Abstract
Background
Angiosarcomas (AS) are rare tumors of vascular origin with a variable behavior and overall poor prognosis in the metastatic setting. We sought to assess the outcomes of patients with metastatic AS treated with systemic chemotherapy in an attempt determine the ideal sequence of available standard agents.
Methods
We performed a retrospective analysis of patients with metastatic AS treated at Memorial Sloan Kettering Cancer Center between 1987 and 2012 and collected their correlative clinical information. Outcomes and efficacy measurements of first-line and subsequent lines of treatment were analyzed.
Results
Among 119 pts with metastatic AS, the median age was 61 years and the female/male ratio was 1.4. The most frequent primary sites were chest wall/breast (n=37, 31%), viscera (n=26, 22%) and head/neck (n=24, 20%). There were 28(24%) patients with radiation -associated AS (RT-AS). The median OS was 12.1 months. We identified 73 (61%) and 46 (39%) patients that received ≥2 lines and ≥3 lines of therapy, respectively. The most commonly used agents included taxanes (T) and anthracyclines (A) with 62% of patients receiving either agent. Median times to tumor progression (TTP) were 3.5m for first line, 3.7m for 2nd line and 2.7m for 3rd line. Overall response rate to 1st line was 30% and less than 10% in subsequent lines. No objective responses were documented in lines 5–7. Doxorubicin, liposomal doxorubicin and taxanes resulted in similar response rates and survival.
Conclusion
Despite reasonable response rates in the first line setting, benefit from systemic therapy is short-lived in metastatic AS and outcomes are poor. Doxorubicin, liposomal doxorubicin and taxanes are reasonable choices for first-line and any sequential use of these drugs in monotherapy is appropriate. Further exploration of the molecular pathophysiology to better identify better therapeutic strategies is essential.
Keywords: angiosarcoma, chemotherapy
Introduction
Angiosarcomas (AS) are rare, malignant endothelial cell tumors of vascular origin which account for <2–3% of all soft tissue sarcomas.1 AS are usually associated with highly heterogeneous patterns of presentation and clinical course. 2,3 It occurs with similar incidence amongst both sexes and is generally more common in older patients.4 Despite its ubiquitous anatomic presentation, the most frequent primary sites include the skin of the head and neck, breast and deep soft tissues. Less frequently, angiosarcomas arise in visceral organs, bone and retroperitoneum.4–7 Clinical course and response to treatment may vary depending on the primary location, and some series have suggested longer survival and higher response rates for cutaneous angiosarcomas arising in the face and scalp,8–10 although this observation was not uniform.11–12 While only 3% of AS can be attributable to a documented predisposing syndrome (Recklinghausen’s disease, Ollier’s disease, etc.),3,4,13 several factors have been associated with an increased risk of developing AS, including chronic lymphedema (Stewart-Treves syndrome), prior exposure to ionizing radiation and exogenous toxins, including thorium dioxide (Thorotrast), polyvinyl chloride and arsenical insecticide.3,4,6,14,15 Histologically, AS are characterized by spindled, polygonal, epithelioid and primitive round cells interspersed with endothelial cells, with expression both vascular and endothelial antigens on immunohistochemistry, including Factor-VIII, CD31, CD34 and VEGF. 4, 5,16
Surgical resection remains the cornerstone of treatment for patients with localized disease, and additional benefit can potentially be derived from adjuvant radiotherapy when indicated, although adjuvant radiation therapy has never demonstrated a survival benefit.16 Overall approximately 30–35% of the patients are alive at 5 years after diagnosis.16,17 Poor prognostic factors at diagnosis, although not uniform across different series, include large tumor size (>5cm), primary site (liver, heart and retroperitoneal disease), radiation-induced AS, deep primary tumor, high mitotic count, margin status, older age, poor performance status and presence of metastases at diagnosis.6, 7, 18–22 Unfortunately, despite adequate locoregional treatment, almost half of the patients initially treated with curative intent will ultimately develop metastatic disease.
AS commonly exhibit an aggressive natural course, and up to 20–40% of the patients have disseminated disease at initial presentation.4,18,19 The median overall survival is approximately 8–14 months for patients with metastatic disease.4,6
Activity of a number of agents has been reported in the metastatic setting, including anthracyclines, taxanes, dacarbazine, gemcitabine and angiogenesis inhibitors, with response rates ranging from 10–60%. 8–11, 23–29 There are few small, non-randomized prospective clinical trials and most data result from retrospective series. 8, 10, 11, 23–29
Although some results suggest higher response rates of cutaneous AS to taxanes, particularly those originating in the head and neck/scalp,8,9 other agents have also been shown to be active and the impact of treatment choice, histology and site of origin on survival is unclear.23,24 Hence, controversy exists regarding the optimal first-line treatment and whether different approaches should be tailored to the diverse subgroups. In addition, there is limited information regarding the results of subsequent lines of treatment and the outcomes of sequential use of different agents for patients with advanced AS.
In this analysis, we sought to evaluate the outcomes in this group of patients with metastatic AS treated at a single institution and the evaluate the efficacy of systemic treatment.
Materials & Methods
Study design and population
Using our institutional sarcoma database and data query system, we identified AS patients treated at Memorial Sloan-Kettering Cancer Center between 1982 and 2012. Eligible patients were required to have a centrally reviewed, histologically confirmed diagnosis of advanced or metastatic AS. Following approval by the Institutional Review Board, relevant information was retrieved from electronic medical record including: gender, age at the time of diagnosis, performance status (KPS), features at presentation (localized versus advanced disease), primary tumor characteristics (location, size, etc.), prior radiation exposure, presence of pulmonary and extrapulmonary visceral metastases, presence of bone metastases, treatment-related variables (type of chemotherapy, duration, number of systemic treatment lines) as well as survival dates, including progression dates for each individual treatment line. Primary sites were divided into 6 categories (head/neck, trunk/breast, extremities, retroperitoneum, visceral and other) based on the classification proposed by Brennan et al and used in the prospectively-maintained database.30 The largest dimension of the primary tumor determined pathologically defined tumor size; it was also stratified as ≤5cm, 5.1–10cm or >10cm. Performance status was stratified as <80% or ≥80%. Grade was not evaluated because all except primary breast angiosarcomas are considered to be high grade tumors.21
For patients with target lesions, radiologic evaluation was performed at baseline and every two to three cycles of systemic therapy using computed tomography (CT) or magnetic resonance imaging (MRI). Partial and complete radiologic responses were determined retrospectively by a reference radiologist according to the Response Evaluation Criteria in Solid Tumors – RECIST v1.1 for those patients whose images could be retrieved. Date of progression was defined either by the date of consultation with attending physician, in the case of clinical deterioration, or the date of imaging tests (whenever a CT or MRI was available).
Since the toxicity profile of treatment regimens used for metastatic AS is well described in the literature, details on adverse events were not pursued.
Statistical considerations
Patient characteristics are presented by frequencies and percentages for categorical variables and median and range for continuous variables. Time to tumor progression (TTP) for each line of treatment was calculated from date of beginning of treatment to radiologic progression or end of treatment. Overall survival (OS) was estimated from date of first systemic treatment to date of death from any cause or last follow up. Univariate analysis was performed for factors influencing TTP and OS. Fisher’s test was used to assess the relationship between response to first line and patients characteristics/treatment variables. Variables that were significant in the univariate setting were added to the multivariate model. Survival curves were estimated using the Kaplan-Meier method. P-values < 0.05 were considered significant. All analysis was done using R version 3.1.1.
Results
Demographics and disease characteristics
We identified 119 patients with metastatic AS treated at MSKCC between 1987 and 2012. (Table 1) There was a slight female preponderance (female/male ratio: 1.4; female 58%; male: 42%). The primary tumor size at the time of diagnosis was ≤5cm in 43 cases (36%), 5.1–10cm in 33 (28%), and >10cm in 22 patients (18%). Size description was missing or unknown for 21 patients (18%). Median age was 61 years, ranging from 19 to 86 years. KPS at the start of chemotherapy was 80–100% for 54 patients (45%), <80% for 18 patients (15%) and missing for 47 patients (40%). Metastatic AS most frequently originated in the trunk (n=37; 31%; 31 primary breast and 6 chest wall), followed by viscera (n=26; 22%) and head/neck (n=24; 20%). Overall, 33(28%) were classified as cutaneous AS originating in the head/neck (n=22), extremities (n=9) and other/unknown (n=2), while 8 (7%) arose from bone. In this analysis, there were 28 patients (24%) that developed AS in a previously irradiated area including the breast (n=21), chest wall (n=2), extremities (n=2) and liver/neck/retroperitoneum (n=1 each). Extrapulmonary visceral involvement occurred in 45 patients (38%) and osseous metastases in 33 (28%).
Table 1.
Demographics and disease characteristics
| Characteristic | Number (%) |
|---|---|
|
| |
| Number of Patients | 119 |
|
| |
| Age-years | |
| Median (range) | 61 (19–86) |
|
| |
| Sex | |
| Male | 50 (42%) |
| Female | 69 (58%) |
|
| |
| KPS at 1st line of treatment | |
| 80–100% | 54 (45%) |
| ≤70% | 18 (15%) |
| Missing data | 47 (40%) |
|
| |
| Primary site | |
| Trunk/breast | 37 (31%)* |
| Visceral primary | 26 (22%) |
| Head/neck | 24 (20%) |
| Extremities | 17 (14%) |
| Retroperitoneum | 2 (2%) |
| Other | 9 (8%)** |
| Missing | 4 (3%) |
|
| |
| Cutaneous origin | |
| Yes | 33 (28%) |
| No | 83 (70%) |
| Not specified | 3 (2%) |
|
| |
| Radiation therapy-associated | |
| Yes | 28 (24%) |
| No | 91 (76%) |
|
| |
| Size – primary tumor | |
| ≤5cm | 43 (36%) |
| 5.1–10cm | 33 (28%) |
| >10cm | 22 (18%) |
| Not specified | 21 (18%) |
|
| |
| Extrapulmonary sites of metastases | |
| Visceral | 45 (38%) |
| Bone | 33 (28%) |
Abbreviations: KPS – Karnofsky Performance Scale;
- Breast primary:31;
-Other: pelvis:5, paraspinal:1, aorta:1, anterior mediastinum:1, acetabulum:1
Treatment details
Most frequently used agents across different lines included doxorubicin (single agent or in combination with ifosfamide), paclitaxel, liposomal doxorubicin and tyrosine kinase inhibitors (TKI) sunitinib and sorafenib. Other treatments included gemcitabine, vinorelbine, everolimus and miscellaneous (platinum agents, dacarbazine, temozolomide, brivanib and bevacizumab.) Overall, 73 patients (61%) received 2 or more lines of chemotherapy and 46 (39%) received at least 3 lines of treatment. Median number of lines of therapy was 2, and 3 patients received up to 7 lines of treatment.
Most patients were exposed to anthracyclines (doxorubicin-based or liposomal doxorubicin) (n=74, 62%) and taxanes (n=74, 62%) at some point during their treatments. (Table 2). Overall, 49 patients (41%) received anthracyclines in the first line setting. There were 28 (24%) patients that received doxorubicin-based regimens either as a single-agent (n=8) or in combination (n=20 cases). In addition, 21 (18%) patients received liposomal doxorubicin. Taxanes (mostly commonly single-agent paclitaxel) were the initial treatment in 45 (38%) cases. Sorafenib or sunitinib were recommended for 9 patients (8%) in the first line.
Table 2.
Treatment details
| Characteristic | Number – Total=119 | |
|---|---|---|
|
| ||
| Number of lines of systemic treatment | ||
| Median (range) | 2 (1–7) | |
| 1 | 46 (39%) | |
| ≥2 | 73 (61%) | |
| ≥3 | 46 (39%) | |
|
| ||
| Agent | ||
| Patients treated in first line (N=119) | Patients treated in second line (N=73) | |
| Doxorubicin-based | 28 (24%) | 11 (15%) |
| Monotherapy | 8 | 5 |
| Combination | 20 | 6 |
| Liposomal doxorubicin | 21 (18%) | 10 (14%) |
| Taxane-based | 45 (38%) | 24 (33%) |
| Monotherapy | 41 | 20 |
| Combination | 4 | 4 |
| Sorafenib/Sunitinib | 9 (8%) | 7 (10%) |
| Other | 16 (13%) | 21 (29%) |
Abbreviations: Lipo-dox – liposomal doxorubicin
Following progression, 73 patients received second-line therapies that included anthracyclines (n=21; 29%), taxanes (n=24; 33%), sorafenib/sunitinib (n=7; 10%) or other drugs (n=21; 29%). Among the 46 patients that received a third line of treatment, 13 (28%) received gemcitabine.
In general, a similar number of patients received 2 or more lines of treatment after progressing on first-line anthracyclines (n=30; 61%) in comparison to first-line taxanes (n=27; 60%). There were 16 (36%) patients that received first-line taxanes who crossed over to anthracyclines anytime during treatment. Similarly, 20 (41%) patients who were initially treated with doxorubicin or liposomal-doxorubicin received taxanes in subsequent lines (p=0.75). There were 45 (38%) of patients without exposure to taxanes or anthracyclines.
Overall survival
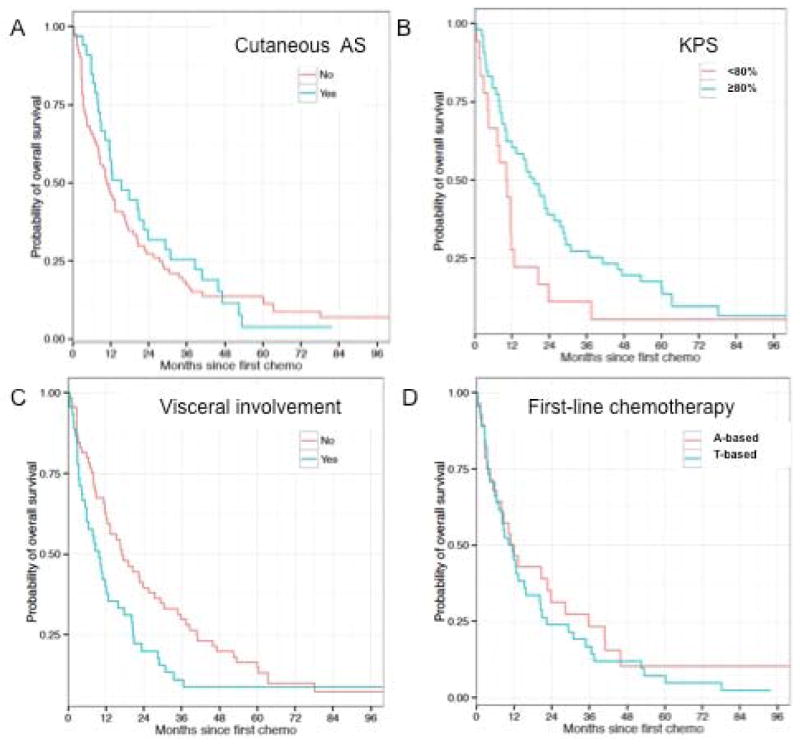
At time of analyses, 106 patients had died and median overall survival was 12.1 months (Supplemental Figure 1). Nine patients were still alive, including 5 without evidence of disease. Median follow up amongst survivors was 93.8 months.
In univariate analysis, larger primary tumor size (>10cm) (p=0.003), KPS at first chemotherapy ≤70% (p=0.049) and presence of visceral involvement (p=0.041) were significantly associated with shorter overall survival. In multivariate analysis, large primary tumor size (>10cm) (p=0.014) was confirmed as a poor independent prognostic factor of decreased overall survival. Due to the large number of patients for whom KPS status was missing (n=47, 40%), this variable was excluded from the multivariate analysis.
Cutaneous AS had a similar median OS versus non-cutaneous AS (15.3mo vs 10.9mo; p=0.498). There was no difference in OS observed between patients with AS arising in previously irradiated areas versus other AS (10.8 vs 12.3mo; p=0.757).
The choice of first-line regimen (anthracyclines versus taxanes versus other) did not impact OS (12.0 vs 11.6 vs 17.8mo, respectively; p=0.193). Similarly, patients treated with polychemotherapy had similar OS to those treated with single-agents in first line (p=0.789 for any single-agent versus any combination).
Time to tumor progression
The median TTP was 3.5mo in first line, 3.7mo in second line and 2.7mo in third line setting. In a univariate analysis, only the presence of visceral involvement predicted a shorter progression-free interval to first-line therapy (p=0.019).
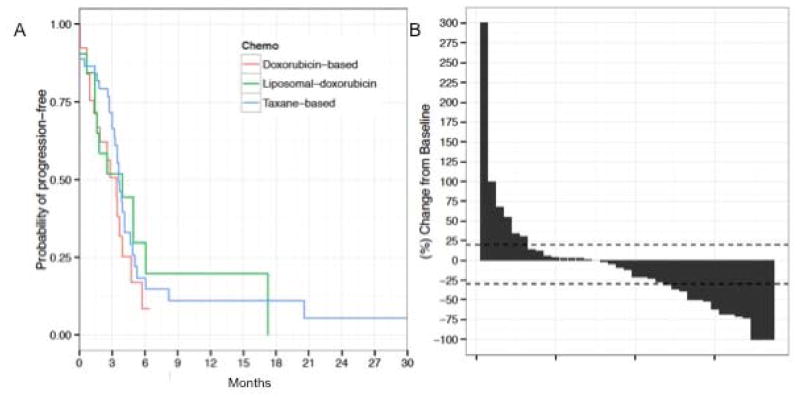
No statistically significant differences in first-line mTTP were observed between anthracyclines (single agent or combination - mTTP 3.4mo), taxanes (single-agent or combination – mTTP 3.6mo) and other agents (mTTP 3.0mo) (p=0.48) in the first-line setting. Of note, mTTP for liposomal doxorubicin was 3.9mo. The choice of single-agent versus combination had did not impact mTTP either (p=0.498) (Figure 2 and Table 4).
Figure 2.
Median TTP curves for different first-line treatments and response to first line treatment
Figure 2. mTTP Kaplan-Meier plots based on type of first-line chemotherapy (A); Waterfall plot for illustrating patterns of response to first-line chemotherapy, each bar represents an individual patient (B).
Table 4.
Treatment outcomes
| Survival - Entire cohort (N=119) | ||||
|---|---|---|---|---|
|
| ||||
| Subgroups | mTTP (months) | mOS (months) | ||
| Entire Cohort | 12.1 | |||
| First line | 3.5 | |||
| Second line | 3.7 | |||
| Third line | 2.7 | |||
|
| ||||
| Type of chemotherapy | First line | |||
| Anthracycline-based | 3.4 | p=0.48 | 12.0 | p=0.193 |
| Taxane-based | 3.6 | 11.6 | ||
| Other | 3.0 | 17.8 | ||
| T or A single-agent | 3.5 | p=0.498 | 11.3 | p=0.463 |
| T or A combination | 3.7 | 12.3 | ||
|
| ||||
| Site | ||||
| Cutaneous | 3.6 | p=0.791 | 15.3 | P=0.498 |
| Non-cutaneous | 3.0 | 10.9 | ||
|
| ||||
| Response to treatment – Evaluable patients (N=48) | ||||
|
| ||||
| Line of treatment | ORR (CR + PR) | SD | ||
| 1st (n=47) | 30% (n=14) | 40%(n=19) | ||
| 2nd (n=24) | 8% (n=2) | 42% (n=10) | ||
| 3rd (n=11) | 9% (n=1) | 27% (n=3) | ||
|
| ||||
| Type of chemotherapy | ORR - First line | |||
| Anthracycline-based (n=20) | 30% (n=6) | p=1.00 | ||
| Taxane-based (n=13) | 31% (n=4) | |||
| T or A single-agent (n=26) | 27% (n=7) | p=0.726 | ||
| T or A combination (n=7) | 43% (n=3) | |||
|
| ||||
| Site | ORR – First line | |||
| Cutaneous (n=8) | 50% (n=4) | p=0.196 | ||
| Non-cutaneous (n=38) | 24% (n=9) | |||
Abbreviations: Lipo-doxo – liposomal doxorubicin; ORR – overall response rate; CR – complete response; PR – partial response; SD – stable disease; T – taxane; A - anthracycline
Response to treatment
Of 119 patients identified, 48 patients were radiographically evaluable for response based on RECIST criteria v1.1 (Table 3). One patient was not evaluable for first line due to inadequate baseline scans. Overall response rate to first line therapy was 30% (N=14) and 19 patients (40%) achieved SD as best response (Figure 2, Panel B); 14 patients (30%) had progression of disease at first response assessment.
Table 3.
Univariate and multivariate analyses for OS
| Univariate analysis | |||
|---|---|---|---|
|
| |||
| Variable | n | Median OS (months) | p-value |
|
| |||
| Age | 119 | - | 0.378 |
|
| |||
| Gender | 0.8606 | ||
| Female | 69 | 12.7 | |
| Male | 50 | 11.9 | |
|
| |||
| Primary site | 0.5588 | ||
| Head/Neck | 24 | 20.5 | |
| Extremities | 17 | 11.6 | |
| Trunk/breast | 37 | 11.3 | |
| Retroperitoneum | 2 | 11.2 | |
| Visceral primary | 26 | 10.0 | |
| Other | 9 | 13.3 | |
|
| |||
| Bone origin | 0.9323 | ||
| Yes | 8 | 12.0 | |
| No | 111 | 12.3 | |
|
| |||
| Cutaneous | 0.4977 | ||
| Yes | 33 | 15.3 | |
| No | 83 | 10.9 | |
|
| |||
| RT-associated | 0.7565 | ||
| Yes | 28 | 10.8 | |
| No | 91 | 12.3 | |
|
| |||
| Primary tumor size | 0.0034 | ||
| ≤5cm | 43 | 15.8 | |
| 5.1–10cm | 33 | 12.0 | |
| >10cm | 22 | 5.9 | |
|
| |||
| Visceral metastases | 0.0405 | ||
| Yes | 45 | 9.7 | |
| No | 66 | 17.2 | |
|
| |||
| Bone metastases | 0.6869 | ||
| Yes | 33 | 11.6 | |
| No | 78 | 16.6 | |
|
| |||
| KPS | 0.0485 | ||
| ≤70 | 18 | 10.5 | |
| 80–100 | 54 | 19.2 | |
|
| |||
| First-line regimen | 0.193 | ||
| Anthracycline-based | 49 | 12.0 | |
| Taxane-based | 45 | 11.6 | |
| Other | 25 | 17.8 | |
| Single-agent | 95 | 12.0 | 0.7894 |
| Combination | 24 | 12.3 | |
|
| |||
| Multivariate analysis | |||
|
| |||
| Variable | HR | 95% CI | p-value |
|
| |||
| Primary tumor size | |||
| 5.1–10cm vs ≤5cm | 0.783 | 0.462–1.327 | 0.363 |
| >10cm vs ≤5cm | 2.199 | 1.230–3.929 | 0.008 |
|
| |||
| Visceral metastases | |||
| Yes vs No | 1.538 | 0.969–2.440 | 0.068 |
Objective responses to second and third lines occurred in less than 10% of the patients and one patient had significant response after treatment with polychemotherapy in 4th line. In the second and third line settings, 42% and 27% had stable disease as best response, respectively. The rates of stable disease were 25% and 20% in 4th and 5th lines, respectively. No objective responses were documented in lines 5–7.
We could not identify any factors predictive of response. Response rate was not significantly influenced by the type of first line therapy (ORR: 25% for doxorubicin-based, 33% for liposomal doxorubicin, 31% for taxanes; p=1.00) Patients receiving anthracyclines and taxanes in combinations achieved ORR of 43% in comparison to 28% for the same agents in monotherapy 28% (p=0.41). Response rates in patients with cutaneous AS were 50% compared to 24% in the non-cutaneous group (p=0.196) (Table 4).
Discussion
Angiosarcomas account for less than 3% of adult soft tissue sarcomas and most patients with metastatic disease live less than 8–14 months.4,6 In this setting, frequently used agents include anthracyclines, taxanes, gemcitabine and tyrosine kinase inhibitors.8,11,23–28 Despite the results of few prospective clinical trials and retrospective series, there is no standard approach for patients with metastatic disease and the benefit of systemic therapy for patients who progress after first line is unclear. Here we present the results of one of the largest retrospective series of patients with metastatic AS and the first to evaluate the details of management after progression on first line chemotherapy.
Baseline characteristics of patients in our analysis are concordant with earlier data, including age, female/male ratio and frequency of patients with RT-associated AS. While distribution varies across different studies, head/neck and chest/breast are typical primary sites for AS.3,4,6,19 We noted that 22% had visceral primary AS; other series have similarly reported a high proportion of patients with deep AS arising in the viscera.19,24
Survival outcomes herein reported (mTTP to fisrt line: 3.5mo; mOS: 12.1mo) are in line with previously published studies (Table 5), in which the median times to progression ranged between 1.7 and 7.6m and the median OS was between 5.5 and 19.5 months with systemic chemotherapy. Primary tumor size >10cm, KPS ≤70% and presence of extrapulmonary visceral involvement were associated with shorter overall survival in univariate analysis. Poor performance status is a well-documented poor prognostic factor in patients with metastatic AS.23,24 However, KPS status at baseline was missing for 40% of the patients and, although significant in the univariate analysis, this variable could not be included in the final multivariate model. We also found that primary tumor size at diagnosis was confirmed as poor prognostic factor in multivariate analysis which could reflect a more aggressive tumor biology. Interestingly, history of prior radiation showed no correlation with survival or response to treatment in the metastatic setting and this confirms prior observations,19, 23,24 and these patients should be managed similarly to those with primary AS.
Table 5.
Retrospective studies and prospective clinical trials in AS
| Author | Year | N | Treatment regimen | ORR | mTTP/mPFS (months) | mOS (months) |
|---|---|---|---|---|---|---|
| Retrospective studies | ||||||
| Schlemmer et al.(8) | 2008 | 32 | Single-agent P (weekly or q3w) | 62% | 7.6 | NI |
| Stacchiotti et al.(11) | 2011 | 25 | Gemcitabine | 64% | 7.0 | 17.0 |
| Penel et al.(24) | 2012 | 149 | - Ant-based (46.9%) -P (31.5%) -Other (Sorafenib, platinum-based, vinorelbine, ifosfamide etc.) |
Ant-based – 30.9% | Ant-based – 3.9 | 11.0 |
| P – 45.5% | P – 5.6 | |||||
| Overall | 3.2 | |||||
| Italiano et al.(23) | 2012 | 117 | Single-agent P (64%)/Single-agent Dox (36%) | Dox – 29.5% | Dox – 3.0 | Dox – 5.5 |
| P – 53% | P – 5.8 | P – 10.3 | ||||
| Overall | 4.9 | 8.5 | ||||
| D’Angelo & Munhoz et al. | 2014 | 119 | - Ant-based - Tax-based -Other (Sorafenib, platinum-based, vinorelbine, ifosfamide etc.) |
Ant-based - 30% | 3.4 | 12.0 |
| Tax-based - 31% | 3.6 | 11.6 | ||||
| Overall - 30% | 3.9 | 12.1 | ||||
| Prospective clinical trials | ||||||
| Penel et al. (27) | 2008 | 30 | WP | 19% | 4.0m | 8.0m |
| Maki R et al.(26) | 2009 | 37 | Sorafenib | 14% | 3.8m | 14.9m |
| Ray-Coquard et al (10) | 2012 | 41 | Sorafenib | 14.6% | 2.0m | 9.7m |
| Agulnik et al. (25) | 2013 | 23 | Bev | 9% | 3.0m | 13.2m |
| D’Angelo et al. (29) | 2014 | 16 | Trebananib (AMG386) | 0% | 1.7m | 7.0m |
| Penel et al. (28) | 2014 | 50 | WP | 50% | 6.8m | 19.5m |
| WP + Bev | 40% | 6.9m | 15.9m | |||
N – total number of patients/Dox – doxorubicin/P – paclitaxel/Ant-anthracyclines/Tax-taxanes/PS – performance status/ORR – objective response rate/mTTP – median time to tumor progression/mOS – median overall survival/Prog. factors – prognostic factors associated with survival on multivariate analysis/WP – weekly paclitaxel/Bev – Bevacizumab/ORR – objective response rate/SD – stable disease rate/mPFS – median progression free survival in months/mOS – median overall survival in months
While both taxanes and anthracyclines are considered active and frequently recommended for AS patients, no randomized, controlled trials have been conducted to date comparing these two classes and controversy still exists regarding the best treatment choice.6 We found similar results in terms of ORR, mTTP and mOS in patients treated with doxorubicin, liposomal doxorubicin and taxanes. It is important to highlight that the present series includes the largest number of AS patients treated with liposomal doxorubicin (n=21 in first line and n=10 in second line), and this resulted in an ORR of 33% in the first-line setting. Consideration of liposomal doxorubicin in the front line setting may be a reasonable front line options for patients metastatic AS based on our retrospective data.
In the largest series published to date that included 149 AS patients treated between 1996 and 2009, Penel et al. reported results very similar to the ones described here.24 In that series, 46.9% of patients were treated with doxorubicin-based combinations; 31.5% weekly-paclitaxel and 10.9% received exclusive palliative-care. There was no significant difference in terms of OS between weekly paclitaxel and doxorubicin-based regimens (11.0 versus 13.1mo; p = 0.81). In another multi-institutional series that include 117 patients published by Italiano et al., 23 response rate was significantly higher in the weekly paclitaxel group than in the doxorubicin group (53% vs 29%; P=0.02). It’s important to highlight patients treated with paclitaxel were more likely to be older and have primary skin AS (89% of the patients with cutaneous AS received paclitaxel). In the subgroup of noncutaneous AS, no significant difference in terms of objective response rate between weekly paclitaxel and doxorubicin was observed (40% vs 26%; p =0.2). In the single-arm phase II ANGIOTAX trial with patients with AS weekly paclitaxel demonstrated an ORR of 19%; the median TTP was 4 months and the median OS was 8 months.27 Better results with weekly paclitaxel were recently presented by the same group in a contemporary phase II trial which randomized patients to receive weekly paclitaxel with or without bevacizumab. Single-agent paclitaxel resulted in a response rate of 40%, mPFS of 6.8 mo and median OS of 19.5 mo. Of note, 32% of the patients had been previously treated with anthracyclines and dose of paclitaxel was slightly higher (90mg/m2 weekly versus 75–80mg/m2 weekly or 175mg/m2 every 3 weeks in most retrospective series and ANGIOTAX trial).28
This is the first series to provide more robust details regarding the management of patients beyond first-line. Taxanes or anthracyclines did not have an impact on survival outcomes; this is likely due to the fact that patiens when on to receive either drug in the second line setting. Overall exposure to either class of drugs was similar: sixty two percent of the patients were exposed to anthracyclines and 62% to taxanes throughout the course of their disease. Rates of cross-over between anthracyclines and taxanes and vice-versa were also comparable (36% from taxanes to anthracyclines and 41% from anthracyclines to taxanes). Median TTP in second and third lines were 3.7mo and 2.7mo, respectively, and, although response rates were inferior to 10%, disease stabilization was achieved in 42% (n=10) in second line and 27% (n=3) in third line settings. In the series by Italiano et al, data regarding the management of patients after first-line chemotherapy were available for only 43 individuals, and only 26 received at least 2 lines of treatment. Chemotherapy regimens beyond second line were diverse and only 3 patients out of 26 (11%) experienced a partial objective response: one was treated with liposomal doxorubicin, one with gemcitabine and one patient from the doxorubicin group who was treated with second-line weekly paclitaxel plus bevacizumab. Median PFS was 1.9 months. 23 Hence, our results provide some insight into the medical management of AS patients. Since both taxanes and anthracyclines are active drugs with no proven superiority of one over the other, the sequential use either agent as monotherapy is acceptable. Taxanes and liposomal doxorubicin are associated with appreciable response rates and have an acceptable toxicity profile. Moreover, the use of polychemotherapy was not associated with improved overall survival when compared to the sequential use of single-agent drugs (p=0.463).
More recently, impressive results were reported by Sttachiotti et al in a retrospective analysis of 25 cases treated with single-agent gemcitabine: 64% of the patients achieved objective responses and median OS was 17 months.11 Unfortunately, very few patients received this drug in 1st or 2nd lines in our series and the activity of gemcitabine could not be adequately described. In addition, only a small number of patients received TKI (8% in first line, 9% in second line). Despite its vascular origin and evidences of pro-angiogenic pathways activation in AS, including overexpression of vascular growth factors and receptors (vascular endothelial growth factor A, C, F; VEGF receptors 1, 3 etc.),31,32 results with anti-angiogenic agents and TKI remain disappointing. In the largest prospective study with sorafenib in patients with soft tissue sarcomas(n=147), Maki et al. reported an ORR of only 14% among 37 evaluable AS patients, with a median progression-free survival of 3.8 months.26 Similarly, modest activity of sorafenib was reported von Mehren et al. and Ray-Coquard et al., with ORR of 0% and 14.6%, respectively.10, 33 Despite some evidence of activity bevacizumab in a single-arm phase II trial that included patients with AS (n=23) and epithelioid hemangioendothelioma (n=7) (four partial responses, ORR=17%; two patients with AS and two patients with epithelioid hemangioendothelioma), 25 there were no statistically significant differences in ORR (40% vs 50%), median PFS (6.8 vs 6.9 mo) and median OS (19.5 vs 15.9 mo) in the ANGIOTAX-PLUS randomized phase II trial of paclitaxel with or without bevacizumab.28
The retrospective nature of this series poses limitations: there was high heterogeneity regarding the types of treatment beyond second line and this limited the power of additional analyses and the population was quite diverse; therefore it is not possible to generalize this data. In addition, the small number of specific subsets limited analyses addressing the best chemotherapy choice for particular subtypes, such as cutaneous AS and visceral AS.
There remains the need to continue to identify additional molecular changes to better characterize this heterogeneous disease and determine biomarkers predictive of response to therapy in the adjuvant and metastatic setting. Already defined molecularly subsets of AS include those with MYC and/or FLT4 (VEGFR-3) alterations and KDR (VEGFR-2) mutations.32,34 We previously reported the efficacy of sorafenib in a small series of radiation-associated breast AS patients.35 Complete and partial responses were seen in patients with co-amplification of MYC and FLT4 treated with sorafenib as 1st or 2nd line therapy. This observation shadows the direction that oncology is heading towards, personalization of medicine based on the presence of targets of therapeutic relevance.
Conclusion
In conclusion, our findings suggest that, although angiosarcomas are chemosensitive malignancies. However, despite objective responses being documented in subsequent lines of treatment, benefit from systemic therapy is short-lived and new approaches for the management of patients with advanced disease remain an unmet need. Although the best treatment order is yet to be determined, doxorubicin, liposomal doxorubicin and taxanes resulted in similar outcomes, and should be used sequentially as single-agents in most circumstances. Further exploration of the molecular pathophysiology and biology to identify better targets for systemic therapy is crucial.
Supplementary Material
Figure 1.
Overall survival curves for specific subgroups.
Figure 1. Kaplan-Meier plots: Cutaneous versus non-cutaneous AS (A); KPS at baseline (B); Status of extrapulmonary visceral involvement (C); type of first-line chemotherapy (D).
Acknowledgments
Murray Brennan, M.D. who created the MSKCC prospective surgical data base and to all that have maintained it since 1982. Nicole Moraco who has assisted in data collection for this analysis.
Footnotes
Disclosures: The authors have declared no conflicts of interest.
References
- 1.Rouhani P, Fletcher CD, Devesa SS, Toro JR. Cutaneous soft tissue sarcoma incidence patterns in the U.S.: an analysis of 12,114 cases. Cancer. 2008;113:616–627. doi: 10.1002/cncr.23571. [DOI] [PubMed] [Google Scholar]
- 2.Weiss SWGJ. Enzinger & Weiss’s soft tissue tumors. 5. Mosby; 2008. [Google Scholar]
- 3.Penel N, Marreaud S, Robin Y, et al. Angiosarcoma: State of the art and perspectives. Crit Rev Oncol Hematol. 2011;80:257–263. doi: 10.1016/j.critrevonc.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 4.Fury MG, Antonescu CR, Van Zee KJ, Brennan MF, Maki RG. A 14-year retrospective review of angiosarcoma: clinical characteristics, prognostic factors, and treatment outcomes with surgery and chemotherapy. Cancer J. 2005;11:241–247. doi: 10.1097/00130404-200505000-00011. [DOI] [PubMed] [Google Scholar]
- 5.Weiss SW, Lasota J, Miettinem MM. Angiosarcoma of soft tissue. In: Fletcher CDM, Unni KK, Mertens F, et al., editors. WHO Classification Tumours of Soft Tissue and Bone. Lyon, France: IARC Press; 2002. pp. 175–177. [Google Scholar]
- 6.Fayette J, Martin E, Piperno-Neumann S, et al. Angiosarcomas, a heterogeneous group of sarcomas with specific behavior depending on primary site: a retrospective study of 161 cases. Ann Oncol. 2007;18:2030–2036. doi: 10.1093/annonc/mdm381. [DOI] [PubMed] [Google Scholar]
- 7.Mark RJ, Poen JC, Tran LM, Fu YS, Juillard GF. Angiosarcoma. A report of 67 patients and a review of the literature. Cancer. 1996;77:2400–2406. doi: 10.1002/(SICI)1097-0142(19960601)77:11<2400::AID-CNCR32>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 8.Schlemmer M, Reichardt P, Verweji J, et al. Paclitaxel in patients with advancedangiosarcomas of soft tissue: a retrospective study of the EORTC soft tissue and bone sarcoma group. Eur J Cancer. 2008;44:3. doi: 10.1016/j.ejca.2008.07.037. [DOI] [PubMed] [Google Scholar]
- 9.Fata F, O’Reilly E, Ilson D, et al. Paclitaxel in the treatment of patients with angiosarcoma of the scalp or face. Cancer. 1999;86:2034–7. [PubMed] [Google Scholar]
- 10.Ray-Coquard I, Italiano A, Bompas E, et al. Sorafenib for Patients with Advanced Angiosarcoma: A Phase II Trial from the French Sarcoma Group (GSF/GETO) Oncologist. 2012;17:260. doi: 10.1634/theoncologist.2011-0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stacchiotti S, Palassini E, Sanfilippo R, et al. Gemcitabine in advanced angiosarcoma: a retrospective case series analysis from the Italian Rare Cancer Network. Ann Oncol. 2012;23:501–508. doi: 10.1093/annonc/mdr066. [DOI] [PubMed] [Google Scholar]
- 12.Farid M, Ong WS, Lee MJF, et al. Cutaneous versus Non-Cutaneous Angiosarcoma:Clinicopathologic Features and Treatment Outcomes in 60 Patients at a Single Asian Cancer Centre. Oncology. 2013;85:182. doi: 10.1159/000354215. [DOI] [PubMed] [Google Scholar]
- 13.Penel N, Grosjean J, Robin YM, Vanseymortier L, Clisant S, Adenis A. Frequency of certain established risk factors in soft tissue sarcomas in adult: a prospective descriptive study of 658 cases. Sarcoma. 2008;2008:459386. doi: 10.1155/2008/459386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meis-Kindblom J, Kindblom LG. Angiosarcoma of soft tissue: a study of 80 cases. Am J Surg Pathol. 1998;22:683–697. doi: 10.1097/00000478-199806000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Thomas LB, Popper H. Pathology of angiosarcomas of the liver among vinyl chloride-polychloride workers. Ann NY Acad Sci. 1975;246:268–285. doi: 10.1111/j.1749-6632.1975.tb51102.x. [DOI] [PubMed] [Google Scholar]
- 16.Young RJ, Brown NJ, Reed MW, et al. Angiosarcoma. Lancet Oncol. 2010;11:983–91. doi: 10.1016/S1470-2045(10)70023-1. [DOI] [PubMed] [Google Scholar]
- 17.Espat NJ, Lewis JJ, Woodruff JM, et al. Confirmed angiosarcoma: prognostic factors and outcome in 50 prospectively followed patients. Sarcoma. 2000;4:173–177. doi: 10.1155/2000/575781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abraham JA, Hornicek FJ, Kaufman AM, et al. Treatment and outcome of 82 patients with angiosarcoma. Ann Surg Oncol. 2007;14:1953–1967. doi: 10.1245/s10434-006-9335-y. [DOI] [PubMed] [Google Scholar]
- 19.Buehler D, Rice SR, Moody JS, et al. Angiosarcoma outcomes and ptognostic factors: a 25 year signle institution experience. Am J Clin Oncol. 2013;00:000. doi: 10.1097/COC.0b013e31827e4e7b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lahat G, Dhuka AR, Hallevi H, et al. Angiosarcoma: clinical and molecular insights. Ann Surg. 2010;251:1098–1106. doi: 10.1097/SLA.0b013e3181dbb75a. [DOI] [PubMed] [Google Scholar]
- 21.Naka N, Ohsawa M, Tomita Y, et al. Prognostic factors in angiosarcoma: a multivariate analysis of 55 cases. J Surg Oncol. 1996;61:170–176. doi: 10.1002/(SICI)1096-9098(199603)61:3<170::AID-JSO2>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 22.Pawlik TM, Paulino AF, McGinn CJ, et al. Cutaneous angiosarcoma of the scalp: a multidisciplinary approach. Cancer. 2003;98:1716–1726. doi: 10.1002/cncr.11667. [DOI] [PubMed] [Google Scholar]
- 23.Italiano A, Cioffi A, Penel N, et al. Comparison of Doxorubicin and Weekly Paclitaxel Efficacy in Metastatic Angiosarcomas. Cancer. 2012;118:3330–3336. doi: 10.1002/cncr.26599. [DOI] [PubMed] [Google Scholar]
- 24.Penel N, Italiano A, Ray-Coquard I, et al. Metastatic angiosarcomas: doxorubicin-based regimens, weekly paclitaxel and metastasectomy significantly improve the outcome. Ann Oncol. 2012;23:517–523. doi: 10.1093/annonc/mdr138. [DOI] [PubMed] [Google Scholar]
- 25.Agulnik M, Yarber JL, Okuno SH, et al. An open-label, multicenter, phase II study of bevacizumab for the treatment of angiosarcoma and epithelioid hemangioendotheliomas. Ann Oncol. 2013;24:257–263. doi: 10.1093/annonc/mds237. [DOI] [PubMed] [Google Scholar]
- 26.Maki RG, D’Adamo DR, Keohan ML, et al. Phase II study of sorafenib in patients with metastatic or recurrent sarcomas. J Clin Oncol. 2009;27:3133–3140. doi: 10.1200/JCO.2008.20.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Penel N, Bui BN, Bay JO, et al. Phase II trial of weekly paclitaxel for unresectable angiosarcoma: the ANGIOTAX Study. J Clin Oncol. 2008;26:5269–5274. doi: 10.1200/JCO.2008.17.3146. [DOI] [PubMed] [Google Scholar]
- 28.Penel N, Blay JY, Mir O, et al. ANGIOTAX-PLUS trial: A randomized phase II trial assessing the activity of weekly paclitaxel (WP) plus or minus bevacizumab (B) in advanced angiosarcoma (AS) J Clin Oncol. 2014;32:5s. (suppl; abstr 10501) [Google Scholar]
- 29.D’Angelo SP, Mahoney MR, Van Tine BA, et al. Alliance A091103: A multicenter phase II study of the angiopoietin-1 and -2 peptibody trebananib (AMG386) for the treatment of angiosarcoma (AS) J Clin Oncol. 2014;32:5s. doi: 10.1007/s00280-015-2689-8. (suppl; abstr 10568) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brennan MF, Antonescu CR, Maki RG. Management of Soft Tissue Sarcomas. 1. New York, NY: Springer; 2013. Vascular Sarcomas. [Google Scholar]
- 31.Itakura E, Yamamoto H, Oda Y, et al. Detection and characterization of vascular endothelia growth factors and their receptors in a series of angiosarcoma. J Surg Oncol. 2008;97:74–81. doi: 10.1002/jso.20766. [DOI] [PubMed] [Google Scholar]
- 32.Antonescu CR, Yoshida A, Guo T, et al. KDR activating mutations in human angiosarcomas are sensitive to specific kinase inhibitors. Cancer Res. 2009;69:7175–7179. doi: 10.1158/0008-5472.CAN-09-2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.von Mehren M, Rankin C, Goldblum JR, et al. Phase 2 Southwest Oncology Group-directed intergroup trial (S0505) of sorafenib in advanced soft tissue sarcomas. Cancer. 2012;118(3):770–6. doi: 10.1002/cncr.26334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guo T, Zhang L, Chang NE, Singer S, Maki RG, Antonescu CR. Consistent MYC and FLT4 gene amplification in radiation-induced angiosarcoma but not in other radiation-associated atypical vascular lesions. Genes Chromosomes Cancer. 2011;50:25–33. doi: 10.1002/gcc.20827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.D’Angelo SPAC, Keohan ML, Carvajal RD, Dickson MA, Gounder M, Moraco N, Singer S, Schwartz G, Tap WD. Activity of sorafenib in radiation-associated breast angiosarcomas harboring MYC and FLT4 amplifications. Journal of Clinical Oncology. 2012:30. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.