Abstract
The aim of the present study was to examine the apoptotic effect of cordycepin (COR) on human THP-1 acute monocytic leukemia cells. THP-1 cells were exposed to different concentrations of COR for 24, 48, 72 or 96 h. The cell viability and apoptotic rate were analyzed. The gene expression of Akt1, Akt2, Akt3, B-cell lymphoma 2 (Bcl-2) and Bcl-2-associated X protein (Bax) were assessed by reverse-transcription quantitative PCR. Western blot analysis was used to detect the protein levels of phosphorylated (p)-Akt, p-extracellular signal-regulated kinase (ERK) and cleaved caspase-3. It was found that the viability of THP-1 cells was inhibited by COR in a dose- and time-dependent manner. After treatment with 200 µM COR for 24 h, the percentage of apoptotic cells was significantly increased. COR also downregulated the levels of Bcl-2, Akt1, Akt2 and Akt3, and elevated the expression of Bax. The protein levels of p-Akt and p-ERK were suppressed and cleaved caspase-3 was increased after treatment of COR. In conclusion, COR was found to induce apoptosis of THP-1 acute monocytic leukemia cells through downregulation of ERK/Akt signaling.
Keywords: cordycepin, THP-1, apoptosis, ERK, Akt, caspase-3
Introduction
Acute myeloid leukemia (AML) is a clonal disorder, which comprises a group of clonal malignant diseases. The leukemia cells originate from the bone marrow, giving rise to an accumulation of abnormal immature myeloid cells in the bone marrow and blood (1,2). AML is one of the most common leukemia types. Over 50% of patients diagnosed with AML are >65 years of age (3). The main therapeutic strategies for AML patients are aggressive chemotherapeutic regimens and hematopioetic stem cell transplantation, and ~40% of AML patients receive chemotherapy within 3 months after diagnosis (4,5). In spite of intensive chemotherapy being able to achieve complete remission in most AML patients, the overall survival rate remains poor and the therapeutic process is usually associated with serious adverse events (6). However, AML comprises the following 8 types: M0, minimally differentiated AML; M1, AML without maturation; M2, AML with maturation; M3, acute pro-myelocytic leukemia; M4, acute myelomonocytic leukemia; M5, acute monocytic leukemia (AMoL); M6, erythroleukemia; and M7, acute megakaryoblastic leukemia. And there is no treatment plan for any of the AML typse (7–10). The major objective of the present study was to identify a drug for curing AMoL.
The development of AMoL is a multistep and multifactorial process. The neoplastic cells are most frequently derived from white blood cells, have a high rate of marrow infiltration and high bad karyotype ratio. As AMoL is insensitive to numerous chemotherapy regimens, affected patients have a low remission rate and short lifetime (11–16). Therefore, a novel and effective drug is urgently required.
In recent years, bioactive natural products have emerged and received a considerable amount of attention from researchers. Cordyceps militaris is a traditional Chinese medicinal plant, which has been widely used for a long time. Recent studies have demonstrated that biologically active components isolated from Cordyceps species have various pharmacological effects (17,18). Cordycepin (COR) is one of the most widely studied active components of Cordyceps militaris and has diverse biological functions, such as anti-tumor (19), anti-invasive (20) and anti-inflammatory effects (21). However, the effects and potential mechanisms of COR in AMoL have largely remained to be elucidated. Therefore, the purpose of the present study was to evaluate the anti-cancer effect of COR on AMoL and investigate the potential underlying mechanisms. The present findings suggested that COR induces apoptosis of the THP-1 AMoL cell line via deactivating Akt and extracellular signal-regulated kinase (ERK) and upregulating the expression of cleaved caspase-3 indicating that COR may be a potential therapeutic drug for AMoL.
Materials and methods
Reagents
COR and phorbol-12-myristate-13-acetate (PMA) were purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). COR was dissolved in dimethyl sulfoxide (DMSO; Sigma-Aldrich; Merck KGaA), and the proportion of DMSO in the culture medium was <0.05%. COR was stored at −20°C. Primary antibodies against phosphorylated (p)-ERK (Cat no. 4348S), total (t)-ERK (Cat no. 4695S), p-Akt (Cat no. 4060S), t-Akt (Cat no. 4685S), cleaved caspase-3 (Cat no. 1050S), β-actin (Cat no. 4970S) as well as Anti-rabbit Immunoglobulin G (IgG) secondary antibodies (Cat no. 5151S) were purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA).
Cell culture
THP-1 AMoL cells were purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). THP-1 cells were cultured in RPMI 1640 medium (Hyclone; GE Healthcare, Little Chalfont, UK) containing 10% fetal bovine serum (Hyclone; GE Healthcare), 100 U/ml penicillin and 100 µg/ml streptomycin (Hyclone; GE Healthcare) in an incubator at 37°C with 5% CO2. All cells we used at a passage of <20.
Measurement of cell viability
A Cell Counting Kit-8 (CCK-8; Dojindo, Kumamoto, Japan) was used to assess the potential therapeutic effect of COR. For measurement of cell proliferation, THP-1 AMoL cells were seeded into each well of a 96-well plate at a density of 20,000 cells/well in 100 µl culture media containing 100 nM PMA to induce adherence, and treated with 0, 25, 50, 100, 150, 200 µmol/l COR for 96 h after adherence (22). Following treatment with COR for 24, 48, 72 and 96 h, 100 µl culture media and 10 µl CCK-8 solution were added to each well, followed by incubation at 37°C for 150 min. The optical density (OD) value at 450 nm was determined using a microplate reader (BioTek Instruments, Inc., Winooski, VT, USA). Each experimental condition was repeated in three wells. The cell viability relative to that in the 0 µmol/l COR group (control) was calculated using the following equation: Cell viability (% of control group)=ODdrug-treated group/ODcontrol group.
Apoptosis assay
THP-1 cells were incubated with various concentrations of COR for 24 h. Subsequently, the cells were washed twice with cold PBS and harvested. The cells were re-suspended in 1X Annexin-binding buffer, and stained with Annexin V-fluorescein isothiocyanate (FITC) and propidium iodide (PI; BD Biosciences, Franklin Lakes, NJ, USA). The apoptotic rate was measured by flow cytometry using a BD FACSCalibur™ analyzer (BD Biosciences, Franklin Lakes, NJ, USA). The total apoptotic rate of cells was considered to be the early apoptotic rate (lower right quadrant in the PI vs. FITC dot plot) plus the late apoptotic rate (upper right quadrant in the dot plot).
RNA isolation and reverse-transcription quantitative polymerase chain reaction (RT-qPCR)
THP-1 cells were cultured in complete medium in the presence of various concentrations of COR for 24 h. The total RNA of the THP-1 cells was then isolated using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) following the manufacturer's instructions. For first-strand complementary DNA (cDNA) synthesis, RT was performed using the 1st Strand cDNA Synthesis kit (Takara Bio Inc., Dalian, China). The relative gene expression was determined by real-time PCR using the SYBR Premix Ex Taq kit (Takara Bio, Inc.) with the ABI Prism 7500 Fast Real-Time PCR system (Applied Biosystems; Thermo Fisher Scientific, Inc.). The real-time PCR thermocycling conditions were as follows: pre-degeneration at 95°C for 30 sec, followed by 40 cycles at 95°C for 5 sec and 60°C for 34 sec, and the dissociation stage was 34 sec at 95°C. The primers were designed and selected using the Primer-BLAST tool (https://www.ncbi.nlm.nih.gov/tools/primer-blast/). Gene expression was quantified using the 2−∆∆Cq method (23). β-actin was used as the internal control. The primer sequences are listed in Table I.
Table I.
Sequences of primers used for polymerase chain reaction.
| Gene | Direction | Primer sequence (5′-3′) |
|---|---|---|
| Akt1 | Forward | GAAGGACGGGAGCAGGC |
| Reverse | CTCACGCGCTCCTCTCAG | |
| Akt2 | Forward | TGCCACCATGAATGAGGTGAA |
| Reverse | GTACCCAATGAAGGAGCCGT | |
| Akt3 | Forward | TTTCTCCAAGTTGGGGGCTC |
| Reverse | CCCCTCTTCTGAACCCAACC | |
| Bcl-2 | Forward | TTTGTGGAACTGTACGGCCC |
| Reverse | GTTGACTTCACTTGTGGCCC | |
| Bax | Forward | AGCAGATCATGAAGACAGGGG |
| Reverse | TGCTCGATCCTGGATGAAACC | |
| β-actin | Forward | CTCACCATGGATGATGATATCGC |
| Reverse | AGGAATCCTTCTGACCCATGC |
Bcl-2, B-cell lymphoma 2; Bax, Bcl-2-associated X protein.
Western blot analysis
THP-1 cells were treated as for the RT-qPCR analysis and then lysed with radioimmunoprecipitation assay buffer (Upstate Biotechnology, Inc., Lake Placid, NY, USA) supplemented with a protease inhibitor cocktail (Roche Diagnostics GmbH, Mannheim, Germany). For western blot analysis, protein concentration was quantified using the BCA Protein Assay kit (Thermo Fisher Scientific, Inc.; catalogue no. 23250) according to manufacturer protocol. Samples were adjusted to the same protein concentration prior to loading (30 µg each sample) and separated by 10% SDS-PAGE for p-Akt, t-Akt, p-ERK, t-ERK and β-actin and 12.5% SDS-PAGE for cleaved caspase-3. Subsequently, proteins were transferred onto a nitrocellulose membrane (Merck Millipore, Billerica, MA, USA). Antibodies against p-Akt, t-Akt, p-ERK, t-ERK and cleaved caspase-3 were applied at the dilutions recommended by the manufacturer (1:1,000) at 4°C overnight. β-actin (1:1,000) was used as a loading control. After 37 min washes in Tris-buffered saline containing Tween-20 (TBST), the membranes were incubated with anti-rabbit second antibody (1:15,000; Cell Signalling Technology, Inc) for 1 h at room temperature. The target proteins were detected using the Odyssey Infrared Imaging System (Li-Cor Biosciences, Lincoln, NE, USA). The quantification of the bands was analyzed by the software of Odyssey Infrared Imaging System (Version 3.0).
Statistical analysis
All of the experiments were repeated 3 times. Values are expressed as the mean ± standard deviation. Statistical analysis was performed by one-way analysis of variance, followed by Duncan's post-hoc test using SPSS 19.0 (International Business Machines, Corp., Armonk, NY, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
COR inhibits THP-1 cell proliferation
To assess the effects of COR on the proliferation of THP-1 cells, a CCK-8 assay using was performed. The inhibitory effect of COR on the proliferation of THP-1 cells was strengthened with the increase of the COR concentration after 24, 48, 72 and 96 h of treatment (Fig. 1). In conclusion, COR inhibited the proliferation of THP-1 cells in a dose- and time-dependent manner.
Figure 1.
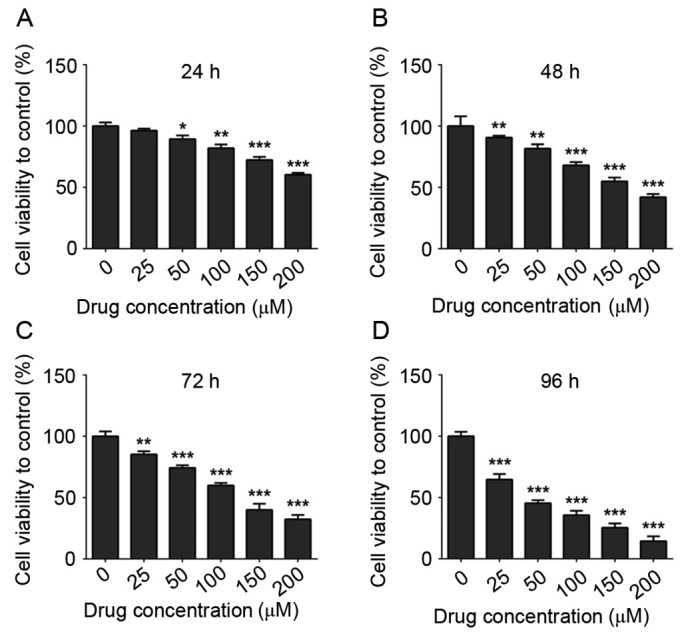
Anti-proliferative effects of COR on THP-1 cells. (A-D) A Cell Counting Kit-8 assay demonstrated the anti-proliferative effects of COR on THP-1 cells after treatment for 24, 48, 72 and 96 h. Values are expressed as the mean ± standard error. *P<0.05; **P<0.01; ***P<0.001 vs. untreated group. COR, cordycepin.
COR concentration-dependently induces apoptosis in THP-1 cells
As presented in Fig. 2, flow cytometric analysis was employed to detect COR-induced apoptosis. Different concentrations of COR increased the total apoptotic rate in a dose-dependent manner (3.35% in the control vs. 11.48, 13.67 and 24.99% after treatment with COR at 100, 150 and 200 µM, respectively; P<0.05; Fig. 2A-G). Quantitative results on the early and late apoptotic rates were consistent with this phenomenon (Fig. 2H-I). To conclude, COR was found to exert its inhibitory function on THP-1 cells by induction of apoptosis, resulting in cell death.
Figure 2.
Apoptosis of THP-1 cells following COR treatment. (A-F) Flow cytometry-based assessment of apoptosis in THP-1 cells following treatment with 0, 25, 50, 100, 150, 200 µM of COR. Annexin V-fluorescein isothiocyanate is displayed on the x-axis. (G-I) Quantitative analysis of total, early and late apoptotic rate. Values are expressed as the mean ± standard error. *P<0.05; **P<0.01; ***P<0.001 vs. untreated group. COR, cordycepin; PI, propidium iodide.
COR regulates apoptosis- and survival-associated gene expression in THP-1 cells
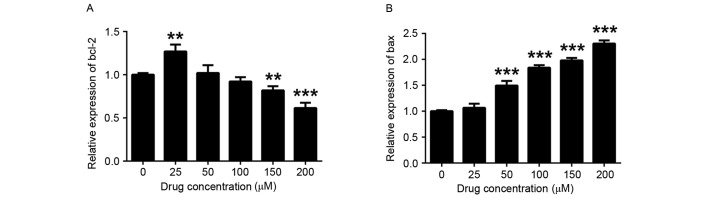
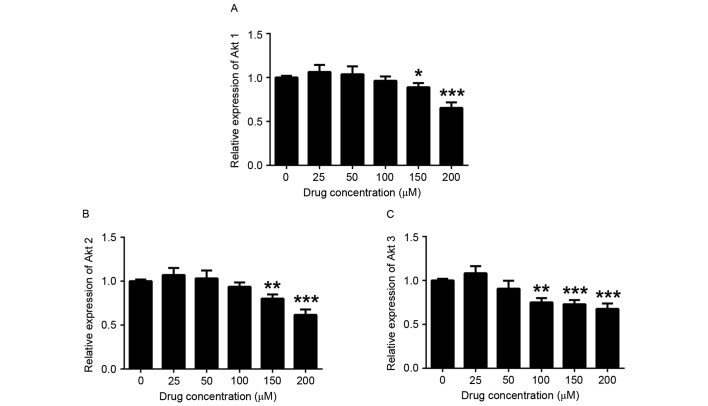
To investigate the in-depth molecular mechanisms of the inhibitory effect of COR on THP-1 human monocytic leukemia cells, RT-qPCR was performed to examine its impact on the expression of apoptosis-associated genes. It was found that the expression levels of Bcl-2 were reduced in COR-treated THP-1 cells in a dose-dependent manner, while the expression of Bax was upregulated (Fig. 3). In addition, Akt signaling is crucial for the initiation and progression of AML and also regulates a number of downstream targets responsible for cell survival and proliferation (24,25). Therefore, the current study detected the mRNA level of three isoforms (Akt1, Akt2 and Akt3) of Akt. The cell survival-associated genes Akt1, Akt2 and Akt3 were downregulated after COR treatment (Fig. 4). Therefore, the phosphorylation of Akt was detected by western blot analysis based on the levels of mRNA (Fig. 4). These results indicated that these genes are potentially involved in signaling pathways downstream of those targeted by COR in monocytic leukemia cells.
Figure 3.
Apoptosis-associated gene expression in THP-1 cells after COR treatment. Reverse-transcription quantitative polymerase chain reaction analysis was employed to detect the expression of (A) Bcl-2 and (B) Bax in COR-treated THP-1 cells. Values are expressed as the mean ± standard error. **P<0.01; ***P<0.001 vs. untreated group. COR, cordycepin; Bcl-2, B-cell lymphoma 2; Bax, Bcl-2-associated X protein.
Figure 4.
Cell survival-associated gene expression in THP-1 cells after COR treatment. Reverse-transcription quantitative polymerase chain reaction analysis was employed to detect the expression of (A) Akt1, (B) Akt2 and (C) Akt3 in COR-treated THP-1 cells. Values are expressed as the mean ± standard error. *P<0.05; **P<0.01; ***P<0.001 vs. untreated group. COR, cordycepin.
Akt and ERK are potential downstream targets of COR
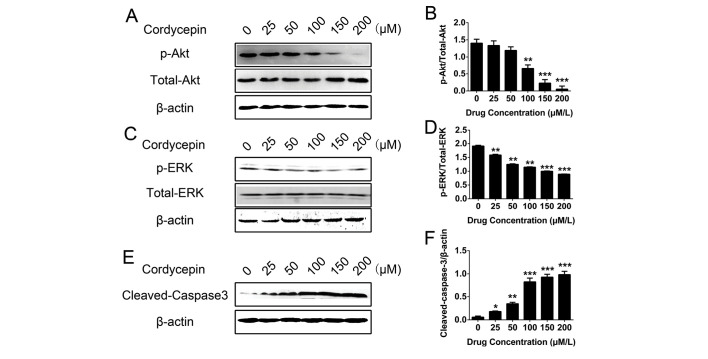
To evaluate the potential involvement of the signal transduction pathways and the mechanisms of the effects of COR on THP-1 cells, the expression of various signaling proteins was determined in THP-1 cells by western blot analysis following treatment with different concentrations of COR. The results demonstrated that COR inhibited the protein expression of p-Akt and p-ERK in a concentration-dependent manner significantly (P<0.05; Fig. 5A-D). However, cleaved caspase-3 was significantly activated by increasing concentrations of COR (P<0.05; Fig. 5E and F). Taken together, these results suggested that Akt and ERK are potential downstream targets of COR in THP-1 cells, and that apoptosis is induced via associated signaling pathways.
Figure 5.
Western blot analysis of p/total-Akt, p-ERK/total-ERK and cleaved caspase-3 levels following apoptosis induction by COR in THP-1 cells. (A-C) Representative western blot displaying the levels of (A) p/total-Akt, (B) quantified, (C) p-ERK/total-ERK, (D) quantifies, (E) cleaved caspase-3 and, (F) quantified in THP-1 cells following COR treatment. Values were normalized to β-actin. Values are expressed as the mean ± standard error. *P<0.05; **P<0.01; ***P<0.001 vs. untreated group. COR, cordycepin; p-ERK, phosphorylated extracellular signal-regulated kinase.
Discussion
The major challenge in the treatment of AML is the high failure and relapse rate due to drug resistance. Application of novel drug treatments is one of the therapeutic approaches for patients with resistance to standard therapies. Clinical evaluation of potential effective chemotherapeutic drugs may provide novel treatments, which may improve the prospects of refractory leukemia patients. AMoL is a rare but distinct disease entity, which is most remarkable due to its clinical course. Cytogenetic characterization is essential for the diagnosis and determination of the prognosis of patients (26–28). There is an urgent requirement to identify less toxic and more effective treatments, and an increasing amount of research has focused on the application of natural products in AMoL treatment.
COR was previously reported to have anti-tumor (29,30), anti-inflammatory (31) and anti-oxidant (32) activities. However, the potential role and mechanisms of COR in the treatment of AMoL have remained to be determined. The aim of the present study was to examine whether COR had anti-cancer activity against AMoL. The results demonstrated that COR exerted a marked inhibitory effect on the THP-1 AMoL cell line in vitro. COR inhibited the spontaneous growth of THP-1 cells in a dose- and time-dependent manner. To further investigate the mechanisms of the anti-AMoL activity of COR, the effects of COR on apoptosis and activation of Akt/ERK survival signaling as well as Bcl-2/Bax/caspase-3 apoptotic pathways in THP-1 cells were assessed. It was revealed that COR induced apoptosis by inhibiting Akt/ERK signaling and activating caspase-3 following disturbance of the balance of the Bcl-2/Bax axis.
Leukemia cells often display continued activation of survival and signaling pathways, such as Akt and ERK, due to gene mutations, including rearrangements and chromosome translocation, and survival signaling pathways have an important role in the proliferation, tumorigenesis, evasion of apoptosis and drug resistance (33–36). The more survival signaling pathways are constitutively active in AML, the poorer the prognosis. The results of the present study demonstrated that in the THP-1 AMoL cell line, Akt and ERK signal transduction pathways were simultaneously suppressed. The apoptotic protein caspase-3 was activated by downregulation of the Bcl-2/Bax ratio after COR treatment. It was speculated that inhibition of the activation of the Akt/ERK pathways and promotion of the activation of caspase-3 by COR may produce an enhanced response in anti-AMoL therapy. Compared with numerous specific inhibitors, which have a single target for the treatment of leukemia, COR may be more effective, as drug resistance frequently emerges due to hyperactivation of alternative signaling pathways under the treatment with a drug that has a single target. As Akt, ERK and caspase-3 signaling are all interconnected and are not separate pathway entities (37) and COR exhibited characteristics of a multi-targeted therapeutic, it may be deduced that it rarely causes resistance in AMoL, that its efficacy may be higher and that the response duration may be longer.
Based on the above, the role of COR in restricting the proliferation and inducing apoptosis of AMoL cells was indicated. COR was found to indirectly or directly affect the viability of AMoL cells via Akt/ERK survival signaling and caspase-associated apoptosis signaling, which are key controllers of cell survival and apoptosis (38–41). However, further research should be performed based on this preliminary research. First, the in-depth molecular mechanisms underlying the COR-mediated inhibition of cell survival and induction of apoptosis-associated signaling pathways should be elucidated. Furthermore, animal experiments should be performed to verify the therapeutic effect of COR in vivo. Finally, the efficacy of COR in patients may be assessed.
In conclusion, the present study revealed that COR was efficacious against AMoL in vitro. Furthermore, COR treatment led to a decrease of p-Akt and p-ERK, while increasing the levels of cleaved caspase-3 via increasing the Bcl-2/Bax ratio. These are likely to be downstream mechanisms, by which COR exerts its inhibitory effects in AMoL. However, further study is required to fully elucidate the underlying mechanisms.
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (nos. 81500392 and 31201010), the Shanghai Committee of Science and Technology of China (nos. 12ZR1419500 and 114119a8700) and the Health Bureau of Shanghai (no. ZYSNXD-CCZDYJ029).
References
- 1.Li S, Mason C, Melnick A. Genetic and epigenetic heterogeneity in acute myeloid leukemia. Curr Opin Genet Dev. 2016;36:100–106. doi: 10.1016/j.gde.2016.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yeh CH, Moles R, Nicot C. Clinical significance of microRNAs in chronic and acute human leukemia. Mol Cancer. 2016;15:37. doi: 10.1186/s12943-016-0518-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khaled S, Al Malki M, Marcucci G. Acute myeloid leukemia: Biologic, prognostic, and therapeutic insights. Oncology (Williston Park) 2016;30:318–329. [PubMed] [Google Scholar]
- 4.Pettersson L, Levéen P, Axler O, Dvorakova D, Juliusson G, Ehinger M. Improved minimal residual disease detection by targeted quantitative polymerase chain reaction in Nucleophosmin 1 type a mutated acute myeloid leukemia. Genes Chromosomes Cancer. 2016;55:750–766. doi: 10.1002/gcc.22375. [DOI] [PubMed] [Google Scholar]
- 5.Brumatti G, Ma C, Lalaoui N, Nguyen NY, Navarro M, Tanzer MC, Richmond J, Ghisi M, Salmon JM, Silke N, et al. The caspase-8 inhibitor emricasan combines with the SMAC mimetic birinapant to induce necroptosis and treat acute myeloid leukemia. Sci Transl Med. 2016;8:339ra69. doi: 10.1126/scitranslmed.aad3099. [DOI] [PubMed] [Google Scholar]
- 6.Yadav M, Singh AK, Kumar H, Rao G, Chakravarti B, Gurjar A, Dogra S, Kushwaha S, Vishwakarma AL, Yadav PN, et al. Epidermal growth factor receptor inhibitor cancer drug gefitinib modulates cell growth and differentiation of acute myeloid leukemia cells via histamine receptors. Biochim Biophys Acta. 2016;1860:2178–2190. doi: 10.1016/j.bbagen.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 7.Hu S, Ueda M, Stetson L, Ignatz-Hoover J, Moreton S, Chakrabarti A, Xia Z, Karan G, de Lima M, Agrawal MK, Wald DN. A novel glycogen synthase kinase-3 inhibitor optimized for acute myeloid leukemia differentiation activity. Mol Cancer Ther. 2016;15:1485–1494. doi: 10.1158/1535-7163.MCT-15-0566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jackson RC, Radivoyevitch T. Evolutionary dynamics of chronic myeloid leukemia progression: The progression-inhibitory effect of imatinib. AAPS J. 2016;18:914–922. doi: 10.1208/s12248-016-9905-2. [DOI] [PubMed] [Google Scholar]
- 9.Foucar K, Anastasi J. Acute myeloid leukemia with recurrent cytogenetic abnormalities. Am J Clin Pathol. 2015;144:6–18. doi: 10.1309/AJCPI9C8UILYQTNS. [DOI] [PubMed] [Google Scholar]
- 10.Banerji L, Sattler M. Targeting mutated tyrosine kinases in the therapy of myeloid leukaemias. Exp Opin Ther Targets. 2004;8:221–239. doi: 10.1517/14728222.8.3.221. [DOI] [PubMed] [Google Scholar]
- 11.Mo J, Lampkin B, Perentesis J, Poole L, Bao L. Translocation (8;18;16)(p11;q21;p13). A new variant of t(8;16)(p11;p13) in acute monoblastic leukemia: Case report and review of the literature. Cancer Genet Cytogenet. 2006;165:75–78. doi: 10.1016/j.cancergencyto.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 12.Prayongratana K, Kulpraneet M, Panichchob P, Tantisiriwat W. Acute monoblastic leukemia with t(10;11)(p12;q23) presenting with pulmonary involvement: A case report and literature review. J Med Assoc Thai. 2008;91:559–563. [PubMed] [Google Scholar]
- 13.Douet-Guilbert N, Morel F, Le Bris MJ, Sassolas B, Giroux JD, De Braekeleer M. Rearrangement of MLL in a patient with congenital acute monoblastic leukemia and granulocytic sarcoma associated with a t(1;11)(p36;q23) translocation. Leuk Lymphoma. 2005;46:143–146. doi: 10.1080/104281904000010783. [DOI] [PubMed] [Google Scholar]
- 14.Tasaka T, Matsuhashi Y, Uehara E, Tamura T, Kakazu N, Abe T, Nagai M. Secondary acute monocytic leukemia with a translocation t(8;16)(p11;p13): Case report and review of the literature. Leuk Lymphoma. 2004;45:621–625. doi: 10.1080/10428190310001593058. [DOI] [PubMed] [Google Scholar]
- 15.Goemans BF, Zwaan CM, Harlow A, Loonen AH, Gibson BE, Hählen K, Reinhardt D, Creutzig U, Heinrich MC, Kaspers GJ. In vitro profiling of the sensitivity of pediatric leukemia cells to tipifarnib: Identification of T-cell ALL and FAB M5 AML as the most sensitive subsets. Blood. 2005;106:3532–3537. doi: 10.1182/blood-2005-04-1640. [DOI] [PubMed] [Google Scholar]
- 16.Riccioni R, Senese M, Diverio D, Riti V, Buffolino S, Mariani G, Boe A, Cedrone M, Lo-Coco F, Foà R, et al. M4 and M5 acute myeloid leukaemias display a high sensitivity to Bortezomib-mediated apoptosis. Br J Haematol. 2007;139:194–205. doi: 10.1111/j.1365-2141.2007.06757.x. [DOI] [PubMed] [Google Scholar]
- 17.Zhang HW, Lin ZX, Tung YS, Kwan TH, Mok CK, Leung C, Chan LS. Cordyceps sinensis (a traditional Chinese medicine) for treating chronic kidney disease. Cochrane Database Syst Rev. 2014;18:Cd008353. doi: 10.1002/14651858.CD008353.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakamura K, Shinozuka K, Yoshikawa N. Anticancer and antimetastatic effects of cordycepin, an active component of Cordyceps sinensis. J Pharmacol Sci. 2015;127:53–56. doi: 10.1016/j.jphs.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 19.Tuli HS, Sharma AK, Sandhu SS, Kashyap D. Cordycepin: A bioactive metabolite with therapeutic potential. Life Sci. 2013;93:863–869. doi: 10.1016/j.lfs.2013.09.030. [DOI] [PubMed] [Google Scholar]
- 20.Nakamura K, Konoha K, Yoshikawa N, Yamaguchi Y, Kagota S, Shinozuka K, Kunitomo M. Effect of cordycepin (3′-deoxyadenosine) on hematogenic lung metastatic model mice. In vivo. 2005;19:137–141. [PubMed] [Google Scholar]
- 21.Li Y, Li K, Mao L, Han X, Zhang K, Zhao C, Zhao J. Cordycepin inhibits LPS-induced inflammatory and matrix degradation in the intervertebral disc. Peer J. 2016;4:e1992. doi: 10.7717/peerj.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith MP, Young H, Hurlstone A, Wellbrock C. Differentiation of THP1 cells into macrophages for transwell co-culture assay with melanoma cells. Bio Protoc. 2015;5 doi: 10.21769/BioProtoc.1638. pii: e1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 24.Bertacchini J, Heidari N, Mediani L, Capitani S, Shahjahani M, Ahmadzadeh A, Saki N. Targeting PI3K/AKT/mTOR network for treatment of leukemia. Cell Mol Life Sci. 2015;72:2337–2347. doi: 10.1007/s00018-015-1867-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barrett D, Brown VI, Grupp SA, Teachey DT. Targeting the PI3K/AKT/mTOR signaling axis in children with hematologic malignancies. Paediatr Drugs. 2012;14:299–316. doi: 10.2165/11594740-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shafer D, Grant S. Update on rational targeted therapy in AML. Blood Rev. 2016;30:275–283. doi: 10.1016/j.blre.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saultz JN, Garzon R. Acute myeloid leukemia: A concise review. J Clin Med. 2016;5 doi: 10.3390/jcm5030033. pii: E33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wouters BJ, Delwel R. Epigenetics and approaches to targeted epigenetic therapy in acute myeloid leukemia. Blood. 2016;127:42–52. doi: 10.1182/blood-2015-07-604512. [DOI] [PubMed] [Google Scholar]
- 29.Zhang P, Huang C, Fu C, Tian Y, Hu Y, Wang B, Strasner A, Song Y, Song E. Cordycepin (3′-deoxyadenosine) suppressed HMGA2, Twist1 and ZEB1-dependent melanoma invasion and metastasis by targeting miR-33b. Oncotarget. 2015;6:9834–9853. doi: 10.18632/oncotarget.3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamamoto K, Shichiri H, Uda A, Yamashita K, Nishioka T, Kume M, Makimoto H, Nakagawa T, Hirano T, Hirai M. Apoptotic effects of the extracts of cordyceps militaris via Erk phosphorylation in a renal cell carcinoma cell line. Phytother Res. 2015;29:707–713. doi: 10.1002/ptr.5305. [DOI] [PubMed] [Google Scholar]
- 31.Yang X, Li Y, He Y, Li T, Wang W, Zhang J, Wei J, Deng Y, Lin R. Cordycepin alleviates airway hyperreactivity in a murine model of asthma by attenuating the inflammatory process. Int Immunopharmacol. 2015;26:401–408. doi: 10.1016/j.intimp.2015.04.017. [DOI] [PubMed] [Google Scholar]
- 32.Ramesh T, Yoo SK, Kim SW, Hwang SY, Sohn SH, Kim IW, Kim SK. Cordycepin (3′-deoxyadenosine) attenuates age-related oxidative stress and ameliorates antioxidant capacity in rats. Exp Gerontol. 2012;47:979–987. doi: 10.1016/j.exger.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 33.Shao X, Liu Y, Li Y, Xian M, Zhou Q, Yang B, Ying M, He Q. The HER2 inhibitor TAK165 sensitizes human acute myeloid leukemia cells to retinoic acid-induced myeloid differentiation by activating MEK/ERK mediated RARα/STAT1 axis. Sci Rep. 2016;6:24589. doi: 10.1038/srep24589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bi L, Wu J, Ye A, Wu J, Yu K, Zhang S, Han Y. Increased Th17 cells and IL-17A exist in patients with B cell acute lymphoblastic leukemia and promote proliferation and resistance to daunorubicin through activation of Akt signaling. J Transl Med. 2016;14:132. doi: 10.1186/s12967-016-0894-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Waibel M, Gregory G, Shortt J, Johnstone RW. Rational combination therapies targeting survival signaling in aggressive B-cell leukemia/lymphoma. Curr Opin Hematol. 2014;21:297–308. doi: 10.1097/MOH.0000000000000045. [DOI] [PubMed] [Google Scholar]
- 36.Guo Y, Shan Q, Gong Y, Lin J, Shi F, Shi R, Yang X. Curcumin induces apoptosis via simultaneously targeting AKT/mTOR and RAF/MEK/ERK survival signaling pathways in human leukemia THP-1 cells. Pharmazie. 2014;69:229–233. [PubMed] [Google Scholar]
- 37.Zhang S, He Y, Tong Q, Chen Q, Wu X, Huang W. Deltonin induces apoptosis in MDA-MB-231 human breast cancer cells via reactive oxygen species-mediated mitochondrial dysfunction and ERK/AKT signaling pathways. Mol Med Rep. 2013;7:1038–1044. doi: 10.3892/mmr.2013.1273. [DOI] [PubMed] [Google Scholar]
- 38.Reynolds C, Roderick JE, LaBelle JL, Bird G, Mathieu R, Bodaar K, Colon D, Pyati U, Stevenson KE, Qi J, et al. Repression of BIM mediates survival signaling by MYC and AKT in high-risk T-cell acute lymphoblastic leukemia. Leukemia. 2014;28:1819–1827. doi: 10.1038/leu.2014.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu GH, Dai HP, Shen Q, Ji O, Zhang Q, Zhai YL. Curcumin induces apoptosis and suppresses invasion through MAPK and MMP signaling in human monocytic leukemia SHI-1 cells. Pharm Biol. 2016;54:1303–11. doi: 10.3109/13880209.2015.1060508. [DOI] [PubMed] [Google Scholar]
- 40.Wang X, Pesakhov S, Harrison JS, Danilenko M, Studzinski GP. ERK5 pathway regulates transcription factors important for monocytic differentiation of human myeloid leukemia cells. J Cell Physiol. 2014;229:856–867. doi: 10.1002/jcp.24513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gonnella R, Santarelli R, Farina A, Granato M, D'Orazi G, Faggioni A, Cirone M. Kaposi sarcoma associated herpesvirus (KSHV) induces AKT hyperphosphorylation, bortezomib-resistance and GLUT-1 plasma membrane exposure in THP-1 monocytic cell line. J Exp Clin Cancer Res. 2013;32:79. doi: 10.1186/1756-9966-32-79. [DOI] [PMC free article] [PubMed] [Google Scholar]