Abstract
Many cancers, including those of the colon, lung, and pancreas, depend upon the signaling pathways induced by mutated and constitutively active Ras. The molecular scaffolds Kinase Suppressor of Ras 1 and 2 (KSR1 and KSR2) play potent roles in promoting Ras-mediated signaling through the Raf/MEK/ERK kinase cascade. Here we summarize the canonical role of KSR in cells, including its central role as a scaffold protein for the Raf/MEK/ERK kinase cascade, its regulation of various cellular pathways mediated through different binding partners, and the phenotypic consequences of KSR1 or KSR2 genetic inactivation. Mammalian KSR proteins have a demonstrated role in cellular and organismal energy balance with implications for cancer and obesity. Targeting KSR1 in cancer using small molecule inhibitors has potential for therapy with reduced toxicity to the patient. RNAi and small molecule screens using KSR1 as a reference standard have the potential to expose and target vulnerabilities in cancer. Interestingly, although KSR1 and KSR2 are similar in structure, KSR2 has a distinct physiological role in regulating energy balance. Although KSR proteins have been studied for two decades, additional analysis is required to elucidate both the regulation of these molecular scaffolds and their potent effect on the spatial and temporal control of ERK activation in health and disease.
Keywords: ERK Signalling, Kinase Suppressor of Ras, KSR
Introduction
The three Ras genes ( K-Ras, H-Ras, and N-Ras) regulate diverse cellular functions through multiple pathways and are also commonly mutated in human cancer to yield constitutively active small GTPases. Activating Ras mutations are found in approximately 25% of human tumors, though these three small GTPases are not mutated at equivalent frequencies in cancer. A total of 85% of Ras-driven cancers have activating mutations in K-Ras, while N-Ras and H-Ras are mutated in 12% and 3%, respectively, of these cancers ( http://cancer.sanger.ac.uk/cosmic). Ras mutations are most common in pancreatic ductal adenocarcinomas (95%), colorectal adenocarcinomas (52%), and lung adenocarcinomas (31%). Intensive analysis revealed that multiple effectors with Ras-binding domains (or Ras association domains) were capable of interacting with the Ras effector loop and mediating its biological effects 1. Observations that activating Ras and Raf mutations are typically mutually exclusive 2– 4, and that only components of the Raf/MEK/ERK pathway rescue growth in “Rasless” mouse embryo fibroblasts (MEFs) 5, suggest that the interaction of Ras with Raf, and the activation of MEK1/2 and ERK1/2, may be most critical to Ras-driven cancers.
Kinase Suppressor of Ras 1 (KSR1) interacts with Raf, MEK, and ERK 6– 12, mediates ERK activation and signaling in a dose-dependent fashion (discussed in greater detail below), and is essential for the transformation of MEFs by oncogenic Ras 8, 13. These discoveries revealed a critical role played by this molecular scaffold in transformation and tumorigenesis. However, KSR1 –/– mice are fertile and show inconsequential developmental alterations 12, 14. These observations suggest that KSR1 may play a prominent role in cancers that are dependent upon Ras and ERK signaling and that it might be exploited therapeutically with minimal toxicity to the patient. Here we review the biochemistry and biology of KSR1 and its paralog, KSR2, and discuss their potential as therapeutic targets.
The role of KSR proteins in the Raf/MEK/ERK cascade
A single ksr gene was identified as necessary for the rough-eye phenotype of activated Ras in Drosophila 15. Two ksr genes ( ksr1 and ksr2) are expressed in Caenorhabditis elegans 16, 17 and mammals 8, 15, 17– 20. KSR1 and KSR2 proteins facilitate Raf phosphorylation of MEK, leading to increased ERK activation in response to Ras activation or calcium influx 8, 9, 12, 15, 21– 25. KSR proteins have properties expected of molecular scaffolds 6– 12. As expected of true scaffolds, increasing KSR1 allows for increased ERK activation until KSR1 reaches an optimal level. Surprisingly, in MEFs the level of KSR1 that maximizes ERK activation and signaling is approximately 12 times the endogenous level of expression. Further increasing KSR1 causes a decrease in ERK activation because the cellular concentration of KSR1 exceeds the amount of scaffold that can coordinate signaling with Raf, MEK, and ERK 8, 26. This suggests that overexpression of KSR1 sequesters individual components of the MAPK cascade such that they are unable to interact, which reduces MAPK signaling. However, overexpressing additional individual components of the MAPK pathway can suppress the inhibitory effects of scaffold excess 9. This observation likely explains why early studies in which ectopic KSR1 was overexpressed suggested that KSR1 inhibited Ras-driven transformation 27– 30. Importantly, the level of KSR1 expression that optimizes ERK activation is the same level that maximizes the transforming activity of oncogenic Ras and the proliferative effects of growth factors 8.
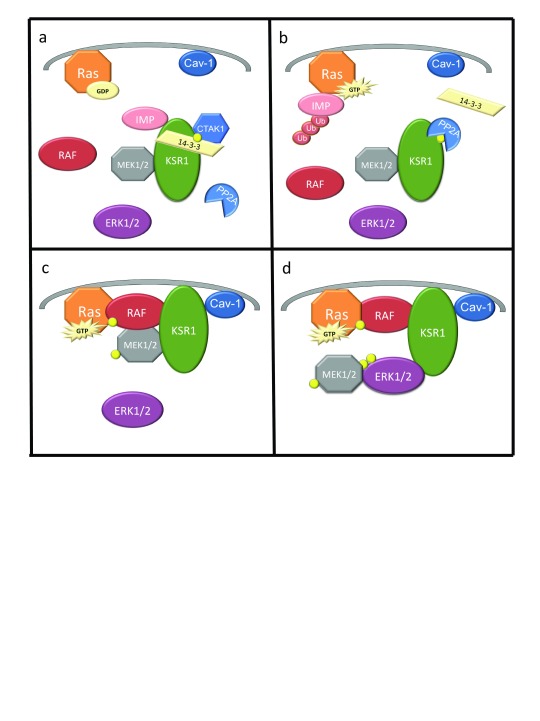
Phosphorylation sites and determinants of protein–protein interaction have been mapped extensively on KSR1 and KSR2 and have been shown to regulate KSR1 in part through subcellular localization 31– 37. Analysis of these phosphorylation sites and interactions with effectors and modifiers suggest dynamic regulation of KSR1 and its scaffold function. Interaction with the E3 ligase IMP promotes the redistribution of KSR1 to Triton-resistant punctate structures that sequester KSR1 and impair ERK activation 38, 39. Phosphorylation of KSR1 on Ser297 and Ser392 (Ser310 and Ser469 in KSR2) by the kinase C-TAK1 (MARK3) creates a 14-3-3 binding site that anchors KSR1 within this subcellular compartment ( Figure 1a) 13, 18, 25, 28, 38, 40– 42. Ras activation catalyzes IMP autopolyubiquitylation and proteasomal destruction ( Figure 1b) 39. Stimuli that promote IMP degradation also promote the dephosphorylation of KSR1 at Ser392 by PP2A, which eliminates 14-3-3 binding ( Figure 1b) 35, 41, 43. Calcineurin dephosphorylates 14-3-3 binding sites on KSR2 25. These events promote the redistribution of KSR1 to the plasma membrane, facilitating the activation of MEK by Raf ( Figure 1c). MEK is bound to KSR1 in the absence of Ras activation 44. Though identified as a loss-of-function mutation on KSR1 in C. elegans 15, 16, 42, the mutation of KSR1 at Cys809 to tyrosine (C809Y) enhances the activation of ERK in mammalian cells 13. These observations suggest that KSR proteins may sequester MEK in an inactivated state and present MEK for phosphorylation by Raf 21, 45. In this model, MEK does not need to be in complex with KSR1 to phosphorylate and activate ERK. However, KSR1 encodes a DEF domain 46, which is essential to KSR1-mediated ERK interaction and critical for competent signal transduction 13, 34. KSR1 (but not KSR2) also encodes a caveolin-binding domain 47, which is required for binding to caveolin-1, localization to the plasma membrane, and ERK activation ( Figure 1c and 1d) 47, 48. Another level of KSR1 regulation exists in its degradation. Recently, it has been shown that KSR1 is polyubiquitinated by praja2, which promotes KSR1 degradation, causing a decrease in ERK signaling 49. Reconciling these observations implies that KSR1 coordinates a dynamic mechanism that provides spatial and temporal control of signaling through the Raf/MEK/ERK kinase cascade.
Figure 1. KSR1 dynamically regulates the Raf/MEK/ERK kinase cascade.
( a) KSR1 is constitutively bound to MEK1/2 and IMP. C-TAK1 phosphorylates (yellow circle) KSR1 at Ser392, allowing for 14-3-3 binding and cytoplasmic localization of KSR1. ( b) Upon Ras activation and GTP binding, IMP dissociates from KSR1, binds Ras, autoubiquitylates, and is degraded. PP2A dephosphorylates KSR1 at Ser392, destroying the 14-3-3 binding site anchoring KSR1. ( c) KSR1 and MEK1/2 translocate to the plasma membrane, where KSR1 interacts with Raf and MEK1/2 is phosphorylated and activated. ( d) MEK1/2 dissociates from KSR1 and ERK1/2 is phosphorylated and associates with KSR1, facilitating signaling. Cav-1, caveolin-1; C-TAK1, Cdc25C-associated kinase 1; ERK, extracellular signal-regulated protein kinase; GTP, guanosine triphosphate; IMP, impedes mitogenic signal propagation; KSR1, Kinase Suppressor of Ras 1; MEK, mitogen-activated protein/extracellular signal-regulated protein kinase kinase; PP2A, protein phosphatase 2.
Regulation of MEK and ERK by KSR and Raf heterodimers
The dimerization of Raf proteins is an essential step in wild-type Raf activation. KSR and Raf proteins share homology within the region required for Raf dimerization, and KSR has been shown to form heterodimers with Raf, particularly B-Raf 24, 36, 50. Based on modeling, KSR dimerization with Raf induces a conformational change in KSR that induces the exposure of the MEK activation loop and facilitates its phosphorylation 24. However, the dimerization of KSR and Raf orients the Raf protein such that the catalytic site of Raf is not in close proximity to its phosphorylation target site on MEK. Therefore, MEK phosphorylation must be completed by another Raf protein 24, 36. KSR2 also forms homodimers through a side-to-side interface that is specifically dependent upon Arg718 36. Mutations at this site suppress Ras signaling, suggesting that the dimerization of KSR proteins is required to promote Ras signaling 24. This observation is consistent with results showing that mutations inhibiting the KSR-Raf heterodimerization decrease Raf activity 36. Additionally, there is potential for KSR1 to directly activate ERK or BRAF and CRAF if Y552 is phosphorylated, as the KSR1 Y552D mutant demonstrated this ability 51. The functional role of KSR homodimers is still incompletely understood, but the ability of IMP to inhibit MEK activation by Raf has been suggested to result from IMP-mediated disruption of KSR1 homodimers and B-Raf/c-Raf heterodimers 52.
Phenotypic effects of KSR1/2 genetic inactivation
Genetic studies in model organisms demonstrate that KSR proteins promote Ras signaling 15– 17. Heterozygous loss of ksr in Drosophila suppresses Ras G12V signaling and prevents the rough-eye phenotype caused by constitutive Ras signaling 15, 28. Similarly, loss-of-function mutations in ksr1 suppressed the multiple vulva phenotype of activated Ras in C. elegans 43. KSR1 plays a similar role in mammals. Apart from minor deficits, ksr1 –/– knockout mice are fertile and developmentally normal. Ksr1 –/– mice have hair follicle defects similar to the phenotype of egfr –/– mice, supporting the suggestion that these proteins function within the same pathway 12, 14, 53. As a result of reduced ERK signaling, ksr1 –/– mice have a marginally impaired immunological response 6, 12, 54, 55. The most profound and translationally significant phenotype of ksr1 –/– mice is resistance to Ras-dependent tumor formation. Skin tumor induction by v-Ha-Ras is lost in ksr1 –/– mice 14, and mammary tumor burden is markedly reduced by KSR1 disruption in mice expressing transgenic polyomavirus Middle T-Antigen 12. These observations demonstrate that KSR modulates Ras signaling in vivo, but it is largely dispensable for normal cell survival. The requirement for KSR1 in Ras-driven tumor formation, but not normal development, reveals KSR1 as a potential target for therapeutic intervention.
In contrast to the mild phenotype of ksr1 –/– mice, ksr2 –/– mice have reduced fertility and become spontaneously obese 56– 59. Pathways regulating adaptive thermogenesis, metabolic rate, and leptin-sensitive food consumption are implicated in KSR2-dependent energy balance 56– 60. Consistent with observations in the knockout mice, humans with ksr2 mutations show severe early onset obesity 60. Ksr2 variants in humans that impair Ras signaling or inhibit KSR2 interaction with AMPK also disrupt glucose metabolism and fatty acid oxidation 60. Interestingly, KSR2 is almost exclusively expressed in the brain and pituitary 19, 58. Brain-specific disruption of KSR2 is sufficient to cause obesity and glucose intolerance in mice, though it does not perfectly recapitulate the phenotype of ksr2 –/– mice 19. These observations show that KSR2 function in the brain plays a potent role in the regulation of energy balance.
Structural properties of KSR proteins
KSR1 and KSR2 proteins are highly conserved in invertebrates and mammals 9, 15. KSR proteins are structurally related to Raf proteins in five conserved areas, CA1–CA5 15. CA1 is located on the N-terminus end. It contains 40 amino acids that contribute to B-Raf binding by KSR1 and encode coiled-coil and sterile-α-motif (SAM) structures that promote KSR1 membrane association 13, 34, 44, 61. CA2 is a proline-rich region without known function. A region in KSR2 between CA2 and CA3 is required for KSR interaction with AMPK, and mutations in this region inhibit this interaction 19, 31, 58. CA3 is a cysteine-rich region containing an atypical C1 motif homologous to the cysteine-rich CR1 region in Raf that also contributes to KSR1 membrane localization 21, 62. CA3 mediates the membrane localization of KSR by recruiting phospholipids but does not react to phorbol esters or ceramide or interact directly with Ras 63. CA4 is a serine/threonine-rich region that mediates interactions with ERK through an FXFP motif 32. This interaction is not constitutive but requires Ras activation 21, 33, 42, 61, 63. The CA5 domain in KSR proteins encodes a kinase (or pseudokinase) domain highly homologous to Raf family CR3 kinase domains 15, 17. Mammalian KSR proteins contain multiple alterations in amino acids typically required for catalytic activity including arginine in place of the lysine that coordinates the gamma phosphate of ATP 15, 61. Substantial effort has been exerted to clarify if KSR can or does phosphorylate cellular substrates and, if so, whether or not this activity contributes to the downstream effects of KSR 21, 24, 37, 64. KSR1 substrates and the biological relevance of any residual phosphotransferase activity have yet to be validated.
The CA5 region contributes to KSR interaction with MEK in both quiescent and growth factor-activated cells 31, 44, 65. Amino acid substitutions within the CA5 region that diminish interaction with MEK also reduce ERK signaling 15– 17, 44, 65. However, these alterations are within or near the ATP-binding domain and may disrupt ATP binding, potentially affecting interaction with MEK secondarily. The CA5 domain also interacts with Raf, but the mechanism is incompletely understood 34. Thus, there may be unidentified dynamic interactions between the CA1 and CA5 domains of KSR proteins and B-Raf that regulate Raf kinase activation, MEK phosphorylation, and signal transduction through the kinase cascade.
KSR proteins as targets for therapy
Given the importance of KSR1 in modulating signaling through the Raf/MEK/ERK kinase cascade in tumor cells and observations that ksr1 –/– mice develop with only inconsequential phenotypic differences, targeting KSR1 or KSR1-dependent signaling pathways in Ras-driven cancers may selectively target cancer cells with reduced toxicity to patients. Supporting this strategy, RNAi approaches depleting cancer cells of KSR1 in vitro and in vivo caused a decrease in tumor growth. Continuous infusion of phosphorothioate antisense oligonucleotides targeting KSR1 mRNA also caused regression of established tumors and inhibited metastases without overt toxicity in Ras-driven PANC-1 pancreatic and A549 non-small-cell lung cancer xenografts 66.
Mutations in KSR that suppress signaling by activated Ras are often adjacent to the ATP-binding pocket 15– 17. Furthermore, KSR1 binds ATP 65, and mutations that prevent that binding impair ERK activation 67. These observations suggest that manipulation of the ATP-binding cleft in KSR1 may be therapeutically effective. The recently discovered small molecule APS-2-79 binds and stabilizes KSR kinase domains in an inactive conformation observed when the KSR2 kinase domain is bound to MEK1 and ATP 24, interferes with KSR:Raf heterodimerization, and inhibits oncogenic Ras signaling 50. The effect of APS-2-79 was also lost when KSR was mutated within the active site (A690F) such that KSR can promote MEK phosphorylation even in the absence of ATP binding. APS-2-79 modestly decreased cell viability in two Ras-mutated cancer cell lines (HCT116 and A549) and did not affect Raf-mutated cancer cells (A375 and SK-MEL-293) but did demonstrate substantial synergy in Ras-mutated cancer cells with MEK inhibitors, suggesting the potential to target both kinase and scaffolding components of the Ras signaling pathway in Ras-dependent cancers 50.
The observation that KSR1 expression was required for tumor-dependent ERK signaling, but not normal development, suggested the possibility that effectors of KSR1-dependent signaling pathways in tumor cells might reveal additional putative targets for cancer therapy that preferentially support tumor cell growth and viability. A gene expression high-throughput screen termed Functional Signature Ontology (FUSION) was developed to detect effectors of KSR1-dependent signaling in Ras-driven tumors and identify small molecules that can target those effectors 68. Recent results from FUSION detected hits that mediate KSR1-dependent signals and promote the viability of human colon tumor cells but have no similar role on non-transformed human colon epithelial cells 69, 70. These observations suggest the existence of multiple effectors that may be used to support the survival in tumor cells in a manner distinct from their role in normal tissue. Careful validation of these potential targets may yield additional therapeutic strategies.
Conclusions
KSR proteins are established as scaffolds for the Raf/MEK/ERK kinase cascade, though a detailed understanding of KSR-dependent temporal and spatial control of ERK signaling is still lacking. The physiology regulated by KSR1 and KSR2 is also incompletely understood, though disruption of KSR2 in mice and its mutation in humans show that it plays an important role in mammalian energy balance. Given the minimal phenotype of ksr1 –/– mice, the normal function of KSR1 is unclear. However, KSR1 is demonstrably physiologically important as ksr1 –/– ksr2 –/– mice do not survive beyond 21 days of age (Costanzo-Garvey and Lewis, unpublished results), while the single gene knockouts survive into adulthood. KSR1 and KSR2 are primarily expressed in brain, though KSR1 is expressed at lower levels throughout the body. Thus, the most profound function of KSR proteins is likely found in the central nervous system. The obesity phenotype caused by brain-specific knockout of KSR2 demonstrates its potent role in cell non-autonomous regulation of energy balance. However, the role of KSR2 as a scaffold and its regulation of ERK signaling in support of brain-regulated development and maintenance of metabolism are unknown. Relevant in vitro cell models that express both KSR1 and KSR2 will be crucial to further our understanding of the cell biology controlled by these molecular scaffolds.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Andrey Shaw, Department of Research Biology, Genentech, South San Francisco, USA
Melanie H. Cobb, Department of Pharmacology, Harold C. Simmons Comprehensive Cancer Center, University of Texas Southwestern Medical Center, Dallas, TX, USA
Aroon Karra, Department of Pharmacology, University of Texas Southwestern Medical Center at Dallas, Dallas, Texas, USA
Courtney Powell, Department of Pharmacology, University of Texas Southwestern Medical Center at Dallas, Dallas, Texas, USA
Funding Statement
Our research is supported by National Cancer Institute (P30 CA036727, R01 CA157774) and National Institute of General Medical Sciences (P20 GM104320)
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; referees: 2 approved]
References
- 1. Cox AD, Der CJ: Ras history: The saga continues. Small GTPases. 2010;1(1):2–27. 10.4161/sgtp.1.1.12178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Witkiewicz AK, Balaji U, Eslinger C, et al. : Integrated Patient-Derived Models Delineate Individualized Therapeutic Vulnerabilities of Pancreatic Cancer. Cell Rep. 2016;16(7):2017–31. 10.1016/j.celrep.2016.07.023 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 3. Foster SA, Whalen DM, Özen A, et al. : Activation Mechanism of Oncogenic Deletion Mutations in BRAF, EGFR, and HER2. Cancer Cell. 2016;29(4):477–93. 10.1016/j.ccell.2016.02.010 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 4. Chen SH, Zhang Y, Van Horn RD, et al. : Oncogenic BRAF Deletions That Function as Homodimers and Are Sensitive to Inhibition by RAF Dimer Inhibitor LY3009120. Cancer Discov. 2016;6(3):300–15. 10.1158/2159-8290.CD-15-0896 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 5. Drosten M, Dhawahir A, Sum EY, et al. : Genetic analysis of Ras signalling pathways in cell proliferation, migration and survival. EMBO J. 2010;29(6):1091–104. 10.1038/emboj.2010.7 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 6. Liu L, Channavajhala PL, Rao VR, et al. : Proteomic characterization of the dynamic KSR-2 interactome, a signaling scaffold complex in MAPK pathway. Biochim Biophys Acta. 2009;1794(10):1485–95. 10.1016/j.bbapap.2009.06.016 [DOI] [PubMed] [Google Scholar]
- 7. Kortum RL, Costanzo DL, Haferbier J, et al. : The molecular scaffold kinase suppressor of Ras 1 (KSR1) regulates adipogenesis. Mol Cell Biol. 2005;25(17):7592–604. 10.1128/MCB.25.17.7592-7604.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kortum RL, Lewis RE: The molecular scaffold KSR1 regulates the proliferative and oncogenic potential of cells. Mol Cell Biol. 2004;24(10):4407–16. 10.1128/MCB.24.10.4407-4416.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Roy F, Laberge G, Douziech M, et al. : KSR is a scaffold required for activation of the ERK/MAPK module. Genes Dev. 2002;16(4):427–38. 10.1101/gad.962902 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 10. Raabe T, Rapp UR: KSR--a regulator and scaffold protein of the MAPK pathway. Sci STKE. 2002;2002(136):pe28. 10.1126/stke.2002.136.pe28 [DOI] [PubMed] [Google Scholar]
- 11. Clapéron A, Therrien M: KSR and CNK: two scaffolds regulating RAS-mediated RAF activation. Oncogene. 2007;26(22):3143–58. 10.1038/sj.onc.1210408 [DOI] [PubMed] [Google Scholar]
- 12. Nguyen A, Burack WR, Stock JL, et al. : Kinase suppressor of Ras (KSR) is a scaffold which facilitates mitogen-activated protein kinase activation in vivo. Mol Cell Biol. 2002;22(9):3035–45. 10.1128/MCB.22.9.3035-3045.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kortum RL, Johnson HJ, Costanzo DL, et al. : The molecular scaffold kinase suppressor of Ras 1 is a modifier of Ras V12-induced and replicative senescence. Mol Cell Biol. 2006;26(6):2202–14. 10.1128/MCB.26.6.2202-2214.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 14. Lozano J, Xing R, Cai Z, et al. : Deficiency of kinase suppressor of Ras1 prevents oncogenic ras signaling in mice. Cancer Res. 2003;63(14):4232–8. [PubMed] [Google Scholar]
- 15. Therrien M, Chang HC, Solomon NM, et al. : KSR, a novel protein kinase required for RAS signal transduction. Cell. 1995;83(6):879–88. 10.1016/0092-8674(95)90204-X [DOI] [PubMed] [Google Scholar]
- 16. Kornfeld K, Hom DB, Horvitz HR: The ksr-1 gene encodes a novel protein kinase involved in Ras-mediated signaling in C. elegans. Cell. 1995;83(6):903–13. 10.1016/0092-8674(95)90206-6 [DOI] [PubMed] [Google Scholar]
- 17. Sundaram M, Han M: The C. elegans ksr-1 gene encodes a novel Raf-related kinase involved in Ras-mediated signal transduction. Cell. 1995;83(6):889–901. 10.1016/0092-8674(95)90205-8 [DOI] [PubMed] [Google Scholar]
- 18. Guo L, Volle DJ, Lewis RE: Identification of a truncated kinase suppressor of Ras 2 mRNA in sperm. FEBS Open Bio. 2014;4(1):420–5. 10.1016/j.fob.2014.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Guo L, Costanzo-Garvey DL, Smith DR, et al. : Kinase Suppressor of Ras 2 (KSR2) expression in the brain regulates energy balance and glucose homeostasis. Mol Metab. 2017;6(2):194–205. 10.1016/j.molmet.2016.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Downward J: KSR: a novel player in the RAS pathway. Cell. 1995;83(6):831–4. 10.1016/0092-8674(95)90198-1 [DOI] [PubMed] [Google Scholar]
- 21. Michaud NR, Therrien M, Cacace A, et al. : KSR stimulates Raf-1 activity in a kinase-independent manner. Proc Natl Acad Sci U S A. 1997;94(24):12792–6. 10.1073/pnas.94.24.12792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Udell CM, Rajakulendran T, Sicheri F, et al. : Mechanistic principles of RAF kinase signaling. Cell Mol Life Sci. 2011;68(4):553–65. 10.1007/s00018-010-0520-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Razidlo GL, Kortum RL, Haferbier JL, et al. : Phosphorylation regulates KSR1 stability, ERK activation, and cell proliferation. J Biol Chem. 2004;279(46):47808–14. 10.1074/jbc.M406395200 [DOI] [PubMed] [Google Scholar]
- 24. Brennan DF, Dar AC, Hertz NT, et al. : A Raf-induced allosteric transition of KSR stimulates phosphorylation of MEK. Nature. 2011;472(7343):366–9. 10.1038/nature09860 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 25. Dougherty MK, Ritt DA, Zhou M, et al. : KSR2 is a calcineurin substrate that promotes ERK cascade activation in response to calcium signals. Mol Cell. 2009;34(6):652–62. 10.1016/j.molcel.2009.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 26. Levchenko A, Bruck J, Sternberg PW: Scaffold proteins may biphasically affect the levels of mitogen-activated protein kinase signaling and reduce its threshold properties. Proc Natl Acad Sci U S A. 2000;97(11):5818–23. 10.1073/pnas.97.11.5818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sugimoto T, Stewart S, Han M, et al. : The kinase suppressor of Ras (KSR) modulates growth factor and Ras signaling by uncoupling Elk-1 phosphorylation from MAP kinase activation. EMBO J. 1998;17(6):1717–27. 10.1093/emboj/17.6.1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Joneson T, Fulton JA, Volle DJ, et al. : Kinase suppressor of Ras inhibits the activation of extracellular ligand-regulated (ERK) mitogen-activated protein (MAP) kinase by growth factors, activated Ras, and Ras effectors. J Biol Chem. 1998;273(13):7743–8. 10.1074/jbc.273.13.7743 [DOI] [PubMed] [Google Scholar]
- 29. Yu W, Fantl WJ, Harrowe G, et al. : Regulation of the MAP kinase pathway by mammalian Ksr through direct interaction with MEK and ERK. Curr Biol. 1998;8(1):56–64. 10.1016/S0960-9822(98)70020-X [DOI] [PubMed] [Google Scholar]
- 30. Denouel-Galy A, Douville EM, Warne PH, et al. : Murine Ksr interacts with MEK and inhibits Ras-induced transformation. Curr Biol. 1998;8(1):46–55. 10.1016/S0960-9822(98)70019-3 [DOI] [PubMed] [Google Scholar]
- 31. Cacace AM, Michaud NR, Therrien M, et al. : Identification of constitutive and ras-inducible phosphorylation sites of KSR: implications for 14-3-3 binding, mitogen-activated protein kinase binding, and KSR overexpression. Mol Cell Biol. 1999;19(1):229–40. 10.1128/MCB.19.1.229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fantz DA, Jacobs D, Glossip D, et al. : Docking sites on substrate proteins direct extracellular signal-regulated kinase to phosphorylate specific residues. J Biol Chem. 2001;276(29):27256–65. 10.1074/jbc.M102512200 [DOI] [PubMed] [Google Scholar]
- 33. Jacobs D, Glossip D, Xing H, et al. : Multiple docking sites on substrate proteins form a modular system that mediates recognition by ERK MAP kinase. Genes Dev. 1999;13(2):163–75. 10.1101/gad.13.2.163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McKay MM, Ritt DA, Morrison DK, et al. : Signaling dynamics of the KSR1 scaffold complex. Proc Natl Acad Sci U S A. 2009;106(27):11022–7. 10.1073/pnas.0901590106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ory S, Zhou M, Conrads TP, et al. : Protein phosphatase 2A positively regulates Ras signaling by dephosphorylating KSR1 and Raf-1 on critical 14-3-3 binding sites. Curr Biol. 2003;13(16):1356–64. 10.1016/S0960-9822(03)00535-9 [DOI] [PubMed] [Google Scholar]
- 36. Rajakulendran T, Sahmi M, Lefrançois M, et al. : A dimerization-dependent mechanism drives RAF catalytic activation. Nature. 2009;461(7263):542–5. 10.1038/nature08314 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 37. Volle DJ, Fulton JA, Chaika OV, et al. : Phosphorylation of the kinase suppressor of ras by associated kinases. Biochemistry. 1999;38(16):5130–7. 10.1021/bi983050d [DOI] [PubMed] [Google Scholar]
- 38. Morrison DK, Davis RJ: Regulation of MAP kinase signaling modules by scaffold proteins in mammals. Annu Rev Cell Dev Biol. 2003;19:91–118. 10.1146/annurev.cellbio.19.111401.091942 [DOI] [PubMed] [Google Scholar]
- 39. Matheny SA, Chen C, Kortum RL, et al. : Ras regulates assembly of mitogenic signalling complexes through the effector protein IMP. Nature. 2004;427(6971):256–60. 10.1038/nature02237 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 40. Xing H, Kornfeld K, Muslin AJ: The protein kinase KSR interacts with 14-3-3 protein and Raf. Curr Biol. 1997;7(5):294–300. 10.1016/S0960-9822(06)00152-7 [DOI] [PubMed] [Google Scholar]
- 41. Müller J, Ory S, Copeland T, et al. : C-TAK1 regulates Ras signaling by phosphorylating the MAPK scaffold, KSR1. Mol Cell. 2001;8(5):983–93. 10.1016/S1097-2765(01)00383-5 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 42. Therrien M, Michaud NR, Rubin GM, et al. : KSR modulates signal propagation within the MAPK cascade. Genes Dev. 1996;10(21):2684–95. 10.1101/gad.10.21.2684 [DOI] [PubMed] [Google Scholar]
- 43. Yoder JH, Chong H, Guan KL, et al. : Modulation of KSR activity in Caenorhabditis elegans by Zn ions, PAR-1 kinase and PP2A phosphatase. EMBO J. 2004;23(1):111–9. 10.1038/sj.emboj.7600025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Stewart S, Sundaram M, Zhang Y, et al. : Kinase suppressor of Ras forms a multiprotein signaling complex and modulates MEK localization. Mol Cell Biol. 1999;19(8):5523–34. 10.1128/MCB.19.8.5523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Koveal D, Schuh-Nuhfer N, Ritt D, et al. : A CC-SAM, for coiled coil-sterile α motif, domain targets the scaffold KSR-1 to specific sites in the plasma membrane. Sci Signal. 2012;5(255):ra94. 10.1126/scisignal.2003289 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 46. Shin S, Dimitri CA, Yoon SO, et al. : ERK2 but not ERK1 induces epithelial-to-mesenchymal transformation via DEF motif-dependent signaling events. Mol Cell. 2010;38(1):114–27. 10.1016/j.molcel.2010.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 47. Kortum RL, Fernandez MR, Costanzo-Garvey DL, et al. : Caveolin-1 is required for kinase suppressor of Ras 1 (KSR1)-mediated extracellular signal-regulated kinase 1/2 activation, H-Ras V12-induced senescence, and transformation. Mol Cell Biol. 2014;34(18):3461–72. 10.1128/MCB.01633-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fernandez MR, Henry MD, Lewis RE: Kinase suppressor of Ras 2 (KSR2) regulates tumor cell transformation via AMPK. Mol Cell Biol. 2012;32(18):3718–31. 10.1128/MCB.06754-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rinaldi L, Delle Donne R, Sepe M, et al. : praja2 regulates KSR1 stability and mitogenic signaling. Cell Death Dis. 2016;7(5):e2230. 10.1038/cddis.2016.109 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 50. Dhawan NS, Scopton AP, Dar AC: Small molecule stabilization of the KSR inactive state antagonizes oncogenic Ras signalling. Nature. 2016;537(7618):112–6. 10.1038/nature19327 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 51. Hu J, Stites EC, Yu H, et al. : Allosteric activation of functionally asymmetric RAF kinase dimers. Cell. 2013;154(5):1036–46. 10.1016/j.cell.2013.07.046 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 52. Chen C, Lewis RE, White MA: IMP modulates KSR1-dependent multivalent complex formation to specify ERK1/2 pathway activation and response thresholds. J Biol Chem. 2008;283(19):12789–96. 10.1074/jbc.M709305200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hansen LA, Alexander N, Hogan ME, et al. : Genetically null mice reveal a central role for epidermal growth factor receptor in the differentiation of the hair follicle and normal hair development. Am J Pathol. 1997;150(6):1959–75. [PMC free article] [PubMed] [Google Scholar]
- 54. Fusello AM, Mandik-Nayak L, Shih F, et al. : The MAPK scaffold kinase suppressor of Ras is involved in ERK activation by stress and proinflammatory cytokines and induction of arthritis. J Immunol. 2006;177(9):6152–8. 10.4049/jimmunol.177.9.6152 [DOI] [PubMed] [Google Scholar]
- 55. Le Borgne M, Filbert EL, Shaw AS: Kinase suppressor of Ras 1 is not required for the generation of regulatory and memory T cells. PLoS One. 2013;8(2):e57137. 10.1371/journal.pone.0057137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Revelli J, Smith D, Allen J, et al. : Profound obesity secondary to hyperphagia in mice lacking kinase suppressor of ras 2. Obesity (Silver Spring). 2011;19(5):1010–8. 10.1038/oby.2010.282 [DOI] [PubMed] [Google Scholar]
- 57. Henry MD, Costanzo-Garvey DL, Klutho PJ, et al. : Obesity-dependent dysregulation of glucose homeostasis in kinase suppressor of ras 2 -/- mice. Physiol Rep. 2014;2(7): pii: e12053. 10.14814/phy2.12053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Costanzo-Garvey DL, Pfluger PT, Dougherty MK, et al. : KSR2 is an essential regulator of AMP kinase, energy expenditure, and insulin sensitivity. Cell Metab. 2009;10(5):366–78. 10.1016/j.cmet.2009.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Guo L, Costanzo-Garvey DL, Smith DR, et al. : Cell non-autonomous regulation of hepatic IGF-1 and neonatal growth by Kinase Suppressor of Ras 2 (KSR2). Sci Rep. 2016;6: 32093. 10.1038/srep32093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Pearce LR, Atanassova N, Banton MC, et al. : KSR2 mutations are associated with obesity, insulin resistance, and impaired cellular fuel oxidation. Cell. 2013;155(4):765–77. 10.1016/j.cell.2013.09.058 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 61. Kolch W: Coordinating ERK/MAPK signalling through scaffolds and inhibitors. Nat Rev Mol Cell Biol. 2005;6(11):827–37. 10.1038/nrm1743 [DOI] [PubMed] [Google Scholar]
- 62. Karthik D, Majumder P, Palanisamy S, et al. : Targeting cysteine rich C1 domain of Scaffold protein Kinase Suppressor of Ras (KSR) with anthocyanidins and flavonoids - a binding affinity characterization study. Bioinformation. 2014;10(9):580–5. 10.6026/97320630010580 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 63. Zhou M, Horita DA, Waugh DS, et al. : Solution structure and functional analysis of the cysteine-rich C1 domain of kinase suppressor of Ras (KSR). J Mol Biol. 2002;315(3):435–46. 10.1006/jmbi.2001.5263 [DOI] [PubMed] [Google Scholar]
- 64. Zhang Y, Yao B, Delikat S, et al. : Kinase suppressor of Ras is ceramide-activated protein kinase. Cell. 1997;89(1):63–72. 10.1016/S0092-8674(00)80183-X [DOI] [PubMed] [Google Scholar]
- 65. Müller J, Cacace AM, Lyons WE, et al. : Identification of B-KSR1, a novel brain-specific isoform of KSR1 that functions in neuronal signaling. Mol Cell Biol. 2000;20(15):5529–39. 10.1128/MCB.20.15.5529-5539.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zhang J, Zafrullah M, Yang X, et al. : Downregulation of KSR1 in pancreatic cancer xenografts by antisense oligonucleotide correlates with tumor drug uptake. Cancer Biol Ther. 2008;7(9):1490–5. 10.4161/cbt.7.9.6472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hu J, Yu H, Kornev AP, et al. : Mutation that blocks ATP binding creates a pseudokinase stabilizing the scaffolding function of kinase suppressor of Ras, CRAF and BRAF. Proc Natl Acad Sci U S A. 2011;108(15):6067–72. 10.1073/pnas.1102554108 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 68. Potts MB, Kim HS, Fisher KW, et al. : Using functional signature ontology (FUSION) to identify mechanisms of action for natural products. Sci Signal. 2013;6(297):ra90. 10.1126/scisignal.2004657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Fisher KW, Das B, Kim HS, et al. : AMPK Promotes Aberrant PGC1β Expression To Support Human Colon Tumor Cell Survival. Mol Cell Biol. 2015;35(22):3866–79. 10.1128/MCB.00528-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. McCall JL, Gehring D, Clymer BK, et al. : KSR1 and EPHB4 Regulate Myc and PGC1β To Promote Survival of Human Colon Tumors. Mol Cell Biol. 2016;36(17):2246–61. 10.1128/MCB.00087-16 [DOI] [PMC free article] [PubMed] [Google Scholar]