Abstract
In-vitro maturation (IVM) of the immature oocytes recovered from the surgically removed ovarian tissue has been considered as a process for fertility preservation in patients with cancer. Fertility preservation for a woman with Mullerian adenocarcinoma. A 37-year-old woman with Mullerian adenocarcinoma was a candidate for ovarian resection. The immature oocytes were retrieved after ovarian resection from a 37-year-old woman with Mullerian adenocarcinoma. The oocytes underwent IVM and were fertilized using intracytoplasmic sperm injection (ICSI). Two healthy embryos were cryopreserved for future use. The immature oocytes from the ovarian tissue can be matured with IVM for generation of embryos after ICSI. The embryos can be vitrified using routine methods for fertility preservation in young women with cancer.
KEYWORDS: Cancer, embryo vitrification, fertility preservation, in-vitro maturation
INTRODUCTION
Worldwide, the number of new cases with cancer is increasing annually, and 8% of these cases are reported from those younger than 40 years.[1] It has been stated that 50% of the young patients with cancer wish to have children after recovery.[2] Besides cancer treatment, fertility preservation is an important issue in young women with cancer. The classical therapy for these patients is surgical resection, which makes it challenging for future pregnancies. Furthermore, it is known that supporting treatments such as radiation and chemotherapeutic agents are gonadotoxic, leading to premature ovarian failure (POF).[1,2]
Cryopreservation of both the ovarian tissue and the oocyte is possible; however, ovarian tissue cryopreservation is still an investigational method for preserving future fertility without a defined sex partner. Recently, with the developing gamete cryopreservation techniques, the survival rate of the frozen–thawed oocyte and the pregnancy rate have been increased to 94% and 46.7%, respectively.[3] Embryo cryopreservation is a routine and recognized method for women with a stable partner in assisted reproduction programs. For this purpose, ovarian stimulation and oocyte collection followed by intracytoplasmic sperm injection (ICSI) are needed. This procedure may, however, postpone the cancer treatment for 3–5 weeks, and this may be harmful in a number of women with hormone-dependent cancers. Alternatively, we did attempt another option by collecting the immature oocytes from the ovarian biopsy tissue followed by in-vitro maturation (IVM) in a patient with cervical cancer.
CASE PRESENTATION
A 37-year-old woman (gravida 1, para 1, and aborta 0) was admitted to our oncology clinic with chief complaints of abnormal vaginal bleeding and vaginal mass. No important familial or medical history and medication were noted. Menarche happened at the age of 14 years and continued by regular menses. She underwent a diagnostic curettage during the previous summer and was diagnosed with cervical polyp. No malignant pathological finding was detected, and the patient was treated by surgical polypectomy three times. She was then referred to our clinic 5 months later due to cervical polyp occurrence. A physical examination showed a cervical polypoid mass and the growth of the tumor into the vagina. A transabdominal ultrasonography demonstrated the cervix as larger than normal and an endometrial lining of 7 mm. A round mass was occupying the cervix with extension to the vagina. The bilateral ovaries were normal in size and shape. Computed tomography revealed a 33-mm, smooth, heterogeneous mass involving the cervix without tumor extension into the bladder or the rectum. The tubes, the ovaries, and the upper abdomen were free of tumor. The laboratory findings including cancer antigen (CA-125), carcinogenic antigen (CA 19-9), carcinoembriogenic antigen, and alpha-fetoprotein were within normal range as 3.8 U/mL, 2.9 U/mL, 0.5 mcg/L, and 0.8 ng/mL, respectively. Consequently, cervical biopsy demonstrated Mullerian adenosarcoma.
The patient and her husband were counseled about the fertility preservation options. They decided to undergo hysterectomy and bilateral salpingectomy. According to the frozen section procedures, no pathological report was mentioned in both the ovaries. The right ovary was saved, and an ovarian wedge resection in the left ovary was performed. A 2 cm × 2 cm × 0.5 cm piece of the ovary in Ham’s F10–HEPES medium at 4°C was transferred to the laboratory [Figure 1]. All detectable antral follicles on the ovarian surface were aspirated with a 20-gauge needle attached to a 10 mL syringe. The extracts were washed in Ham’s F-10–HEPES medium and then searched for the presence of the immature germinal vesicle (GV) oocytes under a dissecting microscope. After follicle aspiration, the ovarian cortex was isolated from the basic stromal tissue in the culture medium and cut into three (2 × 5 × 1) pieces [Figure 2]. Three detected GV oocytes were cultured in a maturation medium (SAGE IVF) supplemented with 75 mIU/mL follicle stimulating hormone (FSH) and 75 mIU/mL luteinizing hormone (LH) (Ferring) at 37°C in an atmosphere of 5% CO2. At 24 h post-IVM, the oocytes were screened for the presence of the first polar body (PB) by a stereo-microscope (Olympus, Japan); the oocytes did not show any sign of maturation [Figure 3]. In consequence additional 24 h, two oocytes reached to Metaphase II stage. ICSI was performed after placing the first PB at the 6 o’clock position using a sperm from the patient’s husband. The injected oocytes were incubated in the droplets of G1 (Vitrolife Co., Sweden) for the subsequent analysis. Fertilization assessment was checked after 16–18 h post-ICSI. Two embryos with grades B and C were cryopreserved using Rapid Vit Cleave (Vitrolife, Goteborg, Sweden) as per instruction. The embryos were then loaded on the cryotop, and the samples were immediately submerged into liquid nitrogen for post-treatment fertility options. Cryopreservation of the ovarian tissue was based on an earlier published method.[4]
Figure 1.
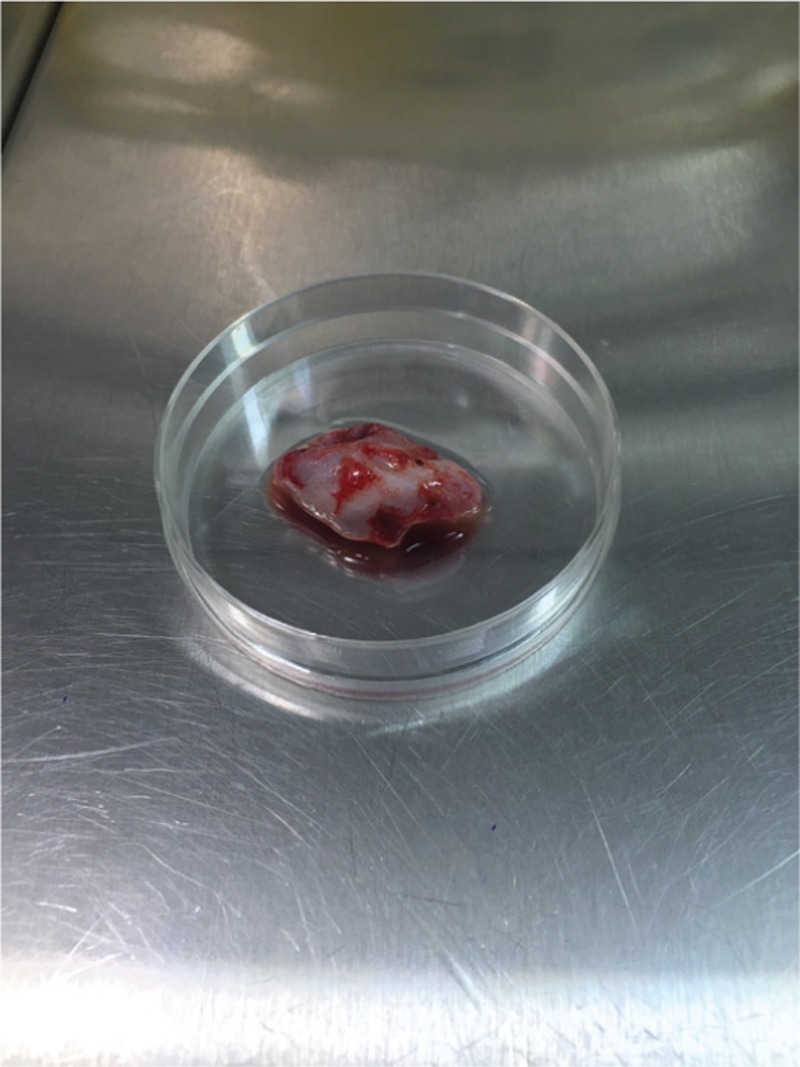
Extracted ovarian tissue
Figure 2.
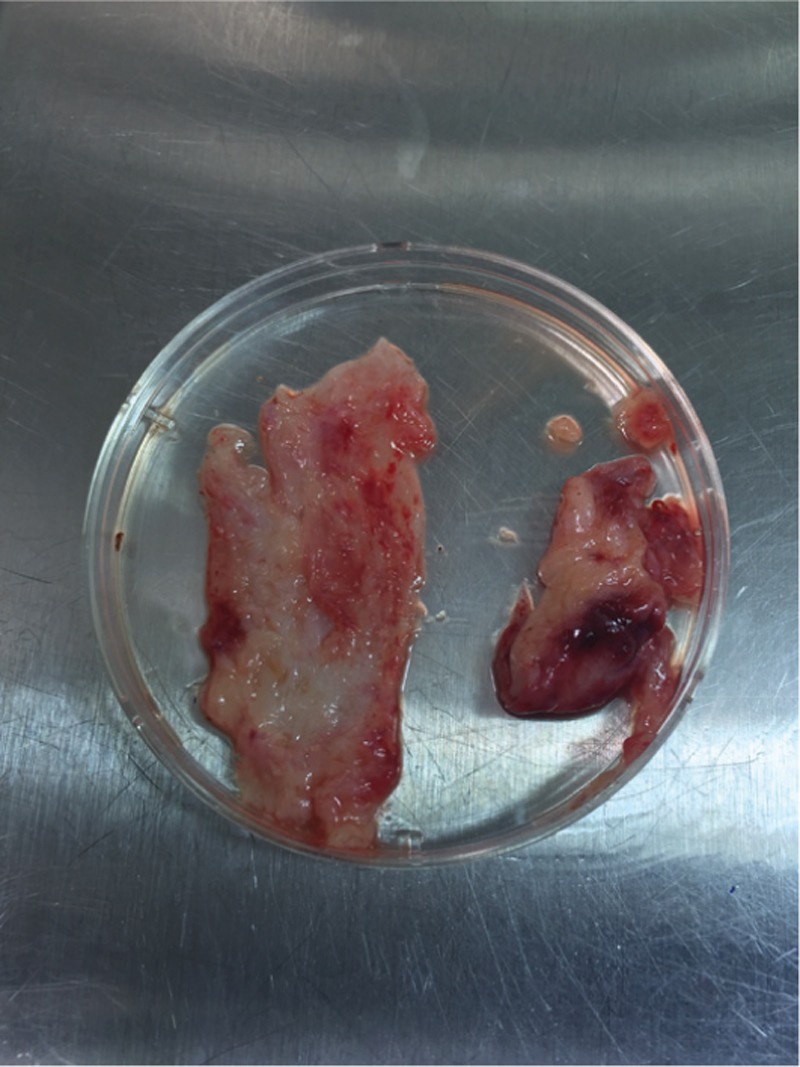
Ovarian cortex isolated from the basic stromal tissue
Figure 3.
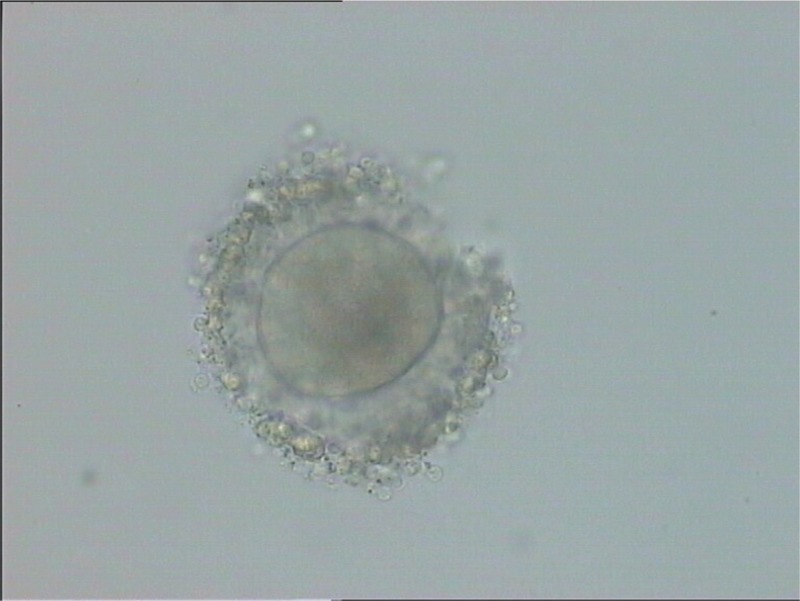
Immature oocytes obtained from ovarian tissue
A written informed consent was obtained from the patient. This study was approved by the Ethics Committee of our institution.
DISCUSSION
It is now accepted that fertility preservation is a safe and valuable option among women in reproductive age with early diagnosed and entirely treatable cancers. Currently, some cryopreservation methods such as ovarian tissue freezing are considered as experimental techniques for preserving future fertility. Furthermore, in some cases, these procedures have been applied in combination with IVM and embryo cryopreservation. The aim of ovarian tissue freezing is to cryopreserve the primordial follicles in the ovarian cortex. The thawed ovarian tissue can be transplanted after cancer remission. Across the world, the usage of this method resulted in at least 40 live births after ovarian tissue transplantation.[5] However, recurrence of the disease may occur following grafting of the ovarian tissue.[6] Another option is to aspirate the antral follicles from the ovarian cortex followed by IVM. The puncture of the immature oocytes allows immediate cancer treatment without ovarian stimulation protocol as well as the ability to retrieve the oocyte at any time of the menstrual cycle. The number of the immature oocytes retrieved from the ovarian tissue depends on the patients’ age, the size of the excised ovarian tissue, and the day of the menstrual cycle.[7]
This case report illustrates the vitrification of two good quality embryos by collecting the GV oocytes from the ovarian tissue followed by IVM in the woman with Mullerian adenosarcoma. To our knowledge, this is the first reported case of oocyte maturation in vitro and cryopreservation of embryos for fertility preservation during surgery for cervical cancer treatment in Iran. The collection of the immature oocytes during ovarian tissue cryopreservation was reported for the first time by Revel et al.[8] In their study, the maturation rate was 50%, and the MII oocytes were inseminated by donor sperms through ICSI, and the harvested embryos were cryopreserved. Isachenko et al.[9] applied IVM for the immature oocytes retrieved through ovarian tissue excision in two cases with Hodgkin lymphoma and cervical cancer and reported 56.6% maturation rate. Slow freezing was used for cryopreservation of 90% of the matured oocytes.[9] Maturation in vitro and oocyte freezing is also a promising approach for fertility preservation among young women with mosaic Turner syndrome.[10]
In a case series, four patients suffering from different types of cancer had undertaken ovarian tissue freezing for the preservation of future fertility. Following IVM, 79% of the immature oocytes reached maturity.[7] Fadini et al.[11] reported the transfer of a single embryo for a woman after the treatment of ovarian adenocarcinoma, with no result of viable pregnancy. This fertilized egg was achieved after insemination of a frozen–thawed oocyte after IVM. Recently, the first live birth was noted from a cryopreserved embryo, which was obtained from an in-vitro matured oocyte after oophorectomy in a patient with ovarian cancer.[12] Moreover, a healthy live birth from aspiration of the immature oocytes followed by IVM and a frozen–thawed embryo transfer after 5 years was documented by Uzelac and colleagues.[13] These results suggest that the GV oocytes from unstimulated ovaries can successfully undergo IVM in selected patients with cancer who are at risk for POF.
CONCLUSION
In conclusion, our study showed that the immature oocytes from the ovarian tissue could undergo IVM for the generation of embryos after ICSI. The embryos could then be vitrified using routine methods for fertility preservation in young women with cancer.
Financial support and sponsorship
This study was supported financially by the research grant of the Research Infertility Center, Yazd Reproductive Sciences Institute, Shahid Sadoughi University of Medical Sciences.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Campos JR, Rosa ES. Cryopreservation and fertility: Current and prospective possibilities for female cancer patients. ISRN Obstet Gynecol. 2011;2011:350813. doi: 10.5402/2011/350813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Imbert R, Moffa F, Tsepelidis S, Simon P, Delbaere A, Devreker F, et al. Safety and usefulness of cryopreservation of ovarian tissue to preserve fertility: A 12-year retrospective analysis. Hum Reprod. 2014;29:1931–40. doi: 10.1093/humrep/deu158. [DOI] [PubMed] [Google Scholar]
- 3.Chian RC, Son WY, Huang JY, Cui SJ, Buckett WM, Tan SL. High survival rates and pregnancies of human oocytes following vitrification: Preliminary report. Fertil Steril. 2005;84:S36. [Google Scholar]
- 4.Kagawa N, Silber S, Kuwayama M. Successful vitrification of bovine and human ovarian tissue. Reprod Biomed Online. 2009;18:568–77. doi: 10.1016/s1472-6483(10)60136-8. [DOI] [PubMed] [Google Scholar]
- 5.Anderson RA, Mitchell RT, Kelsey TW, Spears N, Telfer EE, Wallace WH. Cancer treatment and gonadal function: Experimental and established strategies for fertility preservation in children and young adults. Lancet Diabet Endocrinol. 2015;3:556–67. doi: 10.1016/S2213-8587(15)00039-X. [DOI] [PubMed] [Google Scholar]
- 6.Bastings L, Beerendonk CC, Westphal JR, Massuger LF, Kaal SE, van Leeuwen FE, et al. Autotransplantation of cryopreserved ovarian tissue in cancer survivors and the risk of reintroducing malignancy: A systematic review. Hum Reprod Update. 2013;19:483–506. doi: 10.1093/humupd/dmt020. [DOI] [PubMed] [Google Scholar]
- 7.Huang JY, Tulandi T, Holzer H, Tan SL, Chian RC. Combining ovarian tissue cryobanking with retrieval of immature oocytes followed by in vitro maturation and vitrification: An additional strategy of fertility preservation. Fertil Steril. 2008;89:567–72. doi: 10.1016/j.fertnstert.2007.03.090. [DOI] [PubMed] [Google Scholar]
- 8.Revel A, Koler M, Simon A, Lewin A, Laufer N, Safran A. Oocyte collection during cryopreservation of the ovarian cortex. Fertil Steril. 2003;79:1237–9. doi: 10.1016/s0015-0282(02)04963-4. [DOI] [PubMed] [Google Scholar]
- 9.Isachenko E, Rahimi G, Isachenko V, Nawroth F. In-vitro maturation of germinal-vesicle oocytes and cryopreservation in metaphase I/II: A possible additional option to preserve fertility during ovarian tissue cryopreservation. Reprod Biomed Online. 2004;8:553–7. doi: 10.1016/s1472-6483(10)61102-9. [DOI] [PubMed] [Google Scholar]
- 10.Huang JY, Tulandi T, Holzer H, Lau NM, Macdonald S, Tan SL, et al. Cryopreservation of ovarian tissue and in vitro matured oocytes in a female with mosaic Turner syndrome: Case report. Hum Reprod. 2008;23:336–9. doi: 10.1093/humrep/dem307. [DOI] [PubMed] [Google Scholar]
- 11.Fadini R, Dal Canto M, Mignini Renzini M, Milani R, Fruscio R, Cantu MG, et al. Embryo transfer following in vitro maturation and cryopreservation of oocytes recovered from antral follicles during conservative surgery for ovarian cancer. J Assist Reprod Genet. 2012;29:779–81. doi: 10.1007/s10815-012-9768-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prasath EB, Chan ML, Wong WH, Lim CJ, Tharmalingam MD, Hendricks M, et al. First pregnancy and live birth resulting from cryopreserved embryos obtained from in vitro matured oocytes after oophorectomy in an ovarian cancer patient. Hum Reprod. 2014;29:276–8. doi: 10.1093/humrep/det420. [DOI] [PubMed] [Google Scholar]
- 13.Uzelac PS, Delaney AA, Christensen GL, Bohler HC, Nakajima ST. Live birth following in vitro maturation of oocytes retrieved from extracorporeal ovarian tissue aspiration and embryo cryopreservation for 5 years. Fertil Steril. 2015;104:1258–60. doi: 10.1016/j.fertnstert.2015.07.1148. [DOI] [PubMed] [Google Scholar]


