Abstract
Background
Adipokines are elaborated by adipose tissue and are associated with glycemic, lipid, and vascular traits. We hypothesized that in a cross‐sectional analysis circulating adipokines are altered among subsets of obesity stratified by presence versus absence of metabolic syndrome (MetS) and prospectively predict the incidence of MetS.
Methods and Results
Participants in the community‐based Framingham Third Generation Cohort who attended examination cycle 1 were included in the study (2002–2005; N=3777, mean age, 40 years; 59% women). Circulating adiponectin, leptin, leptin receptor, fetuin‐A, fatty acid–binding protein 4, and retinol binding protein 4 were assayed and related to incident MetS in follow‐up (mean 6 years). The adipokines were compared among individuals with excess body weight (body mass index ≥25 kg/m2) and prevalent MetS, excess body weight without MetS (metabolically healthy obese), and normal‐weight with MetS (metabolically obese, normal‐weight) with normal‐weight participants without MetS as a referent. Metabolically healthy obese individuals (n=1467) had higher circulating levels of fetuin‐A and fatty acid–binding protein 4 but lower levels of leptin, leptin receptor, and adiponectin (P<0.001 for all). The adipokine panel was associated with incident MetS (263 new‐onset cases; P=0.002). Higher circulating concentrations of retinol‐binding protein 4 and fetuin‐A were associated with incidence of MetS (odds ratio per 1‐SD increment log marker, 1.21; 95% CI, 1.03–1.41 [P=0.02] and 1.17; 95% CI, 1.01–1.34 [P=0.03], respectively).
Conclusions
In our community‐based sample of young to middle‐aged adults, metabolically healthy obese individuals demonstrated an adverse adipokine profile. Higher circulating levels of retinol‐binding protein 4 and fetuin‐A marked future cardiometabolic risk.
Keywords: adipokine, epidemiology, metabolic syndrome, obesity, risk factor
Subject Categories: Diabetes, Type 2; Epidemiology; Obesity; Risk Factors
Clinical Perspective
What is New?
Since the association between obesity and MetS traits is variable, we explored the association between circulating adipokines and future incidence of MetS.
We observed that higher levels of RBP4 and fetuin‐A were associated with future incidence of MetS.
What are the Clinical Implications?
With future investigation, circulating adipokines may serve to identify persons at high risk of MetS.
Future studies may also explore the role of adipokines in the pathophysiology of MetS and the association between MetS and atherosclerotic events.
Introduction
Metabolic syndrome (MetS) is associated with an increased risk of diabetes mellitus (DM) and cardiovascular disease (CVD), likely attributable to its constituent constellation of risk factors.1, 2 In addition, there is significant variation in cardiometabolic risk among individuals with similar body mass index (BMI). While some persons with excess weight may have profound metabolic derangements, nearly 40% of obese individuals have a normal metabolic profile (so‐called metabolically healthy obese [MHO]).3 Conversely, some normal‐weight persons may have substantial metabolic derangements (termed metabolically obese, normal weight [MONW]). Since BMI alone cannot consistently predict cardiometabolic risk, researchers have focused on variations in adipose tissue endocrine function to understand the pathogenesis of cardiometabolic risk in obese individuals.4, 5 Adipokines are biologically active compounds elaborated by adipose tissue, with examples including leptin, its counter‐regulatory circulating receptor (LEP‐R), adiponectin, fetuin‐A, fatty acid–binding protein 4 (FABP4), and retinol‐binding protein 4 (RBP4).6 Investigators have reported that some adipokines individually predict cardiometabolic risk, but, to our knowledge, the prospective association of a panel of adipokines for MetS in both MHO and MONW groups has not been previously evaluated.6 We hypothesized that in a cross‐sectional analysis both MHO and MONW individuals have an altered circulating adipokine profile compared with a referent group with normal BMI (<25 kg/m2) and without MetS. We also postulated that in a prospective analysis, an adipokine profile characterized by higher circulating levels of fetuin‐A, FABP4, RBP4, and LEP‐R but lower concentrations of adiponectin and leptin will be prospectively associated with a greater incidence of MetS. We tested these hypotheses using a multimarker adipokine panel in a community‐based sample of young to middle‐aged adults.
Methods
Study Procedures
The design and the selection criteria of the Third Generation Cohort of the Framingham Heart Study have been detailed elsewhere.7 The institutional review board of Boston University approved the study protocol, and all study participants provided written informed consent. At each Framingham Heart Study examination, attendees underwent a routine medical history with a standardized physical examination including anthropometry and blood pressure (BP) measurement. After the participant rested in a seated position for 10 minutes, a physician measured the participant's BP using a standardized protocol with mercury column sphygmomanometer and an appropriately sized cuff. The average of 2 readings constituted the examination BP. Self‐reported use of cigarettes within the year preceding the baseline examination was defined as current smoking.
Attendees underwent laboratory assessment of other risk factors in a fasting state. Phlebotomy was performed after participants rested for 10 minutes in the supine position. Specimens were stored at −80°C without freeze‐thaw cycles until assay. Fasting levels of plasma high‐density lipoprotein cholesterol, triglycerides, insulin, and glucose were measured using standardized assays. Plasma levels of fetuin‐A and FABP4 were measured using sandwich ELISA (BioVendor Reasearch and Diagnostic Products). Plasma levels of leptin, LEP‐R, RBP4, and adiponectin were measured using ELISA (R&D Systems). The average interassay coefficients of variation for the biomarkers were as follows: leptin 4.97%, LEP‐R 4.01%, FABP4 2.38%, RBP4 2.18%, fetuin‐A 2.52%, and adiponectin 2.23%.
Study Samples
For the present study, participants who attended the first examination cycle (2002–2005) were eligible. Of 4095 participants who attended the first baseline examination, 221 individuals were excluded for having missing adipokine data, 53 for missing covariates, 44 for prevalent CVD (defined as coronary heart disease, congestive heart failure, intermittent claudication, or cerebrovascular disease), and one for missing serum creatinine. The remaining 3777 participants (sample 1) were included in the cross‐sectional adipokine analyses (Figure 1).
Figure 1.
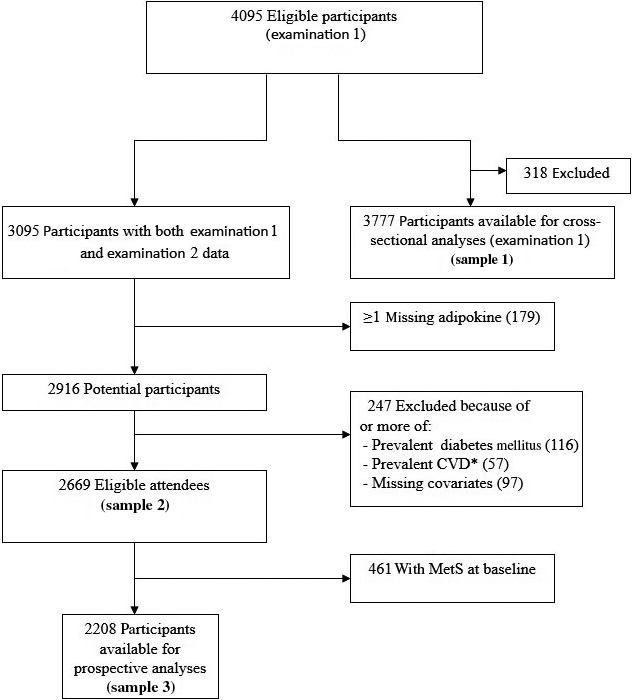
Flow diagram of respective sample inclusion/exclusion criteria. CVD indicates cardiovascular disease; MetS, metabolic syndrome.
For the prospective analyses, we used a 2‐step analysis where longitudinal associations were identified between adipokines and incident MetS at the follow‐up examination in participants without MetS at the baseline examination. Adipokines identified at this step were examined for longitudinal associations with individual change in components of MetS measured between the baseline and follow‐up examinations. Of examination cycle 1 attendees, 2669 participants subsequently attended examination cycle 2 (2008–2011), a mean of 6 years after the baseline examination (sample 2), and were examined for associations with the individual MetS traits. Of sample 2, a total of 461 participants were excluded for prevalent MetS at the baseline examination, prevalent CVD, prevalent DM (use of antiglycemic agents or fasting plasma glucose ≥100 mg/dL), serum creatinine >2.0 mg/dL, missing adipokine data, or missing covariates, leaving 2208 participants (sample 3) eligible for analyses relating adipokine concentrations to the incidence of MetS.
Outcome Definition
Using the National Cholesterol Education Program Adult Treatment Panel III guidelines, MetS was defined as the presence of 3 or more of the following: elevated BP (≥130 mm Hg systolic, ≥85 mm Hg diastolic) or treatment for high BP; hypertriglyceridemia (≥150 mg/dL) or treatment with lipid‐lowering treatment; low high‐density lipoprotein cholesterol (<40 mg/dL in men, <50 mg/dL in women); hyperglycemia (fasting glucose ≥100 mg/dL) or treatment with oral hypoglycemic agents or insulin; and increased waist circumference (≥102 cm for men, ≥88 cm for women).1, 2
Statistical Analyses
Adipokine concentrations were standardized within sex (mean=0, SD=1) to account for sex differences in their distributions. Pairwise Pearson correlation coefficients were calculated to assess the relations among the adipokines. The homeostasis model assessment insulin resistance index (HOMA‐IR) was calculated using the formula: fasting insulin×fasting glucose/22.5.8
Cross‐sectional analyses
Participants were grouped according to BMI <25 kg/m2 versus ≥25 kg/m2 (normal weight and overweight, respectively). Participants were divided into 4 groups: (1) absence of MetS and BMI <25 kg/m2; (2) presence of MetS and BMI <25 kg/m2 (MONW); (3) absence of MetS and BMI ≥25 kg/m2 (MHO); and (4) presence of MetS and BMI ≥25 kg/m2 (at risk for overweight). Using the absence of MetS and BMI <25 kg/m2 as the referent group, we compared the mean levels of adipokines among the 4 groups.
Longitudinal analyses: multimarker panel and incidence of MetS
We used a conservative 2‐step multimarker strategy to relate the adipokines (modeled as sex‐standardized continuous variables) to the incidence of MetS in participants in sample 3. First, we used multivariable logistic regression to relate the entire panel of adipokines to incident MetS. We adjusted for age, sex, and baseline MetS component levels, which included waist circumference. We estimated a chi‐square test–derived global P value using a likelihood ratio test examining the difference between the −2 log‐likelihood models of clinical covariates alone versus clinical covariates plus all adipokines as a panel. If the panel global P value was <0.05, we then used backward elimination (threshold of P<0.05 for retention in the model) to select a final parsimonious set of “informative” adipokines.
In secondary analyses, to explore whether the association of adipokines with incident MetS was confounded by insulin resistance, HOMA‐IR was added as an additional covariate to the multivariable model. A receiver operator curve comparing the models with versus without HOMA‐IR in the prediction of MetS at follow‐up examination was constructed. The MetS prediction C statistic (area under the receiver operating characteristic curve) for participants missing HOMA‐IR data was not significantly different than those with HOMA‐IR (0.82 versus 0.83), therefore participants with missing HOMA‐IR data were included to maximize statistical power. We also used age and sex interaction terms to examine effect modification on the biomarker‐MetS relationship. To ascertain nonlinearity in the adipokine‐MetS association, we constructed restricted cubic splines with 3 knots corresponding to quartile cut points. We assessed the conjoint influence of informative adipokines on incident MetS by cross‐classifying participants according to sex‐standardized median values and determining whether individuals with values above the median (“high”) for ≥1 adipokines had a greater risk of developing MetS compared with those with the concentrations of the informative adipokines at or below the median (“low”). These models were adjusted for age, sex, baseline BMI (except for models with waist circumference as the dependent variable), and baseline level of the individual risk factor analyzed.
Longitudinal analyses: adipokines and longitudinal changes in MetS components
Finally, the association between the baseline informative adipokine levels (modeled together as continuous variables) with longitudinal changes in the levels of individual MetS components separately were examined in the 2669 participants from sample 2, excluding participants using BP‐ or lipid‐lowering treatment at baseline for BP and triglyceride analyses, respectively. In sex‐pooled multivariable logistic regression models, the change in each MetS component was the dependent variable (separate analysis for each component) and informative sex‐standardized, natural logarithmically transformed adipokines as the independent variables. For waist circumference, change was sex‐standardized. For systolic BP, diastolic BP, plasma glucose, and log‐triglycerides, we used censored normal regression to account for initiating treatment of high BP, DM, or dyslipidemia by the follow‐up examination.9 For all analyses, a 2‐sided P value of <0.05 was considered statistically significant. All analyses were performed with SAS (version 9.2, SAS Institute) and R (version 2.12).
Results
Baseline Adipokines and Clinical Characteristics
Baseline clinical characteristics of samples 1, 2, and 3 are shown in Table 1. Mean values of circulating leptin, adiponectin, and FABP4 were higher in women than in men. Mean values of fetuin‐A and RBP4 according to sex and age younger than 40 or 40 years and older among BMI and MetS groups are seen in Figure 2. Compared with nonattendees, sample 2 participants generally had lower BP, rates of obesity, triglyceride levels, proportion of smoking, and blood sugar (Table 2). Modest pairwise correlations were noted among the adipokines (r=−0.30 to +0.55; Table 3). Stronger correlations were demonstrated for leptin and HOMA‐IR or FABP4 (r=0.55 for both).
Table 1.
Baseline Characteristics of Study Sample 1
| Clinical Features | Sample 1 (n=3777) | Sample 2 (n=2669) | Sample 3 (n=2208) | |||
|---|---|---|---|---|---|---|
| Men (n=1747) | Women (n=2030) | Men (n=1202) | Women (n=1467) | Men (n=907) | Women (n=1301) | |
| Age, y | 40.1±837 | 40.0±8.8 | 40.2±8.5 | 40.1±8.7 | 39.3±8.6 | 39.5±8.6 |
| Systolic BP, mm Hg | 120±13 | 113±14 | 120±12 | 113±14 | 118±11 | 111±12 |
| Diastolic BP, mm Hg | 78±9 | 73±9 | 78±9 | 72±9 | 76±9 | 71±9 |
| BP ≥130/85 or treatment, % | 36 | 19 | 18.3 | 9.8 | 9.5 | 5.9 |
| Hypertension, % | 12.0 | 7.8 | 19.0 | 10.0 | 9.5 | 6.1 |
| Treatment for hypertension, % | 9.4 | 7.0 | 8.0 | 5.3 | 3.2 | 3.4 |
| BMI, kg/m² | 27.8±4.6 | 26.0±6.1 | 27.7±4.4 | 25.5±5.5 | 26.5±3.7 | 24.6±4.7 |
| BMI ≥30 kg/m², % | 25.0 | 20.1 | 23.8 | 17.9 | 13.6 | 12.8 |
| Weight, kg | 88.1±15.8 | 70.0±16.7 | 87.6±15.0 | 68.7±15.2 | 83.9±13.0 | 66.4±13.1 |
| Waist circumference, cm | 98.1±12.6 | 88.4±15.7 | 97.6±12.0 | 87.1±14.3 | 94.2±10.4 | 84.8±12.5 |
| Elevated waist circumference, %a | 32.5 | 41.4 | 31.0 | 39.0 | 17.4 | 31.7 |
| Plasma total cholesterol, mg/dL | 193±37 | 185±34 | 194±36 | 184±32 | 191±36 | 183±32 |
| Plasma triglycerides, mg/dL | 134±108 | 97±63 | 129±92 | 92±52 | 104±62 | 82±35 |
| Elevated triglycerides, %b | 29.4 | 13.0 | 27.3 | 10.7 | 12.7 | 4.3 |
| Plasma HDL‐C, mg/dL | 47±12 | 61±16 | 47±12 | 62±16 | 50±11 | 64±15 |
| Low HDL‐C, %c | 28.7 | 23.9 | 27.5 | 21.8 | 14.6 | 15.5 |
| Change in weight, kgd | NA | NA | 3±7 | 3±7 | 3±6 | 3±7 |
| Smoking, % | 15.9 | 14.3 | 15.6 | 13.4 | 15.8 | 12.2 |
| Fasting plasma glucose, mg/dL | 98±17 | 92±18 | 96±8 | 90±8 | 94±7 | 89±7 |
| Impaired fasting glucose, %e | 3.32 | 1.92 | 0.3 | 0.1 | 0.1 | 0.1 |
| HOMA‐IR | 1.4±1.2 | 1.1±0.9 | 1.3±0.9 | 1.0±0.7 | 1.0±0.5 | 0.9±0.5 |
| Biomarkers, median (quartile 1–3) | ||||||
| CRP mg/L | 0.90 (0.4–2.1) | 1.21 (0.5–3.4) | 0.88 (0.4–2.0) | 1.1 (0.4–3.2) | 0.7 (0.3–1.6) | 1.0 (0.4–2.7) |
| Leptin, ng/mL | 6.0 (2.3–7.4) | 18.0 (6.5–23.5) | 4.1 (2.4–7.1) | 11.6 (6.2–22.1) | 3.4 (2.0–5.5) | 10.2 (5.7–19.3) |
| Leptin receptor, ng/mL | 18.9 (12.7–23.5) | 19.9 (13.0–25.0) | 17.4 (11.9–22.9) | 18.3 (12.3–24.7) | 18.5 (12.3–23.6) | 18.9 (12.6–25.3) |
| Fetuin‐A, ng/mL | 442.5 (325.1–524.1) | 469.1 (337.3–570.4) | 409.3 (321.2–521.4) | 438.6 (329.1–571.2) | 408.3 (323.9–516.5) | 434.4 (325.7–571.4) |
| RBP4, ng/mL | 43.9 (39.6–49.6) | 38.2 (30.6–44.7) | 42.8 (36.9–49.4) | 36.6 (30.6–44.6) | 42.2 (36.4–48.1) | 36.1 (30.3–43.6) |
| FABP4, ng/mL | 16.6 (10.9–19.9) | 21.7 (13.2–25.9) | 14.7 (10.6–19.3) | 17.5 (12.8–24.6) | 13.2 (9.9–17.6) | 16.6 (12.3–22.2) |
| Adiponectin, μg/mL | 6.1 (3.3–7.9) | 10.9 (6.5–14.6) | 5.3 (3.4–8.0) | 10.3 (6.9–14.7) | 5.9 (3.9–8.5) | 10.7 (7.6–15.1) |
Values for clinical features are means±SD or percentages and for adipokines are median and 25%–75% interquartile range. BMI indicates body mass index; BP, blood pressure; CRP, C‐reactive protein; FABP4, fatty acid–binding protein 4; HOMA‐IR, homeostasis model assessment insulin resistance index; NA, not available; RBP4, retinol‐binding protein 4.
Waist circumference ≥40.2 inches (102 cm) in men, ≥34.6 inches (88 cm) in women.
Triglycerides ≥150 mg/dL or treatment.
High‐density lipoprotein cholesterol (HDL‐C) <40 mg/dL in men, <50 mg/dL in women.
Change in weight from examination 1 to examination 2.
Glucose ≥100 mg/dL.
Figure 2.
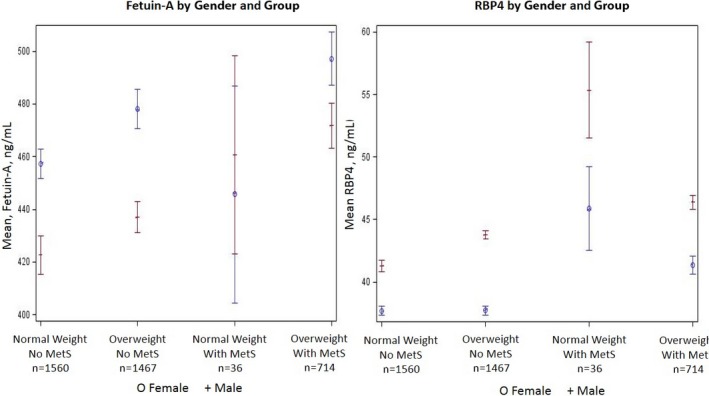
Least square mean and standard error of plasma fetuin and retinol‐binding protein 4 (RBP4). The x axis represents different subgroups (presence/absence of metabolic syndrome [MetS] and normal/overweight). The y axis represents least square mean for fetuin and RBP4.
Table 2.
Baseline Characteristics of Participants in Study Sample 2 vs Nonattendees
| Clinical Features | Men Attending Examination 2 (n=1202) | Men Not Attending Examination 2 (n=710) | P Value | Women Attending Examination 2 (n=1467) | Women Not Attending Examination 2 (n=716) | P Value |
|---|---|---|---|---|---|---|
| Age, y | 40.2 (8.5) | 40.5 (9.4) | 0.481 | 40.1 (8.7) | 39.9 (9.1) | 0.751 |
| Systolic BP, mm Hg | 120.0 (12.1) | 122.2 (13.3) | 0.001 | 112.8 (14.0) | 114.1 (15.1) | 0.050 |
| Diastolic BP, mm Hg | 78.0 (9.2) | 79.0 (9.4) | 0.025 | 72.3 (9.2) | 73.4 (9.2) | 0.009 |
| Hypertension, % | 19.0 | 27.3 | <0.0001 | 10.0 | 16.3 | <0.0001 |
| Treatment for hypertension, % | 8.0 | 14.1 | <0.0001 | 5.3 | 11.2 | <0.0001 |
| BMI, kg/m² | 27.7 (4.4) | 28.4 (5.2) | 0.002 | 25.5 (5.5) | 27.2 (7.1) | <0.0001 |
| BMI ≥30 kg/m², % | 23.8 | 29.5 | 0.005 | 17.9 | 26.9 | <0.0001 |
| Weight, kg | 87.6 (15.0) | 89.4 (17.4) | 0.017 | 68.7 (15.2) | 72.9 (19.4) | <0.0001 |
| Waist circumference, cm | 97.6 (12.0) | 99.5 (13.7) | 0.002 | 87.1 (14.3) | 91.5 (18.0) | <0.0001 |
| Total cholesterol, mg/dL | 193.7 (36.2) | 191.9 (38.9) | 0.335 | 184.3 (32.2) | 187.0 (34.5) | 0.092 |
| Triglycerides, mg/dL | 128.7 (91.9) | 149.2 (135.5) | 0.001 | 91.8 (50.6) | 109.5 (80.3) | <0.0001 |
| HDL‐C, mg/dL | 46.9 (11.9) | 46.5 (13.4) | 0.508 | 61.9 (15.8) | 59.0 (16.3) | <0.0001 |
| Current smoker, % | 15.6 | 23.4 | <0.0001 | 13.4 | 21.9 | <0.0001 |
| Fasting plasma glucose, mg/dL | 95.9 (7.6) | 103.6 (27.6) | <0.0001 | 89.8 (7.5) | 96.9 (29.3) | <0.0001 |
Values for clinical features are means±SD or percentages. BMI indicates body mass index; BP, blood pressure; HDL‐C, high‐density lipoprotein cholesterol; HOMA‐IR, homeostasis model assessment insulin resistance index.
Table 3.
Adipokine Intercorrelations
| Leptin | Leptin Receptor | Fetuin‐A | RBP4 | FABP4 | Adiponectin | CRP | HOMA‐IR | |
|---|---|---|---|---|---|---|---|---|
| Leptin | 1.00 | −0.21a | 0.07a | 0.04 | 0.55a | −0.18a | 0.29a | 0.55a |
| Leptin receptor | 1.00 | −0.04a | 0.11a | −0.12a | 0.17a | −0.09a | −0.17a | |
| Fetuin‐A | 1.00 | 0.06a | 0.04a | −0.02 | 0.05a | −0.10a | ||
| RBP4 | 1.00 | 0.07a | 0.003 | −0.04a | 0.05a | |||
| FABP4 | 1.00 | −0.22a | 0.21a | 0.40a | ||||
| Adiponectin | 1.00 | −0.12a | −0.30a | |||||
| CRP | 1.00 | 0.24a | ||||||
| HOMA‐IR | 1.00 |
CRP indicates C‐reactive protein; FABP4, fatty acid–binding protein 4; HOMA‐IR, homeostasis model assessment insulin resistance index; RBP4, retinol‐binding protein 4.
Values are Pearson correlation coefficients (n=2416) for sex‐standardized, age‐adjusted adipokines in natural units.
Cross‐Sectional Findings
Baseline characteristics according to presence/absence of MetS and by BMI group are summarized in Table 4. Participants with normal weight represented roughly 41% of sample 1, 19% had MetS, 39% were MHO, and 1% were MONW. In age‐ and sex‐adjusted models, leptin, fetuin‐A, RBP4, and FABP4 were higher in the MHO group (compared with the referent group; P value for all <0.01). Leptin‐R and adiponectin were significantly lower in the MHO group than in the referent group (P value for both P<0.001).
Table 4.
Baseline Examination Characteristics Cross‐Classified by Weight vs MetS Category
| Clinical Features | Absence of MetS | Presence of MetS | ||
|---|---|---|---|---|
| BMI <25 (n=1560) | MHO Participants (n=1467) | MONW Participants (n=36) | BMI ≥25 (n=714) | |
| Age, y | 38.4 | 39.8 | 43.0 | 43.7 |
| Systolic BP, mm Hg | 111 | 117 | 125 | 128 |
| Diastolic BP, mm Hg | 71 | 76 | 81 | 83 |
| BP ≥130/85 mm Hg or treatment, % | 13.0 | 20.3 | 77.8 | 74.1 |
| Hypertension, % | 13.0 | 20.3 | 77.8 | 74.1 |
| Treatment for hypertension, % | 3.5 | 3.5 | 22.2 | 17.1 |
| BMI, kg/m² | 22.2 | 29.0 | 23.5 | 32.8 |
| BMI ≥30 kg/m², % | 0 | 27.5 | 0 | 61.8 |
| Weight, kg | 63 | 85 | 68 | 98 |
| Waist circumference, cm | 80 | 98 | 90 | 110 |
| Increased waist circumference, %a | 5.4 | 48.0 | 33.3 | 85.2 |
| Total cholesterol, mg/dL | 180 | 192 | 202 | 202 |
| Triglycerides, mg/dL | 82 | 103 | 252 | 202 |
| High triglycerides, %b | 11.1 | 20.0 | 66.7 | 72.3 |
| HDL‐C, mg/dL | 54 | 54 | 45 | 42 |
| Low HDL‐C, %c | 14.0 | 17.5 | 77.8 | 66.7 |
| Smoking, % | 14.8 | 13.1 | 22.2 | 19.2 |
| Fasting plasma glucose, mg/dL | 90 | 64 | 102 | 108 |
| Impaired fasting glucose, %d | 0.51 | 0.89 | 5.56 | 10.36 |
| Biomarkers, mean (95% CIs) | ||||
| HOMA‐IR | 0.86 | 1.16 | 1.29 | 2.21 |
| Leptin, ng/mL | 4.1 (3.7–4.5) | 16.4 (15.7–17.1) | 7.8 (5.7–9.9) | 22.8 (21.6–24.0) |
| Leptin receptor, ng/mL | 22.1 (21.5–22.6) | 18.4 (18.0–18.8) | 20.4 (18.4–22.4) | 16.5 (16.0–17.1) |
| Fetuin‐A, ng/mL | 439.4 (430.5–488.2) | 458.8 (449.4–468.2) | 455.6 (399.8–511.4) | 490.2 (476.7–503.6) |
| RBP4, ng/mL | 39.7 (39.1–40.2) | 40.6 (40.1–41.1) | 50.1 (45.1–55.1) | 43.6 (42.7–44.5) |
| FABP4, ng/mL | 13.5 (13.1–13.9) | 21.3 (20.8–21.8) | 21.7 (18.0–25.4) | 28.2 (27.2–29.3) |
| Adiponectin, μg/mL | 10.4 (10.1–10.7) | 8.2 (8.0–8.5) | 6.1 (4.9–7.4) | 5.8 (5.5–6.1) |
Values for clinical features are means or percentages, biomarkers are age and sex adjusted means with 95% CI. BMI indicate body mass index; BP, blood pressure; FABP4, fatty acid–binding protein 4; HOMA‐IR, homeostasis model assessment insulin resistance index; MetS, metabolic syndrome; MHO, metabolically healthy obese; MONW, metabolically obese, normal‐weight; RBP4, retinol‐binding protein 4.
Waist circumference ≥40.2 inches (102 cm) in men, ≥34.6 inches (88 cm) in women.
Triglycerides ≥150 mg/dL or treatment.
High‐density lipoprotein cholesterol (HDL‐C) <40 mg/dL in men, <50 mg/dL in women.
Glucose ≥100 mg/dL.
Relation of the Adipokine Panel to Incident MetS
A total of 263 participants (113 women) in sample 2 developed new‐onset MetS on follow‐up. The panel of biomarkers was associated with incidence of MetS (global test, P=0.004). On multivariable logistic regression with backward elimination of adipokines and adjusted for baseline MetS component levels, higher plasma RBP4 (adjusted OR per 1‐SD increment, 1.21; 95% CI, 1.03–1.41 [P=0.02]) and fetuin‐A (1.17; 95% CI, 1.01–1.34 [P=0.03]) were significantly associated with development of new‐onset MetS. Table 5 shows quartile‐based analyses where circulating RBP4 was associated with a 17% increased risk of MetS per quartile increment, whereas fetuin‐A increased risk of future MetS by 16% per quartile increment. Restricted cubic splines demonstrating the association between fetuin‐A or RBP4 and future development of MetS is seen in Figure 3. Analysis of incident MetS risk among binary categories of RBP4 and fetuin‐A dichotomized at the median shows that the individuals with levels of both RBP4 and fetuin‐A above the median experienced a higher risk compared with the group with both adipokines at or below the median (Table 5).
Table 5.
Logistic Regression Analysis Examining Biomarkers in Quartiles and in Combinations to Incidence of MetS
| No. of Cases/No. of People at Riska | Unadjusted Incidence Rates % (95% CI) | Multivariable Adjustedb OR (95% CI) | P Value | |
|---|---|---|---|---|
| RBP4 | ||||
| Quartile 1 | 47/591 | 8.0 (5.7–10.2) | Referent | |
| Quartile 2 | 64/568 | 11.3 (8.5–14.0) | 1.39 (0.90–2.16) | 0.14 |
| Quartile 3 | 70/552 | 12.7 (9.7–15.7) | 1.25 (0.81–1.94) | 0.32 |
| Quartile 4 | 82/497 | 16.5 (12.9–20.1) | 1.75 (1.12–2.78) | 0.02 |
| Trend | ··· | ··· | 1.17 (1.01–1.35) | 0.03 |
| Fetuin‐A | ||||
| Quartile 1 | 50/559 | 8.9 (6.5–11.4) | Referent | |
| Quartile 2 | 68/557 | 12.2 (9.3–15.1) | 1.28 (0.84–1.96) | 0.25 |
| Quartile 3 | 69/550 | 12.5 (9.6–15.5) | 1.47 (0.97–2.24) | 0.07 |
| Quartile 4 | 76/542 | 14.0 (10.9–17.2) | 1.59 (1.05–2.42) | 0.03 |
| Trend | ··· | ··· | 1.16 (1.02–1.33) | 0.03 |
| Marker combination | ||||
| Both ≤ median | 42/579 | 7.3 (5.1–9.4) | Referent | |
| Either RBP4 or fetuin‐A below median | 134/1050 | 12.8 (10.6–14.9) | 1.48 (1.01–2.22) | 0.05 |
| Both above median | 87/579 | 15.0 (11.9–18.2) | 1.70 (1.10–2.65) | 0.02 |
OR indicates odds ratio for log‐transformed adipokine; RBP4, retinol‐binding protein 4.
Excludes participants with metabolic syndrome (MetS) at baseline, and uses sex‐standardized biomarkers (which explains why the number at risk differs for each quartile).
Multivariable models are adjusted for age, sex, baseline body mass index, sex‐standardized waist circumference, systolic and diastolic blood pressure, high‐density lipoprotein cholesterol, glucose, and log of triglycerides.
Figure 3.
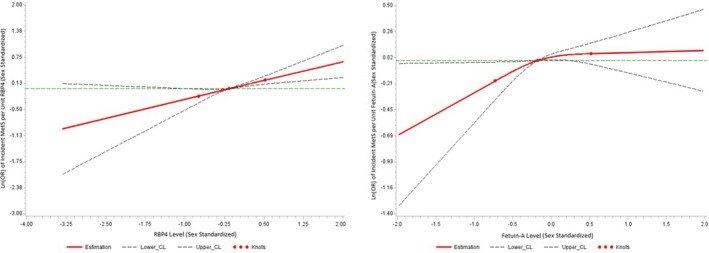
Association between fetuin‐A or retinol‐binding protein 4 (RBP4) and incident metabolic syndrome (MetS) using restricted cubic splines with 3 knots. Knots represent the first, second (median), and third quartile values, with the reference as the first (or lower) quartile value. The x axis represents the range of values for each respective adipokine and the y axis represents the log (odds ratio [OR]) for a 1‐unit increase in the sex‐standardized adipokine. CL indicates 95% confidence limit.
Circulating RBP4 and Fetuin‐A and Longitudinal Tracking of MetS Components
In multivariable‐adjusted logistic regression models, circulating RBP4 and fetuin‐A were associated with longitudinal changes in systolic BP and borderline statistically significant associations with higher fasting glucose (Table 6). In addition, blood RBP4 was significantly associated with an increase in diastolic BP (P=0.007).
Table 6.
Logistic Regression Analysis Examining the Incidence of Individual Risk Factors From Baseline Examination to Follow‐Up Examination According to Biomarkers (Modeled Together)
| Δ MetS Component | No. | Incidence Proportion, % | RBP4 | Fetuin‐A | ||
|---|---|---|---|---|---|---|
| Multivariable Adjusteda OR (95% CI) | P Value | Multivariable Adjusteda OR (95% CI) | P Value | |||
| Waist circumference | 2208 | 916/2208 (41.5%) | 1.05 (0.93–1.18) | 0.42 | 1.04 (0.92–1.18) | 0.5 |
| Fasting glucose | 2207 | 369/2208 (16.7%) | 1.13 (0.99–1.28) | 0.06 | 1.03 (0.90–1.17) | 0.07 |
| Systolic BP | 2206 | 411/2206 (18.6%) | 1.22 (1.06–1.39) | 0.004 | 1.14 (1.00–1.30) | 0.04 |
| Diastolic BP | 2206 | 394/2206 (17.8%) | 1.19 (1.04–1.13) | 0.007 | 1.08 (0.95–1.22) | 0.26 |
| Triglycerides | 2206 | 441/2206 (20.0%) | 1.08 (0.96–1.23) | 0.19 | 0.99 (0.88–1.11) | 0.80 |
| HDL‐C | 2208 | 238/2206 (10.8%) | 1.05 (0.89–1.22) | 0.58 | 1.09 (0.94–1.26) | 0.26 |
BP indicates blood pressure; HDL‐C, high‐density lipoprotein cholesterol; RBP4, retinol‐binding protein 4.
Multivariable‐adjusted beta for change (Δ) in components of metabolic syndrome (MetS) are adjusted for age, sex, baseline body mass index (except waist circumference model), and baseline level of the individual component, and values are per SD increment of log biomarkers.
Discussion
We evaluated circulating concentrations of a panel of adipokines in younger adults with normal weight, MONW individuals, and MHO individuals cross‐sectionally, and the incidence of MetS prospectively. Overall, MHO participants exhibited an abnormal adipokine profile and MONW participants also had trends toward abnormal adipokine profile. Circulating RBP4 and fetuin‐A were associated with incident MetS and longitudinal increase in systolic BP, while RBP4 was associated with significantly higher diastolic BP and a trend toward higher fasting glucose. Overall, our data extend to young to middle‐aged adults in previous work associating certain adipokines to one or more metabolic traits in middle to older adults.10, 11
Cross‐Sectional Data on Adipokine Concentrations in Subsets of Obesity
It is estimated that 28% of the US population older than 20 years is MHO, while the prevalence of MONW is 8.1%.3 The prevalence of metabolically healthy obesity was 39% in our current study, similar to US, Swedish, and prior Framingham estimates, while our rate of MONW was roughly 1%.12, 13 The CVD risk of these respective categories is somewhat controversial. Some studies identify an elevated risk of CVD events in obese persons without cardiometabolic risk factors, while other studies have found no elevated risk.14, 15, 16 These conflicting results may be explained by different follow‐up periods and event rates between studies. On the other hand, there is evidence that persons with cardiometabolic risk factors in the context of normal body weight have an elevated risk for both experiencing a CVD event and developing DM.15
Metabolically abnormal profiles appear to be more frequent with advancing age (10% among persons aged 20 to 34 years; 56% among persons aged 80 years or older), suggesting that metabolically healthy obesity may be an early phenotype that can worsen to greater cardiometabolic risk over time.3 In a Finnish population, 20% of men and 24% of women were MONW, possibly reflecting ethnic differences in the proportion of metabolic derangement compared with our cohort.17 Beyond epidemiological differences, our data suggest key biological distinctions between MHO and MONW groups. The leptin pathway and adiponectin appeared to track with participants with obesity in that the MHO pattern was associated with higher leptin and lower LEP‐R in participants with normal weight. The MONW participants appeared to be intermediate between metabolically healthy obesity and normal weight in leptin and LEP‐R, while overweight participants with MetS had the highest levels of leptin and lowest LEP‐R and adiponectin. Therefore, the monotonically higher free leptin that can be inferred by the gap between measured leptin and LEP‐R levels among categories of normal weight, MONW, metabolically healthy obesity, and overweight with MetS may reflect the dominant role played by weight in driving leptin pathway levels. In contrast, adiponectin levels were highest in normal‐weight individuals, lower in MHO individuals, and lowest in MONW and overweight individuals with MetS, suggesting it may be a strong driver of cardiometabolic risk in these individuals. Comparable levels of fetuin and FABP4 were shown in MHO and MONW persons, which were higher than those in normal‐weight persons but lower than those in overweight individuals with MetS, suggesting a codependent pattern where cardiometabolic risk or excess weight each drive abnormalities in these adipokines. Finally, RBP4 showed a pattern of elevation more consistent with cardiometabolic risk rather than with weight per se, since circulating concentrations in metabolically healthy obesity and normal weight were largely similar.
RBP4 and Risk of MetS
We observed that higher plasma RBP4 concentrations were not only associated with cross‐sectional presence of MetS but also prospectively associated with incident MetS. Higher circulating RBP4 concentrations were specifically associated with higher systolic and diastolic BPs and with borderline higher fasting glucose level on follow‐up. These findings are consistent with previous reports linking RBP4 to dysglycemia, vascular properties, and hypertension.18, 19, 20 RBP4 may be an intriguing predictor of cardiometabolic risk, especially given that serum levels of RBP4 have appeared to be independent of obesity and previously noted to be elevated in nonobese, insulin‐resistant patients.20, 21 RBP4 is a transport protein highly expressed in liver and adipose tissue.22, 23 Overexpression of RBP4 results in insulin resistance, while, conversely, knockout of the protein translates into enhanced insulin sensitivity.20, 24 Serum RBP4 levels have previously been inversely correlated with insulin sensitivity in nondiabetic patients and are increased in patients with impaired glucose tolerance, type 2 DM, fatty liver, cerebral infarction, and components of MetS, particularly triglycerides.20, 25, 26, 27 RBP4 has been shown to be a relevant marker for the prediction of prevalent MetS in cross‐sectional, community‐based samples of elderly patients, although less is known about its effects on younger patient populations or in prospective studies.25, 28 Our data clarify and extend previous observations to younger adult populations.
Fetuin‐A and Risk of MetS
In our sample, plasma fetuin‐A levels were positively associated with MetS cross‐sectionally and with greater risk of incident MetS longitudinally, specifically with elevation in systolic BP and trends toward higher fasting glucose on follow‐up, consistent with previous reports. Fetuin‐A inhibits insulin receptor signaling in liver and muscle via tyrosine kinase activity, thereby promoting hepatic and skeletal muscle insulin resistance.29, 30, 31 Fetuin‐A knockout mice have been shown to have enhanced insulin sensitivity, resistance to weight gain, and lower triglyceride levels.30 Higher serum fetuin‐A levels have been associated with risk of type 2 DM, myocardial infarction and ischemic stroke, MetS, and specific components of MetS.10, 32, 33, 34, 35
Study Strengths and Limitations
The strengths of our investigation include the moderately sized community‐based sample, the routine assessment of several circulating adipokines in a younger adult sample, a design that included cross‐sectional and longitudinal components, the adjustment for several standard clinical covariates in multivariable analyses, and the assessment of the conjoint associations of several adipokines. These adipokines may provide novel insights into the pathophysiological progression to MetS.
The limitations of our investigation include the “single occasion” measurement of adipokines that may not adequately capture their biologic activity or variability over time. The sample size of the MONW group was small (n=36), limiting our statistical power to assess associations for this group. Our sample consists of mostly white participants, therefore generalizability to other ethnicities is limited. The differences between included participants and nonattendees may also limit generalizability of our findings to persons with higher CVD risk. Lastly, the follow‐up period in the current study was 6 years, and we may have underestimated the long‐term metabolic risks associated with adipokine profiles. This, in part, may explain why both circulating RBP4 and fetuin‐A concentrations demonstrated borderline statistically significant associations with fasting blood glucose concentrations.
Conclusions
In our community‐based sample of young to middle‐aged adults, distinctive cross‐sectional patterns of adipokine elevations were observed in normal‐weight, overweight with MetS, MHO, and MONW participants. Longitudinally, circulating fetuin‐A and RBP4 concentrations were associated prospectively with the incidence of MetS and of specific MetS components, even after adjustment for baseline MetS component levels. Additional studies of other age groups and multiethnic samples are warranted to confirm the role of these adipokines in the incipient MetS and cardiometabolic consequences. Future work may identify adipokines as screening tools to identify persons at high risk for MetS and CVD events.
Sources of Funding
This project was supported in part by National Heart, Lung, and Blood Institute HL083781 (Quiroz), N01‐HC‐25195 (Vasan), HHSN268201500001I (Vasan), HL111335 (Zachariah), and NIDDK DK‐080739‐01 (Vasan). The funding agencies had no role in the formulation, editing, or decision to submit this article.
Disclosures
None.
(J Am Heart Assoc. 2017;6:e004974 DOI: 10.1161/JAHA.116.004974.)28713076
Portions of this work were previously presented at the American Heart Association's Scientific Sessions, November 16–20, 2013, in Dallas, TX.
References
- 1. Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC Jr; International Diabetes Federation Task Force on E, Prevention, Hational Heart L, Blood I, American Heart A, World Heart F, International Atherosclerosis S, International Association for the Study of O . Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. [DOI] [PubMed] [Google Scholar]
- 2. Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC Jr, Spertus JA, Costa F; American Heart A, National Heart L, Blood I . Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. [DOI] [PubMed] [Google Scholar]
- 3. Wildman RP, Muntner P, Reynolds K, McGinn AP, Rajpathak S, Wylie‐Rosett J, Sowers MR. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: prevalence and correlates of 2 phenotypes among the US population (NHANES 1999–2004). Arch Intern Med. 2008;168:1617–1624. [DOI] [PubMed] [Google Scholar]
- 4. Cornier MA, Després JP, Davis N, Grossniklaus DA, Klein S, Lamarche B, Lopez‐Jimenez F, Rao G, St‐Onge MP, Towfighi A, Poirier P; American Heart Association Obesity Committee of the Council on N, Physical A, Metabolism, Council on A, Thrombosis, Vascular B, Council on Cardiovascular Disease in the Y, Council on Cardiovascular R, Intervention, Council on Cardiovascular Nursing CoE, Prevention, Council on the Kidney in Cardiovascular D, Stroke C . Assessing adiposity: a scientific statement from the American Heart Association. Circulation. 2011;124:1996–2019. [DOI] [PubMed] [Google Scholar]
- 5. Flier JS. The adipocyte: storage depot or node on the energy information superhighway? Cell. 1995;80:15–18. [DOI] [PubMed] [Google Scholar]
- 6. Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11:85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Splansky GL, Corey D, Yang Q, Atwood LD, Cupples LA, Benjamin EJ, D'Agostino RB Sr, Fox CS, Larson MG, Murabito JM, O'Donnell CJ, Vasan RS, Wolf PA, Levy D. The Third Generation Cohort of the National Heart, Lung, and Blood Institute's Framingham Heart Study: design, recruitment, and initial examination. Am J Epidemiol. 2007;165:1328–1335. [DOI] [PubMed] [Google Scholar]
- 8. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta‐cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. [DOI] [PubMed] [Google Scholar]
- 9. Tobin MD, Sheehan NA, Scurrah KJ, Burton PR. Adjusting for treatment effects in studies of quantitative traits: antihypertensive therapy and systolic blood pressure. Stat Med. 2005;24:2911–2935. [DOI] [PubMed] [Google Scholar]
- 10. Ix JH, Shlipak MG, Brandenburg VM, Ali S, Ketteler M, Whooley MA. Association between human fetuin‐A and the metabolic syndrome: data from the Heart and Soul Study. Circulation. 2006;113:1760–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yang Q, Graham TE, Mody N, Preitner F, Peroni OD, Zabolotny JM, Kotani K, Quadro L, Kahn BB. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature. 2005;436:356–362. [DOI] [PubMed] [Google Scholar]
- 12. Arnlov J, Ingelsson E, Sundstrom J, Lind L. Impact of body mass index and the metabolic syndrome on the risk of cardiovascular disease and death in middle‐aged men. Circulation. 2010;121:230–236. [DOI] [PubMed] [Google Scholar]
- 13. Meigs JB, Wilson PW, Fox CS, Vasan RS, Nathan DM, Sullivan LM, D'Agostino RB. Body mass index, metabolic syndrome, and risk of type 2 diabetes or cardiovascular disease. J Clin Endocrinol Metab. 2006;91:2906–2912. [DOI] [PubMed] [Google Scholar]
- 14. Bell JA, Kivimaki M, Hamer M. Metabolically healthy obesity and risk of incident type 2 diabetes: a meta‐analysis of prospective cohort studies. Obes Rev. 2014;15:504–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Carnethon MR, De Chavez PJ, Biggs ML, Lewis CE, Pankow JS, Bertoni AG, Golden SH, Liu K, Mukamal KJ, Campbell‐Jenkins B, Dyer AR. Association of weight status with mortality in adults with incident diabetes. JAMA. 2012;308:581–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fan J, Song Y, Chen Y, Hui R, Zhang W. Combined effect of obesity and cardio‐metabolic abnormality on the risk of cardiovascular disease: a meta‐analysis of prospective cohort studies. Int J Cardiol. 2013;168:4761–4768. [DOI] [PubMed] [Google Scholar]
- 17. Pajunen P, Kotronen A, Korpi‐Hyovalti E, Keinanen‐Kiukaanniemi S, Oksa H, Niskanen L, Saaristo T, Saltevo JT, Sundvall J, Vanhala M, Uusitupa M, Peltonen M. Metabolically healthy and unhealthy obesity phenotypes in the general population: the FIN‐D2D survey. BMC Public Health. 2011;11:754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zachariah JP, Hwang S, Hamburg NM, Benjamin EJ, Larson MG, Levy D, Vita JA, Sullivan LM, Mitchell GF, Vasan RS. Circulating adipokines and vascular function: cross‐sectional associations in a community‐based cohort. Hypertension. 2016;67:294–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cho YM, Youn BS, Lee H, Lee N, Min SS, Kwak SH, Lee HK, Park KS. Plasma retinol‐binding protein‐4 concentrations are elevated in human subjects with impaired glucose tolerance and type 2 diabetes. Diabetes Care. 2006;29:2457–2461. [DOI] [PubMed] [Google Scholar]
- 20. Graham TE, Yang Q, Bluher M, Hammarstedt A, Ciaraldi TP, Henry RR, Wason CJ, Oberbach A, Jansson PA, Smith U, Kahn BB. Retinol‐binding protein 4 and insulin resistance in lean, obese, and diabetic subjects. N Engl J Med. 2006;354:2552–2563. [DOI] [PubMed] [Google Scholar]
- 21. Kaess BM, Enserro DM, McManus DD, Xanthakis V, Chen MH, Sullivan LM, Ingram C, O'Donnell CJ, Keaney JF, Vasan RS, Glazer NL. Cardiometabolic correlates and heritability of fetuin‐A, retinol‐binding protein 4, and fatty‐acid binding protein 4 in the Framingham Heart Study. J Clin Endocrinol Metab. 2012;97:E1943–E1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tsutsumi C, Okuno M, Tannous L, Piantedosi R, Allan M, Goodman DS, Blaner WS. Retinoids and retinoid‐binding protein expression in rat adipocytes. J Biol Chem. 1992;267:1805–1810. [PubMed] [Google Scholar]
- 23. Zovich DC, Orologa A, Okuno M, Kong LW, Talmage DA, Piantedosi R, Goodman DS, Blaner WS. Differentiation‐dependent expression of retinoid‐binding proteins in BFC‐1 beta adipocytes. J Biol Chem. 1992;267:13884–13889. [PubMed] [Google Scholar]
- 24. Abel ED, Peroni O, Kim JK, Kim YB, Boss O, Hadro E, Minnemann T, Shulman GI, Kahn BB. Adipose‐selective targeting of the GLUT4 gene impairs insulin action in muscle and liver. Nature. 2001;409:729–733. [DOI] [PubMed] [Google Scholar]
- 25. Ingelsson E, Sundstrom J, Melhus H, Michaelsson K, Berne C, Vasan RS, Riserus U, Blomhoff R, Lind L, Arnlov J. Circulating retinol‐binding protein 4, cardiovascular risk factors and prevalent cardiovascular disease in elderly. Atherosclerosis. 2009;206:239–244. [DOI] [PubMed] [Google Scholar]
- 26. Jia W, Wu H, Bao Y, Wang C, Lu J, Zhu J, Xiang K. Association of serum retinol‐binding protein 4 and visceral adiposity in Chinese subjects with and without type 2 diabetes. J Clin Endocrinol Metab. 2007;92:3224–3229. [DOI] [PubMed] [Google Scholar]
- 27. Sasaki M, Otani T, Kawakami M, Ishikawa SE. Elevation of plasma retinol‐binding protein 4 and reduction of plasma adiponectin in subjects with cerebral infarction. Metabolism. 2010;59:527–532. [DOI] [PubMed] [Google Scholar]
- 28. von Eynatten M, Humpert PM. Retinol‐binding protein 4 & atherosclerosis: risk factor or innocent bystander? Atherosclerosis. 2009;206:38–39. [DOI] [PubMed] [Google Scholar]
- 29. Auberger P, Falquerho L, Contreres JO, Pages G, Le Cam G, Rossi B, Le Cam A. Characterization of a natural inhibitor of the insulin receptor tyrosine kinase: cDNA cloning, purification, and anti‐mitogenic activity. Cell. 1989;58:631–640. [DOI] [PubMed] [Google Scholar]
- 30. Mathews ST, Singh GP, Ranalletta M, Cintron VJ, Qiang X, Goustin AS, Jen KL, Charron MJ, Jahnen‐Dechent W, Grunberger G. Improved insulin sensitivity and resistance to weight gain in mice null for the Ahsg gene. Diabetes. 2002;51:2450–2458. [DOI] [PubMed] [Google Scholar]
- 31. Rauth G, Poschke O, Fink E, Eulitz M, Tippmer S, Kellerer M, Haring HU, Nawratil P, Haasemann M, Jahnen‐Dechent W, Muller‐Esterl W. The nucleotide and partial amino acid sequences of rat fetuin. Identity with the natural tyrosine kinase inhibitor of the rat insulin receptor. Eur J Biochem. 1992;204:523–529. [DOI] [PubMed] [Google Scholar]
- 32. Ix JH, Wassel CL, Kanaya AM, Vittinghoff E, Johnson KC, Koster A, Cauley JA, Harris TB, Cummings SR, Shlipak MG; Health ABCS . Fetuin‐A and incident diabetes mellitus in older persons. JAMA. 2008;300:182–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stefan N, Fritsche A, Weikert C, Boeing H, Joost HG, Haring HU, Schulze MB. Plasma fetuin‐A levels and the risk of type 2 diabetes. Diabetes. 2008;57:2762–2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Weikert C, Stefan N, Schulze MB, Pischon T, Berger K, Joost HG, Haring HU, Boeing H, Fritsche A. Plasma fetuin‐A levels and the risk of myocardial infarction and ischemic stroke. Circulation. 2008;118:2555–2562. [DOI] [PubMed] [Google Scholar]
- 35. Xu Y, Xu M, Bi Y, Song A, Huang Y, Liu Y, Wu Y, Chen Y, Wang W, Li X, Ning G. Serum fetuin‐A is correlated with metabolic syndrome in middle‐aged and elderly Chinese. Atherosclerosis. 2011;216:180–186. [DOI] [PubMed] [Google Scholar]


