Abstract
Background
There is strong association between childhood rotavirus, diarrhoea, climate factors and malnutrition. Conversely, a significant nutritional transition (reduced under-nutrition) with a concurrent increasing trend of rotavirus infection in last decade was also observed among under 5 children, especially in developing countries including Bangladesh. Considering the pathophysiology of rotavirus, there might be an interaction of this nutrition transition which plays a pivotal role in increasing rotavirus infection in addition to climate and other man-made factors in urban areas such as Dhaka, Bangladesh.
Methods
Relevant monthly data from 1993–2012 were extracted from the archive of the Diarrhoeal Disease Surveillance System of icddr, b and linked with data collected from the Dhaka station of the Bangladesh Meteorological Department (mean temperature, rainfall, sea level pressure and humidity). Seasonal autoregressive integrated moving average time series models were deployed to determine the association between the monthly proportion of rotavirus infection and underweight, stunting and wasting adjusting for climate, socio-demographic and sanitation factors.
Finding
The proportion of rotavirus cases among all causes diarrhoea increased from 20% in 1993 to 43% in 2012 (Chi squared for trend p = 0.010). In contrast, underweight, stunting and wasting decreased from 59%-29% (p<0.001); 53%-21% (p<0.001) and 32%-22% (p<0.001) respectively over the same period. Mean ambient temperature increased from 25.76°C-26.62°C (p = 0.07); mean rainfall, sea level pressure and mean humidity decreased from 234.92–111.75 mm (p = 0.5), 1008.30–1006.61 mm of hg (p = 0.02) and 76.63%-70.26% (p<0.001), respectively. In the adjusted model, a decrease in monthly proportion of underweight [coef.: -0.189 (95% CI:-0.376, -0.003)] and wasting [-0.265 (-0.455, -0.075)] were significantly and inversely associated with rotavirus infection. However, an inverse but insignificant association was observed for stunting [-0.070 (-0.249, 0.109)].
Interpretation
The reduction of acute childhood malnutrition is significantly associated with increasing rotavirus diarrhoea among under-5 children. Thus mass vaccination in addition to interventions directed at man-made modifiable predictors for prevention and control is warranted.
Introduction
Childhood diarrhoea remains a major concern in developing Bangladesh [1]. There is a strong association between childhood diarrhoeal disease and several climate factors. For example, an increasing number of diarrhoea cases due to Vibrio cholera has been observed with both high and low rainfall [2], while rising temperature as well as high and low rainfall both predict increasing non-cholera cases [3]. These might be closely related to raising of river levels [3] resulting in flooding that effects the most vulnerable groups in poor socio-economic areas and where water-satiation practices are are suboptimal [4]. Flooding is also associated with rapid land coverage (loss of arable land, habitat destruction and the decline in natural vegetation cover) in the Dhaka megacity [5–7]. Moreover, overcrowding, poor sewer systems and waste disposal with water stagnantion, increasing surface temperature and environmental pollution together [7] and concurrently facilitates other infections including typhoid and dengue fever in Dhaka [8–10].
Epidemiological studies suggested a strong association between childhood malnutrition and increased risk of infectious diarrhoea [11]. Among all causes of childhood diarrhoea, rotavirus is one of the most significant attributable pathogens [1] and cellular attachment with healthy cells in the brush border of the intestine is fundamental in the pathophysiology of the rotavirus infection [12–14]. As a result and, compared to undernourished children, rotavirus infection disproportionately affects well-nourished children [15,16].
Global warming with climate change effects ecosystems and might also predict emergence and re-emergence of various infections [17–19] [20]. Data from the long established diarrhoeal disease surveillance system (DDSS) [21] of the Dhaka Hospital at International Centre for Diarrhoeal Disease Research, Bangladesh (icddr,b) observed an association of an increased incidence of rotavirus diarrhoea with high temperature, low humidity and high river-levels [22] with seasonal variability [23, 24]. The facility also experienced a sustained rising trend of rotavirus infection over the last two decades [25] despite a significant improvement of water and sanitation practices in the capital city and its neighbouring catchment areas [24]. Similar to other countries, the profile of childhood malnutrition in Bangladesh has shifted over time with a more recent decrease in under nutrition and increase in over nutrition [24]. Thus, there might be an individual or combined contribution of changing climate factors and decrease in childhood under nutrition to the rising trend of rotavirus infection among children less than 5 years old.
The DDSS has maintained an around the clock diarrhoeal disease surveillance system for the last three decades [15,21,24]. This surveillance system prospectively collects information on diarrhoeal patients and the aetiology, including rotavirus. We have linked this data with different meteorological indicators such as temperature, rainfall, sea level pressure and humidity over the last two decades (1993 to 2012). This enables determination of the long term relationship of the changing pattern of childhood malnutrition, meteorological indicators, and other socio-demographic and sanitation factors with rotavirus diarrhoea in children less than 5 years of age in urban Dhaka, Bangladesh.
Materials and methods
Study site and diarrhoeal disease surveillance system
The Dhaka Hospital of icddr,b is located in Dhaka, the capital city of Bangladesh. Since 1962, icddr,b has operated this large urban diarrhoeal disease facility which currently provides care and treatment to approximately 140,000 patients of all ages each year. Most patients are residents of urban and peri-urban Dhaka and the majority are of poor socio-economic background. In addition to patient care services, clinical research on enteric diseases, other infectious diseases and non-communicable diseases is also conducted. The DDSS of the Dhaka Hospital was established in 1979 to study a systematic 4% sample until 1995, when the increase in patient number enable the change to a 2% sample beginning in 1996 of all patients attending this facility irrespective of age, sex, disease severity or socioeconomic context (details are described elsewhere [21]). For the DDSS and following standard methods, a fresh stool spaciemen is collected from each enrolled patient and tested for rotavirus, Vibrio cholerae, enterotoxigenic Escherichia coli (ETEC) and Shigella spp. following standard laboratory methods, reported elsewhere in detail [26–28]. Relevant data for the last two decades (1993–2012) was extracted from the electronic database of the DDSS for analysis.
Meteorological data
Data on daily maximum and minimum temperature, rainfall, sea level pressure and relative humidity were obtained from the Dhaka station of the Bangladesh Meteorological Department [22]. Meteorological data for months and years were determined from the daily records.
Assessment of nutritional status and malnutrition
Anthropometric measurements of children (weight and length/height) were measured [weight was measured to the nearest 100 gm using a digital scale, length/height was measured using a locally constructed length board or stadiometer with a precision of 0.1 cm] by trained and experienced Research Assistants following standard procedures [29]. All measurements were compared to the WHO 2006 growth standards and the nutritional status defined by z-score underweight (weight-for-age z score < -2 sd), stunting (height-for-age z score < -2 sd), and wasting (weight-for-height z score < -2 sd) [29].
Socio-demographic factors
Monthly mean age and proportion of females under 5 children enrolled in the DSSS, monthly proportion of non-sanitary toilet uses, non-slum residence, greater than one under 5 year children in the household were estimated and included in the analyses with the aim to determine the adjusted association.
Ethical considerations
The Diarrhoeal Disease Surveillance System (DDSS) of icddr,b is an established ongoing activity of the Dhaka Hospital approved by the Research Review Committee (RRC) and Ethical Review Committee (ERC) of icddr,b. At enrollment, verbal consent is taken from the caregivers or guardians on behalf of the patients following the hospital policy. This verbal consent was documented by keeping a check mark in the questionnaire which was again shown to the patient or the parents. Parents or guardians were assured about the non-disclosure of information collected from them, and were also informed about the use of data for analysis and using the results for improving patient care activities, conducting researches as well as publication without disclosing the name or identity of their children. ERC was satisfied with the voluntary participation, maintenance of the rights of the participants and confidential handling of personal information by the hospital physicians and has approved this consent procedure.
Data analysis
All relevant data in the DDSS and meteorological indicators were collected in daily basis. From these daily data, the monthly proportion of rotavirus infection, proportion of malnutrition (underweight, stunting and wasting) among children under 5 years, monthly mean ambient temperature, rainfall, sea level pressure and humidity with other socio-demographic factors were calculated. A time series analysis was performed to explore the relationship between the monthly proportion of rotavirus infection with the monthly proportion of malnutrition (the main exposure), monthly mean ambient temperature, rainfall, sea level pressure and humidity using a seasonal autoregressive integrated moving average (ARIMA) model [30,31]. Firstly, the autocorrelation function (ACF; S1 Fig) and partial autocorrelation function (PACF; S2 Fig) plots of the differenced series were used to identify the number of autoregressive and/or moving average (MA) terms for inclusion in the model. Cross-correlations to lag 20 were performed to assess the similarity of the monthly proportion of rotavirus infection and all types of childhood malnutrition to other meteorological indicators (mean temperature, rainfall, sea level pressure and humidity) (S3 Fig). There was a known strong seasonality in rotavirus infection [23,32, 33] and thus the seasonal Auto-regressive Integrated Moving Average (ARIMA) was finally used. A seasonal ARIMA (p, d, q) (P, D, Q) s model was employed where
p and P are the order of the auto regressive and seasonal autoregressive terms, respectively,
d and Dare the order of non-seasonal and seasonal differencing,
q and Q are the order of the moving average and seasonal moving average terms and
s = 12 represented the length of the seasonal period in months.
All explanatory variables (main exposures; monthly proportion of underweight, stunting and wasting and four meteorological indicators were first centred by calculating the deviation from its mean and these deviations were used as the predictor variables. A series of multivariable models were employed. The initial models are: Model1: adjusted for mean temperature, rainfall, sea level pressure and humidity; Model 2: Model1 with year’s stratum added (two decades: 1993–2002 and 2003–2012). Considering a sharp change in prevalence of rotavirus infection and all the indicators of malnutrition after 2000, the years of observation were categorized into two stratums which is subsequently referred to as years stratum. Model3: Model2 with added socio-demographic characteristics (monthly mean age of under 5 children, proportion of female children, use non-sanitary toilet, non-slum residence, more than one under 5 year children in the household). Next, a series of interaction models were fitted with the aim to determine variability of association between rotavirus infection and childhood malnutrition. Model 4: Model 3 plus interaction between underweight and year stratum; Model 5: Model 3 plus interaction between underweight and mean temperature; Model 6: Model 3 plus the interaction between underweight and mean rainfall; Model 7: Model 3 plus interaction between underweight and mean sea level pressure; Model 8: Model 3 plus interaction between underweight and mean humidity; Model 9: Model 3 plus interaction between underweight, mean temperature and rainfall etc. (details in S4, S5 and S6 Tables)).
To assess the possibility of a lag effect of malnutrition on proportion of rotavirus infection (adjusted for different meteorological indicators lag of one period) was also putatively fitted. AIC and BIC values were calculated to determine whether including or excluding of lag improved the model fit (S2, S4, S5 and S6 Tables). Analyses were performed separately for all three components of malnutrition (underweight, stunting and wasting).
Additionally, the yearly mean distribution of each variable was also estimated using a moving average that used one lagged term, current value and lead term. A Spearman correlation [34] was calculated to estimate the Chi-squared for trend (S1 Table).
All analyses were undertaken using STATA version13 (StataCorp, College Station, TX, USA).
Results
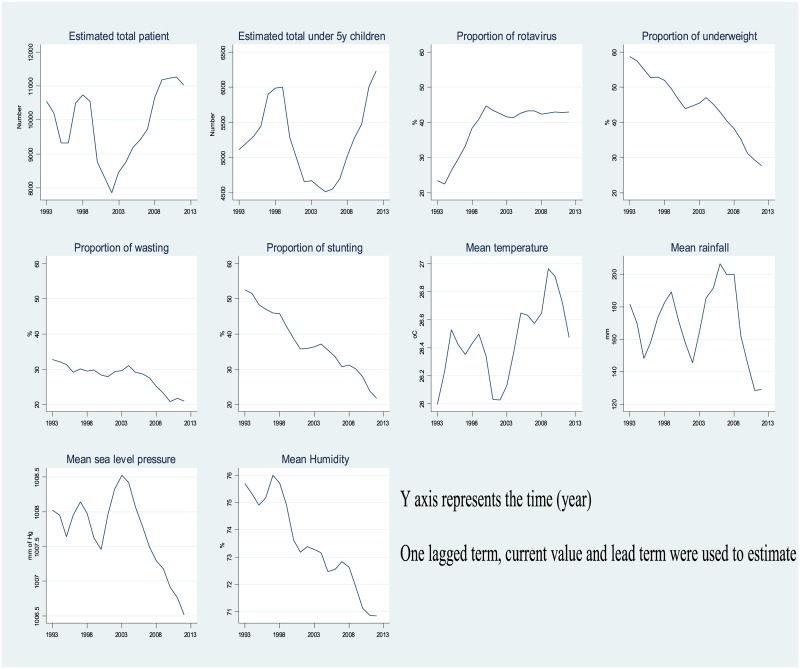
The yearly estimated number of under 5 years children with diarrhoea admitted to the hospital in 1993 was 5,206 and that increased to 6,892 in 2012. Similarly, the proportion of rotavirus cases during this period gradually increased from 20% in 1993 to 43% in 2012 (Chi-square for trend: p = 0.010) (Fig 1). An abrupt increase in rotavirus infection was observed in 1999 and that remained for the rest of the period.
Fig 1. Moving average of yearly estimated to total patients and under-5 years children, proportion of rotavirus, underweight, stunting, wasting, mean temperature, rainfall, sea level pressure and humidity (1993–2012).
In contrast, the proportion of children who were underweight, stunted or wasted decreased from 1993 to 2012 from 58% to 29% (Chi squared for trend: p<0.001), 53% to 21% (p<0.001) and 32% to 22% (p = <0.001) respectively (Fig 1). During this time, the mean temperature increased from 25.76°C to 26.61°C (p = 0.07) while the mean rainfall, sea level pressure and humidity decreased from 234.92 mm to 111.75 mm (p = 0.5), 1008.30 mm of hg to 1006.61 mm of hg (p = 0.02) and 76.63% to 70.26% (p<0.001) respectively.
In the unadjusted ARIMA model (Table 1), there is significant association between monthly proportion of rotavirus infection and underweight. A decrease in one unit of the monthly proportion of underweight coincided with a 0.26 increase in the monthly proportion of rotavirus infection [(95% CI: -0.43, -0.094) p = 0.002]. The association remained when adjusted for monthly mean temperature, rainfall, sea level pressure and humidity (Table 1; model 1). When adjusted for years stratum, the effect of monthly change in proportion of underweight decreased to -0.24 (95% CI: -0.42, -0.06) (Table 1, model 2). Moreover, in the final model (Table 1, model 3) adjusted for other socio-demographic factors, it accounted for a-0.19 (95% CI:-0.376, -0.003). A similar association was also observed for wasting in which one unit decrease in monthly proportion of wasting resulted in an increase of the monthly proportion of rotavirus infection by -0.27 (95% CI: -0.455, -0.075) after adjusting for all the co-variates including meteorological indicators. However, there was no association with stunting (full model for underweight, stunting and wasting are describe in S3 Table).
Table 1. Association between the monthly proportion of rotavirus infection and underweight, stunting, wasting for under-5 children in urban Dhaka, Bangladesh.
Adjusted and unadjusted seasonal ARIMA models were fitted to the proportion of children with rotavirus infection. Covariates are outlined in the model definitions below.
| Underweight | Stunting | Wasting | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coef. | 95% CI | p | Coef. | 95% CI | p | Coef. | 95% CI | p | ||||
| LL | UL | LL | UL | LL | UL | |||||||
| Unadjusted | -0.260 | -0.426 | -0.094 | 0.002 | -0.134 | -0.288 | 0.020 | 0.089 | -0.296 | -0.475 | -0.117 | 0.001 |
| Model 1 | -0.264 | -0.440 | -0.089 | 0.003 | -0.134 | -0.290 | 0.021 | 0.090 | -0.301 | -0.487 | -0.115 | 0.002 |
| Model 2 | -0.239 | -0.417 | -0.062 | 0.008 | -0.122 | -0.281 | 0.036 | 0.131 | -0.288 | -0.476 | -0.099 | 0.003 |
| Model 3 | -0.189 | -0.376 | -0.003 | 0.047 | -0.070 | -0.249 | 0.109 | 0.441 | -0.265 | -0.455 | -0.075 | 0.006 |
Outcome: Proportion of rotavirus infection; main exposure: proportion of underweight/stunting/wasting
Model 1: Unadjusted+ mean centred monthly temperature, rainfall, sea level pressure, humidity
Model 2: Model 1 + year strata (1993–2002 vs. 2003–2012)
Model 3: Model 2 + mean age, proportion female, use non-sanitary toilet, non-slum residence, more than one under 5 year children in the household
Note: All estimates were in monthly basis; Centre value of underweight, mean temperature, rainfall, sea level pressure, humidity were used
When interactions were modelled, the association between monthly rotavirus infection and underweight remained significant with a variation in effect size as expected and effect size ranged between -0.14 to -0.26 with an additional variability which ranged between 0.7% to 1.52% in rotavirus infection with unadjusted model by adding different interaction terms (see S4 Table). For wasting, the effect size ranged between -0.17 and -0.36 with a variability of 0.90% to 1.14% with unadjusted model (S6 Table). Details of interaction modeling for stunting are provided in S5 Table.
Discussion
Findings of the present study indicate a striking inverse relationship of wasting (acute malnutrition) and underweight (mixed acute and chronic malnutrition) with childhood rotavirus infection after controlling for different climate, socio-demographic and sanitation practices among under-5 years children in Dhaka. However, while a similar association with stunting (chronic malnutrition) was also observed it was not statistically significant. This nutrition interaction in which rotavirus disproportionately affects better nourished children, has been previously described [35]. Notably, this is in contrast to childhood malnutrition as an established risk factor for diarrhoeal disease due to most other enteric pathogens [36].
It is evident that rotavirus infection is associated with the variability of climate factors [22] with strong seasonal increases especially during cooler and dries months [23,32,33]. A one degree centigrade increase of temperature above a threshold (29 degrees centigrade) was associated with a 40% increase in rotavirus diarrhoea and there was a linear inverse relationship between the number of cases of rotavirus diarrhoea with relative humidity [22]. To our knowledge, this is the only study that estimated the effect of changing childhood malnutrition and its association with rotavirus infection after controlling for the ambient temperature, rainfall, sea level pressure and humidity (Model 1). These analyses indicate that not only climatic factors but also improved nutrition or over nutrition might have a significant role in the pathogenesis of rotavirus infection [12–14]. However, other man-made influences including unplanned urbanization with rapid land coverage and reduced green spaces in the Dhaka megacity and improper sewerage might be additional contributors to the causal path [5–7,37,38]. After adjustment for host (age and sex) and demographic (residence and under 5 children in the household) characteristics and sanitation practices, the significant association indicates a strong relationship between rotavirus infection and acute and chronic malnutrition.
Rotavirus, a major pathogens responsible for acute watery diarrhoea, perhaps requires healthy intestinal epithelium for attachment and pathogenesis [14]. In animal models, malnutrition results in a decreased number of cells and impaired epithelial proliferation in the small intestinal mucosa [12] that inhibit cellular attachment by the virus [13]. In addition to natural infection, it is plausible that a predilection of rotavirus for healthy, nutritionally intact intestinal epithelium is a factor in the observed immune response to Rotarix oral vaccine in which meant seroconversion rates to Rotarix were 86%, 75%, and 63% in high, middle, and low income countries respectively [39].
However, immune-compromised, severely malnourished children [11] are more prone to have infection rotavirus [16]. A reduction of acute malnutrition and, increases in the proportion of well-nourished children, as well as the recent trend towards an increasing prevalence of childhood obesity among under-5 children at DSSS [24], might positively contributed for an acute infection [15]. On the other hand, genetic variation might predict chronic malnutrition such as stunting [40], which may not be interlinked with acute infection [41].
In the present study, we observed that in the early 1990’s (1993) the proportion of all the malnutrition subtypes (underweight, wasting and stunting) were relatively high among the under5 yeras children and the proportion of rotavirus was low as was mean ambient temperature. However, from 1993 onwards, the proportion of under 5 years children with malnutrition gradually decreased and rotavirus diarrhoea increased. During 1999–2001, there was a sudden peak of rotavirus diarrhoea associated with a decreased mean level of all of the examined climate factors, in addition to reduced malnutrition rates which might have further affected the association. It is perhaps relevant that from 2002 onwards, the proportion of children under 5 years with malnutrition, mean relative humidity, rainfall and sea level pressure all decreased steadily whereas mean ambient temperature gradually increased. We employed a series of interaction models between all three components of malnutrition and climate factors and year stratum (Model 4–13; S4, S5 and S6 Tables) and estimated the variability of associations. The association largely remained significant with some degree of variability in the effect size.
Strengths and limitations
Considering methodologic strengths of the current study are the inclusion of long-term observations with unbiased systematic samples and the quality assessment of nutrition status by trained personnel, and quality laboratory performance with same method of detection of rotavirus over the study period. The adjusting for possible confounding socio-economic factors and performing seasonal ARIMA models with a series of likely interactions are the other strengths. However, we did not consider other potentially important factors like vegetation index, per-capita land, river water level, or land surface temperature which might influence the causal path in transition of rotavirus disease [8,9]. Hospital based data might not adequately represent the population at large and might have a bias towards patients with less severe rotavirus illness and who seek care less often at hospital facilities. Finally, patients enrolled in the DDSS were self-referred and there is no subject-specific information on of their conditions, including spatial variability.
Conclusion
Findings of the present study indicate an inverse association between acute childhood malnutrition and rotavirus diarrhoea among a population of children less than 5 years of age in urban Dhaka, Bangladesh after adjusting for important climate, socio-demographic and sanitation factors. An insignificant inverse association; however, was observed for chronic malnutrition. The relationship between recent nutritional transition and rotavirus infection in the mega city might be additionally influence by other man-made factors. As a result, an alternative intervention other than man made factors may be warranted such as mass vaccination may be needed to prevention and control of childhood rotavirus infection.
Supporting information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(PDF)
(PDF)
(PDF)
Acknowledgments
Hospital surveillance was funded by icddr,b and the Government of the People's Republic of Bangladesh through IHP-HNPRP. icddr,b acknowledges with gratitude the commitment of the Government of the People's Republic of Bangladesh to the icddr,b’s research efforts. icddr,b also gratefully acknowledges the following donors which provided unrestricted support to icddr,b since 1993 from when the data is used for this study: Australian Agency for International Development (AusAID), Government of the People's Republic of Bangladesh, Canadian International Development Agency (CIDA), Embassy of the Kingdom of the Netherlands (EKN), Swedish International Development Cooperation Agency (Sida), Swiss Agency for Development and Cooperation (SDC), and Department for International Development, UK (DFID).
Data Availability
All relevant data are within the paper.
Funding Statement
Hospital surveillance was funded by icddr,b and the Government of the People's Republic of Bangladesh through IHP-HNPRP.
References
- 1.Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, et al. (2013) Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet 382: 209–222. doi: 10.1016/S0140-6736(13)60844-2 [DOI] [PubMed] [Google Scholar]
- 2.Hashizume M, Armstrong B, Hajat S, Wagatsuma Y, Faruque AS, Hayashi T, et al. (2008) The effect of rainfall on the incidence of cholera in Bangladesh. Epidemiology 19: 103–110. doi: 10.1097/EDE.0b013e31815c09ea [DOI] [PubMed] [Google Scholar]
- 3.Hashizume M, Armstrong B, Hajat S, Wagatsuma Y, Faruque AS, Hayashi T, et al. (2007) Association between climate variability and hospital visits for non-cholera diarrhoea in Bangladesh: effects and vulnerable groups. Int J Epidemiol 36: 1030–1037. doi: 10.1093/ije/dym148 [DOI] [PubMed] [Google Scholar]
- 4.Hashizume M, Wagatsuma Y, Faruque AS, Hayashi T, Hunter PR, Armstrong B, et al. (2008) Factors determining vulnerability to diarrhoea during and after severe floods in Bangladesh. J Water Health 6: 323–332. [DOI] [PubMed] [Google Scholar]
- 5.Dewan AM, Yamaguchi Y (2008) Effect of Land Cover Changes on Flooding: Example from Greater Dhaka of Bangladesh. International Journal of Geoinformatics 4. [Google Scholar]
- 6.Dewan AM, Yamaguchi Y (2009) Land use and land cover change in Greater Dhaka, Bangladesh: Using remote sensing to promote sustainable urbanization. Applied Geography 29: 390–401. [Google Scholar]
- 7.Dewan A, Corner R (2013) Dhaka megacity: geospatial perspectives on urbanisation, environment and health: Springer Science & Business Media. [Google Scholar]
- 8.Dewan AM, Corner R, Hashizume M, Ongee ET (2013) Typhoid Fever and its association with environmental factors in the Dhaka Metropolitan Area of Bangladesh: a spatial and time-series approach. PLoS Negl Trop Dis 7: e1998 doi: 10.1371/journal.pntd.0001998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hashizume M, Dewan AM, Sunahara T, Rahman MZ, Yamamoto T (2012) Hydroclimatological variability and dengue transmission in Dhaka, Bangladesh: a time-series study. BMC Infect Dis 12: 98 doi: 10.1186/1471-2334-12-98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharmin S, Viennet E, Glass K, Harley D (2015) The emergence of dengue in Bangladesh: epidemiology, challenges, and future disease risk. Transactions of The Royal Society of Tropical Medicine and Hygiene: trv067. [DOI] [PubMed] [Google Scholar]
- 11.Rodriguez L, Cervantes E, Ortiz R (2011) Malnutrition and gastrointestinal and respiratory infections in children: a public health problem. Int J Environ Res Public Health 8: 1174–1205. doi: 10.3390/ijerph8041174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guiraldes E, Hamilton JR (1981) Effect of chronic malnutrition on intestinal structure, epithelial renewal, and enzymes in suckling rats. Pediatr Res 15: 930–934. doi: 10.1203/00006450-198106000-00010 [DOI] [PubMed] [Google Scholar]
- 13.Hu L, Crawford SE, Czako R, Cortes-Penfield NW, Smith DF, Le Pendu J, et al. (2012) Cell attachment protein VP8* of a human rotavirus specifically interacts with A-type histo-blood group antigen. Nature 485: 256–259. doi: 10.1038/nature10996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramig RF (2004) Pathogenesis of intestinal and systemic rotavirus infection. J Virol 78: 10213–10220. doi: 10.1128/JVI.78.19.10213-10220.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Das SK, Chisti MJ, Huq S, Malek MA, Vanderlee L, Kaur G, et al. (2013) Clinical characteristics, etiology and antimicrobial susceptibility among overweight and obese individuals with diarrhea: observed at a large diarrheal disease hospital, Bangladesh. PLoS One 8: e70402 doi: 10.1371/journal.pone.0070402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Das SK, Chisti MJ, Huq S, Malek MA, Salam MA, Ahmed T, et al. (2013) Etiology of diarrhea among severely malnourished infants and young children: Observation of urban-rural differences over one decade in Bangladesh. Food and Nutrition Sciences 4: 233. [Google Scholar]
- 17.Institute of Medicine Forum on Microbial T (2008) The National Academies Collection: Reports funded by National Institutes of Health Global Climate Change and Extreme Weather Events: Understanding the Contributions to Infectious Disease Emergence: Workshop Summary. Washington (DC): National Academies Press (US) National Academy of Sciences. [PubMed] [Google Scholar]
- 18.Guzman Herrador BR, de Blasio BF, MacDonald E, Nichols G, Sudre B, Vold L, et al. (2015) Analytical studies assessing the association between extreme precipitation or temperature and drinking water-related waterborne infections: a review. Environ Health 14: 29 doi: 10.1186/s12940-015-0014-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lobitz B, Beck L, Huq A, Wood B, Fuchs G, Faruque AS, et al. (2000) Climate and infectious disease: use of remote sensing for detection of Vibrio cholerae by indirect measurement. Proc Natl Acad Sci U S A 97: 1438–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Friel S (2010) Climate change, food insecurity and chronic diseases: sustainable and healthy policy opportunities for Australia. N S W Public Health Bull 21: 129–133. doi: 10.1071/NB10019 [DOI] [PubMed] [Google Scholar]
- 21.Stoll BJ, Glass RI, Huq MI, Khan MU, Holt JE, Banu H (1982) Surveillance of patients attending a diarrhoeal disease hospital in Bangladesh. Br Med J (Clin Res Ed) 285: 1185–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hashizume M, Armstrong B, Wagatsuma Y, Faruque AS, Hayashi T, Sack DA (2008) Rotavirus infections and climate variability in Dhaka, Bangladesh: a time-series analysis. Epidemiol Infect 136: 1281–1289. doi: 10.1017/S0950268807009776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Das SK, Begum D, Ahmed S, Ferdous F, Farzana FD, Chisti MJ, et al. (2014) Geographical diversity in seasonality of major diarrhoeal pathogens in Bangladesh observed between 2010 and 2012. Epidemiol Infect 142: 2530–2541. doi: 10.1017/S095026881400017X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Das SK, Chisti MJ, Malek MA, Das J, Salam MA, Ahmed T, et al. (2015) Changing childhood malnutrition in Bangladesh: trends over the last two decades in urban-rural differentials (1993–2012). Public Health Nutr 18: 1718–1727. doi: 10.1017/S136898001500004X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sarker MH, Das SK, Ahmed S, Ferdous F, Das J, Farzana FD, et al. (2014) Changing characteristics of rotavirus diarrhea in children younger than five years in urban Bangladesh. PLoS One 9: e105978 doi: 10.1371/journal.pone.0105978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Programme for control of diarrheal disease In Manual for laboratory investigation of acute enteric infections. Geneva, Switzerland: World Health Organization; 1987. [Google Scholar]
- 27.Qadri F, Khan AI, Faruque AS, Begum YA, Chowdhury F, Nair GB, et al. (2005) Enterotoxigenic Escherichia coli and Vibrio cholerae diarrhea, Bangladesh, 2004. Emerg Infect Dis 11: 1104–1107. doi: 10.3201/eid1107.041266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rahman M, De Leener K, Goegebuer T, Wollants E, Van der Donck I, Van Hoovels L, et al. (2003) Genetic characterization of a novel, naturally occurring recombinant human G6P[6] rotavirus. J Clin Microbiol 41: 2088–2095. doi: 10.1128/JCM.41.5.2088-2095.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Onyango AW, De Onis M (2008) WHO child growth standards: training course on child growth assessment.
- 30.Box GE, Jenkins GM, Reinsel GC (2008): Wiley. 701–727 p.
- 31.Enders W (2010) Applied Econometric Times Series: Chapter 6. Wiley: 360. [Google Scholar]
- 32.Levy K, Hubbard AE, Eisenberg JN (2009) Seasonality of rotavirus disease in the tropics: a systematic review and meta-analysis. Int J Epidemiol 38: 1487–1496. doi: 10.1093/ije/dyn260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jagai JS, Sarkar R, Castronovo D, Kattula D, McEntee J, Ward H, et al. (2012) Seasonality of rotavirus in South Asia: a meta-analysis approach assessing associations with temperature, precipitation, and vegetation index. PLoS One 7: e38168 doi: 10.1371/journal.pone.0038168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Conover W (1999) Practical nonparametric statistics, 3rd edn Wiley; New York: 250–257. [Google Scholar]
- 35.Unicomb LE, Kilgore PE, Faruque SG, Hamadani JD, Fuchs GJ, Albert MJ, et al. (1997) Anticipating rotavirus vaccines: hospital-based surveillance for rotavirus diarrhea and estimates of disease burden in Bangladesh. Pediatr Infect Dis J 16: 947–951. [DOI] [PubMed] [Google Scholar]
- 36.Faruque AS, Das SK, Chisti MJ, Afroze F, Ashraf H, Hossain MI, et al. (2014) Childhood diarrhea and severe malnutrition; Stein N, editor: Jones & Bartlett Publishers. [Google Scholar]
- 37.Corner RJ, Dewan AM, Hashizume M (2013) Modelling typhoid risk in Dhaka Metropolitan Area of Bangladesh: the role of socio-economic and environmental factors. International journal of health geographics 12: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Byomkesh T, Nakagoshi N, Dewan AM (2012) Urbanization and green space dynamics in Greater Dhaka, Bangladesh. Landscape and Ecological Engineering 8: 45–58. [Google Scholar]
- 39.Patel M, Shane AL, Parashar UD, Jiang B, Gentsch JR, Glass RI (2009) Oral rotavirus vaccines: how well will they work where they are needed most? J Infect Dis 200 Suppl 1: S39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Waterlow J (1994) Introduction. Causes and mechanisms of linear growth retardation (stunting). Eur J Clin Nutr 48: S1–4. [PubMed] [Google Scholar]
- 41.Reinhardt K, Fanzo J (2014) Addressing Chronic Malnutrition through Multi-Sectoral, Sustainable Approaches: A Review of the Causes and Consequences. Front Nutr 1: 13 doi: 10.3389/fnut.2014.00013 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(PDF)
(PDF)
(PDF)
Data Availability Statement
All relevant data are within the paper.