Abstract
Tendons and ligaments provide connections between muscle and bone or bone and bone to enable locomotion. Damage to tendons and ligaments caused by acute or chronic injury or associated with aging and arthritis is a prevalent cause of disability. Improvements in approaches for the treatment of these conditions depend on a better understanding of tendon and ligament development, cell biology and pathophysiology. This review focuses on recent advances in the discovery of transcription factors that control ligament and tendon cell differentiation, how cell and extracellular matrix homeostasis are altered in disease and how this new insight can lead to novel therapeutic approaches.
Keywords: tendon, ligament, arthritis, Mkx, Scx, Egr1/2
INTRODUCTION
Despite the unique and important roles of tendons and ligaments in the musculoskeletal function and musculoskeletal diseases, research in this area is not as advanced as in other skeletal tissues. This is partly because, until recently, the transcription factors critical for the formation and maintenance of these tissues were unknown. The recent identification of Scleraxis (Scx), Mohawk (Mkx) and early growth response factor (Egr1) and their specific roles in tendon and ligament development, maturation, homeostasis and repair of mature tissues has substantially advanced the field of tendon and ligament research. This review summarizes the current knowledge and concepts regarding the development, function and disease-associated changes in tendon and ligament cells and extracellular matrix (ECM) with a focus on Mkx, Scx and Egr1.
Tendons and ligaments are distinct but closely related tissues. Although developmental processes of tendon and ligament tissues and the phenotypes of mice with genetic deletion of the key transcription factors are different (see below section 2 and 3), the basic structures of these tissues and the gene expression profiles of their major cell type (tenocytes) are rather similar.
We therefore focus this report on the common basis between tendons and ligaments uncovered in recent research and use anterior cruciate ligament (ACL) injuries as a representative example of tendon and ligament injuries (see section 1 below).
1. Clinical significance of tendon and ligament tissue damage
Tendon and ligament damage caused by injury or overuse or associated with aging and arthritis is a common clinical problem because damaged tissues heal very slowly and rarely recover completely (1). Damage to the glenohumeral ligaments and the rotator cuff tendons of the shoulder joint, which can lead to shoulder pain and functional limitations, are highly prevalent, with >50% of individuals older than 60 years of age being affected (2). The success of surgical treatment is limited, as up to 50% of rotator cuff repair procedures fail.
The Achilles tendon is the most frequently ruptured tendon in humans (3). Injury and aging-related changes in cells and the ECM of tendon and ligament in the knee joints, particularly the ACL, represent major risk factors for the development of osteoarthritis (OA) (4, 5). The ACL is essential for knee kinematics, especially in rotation, and functions as an anterior/posterior stabilizer (6, 7). In the setting of ACL deficiency, the articular cartilage as well as the menisci are more susceptible to arthritic changes. A large number of OA patients without a history of ligament injury have ACL deficiency at the time of total knee arthroplasty (8), and a correlation between the radiologic OA grade and the histological grade of ACL degeneration has been reported in end-stage OA (9). In addition, ACL rupture is more common among patients with symptomatic knee OA than in those without. It has been reported that fewer than half of subjects with ACL rupture recall a knee injury, suggesting that this risk factor for knee OA is under-recognized (8). These findings indicate that substantial changes occur in tendons and ligaments in arthritic knees that are not related to major trauma but are presumably caused by mediators, such as cytokines or matrix degrading enzymes, in the arthritic joints that affect tendon and ligament cells and the ECM.
2. Biology of mature tendon and ligament tissues and cells
2.1 Structure of mature tendon and ligament tissue
Tendon and ligament are dense, fibrous connective tissues that connect muscle and bone or bone and bone and transmit the mechanical forces that stabilize the skeleton and allow for body movement (10, 11). The ECM of the main part of the tendon and ligament (Figure 1A) consists predominantly of type I collagen (12, 13) and small amounts of other collagens, including types III, IV, V and VI (14, 15). The proteoglycans found in tendon and ligament, include decorin, fibromodulin, biglycan and lumican. Their main function is to organize and lubricate collagen fiber bundles (14). Targeted disruption of these proteoglycans in mice leads to abnormal collagen fibrils in tendons (12, 13). The ECM of tendons and ligaments is hierarchically organized in several distinct layers (Figure 1A) (11, 16). Collagen microfibrils, the primary unit of mature tendon and ligament tissues, are first formed from collagen I molecules, organized as triple-helix polypeptide chains. Lateral and longitudinal stacking of microfibrils leads to the assembly of fibrils, which are arrayed in a parallel fashion to form collagen fibers with increasing diameter and mechanical strength. The fibers are then packed into larger units, termed fascicles, which are bundled by endotenon, and these bundles are wrapped by epitenon to form the complete tendon. Endotenon and epitenon are vascularized and innervated, which is essential for tendon homeostasis and response to injury (11, 16). The hierarchical structure of tendons and ligaments determines their biomechanical properties. The sharp ends of the fibrils, which on microscopy appear in wave-like or crimp patterns, provide tensile strength. The elasticity of tendons and ligaments is due to the large amount of type I collagen (17).
Figure 1.
(A) A schematic drawing showing the hierarchal structure of tendon/ligament tissue. Tropocollagens synthesized in tenocytes covalently bind and become collagen fibrils. The arrays of the collagen fibrils form collagen fibers. Tenocytes reside in the gaps of the fibers, connecting to one another through cellular channels. The collagen fibers are packed into fascicles that are bundled by endotenon. The fascicles are wrapped by epitenon to form the complete tendon/ligament tissue. (B) The expression period of the transcription factors and the signal input during the tendon development. Tenocytes are specified from mesenchymal progenitor cells by signaling cues, such as TGFβ or FGF, from neighboring tissues and marked by the expression of Scx. The expression of Egr1 and Mkx follow soon after and support the differentiation of tenocytes into embryonic tendon tissue. While the expression of Scx decreases after birth, the expression of Egr1 and Mkx persists throughout the post-natal maturation period and is essential for tissue maintenance during adult life. The panels are adopted from Itoh et al. PNAS 2010 and showing in situ hybridization of Mkx that indicates the embryonic tendon (left) and the tail tendon tissue from 10-week old mice (right).
2.2 Biology of mature and progenitor tendon and ligament cells
The principal cell types in tendon and ligament are fibroblast-like cells, termed tenocytes, or ligament fibroblasts that are located between parallel chains of collagen fibrils (14, 21). Under normal conditions, tendon and ligament cells are thought to be quiescent and have very low rates of proliferation, while ECM production is responsive to changes in the mechanical load (22). These upregulated genes in tendon and ligament cells include types I, III, IV, V and VI collagen, which are also produced during embryogenesis to form tendon and ligament tissues. While these matrix genes are not specific to tendon and ligament cells, tenomodulin (Tnmd) is specifically expressed in mature tendon and ligament cells (23). However, its function in tendons and ligaments is not well characterized, and Tnmd knockout mice appear grossly normal (15).
The cell populations in tendon and ligament also include small subsets of progenitor cells (24). The deletion of biglycan and fibromodulin in mice causes the depletion of tendon progenitor cells, suggesting that these extracellular matrix components provide a niche environment for stem cells (12). The identification of specific molecular markers for tendon progenitor cells in adult tissues will help elucidate the contribution of this subset to tendon homeostasis and regeneration.
3. Developmental biology of tendon and ligament tissues and cells
3.1 Developmental biology of tendon and ligament tissues
Differentiation of tenocytes is tightly regulated by a series of growth factors and transcription factors (Figure 1B). The developmental origins of tenocytes and their interacting cells that initiate tenocyte differentiation differ in the trunk, limbs and craniofacial region (16). In the trunk, the tenocyte progenitor cells originate from the sclerotome, which is derived from somite, and they are condensed in a subregion called syndetome where Scx is expressed (25–27). The myotome, which is also derived from somite and the source of myocytes, is essential for the induction of tendon progenitor cells in the syndetome via FGF signals (27–29). In contrast, tenocytes in limbs arise from the lateral plate mesoderm and interact with migrating myoblasts from the ventrolateral lip of the dermomyotome (25, 30). In this process, tenocyte progenitor cell induction does not depend on myocytes or myocyte-derived factors (31). At later stages, however, the absence of myoblasts causes disruption of tenocyte survival and tendon formation (25, 28, 32). In this process, TGFβ and FGF signals in limb play a critical role in tenocyte differentiation by promoting Scx expression (28, 33). In addition, recent findings have revealed that the tendons in autopods differ significantly from those in zeugopods (34). While the development of the tendons in autopods depends on limb cartilage, that in zeugopods requires limb muscles to form properly.
An essential role of TGFβ for tendon progenitor cell recruitment and maintenance during embryogenesis has been revealed by studies in mutant mice (28, 33). Tgfb2 and Tgfb3 double mutant mice or mice with TGFBR2 inactivation show the loss of tendons and ligaments in both the limbs and trunk (33). As TGFβ and FGF are also involved in muscle and cartilage development (35), the specificity of these effects for tenogenic differentiation remains unclear; however, the focal and interactive effects of these multiple growth factor signals are integrated to promote proper tendon development (16). This notion is supported by the findings of a transcriptome analysis of limb tendons, which revealed that the expression of TGFβ and FGF signaling related genes was highly upregulated during tendon differentiation and maturation (36).
Improvements in serial block face-scanning electron microscopy technology have shed new light on how the tendon anlage, which mainly consists of progenitor cells but less ECM, evolves into a mature tendon structure where tenocytes are embedded in collagen fibers. Kalson et al. stated that tendon development could be divided into two distinct stages: during the first stage (embryonic stage), tendon progenitors form cellular channels that define the extracellular space where collagen fibrils are deposited. This serves as the template for the second post-natal stage when the number of cells or collagen bundles remains constant and tissue growth depends on the increase in the diameter of collagen fibers (37).
3.2 Roles of transcription factors during tendon and ligament development
Major advances in research on tendon and ligament biology include the recent identification of three transcription factors Scx, Mkx and Egr1 as critical regulators of tendon and ligament development and potentially homeostasis (Figure 1B). Scx is a helix-loop-helix (bHLH) transcription factor that is expressed in tendon progenitors (25, 26) and is required for the development of limb tendons. Scx−/− mice show severe disruption of force-transmitting tendons (38). While the induction of tendon progenitor cells is normal in Scx−/− mice, the formation of distinct tendons in the limbs, tail or trunk is disrupted. However, short tendons or ligaments that mainly act as anchors of bones or muscles are not affected by the loss of Scx, indicating an as-yet-undefined difference in the formation process of tendons and ligaments (38). The currently known targets of Scx-dependent transcription are Col1a1, Col1a2, Acan and Tnmd (23, 38–40). However, the Scx expression is strongly downregulated after birth (16), suggesting that Scx is essential for early stages of tendon development but may not be as important in the homeostasis of tendons and ligaments in adulthood.
The phenotypes of Scx and Mkx mutant mice are different. While Scx−/− mutants exhibit loss of segments or of complete tendons (38), Mkx−/− mice have reduced tendon mass without a decrease in the number of tendon cells (41–43). These data suggest that Scx is essential for the initiation of tendon differentiation, whereas Mkx plays a critical role in tendon maturation. Recently, Mkx−/− rats have been generated using the CRISPR/Cas9 system (44). Mkx−/− rats showed systemic hypoplasia of tendons similar to Mkx−/− mice, and by virtue of their larger sizes, the physiological functions of tendon and ligament tissues were able to be evaluated in this model animal. A gait analysis revealed a significant decrease in the maximum ankle plantar flexion of Mkx−/− rats (44). In addition, earlier heterotopic ossification of the Achilles tendon was observed in Mkx−/− rats (44), which is discussed further in the chondrogenic/osteogenic alteration of tendon and ligament tissues section below.
Egr1 belongs to a family of zinc finger transcription factors named after the immediate response to growth factor stimulation (45). Among the four family members, Egr1 and 2 are expressed in developing tendon tissue and play essential roles in tendon and ligament formation (46). Mice with mutations in either Egr1 or Egr2 showed a reduction of collagen fibrils numbers in embryonic tendons associated with a reduced expression of tenogenic genes, while misexpression of these genes led to the ectopic expression of Scx and Col1a1 in a chick model (46). Interestingly, while the loss of Egr1 resulted in a reduction in both Scx and Mkx expression in embryonic tendons, only the Scx expression and not the Mkx expression was impaired in adult Egr1−/− injured tendon (47). These results indicate dynamic differences in the mutual regulation of these three transcription factors between embryonic and adult tendon and ligament tissue. Furthermore, no mice with knockout for these transcription factors showed complete loss of tendon or ligament tissue, implying that some functions may be shared among these factors or that as-yet-unknown factors ensuring the formation of tendon/ligament tissues may exist.
3.3 Transition zones between bone and tendon or ligament
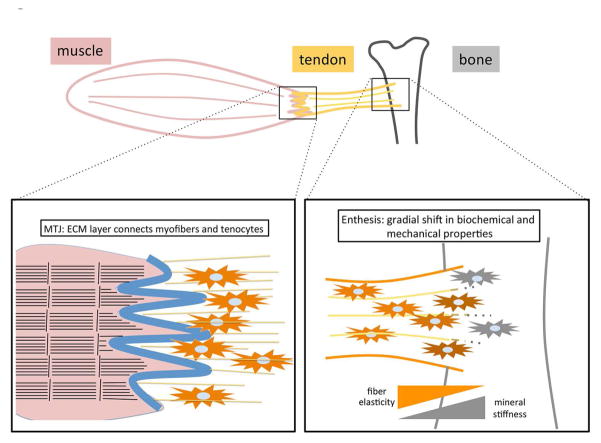
The enthesis, which directly inserts into the metaphysis or diaphysis of long bones, is a heterogeneous composite of four zones (tendon and ligament ECM, fibrocartilage, mineralized fibrocartilage and bone), each having unique ECM and cell phenotypes (18, 19). This gradual change in structure is crucial to avoid rupture between two tissues upon application of a mechanical load. Signaling mechanisms that control the formation of the enthesis are being discovered. During the initiation stage of enthesis formation, Scx-dependent BMP4 expression in tenocytes is essential (49), and subsequent biomechanical stress caused by muscle contraction on this bone ridge area promotes further development of the tendon-bone connection (50). In addition, hedgehog (Hh) signaling is also indispensable for the proper maturation of enthesis tissue. Thomopoulos et al. found that the Hh signal is activated in a subpopulation of cells in the enthesis, and inactivation of Hh signaling in Scx-expressing cells impaired enthesis maturation (51).
Sox9 is a master regulator of chondrocyte differentiation and cartilage development. It starts appearing in mesenchymal condensations at the limb buds and induces chondrocyte progenitors by regulating cartilage-specific genes, such as Col2a1. In limbs, this chondrogenesis status is linked to tenocyte differentiation (52, 53). In the initiation phase, Sox9 and Scx double-positive cells transiently appear in the mesenchyme and then transform to Sox9 or Scx single-positive cells (52, 53). The Sox9-Scx double-positive cells show delayed expression of the chondrocyte marker Col2, and molecular lineage tracing studies have shown that Sox9 and Scx double-positive cells mainly contribute to the tendon-bone ridge area, where Sox9 and Scx expression are specifically and exclusively restricted to chondrocytes and tenocytes at this stage. Loss of Sox9 (52, 53) and Scx (54) impaired the formation of the bone ridge and enthesis, indicating that both transcription factors are functionally essential for the formation of this transitional zone. This suggests that the balance between Sox9 and Scx regulates cell differentiation and the formation of the tendon-bone junction (52, 53).
Recently, Hh-activated and GDF5-positive cells were identified as enthesis progenitors (51, 55). Whether the classification of cell populations based on transcription factors (Sox9/Scx) and signaling molecules (GDF5/Hh) overlaps or represents different subsets is unclear at present. In this regard, GDF5-positive cells were found to be not a stable population but rather dynamically replaced by continuous recruitment and exit during joint formation (influx model) (56). Further investigation based on these findings will provide a more comprehensive understanding of enthesis development.
3.4 Transition zones between muscle and tendon
The transition zone between tendon and muscle is called the myotendinous junction (MTJ), a layer of ECMs including laminin, types I and IV collagen and thrombospondins. Cells in these areas interact with the ECM via transmembrane proteins, such as integrins and utrophin, that mediate intracellular signaling via dystrophin complex, talin/vinculin or integrin-linked kinase (20). Structurally, the MTJ forms a finger-like process between muscle sarcolemma and tendon collagen fibers to increase the attachment interface and provide resistance to the mechanical force. In mammalian MTJ, the ultrastructure of the finger-like process and its post-natal maturation is well described (57) (Figure 2), but the corresponding molecular and cellular mechanisms are less well-understood. Other model organisms, such as Drosophila and zebrafish, provide insight into this process. In zebrafish myosepta, secretion and accumulation of ECM between muscle segments occur first, followed by the recruitment of tenocytes and further maturation of ECM structure and binding with the muscle cells (58). Myosepta can be considered an analog of the mammalian MTJ. Most ECM components are common, and thus molecular mechanisms can also be conserved (58). For example, the functional importance of Col22a1 or thrombospondin4, ECM components found in mammalian MTJ, has also been proven in the zebrafish model system (59, 60). The craniofacial tendon of zebrafish can also serve as a model for mammalian long tendons (61). In addition to tissue formation, the mechanisms underlying the correct positioning and orientation of tendons and muscles, which derive from distinct progenitors, remain unclear in vertebrates (16). In Drosophila, an elaborate guiding system employing secreted Slit protein and Robo receptors governs muscle-tendon attachment (16). Slit ligand secreted from tendon cells is cleaved by the Robo2 receptor and oligomerized/stabilized on the tendon cell membrane, providing a repulsion/halt signal to muscle cells through Robo1/3 receptors (62).
Figure 2.
The transition zones between tendon and muscle (MTJ) and tendon and bone (enthesis). Top panel: a schematic drawing of the muscle-tendon-bone unit. Muscle, tendon and bone are depicted in red, yellow and gray, respectively. Bottom panel, left: MTJ. Muscle and tendon are illustrated on the left and right, respectively. Muscle sarcomeres are depicted as black horizontal lines, with vertical bars indicating Z lines. Collagen fibers are indicated as yellow lines, with tenocytes (orange cells) embedded. MTJ is the ECM layer with finger-like protrusions to increase the surface area between the muscle and tendon and is shown as a blue line. Bottom panel, right: enthesis. Tendon and bone are illustrated on the left and right, respectively. The ECM contents and deposited minerals change gradually within the enthesis, resulting in the continuous shift of tissue elasticity and stiffness.
3.5 Mechanobiology of tendon and ligament cells
Tenocytes respond to mechanical strain by increasing collagen production (22), and mechanical signals promote MSC differentiation into tenocytes (63). Mechanical stimulation also promotes collagen fiber thickening along with increased fiber density (64).
The three major tendon/ligament transcription factors (Scx, Mkx and Egr1) are also responsive to mechanical stimulation (65). Scx expression was promoted in bioartifical tendons composed of mouse mesenchymal stem cells after stretching in vitro and in the epitenon cells of mouse Achilles tendon after treadmill running in vivo (66). Mechanical force and misexpression of Scx synergistically promoted the differentiation of human MSCs to tenocytes (67). Egr1/2 was also increased after short treadmill exercise in rats (68), and the forced expression of Egr1 rescued tendon gene downregulation during tendon healing under conditions of a reduced mechanical load (69).
Mkx is also responsive to mechanical stimulation both in vitro and in vivo. In moderate treadmill exercise, the effects of mechanical loading on collagen fiber thickening are not observed in Mkx-deficient mice, suggesting that Mkx is critical in the response to mechanical stimuli (64). Interestingly, general transcription factor II-I repeat domain-containing protein 1 (Gtf2ird1) was found to play an important role in translating mechanical stress to Mkx expression (64). Gtf2ird1 is localized in the cytoplasm of unstressed tenocytes and translocated into the nucleus upon mechanical stretching to activate the Mkx expression, thereby acting as a mechano-sensor (64). GTF2IRD1 is 1 of 28 genes located in the Williams syndrome critical region (70), implying that this syndrome may be caused in part by dysfunction in this new mechano-signal cascade.
4. Pathogenesis of tendon and ligament tissues and cells
4.1 Aging and arthritis-associated changes in tendon and ligament tissues and cells
Histological abnormalities in ACL are highly prevalent in OA-affected knees and include cystic changes, the disorientation of collagen fibers and mucoid degeneration (9) (Figure 3). ACL degeneration can be initiated before or progress more rapidly than cartilage degeneration, at least in a subpopulation of individuals (71).
Figure 3.
Mkx is relevant for both the differentiation and the identity of tenocytes. Left: During embryonic development, mesenchymal progenitor cells acquire a tendon/ligament or chondrocyte cell fate, depending on lineage-specific transcription factors, such as Mkx or Sox9. In the adult tissue, the tendon/ligament cells continue to express Mkx, which is indispensable for the maintenance of their cellular identity, in part through the repression of Sox9 transcription. Right: When Mkx is genetically deleted or decreased by aging/OA, the SOX9 expression increases in tendon/ligament cells and these cells transform into chondrocyte-like cells.
The earliest and most prevalent age-related change in the ACL extracellular matrix is the disorganization of collagen fibers, which can be seen in ACL from young donors without cartilage degeneration or inflammation (71). An aging-related decrease in the diameter of the collagen fibrils and a corresponding increase in the concentration of small fibrils have also been described for the human ACL (72).
The Mkx expression is maintained in murine and human mature tendon and ligament cells, and its expression is reduced in ACL cells from OA-affected joints (73). Indeed, Mkx knockdown in the ACL cells (annulus fibrosus cell and ligament-like cells in the inter-vertebral disc [IVD]), resulted in decreased tendon ECM gene expression (73, 74) (Figure 3). These findings suggest the potential role of Mkx in maintaining the tendon and ligament cell functions in adult tissues.
4.2 Chondrogenic/osteogenic alteration of tendon and ligament cells
The alteration of cell phenotypes is a main feature of injured or degraded tendons and ligaments (Figure 4). Chondroid metaplasia is a well-known feature of degenerated ACL (9). In this regard, Mkx mutant rats showed early heterotopic ossification in the Achilles tendon with elevated osteogenic and chondrogenic gene expressions (44). An analysis of tendon-derived cells revealed that Mkx deficiency accelerated chondrogenic and osteogenic differentiation, whereas Mkx overexpression suppressed chondrogenic, osteogenic and adipogenic differentiation (44) (Figure 4).
Figure 4.
A. Osteoarthritis (OA)-associated changes in the anterior cruciate ligament (ACL). Histological images of ACL from human knee joints illustrating changes in the collagen fiber orientation and chondroid metaplasia, the two most frequent changes. Chondroid metaplasia represents a shift in the ligament cell phenotype towards a more chondrocytic cell morphology. In normal ACL, only fusiform (white arrow) and ovoid cells (white arrow head) are observed. In mild cases, only a few spheroid cells are seen. In moderate cases, spheroid cells are dominantly observed. In severe cases, only spheroid cells (black arrow) and pseudo-cloning (black arrow head) that suggests chondroid metaplasia is observed and associated with mucoid degeneration. These histological changes in the ACL correlate with the severity of changes in the articular cartilage. B. MKX is expressed in normal ACL ligament cells. The numbers of ligament cells expressing MKX are reduced in OA ACL. HE staining (A, C) and MKX immunohistochemistry (B, D) (modified from Nakahara et. al. Arthritis Rheum 2013).
Under chondrogenic differentiation conditions, the stem/progenitor cells (TSPCs) from Mkx mutant rat tendon tissues (12) showed enhanced osteogenic and chondrogenic differentiation, and Mkx overexpression rescued this phenotype (44). These data suggest that Mkx has a dual function: maintain the production of tendon/ligament ECM components but also prevent the trans-differentiation of tendon/ligament cells to related lineage cells by inhibiting chondrogenic genes, including Sox9, and chondrogenesis. In support of this notion, a chromatin immunoprecipitation-seq analysis with rat primary tendon cells revealed that both tenogenic-related genes, such as Col1a1 and Col3a1, and chondrogenic differentiation-related genes, such as Sox5, Sox6 and Sox9, could be targets of Mkx (44).
4.3 Inflammatory signals in tendon and ligament cells
Biochemical mediators, such as cytokines and growth and differentiation factors, that are part of the abnormal synovial fluid composition in arthritic joints may also lead to alterations in the ligament cell phenotype. For example, IL-33 expression at the onset of tendinopathy leads to early tissue remodeling through type 1 to 3 collagen transition. Interestingly, IL-33 negatively regulates the expression of microRNA29a, which targets the IL-33 receptor mST2 and Col3a1 (75). Other studies have also addressed the role of inflammatory cytokines in tendon/ligament cell homeostasis and regeneration (76), mainly with regard to stimulating the production of inflammatory mediators and ECM-degrading enzymes.
IL-1β is a main OA related inflammatory cytokine and inhibits the expression of Mkx as well as the expression of critical tendon/ligament-specific genes in cultured ligament cells. As described above, the expression of Mkx is reduced in human ACL from aged and OA-affected knees. One potential mechanism behind the suppression of Mkx is the involvement of inflammatory cytokines, such as IL-1 (73). Thus, the loss of Mkx, driven by proinflammatory cytokines such as IL-1β, can lead to the abnormal chondrocyte-like differentiation of ligament cells and a reduced production of ECM with deficient biomechanical properties.
4.4 Pathogenic mechanical stress in tendon and ligament cells
Under ideal conditions, mechanical loading has a positive effect on gene expression and subsequent tendon and ligament tissue homeostasis. Tendon and ligament cells are highly sensitive to mechanical stimuli, and excess mechanical stimulation results in damaging the tendon and ligament tissues with a reduction in the tendon-related gene expressions. Indeed, extreme stretching and intense exercise have been reported to increase osteogenic and chondrogenic marker expression in tendons (66, 77).
While mild stretching of tenocytes in vitro increases the expression of Mkx and tendon-associated genes, such as Col1a1 and Col3a1, the same mechanical stretching stimulation of Mkx−/− tendon derived cells leads to chondrogenic differentiation with increased expression of chondrogenic markers, such as Sox6, Sox9 and Acan (44).
The type of mechanical loading also affects the stiffness of tendons; stochastic strains are more likely to cause microdamage and decreased stiffness than cyclic strains (78). The mechanical loading factors, including amplitude, frequency, duration and period, are coordinately orchestrated for tendon homeostasis, and an imbalance in these mechanical signals leads to tendon and ligament tissue damage, partially via tendon and ligament cell catabolic gene and the Mkx transcription factor network.
5. Tendon and ligament tissue engineering
Although tendon tissue has a regenerative capacity after injury at the neonatal stage, its potential to restore functional tissue is very limited in adults (79). Recent findings in tendon and ligament biology as summarized above have provided new opportunities to develop solid tissue engineering approaches to treating ligaments and tendons in adult (Figure 5). Bone marrow mesenchymal stem cell (MSC) transplantation can promote tendon injury repair (80, 81). To stabilize the transplanted MSCs and accelerate tissue regeneration, collagen scaffolds are successfully utilized both in tendon (80) and ligament (82) injury models.
Figure 5.
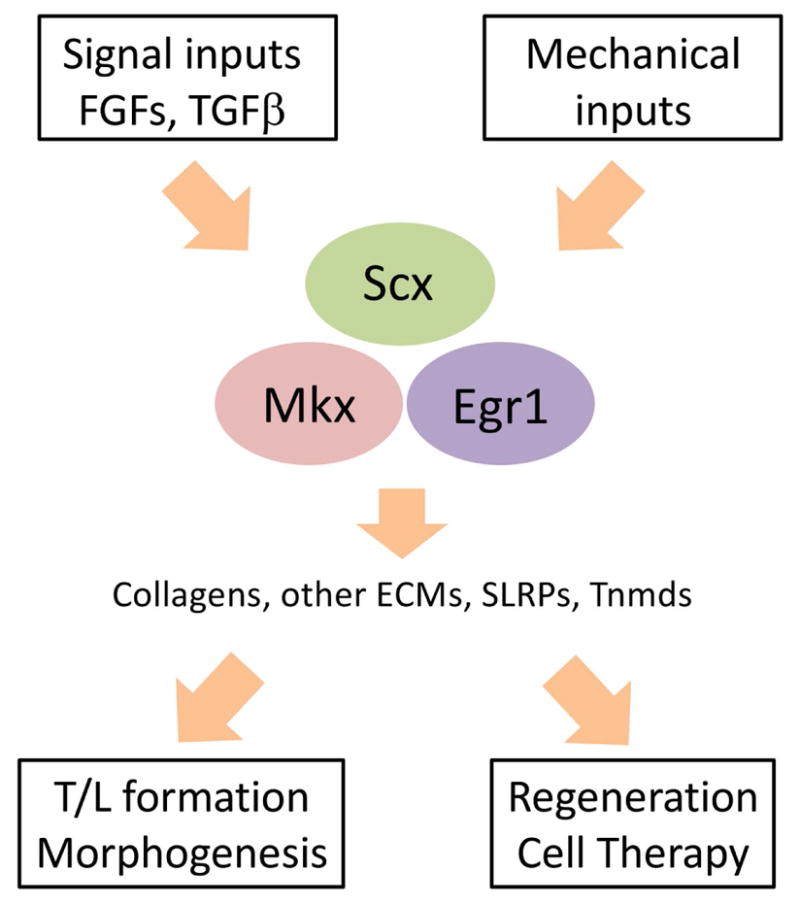
A summary of the tendon/ligament biology discussed in this review. The expressions of Scx, Mkx and Egr1 are induced by signaling cues, such as TGFβ or FGFs (see also Figure 1), and promoted by mechanical cues. These transcription factors activate the transcription of tendon structural elements, such as collagens and SLRPs, which are indispensable for proper tendon and ligament tissue formation during embryonic development as well as tissue regeneration. They also play major roles in the maintenance of the tendon and ligament cell identity during aging or pathogenic processes (see also Figure 3), partly by repressing the factors, which promote other cell fates (see also Figure 4).
To acquire more efficient regeneration of tendon and ligament tissues, pretreatment of MSCs with growth factors such as GDF5 and GDF7 (also known as BMP12) has been used to enhance the tenogenic differentiation of MSCs (80, 83–85).
The introduction of key transcription factors into MSCs is another promising strategy. MSCs with forced expression of Mkx gain tendon and ligament cell shape and gene expression profiles and become resistant to the differentiation cues for the other cell types (74, 86, 87). When Mkx-overexpressing MSCs were transplanted into injured IVDs in vivo, transplanted cells formed collagen fibrils with a similar diameter to the endogenous annulus fibrosus (AF) cells, the ligament-like cells in IVDs, and restored tissues were then protected against the mechanical stress to a similar degree as native tissue (74). These in vitro and in vivo findings indicate the potential utility of Mkx in cell-based tendon and ligament tissue repair and engineering.
Egr1 also plays an important role in tendon and ligament differentiation (40) and in tendon gene expression (47). Furthermore, Egr1-expressing mesenchymal stem cells regenerated tendon tissue in a rat tendon injury model more efficiently than control mesenchymal stem cells (47). Scx, which had been considered to be more involved in the embryonic period than in later periods, also promoted more efficient tendon repair by TDSCs (88).
Mkx, Scx and Egr1 may promote tendon/ligament cell differentiation of MSC not only by promoting tendon- and ligament-specific gene expression profiles but also by changing cellular responsiveness to other signaling cues. Egr1 may therefore contribute to Tgfb2 and Scx but not Mkx expression (47). Mkx-expressing MSCs showed increased responsiveness for TGFβ signaling and reduced responsiveness for BMP signaling, which promotes chondrogenic or osteogenic programs (74). The more optimized utilization of hierarchically or temporally controlled combinations of growth factors and transcription factors may substantially improve the tendon and ligament regeneration.
6. Conclusions and future directions
Recent advances in research on the biology of tendon and ligament cells, especially the identification and functional analyses of tissue-specific transcription factors, have shown novel mechanisms that control their differentiation and activation (Figure 5). However, important questions remain to be answered, such as how the hierarchal structure of tendon and ligament tissue is formed by tenocytes and how the precise and robust tissue integration occurs between tendon and ligament and bone or muscle. Emerging approaches such as mechano-transduction or knockout rat models should also provide new insight in the near future. While the myogenesis molecular program has been clarified by genome-wide analyses of DNA methylation with DNMT3A conditional knockout mice (89), further analyses of the tendon and ligament cell differentiation at the epigenetic level will be needed to establish a basis for tendon physiology and tissue engineering (89, 90). Progress in research on tendon and ligament biology will also shed light on to its future therapeutic applications to musculoskeletal injuries and diseases. For example, correcting abnormal Mkx expression may be a useful new approach to addressing tissue repair after injury and during chronic processes, such as OA. Pharmacological approaches to correcting abnormal differentiation through the inhibition of inflammatory signals or enhancement of differentiation cues can also be applied, such as using Mkx expression as a parameter when screening for drug candidates. Further studies will be necessary, both in basic tendon and ligament biology as well as animal models of injury and disease, to achieve effective approaches for the treatment of tendon and ligament pathology.
Acknowledgments
Funding:
This work was supported by grants from the NIH AR050631 (HA), AR065379 (HA), AG007996 (MKL) CREST funding from AMED (HA), JSPS KAKENHI (Grant Number: 26113008, 15H02560, 15K15544) (HA).
Footnotes
Author contributions:
HA, MI and MKL wrote the paper.
References
- 1.Sharma P, Maffulli N. Biology of tendon injury: healing, modeling and remodeling. Journal of musculoskeletal & neuronal interactions. 2006;6(2):181–90. [PubMed] [Google Scholar]
- 2.Yamaguchi K, Ditsios K, Middleton WD, Hildebolt CF, Galatz LM, Teefey SA. The demographic and morphological features of rotator cuff disease. A comparison of asymptomatic and symptomatic shoulders. The Journal of bone and joint surgery American volume. 2006;88(8):1699–704. doi: 10.2106/JBJS.E.00835. [DOI] [PubMed] [Google Scholar]
- 3.Longo UG, Ronga M, Maffulli N. Acute ruptures of the achilles tendon. Sports medicine and arthroscopy review. 2009;17(2):127–38. doi: 10.1097/JSA.0b013e3181a3d767. [DOI] [PubMed] [Google Scholar]
- 4.Nelson F, Billinghurst RC, Pidoux I, Reiner A, Langworthy M, McDermott M, et al. Early post-traumatic osteoarthritis-like changes in human articular cartilage following rupture of the anterior cruciate ligament. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2006;14(2):114–9. doi: 10.1016/j.joca.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 5.Fleming BC, Hulstyn MJ, Oksendahl HL, Fadale PD. Ligament Injury, Reconstruction and Osteoarthritis. Curr Opin Orthop. 2005;16(5):354–62. doi: 10.1097/01.bco.0000176423.07865.d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fleming BC. Biomechanics of the anterior cruciate ligament. Journal of Orthopaedic and Sports Physical Therapy. 2003;33(8):A13–5. [PubMed] [Google Scholar]
- 7.Woo SL, Livesay GA, Engle C. Biomechanics of the human anterior cruciate ligament. Muscle stabilization and ACL reconstruction. Orthopaedic Review. 1992;21(8):935–41. [PubMed] [Google Scholar]
- 8.Hill CL, Seo GS, Gale D, Totterman S, Gale ME, Felson DT. Cruciate ligament integrity in osteoarthritis of the knee. Arthritis Rheum. 2005;52(3):794–9. doi: 10.1002/art.20943. [DOI] [PubMed] [Google Scholar]
- 9.Mullaji AB, Marawar SV, Simha M, Jindal G. Cruciate ligaments in arthritic knees: a histologic study with radiologic correlation. J Arthroplasty. 2008;23(4):567–72. doi: 10.1016/j.arth.2007.05.024. [DOI] [PubMed] [Google Scholar]
- 10.Birk DE, Trelstad RL. Extracellular compartments in tendon morphogenesis: collagen fibril, bundle, and macroaggregate formation. The Journal of cell biology. 1986;103(1):231–40. doi: 10.1083/jcb.103.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Connizzo BK, Yannascoli SM, Soslowsky LJ. Structure-function relationships of postnatal tendon development: a parallel to healing. Matrix biology : journal of the International Society for Matrix Biology. 2013;32(2):106–16. doi: 10.1016/j.matbio.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bi Y, Ehirchiou D, Kilts TM, Inkson CA, Embree MC, Sonoyama W, et al. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat Med. 2007;13(10):1219–27. doi: 10.1038/nm1630. [DOI] [PubMed] [Google Scholar]
- 13.Jepsen KJ, Wu F, Peragallo JH, Paul J, Roberts L, Ezura Y, et al. A syndrome of joint laxity and impaired tendon integrity in lumican- and fibromodulin-deficient mice. The Journal of biological chemistry. 2002;277(38):35532–40. doi: 10.1074/jbc.M205398200. [DOI] [PubMed] [Google Scholar]
- 14.Kannus P. Structure of the tendon connective tissue. Scandinavian journal of medicine & science in sports. 2000;10(6):312–20. doi: 10.1034/j.1600-0838.2000.010006312.x. [DOI] [PubMed] [Google Scholar]
- 15.Docheva D, Hunziker EB, Fassler R, Brandau O. Tenomodulin is necessary for tenocyte proliferation and tendon maturation. Molecular and cellular biology. 2005;25(2):699–705. doi: 10.1128/MCB.25.2.699-705.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schweitzer R, Zelzer E, Volk T. Connecting muscles to tendons: tendons and musculoskeletal development in flies and vertebrates. Development (Cambridge, England) 2010;137(17):2807–17. doi: 10.1242/dev.047498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thompson MS. Tendon mechanobiology: experimental models require mathematical underpinning. Bulletin of mathematical biology. 2013;75(8):1238–54. doi: 10.1007/s11538-013-9850-5. [DOI] [PubMed] [Google Scholar]
- 18.Cooper RR, Misol S. Tendon and ligament insertion. A light and electron microscopic study. The Journal of bone and joint surgery American volume. 1970;52(1):1–20. [PubMed] [Google Scholar]
- 19.Lu HH, Thomopoulos S. Functional attachment of soft tissues to bone: development, healing, and tissue engineering. Annual review of biomedical engineering. 2013;15:201–26. doi: 10.1146/annurev-bioeng-071910-124656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Charvet B, Ruggiero F, Le Guellec D. The development of the myotendinous junction. A review. Muscles, ligaments and tendons journal. 2012;2(2):53–63. [PMC free article] [PubMed] [Google Scholar]
- 21.Kjaer M. Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiological reviews. 2004;84(2):649–98. doi: 10.1152/physrev.00031.2003. [DOI] [PubMed] [Google Scholar]
- 22.Yang G, Crawford RC, Wang JH. Proliferation and collagen production of human patellar tendon fibroblasts in response to cyclic uniaxial stretching in serum-free conditions. J Biomech. 2004;37(10):1543–50. doi: 10.1016/j.jbiomech.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 23.Shukunami C, Takimoto A, Oro M, Hiraki Y. Scleraxis positively regulates the expression of tenomodulin, a differentiation marker of tenocytes. Dev Biol. 2006;298(1):234–47. doi: 10.1016/j.ydbio.2006.06.036. [DOI] [PubMed] [Google Scholar]
- 24.Steinert AF, Kunz M, Prager P, Barthel T, Jakob F, Noth U, et al. Mesenchymal stem cell characteristics of human anterior cruciate ligament outgrowth cells. Tissue Eng Part A. 2011;17(9–10):1375–88. doi: 10.1089/ten.tea.2010.0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schweitzer R, Chyung JH, Murtaugh LC, Brent AE, Rosen V, Olson EN, et al. Analysis of the tendon cell fate using Scleraxis, a specific marker for tendons and ligaments. Development (Cambridge, England) 2001;128(19):3855–66. doi: 10.1242/dev.128.19.3855. [DOI] [PubMed] [Google Scholar]
- 26.Brent AE, Schweitzer R, Tabin CJ. A somitic compartment of tendon progenitors. Cell. 2003;113(2):235–48. doi: 10.1016/s0092-8674(03)00268-x. [DOI] [PubMed] [Google Scholar]
- 27.Brent AE, Tabin CJ. FGF acts directly on the somitic tendon progenitors through the Ets transcription factors Pea3 and Erm to regulate scleraxis expression. Development (Cambridge, England) 2004;131(16):3885–96. doi: 10.1242/dev.01275. [DOI] [PubMed] [Google Scholar]
- 28.Eloy-Trinquet S, Wang H, Edom-Vovard F, Duprez D. Fgf signaling components are associated with muscles and tendons during limb development. Developmental Dynamics. 2009;238(5):1195–206. doi: 10.1002/dvdy.21946. [DOI] [PubMed] [Google Scholar]
- 29.Brent AE, Braun T, Tabin CJ. Genetic analysis of interactions between the somitic muscle, cartilage and tendon cell lineages during mouse development. Development (Cambridge, England) 2005;132(3):515–28. doi: 10.1242/dev.01605. [DOI] [PubMed] [Google Scholar]
- 30.Tozer S, Duprez D. Tendon and ligament: development, repair and disease. Birth defects research Part C, Embryo today : reviews. 2005;75(3):226–36. doi: 10.1002/bdrc.20049. [DOI] [PubMed] [Google Scholar]
- 31.Edom-Vovard F, Duprez D. Signals regulating tendon formation during chick embryonic development. Developmental Dynamics. 2004;229(3):449–57. doi: 10.1002/dvdy.10481. [DOI] [PubMed] [Google Scholar]
- 32.Edom-Vovard F, Schuler B, Bonnin MA, Teillet MA, Duprez D. Fgf4 positively regulates scleraxis and tenascin expression in chick limb tendons. Dev Biol. 2002;247(2):351–66. doi: 10.1006/dbio.2002.0707. [DOI] [PubMed] [Google Scholar]
- 33.Pryce BA, Watson SS, Murchison ND, Staverosky JA, Dunker N, Schweitzer R. Recruitment and maintenance of tendon progenitors by TGFbeta signaling are essential for tendon formation. Development (Cambridge, England) 2009;136(8):1351–61. doi: 10.1242/dev.027342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang AH, Riordan TJ, Pryce B, Weibel JL, Watson SS, Long F, et al. Musculoskeletal integration at the wrist underlies the modular development of limb tendons. Development (Cambridge, England) 2015;142(14):2431–41. doi: 10.1242/dev.122374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kawakami Y, Rodriguez-Leon J, Izpisua Belmonte JC. The role of TGFbetas and Sox9 during limb chondrogenesis. Current opinion in cell biology. 2006;18(6):723–9. doi: 10.1016/j.ceb.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 36.Havis E, Bonnin MA, Olivera-Martinez I, Nazaret N, Ruggiu M, Weibel J, et al. Transcriptomic analysis of mouse limb tendon cells during development. Development (Cambridge, England) 2014;141(19):3683–96. doi: 10.1242/dev.108654. [DOI] [PubMed] [Google Scholar]
- 37.Kalson NS, Lu Y, Taylor SH, Starborg T, Holmes DF, Kadler KE. A structure-based extracellular matrix expansion mechanism of fibrous tissue growth. eLife. 2015:4. doi: 10.7554/eLife.05958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murchison ND, Price BA, Conner DA, Keene DR, Olson EN, Tabin CJ, et al. Regulation of tendon differentiation by scleraxis distinguishes force-transmitting tendons from muscle-anchoring tendons. Development (Cambridge, England) 2007;134(14):2697–708. doi: 10.1242/dev.001933. [DOI] [PubMed] [Google Scholar]
- 39.Cserjesi P, Brown D, Ligon KL, Lyons GE, Copeland NG, Gilbert DJ, et al. Scleraxis: a basic helix-loop-helix protein that prefigures skeletal formation during mouse embryogenesis. Development (Cambridge, England) 1995;121(4):1099–110. doi: 10.1242/dev.121.4.1099. [DOI] [PubMed] [Google Scholar]
- 40.Lejard V, Brideau G, Blais F, Salingcarnboriboon R, Wagner G, Roehrl MH, et al. Scleraxis and NFATc regulate the expression of the pro-alpha1(I) collagen gene in tendon fibroblasts. The Journal of biological chemistry. 2007;282(24):17665–75. doi: 10.1074/jbc.M610113200. [DOI] [PubMed] [Google Scholar]
- 41.Ito Y, Toriuchi N, Yoshitaka T, Ueno-Kudoh H, Sato T, Yokoyama S, et al. The Mohawk homeobox gene is a critical regulator of tendon differentiation. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(23):10538–42. doi: 10.1073/pnas.1000525107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu W, Watson SS, Lan Y, Keene DR, Ovitt CE, Liu H, et al. The atypical homeodomain transcription factor Mohawk controls tendon morphogenesis. Molecular and cellular biology. 2010;30(20):4797–807. doi: 10.1128/MCB.00207-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Onizuka N, Ito Y, Inagawa M, Nakahara H, Takada S, Lotz M, et al. The Mohawk homeobox transcription factor regulates the differentiation of tendons and volar plates. Journal of orthopaedic science : official journal of the Japanese Orthopaedic Association. 2014;19(1):172–80. doi: 10.1007/s00776-013-0485-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suzuki H, Ito Y, Shinohara M, Yamashita S, Ichinose S, Kishida A, et al. Gene targeting of the transcription factor Mohawk in rats causes heterotopic ossification of Achilles tendon via failed tenogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2016 doi: 10.1073/pnas.1522054113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sheng M, Greenberg ME. The regulation and function of c-fos and other immediate early genes in the nervous system. Neuron. 1990;4(4):477–85. doi: 10.1016/0896-6273(90)90106-p. [DOI] [PubMed] [Google Scholar]
- 46.Lejard V, Blais F, Guerquin MJ, Bonnet A, Bonnin MA, Havis E, et al. EGR1 and EGR2 involvement in vertebrate tendon differentiation. The Journal of biological chemistry. 2011;286(7):5855–67. doi: 10.1074/jbc.M110.153106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guerquin MJ, Charvet B, Nourissat G, Havis E, Ronsin O, Bonnin MA, et al. Transcription factor EGR1 directs tendon differentiation and promotes tendon repair. J Clin Invest. 2013;123(8):3564–76. doi: 10.1172/JCI67521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Subramanian A, Schilling TF. Tendon development and musculoskeletal assembly: emerging roles for the extracellular matrix. Development (Cambridge, England) 2015;142(24):4191–204. doi: 10.1242/dev.114777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Blitz E, Viukov S, Sharir A, Shwartz Y, Galloway JL, Pryce BA, et al. Bone ridge patterning during musculoskeletal assembly is mediated through SCX regulation of Bmp4 at the tendon-skeleton junction. Developmental cell. 2009;17(6):861–73. doi: 10.1016/j.devcel.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thomopoulos S, Kim HM, Rothermich SY, Biederstadt C, Das R, Galatz LM. Decreased muscle loading delays maturation of the tendon enthesis during postnatal development. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2007;25(9):1154–63. doi: 10.1002/jor.20418. [DOI] [PubMed] [Google Scholar]
- 51.Schwartz AG, Long F, Thomopoulos S. Enthesis fibrocartilage cells originate from a population of Hedgehog-responsive cells modulated by the loading environment. Development (Cambridge, England) 2015;142(1):196–206. doi: 10.1242/dev.112714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sugimoto Y, Takimoto A, Akiyama H, Kist R, Scherer G, Nakamura T, et al. Scx+/Sox9+ progenitors contribute to the establishment of the junction between cartilage and tendon/ligament. Development (Cambridge, England) 2013;140(11):2280–8. doi: 10.1242/dev.096354. [DOI] [PubMed] [Google Scholar]
- 53.Blitz E, Sharir A, Akiyama H, Zelzer E. Tendon-bone attachment unit is formed modularly by a distinct pool of Scx- and Sox9-positive progenitors. Development (Cambridge, England) 2013;140(13):2680–90. doi: 10.1242/dev.093906. [DOI] [PubMed] [Google Scholar]
- 54.Killian ML, Thomopoulos S. Scleraxis is required for the development of a functional tendon enthesis. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2016;30(1):301–11. doi: 10.1096/fj.14-258236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dyment NA, Breidenbach AP, Schwartz AG, Russell RP, Aschbacher-Smith L, Liu H, et al. Gdf5 progenitors give rise to fibrocartilage cells that mineralize via hedgehog signaling to form the zonal enthesis. Dev Biol. 2015;405(1):96–107. doi: 10.1016/j.ydbio.2015.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shwartz Y, Viukov S, Krief S, Zelzer E. Joint Development Involves a Continuous Influx of Gdf5-Positive Cells. Cell reports. 2016;15(12):2577–87. doi: 10.1016/j.celrep.2016.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kostrominova TY, Calve S, Arruda EM, Larkin LM. Ultrastructure of myotendinous junctions in tendon-skeletal muscle constructs engineered in vitro. Histology and histopathology. 2009;24(5):541–50. doi: 10.14670/hh-24.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Charvet B, Malbouyres M, Pagnon-Minot A, Ruggiero F, Le Guellec D. Development of the zebrafish myoseptum with emphasis on the myotendinous junction. Cell and tissue research. 2011;346(3):439–49. doi: 10.1007/s00441-011-1266-7. [DOI] [PubMed] [Google Scholar]
- 59.Charvet B, Guiraud A, Malbouyres M, Zwolanek D, Guillon E, Bretaud S, et al. Knockdown of col22a1 gene in zebrafish induces a muscular dystrophy by disruption of the myotendinous junction. Development (Cambridge, England) 2013;140(22):4602–13. doi: 10.1242/dev.096024. [DOI] [PubMed] [Google Scholar]
- 60.Subramanian A, Schilling TF. Thrombospondin-4 controls matrix assembly during development and repair of myotendinous junctions. eLife. 2014:3. doi: 10.7554/eLife.02372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen JW, Galloway JL. The development of zebrafish tendon and ligament progenitors. Development (Cambridge, England) 2014;141(10):2035–45. doi: 10.1242/dev.104067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ordan E, Volk T. A non-signaling role of Robo2 in tendons is essential for Slit processing and muscle patterning. Development (Cambridge, England) 2015;142(20):3512–8. doi: 10.1242/dev.128157. [DOI] [PubMed] [Google Scholar]
- 63.Zhang L, Kahn CJ, Chen HQ, Tran N, Wang X. Effect of uniaxial stretching on rat bone mesenchymal stem cell: orientation and expressions of collagen types I and III and tenascin-C. Cell Biol Int. 2008;32(3):344–52. doi: 10.1016/j.cellbi.2007.12.018. [DOI] [PubMed] [Google Scholar]
- 64.Kayama T, Mori M, Ito Y, Matsushima T, Nakamichi R, Suzuki H, et al. Gtf2ird1-Dependent Mohawk Expression Regulates Mechanosensing Properties of the Tendon. Molecular and cellular biology. 2016;36(8):1297–309. doi: 10.1128/MCB.00950-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Scott A, Sampaio A, Abraham T, Duronio C, Underhill TM. Scleraxis expression is coordinately regulated in a murine model of patellar tendon injury. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2011;29(2):289–96. doi: 10.1002/jor.21220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mendias CL, Gumucio JP, Bakhurin KI, Lynch EB, Brooks SV. Physiological loading of tendons induces scleraxis expression in epitenon fibroblasts. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2012;30(4):606–12. doi: 10.1002/jor.21550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen X, Yin Z, Chen JL, Shen WL, Liu HH, Tang QM, et al. Force and scleraxis synergistically promote the commitment of human ES cells derived MSCs to tenocytes. Scientific reports. 2012;2:977. doi: 10.1038/srep00977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Reumann MK, Strachna O, Yagerman S, Torrecilla D, Kim J, Doty SB, et al. Loss of transcription factor early growth response gene 1 results in impaired endochondral bone repair. Bone. 2011;49(4):743–52. doi: 10.1016/j.bone.2011.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gaut L, Robert N, Delalande A, Bonnin MA, Pichon C, Duprez D. EGR1 Regulates Transcription Downstream of Mechanical Signals during Tendon Formation and Healing. PLoS One. 2016;11(11):e0166237. doi: 10.1371/journal.pone.0166237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pober BR. Williams-Beuren syndrome. N Engl J Med. 2010;362(3):239–52. doi: 10.1056/NEJMra0903074. [DOI] [PubMed] [Google Scholar]
- 71.Hasegawa A, Otsuki S, Pauli C, Miyaki S, Patil S, Steklov N, et al. Anterior cruciate ligament changes in the human knee joint in aging and osteoarthritis. Arthritis Rheum. 2012;64(3):696–704. doi: 10.1002/art.33417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Strocchi R, De Pasquale V, Facchini A, Raspanti M, Zaffagnini S, Marcacci M. Age-related changes in human anterior cruciate ligament (ACL) collagen fibrils. Italian Journal of Anatomy and Embryology. 1996;101(4):213–20. [PubMed] [Google Scholar]
- 73.Nakahara H, Hasegawa A, Otabe K, Ayabe F, Matsukawa T, Onizuka N, et al. Transcription factor mohawk and the pathogenesis of human anterior cruciate ligament degradation. Arthritis Rheum. 2013;65(8):2081–9. doi: 10.1002/art.38020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nakamichi R, Ito Y, Inui M, Onizuka N, Kayama T, Kataoka K, et al. Mohawk promotes the maintenance and regeneration of the outer annulus fibrosus of intervertebral discs. Nat Commun. 2016;(7):12503. doi: 10.1038/ncomms12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Millar NL, Gilchrist DS, Akbar M, Reilly JH, Kerr SC, Campbell AL, et al. MicroRNA29a regulates IL-33-mediated tissue remodelling in tendon disease. Nat Commun. 2015;6(6774) doi: 10.1038/ncomms7774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.John T, Lodka D, Kohl B, Ertel W, Jammrath J, Conrad C, et al. Effect of pro-inflammatory and immunoregulatory cytokines on human tenocytes. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2010;28(8):1071–7. doi: 10.1002/jor.21079. [DOI] [PubMed] [Google Scholar]
- 77.Zhang J, Wang JH. The effects of mechanical loading on tendons--an in vivo and in vitro model study. PLoS One. 2013;8(8) doi: 10.1371/journal.pone.0071740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Steiner TH, Burki A, Ferguson SJ, Gantenbein-Ritter B. Stochastic amplitude-modulated stretching of rabbit flexor digitorum profundus tendons reduces stiffness compared to cyclic loading but does not affect tenocyte metabolism. BMC Musculoskelet Disord. 2012;13(222):1471–2474. doi: 10.1186/1471-2474-13-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Howell K, Chien C, Bell R, Laudier D, Tufa SF, Keene DR, et al. Novel Model of Tendon Regeneration Reveals Distinct Cell Mechanisms Underlying Regenerative and Fibrotic Tendon Healing. Scientific reports. 2017;7:45238. doi: 10.1038/srep45238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lee JY, Zhou Z, Taub PJ, Ramcharan M, Li Y, Akinbiyi T, et al. BMP-12 treatment of adult mesenchymal stem cells in vitro augments tendon-like tissue formation and defect repair in vivo. PLoS One. 2011;6(3):0017531. doi: 10.1371/journal.pone.0017531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chong AK, Ang AD, Goh JC, Hui JH, Lim AY, Lee EH, et al. Bone marrow-derived mesenchymal stem cells influence early tendon-healing in a rabbit achilles tendon model. The Journal of bone and joint surgery American volume. 2007;89(1):74–81. doi: 10.2106/JBJS.E.01396. [DOI] [PubMed] [Google Scholar]
- 82.Vavken P, Fleming BC, Mastrangelo AN, Machan JT, Murray MM. Biomechanical outcomes after bioenhanced anterior cruciate ligament repair and anterior cruciate ligament reconstruction are equal in a porcine model. Arthroscopy. 2012;28(5):672–80. doi: 10.1016/j.arthro.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wolfman NM, Hattersley G, Cox K, Celeste AJ, Nelson R, Yamaji N, et al. Ectopic induction of tendon and ligament in rats by growth and differentiation factors 5, 6, and 7, members of the TGF-beta gene family. J Clin Invest. 1997;100(2):321–30. doi: 10.1172/JCI119537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang QW, Chen ZL, Piao YJ. Mesenchymal stem cells differentiate into tenocytes by bone morphogenetic protein (BMP) 12 gene transfer. J Biosci Bioeng. 2005;100(4):418–22. doi: 10.1263/jbb.100.418. [DOI] [PubMed] [Google Scholar]
- 85.Haddad-Weber M, Prager P, Kunz M, Seefried L, Jakob F, Murray MM, et al. BMP12 and BMP13 gene transfer induce ligamentogenic differentiation in mesenchymal progenitor and anterior cruciate ligament cells. Cytotherapy. 2010;12(4):505–13. doi: 10.3109/14653241003709652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Otabe K, Nakahara H, Hasegawa A, Matsukawa T, Ayabe F, Onizuka N, et al. Transcription factor Mohawk controls tenogenic differentiation of bone marrow mesenchymal stem cells in vitro and in vivo. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2015;33(1):1–8. doi: 10.1002/jor.22750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liu H, Zhang C, Zhu S, Lu P, Zhu T, Gong X, et al. Mohawk promotes the tenogenesis of mesenchymal stem cells through activation of the TGFbeta signaling pathway. Stem Cells. 2015;33(2):443–55. doi: 10.1002/stem.1866. [DOI] [PubMed] [Google Scholar]
- 88.Tan C, Lui PP, Lee YW, Wong YM. Scx-transduced tendon-derived stem cells (tdscs) promoted better tendon repair compared to mock-transduced cells in a rat patellar tendon window injury model. PLoS One. 2014;9(5):e97453. doi: 10.1371/journal.pone.0097453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Naito M, Mori M, Inagawa M, Miyata K, Hashimoto N, Tanaka S, et al. Dnmt3a Regulates Proliferation of Muscle Satellite Cells via p57Kip2. PLoS genetics. 2016;12(7):e1006167. doi: 10.1371/journal.pgen.1006167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Miyata K, Miyata T, Nakabayashi K, Okamura K, Naito M, Kawai T, et al. DNA methylation analysis of human myoblasts during in vitro myogenic differentiation: de novo methylation of promoters of muscle-related genes and its involvement in transcriptional down-regulation. Hum Mol Genet. 2015;24(2):410–23. doi: 10.1093/hmg/ddu457. [DOI] [PMC free article] [PubMed] [Google Scholar]