Abstract
Traditionally, a reduction in floating behavior or immobility in the Porsolt forced swim test is employed as a predictor of anti-depressant efficacy. However, over the past several years, our studies of alcohol withdrawal-induced negative affect consistently indicate the coincidence of increased anxiety-related behaviors on various behavioral tests with reduced immobility in the forced swim test. Further, this behavioral profile correlates with increased mGlu5 protein expression within limbic brain regions. As the role for mGlu5 in anxiety is well established, we hypothesized that the reduced immobility exhibited by alcohol-withdrawn mice when tested in the forced swim test might reflect anxiety, possibly a hyper-reactivity to the acute swim stressor. Herein, we evaluated whether or not the decreased forced swim test immobility during alcohol withdrawal responds to systemic treatment with a behaviorally effective dose of the prototypical anxiolytic, buspirone (5 mg/kg). We also determined the functional relevance of the withdrawal-induced increase in mGlu5 expression for forced swim test behavior by comparing the effects of buspirone to a behaviorally effective dose of the mGlu5 negative allosteric modulator MTEP (3 mg/kg). Adult male C57BL/6J mice were subjected to a 14-day, multi-bottle, binge-drinking protocol that elicits hyper-anxiety and increases glutamate-related protein expression during early withdrawal. Control animals received only water. At 24-h withdrawal, animals from each drinking condition were subdivided into groups and treated with an intraperitoneal injection of buspirone, MTEP, or vehicle, 30 min prior to the forced swim test. Drug effects on general locomotor activity were also assessed. As we reported previously, alcohol-withdrawn animals exhibited significantly reduced immobility in the forced swim test compared to water controls. Both buspirone and MTEP significantly increased immobility in alcohol-withdrawn animals, with a modest increase also seen in water controls. No significant group differences were observed for locomotor activity, indicating that neither anxiolytic was sedating. These results provide predictive validity for increased swimming/reduced immobility in the forced swim test as a model of anxiety and provide novel evidence in favor of mGlu5 inhibition as an effective therapeutic strategy for treating hyper-anxiety during alcohol withdrawal.
Keywords: alcohol, binge drinking, anxiety, nucleus accumbens, adolescence, forced swim test, MTEP, mGlu5, buspirone, behavioral despair
Introduction
The Porsolt forced swim test (FST) was first described by Dr. Roger Porsolt in the late 1970s and has been used traditionally as a behavioral screen with high predictive validity for the clinical efficacy of anti-depressant drugs.1 Compounds with anti-depressant properties have been shown to reduce immobile floating behavior and increase active swimming in the FST.2 Researchers have also used the FST as a behavioral model for depression in which increased immobility is associated with a depressive state.2,3 Immobile floating behavior is thought to reflect the hopelessness or helplessness associated with depression (i.e., behavioral despair).4
Our laboratory has previously employed the FST in a series of studies designed to assay the affective consequences of excessive alcohol consumption.5,6 Chronic excessive alcohol consumption is known to elicit a dysphoric state during withdrawal,7 as reported both in humans8–10 and a variety of animal models.11–14 Our studies demonstrated that even a two-week binge-drinking history is sufficient to increase anxiety-related behaviors in adult animals during early (24 h) withdrawal across various behavioral assays, including the light-dark box and defensive marble burying test.5,6 However, we have also reported that the increase in anxiety-related behaviors consistently coincides with reduced immobility in the FST. These results were unexpected, as according to traditional interpretations, this decrease in floating behavior would suggest an anti-depressant effect of alcohol withdrawal, which is counter-intuitive based on the characterization of alcohol withdrawal described in the extant clinical and basic science literature highlighted above. Based on this collection of observations, we speculated that the reduced immobility exhibited by alcohol-withdrawn mice in the FST5,6 might reflect psychomotor hyper-reactivity to an acute swim stressor, analogous to a panic-like fight or flight response.15
Panic is a state characterized by intense fear and anxiety and is frequently accompanied by elevated heart rate, perspiration, shortness of breath, dizziness, and shaking.16 In the clinical population, panic is frequently a symptom of generalized anxiety disorder but can also itself constitute a discrete anxiety disorder subtype.17 Interestingly, there is also an association between alcohol use disorders and panic in the human population,18 which, theoretically, might be expressed in animal models of excessive alcohol consumption. In laboratory animals, panic is commonly quantified with assessments of stimulus-provoked flight, freezing, and/or defensive attack, typically in response to an unconditioned predator stimulus.19 It is plausible that the acute stress of the FST and the unconditioned fear of drowning presents a similar survival threat, leading to a manifestation of a prolonged fight or flight response in anxious animals.20 Indeed, other reports comparing behavior in the elevated plus-maze and FST noted more active struggling behavior (defined as “a presence of energetic escape-directed movements”) in the FST in animals characterized as “high anxiety” on the plus-maze, compared to “low anxiety” counterparts.21
Through our investigation of the neurobiological correlates of alcohol withdrawal, we identified an increase in mGlu5 receptor protein expression within the nucleus accumbens shell as a potential mediator of withdrawal-induced hyper-anxiety.6 In support of this notion, mGlu5 antagonists such as 2-methyl-6-(phenylethynyl)-pyridine (MPEP) and 1-(3-Chlorophenyl)-3-(3-methyl-5-oxo-4H-imidazol-2-yl)urea (fenobam), and negative allosteric modulators such as 3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]pyridine (MTEP) exert anxiolytic effects in various animals.22–25 Thus, we tested the effects of reducing mGlu5 signaling upon behavior in the FST during early alcohol withdrawal compared to the prototypical anxiolytic buspirone,26 a 5-HT1a partial agonist with efficacy in alleviating symptoms of alcohol withdrawal-induced anxiety.27
Materials and methods
Experimental procedures were similar to those described in our previous studies of alcohol withdrawal-induced anxiety5,6 and are briefly summarized below.
Subjects
This study used 60 adult male C57BL/6J mice (Jackson Laboratories, Sacramento, CA) that were eight weeks of age at the onset of drinking. Animals were housed in groups of four in standard Plexiglas cages, in a temperature-controlled vivarium (23℃), under a 12-h reverse light/dark cycle (lights off at 10 a.m.). Animals were identified using small animal ear tags (Stoelting, Wood Dale, IL). Food and water were available ad libitum, with the exception of the 2-h alcohol-drinking period. The study had a 2 (alcohol or water) × 3 (vehicle, buspirone, or MTEP) factorial design, with n = 10/group. All experiments were conducted in compliance with the National Institutes of Health Guide for Care and Use of Laboratory Animals (NIH Publication No. 80–23, revised 2014) and approved by the IACUC of the University of California, Santa Barbara.
Drinking-in-the-dark procedures
Half of the animals were subjected to 14 consecutive days of binge drinking under modified three-bottle drinking-in-the-dark procedures, while control animals were given a single bottle of water. Alcohol access was restricted to 14 days in this study to be consistent with our more recent studies of the ontogeny of binge drinking.6 Each day prior to the drinking period, animals were separated into individual cages and allowed to acclimate for approximately 45 min. Beginning 3 h into the dark phase of the circadian cycle, corresponding to the peak time of daily fluid intake,28 animals were given concurrent access to 10%, 20%, and 40% (v/v) unsweetened ethanol solutions for 2 h. Animals were returned to their original group housing at the conclusion of the 2-h drinking period. While our prior work employed four-bottle drinking procedures (offering mice access also to a sipper tube containing 5% ethanol), alcohol intake from this solution is negligible, compared to that of 10%, 20%, and 40% ethanol.5,6,29 Thus, the 5% concentration was not included in the present study. The amount of alcohol consumed each day was calculated by bottle weight immediately before and after the drinking period and expressed as a function of the animal’s body weight (in kg).
Blood alcohol sampling
Submandibular blood samples were collected from all alcohol-drinking animals on day 10 of drinking, immediately following the 2-h drinking period. The scheduling of the blood sampling was selected to ensure that the animals’ intakes had stabilized, while also allowing ample time for recovery prior to behavioral testing. Blood alcohol concentrations (BACs) were determined using an Analox alcohol analyzer (model AM1, Analox Instruments USA, Lunenburg, MA), according to the manufacturer’s instructions.
Drugs
Treatments were administered systemically via intraperitoneal injection, 30 min prior to the onset of behavioral testing. Ten animals from each drinking group received a 5 mg/kg injection of buspirone hydrochloride (Sigma-Aldrich, Atlanta, GA) in sterile water. This buspirone dose was selected for study as it elicits anxiolytic effects in other drug-related behavioral paradigms.30–32 An additional 10 animals from each drinking group received a 3 mg/kg injection of the mGlu5 antagonist MTEP in sterile water. This MTEP dose was selected for study based on evidence of anxiolytic efficacy in rodents.33–35 The remaining animals served as vehicle controls and received injections of sterile water (Vol = 0.01 ml/g).
Behavioral testing
Behavioral testing commenced approximately 24 h following the final alcohol presentation and thus occurred during the circadian dark phase.
Porsolt forced swim test
Each animal was placed into an 11-cm diameter cylindrical container filled with room-temperature water deep enough that animals were unable to touch the bottom of the enclosure. The latency to first exhibit immobility (defined as no horizontal or vertical displacement of the animal’s center of gravity for ≥5 s), total time spent immobile, and the numbers of immobile episodes were monitored throughout the entire 6-min trial duration using AnyMaze™ tracking software (Stoelting Co., Wood Dale, IL, USA), as conducted previously in literature.6
Locomotor activity
Following the FST, animals were allowed to dry off and recover for 20 min before being assessed for generalized locomotor effects of the anxiolytic treatments. Animals were placed in a polycarbonate box measuring 24 cm long × 23 cm wide × 24 cm high, and the total distance traveled was monitored for during a 15-min trial using Any-maze™ tracking software (Stoelting Co., Wood Dale, IL). Locomotor testing occurred within 20-min post-FST and thus occurred at approximately 60 min following drug injection.
Statistical analysis
A one-way analysis of variance (ANOVA) was conducted first to ensure that there were no differences in alcohol intake between the three treatment groups. To determine the relationship between alcohol intake and resulting BACs, a Pearson’s correlational analysis was conducted. Behavioral data were analyzed using between-subjects, two-way ANOVAs with Tukey’s multiple comparison tests; α = 0.05. All calculations and analyses were performed using SPSS v.21 statistical software (IBM, 2012).
Results
Alcohol consumption
Across the entire 14-day drinking period, animals had an average alcohol intake of 4.00 ± 0.05 g/kg, which has been demonstrated to results in BACs > 80 mg/dL.28,36 On day 10 of drinking, the animals averaged an intake lower than the 14-day average (3.37 ± 0.16 g/kg), and this intake resulted in an average BAC of 73.19 ± 3.84 mg/dl. Importantly, there was a significant positive correlation between intake and BAC (r = 0.45, p = 0.013; Figure 1). There were no significant differences in alcohol consumption across the three treatment groups, F(2, 27) = 0.84, p = 0.44.
Figure 1.
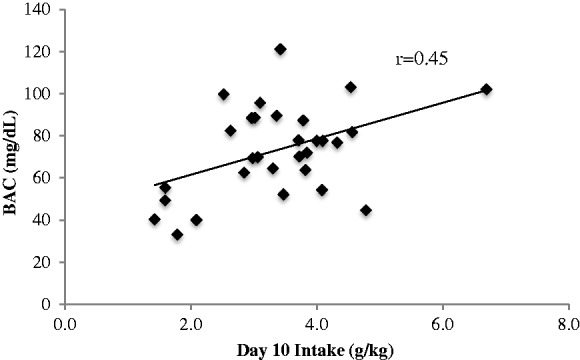
BAC and alcohol consumption. On day 10, animals consumed an average of 3.37 ± 0.16 g/kg with a resulting BAC of 73.19 ± 3.84 mg/dl, n = 30. There was a significant positive correlation between intake and BAC (r = 0.45, p = 0.013).
BAC: blood alcohol concentration.
FST behavior
Withdrawal-induced reduction in immobility
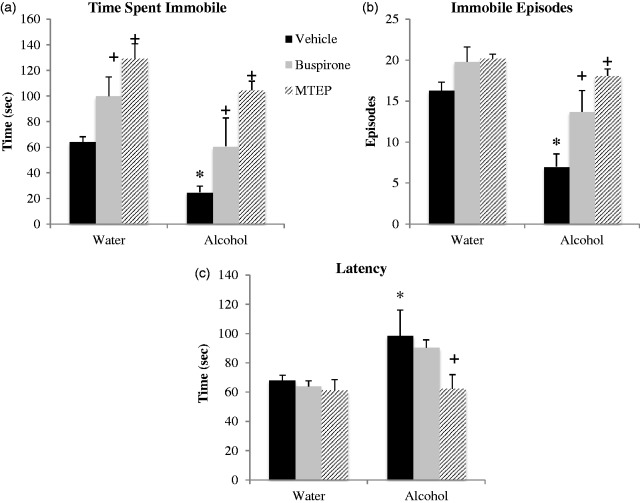
In the FST, we replicated our prior observations that early alcohol withdrawal reduces immobility in adult male mice.5,6 The ANOVA revealed a significant main alcohol effect for all three dependent behavioral measures. Overall, alcohol-drinking mice spent less time immobile, F(1, 54) = 11.89, p = 0.001; Figure 2(a), exhibited fewer immobile episodes, F(1, 54) = 21.33, p < 0.001; Figure 2(b), and a longer latency to first immobility, F(1, 54) = 6.57, p = 0.013; Figure 2(c), compared to water controls. More specifically, Tukey’s post-hoc tests showed that vehicle-treated alcohol-drinking mice spent significantly less time immobile (p = 0.026, Figure 2(a)), had fewer immobile episodes (p = 0.001, Figure 2(b)) and a longer latency to first immobility (p = 0.024, Figure 2(c)) compared to vehicle-treated water control animals, thus confirming the presence of alcohol withdrawal-induced behavioral differences.
Figure 2.
Withdrawal from binge drinking decreases immobility in the FST, an effect reversed by anxiolytics. (a) Vehicle-treated alcohol drinkers spent significantly less time immobile compared to vehicle-treated water controls. Buspirone and MTEP treatment significantly increased time immobile in both water and alcohol drinkers. (b) Vehicle-treated bingers had less immobile episodes compared to vehicle-treated water controls. Buspirone and MTEP treatment significantly increased immobile episodes in alcohol but not water drinkers. (c) Vehicle-treated bingers a longer latency to first immobility compared to vehicle-treated water controls. MTEP but not buspirone reduced latency in bingers only. Neither MTEP nor buspirone affected latency in water drinkers. *Tukey’s p < 0.05 vs. water control; +p < 0.05 vs. vehicle treatment; n = 10/treatment group.
Anxiolytic effects on immobility
There was a significant effect of treatment on time spent immobile, F(2, 54) = 17.57, p < 0.001; Figure 2(a). Relative to vehicle treatment, both buspirone and MTEP significantly increased time spent immobile, and this effect was apparent in alcohol- and water-drinking mice alike (buspirone: for alcohol, p = 0.042; for water, p = 0.044; MTEP: for water, p = 0.001; for alcohol, p < 0.001). A direct comparison of the effects of buspirone versus MTEP upon the time immobile exhibited by alcohol-drinking mice indicated a significantly greater effect of MTEP (p = 0.014), and the data for water controls also exhibited a similar trend toward significance (p = 0.09).
There was also a significant effect of treatment on total immobile episodes, F(2, 54) = 12.26, p < 0.001; Figure 2(b). Buspirone and MTEP both increased immobile episodes in alcohol-drinking mice, relative to vehicle treatment (p = 0.014 and p < 0.001, respectively), although their effects on this measure were not statistically significant in the water controls (p = 0.11 and p = 0.08, respectively). As observed for the time spent immobile, MTEP increased the number of immobile episodes in alcohol-drinking mice to a greater extent than buspirone (p = 0.04), with no drug-related differences noted for water controls (p > 0.10).
There was a strong trend toward a main treatment effect on latency to immobility, F(2, 54) = 2.88, p = 0.06, and inspection of Figure 2(c) suggested that this trend was driven exclusively by an effect of MTEP upon this measure. As the results above suggested that MTEP was a more effective anxiolytic in alcohol-drinking mice than buspirone, Tukey’s post-hoc tests were employed and determined that MTEP did, in fact, exert a statistically significant effect upon the latency to immobility (p = 0.008), while buspirone did not (p = 0.54). Neither compound altered the latency to the first immobile episode in water controls (p’s > 0.10).
General locomotor activity
There were no significant effects of alcohol or anxiolytic treatment on the total distance traveled, when assessed following the FST (one-way ANOVA p > 0.10; Figure 3).
Figure 3.
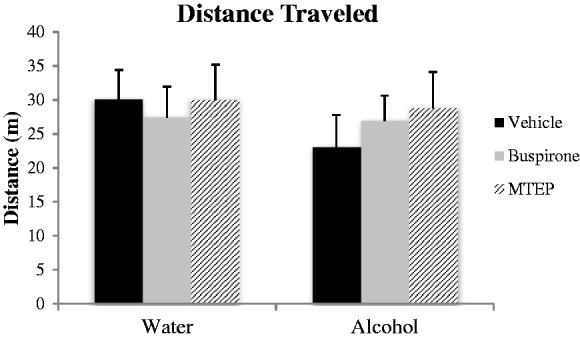
General locomotor activity. There were no significant differences between any of the groups in distance traveled in the activity monitor (p’s > 0.05; n = 10/treatment group).
Discussion
In this study, we replicated our previous findings5,6 showing decreased immobility in the FST during early withdrawal following a 14-day period of binge drinking, as indicated by a longer latency to first become immobile, as well as reduced time spent immobile and reduced number of immobile episodes. Although the average BAC obtained on day 10 of drinking (73.19 ± 3.84 mg/dL) was slightly under the 80 mg/dL specified in the official National Institute on Alcohol Abuse and Alcoholism37 definition of binge drinking,38 alcohol intake was lower on this day than the average alcohol intake observed across the entire two-week drinking period (day 10: 3.37 ± 0.16 vs. average: 4.00 ± 0.05 g/kg). Importantly, the BACs on day 10 of drinking were significantly correlated with alcohol intake on that day, and the average alcohol intake for the entire drinking period was above that reported to result in BACs above the 80 mg/dL National Institute on Alcohol Abuse and Alcoholism criterion for binge drinking.5,6,28,29 Thus, while the animals may not have achieved BACs > 80 mg/dL every day during the two-week drinking period, the alcohol intakes (and resultant BACs) were nonetheless sufficient to elicit signs of behavioral dysregulation during withdrawal, as manifested by reduced immobility/increased swimming in the FST. Although other common methods of high alcohol exposure (e.g., vapor inhalation, gavage, liquid diet, injection) may be capable of eliciting more robust withdrawal symptoms, the present data add to our prior evidence that voluntary excessive alcohol consumption is sufficient to elicit negative affective consequences in mice, while maintaining ethological validity.
In this study, treatment with the prototypical anxiolytic buspirone, as well as the non-conventional anxiolytic MTEP, reversed the effect of alcohol withdrawal upon swimming behavior and increased immobility. Moreover, consistent with the results of a prior report for buspirone,39 both buspirone and MTEP reduced some signs of immobility also in alcohol-naïve, water-drinking, controls. Our finding that buspirone and MTEP pretreatment did not impact the behavior of water controls on all measures obtained from the FST is in line with the results of other studies demonstrating minimal or absent effects of anxiolytic treatment on the behavior of non-anxious animals.40–43 Importantly, our FST results for buspirone, in particular, strongly support our initial hypothesis that the reduction in immobility/increased swimming behavior observed consistently by our laboratory in alcohol-withdrawn animals (Figure 2)5,6 reflects an anxiety-related response.
Neither buspirone nor MTEP altered locomotor activity, when assessed following the 6-min FST. This negative outcome argues against the drug-induced increase in immobility/reduction in swimming behavior observed in the FST being attributable to non-specific motor effects of the doses administered. The fact that the effects of both buspirone and MTEP on the specific dependent variables examined in the FST were either weaker or absent in water controls versus alcohol-withdrawn mice also argues that the drug doses administered were not hypnotic or motor-impairing. It is also unlikely that our results were confounded by an alcohol or treatment-related learning/memory deficit, as animals were tested in a single trial, and the tank diameter is such that it is readily apparent that there is no means of escape or platform to shelter upon. Therefore, we conclude that reduced immobility/increased swimming in the FST reflects the anxiogenic effects of alcohol withdrawal, possibly indicative of panic-like behavioral hyper-reactivity.
In support of our interpretation and consistent with the reduction in swimming produced by acute treatment with buspirone39 and/or MTEP in both alcohol-withdrawn and alcohol-naïve mice, the anxiolytic benzodiazepine diazepam is also reported to significantly increase the duration of immobility, while the anxiogenic drug beta-CCE conversely reduces immobility.31 Furthermore, mGlu2 knock-out mice, reported to exhibit elevated anxiety-like behaviors on more conventional anxiety tests such as the open field test and elevated plus maze,44 also exhibit decreased immobility in the FST compared to wild-type mice but do not differ from wild-type mice with respect to behavior in another test with predictive validity for anti-depressant efficacy—the tail suspension test.45 A similar correlation between anxiety-like behavior and reduced immobility in the FST has also been reported in 5HT1a knock-out mice.38 Thus, both behavioral pharmacological and genetics evidence argue that reduced immobility/increased swimming in the FST can reflect increased anxiety in rodent models.
It is interesting to note that, at the doses tested herein, MTEP treatment was more effective than buspirone at increasing immobility in alcohol-withdrawn animals on all three dependent variables. Other studies have also shown MTEP to out-perform buspirone in anxiety tests such as fear-potentiated startle46 and the anti-conflict test.47 Together, the present findings, coupled with evidence from other laboratories,48,49 suggest that mGlu5 inhibitors may be more effective anxiolytics than conventional treatments such as buspirone, particularly for alleviating alcohol withdrawal-induced anxiety. Early withdrawal from a history of voluntary alcohol-drinking increases mGlu5 signaling throughout the extended amygdala6,29,36,50–52—a neurocircuit critically involved in regulating the anxiogenic/negative affective properties of drug withdrawal,53,54 and recent correlative evidence suggests a relationship between the manifestation of alcohol withdrawal-induced hyper-anxiety and mGlu5 expression, at least within the nucleus accumbens shell.6 The present data for MTEP argue that the upregulation of mGlu5 signaling during early withdrawal from voluntary alcohol consumption may be causally related to withdrawal-induced anxiety, implicating increased mGlu5 signaling in the neurochemical imbalance driving the hyper-anxious state during alcohol withdrawal. However, while the mGlu5 negative allosteric modulator fenobam demonstrates anxiolytic efficacy comparable to benzodiazepines, with an improved side-effect profile with respect to its hypnotic and alcohol-promoting effects,23,47,55 amnesic and psychotomimetic side effects currently limit its clinical utility.56,57 Thus, additional research into the anxiolytic potential of mGlu5 antagonists could provide a beneficial clinical tool for the treatment of anxiety disorders and substance abuse-related anxiety.
As a final point of discussion, it is important to clarify that the intention of this study was not to invalidate the FST as a predictive animal model for anti-depressant efficacy. Rather, we conducted this study to draw attention to alternative interpretations of the behavioral outcomes from this assay and to highlight the importance of testing animals in a variety of paradigms with predictive validity for both anxiolytic and anti-depressant action, as the results from one assay alone are often subject to interpretational debate (see e.g., literature58–62 for debate over interpretation of behavior in the elevated plus-maze and other exploratory animal models). As such, results should be considered carefully, particularly within the context of the animals’ history, and additional measures/assays should be included whenever possible to facilitate distinction between anxiety- versus depression-related behavioral phenotypes in the FST. For examples, other assays with predictive validity for anti-depressant efficacy (e.g., the sucrose preference test,63 tail suspension test,64 or intracranial self-stimulation65), and tests with predictive validity for anxiolytic efficacy (e.g., elevated plus maze,66 light-dark box,67 defensive burying,68 or conflict test69) are well-validated options for consideration when trying to interpret behavioral changes within the FST.
In conclusion, this study provides evidence that reduced immobility in the FST may reflect anxiety, validated by a subsequent increase in immobility following anxiolytic treatment. Within this interpretational context, we also conclude that systemic mGlu5 blockade has robust anxiolytic properties in alcohol-withdrawn animals, providing cause-effect evidence implicating increased mGlu5 signaling in the etiology of withdrawal-induced anxiety. As withdrawal-induced anxiety acts as a negative reinforcer to promote continued drinking, further research into the formulation of mGlu5 inhibitors for alleviating withdrawal-induced anxiety could yield additional non-benzodiazepine anxiolytic options, with lower abuse liability, for patients with a history of alcohol abuse.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work is supported by NIAAA grant AA024044 to Karen K Szumlinski.
References
- 1.Porsolt RD, Bertin A, Jalfre M. Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Thér. 1977; 229(2): 327–336. [PubMed] [Google Scholar]
- 2.Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977; 266(5604): 730–732. [DOI] [PubMed] [Google Scholar]
- 3.Porsolt RD, Brossard G, Hautbois C, et al. Rodent models of depression: forced swimming and tail suspension behavioral despair tests in rats and mice. Curr Protoc Neurosci. 2001; 8: Unit 8.10A, doi: 10.1002/0471142301.ns0810as14. [DOI] [PubMed] [Google Scholar]
- 4.Alloy LB, Abramson LY, Metalsky GI, et al. The hopelessness theory of depression: attributional aspects. Br J Clin Psychol. 1988; 27(Pt 1): 5–21. [DOI] [PubMed] [Google Scholar]
- 5.Lee KM, Coehlo M, McGregor HA, et al. Binge alcohol drinking elicits persistent negative affect in mice. Behav Brain Res. 2015; 291: 385–398. doi:10.1016/j.bbr.2015.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee KM, Coelho MA, McGregor HA, et al. Adolescent mice are resilient to alcohol withdrawal-induced anxiety and changes in indices of glutamate function within the nucleus accumbens. Front Cell Neurosci. 2016; 10: 265, doi:10.3389/fncel.2016.00265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson GT. Alcohol and anxiety. Behav Res Ther. 1988; 26(5): 369–381. doi:10.1016/0005-7967(88)90070-8. [DOI] [PubMed] [Google Scholar]
- 8.Driessen M, Meier S, Hill A, et al. The course of anxiety, depression and drinking behaviours after completed detoxification in alcoholics with and without comorbid anxiety and depressive disorders. Alcohol Alcohol. 2001; 36(3): 249–255. [DOI] [PubMed] [Google Scholar]
- 9.Kiefer F, Horntrich M, Jahn H, et al. Is withdrawal-induced anxiety in alcoholism based on beta-endorphin deficiency? Psychopharmacology. 2002, 162(4): 433–437. doi:10.1007/s00213-002-1118-y. [DOI] [PubMed] [Google Scholar]
- 10.Schuckit MA, Hesselbrock V. Alcohol dependence and anxiety disorders: what is the relationship? Am J Psychiatry. 1994, 151(12): 1723–1734. doi:10.1176/ajp.151.12.1723. [DOI] [PubMed] [Google Scholar]
- 11.Emmett-Oglesby MW, Mathis DA, Moon RT, et al. Animal models of drug withdrawal symptoms. Psychopharmacology. 1990; 101(3): 292–309. [DOI] [PubMed] [Google Scholar]
- 12.Getachew B, Hauser SR, Taylor RE, et al. Desipramine blocks alcohol-induced anxiety- and depressive-like behaviors in two rat strains. Pharmacol Biochem Behav. 2008; 91(1): 97–103. doi:10.1016/J.Pbb.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knapp DJ, Duncan GE, Crews FT, et al. Induction of Fos-like proteins and ultrasonic vocalizations during ethanol withdrawal: further evidence for withdrawal-induced anxiety. Alcohol Clin Exp Res. 1998; 22(2): 481–493. [PubMed] [Google Scholar]
- 14.Valcheva-Kuzmanova S, Kuzmanov K, Tancheva S, et al. Hypoglycemic and hypolipidemic effects of Aronia melanocarpa fruit juice in streptozotocin-induced diabetic rats. Methods Find Exp Clin Pharmacol. 2007; 29(2): 101–105. doi:10.1358/Mf.2007.29.2.1075349. [DOI] [PubMed] [Google Scholar]
- 15.Graeff FG. [Anxiety, panic and the hypothalamic-pituitary-adrenal axis]. Rev Bras Psiquiatr. 2007; 29(Suppl 1): S3–S6. [DOI] [PubMed] [Google Scholar]
- 16.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed Arlington, VA: American Psychiatric Publishing, 2013. [Google Scholar]
- 17.Craske MG, Kircanski K, Epstein A, et al. Panic disorder: a review of DSM-IV panic disorder and proposals for DSM-V. Depress Anxiety. 2010; 27(2): 93–112. doi:10.1002/da.20654. [DOI] [PubMed] [Google Scholar]
- 18.Cosci F, Schruers KRJ, Abrams K, et al. Alcohol use disorders and panic disorder: a review of the evidence of a direct relationship. J Clin Psychiatry. 2007; 68(6): 874–880. [DOI] [PubMed] [Google Scholar]
- 19.Blanchard DC, Griebel G, Blanchard RJ. The mouse defense test battery: pharmacological and behavioral assays for anxiety and panic. Eur J Pharmacol. 2003; 463(1–3): 97–116. [DOI] [PubMed] [Google Scholar]
- 20.Polani PE. Attacks of anxiety, panic and frenzy, and their related depression: a hypothesis. Med Hypotheses. 2004; 63(1): 124–127. doi:10.1016/j.mehy.2004.01.026. [DOI] [PubMed] [Google Scholar]
- 21.Ferre P, Fernandez Teruel A, Escorihuela RM, et al. Struggling and flumazenil effects in the swimming test are related to the level of anxiety in mice. Neuropsychobiology. 1994; 29(1): 23–27. [DOI] [PubMed] [Google Scholar]
- 22.Busse CS, Brodk0in J, Tattersall D, et al. The behavioral profile of the potent and selective mGlu5 receptor antagonist 3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]pyridine (MTEP) in rodent models of anxiety. Neuropsychopharmacology. 2004; 29(11): 1971–1979. doi:10.1038/sj.npp.1300540. [DOI] [PubMed] [Google Scholar]
- 23.Porter RH, Jaeschke G, Spooren W, et al. Fenobam: a clinically validated nonbenzodiazepine anxiolytic is a potent, selective, and noncompetitive mGlu5 receptor antagonist with inverse agonist activity. J Pharmacol Exp Ther. 2005; 315(2): 711–721. doi:10.1124/jpet.105.089839. [DOI] [PubMed] [Google Scholar]
- 24.Tatarczynska E, Klodzinska A, Chojnacka-Wojcik E, et al. Potential anxiolytic- and antidepressant-like effects of MPEP, a potent, selective and systemically active mGlu5 receptor antagonist. Br J Pharmacol. 2001; 132(7): 1423–1430. doi:10.1038/sj.bjp.0703923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Varty GB, Grilli M, Forlani A, et al. The antinociceptive and anxiolytic-like effects of the metabotropic glutamate receptor 5 (mGluR5) antagonists, MPEP and MTEP, and the mGluR1 antagonist, LY456236, in rodents: a comparison of efficacy and side-effect profiles. Psychopharmacology. 2005; 179(1): 207–217. doi:10.1007/s00213-005-2143-4. [DOI] [PubMed] [Google Scholar]
- 26.Batool F. Buspirone and anxiety disorders: a review with pharmacological and clinical perspectives. Internet J Pharmacol. 2007; 5(2). [Google Scholar]
- 27.Lal H, Prather PL, Rezazadeh SM. Anxiogenic behavior in rats during acute and protracted ethanol withdrawal: reversal by buspirone. Alcohol. 1991; 8(6): 467–471. [DOI] [PubMed] [Google Scholar]
- 28.Rhodes JS, Best K, Belknap JK, et al. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol Behav. 2005; 84(1): 53–63. doi:10.1016/j.physbeh.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 29.Cozzoli DK, Courson J, Wroten MG, et al. Binge alcohol drinking by mice requires intact group1 metabotropic glutamate receptor signaling within the central nucleus of the amygdale. Neuropsychopharmacology. 2014; 39(2): 435–444. doi:10.1038/npp.2013.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ettenberg A, Bernardi RE. Anxiolytic-like actions of buspirone in a runway model of intravenous cocaine self-administration. Pharmacol Biochem Behav. 2006; 85(2): 393–399. doi:10.1016/j.pbb.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nishimura H, Tanaka M, Tsuda A, et al. Atypical anxiolytic profile of buspirone and a related drug, SM-3997, in a modified forced swim test employing straw suspension. Pharmacol Biochem Behav. 1993; 46(3): 647–651. [DOI] [PubMed] [Google Scholar]
- 32.Risbrough VB, Brodkin JD, Geyer MA. GABA-A and 5-HT1A receptor agonists block expression of fear-potentiated startle in mice. Neuropsychopharmacology. 2003; 28(4): 654–663. doi:10.1038/sj.npp.1300079. [DOI] [PubMed] [Google Scholar]
- 33.Klodzinska A, Tatarczynska E, Chojnacka-Wojcik E, et al. Anxiolytic-like effects of MTEP, a potent and selective mGlu5 receptor agonist does not involve GABA(A) signaling. Neuropharmacology. 2004; 47(3): 342–350. doi:10.1016/j.neuropharm.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 34.Pietraszek M, Sukhanov I, Maciejak P, et al. Anxiolytic-like effects of mGlu1 and mGlu5 receptor antagonists in rats. Eur J Pharmacol. 2005; 514(1): 25–34. doi:10.1016/j.ejphar.2005.03.028. [DOI] [PubMed] [Google Scholar]
- 35.Stachowicz K, Golembiowska K, Sowa M, et al. Anxiolytic-like action of MTEP expressed in the conflict drinking Vogel test in rats is serotonin dependent. Neuropharmacology. 2007; 53(6): 741–748. doi:10.1016/j.neuropharm.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 36.Cozzoli DK, Goulding SP, Zhang PW, et al. Binge drinking upregulates accumbens mGluR5-Homer2-PI3K signaling: functional implications for alcoholism. J Neurosci. 2009; 29(27): 8655–8668. doi:10.1523/JNEUROSCI.5900-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.National Institute on Alcohol Abuse and Alcoholism. NIAAA council approves definition of binge drinking. NIAAA Newsletter. February 5, 2004, Vol. 3:3.
- 38.Ramboz S, Oosting R, Amara DA, et al. Serotonin receptor 1A knockout: an animal model of anxiety-related disorder. Proc Natl Acad Sci U S A. 1998; 95(24): 14476–14481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kitamura Y, Nagatani T. Buspirone enhances immobility in the forced swim test in mice. Pharmacol Biochem Behav. 1996; 55(3): 445–451. [DOI] [PubMed] [Google Scholar]
- 40.Horváth J, Barkóczi B, Müller G, et al. Anxious and nonanxious mice show similar hippocampal sensory evoked oscillations under urethane anesthesia: difference in the effect of buspirone. Neural Plast. 2015; 2015: 9, doi:10.1155/2015/186323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Landgraf R, Wigger A. High vs low anxiety-related behavior rats: an animal model of extremes in trait anxiety. Behav Genet. 2002; 32(5): 301–314. [DOI] [PubMed] [Google Scholar]
- 42.Liebsch G, Linthorst AC, Neumann ID, et al. Behavioral, physiological, and neuroendocrine stress responses and differential sensitivity to diazepam in two Wistar rat lines selectively bred for high- and low-anxiety-related behavior. Neuropsychopharmacology. 1998; 19(5): 381–396. doi:10.1016/S0893-133X(98)00042-6. [DOI] [PubMed] [Google Scholar]
- 43.Naslund J, Studer E, Pettersson R, et al. Differences in anxiety-like behavior within a batch of wistar rats are associated with differences in serotonergic transmission, enhanced by acute SRI administration, and abolished by serotonin depletion. Int J Neuropsychopharmacol. 2015; 18(8). doi:10.1093/ijnp/pyv018. [DOI] [PMC free article] [PubMed]
- 44.Ceolin L, Kantamneni S, Barker GR, et al. Study of novel selective mGlu2 agonist in the temporo-ammonic input to CA1 neurons reveals reduced mGlu2 receptor expression in a Wistar substrain with an anxiety-like phenotype. J Neurosci. 2011; 31(18): 6721–6731. doi:10.1523/JNEUROSCI.0418-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morishima Y, Miyakawa T, Furuyashiki T, et al. Enhanced cocaine responsiveness and impaired motor coordination in metabotropic glutamate receptor subtype 2 knockout mice. Proc Natl Acad Sci U S A. 2005; 102(11): 4170–4175. doi:10.1073/pnas.0500914102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brodkin J, Busse C, Sukoff SJ, et al. Anxiolytic-like activity of the mGluR5 antagonist MPEP: a comparison with diazepam and buspirone. Pharmacol Biochem Behav. 2002; 73(2): 359–366. [DOI] [PubMed] [Google Scholar]
- 47.Goldberg ME, Salama AI, Patel JB, et al. Novel non-benzodiazepine anxiolytics. Neuropharmacology. 1983; 22(12B): 1499–1504. [DOI] [PubMed] [Google Scholar]
- 48.Kotlinska J, Bochenski M. The influence of various glutamate receptors antagonists on anxiety-like effect of ethanol withdrawal in a plus-maze test in rats. Eur J Pharmacol. 2008; 598(1–3): 57–63. doi:10.1016/j.ejphar.2008.09.026. [DOI] [PubMed] [Google Scholar]
- 49.Kumar J, Hapidin H, Bee YT, et al. Effects of the mGluR5 antagonist MPEP on ethanol withdrawal induced anxiety-like syndrome in rats. Behav Brain Funct. 2013; 9: 43, doi:10.1186/1744-9081-9-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cozzoli DK, Courson J, Caruana AL, et al. Nucleus accumbens mGluR5-associated signaling regulates binge alcohol drinking under drinking-in-the-dark procedures. Alcohol Clin Exp Res. 2012; 36(9): 1623–1633. doi:10.1111/j.1530-0277.2012.01776.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Obara I, Bell RL, Goulding SP, et al. Differential effects of chronic ethanol consumption and withdrawal on homer/glutamate receptor expression in subregions of the accumbens and amygdala of P rats. Alcohol Clin Exp Res. 2009; 33(11): 1924–1934. doi:10.1111/j.1530-0277.2009.01030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Szumlinski KK, Ary AW, Lominac KD, et al. Accumbens Homer2 overexpression facilitates alcohol-induced neuroplasticity in C57BL/6J mice. Neuropsychopharmacology. 2008; 33(6): 1365–1378. doi:10.1038/sj.npp.1301473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Koob GF. Neuroadaptive mechanisms of addiction: studies on the extended amygdala. Eur Neuropsychopharmacol. 2003; 13(6): 442–452. [DOI] [PubMed] [Google Scholar]
- 54.Koob GF. Addiction is a reward deficit and stress surfeit disorder. Front Psychiatry. 2013; 4: 72, doi:10.3389/fpsyt.2013.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pecknold JC, McClure DJ, Appeltauer L, et al. Treatment of anxiety using fenobam (a nonbenzodiazepine) in a double-blind standard (diazepam) placebo-controlled study. J Clin Psychopharmacol. 1982; 2(2): 129–133. [PubMed] [Google Scholar]
- 56.Jacob W, Gravius A, Pietraszek M, et al. The anxiolytic and analgesic properties of fenobam, a potent mGlu5 receptor antagonist, in relation to the impairment of learning. Neuropharmacology. 2009; 57(2): 97–108. doi:10.1016/j.neuropharm.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 57.Palucha A, Pilc A. Metabotropic glutamate receptor ligands as possible anxiolytic and antidepressant drugs. Pharmacol Ther. 2007; 115(1): 116–147. doi:10.1016/j.pharmthera.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 58.Olausson P, Engel JA, Soderpalm B. Behavioral sensitization to nicotine is associated with behavioral disinhibition; counteraction by citalopram. Psychopharmacology. 1999; 142(2): 111–119. [DOI] [PubMed] [Google Scholar]
- 59.Olausson P, Engel JA, Soderpalm B. Effects of serotonergic manipulations on the behavioral sensitization and disinhibition associated with repeated amphetamine treatment. Pharmacol Biochem Behav. 2000; 66(1): 211–220. [DOI] [PubMed] [Google Scholar]
- 60.Ouagazzal AM, Kenny PJ, File SE. Modulation of behaviour on trials 1 and 2 in the elevated plus-maze test of anxiety after systemic and hippocampal administration of nicotine. Psychopharmacology. 1999; 144(1): 54–60. [DOI] [PubMed] [Google Scholar]
- 61.Pellow S. Anxiolytic and anxiogenic drug effects in a novel test of anxiety: are exploratory models of anxiety in rodents valid? Methods Find Exp Clin Pharmacol. 1986, 8(9): 557–565. [PubMed] [Google Scholar]
- 62.Pellow S, Chopin P, File SE, et al. Validation of open: closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985; 14(3): 149–167. [DOI] [PubMed] [Google Scholar]
- 63.Katz RJ. Animal model of depression: pharmacological sensitivity of a hedonic deficit. Pharmacol Biochem Behav. 1982; 16(6): 965–968. [DOI] [PubMed] [Google Scholar]
- 64.Steru L, Chermat R, Thierry B, et al. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology. 1985; 85(3): 367–370. [DOI] [PubMed] [Google Scholar]
- 65.Vogel G, Neill D, Hagler M, et al. Decreased intracranial self-stimulation in a new animal model of endogenous depression. Neurosci Biobehav Rev. 1990; 14(1): 65–68. [DOI] [PubMed] [Google Scholar]
- 66.Walf AA, Frye CA. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nature Protoc. 2007; 2(2): 322–328. doi:10.1038/nprot.2007.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bourin M, Hascoet M. The mouse light/dark box test. Eur J Pharmacol. 2003; 463(1–3): 55–65. [DOI] [PubMed] [Google Scholar]
- 68.File SE, Lippa AS, Beer B, et al. Animal tests of anxiety. Curr Protoc Neurosci. 2004; 8: Unit 8.3, doi:10.1002/0471142301.ns0803s26. [DOI] [PubMed] [Google Scholar]
- 69.Moreira FA, Aguiar DC, Guimaraes FS. Anxiolytic-like effect of cannabidiol in the rat Vogel conflict test. Prog Neuro-Psychopharmacol Biol Psychiatry. 2006; 30(8): 1466–1471. doi:10.1016/j.pnpbp.2006.06.004. [DOI] [PubMed] [Google Scholar]