Abstract
Background
Conventional drug treatments for Generalized Anxiety Disorder (GAD) are often accompanied by substantial side effects, dependence, and/or withdrawal syndrome. A prior controlled study of oral chamomile (Matricaria chamomilla L.) extract showed significant efficacy versus placebo, and suggested that chamomile may have anxiolytic activity for individuals with GAD.
Hypothesis
We hypothesized that treatment with chamomile extract would result in a significant reduction in GAD severity ratings, and would be associated with a favorable adverse event and tolerability profile.
Study Design
We report on the open-label phase of a two-phase randomized controlled trial of chamomile versus placebo for relapse-prevention of recurrent GAD.
Methods
Subjects with moderate to severe GAD received open-label treatment with pharmaceutical-grade chamomile extract 1,500 mg/day for up to 8 weeks. Primary outcomes were the frequency of clinical response and change in GAD-7 symptom scores by week 8. Secondary outcomes included the change over time on the Hamilton Rating Scale for Anxiety, the Beck Anxiety Inventory, and the Psychological General Well Being Index. Frequency of treatment-emergent adverse events and premature treatment discontinuation were also examined.
Results
Of 179 subjects, 58.1% (95% CI: 50.9% to 65.5%) met criteria for response, while 15.6% prematurely discontinued treatment. Significant improvement over time was also observed on the GAD-7 rating (β = −8.4 [95% CI = −9.1 to −7.7]). A similar proportion of subjects demonstrated statistically significant and clinically meaningful reductions in secondary outcome ratings of anxiety and well-being. Adverse events occurred in 11.7% of subjects, although no serious adverse events occurred.
Conclusion
Chamomile extract produced a clinically meaningful reduction in GAD symptoms over 8 weeks, with a response rate comparable to those observed during conventional anxiolytic drug therapy and a favorable adverse event profile. Future comparative effectiveness trials between chamomile and conventional drugs may help determine the optimal risk/benefit of these therapies for patients suffering from GAD.
Keywords: Chamomile, Generalized Anxiety Disorder, Anxiolytic, Herbal, Botanical, Alternative and complementary medicine
Graphical abstract
Clinically significant anxiety improvement
Introduction
Generalized anxiety disorder (GAD) is a common form of anxiety with manifestations including excessive worry, poor concentration, restlessness, muscle tension, irritability, fatigue, and sleep difficulties that have occurred for ≥6 months (American Psychiatric Association, 2000). Lifetime prevalence for GAD ranges from 3.7–9.0% of the population in Europe and the United States (Asnaani et al., 2010; Kessler et al., 2012; Wittchen et al., 2011). It is also one of the most common psychiatric disorders in primary care (Davidson et al., 2010; King et al., 2008). While around 46–56% of GAD patients qualify for remission over the course of 8–12 years, 36–43% of patients will experience relapse (Bruce et al., 2005; Francis et al., 2012; Penninx et al., 2011; Yonkers et al., 2003). Although patients with GAD do experience a decline in psychiatric severity over time, the absolute magnitude of that improvement is modest if untreated (Ramsawh et al., 2008).
One of the most commonly prescribed psychopharmacological therapies for GADsymptoms are benzodiazepine tranquilizers (Baldwin et al., 2012; Reinhold and Rickels, 2015; Rickels and Rynn, 2002; Stahl, 2002). Although effective as a short-term therapy, extended use of benzodiazepines can result in tolerance, habituation, and withdrawal syndrome (Ashton, 2005; Bateson, 2002; Biggio et al., 2003; Bonavita et al., 2002). Furthermore, benzodiazepines result in relatively non-specific suppression of autonomic arousal, causing many to experience neurocognitive impairments (e.g., memory consolidation deficits) while the drug is active (Barker et al., 2004; Lader, 2011; Stewart, 2005), which may make benzodiazepine use prohibited in particular situations of cognitive alertness.
Other classes of medication (e.g. serotonin-1A receptor partial agonists, serotonin reuptake inhibitors) also demonstrate anxiolytic activity (Baldwin et al., 2011; Gelenberg et al., 2000; Mitte et al., 2005). However, the commonly used selective serotonin reuptake inhibitors are often associated with weight gain, insomnia, daytime somnolence, jitteriness, agitation, and sexual side effects (Ferguson, 2001; Goethe et al., 2007). Concerns over side effects may influence a patient’s attempts to treat their anxiety, with around half of treatment-seeking GAD patients tolerating qualifying symptoms for at least 2 years prior to pursuing medical attention (median delay between 6 to 14 years), and a third of those patients ignoring given psychiatric referrals (Baldwin et al., 2012; Kessler et al., 1998). By contrast, many individuals with anxiety and severe depression report attempts to use a complementary or alternative therapy to treat their problem, and endorse a high acceptability of the treatment modality (Kessler et al., 2001; McIntyre et al., 2016).
As one of the most established herbal remedies, chamomile (Matricaria chamomilla L. or Matricaria recutita) has been employed as a carminative (anti-colic), antiseptic, and anxiolytic (Blumenthal et al., 1998; Bruni et al., 1997; Di Stasi et al., 2002; Merzouki et al., 2000; Pieroni et al., 2002; Singh et al., 2011; Srivastava et al., 2010). Although substantial animal data support the anxiolytic properties of chamomile and several of its flavonoid constituents (Avallone et al., 1995; Nakazawa et al., 2003; Paladini et al., 1999; Reis et al., 2006; Yamada et al., 1996; Zanoli et al., 2000), few clinical trials have been undertaken in humans. To date, there has been one randomized, double-blind, placebo-controlled trial of chamomile safety and efficacy in individuals with GAD (Amsterdam et al. 2009). In this small proof-of-concept study, patients experienced a significantly greater reduction in anxiety symptoms when randomly assigned to chamomile versus placebo (p = 0.047).
Building upon its biological plausibility as an active anxiolytic and demonstrated preliminary controlled effects (Amsterdam et al., 2009), we hypothesized that treatment with pharmaceutical grade oral chamomile extract would result in a significant reduction in GAD severity ratings and be associated with a favorable adverse event and tolerability profile. We report acute-phase open-label findings here to allow effect comparisons with the placebo-controlled findings from the original clinical trial of chamomile (Amsterdam et al., 2009), as the open-label conditions in the present trial better resemble how chamomile would be administered in clinical practice. In addition, we decided to separately examine the open-label findings as active drug effects tend to be underestimated in placebo-controlled trials of anxiety and depression. This is due to lowered patient expectancies of treatment efficacy that stem from the knowledge of possibly being treated with an inert compound (Rutherford et al., 2015; Rutherford et al., In press; Rutherford et al., 2009). We provide results on long-term safety and effectiveness with subsequent continuation chamomile therapy versus placebo among responders who remained well for an additional 4 weeks of consolidation therapy after this open-label phase in a related paper [Mao Phytomedicine citation TK].
Methods
Subjects
A detailed description of the study design and procedures (Trial Registration Number NCT01072344) is available (Mao et al., 2014). Subjects were recruited from media and print advertisements approved by the Institutional Review Board (IRB) of the University of Pennsylvania, and from subjects referred from the outpatient Family Medicine clinic at the University of Pennsylvania Medical Center. All study-related procedures were performed at the Depression Research Unit of the University of Pennsylvania Medical Center.
Subject enrollment occurred from March 2010 to November 2014. Inclusion and exclusion criteria are described in the associated continuation-phase randomized controlled trial (RCT) paper [Mao Phytomedicine citation TK].
Study drug
Matricaria chamomilla L. 500 mg dry extract per capsule was pharmaceutical grade. A complete description of active constituents, extraction methods used, certificate of analysis, figure of the High Performance Liquid Chromatography fingerprint, and details on preparation, packaging, and quality control for consistent production of the study drug is provided in our related paper [Mao Phytomedicine citation TK]. Product approval for use in GAD was further granted in a “Safe to Proceed” letter by the Food and Drug Administration on December 17, 2009 (IND 107,206).
Study procedures
After a description of the study was provided to subjects, written informed consent was obtained in accordance with the ethical standards of the IRB of the University of Pennsylvania. The study was conducted using Good Clinical Practice guidelines with oversight by the local Office of Human Research and an independent Data and Safety Monitoring Board.
Psychiatric diagnoses was verified using the Structured Clinical Interview for DSM-IV-TR Axis I Disorders (SCID-I; (First et al., 2001). The best estimate of the number of prior GAD episodes (as defined by DSM-IV criteria) that occurred since onset of the disorder were obtained from subjects at their initial interview using the SCID interview format. Medical history, physical examination, weight and blood pressure, and laboratory tests (including hepatic, renal, and thyroid panels, pregnancy test in women, urine screen for drug abuse, and electrocardiogram) were performed.
Outcome measures
Outcome measures were obtained at baseline and after treatment weeks 2, 4, and 8. The protocol-designated primary outcome was frequency of response at week 8 defined as a ≥50% reduction in baseline GAD-7 score plus a final CGI-S score of 1 (i.e., normal), 2 (i.e., borderline), or 3 (i.e., mild symptoms). Responders at week 8 continued on consolidation chamomile therapy for an additional 4 weeks. Non-response was defined as a <50% reduction in total GAD-7 score or a CGI-S score ≥4 at study week 8. The primary continuous outcome measure was change in GAD-7 scores.
Secondary outcome measures included: change from baseline on the Hamilton Rating Scale for Anxiety (HAM-A) (Hamilton, 1959); change in Beck Anxiety Inventory (BAI) (Beck et al., 1988) score; and change in baseline Psychological General Well Being Index (PGWB; (Wiklund et al., 1992) and PGWB-Anxiety subscale scores.
In addition, a listing of probable and definite reported and elicited adverse events was compiled (NIMH, 1985). Adverse events were ascertained by both spontaneous subject reports and clinician-elicited queries and vital sign monitoring at each study visit.
Treatment
To assure uniform treatment procedures among clinicians, clinical management was conducted in a structured fashion (Fawcett et al., 1987). A fixed-flexible dosing strategy with chamomile 1,500 mg (three 500-mg capsules) daily was employed. This dose was selected based upon prior observation of superior efficacy and tolerability versus placebo at a dose of 1,100 mg daily (Amsterdam et al., 2009). This strategy also afforded subjects the opportunity to receive maximum chamomile dosing from treatment onset and the ability to reduce the daily dose to a minimum of 500 mg (if warranted). Drug accountability and capsule counts were performed at each study visit.
Sample size justification
A full account of sample size determination for the open-label phase portion of the study is described in the associated continuation RCT paper [Mao Phytomedicine citation TK]. The resulting sample size of 180 subjects allowed us to test our primary study hypothesis (i.e., relapse-prevention) with 80% power to detect a difference between chamomile versus placebo at the 0.05 level, as well as any within-subject change of clinical importance (within-person Cohen’s d ≥ 0.8).
Statistical procedures
Linear mixed model tests using restricted estimation of maximum likelihood were employed to estimate change over time in primary and secondary continuous outcome measures. Random slopes and intercepts were used to measure change in symptomatic outcome measures. All available symptom measurements obtained at baseline and weeks 2, 4, and 8 were included in the analyses in an intention-to-treat design using all observations. Time was parameterized in a linear fashion as the percentage of average change over time in study completers occurring cumulatively between each time unit (e.g., between baseline and week 2), summing to 1.
Outcome measurements with missing items had data imputed using a random forest imputation algorithm (Stekhoven and Bühlmann, 2012). 95% likelihood profile confidence intervals were calculated for all mixed model fixed effect coefficient estimates. We used statistical packages “lme4” (Bates et al., 2016) and “lmerTest” (Kuznetsova et al., 2016) in the R statistical computing language (R Development Core Team, 2016).
Results
Enrollment
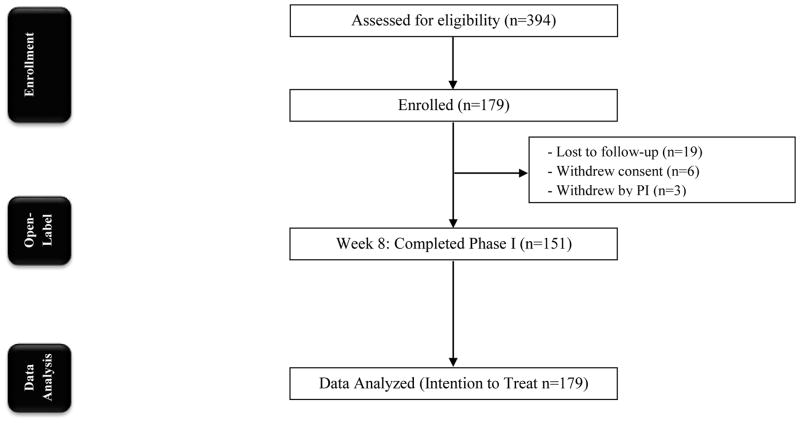
Overall, 180 subjects were enrolled and 179 subjects took at least one dose of study drug. One subject was categorized as a screen failure for not meeting all inclusion criteria.
Table 1 displays baseline clinical and demographic characteristics of the treatment sample. Subjects had a mean (SD) age of 45.7 years (SD 15.3; range 19.7–78.3). Among patients, 119 (66.5%) were women, 134 (74.9%) were Caucasian; 31 (17.3%) were African American; 10 (5.6%) were Asian American; 8 (4.5%) were Hispanic American; and 6 (3.4%) were multiracial.
Table 1.
Baseline characteristics of study subjects (n = 179)
| Baseline Characteristics | No. (%) or Mean (SD) |
|---|---|
| Age | 45.7 (15.3), range (19.7 to 78.3) |
| Gender (% Female) | 119 (66.5%) |
| Race (% Caucasian) | 134 (74.9%) |
| Ethnicity (% Hispanic) | 8 (4.5%) |
| % Unemployed | 31 (17.3%) |
| % Married | 67 (37.4%) |
| Age at first GAD episode | 21.5 (15.4) |
| Duration of current GAD episode (years) | 8.4 (13.9) |
| % Current major depressive episode | 56 (31.3%) |
| Number of psychiatric co-morbidities | 0.7 (0.9) |
| Number of previous treatments for GAD | 1.5 (1.7) |
| GAD-7 | 15.1 (3.1) |
| HAM-A | 14.7 (3.6) |
| BAI | 16.9 (9.4) |
| PGWB-Anxiety | 9.3 (3.9) |
| CGI-S | 4.2 (0.4) |
BAI = Beck Anxiety Inventory; CGI-S = Clinical Global Impression–Severity; GAD-7 = Generalized Anxiety Disorder 7-Item Scale; HAM-A = Hamilton Rating Scale for Anxiety; PGWB = Psychological General Well Being.
The mean (SD) baseline GAD-7 score among all subjects was 15.1 (3.1), with the majority of subjects having moderate severity on the CGI-S score: 151 (84.4%) moderate, 27 (15.1%) marked, and 1 (0.6%) severe. Mean age at illness onset was 21.5 (SD 15.4) years of age, and most subjects reported chronic symptoms (lasting >2 years) with a mean illness duration of 8.4 (SD 13.9) years. Most subjects reported taking at least one prior pharmacological or psychotherapeutic treatment for GAD, with the mean number of prior treatments being 1.5 (SD 1.7). In addition, 56 subjects (31.3%) also met DSM-IV criteria for concurrent major depressive episode, although anxiety symptoms were their primary complaint.
Overall, 28 subjects (15.6%) prematurely discontinued treatment during the initial 8 weeks of therapy (see Fig. 1). Only one subject was withdrawn from the trial for study drug nonadherence of at least 70%.
Figure 1.
CONSORT Diagram
Primary outcome measure: Clinical response
Overall, 104 subjects (58.1%) (95% CI: 50.9% to 65.5%) met criteria for treatment response (according to GAD-7 and CGI-S scores at week 8 endpoint). Among the 151 treatment completers at week 8, 44 (29.1%) had a final CGI -S score of 1 (normal), 45 (29.8%) a CGI-S score of 2 (borderline), 31 (20.5%) a CGI-S score of 3 (mild), 27 (17.9%) a CGI-S score of 4 (moderate), and 2 (1.3%) a final CGI-S score of 5 (marked) symptom.
None of the baseline demographic or clinical variables were significant or trend-level predictors of a response at week 8 (controlling for baseline GAD-7 score; findings available upon request). Baseline GAD-7 scores were also not a significant predictor of response (OR = 1.00 [95% CI = 0.91–1.11], p = 0.938).
Primary outcome measure: Change in GAD-7
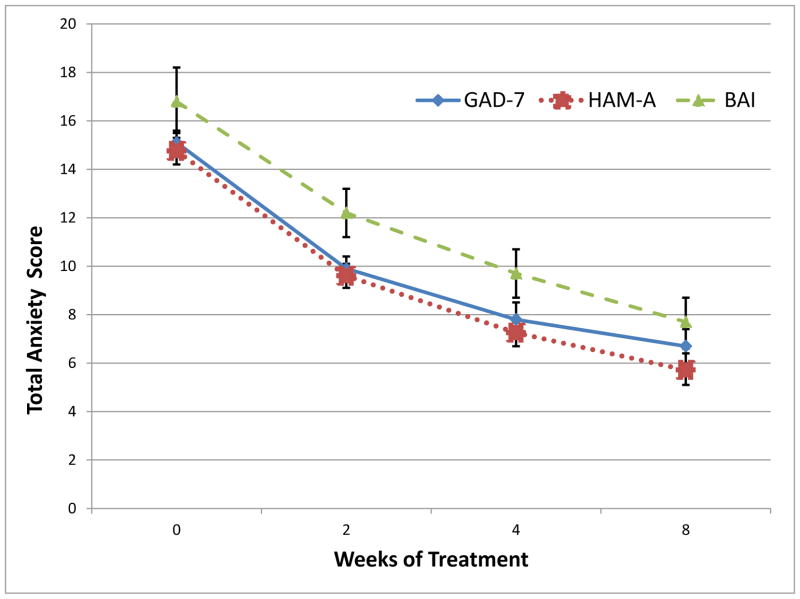
There was a statistically significant and clinically meaningful reduction in GAD-7 total score over time by week 8 (β = −8.4 [95% CI = −9.1 to −7.7], standardized β = −1.6 [95% CI = −1.7 to −1.4], df = 159.9, t = −23.4, p < 0.001; see Fig. 2). The average subject demonstrated a reduction from moderate-to-severe anxiety symptoms (i.e., GAD-7 = 15.1) to mild anxiety symptoms (i.e., GAD-7 = 6.7). Forty-two subjects (23.5%) had a rapid response to chamomile, showing ≥50% reduction from total baseline GAD-7 score after 2 weeks of treatment.
Figure 2. Change in anxiety symptomatology over acute treatment.
All indices of GAD-specific and general anxiety symptomatology improved over the course of treatment (p < .001). GAD-7 = Generalized Anxiety Disorder scale; HAM-A = Hamilton Anxiety Rating Scale; BAI = Beck Anxiety Inventory.
Secondary outcome measures
There was a statistically significant and clinically meaningful reduction over time for the HAM-A score (B = −9.0 [95% CI: −9.7 to −8.4], standardized β = −1.7 [95% CI: −1.8 to −1.6], df = 166.3, t = −26.2, p < 0.001; see Fig. 2). In addition, there was a statistically significant and clinically meaningful reduction in mean self-reported BAI score over time by week 8 (B = −9.2 [95% CI: −10.4 to −7.9], standardized β = −1.0 [95% CI: −1.2 to −0.9], df =170.8, t = −14.5, p < 0.001; see Fig. 2).
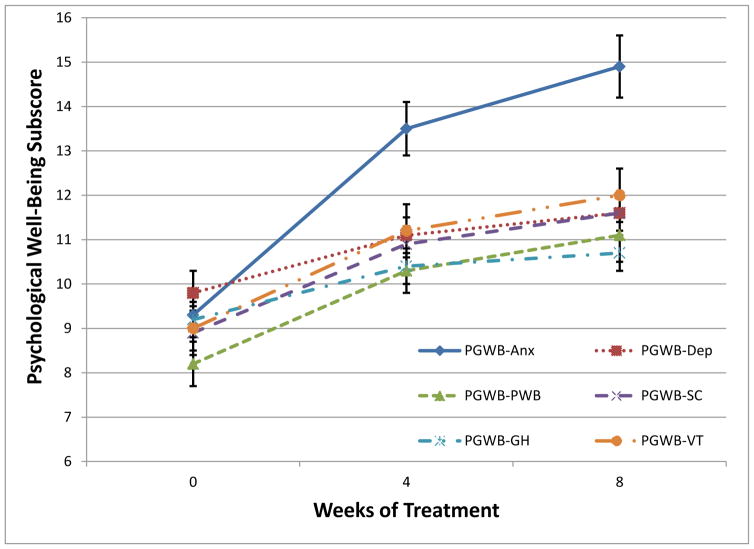
Finally, there was a statistically significant and clinically meaningful improvement by week 8 in PGWB total score (B = 17.5 [95% CI: 15.2 to 19.8], standardized β = 1.0 [0.9 to 1.1], df = 154.83, t = −14.95, p < 0.001; see Fig. 3). An increase in functioning was observed via the PGWB-Anxiety subscale (B = 5.6 [95% CI: 4.8 to 6.3], standardized β = 1.2 [1.0 to 1.3], df = 154.1, t= 14.1, p < 0.001). All other PGWB subscales also demonstrated improvement in functioning, including PGWB-Depression (B = 1.8 [95% CI: 1.4 to 2.1], standardized β = 0.6 [0.5 to 0.7], df = 160.8, t = 9.0, p < 0.001), PGWB-Positive Well-Being (B = 2.9 [95% CI: 2.4 to 3.3], standardized β = 0.8 [0.6 to 0.9], df = 152.2, t = 11.5, p < 0.001), PGWB-Self Control (B = 2.8 [95% CI: 2.3 to 3.2], standardized β = 0.9 [0.8 to 1.0], df = 169.0, t = 12.2, p < 0.001), PGWB-General Health (B = 1.5 [95% CI: 1.1 to 1.9], standardized β = 0.5 [0.4 to 0.7], df = 153.0, t = 6.9, p < 0.001), and PGWB-Vitality (B = 3.0 [95% CI: 2.4 to 3.5], standardized β = 0.8 [0.6 to 0.9], df = 157.1, t = 10.7, p < 0.001).
Figure 3. Change in psychological well-being over acute treatment.
All subscales of the Psychological General Well Being Index improved significantly over the course of 8 weeks of chamomile treatment (p <.001). PGWB-Anx = Anxiety; PGWB-Dep = Depression; PGWB-PWB = Positive well-being; PGWB-SC = Self-control; PGWB-GH = General health; PGWB-VT = Vitality.
Safety and tolerability
No subjects required dose reduction during the trial. Twenty-one subjects (11.7%) experienced at least one adverse event judged as either probably or definitely related to treatment. Exact adverse events reported are displayed in Table 2.
Table 2.
Adverse events potentially related to treatment by type
| Type of Adverse Event | No. (%) of patients reporting (n = 179) |
|---|---|
| Diarrhea | 1 (<1%) |
| Drowsiness | 13 (7.2%) |
| Fatigue | 1 (<1%) |
| Herbal taste lingering | 7 (3.9%) |
| Indigestion | 1 (<1%) |
| Nausea | 2 (1.1%) |
Some patients experienced more than one adverse event.
Subjects reported a mean (SD) of 0.14 (0.41) (range, 0–2) adverse events rated as ‘probably’ or ‘definitely’ related to treatment. Overall only 4 adverse events were classified as moderate severity, while the remainder were classified as mild. No serious adverse events occurred during the trial. The most common adverse events were drowsiness (n = 13; 7.2%) and dysgustia (n = 7; 3.9%).
Discussion
Current psychopharmacological interventions for GAD are not meeting the needs of many individuals. In this open-label trial, we found that chamomile therapy over 8 weeks was associated with a clinically meaningful response in 58.1% of subjects, as well as similar rates of improvement in observer and self-rated outcome measures. Chamomile dosage of 1,500 mg was well-tolerated with no severe adverse events reported. Almost one-quarter of patients were rapid responders to chamomile, attaining at least 50% reduction in GAD-7 symptom scores within 2 weeks of initiating treatment.
The clinical response rate in this study (58.1%) was similar to the 57.1% response rate from our prior placebo-controlled acute-treatment chamomile trial (Amsterdam et al., 2009). In addition, the average reduction in HAM-A score in this trial (9.0 points) was close to that of the placebo-controlled trial (approximately 7.5 points). Thus, there is some indication that the efficacy of chamomile was comparable across both trials, which were conducted at the same clinical site.
Moreover, the absolute response rates reported across both trials are akin to those reported in earlier controlled trials of benzodiazepines and selective serotonin reuptake inhibitors in GAD (Mitte et al., 2005). However, a direct comparison of chamomile versus conventional anxiolytics has not been reported. In addition, the current study demonstrated an exceedingly favorable adverse event and tolerability profile for chamomile, confirming observations from our prior placebo-controlled trial. These findings may make chamomile therapy attractive to individuals with GAD who express concerns over adverse events with conventional anxiolytics (McHugh et al., 2013).
Chamomile’s anxiolytic mode of action is not well-characterized. However, several lines of evidence suggest that several of its flavonoid constituents may produce anxiolytic activity by affecting γ-amino butyric acid (GABA), noradrenalin (NA), dopamine (DA), and serotonin neurotransmission or by modulating hypothalamic-pituitary-adrenocortical axis function (Awad et al., 2007; Hanrahan et al., 2011; Lorenzo et al., 1996; Marder and Paladini, 2002; Reis et al., 2006). In addition, apigenin (a chamomile constituent) has been shown to bind to benzodiazepine receptors and reduce GABA-activated activity in cultured nerve cells, an effect that is blocked by the benzodiazepine receptor antagonist Ro 15-1788 (Avallone et al., 1995). In addition, the semi-synthetic chamomile derivative, 6,3′-dinitro-flavone, has been found to be 30-fold more potent than diazepam at the benzodiazepine receptor (Viola et al., 1995). Given the observed clinical response in this study and past research (Amsterdam et al., 2009), more translational research is needed to elucidate the underlying mechanism of chamomile’s anxiolytic effect.
Several caveats should be considered in the interpretation of the current findings. The overall design of the current study precluded our ability to utilize a placebo comparator group in the present analysis (Mao et al., 2014). This limitation constrained our ability to estimate the anxiolytic efficacy of chamomile versus placebo, and to directly replicate our prior findings (Amsterdam et al., 2009). Thus, it is possible that the observed reduction in anxiety scores in this trial may result from a regression toward the statistical mean, or a placebo effect unrelated to an active effect of chamomile. Nevertheless, in the later phase of the trial in which responders to chamomile were double-blind randomized to chamomile continuation or placebo withdrawal, patients withdrawn from chamomile exhibited significantly worse GAD-7 scores across follow-up [Mao Phytomedicine citation TK].
Finally, it is possible that the apparent beneficial effect of chamomile may be limited to individuals with more moderate GAD, and that more severe GAD symptoms (or anxiety disorders other than GAD) would not benefit from chamomile therapy. However, there was no indication of a significant relationship between intake symptom severity and likelihood of attaining a clinical response, nor between any clinical variables and response rates. Additional studies will be needed to replicate the current observations.
Conclusion
Despite the limitations of the present study, to date, this analysis represents the largest prospective trial of the safety and effectiveness of chamomile extract for moderate to severe GAD. Given the observed effect size, we speculate that chamomile extract may produce a more favorable risk/benefit ratio than conventional anxiolytic agents. However, future comparativestudies comparing chamomile extract to conventional GAD therapies will need to be undertaken.
Acknowledgments
We thank Alexander Panossian, PhD of the Swedish Herbal Institute for his assistance in obtaining IND approval for SHC-1 chamomile extract from the US Food and Drug Administration, and providing expertise concerning the chamomile preparation. We also thank The Swedish Herbal Institute (Gothenburg, Sweden) for providing producing and the standardized chamomile extract for use in the present study. We note that The Swedish Herbal Institute had no role in design, conduct, or report of the study. We also thank Justin Hollm, James Baier, Theresa Tiliakos, Rolma Gano, and Maryanne Giampapa for their dedication to clinical trial management, coordination, data collection and entry. We thank Ingrid Haviland for editorial assistance in the preparation of this manuscript.
Funding Source
This study was supported by grants from the National Institutes of Health/National Center for Complementary and Alternative Medicine (NCCAM) R01 AT005074 and NCCAM K23 AT004112. The study was also supported by NIH/National Cancer Institute Cancer Center Support Grant P30 CA008748. The funding agencies had no role in the design or conduct of the study. Dr. Mao has full access to all the data in the study and had final responsibility for the decision to submit for publication.
Abbreviations
- BAI
Beck Anxiety Inventory self-report scale
- CGI-S
Clinical Global Impression–Severity
- GAD
Generalized Anxiety Disorder
- HAM-A
Hamilton Rating Scale for Anxiety observer scale
- PGWB
Psychological General Well Being self-report scale
Footnotes
Trial Registration: ClinicalTrials.gov Trials Register NCT01072344
Conflicts of interests
None of the authors had any potential conflict of interest to report.
Contribution of Each Author to the Study and Manuscript Preparation
All authors participated in study design and conduct, manuscript preparation, and final approval of the submitted manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amsterdam JD, Li Y, Soeller I, Rockwell K, Mao JJ, Shults J. A randomized, double-blind, placebo-controlled trial of oral Matricaria recutita (chamomile) extract therapy for generalized anxiety disorder. J Clin Psychopharmacol. 2009;29:378–382. doi: 10.1097/JCP.0b013e3181ac935c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashton H. The diagnosis and management of benzodiazepine dependence. Curr Opin Psychiatry. 2005;18:249–255. doi: 10.1097/01.yco.0000165594.60434.84. [DOI] [PubMed] [Google Scholar]
- Asnaani A, Richey JA, Dimaite R, Hinton DE, Hofmann SG. A cross-ethnic comparison of lifetime prevalence rates of anxiety disorders. J Nerv Ment Dis. 2010;198:551–555. doi: 10.1097/NMD.0b013e3181ea169f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avallone R, Zanoli P, Corsi L, Cannazza G, Baraldi M. Benzodiazepine-like compounds and GABA in flower heads of Matricaria chamomilla. Phytotherapy Research. 1995;10:S177–S179. [Google Scholar]
- Awad R, Levac D, Cybulska P, Merali Z, Trudeau VL, Arnason JT. Effects of traditionally used anxiolytic botanicals on enzymes of the gamma-aminobutyric acid (GABA) system. Can J Physiol Pharmacol. 2007;85:933–942. doi: 10.1139/Y07-083. [DOI] [PubMed] [Google Scholar]
- Baldwin D, Woods R, Lawson R, Taylor D. Efficacy of drug treatments for generalised anxiety disorder: systematic review and meta-analysis. Bmj. 2011;342:d1199. doi: 10.1136/bmj.d1199. [DOI] [PubMed] [Google Scholar]
- Baldwin DS, Allgulander C, Bandelow B, Ferre F, Pallanti S. An international survey of reported prescribing practice in the treatment of patients with generalised anxiety disorder. World J Biol Psychiatry. 2012;13:510–516. doi: 10.3109/15622975.2011.624548. [DOI] [PubMed] [Google Scholar]
- Barker MJ, Greenwood KM, Jackson M, Crowe SF. Cognitive Effects of Long-Term Benzodiazepine Use. CNS Drugs. 2004;18:37–48. doi: 10.2165/00023210-200418010-00004. [DOI] [PubMed] [Google Scholar]
- Bates D, Maechler M, Bolker B, Walker S, Christensen RHB, Singmann H, Dai B, Grothendieck G, Green P. lme4: Linear Mixed-Effects Models using ‘Eigen’ and S4, 1.1–12 ed. 2016. [Google Scholar]
- Bateson AN. Basic Pharmacologic Mechanisms Involved in Benzodiazepine Tolerance and Withdrawal. Current Pharmaceutical Design. 2002;8:5–21. doi: 10.2174/1381612023396681. [DOI] [PubMed] [Google Scholar]
- Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56:893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- Biggio G, Dazzi L, Biggio F, Mancuso L, Talani G, Busonero F, Mostallino MC, Sanna E, Follesa P. Molecular mechanisms of tolerance to and withdrawal of GABA(A) receptor modulators. Eur Neuropsychopharmacol. 2003;13:411–423. doi: 10.1016/j.euroneuro.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Blumenthal M, Busse WR, Goldberg A. The Complete Commission E Monographs: Therapeutic Guide to Herbal Medicines. Integrative Medicine Communications; Boston, MA: 1998. [Google Scholar]
- Bonavita CD, Bisagno V, Bonelli CG, Acosta GB, Rubio MC, Wikinski SI. Tolerance to the sedative effect of lorazepam correlates with a diminution in cortical release and affinity for glutamate. Neuropharmacology. 2002;42:619–625. doi: 10.1016/s0028-3908(02)00012-6. [DOI] [PubMed] [Google Scholar]
- Bruce SE, Yonkers KA, Otto MW, Eisen JL, Weisberg RB, Pagano M, Shea MT, Keller MB. Influence of psychiatric comorbidity on recovery and recurrence in generalized anxiety disorder, social phobia, and panic disorder: a 12-year prospective study. Am J Psychiatry. 2005;162:1179–1187. doi: 10.1176/appi.ajp.162.6.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruni A, Ballero M, Poli F. Quantitative ethnopharmacological study of the Campidano Valley and Urzulei district, Sardinia, Italy. J Ethnopharmacol. 1997;57:97–124. doi: 10.1016/s0378-8741(97)00055-x. [DOI] [PubMed] [Google Scholar]
- Davidson JR, Feltner DE, Dugar A. Management of generalized anxiety disorder in primary care: identifying the challenges and unmet needs. Prim Care Companion J Clin Psychiatry. 2010:12. doi: 10.4088/PCC.09r00772blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Stasi LC, Oliveira GP, Carvalhaes MA, Queiroz-Junior M, Tien OS, Kakinami SH, Reis MS. Medicinal plants popularly used in the Brazilian Tropical Atlantic Forest. Fitoterapia. 2002;73:69–91. doi: 10.1016/s0367-326x(01)00362-8. [DOI] [PubMed] [Google Scholar]
- Fawcett J, Epstein P, Fiester SJ, Elkin I, Autry JH. Clinical management--imipramine/placebo administration manual. NIMH Treatment of Depression Collaborative Research Program. Psychopharmacol Bull. 1987;23:309–324. [PubMed] [Google Scholar]
- Ferguson JM. SSRI Antidepressant Medications: Adverse Effects and Tolerability. Prim Care Companion J Clin Psychiatry. 2001;3:22–27. doi: 10.4088/pcc.v03n0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MS, Spitzer L, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV-TR Axis I Disorders (SCID-I/P W/PSY Screen) Biometrics Research, New York State Psychiatric Institute; New York, NY: 2001. [Google Scholar]
- Francis JL, Moitra E, Dyck I, Keller MB. The impact of stressful life events on relapse of generalized anxiety disorder. Depress Anxiety. 2012;29:386–391. doi: 10.1002/da.20919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelenberg AJ, Lydiard RB, Rudolph RL, Aguiar L, Haskins JT, Salinas E. Efficacy of venlafaxine extended-release capsules in nondepressed outpatients with generalized anxiety disorder: A 6-month randomized controlled trial. JAMA. 2000;283:3082–3088. doi: 10.1001/jama.283.23.3082. [DOI] [PubMed] [Google Scholar]
- Goethe JW, Woolley SB, Cardoni AA, Woznicki BA, Piez DA. Selective serotonin reuptake inhibitor discontinuation: side effects and other factors that influence medication adherence. J Clin Psychopharmacol. 2007;27:451–458. doi: 10.1097/jcp.0b013e31815152a5. [DOI] [PubMed] [Google Scholar]
- Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- Hanrahan JR, Chebib M, Johnston GA. Flavonoid modulation of GABA(A) receptors. Br J Pharmacol. 2011;163:234–245. doi: 10.1111/j.1476-5381.2011.01228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Olfson M, Berglund PA. Patterns and predictors of treatment contact after first onset of psychiatric disorders. Am J Psychiatry. 1998;155:62–69. doi: 10.1176/ajp.155.1.62. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Petukhova M, Sampson NA, Zaslavsky AM, Wittchen HU. Twelvemonth and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int J Methods Psychiatr Res. 2012;21:169–184. doi: 10.1002/mpr.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Soukup J, Davis RB, Foster DF, Wilkey SA, Van Rompay MI, Eisenberg DM. The use of complementary and alternative therapies to treat anxiety and depression in the United States. Am J Psychiatry. 2001;158:289–294. doi: 10.1176/appi.ajp.158.2.289. [DOI] [PubMed] [Google Scholar]
- King M, Nazareth I, Levy G, Walker C, Morris R, Weich S, Bellón-Saameño JÁ, Moreno B, Švab I, Rotar D, Rifel J, Maaroos HI, Aluoja A, Kalda R, Neeleman J, Geerlings MI, Xavier M, de Almeida MC, Correa B, Torres-Gonzalez F. Prevalence of common mental disorders in general practice attendees across Europe. The Br J Psychiatry. 2008;192:362–367. doi: 10.1192/bjp.bp.107.039966. [DOI] [PubMed] [Google Scholar]
- Kuznetsova A, Brockhoff PB, Christensen RHB. lmerTest: Tests in Linear Mixed Effects Models, 2.0–30 ed. 2016. [Google Scholar]
- Lader M. Benzodiazepines revisited—will we ever learn? Addiction. 2011;106:2086–2109. doi: 10.1111/j.1360-0443.2011.03563.x. [DOI] [PubMed] [Google Scholar]
- Lorenzo PS, Rubio MC, Medina JH, Adler-Graschinsky E. Involvement of monoamine oxidase and noradrenaline uptake in the positive chronotropic effects of apigenin in rat atria. Eur J Pharmacol. 1996;312:203–207. doi: 10.1016/0014-2999(96)00486-4. [DOI] [PubMed] [Google Scholar]
- Mao JJ, Li QS, Soeller I, Rockwell K, Xie SX, Amsterdam JD. Long-term chamomile therapy of generalized anxiety disorder: A study protocol for a randomized, double-blind, placebo-controlled trial. Clinical Trials. 2014;4:5. doi: 10.4172/2167-0870.1000188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder M, Paladini AC. GABA(A)-receptor ligands of flavonoid structure. Curr Top Med Chem. 2002;2:853–867. doi: 10.2174/1568026023393462. [DOI] [PubMed] [Google Scholar]
- McHugh RK, Whitton SW, Peckham AD, Welge JA, Otto MW. Patient preference for psychological vs pharmacologic treatment of psychiatric disorders: a meta-analytic review. J Clin Psychiatry. 2013;74:595–602. doi: 10.4088/JCP.12r07757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre E, Saliba AJ, Wiener KK, Sarris J. Herbal medicine use behaviour in Australian adults who experience anxiety: a descriptive study. BMC Complement Altern Med. 2016;16:60. doi: 10.1186/s12906-016-1022-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merzouki A, Ed-derfoufi F, Molero Mesa J. Contribution to the knowledge of Rifian traditional medicine. II: Folk medicine in Ksar Lakbir district (NW Morocco) Fitoterapia. 2000;71:278–307. doi: 10.1016/s0367-326x(00)00139-8. [DOI] [PubMed] [Google Scholar]
- Mitte K, Noack P, Steil R, Hautzinger M. A meta-analytic review of the efficacy of drug treatment in generalized anxiety disorder. J Clin Psychopharmacol. 2005;25:141–150. doi: 10.1097/01.jcp.0000155821.74832.f9. [DOI] [PubMed] [Google Scholar]
- Nakazawa T, Yasuda T, Ueda J, Ohsawa K. Antidepressant-like effects of apigenin and 2,4,5-trimethoxycinnamic acid from Perilla frutescens in the forced swimming test. Biol Pharm Bull. 2003;26:474–480. doi: 10.1248/bpb.26.474. [DOI] [PubMed] [Google Scholar]
- NIMH. Treatment emergent symptoms scale. Psychopharmacol Bull. 1985;21:1069–1073. [Google Scholar]
- Paladini AC, Marder M, Viola H, Wolfman C, Wasowski C, Medina JH. Flavonoids and the central nervous system: from forgotten factors to potent anxiolytic compounds. J Pharm Pharmacol. 1999;51:519–526. doi: 10.1211/0022357991772790. [DOI] [PubMed] [Google Scholar]
- Penninx BW, Nolen WA, Lamers F, Zitman FG, Smit JH, Spinhoven P, Cuijpers P, de Jong PJ, van Marwijk HW, van der Meer K, Verhaak P, Laurant MG, de Graaf R, Hoogendijk WJ, van der Wee N, Ormel J, van Dyck R, Beekman AT. Two-year course of depressive and anxiety disorders: results from the Netherlands Study of Depression and Anxiety (NESDA) J Affect Disord. 2011;133:76–85. doi: 10.1016/j.jad.2011.03.027. [DOI] [PubMed] [Google Scholar]
- Pieroni A, Quave C, Nebel S, Heinrich M. Ethnopharmacy of the ethnic Albanians (Arbereshe) of northern Basilicata, Italy. Fitoterapia. 2002;73:217–241. doi: 10.1016/s0367-326x(02)00063-1. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2016. [Google Scholar]
- Ramsawh HJ, Raffa SD, Edelen MO, Rende R, Keller MB. Anxiety in middle adulthood: effects of age and time on the 14-year course of panic disorder, social phobia and generalized anxiety disorder. Psychol Med. 2008;39:615–624. doi: 10.1017/S0033291708003954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhold JA, Rickels K. Pharmacological treatment for generalized anxiety disorder in adults: an update. Expert Opin Pharmacother. 2015;16:1669–1681. doi: 10.1517/14656566.2015.1059424. [DOI] [PubMed] [Google Scholar]
- Reis LS, Pardo PE, Oba E, do Kronka SN, Frazatti-Gallina NM. Matricaria chamomilla CH12 decreases handling stress in Nelore calves. J Vet Sci. 2006;7:189–192. doi: 10.4142/jvs.2006.7.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickels K, Rynn M. Pharmacotherapy of generalized anxiety disorder. J Clin Psychiatry. 2002;63(Suppl 14):9–16. [PubMed] [Google Scholar]
- Rutherford BR, Bailey VS, Schneier FR, Pott E, Brown PJ, Roose SP. Influence of study design on treatment response in anxiety disorder clinical trials. Depress Anxiety. 2015;32:944–957. doi: 10.1002/da.22433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford BR, Pott E, Tandler JM, Wall MM, Roose SP, Lieberman JA. Placebo response in antipsychotic clinical trials: A meta-analysis. JAMA Psychiatry. doi: 10.1001/jamapsychiatry.2014.1319. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford BR, Sneed JR, Roose SP. Does study design influence outcome?. The effects of placebo control and treatment duration in antidepressant trials. Psychother Psychosom. 2009;78:172–181. doi: 10.1159/000209348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh O, Khanam Z, Misra N, Srivastava MK. Chamomile (Matricaria chamomilla L.): An overview. Pharmacogn Rev. 2011;5:82–95. doi: 10.4103/0973-7847.79103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava JK, Shankar E, Gupta S. Chamomile: A herbal medicine of the past with bright future. Mol Med Rep. 2010;3:895–901. doi: 10.3892/mmr.2010.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl SM. Don’t ask, don’t tell, but benzodiazepines are still the leading treatments for anxiety disorder. J Clin Psychiatry. 2002;63:756–757. doi: 10.4088/jcp.v63n0901. [DOI] [PubMed] [Google Scholar]
- Stekhoven DJ, Bühlmann P. MissForest—non-parametric missing value imputation for mixed-type data. Bioinformatics. 2012;28:112–118. doi: 10.1093/bioinformatics/btr597. [DOI] [PubMed] [Google Scholar]
- Stewart SA. The Effects of Benzodiazepines on Cognition. J Clini Psychiatry. 2005;66:9–13. [PubMed] [Google Scholar]
- Viola H, Wasowski C, Levi de Stein M, Wolfman C, Silveira R, Dajas F, Medina JH, Paladini AC. Apigenin, a component of Matricaria recutita flowers, is a central benzodiazepine receptors-ligand with anxiolytic effects. Planta Med. 1995;61:213–216. doi: 10.1055/s-2006-958058. [DOI] [PubMed] [Google Scholar]
- Wiklund I, Berg G, Hammar M, Karlberg J, Lindgren R, Sandin K. Long-term effect of transdermal hormonal therapy on aspects of quality of life in postmenopausal women. Maturitas. 1992;14:225–236. doi: 10.1016/0378-5122(92)90117-m. [DOI] [PubMed] [Google Scholar]
- Wittchen HU, Jacobi F, Rehm J, Gustavsson A, Svensson M, Jönsson B, Olesen J, Allgulander C, Alonso J, Faravelli C, Fratiglioni L, Jennum P, Lieb R, Maercker A, van Os J, Preisig M, Salvador-Carulla L, Simon R, Steinhausen HC. The size and burden of mental disorders and other disorders of the brain in Europe 2010. Eur Neuropsychopharmacol. 2011;21:655–679. doi: 10.1016/j.euroneuro.2011.07.018. [DOI] [PubMed] [Google Scholar]
- Yamada K, Miura T, Mimaki Y, Sashida Y. Effect of inhalation of chamomile oil vapour on plasma ACTH level in ovariectomized-rat under restriction stress. Biol Pharm Bull. 1996;19:1244–1246. doi: 10.1248/bpb.19.1244. [DOI] [PubMed] [Google Scholar]
- Yonkers KA, Bruce SE, Dyck IR, Keller MB. Chronicity, relapse, and illness--course of panic disorder, social phobia, and generalized anxiety disorder: findings in men and women from 8 years of follow-up. Depress Anxiety. 2003;17:173–179. doi: 10.1002/da.10106. [DOI] [PubMed] [Google Scholar]
- Zanoli P, Avallone R, Baraldi M. Behavioral characterisation of the flavonoids apigenin and chrysin. Fitoterapia. 2000;71(Suppl 1):S117–123. doi: 10.1016/s0367-326x(00)00186-6. [DOI] [PubMed] [Google Scholar]