ABSTRACT
The host immune response affects pathogen virulence in Clostridium difficile infection (CDI). Thus, cytokine responses to CDI likely are associated with disease initiation and progression. Understanding the molecular drivers of inflammation and biochemical markers of disease severity is important for developing novel therapies and predicting disease prognosis. In this study, we investigated cytokine production in patients with CDI and evaluated the potential of cytokines to serve as biomarkers for CDI and predictors of disease severity. The systemic cytokine profiles of 36 CDI patients (20 with severe disease) and 8 healthy donors and the toxin-induced cytokine profiles of peripheral blood mononuclear cells (PBMC) were determined. Further, we evaluated glucosyltransferase (GT) activity in regulation of toxin-induced cytokine expression. We found upregulation of the majority of measured cytokines (11/20, 55%) in CDI patients. Interleukin-1β (IL-1β), IL-6, IL-8, IL-17A, and IL-16 were the most upregulated. High serum levels of IL-2 and IL-15 were associated with a poor prognosis in CDI patients, whereas high levels of IL-5 and gamma interferon (IFN-γ) were associated with less severe disease. Both TcdA and TcdB were potent inducers of cytokine responses, as demonstrated by stimulation of a greater number and amount of cytokines. In addition to confirming prior reports on the role of IL-8, IL-1β, and IL-6 in CDI, our data suggest that IL-16 and IL-17A, as well as the IL-1β/Th17 axis, play a key role in driving inflammatory responses in CDI. A functional GT domain of C. difficile toxins was required for the induction of a majority of cytokines investigated.
KEYWORDS: Clostridium difficile infection, cytokine response, disease severity, glucosyltransferase activity
INTRODUCTION
Clostridium difficile infection (CDI) is a common and debilitating health care-associated infection with increased morbidity and mortality over the past 2 decades (1). C. difficile-mediated diarrhea and colitis are mainly triggered by two major toxins, toxin A (TcdA) and toxin B (TcdB), which are released by toxigenic strains (2). CDI has variable clinical manifestations that range from asymptomatic colonization to fulminant colitis and death (3), which likely reflects differences in C. difficile virulence factors as well as differences in immune and inflammatory responses by the host (4).
CDI is characterized by an intense inflammatory response (5). C. difficile toxins cause direct injury to the intestinal epithelium, which initiates host inflammatory responses via eliciting cytokine release and inducing prominent neutrophil activation and recruitment that subsequently result in additional intestinal injury (6). Once the inflammatory response is initiated, antibiotics have minimal impact on disease protection. Recently, Haslam et al. have shown that persistent diarrhea in CDI patients on appropriate antibiotic therapy correlates with intestinal inflammation, not bacterial burden (7). Inhibiting the acute inflammatory response might attenuate intestinal injury (6). Indeed, anti-inflammatory agents diminish intestinal injury in experimental CDI (6, 8), suggesting mitigation of the host inflammatory response is an effective strategy when used in concert with antibiotic or probiotic approaches to CDI therapy and/or prevention. Thus, understanding the molecular drivers of acute inflammation is important for developing novel, host-centered therapies. Furthermore, neutrophil influx to the epithelium and submucosa is a hallmark of severe CDI (9). Therefore, cytokines induced by immune cells in response to CDI might be associated with disease severity and, if they are, could be used in outcome prediction.
In the present study, we investigated cytokine profiles in CDI patients and aimed to detect biomarkers for therapeutic targets and disease prognosis. Furthermore, we characterized the host cytokine responses to the two major virulence factors of C. difficile, TcdA and TcdB. Finally, we demonstrate the molecular basis that endows toxins with inflammatory activity.
RESULTS
Circulating levels of cytokines are associated with disease and disease severity.
Compared to healthy controls, patients were found to respond to C. difficile infection by secreting significant amounts of interleukin-1β (IL-1β), IL-2, IL-5, IL-6, IL-8, IL-10, IL-13, IL-15, IL-16, IL-17A, and tumor necrosis factor alpha (TNF-α), which comprised a majority of the 20 tested cytokines (11/20, 55%) (Table 1). The other 8 cytokines (IL-1a, IL-4, IL-12p70, IL-12p40, granulocyte-macrophage colony-stimulating factor [GM-CSF], gamma interferon [IFN-γ], IFN-β, and vascular endothelial growth factor [VEGF]) had comparable levels in patients and healthy donors. Only IL-7 was found to be lower in CDI patients. The levels of all upregulated cytokines in patients were at least 2-fold higher than that of the controls. The most upregulated cytokines included IL-1β, IL-6, IL-8, IL-16, and IL-17A. The cytokines with the highest magnitude in difference between healthy donors and patients with CDI were IL-1β (0.0149 ± 0.0149 pg/ml versus 2.135 ± 0.9264 pg/ml) and IL-8 (5.693 ± 0.835 pg/ml versus 402.1 ± 91.26 pg/ml), with a 140- and 70-fold upregulation, respectively. Levels for IL-6, IL-17A, and IL-16 were 42.3, 7.3, and 4.54 times higher than those for healthy donors. Four cytokines (IL-2, IL-5, IL-15, and IFN-γ) were associated with disease severity, showing significant differences in levels between patients with mild to moderate disease (Pm) and those with severe disease (Ps). Interestingly, IFN-γ and IL-5 were more commonly elevated in mild-to-moderate CDI, while IL-2 and IL-15 were higher in severe/severe-complicated CDI in these patients (Table 1).
TABLE 1.
Serum cytokine levels in patients with CDI and healthy controls
| Cytokine | Serum cytokine level (pg/ml) ina: |
P value, H vs Pb | Serum cytokine level (pg/ml) ina: |
P value, Pm vs Psb | ||
|---|---|---|---|---|---|---|
| Healthy donors | CDI patients | CDI patients with mild-moderate disease | CDI patients with severe/severe complicated disease | |||
| IL-1α | 1.740 ± 0.9359 (0.03∼6.73), 88% | 0.7975 ± 0.4318 (0.02∼15.49), 75% | 0.1394 | 0.4277 ± 0.1678 (0.02∼2.19), 75% | 1.093 ± 0.7681 (0.02∼15.49), 75% | 0.6017 |
| IL-1β | 0.01490 ± 0.0149 (0.12∼0.12), 13% | 2.135 ± 0.9264 (0.03∼29.9), 83% | 0.0005** | 1.229 ± 0.4367 (0.09∼3.65), 81% | 2.860 ± 1.632 (0.03∼29.9), 85% | 0.3451 |
| IL-2 | 0.1184 ± 0.02514 (0.07∼0.22), 88% | 0.4224 ± 0.08289 (0.02∼2.17), 86% | 0.0155* | 0.1733 ± 0.0315 (0.02∼0.88), 69% | 0.4754 ± 0.1108 (0.04∼2.17), 100% | 0.0362* |
| IL-4 | 0.0004392 ± 0.00033 (0.014∼0.014), 13% | 0.03401 ± 0.0228 (0.02∼0.76), 53% | 0.2988 | 0.01756 ± 0.007 (0.03∼0.087), 44% | 0.08027 ± 0.046 (0.02∼0.76), 60% | 0.5009 |
| IL-5 | 0.2414 ± 0.07368 (0.07∼0.66), 63% | 0.7493 ± 0.1649 (0.025 ∼26.5), 100% | 0.0442* | 1.201 ± 0.3456 (0.15 ∼26.5), 100% | 0.3550 ± 0.0596 (0.025 ∼11.0), 100% | 0.0312* |
| IL-6 | 0.1368 ± 0.03914 (0.03∼0.28), 100% | 5.797 ± 0.9128 (0.05∼19.8), 100% | <0.0001*** | 4.829 ± 1.092 (0.05 ∼0.29), 100% | 6.652 ± 1.421 (0.06 ∼19.8), 100% | 0.3270 |
| IL-7 | 16.27 ± 1.090 (13.4 ∼21.9), 100% | 12.63 ± 1.548 (0.55 ∼56.6), 100% | 0.0324* | 9.811 ± 1.288 (1.0∼17.0), 100% | 20.58 ± 3.691 (0.55∼56.6), 100% | 0.0897 |
| IL-8 | 5.693 ± 0.8350 (3.06∼9.61), 100% | 402.1 ± 91.26 (13.91∼996), 100% | <0.0001*** | 276.5 ± 84.55 (14.5∼921.7), 100% | 308.1 ± 74.22 (13.91∼996), 100% | 0.7805 |
| IL-10 | 0.3802 ± 0.1118 (0.06∼0.87), 100% | 1.728 ± 0.3176 (0.09∼7.13), 100% | 0.002** | 1.224 ± 0.2593 (0.22∼3.55), 100% | 1.836 ± 0.4585 (0.09∼7.13), 100% | 0.6527 |
| IL-12p40 | 77.38 ± 6.549 (54.0∼108.3), 100% | 80.46 ± 10.60 (2.76∼207.6), 100% | 0.9035 | 93.24 ± 17.06 (2.76∼207.6), 100% | 70.38 ± 13.32 (4.94∼178.5), 100% | 0.2998 |
| IL-12p70 | 0.1230 ± 0.04778 (0.03∼0.38), 75% | 0.1876 ± 0.02419 (0.02∼0.49), 92% | 0.2446 | 0.1951 ± 0.0308 (0.03∼0.39), 100% | 0.18632 ± 0.0258 (0.02∼0.49), 85% | 0.4713 |
| IL-13 | 0.7309 ± 0.07545 (0.45∼1.14), 100% | 2.242 ± 0.1941 (0.57∼4.59), 100% | 0.0003** | 2.201 ± 0.3953 (0.57∼4.59), 100% | 2.271 ± 0.1862 (0.73∼3.75), 100% | 0.6742 |
| IL-15 | 2.003 ± 0.08579 (1.63∼2.32), 100% | 4.889 ± 0.4417 (1.80∼10.84), 100% | <0.0001*** | 2.868 ± 0.2057 (1.80∼4.16), 100% | 6.146 ± 0.5689 (2.81∼10.84), 100% | <0.0001*** |
| IL-16 | 155.6 ± 16.09 (104.7∼236.0), 100% | 692.9 ± 79.45 (49.1∼1,581), 100% | <0.0006** | 749.9 ± 137.4 (117.9∼1,581), 100% | 653.0 ± 96.90 (49.1∼1,419.9), 100% | 0.5568 |
| IL-17A | 0.9552 ± 0.2777 (0.20∼1.42), 100% | 6.979 ± 1.548 (0.56∼16.42), 100% | 0.0071** | 4.838 ± 1.153 (0.75∼14.31), 100% | 8.692 ± 2.600 (0.56∼16.42), 100% | 0.7315 |
| IFN-γ | 4.692 ± 0.6091 (3.02∼7.07), 100% | 12.49 ± 2.243 (0.45∼28.41), 100% | 0.2856 | 13.85 ± 2.454 (1.89∼28.41), 100% | 4.190 ± 0.7325 (0.45∼10.07), 100% | 0.006** |
| TNF-α | 2.310 ± 0.1070 (1.86∼2.69), 100% | 4.376 ± 0.5504 (0.63∼15.80), 100% | 0.0296* | 5.260 ± 0.4403 (0.82∼11.73), 100% | 4. 268 ± 0.2058 (0.63∼15.80), 100% | 0.2183 |
| IFN-β | 0.1509 ± 0.02305 (0.06∼0.23), 100% | 0.1261 ± 0.01546 (0.02∼0.32), 100% | 0.3863 | 0.1480 ± 0.0244 (0.03∼0.32), 100% | 0.1044 ± 0.0190 (0.02∼0.32), 100% | 0.1690 |
| GM-CSF | 0.2089 ± 0.07594 (0.04∼0.23), 88% | 0.3533 ± 0.1174 (0.01∼1.2), 83% | 0.5971 | 0.4229 ± 0.2363 (0.01∼1.2), 100% | 0.2622 ± 0.0736 (0.06∼0.72), 70% | 0.3727 |
| VEGF | 80.39 ± 12.19 (49.2∼133.6), 100% | 185.0 ± 25.54 (15.7∼533.7), 100% | 0.0548 | 153.2 ± 33.70 (15.7∼474.1), 100% | 246.9 ± 49.93 (22.0∼533.7), 100% | 0.1308 |
Serum cytokine levels are expressed as means ± SEM. The range of detectable cytokine expression is recorded in parentheses. The value of the expression level of undetectable sample was set to 0 for statistical analysis. The percentages of detectable sample are indicated.
H, healthy donors (n = 8); P, CDI patients (n = 36); Pm, CDI patients with mild-moderate disease (n = 16); Ps, CDI patients with severe/severe complicated disease (n = 20). *, 0.01 < P < 0.05; **, 0.0001 < P < 0.01; ***, P < 0.0001.
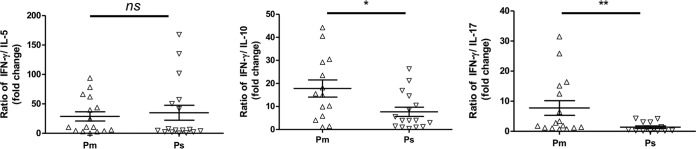
Aberrant T-cell responses are associated with a range of inflammatory diseases, including asthma, allergy, inflammatory bowel disease, and autoimmune diseases. Naive Th cells can be differentiated into Th1, Th2, or Th17 effector cells with distinct cytokine secretion phenotypes. Th1, Th2, and Th17 populations, and the cytokines they release, are antagonistic to each other, and one or the other subtype is dominant in response to a particular pathogen at any one time. To understand the roles of cytokines in CDI pathogenesis, we analyzed the shift of Th1 to Th2 response in the CDI patients by assessing the ratio of IFN-γ or IL-12p70 to IL-10 and IFN-γ or IL-12p70 to IL-5 and the Th1 to Th17 response by assessing the ratio of IFN-γ or IL-12p70 to IL-17A. Impressively, the IFN-γ/IL-17A ratio was significantly lower (5.66-fold decrease) in Ps than in Pm, suggesting a shift of Th1 response to Th17 response in those patients with more severe disease (Fig. 1). Additionally, a lower IFN-γ/IL-10, but not IFN-γ/IL-5, ratio was found in Ps than in Pm. These data matched the change of cytokine levels in Pm and Ps, indicating that a shift of Th1 to Th2 (IL-10) as well as Th1 to Th17 responses was due to a significantly lower level of IFN-γ in severe cases of diseases among these donors (Fig. 1). No significant difference was found in ratios of IL-12p70 to IL-10, IL-5, or IL-17A.
FIG 1.
Shift of Th1 to either Th2 or Th17 immunodominance associated with severe CDI. The ratios of serum level of IFN-γ to that of IL-5, IL-10, and IL-17A were calculated and compared between patients with mild-to-moderate CDI (Pm) and severe/severe complicated CDI (Ps). Unpaired t test was used for analysis. A P value of less than 0.05 was considered statistically significant. ns, not significant; *, P < 0.05; **, P < 0.01.
C. difficile toxin-induced cytokine responses in PBMC.
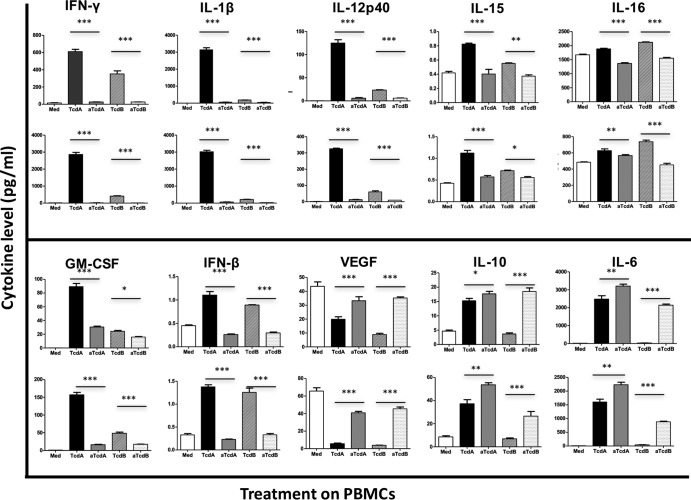
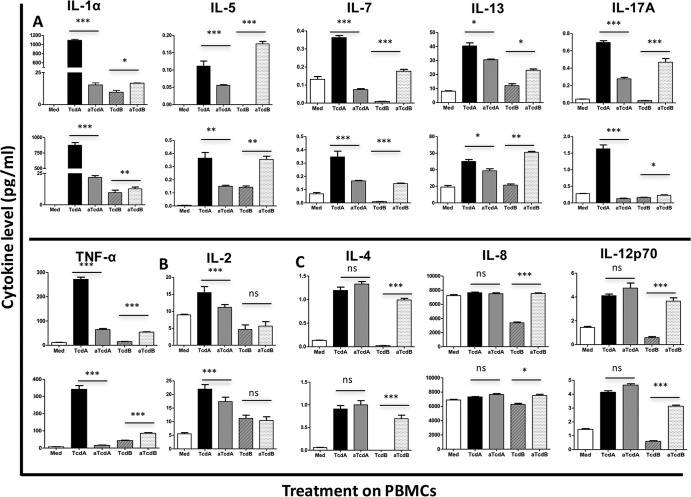
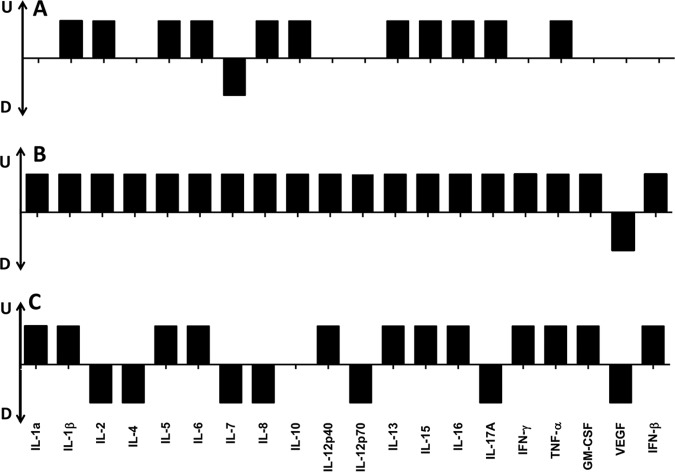
Unstimulated peripheral blood mononuclear cells (PBMC) produced low levels of most tested cytokines, except for IL-8 and IL-16, whose levels were as high as 7,232.29 ± 135.9 and 1,671 ± 21.34 pg/ml (Fig. 2 and 3). IL-1α was undetectable in culture supernatant from PBMC without stimulation (Fig. 3). In response to 100 ng/ml of TcdA, 19 of the 20 (95%) tested cytokines were upregulated, while only VEGF was downregulated (Fig. 2 and 4B). TcdB stimulation at the 100 ng/ml concentration resulted in 12 cytokines being upregulated, 7 downregulated, and only IL-10 remaining unaffected (Fig. 4). Expression of 12 cytokines was upregulated by both TcdA and TcdB, including IFN-γ, IL-1β, IL-13, IL-5, IL-6, TNF-α, GM-CSF, IL-12p40, IL-15, IL-16, IL-1α, and IFN-β (Fig. 2, 3, and 4). Among these cytokines, only IL-16 was preferentially induced by TcdB than TcdA, while the others were more strongly produced in response to TcdA stimulation (Fig. 2 and 3). The level of VEGF was downregulated by both TcdA and TcdB (Fig. 2 and 4). Compared with control samples, IL-10 expression was significantly induced by TcdA but not by TcdB (Fig. 2 and 4). Some cytokines were upregulated by TcdA but downregulated by TcdB, including IL-2, IL-4, IL-7, IL-8, IL-12p70, and IL-17A (Fig. 4). The combined cytokine profiles induced by C. difficile TcdA and TcdB had similarities to the responses observed from serum samples obtained from CDI patients, except a significant upregulation of IL-1α, IL-12p40, GM-CSF, IFN-β, and IFN-γ and a significant downregulation of VEGF by PBMC in response to C. difficile toxins were found, and these were not found to be affected in patients compared to controls (Fig. 4). These results demonstrate that PBMC cytokine responses evoked by C. difficile toxins in vitro overlap yet remain distinct from responses in CDI patients. The difference is likely due to the fact that these are in vitro PBMC responses which do not completely reflect CDI in human.
FIG 2.
GT activity regulates toxin-induced cytokine expression, similar regulation by GT deficiency in TcdA and TcdB. Toxin-mediated expression of IFN-γ, IL-1β, IL-12p40, IL-15, IL-16, GM-CSF, and IFN-β was downregulated by GT-deficient TcdA (aTcdA) and GT-deficient TcdB (aTcdB). Toxin-induced expression of VEGF, IL-10, and IL-6 was upregulated by GT deficiency in TcdA and TcdB. A P value of less than 0.05 was considered statistically significant. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
FIG 3.
GT activity regulates toxin-induced cytokine expression, differential regulation by GT deficiency in TcdA and TcdB. (A) Toxin-mediated expression of IL-1α, IL-5, IL-7, IL-13, IL-17A, and TNF-α were downregulated by GT-deficient TcdA (aTcdA) but upregulated by GT-deficient TcdB (aTcdB). (B) Toxin-induced expression of IL-2 was decreased by GT-deficient TcdA but not affected by GT-deficient TcdB. (C) Toxin-induced production of IL-4, IL-8, and IL-12p70 was not affected by GT deficiency in TcdA but was upregulated by GT-deficient TcdB. A P value of less than 0.05 was considered statistically significant. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
FIG 4.
Cytokine profiles in response to CDI and C. difficile toxins. Cytokine profiles of PBMC in response to CDI (A), TcdA (B), and TcdB (C) are compared. U, upregulation; D, downregulation. Bars not shown means the level of cytokine was unaffected. The heights of bars do not represent expression levels. The regulation of cytokines shown in panel A compares levels in patients to levels in healthy donors; in panels B and C, cytokine levels of PBMC stimulated with toxins (100 ng/ml) are compared to those of PBMC treated with culture medium.
Effect of GT activity on cytokine production.
To understand the relevance of toxin-induced cytokine responses and toxin effector function, we utilized glucosyltransferase (GT)-deficient toxins and observed their influence on cytokine production. GT-deficient counterparts induced lower levels of most of the cytokines (14/19, 74%) (IFN-γ, IL-13, IL-1β, IL-2, TNF-α, GM-CSF, IL-12p40, IL-15, IL-16, IL-17A, IL-1α, IL-5, IL-7, and IFN-β) (Fig. 2 and 3) than wild-type TcdA. Noticeably, IL-1β and IL-17A, which were identified to be highly upregulated in CDI, were decreased by 98% and 60%, respectively (Fig. 2 and 3A). Interestingly, GT-deficient TcdA (aTcdA) elicited a higher production of anti-inflammatory cytokine IL-10 and VEGF (Fig. 2). The expression levels of IL-4, IL-8, and IL-12p70 were not affected by GT deficiency in TcdA (Fig. 3C). Similar to GT-deficient TcdA, GT-deficient TcdB (aTcdB) also induced levels of expression of IFN-γ, IL-1β, GM-CSF, IL-12p40, IL-15, IL-16, and IFN-β which were 20 to 90% lower than those of wild-type TcdB. aTcdB, however, promoted higher levels of VEGF and IL-10 (4 to 6.2 times higher than that induced by wild-type TcdB) (Fig. 2). Some cytokines were differentially regulated by GT deficiency in TcdA and TcdB. Specifically, the production of IL-1α, IL-5, IL-7, IL-13, IL-17A, and TNF-α was lowered by GT-deficient TcdA but elevated by GT-deficient TcdB compared to their corresponding wild-type toxins (Fig. 3A). IL-2 was downregulated by GT-deficient TcdA but was not affected by GT-deficient TcdB (Fig. 3B). The GT-deficient TcdA induced similar levels of IL-4, IL-8, and IL-12p70 as the wild-type toxin, but GT-deficient TcdB increased the expression of these cytokines compared to its wild type (Fig. 3C).
DISCUSSION
Host inflammatory responses to CDI are believed to influence the course of the disease and are garnering increasing interest from the scientific community. Systemic biomarkers in blood, such as C-reactive protein, albumin, and white blood cell count, were recently proposed to be associated with clinical outcomes in CDI (10, 11). Despite appropriate antibiotic therapy, symptomatic cure is not achieved in 20% to 30% of cases (12), and up to 22% of patients with CDI remain symptomatic beyond 10 days of treatment (13), which is associated with intestinal inflammation, not bacterial burden (7). Gaining an understanding of the molecular drivers of acute inflammation and identifying biochemical markers that are predictive of a poor prognosis in CDI are important for the development of novel strategies of therapy and prevention. In this study, we identified biologically relevant markers associated with disease and disease severity in patients with CDI. Our results are consistent with the results of others who have also shown that CDI stimulates the production of multiple cytokines, which is considered a characteristic of CDI (14, 15). Upon infection, we identified a tremendous increase in IL-8 (80-fold), IL-6 (42.3-fold), and IL-1β (143-fold), which suggests they play a vital role in CDI pathogenesis. Importantly, in the current study, IL-17A and IL-16 were also found to be markedly elevated in CDI patients. Thus, these two cytokines may also play key roles in the systemic inflammatory response in CDI and may be critical drivers of CDI manifestations (Table 1).
IL-8, a CXC chemokine, plays a role in recruiting and activating neutrophils in intestinal mucosa via the Ras/mitogen-activated protein kinase (MAPK)/AP-1 (16, 17) and the IκB kinase (IKK)/NF-κB (18, 19) pathways. Additionally, it has been demonstrated that the IL-8 −251A/A genotype affects susceptibility to C. difficile diarrhea (20, 21). IL-1β, which we found to be upregulated 143-fold in CDI patients, is a pleiotropic immune mediator that induces fever, recruits neutrophils, and leads to local tissue destruction during CDI. The role of IL-1β as a Th17 differentiation factor is well established, and the effector IL-17A was found to have a 7.3-fold elevation in CDI patients (Table 1), suggesting that the IL-1β-Th17 axis is a target for therapeutic intervention in C. difficile infection.
IL-15 and IL-16 are pleiotropic cytokines. The former is characterized as being a T cell growth factor and regulates T and natural killer (NK) cell activation and proliferation (22); the latter plays a role in recruiting and activating cells expressing the CD4 molecule, including T cells, monocytes, eosinophils, and dendritic cells (23). Previous studies have shown that several cell types produce IL-8 and IL-1β during CDI, such as peripheral blood monocytes, the monocyte cell line THP-1 (24), and the intestinal epithelial cell lines HT-29 and T-84 (25, 26). Observations in the present study show a robust increase in IL-15 and IL-16, together with a rise in IL-2 (Table 1). These findings suggest that T cells contribute to immunopathology in C. difficile-mediated diseases. This notion is further supported by the finding that expression levels of IL-2 and IL-15 are associated with CDI disease severity (Table 1). These cytokines are reported to be associated with other intestinal diseases and inflammation. For example, IL-15 was overexpressed in inflammatory bowel disease, regulated local T cell-dependent cytokine production, and resulted in chronic inflammation, an abnormal immune response, and tissue damage (27); IL-16 has been found to be elevated in both Crohn's disease and ulcerative colitis, with a positive correlation between level and disease activity (28). Animal studies have also found upregulation of IL-16 during chronic intestinal inflammation, and blocking IL-16 activity ameliorated 2,4,6-trinitrobenzenesulfonic acid (TNBS)-induced colitis (29).
Although it recently has been reported by Steiner et al. that both fecal IL-8 and IL-1β were elevated in patients with severe C. difficile colitis (30), these cytokines in circulation were not associated with CDI severity in our current study (Table 1). Instead, we found systemic IL-2, IL-5, IL-15, and IFN-γ were associated with CDI severity. Not surprisingly, the anti-inflammatory cytokine IL-5 was negatively correlated with CDI disease severity. Interestingly, IFN-γ was also found downregulated in severe cases. Furthermore, the shift of Th1 response to Th17 or Th2 response in patients with severe disease (Fig. 1) was consistent with reduced IFN-γ in these cases. While a previous study using an ileal loop model found a pathogenic role of IFN-γ in TcdA-induced enteritis (31), a recent study using a CDI mouse model showed significantly lower levels of IFN-γ in severely infected mice (32), which is consistent with our observations in CDI patients, highlighting IFN-γ as a central host defense cytokine against C. difficile.
TcdA and TcdB are the major virulence factors of C. difficile, supported by reports demonstrating that C. difficile clinical isolates lacking both toxin genes are nonpathogenic in humans and animals (33). These two toxins have a high degree of similarity in multidomain structure but exhibit different biological functions. TcdA is considered a strong enterotoxin. When purified TcdA was injected into ileal loops, considerable pathology was induced, including marked mucosal edema, villous disruption, fluid secretion, and massive neutrophil infiltration. In comparison, TcdB does not exhibit enterotoxicity in ileal and colonic loops of rabbit (34), hamster (35), or mouse (36), even though it is a more potent cytotoxin than TcdA (37). In this study, we investigated the cytokine response of human PBMC elicited by these toxins. The dose of toxins (100 ng/ml) we chose for PBMC stimulation to evaluate cytokine response considered the following aspects based on literature and our experience: (i) effective immune response (38, 39, 40); (ii) no significant effect on cytotoxicity observed by culture of PBMC with or without toxins at 100 ng/ml (data not shown) and as reported in the literature (38, 40); and (iii) the ranges of toxin concentrations in fecal samples of CDI patients (41). Both TcdA and TcdB are potent inducers of cytokine responses in PBMC. However, TcdA was more proinflammatory at the tested concentration, as demonstrated by its inducing a greater number of different cytokines and at significantly higher levels (Fig. 2 and 3). All of the cytokines upregulated in patients with CDI (Table 1) were also induced by PBMC exposed to TcdA (Fig. 2, 3, and 4). Noticeably, important cytokines associated with CDI and CDI progression, such as IL-2, IL-8, and IL-17A, were only upregulated by TcdA, which is contrary to their downregulation induced by TcdB (Fig. 4). These results suggest that TcdA is the major driver of cytokine responses during CDI. Although both TcdA and TcdB elevated the release of IL-1α, GM-CSF, and IL-12p40, their systemic levels were not affected in patients during CDI (Fig. 4). Taken together, our data reveal that (i) the more potent proinflammatory activity of TcdA relative to TcdB may contribute to the higher enterotoxicity of TcdA seen in animal ileal loop models; (ii) C. difficile TcdA and TcdB may be the chief factors for the induction of inflammation during CDI; and (iii) the molecular mechanisms of cytokine production induced by the two toxins may be different.
The N-terminal domains of TcdA and TcdB show 74% homology, and this homology provides a basis for the similar substrate specificity of these two toxins. The N-terminal catalytic glucosyltransferase domain (GTD) transfers the glucose moiety of UDP-glucose to members of the Rho family of small GTPases, e.g., Rho, Rac, and Cdc42 (42). Glucosylation of Rho proteins by these toxins inactivates these small GTPases and inhibits their molecular switch function, thus blocking Rho GTPase-dependent signaling and resulting in modulation of numerous physiological events in cells which contribute directly to the disease. Previously, we generated GT-deficient holotoxins and demonstrated loss of toxicity as measured by cytotoxicity assay and reduced enterotoxicity in an ileal loop model (43). Therefore, we hypothesized that C. difficile toxin-induced inflammation is at least partially GT activity dependent. To investigate this question, we measured the cytokine production in PBMC induced by GT-deficient toxins and compared it with that mediated by corresponding wild-type toxins. In comparison with wild-type TcdA, 14 of 19 TcdA-upregulated cytokines displayed a decreased expression in PBMC treated with GT-deficient TcdA (Fig. 2, 3A, 3B, and 4B), with especially marked reductions of IL-1β by 98%, IL-17A by 60%, IL-15 by 50%, and IL-16 by 28% (Fig. 2), which are cytokines that we found elevated in CDI patients and to be associated with CDI severity. On the contrary, GT-deficient TcdA induced an increased secretion of IL-10, an anti-inflammatory cytokine, the absence of which aggravates C. difficile colitis in mice challenged with C. difficile spores (44). IL-8 production was not significantly altered by the GT deficiency in TcdA. Therefore, GT activity in TcdA was necessary for inducing a robust inflammation in CDI that is mediated by the upregulation of proinflammatory cytokines and downregulation of anti-inflammatory cytokines. However, our data suggest a more complicated regulation of cytokine responses by GT-deficient TcdB. GT deficiency in TcdB did lower the levels of some cytokines (IFN-γ, IL-1β, GM-CSF, IL-12p40, IL-15, IL-16, and IFN-β), similarly to GT deficiency in TcdA, but the reductions were not as potent as those of the latter, with the exception of that for IL-16 (Fig. 2). These results suggest GT activity of TcdA and TcdB is at least partly responsible for the induction of inflammation. Consistent with this point of view, Xu and coworkers found that the glucosyltransferase-inactive mutant TcdB fails to induce inflammasome stimulation (45), since it is widely accepted that C. difficile toxin-induced inflammation and intestinal injury are mediated by the inflammasome (45). In contrast, Ng et al. thought GT activity is dispensable for inducing inflammasome activation in mice (46). Differential regulation of some cytokines was observed in GT deficiency in TcdA and TcdB, such as IL-13, TNF-α, IL-17A, IL-1a, IL-5, and IL-7, which were downregulated by GT-deficient TcdA but upregulated by GT-deficient TcdB (Fig. 2). This differential regulation might be due to the different eukaryotic target proteins of glucosylation by TcdA and TcdB with resulting differences in downstream signaling, or it could be due to differences in sequence and structure of the GT domain between TcdA and TcdB.
In summary, CDI results in a cascade of systemic cytokine production, including marked upregulation of IL-1β, IL-8, IL-16, and IL-17A, which are considered main cytokines mediating C. difficile-associated disease. The IL-1β/Th17 axis might be a target for therapeutic intervention in C. difficile infection. Once disease is initiated, IL-2 and IL-15 are the key drivers toward severe disease, while IL-5 and IFN-γ appear to provide defense against disease progression. In addition, another characteristic of severe CDI is the shift of Th1 to Th17 response. This report addressed the systemic cytokine markers for C. difficile-associated disease as well as their association with disease severity. Additionally, the significant upregulation of IL-2, IL-15, and IL-16 observed in those patients with CDI or severe CDI suggests a central role of cross talk of T cells with other cell types in pathogenesis of CDI. Both TcdA and TcdB are potent in inducing cytokine responses in human PBMC. The cytokine release mediated by C. difficile toxins is at least partly due to their functional GTD. Most cytokines regulated by GT activities in TcdA or TcdB showed similar trends, but some are somehow differentially affected, and the mechanisms need further investigation. Moreover, studies utilizing other in vitro or ex vivo models, such as colonic biopsy specimens and three-dimensional intestinal organoids, may provide additional insights on the differential roles of the two toxins in the induction of the inflammatory response. Similar to many inflammatory diseases, such as rheumatoid arthritis, inflammatory bowel diseases, and other chronic inflammatory diseases, a skewed pattern of cytokine expression was identified in CDI. While blocking of some inflammatory signaling pathways results in increased bacterial burden and worsening of disease (47), attenuating proinflammatory cytokine responses reduced severity of CDI in animal models (48). Therefore, our study may provide insights into anti-inflammation-based immunotherapies against CDI. Since cytokine responses are complex and their expression is regulated in network, the anti-inflammation-based immunotherapies against CDI ultimately need to be tested in animal disease models and humans.
MATERIALS AND METHODS
Ethics statement.
This study was approved by the Institutional Review Boards of the University of Maryland–Baltimore, Beth Israel Deaconess Medical Center–Boston, and Baylor College of Medicine-St. Luke's Hospital. Discarded laboratory blood specimens and samples obtained from patients who provided written informed consent were collected.
Patients, clinical data, and specimen collection.
Inpatients (n = 213) were consecutively recruited from the above-mentioned clinical centers between January 2012 and May 2014. All CDI cases had a confirmed positive diagnosis (stool test for toxigenic C. difficile, either a DNA amplification assay or a toxin enzyme immunoassay [EIA]) based on clinical verification (10). Healthy volunteer controls (n = 38) were individuals not suffering from diarrhea and with no history of CDI. Baseline characteristics on subject demographics and clinical history, including CDI risk factors and immunosuppression, were collected for every patient. Blood samples were collected within 24 to 48 h of the positive diagnostic test, and serum was separated by centrifugation after clotting, aliquoted, and stored at −80°C until used. In this study, we randomly chose 8 healthy serum samples and 36 samples from immunocompetent CDI patients (16 with mild-to-moderated disease and 20 with severe disease, respectively). Samples obtained from immunosuppressed subjects were not included, since immunosuppression might interfere with cytokine responses.
SHEA/IDSA guidelines on disease severity.
Clinical CDI disease severity was stratified according to criteria set forth in the 2010 SHEA/IDSA guidelines on management of CDI in adults (10). According to the criteria, severe CDI is characterized by leukocytosis with a white blood cell (WBC) count of 15,000 cells/ml or higher or a serum creatinine level greater than or equal to 1.5 times the premorbid level.
Laboratory assays. (i) Serum cytokine responses in CDI patients.
Serum samples from eight healthy donors (H) and 36 CDI patients (16 with mild-to-moderate disease [Pm] and 20 having severe disease [Ps]) were used (Table 1). Twenty cytokines (IL-1α, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12p40, IL-12p70, IL-13, IL-15, IL-16, IL-17A, IFN-γ, IFN-β, TNF-α, GM-CSF, and VEGF) were measured on serum samples in triplicates using the V-PLEX kit (human proinflammatory panel 1, K15049D, and chemokine panel 1, K15050D; Meso Scale Discovery, Rockville, MD, USA) according to the manufacturer's instructions.
(ii) Preparation of C. difficile toxins.
The full-length genes of TcdA and TcdB, amplified from chromosomal DNA of C. difficile strain VPI 10463, were cloned into a pHis-1522 shuttle vector and expressed in Bacillus megaterium. The expressed recombinant wild-type toxins were purified by Ni affinity chromatography as described previously (49). Using this platform, glucosyltransferase (GT)-deficient toxins were generated by introducing point mutations into conserved amino acids that are responsible for substrate uridine diphosphoglucose (UDP-Glc) binding. The details were described previously (50).
(iii) C. difficile toxin-induced cytokine production in PBMC.
PBMC were isolated from the fresh blood of 2 healthy donors for the assay of C. difficile toxin-induced cytokine production using Ficoll-Paque plus (GE Healthcare, Sweden) according to the user manual. PBMC were aliquoted and frozen in nitrogen until used. Frozen PBMC were rapidly thawed in a 37°C water bath and cultured in RPMI 1640 medium (Gibco, USA) containing 10% complement-inactivated fetal bovine serum (Invitrogen, USA) and 100 U/ml penicillin–100 μg/ml streptomycin (Invitrogen, USA) as described previously (51). Cell viability was >93% by trypan blue staining. PBMC were seeded in 96-well plates at 5 × 104 cells/well and then cultured with or without C. difficile wild-type toxins or GT-deficient toxins (100 ng/ml) for 24 h at 37°C in an incubator with 5% CO2. Cell viability was assessed by trypan blue staining, and fresh supernatants were harvested and analyzed by enzyme-linked immunosorbent assay (ELISA) using the same V-PLEX kit as that described above.
Statistical analysis.
Analysis was performed using GraphPad Prism software, version 5.0. Cytokine data are presented as means ± standard errors of the means (SEM). Before group comparisons, outliers were determined by Grubbs' test. A Fisher exact test then was used to assess data distribution. Significance was analyzed next using an unpaired t test if data were normally distributed or by performing a Mann-Whitney test if values were not normally distributed. A P value of less than 0.05 was considered statistically significant.
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health (R01AI088748, R01DK084509, RO1 AI 095256, U19 AI109776, R43AI129044, R01AI100914, R21DK096323, and DK56338).
We have no conflicts of interest to declare.
REFERENCES
- 1.Koon HW, Shih DQ, Hing TC, Yoo JH, Ho S, Chen X, Kelly CP, Targan SR, Pothoulakis C. 2013. Human monoclonal antibodies against Clostridium difficile toxins A and B inhibit inflammatory and histologic responses to the toxins in human colon and peripheral blood monocytes. Antimicrob Agents Chemother 57:3214–3223. doi: 10.1128/AAC.02633-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carter GP, Rood JI, Lyras D. 2010. The role of toxin A and toxin B in Clostridium difficile-associated disease: past and present perspectives. Gut Microbes 1:58–64. doi: 10.4161/gmic.1.1.10768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rupnik M, Wilcox MH, Gerding DN. 2009. Clostridium difficile infection: new developments in epidemiology and pathogenesis. Nat Rev Microbiol 7:526–536. doi: 10.1038/nrmicro2164. [DOI] [PubMed] [Google Scholar]
- 4.Olson A, Diebel LN, Liberati DM. 2013. Effect of host defenses on Clostridium difficile toxin-induced intestinal barrier injury. J Trauma Acute Care Surg 74:983–989. doi: 10.1097/TA.0b013e3182858477. [DOI] [PubMed] [Google Scholar]
- 5.Madan R, Guo X, Naylor C, Buonomo EL, Mackay D, Noor Z, Concannon P, Scully KW, Pramoonjago P, Kolling GL, Warren CA, Duggal P, Petri WA Jr. 2014. Role of leptin-mediated colonic inflammation in defense against Clostridium difficile colitis. Infect Immun 82:341–349. doi: 10.1128/IAI.00972-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kelly CP, Kyne L. 2011. The host immune response to Clostridium difficile. J Med Microbiol 60:1070–1079. doi: 10.1099/jmm.0.030015-0. [DOI] [PubMed] [Google Scholar]
- 7.El Feghaly RE, Stauber JL, Deych E, Gonzalez C, Tarr PI, Haslam DB. 2013. Markers of intestinal inflammation, not bacterial burden, correlate with clinical outcomes in Clostridium difficile infection. Clin Infect Dis 56:1713–1721. doi: 10.1093/cid/cit147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim H, Kokkotou E, Na X, Rhee SH, Moyer MP, Pothoulakis C, Lamont JT. 2005. Clostridium difficile toxin A-induced colonocyte apoptosis involves p53-dependent p21(WAF1/CIP1) induction via p38 mitogen-activated protein kinase. Gastroenterology 129:1875–1888. doi: 10.1053/j.gastro.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 9.Theriot CM, Koumpouras CC, Carlson PE, Bergin II, Aronoff DM, Young VB. 2011. Cefoperazone-treated mice as an experimental platform to assess differential virulence of Clostridium difficile strains. Gut Microbes 2:326–234. doi: 10.4161/gmic.19142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen SH, Gerding DN, Johnson S, Kelly CP, Loo VG, McDonald LC, Pepin J, Wilcox MH. 2010. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Contrl Hosp Epidemiol 31:431–455. doi: 10.1086/651706. [DOI] [PubMed] [Google Scholar]
- 11.Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. 2007. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis 45:302–307. doi: 10.1086/519265. [DOI] [PubMed] [Google Scholar]
- 12.Nelson RL, Kelsey P, Leeman H, Meardon N, Patel H, Paul K, Rees R, Taylor B, Wood E, Malakun R. 2011. Antibiotic treatment for Clostridium difficile-associated diarrhea in adults. Cochrane Database Syst Rev 9:CD004610. [DOI] [PubMed] [Google Scholar]
- 13.Musher DM, Aslam S, Logan N, Nallacheru S, Bhaila I, Borchert F, Hamill RJ. 2005. Relatively poor outcome after treatment of Clostridium difficile colitis with metronidazole. Clin Infect Dis 40:1586–1590. doi: 10.1086/430311. [DOI] [PubMed] [Google Scholar]
- 14.Czepiel J, Biesiada G, Brzozowski T, Ptak-Belowska A, Perucki W, Birczynska M, Jurczyszyn A, Strzalka M, Targosz A, Garlicki A. 2014. The role of local systemic cytokines in patients infected with Clostridium difficile. J Physiol Pharmacol 65:695–703. [PubMed] [Google Scholar]
- 15.Cowardin CA, Kuehne SA, Buonomo EL, Marie CS, Minton NP, Petri WA Jr. 2015. Inflammasome activation contributes to interleukin-23 production in response to Clostridium difficile. mBio 6:e02386-14. doi: 10.1128/mBio.02386-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee JY, Park HR, Oh YK, Kim YJ, Youn J, Han JS, Kim JM. 2007. Effects of transcription factor activator protein-1 on interleukin-8 expression and enteritis in response to Clostridium difficile toxin A. J Mol Med 85:1393–1404. doi: 10.1007/s00109-007-0237-7. [DOI] [PubMed] [Google Scholar]
- 17.Warny M, Keates AC, Keates S, Castagliuolo I, Zacks JK, Aboudola S, Qaman A, Pothoulakis C, LaMont JT, Kelly CP. 2000. p38 MAP kinase activation by Clostridium difficile toxin A mediates monocyte necrosis, IL-8 production, and enteritis. J Clin Investig 105:1147–1156. doi: 10.1172/JCI7545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jefferson KK, Smith MF Jr, Bobak DA. 1999. Roles of intracellular calcium and NF-kappa B in the Clostridium difficile toxin A-induced up-regulation and secretion of IL-8 from human monocytes. J Immunol 163:5183–5191. [PubMed] [Google Scholar]
- 19.Kim JM, Lee JY, Yoon YM, Oh YK, Youn J, Kim YJ. 2006. NF-kappa B activation pathway is essential for the chemokine expression in intestinal epithelial cells stimulated with Clostridium difficile toxin A. Scand J Immunol 63:453–460. doi: 10.1111/j.1365-3083.2006.001756.x. [DOI] [PubMed] [Google Scholar]
- 20.Garey KW, Jiang ZD, Ghantoji S, Tam VH, Arora V, Dupont HL. 2010. A common polymorphism in the interleukin-8 gene promoter is associated with an increased risk for recurrent Clostridium difficile infection. Clin Infect Dis 51:1406–1410. doi: 10.1086/657398. [DOI] [PubMed] [Google Scholar]
- 21.Jiang ZD, DuPont HL, Garey K, Price M, Graham G, Okhuysen P, Dao-Tran T, LaRocco M. 2006. A common polymorphism in the interleukin 8 gene promoter is associated with Clostridium difficile diarrhea. Am J Gastroenterol 101:1112–1116. doi: 10.1111/j.1572-0241.2006.00482.x. [DOI] [PubMed] [Google Scholar]
- 22.Steel JC, Waldmann TA, Morris JC. 2012. Interleukin-15 biology and its therapeutic implications in cancer. Trends Pharmacol Sci 33:35–41. doi: 10.1016/j.tips.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cruikshank WW, Center DM, Nisar N, Wu M, Natke B, Theodore AC, Kornfeld H. 1994. Molecular and functional analysis of a lymphocyte chemoattractant factor: association of biologic function with CD4 expression. Proc Natl Acad Sci U S A 91:5109–5113. doi: 10.1073/pnas.91.11.5109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Linevsky JK, Pothoulakis C, Keates S, Warny M, Keates AC, Lamont JT, Kelly CP. 1997. IL-8 release and neutrophil activation by Clostridium difficile toxin-exposed human monocytes. Am J Physiol 273:G13. [DOI] [PubMed] [Google Scholar]
- 25.Branka JE, Vallette G, Jarry A, Bou-Hanna C, Lemarre P, Van PN, Laboisse CL. 1997. Early functional effects of Clostridium difficile toxin A on human colonocytes. Gastroenterology 112:1887. doi: 10.1053/gast.1997.v112.pm9178681. [DOI] [PubMed] [Google Scholar]
- 26.Mahida YR, Makh S, Hyde S, Gray T, Borriello SP. 1996. Effect of Clostridium difficile toxin A on human intestinal epithelial cells: induction of interleukin 8 production and apoptosis after cell detachment. Gut 38:337. doi: 10.1136/gut.38.3.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu Z, Geboes K, Colpaert S, D'Haens GR, Rutgeerts P, Ceuppens JL. 2000. IL-15 is highly expressed in inflammatory bowel disease and regulates local T cell-dependent cytokine production. J Immunol 164:3608–3615. doi: 10.4049/jimmunol.164.7.3608. [DOI] [PubMed] [Google Scholar]
- 28.Seegert D, Rosenstiel P, Pfahler H, Pfefferkorn P, Nikolaus S, Schreiber S. 2001. Increased expression of IL-16 in inflammatory bowel disease. Gut 48:326–332. doi: 10.1136/gut.48.3.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keates AC, Castagliuolo I, Cruickshank WW, Qiu B, Arseneau KO, Brazer W, Kelly CP. 2000. Interleukin 16 is up-regulated in Crohn's disease and participates in TNBS colitis in mice. Gastroenterology 119:972–982. doi: 10.1053/gast.2000.18164. [DOI] [PubMed] [Google Scholar]
- 30.Steiner TS, Flores CA, Pizarro TT, Guerrant RL. 1997. Fecal lactoferrin, interleukin-1beta, and interleukin-8 are elevated in patients with severe Clostridium difficile colitis. Clin Diagn Lab Immunol 4:719–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ishida Y, Maegawa T, Kondo T, Kimura A, Iwakura Y, Nakamura S, Mukaida N. 2004. Essential involvement of IFN-gamma in Clostridium difficile toxin A-induced enteritis. J Immunol 172:3018–3025. doi: 10.4049/jimmunol.172.5.3018. [DOI] [PubMed] [Google Scholar]
- 32.Pawlowski SW, Calabrese G, Kolling GL, Platts-Mills J, Freire R, Alcantara Warren C, Liu B, Sartor RB, Guerrant RL. 2010. Murine model of Clostridium difficile infection with aged gnotobiotic C57BL/6 mice and a BI/NAP1 strain. J Infect Dis 202:1708–1712. doi: 10.1086/657086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kelly CP, Pothoulakis C, LaMont JT. 1994. Clostridium difficile colitis. N Engl J Med 330:257–262. doi: 10.1056/NEJM199401273300406. [DOI] [PubMed] [Google Scholar]
- 34.Mitchell TJ, Ketley JM, Haslam SC, Stephen J, Burdon DW, Candy DC, Daniel R. 1986. Effect of toxin A and B of Clostridium difficile on rabbit ileum and colon. Gut 27:78–85. doi: 10.1136/gut.27.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Libby JM, Jortner BS, Wilkins TD. 1982. Effects of the two toxins of Clostridium difficile in antibiotic-associated cecitis in hamsters. Infect Immun 36:822–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lonnroth I, Lange S. 1983. Toxin A of Clostridium difficile: production, purification and effect in mouse intestine. Acta Pathol Microbiol Immunol Scand B 91:395–400. [DOI] [PubMed] [Google Scholar]
- 37.von Eichel-Streiber C, Boquet P, Sauerborn M, Thelestam M. 1996. Large clostridial cytotoxins–a family of glycosyltransferases modifying small GTP-binding proteins. Trends Microbiol 4:375–382. doi: 10.1016/0966-842X(96)10061-5. [DOI] [PubMed] [Google Scholar]
- 38.Lee JY, Kim H, Cha MY, Park HG, Kim YJ, Kim IY, Kim JM. 2009. Clostridium difficile toxin A promotes dendritic cell maturation and chemokine CXCL2 expression through p38, IKK, and the NF-kappa B signaling pathway. J Mol Med (Berlin) 87:169–180. doi: 10.1007/s00109-008-0415-2. [DOI] [PubMed] [Google Scholar]
- 39.Rocha MF, Maia ME, Bezerra LR, Lyerly DM, Guerrant RL, Ribeiro RA, Lima AA. 1997. Clostridium difficile toxin A induces the release of neutrophil chemotactic factors from rat peritoneal macrophages: role of interleukin-1beta, tumor necrosis factor alpha, and leukotrienes. Infect Immun 65:2740–2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cowardin CA, Jackman BM, Noor Z, Burgess SL, Feig AL, Petri WA Jr. 2016. Glucosylation drives the innate inflammatory response to Clostridium difficile toxin A. Infect Immun 84:2317–2323. doi: 10.1128/IAI.00327-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ryder AB, Huang Y, Li H, Zheng M, Wang X, Stratton CW, Xu X, Tang YW. 2010. Assessment of Clostridium difficile infections by quantitative detection of tcdB toxin by use of a real-time cell analysis system. J Clin Microbiol 48:4129–4134. doi: 10.1128/JCM.01104-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Voth DE, Ballard JD. 2005. Clostridium difficile toxins: mechanism of action and role in disease. Clin Microbiol Rev 18:247–263. doi: 10.1128/CMR.18.2.247-263.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang Z, Zhang Y, Huang T, Feng H. 2015. Glucosyltransferase activity of Clostridium difficile toxin B is essential for disease pathogenesis. Gut Microbes 6:221–224. doi: 10.1080/19490976.2015.1062965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim MN, Koh SJ, Kim JM, Im JP, Jung HC, Kim JS. 2014. Clostridium difficile infection aggravates colitis in interleukin 10-deficient mice. World J Gastroenterol 20:17084–17091. doi: 10.3748/wjg.v20.i45.17084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu H, Yang J, Gao W, Li L, Li P, Zhang L, Gong YN, Peng X, Xi JJ, Chen S, Wang F, Shao F. 2014. Innate immune sensing of bacterial modifications of Rho GTPases by the Pyrin inflammasome. Nature 513:237–241. doi: 10.1038/nature13449. [DOI] [PubMed] [Google Scholar]
- 46.Ng J, Hirota SA, Gross O, Li Y, Ulke-Lemee A, Potentier MS, Schenck LP, Vilaysane A, Seamone ME, Feng H, Armstrong GD, Tschopp J, Macdonald JA, Muruve DA, Beck PL. 2010. Clostridium difficile toxin-induced inflammation and intestinal injury are mediated by the inflammasome. Gastroenterology 139:542–552. doi: 10.1053/j.gastro.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 47.Madan R, Petri WA Jr. 2012. Immune responses to Clostridium difficile infection. Trends Mol Med 18:658–666. doi: 10.1016/j.molmed.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hansen A, Alston L, Tulk SE, Schenck LP, Grassie ME, Alhassan BF, Veermalla AT, Al-Bashir S, Gendron FP, Altier C, MacDonald JA, Beck PL, Hirota SA. 2013. The P2Y6 receptor mediates Clostridium difficile toxin-induced CXCL8/IL-8 production and intestinal epithelia barrier dysfunction. PLoS One 8:e81491. doi: 10.1371/journal.pone.0081491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang G, Zhou B, Wang J, He X, Sun X, Nie W, Tzipori S, Feng H. 2008. Expression of recombinant Clostridium difficile toxin A and B in Bacillus megaterium. BMC Microbiol 8:192. doi: 10.1186/1471-2180-8-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang H, Sun X, Zhang Y, Li S, Chen K, Shi L, Nie W, Kumar R, Tzipori S, Wang J, Savidge T, Feng H. 2012. A chimeric toxin vaccine protects against primary and recurrent Clostridium difficile infection. Infect Immun 80:2679–2688. doi: 10.1128/IAI.00215-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Katial RK, Sachanandani D, Pinney C, Lieberman MM. 1998. Cytokine production in cell culture by peripheral blood mononuclear cells from immunocompetent host. Clin Diagn Lab Immunol 5:78–81. [DOI] [PMC free article] [PubMed] [Google Scholar]