Significance
The awareness of the negative consequences of biological invasions and the critical importance of evidence-based decision making have led to a persistent effort to understand the factors driving the successful invasion of exotic species and to predict invasion outcome. We assess, taking advantage of an exceptionally comprehensive dataset of exotic birds in the Iberian Peninsula, the role of different factors in the invasion success of current avian introductions. Our findings contrast with the evidence previously found in studies mostly based on deliberate introductions regarding factors influencing the invasion success of birds and show that drivers controlling the establishment and spread stages are markedly different. Our results also highlight certain challenges for managing current invasions.
Keywords: alien species, species traits, propagule pressure, life history, niche similarity
Abstract
Understanding factors driving successful invasions is one of the cornerstones of invasion biology. Bird invasions have been frequently used as study models, and the foundation of current knowledge largely relies on species purposefully introduced during the 19th and early 20th centuries in countries colonized by Europeans. However, the profile of exotic bird species has changed radically in the last decades, as birds are now mostly introduced into the invasion process through unplanned releases from the worldwide pet and avicultural trade. Here we assessed the role of the three main drivers of invasion success (i.e., event-, species-, and location-level factors) on the establishment and spatial spread of exotic birds using an unprecedented dataset recorded throughout the last 100 y in the Iberian Peninsula. Our multimodel inference phylogenetic approach showed that the barriers that need to be overcome by a species to successfully establish or spread are not the same. Whereas establishment is largely related to event-level factors, apparently stochastic features of the introduction (time since first introduction and propagule pressure) and to the origin of introduced species (wild-caught species show higher invasiveness than captive-bred ones), the spread across the invaded region seems to be determined by the extent to which climatic conditions in the new region resemble those of the species’ native range. Overall, these results contrast with what we learned from successful deliberate introductions and highlight that different management interventions should apply at different invasion stages, the most efficient strategies being related to event-level factors.
Exotic species (i.e., nonnative species intentionally or unintentionally introduced by human action in a new geographic area) are now recognized as one of the most important threats to biological diversity and have severe impacts on ecological systems and human health (1). The large number of species transported and the range of pathways by which species move have greatly increased the number and geographical extent of exotic species globally (2). However, many of the species introduced to a new region fail to survive, and of those that survive many do not successfully establish breeding populations and even fewer spread and become invasive, so that the invasion process can be divided into a series of sequential stages (transport, introduction, establishment, and spread) (3, 4). The awareness of the negative consequences of biological invasions and the critical importance of evidence-based decision making have led to a persistent effort to understand the factors driving the successful invasion of exotic species and to predict invasion outcome (5). Answering the question of what characteristics make a species likely to be a successful invader should account for the fact that each invasion stage may have its own dynamics and depend on different factors (4, 5).
A number of factors have been proposed as influential in invasion success, which can broadly be classified into three categories (6). First are event-level factors, which comprise characteristics of the release or escape, such as the number of introduction events and the number of individuals introduced (i.e., propagule pressure) (7), or invasion history (e.g., the time since introduction). Such factors have been shown to have a major effect on invasion success in several taxa (8). Second are location-level factors, which are attributes associated with the novel landscape and should also be relevant for establishment success, in particular the degree of abiotic and biotic similarity to the species’ native range (6, 9, 10). While the idea of climate matching (i.e., the degree to which the introduced location resembles the species’ native range) is implicit in many studies assessing the invasion risks by exotic species using species distribution models (SDMs) (11), very few have explicitly tested its relationship with invasion success. Third are species-level factors, which include attributes of the exotic species (e.g., life-history traits). For example, we expect that species that use a greater array of resources and maintain viable populations within a wider variety of conditions are more likely to establish breeding populations outside of their native range (12). It is also arguable that species with traits that promote fast demographic growth rates (e.g., large clutch size) should be more likely to persist since those traits reduce the risk of extinction due to environmental and demographic stochasticity (13). Similarly, behavioral flexibility, in the form of learning, cognition, and/or rapid adjustment to new conditions, should be an advantage when invading novel habitats (e.g., ref. 14). Although evidence for the effect of each of these factors exists, their relative importance is poorly understood as studies frequently focus on only a subset of factors or fail to account for phylogenetic independence (reviews in refs. 12 and 15). Furthermore, available information is often biased toward successful invasions because accidental introductions are often recorded only when they are successful, which limits our ability to derive absolute probabilities of establishment and spread success from invasiveness models (15). Similarly, the bulk of previous empirical work attempting to model invasion success has often failed to discriminate between stages, so that establishment and spread are often confounded, or has focused on only one of these two stages (e.g., refs. 12 and 16).
Because birds are among the most well-studied taxa in the world, bird invasions have been frequently used as study models (6). Ecological plasticity (12), behavioral flexibility (14), and, in particular, the introduction effort or propagule pressure (6, 17) have been positively related to successful avian invasions. Most of these comparative studies relied on species that were purposefully introduced during the 19th and early 20th centuries (e.g., refs. 12, 14 and 18), which have been rather well documented (19, 20). Consequently, much of our understanding of the determinants of avian establishment and invasion comes from studies of countries colonized by Europeans, such as Australia, New Zealand, and North America, where early acclimatization societies purposefully introduced a wide range of species, mostly from Europe, for hunting, combating plagues, or aesthetic/romantic purposes. However, exotic birds currently become entrained in the invasion process primarily through unplanned releases (mainly accidental) of individuals from the pet and avicultural trade (21–23). Because early introductions by acclimatization societies were biased toward species with particular characteristics, the attributes proposed to account for a species’ chance to establish and spread outside its natural range might be different in ongoing invasions.
Here we assessed the role of different factors in the invasion success of current avian introductions. Notably, we aimed to investigate the factors that influence the establishment success (i.e., self-sustaining, exotic populations) and spatial spread in a region without a history of recent European colonization using a multimodel inference approach in a phylogenetic comparative framework. For this purpose, we used a comprehensive database of exotic birds in the Iberian Peninsula (i.e., mainland Spain and Portugal) that is, to our knowledge, the largest and most complete dataset on exotic birds existing at a regional level (23). Our dataset is based on an unprecedented search for introduced, but not necessarily established, species and covers 100 y.
Results and Discussion
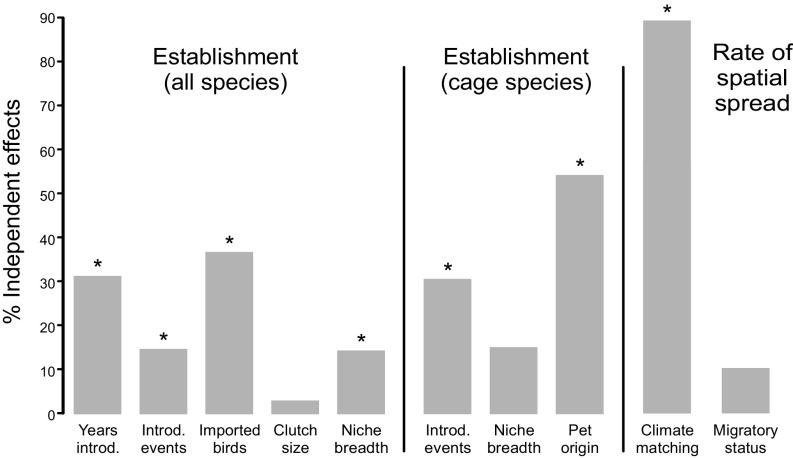
The results of the phylogenetic regressions show that the establishment success and the spatial spread of exotic birds in the study area are controlled to a large extent by different drivers (Fig. 1 and Tables 1 and 2; see also SI Appendix, Tables S1 and S2 for results of nonphylogenetic analyses). Establishment success was mainly influenced by event-level factors and to a lesser extent by species-level factors. Contrastingly, the spatial spread of the subset of established species across the study region was driven by location-level factors with a limited role of event-level factors.
Fig. 1.
Hierarchical partitioning showing independent effects of different factors on establishment success and the rate of spatial spread. The left and middle panels show variance explained by the subset of variables selected in the best PLR models on the establishment success for the whole dataset and considering only cage species, respectively, while the right panel shows variance explained by the subset of variables selected in the best PGLS model on the rate of spatial spread in established species. The asterisk denotes significance at the P < 0.05 level. Abbreviations as follows: Introd. events, number of introduction events; Pet origin, wild-caught/captive-bred status; Years introd., years since first introduction.
Table 1.
Results of the PLRs testing the link between the different predictors and establishment success
| Univariate models | Best model | ||||
| Variables | Coefficients | D2 | Coefficients | D2 | ΣwAIC |
| Event-level factors | |||||
| Years since introduction | 0.776 (0.731, 0.835) | 0.10 | 0.570 (0.540, 0.599) | 0.26 | 1.00 (0.97, 1.00) |
| Introduction events | 0.912 (0.877, 0.947) | 0.08 | 0.566 (0.541, 0.610) | 0.64 (0.57, 0.71) | |
| Imported birds | 0.643 (0.594, 0.682) | 0.07 | 1.012 (0.982, 1.036) | 1.00 (1.00, 1.00) | |
| Species-level factors | |||||
| Clutch size | 0.291 (0.240, 0.355) | 0.01 | 0.560 (0.527, 0.590) | 0.55 (0.43, 0.70) | |
| Brood value | 0.186 (0.149, 0.260) | 0.01 | 0.22 (0.15, 0.32) | ||
| Body mass | −0.347 (−0.403, −0.293) | 0.03 | 0.12 (0.10, 0.14) | ||
| Range size | 0.278 (0.107, 0.339) | 0.00 | 0.10 (0.08, 0.13) | ||
| Brain size | 0.361 (0.372, 0.450) | 0.03 | 0.17 (0.11, 0.30) | ||
| Niche breadth | 0.417 (0.270, 0.439) | 0.03 | 0.669 (0.64, 0.702) | 0.98 (0.96, 1.00) | |
| Migrant | −0.464 (−1.239, −0.141) | 0.02 | 0.12 (0.10, 0.14) | ||
| Location-level factors | |||||
| Climate matching | 0.136 (−0.075, 0.215) | 0.00 | 0.09 (0.07, 0.11) | ||
Standardized regression coefficients are shown for univariate models and for the best multivariate model based on AICc. The parameter estimates that are significantly different from zero (P < 0.05) are indicated by bold numbers. Relative importance of variables (ΣwAIC) based on a multimodel information theory-based approach is shown. Variables with the strongest support (ΣwAIC ≥ 0.8) are shown in bold. Regression coefficients and ΣwAIC values represent the median and the central range that contains 95% of values for 1,000 phylogenetic trees. Goodness-of-model fit, as evaluated by calculating the explained deviance (D2) from the nonphylogenetic logistic regression, is also provided.
Table 2.
Results of the PGLS models testing for the link between the different predictors and rate of spatial spread in established species
| Univariate models | Best model | ||||
| Variables | Coefficients | R2 | Coefficients | R2 | ΣwAIC |
| Event-level factors | |||||
| Years since introduction | 0.409 (0.290, 0.539) | 0.18 | 0.40 | 0.10 (0.06, 0.16) | |
| Introduction events | 0.078 (0.013, 0.277) | 0.20 | 0.08 (0.04, 0.13) | ||
| Imported birds | 0.246 (0.108, 0.646) | 0.00 | 0.12 (0.06, 0.45) | ||
| Species-level factors | |||||
| Clutch size | 0.154 (−0.433, 0.302) | 0.07 | 0.07 (0.04, 0.12) | ||
| Brood value | 0.142 (0.024, 0.251) | 0.04 | 0.11 (0.04, 0.42) | ||
| Body mass | −0.409 (−0.666, −0.025) | 0.02 | 0.06 (0.03, 0.11) | ||
| Range size | 0.149 (0.027, 0.385) | 0.02 | 0.08 (0.04, 0.42) | ||
| Brain size | −0.575 (−0.847, −0.392) | 0.02 | 0.39 (0.17, 0.71) | ||
| Niche breadth | −0.081 (−0.133, −0.004) | 0.03 | 0.06 (0.03, 0.09) | ||
| Migrant | −0.380 (−0.852, −0.271) | 0.01 | −0.525 (−0.800, −0.419) | 0.66 (0.31, 1.00) | |
| Location-level factors | |||||
| Climate matching | 0.475 (0.400, 0.545) | 0.34 | 0.485 (0.414, 0.534) | 1.00 (1.00, 1.00) | |
Standardized regression coefficients are showed for univariate models and for the best multivariate model based on AICc. Relative importance of variables (ΣwAIC) based on a multimodel information theory-based approach is shown. Variables with strong support (ΣwAIC ≥ 0.8) are shown in bold. Regression coefficients and ΣwAIC values represent the median and the central range that contains 95% of values for 1,000 phylogenetic trees. The parameter estimates that are significantly different from zero at the 0.05 level are indicated by bold numbers. Goodness-of-model fit (R2) from ordinary least squares regression is also provided.
The results of the phylogenetic logistic regressions show that the establishment success of exotic birds in the study area is mainly influenced by two event-level factors: years since first introduction and propagule pressure (Fig. 1 and Table 1). There is a bulk of empirical and statistical evidence showing propagule pressure or introduction effort as important determinants of the successful establishment and spread of exotic species, including birds (e.g., refs. 4, 6, 7, and 24, but see ref. 25). Small populations are more likely to suffer from effects of demographic and genetic stochasticity, to be extirpated by environmental stochasticity, and to suffer from the Allee effect, so increasing propagule pressure enhances establishment probability. Furthermore, the delay between initial colonization of a species, measured in years since first introduction, and its success in establishing a viable population is a common feature of biological invasions (6, 26). This variable can be seen as a proxy of propagule pressure, since longer time since introduction would allow higher numbers of cumulative released individuals and, as result, larger populations. However, the link between years since introduction and establishment success can be also related to additional population and evolutionary processes not associated with propagule pressure. Exotic birds commonly show lag phases in population growth (27), indicating that several cycles of survival and reproduction are likely to be necessary to ensure that a viable population is established (4, 28). Lag times are also expected if evolutionary change is an important part of the colonization process, which would include the evolution of adaptations to the new habitat, the evolution of invasive life-history characteristics, or the purging of genetic load responsible for inbreeding depression (28).
Remarkably, phylogenetic generalized least squares (PGLS) models showed that event-level processes have a limited effect on the rate of spatial spread for the subset of established species (Fig. 1 and Table 2), in contrast with the findings for the establishment success. Nevertheless, and as expected, our models showed that years since introduction was a key predictor of the size of the invaded range, as estimated from the number of occupied spatial cells (SI Appendix, Fig. S1 and Table S3). The longer the time since introduction, the greater the likelihood of new accidentally released individuals at different locations or new colonizations of dispersers from previously occupied sites, which would imply a greater number of cumulative occupied cells. While it has become widely recognized that years since introduction is an important determinant of the geographical range size of exotic plants (29), previous evidence has been mixed for birds (17, 30, 31).
As with event-level factors, the role of location-level factors was strikingly different between the establishment and spread stages. Surprisingly, we did not find any relationship between establishment success and climate matching (Table 1), suggesting that the similarity in environmental conditions between the native and nonnative ranges is not a significant predictor of establishment success in ongoing bird invasions (this result was consistent across the different measures of niche similarity used; see SI Appendix, Table S4). On the contrary, climate matching between introduced and native regions was the main predictor of the rate of spatial spread and the invaded range size (Fig. 1 and Table 2; see also SI Appendix, Fig. S1 and Table S3). Thus, our findings suggest that, once initial colonization and establishment have occurred, the degree to which the target region resembles the species’ native range is a critical factor regulating the spread.
The potential invasive success of exotic species is thought to be associated with similarity in climate between the native and the invaded ranges (6), so that introduced bird populations would fail to establish simply because they are introduced into environments to which they are completely maladapted. While the results of several studies of bird introductions are consistent with a role for environmental differences in establishment success (e.g., refs. 9, 10, and 32), others have shown that exotic species are able to occupy climate niches in the new range that differ substantially from those of the native range (e.g., refs. 33 and 34, but see, e.g., ref. 35). This is particularly the case for species with small native ranges, those that occupy a narrow range of climate conditions, or those that primarily occupy marginal climates in their native region (36). Similarly, the association of some species with humans may also allow them to overcome climatic constraints (37). The “urban heat island” effect is one of the best-documented climatic feature of cities (38), referring to the higher temperatures of urban areas compared with their surroundings, so human settlements may be especially favorable for birds during winter when climatic conditions are harsh and food is in poor supply. However, only 10 out of the 26 established species (38%) were initially established in urban habitats, the rest establishing in natural and rural habitats. All these 10 species were popular cage-bird species (seven parrots and three passerines), so their initial establishment in cities may be rather related to a higher abundance of cage birds in populated cities and thus a higher risk of accidental escapes (i.e., a larger propagule pressure in cities). Thus, while a high propagule pressure and other potential local factors (e.g., association with humans) can allow for the establishment of self-sustaining populations for some exotic species in areas with suboptimal climatic conditions, the availability of climatically suitable areas in the target region determines the rate of spatial spread and the invaded range size. It should be noted here that our measure of niche similarity refers to the target region (i.e., the Iberian Peninsula). Nevertheless, using the records for each species in the invaded region (i.e., the 5- × 5-km cells in which the species has been recorded), niche overlap measures highly correlated with those obtained when considering all grid cells of the Iberian Peninsula (Supporting Information). Because SDMs are often used in invasion and conservation biology to predict the potential establishment of exotic species in novel climates in time or space (11, 39), our results have important implications for the use and interpretation of SDMs. SDMs might be useful tools to predict the spread, not the establishment success, of invasive species.
Additionally, one of the central themes of invasion biology has been to identify those species traits that make species more successful invaders (e.g., refs. 3, 5, and 12). We found no significant influence for most of the studied species-level factors, with only niche breadth and, to a lesser extent, clutch size showing some relevance for establishment success (Fig. 1 and Table 1), and migratory status for spread rate. The positive correlation between niche breadth and invasion success represents one of the first attempts for generalization in invasion biology; species with broader niches (i.e., “generalists”) are more likely to invade than species with narrower niches (i.e., “specialists”), because they are more likely to find the necessary resources or conditions in the novel environment (40). In agreement with previous studies of bird introductions (6, 14), our findings support the “niche breadth-invasion success” hypothesis, which suggests that variability in resource use, as estimated from climatic niche breadth, can be important for responding to novel environments in ongoing bird invasions after controlling for event-level factors. Interestingly, native range size, estimated as extent of occurrence, was not significantly associated with invasion success, which suggests that our measure of niche breadth, focused on climatic niche, provides more accurate information than just the size of the geographic range.
Life-history traits have also been suggested to affect the ability of animals to establish viable populations in new environments, although previous studies provide contrasting results. While some studies have reported positive relationships between clutch size and establishment success (41), in agreement with the theoretical prediction that “fast” life histories facilitate establishment by promoting faster population growth, others have reported negative relationships (13), or no relation at all (12, 32). We found very little support for a positive effect of clutch size, as a proxy of fast-slow continuum, on establishment success (Table 1). The same was true for body mass. As an alternative to the population growth hypothesis, Sol et al. (13) suggested that successful invaders are better characterized by life-history strategies that prioritize future over current reproduction; however, our results do not support this view either. Furthermore, previous evidence based on deliberate introductions suggested that avian species with larger brains relative to their body mass tend to be more successful at establishing themselves in novel environments (13, 14). Contrary to this “behavioral flexibility” hypothesis for establishment success, which says that large brains confer an advantage when responding to variable, unpredictable, and novel ecological demands through enhanced behavioral flexibility, learning, and innovation, we did not find support for the role of relative brain size in establishment success in ongoing bird invasions (Table 1).
It should also be noted that factors associated with recent unintentional introductions might obscure the role of some of the studied species- and site-level factors and explain the discrepancy with previous studies based mostly on past, deliberate introductions. The subset of established species was significantly biased to cage species (20 of 26; χ2 = 6.06, df = 1, P < 0.014). When this subset of cage species (orders Passeriformes and Psittaciformes) was separately analyzed, the pet origin was the main explanatory variable in establishment success (Fig. 1 and SI Appendix, Table S5). In agreement with Carrete and Tella (21), wild-caught species were more likely to establish viable populations than captive-bred ones, even after controlling for other event-level factors such as the number of imported birds or their availability in the pet market. Carrete and Tella (21) postulated that the ability to cope with new environments seems to have been lost in species bred in captivity over a long period, as a consequence of the detrimental effects of inbreeding depression (captive-bred birds often descend from a small pool of individuals) and the erosion in captivity of their antipredatory and foraging behaviors (42). Furthermore, as pointed out above, it should be noted that past introductions were mostly the result of organized and concerted efforts by early acclimatization societies to introduce species with particular characteristics, which may not match with those from current unintentional introductions. Moreover, the repeated introduction of large amounts of individuals of particular species should have overcome population demographic processes (e.g., Allee effects) and obscured the role of other species- and site-level factors that can influence the successful establishment and spread of a species outside its natural range. As a consequence, the specific traits that may be advantageous for successfully invading new environments could vary when studying unintentional introductions. Finally, another factor that can explain the discrepancies with previous research on avian invasions is the particularities of our study region, which has a relatively mild climate. Thus, the extent to which the results of our work are applicable to other regions, or particular groups of taxa, remains to be investigated.
Overall, our findings show contrasting evidence about the factors influencing the invasion success of birds from that provided by previous studies, mostly based on deliberate introductions (see ref. 6 for a review). Furthermore, they highlight that the barriers that need to be overcome by a species to successfully establish or spread are not the same, which is in agreement with previous frameworks proposed for biological invasions (4, 5). In the establishment stage these barriers are related to survival and reproduction, so that success seems to be the result of propagule pressure and species' variability in resource use. In the spread stage, in contrast, barriers are likely related to dispersal, so that success seems to be mainly influenced by the extent to which climatic conditions in this region resemble those from the species' native range. Our results pose certain challenges for managing biological invasions, showing that different management interventions should apply at different invasion stages, as factors influencing establishment and spread are not the same. Importantly, our findings underline the difficulty of preintroduction invasion risk assessments (43), which often rely on the assumption that it is possible to predict the establishment success of a species based on its characteristics and the characteristics of the recipient environments. Because establishment success is mainly influenced by event-level factors, limiting the transport and accidental release of exotic species would be the most effective strategy (44). Thus, enhancing the security of bird-keeping enclosures in public and private facilities would reduce accidental introductions (23). Furthermore, given that captive-bred birds are much less likely to establish in the wild relative to wild-caught birds (21), one of the most effective actions for preventing avian invasions may be blocking the transport of wild-caught individuals. Our findings also show that invasion-related lags are critical for our efforts to manage invaders, as they may lead to inaccurate assessments of the risks posed by invaders as well as miss critical windows for action (26, 45). Climatic niche models, which have been pervasively used for invasion prediction and management (e.g., ref. 11), would only be useful to predict the probability of spread of already successfully established species.
Materials and Methods
Specific details of all methods are provided in SI Appendix, SI Materials and Methods.
Dataset.
We obtained data on introduced birds in the Iberian Peninsula from a comprehensive database of exotic birds in Spain and Portugal (23), which compiles records of exotic species observed in the wild in both countries from 1912 to 2012. This dataset, which is based on a systematic review of scientific and gray literature, complemented with our own data and unpublished observations from other researchers (see dataset details in ref. 23), includes over 11,200 records for 335 exotic birds in the Iberian Peninsula. To avoid a bias toward anecdotal introductions, we focused only on those species with at least five georeferenced records, so our final dataset consisted of 107 bird species (Dataset S1). Established species were those that had established self-sustaining populations or, at least, whose reproduction in the wild had been regularly verified (n = 26) (23). For the subset of established species, we estimated the increase through time in the number of occupied 5- × 5-km cells, as an estimate of the rate of spatial spread (SI Appendix, Fig. S2). While the increase in the number of new occupied cells per year may not be just the result of a population spread process, but can also be partially influenced by the release/escape of new individuals, it provides a reliable estimate to further investigate factors explaining differences in this spread rate. Additionally, we also calculated the degree of invasion or spread by quantifying the number of occupied 5- × 5-km cells in the study area as a measure of invaded range size.
Event-Level Factors.
Years since first introduction (i.e., the number of years since the species was first recorded as introduced relative to 2012) was used as a variable reflecting introduction history. Propagule pressure was estimated as the total number of live birds reported by the Convention on International Trade in Endangered Species of Wild Fauna and Flora (https://www.cites.org/) that have been legally traded from each of the native ranges to Spain and Portugal. We also used the number of introduction (or escape) events as a proxy of propagule pressure. For each species, the number of introduction events was estimated using graph theory from the geographic locations of their records. The igraph R package (46) was used to obtain a network in which any two nodes (georeferenced records) were deemed connected by an edge if they were separated by a geographic distance lower than 100 km. The number of isolated or nonconnected subnetworks present in the whole graph was then assimilated to the number of independent introduction events.
For passerines (songbirds) and parrots (orders Passeriformes and Psittaciformes, respectively), which encompass most pet or cage birds, we included two additional variables. First, we used information of their abundance in the pet market (i.e., market availability) from Carrete and Tella (21) as an additional surrogate of propagule pressure. Second, cage birds were classified as wild-caught or captive-bred species according to their main origin (hereafter “pet origin”; see classification in ref. 21).
Location-Level Factors.
We assessed the degree to which the introduced location resembles the species’ native range (i.e., climate matching between the regions of introduction and origin of the species) using two different approaches. First, we measured niche overlap between native and invaded ranges using the same approach as Broennimann et al. (47). A principal components analysis (PCA) was calibrated using global climate conditions from 19 bioclimatic variables at a five arc minutes spatial resolution. The first two axes of this PCA were then used to examine the overlap between the species’ native niche and Iberian conditions, taking into account the densities of occurrence records and climatic conditions within the species’ ranges. Two metrics of niche overlap, Schoener’s D and Hellinger’s distance (I), were calculated from the occupancies in the environmental space depicted by the two first axes of the PCA (47). An alternative measure of niche climatic matching was calculated as the distance in the environmental space between the centroid of species’ scores along PCA axes (an estimate of the center of the distribution for each species along an axis) and the centroid of Iberian conditions (scores along PCA axes).
Second, we also used SDMs to measure niche overlap between native and invaded ranges. SDMs were calibrated using occurrence data in each native range and a set of bioclimatic variables as predictors, using the Maxent modeling algorithm (48). We used seven bioclimatic variables commonly used in avian distribution modeling to denote bioclimatic controls (e.g., ref. 10) representing average and extreme climatic conditions. Results of Maxent models are summarized in Dataset S1. Because results using the different measures of climatic matching were qualitatively congruent (SI Appendix, Table S4), we report here the outputs for the measure of niche overlap between native and invaded ranges using Schoener’s D from the PCA procedure.
Species-Level Factors.
We considered several factors related to species traits.
Relative brain size.
We compiled brain-size information for a total of 1,357 bird species (both species introduced and species that have never been introduced), including 74 of those in our dataset of exotic birds, from different literature sources (see reference list in Dataset S1). To control for the allometric effect of body size on brain size, we used the residuals of log-log regressions against body mass. Then, for those species in our dataset of exotic birds for which brain mass was not available (n = 33), we used the average brain residual of the species from the same genus.
Life-history strategies.
We collected information for a set of life-history traits, namely clutch size, number of broods per year, fecundity, egg mass, incubation period, fledgling period, lifespan, and age at first breeding (sources are detailed in Supporting Information) to estimate the fast–slow continuum of life-history strategies of the different species. We performed a factor analysis to simplify the pattern of covariation among traits by positing latent variables underlying the data on information for both species introduced and species that have never been introduced. A total of 253 species, for which information was available for all of the eight traits, were used in the factor analysis, including 52 of those in our dataset of exotic birds. The confounding effect of body size was removed by regressing life‐history variables on body mass after log transformation, using ordinary least squares, and computed residuals for use in the factor analysis. The first factor was retained as an estimate of the fast–slow continuum. However, because clutch size (i.e., the residuals of log-log regression against body mass) was highly correlated with this derived variable (r = 0.91) and was available for all of the target species, we used it as a surrogate of the fast–slow continuum to maximize the number of species included in the analysis. Furthermore, as an additional proxy of the slow–fast axis, we also explored body mass, as obtained from several sources (Dataset S1). Finally, as an alternative life-history strategy we computed a brood value for each species, which accounts for the ability of species to prioritize current survival over future reproduction (13), expressed as log10(1/[number of broods per year × reproductive life span]). For species for which either or both of these parameters were unavailable (n = 25) values were extrapolated from the mean for congeners.
Niche breadth.
For each species, an estimate of niche breadth was calculated using the area of the PCA envelope surrounding the native distribution points in the global PCA climate space (discussed above) after excluding the 5% of most extreme values. Additionally, geographic range size in native areas (49) was also used as a proxy of niche breadth.
Migratory status.
Species were classified as migratory (i.e., species for which a substantial proportion of the global or regional population makes regular or seasonal cyclical movements beyond the breeding range, with predictable timing and destinations) or nonmigratory (49).
Modeling Invasion Success.
To test for the link between the different predictors and establishment success we conducted logistic regressions, in which the outcome of the introduction was the dependent variable, taking a value of 0 when the species failed in establishing self-sustaining populations and 1 when it succeeded. Most predictor variables were log-transformed to improve compliance with normality, and all of the continuous predictors were standardized to allow comparisons among estimates. We performed our analyses in a phylogenetic comparative framework using dated phylogenies of all extant bird species (50). We used phylogenetic logistic regression (PLR) (51) to assess the relationship between single predictor variables and establishment success. Then, we examined the combined influence of predictor variables on establishment success in phylogenetic multiple logistic regression. We used a multimodel approach based on Akaike’s information criterion adjusted for small sample sizes (AICc) to evaluate the parameter estimates and the relative importance of predictor variables in a likelihood-based framework. We identified the best model based on AICc and calculated the relative importance of each predictor variable as the sum of the AICc weights of all models that included this variable in the set of most likely models (ΔAICc < 4). To account for phylogenetic uncertainty, we conducted this approach for a set of 1,000 pseudoposterior samples of the global bird phylogenies (50). Goodness-of-model fit was evaluated by calculating the explained deviance (D2). It is not currently possible to obtain D2 for PLRs (51) so we relied on the results of nonphylogenetic logistic regression.
We also tested the link between the different predictors and the rate of spatial spread in the study area for the subset of established species using PGLS models. PGLS models were constructed assuming a Brownian motion model of evolution and with the rate of spatial spread (increment in the number of occupied 5- × 5-km cells over time, log-transformed) as response variable. Similarly, we also tested how the different factors explained the size of the invaded range (number of occupied 5- × 5-km cells in the study area, also log-transformed). As for PLR, we fitted univariate PGLS models and all possible PGLS multivariate models from predictor variables to identify the most likely models and to calculate variable importance based on AICc and model averaging.
The relative independent effect of the explanatory variables was evaluated with a hierarchical partitioning (52) on the subsets of variables selected in the final best PLR and PGLS models. A 1,000-randomization procedure was carried out to test the statistical significance of the independent effects of each predictor (52). Because this analysis does not support PLR or PGLS models, we relied on the results of nonphylogenetic models.
Supplementary Material
Acknowledgments
We thank A. Brewer for kindly editing the English and two anonymous referees for useful comments on an earlier version of the manuscript. This work was funded by the Division of Mathematics and Natural Sciences of Queens College, City University of New York, and by Excellence Projects P07RNM 02918 and P08-RNM-4014 (Junta de Andalucía), Fundación Repsol, the Project Estación Biológica de Doñana-Severo Ochoa (Grant SEV-2012-0262), and AIC-A-2011-0706.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 9237.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1704815114/-/DCSupplemental.
References
- 1.Hassan RM, Scholes R, Ash N. Ecosystems and Human Well-Being: Current State and Trends. Island; Washington, DC: 2005. [Google Scholar]
- 2.Hulme PE. Trade, transport and trouble: Managing invasive species pathways in an era of globalization. J Appl Ecol. 2009;46:10–18. [Google Scholar]
- 3.Lockwood JL, Hoopes MA, Marchetti MP. Invasion Ecology. Blackwell; Malden, MA: 2007. [Google Scholar]
- 4.Blackburn TM, et al. A proposed unified framework for biological invasions. Trends Ecol Evol. 2011;26:333–339. doi: 10.1016/j.tree.2011.03.023. [DOI] [PubMed] [Google Scholar]
- 5.Kolar CS, Lodge DM. Progress in invasion biology: Predicting invaders. Trends Ecol Evol. 2001;16:199–204. doi: 10.1016/s0169-5347(01)02101-2. [DOI] [PubMed] [Google Scholar]
- 6.Blackburn T, Lockwood J, Cassey P. Avian Invasions: The Ecology and Evolution of Exotic Birds. Oxford Univ Press; Oxford: 2009. [Google Scholar]
- 7.Lockwood JL, Cassey P, Blackburn T. The role of propagule pressure in explaining species invasions. Trends Ecol Evol. 2005;20:223–228. doi: 10.1016/j.tree.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 8.Colautti RI, Grigorovich IA, MacIsaac HJ. Propagule pressure: A null model for biological invasions. Biol Invasions. 2006;8:1023–1037. [Google Scholar]
- 9.Duncan RP, Bomford M, Forsyth DM, Conibear L. High predictability in introduction outcomes and the geographical range size of introduced Australian birds: A role for climate. J Anim Ecol. 2001;70:621–632. [Google Scholar]
- 10.Cardador L, Carrete M, Gallardo B, Tella JL. Combining trade data and niche modelling improves predictions of the origin and distribution of non-native European populations of a globally invasive species. J Biogeogr. 2016;43:967–978. [Google Scholar]
- 11.Thuiller W, et al. Niche-based modelling as a tool for predicting the risk of alien plant invasions at a global scale. Glob Chang Biol. 2005;11:2234–2250. doi: 10.1111/j.1365-2486.2005.001018.x. [DOI] [PubMed] [Google Scholar]
- 12.Blackburn TM, Cassey P, Lockwood JL. The role of species traits in the establishment success of exotic birds. Glob Chang Biol. 2009;15:2852–2860. [Google Scholar]
- 13.Sol D, et al. Unraveling the life history of successful invaders. Science. 2012;337:580–583. doi: 10.1126/science.1221523. [DOI] [PubMed] [Google Scholar]
- 14.Sol D, Duncan RP, Blackburn TM, Cassey P, Lefebvre L. Big brains, enhanced cognition, and response of birds to novel environments. Proc Natl Acad Sci USA. 2005;102:5460–5465. doi: 10.1073/pnas.0408145102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sol D, Vilà M, Kühn I. The comparative analysis of historical alien introductions. Biol Invasions. 2008;10:1119–1129. [Google Scholar]
- 16.Byers JE, et al. Invasion expansion: Time since introduction best predicts global ranges of marine invaders. Sci Rep. 2015;5:12436. doi: 10.1038/srep12436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blackburn TM, Lockwood JL, Cassey P. The influence of numbers on invasion success. Mol Ecol. 2015;24:1942–1953. doi: 10.1111/mec.13075. [DOI] [PubMed] [Google Scholar]
- 18.Cassey P, Blackburn TM, Duncan RP, Loockwood JL. Lessons from the establishment of exotic species: A meta-analytical case study using birds. J Anim Ecol. 2005;74:250–258. [Google Scholar]
- 19.Long JL. Introduced Birds of the World: The Worldwide History, Distribution and Influence of Birds Introduced to New Environments. David and Charles; London: 1981. [Google Scholar]
- 20.Lever C. Naturalised Birds of the World. Poyser; London: 2005. [Google Scholar]
- 21.Carrete M, Tella J. Wild-bird trade and exotic invasions: A new link of conservation concern? Front Ecol Environ. 2008;6:207–211. [Google Scholar]
- 22.Blackburn TM, Gaston KJ, Parnell M. Changes in non-randomness in the expanding introduced avifauna of the world. Ecography. 2010;33:168–174. [Google Scholar]
- 23.Abellán P, Carrete M, Anadón JD, Cardador L, Tella JL. Non-random patterns and temporal trends (1912–2012) in the transport, introduction and establishment of exotic birds in Spain and Portugal. Divers Distrib. 2016;22:263–273. [Google Scholar]
- 24.Simberloff D. The role of propagule pressure in biological invasions. Annu Rev Ecol Evol Syst. 2009;40:81–102. [Google Scholar]
- 25.Moulton MP, Cropper WP, Moulton LE, Avery ML, Peacock D. A reassessment of historical records of avian introductions to Australia: No case for propagule pressure. Biodivers Conserv. 2012;21:155–174. [Google Scholar]
- 26.Crooks JA. Lag times and exotic species: The ecology and management of biological invasions in slow-motion. Ecoscience. 2005;12:316–329. [Google Scholar]
- 27.Aagaard K, Lockwood J. Exotic birds show lags in population growth. Divers Distrib. 2014;20:547–554. [Google Scholar]
- 28.Sakai AK, et al. The population biology of invasive species. Annu Rev Ecol Syst. 2001;32:305–332. [Google Scholar]
- 29.Wilson JRU, et al. Residence time and potential range: Crucial considerations in modelling plant invasions. Divers Distrib. 2007;13:11–22. [Google Scholar]
- 30.Duncan RP, Blackburn TM, Veltman CJ. Determinants of geographical range sizes: A test using introduced New Zealand birds. J Anim Ecol. 1999;68:963–975. [Google Scholar]
- 31.Dyer EE, et al. A global analysis of the determinants of alien geographical range size in birds. Glob Ecol Biogeogr. 2016;25:1346–1355. [Google Scholar]
- 32.Blackburn TM, Duncan RP. Determinants of establishment success in introduced birds. Nature. 2001;414:195–197. doi: 10.1038/35102557. [DOI] [PubMed] [Google Scholar]
- 33.Broennimann O, et al. Evidence of climatic niche shift during biological invasion. Ecol Lett. 2007;10:701–709. doi: 10.1111/j.1461-0248.2007.01060.x. [DOI] [PubMed] [Google Scholar]
- 34.Tingley R, Vallinoto M, Sequeira F, Kearney MR. Realized niche shift during a global biological invasion. Proc Natl Acad Sci USA. 2014;111:10233–10238. doi: 10.1073/pnas.1405766111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Strubbe D, Beauchard O, Matthysen E. Niche conservatism among non-native vertebrates in Europe and North America. Ecography. 2015;38:321–329. [Google Scholar]
- 36.Early R, Sax DF. Climatic niche shifts between species’ native and naturalized ranges raise concern for ecological forecasts during invasions and climate change. Glob Ecol Biogeogr. 2014;23:1356–1365. [Google Scholar]
- 37.Strubbe D, Matthysen E. Establishment success of invasive ring-necked and monk parakeets in Europe. J Biogeogr. 2009;36:2264–2278. [Google Scholar]
- 38.Collins JP, et al. A new urban ecology: Modeling human communities as integral parts of ecosystems poses special problems for the development and testing of ecological theory. Am Sci. 2000;88:416–425. [Google Scholar]
- 39.Guisan A, Petitpierre B, Broennimann O, Daehler C, Kueffer C. Unifying niche shift studies: Insights from biological invasions. Trends Ecol Evol. 2014;29:260–269. doi: 10.1016/j.tree.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 40.Vazquez D. Exploring the relationship between niche breadth and invasion success. In: Cadotte MW, McMahon SM, Fukami T, editors. Conceptual Ecology and Invasion Biology: Reciprocal Approaches to Nature. Springer; New York: 2006. pp. 307–322. [Google Scholar]
- 41.Jeschke JM, Strayer DL. Are threat status and invasion success two sides of the same coin? Ecography. 2008;31:124–130. [Google Scholar]
- 42.Carrete M, Tella JL. Rapid loss of antipredatory behaviour in captive-bred birds is linked to current avian invasions. Sci Rep. 2015;5:18274. doi: 10.1038/srep18274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hulme PE. Weed risk assessment: A way forward or a waste of time? J Appl Ecol. 2012;49:10–19. [Google Scholar]
- 44.Leung B, et al. An ounce of prevention or a pound of cure: Bioeconomic risk analysis of invasive species. Proc Biol Sci. 2002;269:2407–2413. doi: 10.1098/rspb.2002.2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Edelaar P, Tella JL. Managing non-native species: Don’t wait until their impacts are proven. Ibis. 2012;154:635–637. [Google Scholar]
- 46.Csardi G, Nepusz T. The igraph software package for complex network research. InterJournal, Complex Syst. 2006;1695:1–9. [Google Scholar]
- 47.Broennimann O, et al. Measuring ecological niche overlap from occurrence and spatial environmental data. Glob Ecol Biogeogr. 2012;21:481–497. [Google Scholar]
- 48.Phillips SJ, Anderson RP, Schapire RE. Maximum entropy modeling of species geographic distributions. Ecol Modell. 2006;190:231–259. [Google Scholar]
- 49.BirdLife International 2012 Species factsheets. Available at www.birdlife.org. Accessed February 1, 2016.
- 50.Jetz W, Thomas GH, Joy JB, Hartmann K, Mooers AO. The global diversity of birds in space and time. Nature. 2012;491:444–448. doi: 10.1038/nature11631. [DOI] [PubMed] [Google Scholar]
- 51.Ives AR, Garland T., Jr Phylogenetic logistic regression for binary dependent variables. Syst Biol. 2010;59:9–26. doi: 10.1093/sysbio/syp074. [DOI] [PubMed] [Google Scholar]
- 52.Mac Nally R. Multiple regression and inference in ecology and conservation biology: Further comments on identifying important predictor variables. Biodivers Conserv. 2002;11:1397–1401. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.