Abstract
B-1 cells produce natural antibodies which provide an integral first line of defense against pathogens while also performing important homeostatic housekeeping functions. In this study, we demonstrate programmed cell death 1 ligand 2 (PD-L2) regulates the production of natural antibodies against phosphorylcholine (PC). Naïve PD-L2-deficient (PD-L2−/−) mice produced significantly more PC-reactive IgM and IgA. This afforded PD-L2−/− mice with selectively enhanced protection against PC-expressing non-typeable Haemophilus influenzae (NTHi), but not PC-negative NTHi, relative to wild type mice. PD-L2−/− mice had significantly increased PC-specific CD138+ splenic plasmablasts bearing a B-1a phenotype, and produced PC-reactive Abs largely of the T15 idiotype. Importantly, PC-reactive B-1 cells expressed PD-L2 and irradiated chimeras demonstrated B cell-intrinsic PD-L2 expression regulated PC-specific Ab production. In addition to increased PC-specific IgM, naïve PD-L2−/− mice and irradiated chimeras reconstituted with PD-L2−/− B cells had significantly higher levels of IL-5 – a potent stimulator of B-1 cell Ab production. PDL2 mAb blockade of wild type B-1 cells in culture significantly increased CD138 and Blimp1 expression and PC-specific IgM, but did not affect proliferation. PDL2 mAb blockade significantly increased IL-5+ T cells in culture. Both IL-5 neutralization and STAT5 inhibition blunted the effects of PDL2 mAb blockade on B-1 cells. Thus, B-1 cell-intrinsic PD-L2 expression inhibits IL-5 production by T cells and thereby limits natural Ab production by B-1 cells. These findings have broad implications for the development of therapeutic strategies aimed at altering natural Ab levels critical for protection against infectious disease, autoimmunity, allergy, cancer, and atherosclerosis.
Introduction
Natural antibody (Ab) bridges the innate and adaptive immune response, providing an initial defense against pathogenic microorganisms (1–3). These immunoglobulins are produced in the sera of normal mice and humans in the absence of immunization or infection (4–8). Natural Abs serve important functions in tissue homeostasis and clearance of senescent and cancerous cells, autoimmune disease processes, infection, B cell development, and protection against atherosclerosis (4, 5, 9–22). The B-1 cell subset produces the majority of natural Abs in the serum, of which IgM is the principal isotype (4, 5, 23–26). Natural Abs are directed against multiple specificities, including oxidized LDL (OxLDL), phosphatidylcholine (PtC), phosphorylcholine (PC), malondialdehyde (MDA), oxidized cardiolipin (OxCL), 4-hydroxynonenal (4-HNE), and Thy-1 (CD90), as well as tumor associated antigens (10, 11, 13, 27).
One of the most well-studied natural Ab specificities is PC. In particular, the regulation and function of PC-specific Ab of the T15 idiotype (Id) has been extensively studied. T15 is a protective natural Ab encoded by germline sequence of the BCR in multiple mouse strains (18, 20, 28) and recognizes PC expressed on the surface of Streptococcus pneumoniae (the pneumococcus) as a component of the teichoic and lipoteichoic acids, as well as PC expressed in OxLDL. B-1 B cell derived T15 anti-PC Ab is atheroprotective (9, 22, 29, 30). Context is equally important, as the majority of natural Ab in the naïve mouse is IgM, but in response to pneumococcal infection, T15 IgG is more protective than T15 IgM (31). Recent work by the Kearney lab has shown that T15 Id+ B cells suppress allergic disease (8, 32). Although considered a protective Ab, under conditions favoring autoimmunity, PC-reactive Ab has been shown to cross-react to dsDNA (33–35) and somatic mutation of the T15 Id can specifically contribute to increased dsDNA reactivity (36). Thus, regulation of PC-specific Ab production is of critical importance to protection against infectious disease, autoimmunity, allergy, and atherosclerosis.
B-1 B cells producing the vast preponderance of natural Ab are efficiently generated from precursors in fetal liver and bone marrow, the former being a rich source of B-1 progenitors (4, 14, 25, 37–41). B-1 cells are enriched in the pleural and peritoneal space of mice and primates (42, 43), but for reasons not yet fully understood, are suppressed from producing Abs within this environment (44). B-1 cells consist of B-1a cells and B-1b cells, which are phenotypically distinguished by CD5 expression on B-1a cells. The B-1a cell (CD19+CD5+) repertoire consists of cross-reactive receptors which bind self and non-self antigens (24). The presence of potentially auto-reactive B cells requires stringent mechanisms that control their activation and differentiation to Ab-producing cells. Activation signals induce B-1a cell trafficking to the spleen and bone marrow (5), enabling differentiation of B-1a cells into ASCs (45). While signals controlling B-1a cell activation and peritoneal exit in response to TLR agonists have been investigated, the mechanism controlling spontaneous or natural Ab production, including PC-specific Ab, is less clear. The distinctive expression of cell surface regulators, such as programmed cell death 1 (PD-1) ligand 2 (commonly, PD-L2 or B7DC) (46, 47), by this unique cell subset suggests potential pathways of regulation.
Our recent work has demonstrated PD-1 and its ligands, PD-L1 (B7H1) and PD-L2 (B7DC), are important negative regulators of the adaptive immune response to the polysaccharide capsule of Streptococcus pneumoniae. B cell-expressed PD-1 plays a major role in suppressing Ab production to pneumococcal polysaccharide, as well as other T cell– independent type 2 antigens (TI-2 Ags) and tumor-associated carbohydrate Ags (48–50). Evidence supports that PD-1 specifically induced on Ag-specific B-1b cells suppresses B cell proliferation and differentiation to IgG-producing cells (48). Nonetheless, spontaneous PC-specific Ab production, as well as adaptive immune responses to PC may be differentially regulated (23, 30, 51). The potential of the PD-1:PD-L regulatory axis to control natural or adaptive PC-specific Ab responses is not known.
In this study, PD-L2 was found to regulate the production of natural Ab against PC, as well as PtC. Mice deficient for PD-L2 produced significantly more anti-PC IgM, specifically the T15 Id, and had significantly increased PC-reactive plasmablasts bearing a B-1a phenotype. The increased PC-specific Ab in these mice was associated with significantly increased protection against a constitutive PC-expressing mutant of non-typeable Haemophilus influenzae (NTHi), but not an isogenic mutant lacking surface PC. Importantly, B cell-expressed PD-L2 was found to suppress PC-specific Ab production as well as systemic IL-5 levels in vivo. In vitro culture assay demonstrated that PD-L2 suppressed B-1 cell differentiation into ASC via suppressing T cell production of the critical B-1 cell ASC differentiation factor, IL-5. Thus, this study reveals a novel PD-L2–dependent pathway that regulates natural Ab production by B-1 cells.
Materials & Methods
Mice
Mice were bred in-house and included WT and muMT (The Jackson Laboratory) mice as well as PD-1−/−, PD-L2−/−, and PD-L1−/− on the C57BL/6 background as previously described (48). Mice were housed in specific pathogen-free conditions, except during infection experiments. Mice (used at 2–4 months of age) were age-matched for experiments. In some experiments, mice treated with drinking water supplemented with vancomycin (0.5g/L), neomycin (1g/L), metronidazole (1g/L), and ampicillin (1g/L). All studies and procedures were approved by the Wake Forest Animal Care and Use Committee.
Infections
NTHi 86-028NP licON or NTHi licD mutant strains, which are exclusively PC+ (licON) or PC− (licD) populations (52, 53) were cultured overnight on brain-heart infusion agar (Difco) supplemented with hemin (10 μg/mL, ICN Biochemical) and nicotinamide adenine dinucleotide (10 μg/mL, Sigma). Bacteria were harvested from plates with a sterile swab and suspended in phosphate-buffered saline to an OD600 ~0.1. Mice were infected via the intratracheal (i.t.) route with ~5 × 107 CFU. Nasal wash fluid (NWF) and broncho-alveolar lavage were collected as described previously (33, 48), serially diluted in sterile PBS, and plated onto supplemented BHI agar for plate counts.
ELISA and ELISPOT
ELISAs were performed as previously described (48). Samples were diluted in TBS containing 1% BSA (TBS-BSA) and analyzed for total and PC-specific IgM, IgG, and IgA. Diluted serum samples were added to Costar plates coated with 0.625 μg/mL PC(4)BSA (Biosearch Technologies) or 5 μg/mL anti-mouse Ig(H+L) (Southern Biotechnology Associates) or AB1.2 (anti-T15 Id) Ab and blocked with TBS-BSA. Alkaline phosphatase-conjugated polyclonal goat anti-mouse IgM, IgG, IgG3, and IgA Abs (Southern Biotech) and pNPP (Sigma) were used to detect Ab. A PC-specific IgM hybridoma was used for quantitation of the PC IgM response. IL-4, IL-5, IL-6, IL-10, IFNγ, TNFα, and GM-CSF ELISAs were performed according to manufacturer’s instructions (BioLegend). Malondialdehyde-specific ELISAs used plates coated with MDA-BSA (Cell Biolabs, Inc) at 5 ug/ml. Phosphatidylcholine ELISAs were performed on air-dried plates that had been coated with L-alpha-phosphatidylcholine (Sigma) at 30 ug/ml in hexane. ELISPOT was performed as previously described (33), using both Image Acquisition 4.5 and ImmunoSpot software for analysis of spot number and size.
Bone marrow and neonatal liver chimeric mice
For bone marrow chimeras, WT recipient mice were lethally irradiated (950 rad) and reconstituted with either muMT:WT bone marrow (BM) or muMT:PD-L2−/− BM (20% WT or PD-L2−/− plus 80% muMT BM). For neonatal liver chimeras, recipient muMT mice were lethally irradiated (950 rad) and reconstituted with neonatal WT or PD-L2−/− liver from 2-day old mice mixed with adult muMT BM (using a 20:80 ratio). 107 cells were delivered intravenously to recipient mice. Recipient mice were rested for three weeks and serum was analyzed for anti-PC IgM.
In vitro assay and MACS enrichment
B-1 cells from naïve WT mice were enriched from PerC using CD19+ (Miltenyi) and biotinylated CD23 mAb + streptavidin (Dynal) magnetic activated cell sorting (MACS). Purity of CD19+CD23− B-1 cells was 96.0 ± 0.3% as assessed by IgM staining. Purified B-1 cells (CD19+23−) were cultured in the absence of stimulation with muMT splenocytes at a 1:1 ratio in cRPMI + 10% FCS supplemented with 50 μM β-mercaptoethanol (Sigma) and 50 ng/mL BLyS (R&D Systems) in the presence of either PDL2 monoclonal antibody (mAb) blockade (TY25, InVivo mAb, BioXCell) or control mAb (2A3, InVivo mAb, BioXCell) at 5 μg/mL concentration. For in vitro cultures, a STAT5 inhibitor (CAS 285986-31-4, Calbiochem) was used at 50 μg/mL and anti-IL-5 mAb (TRFK5, BioLegend) was used at 2.5 μg/mL. Cultures were harvested at day 2 or 4 for ELISA and flow cytometry analysis.
Flow cytometry
Single-cell suspensions (2 × 107/mL) were washed with PBS containing 2% newborn calf serum and then incubated with Fc Block (eBioscience) for 15 minutes, followed by staining with a combination of the following fluorochrome-conjugated Abs: CD5 (53–7.3), B220 (RA3-6B2), CD86 (GL-1), CD11b (M1/70), CD1d (1B1), CD21/35 (7E9) (BioLegend); CD19 (1D3), CD138 (281–2), PD-L2 (TY25) (eBioscience); CD131 (common β chain; JORO50), CD125 (IL-5Rα, T21) (BD); and goat F(ab′)2 anti-mouse IgM (Southern Biotech). Intracellular cytokine and transcription factor staining was performed as previously described (48), and used Blimp1 (5E7) and Pax5 (1H9) (eBioscience); IL-4 (11B11), IFNγ (XMG1.2), TNFα (MP6-XT22), and IL-5 (TRFK5, BioLegend). Fluorochrome-labeled isotype controls were used to determine background staining levels. PC-reactive B cells were detected as previously described (42). Briefly, following Fc block incubation, cells were incubated with PC-Fluorescein-BSA (Biosearch Technologies; 20 μg/ml), BSA-Alexafluor-647 (20 μg/ml), and fluorochrome labeled mAbs in PBS containing 2% normal calf serum at room temperature for 25 min. Cells were washed and fixed. BSA-binding cells were gated out prior to analysis of PC-BSA-binding B cells. Stained cells were analyzed using a FACS Canto II cytometer (BD Biosciences) with forward light scatter (FSC)-A/FSC-H doublet exclusion. Data were analyzed using FlowJo analysis software (Tree Star).
Statistical analysis
Data are shown as mean ± SEM with differences assessed using Student’s t test or one-way Analysis of Variance (ANOVA) with Dunnett Post Test, as indicated in figure legends. Differences in in vitro assay conditions were assessed using pairwise Student’s t test.
Results
Anti-PC Ab is selectively increased in PD-L2−/− mice
We assessed total Ig levels in PD-1−/−, PD-L1−/−, and PD-L2−/− mice and found that PD-L2−/− mice had significantly decreased total serum IgM, but significantly increased total serum IgG and IgA compared to WT mice (Fig. 1A). PD-1−/− and PD-L1−/− mice similarly exhibited significant increases in serum IgG, as previously described (54), but had normal or increased serum IgM levels, in contrast to PD-L2−/− mice. IgA was increased in PD-1−/−, but not PD-L1−/− mice. These data indicate PD-1 and its ligands have distinct roles in regulating total Ig levels, with PD-L2, but not PD-1 or PD-L1, required for maintaining optimal IgM serum levels.
Figure 1. PC-specific Ab levels in PD-L2−/− mice are selectively increased.
A) Total serum Ig levels in WT, PD-1−/−, PD-L2−/−, and PD-L1−/− mice. B) PC-specific serum IgM, IgG, and IgA levels in WT, PD-1−/−, PD-L2−/−, and PD-L1−/− mice (top: RAU = relative absorbance units; bottom left: reciprocal serum IgM titers). C) PC-specific IgM and IgA levels in broncho-alveolar lavage (BAL) of WT and PD-L2−/− mice. D) MDA-specific serum IgM levels in WT and PD-L2−/− mice. E) PtC-specific serum IgM levels (RAU and reciprocal serum titers) in WT and PD-L2−/− mice. F) NTHi burden in WT and PD-L2−/− mice 48 hours after inoculation with NTHi 86-028NP licON or NTHi 86-028NP licD. N ≥ 5 mice/group; A-B analyzed by ANOVA with Dunnett Post Test; reciprocal titer data analyzed by Student’s t test; C-F analyzed by Student’s t test (* p<0.05, ** p<0.005, *** p<0.001).
The significantly decreased total IgM in PD-L2−/− mice led us to hypothesize that natural Ab would be similarly decreased. We therefore assayed PC-, PtC-, and MDA-specific Ab levels. Strikingly, serum PC-specific IgM levels in naïve mice were significantly higher in PD-L2−/− mice, with reciprocal titers 3.1-fold higher than that of WT mice (Fig. 1B). PC-specific Ab levels were slightly increased in PD-1−/− and PD-L1−/− mice, but were not significantly different from WT mice (Fig. 1B). Serum PC-specific IgG and IgA were also significantly elevated in PD-L2−/− mice over WT mice (Fig. 1B). PC-specific IgM and IgA were also significantly increased in broncho-alveolar lavage (BAL) of PD-L2−/− mice (Fig. 1C). Interestingly, IgM against MDA, a specificity found among B-1b and B-1a cells (22), was significantly decreased in PD-L2−/− mice (Fig. 1D). However, serum PtC-specific IgM, a specificity attributed to B-1a cells, was significantly increased in PD-L2−/− mice, with reciprocal titers 3.5-fold higher than that of WT mice (Fig. 1E), similar to PC-specific IgM. Natural IgM reactive against pneumococcal polysaccharide type 3 (PPS3) was comparable between WT and PDL2−/− naïve mice and was slightly reduced in PD-L2−/− mice following native PPS immunization (Pneumovax-23). However, IgM produced against protein-conjugated PPS (Prevnar-13) was significantly reduced in PD-L2−/− mice (Supplemental Fig. 1A). Thus, PD-L2 has different effects on IgM production, depending upon antigen specificity. In contrast to PD-1 and PD-L1, which moderately influence PC-specific Ig levels and are dispensable for maintenance of total serum IgM, PD-L2 promotes maintenance of total serum IgM levels and limits production of natural IgM against PC and PtC, two well-defined specificities produced by B-1a cells.
PC-expressing NTHi strains are cleared more rapidly from the lungs of PD-L2−/− mice
We assessed the physiological relevance of increased PC-specific Ab levels in the BAL and lungs of naïve adult PD-L2−/− mice by examining acute clearance of PC-expressing and non-PC-expressing isolates of non-typeable Haemophilus influenzae (NTHi) 86-028NP. NTHi 86-028NP licON constitutively expresses PC on its lipooligosaccharide (LOS) layer, whereas NTHi 86-028NP licD lacks the phosphorylcholine transferase, and thus lacks PC on its surface (52). Two days post-challenge, PD-L2−/− mice exhibited significantly lower burden of NTHI 86-028NP licON compared to WT mice. NTHi 86-028NP licD burden was similar in WT and PD-L2−/− mice (Fig. 1F). These data suggest that significantly increased PC-specific natural Ab in naïve PD-L2−/− mice promotes enhanced clearance of PC-expressing respiratory pathogens.
Broad spectrum antibiotics do not alter elevated PC-specific Ab levels in PD-L2−/− mice
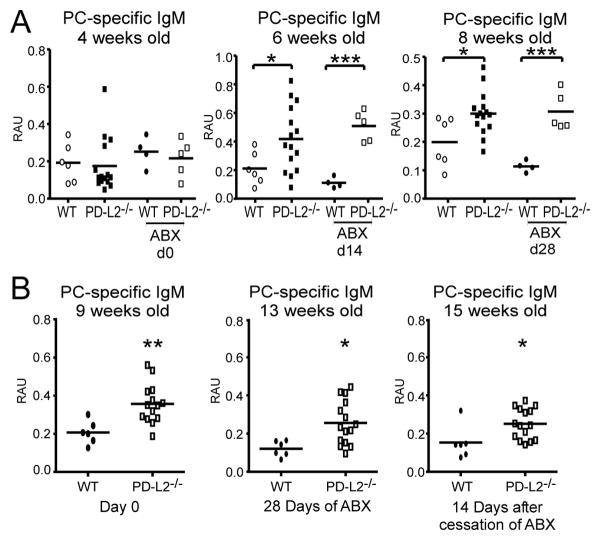
The relationship between host and microbiota contributes to the development of the immune system (55, 56), and recent evidence suggests that PD-1 influences the selection of IgA+ plasma cell repertoires and gut flora regulation (57, 58). We therefore sought to determine if the microbiota influenced the natural PC-specific Ab levels in PD-L2−/− mice using broad-spectrum antibiotic (ABX) therapy as previously described (57). We evaluated PC-specific IgM levels in mice in which therapy was initiated at: 1) 4 weeks of age (Fig. 2A); and 2) 9 weeks of age (Fig. 2B). Interestingly, increased PC-specific IgM was not observed in PD-L2−/− mice until 6 weeks of age. Treatment of 4-week old mice with ABX for 14 or 28 days did not alter the significantly increased levels of PC-specific IgM that were observed in PD-L2−/− mice at 6 and 8 weeks of age (Fig. 2A).
Figure 2. Broad spectrum antibiotics do not alter elevated levels of PC-specific Ab in PD-L2 deficiency.
A-B) PC-specific serum IgM levels in WT and PD-L2−/− mice. A) Mice were placed on ABX therapy at weaning (4 weeks old) for 28 days. Age-matched untreated control WT and PD-L2−/− mice were included. B) Untreated WT and PD-L2−/− mice from (A) were placed on ABX therapy at adulthood (9 weeks old) for 28 days. PC-specific IgM levels were measured by ELISA. Data analyzed by Student’s t test (* p<0.05, ** p<0.005, *** p<0.001).
Adult (9-week old) PD-L2−/− mice had significantly elevated PC-specific Ab levels prior to, during, and after cessation of ABX therapy compared to WT mice (Fig. 2B). Notably, all treated mice displayed significant reductions in total serum IgA levels after 14 days of ABX therapy (Suppl. Fig. 1B), as expected for this treatment regimen (59–61). Thus, while PD-L2−/− and WT weanling mice (4 weeks of age) begin with equivalent PC-specific Ab levels in the serum, altered host microbiota is unlikely to explain the appearance or maintenance of enhanced PC-specific Ab levels in PD-L2−/− mice.
PD-L2 deficiency results in increased PC-specific ASC number and output
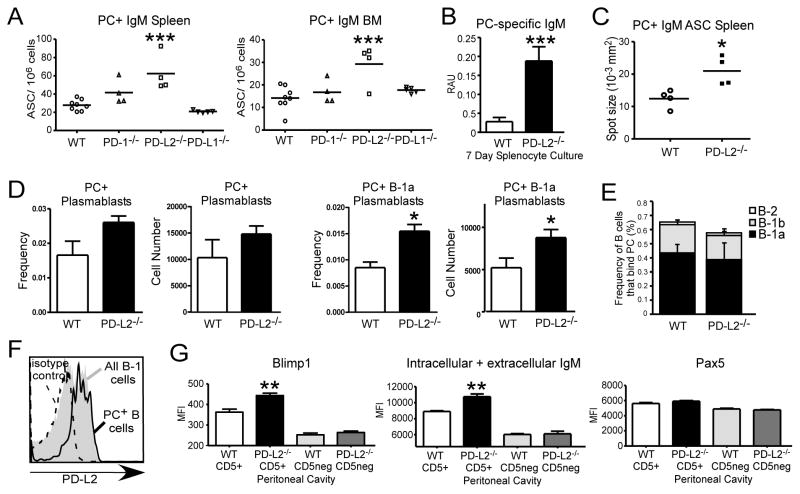
Increased PC-specific Ab in the context of PD-L2 deficiency may potentially be explained by increased Ab secreting cells (ASC) or increased output on a per cell basis. We therefore analyzed bone marrow (BM) and spleen ASC via ELISPOT. PD-L2−/− mice had significantly increased PC-specific ASC in the BM and spleen compared with WT mice (Fig. 3A, Supplemental Figure 1C–D). Interestingly, splenic PC-specific IgM ASC numbers were moderately increased in PD-1−/− mice, albeit not significantly different than WT mice. PC-specific IgM ASC numbers were at WT levels in PD-L1−/− mice. Consistent with these findings, supernatant from 7-day cultures of unstimulated, naïve PD-L2−/− splenocytes had significantly increased PC-specific IgM compared to WT splenocytes (Fig. 3B). Further, there was a significant increase in the spot size generated by PD-L2−/− splenocytes for both PC-specific IgM (Fig. 3C) and IgA (Suppl. Fig. 1D). These data suggest that an increase in both ASC number and output contributes to elevated PC-specific Ab levels in PD-L2−/− mice.
Figure 3. PD-L2 deficiency results in increased PC-specific ASC numbers in the spleen and bone marrow.
A) ELISPOT analysis of PC-specific IgM+ ASC number in the BM and spleen of naïve WT, PD-1−/−, PD-L2−/−, and PD-L1−/− mice. B) ELISA analysis of PC-specific IgM in supernatant from unstimulated, naïve 7-day splenocyte cultures. C) ELISPOT analysis of splenic PC-specific IgM+ ASC size in WT and PD-L2−/− mice. D) Frequency and number of splenic PC-specific total plasmablasts and PC-specific B-1a plasmablasts (CD5+19+138+) in WT and PD-L2−/− mice. E) Frequencies of peritoneal B cells (CD19+CD11b+/−) that bind PC in WT and PD-L2−/− mice, with distribution among B-1a, B-1b, and B-2 cells indicated. F) PD-L2 expression on PC-specific peritoneal B cells in WT mice relative to total B-1 cell PD-L2 expression and isotype control staining. G) Blimp1, total IgM (intracellular + extracellular), and Pax5 levels in peritoneal B cells (CD5+19+ vs CD5neg19+) in WT and PD-L2−/− mice. N ≥ 4 mice/group; for A, analyzed by ANOVA with Dunnett Post Test; B-G analyzed by Student’s t test (* p<0.05, ** p<0.005, *** p<0.001).
PD-L2−/− mice have significantly increased PC-specific B-1a cells with plasmablast phenotype and PC Ab of T15 specificity
We did not detect a difference in the frequency of total splenic PC-specific B cells in PD-L2−/− mice (0.31 ± 0.06% WT, 0.25 ± 0.02% PD-L2−/−). However, PD-L2−/− mice displayed an increase in the frequency of PC-reactive (CD138+) plasmablasts compared to WT mice (Fig. 3D). In particular, PD-L2−/− mice exhibited a significant increase in the frequency and number of CD19+CD5+CD138+ PC-binding B-1a cells in spleens compared to WT mice (Fig. 3D). We did not detect differences in the overall frequency of peritoneal B cells that bound PC in the peritoneal cavity or in the distribution of B-1a, B-1b, or B-2 cells binding to PC (Fig. 3E). Notably, approximately half of peritoneal B-1 cells expressed PD-L2 as previously reported (26) and 83 ± 1% (n=5) of PC-specific peritoneal B cells expressed PD-L2 (Fig. 3F and data not shown). The minor fraction of PC-binding B cells that were PD-L2lo/− cells largely belonged to the B-1b cell subset (82 ± 4%; data not shown). Notably, peritoneal cavity (PerC) CD5+ (B-1a) B cells in PD-L2−/− mice had significantly increased Blimp1 and total (intracellular + extracellular) IgM staining but normal Pax5 levels (Fig. 3G). In contrast, PD-L2−/− peritoneal CD5− B cells were similar to WT mice. Thus, PD-L2−/− mice have PerC B-1a cells that are poised for Ab secretion as well as significantly increased splenic B-1a plasmablasts dedicated to secreting PC-specific IgM.
Given the increase in PC-specific B-1a cells, we assessed the contribution of the T15 Id to PC-specific Ab levels in WT and PD-L2−/− mice using the AB1.2 (anti-T15 idiotype) Ab as described previously (20). Blocking with AB1.2 caused a dramatic dose-dependent decrease in bound PC-specific IgM in PD-L2−/− sera whereas WT sera binding were only slightly diminished (Fig. 4A). 50 μg/mL AB1.2 inhibited 77.3 ± 2.3% of PC-binding IgM in PD-L2−/− serum, but only 24.5 ± 9.9% binding in WT serum (p=0.001). Consistent with this finding, significantly increased PC-specific IgM concentrations in PD-L2−/− mice (Fig. 4B) corresponded with significantly increased T15 Id PC-specific IgM concentrations (Fig. 4C).
Figure 4. PD-L2 deficiency results in selective increase of serum T15-Id IgM.
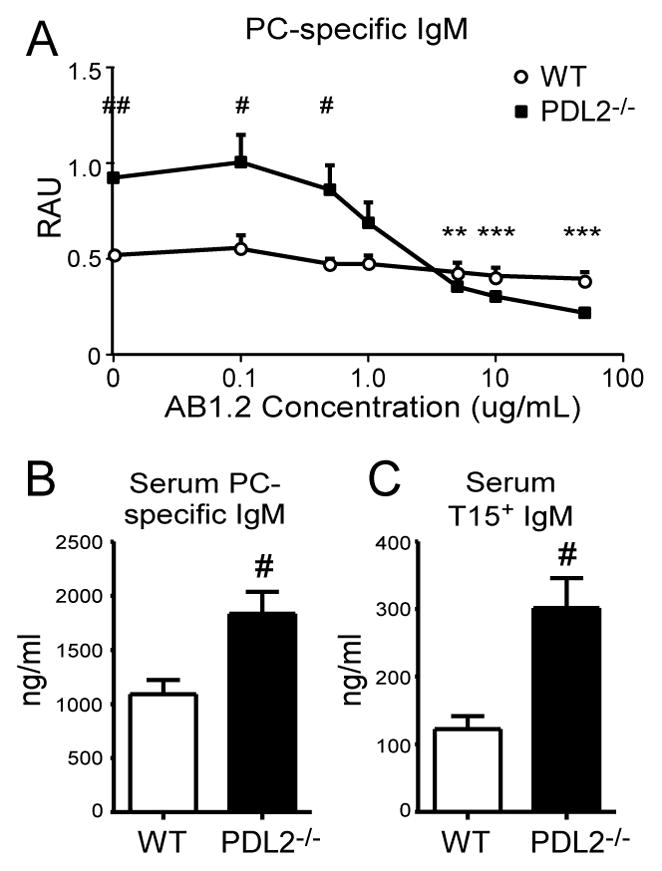
A) Dose-response AB1.2 mAb (anti-T15 idiotype) inhibition ELISA of PC-specific serum IgM from naïve WT and PD-L2−/− mice. B) Serum PC-specific IgM concentrations from naïve WT and PD-L2−/− mice. C) Serum T15 Id-specific IgM concentrations from naïve WT and PD-L2−/− mice. N ≥ 4 mice/group; A-C, WT sera versus PD-L2−/− sera data analyzed by Student’s t test (# p<0.05, ## p<0.01); also for A, PD-L2−/− anti-T15 Id treated sera versus PD-L2−/− sera no treatment data analyzed by pairwise Student’s t test, (* p<0.05, ** p<0.01, *** p<0.001).
B cell-intrinsic expression of PD-L2 regulates PC-specific Ab production
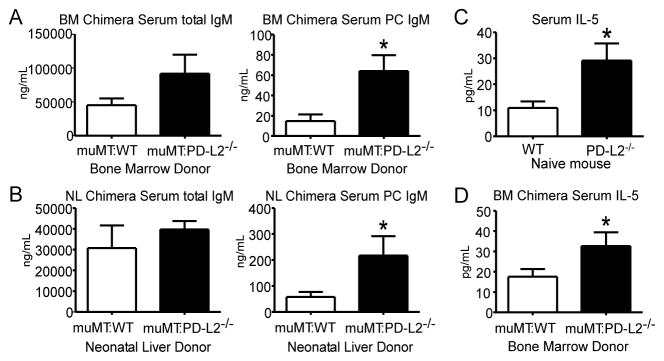
To test whether PD-L2 expression by B cells regulates their ability to produce PC-specific Ab, we generated chimeric mice selectively lacking PD-L2 on B cells using donor BM of WT or PD-L2−/− mice mixed with muMT BM. Three weeks after reconstitution, there was a significant increase in PC-specific IgM in muMT:PD-L2−/− chimeras as compared to muMT:WT chimeras (Fig. 5A). Similar results were obtained when neonatal WT or PD-L2−/− liver (enriched for B-1 progenitors) was used as a source of B cells (Fig. 5B). Notably, total serum IgM levels were not significantly different between groups, although bone marrow chimeras reconstituted with PD-L2−/− B cells had increased total serum IgM in contrast to the decreased levels observed in PD-L2−/− mice. These data demonstrate that B cell-intrinsic PD-L2 expression suppresses PC-specific natural IgM production.
Figure 5. B cell–intrinsic PD-L2 expression regulates serum PC-specific Ab and IL-5 levels.
A-B) Total and PC-specific serum IgM levels in irradiated chimera mice 3 weeks after reconstitution with WT or PD-L2−/− bone marrow (BM) mixed with muMT BM (A) or neonatal (2-day old) liver (NL) of WT or PD-L2−/− mice mixed with muMT BM (B). C) Serum IL-5 levels in naïve WT and PD-L2−/− mice. D) Serum IL-5 levels in BM chimera mice. N = 3–5 mice/group; data analyzed by Student’s t test (* p<0.05).
B cell-intrinsic expression of PD-L2 contributes to IL-5 regulation
We assessed whether PD-L2−/− mice had alterations in cytokines known to play a role in supporting B-1 cell Ab production, including GM-CSF and IL-5 (30, 62–68). Whereas serum GM-CSF was not significantly different between WT and PD-L2−/− mice (Suppl. Fig. 2C), PD-L2−/− mice had a 2.5-fold increase in serum IL-5 levels (Fig. 5C). Serum IL-5 was also significantly increased in muMT:PD-L2−/− BM chimeras relative to muMT:WT BM chimeras (Fig. 5D). Collectively, these data suggest that B cell-intrinsic PD-L2 expression suppresses IL-5 production.
PD-L2 regulates ASC differentiation
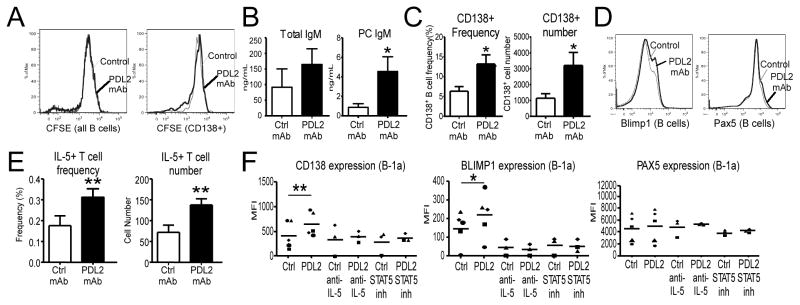
We developed an in vitro assay to elucidate the mechanism by which PD-L2 regulates B-1 cells. Purified WT PerC B-1 cells were cultured with muMT splenocytes in the absence of stimulation for 4 days with PDL2 mAb (TY25) or control mAb (2A3), and analyzed by flow cytometry for differences in division and differentiation and by ELISA for Ab secretion. PDL2 mAb blockade had no effect on B-1 cell division (Fig. 6A). However, PDL2 mAb increased B-1 cell total IgM secretion and significantly increased PC-specific IgM production (Fig. 6B). Consistent with this, PDL2 mAb significantly increased the frequency and number of differentiated CD138+ plasmablasts recovered compared to control mAb (Fig. 6C). Blimp1 up-regulation was also observed with PDL2 blockade (Fig. 6D). Notably, PD-L2−/− PerC B-1 cells in culture did not show enhanced CD138 or Blimp1 expression with PDL2 mAb blockade (data not shown). Collectively, these data suggest that PD-L2 regulates B-1 cell natural IgM production through suppressing differentiation, as opposed to proliferation, of ASC.
Figure 6. PD-L2 regulates B-1 ASC differentiation in an IL-5–, Stat5-dependent manner and increases T cell IL-5 production.
A) CFSE-labeled WT peritoneal cavity (PerC) B-1 cells cultured (unstimulated) for 4 days ± PDL2 mAb blockade with muMT splenocytes were analyzed for differences in division (bold line is PDL2 (TY25) mAb, solid line is control (2A3) mAb; representative histogram of 4 independent experiments in which 13 total mice were used). B) Total and PC-specific IgM levels in supernatants of 4-day cultures of naïve unstimulated WT PerC B-1 cells and muMT splenocytes. C) ASC (CD19+CD138+) frequency and number in 4-day cultures of naïve unstimulated WT PerC B-1 cells incubated with PDL2 or control (2A3) mAb. D) Blimp1 and Pax5 expression in unstimulated WT PerC B-1 cells following culture with muMT splenocytes and PDL2 (bold line) or control (thin line) mAb. E) Frequency and number of IL-5+ T cells among muMT splenocytes cultured with WT PerC B-1 cells for 2 days in the presence of PDL2 or control mAb. F) CD138, Blimp1, and Pax5 expression (MFI) in naïve WT PerC B-1a cells cultured for 2 days with muMT splenocytes in the presence of PDL2 or control mAb ± IL-5 neutralizing Ab or STAT5 inhibitor. Results are from four independent experiments in which a total of 13 mice were used (B-C), a representative experiment of results from four independent experiments (D), two independent experiments in which a total of 12 mice were used (E), or 3 independent experiments in which 3–5 mice were used (F); data analyzed by pairwise Student’s t test (* p<0.05, ** p<0.01, *** p<0.001).
B-1 cell PD-L2 regulates T cell IL-5 production
Our BM chimera results (Fig. 5) led us to hypothesize that B-1 cell-expressed PD-L2 could suppress IL-5 production, and this could explain the significant increases in B-1 plasmablast differentiation and PC-specific IgM production in cultures in which PD-L2 was blocked. Purified WT PerC B-1 cells were cultured with muMT splenocytes in the absence of stimulation for 2 days with PDL2 mAb or control mAb, and analyzed by flow cytometry. As demonstrated in Fig. 6E, PDL2 mAb in cultures of WT B-1 cells and muMT splenocytes significantly increased the frequency and number of IL-5+ CD5+ T cells compared to control mAb. These changes induced by PDL2 mAb blockade corresponded with significantly increased B-1a cell-expression of CD138 (MFI values increased by 48 ± 17%) and Blimp1 (MFI values increased by 43 ± 22%), but unchanged Pax5 levels (2 ± 6%) (Fig. 6F). Notably, B-1a cells are more responsive to IL-5 than other subsets as they have significantly higher expression of IL-5Rα and CD131 (common β chain signaling component) than B-1b and B-2 cells (Supplemental Fig. 2A). In contrast to the increase observed in IL-5–producing T cell frequencies, IFNγ-producing T cells were no longer detectable in cultures following PDL2 mAb blockade (Supplemental Fig. 2B). IL-4- and TNFα–producing T cell frequencies were unchanged or increased, respectively. A similar trend was observed in PD-L2−/− mice—serum IL-4 was modestly increased whereas IFNγ was decreased, albeit not significantly (Supplemental Fig. 2C). Interestingly, serum TNFα levels, as well as IL-6, were increased in PD-L2−/− mice.
To assess whether increased IL-5 in cultures treated with PDL2 mAb played a role in increased B-1a cell differentiation to ASC, we added a neutralizing IL-5 mAb (TRFK5) to cultures. As shown in Fig. 6F, IL-5 neutralization in cultures inhibited the effects of PDL2 mAb blockade on promoting B-1a cell differentiation to ASC, including inhibition of increases in CD138 and Blimp1 levels. A STAT5 inhibitor, which negates IL-5Rα:CD131 signal activation, similarly ablated the effects of PDL2 mAb blockade on B-1a cell differentiation to ASC (Fig. 6F). Taken together, these data demonstrate B cell–intrinsic PD-L2 suppresses IL-5 production in T cells, a cytokine utilized by IL-5R–expressing B-1 cells to differentiate to ASC.
Discussion
Natural antibody production is critical for protection against infectious diseases, allergy, cancer, and atherosclerosis (8, 10, 11, 69, 70). B-1 cells play a crucial role in producing natural Abs, including phosphorylcholine (PC)–specific Ab (4). The regulation of natural Ab production by B-1 cells is not completely understood, although it is evident that these cells are regulated differently than conventional B cells. In this study, we identified a novel role for PD-L2 in regulating B-1 cell natural Ab production. Specifically, we demonstrate B-1 cell–expressed PD-L2 suppresses the ability of B-1 cells to differentiate into ASC through regulating IL-5 production by T cells. In the absence of PD-L2, PC-specific B-1a cell plasmablasts, T15 Id IgM, and serum IL-5 levels were significantly increased. Concurrently, we observed significantly increased clearance of PC-expressing, but not PC-deficient, NTHi isolates from the lungs of mice lacking PD-L2. Our study thereby demonstrates a unique pathway whereby PD-L2 regulates innate-like B cells to secrete natural Ab important for protection against a broad range of diseases.
Phosphorylcholine, a component of mammalian cell membranes, is also a component of the cell walls of the pneumococcus and Haemophilus influenzae, among other pathogens. PC-specific Ab has demonstrated protective effects against bacterial pathogens and atherosclerosis (1, 20, 52, 71–73). The T15 Id of PC-specific natural Ab is particularly well-known for its protection against Streptococcus pneumoniae infection (1, 20, 23, 73–75) and atherosclerosis (18, 76, 77). Although follicular (FO), marginal zone (MZ), and B-1b B cells have the capacity to produce natural Ab (78, 79), the B-1a cell subset plays a critical role (8, 24, 26). Naturally-produced Ab against PC is highly represented by IgM, particularly of the cross-protective T15 Id, whereas adaptive PC-specific Ab is more commonly IgG or IgA, and does not efficiently recognize PC expressed by the pneumococcus (2, 11, 18, 20, 24, 34, 74, 80). While considerable work has served to elucidate the genetic makeup of natural Abs, such as the T15 Id, the intricate contributions of cell types and surface molecules to the regulation of natural Ab is unclear (1, 2, 17–20, 23, 73–75), although IL-5 in particular has been shown to promote T15 Id Ab production (30). Indeed, the contribution of IL-5 to natural antibody producing B-1 cells is well-known (63, 81). IL-5Rα is constitutively expressed on B-1 cells (82–84) and is important to development, differentiation, and self-renewal of B-1 cells (85). Our data demonstrate that the PC-specific IgM produced in PD-L2−/− mice is highly represented by the T15 Id, potentially identifying a unique clonal specificity through which PD-L2 exerts regulatory control, especially upon egress to the spleen, given the predilection of B-1 cells for the spleen for the purpose of Ab secretion (25, 45, 86–90). Notably, our work shows PC-specific B-1 cells constitutively express PD-L2 and work from others has shown depletion of PD-L2+ B-1 cells significantly decreases PC-specific IgM levels (26), thereby demonstrating the key role PD-L2+ PC-specific B-1 cells play in maintaining PC-specific Ab levels. Interestingly, it is possible that use of the same transcriptional regulator for IL-5Rα and PD-L2 expression (Oct2) in these B-1 cells serves as a means of input/output control (46, 91–93). Thus, our work highlights a key and novel relationship between PD-L2, IL-5, and T15 Ab production.
Our data suggest PD-L2 differentially regulates IgM production by distinct B cell subsets. Similar to PC-specific IgM, serum IgM levels against PtC, another specificity found among PD-L2+ B-1a cells (47), were significantly higher in naïve PD-L2−/− mice. However, total serum IgM levels in naïve PD-L2−/− mice were significantly lower, whereas chimeric mice lacking PD-L2 only on B cells had normal levels of serum IgM. Production of natural IgM against MDA, which has recently been shown to involve B-1b cells (22), was also significantly lower in naïve PD-L2−/− mice. In contrast to normal IgM responses to native PPS3, IgM responses to PPS3 following immunization with the pneumococcal polysaccharide-protein conjugate vaccine were significantly lower in PD-L2−/− mice. The reduction in IgM production in response to T cell dependent antigens (NP-CGG) has been previously reported for PD-L2−/− mice (94). Although B-1a cells play a major role in contributing to natural IgM production, other subsets are also known to contribute. That PD-L2 may only suppress a fraction of these cells, and has an opposing role in promoting IgM secretion, including natural IgM secretion by B-1b and marginal zone (MZ) B cells, offers a potential explanation for these findings. We hypothesize that PD-L2 on non-B cells, such as dendritic cells and macrophages, may in some way promote IgM production by B-1b cells, MZ B cells, and/or B-2 cells either directly or indirectly. The mechanism(s) by which this might occur is not yet clear, although PD-L2–mediated support of T follicular helper cells or other accessory cells could play a role. Future work will address this and other possibilities.
PD-L2 is an enigmatic molecule reported to positively and negatively regulate T cells (95–101); its effects on B cells are less well-defined. PD-L2 expression is restricted to B-1 cells, memory B cells raised in response to T cell–dependent Ags, macrophages, and dendritic cells (47, 101, 102). That PD-L2 deficiency on B cells alone can significantly influence basal IL-5 levels and PC-specific IgM production is therefore striking. The extent to which IL-5 regulation is attributed to B-1 cells, as opposed to conventional memory B cells, is unclear. PD-L2 expressed by germinal center B cells has been proposed to promote T follicular helper cell survival and function, and thereby support the development of long-lived plasma cells (94). Therefore, the suppression of T cell cytokine production by PD-L2 in the context of the germinal center reaction may be unlikely. On the other hand, B-1a cells are known to promote Th1 and Th17 responses, although the ability to polarize Th1/Th17 differentiation has been reported to be independent of PD-L2 (103) since PD-L2− and PD-L2+ B-1 cells have similar efficacy in eliciting differentiation (104, 105). Nonetheless, our work indicates further investigation into B-1 cell PD-L2–mediated regulation of cytokine production is certainly warranted.
PD-L2 has been shown in other systems to suppress production of IL-5 and other Th2 cytokines, and to promote Th1 responses. Macrophage expression of PD-L2 has been shown to contribute to Th2 hypo-responsiveness in the context of infection (106). Further, PD-L2 has been shown to augment Th1 cytokine production in a tumor model (107). In a model of asthma, PD-L2 was shown to inhibit IL-4, IL-5, and IL-13, and support allergen–induced airway hyper-responsiveness in a PD-1–independent manner (101, 108). Interestingly, inhibition of the PD-L2 ligand RGMb (DRAGON) was shown to impair tolerization in an OVA airway model, resulting in increased IL-4 and IL-5 (109). Further work by Shin and colleagues demonstrated that PD-L2 inhibits Th2 responses induced by the nematode Nippostrongylus brasiliensis in a PD-1–independent manner (110). Thus, there is strong evidence for PD-L2 suppression of Th2 responses through PD-1–independent mechanisms (111). Indeed, the distinct phenotypes of PD-1−/− mice (moderate increases in PC-specific IgM and total IgM levels) and PD-L2−/− mice (significant increases in PC-specific IgM, significant decreases in total IgM levels) raise the question of whether PD-L2 regulation of PC-specific Ab secretion is also PD-1–independent. Further work will determine whether PD-L2 regulation of B-1 cells proceeds via PD-1, RGMb (DRAGON) (109), or an as-yet unknown ligand.
B-1 cell natural Ab production is differentially regulated from B-2 cells. Whereas B-2 cells rely heavily on cognate MHC Class II:TCR interactions (among others), B-1 cells secrete Abs in the absence of MHC-dependent signals. Natural Ab production is thought to be driven by endogenous Ags and regulated via anti-idiotype Abs (2, 14, 61, 75, 112–115) and specific cytokines like IL-5 (30, 81, 116). Progenitor population and anatomical location play an important role in B-1 cell development, and the existence of other mechanisms of control include microenvironment, surface molecules, and intracellular signal transducers (23, 89, 117–124). Soluble and cell-bound signals from other innate cells, including DC, macrophages, and NK cells, are adept at stimulating B-1 cell Ab production. These include BlyS (BAFF), APRIL, and GM-CSF; nonetheless, cytokines such as IL-4 and IL-5 produced by T cells promote B-1 cell Ab secretion (66, 125–128). Although bystander activation by T cells is possible, it should be noted that induced Ab responses to bacteria-associated PC involves non-TCR-specific, B7–dependent co-stimulation (via cell:cell interactions) from T cells (51). Thus, it is conceivable that PD-L2 on PC-reactive B-1 cells interacts with PD-1 or another ligand on T or NKT cells, and with other signals (such as B7:CD28 interactions), this tightly balances IL-5 production and subsequent PC-specific Ab secretion by B-1 cells. An important consideration here is the existence of this phenomenon in the absence of known Ag stimulation, although it is possible that PC exposed on dying cells in culture and in vivo could contribute to this effect. Interestingly, our 2-day culture demonstrates very early differences in IL-5 production by T cells, leading us to hypothesize that the mechanism by which PD-L2 exerts regulatory control of B-1 cells is by modulating IL-5 secretion by previously differentiated Th2 or NKT cells (30, 129). Further investigations will be required to determine the extent to which cell:cell interactions mediated via Ag-specific or non-specific interactions contribute to PD-L2–mediated regulation of B cells.
In summary, this study identifies a novel regulatory role for PD-L2 expressed on B-1 cells in suppressing IL-5 production by T cells, which ultimately limits natural Ab production, the specificities against which IgM may lead to protective effects against infectious bacteria and parasites, atherosclerotic antigens, allergens, and even cancers (130–132) expressing high levels of PtC, PC, and perhaps related antigens. Absent a demonstrable signaling capacity, PD-L2 seems likely to fine-tune the provision of key signals (such as IL-5) by select cell types. Given the role of IL-5 in expansion of B-1 cells, it is logical to expect a mechanism whereby these cells are conditioned to differentiate and produce an appropriate homeostatic level of natural Ab to perform vital functions while limiting the potential for autoimmune disease development. The potential for PD-L2 to interact with other molecules opens the door to alternative regulatory events not yet appreciated, and the capacity for PD-L2 manipulation in humans represents a potential strategy to improve vaccines and treatments for infectious diseases, atherosclerosis, allergy, cancer, and autoimmunity.
Supplementary Material
Acknowledgments
The authors wish to thank Dr. Jason Grayson, Beth Holbrook, and Mingyong Liu for their help involving T cell intracellular cytokine staining.
References
- 1.Briles DE, Nahm M, Schroer K, Davie J, Baker P, Kearney J, Barletta R. Antiphosphocholine antibodies found in normal mouse serum are protective against intravenous infection with type 3 Streptococcus pneumoniae. J Exp Med. 1981;153:694–705. doi: 10.1084/jem.153.3.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benedict CL, Kearney JF. Increased junctional diversity in fetal B cells results in a loss of protective anti-phosphorylcholine antibodies in adult mice. Immunity. 1999;10:607–17. doi: 10.1016/s1074-7613(00)80060-6. [DOI] [PubMed] [Google Scholar]
- 3.Ochsenbein AF, Fehr T, Lutz C, Suter M, Brombacher F, Hengartner H, Zinkernagel RM. Control of Early Viral and Bacterial Distribution and Disease by Natural Antibodies. Science. 1999;286:2156–2159. doi: 10.1126/science.286.5447.2156. [DOI] [PubMed] [Google Scholar]
- 4.Baumgarth N. The double life of a B-1 cell: self-reactivity selects for protective effector functions. Nat Rev Immunol. 2011;11:34–46. doi: 10.1038/nri2901. [DOI] [PubMed] [Google Scholar]
- 5.Choi YS, Dieter JA, Rothaeusler K, Luo Z, Baumgarth N. B-1 cells in the bone marrow are a significant source of natural IgM. Eur J Immunol. 2012;42:120–129. doi: 10.1002/eji.201141890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haury M, Sundblad A, Grandien A, Barreau C, Coutinho A, Nobrega A. The repertoire of serum IgM in normal mice is largely independent of external antigenic contact. Eur J Immunol. 1997;27:1557–1563. doi: 10.1002/eji.1830270635. [DOI] [PubMed] [Google Scholar]
- 7.Dai H, Zhang Y, Lv P, Gao XM. A study on the glycan specificity of natural antibody repertoires in rodents. Cell Mol Immunol. 2009;6:453–9. doi: 10.1038/cmi.2009.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kearney JF, Patel P, Stefanov EK, King RG. Natural Antibody Repertoires: Development and Functional Role in Inhibiting Allergic Airway Disease. Ann Rev Immunol. 2015;33:475–504. doi: 10.1146/annurev-immunol-032713-120140. [DOI] [PubMed] [Google Scholar]
- 9.Gronwall C, Vas J, Silverman GJ. Protective Roles of Natural IgM Antibodies. Front Immunol. 2012;3:66. doi: 10.3389/fimmu.2012.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Díaz-Zaragoza M, Hernández-Avila R, Viedma-Rodríguez R, Arenas-Aranda D, Ostoa-Saloma P. Natural and adaptive IgM antibodies in the recognition of tumor-associated antigens of breast cancer. Oncol Rep. 2015;34:1106–1114. doi: 10.3892/or.2015.4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Binder CJ, Silverman GJ. Natural antibodies and the autoimmunity of atherosclerosis. Springer Semin Immunopathol. 2005;26:385–404. doi: 10.1007/s00281-004-0185-z. [DOI] [PubMed] [Google Scholar]
- 12.Boyden SV. Natural antibodies and the immune response. Adv Immunol. 1964;5:1–28. doi: 10.1016/s0065-2776(08)60271-0. [DOI] [PubMed] [Google Scholar]
- 13.Chou MY, Fogelstrand L, Hartvigsen K, Hansen LF, Woelkers D, Shaw PX, Choi J, Perkmann T, Backhed F, Miller YI, Horkko S, Corr M, Witztum JL, Binder CJ. Oxidation-specific epitopes are dominant targets of innate natural antibodies in mice and humans. J Clin Invest. 2009;119:1335–49. doi: 10.1172/JCI36800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ehrenstein MR, Notley CA. The importance of natural IgM: scavenger, protector and regulator. Nat Rev Immunol. 2010;10:778–86. doi: 10.1038/nri2849. [DOI] [PubMed] [Google Scholar]
- 15.Guilbert B, Dighiero G, Avrameas S. Naturally occurring antibodies against nine common antigens in human sera. I. Detection, isolation, and characterization. J Immunol. 1982;128:2779–2787. [PubMed] [Google Scholar]
- 16.Hardy RR. B-1 B cells: development, selection, natural autoantibody and leukemia. Curr Opin Immunol. 2006;18:547–555. doi: 10.1016/j.coi.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 17.Kawahara T, Ohdan H, Zhao G, Yang YG, Sykes M. Peritoneal cavity B cells are precursors of splenic IgM natural antibody-producing cells. J Immunol. 2003;171:5406–5414. doi: 10.4049/jimmunol.171.10.5406. [DOI] [PubMed] [Google Scholar]
- 18.Shaw PX, Horkko S, Chang M-K, Curtiss LK, Palinski W, Silverman GJ, Witztum JL. Natural antibodies with the T15 idiotype may act in atherosclerosis, apoptotic clearance, and protective immunity. J Clin Invest. 2000;105:1731–1740. doi: 10.1172/JCI8472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thornton BP, Vetvicka V, Ross GD. Natural antibody and complement-mediated antigen processing and presentation by B lymphocytes. J Immunol. 1994;152:1727–1737. [PubMed] [Google Scholar]
- 20.Vale AM, Kapoor P, Skibinski GA, Elgavish A, Mahmoud TI, Zemlin C, Zemlin M, Burrows PD, Nobrega A, Kearney JF, Briles DE, Schroeder HW., Jr The link between antibodies to OxLDL and natural protection against pneumococci depends on D(H) gene conservation. J Exp Med. 2013;210:875–90. doi: 10.1084/jem.20121861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nguyen TT, Elsner RA, Baumgarth N. Natural IgM prevents autoimmunity by enforcing B cell central tolerance induction. J Immunol. 2015;194:1489–502. doi: 10.4049/jimmunol.1401880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosenfeld SM, Perry HM, Gonen A, Prohaska TA, Srikakulapu P, Grewal S, Das D, McSkimming C, Taylor AM, Tsimikas S, Bender TP, Witztum JL, McNamara CA. B-1b Cells Secrete Atheroprotective IgM and Attenuate Atherosclerosis. Circ Res. 2015;117:28–39. doi: 10.1161/CIRCRESAHA.117.306044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haas KM, Poe JC, Steeber DA, Tedder TF. B-1a and B-1b cells exhibit distinct developmental requirements and have unique functional roles in innate and adaptive immunity to S. pneumoniae. Immunity. 2005;23:7–18. doi: 10.1016/j.immuni.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 24.Masmoudi H, Mota-Santos T, Huetz F, Coutinho A, Cazenave PA. All T15 Id-positive antibodies (but not the majority of VHT15+ antibodies) are produced by peritoneal CD5+ B lymphocytes. Int Immunol. 1990;2:515–520. doi: 10.1093/intimm/2.6.515. [DOI] [PubMed] [Google Scholar]
- 25.Holodick NE, Vizconde T, Rothstein TL. Splenic B-1a Cells Expressing CD138 Spontaneously Secrete Large Amounts of Immunoglobulin in Naive Mice. Front Immunol. 2014;5:129. doi: 10.3389/fimmu.2014.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee RA, Mao C, Vo H, Gao W, Zhong X. Fluorescence tagging and inducible depletion of PD-L2-expressing B-1 B cells in vivo. Ann N Y Acad Sci. 2015;1362:77–85. doi: 10.1111/nyas.12865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kulik L, Fleming SD, Moratz C, Reuter JW, Novikov A, Chen K, Andrews KA, Markaryan A, Quigg RJ, Silverman GJ, Tsokos GC, Holers VM. Pathogenic natural antibodies recognizing annexin IV are required to develop intestinal ischemia-reperfusion injury. J Immunol. 2009;182:5363–73. doi: 10.4049/jimmunol.0803980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cancro MP, Sigal NH, Klinman NR. Differential expression of an equivalent clonotype among BALB/c and C57BL/6 mice. J Exp Med. 1978;147:1–12. doi: 10.1084/jem.147.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Binder CJ, Shaw PX, Chang M-K, Boullier A, Hartvigsen K, Hörkkö S, Miller YI, Woelkers DA, Corr M, Witztum JL. Thematic review series: The Immune System and Atherogenesis. The role of natural antibodies in atherogenesis. J Lipid Res. 2005;46:1353–1363. doi: 10.1194/jlr.R500005-JLR200. [DOI] [PubMed] [Google Scholar]
- 30.Binder CJ, Hartvigsen K, Chang M-K, Miller M, Broide D, Palinski W, Curtiss LK, Corr M, Witztum JL. IL-5 links adaptive and natural immunity specific for epitopes of oxidized LDL and protects from atherosclerosis. J Clin Invest. 2004;114:427–437. doi: 10.1172/JCI20479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Briles DE, Claflin JL, Schroer K, Forman C. Mouse Igg3 antibodies are highly protective against infection with Streptococcus pneumoniae. Nature. 1981;294:88–90. doi: 10.1038/294088a0. [DOI] [PubMed] [Google Scholar]
- 32.Patel PS, Kearney JF. Neonatal Exposure to Pneumococcal Phosphorylcholine Modulates the Development of House Dust Mite Allergy During Adult Life. J Immunol. 2015;194:5838–5850. doi: 10.4049/jimmunol.1500251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yammani RD, Leyva MA, Jennings RN, Haas KM. C4 Deficiency is a predisposing factor for Streptococcus pneumoniae-induced autoantibody production. J Immunol. 2014;193:5434–43. doi: 10.4049/jimmunol.1401462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Limpanasithikul W, Ray S, Diamond B. Cross-reactive antibodies have both protective and pathogenic potential. J Immunol. 1995;155:967–973. [PubMed] [Google Scholar]
- 35.Ray SK, Putterman C, Diamond B. Pathogenic autoantibodies are routinely generated during the response to foreign antigen: a paradigm for autoimmune disease. Proc Natl Acad Med USA. 1996;93:2019–2024. doi: 10.1073/pnas.93.5.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cohen-Solal J, Diamond B. Lessons from an anti-DNA autoantibody. Mol Immunol. 2011;48:1328–31. doi: 10.1016/j.molimm.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Holodick NE, Vizconde T, Rothstein TL. B-1a Cell Diversity: Nontemplated Addition in B-1a Cell Ig Is Determined by Progenitor Population and Developmental Location. J Immunol. 2014;192:2432–2441. doi: 10.4049/jimmunol.1300247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dorshkind K, Montecino-Rodriguez E. Fetal B-cell lymphopoiesis and the emergence of B-1-cell potential. Nat Rev Immunol. 2007;7:213–219. doi: 10.1038/nri2019. [DOI] [PubMed] [Google Scholar]
- 39.Yang Y, Ghosn EEB, Cole LE, Obukhanych TV, Sadate-Ngatchou P, Vogel SN, Herzenberg LA, Herzenberg LA. Antigen-specific memory in B-1a and its relationship to natural immunity. Proc Natl Acad Med USA. 2012;109:5388–5393. doi: 10.1073/pnas.1121627109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Duan B, Morel L. Role of B-1a cells in autoimmunity. Autoimmun Rev. 2006;5:403–408. doi: 10.1016/j.autrev.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 41.Fagarasan S, Watanabe N, Honjo T. Generation, expansion, migration and activation of mouse B1 cells. Immunol Rev. 2000;176:205–215. doi: 10.1034/j.1600-065x.2000.00604.x. [DOI] [PubMed] [Google Scholar]
- 42.Yammani RD, Haas KM. Primate B-1 cells generate antigen-specific B cell responses to T cell-independent type 2 antigens. J Immunol. 2013;190:3100–8. doi: 10.4049/jimmunol.1203058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Montecino-Rodriguez E, Dorshkind K. B-1 B cell development in the fetus and adult. Immunity. 2012;36:13–21. doi: 10.1016/j.immuni.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chace JH, Fleming AL, Gordon JA, Perandones CE, Cowdery JS. Regulation of differentiation of peritoneal B-1a (CD5+) B cells. Activated peritoneal macrophages release prostaglandin E2, which inhibits IgM secretion by peritoneal B-1a cells. J Immunol. 1995;154:5630–6. [PubMed] [Google Scholar]
- 45.Yang Y, Tung JW, Ghosn EE, Herzenberg LA. Division and differentiation of natural antibody-producing cells in mouse spleen. Proc Natl Acad Sci U S A. 2007;104:4542–4546. doi: 10.1073/pnas.0700001104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kaku H, Rothstein TL. Octamer binding protein 2 (Oct2) regulates PD-L2 gene expression in B-1 cells through lineage-specific activity of a unique, intronic promoter. Genes Immun. 2010;11:55–66. doi: 10.1038/gene.2009.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhong X, Tumang JR, Gao W, Bai C, Rothstein TL. PD-L2 expression extends beyond dendritic cells/macrophages to B1 cells enriched for V(H)11/V(H)12 and phosphatidylcholine binding. Eur J Immunol. 2007;37:2405–10. doi: 10.1002/eji.200737461. [DOI] [PubMed] [Google Scholar]
- 48.McKay JT, Egan RP, Yammani RD, Chen L, Shin T, Yagita H, Haas KM. PD-1 suppresses protective immunity to Streptococcus pneumoniae through a B cell-intrinsic mechanism. J Immunol. 2015;194:2289–99. doi: 10.4049/jimmunol.1401673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Haas KM. Programmed cell death 1 suppresses B-1b cell expansion and long-lived IgG production in response to T cell-independent type 2 antigens. J Immunol. 2011;187:5183–5195. doi: 10.4049/jimmunol.1101990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Haro MA, Littrell CA, Yin Z, Huang X, Haas KM. PD-1 Suppresses Development of Humoral Responses That Protect against Tn-Bearing Tumors. Cancer Immunol Res. 2016;4:1027–1037. doi: 10.1158/2326-6066.CIR-16-0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu ZQ, Shen Y, Khan AQ, Chu CL, Riese R, Chapman HA, Kanagawa O, Snapper CM. The mechanism underlying T cell help for induction of an antigen-specific in vivo humoral immune response to intact Streptococcus pneumoniae is dependent on the type of antigen. J Immunol. 2002;168:5551–7. doi: 10.4049/jimmunol.168.11.5551. [DOI] [PubMed] [Google Scholar]
- 52.Hong W, Pang B, West-Barnette S, Swords WE. Phosphorylcholine expression by nontypeable Haemophilus influenzae correlates with maturation of biofilm communities in vitro and in vivo. J Bacteriol. 2007;189:8300–7. doi: 10.1128/JB.00532-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hong W, Juneau RA, Pang B, Swords WE. Survival of bacterial biofilms within neutrophil extracellular traps promotes nontypeable Haemophilus influenzae persistence in the chinchilla model for otitis media. J Innate Immun. 2009;1:215–24. doi: 10.1159/000205937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nishimura H, Minato N, Nakano T, Honjo T. Immunological studies on PD-1 deficient mice: implication of PD-1 as a negative regulator for B cell responses. Int Immunol. 1998;10:1563–1572. doi: 10.1093/intimm/10.10.1563. [DOI] [PubMed] [Google Scholar]
- 55.Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science. 2012;336:1268–73. doi: 10.1126/science.1223490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kato LM, Kawamoto S, Maruya M, Fagarasan S. The role of the adaptive immune system in regulation of gut microbiota. Immunol Rev. 2014;260:67–75. doi: 10.1111/imr.12185. [DOI] [PubMed] [Google Scholar]
- 57.Kawamoto S, Tran TH, Maruya M, Suzuki K, Doi Y, Tsutsui Y, Kato LM, Fagarasan S. The inhibitory receptor PD-1 regulates IgA selection and bacterial composition in the gut. Science. 2012;336:485–9. doi: 10.1126/science.1217718. [DOI] [PubMed] [Google Scholar]
- 58.Kato LM, Kawamoto S, Maruya M, Fagarasan S. Gut TFH and IgA: key players for regulation of bacterial communities and immune homeostasis. Immunol Cell Biol. 2014;92:49–56. doi: 10.1038/icb.2013.54. [DOI] [PubMed] [Google Scholar]
- 59.Peterson DA, McNulty NP, Guruge JL, Gordon JI. IgA Response to Symbiotic Bacteria as a Mediator of Gut Homeostasis. Cell Host & Microbe. 2007;2:328–339. doi: 10.1016/j.chom.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 60.Bos NA, Kimura H, Meeuwsen CG, De Visser H, Hazenberg MP, Wostmann BS, Pleasants JR, Benner R, Marcus DM. Serum immunoglobulin levels and naturally occurring antibodies against carbohydrate antigens in germ-free BALB/c mice fed chemically defined ultrafiltered diet. Eur J Immunol. 1989;19:2335–9. doi: 10.1002/eji.1830191223. [DOI] [PubMed] [Google Scholar]
- 61.Lino AC, Mohr E, Demengeot J. Naturally secreted immunoglobulins limit B1 and MZ B-cell numbers through a microbiota-independent mechanism. Blood. 2013;122:209–218. doi: 10.1182/blood-2012-08-447136. [DOI] [PubMed] [Google Scholar]
- 62.Kantor AB. The development and repertoire of B-1 cells (CD5 B cells) Immunology Today. 1991;12:389–391. doi: 10.1016/0167-5699(91)90136-H. [DOI] [PubMed] [Google Scholar]
- 63.Moon BG, Takaki S, Miyake K, Takatsu K. The role of IL-5 for mature B-1 cells in homeostatic proliferation, cell survival, and Ig production. J Immunol. 2004;172:6020–9. doi: 10.4049/jimmunol.172.10.6020. [DOI] [PubMed] [Google Scholar]
- 64.Snapper CM, Moorman MA, Rosas FR, Kehry MR, Maliszewski CR, Mond JJ. IL-3 and granulocyte-macrophage colony-stimulating factor strongly induce Ig secretion by sort-purified murine B cell activated through the membrane Ig, but not the CD40, signaling pathway. J Immunol. 1995;154:5842–50. [PubMed] [Google Scholar]
- 65.Rauch PJ, Chudnovskiy A, Robbins CS, Weber GF, Etzrodt M, Hilgendorf I, Tiglao E, Figueiredo JL, Iwamoto Y, Theurl I, Gorbatov R, Waring MT, Chicoine AT, Mouded M, Pittet MJ, Nahrendorf M, Weissleder R, Swirski FK. Innate response activator B cells protect against microbial sepsis. Science. 2012;335:597–601. doi: 10.1126/science.1215173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Weber GF, Chousterman BG, Hilgendorf I, Robbins CS, Theurl I, Gerhardt LM, Iwamoto Y, Quach TD, Ali M, Chen JW, Rothstein TL, Nahrendorf M, Weissleder R, Swirski FK. Pleural innate response activator B cells protect against pneumonia via a GM-CSF-IgM axis. J Exp Med. 2014;211:1243–56. doi: 10.1084/jem.20131471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Thies FG, Laurindo MF, Perez EC, Novaes e Brito RR, Mariano M, Popi AF. Cross talk between peritoneal macrophages and B-1 cells in vitro. PLoS ONE. 2013;8:e62805. doi: 10.1371/journal.pone.0062805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sakiyama T, Ikuta K, Nisitani S, Takatsu K, Honjo T. Requirement of IL-5 for induction of autoimmune hemolytic anemia in anti-red blood cell autoantibody transgenic mice. Int Immunol. 1999;11:995–1000. doi: 10.1093/intimm/11.6.995. [DOI] [PubMed] [Google Scholar]
- 69.Zola TA, Lysenko ES, Weiser JN. Natural antibody to conserved targets of Haemophilus influenzae limits colonization of the murine nasopharynx. Infect Immun. 2009;77:3458–65. doi: 10.1128/IAI.01564-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Baumgarth N, Tung JW, Herzenberg LA. Inherent specificities in natural antibodies: a key to immune defense against pathogen invasion. Springer Semin Immunopathol. 2005;26:347–62. doi: 10.1007/s00281-004-0182-2. [DOI] [PubMed] [Google Scholar]
- 71.Clark SE, Weiser JN. Microbial modulation of host immunity with the small molecule phosphorylcholine. Infect Immun. 2013;81:392–401. doi: 10.1128/IAI.01168-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Magee AD, Yother J. Requirement for Capsule in Colonization by Streptococcus pneumoniae. Infect Immun. 2001;69:3755–3761. doi: 10.1128/IAI.69.6.3755-3761.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yother J, Forman C, Gray BM, Briles DE. Protection of mice from infection with Streptococcus pneumoniae by anti-phosphocholine antibody. Infect Immun. 1982;36:184–8. doi: 10.1128/iai.36.1.184-188.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kenny JJ, Wicker LS, Guelde G, Scher I. Regulation of T15 idiotype dominance. I. Mice expressing the xid immune defect provide normal help to T15+ B cell precursors. J Immunol. 1982;129:1534–8. [PubMed] [Google Scholar]
- 75.Kearney JF, Vakil M. Idiotype-directed interactions during ontogeny play a major role in the establishment of the adult B cell repertoire. Immunol Rev. 1986;94:39–50. doi: 10.1111/j.1600-065x.1986.tb01163.x. [DOI] [PubMed] [Google Scholar]
- 76.Horkko S, Bird DA, Miller E, Itabe H, Leitinger N, Subbanagounder G, Berliner JA, Friedman P, Dennis EA, Curtiss LK, Palinski W, Witztum JL. Monoclonal autoantibodies specific for oxidized phospholipids or oxidized phospholipid–protein adducts inhibit macrophage uptake of oxidized low-density lipoproteins. J Clin Invest. 1999;103:117–128. doi: 10.1172/JCI4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Binder CJ, Chang MK, Shaw PX, Miller YI, Hartvigsen K, Dewan A, Witztum JL. Innate and acquired immunity in atherogenesis. Nat Med. 2002;8:1218–26. doi: 10.1038/nm1102-1218. [DOI] [PubMed] [Google Scholar]
- 78.Martin F, Oliver AM, Kearney JF. Marginal zone and B1 B cells unite in the early response against T-independent blood-borne particulate antigens. Immunity. 2001;14:617–629. doi: 10.1016/s1074-7613(01)00129-7. [DOI] [PubMed] [Google Scholar]
- 79.Ohdan H, Swenson KG, Kruger Gray HS, Yang YG, Xu Y, Thall AD, Sykes M. Mac-1-negative B-1b phenotype of natural antibody-producing cells, including those responding to Gal alpha 1,3Gal epitopes in alpha 1,3-galactosyltransferase-deficient mice. J Immunol. 2000;165:5518–5129. doi: 10.4049/jimmunol.165.10.5518. [DOI] [PubMed] [Google Scholar]
- 80.Zhong X, Rothstein TL. L2pB1: a new player in autoimmunity. Mol Immunol. 2011;48:1292–300. doi: 10.1016/j.molimm.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kopf M, Brombacher F, Hodgkin PD, Ramsay AJ, Milbourne EA, Dai WJ, Ovington KS, Behm CA, Kohler G, Young IG, Matthaei KI. IL-5-deficient mice have a developmental defect in CD5+ B-1 cells and lack eosinophilia but have normal antibody and cytotoxic T cell responses. Immunity. 1996;4:15–24. doi: 10.1016/s1074-7613(00)80294-0. [DOI] [PubMed] [Google Scholar]
- 82.Hitoshi Y, Yamaguchi N, Mita S, Sonoda E, Takaki S, Tominaga A, Takatsu K. Distribution of IL-5 receptor-positive B cells. Expression of IL-5 receptor on Ly-1(CD5)+ B cells. J Immunol. 1990;144:4218–25. [PubMed] [Google Scholar]
- 83.Weber JD, Isakson PC, Purkerson JM. IL-5 receptor expression and Ig secretion from murine B lymphocytes requires coordinated signaling by membrane Ig, IL-4, and IL-5. J Immunol. 1996;157:4428–35. [PubMed] [Google Scholar]
- 84.Kouro T, Takatsu K. IL-5- and eosinophil-mediated inflammation: from discovery to therapy. Int Immunol. 2009;21:1303–9. doi: 10.1093/intimm/dxp102. [DOI] [PubMed] [Google Scholar]
- 85.Kouro T, Ikutani M, Kariyone A, Takatsu K. Expression of IL-5Ralpha on B-1 cell progenitors in mouse fetal liver and involvement of Bruton’s tyrosine kinase in their development. Immunol Lett. 2009;123:169–78. doi: 10.1016/j.imlet.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 86.Dal Porto JM, Burke K, Cambier JC. Regulation of BCR signal transduction in B-1 cells requires the expression of the Src family kinase Lck. Immunity. 2004;21:443–453. doi: 10.1016/j.immuni.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 87.Ghosn EE, Sadate-Ngatchou P, Yang Y, Herzenberg LA. Distinct progenitors for B-1 and B-2 cells are present in adult mouse spleen. Proc Natl Acad Sci U S A. 108:2879–84. doi: 10.1073/pnas.1019764108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Itakura A, Szczepanik M, Campos RA, Paliwal V, Majewska M, Matsuda H, Takatsu K, Askenase PW. An hour after immunization peritoneal B-1 cells are activated to migrate to lymphoid organs where within 1 day they produce IgM antibodies that initiate elicitation of contact sensitivity. J Immunol. 2005;175:7170–8. doi: 10.4049/jimmunol.175.11.7170. [DOI] [PubMed] [Google Scholar]
- 89.Wardemann H, Boehm T, Dear N, Carsetti R. B-1a B cells that link the innate and adaptive immune responses are lacking in the absence of the spleen. J Exp Med. 2002;195:771–780. doi: 10.1084/jem.20011140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Whitmore AC, Neely HR, Diz R, Flood PM. Rapid induction of splenic and peritoneal B-1a cells in adult mice by thymus-independent type-2 antigen. J Immunol. 2004;173:5406–5414. doi: 10.4049/jimmunol.173.9.5406. [DOI] [PubMed] [Google Scholar]
- 91.Emslie D, D’Costa K, Hasbold J, Metcalf D, Takatsu K, Hodgkin PO, Corcoran LM. Oct2 enhances antibody-secreting cell differentiation through regulation of IL-5 receptor alpha chain expression on activated B cells. J Exp Med. 2008;205:409–21. doi: 10.1084/jem.20072049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Corcoran LM, Koentgen F, Dietrich W, Veale M, Humbert PO. All known in vivo functions of the Oct-2 transcription factor require the C-terminal protein domain. J Immunol. 2004;172:2962–9. doi: 10.4049/jimmunol.172.5.2962. [DOI] [PubMed] [Google Scholar]
- 93.Humbert PO, Corcoran LM. oct-2 gene disruption eliminates the peritoneal B-1 lymphocyte lineage and attenuates B-2 cell maturation and function. J Immunol. 1997;159:5273–84. [PubMed] [Google Scholar]
- 94.Good-Jacobson KL, Szumilas CG, Chen L, Sharpe AH, Tomayko MM, Shlomchik MJ. PD-1 regulates germinal center B cell survival and the formation and affinity of long-lived plasma cells. Nat Immunol. 2010;11:535–542. doi: 10.1038/ni.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ghiotto M, Gauthier L, Serriari N, Pastor S, Truneh A, Nunès JA, Olive D. PD-L1 and PD-L2 differ in their molecular mechanisms of interaction with PD-1. Int Immunol. 2010;22:651–660. doi: 10.1093/intimm/dxq049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, Iwai Y, Long AJ, Brown JA, Nunes R, Greenfield EA, Bourque K, Boussiotis VA, Carter LL, Carreno BM, Malenkovich N, Nishimura H, Okazaki T, Honjo T, Sharpe AH, Freeman GJ. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. 2001;2:261–268. doi: 10.1038/85330. [DOI] [PubMed] [Google Scholar]
- 97.Saunders PA, Hendrycks VR, Lidinsky WA, Woods ML. PD-L2:PD-1 involvement in T cell proliferation, cytokine production, and integrin-mediated adhesion. Eur J Immunol. 2005;35:3561–9. doi: 10.1002/eji.200526347. [DOI] [PubMed] [Google Scholar]
- 98.McAlees JW, Lajoie S, Dienger K, Sproles AA, Richgels PK, Yang Y, Khodoun M, Azuma M, Yagita H, Fulkerson PC, Wills-Karp M, Lewkowich IP. Differential control of CD4(+) T-cell subsets by the PD-1/PD-L1 axis in a mouse model of allergic asthma. Eur J Immunol. 2015;45:1019–29. doi: 10.1002/eji.201444778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shin T, Kennedy G, Gorski K, Tsuchiya H, Koseki H, Azuma M, Yagita H, Chen L, Powell J, Pardoll D, Housseau F. Cooperative B7-1/2 (CD80/CD86) and B7-DC Costimulation of CD4(+) T Cells Independent of the PD-1 Receptor. J Exp Med. 2003;198:31–38. doi: 10.1084/jem.20030242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang S, Bajorath J, Flies DB, Dong H, Honjo T, Chen L. Molecular Modeling and Functional Mapping of B7-H1 and B7-DC Uncouple Costimulatory Function from PD-1 Interaction. J Exp Med. 2003;197:1083–1091. doi: 10.1084/jem.20021752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lewkowich IP, Lajoie S, Stoffers SL, Suzuki Y, Richgels PK, Dienger K, Sproles AA, Yagita H, Hamid Q, Wills-Karp M. PD-L2 modulates asthma severity by directly decreasing dendritic cell IL-12 production. Mucosal Immunol. 2013;6:728–39. doi: 10.1038/mi.2012.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zuccarino-Catania GV, Sadanand S, Weisel FJ, Tomayko MM, Meng H, Kleinstein SH, Good-Jacobson KL, Shlomchik MJ. CD80 and PD-L2 define functionally distinct memory B cell subsets that are independent of antibody isotype. Nat Immunol. 2014;15:631–7. doi: 10.1038/ni.2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang Y, Rothstein TL. Induction of Th17 cell differentiation by B-1 cells. Front Immunol. 2012;3:281. doi: 10.3389/fimmu.2012.00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhong X, Gao W, Degauque N, Bai C, Lu Y, Kenny J, Oukka M, Strom TB, Rothstein TL. Reciprocal generation of Th1/Th17 and Treg cells by B1 and B2 B cells. European J Immunol. 2007;37:2400–2404. doi: 10.1002/eji.200737296. [DOI] [PubMed] [Google Scholar]
- 105.Muzzio DO, Soldati R, Rolle L, Zygmunt M, Zenclussen AC, Jensen F. B-1a B Cells Regulate T Cell Differentiation Associated with Pregnancy Disturbances. Front Immunol. 2014;5:6. doi: 10.3389/fimmu.2014.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.van der Werf N, Redpath SA, Azuma M, Yagita H, Taylor MD. Th2 cell-intrinsic hypo-responsiveness determines susceptibility to helminth infection. PLoS Pathog. 2013;9:e1003215. doi: 10.1371/journal.ppat.1003215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shin T, Yoshimura K, Shin T, Crafton EB, Tsuchiya H, Housseau F, Koseki H, Schulick RD, Chen L, Pardoll DM. In vivo costimulatory role of B7-DC in tuning T helper cell 1 and cytotoxic T lymphocyte responses. J Exp Med. 2005;201:1531–41. doi: 10.1084/jem.20050072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Akbari O, Stock P, Singh AK, Lombardi V, Lee WL, Freeman GJ, Sharpe AH, Umetsu DT, Dekruyff RH. PD-L1 and PD-L2 modulate airway inflammation and iNKT-cell-dependent airway hyperreactivity in opposing directions. Mucosal Immunol. 2010;3:81–91. doi: 10.1038/mi.2009.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Xiao Y, Yu S, Zhu B, Bedoret D, Bu X, Francisco LM, Hua P, Duke-Cohan JS, Umetsu DT, Sharpe AH, DeKruyff RH, Freeman GJ. RGMb is a novel binding partner for PD-L2 and its engagement with PD-L2 promotes respiratory tolerance. J Exp Med. 2014;211:943–59. doi: 10.1084/jem.20130790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ishiwata K, Watanabe N, Guo M, Tomihara K, Brumlik MJ, Yagita H, Pardoll D, Chen L, Shin T. Costimulator B7-DC attenuates strong Th2 responses induced by Nippostrongylus brasiliensis. J Immunol. 2010;184:2086–94. doi: 10.4049/jimmunol.0804051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nie X, Chen W, Zhu Y, Huang B, Yu W, Wu Z, Guo S, Zhu Y, Luo L, Wang S, Chen L. B7-DC (PD-L2) costimulation of CD4+ T-helper 1 response via RGMb. Cell Mol Immunol ePub. 2017 doi: 10.1038/cmi.2017.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ehrenstein MR, O’Keefe TL, Davies SL, Neuberger MS. Targeted gene disruption reveals a role for natural secretory IgM in the maturation of the primary immune response. Proc Natl Acad Sci U S A. 1998;95:10089–10093. doi: 10.1073/pnas.95.17.10089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Boes M, Esau C, Fischer MB, Schmidt T, Carroll M, Chen J. Enhanced B-1 cell development, but impaired IgG antibody responses in mice deficient in secreted IgM. J Immunol. 1998;160:4776–87. [PubMed] [Google Scholar]
- 114.Macpherson AJ, Gatto D, Sainsbury E, Harriman GR, Hengartner H, Zinkernagel RM. A Primitive T Cell-Independent Mechanism of Intestinal Mucosal IgA Responses to Commensal Bacteria. Science. 2000;288:2222–2226. doi: 10.1126/science.288.5474.2222. [DOI] [PubMed] [Google Scholar]
- 115.Fagarasan S, Honjo T. T-Independent immune response: new aspects of B cell biology. Science. 2000;290:89–92. doi: 10.1126/science.290.5489.89. [DOI] [PubMed] [Google Scholar]
- 116.Yoshida T, Ikuta K, Sugaya H, Maki K, Takagi M, Kanazawa H, Sunaga S, Kinashi T, Yoshimura K, Miyazaki J, Takaki S, Takatsu K. Defective B-1 cell development and impaired immunity against Angiostrongylus cantonensis in IL-5R alpha-deficient mice. Immunity. 1996;4:483–94. doi: 10.1016/s1074-7613(00)80414-8. [DOI] [PubMed] [Google Scholar]
- 117.Kerner JD, Appleby MW, Mohr RN, Chien S, Rawlings DJ, Maliszewski CR, Witte ON, Perlmutter RM. Impaired expansion of mouse B cell progenitors lacking Btk. Immunity. 1995;3:301–312. doi: 10.1016/1074-7613(95)90115-9. [DOI] [PubMed] [Google Scholar]
- 118.Khan WN, Alt FW, Gerstein RM, Malynn BA, Larsson I, Rathbun G, Davidson L, Müller S, Kantor AB, Herzenberg LA, Rosen FS, Sideras P. Defective B cell development and function in Btk-deficient mice. Immunity. 1995;3:283–299. doi: 10.1016/1074-7613(95)90114-0. [DOI] [PubMed] [Google Scholar]
- 119.Satterthwaite AB, Li Z, Witte ON. Btk function in B cell development and response. Semin Immunol. 1998;10:309–316. doi: 10.1006/smim.1998.0123. [DOI] [PubMed] [Google Scholar]
- 120.Ahearn JM, Fischer MB, Croix D, Goerg S, Ma M, Xia J, Zhou X, Howard RG, Rothstein TL, Carroll MC. Disruption of the Cr2 locus results in a reduction in B-1a cells and in an impaired B cell response to T-dependent antigen. Immunity. 1996;4:251–262. doi: 10.1016/s1074-7613(00)80433-1. [DOI] [PubMed] [Google Scholar]
- 121.Montecino-Rodriguez E, Dorshkind K. New perspectives in B-1 B cell development and function. Trends Immunol. 2006;27:428–433. doi: 10.1016/j.it.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 122.Nakashima H, Hamaguchi Y, Watanabe R, Ishiura N, Kuwano Y, Okochi H, Takahashi Y, Tamaki K, Sato S, Tedder TF, Fujimoto M. CD22 expression mediates the regulatory functions of peritoneal B-1a cells during the remission phase of contact hypersensitivity reactions. J Immunol. 2010;184:4637–4645. doi: 10.4049/jimmunol.0901719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ansel KM, Harris RB, Cyster JG. CXCL13 is required for B1 cell homing, natural antibody production, and body cavity immunity. Immunity. 2002;16:67–76. doi: 10.1016/s1074-7613(01)00257-6. [DOI] [PubMed] [Google Scholar]
- 124.Stoermann B, Kretschmer K, Duber S, Weiss S. B-1a cells are imprinted by the microenvironment in spleen and peritoneum. Eur J Immunol. 2007;37:1613–20. doi: 10.1002/eji.200636640. [DOI] [PubMed] [Google Scholar]
- 125.Vogel LA, Lester TL, Van Cleave VH, Metzger DW. Inhibition of murine B1 lymphocytes by interleukin-12. Eur J Immunol. 1996;26:219–23. doi: 10.1002/eji.1830260134. [DOI] [PubMed] [Google Scholar]
- 126.Erickson LD, Foy TM, Waldschmidt TJ. Murine B1 B cells require IL-5 for optimal T cell-dependent activation. J Immunol. 2001;166:1531–9. doi: 10.4049/jimmunol.166.3.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.El Shikh ME, El Sayed RM, Szakal AK, Tew JG. T-independent antibody responses to T-dependent antigens: a novel follicular dendritic cell-dependent activity. J Immunol. 2009;182:3482–91. doi: 10.4049/jimmunol.0802317. [DOI] [PubMed] [Google Scholar]
- 128.Schneider P, MacKay F, Steiner V, Hofmann K, Bodmer JL, Holler N, Ambrose C, Lawton P, Bixler S, Acha-Orbea H, Valmori D, Romero P, Werner-Favre C, Zubler RH, Browning JL, Tschopp J. BAFF, a novel ligand of the tumor necrosis factor family, stimulates B cell growth. J Exp Med. 1999;189:1747–1756. doi: 10.1084/jem.189.11.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nakayama T, Hirahara K, Onodera A, Endo Y, Hosokawa H, Shinoda K, Tumes DJ, Okamoto Y. Th2 Cells in Health and Disease. Ann Rev Immunol. 2017;35:53–84. doi: 10.1146/annurev-immunol-051116-052350. [DOI] [PubMed] [Google Scholar]
- 130.Hilvo M, Denkert C, Lehtinen L, Muller B, Brockmoller S, Seppanen-Laakso T, Budczies J, Bucher E, Yetukuri L, Castillo S, Berg E, Nygren H, Sysi-Aho M, Griffin JL, Fiehn O, Loibl S, Richter-Ehrenstein C, Radke C, Hyotylainen T, Kallioniemi O, Iljin K, Oresic M. Novel theranostic opportunities offered by characterization of altered membrane lipid metabolism in breast cancer progression. Cancer Res. 2011;71:3236–45. doi: 10.1158/0008-5472.CAN-10-3894. [DOI] [PubMed] [Google Scholar]
- 131.Eliyahu G, Kreizman T, Degani H. Phosphocholine as a biomarker of breast cancer: molecular and biochemical studies. Int J Cancer. 2007;120:1721–30. doi: 10.1002/ijc.22293. [DOI] [PubMed] [Google Scholar]
- 132.Dueck DA, Chan M, Tran K, Wong JT, Jay FT, Littman C, Stimpson R, Choy PC. The modulation of choline phosphoglyceride metabolism in human colon cancer. Mol Cell Biochem. 1996;162:97–103. doi: 10.1007/BF00227535. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.