SUMMARY
Intermediate filaments (IFs) comprise a diverse group of flexible cytoskeletal structures, the assembly, dynamics, and functions of which are regulated by posttranslational modifications. Characteristically, the expression of IF proteins is specific for tissues, differentiation stages, cell types, and functional contexts. Recent research has rapidly expanded the knowledge of IF protein functions. From being regarded as primarily structural proteins, it is now well established that IFs act as powerful modulators of cell motility and migration, playing crucial roles in wound healing and tissue regeneration, as well as inflammatory and immune responses. Although many of these IF-associated functions are essential for tissue repair, the involvement of IF proteins has been established in many additional facets of tissue healing and regeneration. Here, we review the recent progress in understanding the multiple functions of cytoplasmic IFs that relate to cell motility in the context of wound healing, taking examples from studies on keratin, vimentin, and nestin. Wound healing and regeneration include orchestration of a broad range of cellular processes, including regulation of cell attachment and migration, proliferation, differentiation, immune responses, angiogenesis, and remodeling of the extracellular matrix. In this respect, IF proteins now emerge as multifactorial and tissue-specific integrators of tissue regeneration, thereby acting as essential guardian biopolymers at the interface between health and disease, the failing of which contributes to a diverse range of pathologies.
Once regarded as primarily structural proteins, intermediate filaments are now known to act as powerful modulators of cell motility and migration, playing crucial roles in wound healing and related processes.
1. Introduction
Although fully differentiated cells are often more or less stationary, they have shown an amazing ability to move at some stage of their lives, including migration along different types of surfaces, migration and invasion through tissues, and even transcellular migration through different cell types. The basis for cell motility arises from the internal skeleton comprising actin microfilaments, microtubules, and intermediate filaments (IFs) (Huber et al. 2015). Cell motility requires careful spatiotemporal coordination between these three distinct cytoskeletal systems. Although less is known about the molecular functions of IFs in cell motility, as compared with the two other cytoskeletal systems, evidence is accumulating that IFs play direct or indirect roles in cytoskeletal rearrangement, cell shape changes, cell adhesion, and cell mechanical and motile properties, as well as the intracellular signaling that regulates cell motility (Leduc and Etienne-Manneville 2015).
Cell motility and migration are essential for the development and maintenance of multicellular organisms and are also required in many important physiological processes such as embryological development, axon guidance, wound healing, tissue regeneration, and dissemination of malignant tumors. During the life of a fully differentiated organism, effective and fast healing and regeneration of an acquired wound is among the most crucial challenges for survival. It has been long known that IFs are important for successful healing and restoration of tissue barriers. As recent research has shown, this importance, to a significant extent, originates from motility-related IF functions; wound healing is a perfect context to illustrate the roles of IFs in cell motility. Hence, this review focuses on wound healing as a conceptual platform to elucidate the multiple functions of IFs in cell motility.
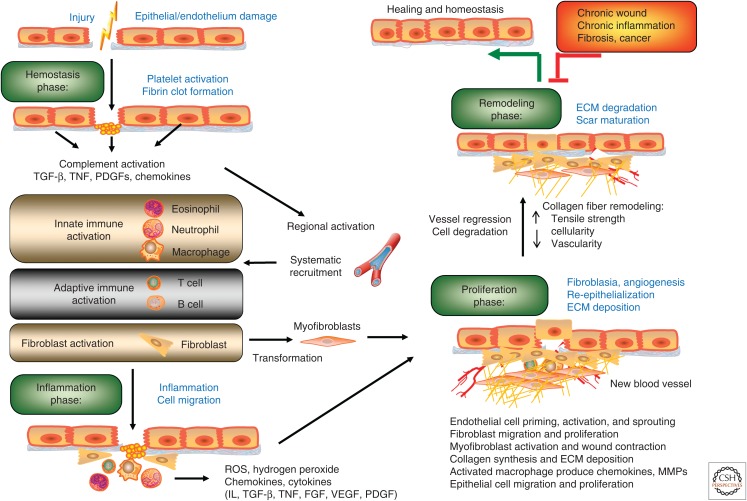
There are great similarities in the restorative healing responses to injury of different tissue types. Taking dermal regeneration as an example, healing in acute wounds occurs as four tightly coordinated and overlapping phases: hemostasis, inflammation, tissue formation (proliferation), and remodeling (Fig. 1) (Gurtner et al. 2008). Immediately upon tissue injury, wounded vessels constrict rapidly and the coagulation cascade is activated to limit blood loss, leading to the formation of a clot, providing a provisional matrix for cellular migration and platelet aggregation. Following hemostasis, there is an inflammation stage spanning the first few days after the injury. During this period, complementary clotting components and cytokines attract inflammatory and immune cells migrating to the site of the wound, both from neighboring tissues and from the circulation, to clear the cell debris and bacteria. At the later stages of the inflammatory phase, macrophages and lymphocytes become key regulatory cells for repair, releasing further cytokines and growth factors to attract fibroblasts, keratinocytes, and endothelial cells into the wound. The inflammatory and immune responses are accompanied by a coincident activation of surrounding tissue, characterized by replacement of the provisional fibrin/fibronectin matrix with newly formed granulation tissue.
Figure 1.
Characteristic stages of wound healing. Epithelial wound repair starts (1) from clot formation (hemostasis phase), followed by (2) an inflammation phase, (3) a proliferation phase, and (4) a tissue-remodeling phase. Proper wound repair requires a close coordination of different cell components such that they are at the right places at the right times. Any significant delay of this self-limiting process is likely to result in pathogenesis. ECM, extracellular matrix; FGF, fibroblast growth factor; IL, interleukin; MMP, matrix metalloprotease; PDGF, platelet-derived growth factor; ROS, reactive oxygen species; TGF-β, transforming growth factor β; TNF, tumor necrosis factor; VEGF, vascular endothelial growth factor.
Upon injury, epithelial cells at the wound edge, stimulated by the various signaling molecules of the injured tissue, often become motile and mitotically active. This activation leads to the disengagement or alteration of cell–cell and cell–matrix attachments, allowing these epithelial cells to migrate to the wound surface, proliferate, and differentiate to reestablish coverage and barrier functions of the wound bed—a complex process termed reepithelialization (Hudson et al. 2009; Reinke and Sorg 2012). Fibroblasts and myofibroblasts are also stimulated to migrate into the wound, proliferate, and produce collagen and other matrix proteins to further support cells growing into the wound. In addition, angiogenic capillary sprouts invade the provisional matrix and organize into a microvascular network throughout the newly formed granulation tissue. Once the tissue deficit has been addressed, the final stage of wound healing—the remodeling phase—will commence. At this stage, collagen synthesis and degradation will be regulated in a concerted way to allow underlying contractile connective tissue to shrink in size and bring the wound margins closer together. With continued remodeling, the outgrowth of capillaries is halted and the density of macrophages and fibroblasts is reduced by apoptosis, finally leading to an acellular, avascular scar at the central area of the wound (reviewed in Gurtner et al. 2008; Reinke and Sorg 2012).
As a diverse group of evolutionarily conserved cytoskeletal structures, IFs form an extensive and elaborate network, which connects the cell cortex to intracellular organelles, with context-dependent, tissue-dependent, and cell type–dependent expression patterns and properties. How IFs affect cell movement during wound repair will depend on the specific cell types affected, the tissue setting, which phase of the wound repair is involved, and the IF expression pattern. The feature mentioned above, of cells around a skin wound being activated, is not limited to skin wounds and is a general feature of harmed tissues. Upon injury of various tissues, cells at the edge of the injury become activated by different injury-specific signaling molecules, thereby being transformed into a more motile and mitotically active phenotype (King and Newmark 2012). Although the activation leads to generic changes in the characteristics of the affected cells (e.g., the dissociated/altered cell–cell and cell–matrix attachments, redemption of migratory properties, often mitotic activation), it is often accompanied by a specific change in the IF gene expression pattern, resulting in IF gene expression shifts that are specific for that particular tissue (Lutolf and Hubbell 2005; Herrmann et al. 2009). Once the tissue deficit has been addressed and the remodeling phase begins, cells tend to adopt a regular, fixed cell shape, with IF networks that provide a greater level of mechanical resistance when assembled, which replace the IFs of the remodeling phase (Herrmann et al. 2009). Hence, there is a dynamic shift back and forth between the IF expression pattern of a terminally differentiated tissue and that of a tissue in a dynamic regeneration stage.
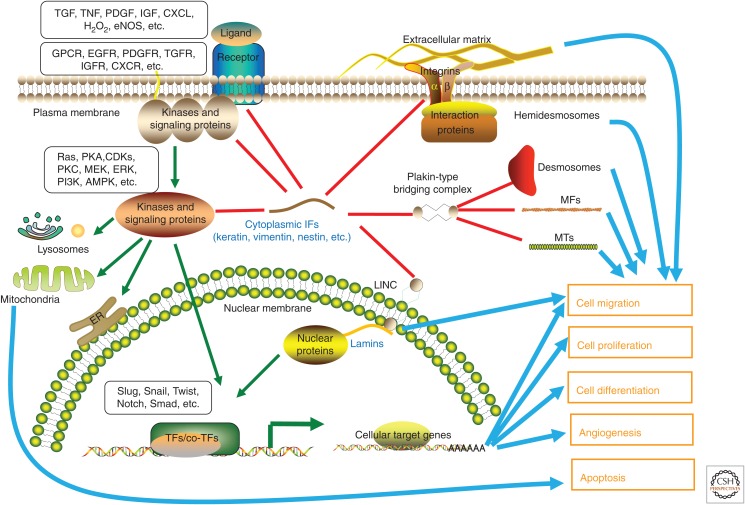
As mentioned above, a number of functional roles have emerged for IFs that are obviously of significance for wound repair. These include modulation of cell migration, proliferation, survival, angiogenesis, collagen synthesis, and remodeling of matrix components. As these activities are regulated as a systematic cascade, which correlates with the appearance of different cell types at the site of injury throughout the different stages of the healing process, IF proteins constitute a perfect match as an organizing structural component, as they have tissue-specific expression patterns, as well as cell-type-specific mechanical and signaling functions. Figure 2 shows an overview of the recently revealed specific molecular mechanisms underlying the IF-mediated regulation of cell motility during wound repair.
Figure 2.
The roles of intermediate filaments (IFs) in cell motility and cell-fate decision-making during wound repair. This overview shows some of the separate and shared functions of cytoplasmic IF proteins (keratin, vimentin, and nestin) in the organization and regulation of signal transduction in a standardized cell. These IFs are also coupled to microfilaments (MFs), microtubules (MTs), IF-anchoring plaques of cell–cell junctions (desmosomes), and cell–matrix junctions (hemidesmosomes) by the plakin-type protein complexes. Focal adhesions are formed through IF complexes and integrins at the base to the extracellular matrix (ECM). Cytoskeletal filaments are, furthermore, coupled to both inner and outer nuclear membranes and lamins by the linker of nucleoskeleton and cytoskeleton (LINC) complex. During wound repair, the alterations in IF homeostasis and dynamics help to promote the correct cellular response at any given time, determining when cells should change their motility to migrate, divide, differentiate, or die. AMPK, AMP-activated protein kinase; CDK, cyclin-dependent kinase; CXCL, C-X-C chemokine ligand; CXCR, C-X-C chemokine receptor; EGFR, epidermal growth factor receptor; eNOS, endothelial nitric oxide synthase; ERK, extracellular signal-regulated kinase; GPCR, G-protein-coupled receptor; H2O2, hydrogen peroxide; IGF, insulin-like growth factor; IGFR, insulin-like growth factor receptor; MEK, MAP kinase kinase; PDGF, platelet-derived growth factor; PDGFR, platelet-derived growth factor receptor; PI3K, phosphoinositide 3-kinase; PKA, protein kinase A; PKC, protein kinase C; TF, transcription factor; TGF, transforming growth factor; TGFR, transforming growth factor receptor; TNF, tumor necrosis factor.
2. IFs in cell adhesion and migration
Cell migration is a fundamental biological process that requires active remodeling of the cytoskeleton. The repair of wounded tissue often induces a shift in the expression of genes encoding IFs that facilitate formation of an IF network to a set that is more suitable for motility, migration, and dynamic reorganization of the tissue. There are numerous reports describing how induction or perturbation of keratin and vimentin IFs affects cell migration. These effects have been extensively reviewed (Chernoivanenko and Minin 2013; Chung et al. 2013; Pan et al. 2013).
The IF protein vimentin is found in cells of mesenchymal, endothelial, and hematopoietic origin. Strongly elevated expression of vimentin is a hallmark of injury in many different tissues (e.g., epithelial, muscle, central nervous system, and various connective tissues) (Ivaska 2011; Satelli and Li 2011). Vimentin is a powerful enhancer of cell migration in normal tissues and during wound repair. Vimentin can act as a signal scaffold and a functional determinant for various signaling molecules involved in cell migration (Chernoivanenko and Minin 2013; Chung et al. 2013). The interaction with signaling complexes is in agreement with the active regulation of vimentin through phosphorylation (Eriksson et al. 2004; Pallari and Eriksson 2006; Hyder et al. 2008). This constitutive regulation by phosphorylation-based posttranslational modifications (PTMs) is also a prerequisite for the dynamic turnover, remodeling, and reorganization of vimentin during migratory processes (Pallari and Eriksson 2006; Eriksson et al. 2009). Vimentin IFs have also been shown to interact with cell–matrix adhesions and to strengthen the adhesion sites (Bhattacharya et al. 2009; Burgstaller et al. 2010; Lynch et al. 2013). For instance, vimentin IFs regulate the specificity of focal adhesion–extracellular matrix (ECM) interactions through vimentin-associated matrix adhesions (VMAs), which assemble in actively migrating cells, interacting with actin microfilaments through vinculin and with vimentin IFs through plectin (Burgstaller et al. 2010). Vimentin IF organization can also modulate the formation of lamellipodia (Helfand et al. 2011). Recently, it has been revealed that vimentin IFs interact with contractile actomyosin arcs, consequently, regulating the localization of arcs and morphogenesis of flat lamellae in migrating cells (Jiu et al. 2015). Emerging evidence suggests that vimentin is involved in cytoskeleton-regulated mechanosensing, a feature that is fundamental for controlled cell motility (Gregor et al. 2014).
In agreement with the strong up-regulation of vimentin IFs following injury to various tissues, when vimentin-deficient (Vim−/−) knockout mice (Colucciguyon et al. 1994) were studied for their regenerative capacity, it was found that the loss of vimentin led to delayed wound healing during both embryogenesis and in adults owing to impaired directional migration and contraction (Eckes et al. 1998; Eckes et al. 2000). Interestingly, a recent report using an ex vivo lens wound model revealed that vimentin IFs in mesenchymal repair cells are associated with myosin IIB and regulate the collective migration of wounded lens epithelia (Menko et al. 2014). Induction of vimentin by the transforming growth factor β1 (TGFβ1)–Smad pathway in alveolar epithelial cells is necessary for wound repair following lung injury (Rogel et al. 2011). Notably, cell migration and wound closure were inhibited by either reducing or increasing vimentin expression, suggesting a vital role for vimentin IF in the injured lung (Rogel et al. 2011). During wound healing, a crucial differentiation program called the epithelial–mesenchymal transition (EMT) has been shown to induce the transition of secondary epithelial cells to resident tissue fibroblasts, a process vital for rapid regeneration of injured epithelium (Kalluri and Weinberg 2009; Weber et al. 2012a). Emerging evidence suggests that vimentin IFs play a functional role in the EMT, and are involved in the cytoskeletal rearrangements, cell migration, and morphological changes of cells undergoing the transition (Ivaska et al. 2007; Mendez et al. 2010; Vuoriluoto et al. 2011; Virtakoivu et al. 2015). However, these studies have been on models related to cancer, in which the EMT plays an important role in rendering epithelial tumor cells their more invasive and metastatic phenotypes, ultimately resulting in a highly aggressive cancer phenotype. Related specifically to wound healing, a recent study revealed a crucial role for vimentin in the coordination and organization of the cellular signaling that takes place during wound healing (Cheng et al. 2016). In this study, vimentin is essential for a wound-healing-mediated EMT, and TGF-β serves as the activator of the vimentin–Slug signaling complex that triggers the EMT and keratinocyte migration (Cheng et al. 2016).
Cell migration entails dynamic changes in the position and shape of the nucleus, the largest and stiffest cell organelle. Emerging evidence suggests that IFs contribute to mechanical adaptation of the nucleus during migration through the confining tissue space, such as skin (Friedl et al. 2011). Recent studies suggested the possibility that IFs are tethered to the “linker of nucleoskeleton and cytoskeleton” (LINC) complex, a protein complex present at the nuclear envelope that participates in anchoring the nuclear lamina to cytoskeletal proteins on the cytoplasmic side, and regulates the dynamics of the nucleus to optimize cell migration (Dupin et al. 2011; Lombardi et al. 2011; Morgan et al. 2011). Based on these studies, it seems plausible that the vimentin that is tethered to LINC thereby participates in regulating nuclear dynamics to optimize cell migration. In agreement with this notion, astrocytes deficient in vimentin and the IF protein glial fibrillary acidic protein (GFAP) show nuclear morphological changes (Dupin et al. 2011) and defects in cell motility (Lepekhin et al. 2001).
Keratin proteins can be divided into type I (K9–K20, K23–K28, K31–K40) and type II proteins (K1–K8, K71–K86), which together form obligate heteropolymers. Each cell type expresses a unique pattern of type I–type II keratin pairs (Moll et al. 2008; Jacob et al. 2016). In a broad array of tissues and organs, there is a coordinated alteration of the keratin expression pattern in surviving cell compartments close to the wound edge. Interestingly, these coordinated alterations resemble stages that have already taken place during organ development and differentiation. If skin is taken as an example, keratins 1 and 10 (K1 and K10) are the predominant pair in terminally differentiated suprabasal layers of the epidermis in homeostasis. K2e is typically expressed during later stages of differentiation in suprabasal keratinocytes, and K5 and K14 are the major pair in keratinocytes of the mitotically active basal layer of the epidermis. Correspondingly, migratory keratinocytes at wound margins in a coordinated cell sheet show decreased expression of the differentiation-associated K1 and K10 and instead de novo production of the specific injury-associated keratins K6, K16, and K17 (reviewed by DePianto and Coulombe 2004 and Jacob et al. 2016).
Keratin IFs regulate cell shape, cell adhesion, and mechanotransduction of intracellular and intercellular tension during cell migration through their association with cell–cell (desmosomal) and cell–matrix (hemidesmosomal) junctions, and other cytoskeleton proteins. Wound healing assays using different cell types further showed faster wound closure on keratin down-regulation or knockout, accompanied by a loss of cell–cell contacts (Long et al. 2006; Seltmann et al. 2013b). In the absence of the entire keratin cytoskeleton, murine keratinocytes display a disorganized plectin, which is an important component of the hemidesmosome. This results in higher cell–matrix adhesion and enhanced migratory properties of keratinocytes in wound healing. Reexpression of the K5–K14 basal keratin pair alone is sufficient to reverse the phenotype (Seltmann et al. 2013a, 2013b). K16 and K17 expression is induced at the wound edge after tissue injury. In this respect, varying the levels of K16 ectopic expression has also been shown to influence the cell adhesion and migration in explant culture assays (Wawersik et al. 2001). A recent study suggests that keratins and the associated protein plakoglobin are positioned at the cellular pole opposite to the direction of migration to define polarization and directional migration of multicellular epithelial assemblies (Weber et al. 2012b; reviewed by Jones et al. 2016).
There is mounting evidence to substantiate the importance of specific keratin responses to a given injury, as genetic manipulations of the involved keratin genes lead to altered wound-healing outcomes. There is a complex interplay between the involved keratins, some acting as brakes and some as accelerators in the complex processes required for proper healing to take place. K6a and K6b, two isoforms of K6, are induced soon after injury to the skin in keratinocytes proximal to the wound and are maintained during the reepithelialization process (Takahashi et al. 1998). In this respect, it was surprising that genetic ablation of K6a and K6b led to an enhanced migration of keratinocytes. It turns out that Src kinase, which is a positive regulator of keratinocyte migration, is normally physically associated with K6, which results in Src kinase inhibition. When K6a and K6b are disrupted, there is a robust release of active Src into the cytoplasm, which drives cell migration (Rotty and Coulombe 2012). The role of these keratins, therefore, seems to be one of organizing and restricting Src activity, to enable controlled migration. K8 seems to have a similar role, albeit, with an opposing effect when compared with K6a and K6b. The knockdown of K8 in simple epithelial cells down-regulates Src activity by disrupting a large Src complex comprising keratins, plectin, protein kinase C (PKC), and integrins, leading to impaired cell migration in a scratch-wound assay (Bordeleau et al. 2010). Therefore, the role of individual keratins in tissue regeneration depends on the specific cell types affected, the tissue setting, and the corresponding IF expression pattern.
3. IFs in inflammation and immune responses
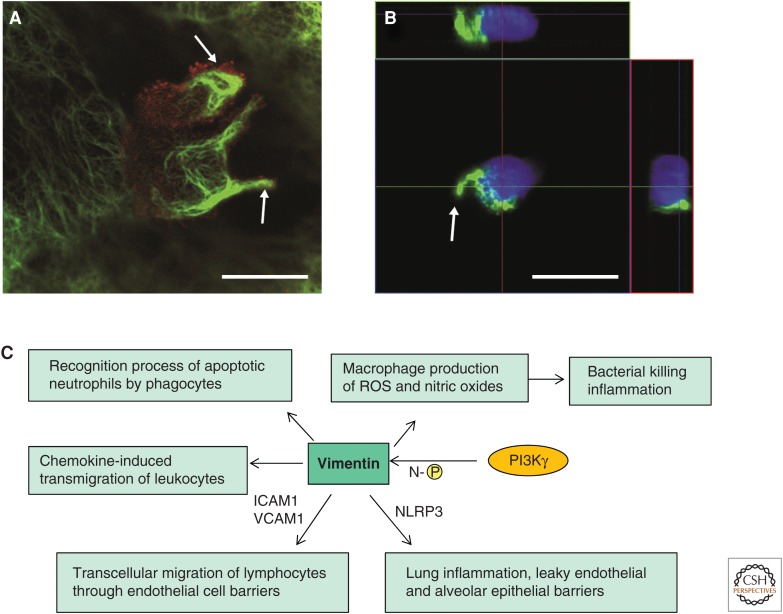
The remarkable capability of immune cells to migrate allows them to effectively recognize and quickly reach the place of injury. One key stage of leukocyte migration is their transmigration through the endothelium of blood vessels and lymphatic vessels. Vimentin IFs form dynamic structures that have been found to participate in the transmigratory process both in the transmigrating leukocytes (Nieminen et al. 2006; Ivaska et al. 2007) and in the endothelial cells through which the leukocytes cross (Carman and Springer 2004; Nieminen et al. 2006). The Vim−/− mouse model (Colucciguyon et al. 1994), referred to above, was helpful when elucidating the role of vimentin in the process of leukocyte transmigration and extravasation. When the involved cells were examined in Vim−/− mice, it was observed that there is a disturbed distribution of the adhesion molecules in both the migrating and the receiving cells—that is, integrins in the lymphocytes and intercellular cell-adhesion molecule 1 (ICAM-1) and vascular cell-adhesion molecule 1 (VCAM-1) in the endothelial cells. Therefore, the lymphocytes and endothelial cells that lack vimentin interact with each other much more poorly, resulting in a severely impaired transcellular migration of lymphocytes through endothelial cell barriers into the tissues, lymph nodes, and the spleen (Fig. 3) (Nieminen et al. 2006). In addition to organizing these crucial surface molecules, vimentin has been implicated as a source of structural support in lymphocytes to limit mechanical deformation on chemokine-induced polarization of the cell and to stimulate cell migration through size-limited endothelium pores. In this respect, extravasation of circulating lymphocytes requires pronounced reorganization of the vimentin network (Brown et al. 2001). In agreement with this concept, a recent report suggests that amino-terminal phosphorylation and reorganization of vimentin by the phosphoinositide 3-kinase γ (PI3Kγ) signaling pathway are required for chemokine-induced transmigration of leukocytes to the inflammation sites (Fig. 3C) (Barberis et al. 2009). Furthermore, vimentin IFs have been found in macrophage filopodia and podosomes, which are highly dynamic cell-adhesion structures that are involved in the directional motility and transmigration of myeloid cells (Calle et al. 2006).
Figure 3.
Vimentin intermediate filaments (IFs) function in leukocyte transcellular migration. (A) Reorganization of vimentin IFs (green) of a migrating peripheral blood mononuclear cell (PBMC) toward the uropods (white arrows) of two migrating PBMCs. The staining by antibody against CD44 (red), a membrane marker for the PBMC, outlines the borders of the PBMC. The focal plane is at the upper level of the endothelial cell (EC), so only parts of the EC vimentin IFs can be seen around the migrating cell. Scale bar, 10 μm. (B) A similar polarization and reorganization of vimentin IFs (green) also occur when the cells are incubated for 30 min on gelatin-coated coverslips. z-axis side views are shown from two different planes (colored lines on the top and on the right-hand side of the large image show the maximum projection). Scale bar, 10 μm. (C) Vimentin IFs have the ability to bind and modulate the activity of signaling proteins, thereby influencing inflammatory signals in response to injury. The figure summarizes the key vimentin-controlled interactions and signaling pathways that influence innate immunity and inflammation. Vimentin itself can be phosphorylated by phosphoinositide 3-kinase γ (PI3Kγ) at its amino terminus (N-[P]). ICAM1, intercellular adhesion molecule 1; NLRP3, NLRP3 inflammasome; ROS, reactive oxygen species; VCAM1, vascular cell-adhesion molecule 1. (A,B, Reprinted, with permission, from Nieminen et al. 2006.)
4. IFs in angiogenesis
Angiogenesis is a process that requires carefully coordinated cell migration and invasion. There are a number of reports implicating direct roles of vimentin in angiogenesis. Hypoxia, a common state in injured tissue, triggers a concerted response in endothelial cells to direct the angiogenesis. Interestingly, it was shown that exposure of endothelial cells to hypoxia induces perinuclear redistribution of vimentin to a more insoluble and extensive filamentous network that was suggested to play a role in endothelial barrier stabilization (Liu et al. 2010). Furthermore, vimentin has been shown to be crucial in regulating stress-induced retinal angiogenesis (Lundkvist et al. 2004). In that study, deficiency of vimentin or vimentin and GFAP caused increased retina fragility and defective angiogenic responses to oxygen-induced retinopathy (Lundkvist et al. 2004). Related to these observations, it was also shown that vimentin-deficient mice have leaky endothelia (Nieminen et al. 2006). Recent data highlight the interplay between vimentin, calpains, and matrix metalloprotease MT1-MMP in regulating successful angiogenic responses in a three-dimensional spheroid-sprouting model. Activation of the cysteine protease calpain by proangiogenic stimuli leads to vimentin cleavage, and the subsequent increased soluble truncated vimentin protein was shown to bind the cytoplasmic tail of MT1-MMP. This binding facilitates proper translocation of MT1-MMP to the endothelial cell surface, allowing matrix degradation and successful angiogenic sprouting (Kwak et al. 2012). Inhibition of vimentin expression blocked the surface localization of MT1-MMP and was correlated with significantly reduced sprouting responses (Kang et al. 2011).
Nestin, a type VI IF, has also been correlated with angiogenesis, although its precise roles are unclear. Nestin is expressed in metabolically active angiogenic vasculature following myocardial infarct, particularly in arteriovenous malformations (Mokry et al. 2004; Shimizu et al. 2006; Sokmensuer and Sokmensuer 2007; Mokry et al. 2008). However, nestin IF expression in neovascularization is transient during wound repair (Suzuki et al. 2010). For instance, on pituitary infarcts, nestin is expressed in capillary neovascularization in the pituitary gland, but its level goes down when infarcted tissue transforms to fibrotic tissue (Salehi et al. 2008). Interestingly, as nestin is down-regulated, vimentin is up-regulated in the neovessels, suggesting that elevation of nestin expression in the process of angiogenesis is likely to correspond to reorganization of IF networks in activated endothelial cells to adapt to the changes in growing tissues (Brychtova et al. 2007; Mokry et al. 2008).
5. IFs in cell growth and survival
When different tissues are injured, the wound-activated signal mechanisms that switch the involved epithelial cells and fibroblasts into a more migratory mode will also make them mitotically active (King and Newmark 2012). In contrast, at the end of tissue repair, when migration is ceasing, unnecessary inflammatory cells, fibroblasts, and endothelial cells have to be removed by apoptosis or autophagy. Although the aspects of accelerated cell growth and survival are closely related to the motility of cells, we will only briefly describe specific roles of IFs in wound-healing-associated cell growth and death, as the general role of IF proteins in modulating cell growth and death have been reviewed elsewhere (Eriksson et al. 2009; Toivola et al. 2010; Pan et al. 2013).
It turns out that, during wound healing, when the regulation of cell growth and cell death requires strict orchestration, IFs can either stimulate or inhibit cell proliferation. Depending on the context, a specific IF protein can even have multiple roles. In many cases, PTMs can act as important functional determinants. For example, the interaction between IFs and individual signaling molecules is often phosphorylation dependent, as in the case of keratin IFs that bind to 14-3-3 in a phosphorylation-dependent manner. 14-3-3 comprises a family of adaptor proteins that modulate the functions of a variety of signaling proteins through direct phosphorylation-dependent interactions. For instance, K17, which is rapidly induced in wounded stratified epithelia (see above), regulates cell size and growth by activating the Akt–mTOR pathway through 14-3-3 ε (Kim et al. 2006; Vijayaraj et al. 2009). Vimentin also regulates 14-3-3 complexes and controls various proliferation and cell-cycle control pathways (Satelli and Li 2011). Nestin was also found to regulate DNA synthesis and proliferation in myofibroblasts, thereby accelerating the healing process following ischemia, although the underlying molecular mechanism is still to be elaborated (Beguin et al. 2012).
Several reports show that IFs can also regulate cellular apoptosis to coordinate tissue regeneration during wound healing. K8 can protect colon, liver, and pancreatic epithelium from tissue injury by modulating a variety of death receptors (tumor necrosis factor TNF receptors, Fas receptors) to exert an antiapoptotic, prosurvival influence (Caulin et al. 2000; Gilbert et al. 2001; Inada et al. 2001; He et al. 2002; Yoneda et al. 2004; Gilbert et al. 2012), or by acting as “PTM sponges” to absorb excessive stress or proapoptotic signals (Ku et al. 1998a; Ku et al. 1998b; Ku and Omary 2006). Site-specific phosphorylation of K8 and K18 are instrumental in hepatocyte survival in response to a broad variety of metabolic, oxidative, and chemical stresses (Toivola et al. 2004; Zhou et al. 2005; Ku et al. 2007). A recent study revealed that, in addition to phosphorylation, site-specific O-glycosylation of K18 (at serine residues 30, 31, or 49) serves to positively regulate the activity of prosurvival kinases, such as Akt and PKC, thereby protecting cells against apoptosis and promoting their adaptation to stresses (Ku et al. 2010). However, keratin function in cell survival is context dependent and influenced by environmental modifiers. For example, transcriptional profiling in colons of K8-null mice revealed an unexpected up-regulation of various prosurvival factors and pathways, suggesting a proapoptotic effect of K8 in the colonic epithelia, which might depend on the cross talk with microflora (Habtezion et al. 2011).
The interaction between nestin and cyclin-dependent kinase Cdk5 is a good example of one interaction having multiple effects, related to survival, differentiation, migration, and apoptosis (Sahlgren et al. 2001; Sahlgren et al. 2003; Sahlgren et al. 2006; de Thonel et al. 2010; Pallari et al. 2011). The degradation of nestin is necessary to sensitize cells to Cdk5-mediated proapoptotic activity (Sahlgren et al. 2006; Liu et al. 2012). In contrast, under certain stress conditions, up-regulated nestin is able to attenuate apoptosis, presumably by sequestering Cdk5 (Huang et al. 2009; Liu et al. 2013). In most of the above studies, nestin preferentially coassembles with vimentin, but it is not known whether vimentin IFs also contribute to the regulation of nestin in Cdk5-mediated apoptosis.
Finally, autophagy is an alternative mechanism to degrade unnecessary or dysfunctional cellular components through the actions of lysosomes. It has been found that vimentin forms a complex with 14-3-3 and beclin 1 to inhibit autophagy through an Akt-dependent mechanism (Steinmetz et al. 2011).
6. IFs in matrix remodeling
The ECM is important at all stages of wound repair, promoting communication between epithelial cells and fibroblasts, an obviously important factor in determining the motility of cells, and a sensor and determinant of both lost and regained tissue integrity. Through modulation of RhoA–ROCK signaling and actin cytoskeleton dynamics, keratins K8 and K18 have been shown to regulate the interplay between cell stiffness and rigidity of the ECM. This has been suggested to be the reason why K8/K18 IF-knockdown rat hepatoma cells lose their ability to spread on seeding on a low-rigidity substratum (Bordeleau et al. 2012). Activated fibroblasts or transformed myofibroblasts migrate into the site of injury and are responsible for laying down a collagen-rich matrix. IFs might also have a direct role in the expression of ECM, which has been illustrated by observations showing that vimentin IFs can stabilize type I collagen messenger RNAs (mRNAs) and that, consequently, loss of vimentin leads to reduced type I collagen production in fibroblasts (Challa and Stefanovic 2011). There is ongoing collagen synthesis and breakdown in response to wounding, as the ECM is continually remodeled, ultimately equilibrating to a steady state at the end of healing. ECM remodeling, including degradation of collagen and other ECM components, is achieved by a concerted interaction between plasminogen and specific MMPs that are produced by many cells at the wound site, including fibroblasts, granulocytes, and macrophages (Cox and Erler 2011). Keratins participate actively in ECM remodeling. For example, in hepatocytes, K8 acts as a cell-surface receptor for the urokinase-type plasminogen activator (uPA), which in turn then activates plasminogen into a major ECM-remodeling enzyme—the serine protease plasmin (Hembrough et al. 1995; Obermajer et al. 2009).
7. IFs in motility-related pathogenesis
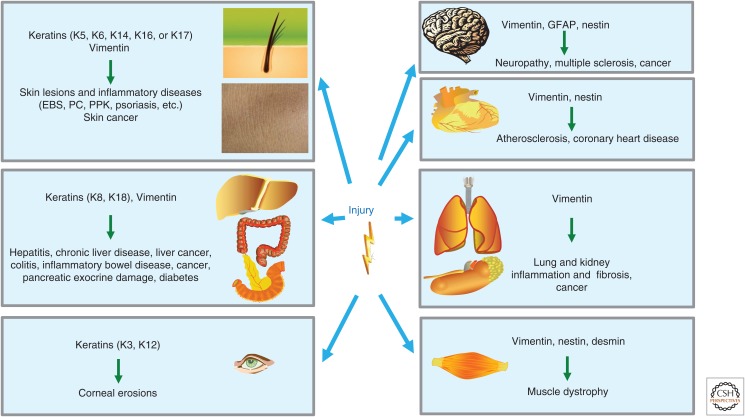
Physiological wound healing, chronic inflammatory and immune diseases, fibrosis, and cancer all depend on similar types of communication among the key cellular components to determine proliferation, differentiation, survival, ECM production, angiogenesis, as well as immune and inflammatory responses. In this way, the same components and processes can contribute to either a physiological process or to pathogenesis (Dvorak 1986; Schafer and Werner 2008; Lopez-Novoa and Nieto 2009; Arwert et al. 2012). There is mounting evidence to suggest that the cellular processes that are controlled by IFs will modulate key functions of epithelial cells, mesenchymal cells, recruited hematopoietic cells, and endothelial cells. In turn, these IF-mediated control functions have influences on a variety of important human diseases. However, although the connection has been outlined, the exact molecular causes underlying the contribution of IFs to chronic inflammatory diseases, fibrosis, and cancer are still poorly understood. In the following section, we will primarily describe how genetic or experimental modification of IFs has shed light on how IF-regulated cell motility might be associated with various kinds of pathogenesis (Fig. 4).
Figure 4.
Involvement of intermediate filament (IF) expression in different tissues on injury and relevant associated disease. IF proteins can be expressed in different compositions and combinations depending on the cell type, differentiation state, and functional settings. The broad and complex distribution of IFs regulates cellular activities in different organs on specific physiological stimuli, especially during tissue repair. The mutation of genes encoding IFs, or disturbance of IF functions, can ultimately lead to tissue-wide pathologic alterations, ranging from skin lesions to cancer of various kinds. EBS, epidermolysis bullosa simplex; GFAP, glial fibrillary acidic protein; K, keratin; PC, pachyonychia congenital; PPK, palmoplantar keratoderma.
Chronic wounds can lead to tumor formation if inflammation cannot be blocked. There are striking similarities between growth factors, cytokines, and chemokines present in a wound compared with what is found in the tumor microenvironment (Dave and Bayless 2014). In this context, tumor formation is characterized by continuous deregulation of the same molecular mechanisms and pathways that are normally involved in wound healing (Arwert et al. 2012). Similar to wound healing, carcinogenesis involves alterations from the signaling pathways that characterize terminally differentiated cells. Correspondingly, a change in the IF expression pattern typically occurs during tumorigenesis. This involves a shift from an IF pattern specific for late-stage differentiation to a pattern characteristic of an early-stage differentiation. Given that a specific terminally differentiated cell type expresses a specific set of IF proteins, IF profiling can be used for diagnosis, prognosis, and assessing therapeutic strategies in various tumors (Moll et al. 2008; Karantza 2011; Satelli and Li 2011). It is also reasonable to assume that alterations in IF expression or even IF mutations would impact a number aspects of tumor biology, especially related to the motility and invasion of cancer cells.
Several studies have provided evidence supporting there being an active role for keratins in cancer cell growth and invasion. Keratin 17 is up-regulated in basaloid skin tumors, with high K17 levels being associated with aggressive growth and poor patient prognosis. In a mouse model of basal cell carcinoma, genetic ablation of K17 affects tumor initiation and growth, correlating with reduced inflammation (DePianto et al. 2010). Related to K17, a recent genome-wide association study (GWAS) identified a single-nucleotide polymorphism (SNP) in the locus of K5, the type I keratin partner of K17, as being associated with a predisposition to human basal cell carcinoma (Stacey et al. 2009; Jacob et al. 2016). Similarly to K17, ablation of basal keratin K14 inhibits keratinocyte proliferation and differentiation, and yields a significant reduction in tumorigenicity in mouse models (Alam et al. 2011b). In further support of an active role for K14 in tumor promotion, it was shown that K14 is activated in collective invasion of mammary epithelial cancer cells. Furthermore, silencing of K14 is sufficient to block the collective invasion of these cancer cells (Cheung et al. 2013). As the basal cytokeratin differentiation program is found in many epithelial organs, it is plausible that these keratins could become potential targets to limit invasion of solid tumors.
Keratin expression could have a positive or negative effect on modulating tumor growth and invasion, depending on the developmental stage, tissue setting, the expression pattern of partner IFs, and the tumor cell type. These aspects are likely to explain the seemingly contradicting results that have been obtained with different keratins. For example, silencing of K8 or K18 expression results in increased invasion of lung cancer cells (Kanaji et al. 2011). However, in another study, K8 loss led to reduced cell invasion in an oral-tumor-derived cell line, which could be rescued by reexpression of small hairpin RNA (shRNA)-resistant K8 into these cells, leading to enhanced tumorigenicity (Alam et al. 2011a). Similarly, K8-deficient FVB mice showed a shorter tumor latency in a breast cancer model (Baribault et al. 1997), but these mice showed activated wound healing and dramatically increased numbers of colon tumors in the azoxymethane (AOM) and ApcMin/+ colorectal cancer models (Misiorek et al. 2016). A cancer-promoting role for K8 was found when it was observed that overexpression resulted in early neoplastic proliferation in the pancreas (Casanova et al. 1999), which also correlated with the extent of spontaneous pancreatic injury (Toivola et al. 2008). Similarly, ectopic expression of K8 in the skin causes epidermal hyperplasia in young mice, preneoplastic changes in aging mice, and malignant progression of skin tumors induced by chemical carcinogenesis (Casanova et al. 2004). A further role for K8 and K18 in regulation of the cell cycle was indicated in a study showing giant multinuclear cells, with tens or even hundreds of nuclei in livers from K8−/− and K18−/− mice (Toivola et al. 2001). In light of these results, it is obvious that early-differentiation-stage keratins participate in regulation of cancer cell invasion. However, it is not possible to define a unifying in vivo role for these keratins in cancer, as the results are so variable in the different cancer models.
Vimentin expression is closely associated with many highly aggressive cancers. In fact, the mechanisms by which vimentin regulates regeneration in wound healing might also be advantageous for cancer cell proliferation, motility, and invasion (Kidd et al. 2014). Vimentin is a signaling hub for the EMT, which is an important differentiation program in both wound healing and malignant transformation (Ivaska 2011; Rodriguez et al. 2013). Vimentin up-regulation by Slug and K-Ras mediates the expression of the receptor tyrosine kinase Axl, which is necessary for vimentin-mediated EMT migration and invasion capabilities (Vuoriluoto et al. 2011). Consistently, inhibition of vimentin expression in tumor cells has been shown to inhibit motility and invasiveness (McInroy and Maatta 2007). Vimentin is also involved in regulating sphingolipid signaling, which plays an important role in determining the invasiveness of many cancer types. The results imply that the sphingolipid–vimentin signaling axis exerts “brake and throttle” functions in the regulation of cell migration, with a shift toward a migration-promoting mode in invasive cancers (Hyder et al. 2015). Given the increasing number of GWASs of human and animal models for different cancers, it is possible that vimentin mutants that could influence specific cancers will be discovered in the near future, with possibilities to unravel the molecular mechanisms underlying the observed migration-promoting effects. Interestingly, the vimentin partner nestin regulates prostate cancer cell invasion by influencing the localization and functions of focal adhesion kinase (FAK) and integrins (Hyder et al. 2014), providing a further IF-mediated mechanism to regulate the invasiveness of cancer cells expressing both vimentin and nestin.
Fibrosis is a normal aspect of scar formation, but is also a pathological outcome of most chronic inflammatory and autoimmune diseases. Fibrosis could be regarded to be a process leading to the opposite outcome from the above-described processes that all are related to elevated migration and increased invasion. Fibrosis is characterized by loss of migratory cells and accumulation of excess ECM components, eventually leading to organ malfunction and death (Wynn and Ramalingam 2012). Idiopathic pulmonary fibrosis (IPF) is defined by alveolar epithelial cell injury, hyperplasia, fibroblast accumulation, collagen deposition, and pathological scar formation. Interestingly, there is a connection between vimentin and fibrosis, as vimentin-null mice are protected from lung inflammation and fibrosis after challenge with the fibrotic agents bleomycin, asbestos, and lipopolysaccharide (LPS)/ATP (Gimena Dos et al. 2013), which act through controlling the NLRP3 inflammasome required for the initiation and progression of IPF (Dos Santos et al. 2015). As fibrosis is another outcome of the EMT, and vimentin serves as such a prominent regulator of the EMT, it is not surprising that vimentin has a prominent effect on fibrotic processes. Although it is clear that vimentin has a key role in regulating EMT-related migratory processes, the details of the molecular mechanism of this vimentin-based regulation still remain to be characterized.
8. Conclusion
Recent advances have added significantly to our current understanding of the complex role of IFs in cell motility, especially in the context of wound repair. Taken together, these findings show that IFs are not merely intrinsic determinants of cellular micromechanical properties, but act as key players in the dynamic migratory processes in response to environmental changes. We are just starting to understand the coordination of different IFs in cell proliferation, migration, and invasion. Now there are a great number of in silico, genetic engineering and imaging tools that can be exploited to understand the roles of IFs, ensuring the correct recruitment and targeting of signaling molecules in the migrating cells. In the future, a better knowledge of the temporal and context-dependent impact of IFs in cell motility in physiological and disease settings is likely to yield new therapies and diagnostic and molecular tools that will use IF-related functions.
Footnotes
Editors: Thomas D. Pollard and Robert D. Goldman
Additional Perspectives on The Cytoskeleton available at www.cshperspectives.org
REFERENCES
*Reference is also in this collection.
- Alam H, Kundu ST, Dalal SN, Vaidya MM. 2011a. Loss of keratins 8 and 18 leads to alterations in α6β4-integrin-mediated signalling and decreased neoplastic progression in an oral-tumour-derived cell line. J Cell Sci 124: 2096–2106. [DOI] [PubMed] [Google Scholar]
- Alam H, Sehgal L, Kundu ST, Dalal SN, Vaidya MM. 2011b. Novel function of keratins 5 and 14 in proliferation and differentiation of stratified epithelial cells. Mol Biol Cell 22: 4068–4078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arwert EN, Hoste E, Watt FM. 2012. Epithelial stem cells, wound healing and cancer. Nat Rev Cancer 12: 170–180. [DOI] [PubMed] [Google Scholar]
- Barberis L, Pasquali C, Bertschy-Meier D, Cuccurullo A, Costa C, Ambrogi C, Vilbois F, Chiarle R, Wymann M, Altruda F, et al. 2009. Leukocyte transmigration is modulated by chemokine-mediated PI3K γ-dependent phosphorylation of vimentin. Eur J Immunol 39: 1136–1146. [DOI] [PubMed] [Google Scholar]
- Baribault H, Wilson-Heiner M, Muller W, Penner J, Bakhiet N. 1997. Functional analysis of mouse keratin 8 in polyoma middle T-induced mammary gland tumours. Transgenic Res 6: 359–367. [DOI] [PubMed] [Google Scholar]
- Beguin PC, Gosselin H, Mamarbachi M, Calderone A. 2012. Nestin expression is lost in ventricular fibroblasts during postnatal development of the rat heart and re-expressed in scar myofibroblasts. J Cell Physiol 227: 813–820. [DOI] [PubMed] [Google Scholar]
- Bhattacharya R, Gonzalez AM, Debiase PJ, Trejo HE, Goldman RD, Flitney FW, Jones JC. 2009. Recruitment of vimentin to the cell surface by β3 integrin and plectin mediates adhesion strength. J Cell Sci 122: 1390–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordeleau F, Galarneau L, Gilbert S, Loranger A, Marceau N. 2010. Keratin 8/18 modulation of protein kinase C–mediated integrin-dependent adhesion and migration of liver epithelial cells. Mol Biol Cell 21: 1698–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordeleau F, Myrand Lapierre ME, Sheng Y, Marceau N. 2012. Keratin 8/18 regulation of cell stiffness-extracellular matrix interplay through modulation of Rho-mediated actin cytoskeleton dynamics. PLoS ONE 7: e38780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MJ, Hallam JA, Colucci-Guyon E, Shaw S. 2001. Rigidity of circulating lymphocytes is primarily conferred by vimentin intermediate filaments. J Immunol 166: 6640–6646. [DOI] [PubMed] [Google Scholar]
- Brychtova S, Fiuraskova M, Hlobilkova A, Brychta T, Hirnak J. 2007. Nestin expression in cutaneous melanomas and melanocytic nevi. J Cutan Pathol 34: 370–375. [DOI] [PubMed] [Google Scholar]
- Burgstaller G, Gregor M, Winter L, Wiche G. 2010. Keeping the vimentin network under control: Cell-matrix adhesion-associated plectin 1f affects cell shape and polarity of fibroblasts. Mol Biol Cell 21: 3362–3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calle Y, Burns S, Thrasher AJ, Jones GE. 2006. The leukocyte podosome. Eur J Cell Biol 85: 151–157. [DOI] [PubMed] [Google Scholar]
- Carman CV, Springer TA. 2004. A transmigratory cup in leukocyte diapedesis both through individual vascular endothelial cells and between them. J Cell Biol 167: 377–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova ML, Bravo A, Ramirez A, Morreale de Escobar G, Were F, Merlino G, Vidal M, Jorcano JL. 1999. Exocrine pancreatic disorders in transgenic mice expressing human keratin 8. J Clin Invest 103: 1587–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova ML, Bravo A, Martinez-Palacio J, Fernandez-Acenero MJ, Villanueva C, Larcher F, Conti CJ, Jorcano JL. 2004. Epidermal abnormalities and increased malignancy of skin tumors in human epidermal keratin 8-expressing transgenic mice. FASEB J 18: 1556–1558. [DOI] [PubMed] [Google Scholar]
- Caulin C, Ware CF, Magin TM, Oshima RG. 2000. Keratin-dependent, epithelial resistance to tumor necrosis factor-induced apoptosis. J Cell Biol 149: 17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challa AA, Stefanovic B. 2011. A novel role of vimentin filaments: Binding and stabilization of collagen mRNAs. Mol Cell Biol 31: 3773–3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng F, Shen Y, Mohanasundaram P, Lindström M, Ivaska J, Ny T, Eriksson JE. 2016. Vimentin coordinates fibroblast proliferation and keratinocyte differentiation in wound healing via TGF-β–Slug signaling. Proc Natl Acad Sci 113: E4320–E4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernoivanenko IS, Minin AA. 2013. [Role of vimentin in cell migration]. Ontogenez 44: 186–202. [DOI] [PubMed] [Google Scholar]
- Cheung KJ, Gabrielson E, Werb Z, Ewald AJ. 2013. Collective invasion in breast cancer requires a conserved basal epithelial program. Cell 155: 1639–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung BM, Rotty JD, Coulombe PA. 2013. Networking galore: Intermediate filaments and cell migration. Curr Opin Cell Biol 25: 600–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colucciguyon E, Portier MM, Dunia I, Paulin D, Pournin S, Babinet C. 1994. Mice lacking vimentin develop and reproduce without an obvious phenotype. Cell 79: 679–694. [DOI] [PubMed] [Google Scholar]
- Cox TR, Erler JT. 2011. Remodeling and homeostasis of the extracellular matrix: Implications for fibrotic diseases and cancer. Dis Model Mech 4: 165–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dave JM, Bayless KJ. 2014. Vimentin as an integral regulator of cell adhesion and endothelial sprouting. Microcirculation 21: 333–344. [DOI] [PubMed] [Google Scholar]
- DePianto D, Coulombe PA. 2004. Intermediate filaments and tissue repair. Exp Cell Res 301: 68–76. [DOI] [PubMed] [Google Scholar]
- DePianto D, Kerns ML, Dlugosz AA, Coulombe PA. 2010. Keratin 17 promotes epithelial proliferation and tumor growth by polarizing the immune response in skin. Nat Genet 42: 910–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Thonel A, Ferraris SE, Pallari HM, Imanishi SY, Kochin V, Hosokawa T, Hisanaga S, Sahlgren C, Eriksson JE. 2010. Protein kinase Cζ regulates Cdk5/p25 signaling during myogenesis. Mol Biol Cell 21: 1423–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dos Santos G, Rogel MR, Baker MA, Troken JR, Urich D, Morales-Nebreda L, Sennello JA, Kutuzov MA, Sitikov A, Davis JM, et al. 2015. Vimentin regulates activation of the NLRP3 inflammasome. Nat Commun 6: 6574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupin I, Sakamoto Y, Etienne-Manneville S. 2011. Cytoplasmic intermediate filaments mediate actin-driven positioning of the nucleus. J Cell Sci 124: 865–872. [DOI] [PubMed] [Google Scholar]
- Dvorak HF. 1986. Tumors: Wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med 315: 1650–1659. [DOI] [PubMed] [Google Scholar]
- Eckes B, Dogic D, Colucci-Guyon E, Wang N, Maniotis A, Ingber D, Merckling A, Langa F, Aumailley M, Delouvee A, et al. 1998. Impaired mechanical stability, migration and contractile capacity in vimentin-deficient fibroblasts. J Cell Sci 111: 1897–1907. [DOI] [PubMed] [Google Scholar]
- Eckes B, Colucci-Guyon E, Smola H, Nodder S, Babinet C, Krieg T, Martin P. 2000. Impaired wound healing in embryonic and adult mice lacking vimentin. J Cell Sci 113: 2455–2462. [DOI] [PubMed] [Google Scholar]
- Eriksson JE, He T, Trejo-Skalli AV, Harmala-Brasken AS, Hellman J, Chou YH, Goldman RD. 2004. Specific in vivo phosphorylation sites determine the assembly dynamics of vimentin intermediate filaments. J Cell Sci 117: 919–932. [DOI] [PubMed] [Google Scholar]
- Eriksson JE, Dechat T, Grin B, Helfand B, Mendez M, Pallari HM, Goldman RD. 2009. Introducing intermediate filaments: From discovery to disease. J Clin Invest 119: 1763–1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedl P, Wolf K, Lammerding J. 2011. Nuclear mechanics during cell migration. Curr Opin Cell Biol 23: 55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert S, Loranger A, Daigle N, Marceau N. 2001. Simple epithelium keratins 8 and 18 provide resistance to Fas-mediated apoptosis. The protection occurs through a receptor-targeting modulation. J Cell Biol 154: 763–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert S, Loranger A, Lavoie JN, Marceau N. 2012. Cytoskeleton keratin regulation of FasR signaling through modulation of actin/ezrin interplay at lipid rafts in hepatocytes. Apoptosis 17: 880–894. [DOI] [PubMed] [Google Scholar]
- Gimena Dos S, David AV, Margaret AB, Paul C, David K, Karen MR. 2013. Vimentin is required for asbestos-induced lung injury. In C39 Lung injury and repair, American Thoracic Society International Conference, May 17–22, 2013, Philadelphia, meeting abstracts, Vol. 187 American Thoracic Society, New York. [Google Scholar]
- Gregor M, Osmanagic-Myers S, Burgstaller G, Wolfram M, Fischer I, Walko G, Resch GP, Jorgl A, Herrmann H, Wiche G. 2014. Mechanosensing through focal adhesion-anchored intermediate filaments. FASEB J 28: 715–729. [DOI] [PubMed] [Google Scholar]
- Gurtner GC, Werner S, Barrandon Y, Longaker MT. 2008. Wound repair and regeneration. Nature 453: 314–321. [DOI] [PubMed] [Google Scholar]
- Habtezion A, Toivola DM, Asghar MN, Kronmal GS, Brooks JD, Butcher EC, Omary MB. 2011. Absence of keratin 8 confers a paradoxical microflora-dependent resistance to apoptosis in the colon. Proc Natl Acad Sci 108: 1445–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He T, Stepulak A, Holmstrom TH, Omary MB, Eriksson JE. 2002. The intermediate filament protein keratin 8 is a novel cytoplasmic substrate for c-Jun N-terminal kinase. J Biol Chem 277: 10767–10774. [DOI] [PubMed] [Google Scholar]
- Helfand BT, Mendez MG, Murthy SN, Shumaker DK, Grin B, Mahammad S, Aebi U, Wedig T, Wu YI, Hahn KM, et al. 2011. Vimentin organization modulates the formation of lamellipodia. Mol Biol Cell 22: 1274–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hembrough TA, Vasudevan J, Allietta MM, Glass WF 2nd, Gonias SL. 1995. A cytokeratin 8-like protein with plasminogen-binding activity is present on the external surfaces of hepatocytes, HepG2 cells and breast carcinoma cell lines. J Cell Sci 108: 1071–1082. [DOI] [PubMed] [Google Scholar]
- Herrmann H, Strelkov SV, Burkhard P, Aebi U. 2009. Intermediate filaments: Primary determinants of cell architecture and plasticity. J Clin Invest 119: 1772–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YL, Wu CM, Shi GY, Wu GC, Lee H, Jiang MJ, Wu HL, Yang HY. 2009. Nestin serves as a prosurvival determinant that is linked to the cytoprotective effect of epidermal growth factor in rat vascular smooth muscle cells. J Biochem 146: 307–315. [DOI] [PubMed] [Google Scholar]
- Huber F, Boire A, Lopez MP, Koenderink GH. 2015. Cytoskeletal crosstalk: When three different personalities team up. Curr Opin Cell Biol 32: 39–47. [DOI] [PubMed] [Google Scholar]
- Hudson LG, Newkirk KM, Chandler HL, Choi C, Fossey SL, Parent AE, Kusewitt DF. 2009. Cutaneous wound reepithelialization is compromised in mice lacking functional Slug (Snai2). J Dermatol Sci 56: 19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyder CL, Pallari H-M, Kochin V, Eriksson JE. 2008. Providing cellular signposts—Post-translational modifications of intermediate filaments. FEBS Lett 582: 2140–2148. [DOI] [PubMed] [Google Scholar]
- Hyder CL, Lazaro G, Pylvanainen JW, Roberts MW, Qvarnstrom SM, Eriksson JE. 2014. Nestin regulates prostate cancer cell invasion by influencing the localisation and functions of FAK and integrins. J Cell Sci 127: 2161–2173. [DOI] [PubMed] [Google Scholar]
- Hyder CL, Kemppainen K, Isoniemi KO, Imanishi SY, Goto H, Inagaki M, Fazeli E, Eriksson JE, Tornquist K. 2015. Sphingolipids inhibit vimentin-dependent cell migration. J Cell Sci 128: 2057–2069. [DOI] [PubMed] [Google Scholar]
- Inada H, Izawa I, Nishizawa M, Fujita E, Kiyono T, Takahashi T, Momoi T, Inagaki M. 2001. Keratin attenuates tumor necrosis factor-induced cytotoxicity through association with TRADD. J Cell Biol 155: 415–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivaska J. 2011. Vimentin: Central hub in EMT induction? Small GTPases 2: 51–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivaska J, Pallari HM, Nevo J, Eriksson JE. 2007. Novel functions of vimentin in cell adhesion, migration, and signaling. Exp Cell Res 313: 2050–2062. [DOI] [PubMed] [Google Scholar]
- *.Jacob JT, Coulomb PA, Kwan R, Omary MB. 2016. Types I and II keratin intermediate filaments. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a018275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiu Y, Lehtimaki J, Tojkander S, Cheng F, Jaalinoja H, Liu X, Varjosalo M, Eriksson JE, Lappalainen P. 2015. Bidirectional interplay between vimentin intermediate filaments and contractile actin stress fibers. Cell Rep 11: 1511–1518. [DOI] [PubMed] [Google Scholar]
- *.Jones JCR, Kam CY, Harmon RM, Woychek AV, Hopkinson SB, Green KJ. 2016. Intermediate filaments and the plasma membrane. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a025866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalluri R, Weinberg RA. 2009. The basics of epithelial–mesenchymal transition. J Clin Invest 119: 1420–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaji N, Bandoh S, Ishii T, Fujita J, Ishida T, Matsunaga T, Kubo A. 2011. Cytokeratins negatively regulate the invasive potential of lung cancer cell lines. Oncol Rep 26: 763–768. [DOI] [PubMed] [Google Scholar]
- Kang H, Kwak HI, Kaunas R, Bayless KJ. 2011. Fluid shear stress and sphingosine 1-phosphate activate calpain to promote membrane type 1 matrix metalloproteinase (MT1-MMP) membrane translocation and endothelial invasion into three-dimensional collagen matrices. J Biol Chem 286: 42017–42026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karantza V. 2011. Keratins in health and cancer: More than mere epithelial cell markers. Oncogene 30: 127–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd ME, Shumaker DK, Ridge KM. 2014. The role of vimentin intermediate filaments in the progression of lung cancer. Am J Respir Cell Mol Biol 50: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Wong P, Coulombe PA. 2006. A keratin cytoskeletal protein regulates protein synthesis and epithelial cell growth. Nature 441: 362–365. [DOI] [PubMed] [Google Scholar]
- King RS, Newmark PA. 2012. The cell biology of regeneration. J Cell Biol 196: 553–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku NO, Omary MB. 2006. A disease- and phosphorylation-related nonmechanical function for keratin 8. J Cell Biol 174: 115–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku NO, Liao J, Omary MB. 1998a. Phosphorylation of human keratin 18 serine 33 regulates binding to 14-3-3 proteins. EMBO J 17: 1892–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku NO, Michie SA, Soetikno RM, Resurreccion EZ, Broome RL, Omary MB. 1998b. Mutation of a major keratin phosphorylation site predisposes to hepatotoxic injury in transgenic mice. J Cell Biol 143: 2023–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku NO, Strnad P, Zhong BH, Tao GZ, Omary MB. 2007. Keratins let liver live: Mutations predispose to liver disease and crosslinking generates Mallory–Denk bodies. Hepatology 46: 1639–1649. [DOI] [PubMed] [Google Scholar]
- Ku NO, Toivola DM, Strnad P, Omary MB. 2010. Cytoskeletal keratin glycosylation protects epithelial tissue from injury. Nat Cell Biol 12: 876–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak HI, Kang H, Dave JM, Mendoza EA, Su SC, Maxwell SA, Bayless KJ. 2012. Calpain-mediated vimentin cleavage occurs upstream of MT1-MMP membrane translocation to facilitate endothelial sprout initiation. Angiogenesis 15: 287–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leduc C, Etienne-Manneville S. 2015. Intermediate filaments in cell migration and invasion: The unusual suspects. Curr Opin Cell Biol 32: 102–112. [DOI] [PubMed] [Google Scholar]
- Lepekhin EA, Eliasson C, Berthold CH, Berezin V, Bock E, Pekny M. 2001. Intermediate filaments regulate astrocyte motility. J Neurochem 79: 617–625. [DOI] [PubMed] [Google Scholar]
- Liu T, Guevara OE, Warburton RR, Hill NS, Gaestel M, Kayyali US. 2010. Regulation of vimentin intermediate filaments in endothelial cells by hypoxia. Am J Physiol Cell Physiol 299: C363–C373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Zhang Y, Hao J, Liu S, Liu Q, Zhao S, Shi Y, Duan H. 2012. Nestin protects mouse podocytes against high glucose-induced apoptosis by a Cdk5-dependent mechanism. J Cell Biochem 113: 3186–3196. [DOI] [PubMed] [Google Scholar]
- Liu W, Zhang Y, Liu S, Liu Q, Hao J, Shi Y, Zhao S, Duan H. 2013. The expression of intermediate filament protein nestin and its association with cyclin-dependent kinase 5 in the glomeruli of rats with diabetic nephropathy. Am J Med Sci 345: 470–477. [DOI] [PubMed] [Google Scholar]
- Lombardi ML, Jaalouk DE, Shanahan CM, Burke B, Roux KJ, Lammerding J. 2011. The interaction between nesprins and sun proteins at the nuclear envelope is critical for force transmission between the nucleus and cytoskeleton. J Biol Chem 286: 26743–26753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long HA, Boczonadi V, McInroy L, Goldberg M, Maatta A. 2006. Periplakin-dependent re-organisation of keratin cytoskeleton and loss of collective migration in keratin-8-downregulated epithelial sheets. J Cell Sci 119: 5147–5159. [DOI] [PubMed] [Google Scholar]
- Lopez-Novoa JM, Nieto MA. 2009. Inflammation and EMT: An alliance towards organ fibrosis and cancer progression. EMBO Mol Med 1: 303–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundkvist A, Reichenbach A, Betsholtz C, Carmeliet P, Wolburg H, Pekny M. 2004. Under stress, the absence of intermediate filaments from Muller cells in the retina has structural and functional consequences. J Cell Sci 117: 3481–3488. [DOI] [PubMed] [Google Scholar]
- Lutolf MP, Hubbell JA. 2005. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol 23: 47–55. [DOI] [PubMed] [Google Scholar]
- Lynch CD, Lazar AM, Iskratsch T, Zhang X, Sheetz MP. 2013. Endoplasmic spreading requires coalescence of vimentin intermediate filaments at force-bearing adhesions. Mol Biol Cell 24: 21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McInroy L, Maatta A. 2007. Down-regulation of vimentin expression inhibits carcinoma cell migration and adhesion. Biochem Biophys Res Commun 360: 109–114. [DOI] [PubMed] [Google Scholar]
- Mendez MG, Kojima SI, Goldman RD. 2010. Vimentin induces changes in cell shape, motility, and adhesion during the epithelial to mesenchymal transition. FASEB J 24: 1838–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menko AS, Bleaken BM, Libowitz AA, Zhang L, Stepp MA, Walker JL. 2014. A central role for vimentin in regulating repair function during healing of the lens epithelium. Mol Biol Cell 25: 776–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misiorek JO, Lahdeniemi IA, Nystrom JH, Paramonov VM, Gullmets JA, Saarento H, Rivero-Muller A, Husoy T, Taimen P, Toivola DM. 2016. Keratin 8-deletion induced colitis predisposes to murine colorectal cancer enforced by the inflammasome and IL-22 pathway. Carcinogenesis 37: 777–786. [DOI] [PubMed] [Google Scholar]
- Mokry J, Cizkova D, Filip S, Ehrmann J, Osterreicher J, Kolar Z, English D. 2004. Nestin expression by newly formed human blood vessels. Stem Cells Dev 13: 658–664. [DOI] [PubMed] [Google Scholar]
- Mokry J, Ehrmann J, Karbanova J, Cizkova D, Soukup T, Suchanek J, Filip S, Kolar Z. 2008. Expression of intermediate filament nestin in blood vessels of neural and non-neural tissues. Acta Medica 51: 173–179. [DOI] [PubMed] [Google Scholar]
- Moll R, Divo M, Langbein L. 2008. The human keratins: Biology and pathology. Histochem Cell Biol 129: 705–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JT, Pfeiffer ER, Thirkill TL, Kumar P, Peng G, Fridolfsson HN, Douglas GC, Starr DA, Barakat AI. 2011. Nesprin-3 regulates endothelial cell morphology, perinuclear cytoskeletal architecture, and flow-induced polarization. Mol Biol Cell 22: 4324–4334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieminen M, Henttinen T, Merinen M, Marttila-Ichihara F, Eriksson JE, Jalkanen S. 2006. Vimentin function in lymphocyte adhesion and transcellular migration. Nat Cell Biol 8: 156–162. [DOI] [PubMed] [Google Scholar]
- Obermajer N, Doljak B, Kos J. 2009. Cytokeratin 8 ectoplasmic domain binds urokinase-type plasminogen activator to breast tumor cells and modulates their adhesion, growth and invasiveness. Mol Cancer 8: 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallari HM, Eriksson JE. 2006. Intermediate filaments as signaling platforms. Sci STKE 2006: e53. [DOI] [PubMed] [Google Scholar]
- Pallari HM, Lindqvist J, Torvaldson E, Ferraris SE, He T, Sahlgren C, Eriksson JE. 2011. Nestin as a regulator of Cdk5 in differentiating myoblasts. Mol Biol Cell 22: 1539–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan X, Hobbs RP, Coulombe PA. 2013. The expanding significance of keratin intermediate filaments in normal and diseased epithelia. Curr Opin Cell Biol 25: 47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinke JM, Sorg H. 2012. Wound repair and regeneration. Eur Surg Res 49: 35–43. [DOI] [PubMed] [Google Scholar]
- Rodriguez MI, Peralta-Leal A, O’Valle F, Rodriguez-Vargas JM, Gonzalez-Flores A, Majuelos-Melguizo J, Lopez L, Serrano S, de Herreros AG, Rodriguez-Manzaneque JC, et al. 2013. PARP-1 regulates metastatic melanoma through modulation of vimentin-induced malignant transformation. PLoS Genet 9: e1003531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogel MR, Soni PN, Troken JR, Sitikov A, Trejo HE, Ridge KM. 2011. Vimentin is sufficient and required for wound repair and remodeling in alveolar epithelial cells. FASEB J 25: 3873–3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotty JD, Coulombe PA. 2012. A wound-induced keratin inhibits Src activity during keratinocyte migration and tissue repair. J Cell Biol 197: 381–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahlgren CM, Mikhailov A, Hellman J, Chou YH, Lendahl U, Goldman RD, Eriksson JE. 2001. Mitotic reorganization of the intermediate filament protein nestin involves phosphorylation by cdc2 kinase. J Biol Chem 276: 16456–16463. [DOI] [PubMed] [Google Scholar]
- Sahlgren CM, Mikhailov A, Vaittinen S, Pallari HM, Kalimo H, Pant HC, Eriksson JE. 2003. Cdk5 regulates the organization of Nestin and its association with p35. Mol Cell Biol 23: 5090–5106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahlgren CM, Pallari HM, He T, Chou YH, Goldman RD, Eriksson JE. 2006. A nestin scaffold links Cdk5/p35 signaling to oxidant-induced cell death. EMBO J 25: 4808–4819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehi F, Kovacs K, Cusimano MD, Horvath E, Bell CD, Rotondo F, Scheithauer BW. 2008. Immunohistochemical expression of nestin in adenohypophysial vessels during development of pituitary infarction. J Neurosurg 108: 118–123. [DOI] [PubMed] [Google Scholar]
- Satelli A, Li SL. 2011. Vimentin in cancer and its potential as a molecular target for cancer therapy. Cell Mol Life Sci 68: 3033–3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer M, Werner S. 2008. Cancer as an overhealing wound: An old hypothesis revisited. Nat Rev Mol Cell Biol 9: 628–638. [DOI] [PubMed] [Google Scholar]
- Seltmann K, Fritsch AW, Kas JA, Magin TM. 2013a. Keratins significantly contribute to cell stiffness and impact invasive behavior. Proc Natl Acad Sci 110: 18507–18512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seltmann K, Roth W, Kroger C, Loschke F, Lederer M, Huttelmaier S, Magin TM. 2013b. Keratins mediate localization of hemidesmosomes and repress cell motility. J Invest Dermatol 133: 181–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu T, Sugawara K, Tosaka M, Imai H, Hoya K, Takeuchi T, Sasaki T, Saito N. 2006. Nestin expression in vascular malformations: A novel marker for proliferative endothelium. Neurol Med Chir 46: 111–117. [DOI] [PubMed] [Google Scholar]
- Sokmensuer LK, Sokmensuer C. 2007. Nestin expression in neoplastic, fetal, and adult pancreatic endocrine cells. Hepatogastroenterology 54: 2177–2180. [PubMed] [Google Scholar]
- Stacey SN, Sulem P, Masson G, Gudjonsson SA, Thorleifsson G, Jakobsdottir M, Sigurdsson A, Gudbjartsson DF, Sigurgeirsson B, Benediktsdottir KR, et al. 2009. New common variants affecting susceptibility to basal cell carcinoma. Nat Genet 41: 909–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz NF, Cho CF, Ablack A, Lewis JD, Manchester M. 2011. Cowpea mosaic virus nanoparticles target surface vimentin on cancer cells. Nanomedicine (Lond) 6: 351–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S, Namiki J, Shibata S, Mastuzaki Y, Okano H. 2010. The neural stem/progenitor cell marker nestin is expressed in proliferative endothelial cells, but not in mature vasculature. J Histochem Cytochem 58: 721–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Yan B, Yamanishi K, Imamura S, Coulombe PA. 1998. The two functional keratin 6 genes of mouse are differentially regulated and evolved independently from their human orthologs. Genomics 53: 170–183. [DOI] [PubMed] [Google Scholar]
- Toivola DM, Nieminen MI, Hesse M, He T, Baribault H, Magin TM, Omary MB, Eriksson JE. 2001. Disturbances in hepatic cell-cycle regulation in mice with assembly-deficient keratins 8/18. Hepatology 34: 1174–1183. [DOI] [PubMed] [Google Scholar]
- Toivola DM, Ku NO, Resurreccion EZ, Nelson DR, Wright TL, Omary MB. 2004. Keratin 8 and 18 hyperphosphorylation is a marker of progression of human liver disease. Hepatology 40: 459–466. [DOI] [PubMed] [Google Scholar]
- Toivola DM, Nakamichi I, Strnad P, Michie SA, Ghori N, Harada M, Zeh K, Oshima RG, Baribault H, Omary MB. 2008. Keratin overexpression levels correlate with the extent of spontaneous pancreatic injury. Am J Pathol 172: 882–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toivola DM, Strnad P, Habtezion A, Omary MB. 2010. Intermediate filaments take the heat as stress proteins. Trends Cell Biol 20: 79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayaraj P, Kroger C, Reuter U, Windoffer R, Leube RE, Magin TM. 2009. Keratins regulate protein biosynthesis through localization of GLUT1 and -3 upstream of AMP kinase and Raptor. J Cell Biol 187: 175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virtakoivu R, Mai A, Mattila E, De Franceschi N, Imanishi SY, Corthals G, Kaukonen R, Saari M, Cheng F, Torvaldson E, et al. 2015. Vimentin-ERK signaling uncouples Slug gene regulatory function. Cancer Res 75: 2349–2362. [DOI] [PubMed] [Google Scholar]
- Vuoriluoto K, Haugen H, Kiviluoto S, Mpindi JP, Nevo J, Gjerdrum C, Tiron C, Lorens JB, Ivaska J. 2011. Vimentin regulates EMT induction by Slug and oncogenic H-Ras and migration by governing Axl expression in breast cancer. Oncogene 30: 1436–1448. [DOI] [PubMed] [Google Scholar]
- Wawersik MJ, Mazzalupo S, Nguyen D, Coulombe PA. 2001. Increased levels of keratin 16 alter epithelialization potential of mouse skin keratinocytes in vivo and ex vivo. Mol Biol Cell 12: 3439–3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber CE, Li NY, Wai PY, Kuo PC. 2012a. Epithelial–mesenchymal transition, TGF-β, and osteopontin in wound healing and tissue remodeling after injury. J Burn Care Res 33: 311–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber GF, Bjerke MA, DeSimone DW. 2012b. A mechanoresponsive cadherin-keratin complex directs polarized protrusive behavior and collective cell migration. Dev Cell 22: 104–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn TA, Ramalingam TR. 2012. Mechanisms of fibrosis: Therapeutic translation for fibrotic disease. Nat Med 18: 1028–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneda K, Furukawa T, Zheng YJ, Momoi T, Izawa I, Inagaki M, Manabe M, Inagaki N. 2004. An autocrine/paracrine loop linking keratin 14 aggregates to tumor necrosis factor α-mediated cytotoxicity in a keratinocyte model of epidermolysis bullosa simplex. J Biol Chem 279: 7296–7303. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Ji X, Chen L, Greenberg HB, Lu SC, Omary MB. 2005. Keratin mutation primes mouse liver to oxidative injury. Hepatology 41: 517–525. [DOI] [PubMed] [Google Scholar]