Abstract
Objectives
Cannabis is the most commonly used illicit drug; a substantial minority of users develop dependence. The current lack of pharmacological treatments for cannabis dependence warrants the use of novel approaches and further investigation of promising pharmacotherapy. In this case series, we assessed the use of self-titrated dosages of Sativex (1:1, Δ9-tetrahydrocannabinol (THC)/cannabidiol (CBD) combination) and Motivational Enhancement Therapy and Cognitive Behavioral Therapy (MET/CBT) for the treatment of cannabis dependence among five treatment-seeking community-recruited cannabis-dependent subjects.
Methods
Participants underwent a 3-month open-label self-titration phase with Sativex (up to 113.4 of THC/105 mg of CBD) and weekly MET/CBT, with a 3-month follow-up.
Results
Sativex was well tolerated by all participants (average dosage 77.5 THC/71.7 mg CBD). The amount of cannabis use decreased with no significant increases in withdrawal. Craving scores increased during the first 2 weeks but progressively returned to baseline levels from the third week of treatment. THC/CBD metabolites’ concentration indicated reduced cannabis use and compliance with medication.
Conclusions
In summary, this pilot study found that with Sativex in combination with MET/CBT cannabis use decreased and withdrawal did not increase in the four participants completing the study. Further systematic exploration of Sativex as a pharmacological treatment option for cannabis dependence should be performed.
Keywords: Cannabis, Marijuana, Withdrawal, THC, Cannabidiol, Abstinence
Introduction
Cannabis is the most widely used illicit substance worldwide (Crime 2010). Research indicates that about 7–9% of those who ever use cannabis develop cannabis dependence (Anthony et al. 1994, Lev-Ran et al. 2013, Volkow et al. 2014). Cannabinoid agonists are among the most promising approaches for treatment of cannabis use disorders (CUD), but have not yet demonstrated their efficacy (Marshall et al. 2014). Due to the large potential impact of cannabis use problems on individuals and society, several studies investigated the use of cannabinoid receptor agonist/partial agonist as potential treatments of cannabis dependence treatment, using a less addictive agonist/partial agonist (Budney et al. 2007, Nordstrom and Levin 2007, Elkashef et al. 2008, Vandrey and Haney 2009, Allsop et al. 2014, Marshall et al. 2014). In addition, avoidance of combustible forms of drug delivery and second hand smoke exposure are also eliminated when oral formulations are used.
Sativex is a 1:1 Δ9-tetrahydrocannabinol (THC)/cannabidiol (CBD) orally bioavailable combination, currently approved for the treatment of spasticity due to multiple sclerosis, that was found to reduce cannabis withdrawal and improve retention in treatment in absence of evident intoxicating effects (Allsop et al. 2014, Trigo et al. 2016). THC and CBD appear to have different properties. THC is a CB1 and CB2 cannabinoid receptor partial agonist, whereas CBD is a CB1/2 antagonist with activity at multiple targets in the “expanded endocannabinoid system” (Pertwee 2008, McPartland et al. 2015). Differential effects were observed following THC and CBD administration in humans. Under certain conditions THC produced psychotic-like and anxiogenic effects (D'Souza et al. 2004, D'Souza et al. 2005, D'Souza et al. 2008). On the other hand, it was suggested that CBD may have anti-psychotic effects in humans (Zuardi et al. 2006) and anxiolytic properties in preclinical studies (Guimaraes et al. 1990). In addition, CBD seems to exert effects on the extinction of cocaine/amphetamine (Parker et al. 2004) and cue-induced reinstatement of heroin seeking (Ren et al. 2009). However, the possible effects of CBD or nearly 1:1 combinations of THC and CBD on cannabis-related addictive behaviors remains unclear (Karschner et al. 2011, Prud'homme et al. 2015).
The objective of this pilot study was to determine whether a self-titrated dosage of Sativex was well-tolerated and sufficient to result in effects on cannabis use, craving and withdrawal in participants seeking treatment for cannabis dependence.
Case Series
Inclusion Criteria were a) adult male or female; b) understand and willing to comply with study requirements and restrictions; c) willing to use appropriate contraceptive method throughout the study; d) otherwise healthy as judged by investigator based on medical history, physical exam, vitals, ECG and labs; e) DSM-IV criteria for current marijuana dependence; f) report marijuana as primary drug of abuse; g) report using marijuana at least 5 days a week for at least one month; h) have marijuana positive urine drug screen; i) treatment seeking cannabis smoker; j) smoke less than or equal to the equivalent of 4 joints per day (or four grams per day if participants smokes cannabis in other forms).
Exclusion criteria were a) meets DSM-IV criteria for a current axis I disorder including substance use disorder other than cannabis, nicotine or caffeine dependence; b) first-degree relative with schizophrenia; c) history of seizures; d) history of cardiovascular disease; e) history of pulmonary disease such as asthma, COPD; f) clinically significant pathology in oral cavity and poor oral hygiene; g) known sensitivity to dronabinol, cannabidiol, propylene glycole, ethanol or peppermint oil (used in Sativex buccal spray); h) unstable medical conditions; i) pregnant or breast-feeding; j) currently taking psychotropic medication with benefit for any other illness than treatment of insomnia; k) holding a job that involves driving, operating heavy machines.
Procedure
This open-label NIH funded pilot study was performed to assess feasibility for a larger double-blind study and Sativex tolerability (GW Pharmaceuticals, Cambridge, UK). Five participants, meeting DSM-IV criteria for current cannabis dependence (but otherwise healthy), underwent 12 weeks treatment (self-titrated Sativex gradually increasing –days 1 to 10- maximum of 42 oromucosal sprays, equal to 113.4 mg THC/105 mg CBD) and Motivational Enhancement Therapy and Cognitive Behavioral Therapy (MET/CBT). The target day to quit cannabis was day 21. Treatment was followed by a 12-week follow-up phase with 4 weekly visits (weeks 13–16) and 2 subsequent monthly visits (weeks 20 and 24).
Participants, recruited by media advertisements and flyers placed within the Greater Toronto area (Canada), underwent pre-screening via a telephone interview. Those meeting initial study criteria were invited to an in-person screening visit where informed consent was obtained and eligibility criteria assessed (baseline).
Methods
This study corresponds to the pilot phase of a double blind placebo controlled trial and was conducted in compliance with Good Clinical Practice guidelines and applicable regulatory requirements. The study was approved by the institutional research ethics board (#144/2011), Health Canada (control #152049) and registered on Clinical Trials.gov (ID# NCT 01747850).
Eligible subjects began treatment on the first visit following the baseline visit. Participants were instructed in the use of the study medication and were observed for a period of two hours following their first dose to ensure medication tolerability. Participants were also instructed in the completion of a smoking diary during the first study visit, to monitor the frequency and quantity of cannabis and medication use, each study day. Use of cannabis was recorded for all forms that could be used (joints, pipes, ingested, etc.) (Mason et al. 2012). New Sativex vials were provided to subjects during the weekly visits. The vials were weighed (before and after) to assess usage.
All participants completed two weekly assessment visits Monday to Friday. On one of the visits, participants underwent individual MET/CBT (approximately 1 hour in duration) with a trained psychologist for the full 12 weeks of the medication phase. During the other weekly visit, participants underwent several assessments including: Cannabis Withdrawal Checklist (CWC) (Budney et al. 1999), Cannabis Craving Questionnaire (CCQ) (Heishman et al. 2001), Timeline Followback (TLFB) for cannabis, tobacco, caffeine and alcohol (Sobell et al. 1988). Participants provided urine samples two times per week (one per visit) for a ten-panel urine drug test to assess for illicit drug use. These urine samples also were quantified for THC and CBD at the Chemistry and Drug Metabolism Section, National Institute on Drug Abuse using previously described methodology (Lowe et al. 2007, Schwope et al. 2011). Participants were considered abstinent based on self-reports from TLFB, smoking diaries, and preliminary urine drug tests (QuickScreen™ Cup Multi Drug Screening Test, Confirm Biosciences, San Diego, CA, USA).
Participants received up to a maximum of $855CAD in compensation for their participation, including public transit cost reimbursement, and small prizes (0–50$) implemented to engage participants and to maximize returns to the laboratory for study visits.
The Sativex medication spray was provided free-of-charge by GW Pharmaceuticals.
Results
A total of 33 participants were invited to a screening visit between 05–2013 and 05–2014. Twelve participants did not attend or lost interest before the screening visit; 14 participants were ineligible (3 due to a medical condition, 11 due to axis I disorder). A total of 7 participants were deemed eligible to receive Sativex but only 5 participants initiated treatment (2 participants were eligible but no longer interested following completion of screening procedures). Case #1 was a Black North American male, age 30, with non-finished high school education and on welfare at the time of enrollment. He reported 8–10 years history of use of cannabis (≥5 days/week) with first use of cannabis age 12. He connected with the study team seeking for treatment to quit or cut down his cannabis use. Case #2 was a White North American female, age 24, with completed high school education and not employed at the time of enrollment. She reported current use of cannabis ≥5 days/week and first/regular use of cannabis around age 12. She connected with the study team seeking for treatment to quit cannabis. Case #3 was an Asian female, age 43, with college degree completed education and self-employed. She reported several years history of use of cannabis (≥5 days/week) and first/regular use of cannabis at age 24. She connected with the study team seeking for treatment to quit cannabis after being unable to quit on her own. Case #4 was a Caucasian male, age 43, with completed university education and working in part time jobs. He reported 2 years history of use of cannabis ≥5 days/week and first use of cannabis at age 25. He connected with the study team seeking for treatment to quit cannabis after many unsuccessful attempts to quit on his own. Case #5 was a White European male, age 21, with completed high school education, not employed. He reported use of cannabis ≥5 days/week since he was 19–20 years old and first use of cannabis around age 16. He was referred by his parents as he was unable to hold down jobs because of his cannabis use.
Four participants completed the entire experimental sequence (6 months). One participant was excluded from the study before completing the treatment phase (incompatible schedule with the study) (case #5). Study completers reported using cannabis 6.5 days (SD=1) per week (92.9%) (range 5 – 7 days/week) at baseline, consuming an average 9.5 g (SD=6.3) per week (case #1 1 g/day, #2 2.7 g/day, #3 0.9 g/day, #4 0.9 g/day, mean values) (see Table 1 for demographics, substance use assessments and psychosocial functioning scores).
Table 1.
Demographics
| Characteristics | Completers n = 4 |
|---|---|
| Demographics, No. (%) | |
| Age, years, mean (SD) | 35.0 (9.6) |
| Male | 2 (50%) |
| White North American | 1 (25%) |
| Black North American | 1 (25%) |
| Asian | 1 (25%) |
| Caucasian | 1 (25%) |
| College/University degree | 2 (50%) |
| Full-time employed | 0 (0%) |
| Married | 1 (25%) |
| Substance Abuse Assessment, mean (SD) | |
| Addiction Severity Index | |
| Employment | 0.2 (0.3) |
| Medical Status | 0.0 (0.0) |
| Psychiatric Status | 0.0 (0.0) |
| Family/Social | 0.0 (0.1) |
| Alcohol Use | 0.0 (0.1) |
| Drug Use | 0.1 (0.1) |
| Legal Status | 0.0 (0.0) |
| Fagerstrom Test for Nicotine Dependence | 0.2 (0.4) |
| Psychosocial functioning scores, mean (SD) | |
| Hamilton Anxiety | 2.5 (2.6) |
| Hamilton Depression Rating Scale | 1.5 (2.4) |
| Beck Depression Inventory | 2.5 (1.3) |
| Brief Psychiatric Rating Scale | 19.8 (2.9) |
| Profile of Mood States | 0.8 (11.9) |
| St. Mary’s Sleep Questionnaire | |
| Sleep Latency (min) | 17.5 (15.0) |
| Sleep Duration (min) | 437.5 (95.0) |
| Sleep Quality | 15.3 (1.3) |
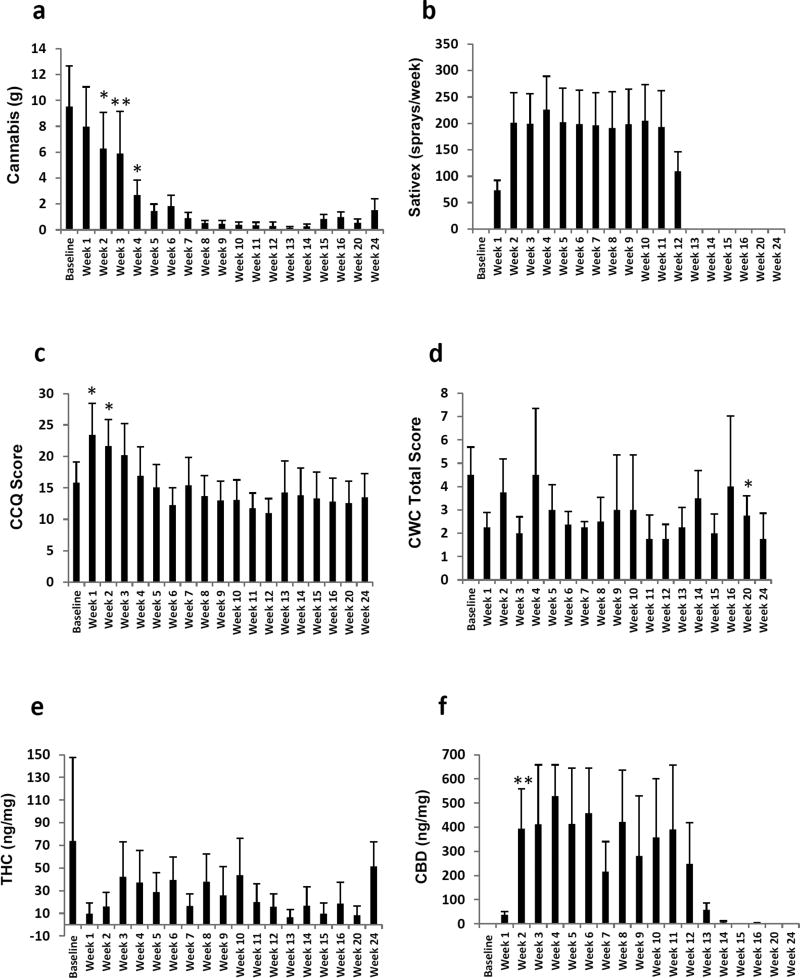
Daily cannabis/medication use was assessed via self-report using the TLFB questionnaire and a smoking diary (Figure 1). TLFB results for cannabis intake were consistent with smoking diary entries. Repeated measures analyses of variance (ANOVA) for use of cannabis according to the TLFB showed a significant effect for time (F(18,54)=4.663, p<0.001), indicating a reduction in cannabis intake over the course of treatment (Figure 1a). Additionally, TLFB showed no compensatory increases, in use of other substances (tobacco, caffeine and alcohol), when participants reduced their cannabis use or remained abstinent (all p, s ns; data not shown). One of four participants in the study achieved cannabis abstinence by target day (day 21) and remained abstinent during the study (case #3). The remaining participants (three) reduced their cannabis use more gradually and two were able to remain abstinent up to four consecutive weeks (case #2 and 3). Two participants (case #2 and 3) showed seven-day point prevalence of cannabis abstinence one week after the end of treatment. The average use of cannabis one week after the end of treatment was 17.9% days/week (range 0 – 4 days/week) vs 92.9% at baseline (case #1, 2, 3 and 4).
Figure 1. Effects of Sativex and Motivational Enhancement Therapy / Cognitive Behavioral Therapy (MET/CBT) intervention on cannabis dependence.
Columns represent average values (+SEM) for the four participants completing treatment and follow-up phases: a) cannabis intake (g) per week as reported on the timeline followback (TLFB), b) Sativex self-titration (sprays/week) as reported in the smoking diary, c) craving for cannabis was determined using the Cannabis Craving Questionnaire (CCQ), d) cannabis withdrawal was measured using the Cannabis Withdrawal Checklist (CWC). Cannabinoids of interest in urine were quantified using a two-dimensional gas chromatography-mass spectrometry method (2D-GCMS), e) creatinine-normalized urine ∆9-tetrahydrocannabinol (THC) concentrations, f) creatinine-normalized urine cannabidiol (CBD) concentrations. * (p<0.05), ** (p<0.01) vs baseline. Repeated measures ANOVA followed by pairwise comparisons.
Participants’ self-reported Sativex compliance by daily recording the number of sprays in the smoking diary provided. Self-reports on medication use was collected in smoking diaries (Figure 1b), which were corroborated by the vials’ weight recordings (data not shown). Participants in this study self-titrated on average 28.7 sprays/day (equivalent to 77.5 mg THC/71.7mg CBD; range 0 – 42 sprays/day). The information contained in the smoking diary regarding other illegal drug use was verified through ten-panel drug tests performed on site at the time of the visits. No other use of illegal drugs was reported (i.e. in the smoking diary) and none were found on the drug checks performed on site.
Craving for cannabis was determined using the CCQ (Figure 1c) and the craving scores on the CWC. Repeated measures ANOVA showed significant changes in craving during the study on the CCQ (F(18,54)= 7.091, p<0.001). Pairwise comparisons showed increased craving scores during the first 2 weeks of treatment as compared to baseline. Craving scores were not different from baseline from the third week of treatment or following treatment completion. However, craving scores on the CWC showed decreased craving scores from week 9 and no significant increases on craving in the initial weeks of treatment (data not shown).
The effects of Sativex + MET/CBT on cannabis withdrawal were determined using the CWC. Repeated measures ANOVA showed no significant differences on withdrawal scores along the study (F(18,54)=0.805, p ns) (Figure 1d).
Creatinine-normalized urine cannabinoid concentrations are shown in Figure 1(e-f). A tendency for decreased THC concentrations can be observed from week 1 of treatment. On the other hand, CBD concentrations progressively increased once participants started medication and decreased after the treatment phase. These results are consistent with the adherence of participants to the experimental conditions (i.e. Sativex intake) and the reduction on the use of cannabis as reported in the Smoking diary and TLFB, respectively.
We did not observe serious adverse events associated with Sativex. Adverse events observed in the study included events not related to the study intervention (e.g. mild cold, skin rash, mild headache) and moderate/mild expected side effects, such as nausea, dry mouth, sleep problems or diarrhea.
Discussion
In this study we observed good tolerability of self-titrated Sativex dosages in treatment seeking adults with cannabis dependence. We were expecting that this dosage (up to 113.4 of THC/105 mg of CBD) might be well-tolerated, since the subjects already had developed tolerance due to their cannabis use. In a previous study, we observed that high fixed dosages of Sativex (108 mg THC and 100 mg CBD, equivalent to 40 sprays) were also well-tolerated in non-treatment seeking cannabis dependent users (Trigo et al. 2016). On the other hand, the self-titration regimen might allow participants to adjust their intake based on tolerability. The amount of Sativex self-titrated in this study is similar to the 29.7 sprays/day average intake in non-treatment seeker individuals during cannabis abstinence (Trigo et al. 2016). Both participants’ self-reports and metabolite concentrations indicate compliance with study medication and reported abstinence.
In our study, participants significantly reduced cannabis use that persisted at the end of the 3 months follow-up. A recent study with Sativex also showed a significant reduction in cannabis use following Sativex treatment (Allsop et al. 2014), although differences in the cannabis use in the Allsop et al. study did not differ with respect to the placebo group.
In this pilot study the combination Sativex + MET/CBT prevented cannabis withdrawal when participants quit or reduced their cannabis use. This finding is in agreement with recent studies showing reduced cannabis withdrawal following Sativex (Allsop et al. 2014, Trigo et al. 2016). On the other hand, our results in craving are somewhat contradictory, depending on the craving scale used. Namely, CCQ showed an initial 2-weeks’ increase in craving, while the CWC showed decreased craving scores from week 9 with no increased craving during the initial weeks of treatment. All in all, our results suggest the feasibility of our approach and that the combination Sativex + MET/CBT might help to cope with craving among cannabis-dependent subjects in the long term. Accordingly, previous studies showed a reduction in craving scores on the CWC following treatment with Sativex (Allsop et al. 2014).
A limitation of this study is that we did not include placebo or no medication groups. All participants included in this pilot study were treatment seeking but otherwise physically and psychologically healthy. Therefore, our results might not generalize outside of this sample and it is unclear what outcomes we might have obtained when compared with a control group.
Conclusions
In summary, our results indicate a substantial reduction in the amount of cannabis consumed that was not accompanied by increases in withdrawal. Transitory increases in craving early during the quitting process may be possible and seem to normalize during long term treatment. Sativex dosage compliance and tolerability in dosed participants in this open-label pilot study suggest it will be feasible to carry out larger double-blind studies. Overall our results suggest that the combination Sativex + MET/CBT should be explored further for its potential as a novel treatment approach for cannabis dependence.
Acknowledgments
Authors would like to thank the co-op students and volunteers that helped on the study. Research reported in this publication was supported by the National Institute On Drug Abuse of the National Institutes of Health under Award Number R21DA031906 (to Dr. Le Foll) and in part by Intramural Research Program of National Institute on Drug Abuse, NIH, DHHS. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- Allsop DJ, Copeland J, Lintzeris N, et al. Nabiximols as an agonist replacement therapy during cannabis withdrawal: a randomized clinical trial. JAMA Psychiatry. 2014;71:281–291. doi: 10.1001/jamapsychiatry.2013.3947. [DOI] [PubMed] [Google Scholar]
- Anthony JC, Warner LA, Kessler RC. Comparative epidemiology of dependence on tobacco, alcohol, controlled substances, and inhalants: Basic findings from the National Comorbidity Survey. Experimental and Clinical Psychopharmacology. 1994;2:244–268. [Google Scholar]
- Budney AJ, Novy PL, Hughes JR. Marijuana withdrawal among adults seeking treatment for marijuana dependence. Addiction. 1999;94:1311–1322. doi: 10.1046/j.1360-0443.1999.94913114.x. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Roffman R, Stephens RS, et al. Marijuana dependence and its treatment. Addict Sci Clin Pract. 2007;4:4–16. doi: 10.1151/ascp07414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crime UNOoDa. World Drug Report 2010. Vienna; Austria: 2010. [Google Scholar]
- D'Souza DC, Abi-Saab WM, Madonick S, et al. Delta-9-tetrahydrocannabinol effects in schizophrenia: implications for cognition, psychosis, and addiction. Biol Psychiatry. 2005;57:594–608. doi: 10.1016/j.biopsych.2004.12.006. [DOI] [PubMed] [Google Scholar]
- D'Souza DC, Perry E, MacDougall L, et al. The psychotomimetic effects of intravenous delta-9-tetrahydrocannabinol in healthy individuals: implications for psychosis. Neuropsychopharmacology. 2004;29:1558–1572. doi: 10.1038/sj.npp.1300496. [DOI] [PubMed] [Google Scholar]
- D'Souza DC, Ranganathan M, Braley G, et al. Blunted psychotomimetic and amnestic effects of delta-9-tetrahydrocannabinol in frequent users of cannabis. Neuropsychopharmacology. 2008;33:2505–2516. doi: 10.1038/sj.npp.1301643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkashef A, Vocci F, Huestis M, et al. Marijuana neurobiology and treatment. Subst Abus. 2008;29:17–29. doi: 10.1080/08897070802218166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimaraes FS, Chiaretti TM, Graeff FG, et al. Antianxiety effect of cannabidiol in the elevated plus-maze. Psychopharmacology (Berl) 1990;100:558–559. doi: 10.1007/BF02244012. [DOI] [PubMed] [Google Scholar]
- Heishman SJ, Singleton EG, Liguori A. Marijuana Craving Questionnaire: development and initial validation of a self-report instrument. Addiction. 2001;96:1023–1034. doi: 10.1046/j.1360-0443.2001.967102312.x. [DOI] [PubMed] [Google Scholar]
- Karschner EL, Darwin WD, McMahon RP, et al. Subjective and physiological effects after controlled Sativex and oral THC administration. Clin Pharmacol Ther. 2011;89:400–407. doi: 10.1038/clpt.2010.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lev-Ran S, Le Strat Y, Imtiaz S, et al. Gender differences in prevalence of substance use disorders among individuals with lifetime exposure to substances: results from a large representative sample. Am J Addict. 2013;22:7–13. doi: 10.1111/j.1521-0391.2013.00321.x. [DOI] [PubMed] [Google Scholar]
- Lowe RH, Karschner EL, Schwilke EW, et al. Simultaneous quantification of Delta9-tetrahydrocannabinol, 11-hydroxy-Delta9-tetrahydrocannabinol, and 11-nor-Delta9-tetrahydrocannabinol-9-carboxylic acid in human plasma using two-dimensional gas chromatography, cryofocusing, and electron impact-mass spectrometry. J Chromatogr A. 2007;1163:318–327. doi: 10.1016/j.chroma.2007.06.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall K, Gowing L, Ali R, et al. Pharmacotherapies for cannabis dependence. Cochrane Database Syst Rev. 2014;12:CD008940. doi: 10.1002/14651858.CD008940.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason BJ, Crean R, Goodell V, et al. A proof-of-concept randomized controlled study of gabapentin: effects on cannabis use, withdrawal and executive function deficits in cannabis-dependent adults. Neuropsychopharmacology. 2012;37:1689–1698. doi: 10.1038/npp.2012.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPartland JM, Duncan M, Di Marzo V, et al. Are cannabidiol and Delta(9) -tetrahydrocannabivarin negative modulators of the endocannabinoid system? A systematic review. Br J Pharmacol. 2015;172:737–753. doi: 10.1111/bph.12944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordstrom BR, Levin FR. Treatment of cannabis use disorders: a review of the literature. Am J Addict. 2007;16:331–342. doi: 10.1080/10550490701525665. [DOI] [PubMed] [Google Scholar]
- Parker LA, Burton P, Sorge RE, et al. Effect of low doses of delta9-tetrahydrocannabinol and cannabidiol on the extinction of cocaine-induced and amphetamine-induced conditioned place preference learning in rats. Psychopharmacology (Berl) 2004;175:360–366. doi: 10.1007/s00213-004-1825-7. [DOI] [PubMed] [Google Scholar]
- Pertwee RG. Ligands that target cannabinoid receptors in the brain: from THC to anandamide and beyond. Addict Biol. 2008;13:147–159. doi: 10.1111/j.1369-1600.2008.00108.x. [DOI] [PubMed] [Google Scholar]
- Prud'homme M, Cata R, Jutras-Aswad D. Cannabidiol as an Intervention for Addictive Behaviors: A Systematic Review of the Evidence. Subst Abuse. 2015;9:33–38. doi: 10.4137/SART.S25081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Y, Whittard J, Higuera-Matas A, et al. Cannabidiol, a nonpsychotropic component of cannabis, inhibits cue-induced heroin seeking and normalizes discrete mesolimbic neuronal disturbances. J Neurosci. 2009;29:14764–14769. doi: 10.1523/JNEUROSCI.4291-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwope DM, Scheidweiler KB, Huestis MA. Direct quantification of cannabinoids and cannabinoid glucuronides in whole blood by liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem. 2011;401:1273–1283. doi: 10.1007/s00216-011-5197-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB, Leo GI, et al. Reliability of a timeline method: assessing normal drinkers' reports of recent drinking and a comparative evaluation across several populations. Br J Addict. 1988;83:393–402. doi: 10.1111/j.1360-0443.1988.tb00485.x. [DOI] [PubMed] [Google Scholar]
- Trigo JM, Lagzdins D, Rehm J, et al. Effects of fixed or self-titrated dosages of Sativex on cannabis withdrawal and cravings. Drug and Alcohol Dependence. 2016 doi: 10.1016/j.drugalcdep.2016.02.020. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandrey R, Haney M. Pharmacotherapy for cannabis dependence: how close are we? CNS Drugs. 2009;23:543–553. doi: 10.2165/00023210-200923070-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Baler RD, Compton WM, et al. Adverse health effects of marijuana use. N Engl J Med. 2014;370:2219–2227. doi: 10.1056/NEJMra1402309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuardi AW, Crippa JA, Hallak JE, et al. Cannabidiol, a Cannabis sativa constituent, as an antipsychotic drug. Braz J Med Biol Res. 2006;39:421–429. doi: 10.1590/s0100-879x2006000400001. [DOI] [PubMed] [Google Scholar]