Abstract
Objective
Alterations in sleep quality and metabolism during menopause are improved by menopausal hormone therapy (MHT). The mechanisms mediating these effects remain unclear. Orexin A (OxA) is a neuro-peptide that regulates sleep/wakefulness, food intake and metabolism. This study examined changes in plasma OxA levels during and after treatment in women from the Kronos Early Estrogen Prevention Study (KEEPS).
Methods
KEEPS randomized women within three years of menopause to: oral conjugated equine estrogen (o-CEE, 0.45 mg/day), transdermal 17β estradiol (t-E2, 50 μg/day), or placebo pills and patches for four years. Plasma OxA levels were measured by enzyme immunoassays in fasting blood samples collected annually from KEEPS participants at Mayo Clinic during and three years after MHT. Changes in menopausal symptoms and plasma OxA levels were assessed for treatment differences.
Results
During treatment, OxA levels increased more in women randomized to o-CEE compared with the other groups. Women randomized to either form of MHT demonstrated smaller increases in BMI than those on placebo. Insomnia severity decreased similarly among treatment groups. However, neither changes in sleep nor changes in BMI correlated with changes in plasma OxA levels. Changes in waist circumference correlated positively with changes in plasma OxA levels three years after discontinuation of study treatments.
Conclusions
Although OxA levels increased only in women randomized to o-CEE, these changes did not correlate with changes in sleep quality or BMI. The modest correlation of OxA levels with waist circumference once study treatments were discontinued suggests that OxA may be modulated through multiple intermediary pathways affected by metabolites of 17β-estradiol.
Keywords: Appetite, Conjugated equine estrogen, 17β estradiol, Hypocretin, Leptin, KEEPS, Sleep
1. Introduction
Orexin A (OxA or hypocretin 1) and Orexin B (OxB or hypocretin 2) are neuropeptides implicated in regulation of sleep/wakefulness, food intake and energy expenditure [1]. OxA and OxB are produced from a single protein precursor, prepro-orexin, in the lateral hypothalamus, and share 46% amino acid sequence homology [2]. Both peptides are endogenous ligands to G-protein coupled receptors; Orexin 1 (Ox1R) and Orexin 2 (Ox2R), with OxA having a 10 fold stronger affinity to the Ox1R [1]. Ox1R is distributed mainly in cortical regions and brainstem nuclei, with OxA neurons having dense projections to brain regions associated with control of arousal and metabolism [3].
Although the main focus of research concerning OxA has been in the central nervous system, OxA (and not OxB) is detected in the peripheral circulation. The Ox1R is expressed in peripheral tissues, through which OxA regulates insulin and glucagon secretion, affects intestinal motility and energy metabolism [4].
The measurement of plasma OxA remains a challenge given that levels in the peripheral circulation are 1/5 to 1/8 those measured in the cerebrospinal fluid [5]. However, plasma OxA increased with age, with the highest levels measured in persons >60 years of age [6]. Sex differences in plasma OxA have yet to be explored in humans, although evidence in rats suggests a sexual dimorphic expression of orexin receptors [7].
When considering women-specific life events, circulating levels of OxA were lower in women with gestational diabetes mellitus [8] and polycystic ovarian syndrome [9] compared to age-matched women without these conditions. In addition, plasma OxA was lower in overweight and obese women than non-obese women, and showed a negative correlation with plasma leptin concentrations [10]. Leptin is a cytokine produced by adipose tissue that suppresses food intake and regulates sleep. Sleep restriction increased plasma leptin concentration and food intake in healthy young men [11]. These associations have yet to be examined in women.
Little is known about how plasma OxA changes with menopause and the association of plasma OxA with menopausal symptoms [12]. At menopause, women experience disturbances in sleep [13], declines in energy metabolism and expenditure and increases in central adiposity [14]. Menopausal hormone therapy (MHT) is beneficial in treating menopause-related symptoms. However, the impact of MHT on plasma OxA is unclear, and little is known about OxA fluctuations after MHT discontinuation.
The objective of this study was to compare the effects of two formulations of MHT with placebo on plasma levels of OxA in healthy, recently menopausal women during and following discontinuation of study treatment. In addition, the study explored the relationship of OxA with leptin, clinical and self-reported sleep and appetite/physical behaviors. Given findings of increased plasma OxA in patients with sleep restriction, narcolepsy or obstructive sleep apnea, and the reduction in sleep quality during menopause that is alleviated by MHT, we hypothesized that OxA would be reduced by MHT.
2. Methods
2.1. Protocol approval
This study was approved by the Mayo Clinic Institutional Review Board (IRB protocol # 2241). All participants gave written informed consent.
2.2. Participants
This study evaluated a subgroup of white participants (n = 74) from the Kronos Early Estrogen Prevention Study (KEEPS; NCT00154180) Cognitive and Affective Ancillary study (KEEPS-Cog) at Mayo Clinic, Rochester MN [15]. KEEPS was a randomized, double-blind, placebo-controlled trial studying the effects of MHT, started within 3 years of menopause, on progression of cardiovascular disease. [16] Briefly, women were included in KEEPS-Cog if their last spontaneous menses occurred between 42 and 58 years of age with >6 months to <3 years of amenorrhea, plasma follicle-stimulating hormone ≥35 ng/mL and/or E2 < 40 pg/mL. Women were excluded if they had a coronary arterial calcification (CAC) score of >50 Agatston Units, a history of cardiovascular disease, a body mass index (BMI) > 35 kg/m2, low-density lipoprotein cholesterol (LDL) > 190 mg/dL, triglycerides (Tg) > 400 mg/dL, blood glucose > 126 mg/dL, uncontrolled hypertension (systolic blood pressure >150 mmHg and/or diastolic blood pressure >95 mmHg), current or recent (6 months) use of cholesterol-lowering medications (statins, fibrate, or >500 mg/day niacin) and if they smoked more than 10 cigarettes per day.
2.3. Study design
Participants were randomized to one of the following: oral conjugated equine estrogens (o-CEE; Premarin, 0.45 mg/day) plus a placebo transdermal patch, transdermal 17β-estradiol (t-E2; Climara 50 μg/day) plus a placebo pill, or placebo (PL) pills and patch. Women in the active treatment groups also received oral micronized progesterone (Prometrium, 200 mg) for the first 12 days of each month. A random number table was used to assign treatment. Study drugs were supplied to centers identified only by the women’s ID number, with both research participants and investigators blinded to treatment.
Treatment was given for four years with visits prior to randomization (baseline) and then annually at 12, 24, 36 and 48 months. In a follow-up study, participants were studied three years after the cessation of study treatment and were compared to their final on-treatment measurements (from 48 to 84 months).
2.4. Blood collection and plasma preparation
At each visit (baseline, 12, 24, 36, 48, and 84 months), morning fasting venous blood was collected in tubes containing 7.5% ethylenediamine tetra-acetic acid tri-potassium salt [EDTA (k3)] anti-coagulant. Aliquots of plasma were stored immediately at −80 °C until analysis. The range of storage time for plasma samples was from 2 to 7 years.
2.5. Measurements of plasma orexin a and leptin
OxA was measured in diluted (1:2 in standard diluent supplied by manufacturer) plasma using a commercially available extraction free peptide enzyme immunoassay kit (EIA, Catalog # S-1374; Peninsula Laboratories, California, USA). Each plasma sample was measured in duplicate and the mean of these two measurements was used for analysis. The lowest detection limit of the assay was 0.5 ng/mL with intra- and inter-assay coefficients of variation of <6% and <15%, respectively. The cross-reactivity of OxA with OxB was reported to be 18%.
Leptin was measured in plasma samples collected at baseline and at 48- and 84-month follow-up visits using the human leptin double antibody radioimmunoassay kit (Linco Research, Inc. St. Louis, MO 63304). Intra –assay coefficients of variation were 6.1%, 7.7%, and 6.3% at 39.7, 21.6, 3.8 ng/mL, respectively; inter-assay coefficients of variation of 11%, and 13% at 20.4, and 3.0 ng/mL, respectively.
2.6. Sleep and energy metabolism outcomes
BMI and waist circumference (WC), were used as surrogates for changes in energy metabolism. In addition, information regarding appetite and physical well-being were obtained through the Health Quality of Life (QOL) domain within the Utian Quality of Life Scale. The Utian Quality of Life Scale incorporates sense of well-being into an assessment tool that is specific to the menopausal population [17]. To address changes in sleep quality, participants completed a questionnaire reporting menopausal insomnia symptoms over the last 3 months, ranking them on a 4-point numerical scale as none, mild, moderate or severe (0–3). BMI and WC were measured at baseline and at end of the intervention (48 months), and at 3 years following discontinuation of treatments (84 months). Health QOL responses were collected at baseline, at 18-, 36- and 48-months during study treatment, and at the three year discontinuation follow up visit (84 months). The insomnia severity scale was collected at baseline, 6 months, and then annually during study treatment.
2.7. Statistical analysis
Descriptive statistics are presented as median and interquartile range (IQR) for continuous or ordinal variables, and as mean and standard deviation or 95% confidence interval (CI) for measures of change over time. With the exception of OxA and leptin, within-subject changes in the response from baseline to 48 months (on-treatment period) and from 48 to 84 months (post-treatment period) are reported as the difference in means between initial and final measurements. Distributions of OxA and leptin were skewed toward large values, and for both outcomes the measure of response is reported as a fold change based on the ratio of geometric means of the initial and final measurements. Mean and 95% CI of the fold change were computed on logarithmic scale and transformed back to original scale using the antilog function. For OxA, health QOL and insomnia severity outcomes, the measures of change were refined so that all multiple repeated measurements during the on-treatment period could be used. Specifically, linear slopes via regression models fitted separately on each subject were obtained and used to estimate changes in OxA and in health QOL over each of the two periods (described in detail in the paragraph below). For insomnia severity score, changes from baseline were averaged over all on-treatment measurements for a given subject due to observation of non-linear trends. For all outcome data, the measures of change at 48 and at 84 months were tested for significance within treatment groups using the Wilcoxon signed rank test, while the difference in changes between treatment groups was assessed with the Kruskal-Wallis test. Between-treatment comparisons were based on a 2 ° of freedom test that assessed if there were any differences among the 3 groups; individual pair-wise comparisons were done only if the overall test revealed evidence of any difference (if P ≤ 0.05).
For serially-collected responses of OxA, a two-stage longitudinal analysis was performed by first reducing these repeated measurements into a single measure of response per individual, and then using the response measure in a second step to test for a difference between treatments. For this, linear regressions of time vs. OxA were fitted separately for each woman (on as many as 5 measurements), from which the least squares slope was obtained and used to summarize that individual’s trend. OxA values were not normally distributed and were transformed to the natural logarithmic scale (with an additive correction factor of 1) before performing this analysis to satisfy the linear modeling assumptions. Slopes from the on-treatment regressions were used to estimate the women’s predicted responses at 48 months. The ratio of geometric means corresponding to the measurement at baseline and the prediction at 48 months was computed to express the fold change in OxA level during treatment. Similarly, the ratio of geometric means of 48-month predicted and 84-month measured values was used to estimate the fold change in OxA during the post-treatment period.
A secondary longitudinal analysis to assess treatment-related changes in OxA was performed using a linear mixed-effects model. All available repeated measurements during the combined on-treatment and post-treatment period (transformed to the natural logarithmic scale) were fitted as responses in the model, which was formulated with two separate linear slopes to describe the time-response profile. In particular, time since the start of treatment took the form of a piecewise linear function (single breakpoint set at the 48-month time point) to delineate the on-treatment and post-treatment intervals. Group difference in linear trends of logarithmically-transformed OxA was determined by testing for a significant treatment-by-time interaction within each of the two intervals. Additional secondary analyses were performed to assess the relation between change in plasma OxA levels and changes in leptin, clinical biomarkers and self-reported sleep quality and health QOL scores, based on nonparametric Spearman’s rank correlation coefficient (rs). All data analyses were carried out with the statistical software package SAS, version 9.4 (SAS Institute, Cary, NC, USA).
3. Results
3.1. General characteristics
In the subset of KEEPS participants (n = 74), who came back three years after discontinuation of the study, the median age at randomization was 53 years with a median of 19 months from menopause. Prior to randomization (baseline), clinical characteristics did not differ among the treatment groups (Table 1).
Table 1.
Baseline characteristics of participants by treatment assignment.
| Variable | PL (n = 31) | t-E2 (n = 23) | o-CEE (n = 20) | P-value |
|---|---|---|---|---|
| Age (years)a | 53.2 (51.8, 54.4) | 53.1 (51.6, 54.6) | 54.0 (52.0, 54.9) | 0.463 |
| Time from menopause (months)a | 12.8 (10.3, 21.4) | 18.4 (14.1, 28.6) | 23.9 (17.9, 28.9) | 0.066 |
| Systolic blood pressure (mmHg) | 121.5 (114.0, 128.0) | 113.5 (104.0, 121.0) | 120.5 (111.2, 130.2) | 0.135 |
| Diastolic blood pressure (mmHg) | 76.0 (70.0, 82.0) | 72.5 (65.5, 77.0) | 77.5 (70.2, 82.5) | 0.078 |
| Total cholesterol (mg/dL) | 217.0 (200.0, 232.0) | 223.0 (202.0, 248.0) | 208.5 (187.5, 236.0) | 0.291 |
| HDL Cholesterol (mg/dL) | 58.0 (50.0, 65.0) | 64.0 (53.0, 72.0) | 60.0 (51.0, 71.5) | 0.514 |
| LDL Cholesterol (mg/dL) | 135.8 (116.6, 152.8) | 148.4 (108.4, 168.4) | 128.5 (118.7, 149.1) | 0.478 |
| Triglyceride (mg/dL) | 77.0 (64.0, 131.0) | 83.0 (66.0, 115.0) | 84.5 (57.0, 113.5) | 0.915 |
| Fasting Blood Glucose (mg/dL) | 90.0 (87.0, 97.0) | 95.0 (88.0, 99.0) | 87.5 (81.5, 96.0) | 0.064 |
| Body mass index (kg/m2) | 25.9 (24.6, 30.9) | 26.3 (21.6, 30.6) | 27.2 (24.5, 31.8) | 0.550 |
| Waist Circumference (cm)b | 85.0 (77.0, 92.0) | 81.0 (72.0, 93.0) | 83.0 (75.0, 92.0) | 0.596 |
| Health Quality of Lifeb | 25.0 (21.0, 29.0) | 26.0 (24.0, 29.0) | 28.0 (23.5, 30.0) | 0.416 |
| Insomnia Severity | 1 (0, 1) | 1 (0, 2) | 1 (0, 2) | 0.791 |
Data presented as median (25th, 75th percentiles); P-value from Kruskal-Wallis test.
Abbreviations: PL, placebo; t-E2, transdermal; o-CEE, oral; HDL, High density lipoprotein; LDL, low density lipoprotein.
Participants age and time since menopause measured at the time of their KEEPS study randomization.
One subject was missing a baseline measurement for waist circumference, and 2 subjects had no baseline domain score of health Quality of Life available.
3.2. Effects of MHT on plasma OxA
Baseline plasma OxA did not differ among treatment groups (p = 0.127), with median (25th, 75th percentiles) of 2.54 ng/mL (1.78, 4.29) in o-CEE, 4.03 ng/mL (3.56, 4.47) in t-E2 and 3.24 ng/mL (2.22, 6.11) in PL. Based on a summary measure of linear trend of OxA during the MHT intervention, there was a significant increase in plasma levels of OxA in participants who were randomized to o-CEE (1.22-fold increase; P = 0.017; Fig. 1). Between-group comparison of changes in OxA during the on-treatment period demonstrated an overall difference among the 3 treatments (P = 0.015), with the increase in OxA in the o-CEE group significantly higher than that in either the t-E2 (P = 0.033) or placebo (P = 0.008) groups (Table 2).
Fig. 1.
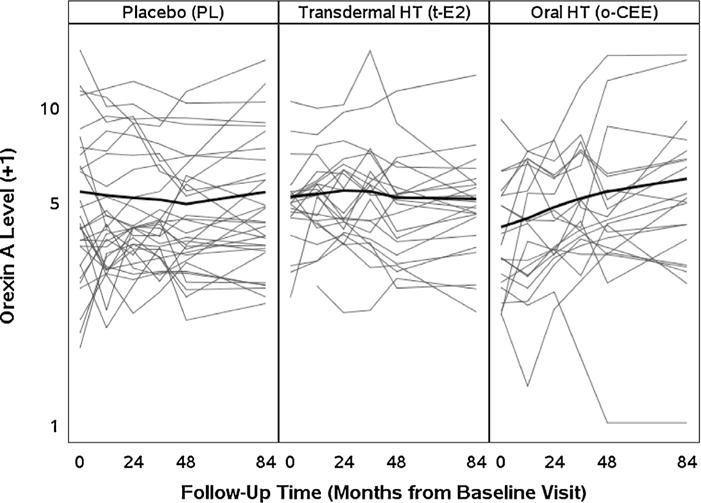
Trend analysis of Orexin-A across treatment groups from Baseline (t = 0) to 84 months using a two slope mixed model. Each individual line represents plasma OXA levels (ng/mL) from each study participant over time by treatment groups. Treatment exposure was from Baseline to 48 months with assessment following discontinuation of treatment at 84 months.
Table 2.
Fold changes in Orexin A and Leptin levels during and after treatment.
| Outcome Variable | Overall | PL | t-E2 | o-CEE | P-value |
|---|---|---|---|---|---|
| Orexin A | |||||
| On-treatment: fold-change, BL-48 mo | 1.01 (0.92–1.11) | 0.91 (0.75–1.06) | 1.00 (0.92–1.08) | 1.22 (1.01–1.44)* | 0.015a |
| Post-treatment: fold-change,48–84 mo | 1.05 (0.99–1.11) | 1.10 (0.98–1.22) | 0.97 (0.89–1.05) | 1.07 (0.97–1.18) | 0.537 |
| Leptinb | |||||
| On-treatment: fold-change, BL-48 mo | 1.20 (1.07–1.33)* | 1.22 (1.04–1.39) | 1.20 (0.91–1.49) | 1.18 (0.95–1.42) | 0.921 |
| Post-treatment: fold-change,48–84 mo | 1.04 (0.94–1.15) | 1.04 (0.89–1.20) | 1.01 (0.86–1.17) | 1.07 (0.83–1.31) | 0.896 |
Reported as mean (95% confidence interval), fold changes were estimated as the ratio of geometric means of initial and final measurements of the on-/post-treatment period (details described in methods section); Kruskal-Wallis test was used to test for any difference in fold changes between the 3 treatment groups.
Abbreviations: PLplacebo; t-E2transdermal; o-CEEoral; BLbaseline; momonths.
P < 0.05 from Wilcoxon signed-rank test indicates a significant change over time within that group.
Based on pairwise comparisons from post-hoc testing, the fold change in Orexin A during treatment in the o-CEE group was significantly greater than that in both the PL (P = 0.008) and t-E2 (P = 0.033) groups.
Due to missing leptin data, fold changes were estimated on N = 49 on-treatment and N = 61 post-treatment.
At three years after study completion, there was no significant post-treatment change in plasma OxA levels within any of the treatment groups (Fig. 1), nor was there a difference in changes in OxA levels among groups (P = 0.537; Table 2). Secondary analysis based on a repeated measures mixed effects model formulated with two within-interval time slopes also showed a group difference in on-treatment trend (P = 0.016 from testing interaction between treatment group and time) and no significant group difference in post-treatment trend (P = 0.312).
3.3. Effects of MHT on plasma leptin
In the subgroup of women (n = 51) with baseline measurements available, plasma values for leptin were similar across treatment groups (p = 0.799), [o-CEE, 19.0 ng/mL (12.8–37.1); t-E2, 18.4 ng/mL (10.5–37.5); and PL, 16.3 ng/mL (11.8–38.3)]. In women who had measurements both at baseline and 48-month of study treatment(n = 49), changes in leptin were significant only within the group as a whole (1.20-fold increase; P = 0.003) with similar changes observed across treatment groups (o-CEE, 1.18-fold increase; t-E2, 1.20-fold increase; and PL, 1.22-fold increase; between-group comparison, P = 0.921).
Among 61 participants with paired measurements at 48 months of study treatment and 3 years following discontinuation, there was no post-treatment change in leptin in this group as a whole (1.04-fold increase; P = 0.232) and no differences in change among treatments (P = 0.896). In addition, there was no correlation between changes in leptin and OxA at 48 months (rs = −0.033, P = 0.822), or during the post-treatment period (rs = 0.183, P = 0.160).
3.4. Relationships of changes in plasma OxA and other outcomes
Between baseline and 48 months, BMI increased on average by 1.08 kg/m2 among women in the placebo group (P < 0.001), but did not significantly change among women in the o-CEE or t-E2 groups. The overall difference among treatment groups in the 48-month change in BMI was significant (P = 0.007), and pair wise comparisons revealed that the average increase in women randomized to PL was greater than those with t-E2 (P = 0.015) or o-CEE (P = 0.005; Table 3). In the combined set of participants, there was no correlation between the changes in BMI and plasma OxA at 48 months (rs = 0.043, P = 0.725) or after discontinuation of study treatments at 84 months (rs = 0.120, P = 0.318). Changes in waist circumference during the active treatment period were not significantly different among treatment groups (P = 0.079), and were not associated with corresponding changes in plasma OxA (rs = 0.143, P = 0.251). Over the 3 years following study treatment, changes in waist circumference correlated with changes in OxA (rs = 0.248, P = 0.041) in the group as a whole but showed no group difference.
Table 3.
Mean changes in clinical and self-reported parameters of a subset of KEEPS participants during and following treatment.
| Outcome Variable | Overall | PL | t-E2 | o-CEE | P-value | |
|---|---|---|---|---|---|---|
| Changes during intervention | ||||||
| BMI (kg/m2, change from BL to 48 mo) | N = 71 | 0.47 ± 1.39* | 1.08 ± 1.52* | 0.16 ± 1.28 | −0.05 ± 1.00 | 0.007a |
| WC (cm, change from BL to 48 mo) | N = 67 | 3.81 ± 6.95* | 5.77 ± 7.28* | 2.74 ± 6.57 | 1.56 ± 6.13 | 0.079 |
| Health QOL (change in score from BL to 48 mo) | N = 74 | −4.58 ± 4.83* | −3.72 ± 4.96* | −5.11 ± 4.90* | −5.32 ± 4.56* | 0.365 |
| Insomnia severity (change in score from BL to 48 mo) | N = 74 | −0.51 ± 0.76* | −0.43 ± 0.73* | −0.62 ± 0.85* | −0.52 ± 0.73* | 0.612 |
| Changes over 3 years after HT intervention | ||||||
| BMI (kg/m2, change from 48 to 84 mo) | N = 71 | 0.51 ± 1.97* | 0.65 ± 2.22 | 0.61 ± 1.28* | 0.19 ± 2.26 | 0.453 |
| WC (cm, change from 48 to 84 mo) | N = 68 | 2.36 ± 7.11* | 1.82 ± 7.01 | 1.87 ± 7.13 | 3.92 ± 7.45 | 0.555 |
| Health QOL (Change in score from 48 to 84 mo) | N = 71 | −1.26 ± 3.51* | −1.85 ± 3.53* | −0.74 ± 3.76 | −0.93 ± 3.21 | 0.687 |
Changes are reported as mean ± standard deviation; Kruskal-Wallis test was used to test for any difference in changes between the 3 treatment groups.
Abbreviations: PL, placebo; t-E2, transdermal; o-CEE, oral; HT, hormone therapy; BL, baseline; mo, months; BMI, body mass index; WC, waist circumference; QOL, quality of life.
P < 0.05 from Wilcoxon signed-rank test indicates a significant change over time within that group.
Based on pairwise comparisons from post-hoc testing, change in BMI in both treatment groups was significantly less than that in placebo group (t-E2 vs. PL, P = 0.015; o-CEE vs PL, P = 0.005).
Baseline insomnia symptom scores and QOL scores of self-perceived health, relating to appetite and physical behavior, were comparable among groups. After four years of treatment, insomnia symptom scores improved on average by one-half point on the severity scale across all participants (mean change from baseline, −0.51 points; P < 0.001), but these changes did not differ between treatment groups (P = 0.612) nor did they correlate with changes in plasma OxA levels (rs = 0.069, P = 0.556). At 48 months, health QOL score decreased on average by 4.58 points in the group as a whole (P < 0.001), though this decline in QOL was not significantly associated with treatment group (P = 0.365) nor with changes in plasma OxA (rs = −0.207, P = 0.077). Similar results were found for post-treatment changes in health QOL, with no difference among prior treatment assignment (P = 0.896) and no significant correlation with changes in OxA (rs = −0.215, P = 0.072).
4. Discussion
This study evaluated plasma levels of OxA during (four years) and following (after three years) treatment with two formulations of MHT or placebo in recently menopausal women. As expected, changes in BMI were less in women randomized to MHT compared to placebo. However, OxA increased only in women randomized to o-CEE. Unexpectedly, these changes in BMI did not associate with changes in plasma OxA nor were changes in OxA associated with other outcomes (waist circumference, appetite/physical behavior, or insomnia scores). Only after discontinuation of study treatments did OxA associate with changes in WC. These results suggest that there may not be a direct effect of MHT on regulation of OxA and that intermediary processes may be inhibited by 17β estradiol or stimulated by metabolites of estrogen found in o-CEE. o –CEE contains more than 10 estrogen metabolites including, estrone, estrone sulfate, 17β-estradiol, and unique ring B unsaturated steroids, which have higher affinity for the estrogen receptor beta (ER-β) than classical estrogens [18].
Two prospective cohort studies that assessed the relationship of MHT and OxA reported conflicting results. In one study, median OxA levels were higher among post-menopausal women not taking MHT compared to those taking MHT or premenopausal controls [19]. In the second study, there were no differences in average plasma OxA levels in menopausal women who did and did not take MHT [20]. The differences in results between these two studies could be due to the different formulations of MHT (o-CEE plus norgestrel and E2 plus drospirenone, respectively), different study populations and/or methodological used to measure OxA (radio-immuno and enzyme-immuno assay, respectively). In addition, both studies had a limited follow up period of 6 months compared to the present study of 4 years of treatment and 3 years of discontinuation of treatment.
OxA plasma concentrations reported in these studies prior to treatment varied from a mean of 2430 ng/mL (SD, 688 ng/mL) [19] to a median of 0.00065 ng/mL (IQR, 0.00043–0.00109) [20], respectively. In comparison to these prior studies, the current study evaluated samples and outcomes from a randomized clinical trial, reducing the risk of selection bias inherent in cohort studies. The median baseline OxA across groups in the present study was 3.64 ng/mL (range, 0.76–14.26); values that fall within the range reported in other studies. [21,22]
Higher levels of OxA were reported in patients with obstructive sleep apnea who experienced excessive daytime sleepiness when compared to patients with obstructive sleep apnea patients who did not experience daytime sleepiness [22]. Healthy men with disrupted sleep also had higher OxA levels than those without disrupted sleep [11]. In the present study, changes in OxA did not correlate with changes in self-reported severity of sleep disturbances. Associations with sleep apnea or narcolepsy remain inconclusive, as this study did not have a baseline assessment of either condition.
In previous studies, plasma OxA negatively correlated with plasma leptin in non-obese men and women [23]. However, in the present study, there was no association of changes in plasma OxA with changes in plasma leptin, nor with changes in metabolic markers (BMI and WC) other than a modest association with post-treatment changes in WC despite prior evidence of either an inverse or direct association between plasma OxA and metabolic risk factors [24,25]. These differences in results among studies may reflect differences in the ages of the populations, differences in comorbid conditions and the absence of stratification of results by sex.
In conclusion, the variability of OxA during menopause has not been explored in detail. To our knowledge, this is the first study to explore changes in OxA both during and after use of MHT. This is an important issue to consider, as studies have reported a greater frequency of sleep problems associated with age at discontinuation, type and duration of MHT [26,27]. Differences in various physiological parameters between o-CEE and t-E2 have been observed in women participating in KEEPS including differences in effects on lipids, glucose metabolism [28], contents of vasoactive agents in circulating platelets [15], mood [29] and brain volumes [30]. Therefore, our results add to the accumulating evidence that various types of MHT are not equal and precision is required in discussing their efficacy relative to target outcome, mode of delivery, composition and dose.
Some evidence suggests that plasma OxA levels parallel plasma estradiol and leptin levels as they reflect physiological mechanisms that control energy balance [31]. It was not possible to provide a clear association with circulating levels of estradiol in the present study given the narrow range of estradiol at baseline and following treatment [32]. OxA increased only in the o-CEE group and further increases after discontinuation of treatment were not statistically significant. The mechanisms involved with these associations remain to be clarified. Furthermore, increases in OxA were dependent on the type of MHT, and did not correlate with any metabolic/sleep outcomes. Only after randomized treatment was discontinued did OxA correlate with WC. Given the literature on the relationship of sleep/wakefulness and OxA and the sleep complaints during menopause, it was hypothesized that OxA would associate with sleep disturbances at menopause and decrease with MHT, but this was not observed. Therefore, further research understanding fluctuations of this peptide in women experiencing menopausal symptoms is necessary.
5. Limitations
This study has several limitations. First, the number of participants in each treatment group was small, and is a subset of women who participated in the original KEEPS. Second, the plasma concentrations of OxA are only an estimate of synthesis of this peptide in the brain with levels reported to be one fifth to one eighth of those measured in cerebral spinal fluid [5]. In addition, longitudinal assessments of OxA levels may not represent an appropriate temporal relationship to shorter term regulatory processes associated with energy metabolism. Third, KEEPS participants were healthy white women, and, therefore, the generalization of these results to other population of women with different sleep and energy metabolic risk factors may not be applicable. Fourth, sleep quality, eating and physical behavior were assessed using subjective, self-report methods at annual visits with a risk for recall bias. Fifth, sleep disorders such as obstructive sleep apnea were not assessed in KEEPS screening, with this respiratory sleep disorder having a significant impact on OxA independent of menopausal transition [22]. Finally, there was an absence of effect of MHT on overall sleep disturbance and appetite/physical behavior scores in this subset of participants, limiting the ability to study relationships between OxA and menopausal symptoms.
Acknowledgments
Funding
This work was supported by the National Institutes of HealthTL1 TR000137 and AG 44170 and the Mayo Clinic Office of Health Disparities Research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Clinical Trial Registration for KEEPS: NCT00154180
Contributors
DC designed the study, performed the experiments, collected the data and drafted and finalized the manuscript.
JPB performed experiments and edited the manuscript.
KRB and BDL performed and oversaw the statistical analyses.
MJ participated in the design of the study, and oversaw the quality control for the OxA assays and editing of the manuscript.
VMM contributed to the design of the study, interpretation of results and editing of the manuscript.
All authors saw and approved the final version of the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was approved by the Mayo Clinic Institutional Review Board (IRB protocol # 2241). All participants gave written informed consent.
Provenance and peer review
This article has undergone peer review.
References
- 1.Xu TR, Yang Y, Ward R, Gao L, Liu Y. Orexin receptors: multi-functional therapeutic targets for sleeping disorders, eating disorders, drug addiction, cancers and other physiological disorders. Cell Signal. 2013;25(12):2413–2423. doi: 10.1016/j.cellsig.2013.07.025. [DOI] [PubMed] [Google Scholar]
- 2.Sakurai T, Mieda M, Tsujino N. The orexin system: roles in sleep/wake regulation. Ann N Y Acad Sci. 2010;1200:149–161. doi: 10.1111/j.1749-6632.2010.05513.x. [DOI] [PubMed] [Google Scholar]
- 3.Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92(4):573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- 4.Heinonen MV, Purhonen AK, Makela KA, Herzig KH. Functions of orexins in peripheral tissues. Acta Physiol (Oxf) 2008;192(4):471–485. doi: 10.1111/j.1748-1716.2008.01836.x. [DOI] [PubMed] [Google Scholar]
- 5.Snow A, Gozal E, Malhotra A, Tiosano D, Perlman R, Vega C, et al. Severe hypersomnolence after pituitary/hypothalamic surgery in adolescents: clinical characteristics and potential mechanisms. Pediatrics. 2002;110(6):e74. doi: 10.1542/peds.110.6.e74. [DOI] [PubMed] [Google Scholar]
- 6.Matsumura T, Nakayama M, Nomura A, Naito A, Kamahara K, Kadono K, et al. Age-related changes in plasma orexin-A concentrations. Exp Gerontol. 2002;37(8–9):1127–1130. doi: 10.1016/s0531-5565(02)00092-x. [DOI] [PubMed] [Google Scholar]
- 7.Johren O, Neidert SJ, Kummer M, Dendorfer A, Dominiak P. Prepro-orexin and orexin receptor mRNAs are differentially expressed in peripheral tissues of male and female rats. Endocrinology. 2001;142(8):3324–3331. doi: 10.1210/endo.142.8.8299. [DOI] [PubMed] [Google Scholar]
- 8.Yilmaz E, Celik O, Celik N, Celik E, Turkcuoglu I, Simsek Y, et al. Maternal and fetal serum orexin-A levels in gestational diabetes mellitus. J Obstet Gynaecol Res. 2013;39(1):139–145. doi: 10.1111/j.1447-0756.2012.01955.x. [DOI] [PubMed] [Google Scholar]
- 9.Yilmaz E, Celik O, Celik N, Simsek Y, Celik E, Yildirim E. Serum orexin-A (OXA) level decreases in polycystic ovarian syndrome. Gynecol Endocrinol. 2013;29(4):388–390. doi: 10.3109/09513590.2012.754874. [DOI] [PubMed] [Google Scholar]
- 10.Baranowska B, Wolinska-Witort E, Martynska L, Chmielowska M, Baranowska-Bik A. Plasma orexin A, orexin B, leptin, neuropeptide Y (NPY) and insulin in obese women. Neuro Endocrinol Lett. 2005;26(4):293–296. [PubMed] [Google Scholar]
- 11.Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. 2004;141(11):846–850. doi: 10.7326/0003-4819-141-11-200412070-00008. [DOI] [PubMed] [Google Scholar]
- 12.Messina G, Viggiano A, De Luca V, Messina A, Chieffi S, Monda M. Hormonal changes in menopause and orexin-a action. Obstet Gynecol Int. 2013;2013:209812. doi: 10.1155/2013/209812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jehan S, Masters-Isarilov A, Salifu I, Zizi F, Jean-Louis G, Pandi-Perumal SR, et al. Sleep disorders in postmenopausal women. J Sleep Disord Ther. 2015;4(5) [PMC free article] [PubMed] [Google Scholar]
- 14.Poehlman ET. Menopause energy expenditure, and body composition. Acta Obstet Gynecol Scand. 2002;81(7):603–611. doi: 10.1034/j.1600-0412.2002.810705.x. [DOI] [PubMed] [Google Scholar]
- 15.Raz L, Hunter LV, Dowling NM, Wharton W, Gleason CE, Jayachandran M, et al. Differential effects of hormone therapy on serotonin, vascular function and mood in the KEEPS. Climacteric. 2016;19(1):49–59. doi: 10.3109/13697137.2015.1116504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harman SM, Brinton EA, Cedars M, Lobo R, Manson JE, Merriam GR, et al. KEEPS: the kronos early estrogen prevention study. Climacteric. 2005;8(1):3–12. doi: 10.1080/13697130500042417. [DOI] [PubMed] [Google Scholar]
- 17.Utian WH, Janata JW, Kingsberg SA, Schluchter M, Hamilton JC. The Utian Quality of Life (UQOL) Scale: development and validation of an instrument to quantify quality of life through and beyond menopause. Menopause. 2002;9(6):402–410. doi: 10.1097/00042192-200211000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Bhavnani BR, Stanczyk FZ. Pharmacology of conjugated equine estrogens: efficacy, safety and mechanism of action. J Steroid Biochem Mol Biol. 2014;142:16–29. doi: 10.1016/j.jsbmb.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 19.El-Sedeek M, Korish AA, Deef MM. Plasma orexin-A levels in postmenopausal women: possible interaction with estrogen and correlation with cardiovascular risk status. BJOG. 2010;117(4):488–492. doi: 10.1111/j.1471-0528.2009.02474.x. [DOI] [PubMed] [Google Scholar]
- 20.Karakus M, Gelisgen R, Topcuoglu A, Guralp O, Topcuoglu D, Simsek G, et al. The effects of 17beta-estradiol plus drospirenone on anthropometric and biochemical measures of adiposity in menopausal women. Arch Gynecol Obstet. 2012;286(5):1233–1239. doi: 10.1007/s00404-012-2437-9. [DOI] [PubMed] [Google Scholar]
- 21.Hao YY, Yuan HW, Fang PH, Zhang Y, Liao YX, Shen C, et al. Plasma orexin-A level associated with physical activity in obese people, Eat. Weight Disord. 2016:1–9. doi: 10.1007/s40519-016-0271-y. http://dx.doi.org/10.1007/s40519-016-0271-y. [DOI] [PubMed]
- 22.Sanchez-de-la-Torre M, Barcelo A, Pierola J, Esquinas C, de la Pena M, Duran-Cantolla J, et al. Plasma levels of neuropeptides and metabolic hormones, and sleepiness in obstructive sleep apnea. Respir Med. 2011;105(12):1954–1960. doi: 10.1016/j.rmed.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 23.Komaki G, Matsumoto Y, Nishikata H, Kawai K, Nozaki T, Takii M, et al. Orexin-A and leptin change inversely in fasting non-obese subjects. Eur J Endocrinol. 2001;144(6):645–651. doi: 10.1530/eje.0.1440645. [DOI] [PubMed] [Google Scholar]
- 24.Gupta V, Mishra S, Kumar S, Mishra S. Association of circulating orexin-A level with metabolic risk factors in north indian pre menopausal women. Indian J Physiol Pharmacol. 2015;59(4):422–427. [PubMed] [Google Scholar]
- 25.Kawada Y, Hayashibe H, Asayama K, Dobashi K, Kodera K, Uchida N, et al. Plasma levels of orexin-a and leptin in obese children. Clin Pediatr Endocrinol: Case Rep Clin Investig. 2004;13(1):47–53. doi: 10.1297/cpe.13.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tom SE, Anderson ML, Landis CA, Bowles EJ, Woods NF, Reed SD, et al. Sleep problems after short-term hormone therapy suspension: secondary analysis of a randomized trial. Menopause. 2011;18(11):1184–1190. doi: 10.1097/gme.0b013e31821d6d33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao L, Woody SK, Chhibber A. Estrogen receptor beta in Alzheimer’s disease: from mechanisms to therapeutics. Ageing Res Rev. 2015;24(Pt B):178–190. doi: 10.1016/j.arr.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harman SM, Black DM, Naftolin F, Brinton EA, Budoff MJ, Cedars MI, et al. Arterial imaging outcomes and cardiovascular risk factors in recently menopausal women: a randomized trial. Ann Intern Med. 2014;161(4):249–260. doi: 10.7326/M14-0353. [DOI] [PubMed] [Google Scholar]
- 29.Gleason CE, Dowling NM, Wharton W, Manson JE, Miller VM, Atwood CS, et al. Effects of hormone therapy on cognition and mood in recently postmenopausal women: findings from the randomized, controlled KEEPS-cognitive and affective study. PLoS Med. 2015;12(6):e1001833. doi: 10.1371/journal.pmed.1001833. discussion e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kantarci K, Tosakulwong N, Lesnick TG, Zuk SM, Gunter JL, Gleason CE, et al. Effects of hormone therapy on brain structure: a randomized controlled trial. Neurology. 2016;87(9):887–896. doi: 10.1212/WNL.0000000000002970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Isidori AM, Strollo F, More M, Caprio M, Aversa A, Moretti C, et al. Leptin and aging: correlation with endocrine changes in male and female healthy adult populations of different body weights. J Clin Endocrinol Metab. 2000;85(5):1954–1962. doi: 10.1210/jcem.85.5.6572. [DOI] [PubMed] [Google Scholar]
- 32.Wharton W, Gleason CE, Dowling NM, Carlsson CM, Brinton EA, Santoro MN, et al. The KEEPS-Cognitive and Affective Study: baseline associations between vascular risk factors and cognition. J Alzheimers Dis. 2014;40(2):331–341. doi: 10.3233/JAD-130245. [DOI] [PMC free article] [PubMed] [Google Scholar]


