Abstract
Objective
To estimate expenditures for fall‐related injuries (FRIs) among older Medicare beneficiaries.
Data Sources
The 2007–2009 Medicare claims and 2008 Health and Retirement Study (HRS) data for 5,497 (228 FRI and 5,269 non‐FRI) beneficiaries.
Study Design
FRIs were indicated by inpatient/outpatient ICD‐9 diagnostic codes for fractures, trauma, dislocations, and by e‐codes. A pre‐post comparison group design was used to estimate the differential change in pre‐post expenditures for the FRI relative to the non‐FRI cohort (FRI expenditures). Out‐of‐pocket (OOP) costs, service category total annual FRI‐related Medicare expenditures, expenditures related to the type of initial FRI treatment (inpatient, ED, outpatient), and the risk of persistently high expenditures (4th quartile for each post‐FRI quarter) were estimated.
Principal Findings
Estimated FRI expenditures were $9,389 (95 percent CI: $5,969–$12,808). Inpatient, physician/outpatient, skilled nursing facility, and home health comprised 31, 18, 39, and 12 percent of the total. OOP costs were $1,363.0 (95 percent CI: $889‐$1,837). Expenditures for FRIs initially treated in inpatient/ED/outpatient settings were $21,424/$6,142/$8,622. The FRI cohort had a 64 percent increased risk of persistently high expenditures. Total Medicare expenditures were $13 billion (95 percent CI: $9–$18 billion).
Conclusions
FRIs are associated with substantial, persistent Medicare expenditures. Cost‐effectiveness of multifactorial falls prevention programs should be assessed using these expenditure estimates.
Keywords: Medicare, falls, fall‐related injuries, elderly, direct medical expenditures
Fall‐related injuries (FRIs) are common among older adults. Among fallers, 20–30 percent experience moderate or serious FRIs (Rubenstein and Josephson 2002) (such injuries can include fractures or serious lacerations) (Rubenstein and Josephson 2002), which are the leading cause of injury‐related ED visits and hospitalization among seniors (Owens et al. 2009). The effects of falls can also be long term, with declines in functioning and well‐being following a fall (Richmond et al. 2002; Inaba et al. 2003; Boonen et al. 2004) that often result in loss of independence and increased health care utilization. Falls play a major role in nursing home admissions (Blank et al. 2011), with nearly two‐thirds of seniors hospitalized for FRI later admitted to a long‐term care facility (Owens et al. 2009). An accurate estimate of the cost of FRIs can assist policy makers in determining how to allocate resources for prevention efforts among older adults.
A 2010 systematic review of 32 studies provided evidence of considerable variation in average estimated costs: average cost per fall ($1,059–$10,913), per‐faller ($2,044–$25,955), and per fall‐related hospitalization ($5,654–$42,840) (Heinrich et al. 2010). One‐third of the studies were conducted prior to 2000 and thus may not be relevant to today's U.S. health care system, with its increasingly complex payment structures. Also, 14 of the studies were conducted outside the United States. Several of the studies included intentional falls (Corso et al. 2006; Stevens et al. 2006), which are different in origin than unintentional falls (e.g., they do not result from incidents such as a stroke or being pushed by someone else) (Tinetti, Speechley, and Ginter 1988; Currie 2008) and thus potentially not generalizable to the Medicare population. Furthermore, only five studies—three of which were United States–based studies—focused on the community‐dwelling population. Using different approaches, the U.S. studies’ estimates (in 2008 dollars) were $3,163, $7,131, and $30,999 (Rizzo et al. 1998; Carroll, Slattum, and Cox 2005; Shumway‐Cook et al. 2009).
Wide variations in prior estimates may be due to variability in study populations, data used, approaches used to identify FRIs, and study design. Study settings were often within a city (Rizzo et al. 1998) or state (Alexander, Rivara, and Wolf 1992; Mahoney et al. 2005; Takanishi, Yu, and Morita 2008; Bohl et al. 2010, 2012), or abroad (Hendrie et al. 2004; Gannon, O'Shea, and Hudson 2008; Hartholt et al. 2011). In United–States based studies, researchers used hospital discharge data (Alexander, Rivara, and Wolf 1992; Stevens et al. 2006), private health plan patient discharge data (Roudsari et al. 2005; Bohl et al. 2010, 2012), and survey data (Rizzo et al. 1998; Stevens et al. 2006). Only a few included national, Medicare data (Finkelstein et al. 2005; Finkelstein, Prabhu, and Chen 2007). Thus, several of the studies have limited generalizability to the overall, U.S. older adult population. In terms of identifying FRIs, some domestic and international studies used self‐reported falls (Rizzo et al. 1998; Carroll, Slattum, and Cox 2005; Shumway‐Cook et al. 2009), while others used various claims‐based approaches (Alexander, Rivara, and Wolf 1992; Scuffham, Chaplin, and Legood 2003; Hendrie et al. 2004; Finkelstein et al. 2005; Mahoney et al. 2005; Roudsari et al. 2005; Stevens et al. 2006; Finkelstein, Prabhu, and Chen 2007; Gannon, O'Shea, and Hudson 2008; Bohl et al. 2010, 2012). Such approaches likely vary in terms of sensitivity and specificity in identifying FRIs, potentially affecting cost estimates. Many studies were limited by the lack of control variables in administrative claims data (Finkelstein, Prabhu, and Chen 2007; Bohl et al. 2010, 2012) or did not control for any sociodemographic or health variables that could affect FRI cost estimates (Roudsari et al. 2005).
Prior studies’ estimates of total, annual fall‐related spending range from $10 to $29 billion in 2008 dollars (Carroll, Slattum, and Cox 2005; Stevens et al. 2006). The study with the lowest estimate used “prevalence‐based costing” (Carroll, Slattum, and Cox 2005), which ascribes all annual fall‐related medical costs to individuals falling in a given year—an approach that may confound costs occurring before and after an FRI; the study with the highest estimate used a strong study design, but used e‐codes only to identify FRIs and did not use a comparison group, meaning some of the cost increases attributed to an FRI may have resulted from non‐FRI aging‐related health declines (potentially leading to overestimates) (Stevens et al. 2006). A recent analysis that estimated lifetime costs associated with FRIs also relied upon a method that uses only e‐codes to identify FRIs (Verma et al. 2016).
This study builds upon and extends earlier work by using Medicare claims with linked survey data (allowing for inclusion of a robust set of model predictors) and an adaptation of a new FRI identification algorithm that may have benefits in terms of sensitivity and specificity compared to prior methods (Ganz et al. 2015; Kim et al. 2016) to provide estimates of per‐faller annual FRI expenditures (including patient out‐of‐pocket—OOP—and service component expenditures, which have not previously been provided in the falls literature) and total annual FRI Medicare spending. Unlike earlier studies, we are able to control for factors that might affect expenditure estimates, including area differences in the local price of labor and a broad set of beneficiary sociodemographic and health characteristics. The study also assesses the risk of persistently high medical expenditures among fallers in the four quarters following the FRI. A number of sensitivity analyses are included in order to compare the estimates produced using this study's approach with varying approaches used in prior FRI cost studies.
Methods
Data and Study Population
This study used 2007–2009 Medicare claims linked to 2008 Health and Retirement Study (HRS) data for 10,240 older (≥65 years) community‐dwelling beneficiaries living during the entire study period. The HRS is a national, longitudinal study of the economic, health, and family status of older Americans (HRS, 2010). Linked Medicare data are available for respondents who were eligible for Medicare, provided their Medicare beneficiary numbers to HRS, and who were enrolled in Medicare Parts A or B. The dataset includes the Beneficiary Summary, Carrier, Denominator, Inpatient, Outpatient, Durable Medical Equipment (DME), Home Health (HH), Skilled Nursing Facility (SNF), Hospice, and MedPAR Standard Analytic Files. Respondents were excluded if they died in the year following the identified index date (defined below; n = 276), were enrolled in Medicare Part C (n = 3,326), or did not have continuous Parts A/B coverage (n = 506). The final analytic sample included 5,503 individuals: 167 in the FRI (3 percent) and 5,336 in the non‐FRI cohort (97 percent). Compared to the non‐FRI cohort individuals, a greater proportion of individuals in the FRI cohort were female; they were also older and had a greater number of functional limitations and chronic conditions (such as stroke and heart disease, as discussed below) on average. However, the two cohorts were similar in terms of race/ethnicity, educational level, income and wealth, and other indicators of health and health insurance status. County provider rates from the Area Health Resource File and wage index data from the FY2008 Medicare Impact File were linked to HRS‐Medicare data using FIPS codes and provider identification numbers, respectively.
Identifying FRIs
The study adapted a UCLA/RAND algorithm (Ganz et al. 2015; Hoffman et al. 2016; Kim et al. 2016) to identify five types of serious FRIs—hip fractures, other nonvertebral fractures, head trauma, joint dislocations plus fall injuries indicated by e‐codes 880/881/882/884/885/888. The study identified fractures, trauma, and dislocation injuries using inpatient (hospital and SNF) ICD‐9 primary diagnosis codes plus outpatient ICD‐9 diagnostic and Current Procedural Terminology imaging and repair procedure codes. Individuals in the FRI cohort were classified as having been (1) admitted for inpatient (hospital or SNF) treatment if the index FRI involved initial inpatient treatment or an admission within 10 days of discharge from the emergency department (ED), (2) treated in the ED only (without admission), or (3) treated in an outpatient setting for the index FRI.
This methodology has potential benefits compared to existing FRI identification methods that use (1) only e‐codes (which may be neither sensitive nor specific in identifying FRIs) and (2) another method referred that has been used in the FRI cost literature that attributes (in addition to these fracture, trauma, and dislocation injury types) diagnostically indicated sprains, strains, and contusions to falls (Roudsari et al. 2005; Bohl et al. 2010, 2012) (which may be sensitive but nonspecific, as not all such injuries are necessarily due to falls). Using the current study's data and those two alternative FRI identification methods would have resulted in very different FRI cohorts, representing 1 percent and 14 percent—versus this study's 3 percent—of the sample, respectively.
Study Design
To isolate FRI expenditures, a pre‐post analysis with comparison group design was used involving two cohorts: (1) an FRI cohort including those with a first FRI in 2008 but no FRIs in the rolling prior year (“washout period”) and (2) a non‐FRI cohort including those with no FRIs in 2007, 2008, or the first half of 2009. Each eligible individual contributed a single observation to the analysis. FRI cohort individuals received an index date—the date of their first qualifying FRI in 2008. Non‐FRI cohort individuals received an index date of July 1, 2008. Medical expenditures for both cohorts were measured during the year prior to (“preindex”) and following the index date (“postindex”). Expenditures were defined as total, direct, medical expenditures from the perspective of the Medicare program and Medicare beneficiary, excluding Medicaid, private supplemental insurance policies, and other third‐party payers. Medical expenditures include the amount of payment paid by Medicare to the provider for all treatments, services, and equipment utilized by beneficiaries. Provider costs billed to (1) Medicare, per the contracted rate and (2) the beneficiary, in the form of OOP costs are also included while reimbursement by third‐party payers is excluded as it is not included in the Medicare claims data.
To isolate FRI‐related expenditures, OLS regression models were used to assess the difference between the FRI and non‐FRI cohorts in expenditure “change scores,” or the difference between preindex medical spending and postindex spending. The resulting difference between the two cohorts’ change scores was then regressed on model covariates where the predictor of interest is an indicator for whether the observation is from the FRI versus non‐FRI cohort. The estimated marginal effect of this indicator, or the beta coefficient, then reflects the differential change in expenditures experienced over time between individuals who did and did not have an FRI. The OLS specification was chosen given that change scores were normally distributed and diagnostic tests did not suggest the need for transformation of the outcome variable. Prior studies have often examined postindex costs (as opposed to such cost changes over time), which are more likely to be nonnormally distributed and thus conversely require use of alternative specifications such as generalized linear model (GLM) or generalized estimating equations (GEEs) (Finkelstein et al. 2005; Stevens et al. 2006; Bohl et al. 2010).
Using this methodology involving comparative change scores is a “case‐crossover” (Finkelstein et al. 2005) design where study respondents act as their own controls to account for unmeasurable health differences that are constant between the pre‐ and postperiods. However, unlike prior case‐crossover designs used in FRI cost estimates (Finkelstein et al. 2005; Stevens et al. 2006), the current study employs a comparison group and a robust set of predictor variables. Because this methodology controls for measurable and unmeasurable confounders, the difference in change scores between the FRI and non‐FRI cohort can be interpreted as annual FRI‐related expenditures (hereafter, we refer to these estimates as FRI‐related expenditures as opposed to change score differences). The study separately estimates patient OOP (including deductible and coinsurance) costs and expenditures by service category (hospital, outpatient/carrier, SNF, HH, DME, hospice). Separate models were estimated for OOP cost changes and for expenditure changes for each service category. The study also estimates expenditures according to whether treatment for the index FRI initially occurred in an inpatient (hospital/SNF), ED (without transfer to inpatient), or outpatient setting. Additionally, total annual Medicare expenditures were estimated for the 12‐month period following an FRI index date. Similar to Garber, MaCurdy, and McClellan (1998), persistently high expenditures were measured. In this study, they were defined as expenditures in the 4th quartile (and, in a sensitivity analysis, ≥95th percentile) in each of the four quarters following the index date.
Risk Adjustment Variables
The study controlled for individual and contextual factors that are associated with falls in the falls literature and health services’ price and/or quantity and thus might confound the falls–expenditure relationship (Deandrea et al. 2010). These include total household income and wealth, age, gender, race/ethnicity (Hispanic, non‐Hispanic white, Black, Asian/Pacific Islander, Other), and educational level (<high school, high school, some college, >college), indices for chronic health conditions (Fauth et al. 2007) (0–5, with one point for each of osteoarthritis, stroke, heart disease, high blood pressure, and diabetes) and functional limitations (0–12, with one point for each limitation, e.g., difficulties with activities like walking several blocks and walking across a room), and self‐rated eyesight (1–6: 1 = legally blind; 2 = poor; 3 = fair; 4 = good; 5 = very good; 6 = excellent) and hearing (1–5: 1 = poor; 2 = fair; 3 = good; 4 = very good; 5 = excellent). When a model was estimated using dummy variables for each of the five chronic conditions and 12 functional limitations rather than using indices for those measures of health status, the expenditure estimates obtained were ~1 percent lower. Low cognitive status was a score of ≤6 on HRS's Telephone Interview of Cognitive Status (0–15; 0 = lowest, 15 = highest status) (Dal Forno et al. 2006) and disability was whether a respondent reported ever applying for Supplemental Security Income or Social Security Disability Insurance. We measured whether a respondent used psychiatric medications and (to account for differences in OOP costs) had supplemental Medicaid coverage. These predictors were taken from the 2008 wave of the HRS. To measure area‐level availability and price of medical care, we used a county's physicians/100,000 older adults ratio and Medicare wage index, respectively.
To compare unadjusted characteristics of the two cohorts, ANOVA, Kruskal–Wallis, and chi‐squared tests were used to assess interval, ordinal, and nominal variables, respectively. Average, unadjusted expenditures by injury type (e.g., hip and other nonvertebral fractures, head trauma, joint dislocations) for those injuries that were coded as index FRIs are presented.
To assess the risk of persistently high expenditures, a logistic regression model was estimated controlling for the same predictor variables as used above as well as an individual's preindex expenditures. Treatment expenditures were allocated to the quarter during which the beneficiary received treatment. A quarter was defined as the date the quarter began plus 91 days. For care episodes greater than one quarter, expenditures were allocated proportionally to the amount of time of the episode falling within each quarter. Estimates from the logistic regression model were used to calculate adjusted risks for each cohort and then to a marginal risk difference (i.e., the difference in the probability, compared to the non‐FRI cohort, that the FRI cohort individuals would have persistently high expenditures).
The robustness of the expenditure estimates was examined by using (1) preindex expenditures as a predictor of change scores (i.e., annual expenditures), given that change scores may vary depending on the beginning expenditure level; (2) individuals who died during the postindex year by inflating their expenditures to the 12‐month equivalent but downweighting those observations by the proportion of the postindex year lived; (3) a 6‐ rather than 12‐month washout period; (4) a case–control approach (a GLM model with a gamma distribution and log link with postindex expenditures rather than the change in expenditures between the pre‐ and postindex periods as the outcome); (5) a propensity‐score matching technique examining the average treatment of effect after accounting for the probability of individuals being in each of the two study cohorts; and (6) inpatient‐related FRI expenditures using (a) a case‐crossover approach without controlling for predictor variables (similar to Finkelstein et al. 2005) and (b) a case–control model controlling only for age and gender (similar to Bohl et al. 2012).
Results
Unadjusted Results
Individuals in the FRI compared to the non‐FRI cohort were slightly older (78 vs. 76). Fewer individuals in the FRI compared to the non‐FRI cohort were male (32 percent vs. 42 percent), but race‐ethnicity, educational levels, income, and wealth were similar across cohorts (Table 1). Health characteristics measured in 2008 were generally similar across cohorts, though the number of functional limitations was higher (6 vs. 4) among those in the FRI cohort. Of those in the FRI cohort, medical treatment initially involved inpatient care for 25 (11 percent), ED without subsequent admission for 58 (25 percent), and outpatient care for 145 (64 percent) beneficiaries.
Table 1.
Unadjusted Descriptive Statistics for Analytic Sample of Older Medicare Community‐Dwelling Fee‐for‐Service Beneficiaries, 2007–2009
| Overall Sample, %/Mean (SD) (n = 5,497) | FRI Cohort, %/Mean (SD) (n = 228) | Non‐FRI Cohort, %/Mean (SD) (n = 5,269) | |
|---|---|---|---|
| Expenditures ($) | |||
| Preindexa | 7,801 (14,682) | 11,575 (18960) | 7,638 (14,448) |
| Postindexa | 10,091 (19,826) | 23,151 (25,977) | 9,515 (19,321) |
| Changea | 2,271 (19,770) | 13,857 (25,224) | 1,908 (19,467) |
| Persistently high expenditures (%)a | 11 | 25 | 11 |
| Agea | 76 (7) | 78 (7) | 76 (7) |
| Male (%)a | 42 | 32 | 42 |
| Race/ethnicity (%) | |||
| White | 82 | 86 | 82 |
| African American | 11 | 7 | 11 |
| Hispanic | 4 | 3 | 4 |
| Other | 3 | 4 | 3 |
| Education (%) | |||
| <High school | 22 | 23 | 22 |
| High school | 37 | 36 | 37 |
| Some college | 20 | 15 | 20 |
| College | 21 | 26 | 20 |
| Income ($1,000) | 55 (110) | 54 (73) | 55 (111) |
| Wealth ($1,000) | 563 (1,307) | 634 (1,200) | 559 (1,312) |
| Eyesight (1–6) | 3 (1) | 3 (1) | 3 (1) |
| Hearing (1–5) | 3 (1) | 3 (1) | 3 (1) |
| Cognitive impairment (%) | 2 | 3 | 2 |
| Number of functional limitations (0–12)a | 4 (3) | 6 (3) | 4 (3) |
| Number of chronic conditions (0–6)a | 2 (1) | 2 (1) | 2 (1) |
| Psychiatric medication (%) | 9 | 12 | 9 |
| Disability (%) | 12 | 13 | 12 |
| Medicaid (%) | 9 | 8 | 9 |
| Area wage index | 0.96 (0.15) | 0.96 (0.16) | 0.96 (0.15) |
| Physicians/10,000 older adults | 188 (141) | 181 (133) | 188 (141) |
Compared to non‐FRI, FRI cohort individuals had higher preindex expenditures ($11,575 vs. $7,638) with the expenditure differential increased in the year after the index date ($23,151 vs. 9,515). Thus, the unadjusted expenditure change was greater for the FRI ($13,857) than the non‐FRI cohort ($1,908) (see Figure S1); unadjusted postindex expenditures were greater for those with an FRI‐related inpatient admission ($34,761) compared to those receiving ED treatment only ($18,093) or outpatient treatment ($23,173) (Figure 1). A greater proportion of those in the FRI (25 percent) compared to the non‐FRI (11 percent) cohort incurred persistently high expenditures (Table 1).
Figure 1.
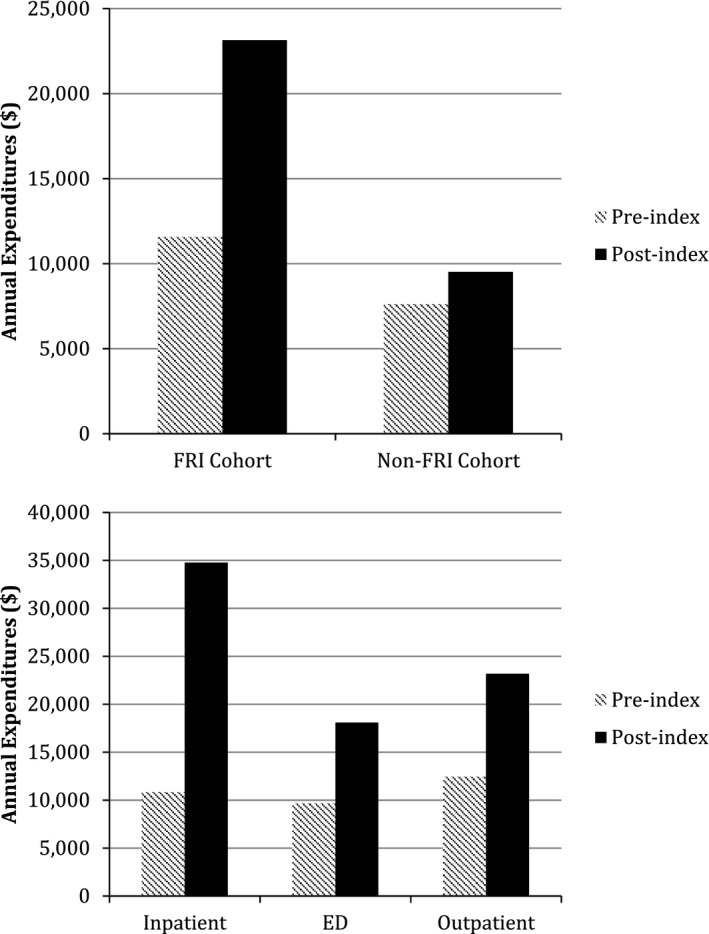
Comparison of Unadjusted Annual Medical Expenditures for Older Medicare Beneficiaries (A) with and without Fall‐Related Injuries and (B) by Type of Index Injury for Those with a Fall‐Related Injury, 2007–2009
- Notes. Unadjusted expenditures for the 5,497 individuals in the analytic sample of the main model (using the case‐crossover with comparison group study design). Injuries are those that were identified as index FRIs in the analysis.
Unadjusted expenditures for injury types among those in the FRI cohort are presented (Table 2). Fractures such as rib, femur, and patella ($18,124, $22,959, and $20,051, respectively) and head injuries such as face and skull fractures ($12,683 and $16,198) had average preindex expenditures >$10,000, while dislocations of the shoulder, elbow, and knee ($2,429, $743, and $6,878, respectively) had preindex expenditures less than $7,000. A number of injuries had average postindex expenditures >$20,000, including fractures of the hip ($46,751), pelvis ($35,430), humerus ($28,119), radius ($20,345), carpal ($21,809), and face ($40,076), among others. The average change in expenditures (between the pre‐ and postindex periods) ranged widely from −$6,837 (patella fracture) and −$2,303 (skull fracture) to $27,394 (face fracture) and $34,449 (hip fracture). Dislocations had average expenditure changes of $5,557 (shoulder) to $7,432 (elbow).
Table 2.
Unadjusted Preindex, Postindex, and Changes in Expenditures for Selected Fall‐Related Injuries among Older Adults, 2007–2009
| Injury | n | Unadjusted Expenditures: $ (SD) | ||
|---|---|---|---|---|
| Preindex | Postindex | Change | ||
| Hip | 15 | 12,917 (15,368) | 52,711 (24,785) | 39,794 (28,444) |
| Pelvis | 3 | 15,013 (12,534) | 38,486 (29,520) | 23,473 (20,817) |
| Rib | 20 | 18,339 (24,675) | 9,852 (9,599) | −8,487 (21,980) |
| Humerus | 18 | 9,541 (11,209) | 25,366 (32,274) | 15,825 (33,014) |
| Radius | 18 | 9,424 (22,517) | 21,474 (27,985) | 12,050 (23,365) |
| Carpal | 5 | 4,311 (3,666) | 21,809 (28,991) | 17,398 (28,843) |
| Metacarpal | 5 | 7,089 (8,356) | 5,340 (2,640) | −1,749 (6,658) |
| Phalanges | 8 | 11,762 (13,595) | 25,552 (48,290) | 13,790 (35,720) |
| Femur | 6 | 27,371 (60,353) | 43,199 (22,844) | 15,828 (51,809) |
| Patella | 6 | 7,957 (16,553) | 12,797 (18,570) | 4,839 (21,021) |
| Ankle | 16 | 9,426 (7,676) | 19,480 (22,676) | 10,053 (19,218) |
| Face | 13 | 16,475 (21,897) | 45,197 (27,421) | 28,722 (30,419) |
| Skull | 8 | 12,138 (16,485) | 15,298 (15,133) | 3,160 (22,939) |
| Head trauma | 17 | 5,513 (5,136) | 20,446 (17,889) | 14,933 (18,272) |
| Shoulder | 5 | 2,429 (2,680) | 7,996 (6,69) | 5,567 (5,620) |
| Elbow | 3 | 743 (425) | 8,175 (6,591) | 7,432 (6,612) |
| Knee | 26 | 6,780 (11,648) | 12,913 (10,464) | 6,133 (13,789) |
Unadjusted expenditures for the 5,497 individuals in the analytic sample of the main model (using the case‐crossover with comparison group study design). Injuries are those that were identified as index FRIs in the analysis.
Adjusted Results
FRI Expenditure Estimates
The estimated expenditure for an FRI (i.e., the adjusted difference in the pre/post change in expenditures between the FRI and non‐FRI cohorts) was $9,389 (95 percent CI: $5,969–$12,808) (Table 3). See Figure S2 for the distribution of adjusted change scores for the FRI and non‐FRI cohorts. Most model predictors were not associated with changes in expenditures between the pre‐ and post years. This was an expected result; these characteristics would likely be associated with postindex expenditures, but not expenditure differences over time. Estimated beneficiary OOP FRI costs were $1,363 (95 percent CI: $889–$1,837), or approximately 15 percent of total FRI‐related expenditures. Deductibles and coinsurance represented 18 percent and 82 percent of the estimated OOP costs, respectively.
Table 3.
Adjusted Expenditures for Fall‐Related Injuries among Older Medicare Community‐Dwelling Fee‐for‐Service Beneficiaries, 2007–2009 (n = 5,497)
| Marginal Change in Pre‐Postexpenditures Attributable to FRI | Risk of Persistently High Expenditures | |||||
|---|---|---|---|---|---|---|
| β | 95% CI | p | Marginal Difference | 95% CI | p | |
| FRI cohort | 9,389 | 5,659–12,808 | <.001 | 0.07 | 0.04–0.10 | <.001 |
| Age | 60 | −21 to 141 | .15 | 0.00 | 0.00–0.00 | .08 |
| Male | 338 | −805 to 1,482 | .58 | 0.00 | −0.02 to 0.02 | .94 |
| Race/ethnicity (reference: White) | ||||||
| African American | 2,067 | −221 to 4,356 | .08 | 0.03 | 0.00–0.06 | .03 |
| Hispanic | 893 | −2,739 to 4,525 | .63 | 0.09 | 0.04–0.14 | <.001 |
| Other | −1,411 | −4,018 to 1,197 | .29 | −0.02 | −0.06 to 0.01 | .23 |
| Education (reference: <high school) | ||||||
| High school | 465 | −1,157 to 2,088 | .57 | 0.00 | −0.02 to 0.02 | .95 |
| Some college | −746 | −2,546 to 1,054 | .42 | 0.01 | −0.02 to 0.03 | .54 |
| College | −59 | −1,854 to 1,736 | .95 | 0.03 | 0.00–0.05 | .06 |
| Income ($100,000) | −53 | −342 to 236 | .72 | 0.00 | −0.01 to 0.01 | .76 |
| Wealth ($100,000) | 14 | −42 to 70 | .62 | 0.00 | 0.00–0.00 | .17 |
| Eyesight | −516 | −1,094 to 62 | .08 | 0.00 | 0.00 –0.01 | .33 |
| Hearing | −123 | −639 to 393 | .64 | 0.00 | −0.01 to 0.01 | .92 |
| Cognitive impairment | 115 | −5,544 to 5,773 | .97 | 0.02 | −0.03 to 0.06 | .43 |
| Number of functional limitations | 189 | −46 to 423 | .11 | 0.01 | 0.01–0.01 | <.001 |
| Number of chronic conditions | 364 | −145 to 873 | .16 | 0.02 | 0.01–0.03 | <.001 |
| Psychiatric medication | 1,198 | −925 to 3,321 | .27 | 0.04 | 0.01–0.06 | .01 |
| Disability | −437 | −2,834 to 1,960 | .72 | 0.02 | −0.01 to 0.04 | .15 |
| Medicaid | 2,403 | 290–5,095 | .08 | 0.04 | 0.01–0.07 | .01 |
| Area wage index | 343 | −3,581 to 4,268 | .86 | 0.04 | −0.01 to 0.10 | .13 |
| Physicians/10,000 older adults | 1 | −2 to 5 | .49 | 0.00 | 0.00–0.00 | .93 |
FRIs identified using the adapted UCLA/RAND algorithm (Ganz et al. 2015; Kim et al. 2016) in which serious FRIs are identified using inpatient (hospital and SNF) ICD‐9 primary diagnoses and external cause of injury codes and outpatient diagnoses and procedural codes. Models were estimated using OLS (expenditure change scores) or logistic regression (persistently high expenditures, controlling for preindex expenditures) with robust standard errors.
Expenditure Components
The hospital expenditure estimate of $2,864 (p = .003) represented 31 percent of total FRI‐related expenditures ($9,389), while physician/outpatient ($1,735, p = .001), SNF ($3,667, p < .001), and HH ($1,130, p < .001) represented 18, 39, and 12 percent of the total expenditure increase, respectively (Table 4). In a separate model, expenditures for index FRIs initially involving inpatient admissions ($21,424, 95 percent CI: $11,567–$31,281), ED treatment only ($6,142, 95 percent CI: $1,314–$10,970), and outpatient treatment ($8,622, 95 percent CI: $3,991–$13,254) were estimated (Table 4).
Table 4.
Adjusted Expenditure Components of Fall‐Related Injuries among Older Medicare Community‐Dwelling Fee‐for‐Service Beneficiaries, 2007–2009 (n = 5,497)
| β | 95% CI | p | % Total | |
|---|---|---|---|---|
| Total | 9,389 | 5,969–12,808 | <.001 | 100 |
| Type of expenditure | ||||
| Hospital | 2,864 | 980–4,748 | .003 | 31 |
| Outpatient | 1,735 | 750–2,719 | .001 | 18 |
| Skilled nursing facility | 3,667 | 2,265–5,070 | <.001 | 39 |
| Home health | 1,130 | 612–1,648 | <.001 | 12 |
| Durable medical equipment | 53 | −85 to 191 | .45 | 1 |
| Hospice | −60 | −157 to 36 | .22 | 0 |
| Source of payment | ||||
| Patient out‐of‐pocket | 1,363 | 889–1,837 | <.001 | 100 |
| Deductible | 252 | 134–371 | <.001 | 18 |
| Coinsurance | 1,111 | 695–1,526 | <.001 | 82 |
| Index FRI type | ||||
| Inpatient | 21,424 | 11,567–31,281 | <.001 | – |
| ED only | 6,142 | 1,315–10,970 | .013 | – |
| Outpatient | 8,622 | 3,391–13,254 | <.001 | – |
FRIs identified using the adapted UCLA/RAND algorithm (Ganz et al. 2015; Kim et al. 2016) in which FRIs are identified using inpatient (hospital and SNF) ICD‐9 primary diagnoses and external cause of injury codes and outpatient diagnoses and procedural codes. Models were estimated using OLS regression with robust standard errors. The sample size for each of the separately estimated models was 5,497, the analytic sample from the model estimating total medical FRI‐related expenditures. The model does not include individuals who died during the follow‐up period.
Risk of Persistently High Expenditures during Postindex Year
Those in the FRI compared to the non‐FRI cohort had a 7 (95 percent CI: 4–10) percentage point greater risk of persistently high expenditures (Table 3), which compared with the predicted probability of such high expenditures among individuals from the non‐FRI cohort of 0.11 (this is the average of the adjusted risk across all individuals in the non‐FRI cohort after controlling for all model risk predictors), translates to an 64 percent (0.07/0.11) increased risk of high spending in each of the four quarters following the index date for those in the FRI compared to the non‐FRI cohort.
Total Medicare Expenditures
Using the study's per‐faller annual expenditures, it is possible to estimate FRI‐related Medicare FFS expenditures. In 2008, 34.3 million older Medicare beneficiaries had Medicare Parts A and B coverage (CMS, 2008). With 3 percent of this study's community‐dwelling older Medicare beneficiaries experiencing a serious FRI, at an average annual expenditure of $9,389 per FRI, the estimated Medicare FFS expenditure is $13 billion (95 percent CI: $9–$18 billion), with 15 percent (or $1–$3 billion) in beneficiary OOP expenditures. (A survey‐weighted expenditure estimate was higher, at $15 billion.) Spending on inpatient and SNF treatment accounted for $4 and $5 billion of the total, respectively.
Sensitivity Analyses
Expenditure estimates were slightly higher when including preindex expenditures or those who died in the analysis (~4 percent), while the marginal risk of persistently high expenditures was similar (a 1‐percentage point change). Use of a 6‐month washout period yielded similar estimates. The case–control and propensity‐score matching estimates were $12,459 (95 percent CI: $6,878–$18,039) and $7,337 (95 percent CI: $3,819–$10,856), respectively. Respective estimates from the case‐crossover without predictors and case–control controlling for age and gender only were $10,542 (95 percent CI: $7,447–$13,638) and $13,263 (95 percent CI: $7,320–$19,207).
Limitations
There were several limitations in this study. First, by using a 12‐month washout period, we excluded individuals with multiple FRIs in 2007/2008. This means that the FRI cohort may have had better‐than‐average health compared to a cohort defined using a shorter washout. However, results were similar when we used a shorter, 6‐month washout period. Second, as with prior studies (Carroll, Slattum, and Cox 2005; Finkelstein et al. 2005; Roudsari et al. 2005; Stevens et al. 2006; Shumway‐Cook et al. 2009; Bohl et al. 2010, 2012; Hoffman et al. 2016), the present study did not have the use of long‐stay nursing home or personal care services expenditures (available in Medicaid claims data) that often result from FRIs (Rizzo et al. 1998)—again likely underestimating expenditures; however, approximately only 9 percent of the sample reported having Medicaid supplemental coverage. Third, the adapted UCLA/RAND method (Ganz et al. 2015; Hoffman et al. 2016; Kim et al. 2016) refines commonly used FRI identification approaches. Although it is potentially an improvement over prior approaches because it uses a more sensitive and specific approach involving inpatient and outpatient diagnoses/procedures, it needs to be further evaluated. Additionally, this algorithm is intended to identify serious FRIs (which are relatively costly but rarer than less serious FRIs that can include contusions and sprains); thus, it may overstate per‐FRI expenditures but understate the total FRI expenditures. However, because it includes both inpatient and outpatient FRIs, this algorithm results in estimates that are relatively low compared to those produced in studies assessing FRIs treated in inpatient settings only. Fourth, the study did not include ~10 million Medicare Advantage (MA) beneficiaries (KFF, 2008) or those <65—potentially healthier groups than older non‐MA Medicare beneficiaries—potentially overestimating per‐faller but underestimating total annual FRI‐related Medicare expenditures. Finally, the study did not use survey weights in the analyses, which may affect the generalizability of the findings; when HRS's individual‐level weights were used in the main analysis, estimates slightly increased to $10,546 (from $9,389), which was well within the confidence interval of the main estimates; the survey‐weighted estimate for FRIs resulting in inpatient admission ($20,899) was <3 percent different from the main estimate ($21,424), while those for ED and outpatient‐treated FRIs were both slightly higher than the main estimates. If anything, the overall per‐FRI and total expenditure estimates are underestimated.
Discussion
This study suggests that FRIs result in substantial and persistent Medicare expenditures for older, community‐dwelling beneficiaries. Using an adaptation of a recently developed FRI identification algorithm, we found that FRIs resulted in a $9,389 increase in annual medical expenditures. Expenditures increased across the care spectrum and were particularly high for treatment and rehabilitation expenditures in hospital, SNF, outpatient, and HH settings. Others have observed similar component spending increases following a fall (Bohl et al. 2010, 2012). The large increase in SNF spending also comports with earlier findings regarding increases in institutional care use after a fall (Blank et al. 2011) or other injuries (Carter and Porell 2011).
Also, as found previously (Bohl et al. 2010, 2012), FRI expenditures did not spike and then immediately level off: FRIs appear to have persistent utilization implications across each of the fours quarter during the year following the initial injury. The study also provides the first estimates of patient OOP cost associated with FRIs (15 percent, or >$1,300), which are costs over and above annual premiums and cost‐sharing for other Medicare services. These payments are due to a combination of hospital and SNF deductible and primarily outpatient coinsurance payments. This finding suggests that falls prevention efforts reducing FRIs would have financial implications not only for payers such as Medicare but also for program beneficiaries; this may be particularly relevant given concerns regarding the impact of patient cost‐sharing on older Medicare beneficiaries (Rice and Matsuoka 2004).
Average, unadjusted postindex expenditures for all respondents ($10,901) were in line with annual per‐beneficiary Medicare Parts A/B spending in 2008–2010 ($9,441, $9,902, and $9,973) (Boards of Trustees, 2011). However, as noted, this study's inpatient‐related FRI expenditure estimate of $21,424 (which was estimated in 2008 dollars) is lower than the $29,185 (converted to 2008 dollars using the medical CPI) from Finkelstein et al.'s (2005) study using a “case‐crossover” design similar to this study's design (Finkelstein et al. 2005) and the $35,144 ($39,570 in 2008 dollars) costs‐attributable‐to‐fall estimate from a more recent study using 2004–2006 data (Bohl et al. 2010). There are several likely explanations for these discrepancies. Notably, the first of those studies identified FRIs with e‐codes—which likely identify serious/costly FRIs. The accuracy of e‐codes has been called into account even where e‐codes are reported, potentially due to an absence of quality assurance activities to monitor the completeness and validity of e‐codes (LeMier, Cummings, and West 2001; Langley et al. 2006; Annest et al. 2008; McKenzie et al. 2009; Hoffman et al. 2016). The second study did not account for prebaseline costs and had limited risk adjustment predictors. Also, the UCLA/RAND algorithm uses SNF treatment to identify FRIs so here the inpatient‐treated expenditure includes both hospital and SNF treatment (which is likely relatively less costly). Conversely, both comparison studies estimated only hospitalized FRI costs. Given interest from practitioners and policy makers in the cost and effectiveness of prevention efforts targeting all FRIs and not only those resulting in hospital treatment, this study offers an alternative approach to exploring FRI expenditures.
Compared to estimates from Finkelstein et al. (2005), while this study's expenditures by type of injury are lower for inpatient ($21,424 vs. $29,185), they are higher for ED ($6,142 vs. $4,506) and outpatient/office‐treated FRIs ($8,622 vs. $5,859). This could reflect changing modalities of treatment. Certain injuries once cared for in inpatient settings may now be assessed in outpatient settings, resulting in diminished inpatient but increased ED and outpatient expenditures on average. Or this could reflect increased costs of patient care. Finally, the divergent findings could reflect different approaches to controlling for confounding and for identifying FRIs in claims data. Further examination of differing cost estimates using various FRI identification techniques is warranted to ascertain how estimates are affected by choice of e‐codes versus diagnostic codes.
Like the prior study (Finkelstein et al. 2005), this study also finds that the case–control approach (using postindex expenditures as the outcome) results in higher estimates compared to the case‐crossover (using expenditure change as the outcome) approach. That study recommended using the case‐crossover (or propensity‐score matching) in future FRI cost estimate studies. This study additionally finds that inclusion of a robust set of predictor variables results in lower relative estimates; these variables may help control for exogenous factors associated with increased expenditures during the study period. The propensity‐score matching analysis further reduces the expenditure estimate. As robust predictors are required for each of these methods, it may be appropriate (if difficult in terms of obtaining data) to use linked claims‐survey data such as the linked Medicare‐HRS data or the Medicare Current Beneficiary Survey (which has linked survey and claims data) in future analyses.
This study's relatively low per‐faller estimates also translate to relatively modest total annual Medicare spending estimates. The estimate of total annual Medicare expenditures falls within a confidence interval of $9–$18 billion. These estimates are substantially lower than those obtained for adults ages 65 and older in a recent analysis that used e‐codes only to assess the medical and societal costs of FRIs (Verma et al. 2016). The estimate of $13 billion (derived using the point estimate of ~$9,000 in per‐faller annual expenditures) should be interpreted with caution given the small sample size of the study and small proportion of individuals in the study's FRI cohort (~3 percent). Had the study used a broader definition of FRIs (such as the method used by Bohl et al.), the proportion in the FRI cohort would have been 14 percent (though the per‐faller annual expenditure would have been $5,836), resulting in substantially higher total annual expenditure estimates (~$28 billion), or more than half the cost of treating diabetes in the Medicare‐eligible population (Huang et al. 2009). Moreover, had MA beneficiaries been included in the analysis (assuming a similar proportion of fallers in that population), estimated expenditures would be even higher because the estimates obtained above include just the 34.3 million Medicare beneficiaries with Parts A/B and not those with MA (another ~11 million); multiplying the average expenditure obtained here by the ~47 million Medicare population would result in higher total annual estimates. Additional explanations for differences between these and previous estimates of annual total Medicare fall‐related expenditures could involve this study's inclusion as models predictors of a broad set of sociodemographic and health characteristics not typically available in claims‐based studies and of area differences in labor prices.
With the aging of the U.S. population and growing morbidity among aging adults (Martin et al. 2009), Medicare expenditures on FRIs may increase. These substantial expenditures are concerning for Medicare. Yet, though existing fall prevention programs are effective (Gillespie et al. 2012), prior FP cost‐effectiveness studies (Tinetti et al. 2006; Hendriks et al. 2008; Frick et al. 2010; Wu et al. 2010) have had mixed findings due to lack of generalizable data (Dolan and Torgerson 1998; Rizzo et al. 1998; RAND, 2003; Fleurence 2004; Frick et al. 2010; Wu et al. 2010). An important next step is providing updated C/E estimates using newer FRI expenditures estimates, such as those reported here. Our study had a relatively small sample size that evidenced considerable variability in expenditures across beneficiaries and across types of FRIs. Future work might use larger Medicare datasets to verify our findings in order to provide estimates for use in C/E studies that can utilize costs for specific injury types and individuals with different levels of expenditures. Though FRIs are less common than noninjurious falls, due to high costs and associated morbidity, their prevention may be paramount (Quigley et al. 2012). Policy makers and researchers should continue to focus on ways to develop a population‐wide, cost‐effective approach to preventing such costly injuries in older adults.
Supporting information
Appendix SA1: Author Matrix.
Figure S1: Distribution of Unadjusted Expenditures for Fall‐Related Injury (FRI) and Non‐FRI Cohorts of Older Medicare Community‐Dwelling Fee‐for‐Service Beneficiaries, 2007–2009.
Figure S2: Distribution of Adjusted Expenditures for Fall‐Related Injury (FRI) and Non‐FRI Cohorts of Older Medicare Community‐Dwelling Fee‐for‐Service Beneficiaries, 2007–2009.
Acknowledgments
Joint Acknowledgment/Disclosure Statement: The authors received the following financial support for the research, authorship, and/or publication of this article: Dr. Hoffman was supported by the NIH/National Center for Advancing Translational Science UCLA CTSI (no. TL1TR000121). Dr. Hays was supported in part by grants from the National Cancer Institute (1 U2‐CCA186878‐01), the National Institute on Aging (P30‐AG021684), and the National Institute on Minority Health and Health Disparities (P20‐MD000182). A version of this manuscript was presented at the 2015 American Public Health Association annual meeting in Chicago, IL.
Disclosures: None.
Disclaimers: None.
References
- Alexander, B. H. , Rivara F. P., and Wolf M. E.. 1992. “The Cost and Frequency of Hospitalization for Fall‐Related Injuries in Older Adults.” The American Journal of Public Health 82 (7): 1020–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annest, J. L. , Fingerhut L. A., Gallagher S. S., Grossman D. C., Hedegaard H., Johnson R. L., Kohn M., Pickett D., Thomas K. E., and Trent R. B.. 2008. “Strategies to Improve External Cause‐of‐Injury Coding in State‐Based Hospital Discharge and Emergency Department Data Systems: Recommendations of the CDC Workgroup for Improvement of External Cause‐of‐Injury Coding.” MMWR—Recommendations and Reports 57 (RR‐1): 1–15. [PubMed] [Google Scholar]
- Blank, W. A. , Freiberger E., Siegrist M., Landendoerfer P., Linde K., Schuster T., Pfeifer K., Schneider A., and Halle M.. 2011. “An Interdisciplinary Intervention to Prevent Falls in Community‐Dwelling Elderly Persons: Protocol of a Cluster‐Randomized Trial [PreFalls].” BMC Geriatrics 11: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boards of Trustees . 2011. “2011 Annual Report of the Boards of Trustees of the Federal Hospital Insurance and Federal Supplementary Medical Insurance Trust Funds.” The Boards of Trustees, Federal Hospital Insurance and Federal Supplementary Medical Insurance Trust Funds. [Google Scholar]
- Bohl, A. A. , Fishman P. A., Ciol M. A., Williams B., Logerfo J., and Phelan E. A.. 2010. “A Longitudinal Analysis of Total 3‐year Healthcare Costs for Older Adults Who Experience a Fall Requiring Medical Care.” Journal of the American Geriatrics Society 58 (5): 853–60. [DOI] [PubMed] [Google Scholar]
- Bohl, A. A. , Phelan E. A., Fishman P. A., and Harris J. R.. 2012. “How Are the Costs of Care for Medical Falls Distributed? The Costs of Medical Falls by Component of Cost, Timing, and Injury Severity.” The Gerontologist 52 (5): 664–75. [DOI] [PubMed] [Google Scholar]
- Boonen, S. , Autier P., Barette M., Vanderschueren D., Lips P., and Haentjens P.. 2004. “Functional Outcome and Quality of Life Following Hip Fracture in Elderly Women: A Prospective Controlled Study.” Osteoporos International 15 (2): 87–94. [DOI] [PubMed] [Google Scholar]
- Carroll, N. V. , Slattum P. W., and Cox F. M.. 2005. “The Cost of Falls among the Community‐Dwelling Elderly.” Journal of Managed Care & Specialty Pharmacy 11 (4): 307–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter, M. W. , and Porell F. W.. 2011. “The Effect of Sentinel Injury on Medicare Expenditures over Time.” Journal of the American Geriatrics Society 59 (3): 406–16. [DOI] [PubMed] [Google Scholar]
- CMS . 2008. “Medicare Enrollment—Aged Beneficiaries as of July 2008.” Medicare Enrollment Reports [accessed on March 2, 2008]. Available at https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/MedicareEnrpts/Downloads/08Aged.pdf
- Corso, P. , Finkelstein E., Miller T., Fiebelkorn I., and Zaloshnja E.. 2006. “Incidence and Lifetime Costs of Injuries in the United States.” Injury Prevention 12 (4): 212–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie, L. 2008. “Fall and Injury Prevention” In Patient Safety and Quality: An Evidence‐Based Handbook for Nurses, edited by Hughes R. G., pp. 1–56. Rockville, MD: Agency for Healthcare Research and Quality. [PubMed] [Google Scholar]
- Dal Forno, G. , Chiovenda P., Bressi F., et al. 2006. “Use of an Italian Version of the Telephone Interview for Cognitive Status in Alzheimer's Disease.” International Journal of Geriatric Psychiatry 21 (2): 126–33. [DOI] [PubMed] [Google Scholar]
- Deandrea, S. , Lucenteforte E., Bravi F., Foschi R., La Vecchia C., and Negri E.. 2010. “Risk Factors for Falls in Community‐Dwelling Older People: A Systematic Review and Meta‐Analysis.” Epidemiology 21 (5): 658–68. [DOI] [PubMed] [Google Scholar]
- Dolan, P. , and Torgerson D. J.. 1998. “The Cost of Treating Osteoporotic Fractures in the United Kingdom Female Population.” Osteoporosis International 8 (6): 611–7. [DOI] [PubMed] [Google Scholar]
- Fauth, E. B. , Zarit S. H., Malmberg B., and Johansson B.. 2007. “Physical, Cognitive, and Psychosocial Variables from the Disablement Process Model Predict Patterns of Independence and the Transition into Disability for the Oldest‐Old.” The Gerontologist 47 (5): 613–24. [DOI] [PubMed] [Google Scholar]
- Finkelstein, E. , Prabhu M., and Chen H.. 2007. “Increased Prevalence of Falls among Elderly Individuals with Mental Health and Substance Abuse Conditions.” The American Journal of Geriatric Psychiatry 15 (7): 611–9. [DOI] [PubMed] [Google Scholar]
- Finkelstein, E. A. , Chen H., Miller T. R., Corso P. S., and Stevens J. A.. 2005. “A Comparison of the Case‐Control and Case‐Crossover Designs for Estimating Medical Costs of Nonfatal Fall‐Related Injuries among Older Americans.” Medical Care 43 (11): 1087–91. [DOI] [PubMed] [Google Scholar]
- Fleurence, R. L. 2004. “Cost‐Effectiveness of Fracture Prevention Treatments in the Elderly.” The International Journal of Technology Assessment in Health Care 20 (2): 184–91. [DOI] [PubMed] [Google Scholar]
- Frick, K. D. , Kung J. Y., Parrish J. M., and Narrett M. J.. 2010. “Evaluating the Cost‐Effectiveness of Fall Prevention Programs that Reduce Fall‐Related Hip Fractures in Older Adults.” Journal of the American Geriatrics Society 58 (1): 136–41. [DOI] [PubMed] [Google Scholar]
- Gannon, B. , O'Shea E., and Hudson E.. 2008. “Economic Consequences of Falls and Fractures among Older People.” Irish Medical Journal 101 (6): 170–3. [PubMed] [Google Scholar]
- Ganz, D. A. , Kim S.‐B., Zingmond D. S., Ramirez K. D., Roth C. P., Jennings L. A., Mori T., Keeler E. B., Wenger N. S., and Reuben D. B.. 2015. “Effect of a Falls Quality Improvement Program On Serious Fall‐Related Injuries.” Journal of the American Geriatrics Society 63 (1): 63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber, A. M. , MaCurdy T. E., and McClellan M. B.. 1998. “Persistence of Medicare Expenditures among Elderly Beneficiaries” In Frontiers in Health Policy Research, Vol. 1, edited by Garber A. M., pp. 153–80. Cambridge, MA: MIT. [Google Scholar]
- Gillespie, L. D. , Robertson M. C., Gillespie W. J., Sherrington C., Gates S., Clemson L. M., and Lamb S. E.. 2012. “Interventions for Preventing Falls in Older People Living in the Community.” Cochrane Database of Systematic Reviews 9: CD007146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartholt, K. A. , Oudshoorn C., Zielinski S. M., Burgers P. T., Panneman M. J., van Beeck E. F., Patka P., and van der Cammen T. J.. 2011. “The Epidemic of Hip Fractures: Are We on the Right Track?” PLoS ONE 6 (7): e22227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich, S. , Rapp K., Rissmann U., Becker C., and Konig H. H.. 2010. “Cost of Falls in old Age: A Systematic Review.” Osteoporosis International 21 (6): 891–902. [DOI] [PubMed] [Google Scholar]
- Hendrie, D. , Hall S. E., Arena G., and Legge M.. 2004. “Health System Costs of Falls of Older Adults in Western Australia.” Australian Health Review 28 (3): 363–73. [DOI] [PubMed] [Google Scholar]
- Hendriks, M. R. , Evers S. M., Bleijlevens M. H., van Haastregt J. C., Crebolder H. F., and van Eijk J. T.. 2008. “Cost‐Effectiveness of a Multidisciplinary Fall Prevention Program in Community‐Dwelling Elderly People: A Randomized Controlled Trial.” The International Journal of Technology Assessment in Health Care 24 (2): 193–202. [DOI] [PubMed] [Google Scholar]
- Hoffman, G. J. , Hays R. D., Shapiro M. F., Wallace S. P., and Ettner S. L.. 2016. “Claims‐Based Identification Methods and the Cost of Fall‐Related Injuries among US Older Adults.” Medical Care 54 (7): 664–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HRS . 2010. “Health and Retirement Study: 2010 Core Data Description and Usage.” Ann Arbor, MI: University of Michigan. [Google Scholar]
- Huang, E. S. , Basu A., O'Grady M., and Capretta J. C.. 2009. “Projecting the Future Diabetes Population Size and Related Costs for the U.S.” Diabetes Care 32 (12): 2225–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba, K. , Goecke M., Sharkey P., and Brenneman F.. 2003. “Long‐Term Outcomes after Injury in the Elderly.” The Journal of Trauma 54 (3): 486–91. [DOI] [PubMed] [Google Scholar]
- KFF . 2008. “Medicare Advantage: Total Enrollment.” State Health Facts [accessed on April 1, 2015]. Available at http://kff.org/medicare/state-indicator/total-enrollment-2/ [Google Scholar]
- Kim, S. B. , Zingmond D. S., Keeler E. B., Jennings L. A., Wenger N. S., Reuben D. B., and Ganz D. A.. 2016. “Development of an Algorithm to Identify Fall‐Related Injuries and Costs in Medicare Data.” Injury Epidemiology 3: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley, J. , Stephenson S., Thorpe C., and Davie G.. 2006. “Accuracy of Injury Coding Under ICD‐9 for New Zealand Public Hospital Discharges.” Injury Prevention 12 (1): 58–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeMier, M. , Cummings P., and West T. A.. 2001. “Accuracy of External Cause of Injury Codes Reported in Washington State Hospital Discharge Records.” Injury Prevention 7 (4): 334–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney, J. E. , Glysch R. L., Guilfoyle S. M., Hale L. J., and Katcher M. L.. 2005. “Trends, Risk Factors, and Prevention of Falls in Older Adults in Wisconsin.” Wisconsin Medical Society 104 (1): 22–8. [PubMed] [Google Scholar]
- Martin, L. G. , Freedman V. A., Schoeni R. F., and Andreski P. M.. 2009. “Health and Functioning among Baby Boomers Approaching 60.” The Journals of Gerontology. Series B, Psychological Sciences and Social Sciences 64 (3): 369–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie, K. , Enraght‐Moony E. L., Walker S. M., McClure R. J., and Harrison J. E.. 2009. “Accuracy of External Cause‐of‐Injury Coding in Hospital Records.” Injury Prevention 15 (1): 60–4. [DOI] [PubMed] [Google Scholar]
- Owens, P. L. , Russo C. A., Spector W., and Mutter R.. 2009. Healthcare Cost and Utilization (HCUP) Statistical Brief #80: Emergency Department Visits for Injurious Falls among the Elderly, 2006. Rockville, MD: Agency for Health Care Policy and Research. [PubMed] [Google Scholar]
- Quigley, P. A. , Campbell R. R., Bulat T., Olney R. L., Buerhaus P., and Needleman J.. 2012. “Incidence and Cost of Serious Fall‐Related Injuries in Nursing Homes.” Clinical Nursing Research 21 (1): 10–23. [DOI] [PubMed] [Google Scholar]
- RAND . 2003. Falls Prevention Interventions in the Medicare Population. Baltimore, MD: The RAND Corporation, prepared for the U.S. Department of Health and Human Services. [Google Scholar]
- Rice, T. , and Matsuoka K. Y.. 2004. “The Impact of Cost‐Sharing on Appropriate Utilization and Health Status: A Review of the Literature on Seniors.” Medical Care Research and Review 61 (4): 415–52. [DOI] [PubMed] [Google Scholar]
- Richmond, T. S. , Kauder D., Strumpf N., and Meredith T.. 2002. “Characteristics and Outcomes of Serious Traumatic Injury in Older Adults.” Journal of the American Geriatrics Society 50 (2): 215–22. [DOI] [PubMed] [Google Scholar]
- Rizzo, J. A. , Friedkin R., Williams C. S., Nabors J., Acampora D., and Tinetti M. E.. 1998. “Health Care Utilization and Costs in a Medicare Population by Fall Status.” Medical Care 36 (8): 1174–88. [DOI] [PubMed] [Google Scholar]
- Roudsari, B. S. , Ebel B. E., Corso P. S., Molinari N. A., and Koepsell T. D.. 2005. “The Acute Medical Care Costs of Fall‐Related Injuries among the U.S. Older Adults.” Injury 36 (11): 1316–22. [DOI] [PubMed] [Google Scholar]
- Rubenstein, L. Z. , and Josephson K. R.. 2002. “The Epidemiology of Falls and Syncope.” Clinics in Geriatric Medicine 18 (2): 141–58. [DOI] [PubMed] [Google Scholar]
- Scuffham, P. , Chaplin S., and Legood R.. 2003. “Incidence and Costs of Unintentional Falls in Older People in the United Kingdom.” Journal of Epidemiology and Community Health 57 (9): 740–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shumway‐Cook, A. , Ciol M. A., Hoffman J., Dudgeon B. J., Yorkston K., and Chan L.. 2009. “Falls in the Medicare Population: Incidence, Associated Factors, and Impact on Health Care.” Physical Therapy 89 (4): 324–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens, J. A. , Corso P. S., Finkelstein E. A., and Miller T. R.. 2006. “The Costs of Fatal and Non‐Fatal Falls among Older Adults.” Injury Prevention 12 (5): 290–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takanishi Jr, D. M. , Yu M., and Morita S. Y.. 2008. “Increased Fatalities and Cost of Traumatic Injuries in Elderly Pedestrians in Hawaii: A Challenge for Prevention and Outreach.” Asia Pacific Journal of Public Health 20 (4): 327–39. [DOI] [PubMed] [Google Scholar]
- Tinetti, M. E. , Speechley M., and Ginter S. F.. 1988. “Risk Factors for Falls among Elderly Persons Living in the Community.” The New England Journal of Medicine 319 (26): 1701–7. [DOI] [PubMed] [Google Scholar]
- Tinetti, M. E. , Gordon C., Sogolow E., Lapin P., and Bradley E. H.. 2006. “Fall‐Risk Evaluation and Management: Challenges in Adopting Geriatric Care Practices.” The Gerontologist 46 (6): 717–25. [DOI] [PubMed] [Google Scholar]
- Verma, S. K. , Willetts J. L., Corns H. L., Marucci‐Wellman H. R., Lombardi D. A., and Courtney T. K.. 2016. “Falls and Fall‐Related Injuries among Community‐Dwelling Adults in the United States.” PLoS ONE 11 (3): e0150939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, S. , Keeler E. B., Rubenstein L. Z., Maglione M. A., and Shekelle P. G.. 2010. “A Cost‐Effectiveness Analysis of a Proposed National Falls Prevention Program.” Clinics in Geriatric Medicine 26 (4): 751–66. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix SA1: Author Matrix.
Figure S1: Distribution of Unadjusted Expenditures for Fall‐Related Injury (FRI) and Non‐FRI Cohorts of Older Medicare Community‐Dwelling Fee‐for‐Service Beneficiaries, 2007–2009.
Figure S2: Distribution of Adjusted Expenditures for Fall‐Related Injury (FRI) and Non‐FRI Cohorts of Older Medicare Community‐Dwelling Fee‐for‐Service Beneficiaries, 2007–2009.


