PRISM software. Differences were considered to be significant at P < 0.05. All experiments were done at least in triplicate.
RESULTS
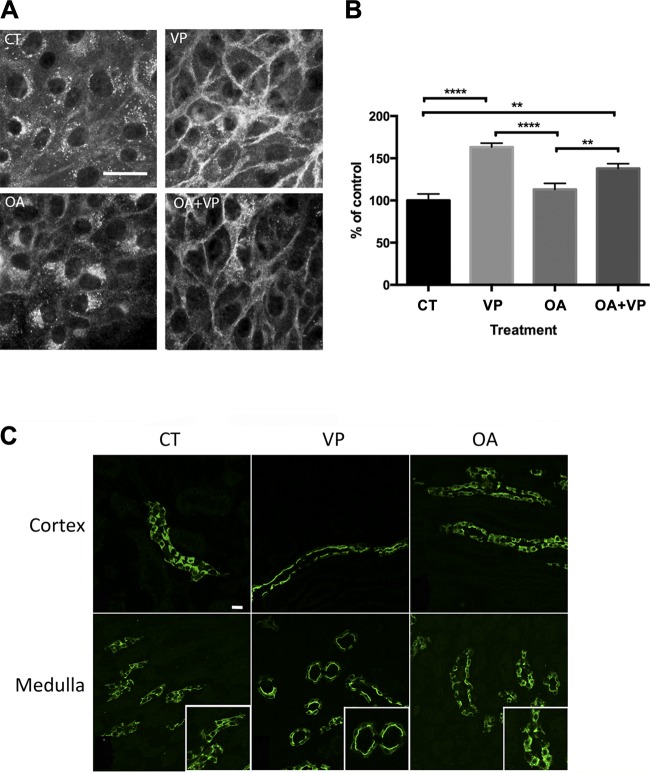
As described previously, AQP2 accumulated on the basolateral plasma membrane after VP treatment of LLC-AQP2 cells (20, 44). In contrast to a previous study using AQP2-transfected renal CD8 cells from rabbit cortical collecting ducts (39), we found that incubation with OA did not result in AQP2 membrane accumulation in our LLC-AQP2 cells. Instead, AQP2 accumulated in the perinuclear region (Fig. 1A). Quantification showed that AQP2 accumulated on the plasma membrane only when cells were exposed to VP. Pretreatment with OA did not affect the extent of the VP-induced AQP2 plasma membrane accumulation (Fig. 1B). These results were confirmed in Sprague-Dawley rat kidney slices. In our in situ model, OA-treated tissues exhibited a cytoplasmic AQP2 staining similar to control tissues (Fig. 1C). In contrast, VP treatment led to a clear apical AQP2 staining in the cortical and medullary regions of the collecting duct (Fig. 1C).
Fig. 1.
Okadaic acid (OA) treatment produces a perinuclear patch of aquaporin 2 (AQP2) but does not cause AQP2 accumulation on the plasma membrane. A: LLC-AQP2 cells were treated for 40 min with OA (1 μM). Before the end of OA treatment, some cells were incubated with vasopressin (VP, 10 nM) for 10 min and others were treated with DMSO. Images are representative of ≥3 independent experiments. B: quantification of images in A shows significantly increased AQP2 plasma membrane accumulation only in cells treated with VP alone or OA + VP. OA alone did not affect AQP2 accumulation on the plasma membrane but resulted in a marked perinuclear accumulation of AQP2. Data were analyzed using 1-way ANOVA with Tukey’s test. Values are means ± SE (n = 3). **P < 0.01, ****P < 0.0001. C: AQP2 did not accumulate on the apical plasma membrane of cortical or medullary collecting duct principal cells after OA treatment of tissue slices in vitro. Kidney slices from adult Sprague-Dawley rats were incubated with OA (1 μM) for 40 min or VP (100 nM) for 15 min. In the cortex and outer medulla, AQP2 accumulated on the apical plasma membrane after VP treatment. In contrast, control (CT) and OA-treated slices showed mostly cytoplasmic AQP2 staining. Scale bar = 25 μm. Images are representative of 3 independent experiments.
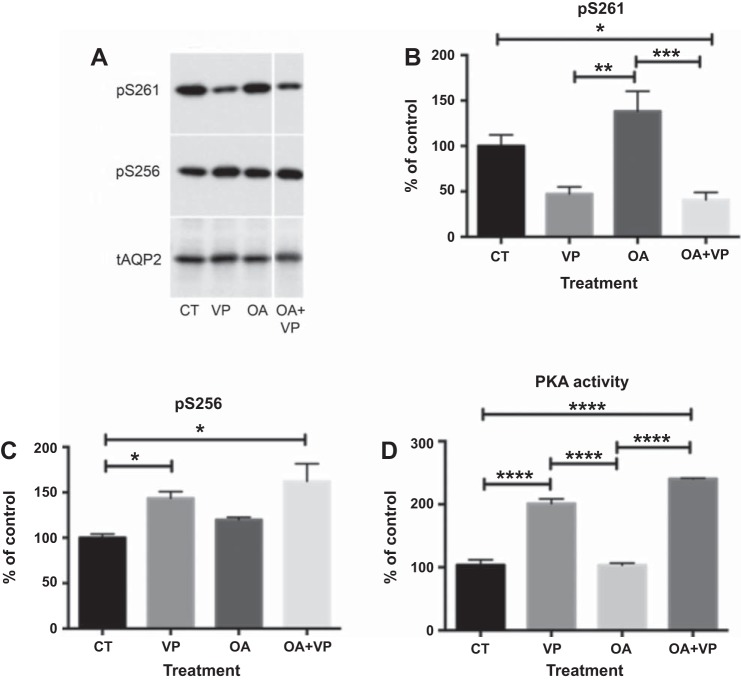
Western blot analysis was used to determine the phosphorylation state of S261 and S256 (Fig. 2, A–C). Specific phospho-antibodies revealed that OA treatment alone did not significantly affect phosphorylation of S261 and that OA did not prevent VP-induced dephosphorylation of S261 (Fig. 2A). While phosphorylation of S256 was slightly increased, this difference was not significant. PKA activity analysis suggested that OA alone did not affect PKA activity and OA did not affect the activation of PKA by VP (Fig. 2D). To determine which phosphatase is responsible for the VP-induced dephosphorylation of S261, we searched for OA-insensitive serine/threonine protein phosphatases. Two phosphatases were reported to be OA-insensitive: PP2B (PPP3CB) in the PPP family and PP2C (PPM1A) in the PPM family. We used CsA (10, 11) for PP2B inhibition and sanguinarine for PP2C inhibition (1).
Fig. 2.
OA does not affect phosphorylation of AQP2 at S256 and S261, nor does it inhibit VP-induced S261 dephosphorylation. A: specific anti-phosphorylated AQP2 antibodies were used on Western blots with cell lysates from LLC-AQP2 cells treated with VP (10 nM), OA (1 μM), or OA + VP. B and C: intensities of phosphoserine bands were normalized to the band intensity of total AQP2 (tAQP2). OA slightly, but not significantly, increased S256 phosphorylation. OA did not prevent VP-induced S261 dephosphorylation. Values are means ± SE (n = 4). *P < 0.05, **P < 0.01, ***P < 0.001 (1-way ANOVA with Tukey’s test). D: PKA activity was not altered by OA. ELISA showed an increase in PKA activity in cells treated with VP, but not after OA pretreatment. Results represent average of 3 independent experiments performed in triplicate. Values are means ± SE (n = 3). ****P < 0.0001 (by 1-way ANOVA with Tukey’s test).
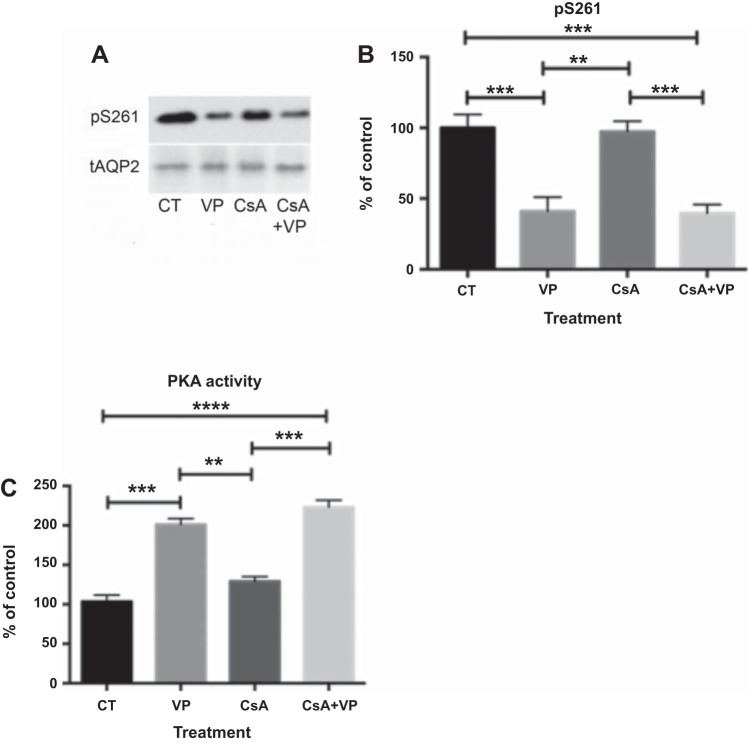
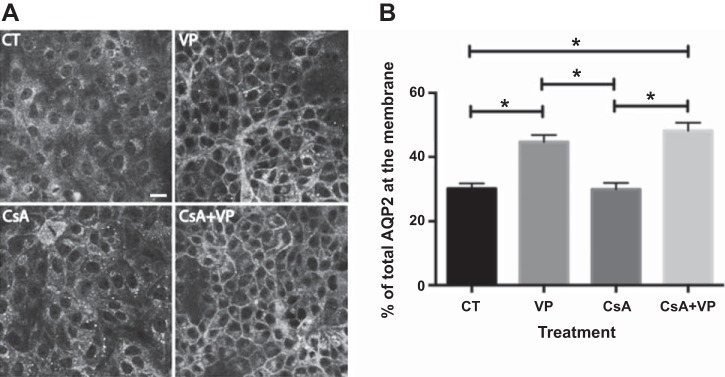
Western blotting with specific phospho-antibodies against S261 showed that, similar to OA, CsA alone did not affect phosphorylation of S261, nor did it inhibit VP-induced S261 dephosphorylation (Fig. 3, A and B). Similar to OA, CsA alone did not increase PKA activity, nor did it inhibit VP-induced activation of PKA. There was no difference in PKA activity between cells treated with VP alone and cells pretreated with CsA before VP stimulation (Fig. 3C). In LLC-AQP2 cells treated with CsA, cytoplasmic AQP2 staining was similar to control cells, and AQP2 accumulated on the plasma membrane in cells treated with VP and CsA + VP (Fig. 4A). Quantification of membrane fluorescence confirmed that CsA alone did not induce membrane accumulation of AQP2, nor did it inhibit VP-induced AQP2 membrane accumulation (Fig. 4B).
Fig. 3.
Cyclosporine A (CsA) does not prevent VP-induced dephosphorylation of S261. A: specific antibodies against pS261 were used on Western blots with cell lysates of LLC-AQP2 cells treated with CsA (70 nM), VP (10 nM), or CsA + VP. B: intensities of phosphoserine bands were normalized to the band intensity of total AQP2. CsA did not prevent dephosphorylation of S261 after VP treatment. Values are means ± SE (n = 5). **P < 0.01, ***P < 0.001. C: PKA activity was not altered by CsA. Values are means ± SE (n = 3). **P < 0.01, ***P < 0.001, ****P < 0.0001 (by 1-way ANOVA with Tukey’s test).
Fig. 4.
CsA does not affect AQP2 membrane accumulation. A: LLC-AQP2 cells were treated with CsA (70 nM) or DMSO for 40 min. After 30 min of pretreatment, cells were incubated with buffer alone or VP (10 nM) for a further 10 min. Cells treated with VP alone were used as a positive control. Confocal images are representative of ≥3 independent experiments. Scale bar = 25 μm. B: quantification of images in A shows significantly increased AQP2 plasma membrane accumulation only in cells treated with VP alone or CsA + VP. CsA treatment before VP stimulation did not prevent AQP2 plasma membrane accumulation. Values are means ± SE (n = 2). *P < 0.05 (by 1-way ANOVA with Tukey’s test).
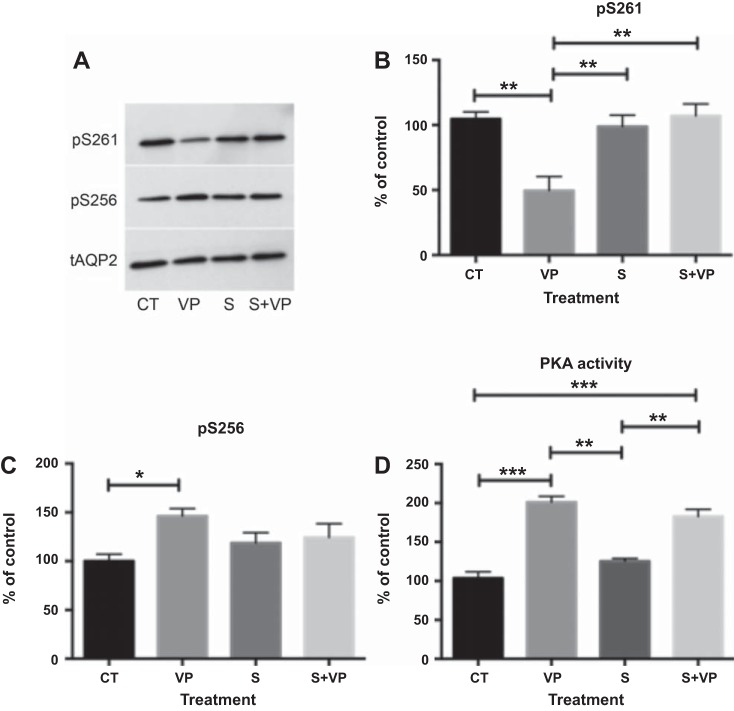
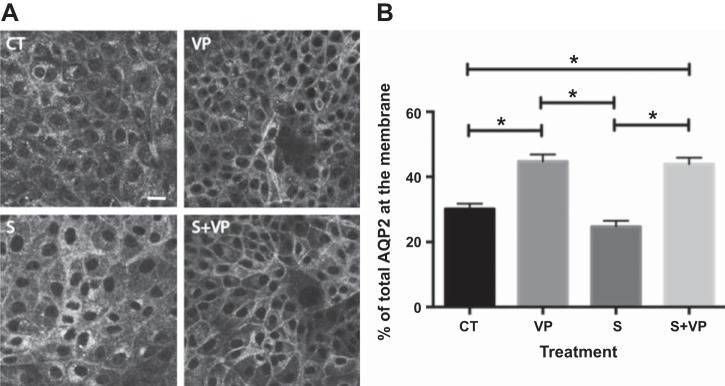
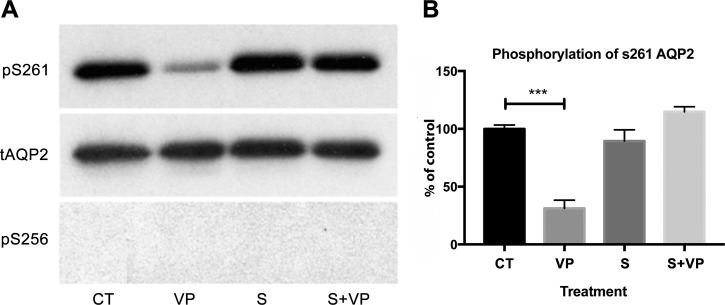
Based on our analysis of the inhibition profiles of known phosphatases, we hypothesized that the OA- and CsA-insensitive phosphatase PP2C might be responsible for VP-induced dephosphorylation of S261. Previous data have shown that PP2C is expressed in collecting duct principal cells (38), and using RT-quantitative PCR, we found that LLC-AQP2 cells also highly express PP2C (PPM1A) (data not shown). Therefore, we treated LLC-AQP2 cells with the specific PP2C inhibitor sanguinarine (25 μM) for 30 min (1). We found that treatment with sanguinarine before VP stimulation completely inhibited the VP-induced dephosphorylation of S261 (Fig. 5, A and B). Sanguinarine alone did not increase phosphorylation of S261 compared with control cells (Fig. 5A), nor did it affect phosphorylation of S256 compared with control cells (Fig. 5, A and C). While sanguinarine did not affect the level of S256 phosphorylation under basal conditions or upon VP stimulation (Fig. 5C), we were interested to find whether the VP/PKA signaling pathway was intact or whether sanguinarine activated another pathway that phosphorylates S256. We found that sanguinarine alone did not increase PKA activity and that pretreatment with sanguinarine did not block VP-induced activation of PKA (Fig. 5D), indicating that the cellular VP signaling machinery remains intact with sanguinarine treatment. We conclude that inhibition of PP2C by sanguinarine independently blocked dephosphorylation of S261, while S256 phosphorylation continued to be induced by VP. This was confirmed by immunocytochemistry. Cytoplasmic AQP2 staining in cells treated with sanguinarine alone was comparable to that in control cells (Fig. 6A). AQP2 accumulated on the plasma membrane in VP-treated cells as expected, and sanguinarine pretreatment did not abolish VP-induced membrane accumulation (Fig. 6B), again indicating that VP signaling remains intact in cells treated with sanguinarine. In addition, sanguinarine also blocked dephosphorylation of S261 in LLC-AQP2 S256A mutant cells, suggesting that dephosphorylation of S261 is S256-independent (Fig. 7).
Fig. 5.
Sanguinarine (S) inhibits VP-induced dephosphorylation of S261. A: specific anti-phosphorylated AQP2 antibodies were used on Western blots to determine the phosphorylation state of S261 and S256. B and C: cell lysates from LLC-AQP2 cells were treated with VP (10 nM), sanguinarine (25 μM), or sanguinarine + VP, and intensity of phosphoserine bands was normalized to the band intensity of total AQP2. Pretreatment for 30 min with sanguinarine prevented VP-induced S261 dephosphorylation. Values are means ± SE (n = 4). *P < 0.05, **P < 0.01 (by 1-way ANOVA with Tukey’s test). D: PKA activity was not altered by sanguinarine. Only VP alone or sanguinarine + VP increased PKA activity. Values are means ± SE (n = 3). **P < 0.01, ***P < 0.001 (by 1-way ANOVA with Tukey’s test).
Fig. 6.
Sanguinarine did not alter AQP2 membrane accumulation. A: LLC-AQP2 cells were treated with sanguinarine (25 μM) for 40 min. After 30 min of incubation, cells were treated with DMSO or VP (10 nM). Cells treated with VP alone were used as a positive control. Images were obtained using a confocal microscope and are representative of ≥3 independent experiments. Scale bar = 25 μm. B: quantification of images in A shows significantly increased AQP2 plasma membrane accumulation only in cells treated with VP alone or sanguinarine + VP. Values are means ± SE (n = 3). *P < 0.05 (by 1-way ANOVA with Tukey’s test).
Fig. 7.
Sanguinarine inhibits VP-induced dephosphorylation of AQP2 at S261 in LLC-AQP2 S256A cells. A: specific anti-phosphorylated AQP2 antibodies were used on Western blots with cell lysates from LLC-AQP2 S256A cells treated with VP (10 nM), sanguinarine (25 μM), or sanguinarine + VP. B: intensities of phosphoserine bands were normalized to the band intensity of total AQP2. Sanguinarine prevents VP-induced S261 dephosphorylation in these cells. Results represent average of 4 independent experiments performed in triplicate. Values are means ± SE (n = 4) ***P < 0.001 (by 1-way ANOVA with Tukey’s test).
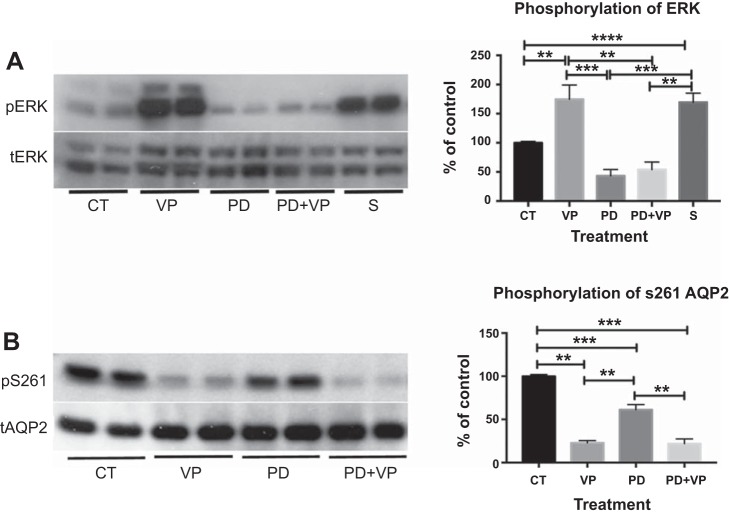
Bioinformatic analysis (5) and phosphoproteomic studies (33) suggested that S261 could be phosphorylated by ERK; therefore, we asked whether sanguinarine has an effect on ERK phosphorylation. We found that sanguinarine alone significantly increases ERK phosphorylation (Fig. 8A), suggesting that PP2C could play a role in regulating the phosphorylation state of ERK. We also found that VP treatment significantly increases ERK phosphorylation (Fig. 8A). To investigate whether ERK is indeed involved in S261 phosphorylation, we used a specific MAPK/ERK inhibitor, PD98059, to evaluate its effects on S261 phosphorylation. PD98059 suppressed ERK phosphorylation and decreased S261 phosphorylation under baseline conditions compared with control. However, the decrease in phosphorylated S261 did not reach the very low level in cells after VP treatment, even though PD98059 completely abolished VP-induced ERK phosphorylation, indicating that the inhibitor was fully active under these conditions (Fig. 8).
Fig. 8.
Sanguinarine and VP increase ERK phosphorylation, and inhibition of ERK decreases S261 phosphorylation. A: treatment of LLC-AQP2 cells with sanguinarine (25 μM) or VP significantly increases ERK phosphorylation. VP-induced ERK phosphorylation was significantly suppressed by the MAPK/ERK inhibitor PD98059 (PD). Values are means ± SE (n = 3). B: phosphorylation of S261AQP2 is decreased significantly with PD, but not to the same low level as VP-induced dephosphorylation of S261. Addition of VP to cells treated with PD98059 for 30 min did not cause further dephosphorylation of S261 compared with VP alone. Values are means ± SE (n = 5). Experiments were performed in duplicate. **P < 0.01, ***P < 0.001, ****P < 0.0001 (by 1-way ANOVA with Tukey’s test).
Using kidney tissue slice preparations, we confirmed the results seen in LLC-AQP2 cells. Pretreatment with the phosphatase inhibitors CsA and sanguinarine did not inhibit VP-induced AQP2 accumulation in the apical membrane of cortical and medullary collecting duct principal cells (Fig. 9).
Fig. 9.
Pretreatment with sanguinarine or CsA does not prevent VP-induced AQP2 plasma membrane accumulation in principal cells of the collecting duct. Kidney slices from adult Sprague-Dawley rats were incubated with sanguinarine (25 μM) or CsA (140 nM) for 40 min. At 15 min before the end of the experiment, VP (100 nM) was added to the kidney slices. Top: in the cortex, AQP2 accumulates on the apical plasma membrane after VP treatment. Pretreatment with CsA or sanguinarine did not affect this AQP2 accumulation on the apical plasma membrane. Cytoplasmic AQP2 staining is visible in control (CT) slices. Bottom: similar results in the inner medulla. Images are representative of 3 independent experiments. Scale bar = 25 μm.
DISCUSSION
Our study shows that dephosphorylation of the S261 COOH-terminal residue of AQP2 is achieved via the activity of PP2C, which appears to act by directly dephosphorylating S261 and by decreasing phosphorylation of ERK. We also show that dephosphorylation of S261 AQP2 induced by VP does not require phosphorylation of S256 AQP2 and that the phosphorylation state of S261 does not have a detectable impact on acute AQP2 membrane accumulation.
While the mechanistic pathway linking VP to PP2C is unclear, in rat parotid acinar cells, it is thought that PP2C is activated by elevated cAMP levels (43). Therefore, a similar mechanism may also exist in the VP signaling pathway: as intracellular cAMP increases with VP stimulation, PP2C would be activated to dephosphorylate S261. PP2C has been described in other signaling pathways to indirectly terminate some effectors, such as bone morphogenetic protein and TNF-α, by acting on their respective key kinases, such as SMAD and IKKβ (22, 35). PP2C has also been shown to deactivate p38, JNK, and MAP kinases (9, 36), which were previously implicated in phosphorylation of S261 (28). Our present study shows that the PP2C inhibitor sanguinarine significantly increases phosphorylation of ERK and, presumably, activates this kinase, which can also phosphorylate S261 (5, 33). Furthermore, PD98059, a specific MAPK/ERK inhibitor, causes a partial decrease in S261 phosphorylation under basal (no VP) conditions, again supporting a role of ERK in S261 phosphorylation. Our finding that sanguinarine increases ERK phosphorylation significantly under basal conditions also suggests that PP2C is at least partially active in the absence of VP stimulation. However, although inhibiting ERK by PD98059 decreases S261 phosphorylation, it does not cause the more extensive dephosphorylation seen after VP treatment. This suggests that ERK is not the only kinase involved in S261 phosphorylation, as also indicated above. Increasing the complexity of this system is our finding that VP greatly increases phosphorylation of ERK (as much as PP2C inhibition by sanguinarine) but still induces a drastic dephosphorylation of S261. This suggests that, in the presence of VP, activation of PP2C can overcome increased ERK activity (and possibly the action of other kinases) to favor the ultimate dephosphorylation of S261. Together, our data suggest that PP2C is involved in S261 dephosphorylation in two ways: it can directly dephosphorylate S261, and it can act indirectly by decreasing ERK phosphorylation, which may contribute to the decrease in S261 phosphorylation.
PP2C does not seem to play a direct role in the canonical V2R signaling pathway, as sanguinarine does not inhibit the VP-induced cAMP increase, PKA activation, or S256 phosphorylation. Furthermore, sanguinarine alone does not induce AQP2 plasma membrane accumulation, nor does it affect VP-induced AQP2 membrane trafficking. This confirms previous findings (24) that the phosphorylation state of S261 does not affect AQP2 trafficking, at least acutely. In addition, we showed that dephosphorylation of S261 is not dependent on S256 phosphorylation, and this may again imply that S261, unlike other serine residues, e.g., S264 and S269, has regulatory role(s) in AQP2 function other than membrane trafficking (5, 25, 26). In this respect, PP2C is a magnesium- or manganese-dependent protein phosphatase, and members of this family have been implicated in stress signaling, protein ubiquitination, death, and survival signaling (23). Indeed, Tamma et al. showed that S261 phosphorylation regulates degradation of AQP2 by stabilizing K279 ubiquitination (37), and S261 seems to play a role in the long-term regulation of AQP2 (25).
We also conducted a parallel investigation of the effect of phosphatase inhibitors on phosphorylation of S256. Some studies have reported that phosphatases such as PP1A, PP2A, and PP2B affect AQP2 trafficking in cell cultures. Valenti et al. showed that OA, an inhibitor of PP1 and PP2A, causes AQP2 membrane accumulation in cultured rabbit collecting duct cells (39), and Gooch et al. suggested that PP2B signaling plays a role in AQP2 localization and phosphorylation in inner medullary collecting duct cells (13). We were not able to reproduce their AQP2 trafficking results in our LLC-AQP2 cells, nor were we able to detect a significant increase in S256 phosphorylation with OA or cyclosporine treatment (data not shown). Instead, we found that OA treatment resulted in a marked perinuclear accumulation of AQP2 in LLC-AQP2 cells. The cellular compartment involved in this OA-induced process has not been identified. One possible explanation for this discrepancy may be that the distribution of phosphatases and phosphorylation patterns of AQP2 could vary in different segments of the collecting duct and other cell types (14, 33), thus producing inconsistent results across different cell lines (14, 33, 41, 42). We validated our results obtained from LLC-AQP2 cells by examining bona fide principal cells in in situ rat kidney slices that have allowed us to uncover an alternative signaling pathway in previous studies (3, 4). We were again unable to observe accumulation of AQP2 at the plasma membrane or inhibition of VP-induced AQP2 membrane accumulation in the presence of any of the phosphatase inhibitors used in this study, thus confirming our results obtained from cells. More recently, Ren et al. showed that calyculin A, another inhibitor of PP1 and PP2A, increased AQP2 membrane accumulation in rats and increased phosphorylation of S256. However, as noted by Ren et al., because of its systemic toxicity, calyculin A infusion through the iliac artery was followed by clamping of the renal arteries and veins for 30 min, and it was difficult to rule out the effect of tissue ischemia on AQP2 localization. Ren et al. also showed that tacrolimus, another PP2B or calcineurin inhibitor, increased AQP2 membrane accumulation 45 min after systemic administration in rats (31), which was not observed in our cell and tissue models. The reason for such a discrepancy is unclear, but systemic administration of tacrolimus can acutely cause afferent arteriolar vasoconstriction (27), mimicking ischemia, similar to the method used for calyculin A administration. Our tissue slice model, however, eliminates this possible confounder. In addition, similar to our data, Ren et al. showed that PP2B inhibition by tacrolimus did not induce a significant increase in S256 phosphorylation, but unlike our study, tacrolimus caused an increase in S261 phosphorylation. This inconsistency is not well understood but may provide some hints about the physiological role of S261 AQP2, as CsA and tacrolimus, although they inhibit calcineurin and NFAT pathways, have different clinical efficacies and side effect profiles.
In conclusion, PP2C activity results in S261 AQP2 dephosphorylation upon VP stimulation, and this effect occurs independent of S256 phosphorylation. Understanding the potential pathways involved in modulating PP2C could help us obtain a deeper understanding of the physiological role of S261 in cellular events involving AQP2.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Grant DK-096586 (D. Brown). L. Ueberdiek was supported by a Kolff Student Fellowship Abroad Grant from the Dutch Kidney Foundation. P. W. Cheung was supported by NIDDK Grant T32 5T32 DK-007540-29. J. Day was supported by an undergraduate summer research award from the American Physiological Society. R. Bouley was supported by a Massachusetts General Hospital Executive Committee on Research (MGH/ECOR) interim support fund. The Nikon A1R confocal microscope in the Program in Membrane Biology Microscopy Core was purchased using National Institutes of Health Shared Instrumentation Grant S10 RR-031563-01 (D. Brown). Additional support for the Program in Membrane Biology Microscopy Core was provided by the Boston Area Diabetes and Endocrinology Research Center (NIDDK Grant DK-057521) and the Massachusetts General Hospital Center for the Study of Inflammatory Bowel Disease (NIDDK Grant DK-043351).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
P.W.C., L.U., J.D., R.B., and D.B. conceived and designed research; P.W.C., L.U., J.D., and R.B. performed experiments; P.W.C., L.U., J.D., R.B., and D.B. analyzed data; P.W.C., L.U., J.D., R.B., and D.B. interpreted results of experiments; P.W.C., L.U., J.D., and R.B. prepared figures; P.W.C., L.U., J.D., R.B., and D.B. drafted manuscript; P.W.C., R.B., and D.B. edited and revised manuscript; P.W.C., L.U., J.D., R.B., and D.B. approved final version of manuscript.
REFERENCES
- 1.Aburai N, Yoshida M, Ohnishi M, Kimura K. Sanguinarine as a potent and specific inhibitor of protein phosphatase 2C in vitro and induces apoptosis via phosphorylation of p38 in HL60 cells. Biosci Biotechnol Biochem 74: 548–552, 2010. doi: 10.1271/bbb.90735. [DOI] [PubMed] [Google Scholar]
- 2.Andreeva AV, Kutuzov MA. PPP family of protein Ser/Thr phosphatases: two distinct branches? Mol Biol Evol 18: 448–452, 2001. doi: 10.1093/oxfordjournals.molbev.a003823. [DOI] [PubMed] [Google Scholar]
- 3.Bouley R, Breton S, Sun T, McLaughlin M, Nsumu NN, Lin HY, Ausiello DA, Brown D. Nitric oxide and atrial natriuretic factor stimulate cGMP-dependent membrane insertion of aquaporin 2 in renal epithelial cells. J Clin Invest 106: 1115–1126, 2000. doi: 10.1172/JCI9594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bouley R, Lu HA, Nunes P, Da Silva N, McLaughlin M, Chen Y, Brown D. Calcitonin has a vasopressin-like effect on aquaporin-2 trafficking and urinary concentration. J Am Soc Nephrol 22: 59–72, 2011. doi: 10.1681/ASN.2009121267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown D, Hasler U, Nunes P, Bouley R, Lu HA. Phosphorylation events and the modulation of aquaporin 2 cell surface expression. Curr Opin Nephrol Hypertens 17: 491–498, 2008. doi: 10.1097/MNH.0b013e3283094eb1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen P. Classification of protein-serine/threonine phosphatases: identification and quantitation in cell extracts. Methods Enzymol 201: 389–398, 1991. doi: 10.1016/0076-6879(91)01035-Z. [DOI] [PubMed] [Google Scholar]
- 7.Deen PM, Verdijk MA, Knoers NV, Wieringa B, Monnens LA, van Os CH, van Oost BA. Requirement of human renal water channel aquaporin-2 for vasopressin-dependent concentration of urine. Science 264: 92–95, 1994. doi: 10.1126/science.8140421. [DOI] [PubMed] [Google Scholar]
- 8.Fenton RA, Moeller HB, Hoffert JD, Yu MJ, Nielsen S, Knepper MA. Acute regulation of aquaporin-2 phosphorylation at Ser-264 by vasopressin. Proc Natl Acad Sci USA 105: 3134–3139, 2008. doi: 10.1073/pnas.0712338105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flajolet M, Rakhilin S, Wang H, Starkova N, Nuangchamnong N, Nairn AC, Greengard P. Protein phosphatase 2C binds selectively to and dephosphorylates metabotropic glutamate receptor 3. Proc Natl Acad Sci USA 100: 16006–16011, 2003. doi: 10.1073/pnas.2136600100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fruman DA, Bierer BE, Benes JE, Burakoff SJ, Austen KF, Katz HR. The complex of FK506-binding protein 12 and FK506 inhibits calcineurin phosphatase activity and IgE activation-induced cytokine transcripts, but not exocytosis, in mouse mast cells. J Immunol 154: 1846–1851, 1995. [PubMed] [Google Scholar]
- 11.Fruman DA, Klee CB, Bierer BE, Burakoff SJ. Calcineurin phosphatase activity in T lymphocytes is inhibited by FK 506 and cyclosporin A. Proc Natl Acad Sci USA 89: 3686–3690, 1992. doi: 10.1073/pnas.89.9.3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fushimi K, Sasaki S, Marumo F. Phosphorylation of serine 256 is required for cAMP-dependent regulatory exocytosis of the aquaporin-2 water channel. J Biol Chem 272: 14800–14804, 1997. doi: 10.1074/jbc.272.23.14800. [DOI] [PubMed] [Google Scholar]
- 13.Gooch JL, Guler RL, Barnes JL, Toro JJ. Loss of calcineurin A results in altered trafficking of AQP2 and in nephrogenic diabetes insipidus. J Cell Sci 119: 2468–2476, 2006. doi: 10.1242/jcs.02971. [DOI] [PubMed] [Google Scholar]
- 14.Gunaratne R, Braucht DW, Rinschen MM, Chou CL, Hoffert JD, Pisitkun T, Knepper MA. Quantitative phosphoproteomic analysis reveals cAMP/vasopressin-dependent signaling pathways in native renal thick ascending limb cells. Proc Natl Acad Sci USA 107: 15653–15658, 2010. doi: 10.1073/pnas.1007424107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gustafson CE, Katsura T, McKee M, Bouley R, Casanova JE, Brown D. Recycling of AQP2 occurs through a temperature- and bafilomycin-sensitive trans-Golgi-associated compartment. Am J Physiol Renal Physiol 278: F317–F326, 2000. [DOI] [PubMed] [Google Scholar]
- 16.Hoffert JD, Fenton RA, Moeller HB, Simons B, Tchapyjnikov D, McDill BW, Yu MJ, Pisitkun T, Chen F, Knepper MA. Vasopressin-stimulated increase in phosphorylation at Ser269 potentiates plasma membrane retention of aquaporin-2. J Biol Chem 283: 24617–24627, 2008. doi: 10.1074/jbc.M803074200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoffert JD, Nielsen J, Yu MJ, Pisitkun T, Schleicher SM, Nielsen S, Knepper MA. Dynamics of aquaporin-2 serine-261 phosphorylation in response to short-term vasopressin treatment in collecting duct. Am J Physiol Renal Physiol 292: F691–F700, 2007. doi: 10.1152/ajprenal.00284.2006. [DOI] [PubMed] [Google Scholar]
- 18.Hoffert JD, Pisitkun T, Wang G, Shen RF, Knepper MA. Quantitative phosphoproteomics of vasopressin-sensitive renal cells: regulation of aquaporin-2 phosphorylation at two sites. Proc Natl Acad Sci USA 103: 7159–7164, 2006. doi: 10.1073/pnas.0600895103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katsura T, Gustafson CE, Ausiello DA, Brown D. Protein kinase A phosphorylation is involved in regulated exocytosis of aquaporin-2 in transfected LLC-PK1 cells. Am J Physiol Renal Physiol 272: F816–F822, 1997. [PubMed] [Google Scholar]
- 20.Katsura T, Verbavatz JM, Farinas J, Ma T, Ausiello DA, Verkman AS, Brown D. Constitutive and regulated membrane expression of aquaporin 1 and aquaporin 2 water channels in stably transfected LLC-PK1 epithelial cells. Proc Natl Acad Sci USA 92: 7212–7216, 1995. doi: 10.1073/pnas.92.16.7212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuwahara M, Fushimi K, Terada Y, Bai L, Marumo F, Sasaki S. cAMP-dependent phosphorylation stimulates water permeability of aquaporin-collecting duct water channel protein expressed in Xenopus oocytes. J Biol Chem 270: 10384–10387, 1995. doi: 10.1074/jbc.270.18.10384. [DOI] [PubMed] [Google Scholar]
- 22.Li R, Gong Z, Pan C, Xie DD, Tang JY, Cui M, Xu YF, Yao W, Pang Q, Xu ZG, Li MY, Yu X, Sun JP. Metal-dependent protein phosphatase 1A functions as an extracellular signal-regulated kinase phosphatase. FEBS J 280: 2700–2711, 2013. doi: 10.1111/febs.12275. [DOI] [PubMed] [Google Scholar]
- 23.Lu G, Wang Y. Functional diversity of mammalian type 2C protein phosphatase isoforms: new tales from an old family. Clin Exp Pharmacol Physiol 35: 107–112, 2008. doi: 10.1111/j.1440-1681.2007.04843.x. [DOI] [PubMed] [Google Scholar]
- 24.Lu HJ, Matsuzaki T, Bouley R, Hasler U, Qin QH, Brown D. The phosphorylation state of serine 256 is dominant over that of serine 261 in the regulation of AQP2 trafficking in renal epithelial cells. Am J Physiol Renal Physiol 295: F290–F294, 2008. doi: 10.1152/ajprenal.00072.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moeller HB, Aroankins TS, Slengerik-Hansen J, Pisitkun T, Fenton RA. Phosphorylation and ubiquitylation are opposing processes that regulate endocytosis of the water channel aquaporin-2. J Cell Sci 127: 3174–3183, 2014. doi: 10.1242/jcs.150680. [DOI] [PubMed] [Google Scholar]
- 26.Moeller HB, Knepper MA, Fenton RA. Serine 269 phosphorylated aquaporin-2 is targeted to the apical membrane of collecting duct principal cells. Kidney Int 75: 295–303, 2009. doi: 10.1038/ki.2008.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Naesens M, Kuypers DR, Sarwal M. Calcineurin inhibitor nephrotoxicity. Clin J Am Soc Nephrol 4: 481–508, 2009. doi: 10.2215/CJN.04800908. [DOI] [PubMed] [Google Scholar]
- 28.Nedvetsky PI, Tabor V, Tamma G, Beulshausen S, Skroblin P, Kirschner A, Mutig K, Boltzen M, Petrucci O, Vossenkämper A, Wiesner B, Bachmann S, Rosenthal W, Klussmann E. Reciprocal regulation of aquaporin-2 abundance and degradation by protein kinase A and p38-MAP kinase. J Am Soc Nephrol 21: 1645–1656, 2010. doi: 10.1681/ASN.2009111190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nielsen S, Chou CL, Marples D, Christensen EI, Kishore BK, Knepper MA. Vasopressin increases water permeability of kidney collecting duct by inducing translocation of aquaporin-CD water channels to plasma membrane. Proc Natl Acad Sci USA 92: 1013–1017, 1995. doi: 10.1073/pnas.92.4.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nishimoto G, Zelenina M, Li D, Yasui M, Aperia A, Nielsen S, Nairn AC. Arginine vasopressin stimulates phosphorylation of aquaporin-2 in rat renal tissue. Am J Physiol Renal Physiol 276: F254–F259, 1999. [DOI] [PubMed] [Google Scholar]
- 31.Ren H, Yang B, Ruiz JA, Efe O, Ilori TO, Sands JM, Klein JD. Phosphatase inhibition increases AQP2 accumulation in the rat IMCD apical plasma membrane. Am J Physiol Renal Physiol 311: F1189–F1197, 2016. doi: 10.1152/ajprenal.00150.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rice WL, Zhang Y, Chen Y, Matsuzaki T, Brown D, Lu HA. Differential, phosphorylation dependent trafficking of AQP2 in LLC-PK1 cells. PLoS One 7: e32843, 2012. doi: 10.1371/journal.pone.0032843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rinschen MM, Yu MJ, Wang G, Boja ES, Hoffert JD, Pisitkun T, Knepper MA. Quantitative phosphoproteomic analysis reveals vasopressin V2-receptor-dependent signaling pathways in renal collecting duct cells. Proc Natl Acad Sci USA 107: 3882–3887, 2010. doi: 10.1073/pnas.0910646107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shi Y. Serine/threonine phosphatases: mechanism through structure. Cell 139: 468–484, 2009. doi: 10.1016/j.cell.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 35.Sun W, Yu Y, Dotti G, Shen T, Tan X, Savoldo B, Pass AK, Chu M, Zhang D, Lu X, Fu S, Lin X, Yang J. PPM1A and PPM1B act as IKKβ phosphatases to terminate TNFα-induced IKKβ-NF-κB activation. Cell Signal 21: 95–102, 2009. doi: 10.1016/j.cellsig.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takekawa M, Maeda T, Saito H. Protein phosphatase 2Cα inhibits the human stress-responsive p38 and JNK MAPK pathways. EMBO J 17: 4744–4752, 1998. doi: 10.1093/emboj/17.16.4744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tamma G, Robben JH, Trimpert C, Boone M, Deen PM. Regulation of AQP2 localization by S256 and S261 phosphorylation and ubiquitination. Am J Physiol Cell Physiol 300: C636–C646, 2011. doi: 10.1152/ajpcell.00433.2009. [DOI] [PubMed] [Google Scholar]
- 38.Uawithya P, Pisitkun T, Ruttenberg BE, Knepper MA. Transcriptional profiling of native inner medullary collecting duct cells from rat kidney. Physiol Genomics 32: 229–253, 2008. doi: 10.1152/physiolgenomics.00201.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Valenti G, Procino G, Carmosino M, Frigeri A, Mannucci R, Nicoletti I, Svelto M. The phosphatase inhibitor okadaic acid induces AQP2 translocation independently from AQP2 phosphorylation in renal collecting duct cells. J Cell Sci 113: 1985–1992, 2000. [DOI] [PubMed] [Google Scholar]
- 40.Wilson JL, Miranda CA, Knepper MA. Vasopressin and the regulation of aquaporin-2. Clin Exp Nephrol 17: 751–764, 2013. doi: 10.1007/s10157-013-0789-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang CR, Raghuram V, Emamian M, Sandoval PC, Knepper MA. Deep proteomic profiling of vasopressin-sensitive collecting duct cells. II. Bioinformatic analysis of vasopressin signaling. Am J Physiol Cell Physiol 309: C799–C812, 2015. doi: 10.1152/ajpcell.00214.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang CR, Tongyoo P, Emamian M, Sandoval PC, Raghuram V, Knepper MA. Deep proteomic profiling of vasopressin-sensitive collecting duct cells. I. Virtual Western blots and molecular weight distributions. Am J Physiol Cell Physiol 309: C785–C798, 2015. doi: 10.1152/ajpcell.00213.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yokoyama N, Kobayashi T, Tamura S, Sugiya H. PP2C phosphatase activity is coupled to cAMP-mediated pathway in rat parotid acinar cells. Biochem Mol Biol Int 36: 845–853, 1995. [PubMed] [Google Scholar]
- 44.Yui N, Lu HA, Chen Y, Nomura N, Bouley R, Brown D. Basolateral targeting and microtubule-dependent transcytosis of the aquaporin-2 water channel. Am J Physiol Cell Physiol 304: C38–C48, 2013. doi: 10.1152/ajpcell.00109.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]