Abstract
KRN7000 is an important ligand identified for CD1d protein of APC, and KRN7000/CD1d complex can stimulate NKT cells to release a broad range of bioactive cytokines. In an effort to understand the structure–activity relationships, we have carried out syntheses of 26 new KRN7000 analogues incorporating aromatic residues in either or both side chains. Structural variations of the phytosphingosine moiety also include varying stereochemistry at C3 and C4, and 4-deoxy and 3,4-dideoxy versions. Their biological activities are described.
Keywords: Glycolipids, KRN7000, α-GalCer, NKT cell, CD1d
In the early 1990s potent antitumor activity was found with agelasphins, the glycolipids extracted from Okinawan sponge Agelas mauritianus by Kirin Pharmaceuticals, and synthetic α-galactosyl ceramide (α-GalCer) named KRN7000 was identified to have the best antitumor activity in a series of structurally modified glycolipids.1 Subsequent studies have shown that T cell receptors (TCRs) of NKT cells recognize the KRN7000 molecule presented by CD1d of antigen presenting cells (APC). The CD1 family of the antigen presenting glycoproteins mediates T-cell responses through the presentation of foreign lipids, glycolipids, lipoproteins and amphiphilic small molecules to TCR in a manner analogous to the peptide presentation by major histocompatibility complex (MHC) class 1 and ll molecules. Various CD1 isoforms (CD1a, -b, -c, -d, and -e) have been identified in humans, and their crystal structures have been studied.2 Upon recognition of KRN7000/CD1d complex, NKT cells release a number of cytokines, including pro-inflammatory T helper 1 (Th1) cytokines such as interferon-γ IFN-γ and tumor necrosis factor-α, and anti-inflammatory Th2 cytokines such as interleukin-4, -10, and -13 (IL-4, -10, and -13).3 Th1 cytokines are thought to correlate with the antitumor, antiviral and antibacterial activities, whereas Th2 cytokine are believed to promote immune tolerance and modulate the onset of autoimmune diseases such as type-1 diabetes.4 However, the opposing activities displayed by Th1 and Th2 cytokines induced by α-GalCer limits its value as a potential therapeutic agent.
A number of KRN7000 (α-GalCer) analogues were prepared and their biological properties evaluated in order to shed some light on the binding interaction between α-GalCer and CD1d, and also to selectively control the cytokine release profile by NKT cells either toward Th1 or Th2. Modification studies focused on the sugar moiety of α-GalCer suggested that the α-anomeric galactose group is critical for α-GalCer to bind CD1d and to active NKT cells through their TCR.5 The equatorial C2–OH group of the galactose was also found to be crucial for the activity of α-GalCer; any modification at this position largely abolished activity.6 Modifications at the C3- and C6–OH groups of the galactose was found to be tolerated.7 The structure–activity relationship (SAR) studies centered on the ceramide moiety of α-GalCer may be grouped into modifications of the acyl and phytosphingosine chains, and of the polar portion of the ceramide. It was found that truncations of either the phytosphingosine or acyl chain resulted in α-GalCer that biased NKT cells toward release of Th2 cytokines (IL-4),8 and insertion of double bonds into the acyl chain of α-GalCer (C20:2) also biased toward Th2 responses.9 On the other hand, introduction of an aromatic residue into either the acyl or phytosphingosine chain enhanced the Th1 cytokine profiles.10 The C-glycoside variant of KRN7000 (α-C-GalCer, in which the glycosidic oxygen was replaced by a methylene group) stimulated strong Th1 responses in vivo from NKT cells.11a–c The carbasugar analogues of KRN7000, in which the oxygen in the sugar ring was replaced by methylene, also showed to release Th1-biased cytokines, that is, IFN-γ in mice.11d The C3- and C4-OH groups of the phytosphingosine were reported to contribute to the activity of α-GalCer, although the C3–OH group appeared to be more important than the C4–OH group.5 The observed results have been rationalized in relation to the structures of the binding domains of the CD1d and NKT cell TCR proteins as determined by X-ray crystallography. Two X-ray studies of the CD1d/α-GalCer complexes have shown that the lipid chains of α-GalCer fit tightly into the CD1d binding groove (A′ and F′ pockets). In addition, hydrogen-bonding interactions were indicated between CD1d and α-GalCer [2-OH group of the galactose group and Asp151 (mouse Asp153) of the CD1d; 3-OH group of the phytosphingosine and Asp80 of CD1d]. These bonds serve to anchor α-GalCer in a distinct orientation and position it in the lipid binding groove.12,13 The recent crystal structure of human NKT TCR-CD1d-α-GalCer complex showed that α-GalCer protrudes minimally from the CD1d cleft with only the galactose group and portions of the proximal polar head of the phytosphingosine exposed for recognition by the NKT TCR.14
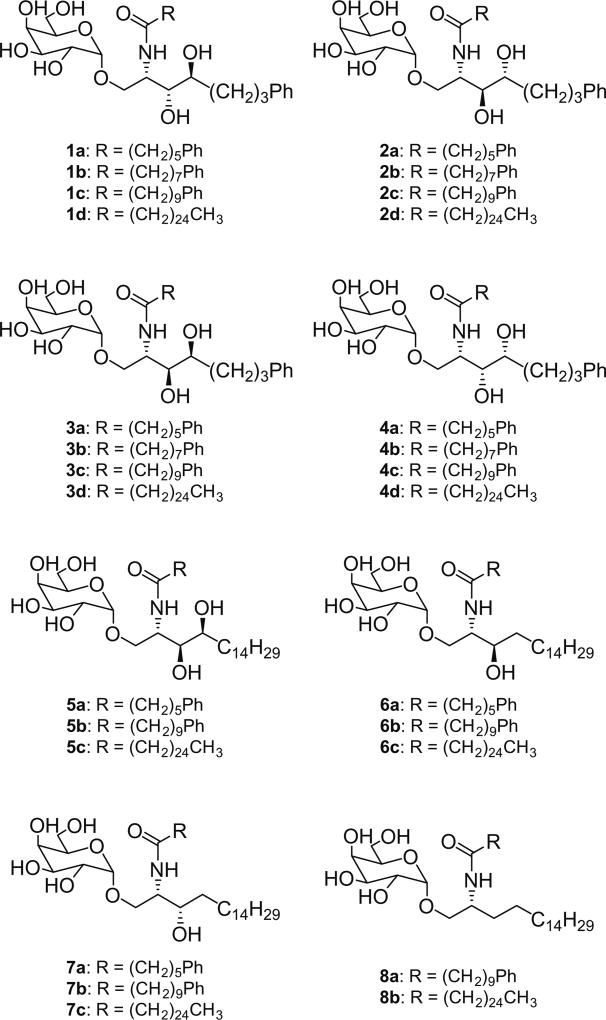
In the related efforts we have previously carried out synthesis of a complete set of the eight KRN7000 stereoisomers, and examined their biological activities, since a systematic study of phytosphingosine core-modified KRN7000 stereoisomers had not been carried out before. With both mouse and human iNKT cells, the stereoisomers with 2S (derived from l-serine) generally showed much higher potency (IFN-γ, IL-4/IL-13 production) than the isomers with 2R (derived from d-serine). However, the 2S isomer having the inverted C4–OH group stereochemistry (d-lyxo based on phytosphingosine) displayed comparable potency (proliferation and cytokine secretion) with those of KRN7000.15 These data suggest that the spatial orientations of C2–NH2 group and C3–OH group of phytosphingosine are both important for the activity, with the configuration of C2–NH2 group having a greater impact than that of C3–OH group. In contrast, stereochemical variation of the C4–OH group seems to have at most a minor effect which is less significant than what was suggested in some earlier studies.5 Based on our stereochemical studies of the phytosphingosine backbone and the earlier results obtained by Wong et al. from the analogues having an aromatic residue in the acyl or phytosphingosine chain,10 we have now prepared a series of compounds in which various combinations of the aromatic residue in both chains and the backbone stereochemistry have been made, and examined their biological properties (Fig. 1).
Figure 1.
Structures of KRN7000 analogues.
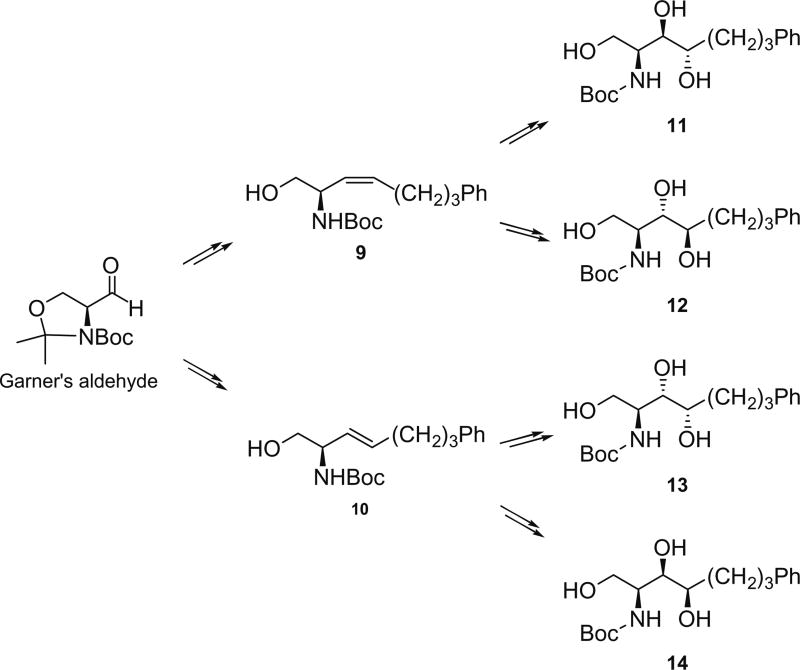
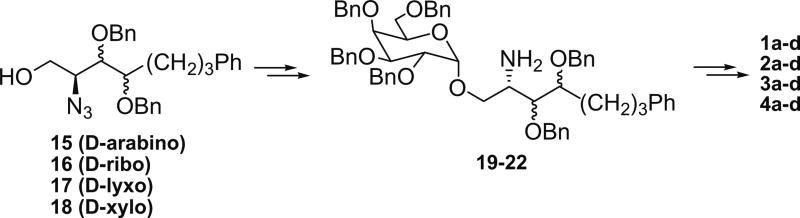
Our syntheses of new KRN7000 analogues commenced with the Garner’s aldehyde, which was readily prepared from l-serine. The Wittig reaction with tripheny-(4-phenylbutyl)-phosphonium bromide in the presence of n-BuLi provided predominantly the (Z)-olefin (Z/E = 22/1) in 88% yield. Removal of the oxazoline protecting group in aq acetic acid gave 9 in 95%. On the other hand, the Wittig reaction in the presence of KHMDS followed by quenching with excess methanol at −78 °C gave the corresponding (E)-olefin (10) in 93%. The Sharpless dihydroxylation16 of 9 and 10 each with the AD-mix-α and AD-mix-β conditions provided in good yields all four diastereomers corresponding to d-arabino (11), d-ribo (12), d-lyxo (13), and d-xylo (14) configurations (Scheme 1). The stereoselectivities observed in the dihydroxylation were found to be about 10:1 for products 11 and 13, and about 5:1 for 12 and 14; they were separated to pure diastereomers. Each of compounds 11–14, after removal of the Boc group, was treated with TfN3, CuSO4 and K2CO3 for diazo transfer reaction (ca. 90% over two steps), in which the amino group was converted to azido functionality.17 The 1°-OH group was protected with trityl chloride and Et3N, and the 2°-OH with benzyl bromide and NaH, respectively. Removal of the trityl group followed by glycosylation with perbenzylated galactosyl iodide with TBAI as promoter exclusively provided the desired α-glycoside in better than 90%.18 The azide group was reduced under the Staudinger conditions,19 and the resulting amine (19–22) was efficiently acylated with four different N-hydroxysuccinimide activated esters.20 In the final step all benzyl protecting groups were removed with H2 over Pd(OH)2/C to yield 16 analogues (1–4) of α-GalCer (Scheme 2).
Scheme 1.
Preparation of truncated phytosphingosine skeletons.
Scheme 2.
α-Glycosylation and N-acylation to analogues 1–4.
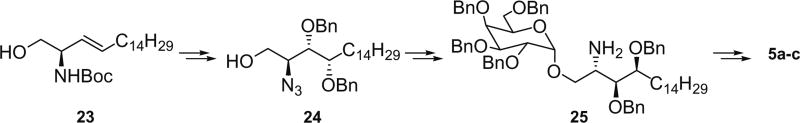
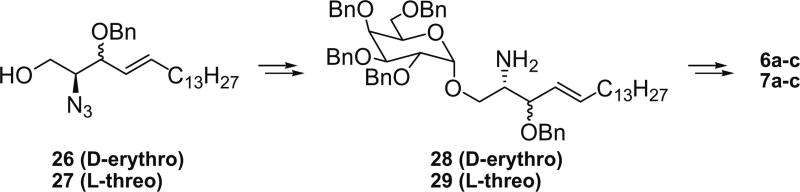
Reaction of the Garner’s aldehyde with tripheny-pentadecylphosphonium bromide in the presence of KHMDS, followed by quenching with excess methanol at −78 °C gave the (E)-olefin product in a good yield, and the subsequent hydrolysis of the oxazoline ring provided 23. The Sharpless dihydroxylation of 23 with Ad-mix-α provided two diastereomeric products in 4:1 ratio. The major product was separated and the Boc group was removed, and the amino functionality was converted to the azido group. Selective manipulations of the 1° and 2° hydroxyl groups as described in the previous series yielded compound 24. The terminal OH group was glycosylated, and the azide was reduced to amino functionality with trimethylphosphine to give 25. Compound 25 was acylated with three different N-hydroxysuccinimide activated esters, and all benzyl groups were removed to yield 3 analogues (5a–c) of α-GalCer. (Scheme 3) The remaining analogues were prepared as follows. d-erythro- and l-threo-sphingosine21 were separately converted to the corresponding azide with N3Tf and CuSO4, and the 2°-OH group in the products was protected as benzyl ether (26 and 27). The terminal OH group was glycosylated, and the azide reduced to the amino group to give 28 and 29. The amino group was acylated with three different N-hydroxysuccinimide activated esters, and removal of all benzyl groups together with saturation of the double bond were accomplished by using H2 and Pd(OH)2/C in MeOH and CH2Cl2 to yield 3 analogues each (6 and 7) of α-GalCer (Scheme 4). A second product was obtained in the last reduction step with H2 over Pd(OH)2 catalyst, and it turned out to be the hydrogenolysis product of the benzyl ether at the allylic position, namely compound 8.
Scheme 3.
Synthesis of analogues 5.
Scheme 4.
Synthesis of analogues 6 and 7.
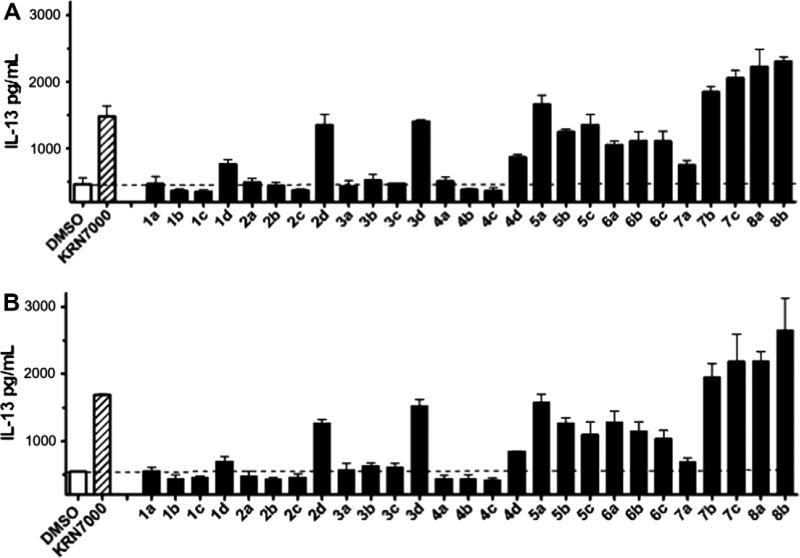
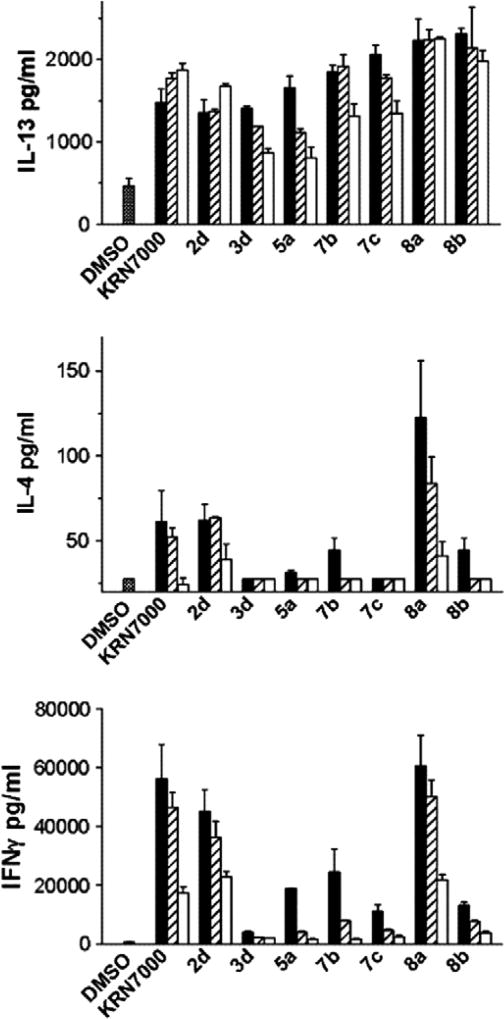
The biological activity of these phenyl-containing α-GalCer analogues for stimulation of human NKT cells was evaluated by induction of cytokines such as INF-γ, IL-4 and IL-13 (Figs. 2 and 3). Fig. 2 summarizes IL-13 releasing activity of all analogues (at 400 nM concentration), which was used as the primary screen since IL-13 production is known to be an extremely sensitive indicator of iNKT cell activation.22 Panel A shows IL-13 production from a representative CD4+ T cell clone and Panel B shows a similar analysis using a representative CD4− T-cell clone. A subset of compounds were observed to be strongly stimulatory, while others were weakly active or inactive. Figure 3 shows expanded analysis (IL-4, IL-13 and IFN-γ) of a human CD4+ iNKT cell clone stimulated with various concentrations (400 nM, 100 nM and 25 nM) of the most active compounds identified in the initial screen. Compounds such as 3d, 5a, 7b, 7c and 8b were highly active even at low dosage, and also showed some selectivity toward stimulation of IL-13 (a Th2 cytokine), whereas compound 2d showed activity more similar to KRN7000. It is also of interest to note the relatively strong activity of compounds 8a and 8b, which have a simple α-amino alcohol skeleton, indicating that the C3–OH group of the sphingosine skeleton was not essential for activity in this series of compounds. In fact, the presence of an aromatic ring in the acyl chain of compound 8a appeared to completely rescue any attenuation of activity due to the lack of a C3–OH group of the sphingosine skeleton. A more extensive study of the biological activities of these KRN7000 analogues and further structural modifications including their conversion to the carbasugar analogues are in progress.
Figure 2.
IL-13 production by human iNKT cell clones as primary screen for activity. Panel A shows IL-13 production by cultures in which a CD4+ NKT cell clone (HDD11) was cultured with antigen presenting cells (CD1d-transfected HeLa cells) and the indicated glycolipids at a concentration of 400 nM. Panel B shows the same analysis using a CD4− NKT cell clone (HDA7). Cultures were done in 96 well microtiter plates, and medium was RPMI-1640 supplemented with 10% fetal bovine serum. 2.5 × 104 CD1d-transfected HeLa cells per well in 0.2 ml medium with the indicated glycolipids were cultured for 18 h at 37 °C, followed by removal of the medium, a brief wash with medium and then replacement with 0.3 ml fresh medium (without glycolipids) containing 2.5 × 104 cloned human iNKT cells. IL-13 levels were determined by standard capture ELISA in culture supernatants harvested after 18 h of incubation at 37 °C.
Figure 3.
Dose dependence of production of IL-13, IL-4 and IFN-γ by human NKT cells in response to selected compounds. Human CD4+ NKT cell clone (HDD11) was stimulated in cultures as described in Figure 2 using the indicated glycolipids at a range of concentrations (black bar, 400 nM; hatched bar 100 nM; open bar 25 nM).
Supplementary Material
Acknowledgments
This work was supported by the Korea Research Foundation grant funded from the Korean Government (MOEHRD: KRF-2005-070-C00078), and by a grant from the NIH/NIAID (RO1 AI45889).
Footnotes
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.bmcl.2009.12.103.
References and notes
- 1.Morita M, Motoki K, Akimoto K, Natori T, Sakai T, Sawa E, Yamaji K, Koezuka Y, Kobayashi E, Fukushima H. J. Med. Chem. 1995;38:2176. doi: 10.1021/jm00012a018. [DOI] [PubMed] [Google Scholar]
- 2.Wu D, Fujio M, Wong C-H. Bioorg. Med. Chem. 2008;16:1073. doi: 10.1016/j.bmc.2007.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.(a) Kronenberg M. Annu. Rev. Immunol. 2005;23:877. doi: 10.1146/annurev.immunol.23.021704.115742. [DOI] [PubMed] [Google Scholar]; (b) Savage PB, Teyton L, Bendelac A. Chem. Soc. Rev. 2006;35:771. doi: 10.1039/b510638a. [DOI] [PubMed] [Google Scholar]; (c) Tsuji M. Cell. Mol. Life Sci. 2006;63:1889. doi: 10.1007/s00018-006-6073-z. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Stronge VS, Salio M, Jones EY, Cerundolo V. Trends Immunol. 2007;28:455. doi: 10.1016/j.it.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 4.(a) Kakimi K, Guidotti LG, Koezuka Y, Chisari FV. J. Exp. Med. 2000;192:921. doi: 10.1084/jem.192.7.921. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Fuji N, Ueda Y, Fujiwara H, Itoh T, Yoshimura T, Yamagishi H. Clin. Cancer Res. 2000;6:3380. [PubMed] [Google Scholar]; (c) Wang B, Geng Y-B, Wang C-R. J. Exp. Med. 2001;194:313. doi: 10.1084/jem.194.3.313. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Van Kaer L. Nat. Rev. Immunol. 2005;5:31. doi: 10.1038/nri1531. [DOI] [PubMed] [Google Scholar]
- 5.(a) Motoki K, Kobayashi E, Uchida T, Fukushima H, Koezuka Y. Bioorg. Med. Chem. Lett. 1995;5:705. doi: 10.1016/0968-0896(96)00049-1. [DOI] [PubMed] [Google Scholar]; (b) Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, Ueno H, Nakagawa R, Sato H, Konodo E, Koseki H, Taniguchi M. Science. 1997;278:1626. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]; (c) Brossay L, Naidenko O, Burdin N, Matsuda J, Sakai T, Kronengerg M. J. Immunol. 1998;161:5124. [PubMed] [Google Scholar]; (d) Sidobre S, Hammond KJL, Sidobre LB, Maltsev SD, Richardson SK, Ndonye RM, Howell AR, Sakai T, Besra GS, Porcelli SA, Kronenberg M. Proc. Natl. Acad. Sci. U.S.A. 2004;101:12254. doi: 10.1073/pnas.0404632101. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Trappeniers M, Goormans S, Van Beneden K, Decruy T, Linclau B, Al-Shamkhani A, Elliot T, Ottensmeier C, Werner JM, Elewaut D, Van Calenbergh S. ChemMedChem. 2008 doi: 10.1002/cmdc.200800021. [DOI] [PubMed] [Google Scholar]
- 6.(a) Barbieri L, Costantino V, Fattorusso E, Mangoni A, Aru E, Parapini S, Taramelli D. Eur. J. Org. Chem. 2004:468. [Google Scholar]; (b) Barbieri L, Costantino V, Fattorusso E, Mangoni A, Basilico N, Mondani M, Taramelli D. Eur. J. Org. Chem. 2005:3279. [Google Scholar]
- 7.(a) Zhou X-T, Forestier C, Goff RD, Li C, Teyton L, Bendelac A, Savage PB. Org. Lett. 2002;4:1267. doi: 10.1021/ol025565+. [DOI] [PubMed] [Google Scholar]; (b) Xing G-W, Wu D, Poles MA, Horowitz A, Tsuji M, Ho DD, Wong C-H. Bioorg. Med. Chem. 2005;13:2907. doi: 10.1016/j.bmc.2005.02.018. [DOI] [PubMed] [Google Scholar]; (c) Wu D, Xing G-W, Poles MA, Horowitz A, Kinjo Y, Sullivan B, Bodmer-Narkevitch V, Plettenburg O, Kronenberg M, Tsuji M, Ho DD, Wong C-H. Proc. Natl. Acad. Sci. U.S.A. 2005;102:1351. doi: 10.1073/pnas.0408696102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.(a) Miyamoto K, Miyake S, Yamamura T. Nature. 2001;413:531. doi: 10.1038/35097097. [DOI] [PubMed] [Google Scholar]; (b) Goff RD, Gao Y, Mattner J, Zhou D, Yin N, Cantu C, III, Teyton L, Bendelac A, Savage PB. J. Am. Chem. Soc. 2004;126:13602. doi: 10.1021/ja045385q. [DOI] [PubMed] [Google Scholar]
- 9.Yu KOA, Im JS, Molano A, Dutronc Y, Illarionov PA, Forestier C, Fujiwara N, Arias I, Miyake S, Yamamura T, Chang YT, Besra GS, Porcelli SA. Proc. Natl. Acad. Sci. U.S.A. 2005;102:3383. doi: 10.1073/pnas.0407488102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.(a) Fujio M, Wu D, Garcia-Navarro R, Ho DD, Tsuji M, Wong C-H. J. Am. Chem. Soc. 2006;128:9022. doi: 10.1021/ja062740z. [DOI] [PubMed] [Google Scholar]; (b) Chang YJ, Huang JR, Tsai YC, Hung JT, Wu D, Fujio M, Wong CH, Yu AL. Proc. Natl. Acad. Sci. U.S.A. 2007;104:10299. doi: 10.1073/pnas.0703824104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.(a) Franck RW, Tsuji M. Acc. Chem. Rev. 2006;39:692. doi: 10.1021/ar050006z. [DOI] [PubMed] [Google Scholar]; (b) Li X, Chen G, Garcia-Navarro R, Franck RW, Tsuji M. Immunology. 2008;127:216. doi: 10.1111/j.1365-2567.2008.02943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Li X, Shiratsuchi T, Chen G, Dellabona P, Casorati G, Franck RW, Tsuji M. J. Immunol. 2009;183:4415. doi: 10.4049/jimmunol.0901021. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Tashiro T, Nakagawa R, Hirokawa T, Inoue S, Watarai H, Taniguchi M, Mori K. Bioorg. Med. Chem. 2009;17:6360. doi: 10.1016/j.bmc.2009.07.025. [DOI] [PubMed] [Google Scholar]
- 12.Zajonc DM, Cantu C, III, Mattner J, Zhou D, Savage PB, Bendelac A, Wilson IA, Teyton L. Nat. Immunol. 2005;6:810. doi: 10.1038/ni1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koch M, Stronge VS, Shepherd D, Gadola SD, Mathew B, Ritter G, Fersht AR, Besra GS, Schmidt RR, Jones EY, Cerundolo V. Nat. Immunol. 2005;6:819. doi: 10.1038/ni1225. [DOI] [PubMed] [Google Scholar]
- 14.(a) Borg NA, Wun KS, Kjer-Nielsen L, Wilce MCJ, Pellicci DG, Koh R, Besra GS, Bharadwaj M, Godfrey DI, McCluskey J, Rossjohn J. Nature. 2007;448:44. doi: 10.1038/nature05907. [DOI] [PubMed] [Google Scholar]; (b) Fellici DG, Patel O, Kjer-Nielsen L, Pang SS, Sullivan L, Kyparissoudis K, Brooks AG, Reid HH, Gras S, Lucet IS, Koh R, Smyth M, Mallevaey T, Matsuda JL, Gapin L, McClusky J, Godfrey DI, Rossjohn J. Immunity. 2009;31:47. doi: 10.1016/j.immuni.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park JJ, Lee JH, Ghosh SC, bricard G, Venkataswamy MM, Porcelli SA, Chung SK. Bioorg. Med. Chem. Lett. 2008;18:3906. doi: 10.1016/j.bmcl.2008.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharpless KB, Amberg W, Bennani YL, Crispino GA, Hartung J, Jeong KS, Kwong HL, Morikawa K, Wang ZM, Xu D, Zhang XL. J. Org. Chem. 1992;57:2768. [Google Scholar]
- 17.Alper PB, Hung SC, Wong CH. Tetrahedron Lett. 1996;34:6029. [Google Scholar]
- 18.(a) Gervay J, Nguyen TN, Hadd ML. Carbohydr. Res. 1997;300:119. [Google Scholar]; (b) Du W, Gervay-Hague J. Org. Lett. 2005;7:2063. doi: 10.1021/ol050659f. [DOI] [PubMed] [Google Scholar]
- 19.Lee A, Farrand KJ, Dickgreber N, Hayman CM, Jurs S, Hermansb IF, Painterra GF. Carbohydr. Res. 2006;341:2785. doi: 10.1016/j.carres.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 20.(a) Chang YT, Choi J, Ding S, Prieschl EE, Baumruker T, Lee JM, Chung SK, Schultz PG. J. Am. Chem. Soc. 2002;124:1856. doi: 10.1021/ja017576o. [DOI] [PubMed] [Google Scholar]; (b) Park JJ, Lee JH, Li Q, Diaz K, Chang YT, Chung SK. Bioorg. Chem. 2008;36:220. doi: 10.1016/j.bioorg.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 21.(a) Chung SK, Lee JM. Tetrahedron: Asymmetry. 1999;10:1441. [Google Scholar]; (b) Lee JM, Lim HS, Chung SK. Tetrahedron: Asymmetry. 2002;13:343. [Google Scholar]; (c) Lee JM, Lim HS, Seo KC, Chung SK. Tetrahedron: Asymmetry. 2003;14:3639. [Google Scholar]
- 22.Wang X, Chen X, Rodenkirch L, Simonson W, Wernimont S, Ndonye RM, Veerapen N, Gibson D, Howell AR, Besra GS, Painter GF, Huttenlocher A, Gumperz JE. Blood. 2008;112:4128. doi: 10.1182/blood-2008-05-157529. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.