Significance
Specialized protein complexes transport and integrate peptides into membranes in all living cells. Protein transport within the universally conserved Sec system requires the formation of an initiator protein substrate hairpin comprised of the signal peptide and adjacent region. Fundamental questions remain regarding when and how this hairpin structure forms. Here, we show that the SecA two-helix finger templates the hairpin within the preformed SecYEG-bound SecA complex prior to its insertion into the SecY channel. In addition to capturing a novel preinsertion intermediate state, our study expands the role of the SecA two-helix finger, which has previously been suggested to be an ATP-powered ratchet that drives cycles of protein transport.
Keywords: protein transport, FRET mapping, Sec system
Abstract
A conserved hairpin-like structure comprised of a signal peptide and early mature region initiates protein transport across the SecY or Sec61α channel in Bacteria or Archaea and Eukarya, respectively. When and how this initiator substrate hairpin forms remains a mystery. Here, we have used the bacterial SecA ATPase motor protein and SecYEG channel complex to address this question. Engineering of a functional miniprotein substrate onto the end of SecA allowed us to efficiently form ternary complexes with SecYEG for spectroscopic studies. Förster resonance energy transfer mapping of key residues within this ternary complex demonstrates that the protein substrate adopts a hairpin-like structure immediately adjacent to the SecA two-helix finger subdomain before channel entry. Comparison of ADP and ATP-γS–bound states shows that the signal peptide partially inserts into the SecY channel in the latter state. Our study defines a unique preinsertion intermediate state where the SecA two-helix finger appears to play a role in both templating the substrate hairpin at the channel entrance and promoting its subsequent ATP-dependent insertion.
Protein transport into and across the plasma membrane of Archaea and Bacteria or the endoplasmic reticular membrane of Eukarya occurs through a universally conserved protein-conducting channel termed the SecY or Sec61 complex, respectively [reviewed in Park and Rapoport (1)]. These channels display remarkably similar hourglass-shaped structures that are doubly gated: they open vertically to allow protein transport across the membrane or open laterally to allow insertion of integral membrane proteins into the lipid bilayer (2–10). The SecYEG channel consists of 15 transmembrane helices with a short helical region that blocks the channel, referred to as the plug domain. Vertical opening is accomplished at least in part by rearrangement of the plug domain of SecY/Sec61α to open the channel interior, while lateral opening requires transverse movement of SecY/Sec61α helices that reside at the lateral gate to create a path for exit from the channel interior into the lipid bilayer.
Substrate proteins can initiate their transport either cotranslationally or posttranslationally depending on a given substrate and organism. For cotranslational transport, the signal recognition particle and its receptor promote the targeting of appropriate nascent chain-bearing polysomes to the channel complex, while for posttranslational transport species-specific proteins are responsible for such targeting. In many cases, a signal sequence located at the N terminus of the substrate protein contains information regarding the ultimate location of the protein and is needed for translocation. The degree of hydrophobicity of this signal sequence appears to control which of these two pathways is used (11, 12).
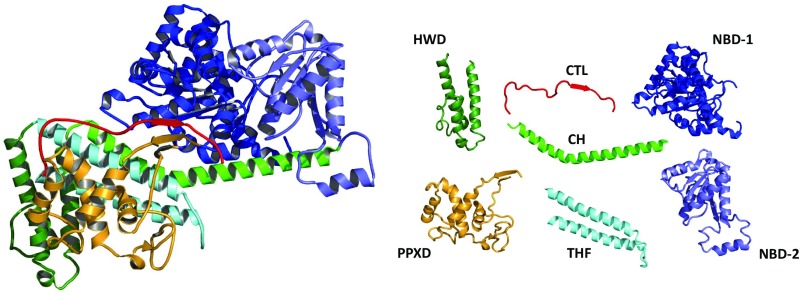
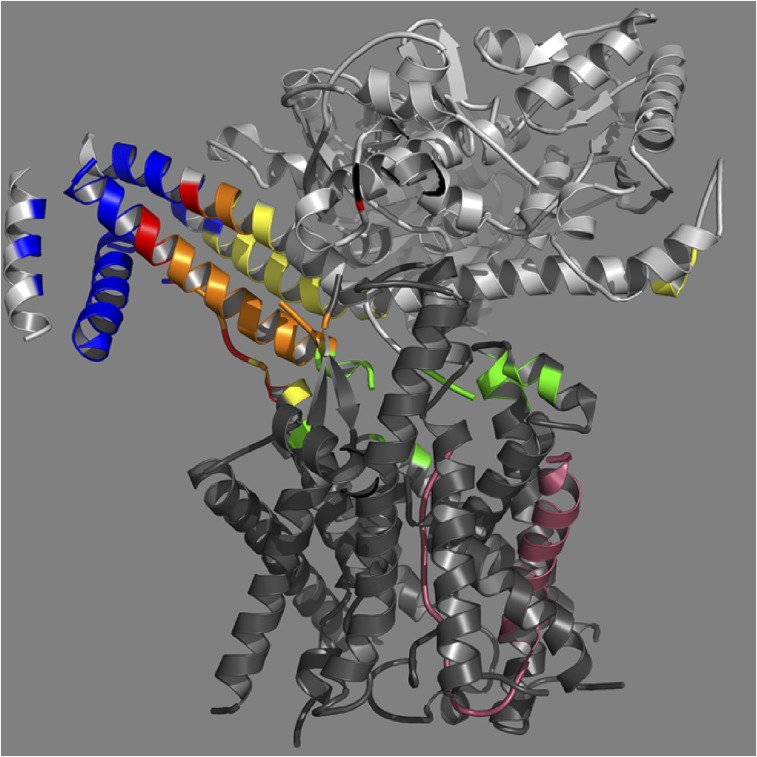
In Bacteria, SecA protein recognizes both signal peptides and SecY protein, and serves to target secretory preproteins to the SecY complex (13). SecA is a multidomain protein with two ATP binding domains (NBD-1, NBD-2), a preprotein cross-linking domain (PPXD) named for its ability to cross-link to preprotein substrates (14), central helix (CH) and two-helix finger (THF) subdomains, as well as helical wing domain (HWD) and carboxyl-terminal linker (CTL) domains (Fig. 1). As an ATPase motor protein, SecA uses ATP-driven hydrolytic cycles to promote domain movements required for substrate protein insertion into the SecY channel as well as subsequent processive protein transport through the channel. Several models of SecA action have been proposed [summarized in Kusters and Driessen (15)]; however, the precise mechanism of SecA-driven protein transport has yet to be fully elucidated. Recent structural and biochemical analysis have suggested that the THF subdomain located in the central region of SecA potentially acts as a molecular ratchet driving substrate proteins into the SecY channel (16, 17) (Fig. 1).
Fig. 1.
Ribbon representation of the B. subtilis SecA protein colored by domain (Left) with the individual domains shown on the Right (PDB ID code 1M6N). They include (N-terminal to C-terminal on SecA): the nucleotide-binding domain-1 (NBD-1) (blue), the preprotein cross-linking domain (PPXD) (gold), the nucleotide-binding domain-2 (NBD-2) (light blue), the central helix subdomain (CH) (green), the helical wing domain (HWD) (dark green), the two helix-finger subdomain (THF) (cyan), and the carboxyl-terminal linker (CTL). The CTL is depicted in red and serves as a model of PhoA signal peptide bound to B. subtilis SecA based on the FRET mapping study of Zhang et al. (21).
Protein transport occurs via a loop model in which the signal peptide and early mature region of the preprotein form a hairpin-like structure within the membrane to initiate the transport process (18, 19). In this topology, the signal peptide remains relatively fixed, while the polypeptide region that follows is processively threaded across the membrane to accommodate increasing larger translocation intermediates. A recent X-ray structure has visualized this substrate protein hairpin within the SecY complex and found that the signal peptide resides in a groove immediately outside of the lateral gate, while the early mature region resides within the channel proper (10), augmenting earlier electron cryomicroscopy studies where only the signal sequence was visible (5–7).
Previously, we used Förster resonance energy transfer (FRET)-based methods to map the location of the SecA signal peptide-binding site using chimeras containing SecA and the alkaline phosphatase (PhoA) or lambda receptor variant (KRRLamB) signal peptides (20, 21). We found that the signal peptide interacts with both the PPXD and THF subdomain and adopts a parallel binding orientation along the long axis of THF mimicking the location of the carboxyl-terminal tail of SecA in the Bacillus subtilis X-ray crystal structure (shown in red, Fig. 1). We also demonstrated that the lambda receptor signal peptide, KRRLamB, bound to SecA in the same location and orientation as its PhoA counterpart, indicative of a common binding site and orientation (21).
In this report, we have used a similar approach to address a critical and unresolved question concerning the initiation of protein transport in the SecA–SecYEG complex: Does the protein substrate hairpin-like structure that initiates protein transport form before or after insertion of the substrate into the membrane? We now report that the hairpin-like structure forms and binds to the SecA THF subdomain before entry into the SecY channel. Given the importance of the substrate hairpin loop in channel activation, templating of the hairpin before channel insertion may be a conserved mechanism for the cotranslational protein transport pathway as well.
Results
To determine the location of the signal peptide and early mature region of the protein substrate within the purified SecYEG-bound SecA complex, we used our genetically engineered SecA–PhoA chimeras and FRET mapping approach as reported previously (21). We have demonstrated that the SecA and PhoA signal peptide portions of the chimera were functional in vivo and in vitro, and that the attached PhoA signal peptide bound specifically to SecA at the previously mapped binding site (20). In the present study, a longer chimera was created in a similar manner by genetically fusing the PhoA substrate to SecA in which we removed the dispensable carboxyl-terminal 67-residue linker domain of SecA and replaced it with a short glycine–serine linker followed by the first 68 residues of PhoA. The PhoA portion contained the 21-residue signal peptide and an additional 47 residues of the early mature region terminated by a hexahistidine tag (Table S1). To achieve differential labeling between SecA and PhoA, dye labels were introduced into the PhoA portion of the chimera by in vivo incorporation of azido-phenylalanine at engineered amber codons followed by protein purification and dye incorporation at these sites using click chemistry (22). In contrast, labeling of selected residues within SecA or SecY was achieved by genetically engineering unique cysteine residues and labeling them using maleimide chemistry. Efficient assembly of the relevant PhoA substrate-bound SecA–SecYEG complex was improved by this approach, as it ensures a one-to-one stoichiometry of bound substrate to SecA–SecYEG complex, and avoids solubility issues associated with high concentrations of free substrate peptides or their premature folding or aggregation in the case of larger protein substrates (20).
Table S1.
Plasmids used in the study
| Plasmid* | Construction/comments† |
| pT7SecA | E. coli secA gene under T7 promoter control (44) |
| pT7SecA(Cys0) | pT7SecA derivative with all four cysteine codons changed to serine |
| pT7SecA(Cys321) | SecA codon 321 on pT7SecA(Cys0) was changed to a cysteine codon |
| pT7SecA(Amber721, Cys321) | SecA codon 721 on pT7SecA(Cys321) was changed to an amber codon |
| pT7SecA834 | Deletion of SecA codons 835–901 of pT7SecA |
| pT7SecA834–PhoA68 | Insertion of first 68 codons of PhoA between SecA codon 834 and his tag of pT7SecA834 in multiple stages: codons 1–7, 8–15, 16–21, 22–28, 29–34, 35–41, 42–48, 48–54, 55–61, and 62–68 |
| pT7SecA834(Cys0)–PhoA68 | SecA codon 98 on pT7SecA834–PhoA68 was changed to a serine codon |
| pT7SecA834(Cys0)–GS–PhoA68 | A linker sequence comprised of glycine and serine residues (SSGGSG) was inserted between SecA and PhoA on pT7SecA834(Cys0)–PhoA68 |
| pT7SecA834(Cys321)–GS–PhoA68 | SecA codon 321 on pT7SecA834(Cys0)–GS–PhoA68 was changed to a cysteine codon |
| pT7SecA834(Cys321)–GS–PhoA68(Amber2) | PhoA codon 2 on pT7SecA834(Cys321)–GS–PhoA68 was changed to an amber codon |
| pT7SecA834(Cys321)–GS–PhoA68(Amber22) | PhoA codon 22 on pT7SecA834(Cys321)–GS–PhoA68 was changed to an amber codon |
| pT7SecA834(Cys321)–GS–PhoA68(Amber37) | PhoA codon 37 on pT7SecA834(Cys321)–GS–PhoA68 was changed to an amber codon |
| pT7SecA834(Cys321)–GS–PhoA68(Amber45) | PhoA codon 45 on pT7SecA834(Cys321)–GS–PhoA68 was changed to an amber codon |
| pT7SecA834(Cys37)–GS–PhoA68 | SecA codon 37 on pT7SecA834(Cys0)–GS–PhoA68 was changed to a cysteine codon |
| pT7SecA834(Cys37)–GS–PhoA68(Amber2) | PhoA codon 2 on pT7SecA834(Cys37)–GS–PhoA68 was changed to an amber codon |
| pT7SecA834(Cys37)–GS–PhoA68(Amber 22) | PhoA codon 22 on pT7SecA834(Cys37)–GS–PhoA68 was changed to an amber codon |
| pT7SecA834(Cys37)–GS–PhoA68(Amber37) | PhoA codon 37 on pT7SecA834(Cys37)–GS–PhoA68 was changed to an amber codon |
| pT7SecA834(Cys37)–GS–PhoA68(Amber45) | PhoA codon 45 on pT7SecA834(Cys37)–GS–PhoA68 was changed to an amber codon |
| pBAD22 SecE–SecY(Cys0)–SecG | E. coli SecYEG lacking any cysteine under araBAD promoter control; courtesy of Tom Rapoport, Harvard Medical School, Boston |
| pBAD22 SecE–SecY(Cys292)–SecG | SecY codon 292 on pBAD22 SecE–SecY(Cys0)–SecG was changed to a cysteine codon; courtesy of Tom Rapoport |
| pEVOL–pAzF | Plasmid for incorporation of H-4-Azido-Phe-OH at amber codons (22) |
All SecA or SecA–PhoA chimeras contained a carboxyl-terminal hexahistidine tag, while the SecYEG-containing plasmids contained an amino-terminal hexahistidine tag on SecE.
QuikChange mutagenesis was used for all plasmid construction as described by the manufacturer. Plasmid DNA sequence was verified by the University of Pennsylvania DNA-Sequencing Facility.
To capture an early intermediate in the transport process, we chose to use n-dodecyl-β-D-maltopyranoside (DDM)-solubilized SecYEG protein for our study, since SecA binds this form of SecYEG with high affinity and the binding stimulates SecA ATPase activity (23, 24). In addition, this system is comparable to that used in the Thermotoga maritima SecA–SecYEG X-ray cocrystal structure (16), which we use for modeling our results. Robust site-specific in vivo photo–cross-linking has demonstrated that the Escherichia coli SecA–SecY complex is structurally similar to its T. maritima counterpart (25). By avoiding the use of a SecYEG-containing proteoliposome system, we further simplified our approach and mitigated concerns over a mixed topology of SecYEG protein within the bilayer, its potential for dimerization, or that a fraction of SecA might bind solely to phospholipids using its known lipid-binding activity.
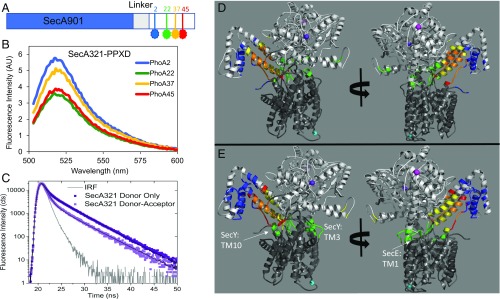
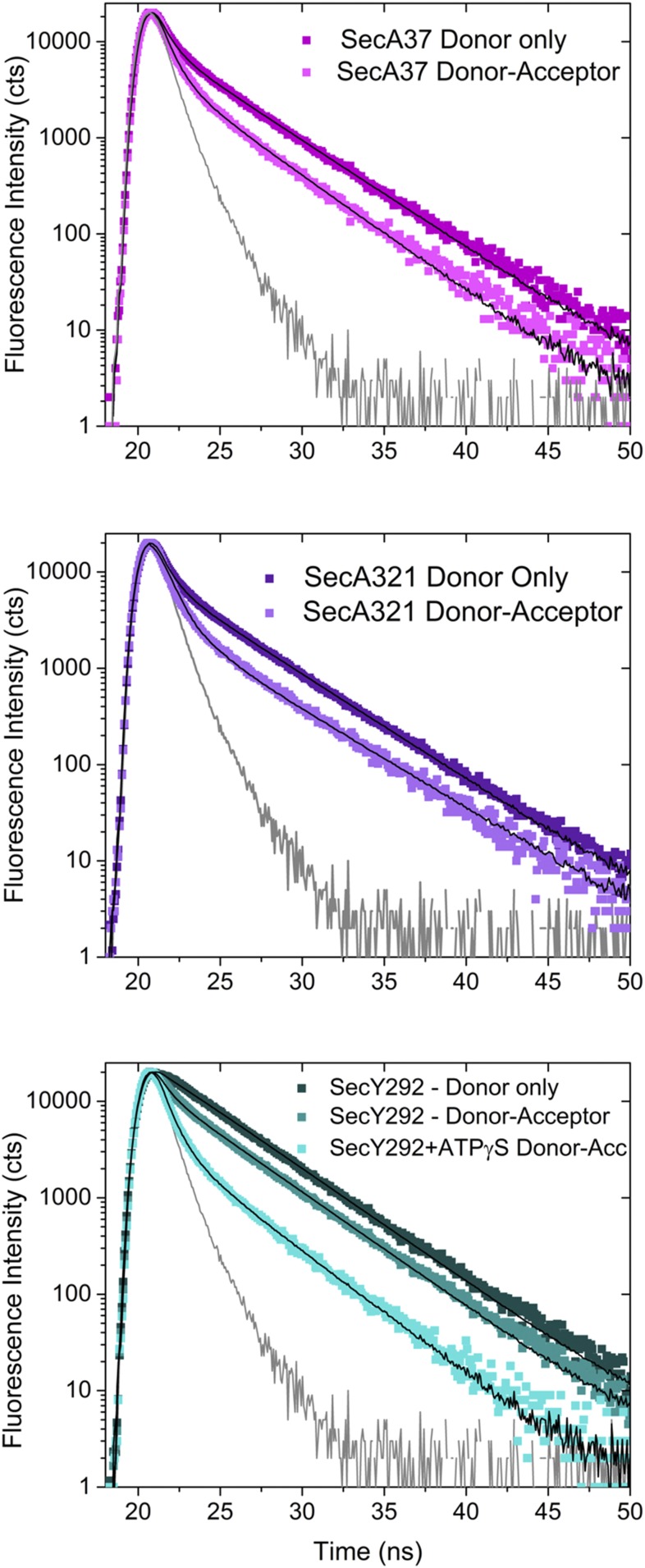
To track the location of the signal peptide and early mature region of the protein substrate in the SecA–SecYEG complex, we introduced dye labels periodically along its length (Fig. 2A). We selected residues at the beginning and end of the signal peptide (residues 2 and 22, respectively) as well as at two positions within the early mature region (residues 37 and 45). We avoided placing a dye within the hydrophobic core of the signal peptide, since we found previously that it perturbed SecA signal peptide binding (20). To accurately map the location of the protein substrate, we also introduced dye labels at three distinct locations in the SecA–SecYEG complex: two within SecA, located within the NBD-1 and the PPXD, and one within SecYEG (shown as spheres in Fig. 2 D and E). The residues selected for labeling the SecA–SecYEG complex needed to satisfy several criteria: (i) be structurally well distributed throughout the complex but within accurate range for FRET measurements, (ii) be surface accessible for good labeling efficiency, and (iii) reside near the ends of well-structured regions for greater accuracy in distance determinations with minimal structural and functional perturbations. As the three residues chosen (SecA37, SecA321, and SecY292) are positioned in distinct regions of the complex, the location of the signal peptide and early mature region on the SecA–SecYEG complex in the presence of either ADP or ATP-γS could be determined from the measured distances (Fig. 2).
Fig. 2.
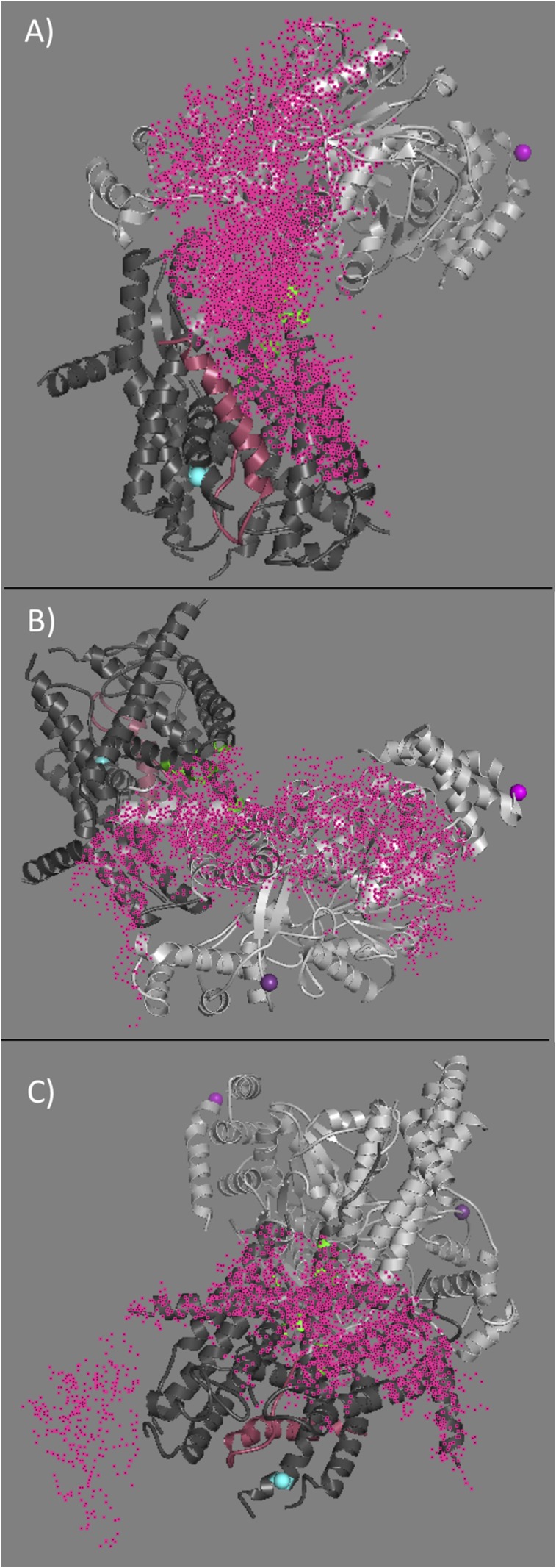
Mapping of PhoA signal peptide and early mature region on to the SecA–SecYEG complex. (A) Schematic of the SecA–PhoA chimera construct in which the PhoA substrate peptide is genetically fused to SecA after a Gly–Ser linker (not drawn to scale). Cys residues were introduced for dye labeling at the indicated positions and are depicted in blue, green, yellow, and red. (B) Representative fluorescence spectra of the doubly labeled SecA–PhoA chimeras in the presence of SecYEG and ADP, with the donor dye positioned at different points within PhoA and the acceptor dye positioned at SecA residue 321. The spectrum generated with the donor dye at PhoA position 22 has the lowest intensity and highest transfer efficiency. Data were acquired and analyzed as described in SI Materials and Methods. (C) Time-resolved fluorescence decay spectra of the SecA–PhoA chimera labeled with the donor dye at PhoA position 22 and with the acceptor dye at SecA residue 321. A donor-only decay is shown in dark violet and a donor–acceptor decay is shown in light violet. The instrument response function (IRF) is given in gray. The donor–acceptor decay yields a shorter lifetime indicative of energy transfer. Data were acquired and analyzed as described in SI Materials and Methods. (D and E) The T. maritima SecA–SecYEG complex (PDB ID code 3DIN) is shown as a ribbon diagram with SecA and SecYEG in light and dark gray, respectively. The locations of T. maritima residues homologous to E. coli SecA37, SecA321, and SecY292 are shown by magenta-, violet-, or cyan-colored spheres, respectively. Mapped location of the PhoA substrate within the SecA–SecYEG complex in the presence of ADP (D) or ATP-γS (E). The region of overlap of the structure with the FRET data for PhoA residue 2, 22, 37, or 45 is shown in blue, green, yellow, or red, respectively. Overlap regions of PhoA residues 22, 37, and 45 are shown in olive, and overlap regions of 37 and 45 are shown in orange. Figures on the Right are rotated by ≈180°.
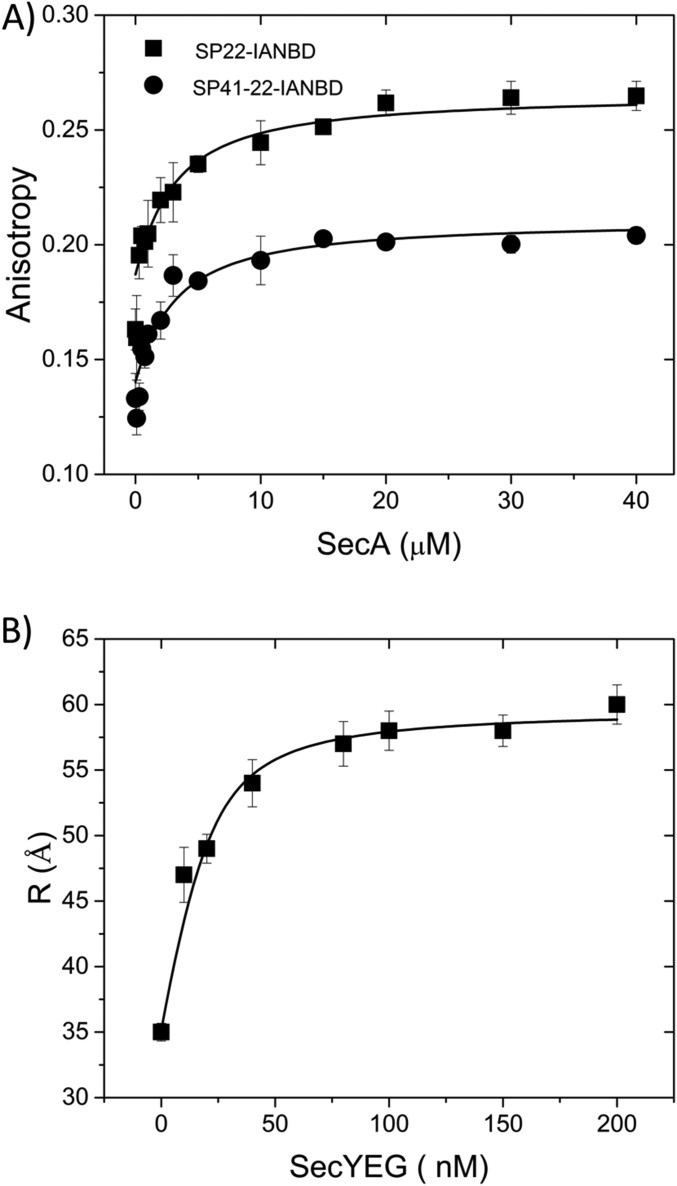
To ensure that both SecA and SecYEG were in their monomeric forms and avoid interprotomer FRET, a buffer system containing 300 mM KCl and 0.1% DDM was used. At this salt concentration, less than 3% of SecA dimer was detected (26), while SecYEG dimer was undetectable at this detergent concentration (27). SecA affinity for exogenous PhoA signal peptide or an extended signal peptide that also contained the early mature region was ∼2 μM at this salt concentration similar to previous reports (20) (Fig. S1A). SecA also bound SecYEG with high affinity in this buffer system with a Kd of 6 nM (Fig. S1B) similar to previous measurements where the complex was shown to consist almost entirely of SecA monomers bound to SecYEG at this salt concentration (28). As expected, the ATPase activity of our various dye-labeled SecA–PhoA chimeras increased in the presence of SecYEG, exhibiting a range of activity from 30 to 160%. We have shown previously that the presence and position of dyes within SecA can affect ATPase activities without necessarily compromising function (20). In addition, we found that the various SecA–PhoA chimeras bound SecYEG with roughly similar affinities based on the similar strength of the FRET signals (see below as well as in SI Materials and Methods).
Fig. S1.
Verification of normal ligand binding in the presence of higher salt concentration. (A) SecA binding to the PhoA signal peptide or extended signal peptide labeled with dye at position 22 (SP22 or SP41, respectively) at 300 mM KCl is depicted using a fluorescence anisotropy binding assay as described in SI Materials and Methods. (B) SecA binding to SecYEG in the presence of 300 mM KCl and 0.1% DDM is depicted using a FRET assay that relies on PPXD-HWD separation (given in angstroms) induced by complex formation. For this purpose, 20 nM SecA labeled at residues 321 and 721 within PPXD and HWD, respectively, with appropriate donor and acceptor dyes was mixed with SecYEG at the final indicated concentration in high-salt TKM buffer containing 0.1% DDM at 20 °C. The calculated distances were determined as described in SI Materials and Methods.
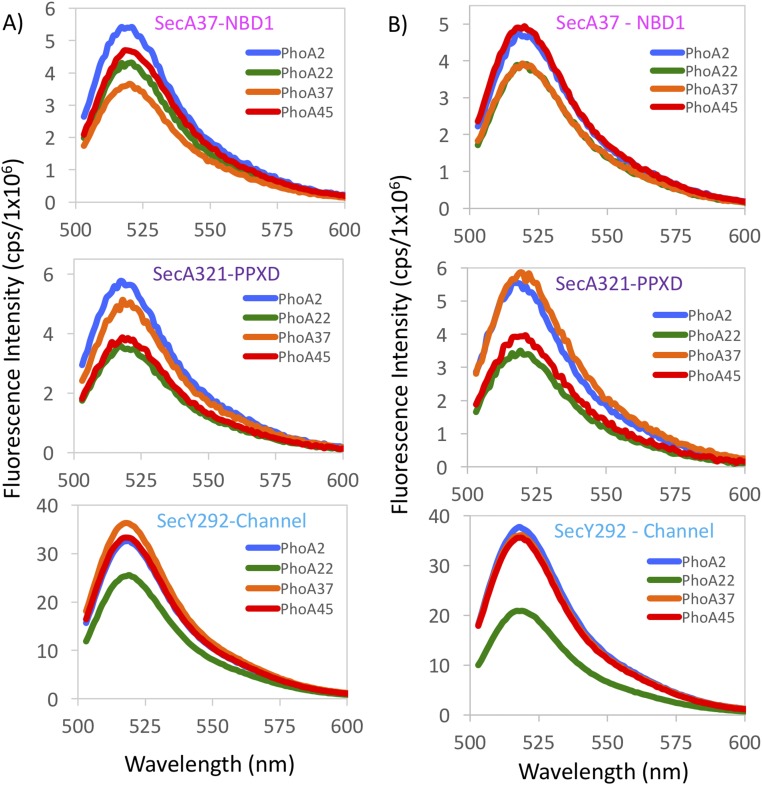
By using four positions on PhoA (Fig. 2A) and three positions on the SecA–SecYEG complex, we measured 12 different distances to position the PhoA substrate within the complex. To observe membrane-retracted or membrane-inserted states of SecA, spectra were collected in the presence of ADP or ATP-γS, respectively (Fig. S2) (29). For two sets of these measurements, the acceptor dye was located on the SecA portion of the chimera (residue 37 or 321 shown as violet or magenta spheres, respectively; Fig. 2 D and E), and the donor dye was located on the PhoA portion of the chimera. In the third set of measurements, the donor dye was on SecY adjacent to its plug domain (residue 292 shown as a cyan sphere, Fig. 2 D and E), and the acceptor dye was located on the PhoA portion of the chimera. Representative steady-state fluorescence spectra from a doubly labeled system are shown in Fig. 2B and demonstrate visually that the highest transfer efficiencies (lowest donor intensities) were observed for the dye located at PhoA residue 22 of the chimera, while the lowest transfer efficiencies (highest donor intensities) were observed for the dye located at PhoA residue 2. Fluorescence spectra of doubly labeled species from all three locations exhibited similar trends where the measured efficiencies were not linearly proportional to the distance of the label from the start of PhoA in the chimera, consistent with the formation of a hairpin loop (Fig. S2).
Fig. S2.
Fluorescence spectra of donor–acceptor labeled SecA–PhoA–SecYEG complexes generated with a 488-nm excitation wavelength. For all three sets of FRET pairs examined, the FRET efficiency is not linearly proportional to the position of the label on the PhoA portion of the chimera, consistent with a hairpin loop configuration. (A) All spectra were obtained in the presence of ADP. (Top) Spectra were generated with SecA37–AF647 as the acceptor, and the donor dye (AF488) was located at four different positions on the PhoA portion of the chimera: PhoA2 (blue), PhoA22 (green), PhoA37 (orange), or PhoA45 (red). (Middle) Spectra were generated with SecA321–AF647 as the acceptor and donor dye (AF488) positioned at either PhoA2 (blue), PhoA22 (green), PhoA37 (orange), or PhoA45 (red). (Bottom) The donor dye was located at SecY292–AF488, and the acceptor dye (AF647) was located at either PhoA2 (blue), PhoA22 (green), PhoA37 (orange), or PhoA45 (red). (B) Same as Fig. S2A, except all spectra were generated in the presence of ATP-γS. Spectral acquisition and analysis are described in SI Materials and Methods. FRET efficiencies, distances, and the degree of labeling are given in Tables S2–S4.
Energy transfer efficiencies were also determined using time-resolved fluorescence spectroscopy to verify the homogeneity of the transfer (Fig. 2C). Donor-only spectra were obtained in the presence of unlabeled proteins to control for any quenching associated with SecA–SecYEG complex formation. Analysis of these decays yielded one dominant lifetime, consistent with only one species participating in energy transfer for a given donor–acceptor pair. The presence of the acceptor reduced the donor lifetime, indicative of energy transfer (Fig. 2C and Fig. S3), and the calculated efficiencies were in excellent agreement with steady-state measurements (Tables S2–S5). The steady-state FRET efficiencies (Tables S2–S4) are all within the linear efficiency range of the dye pairs used (0.2–0.8). The determined distances have a larger error than that obtained experimentally for the efficiencies alone to account for the error introduced by the uncertainty in dye position as determined from steady-state anisotropy values (21, 30, 31).
Fig. S3.
Fluorescence intensity decay spectra of SecA–PhoA–SecYEG complexes obtained with 490-nm excitation. Donor-only decays are shown in darker colors, and lighter-colored decays depict decays obtained in the presence of acceptor. In all cases, the presence of the acceptor leads to a faster decay consistent with energy transfer. (Top) The donor dye (AF488) is located at PhoA22, and the acceptor dye is located at SecA37 (AF647). Donor only is shown in dark magenta and the donor–acceptor decay is given in light magenta. Decays were obtained in the presence of ADP. (Middle) The donor dye (AF488) is located at PhoA22, and the acceptor dye is located at SecA321 (AF647). Donor-only is shown in dark violet, and the donor–acceptor decay is given in light violet. Decays were obtained in the presence of ADP. (Bottom) The donor dye was located at SecY292–AF488, and the acceptor dye (AF647) was located at PhoA22. The donor only decay is shown in dark cyan and donor–acceptor decay is shown in cyan. Both decays were obtained in the presence of ADP. The donor–acceptor decay shown in light cyan was obtained in the presence of ATP-γS. For all spectra depicted, the instrument response function (IRF) is shown in gray. Parameters obtained from the fitting of the decays are given in Table S5.
Table S2.
FRET efficiencies and distances determined from SecA37 FRET pairs on SecA–PhoA–SecYEG complex
| Labeled site on SecA | Labeled site on PhoA peptide portion of SecA–PhoA chimera | |||||||
| SecA37–AF647–PhoA | PhoA2–AF488 | PhoA22–AF488 | PhoA37–AF488 | PhoA45–AF488 | ||||
| R0*: 57 | R0*: 58 | R0*: 57 | R0*: 60 | |||||
| fD = 0.40, fA = 0.58† | fD = 0.70, fA = 0.88† | fD = 0.72, fA = 1.00† | fD = 0.68, fA = 0.80† | |||||
| EFRET‡ | Distance† | EFRET‡ | Distance† | EFRET‡ | Distance† | EFRET‡ | Distance† | |
| ADP and SecYEG | 0.27 ± 0.003 | 67 ± 15 | 0.52 ± 0.02 | 56 ± 12 | 0.39 ± 0.03 | 62 ± 13 | 0.36 ± 0.03 | 66 ± 15 |
| ATP-γS and SecYEG | 0.20 ± 0.06 | 72 ± 16 | 0.42 ± 0.02 | 60 ± 13 | 0.32 ± 0.04 | 65 ± 14 | 0.21 ± 0.04 | 75 ± 16 |
R0 values given in angstroms were calculated as previously described (20).
The donor−acceptor distances (R) given in angstroms were calculated as described in SI Materials and Methods and consider the fractional labeling of the donor (fD) and acceptor (fA) in the doubly labeled complex. The larger error in the distances results from a consideration of the steady-state fluorescence anisotropy values of the dyes.
The FRET efficiency (EFRET) was calculated from the decrease of donor fluorescence intensity in the presence of the acceptor as described in SI Materials and Methods. The indicated error is determined from three independent measurements.
Table S5.
Fit parameters from analysis of time-resolved fluorescence decays of SecA–PhoA–SecYEG complexes
| FRET pairs | ||||||||
| Fit parameters* | SecA37–AF647–PhoA22–AF488 + ADP + SecYEG | SecA321–AF647–PhoA22–AF488 + ADP + SecYEG | SecA321C–PhoA22–AF647 + SecY292–AF488 + SecEG + ADP | SecA321C–PhoA22–AF647 + SecY292–AF488 + SecEG + ATP-γS | ||||
| Donor only | Donor–acceptor | Donor only | Donor–acceptor | Donor only | Donor–acceptor | Donor only | Donor–acceptor | |
| α1 | 0.40 | 0.27 | 0.17 | 0.18 | 0.63 | 0.38 | 0.58 | 0.25 |
| τ1 | 3.80 | 3.04 | 2.85 | 1.21 | 3.75 | 3.77 | 3.69 | 3.38 |
| <τ>† | 1.50 | 0.82 | 0.48 | 0.21 | 2.38 | 1.45 | 2.16 | 0.86 |
| χ2 | 1.21 | 1.11 | 1.17 | 1.02 | 2.78‡ | 1.33 | 2.78‡ | 0.966 |
| EFRET§ | 0.46 | 0.56 | 0.39 | 0.60 | ||||
Fluorescence intensity decays (Fig. S3) were fit to a sum of exponentials using the following expression: I(t) = . Decays were well described by a single exponential, as judged by the χ2 value and a visual inspection of the residuals. Acquisition and analysis details are given in SI Materials and Methods.
The amplitude-weighted lifetime is proportional to the steady-state intensity and is calculated using the following expression: .
This sample exhibited a higher χ2 value due to some precipitation of the sample during spectral acquisition.
EFRET is calculated as described in SI Materials and Methods.
Table S4.
FRET efficiencies and distances determined from SecY292 FRET pairs on SecA–PhoA–SecYEG complex
| Labeled site on SecYEG | Labeled site on PhoA peptide portion of SecA–PhoA chimera | |||||||
| SecA–PhoA, SecYEG–SecY292–AF488 | PhoA2–AF647 | PhoA22–AF647 | PhoA37–AF647 | PhoA45–AF647 | ||||
| R0*: 57 | R0*: 50 | R0*: 53 | R0*: 54 | |||||
| fD = 1.00, fA = 0.61† | fD = 1.00, fA = 0.56† | fD = 1.00, fA = 0.51† | fD = 1.00, fA = 0.61† | |||||
| EFRET‡ | Distance† | EFRET‡ | Distance† | EFRET‡ | Distance† | EFRET‡ | Distance† | |
| ADP | 0.25 ± 0.06 | 68 ± 15 | 0.46 ± 0.06 | 51.3 ± 9.2 | 0.24 ± 0.05 | 64 ± 14 | 0.30 ± 0.01 | 62 ± 13 |
| ATP-γS | 0.19 ± 0.03 | 72 ± 17 | 0.71 ± 0.01 | 42.9 ± 8.6 | 0.30 ± 0.03 | 61 ± 13 | 0.27 ± 0.01 | 63 ± 13 |
R0 values given in angstroms were calculated as previously described (20).
The donor−acceptor distances (R) given in angstroms were calculated as described in SI Materials and Methods and consider the fractional labeling of the donor (fD) and acceptor (fA) in the doubly labeled complex. The larger error in the distances results from a consideration of the steady-state fluorescence anisotropy values of the dyes.
The FRET efficiency (EFRET) was calculated from the decrease of donor fluorescence intensity in the presence of the acceptor as described in SI Materials and Methods. The indicated error is determined from three independent measurements.
Table S3.
FRET efficiencies and distances determined from SecA321 FRET pairs on SecA–PhoA–SecYEG complex
| Labeled site on SecA | Labeled site on PhoA peptide portion of SecA–PhoA chimera | |||||||
| SecA321–AF647–PhoA | PhoA2–AF488 | PhoA22–AF488 | PhoA37–AF488 | PhoA45–AF488 | ||||
| R0*: 40 | R0*: 38 | R0*: 37 | R0*: 38 | |||||
| fD = 0.50, fA = 1.00† | fD = 0.48, fA = 0.85† | fD = 0.50, fA = 0.90† | fD = 0.32, fA = 0.75† | |||||
| EFRET‡ | Distance† | EFRET‡ | Distance† | EFRET‡ | Distance† | EFRET‡ | Distance† | |
| ADP and SecYEG | 0.16 ± 0.04 | 53 ± 11 | 0.63 ± 0.004 | 35 ± 7.3 | 0.39 ± 0.02 | 39.7 ± 7.9 | 0.43 ± 0.06 | 39.7 ± 7.5 |
| ATP-γS and SecYEG | 0.22 ± 0.06 | 50 ± 11 | 0.50 ± 0.04 | 38 ± 8 | 0.49 ± 0.01 | 37.2 ± 7.4 | 0.44 ± 0.04 | 39.5 ± 7.5 |
R0 values given in angstroms were calculated as previously described (20).
The donor−acceptor distances (R) given in angstroms were calculated as described in SI Materials and Methods and consider the fractional labeling of the donor (fD) and acceptor (fA) in the doubly labeled complex. The larger error in the distances results from a consideration of the steady-state fluorescence anisotropy values of the dyes.
The FRET efficiency (EFRET) was calculated from the decrease of donor fluorescence intensity in the presence of the acceptor as described in SI Materials and Methods. The indicated error is determined from three independent measurements.
Using this information, we could position the signal peptide and early mature region of PhoA within the SecA–SecYEG complex. To do this, we considered each of the three distances measured between a given PhoA residue and their corresponding FRET partner on either SecA or SecY as a radius for a spherical shell (Fig. S4). The intersecting region of the three shells defined the position of a given PhoA residue within the complex, where the width of each shell corresponded to our uncertainty in the distance (Tables S2–S4). Thus, in Fig. 2 D and E, the mapped location of the second residue of the PhoA signal peptide (PhoA2) is shown in blue. Similarly, the mapped regions for PhoA residues 22 (PhoA22), 37 (PhoA37), and 45 (PhoA45) are shown in green, yellow, and red, respectively. There is considerable overlap of the PhoA22, PhoA37, and PhoA45 regions, which is shown in olive, while overlapping regions of PhoA37 and PhoA45 are shown in orange. In the ADP-bound form (Fig. 2D), the region mapped to PhoA2 is located mainly on the HWD and lies at the end of the THF farthest from the mouth of the channel. In contrast, the regions mapped solely by PhoA22, shown in green, are primarily found positioned on the loop of THF and lie directly over the mouth of the channel, poised for translocation. The PhoA22 region also maps to one of the helices of the THF along with the regions defined by PhoA37 and PhoA45. This finding supports a model in which the PhoA37 and PhoA45 residues are close to each other in space and are sandwiched in between PhoA22 and PhoA2. This spatial distribution strongly points to the PhoA signal peptide and early mature region forming a hairpin-like structure immediately adjacent to and paralleling the SecA THF subdomain (Fig. 2 D and E). The hairpin loop corresponding to the junction between the end of the signal peptide (PhoA22) and the beginning of the early mature region is formed at the mouth of the channel while the ends of the hairpin (PhoA2 and PhoA45) lie at the end of the THF near to the wing domain, far from the channel mouth. The relatively large area defined by the PhoA2 FRET measurements suggests the N-terminal end of the signal peptide is fairly flexible.
Fig. S4.
Depiction of the spherical shells (pink dots) used to identify the FRET overlap within the SecA–SecYE structure for PhoA residue 22 within the SecA–PhoA chimera. The shells were generated using distance values obtained in the presence of ATP-γS and are shown on the SecA–SecYE cocrystal structure PDB ID 5EUL. SecA is shown in light gray, SecYE is shown in dark gray, and the OmpA peptide is shown in pink. The width of the shells corresponds to the uncertainty in the FRET distance measurement (given in Tables S2–S4). (A) The shell determined from SecA residue 37 (magenta sphere) within NDB-1, (B) the shell determined from SecA residue 321 (violet sphere) within the PPXD, and (C) the shell determined from SecY residue 292 (cyan sphere) at the bottom of the channel. The intersection of the three spherical shells defines the region ascribed to PhoA residue 22 (green) in this case. The script for determining this intersection in given in SI Materials and Methods.
In the ATP-γS–bound state (Fig. 2E), the regions defined solely by PhoA22 (shown in green) primarily map to SecYEG and the top of channel. Exclusive mapping is observed at the top (cytosolic side) of SecY on TM3 and TM10 as well as TM1 of SecE. The loop or tip of the finger is also mapped to PhoA22 only. These results are in excellent agreement with increased insertion of substrates into the channel in the ATP-bound state (32, 33) and are consistent with the highest transfer efficiencies observed between the PhoA22 and SecY292 FRET pair in the presence of ATP-γS (Fig. 2C and Fig. S2B). The regions mapped by PhoA37 and PhoA45 remain along the THF and the CH subdomains as well as the C terminus of SecY (often termed the C6 cytosolic domain of SecY). The relative progression and orientation of the signal peptide and early mature region within the SecA–SecYEG complex can be visualized along the THF, in which the color change from green to red (green, olive, yellow, orange, red) signifies mapping of the regions from PhoA22 to PhoA45. The more orderly structure of the substrate in its ATP-γS–bound versus ADP-bound state suggests that it becomes more templated for translocation upon ATP binding. Although there is still considerable flexibility at the substrate ends as shown by their relatively large mapped areas, we note that these two regions, PhoA2 and PhoA45 (blue and red, respectively), are proximal to one another and far from the channel entry. Taken together, these findings strongly support a model where formation of the initial substrate hairpin is templated by the THF subdomain before its insertion into the channel.
Comparison of our result with the recent X-ray structure of the SecA–SecYEG complex with substrate inserted into the channel (10) (Fig. S5) clearly suggests that our complex represents a preinsertion intermediate state, while the latter structure represents a more mature, postinsertion, translocation-intermediate state. Remarkably, the signal peptide and early mature regions of the substrate adopt similar hairpin-like structures in both cases despite the fact that they are different substrates and were fused to SecA very differently: the OmpA signal peptide and early mature region were inserted at the end of the THF finger in the Li et al. (10) X-ray structure, while in our study the PhoA peptide was fused onto the C-terminal end of SecA. The similar nature of the results strongly implies that the initial substrate hairpin-like structure preexists outside of the SecYEG channel, is nucleated and/or stabilized by the assembled SecA–SecYEG complex, and is a conserved and fundamental unit for initiating transported protein substrates.
Fig. S5.
The B. subtilis SecA–G. thermodenitrificans SecYE cocrystal structure (PDB ID code 5EUL). SecA is shown in light gray, SecYE is in dark gray, and the OmpA peptide substrate inserted at the end of the THF is shown in pink. For clarity, the nanobody crystallized with the complex has been omitted (10). The FRET-mapped regions in the presence of ATP-γS of the beginning portions of the PhoA substrate for residues 2, 22, 37, or 45 are shown in blue, green, yellow, or red, respectively. Mapping was done as described in Fig. S4. Overlap regions of PhoA residues 22, 37, and 45 are shown in olive, and overlap regions of 37 and 45 are shown in orange.
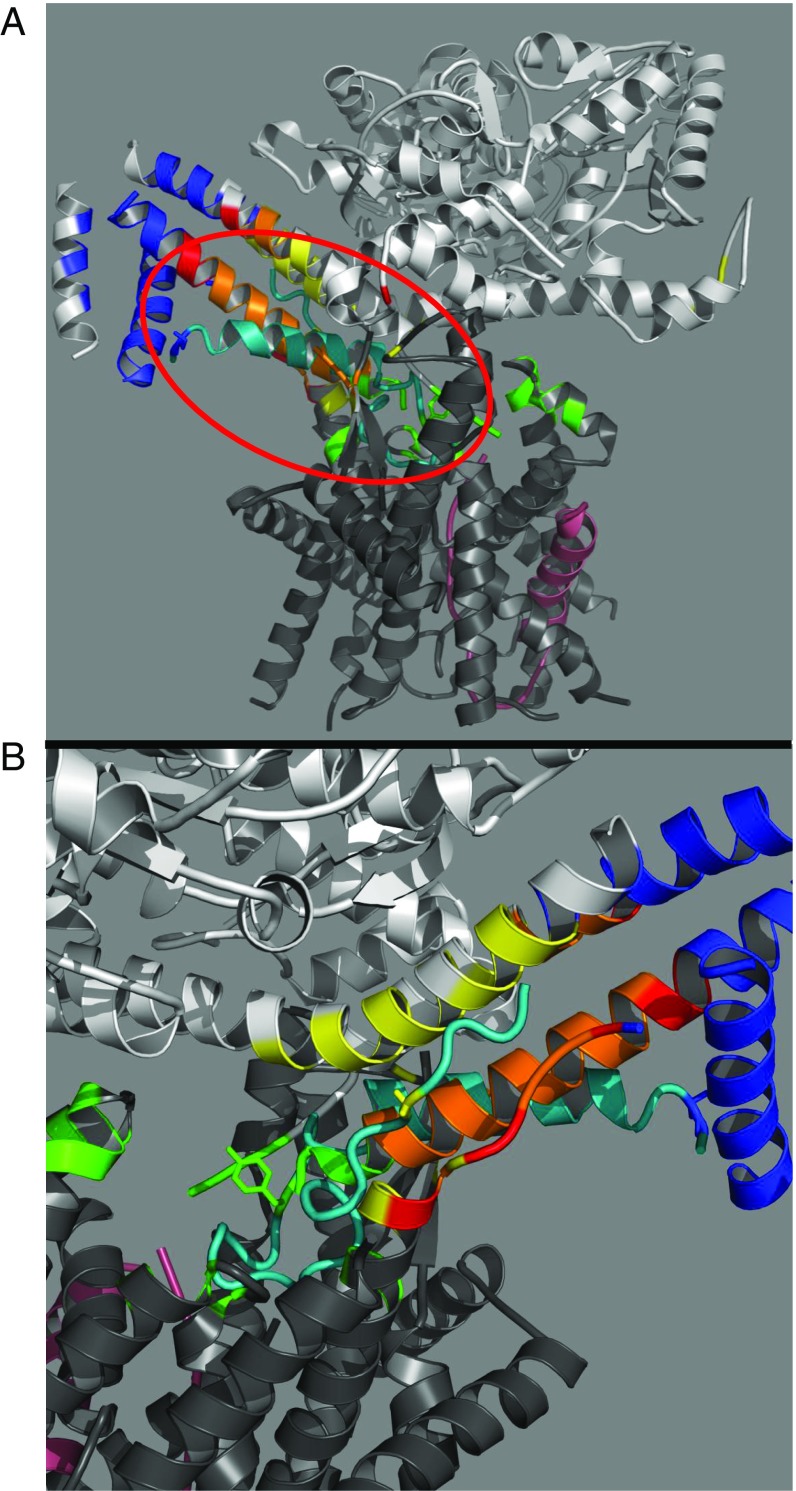
As shown in Fig. 3, we have used the OmpA signal peptide and early mature region hairpin (depicted in pink) from the Li et al. structure to model the location of its PhoA counterpart (depicted in cyan) in the preinsertion state. To generate this structure, the structurally unaltered OmpA hairpin was modeled into our FRET-identified locations. The relative accuracy of the proposed structure can be assessed by comparing specific locations along the OmpA hairpin with their mapped PhoA counterparts. As shown in Fig. 3B, the residues at positions 2 (Lys), 22 (Tyr), and 37 (Gly) of the OmpA hairpin are shown in blue, green, and yellow, respectively, and they match up exactly with the regions identified by our FRET measurements. Although the OmpA peptide is truncated at position 41, if it were extended, the additional segment would completely agree with our predicted position for PhoA45. In fact, the unstructured C-terminal end of SecY (which lies parallel to the unstructured region of the OmpA hairpin) also provides a model demonstrating how the early mature region of the peptide substrate could be binding within this region. The modeled-in hairpin structure further illustrates how the regions defined by PhoA2 and PhoA45 lie proximal to one another and distal from the channel opening. The excellent agreement observed between the structure of the OmpA signal peptide and early mature region and our FRET-based predictions further supports a model in which the initial substrate hairpin is templated along the THF before insertion into the channel.
Fig. 3.
(A) FRET-mapped regions projected on the B. subtilis SecA–Geobacillus thermodenitrificans SecYE cocrystal structure (PDB ID code 5EUL). SecA is shown in light gray, SecYE is in dark gray, and the OmpA peptide substrate inserted at the end of the THF is shown in pink. For clarity, the nanobody crystallized with the complex has been omitted (10). Generation of FRET-mapped regions and their associated colors in the presence of ATP-γS was done as described in Fig. 2. Circled in red is the peptide substrate (residues 749–791) (shown in cyan) excised from the original 5EUL PDB structure and modeled into the mapped regions without any alteration of the original structure. (B) Enlarged view of the modeled peptide (cyan) and mapped locations. Residues 2 (Lys), 22 (Tyr), and 37 (Gly) of the OmpA peptide are shown in a stick representation in blue, green, and yellow, respectively, and exhibit excellent agreement with the PhoA-mapped locations. Note the adjacent C-terminal portion of SecY discussed in the text.
SI Materials and Methods
Materials.
LB (Miller) broth and agar were obtained from Fisher Scientific and Difco, respectively. Other common chemicals were obtained from Sigma or a comparable supplier and were reagent- or spectroscopic-grade quality. Alexa Fluor 488 DIBO Alkyne or maleimide and Alexa Fluor 647 DIBO Alkyne or maleimide were purchased from Life Technologies. H-4-Azido-Phe-OH was purchased from BACHEM. DDM was purchased from Anatrace. E. coli alkaline phosphatase signal peptide SP22, MKQSTIALALLPLLFTPVTKAC-NH2, or extended signal peptide SP41, MCKQSTIALALLPLLYTPVTKARTPEMPVLENRAAQGDITA-NH2, were synthesized (Biomolecules Midwest), HPLC purified, and labeled at cysteine with IANBD ester (N-[2-(iodoacetoxy)ethyl]-N-methyl-amino-7-nitrobenz-2-oxa-1,3-diazole) (Molecular Probes); the carboxyl terminus of the peptide was capped with an amide to prevent an unnatural negative charge; and they were repurified as previously described (42). Peptide identity was verified with electrospray ionization mass spectrometry at the Keck Biotechnology Resource Laboratory at Yale University, and peptide concentration was determined by amino acid analysis at the Keck Biotechnology Resource Laboratory at Yale University or Molecular Biology Core Facilities at Dana Farber Cancer Institute.
Expression and Purification of SecA Protein and SecA–PhoA Chimeras.
Plasmid-containing strains were subcultured by a 50-fold dilution from overnight cultures prepared in LB-containing appropriate antibiotics (100 µg/mL ampicillin and 25 µg/mL chloramphenicol, where needed) and 1 mM H-4-azido-Phe-OH grown at 37 °C. Cultures were induced at an A600 of 0.4 with 0.5 mM IPTG and 0.02% arabinose and grown for an additional 1 h. Cells were harvested by sedimentation at 15,000 × g for 15 min at 4 °C, resuspended in TKM buffer (10 mM Tris⋅HCl, pH 7.5, 50 mM KCl, 10 mM MgOAc, and 1 mM PMSF), and broken in the French press at 8,000–10,000 psi, and unbroken cells and membranes were removed by sedimentation at 125,000 × g for 30 min at 4 °C. Proteins were purified using a His-Bind resin column (Novagen) according to the manufacturer’s protocol. Purified proteins were dialyzed in TKE buffer (25 mM Tris⋅HCl, pH 7.5, 25 mM KCl, 0.5 mM EDTA, and 0.5 mM PMSF) overnight, and protein concentration was measured by protein absorbance at 280 nm with an extinction coefficient of 75,750 M−1⋅cm−1. The SecA and SecA–PhoA chimeras were analyzed by SDS/PAGE for purity (Fig. S7).
Fig. S7.
SDS/PAGE analysis of purified SecA, SecA–PhoA chimeras, and SecYEG protein. Proteins were purified as described in SI Materials and Methods. (A) SecA purified through His-bind resin, where the lysate, flow-through, first and second washes, and specific eluate (L, FT, W1, W2, and E, respectively) are indicated. (B) Purified SecA834(Cys321)–GS–PhoA chimeras with azidophenylalanine incorporation at PhoA residue 2, 22, 37, 45, or the chimera lacking azidophenylalanine (lanes 1–5, respectively) before dye labeling. (C) SecYEG protein purified through His-bind resin followed by gel filtration through a Superdex S200 column. SecE and SecG proteins, which are both under 20 kDa, were not well resolved in this gel system. Molecular-weight protein standards are given in the left lane of each panel, which is labeled “Marker” along with arrows pointing to specific sizes.
Expression and Purification of SecYEG Protein.
Strains were subcultured by a 50-fold dilution from overnight cultures prepared in LB-containing appropriate antibiotics (100 µg/mL ampicillin) and grown at 37 °C. Cultures were induced at an A600 of 0.4 with 0.02% arabinose and grown for 4 h. Four liters of cell culture was harvested by sedimentation at 15,000 × g for 15 min at 4 °C, resuspended in 80 mL of TKM buffer, and broken in the French press at 8,000–10,000 psi. Unbroken cells were removed by sedimentation at 3,000 × g for 15 min at 4 °C, and membranes were harvested by sedimentation at 125,000 × g for 1 h at 4 °C. Membrane pellets were resuspended in 150 mL of TNG buffer (20 mM Tris⋅HCl, pH 7.5, 200 mM NaCl, 10% glycerol) with 1% DDM, and solubilized by shaking for 2 h at 4 °C, followed by resedimentation at 125,000 × g for 30 min at 4 °C to remove any insoluble material. The supernatant was mixed with 10 mL of His-bind resin and shaken for 2 h at 4 °C before loading onto the column with gravity flow. The column was first washed with 50 mL of TNG buffer with 0.1% DDM, followed by washing with 200 mL of TNG buffer with 0.1% DDM and 20 mM imidazole. His-tagged SecYEG was eluted with 50 mL of TNG buffer with 0.1% DDM and 250 mM imidazole. SecYEG protein was further purified by a size exclusion column (Superdex 200, 10/300 GL, GE-AKTA purifier; 0.5 mL/min flow rate at 23 °C) using a 1-mL load volume and eluted with TNG buffer with 0.1% DDM, supplemented with 1× protease inhibitor mixture (Sigma). Protein concentration was measured by absorbance at 280 nm with an extinction coefficient of 71,000 M−1⋅cm−1 for SecYEG protein. The SecYEG complex was analyzed by SDS/PAGE for purity (Fig. S7).
Protein Labeling.
Each purified protein was labeled with dye on ice at a protein to dye molar ratio of 1:10 for 2–4 h in TKE buffer for SecA or TNG buffer with 0.1% DDM for SecYEG protein. Free dye was removed from SecA using extensive overnight dialysis in TKM buffer at 4 °C followed by use of an Amicon Ultra-4 Centrifugal filter (50-kDa Mr cutoff) (Pierce). Free dye was removed from SecYEG protein by size exclusion chromatography (as described above) followed by use of an Amicon Ultra-4 Centrifugal filter (50-kDa Mr cutoff). Labeled SecA or SecA–PhoA chimeras were used directly in experiments, while SecYEG protein was often stored at −80 °C after rapid freezing in liquid nitrogen. Labeling efficiency was calculated as described in the manufacturer’s protocol (Molecular Probes) and was found to be less than 2% for nonspecific labeling.
Preparation of Samples for Fluorescence.
A series of doubly labeled SecA834–GS–PhoA68 chimera proteins were generated where PhoA residue 2, 22, 37, or 45 was labeled with Alexa Fluor 488 (AF488) using click chemistry, while SecA Cys321 or Cys37 was labeled with Alexa Fluor 647 (AF647) using maleimide chemistry. In one experiment, 100 nM doubly labeled chimera was incubated with 400 nM SecYEG in high-salt TKM buffer with 0.1% DDM. In another experiment, 50 nM labeled SecYEG (SecY Cys292–AF488) was incubated with 400 nM SecA–PhoA chimera proteins (labeled with AF647 at PhoA residue 2, 22, 37, or 45) in the same buffer as indicated above. FRET distances were calculated from the steady-state fluorescence data as described below.
Fluorescence Intensity Measurements.
Steady-state fluorescence spectra were collected on a Fluoromax 4 (Horiba) spectrofluorometer with a programmable water bath (Thermo Scientific). Samples were placed in a 3-mm quartz cuvette with 200-µL volume at the indicated temperature. The excitation and emission slits were set at a bandpass of 2 and 4 nm, respectively, for the Alexa Fluor dye pairs. For these dye pairs, the sample was excited at 493 nm and emission spectra were recorded from 508 to 750 nm. Data were collected at 0.1 s/data point and 1 nm/data point. Final results were obtained from at least three individual experiments.
Fluorescence Anisotropy Measurements.
These measurements were obtained with the same instrument as the fluorescence intensity data using 200-µL samples in a 3-mm square quartz cuvette at 293 K. Samples contained 1 µM IANBD-labeled SP22 or SP41 in a high-salt TKE buffer, and SecA protein was titrated over a concentration range from 0 to 40 µM with an incubation time of 45 min before data collection. Samples were excited at 465 nm and measured at 550 nm. The spectral bandwidths of the excitation and emission silts were set at 4 and 6 nm, respectively. Data were fit assuming a 1:1 binding interaction using ORIGIN (version 9.1) software with the following equation:
| [S1] |
where [SP] is the total concentration of signal peptide, [P] is the total concentration of SecA or SecA–PhoA protein, Kd is the equilibrium dissociation constant, A0 is the anisotropy of the signal peptide in the absence of SecA or SecA–PhoA protein, and Ai is the anisotropy under saturating binding conditions.
Steady-State FRET Calculation.
All spectra were corrected for background contributions of the buffer. Donor- or acceptor-only spectra were collected in the presence of the unlabeled counterpart to correct for any changes in fluorescence intensity as a consequence of binding. The FRET efficiency, E, was calculated from the ratio of the donor fluorescence intensity in the FRET complex relative to the donor-only emission in the presence of unlabeled ligand, using the following equation (43):
| [S2] |
where FDA and are the fluorescence intensities of the doubly labeled and donor-only samples; fA is the acceptor labeling efficiency in the doubly labeled complex. The fluorescence of the donor is corrected for any differences in labeling with the doubly labeled sample using the following equation:
| [S3] |
where is the labeling efficiency of the donor-only molecule and fD1 is the labeling efficiency of the donor on the doubly labeled molecule. Sufficient concentrations of the SecA chimera and SecYEG were used to ensure that the binding was saturated.
In the case of the acceptor-labeled SecA–PhoA chimera and donor-labeled SecYEG, transfer efficiency was calculated in the same manner. For the donor-labeled SecYEG, the labeling efficiency was close to 100% and all donor molecules were saturated by acceptor binding.
The efficiency of energy transfer is related to R0, the Förster distance, and R, the distance between donor and acceptor, by the following equation:
| [S4] |
R0 is defined as the distance at which the transfer is 50% efficient and was calculated (in angstroms) as follows (43):
| [S5] |
In Eq. S5, n is the refractive index [assumed to be 1.4 for biomolecules in aqueous solution (43)], κ is the orientation factor (κ2 was assumed to be 2/3 for a randomly oriented, mobile donor and acceptor pair), and QD is the quantum yield of the donor in the absence of acceptor. J(λ) is the overlap integral between donor emission and acceptor absorption. J(λ), QD, and errors resulting from orientation factors (κ2max) were calculated based on the methods described previously (21, 30, 31).
Time-Resolved Fluorescence Intensity Decay Spectra.
Time-resolved fluorescence measurements were performed using time-correlated single-photon counting methodology (PTI TimeMaster instrument). Samples were excited at 490 nm using a 490-nm LED-laser (Horiba 1684-LED) with a repetition rate of 180 kHz. The instrument response function (IRF) was measured to have a FWHM of 1.8 ns. Emission was detected at 520 nm with a time window of 75 ns. Spectra were collected to 20,000 in the peak channel with a 20-nm spectral bandpass for the excitation and emission slits. TR-FRET data were obtained on donor-only (AF488) singly labeled and donor–acceptor (AF488 and AF647) doubly labeled samples. Samples were prepared using the same concentrations and binding conditions used for the steady-state fluorescence measurements as described above. Samples were continuously stirred during acquisition and maintained at 293 K with a temperature bath.
The fluorescence intensity (I) as a function of time, t, was modeled using a multiexponential decay equation:
| [S6] |
where αi is a preexponential factor for the ith component and τi is the lifetime. Analysis of the decays yielded essentially one lifetime component and the quality of the fits was judged by the χ2 values and visual inspection of the residuals (Table S5).
Energy transfer can be calculated from the fluorescence lifetimes using the following equation:
| [S7] |
where τDA is the lifetime of the donor in the presence of the acceptor and τD is the lifetime of the donor only. For this study, the amplitude-weighted lifetime was used, as it is proportional to the area under the decay curve and the steady-state fluorescence intensity (43):
| [S8] |
The average transfer efficiency <E> was calculated from the amplitude-weighted lifetimes using the following equation:
| [S9] |
where IDA and ID are the donor excited-state decay in presence and absence of the acceptor, respectively. Data fitting and analysis were performed using PTI Felix GX 4.1.0.4096 (Horiba) and GlobalsWE (Laboratory for Fluorescence Dynamics, University of California, Irvine) data acquisition programs. Comparable fits were obtained from both software packages. To model the FRET distributions without any bias, the donor-only and the donor–acceptor decays were globally fit using an iterative reconvolution method linking decay terms and amplitudes between models. The χ2 value was used to monitor the “goodness of fit” along with visual inspection of the residuals. All fits reported have χ2 values less than 1.3, unless otherwise reported. Models including multiple decay components were evaluated; however, inclusion of a second decay component did not improve the fit. Values from the fit of the fluorescence decay curves were determined from a minimum of two separate experiments, and the plots were generated using Origin 9.2.214 (MicroCal).
Mapping of Substrate Sites Within the SecA–SecYEG Complex.
The overlap regions between the SecA–SecYEG structure (PDB ID code 3DIN) and the FRET dataset (Tables S2–S4) were determined from the intersection of the three spherical shells generated from the FRET distances for a specific labeled position on the SecA–PhoA chimera protein (shown in Fig. S4 for PhoA22) and the selected atoms of the SecA–SecYEG structure. The thickness of the shell was defined by the maximum and minimum distances determined for that FRET pair based on the measured error. The shells were generated using standard selection algebra commands in the program PyMOL (Schrodinger) (https://pymolwiki.org/index.php/Selection_Algebra). For each shell, we made use of the “around” command for the maximum distance and “beyond” commands for the minimum distance and the Boolean “and” logical operator to create the set of atoms. A sample script for identifying the overlapping region would be as follows:
PyMOL > select a37sp2-1, chain a and resi 30 around 87.9
Selector: selection “a37sp2-1” defined with 16664 atoms.
select a37sp2res, a37sp2-1 beyond 56.18 of chain a and resi 30
Selector: selection “a37sp2res” defined with 10500 atoms.
PyMOL > select a321sp2-1, chain a and resi 346 around 60.6
Selector: selection “a321sp2-1” defined with 10562 atoms.
PyMOL > select a321sp2res, a321sp2-1 beyond 38.6 of chain a and resi 346
Selector: selection “a321sp2res” defined with 5512 atoms.
select y292sp2-1, chain c and resi 289 around 88.7
Selector: selection “y292sp2-1” defined with 9822 atoms.
PyMOL > select y292sp2res, y292sp2-1 beyond 55.5 of chain c and resi 289
Selector: selection “y292sp2res” defined with 5670 atoms.
PyMOL > select sp2atpres, a30sp2res and a321sp2res and y292sp2res
Selector: selection “sp2atpres” defined with 656 atoms.
We did observe some of the PhoA residue overlap occurring on the opposite end of the SecA central helix from our assigned substrate-binding site. These mapped regions are small and occur only for one PhoA residue and could arise from our low-resolution method for determining the overlap area or the inherent flexibility of the substrate.
Discussion
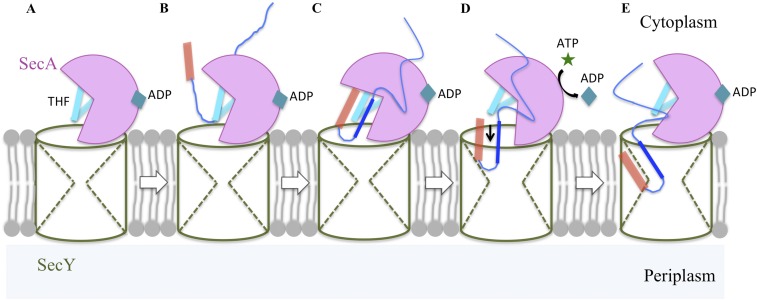
The major goal of our study was to determine the location of the amino-terminal portion of the substrate protein within the SecA–SecYEG complex in a preinsertion state. Previously, it was unclear (i) whether SecA contains two distinct signal peptide-binding sites: one for initial recognition by SecA in solution and another for later use within the SecA–SecYEG complex, (ii) whether SecA also contains a region for binding the early mature region of the substrate that is distinct from its signal peptide-binding site(s), and (iii) whether the initial substrate hairpin-like structure that is requisite for the initiation of protein transport forms before or concurrent with substrate entry into the channel. Our study satisfactorily addresses all three questions. In particular, we have visualized a unique preinsertion intermediate state before the deeper penetration of substrate into the channel proper. In this unique form, the signal peptide and early mature region of the substrate form a hairpin-like conformation lying along the SecA THF subdomain with their two structures in approximate register. The agreement between the PhoA and OmpA (shown in Fig. 3) substrate topologies is striking and attests to the presumably universal nature of the hairpin conformation for substrate entry into the Sec-dependent protein transport pathway. Our results expand the proposed function of the THF subdomain as a molecular ratchet (16, 17), suggesting that it first serves to template the substrate into a hairpin for subsequent entry into the channel (Fig. S6).
Fig. S6.
Schematic representation of SecA priming of protein transport. Stage A represents ADP-bound SecA binding to SecYEG, thus forming an inactive SecA–SecYEG binary complex; stages B and C correspond to the recognition and binding of the preprotein substrate to SecA–SecYEG to form the ternary complex whereby the signal peptide (in orange) and early mature region (in dark blue) of the substrate bind to the THF (in cyan) of SecA to adopt their hairpin structure characteristic of the preinsertion state; stages D and E depict the activation of the ternary complex whereby nucleotide exchange and ATP hydrolysis at SecA allows insertion of the substrate hairpin into the SecY channel to adopt the postinsertion state. Subsequent ATP hydrolytic cycles promote SecA ratcheting function, which along with Brownian motion, drive substrate proteins across the SecY channel.
Our results suggest an obvious model where the THF and adjacent regions form two conjoined substrate-binding sites: one for the signal peptide and another for the early mature region that lie on opposite sides of the finger (shown in Fig. 3 A and B, respectively). We note that the first site coincides with our previously mapped solution state SecA-signal peptide-binding site (20) (Fig. 1), indicating this portion of the substrate does not reposition itself after SecYEG association. We also note that both sites have potential alternative binding partners in the absence of substrate, namely the C-terminal end of SecA (shown in red in Fig. 1) for the signal peptide-binding site and the C-terminal end of SecY (shown in FRET-mapped colors in Fig. 3B) for the early mature region-binding site. It appears likely that these alternative partners occupy these sites in the absence of substrate and potentially play regulatory roles in controlling substrate binding or channel insertion based on the existing literature (21, 34, 35).
The identification of an early mature region-binding site within SecA provides a structural handle to potentially understand how substrates with defective or missing signal peptides are accommodated for transport in certain Sec (Prl) mutants or why substitutions of multiple positively charged amino acid residues within the early mature region of substrates (so-called Sec-avoidance sequences) strongly inhibit their transport (36, 37). Regarding the proofreading activity of the translocon, we note that the existence of a preinsertion intermediate state that is linked to the SecA ATP binding and hydrolysis cycle allows the SecA–SecYEG complex to “scan” the substrate during the templating process and potentially “reject” it in a more readily reversible fashion than later on when the substrate has inserted into the channel proper. Our results imply that both formation of the proper SecA–SecYEG complex and correct placement of the substrate loop within it would be required for activation of SecA ATPase and SecA–SecYEG proofreading activities. The ADP and ATP-γS–bound preinsertion states depicted here could represent good working models of such proofreading steps, which have remained elusive.
Our data also point to the importance of the CH of SecA in binding and positioning the tip of the hairpin (PhoA22, green, Fig. 2D) adjacent to the channel opening, particularly in the ADP-bound state. Previous work has demonstrated the importance of this region of SecA for coupling its ATPase activity to substrate translocation (38). In this context, substrate contact with one end of the remarkably long CH subdomain, which is also in contact with NBD-1, NBD-2, and SecY protein, could provide the appropriate signaling for activation of SecA translocation ATPase activity and its coupling to substrate translocation. The role of the CH subdomain in potentially integrating these different events now deserves further study.
The preinsertion complex visualized in our study extends the SecA power stroke or ratchet model that relies on the THF subdomain for positioning and pushing substrate into and across the SecY channel in a processive manner powered by the SecA DEAD ATPase motor (discussed with references in ref. 15). The ADP and ATP-γS–bound states characterized here indeed depict modest movement of the substrate into the channel, but are clearly insufficient to explain the observed ∼20-aa step size for substrate transport during a single ATP turnover cycle. However, step size may differ significantly for the SecA–SecYEG complex in its preinsertion versus postinsertion states. In addition, protein translocation in this system appears to use both SecA-dependent pushing and Brownian motion-dependent sliding to achieve efficient transport (33). Since the mobility and role of the THF subdomain in protein transport remain controversial (39, 40), additional studies will be required to resolve this matter. Further studies can now address the biochemical and structural requirements needed to transition from the preinitiation to postinitiation states. In that regard, our FRET-based mapping methodology that makes use of functional protein chimeras coupled with site specific dye labeling provides a compelling approach to address this problem.
Materials and Methods
Construction, Expression, and Purification of SecA, SecA–PhoA Chimeras, and SecYEG Proteins.
A series of E. coli SecA mutants or SecA–PhoA chimeras were constructed as described in Table S1. DH5α [F− ϕ80lacZΔ M15Δ (lacZYA-argF)U169 recA1 endA1 hsdR17(rK− mK+) phoA supE44 thi-1 gyrA96 relA1] was used for all plasmid construction and purification, and DNA sequence analysis was performed at the University of Pennsylvania DNA-Sequencing Facility. E. coli BL21.19 [secA13(Am) supF(Ts) trp(Am) zch::Tn10 recA::CAT clpA::KAN] is derived from BL21(λDE3) (41) and was used as the host for all SecA- or SecA–PhoA-containing plasmids. Expression of SecA or SecA–PhoA mutants requiring incorporation of H-4-azido-Phe-OH at amber codons used the pEVOL-pAzF plasmid along with the appropriate pT7SecA or pT7SecA–PhoA plasmid (22) in E. coli BLR(λDE3). C43(DE3) [ompT hsdSB (rB− mB−) gal dsm (λDE3)] was used as the host for all SecYEG-containing plasmids. Expression and purification of SecA, SecA–PhoA chimeras, and SecYEG proteins are described in the SI Materials and Methods and are shown in Fig. S7.
Fluorescence Measurements.
Steady-state fluorescence spectra were collected on a Fluoromax 4 (Horiba) spectrofluorometer. Time-resolved fluorescence decays were collected by the time-correlated single-photon counting method (PTI Timemaster) using a 490-nm LED laser (rep rate, 180 kHz). Acquisition and analysis details are given in SI Materials and Methods.
SecA ATPase Activity.
SecA ATPase activity was measured for all of the chimeras and was determined by the Malachite green method with the modifications described previously (20). In addition to using conventional inverted membrane vesicles, we used purified SecYEG protein and high-salt TKM buffer with 0.1% DDM in our assay. The PhoA portion of the SecA–PhoA chimera served as the substrate protein in lieu of additional proOmpA for measurements performed with the chimera.
Acknowledgments
We thank T. Rapoport (Harvard Medical School) and Peter Schultz (Scripps) for provision of the SecYEG-producing and azido-phenylalanine–incorporating plasmids, respectively. Q.Z. thanks his committee members Manju Hingorani and Richard Olson for their helpful suggestions. This work was supported by National Institutes of Health Grant GM110552 (to D.O.) and National Science Foundation Grant MCB-0843656 (to I.M.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1702201114/-/DCSupplemental.
References
- 1.Park E, Rapoport TA. Mechanisms of Sec61/SecY-mediated protein translocation across membranes. Annu Rev Biophys. 2012;41:21–40. doi: 10.1146/annurev-biophys-050511-102312. [DOI] [PubMed] [Google Scholar]
- 2.Van den Berg B, et al. X-ray structure of a protein-conducting channel. Nature. 2004;427:36–44. doi: 10.1038/nature02218. [DOI] [PubMed] [Google Scholar]
- 3.Tsukazaki T, et al. Conformational transition of Sec machinery inferred from bacterial SecYE structures. Nature. 2008;455:988–991. doi: 10.1038/nature07421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Egea PF, Stroud RM. Lateral opening of a translocon upon entry of protein suggests the mechanism of insertion into membranes. Proc Natl Acad Sci USA. 2010;107:17182–17187. doi: 10.1073/pnas.1012556107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frauenfeld J, et al. Cryo-EM structure of the ribosome-SecYE complex in the membrane environment. Nat Struct Mol Biol. 2011;18:614–621. doi: 10.1038/nsmb.2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hizlan D, et al. Structure of the SecY complex unlocked by a preprotein mimic. Cell Rep. 2012;1:21–28. doi: 10.1016/j.celrep.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park E, et al. Structure of the SecY channel during initiation of protein translocation. Nature. 2014;506:102–106. doi: 10.1038/nature12720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tanaka Y, et al. Crystal structures of SecYEG in lipidic cubic phase elucidate a precise resting and a peptide-bound state. Cell Rep. 2015;13:1561–1568. doi: 10.1016/j.celrep.2015.10.025. [DOI] [PubMed] [Google Scholar]
- 9.Voorhees RM, Hegde RS. Structure of the Sec61 channel opened by a signal sequence. Science. 2016;351:88–91. doi: 10.1126/science.aad4992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li L, et al. Crystal structure of a substrate-engaged SecY protein-translocation channel. Nature. 2016;531:395–399. doi: 10.1038/nature17163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ng DT, Brown JD, Walter P. Signal sequences specify the targeting route to the endoplasmic reticulum membrane. J Cell Biol. 1996;134:269–278. doi: 10.1083/jcb.134.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee HC, Bernstein HD. The targeting pathway of Escherichia coli presecretory and integral membrane proteins is specified by the hydrophobicity of the targeting signal. Proc Natl Acad Sci USA. 2001;98:3471–3476. doi: 10.1073/pnas.051484198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lycklama A, Nijeholt J, Driessen A. The bacterial Sec-translocase: Structure and mechanism. Philos Trans R Soc Lond B Biol Sci. 2012;367:1016–1028. doi: 10.1098/rstb.2011.0201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kimura E, Akita M, Matsuyama S, Mizushima S. Determination of a region in SecA that interacts with presecretory proteins in Escherichia coli. J Biol Chem. 1991;266:6600–6606. [PubMed] [Google Scholar]
- 15.Kusters I, Driessen AJ. SecA, a remarkable nanomachine. Cell Mol Life Sci. 2011;68:2053–2066. doi: 10.1007/s00018-011-0681-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zimmer J, Nam Y, Rapoport TA. Structure of a complex of the ATPase SecA and the protein-translocation channel. Nature. 2008;455:936–943. doi: 10.1038/nature07335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Erlandson KJ, et al. A role for the two-helix finger of the SecA ATPase in protein translocation. Nature. 2008;455:984–987. doi: 10.1038/nature07439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duffaud GD, Lehnhardt SK, March PE, Inouye M. Structure and function of the signal peptide. In: Cook J, editor. Current Topics in Membranes and Transport. Vol 24. Academic; New York: 1985. pp. 65–104. [Google Scholar]
- 19.Plath K, Mothes W, Wilkinson BM, Stirling CJ, Rapoport TA. Signal sequence recognition in posttranslational protein transport across the yeast ER membrane. Cell. 1998;94:795–807. doi: 10.1016/s0092-8674(00)81738-9. [DOI] [PubMed] [Google Scholar]
- 20.Auclair SM, et al. Mapping of the signal peptide-binding domain of Escherichia coli SecA using Förster resonance energy transfer. Biochemistry. 2010;49:782–792. doi: 10.1021/bi901446r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Q, Li Y, Olson R, Mukerji I, Oliver D. Conserved SecA signal peptide-binding site revealed by engineered protein chimeras and Förster resonance energy transfer. Biochemistry. 2016;55:1291–1300. doi: 10.1021/acs.biochem.5b01115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chin JW, et al. Addition of p-azido-l-phenylalanine to the genetic code of Escherichia coli. J Am Chem Soc. 2002;124:9026–9027. doi: 10.1021/ja027007w. [DOI] [PubMed] [Google Scholar]
- 23.Duong F. Binding, activation and dissociation of the dimeric SecA ATPase at the dimeric SecYEG translocase. EMBO J. 2003;22:4375–4384. doi: 10.1093/emboj/cdg418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robson A, Booth AE, Gold VA, Clarke AR, Collinson I. A large conformational change couples the ATP binding site of SecA to the SecY protein channel. J Mol Biol. 2007;374:965–976. doi: 10.1016/j.jmb.2007.09.086. [DOI] [PubMed] [Google Scholar]
- 25.Das S, Oliver DB. Mapping of the SecA·SecY and SecA·SecG interfaces by site-directed in vivo photocross-linking. J Biol Chem. 2011;286:12371–12380. doi: 10.1074/jbc.M110.182931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Das S, Stivison E, Folta-Stogniew E, Oliver D. Reexamination of the role of the amino terminus of SecA in promoting its dimerization and functional state. J Bacteriol. 2008;190:7302–7307. doi: 10.1128/JB.00593-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bessonneau P, Besson V, Collinson I, Duong F. The SecYEG preprotein translocation channel is a conformationally dynamic and dimeric structure. EMBO J. 2002;21:995–1003. doi: 10.1093/emboj/21.5.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kusters I, et al. Quaternary structure of SecA in solution and bound to SecYEG probed at the single molecule level. Structure. 2011;19:430–439. doi: 10.1016/j.str.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 29.Economou A, Wickner W. SecA promotes preprotein translocation by undergoing ATP-driven cycles of membrane insertion and deinsertion. Cell. 1994;78:835–843. doi: 10.1016/s0092-8674(94)90582-7. [DOI] [PubMed] [Google Scholar]
- 30.Ivanov V, Li M, Mizuuchi K. Impact of emission anisotropy on fluorescence spectroscopy and FRET distance measurements. Biophys J. 2009;97:922–929. doi: 10.1016/j.bpj.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Auclair SM, Oliver DB, Mukerji I. Defining the solution state dimer structure of Escherichia coli SecA using Förster resonance energy transfer. Biochemistry. 2013;52:2388–2401. doi: 10.1021/bi301217t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schiebel E, Driessen AJM, Hartl F-U, Wickner W. Delta mu H+ and ATP function at different steps of the catalytic cycle of preprotein translocase. Cell. 1991;64:927–939. doi: 10.1016/0092-8674(91)90317-r. [DOI] [PubMed] [Google Scholar]
- 33.Bauer BW, Shemesh T, Chen Y, Rapoport TA. A “push and slide” mechanism allows sequence-insensitive translocation of secretory proteins by the SecA ATPase. Cell. 2014;157:1416–1429. doi: 10.1016/j.cell.2014.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gelis I, et al. Structural basis for signal-sequence recognition by the translocase motor SecA as determined by NMR. Cell. 2007;131:756–769. doi: 10.1016/j.cell.2007.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chiba K, Mori H, Ito K. Roles of the C-terminal end of SecY in protein translocation and viability of Escherichia coli. J Bacteriol. 2002;184:2243–2250. doi: 10.1128/JB.184.8.2243-2250.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Derman AI, Puziss JW, Bassford PJJ, Jr, Beckwith J. A signal sequence is not required for protein export in prlA mutants of Escherichia coli. EMBO J. 1993;12:879–888. doi: 10.1002/j.1460-2075.1993.tb05728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li P, Beckwith J, Inouye H. Alteration of the amino terminus of the mature sequence of a periplasmic protein can severely affect protein export in Escherichia coli. Proc Natl Acad Sci USA. 1988;85:7685–7689. doi: 10.1073/pnas.85.20.7685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mori H, Ito K. The long α-helix of SecA is important for the ATPase coupling of translocation. J Biol Chem. 2006;281:36249–36256. doi: 10.1074/jbc.M606906200. [DOI] [PubMed] [Google Scholar]
- 39.Whitehouse S, et al. Mobility of the SecA 2-helix-finger is not essential for polypeptide translocation via the SecYEG complex. J Cell Biol. 2012;199:919–929. doi: 10.1083/jcb.201205191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Allen W, et al. Two-way communication between SecY and SecA suggest a Brownian ratchet mechanism for protein translocation. eLife. 2016;5:15598. doi: 10.7554/eLife.15598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Studier FW, Rosenberg AH, Dunn JJ, Dubendorff JW. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- 42.Musial-Siwek M, Rusch SL, Kendall DA. Probing the affinity of SecA for signal peptide in different environments. Biochemistry. 2005;44:13987–13996. doi: 10.1021/bi050882k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lakowicz JR. Principles of Fluorescence Spectroscopy. 3rd Ed Springer; New York: 2006. [Google Scholar]
- 44.Jilaveanu LB, Zito CR, Oliver D. Dimeric SecA is essential for protein translocation. Proc Natl Acad Sci USA. 2005;102:7511–7516. doi: 10.1073/pnas.0502774102. [DOI] [PMC free article] [PubMed] [Google Scholar]