Abstract
Background
Inconsistent findings have been obtained for previous studies evaluating the association between antihypertensive medication (AHM) adherence and the risk of stroke. This dose‐response meta‐analysis was designed to investigate the association between AHM adherence and stroke risk.
Methods and Results
MEDLINE and Embase databases were systematically searched to identify relevant studies. The quantification of adherence to AHM was calculated as the percentage of the sum of days with AHM actually taken divided by the total number of days in a specific period. Summary relative risks (RR) and 95% CIs were estimated using a random‐effects model. Stratified and dose‐response analyses were also performed. A total of 18 studies with 1 356 188 participants were included. The summary RR of stroke for the highest compared with the lowest AHM adherence level was 0.73 (95% CI, 0.67–0.79). Stratified by stroke subtype, a higher AHM adherence was associated with lower risks of ischemic stroke (RR, 0.74; 95% CI, 0.69–0.79) and hemorrhagic stroke (RR, 0.55; 95% CI, 0.42–0.72). Moreover, both fatal (RR, 0.51; 95% CI, 0.36–0.73) and nonfatal stroke (RR, 0.52; 95% CI, 0.28–0.94) were lower in participants with higher AHM adherence. The results of a dose‐response analysis indicated that a 20% increment in AHM adherence level was associated with a 9% lower risk of stroke (RR, 0.91; 95% CI, 0.86–0.96).
Conclusions
Higher AHM adherence is dose‐dependently associated with a lower risk of stroke in patients with hypertension.
Keywords: antihypertensive medication, dose response, medication adherence, meta‐analysis, stroke
Subject Categories: Hypertension, Ischemic Stroke, Intracranial Hemorrhage, Primary Prevention, Secondary Prevention
Clinical Perspective
What Is New?
This meta‐analysis reveals a significant inverse association between antihypertensive medication (AHM) adherence and stroke risk.
Higher AHM adherence is beneficial for the prevention of ischemic stroke and hemorrhagic stroke.
Higher AHM adherence is associated with lower risks of both fatal and nonfatal stroke.
The dose‐response analysis suggests that a 20% increment in the AHM adherence level is associated with a 9% lower risk of stroke.
What Are the Clinical Implications?
This meta‐analysis strengthens and extends the understanding of the positive impact of AHM adherence on stroke prevention, further supporting the notion that improved AHM adherence is associated with improve stroke prevention in patients with hypertension.
Introduction
Despite significant improvements in diagnosis and treatment, stroke remains one of the most important causes of mortality worldwide.1 Patients with stroke typically experience a loss of body function, which finally contributes to long‐term morbidity and disability. Therefore, an increased risk of stroke and subsequent adverse events are associated with increased burden for the patients themselves, their family members, and healthcare systems, particularly in low‐ and middle‐income countries.1, 2
Hypertension is a reversible risk factor underlying the pathogenesis of stroke.3, 4 Previous clinical trials have confirmed the role of antihypertensive medications (AHMs) for the prevention of stroke.3, 4 AHM adherence, defined as the extent to which patients take medications as prescribed by their physicians, is also an important determinant for the preventative effect of AHM for stroke.5 Previous studies have reported that poor adherence to AHM appeared to be associated with an increased risk for stroke incidence or recurrence in patients with hypertension.6, 7 However, inconsistent findings were retrieved for previous studies evaluating the association between AHM adherence and the risk of stroke.
A systematic evaluation of the association between AHM adherence and the risk of stroke is of significance for understanding the role of AHM adherence in stroke prevention. Importantly, confirmation of the dependent association between the AHM adherence magnitude and the preventative efficacy of stroke may provide more detailed guidelines and education information for patients with hypertension who are taking AHM to reduce the risk of stroke. A quantitative analysis of AHM adherence and stroke risk can also provide critical information for the design of future large‐scale prospective cohort studies to explore the association between AHM adherence and the risk of stroke. However, a systematic evaluation of the association between AHM adherence and the risk of stroke is rare, and whether AHM adherence is dose‐dependently associated with stroke risk remains to be determined. Therefore, we investigated the association between AHM adherence and risk of stroke in a quantitative dose‐response meta‐analysis.
Methods
We followed the previously published guidelines for a Meta‐Analysis of Observational Studies in Epidemiology for conducting meta‐analyses and reporting the results.8
Literature Search and Study Selection
The MEDLINE and Embase electronic databases were searched using predefined terms and search criteria (Data S1). The latest search was conducted on January 30, 2017. Studies were included if they met all of the following criteria: (1) publication in the English language; (2) studies with either cohort, case‐control, or controlled trial design; (3) the exposure of interest was AHM adherence in patients with hypertension; (4) the outcome of interest was fatal/nonfatal stroke; and (5) reported the relative risk (RR) and the corresponding 95% CI for the association between AHM adherence and stroke risk (or these data could be estimated). Nonoriginal articles, articles with insufficient data or irrelevant outcomes, or case reports were excluded. No restriction on time of publication was applied.
Data Extraction, Exposure Assessment, and Quality Assessment
The following data were extracted from each study independently by 2 of the authors (T.X. and S.O.): first author, publication year, location, population demographics, data source, stroke type, follow‐up time, primary or secondary prevention for stroke, strategies for the assessment of AHM adherence, RR from the most fully adjusted model for the categories with the highest compared with the lowest adherence level to AHM and the corresponding 95% CI, and confounders adjusted for in the multivariate analysis. According to previous definitions, AHM adherence was primarily assessed by quantifying the adherence level or determining whether AHM was persistently taken during the treatment period.9 Medication adherence is defined as the extent to which a patient participates in a treatment regimen after this patient agrees to that regimen.9 Both low adherence level and the discontinuation of AHM use were considered poor adherence to AHM.9 The AHM adherence level usually refers to data from a quantitative analysis, and a low AHM adherence level was considered nonpersistence rather than the discontinuation of AHM.5, 9 Different studies may have different names for the quantitative assessment of AHM adherence (eg, proportion of days covered,10 medication possession ratio,11 cumulative medication adherence,12 and medication refill adherence13). However, proportion of days covered, medication possession ratio, cumulative medication adherence, and medication refill adherence were similarly obtained by calculating the percentage of days exposed to AHM in a given follow‐up period.9 Therefore, proportion of days covered, medication possession ratio, cumulative medication adherence, and medication refill adherence were considered equally for the evaluation of AHM adherence level. Adherence level of AHM ranges from 0% to 100%, and a higher percentage reflects better adherence. A 9‐star system based on the Newcastle‐Ottawa scale was used to assess the quality of the included studies.14 The full score was 9 stars, and a high‐quality study was defined as a study with ≥8 awarded stars.
Statistical Analysis
We used RRs with 95% CIs for the highest versus lowest adherence level to assess the association between adherence to AHM and the risk of stroke.15 For studies that assessed the exposure of interest based on whether AHM was persistently taken during a specific period, the estimation from persistent AHM use compared with discontinuation of AHM use was only used to calculate the pooled RRs from the highest compared with the lowest AHM adherence level. The heterogeneity between the included studies was evaluated with the Cochrane Q statistic. The I 2 statistic was used to quantify magnitude.16 We recognize the potential heterogeneity between the included studies; thus, we used a random‐effects model to pool the estimates.17 We conducted predefined subgroup analyses according to stroke subtype (ischemic stroke [IS] and hemorrhagic stroke [HS]), stroke outcome (fatal and nonfatal stroke), geographic region, follow‐up time, age distribution, sex, and quality score to evaluate the potential effect of these variables on the results. We also assessed the relationship between AHM adherence and stroke risk in the primary prevention or secondary prevention of stroke. Primary prevention was defined as patients taking AHM to prevent new‐onset stroke, and secondary prevention was defined as patients with a history of stroke events taking AHM to prevent stroke recurrence.18 Moreover, meta‐regression analyses were also performed to investigate the influence of the above predefined variables on the heterogeneity of the studies, and P interaction was used to assess the heterogeneity between subgroups.19 Subsequently, a dose‐response meta‐analysis was performed in accordance with the methods proposed by Greenland and Longnecker20 and Berlin et al21 to compute the trend from the correlated log RR estimates across the category of adherence level. For each study, the adjusted RR with a corresponding 95% CI for each median or mean level of exposure was used. If the median or mean adherence level per category was not available, the midpoint of the upper and lower boundaries was considered the dose of each category. If the highest category was open‐ended, the midpoint of the category was set to 1.2‐fold the lower boundary. We examined a potential nonlinear relationship between adherence level and stroke risk by modeling the adherence level using restricted cubic splines with 3 knots at percentiles 10%, 60%, and 90% of the distribution. We calculated the P value for nonlinearity by testing the null hypothesis that the coefficient of the solid line is equal to 0. A P value <0.050 was considered statistically significant. Publication bias was investigated visually with funnel plots and statistically with Begg's tests.22 STATA version 12.0 (StataCorp) was used for the statistical analyses.
Results
Literature Search and Characteristics of Studies
A flow chart of the selection procedure is shown in Figure 1. A total of 18 studies met our inclusion criteria and were eligible for meta‐analysis.10, 11, 12, 13, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36 The characteristics of the included studies are summarized in Table 1. A total of 18 studies involving 1 356 188 participants were examined. Seventeen studies were cohort studies, and only 1 had a nested case‐control design.11 The exposure measure was the percentage of days covered by prescribed AHM (proportion of days covered [n=9], medication possession ratio [n=5], medication refill adherence [n=1], cumulative medication adherence [n=1]) in 16 studies. In one study,34 the AHM adherence level was calculated as the percentage of months covered by prescribed AHM, and in another study,36 the adherence to AHM was evaluated based on whether AHM was taken persistently during a follow‐up period. The data source, disease classification, and confounders adjusted in the multivariate analysis for each study are listed in Tables S1 and S2. Quality scores of the included studies are listed in Table S3. The mean score of the included studies was 8.06 (SD, 1.06; range, 6–9).
Figure 1.
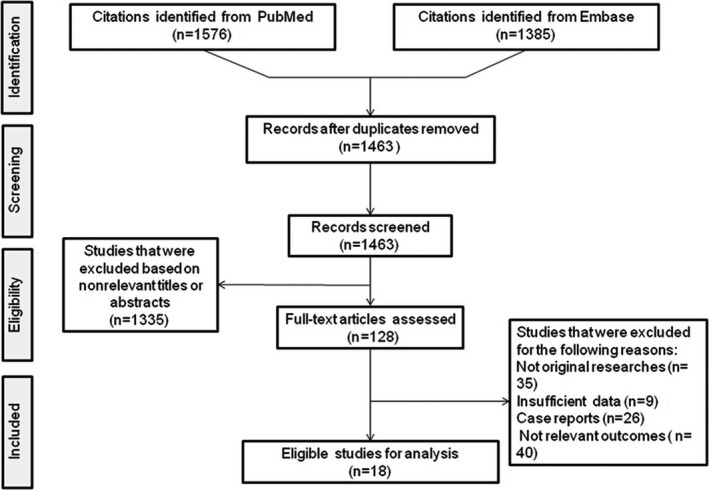
Flowchart of the literature search process.
Table 1.
Characteristics of the Studies Included in the Meta‐Analysis
| First Author, y of Publication | Design | Country | Study Period | Age, y/Women, %/No. in Cohort | Assessment of AHM Adherence | Levels of Prevention and Stroke Type | Outcomes |
|---|---|---|---|---|---|---|---|
| Yang 201623 | CS | United States | 2007–2012 | 18–62/67.3/59 037 | MPR | PP for all stroke | Stroke events |
| Kim 201612 | CS | South Korea | 2002–2010 | ≥20/53.4/33 728 | CMA | PP for all stroke, IS, HS | Stroke events; fatal stroke events |
| Herttua 201624 | CS | Finland | 1995–2007 | ≥30/54.0/58 266 | PDC | PP for all stroke | Fatal stroke events |
| Krousel–Wood 201525 | CS | United States | 2006–2011 | ≥65/59.8/2075 | MPR | PP for all stroke | Stroke events |
| Gosmanova 201526 | CS | United States | 2004–2013 | 53.8 (mean age)/0.9/312 489 | PDC | PP for all stroke | Stroke events |
| Xu 201327 | CS | China | 2007–2008 | ≥18/40.0/8409 | PDC | SP for IS | IS events; fatal IS |
| Wong 201328 | CS | China | 2001–2012 | All age group/54.9/218 047 | PDC | PP for all stroke | Fatal stroke events |
| Shin 201329 | CS | China | 2003–2007 | ≥18/49.7/40 408 | MPR | PP for all stroke | Stroke events |
| Herttua 201330 | CS | Finland | 1995–2007 | ≥30/54.0/73 527 | PDC | PP for all stroke | Stroke events; nonfatal and fatal stroke events |
| Perreault 201211 | NCCS | Canada | 1999–2007 | ≥65/46/14 227 | MPR | PP for all stroke, IS | Stroke events; nonfatal and fatal stroke events |
| Degli Esposti 201131 | CS | Italy | 2004–2006 | ≥18/52.0/31 306 | PDC | PP for all stroke | Stroke events |
| Corrao 201132 | CS | Italy | 2000–2007 | ≥18/56.0/242 594 | PDC | PP for all stroke | Stroke events |
| Khan 201010 | CS | Canada | 2003–2006 | ≥66/51.6/3571 | PDC | SP for all stroke | Fatal stroke events |
| Bailey 201013 | CS | United States | 1994–2000 | 18–64/67.7/49 479 | MRA | PP for all stroke | Stroke events; fatal stroke events |
| Mazzaglia 200933 | CS | Italy | 2000–2005 | ≥35/58.4/18 806 | PDC | PP for all stroke | Stroke events |
| Liu 200934 | CS | China | 1999–2004 | ≥30/48.2/29 759 | PMC | PP for all stroke, IS | Stroke events |
| Kettani 200935 | CS | Canada | 1999–2004 | 45–85/62.7/83 267 | MPR | PP for all stroke, IS, and HS | Stroke events |
| Breekveldt–Postma 200836 | CS | Netherlands | 1993–2002 | ≥18/59.9/77 193 | Discontinuation or not | PP for all stroke | Stroke events |
AHM indicates antihypertensive medication; CMA, cumulative medication adherence; CS, cohort study; HS, hemorrhagic stroke; IS, ischemic stroke; MPR, medication possession ratio; MRA, medication refill adherence; NCCS, nested case‐control study; PDC, proportion of days covered by prescribed AHM; PMC, proportion of months covered by prescribed AHM; PP, primary prevention; SP, secondary prevention.
AHM Adherence and Stroke Risk
The estimation for each study and the pooled RR for the highest compared with the lowest categories of AHM adherence level are shown in Figure 2.10, 11, 12, 13, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36 Overall, compared with participants in the lowest AHM adherence categories, those in the highest categories had a significantly lowered risk of stroke events (RR, 0.73; 95% CI, 0.67–0.79).
Figure 2.
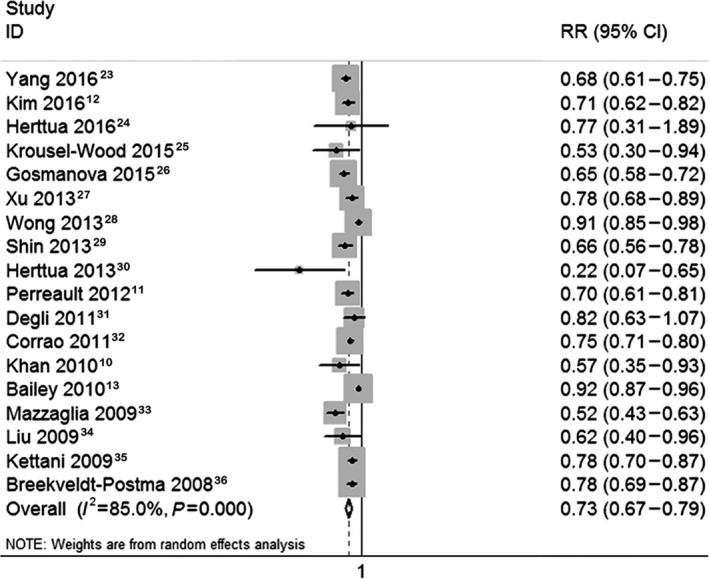
Forest plot of the association between antihypertensive medication adherence and stroke risk. RR indicates relative risk.
Stratification analyses for the association between AHM adherence and stroke risk are shown in Table 2. A higher AHM adherence rate was associated with lower risks for both the IS (RR, 0.74; 95% CI, 0.69–0.79) and HS (RR, 0.55; 95% CI, 0.42–0.72), and the protective effect of high AHM adherence was more remarkable in HS than IS (45% reduction in HS versus 26% reduction in IS). Improved AHM adherence was associated with lower risks of both nonfatal stroke (RR, 0.52; 95% CI, 0.28–0.94) and fatal stroke (RR, 0.51; 95% CI, 0.36–0.73). For age distribution, the preventative effect of improved AHM adherence against stroke was more significant in patients 65 years and older compared with those younger than 65 years (32% reduction in those ≥65 years versus 13% reduction in those <65 years). Subsequent analyses indicated that a higher AHM adherence was associated with a reduced risk of stroke regardless of geographic region, follow‐up duration, sex, and levels of stroke prevention (primary prevention or secondary prevention).
Table 2.
Stratification Analyses of AHM Adherence and Stroke Riska
| Group | No. of Studies | RR | 95% CI | I 2, % | PI |
|---|---|---|---|---|---|
| Stroke type | 0.090 | ||||
| Ischemic stroke | 5 | 0.74 | 0.69–0.79 | 11.7 | |
| Hemorrhagic stroke | 2 | 0.55 | 0.42–0.72 | 0.0 | |
| Stroke outcome | 0.999 | ||||
| Nonfatal | 2 | 0.52 | 0.28–0.94 | 96.4 | |
| Fatal | 8 | 0.51 | 0.36–0.73 | 98.2 | |
| Geographic region | 0.781 | ||||
| Europe | 6 | 0.70 | 0.60–0.81 | 73.3 | |
| North America | 7 | 0.72 | 0.61–0.83 | 90.3 | |
| Eastern Asia | 5 | 0.75 | 0.65–0.88 | 81.3 | |
| Follow‐up time | 0.594 | ||||
| <5 y | 3 | 0.77 | 0.69–0.87 | 0.0 | |
| 5 to 9 y | 11 | 0.70 | 0.63–0.78 | 89.0 | |
| ≥10 y | 4 | 0.81 | 0.67–0.98 | 73.1 | |
| Age distribution | 0.337 | ||||
| <65 y | 2 | 0.87 | 0.83–0.91 | 96.5 | |
| ≥65 y | 3 | 0.68 | 0.60–0.78 | 0.0 | |
| Women, % | 0.419 | ||||
| <50 | 6 | 0.69 | 0.65–0.74 | 2.5 | |
| ≥50 | 12 | 0.74 | 0.67–0.82 | 87.5 | |
| Levels of prevention | 0.888 | ||||
| PP for stroke | 15 | 0.73 | 0.66–0.80 | 87.1 | |
| SP for stroke | 3 | 0.73 | 0.66–0.81 | 9.2 | |
| Quality score | 0.065 | ||||
| <8 | 5 | 0.81 | 0.72–0.91 | 82.0 | |
| ≥8 | 13 | 0.70 | 0.65–0.75 | 58.1 |
AHM indicates antihypertensive medication; PI, P interaction; PP, primary prevention; SP, secondary prevention.
Pooled relative risks (RRs) and 95% CIs were estimated using a random‐effects model.
Dose‐Response Meta‐Analysis
Ten studies were included in the dose‐response analysis of the association between AHM adherence level and risk of stroke.12, 23, 25, 27, 28, 30, 31, 32, 33, 34 Using a restricted cubic splines model, we found no evidence for the nonlinear relationship between the AHM adherence level and the risk of stroke (P trend<0.001, P nonlinearity=0.755). The dose‐response analysis indicated that a 20% increment in AHM adherence level was associated with a 9% lower risk of stroke (RR, 0.91; 95% CI, 0.86–0.96) (Figure 3). Three studies were included to estimate the dose‐response analysis of AHM adherence and risk of IS.12, 27, 34 We also did not detect a nonlinear relationship between AHM adherence and the risk of IS (P trend<0.001, P nonlinearity=0.629), and a 20% increment in the AHM adherence level was associated with a 6% lower risk of IS (RR, 0.94; 95% CI, 0.91–0.96) (Figure 4).
Figure 3.
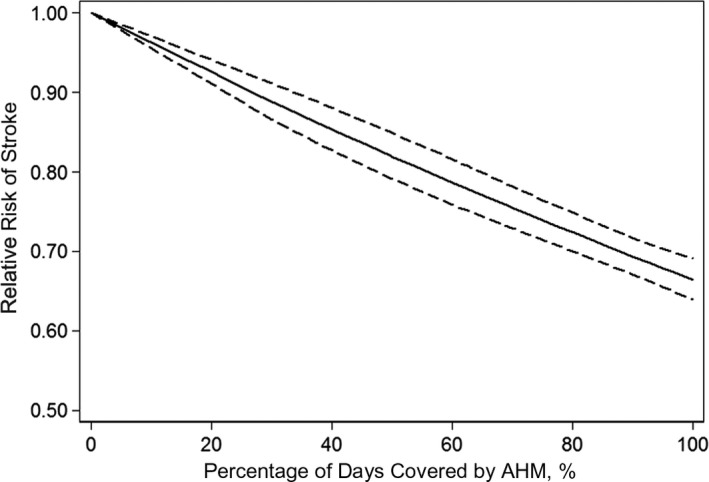
Pooled dose‐response analysis of antihypertensive medication (AHM) adherence and total stroke risk (solid line). Dashed lines represent the 95% CI.
Figure 4.
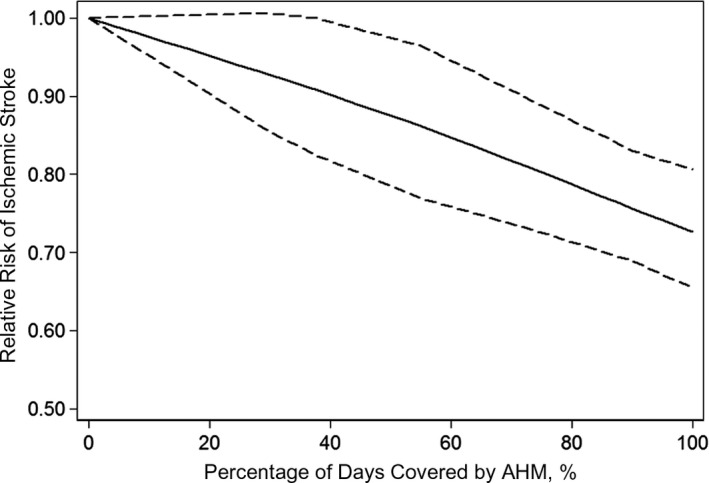
Pooled dose‐response analysis of antihypertensive medication (AHM) adherence and ischemic stroke risk (solid line). Dashed lines represent the 95% CI.
Heterogeneity Assessment and Sensitivity Analyses
A meta‐regression analysis was conducted to explore the potential sources of heterogeneity. The varied stroke subtype, quality of the included studies, and age distribution accounted for the main heterogeneity among the included studies, respectively. Adherence to AHM does not represent the only factor influencing the risk of stroke. The number and class of prescribed AHMs and the use of other cerebrovascular preventive medications (eg, antiplatelet agents, anticoagulants, lipid‐lowering agents, and antidiabetic agents) were also primary factors that may influence stroke risk2 When we excluded the studies that did not report details of adjusted confounders in multivariate analysis, the inverse association between high AHM adherence and stroke risk remained significant (Table S4).
Publication Bias
The funnel plot was asymmetric (Figure S1) on visual inspection. However, Begg's test showed no evidence of publication bias in our meta‐analysis (P=0.495) (Figure S2).
Discussion
To the best of our knowledge, this is the first dose‐response meta‐analysis to investigate the association between AHM adherence and stroke risk. By incorporating 18 observational studies involving 1 356 188 patients, we found a significant inverse association between AHM adherence and stroke risk. The dose‐response analysis suggested that a 20% increment in the AHM adherence level was associated with a 9% lower risk of stroke. This meta‐analysis strengthens and extends the understanding of the positive impact of AHM adherence on stroke prevention, further supporting the notion that improved AHM adherence may be associated with improve stroke prevention in patients with hypertension.
Adherence to certain medication has been defined as the extent to which a patient takes medication as prescribed by their healthcare providers, which has a direct influence on the prognosis of a disease, especially for the prevention or treatment of chronic diseases.5 Hypertension, one of the most prevalent chronic diseases worldwide, could cause serious and irreversible vasculopathy in the brain, thereby representing a known risk factor and treatment target for stroke.2, 3 AHM can effectively control blood pressure and has been confirmed as a major strategy for stroke prevention among patients with hypertension.3, 30 However, in both developed and developing countries, poor adherence to AHM has been raised as a serious concern.7, 12, 30 Notably, a high prevalence rate of poor adherence to AHM has been reported in previous studies (ranging from 20% to 60% among the included studies of this meta‐analysis), primarily because of the deficiencies in health systems, poor health education, and issues related to the patient's income.7 Poor adherence to AHM limited the efficacy of AHM against stroke and has been highlighted as a remarkable obstacle to achieve better stroke prevention outcomes.11, 30, 37 Therefore, better adherence to AHM treatment should be highlighted in the clinical prevention of stroke.
This meta‐analysis was based on studies in real‐world practice, and its results support the importance of improved AHM adherence in preventing stroke among patients with hypertension. Increasing evidence has indicated that AHM not only could ameliorate hypertension and improve vascular structure and function but also have neuroprotective effects, such as the regulation of endothelial NO synthase, anti‐inflammatory effects, and cerebral hemodynamics‐improving effects, which may be potential mechanisms underlying their preventative effects against stroke.3, 38, 39 The results of stratified analyses indicated that higher AHM adherence was associated with lowered risks of both IS and HS, but more remarkably in HS. This is consistent with a previous study showing that hypertension was more associated with HS than IS.2 In addition, the results of our subgroup analyses showed that higher AHM adherence was associated with lower risks of both fatal and nonfatal stroke. This is important considering that stroke has become one of the most important causes of mortality all over the world.1 The subgroup analysis also indicated that the stroke prevention effect of high AHM adherence was more significant in patients 65 years and older compared with patients younger than 65 years, partly because patients from the 2 age groups had different stroke causes, risk factors, and comorbidities.
We subsequently evaluated whether the association between AHM adherence and stroke risk was dose dependent. The inverse association between AHM adherence and stroke risk was linear, and a 20% increment in AHM adherence level was associated with a 9% reduction in stroke risk. Moreover, a 20% increment in AHM adherence level was associated with a 6% reduction in IS risk. Based on our comprehensive review of the literature, only 2 studies12, 35 to date have reported a significant benefit for improved AHM adherence in the prevention of HS, and only 1 of these studies12 found a dose‐response relationship between AHM adherence and the risk of HS. Therefore, whether the association between AHM adherence and risk of HS is dose dependent deserves further investigation. In addition to the quantification of AHM adherence, the discontinuation of AHM use also indicated poor AHM adherence. Of the studies included in this meta‐analysis, the study by Breekveldt‐Postma et al36 investigated the effect of AHM discontinuation on the risk of stroke, and the results of this study suggested that AHM discontinuation was significantly associated with a higher risk of stroke.
Study Limitations
Several limitations of this meta‐analysis should be considered when interpreting the results. First, 4 of the included studies evaluated the risk of cardiovascular morbidity in general, including the risk of stroke, but not exclusively on the risk of stroke.25, 28, 32, 33 Sensitivity analysis indicates that when the 4 studies were excluded the inverse association between AHM adherence and stroke risk was also significant (RR, 0.73; 95% CI, 0.66–0.80). Second, the information regarding AHM adherence was primarily from administrative data and not directly from face‐to‐face interviews with patients. These administrative data do not necessarily indicate whether the patients actually took the AHM, which may cause a misclassification of the AHM adherence level. In addition, of the included studies, 2 had relatively different assessments of AHM adherence compared with the others.34, 36 The sensitivity analysis indicates that when the 2 studies were excluded, the pooled estimation had no significant change. Third, a detailed category of the adherence level is important for a dose‐response meta‐analysis. However, the long interval between the boundaries of the category of the adherence level in several studies may increase the heterogeneity of the results from dose‐response meta‐analysis and lead to an underestimation of the true association between AHM adherence level and stroke risk. Fourth, different classes of AHM may have different effects on stroke prevention; therefore, a different class of AHM is a key confounder for the association between AHM adherence and stroke risk. It is critical to make a separately quantitative assessment for the associations of different classes of AHM. However, none of the included studies in this meta‐analysis reported the association in different classes of AHM. Of the included studies in this meta‐analysis, 5 made an adjustment for the class of AHM in their analysis,13, 27, 28, 29, 36 and the pooled estimation of these studies indicated that the inverse association between AHM adherence and stroke risk was also significant. Fifth, the adherence to AHM is not the only factor that influences the effect of AHM on the risk of stroke. Other confounders (eg, baseline blood pressure, severity of hypertension, and whether target blood pressure values in patients with hypertension were achieved) also influence stroke prevention in patients with hypertension. However, most of the included studies did not report whether they made adjustments for these confounders, which may have reduced the strength of our results. Only the study by Gosmanova et al26 reported an adjustment for the baseline blood pressure in their analysis, and the results of this study also indicated a significantly inverse association between AHM adherence and stroke risk.
Conclusions
Higher AHM adherence is dose‐dependently associated with lower risk of stroke in patients with hypertension. These findings highlight the need to optimize AHM treatment strategies to maximize the beneficial effects of AHM for the prevention of stroke. According to the data reported by the included studies in this meta‐analysis, the prevalence of poor adherence to AHM was high in patients with hypertension (ranging from 20% to 60%). Therefore, more efforts should be encouraged to improve adherence to AHM, which may provide significant long‐term benefits for patients with hypertension.
Sources of Funding
This work was supported by the National Science Foundation of China (No. 81571259).
Disclosures
None.
Supporting information
Data S1. Literature search terms.
Table S1. The Data Source and Diseases Classification in Each Included Study
Table S2. The Confounders Adjusted for the Multivariate Analysis in Each Included Study
Table S3. Quality Assessment of the Included Studies*
Table S4. Sensitivity Analysis for the Main Confounders*
Figure S1. Funnel plot for publication bias test.
Figure S2. Publication bias test for the association between antihypertensive agents adherence and stroke risk. Begg's test, z=0.680 (continuity corrected); P>|z|=0.495 (continuity corrected).
(J Am Heart Assoc. 2017;6:e006371 DOI: 10.1161/JAHA.117.006371.)28743788
References
- 1. Global, regional, and national life expectancy, all‐cause mortality, and cause‐specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1459–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. O'Donnell MJ, Chin SL, Rangarajan S, Xavier D, Liu L, Zhang H, Rao‐Melacini P, Zhang X, Pais P, Agapay S, Lopez‐Jaramillo P, Damasceno A, Langhorne P, McQueen MJ, Rosengren A, Dehghan M, Hankey GJ, Dans AL, Elsayed A, Avezum A, Mondo C, Diener HC, Ryglewicz D, Czlonkowska A, Pogosova N, Weimar C, Iqbal R, Diaz R, Yusoff K, Yusufali A, Oguz A, Wang X, Penaherrera E, Lanas F, Ogah OS, Ogunniyi A, Iversen HK, Malaga G, Rumboldt Z, Oveisgharan S, Al Hussain F, Magazi D, Nilanont Y, Ferguson J, Pare G, Yusuf S. Global and regional effects of potentially modifiable risk factors associated with acute stroke in 32 countries (INTERSTROKE): a case‐control study. Lancet. 2016;388:761–775. [DOI] [PubMed] [Google Scholar]
- 3. Ettehad D, Emdin CA, Kiran A, Anderson SG, Callender T, Emberson J, Chalmers J, Rodgers A, Rahimi K. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta‐analysis. Lancet. 2016;387:957–967. [DOI] [PubMed] [Google Scholar]
- 4. Turnbull F. Effects of different blood‐pressure‐lowering regimens on major cardiovascular events: results of prospectively‐designed overviews of randomised trials. Lancet. 2003;362:1527–1535. [DOI] [PubMed] [Google Scholar]
- 5. Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–497. [DOI] [PubMed] [Google Scholar]
- 6. Hedegaard U, Kjeldsen LJ, Pottegard A, Henriksen JE, Lambrechtsen J, Hangaard J, Hallas J. Improving medication adherence in patients with hypertension: a randomized trial. Am J Med. 2015;128:1351–1361. [DOI] [PubMed] [Google Scholar]
- 7. Tsiantou V, Pantzou P, Pavi E, Koulierakis G, Kyriopoulos J. Factors affecting adherence to antihypertensive medication in Greece: results from a qualitative study. Patient Prefer Adherence. 2010;4:335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta‐analysis of observational studies in epidemiology: a proposal for reporting. Meta‐analysis of observational studies in epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. [DOI] [PubMed] [Google Scholar]
- 9. Vrijens B, De Geest S, Hughes DA, Przemyslaw K, Demonceau J, Ruppar T, Dobbels F, Fargher E, Morrison V, Lewek P, Matyjaszczyk M, Mshelia C, Clyne W, Aronson JK, Urquhart J. A new taxonomy for describing and defining adherence to medications. Br J Clin Pharmacol. 2012;73:691–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Khan NA, Yun L, Humphries K, Kapral M. Antihypertensive drug use and adherence after stroke: are there sex differences? Stroke. 2010;41:1445–1449. [DOI] [PubMed] [Google Scholar]
- 11. Perreault S, Yu AY, Cote R, Dragomir A, White‐Guay B, Dumas S. Adherence to antihypertensive agents after ischemic stroke and risk of cardiovascular outcomes. Neurology. 2012;79:2037–2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kim S, Shin DW, Yun JM, Hwang Y, Park SK, Ko YJ, Cho B. Medication adherence and the risk of cardiovascular mortality and hospitalization among patients with newly prescribed antihypertensive medications. Hypertension. 2016;67:506–512. [DOI] [PubMed] [Google Scholar]
- 13. Bailey JE, Wan JY, Tang J, Ghani MA, Cushman WC. Antihypertensive medication adherence, ambulatory visits, and risk of stroke and death. J Gen Intern Med. 2010;25:495–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stang A. Critical evaluation of the Newcastle‐Ottawa scale for the assessment of the quality of nonrandomized studies in meta‐analyses. Eur J Epidemiol. 2010;25:603–605. [DOI] [PubMed] [Google Scholar]
- 15. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials. 1986;7:177–188. [DOI] [PubMed] [Google Scholar]
- 16. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ. 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Borenstein M, Higgins JP, Hedges LV, Rothstein HR. Basics of meta‐analysis: I 2 is not an absolute measure of heterogeneity. Res Synth Methods. 2017;8:5–18. [DOI] [PubMed] [Google Scholar]
- 18. Patterson C, Chambers LW. Preventive health care. Lancet. 1995;345:1611–1615. [DOI] [PubMed] [Google Scholar]
- 19. Thompson SG, Higgins JP. How should meta‐regression analyses be undertaken and interpreted? Stat Med. 2002;21:1559–1573. [DOI] [PubMed] [Google Scholar]
- 20. Greenland S, Longnecker MP. Methods for trend estimation from summarized dose‐response data, with applications to meta‐analysis. Am J Epidemiol. 1992;135:1301–1309. [DOI] [PubMed] [Google Scholar]
- 21. Berlin JA, Longnecker MP, Greenland S. Meta‐analysis of epidemiologic dose‐response data. Epidemiology. 1993;4:218–228. [DOI] [PubMed] [Google Scholar]
- 22. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 23. Yang Z, Howard DH, Will J, Loustalot F, Ritchey M, Roy K. Association of antihypertensive medication adherence with healthcare use and Medicaid expenditures for acute cardiovascular events. Med Care. 2016;54:504–511. [DOI] [PubMed] [Google Scholar]
- 24. Herttua K, Martikainen P, Batty GD, Kivimaki M. Poor adherence to statin and antihypertensive therapies as risk factors for fatal stroke. J Am Coll Cardiol. 2016;67:1507–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Krousel‐Wood M, Holt E, Joyce C, Ruiz R, Dornelles A, Webber LS, Morisky DE, Frohlich ED, Re RN, He J, Whelton PK, Muntner P. Differences in cardiovascular disease risk when antihypertensive medication adherence is assessed by pharmacy fill versus self‐report: the Cohort Study of Medication Adherence among Older Adults (CoSMO). J Hypertens. 2015;33:412–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gosmanova EO, Molnar MZ, Alrifai A, Lu JL, Streja E, Cushman WC, Kalantar‐Zadeh K, Kovesdy CP. Impact of non‐adherence on renal and cardiovascular outcomes in US Veterans. Am J Nephrol. 2015;42:151–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xu J, Zhao X, Wang Y, Wang C, Liu L, Sun B, Wang A, Wang Y. Impact of a better persistence with antihypertensive agents on ischemic stroke outcomes for secondary prevention. PLoS One. 2013;8:e65233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wong MC, Tam WW, Cheung CS, Wang HH, Tong EL, Sek AC, Yan BP, Cheung NT, Leeder S, Yu CM, Griffiths S. Drug adherence and the incidence of coronary heart disease‐ and stroke‐specific mortality among 218,047 patients newly prescribed an antihypertensive medication: a five‐year cohort study. Int J Cardiol. 2013;168:928–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shin S, Song H, Oh SK, Choi KE, Kim H, Jang S. Effect of antihypertensive medication adherence on hospitalization for cardiovascular disease and mortality in hypertensive patients. Hypertens Res. 2013;36:1000–1005. [DOI] [PubMed] [Google Scholar]
- 30. Herttua K, Tabak AG, Martikainen P, Vahtera J, Kivimaki M. Adherence to antihypertensive therapy prior to the first presentation of stroke in hypertensive adults: population‐based study. Eur Heart J. 2013;34:2933–2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Degli Esposti L, Saragoni S, Benemei S, Batacchi P, Geppetti P, Di Bari M, Marchionni N, Sturani A, Buda S, Degli Esposti E. Adherence to antihypertensive medications and health outcomes among newly treated hypertensive patients. Clinicoecon Outcomes Res. 2011;3:47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Corrao G, Parodi A, Nicotra F, Zambon A, Merlino L, Cesana G, Mancia G. Better compliance to antihypertensive medications reduces cardiovascular risk. J Hypertens. 2011;29:610–618. [DOI] [PubMed] [Google Scholar]
- 33. Mazzaglia G, Ambrosioni E, Alacqua M, Filippi A, Sessa E, Immordino V, Borghi C, Brignoli O, Caputi AP, Cricelli C, Mantovani LG. Adherence to antihypertensive medications and cardiovascular morbidity among newly diagnosed hypertensive patients. Circulation. 2009;120:1598–1605. [DOI] [PubMed] [Google Scholar]
- 34. Liu PH, Hu FC, Wang JD. Differential risks of stroke in pharmacotherapy on uncomplicated hypertensive patients? J Hypertens. 2009;27:174–180. [DOI] [PubMed] [Google Scholar]
- 35. Kettani FZ, Dragomir A, Cote R, Roy L, Berard A, Blais L, Lalonde L, Moreau P, Perreault S. Impact of a better adherence to antihypertensive agents on cerebrovascular disease for primary prevention. Stroke. 2009;40:213–220. [DOI] [PubMed] [Google Scholar]
- 36. Breekveldt‐Postma NS, Penning‐van Beest FJ, Siiskonen SJ, Falvey H, Vincze G, Klungel OH, Herings RM. The effect of discontinuation of antihypertensives on the risk of acute myocardial infarction and stroke. Curr Med Res Opin. 2008;24:121–127. [DOI] [PubMed] [Google Scholar]
- 37. Xu J, Wang CX, Wang YL, Zhao XQ, Liu LP, Wang AX, Wang YJ. Persistence with antihypertensive agents for 12 months after ischemic stroke reduces the rates of death and dependency. CNS Neurosci Ther. 2013;19:142–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cohuet G, Struijker‐Boudier H. Mechanisms of target organ damage caused by hypertension: therapeutic potential. Pharmacol Ther. 2006;111:81–98. [DOI] [PubMed] [Google Scholar]
- 39. Michel MC, Brunner HR, Foster C, Huo Y. Angiotensin II type 1 receptor antagonists in animal models of vascular, cardiac, metabolic and renal disease. Pharmacol Ther. 2016;164:1–81. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Literature search terms.
Table S1. The Data Source and Diseases Classification in Each Included Study
Table S2. The Confounders Adjusted for the Multivariate Analysis in Each Included Study
Table S3. Quality Assessment of the Included Studies*
Table S4. Sensitivity Analysis for the Main Confounders*
Figure S1. Funnel plot for publication bias test.
Figure S2. Publication bias test for the association between antihypertensive agents adherence and stroke risk. Begg's test, z=0.680 (continuity corrected); P>|z|=0.495 (continuity corrected).


