Abstract
The majority of rehabilitation research focuses on the comparative effectiveness of different interventions in groups of patients, while much less is currently known regarding individual factors that predict response to rehabilitation. In a recent article, authors presented a prognostic model to identify the sensorimotor characteristics predictive of the extent of motor recovery after Constraint-Induced Movement (CI) therapy amongst individuals with chronic mild-to-moderate motor deficit using the enhanced probabilistic neural network (EPNN). This follow-up paper examines which participant characteristics are robust predictors of rehabilitation response irrespective of the training modality. To accomplish this, EPNN was first applied to predict treatment response amongst individuals who received a virtual-reality gaming intervention (utilizing the same enrollment criteria as the prior study). The combinations of predictors that yield high predictive validity for both therapies, using their respective datasets, were then identified. High predictive classification accuracy was achieved for both the gaming (94.7%) and combined datasets (94.5%). Though CI therapy employed primarily fine-motor training tasks and the gaming intervention emphasized gross-motor practice, larger improvements in gross motor function were observed within both datasets. Poorer gross motor ability at pre-treatment predicted better rehabilitation response in both the gaming and combined datasets. The conclusion of this research is that for individuals with chronic mild-to-moderate upper extremity hemiparesis, residual deficits in gross motor function are highly responsive to motor restorative interventions, irrespective of the modality of training.
Introduction
Motor restorative therapies aim to restore motor function by emphasizing practice with the more affected upper extremity while minimizing compensatory movement by the less affected upper extremity. Recently, the authors showed that this therapeutic approach may not be appropriate for all individuals who have sufficient motor ability to participate (George et al., 2017). George et al. (2017) presented a novel prognostic computational model to identify which baseline sensorimotor characteristics predicted the extent of motor recovery during Constraint-Induced Movement (CI) therapy, an established motor restorative intervention (Taub et al., 1993; Wolf et al., 2006; Taub et al. 2006), employing the enhanced probabilistic neural network (EPNN) model of Ahmadlou and Adeli (2010). They found that the extent of motor restoration, as measured by the Wolf Motor Function Test (WMFT) (Taub et al. 1993, Taub et al. 2006, Wolf et al. 2006), varied markedly among individuals and was generally poor amongst those with higher baseline ability.
The purpose of this follow-up research is to determine robust predictors of motor restoration irrespective of the type of motor training. This is accomplished by applying the aforementioned machine learning-based model to a very different treatment modality: motor training delivered at home via Recovery Rapids, a Kinect-based video game (Maung et al. 2013, Fuhry et al. 2016). Like CI therapy, this motor restorative video game-based intervention involves high repetition practice with the more affected upper extremity for several hours per day over two weeks, progressive shaping of motor tasks, and an emphasis on carry-over of motor gains to daily activities (Gauthier et al. in press, Morris et al. 2006). There are several important differences between the two types of therapies, however. Recovery Rapids harnesses the benefits of a virtual world (i.e., no task set-up time) to dramatically increase task variability. As such, the client switches rapidly between different types of motor movements. In contrast, CI therapy utilizes blocked practice, in which the same task is practiced repeatedly for a period of about 10-20 minutes. Game-based therapy through Recovery Rapids also involves substantially more repetitions per time (> 1000 per hour on average), is largely delivered at home without direct therapist supervision, incorporates limited tactile feedback (participants do not touch objects), and distal (fine-motor) training comprises a smaller percentage of tasks (∼30% versus >90%).
The authors hypothesized that there are likely to be some commonalities in individual sensorimotor presentation that would make that individual a better candidate overall for motor restorative therapies, irrespective of therapeutic modality. Additionally, they expected that training-related factors would interact with individual characteristics to produce different patterns of poor versus good responders for the two different interventions. Specifically, they hypothesized that those with poorer function on the domain being trained would benefit more from the intervention. Consistent with this hypothesis, George et al. (2017) found that those with relatively greater fine-motor ability at baseline benefited less from CI therapy, an intervention that targets fine motor tasks. In keeping with this hypothesis and the authors' prior findings, we hypothesized that those with poorer gross-motor performance at baseline would be better candidates for gaming therapy, as this approach does not provide as many fine-motor training opportunities. To test this hypothesis, the best combination of predictors from the prior paper will be compared with an identical analysis for the Recovery Rapids gaming therapy. To determine which elements of sensorimotor presentation predict more favorable outcome irrespective of therapeutic modality, the most predictive combinations of baseline sensorimotor ability for both gaming therapy and CI therapy will be identified.
This research aims to identify those individual characteristics at baseline that can predict response to two different motor restorative therapies. Improved predictions of treatment response based on a person's individual characteristics at baseline will enable therapists to devise cost-efficient personalized care plans with the goal of balancing restorative versus compensatory intervention approaches to maximize the motor functions of their patients.
Methodology
Participants
Participants were 19 individuals with chronic (>6 months) mild to moderate upper extremity hemiparesis who had experienced a stroke of any etiology. All participants met the motor inclusion criteria utilized in the EXCITE trial of CI therapy (Wolf et al., 2006), but were enrolled largely irrespective of cognitive or mobility status. The sample utilized in this analysis is thus more inclusive than in prior CI therapy trials. Those who were unable to provide informed consent, or who had received Botox treatment in the past 12 weeks were excluded. Inclusion criteria and recruitment approaches for this study were the same as those used in the earlier study by the authors (George et al. 2017). See Table 1 for participant demographics.
Table 1. Demographic and clinical characteristics of 19 participants in gaming therapy.
| Item | Number of case (Average) | Minimum | Maximum |
|---|---|---|---|
| Age | 47.5 | 14.1 | 69.6 |
| Sex | 8 females | N/A | N/A |
| Chronicity (years) | N/A | 0.55 | 5.34 |
| Stroke affected side | 8 left | N/A | N/A |
| Handedness | At least 6 right | N/A | N/A |
| Affected side was dominant | At least 4 participants | N/A | N/A |
Intervention
The gaming therapy intervention was designed such that physical/occupational therapists manage patients in a consultative role with the majority of the motor practice occurring through Recovery Rapids, an in-home gaming rehabilitation system (Maung et al. 2013). The gaming system utilizes the KinectOne™ sensor to capture particular therapeutic movements (gestures), each of which is tied to a game objective. Gestures include elbow flexion/extension, shoulder flexion with elbow extension, shoulder abduction, shoulder adduction, overhead reaching, forearm supination, grasp release, and wrist extension. The CI therapy principal of shaping (progressively increasing task difficulty as a person improves) is incorporated. In just one example, the user attempts to capture parachutes as they fall from above. An introductory difficulty level for this gesture may require only 30 degrees of shoulder flexion. As a user demonstrates the capacity to perform more difficult movements, the software requires greater shoulder flexion, then increased concurrent elbow extension and forearm supination to accomplish the same game objective. See Figure 1 for a depiction of the gaming environment (http://gamesthatmoveyou.com/). Carry-over of motor improvements to daily life is promoted through an interactive Motor Activity Log problem-solving module that occurs after each 15-20 minutes of the game play.
Figure 1.

Screenshot of the CI therapy game. See http://youtu.be/uAysIGueN9U for a video demonstration.
Five therapist/patient contact hours occurred over 4 home visits. The first session (2 hours) involved instruction in game play, customizing the game to the participant, establishing the treatment contract, and establishing the home program (target functional activities to accomplish daily). Thereafter, sessions focused on review of progress with the home program, modifying game customization as needed, and on “transfer package” elements that could not be readily addressed through the game (Morris et al. 2006, Taub et al. 2013). “Transfer package” elements include reviewing the treatment contract, daily self-assessment of arm use, guided problem-solving to increase the use of the weaker upper extremity for activities of daily living, and collaboratively establishing a home program focused on functional task practice. Participants agreed to play Recovery Rapids for 30 hours over a two-week period.
Outcome Measures
Three outcome measures were utilized: the WMFT, the Brief Kinesthesia Test (BKT), and Touch Test Monofilaments (TM). The WMFT was utilized to assess the motor function of the upper limbs (Taub et al. 1993, Taub et al. 2006, Wolf et al. 2006). As in George et al. (2017), the WMFT scores, recorded in seconds, were natural-log-transformed to account for the non-uniform interpretation of performance time improvement (i.e., an improvement from 5s to 3s is greater than an improvement from 105 to 103s). The BKT is a measure of error in guided reaching with visual occlusion considered to represent upper limb kinesthetic sense (Borstad and Nichols-Larsen, 2016). TM is sensitive to tactile impairment; it identifies the lightest force in grams perceived consistently by an individual on the index finger (Callahan et al. 1995). These same sensorimotor measures were used in the authors' earlier research (George et al. 2017) and are summarized in Table 2
Table 2. Behavioral measures used in the prognostic model.
| Type of test | Number of items | Function assessed | Behavioral measures | Fine or Gross motor |
|---|---|---|---|---|
| Motor | 15 | Upper limb functional performance (timed) | Forearm to table (side) | Gross |
| Forearm to box (side) | Gross | |||
| Extend elbow (side) | Gross | |||
| Hand to table (front) | Gross | |||
| Hand to box (front) | Gross | |||
| Extend Elbow Weight | Gross | |||
| Reach and retrieve | Gross | |||
| Lift can | Fine | |||
| Lift pencil | Fine | |||
| Lift paper clip | Fine | |||
| Stack checkers | Fine | |||
| Flip card | Fine | |||
| Turnkey in lock | Fine | |||
| Fold towel | Fine | |||
| Lift Basket | Fine | |||
| Somatosensory | 2 | Reaching error with visual occlusion (proprioception) | Brief kinesthesia test (affected side) | N/A |
| Touch perception threshold | Touch test monofilaments (affected side) | N/A |
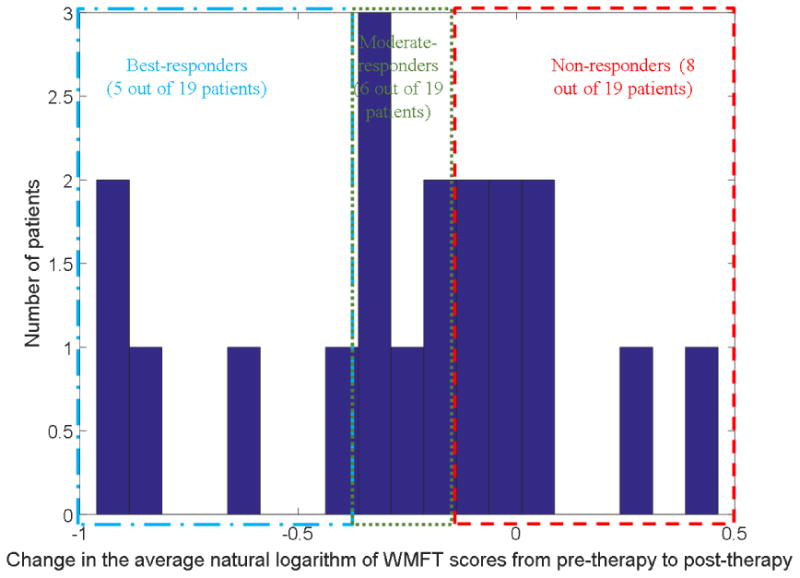
Table 3 summarizes the collected patient data used for the prognostic computational EPNN model. From these data, there were 2 missing values corresponding to the somatosensory measures of only one participant. These were replaced using a simple regression analysis. Each participant was categorized based on their natural-log-transformed WMFT treatment change score as either a non-responder (>-0.15; class 1), moderate-responder (-0.15:-0.40; class 2), or best responder (<-0.40; class 3). Classification thresholds are consistent with the earlier research (George et al. 2017). These categories are represented in the last column of Table 3. A histogram of WMFT change is shown in Figure 2.
Table 3. Participant data used to train and test the EPNN model.
| P# | Predictors | OT | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. SA | 2. BT | 3. CN | 4. EE | 5. EW | 6. FC | 7. FB | 8. FT | 9. HB | 10. HT | 11. KY | 12. LC | 13. PL | 14. SC | 15. RR | 16. TL | 17. BK | 18. TM | ||
| 1 | 0 | 1.16 | 1.48 | 0.00 | 0.10 | 2.62 | 0.26 | 0.34 | 0.41 | 0.34 | 2.17 | 1.81 | 1.44 | 1.74 | 0.34 | 2.12 | 1.84 | -0.92 | 2 |
| 2 | 1 | 1.90 | 1.28 | 0.10 | -0.22 | 3.31 | 0.64 | 0.11 | 0.47 | 0.53 | 2.37 | 3.87 | 2.13 | 3.48 | 0.18 | 3.99 | 1.39 | -3.22 | 2 |
| 3 | 0 | 3.48 | 2.14 | 1.60 | 1.39 | 3.66 | 1.03 | 0.71 | 1.15 | 0.94 | 3.03 | 1.40 | 3.12 | 4.79 | 0.99 | 3.04 | 3.22 | 1.39 | 1 |
| 4 | 0 | 1.86 | 1.41 | 0.59 | 0.41 | 2.47 | 1.06 | 0.26 | 0.47 | 0.26 | 1.46 | 1.03 | 1.06 | 1.82 | 0.59 | 2.56 | 1.90 | 0.69 | 3 |
| 5 | 1 | 0.99 | 0.73 | 0.46 | 0.77 | 2.41 | 0.83 | -0.21 | 0.19 | 0.02 | 1.57 | 1.05 | 0.48 | 1.81 | 0.39 | 2.16 | 2.08 | -0.92 | 2 |
| 6 | 0 | 1.83 | 3.33 | 1.00 | 1.48 | 4.79 | 0.58 | -0.29 | -0.08 | -0.60 | 4.79 | 4.79 | 4.79 | 4.79 | 0.12 | 3.04 | 2.63 | -0.92 | 2 |
| 7 | 1 | 3.68 | 4.79 | 0.34 | 0.00 | 4.79 | 1.41 | 0.34 | 0.00 | 0.18 | 4.79 | 4.79 | 3.47 | 4.79 | 0.88 | 4.79 | 3.37 | 0.10 | 1 |
| 8 | 1 | 3.00 | 4.79 | 0.39 | 1.90 | 4.79 | 0.21 | 0.38 | 0.41 | 0.36 | 4.79 | 4.79 | 4.79 | 4.79 | 4.79 | 3.70 | 2.32 | -1.83 | 3 |
| 9 | 0 | 4.79 | 1.77 | 1.27 | 1.28 | 2.31 | 1.75 | -0.40 | -0.67 | -0.31 | 2.10 | 2.25 | 1.11 | 1.95 | 0.63 | 2.92 | 2.22 | -1.83 | 1 |
| 10 | 0 | 2.58 | 4.79 | 1.35 | 0.00 | 4.79 | 1.33 | -0.08 | 1.04 | 0.41 | 4.79 | 4.79 | 4.79 | 4.79 | 0.00 | 4.75 | 2.80 | -1.83 | 3 |
| 11 | 1 | 0.99 | 0.96 | -0.11 | 0.18 | 1.97 | -0.11 | 0.18 | -0.11 | -0.11 | 1.03 | 1.03 | 0.92 | 1.65 | -0.11 | 2.35 | 2.05 | -0.92 | 2 |
| 12 | 0 | 1.77 | 1.11 | 0.61 | 1.17 | 2.63 | 0.59 | -1.24 | 0.68 | 0.52 | 1.54 | 1.09 | 0.97 | 1.91 | 0.19 | 2.40 | 2.00 | -1.83 | 2 |
| 13 | 0 | 3.68 | 4.79 | 1.99 | 1.57 | 4.52 | 1.25 | 0.18 | 0.59 | 0.41 | 2.80 | 4.79 | 4.79 | 4.79 | 0.26 | 3.43 | 2.42 | -3.22 | 1 |
| 14 | 0 | 1.56 | 2.35 | 1.88 | 1.83 | 3.14 | 0.56 | 0.13 | 0.50 | 0.69 | 3.33 | 1.95 | 2.26 | 4.33 | 1.84 | 3.58 | 2.46 | -2.66 | 1 |
| 15 | 1 | 4.79 | 4.79 | 4.79 | 4.79 | 4.79 | 4.79 | 1.35 | 4.79 | 1.75 | 4.79 | 4.79 | 4.79 | 4.79 | 4.79 | 4.79 | 1.76 | 5.70 | 3 |
| 16 | 1 | 1.26 | 2.20 | -0.07 | -0.07 | 2.83 | 0.98 | 0.26 | 0.54 | 0.18 | 2.65 | 4.79 | 4.79 | 4.79 | -0.53 | 3.54 | 3.05 | 1.39 | 1 |
| 17 | 0 | 0.88 | 0.25 | -0.43 | -0.25 | 1.59 | 0.00 | 0.10 | -0.51 | -0.46 | 1.50 | 0.10 | 0.41 | 1.43 | -0.53 | 1.05 | 1.70 | -1.83 | 1 |
| 18 | 1 | 1.18 | 1.14 | 0.03 | 4.79 | 4.79 | 0.11 | 0.39 | 0.03 | 0.00 | 2.12 | 4.79 | 4.79 | 1.91 | 0.47 | 2.43 | 1.69 | -0.92 | 3 |
| 19 | 0 | 4.79 | 4.79 | 4.79 | 4.79 | 4.79 | 0.96 | 0.50 | 4.79 | 0.63 | 4.79 | 4.79 | 4.79 | 4.79 | -0.42 | 4.79 | 1.39 | -0.92 | 1 |
| A | N/A | 2.43 | 2.57 | 1.08 | 1.36 | 3.52 | 0.96 | 0.16 | 0.77 | 0.30 | 2.97 | 3.09 | 2.93 | 3.43 | 0.78 | 3.23 | 2.23 | -0.76 | N/A |
| S | N/A | 0.49 | 1.35 | 1.64 | 1.44 | 1.64 | 1.13 | 1.03 | 0.50 | 1.45 | 0.52 | 1.35 | 1.76 | 1.75 | 1.44 | 1.48 | 1.04 | 0.57 | N/A |
EPNN: Enhanced probabilistic neural networks; P#: Participant number; SA: Stroke affected side; BT: WMFT basket; CN: WMFT can; EE: WFMT extend elbow; EW: WMFT extend elbow weight; FC: WMFT flip cards; FB: WMFT forearm to box; FT: WMFT forearm to table; HB: WMFT hand to box; HT: WMFT hand to table; KY: WMFT key; LC: WMFT lift paper clip; PL: WMFT pencil; SC: WMFT stack checkers; RR: WMFT reach retrieve; TL: WMFT towel; BK: BKT; WMFT: Wolf motor function test; TM: Touch monofilament; OT: Output; A: Average; S: CI therapy deviation.
Figure 2. The histogram of the participants' change in the natural log of WMFT scores from pre to post gaming therapy.
Sensitivity Analysis and Prognosis Model
In order to identify the best method of classification, the authors previously used three different algorithms to classify participants: k-nearest neighbors (Siddique and Adeli, 2013), the probabilistic neural network, and the enhanced probabilistic neural network (EPNN) (Ahmadlou and Adeli, 2010) and reported the most accurate results by EPNN (George et al. 2017). Therefore, EPNN was also utilized in this follow-up research. Using the available data a total of 262,125 combinations of 18 motor, somatosensory, and stroke-affected side predictors exist.
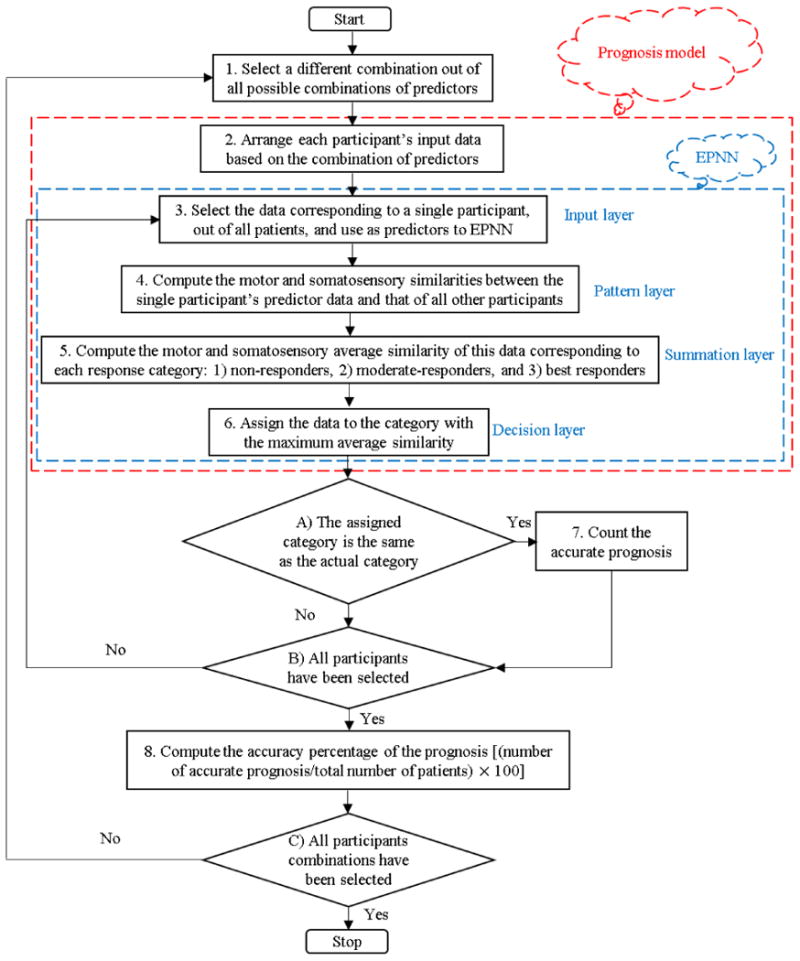
Sensitivity analyses were performed to identify the prediction accuracy of each predictive model. The flowchart of the sensitivity analysis is presented in Figure 3. It consists of 8 steps and 3 decision diamonds. In step 1, one out the 262,125 combinations is selected. In step 2, each participant's motor and somatosensory data are arranged based on the selected combination. In step 3, the data corresponding to a single participant is input to the EPNN (predictor layer in EPNN). In step 4, the motor and somatosensory data of the single participant is compared statistically to the same predictor data of all other patients in the pattern layer of EPNN. In step 5, the summation layer determines how similar the single participant's predictor data are to the average values in each of the three categories: non-, moderate-, and best-responders. In step 6, the predictor data are assigned the category with the maximum average similarity (decision layer in EPNN). In step 7, if the participant was classified correctly, an accurate prognosis is counted. Steps 3 to 7 are repeated for every patient. Once the data for all participants have been analyzed in this manner, the accuracy percentage of the prognosis corresponding to the selected combination is computed in step 8 as follows: (number of accurate prognosis/total number of participants)× 100. This process is repeated for every combination (the outer loop in Figure 3).
Figure 3. Flowchart of the sensitivity analysis using the prognostic computational model for predicting the extent of motor recovery during CI therapy.
Implementation
All possible combinations of motor and somatosensory predictors (Table 2) are considered to identify the combinations with the most accurate prognosis. Because the stroke patient data available for training a sophisticated neural network classification model (EPNN) were limited in this research, the model was trained and tested 19 times (equal to the number of stroke participants), each time using the data for a different patient for testing and the remaining 18 sets of data for training (steps 3 to 6 in Figure 3). This results in an RTT (rate of testing to training) of about 5.0%. The accuracy values reported in this research are the average of testing accuracies of the 19 trials (step 8 in Figure 3).
Results
Accuracy of the Gaming Models and Rates of Selection
Within the gaming therapy cohort, EPNN yielded maximum classification accuracies of 94.7% for 8 out of the 262,125 combinations. The next highest accuracy obtained was 89.5% for 80 out of 262,125 combinations. The most frequently selected predictor in the 8 combinations with the highest accuracy was WMFT forearm to table (gross motor), which was selected in all eight combinations. The next most frequently selected predictors comprised mainly gross motor predictors: WMFT extend elbow weight (gross motor), and WMFT hand to table (gross motor), WMFT basket (gross motor), and stroke-affected side, which were selected 6 times. Somatosensory predictors, BKT and TM, were selected in 1 and 3 combinations, respectively. Table 4 presents the 8 different combinations of predictors with average accuracies of 94.7%.
Table 4. Different combinations of 18 predictors for EPNN resulting in an average accuracy of 94.7% in the current study (1: selected; 0: not-selected).
| C# | Predictors | LR | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. SA | 2. BT | 3. CN | 4. EE | 5. EW | 6. FC | 7. FB | 8. FT | 9. HB | 10. HT | 11. KY | 12. LC | 13. PL | 14. SC | 15. RR | 16. TL | 17. BK | 18. TM | ||
| 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.00 |
| 2 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.08 |
| 3 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.12 |
| 4 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.59 |
| 5 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0.61 |
| 6 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.62 |
| 7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0.76 |
| 8 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.00 |
| TS | 6 | 6 | 1 | 0 | 6 | 0 | 2 | 8 | 3 | 6 | 0 | 0 | 0 | 0 | 2 | 2 | 1 | 3 | N/A |
EPNN: Enhanced probabilistic neural networks; C#: Combination number; SA: Stroke affected side; BT: WMFT basket; CN: WMFT can; EE: WFMT extend elbow; EW: WMFT extend elbow weight; FC: WMFT flip cards; FB: WMFT forearm to box; FT: WMFT forearm to table; HB: WMFT hand to box; HT: WMFT hand to table; KY: WMFT key; LC: WMFT lift paper clip; PL: WMFT pencil; SC: WMFT stack checkers; RR: WMFT reach retrieve; TL: WMFT towel; BK: BKT; WMFT: Wolf motor function test; TM: Touch monofilament; OT: Output; TS: Times selected; LR: Likelihood ratio.
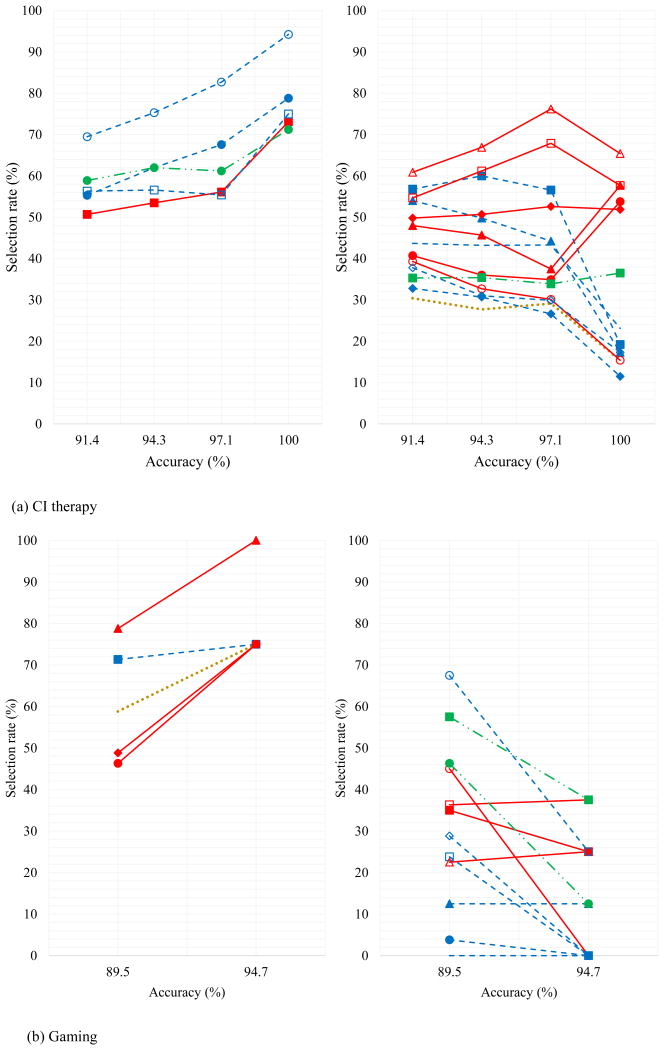
Table 5 presents a comparison of the rates of selection of predictors for the combinations with accuracy of about 90% and greater using the CI and gaming therapies. For the gaming therapy, the most frequently selected predictors consist of a combination of gross, fine and somatosensory predictors: WMFT forearm to table (gross motor), WMFT basket (fine and gross motor), WMFT towel (fine motor), stroke affected side, and TM (somatosensory), with rates of selection of 80.7%, 71.6%, 63.6%, 60.2%, and 55.7%, respectively. The least selected predictors consist of only fine motor tasks: WMFT stack checkers (fine motor), WMFT key (fine motor), WMFT pencil (fine motor), WMFT can, and WMFT lift paper clip (fine motor) with rates of selection of 0.0%, 0.0%, 3.4%, 12.5%, and 21.6%, respectively.
Table 5. Comparison of the rates of selection of predictors for the combinations with accuracy of about 90% and greater using the CI and gaming CI therapies.
| Predictors | Rate of selection (%) | ||
|---|---|---|---|
| Current study (Gaming therapy) |
CI therapy (Hulbert at al. 2017) |
Difference (Gaming –CI therapy) |
|
| 8.WMFT forearm to table (gross motor) | 80.7 | 46.9 | 33.8 |
| 2.WMFT basket (fine motor) | 71.6 | 57.4 | 14.2 |
| 16.WMFT towel (fine motor) | 63.6 | 71.5 | -7.9 |
| 1.Stroke Affected Side | 60.2 | 29.6 | 30.6 |
| 18.TM (somatosensory) | 55.7 | 35.3 | 20.4 |
| 10.WMFT hand to table (gross motor) | 51.1 | 50.2 | 0.9 |
| 5.WMFT extend elbow weight (gross motor) | 48.9 | 39.4 | 9.5 |
| 17.BKT (somatosensory) | 43.2 | 59.8 | -16.6 |
| 4.WFMT extend elbow (gross motor) | 40.9 | 37.2 | 3.7 |
| 9.WMFT hand to box (gross motor) | 36.4 | 56.9 | -20.5 |
| 7.WMFT forearm to box (gross motor) | 34.1 | 51.6 | -17.5 |
| 6.WMFT flip cards (fine motor) | 26.1 | 35.8 | -9.7 |
| 15.WMFT reach retrieve (gross motor) | 22.7 | 63.0 | -40.3 |
| 12.WMFT lift paper clip (fine motor) | 21.6 | 56.4 | -34.8 |
| 3.WMFT can (fine motor) | 12.5 | 52.4 | -39.9 |
| 13.WMFT pencil (fine motor) | 3.4 | 57.5 | -54.1 |
| 11.WMFT key (fine motor) | 0.0 | 43.6 | -43.6 |
| 14.WMFT stack checkers (fine motor) | 0.0 | 32.0 | -32.0 |
WMFT: Wolf motor function test; BKT: Brief kinesthesia test; TM: Touch monofilament
As a point of comparison, in the earlier research for CI therapy, EPNN yielded maximum classification accuracies of 100% (52 combinations), with fine motor tasks comprising the most frequently selected predictors (George et al. 2017). Selection rates for these, along with other combinations achieving accuracies of at least 90% are also included in Table 5.
Table 6 summarizes the rates of selection of 18 predictors in the prognosis model for the gaming and CI therapies, and the combined model with accuracies of about 90% and greater.
Table 6. Rates of selection of 18 predictors in the prognosis model for the gaming and CI therapies, and the combined approach.
| # | Predictors | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. SA | 2. BT | 3. CN | 4. EE | 5. EW | 6. FC | 7. FB | 8. FT | 9. HB | 10. HT | 11. KY | 12. LC | 13. PL | 14. SC | 15. RR | 16. TL | 17. BK | 18. TM | |||
| Gaming | ||||||||||||||||||||
| 1 | 75.0 | 75.0 | 12.5 | 0.0 | 75.0 | 0.0 | 25.0 | 100. 0 | 37.5 | 75.0 | 0.0 | 0.0 | 0.0 | 0.0 | 25.0 | 25.0 | 12.5 | 37.5 | 8 | 94.7 |
| 2 | 58.8 | 71.3 | 12.5 | 45.0 | 46.3 | 28.8 | 35.0 | 78.8 | 36.3 | 48.8 | 0.0 | 23.8 | 3.8 | 0.0 | 22.5 | 67.5 | 46.3 | 57.5 | 80 | 89.5 |
| A | 66.9 | 73.1 | 12.5 | 22.5 | 60.6 | 14.4 | 30.0 | 89.4 | 36.9 | 61.9 | 0.0 | 11.9 | 1.9 | 0.0 | 23.8 | 46.3 | 29.4 | 47.5 | N/A | N/A |
| CI therapy | ||||||||||||||||||||
| 1 | 15.4 | 19.2 | 17.3 | 15.4 | 53.8 | 17.3 | 73.1 | 57.7 | 57.7 | 51.9 | 23.1 | 75.0 | 78.8 | 11.5 | 65.4 | 94.2 | 71.2 | 36.5 | 52 | 100 |
| 2 | 29.1 | 56.6 | 44.2 | 30.1 | 34.9 | 29.9 | 56.1 | 37.5 | 67.9 | 52.6 | 43.3 | 55.4 | 67.6 | 26.6 | 76.2 | 82.7 | 61.2 | 33.9 | 1014 | 97.1 |
| 3 | 27.7 | 60.0 | 49.8 | 32.7 | 36.0 | 31.0 | 53.5 | 45.7 | 61.2 | 50.7 | 43.2 | 56.6 | 62.0 | 30.7 | 66.9 | 75.3 | 62.0 | 35.4 | 4828 | 94.3 |
| 4 | 30.4 | 56.8 | 54.0 | 39.3 | 40.7 | 37.8 | 50.7 | 48.0 | 54.7 | 49.8 | 43.7 | 56.3 | 55.3 | 32.8 | 60.9 | 69.5 | 58.9 | 35.3 | 15052 | 91.4 |
| A | 25.6 | 48.2 | 41.3 | 29.3 | 41.4 | 29.0 | 58.3 | 47.2 | 60.4 | 51.3 | 38.3 | 60.8 | 65.9 | 25.4 | 67.3 | 80.4 | 63.3 | 35.3 | N/A | N/A |
| Combined | ||||||||||||||||||||
| 1 | 100 | 100 | 0 | 0.0 | 100 | 0.0 | 100 | 100 | 0.0 | 100 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 100 | 1 | 94.5 |
| 2 | 100 | 100 | 0. | 0.0 | 50.0 | 50.0 | 100 | 100 | 0.0 | 50.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 50.0 | 0.0 | 100 | 1 | 93.3 |
| 3 | 100 | 100 | 0.0 | 0.0 | 66.7 | 33.3 | 66.7 | 100 | 33.3 | 66.7 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 33.3 | 0.0 | 100 | 1 | 93.1 |
| 4 | 66.7 | 66.7 | 0.0 | 0.0 | 33.3 | 33.3 | 50.0 | 66.7 | 66.7 | 66.7 | 0.0 | 33.3 | 16.7 | 0.0 | 33.3 | 66.7 | 50.0 | 100 | 3 | 92.1 |
| 5 | 60.0 | 50.0 | 0.0 | 10.0 | 30.0 | 40.0 | 50.0 | 50.0 | 60.0 | 40.0 | 0.0 | 30.0 | 20.0 | 0.0 | 20.0 | 80.0 | 50.0 | 100 | 4 | 91.9 |
| 6 | 66.7 | 58.3 | 0.0 | 8.3 | 41.7 | 33.3 | 41.7 | 58.3 | 58.3 | 50.0 | 0.0 | 25.0 | 16.7 | 0.0 | 16.7 | 66.7 | 41.7 | 91.7 | 2 | 91.7 |
| 7 | 59.7 | 29.0 | 6.5 | 9.7 | 37.1 | 35.5 | 61.3 | 51.6 | 54.8 | 41.9 | 0.0 | 54.8 | 35.5 | 0.0 | 48.4 | 83.9 | 62.9 | 74.2 | 50 | 90.7 |
| 8 | 59.2 | 33.8 | 5.6 | 9.9 | 38.0 | 33.8 | 60.6 | 53.5 | 54.9 | 42.3 | 0.0 | 53.5 | 33.8 | 0.0 | 43.7 | 84.5 | 64.8 | 73.2 | 9 | 90.5 |
| 9 | 58.3 | 33.3 | 6.9 | 9.7 | 37.5 | 33.3 | 59.7 | 54.2 | 55.6 | 41.7 | 0.0 | 52.8 | 33.3 | 0.0 | 44.4 | 84.7 | 63.9 | 72.2 | 1 | 90.2 |
| A | 74.5 | 63.5 | 2.1 | 5.3 | 48.3 | 32.5 | 65.5 | 70.5 | 42.6 | 55.5 | 0.0 | 27.7 | 17.3 | 0.0 | 22.9 | 61.1 | 37.0 | 90.1 | N/A | N/A |
EPNN: Enhanced probabilistic neural networks; R#: Row number; SA: Stroke affected side; BT: WMFT basket; CN: WMFT can; EE: WFMT extend elbow; EW: WMFT extend elbow weight; FC: WMFT flip cards; FB: WMFT forearm to box; FT: WMFT forearm to table; HB: WMFT hand to box; HT: WMFT hand to table; KY: WMFT key; LC: WMFT lift paper clip; PL: WMFT pencil; SC: WMFT stack checkers; RR: WMFT reach retrieve; TL: WMFT towel; BK: BKT; WMFT: Wolf motor function test; TM: Touch monofilament; OT: Output; TS: Times selected; AC: Accuracy percentage; #C: Number of combinations associated with AC; A: Average.
Robust Predictors Across Intervention Type
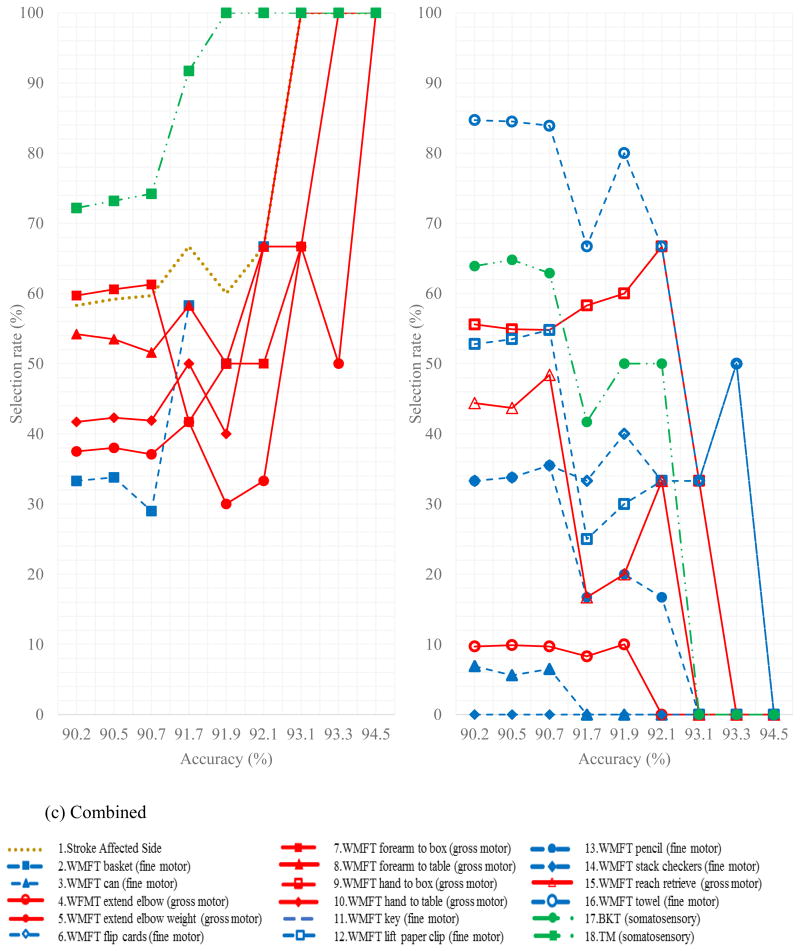
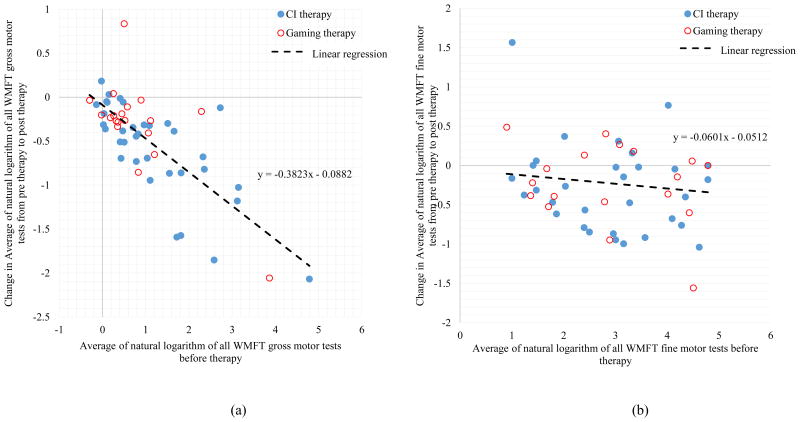
To identify the most robust sensorimotor predictors of motor restoration irrespective of training modality, 72 combinations from the combined CI therapy and gaming therapy data sets with average accuracies above 90%, were identified. To determine the direction of effect for each predictor, scatterplots of baseline scores versus change in the WMFT are created and shown in Figure 4. This figure demonstrates how the selection rates of various predictors vary with overall accuracy of the model. The direction of effect was consistent for all predictors whereby poorer baseline performance yielded greater improvement. To illustrate this point, Figure 5 shows the change in fine and gross motor performance as a function of the initial motor performance.
Figure 4.
a) The selection rates of predictors that were not selected in the best combination in Table 6 versus accuracy including the combinations with at least that accuracy or more and b) The selection rates of predictors that were selected in the best combination in Table 6 versus accuracy including the combinations with at least that accuracy or more
Figure 5.
a) Change in average of natural logarithm of all WMFT gross motor tests from pre therapy to post therapy (R2 = 0.55) versus average of natural logarithm of all WMFT gross motor tests before therapy; b) Change in average of natural logarithm of all WMFT fine motor tests from pre therapy to post therapy (R2 = 0.02) versus average of natural logarithm of all WMFT fine motor tests before therapy
Parsimonious Combinations
In the current study using gaming therapy, among the 8 combinations with the maximum accuracy of 94.7%, three were found to be the most parsimonious, meaning they achieved the highest accuracy with the fewest predictors. They are combination numbers 4, 5, and 7 in Table 4 (bolded and shaded). Combination number 7 includes only four predictors: WMFT forearm to table (gross motor), WMFT reach retrieve (gross motor), WMFT towel (fine motor), and BKT (somatosensory). Combination number 5 includes 5 predictors: WMFT forearm to table (gross motor), WMFT hand to box (gross motor), WMFT reach retrieve (gross motor), WMFT can (fine motor), and WMFT towel (fine motor). Combination number 4 also includes 5 predictors. Interestingly, this combination is contained in the remaining 5 combinations in Table 4. These predictors are: WMFT extend elbow weight (gross motor), WMFT forearm to table (gross motor), WMFT hand to table (gross motor), WMFT basket (fine and gross motor), and stroke affected side.
Note that a parsimony analysis is irrelevant for the combined gaming and CI therapy approach because only one combination was found to have the maximum accuracy of 94.5% (first row in Table 6 under the Combined section).
Sensitivity Analysis on the Parsimonious Combinations
In the current study, in order to identify the influence of each selected predictor on the prediction accuracy, another sensitivity analysis was performed on the three most parsimonious combinations for gaming therapy. This sensitivity analysis is similar to that reported in the earlier work of the authors (George et al. 2017). For each combination of interest, each selected predictor is removed, one at a time, and the classification accuracy is computed each time. Those accuracies are then compared with the one combining all 18 predictors. For the first combination (combination number 4 in Table 4), the model was run 5 times, each time removing one of the 5 included predictors. The accuracy of the prediction drops from 94.7% to 63.2%, 78.9%, 84.2%, 84.2, and 68.4%, by removing each predictor in turn one at a time: WMFT extend elbow weight (gross motor), WMFT forearm to table (gross motor), WMFT hand to table (gross motor), WMFT basket (fine motor), and stroke affected side respectively.
For the second combination (combination number 5 in Table 4), the model was run 5 times, each time removing one of the 5 included predictors. The accuracy of the prediction drops from 94.7% to 89.4%, 89.4%, 63.2%,73.7%, and 84.2%, by removing WMFT forearm to table (gross motor), WMFT hand to box (gross motor), WMFT reach retrieve (gross motor), WMFT towel (fine motor), and WMFT can (fine motor), respectively.
For the third combination (combination number 7 in Table 4), the model was run 4 times, each time removing one of the 4 included predictors. The accuracy of the prediction drops from 94.7% to 84.2%, 63.2%, 73.7%, and 84.2% by removing WMFT forearm to table (gross motor), WMFT reach retrieve (gross motor), WMFT towel (fine motor), and BKT (somatosensory), respectively.
A similar sensitivity analysis was performed on the single combination with the highest average accuracy of the combined approach. This combination includes 7 predictors. The accuracy of the prediction drops from 94.5% to 78.7%, 91.7%, 85.2%, 85.0%, 85.2%, 88.8%, and 78.5%, by removing WMFT extend elbow weight (gross motor), WMFT forearm to box (gross motor), WMFT forearm to table (gross motor), WMFT hand to table (gross motor), WMFT basket (fine and gross motor), TM (somatosensory), and stroke affected side, respectively. Again, among the motor predictors, the accuracy tends to drop equally or more upon removal of a gross motor predictor compared to fine motor predictors.
Discussion
The enhanced probabilistic neural network was used 1) to predict the extent of motor recovery following gaming therapy and 2) to investigate which baseline sensorimotor characteristics were robust predictors of motor restoration, irrespective of therapeutic modality. Concerning the first point, and consistent with our hypothesis, poorer baseline ability on the characteristics most heavily trained during the intervention (i.e., fine motor tasks for in-clinic CI therapy and gross motor tasks for gaming therapy) predicted greater motor restoration. That is, if a participant has a poor baseline score on gross motor tasks and undergoes gaming therapy, our models suggest that person will be a good responder to therapy and have good motor restoration. Accordingly, poorer baseline scores on fine motor tasks predicted better response to CI therapy. This effect does not result from different magnitudes of fine or gross motor improvement between the two interventions.
Concerning the second point, baseline ability on the gross motor tasks of the WMFT, compared to the fine motor tasks, seemed to be the most robust predictors of motor restoration across both datasets. This finding is consistent with Lee et al. (2015), who reported that in 174 chronic stroke patients, proximal joint movement at baseline could significantly predict improvement after both CI therapy (emphasizes fine motor movements) and a specialized robot-assisted therapy (emphasizes gross motor movements). A possible explanation is that the most robust predictors of outcome are those that are most consistently measured over time (least susceptible to variability in performance). However, according to Fritz et al. (2009), the most robust WMFT predictors in the combined approach had reliability (intraclass correlation) coefficients that were rank-ordered amongst the middle of the 15 items, lending little support for this potential explanation.
One partial explanation appears to be a ceiling effect, whereby poorer performers had a larger range of possible improvement. In support of this explanation, those with less gross motor ability at baseline were able to achieve the largest motor gains, which appear to be accounted for by dramatic gains in gross motor ability (Figure 5). It appears that many individuals had not yet achieved their maximum potential even several years post-stroke, whereas those with better baseline ability may have already approached their maximal possible recovery. However, although ceiling effects explain why poorer baseline gross motor ability predicts better gross motor gains (i.e. the direction of prediction), they do not account for why baseline gross motor ability was a more robust predictor of motor restoration than baseline fine motor ability. In fact, there was more potential for motor restoration of fine-motor ability than gross motor ability; participant performance on fine motor tasks was worse overall and, moreover, fine-motor tasks on the WMFT are least susceptible to ceiling effects (Woodbury et al. 2010).
The most compelling reason for the selection of gross motor tasks being the most robust predictors of motor restoration, in the authors' opinion, is that loss of fine-motor control is inherently more difficult to rehabilitate than loss of gross motor function. This idea is supported by the fact that amongst individuals who had considerable potential for improvement at baseline, the extent of improvement on fine motor tasks was smaller than it was for the gross motor tasks. Using the formulation for computing improvement percentage proposed by Lin et al. (2009), amongst all participants, the average improvement on gross motor tasks was 28.91%, whereas average improvement on fine motor tasks was only 9.12%. It was important, however, to consider that some of our population started with baseline values near normal values (Wolf et al. 2006) for some tasks, particularly for gross motor items. After excluding participants who performed within one standard deviation of normal ability at baseline, mean improvements on individual gross motor tasks were between about 40% and 60%, whereas mean improvements on individual fine motor tasks were typically between about 8% and 20%. This trend is consistent with other reports in the literature (Lee et al. 2011, 2015; Myrhaug and Ostensjo 2014).
One physiological explanation for gross motor ability being easier to rehabilitate is that gross motor function can be mitigated by multiple neural pathways originating in different regions of the brain (Lawrence and Kuypers 1968, Baker et al. 2015), whereas fine motor function is thought to be more locally controlled in the motor cortex and descending corticospinal tract (Robert and Lemon 1993, Hoffman et al. 1995, Kobayashi et al. 2003, Lang 2004,). The reticulospinal tract is the most widely studied alternative pathway for gross motor control of the upper limbs (Schepens and Drew 2004; Drew and Rossingol 1984; Davidson and Buford 2004, 2006; Hirschauer and Buford, 2015). In the cases of neurological insult to the corticospinal motor tract, this alternative pathway may facilitate recovery of gross motor function (Herbert et al.,2015a; Ortiz-Rosario et al., 2014; Hulbert et al. 2015). However, the reticulospinal tract appears inefficient for producing fine motor movements, at best demonstrating involvement only in whole-hand grasping (Baker et al. 2015). Another alternative pathway for control of gross motor movements is the uncrossed rubrospinal tract, which may serve as a potential mechanism for the less affected hemisphere to contribute to recovery of the more affected upper extremity; however, its projections cannot be traced to spinal segments below C3 in humans, suggesting that the rubrospinal tract is more likely to influence motor neurons involved in proximal movement (Nathan and Smith 1982). In sum, there appears to be considerably more neurological substrate (corticospinal pathways, reticulospinal pathways, and rubrospinal pathways) that can be harnessed for rehabilitation of gross motor function, whereas recovery of fine motor ability is primarily mitigated by corticospinal pathways alone.
Study Limitations
The main limitation is the small sample size of this study. Another limitation when comparing these findings with the authors' earlier research (George et al. 2017) is that both datasets involved a prospective cohort design and, as such, there is risk of selection bias when directly comparing findings from the two studies. Given these limitations, these findings will need to be replicated on a larger sample, such as that being currently collected in a multisite randomized controlled trial of the gaming therapy system versus CI therapy (Gauthier et al., in press). Despite these limitations, there is some consistency between the two datasets, namely that gross motor function is most strongly influenced by both treatments and that tactile information does not appear to be a robust predictor of neurorestoration following either game-based or CI therapy.
Conclusion
Results of this study suggest that those with near-normal gross motor function at baseline are least likely to benefit from motor restorative training. Though small improvements in fine-motor functioning can be realized through the motor restorative interventions studied here, the potential for dramatic improvement of fine motor abilities appears more limited than for gross motor abilities. These findings suggest that for individuals with near-normal proximal movement and residual distal impairment, interventions that focus primarily on overcoming non-use (such as the transfer package of CI therapy (Morris et al. 2006)), rather than reducing impairment, may be the most appropriate. For individuals with mild to moderate impairment on gross motor tasks (and at least some distal upper extremity movement), interventions emphasizing intense motor practice in conjunction with overcoming non-use would appear to be highly beneficial.
Acknowledgments
Financial support for data analysis was obtained through The Ohio State University Office of the Provost Chronic Brain Injury Discovery Theme initiative. Data collection was supported by American Heart Association 12SDG12200013 and Patient Centered Outcomes Research Institute. Additional support was obtained from Grant UL1TR001070 from the National Center For Advancing Translational Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding sources. The computations were performed on the supercomputers at the Ohio Supercomputer Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Sarah Hulbert George, Department of Biophysics, The Ohio State University, 1012 Wiseman Hall, 400 W. 12th Ave, Columbus, OH 43210 U.S.A..
Mohammad Hossein Rafiei, Department of Civil, Environmental and Geodetic Engineering, The Ohio State University, 470 Hitchcock Hall, 2070 Neil Ave., Columbus, OH 43220 U.S.A..
Alexandra Borstad, Department of Physical Therapy, The College of St. Scholastica, 1200 Kenwood Avenue, Duluth, MN 55811 U.S.A.
Hojjat Adeli, Departments of Civil, Environmental and Geodetic Engineering, Biomedical Informatics, Biomedical Engineering, Neurology, and Neuroscience, The Ohio State University, 470 Hitchcock Hall, 2070 Neil Ave., Columbus, OH 43220 U.S.A..
Lynne V. Gauthier, Physical Medicine and Rehabilitation, The Ohio State University, 480 Medical Center Drive, Columbus OH 43210, USA.
References
- Ahmadlou M, Adeli H. Enhanced probabilistic neural network with local decision circles: A robust classifier. Integrated Computer-Aided Engineering. 2010;17(3):197–210. [Google Scholar]
- Baker SN, Zaaimi B, Fisher KM, Edgley SA, Soteropoulos DS. Pathways mediating functional recovery. Progress in Brain Research. 2015;218:389–412. doi: 10.1016/bs.pbr.2014.12.010. [DOI] [PubMed] [Google Scholar]
- Borstad A, Nichols-Larsen DS. The brief kinesthesia test is feasible and sensitive: A study in stroke. Brazilian Journal of Physical Therapy. 2016;20(1):81–86. doi: 10.1590/bjpt-rbf.2014.0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan AD, Hunter JM, Mackin E. Rehabilitation of the hand: Surgery and therapy Mosby 1995 [Google Scholar]
- Davidson AG, Buford JA. Bilateral actions of the reticulospinal tract on arm and shoulder muscles in the monkey: Stimulus triggered averaging. Experimental Brain Research. 2006;173(1):25–39. doi: 10.1007/s00221-006-0374-1. [DOI] [PubMed] [Google Scholar]
- Davidson AG, Buford JA. Motor outputs from the primate reticular formation to shoulder muscles as revealed by stimulus-triggered averaging. Journal of Neurophysiology. 2004;92(1):83–95. doi: 10.1152/jn.00083.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew T, Rossignol S. Phase-dependent responses evoked in limb muscles by stimulation of medullary reticular formation during locomotion in thalamic cats. Journal of Neurophysiology. 1984;52(4):653–675. doi: 10.1152/jn.1984.52.4.653. [DOI] [PubMed] [Google Scholar]
- Fritz SL, Blanton S, Uswatte G, Taub E, Wolf SL. Minimal detectable change scores for the wolf motor function test. Neurorehabilitation and Neural Repair. 2009;23(7):662–667. doi: 10.1177/1545968309335975. [DOI] [PubMed] [Google Scholar]
- Gauthier LV, Kane C, Borstad A, Strahl N, Uswatte G, Taub E, Morris D, Hall A, Arakelian M, Mark V. Video game rehabilitation for outpatient stroke (VIGoROUS): Protocol for a multi-center comparative effectiveness trial of in-home gamified constraint-induced movement therapy for rehabilitation of chronic upper extremity hemiparesis. BMC Neurology. 2017;17(1):109. doi: 10.1186/s12883-017-0888-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George SH, Rafiei MH, Gauthier L, Borstad A, Buford JA, Adeli H. Computer-aided prediction of extent of motor recovery following constraint-induced movement therapy in chronic stroke. Behavioural Brain Research. 2017;329:191–199. doi: 10.1016/j.bbr.2017.03.012. [DOI] [PubMed] [Google Scholar]
- Herbert WJ, Powell K, Buford JA. Evidence for a role of the reticulospinal system in recovery of skilled reaching after cortical stroke: Initial results from a model of ischemic cortical injury. Experimental Brain Research. 2015;233(11):3231–3251. doi: 10.1007/s00221-015-4390-x. [DOI] [PubMed] [Google Scholar]
- Hirschauer TJ, Buford JA. Bilateral force transients in the upper limbs evoked by single-pulse microstimulation in the pontomedullary reticular formation. Journal of Neurophysiology. 2015;113(7):2592–2604. doi: 10.1152/jn.00852.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman DS, Strick PL. Effects of a primary motor cortex lesion on step-tracking movements of the wrist. Journal of Neurophysiology. 1995;73(2):891–895. doi: 10.1152/jn.1995.73.2.891. [DOI] [PubMed] [Google Scholar]
- Hulbert S, Adeli H, Buford J. 2015 Neuroscience Meeting Planner. Washington, DC: Society for Neuroscience; 2015. Interactions between corticospinal and reticulospinal outputs determine muscle response in the upper limbs and trunk as revealed with stimulus-triggered averaging. Program No. 606.09. Online. [Google Scholar]
- Kobayashi M, Hutchinson S, Schlaug G, Pascual-Leone A. Ipsilateral motor cortex activation on functional magnetic resonance imaging during unilateral hand movements is related to interhemispheric interactions. Neuroimage. 2003;20(4):2259–2270. doi: 10.1016/s1053-8119(03)00220-9. [DOI] [PubMed] [Google Scholar]
- Lang CE, Schieber MH. Reduced muscle selectivity during individuated finger movements in humans after damage to the motor cortex or corticospinal tract. Journal of Neurophysiology. 2004;91(4):1722–1733. doi: 10.1152/jn.00805.2003. [DOI] [PubMed] [Google Scholar]
- Lawrence DG, Kuypers HG. The functional organization of the motor system in the monkey 1: I. the effects of bilateral pyramidal lesions. Brain. 1968;91(1):1–14. doi: 10.1093/brain/91.1.1. [DOI] [PubMed] [Google Scholar]
- Lee Y, Hsieh Y, Wu C, Lin K, Chen C. Proximal fugl-meyer assessment scores predict clinically important upper limb improvement after 3 stroke rehabilitative interventions. Archives of Physical Medicine and Rehabilitation. 2015;96(12):2137–2144. doi: 10.1016/j.apmr.2015.07.019. [DOI] [PubMed] [Google Scholar]
- Lin K, Huang Y, Hsieh Y, Wu C. Potential predictors of motor and functional outcomes after distributed constraint-induced therapy for patients with stroke. Neurorehabilitation and Neural Repair. 2009;23(4):336–342. doi: 10.1177/1545968308321773. [DOI] [PubMed] [Google Scholar]
- Maung D, Crawfis R, Gauthier LV, Worthen-Chaudhari L, Lowes LP, Borstad A, McPherson RJ. Games for therapy: Defining a grammar and implementation for the recognition of therapeutic gestures. Fdg. 2013:314–321. [Google Scholar]
- Morris D, Taub E, Mark V. Constraint-induced movement therapy: Characterizing the intervention protocol. Europa Medicophysica. 2006;42(3):257. [PubMed] [Google Scholar]
- Myrhaug HT, Ostensjo S. Motor training and physical activity among preschoolers with cerebral palsy: A survey of parents' experiences. Physical & Occupational Therapy in Pediatrics. 2014;34(2):153–167. doi: 10.3109/01942638.2013.810185. [DOI] [PubMed] [Google Scholar]
- Nathan PW, Smith MC. The rubrospinal and central tegmental tracts in man. Brain : A Journal of Neurology. 1982;105(Pt 2):223–269. doi: 10.1093/brain/105.2.223. [DOI] [PubMed] [Google Scholar]
- Ortiz-Rosario A, Berrios-Torres I, Adeli H, Buford JA. Combined corticospinal and reticulospinal effects on upper limb muscles. Neuroscience Letters. 2014;561:30–34. doi: 10.1016/j.neulet.2013.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepens B, Drew T. Independent and convergent signals from the pontomedullary reticular formation contribute to the control of posture and movement during reaching in the cat. Journal of Neurophysiology. 2004;92(4):2217–2238. doi: 10.1152/jn.01189.2003. [DOI] [PubMed] [Google Scholar]
- Siddique N, Adeli H. Computational intelligence: Synergies of fuzzy logic, neural networks and evolutionary computing. John Wiley & Sons; 2013. [Google Scholar]
- Taub E, Uswatte G, Mark V, Morris D. The learned nonuse phenomenon: Implications for rehabilitation. Eura Medicophys. 2006;42:241–255. [PubMed] [Google Scholar]
- Taub E, Miller NE, Novack TA, Cook EW, 3rd, Fleming WC, Nepomuceno CS, Connell JS, Crago JE. Technique to improve chronic motor deficit after stroke. Archives of Physical Medicine and Rehabilitation. 1993;74(4):347–354. [PubMed] [Google Scholar]
- Taub E, Uswatte G, King DK, Morris D, Crago JE, Chatterjee A. A placebo-controlled trial of constraint-induced movement therapy for upper extremity after stroke. Stroke. 2006;37(4):1045–1049. doi: 10.1161/01.STR.0000206463.66461.97. [DOI] [PubMed] [Google Scholar]
- Taub E, Uswatte G, Mark VW, Morris DM, Barman J, Bowman MH, Bryson C, Delgado A, Bishop-McKay S. Method for enhancing real-world use of a more affected arm in chronic stroke: Transfer package of constraint-induced movement therapy. Stroke. 2013;44(5):1383–1388. doi: 10.1161/STROKEAHA.111.000559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf SL, Winstein CJ, Miller JP, Taub E, Uswatte G, Morris D, Giuliani C, Light KE, Nichols-Larsen D, Excite Investigators Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: The EXCITE randomized clinical trial. Jama. 2006;296(17):2095–2104. doi: 10.1001/jama.296.17.2095. [DOI] [PubMed] [Google Scholar]
- Woodbury M, Velozo CA, Thompson PA, Light K, Uswatte G, Taub E, Winstein CJ, Morris D, Blanton S, Nichols-Larsen DS. Measurement structure of the wolf motor function test: Implications for motor control theory. Neurorehabilitation and Neural Repair. 2010;24(9):791–801. doi: 10.1177/1545968310370749. [DOI] [PMC free article] [PubMed] [Google Scholar]