Abstract
Hydrogen peroxide (H2O2) is an important signaling molecule in cancer cells. However, the significant secretion of H2O2 by cancer cells have been rarely observed. Cold atmospheric plasma (CAP) is a near room temperature ionized gas composed of neutral particles, charged particles, reactive species, and electrons. Here, we first demonstrated that breast cancer cells and pancreatic adenocarcinoma cells generated micromolar level H2O2 during just 1 min of direct CAP treatment on these cells. The cell-based H2O2 generation is affected by the medium volume, the cell confluence, as well as the discharge voltage. The application of cold atmospheric plasma (CAP) in cancer treatment has been intensively investigated over the past decade. Several cellular responses to CAP treatment have been observed including the consumption of the CAP-originated reactive species, the rise of intracellular reactive oxygen species, the damage on DNA and mitochondria, as well as the activation of apoptotic events. This is a new previously unknown cellular response to CAP, which provides a new prospective to understand the interaction between CAP and cells in vitro and in vivo. The short-lived reactive species in CAP may activate cells in vivo to generate long-lived reactive species such as H2O2, which may trigger immune attack on tumorous tissues via the H2O2-mediated lymphocyte activation.
Introduction
H2O2 is an important signaling molecule in cancer cells1. The production of nanomolar (nM) level of H2O2 by several cancer cell lines including melanomas, neuroblastoma, colon carcinoma, and ovarian carcinoma have been observed two decades ago2. H2O2 may increase the genetic instability of cancer cells by inducing DNA strand breaks, damage on guanine or thymine bases, and the sister chromatid exchanges, which may facilitate the malignant process of cancer cells, such as proliferation, apoptosis resistance, metastasis, angiogenesis and hypoxia-inducible factor 1 activation1, 2. On the other hand, H2O2 alone with a relative high concentration or as the mediator of a series of anticancer drugs can selectively induce apoptosis in cancer cells1, 3–5. H2O2 may have promising application in cancer treatment at least as a mediator of series of physical or chemical strategies.
Cold atmospheric plasma (CAP), a near room temperature ionized gas composed of charged particles, neutral particles and electrons, has shown its promising application in cancer treatment over the past decade6–11. CAP not only effectively decreases the growth of many cancer cell lines in vitro through reactive species-triggered cell death but also significantly inhibits or halts the growth of subcutaneous xenograft tumors or melanoma in mice by the direct CAP treatment just above skin8, 12–15. The reactive oxygen species (ROS) and the reactive nitrogen species (RNS) have been regarded as the main factors contributing to the complicate interaction between CAP and cancer cells in vitro and in vivo 16, 17. Many studies conclude that the death of the CAP-treated cancer cells in vitro is due to the apoptosis triggered by the significant rise of intracellular ROS, DNA damage, as well as mitochondrial damage7, 11, 18–21.
Among dozens of CAP-originated species in aqueous solutions, H2O2 has been proven to be a main factor triggering the death of cancer cells in vitro 22–26. H2O2 has not been detected in the emission spectra of CAP in gas phase19. The H2O2 in aqueous solution may be due to the recombination reactions between short-lived species OH27, 28. To date, CAP is the only confirmed extracellular H2O2 source in plasma medicine. Cells have just been regarded as a consumer for the CAP-originated H2O2 11, 24. However, using a solution with H2O2 alone does not cause the same decrease in cancer cells viability as seen following the CAP treatment29, 30. Thus, the CAP treatment on cancer cells cannot be simply regarded as a H2O2-treatment.
In addition to the direct CAP treatment, another strategy using the CAP-stimulated solutions (PSS) to inhibit the growth of cancer cells in vitro or to inhibit the growth of tumorous tissues in mice through injection has been also demonstrated recently31–34. PSS is also named as the indirect CAP treatment or the CAP-activated solutions24, 35. For the direct CAP treatment in vitro, a thin layer of cell culture medium is used to cover cancer cells36. This medium layer facilitates the transition of the reactive species in the gas phase into the dissolved reactive species in medium36. The direct CAP treatment can be regarded essentially the same as the indirect CAP treatment if we assume that the finally formed long-lived reactive species such as H2O2, NO2 −, ONOO− in the CAP-treated solution is the sole factor leading to decreased tumor cell viability. However, considering the potential interaction between the short-lived species such as superoxide (O2 −), hydroxyl radicals (OH.) in CAP and cells, the direct CAP treatment should be different from the indirect CAP treatment. PSS will not contain short-lived species. So far, no direct experimental evidence has been found to describe such essential difference neither qualitatively nor quantitatively.
In this study, we first provide the experimental evidence that the 1 min of CAP treatment can trigger μM level cancer cell-based H2O2 generation through comparing the H2O2 generation in the CAP-treated cell-free medium and the CAP-treated cancer cells covered by a thin layer of medium. Such cell-based H2O2 generation can be regulated by controlling the volume of the medium layer, the cell confluence, as well as the discharge voltage. This study provides the first evidence that cells can not only quickly consume H2O2 but also significantly generate H2O2 through CAP treatment. This is a novel perspective to understand the interaction between CAP and cells.
Results
Consuming the extracellular H2O2 is a basic response of cancer cells cultured in an H2O2-containing environment11, 24. We have demonstrated that the CAP-originated H2O2 could be completely consumed just several hours after using the CAP-stimulated DMEM to culture glioblastoma (U87MG) cells11. In this study, we investigated the evolution of extracellular H2O2 in PSM and H2O2-DMEM, which have been used to culture cancer cell lines. All solutions contained 36.3 μM H2O2. The H2O2 concentration in PSM and H2O2-DMEM decreases as the culture time increases (Fig. S1). At a higher cell confluence, a faster H2O2 consumption rate is noted. To date, all three cancer cell lines we studied have shown a similar feature that cells quickly consume the extracellular H2O2 in just 3 hr. This observation is consistent with the observation that the rise of intracellular ROS started immediately after the CAP treatment and ended just about 3 hr post the CAP treatment37.
To date, the direct CAP treatment is the main strategy to investigate the toxicity of CAP on cancer cells in vitro 8, 31, 32. In this study, we measured the H2O2 concentration in a thin layer of DMEM which has been used to immerse cells during the direct CAP treatment. Without the protection of such medium layer, cancer cells are killed immediately due to the dehydration caused by the helium flow from the CAP jet tube. Based on the preliminary test, 20 μL per well on a 96-wells plate was the minimum volume to preclude the impact of helium flow (Fig. S2). In addition, the volume effect of this thin DMEM layer was also investigated through increasing the volume from 20 μL to 100 μL. Three general trends have been observed when we immediately measured the H2O2 concentration in DMEM after the treatment. First, the concentration of H2O2 in all cases increases as the volume of DMEM decreases (Fig. 1a). This result may be due to the principle that a salute will form in a larger concentration when the volume of solvent (reactive species) is smaller. Second, the direct CAP treatment on pancreatic adenocarcinoma cells (PA-TU-8988T) and breast adenocarcinoma cells (MDA-MB-231) rather than on glioblastoma cells (U87MG) generates significantly more H2O2 than the same CAP treatment on DMEM in the same volume (Fig. 1a). This trend is more obvious when the volume of DMEM is smaller. U87MG cells will show similar feature only at some specific conditions such as at a medium volume of 80 μL and 100 μL. The generation of H2O2 is also illustrated as the change in H2O2 concentration between the CAP-treated DMEM with and without cells (Fig. 1b). The volume of DMEM is also a key factor to affect the cell-based H2O2 generation. The maximum relative H2O2 generation from MDA-MB-231 cells, PA-TU-8988 cells, and U87MG occurs when the volume of DMEM is 50 μL, 100 μL, and 80 μL, respectively (Fig. 1b). More importantly, only the direct CAP treatment on cancer cells can cause such cell-based H2O2 generation. The CAP-stimulated DMEM cannot generate the similar response on all three cell lines (Fig. 1c). The response of cancer cells to PSM is just consuming H2O2, as seen in Fig. S1. In addition, all three cell lines do not generate H2O2 without the CAP treatment.
Figure 1.
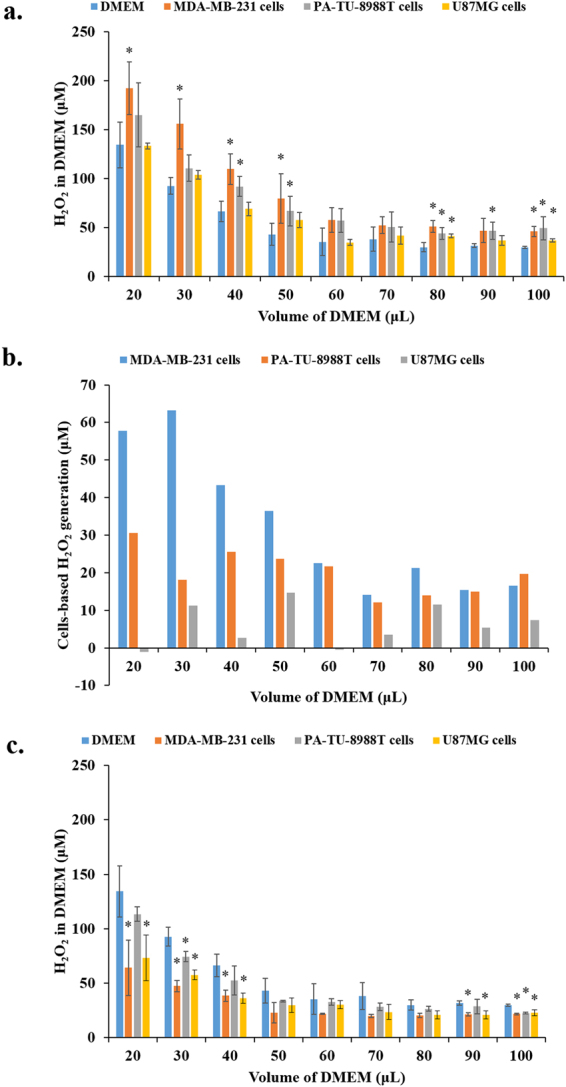
Only the direct CAP treatment can trigger the H2O2 production of specific cancer cell lines in vitro. (a) The H2O2 concentration in DMEM after the CAP treatment on just DMEM (control), on pancreatic adenocarcinoma cells (PA-TU-8988T) immerged in DMEM, on breast adenocarcinoma cells (MDA-MB-231) immerged in DMEM, as well as on glioblastoma cells (U87MG) immerged in DMEM. (b) The cells-based H2O2 concentration. The data is calculated based on the following formula. Cells-based H2O2 concentration = H2O2 concentration in the DMEM which has been used to immerse cancer cells during the CAP treatment – H2O2 concentration in the CAP-treated DMEM (control). The H2O2 concentration corresponds to the mean of each bar shown in 1a. (c) The indirect CAP treatment will not stimulate cancer cells to generate H2O2. All H2O2 concentration was measured immediately (about 1 min) after the treatment. Results are presented as the mean ± s.d. of three independently repeated experiments. Student’s t-test was performed between the data based on cells immersed in specific volume of DMEM and the data just based on the DMEM with the same volume. The significance is indicated as *p < 0.05.
To investigate whether the CAP-treated cancer cells will continue to generate H2O2 post the CAP treatment, the evolution of H2O2 concentration in DMEM after the direct CAP treatment on cancer cells and the CAP-treated DMEM has been studied. For all cell lines, the H2O2 concentration in DMEM gradually decreases post CAP treatment (Fig. 2). Such a decay trend has not been observed during the evolution of the CAP-treated DMEM. Thus, the decreased H2O2 concentration is due to the consumption of cancer cells on extracellular H2O2. The cell-based H2O2 generation occurs only when the direct CAP treatment is performed. Once the CAP treatment ceases, the H2O2 generation stops. When the H2O2 concentration measurement was performed at the tenth min after CAP treatment, the cell-based H2O2 generation was no longer observed (Fig. 2). This may explain why the cell-based H2O2 generation has not been observed in all previous studies.
Figure 2.
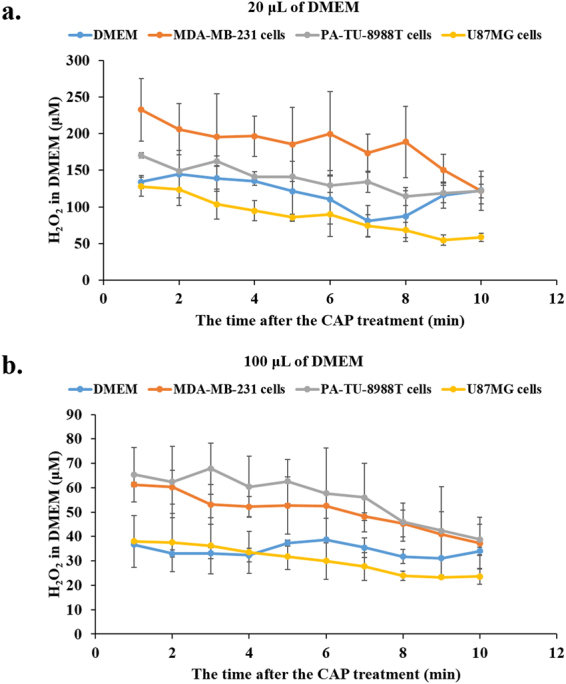
H2O2 evolution in the DMEM surrounding cancer cells after the direct CAP treatment. (a) Cancer cells immersed in 20 μL of DMEM. (b) Cancer cells immersed in 100 μL of DMEM. CAP was used to treat DMEM (control) or cancer cells (MDA-MB-231 cells, PA-TU-8988T cells, and U87MG cells) immersed in DMEM. In each experiment, the 1 min of CAP treatment was performed on the cells immersed in 20 μL or 100 μL of DMEM. The H2O2 concentration was measured very minute after the CAP treatment until the tenth minute. Cancer cells in 96-wells plate were cultured overnight before the CAP treatment. The cell confluence was 3 × 104 cells/mL. Results are presented as the mean ± s.d. of three independently repeated experiments.
In addition to the volume of DMEM used to cover cancer cells during CAP treatment, the cell confluence and the discharge voltage can also affect the cell-based H2O2 generation. Under the same experimental conditions, the maximum of the cell-based H2O2 generation of MDA-MB-231 cells, and PA-TU-8988T cells appears when the cell confluence is 1 × 104 cells/mL and 3 × 104 cells/mL, respectively (Fig. 3a). For U87MG cells, the significant effect of the cell confluence on the cell-based H2O2 generation was not observed (Fig. 3a). The discharge voltage is an important physical factor during the CAP treatment. The change of discharge voltage in a short range can significantly change the chemical composition of CAP. According to prior research, the increase of output (discharge) AC voltage from 2.56 kV to 3.80 kV significantly increased the generation of the nitrogen-based species such as N2 + and NO/N2 in the gas phase of CAP38. The generation of the oxygen-based species such as O and OH. just slightly increased during the same process38. Thus, the ratio between the oxygen-based species and the nitrogen-based species decreases as the output voltage increased. We observed the same trend in the CAP-stimulated PBS that the concentration ratio between H2O2 and NO2 − decreases from 11.2 to 5.5 as the output voltage increased from 3.02 kV to 3.85 kV. In this study, the increase of output voltage significantly weakens the cell-based H2O2 generation (Fig. 3b). A large output voltage will completely inhibit the cell-based H2O2 generation for all three cell lines.
Figure 3.
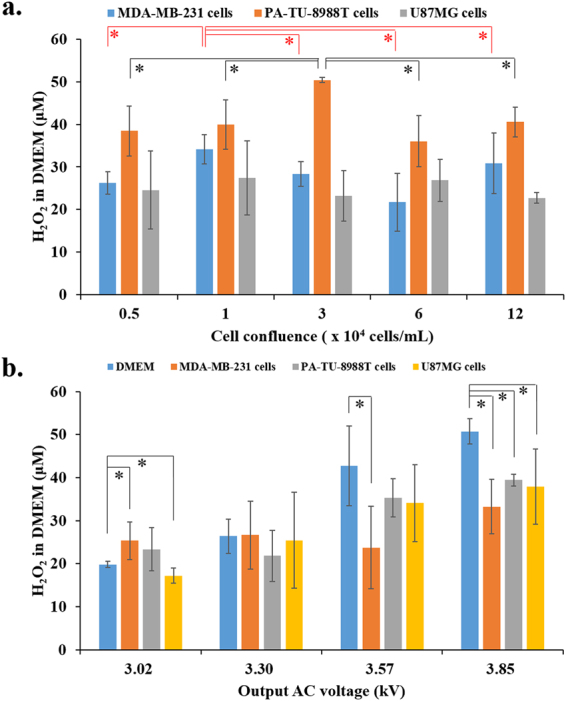
The cell confluence and the output voltage affect the cell-based H2O2 generation. (a) A relative small confluence can achieve the maximum cell-based H2O2 generation. (b) High output AV voltage significantly weakens the cell-based H2O2 generation. In each experiment, a 1 min of CAP treatment was used to treat DMEM (control) or cancer cells (MDA-MB-231 cells, PA-TU-8988T cells, and U87MG cells) immersed in 100 μL of DMEM. The H2O2 concentration was measured immediately (about 1 min) after the treatment. Cancer cells were cultured overnight before the treatment. The cell confluence for (b) was 3 × 104 cells/mL. Results are presented as the mean ± s.d. of three independently repeated experiments. Student’s t-test was performed and the significance is indicated as *p < 0.05.
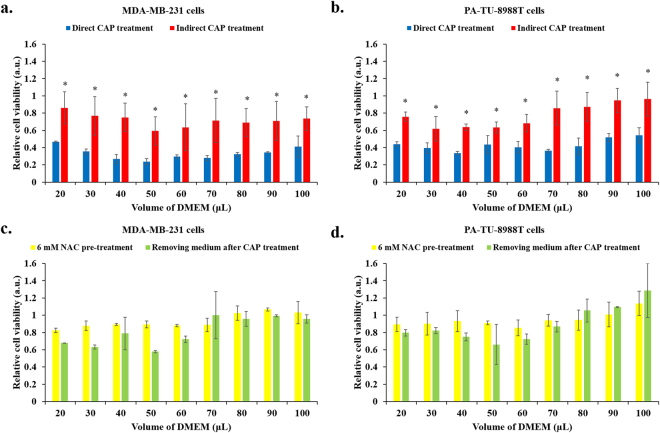
H2O2 has been regarded as a main anti-cancer reactive species in the CAP treatment in vitro 24–26. Because the observation that the direct CAP treatment on cancer cells immersed in a thin layer of DMEM causes a significantly stronger H2O2 generation than that seen with the indirect CAP treatment on DMEM, it is reasonable to speculate that the direct CAP treatment will cause a stronger anti-cancer effect on cancer cells than the indirect CAP treatment does under the same experimental conditions. Such a comparison was performed on MDA-MB-231 cells and PA-TU-8988T cells. As shown in Fig. 4a and b, both cell lines are much more vulnerable to the direct CAP treatment than the indirect CAP treatment. In addition, it was further demonstrated that the strong anti-cancer effect of the direct CAP treatment on the two cell lines could nearly be completely counteracted by pre-treating cancer cells with 6 mM intracellular ROS scavenger NAC or by removing the DMEM immersing cancer cells and replacing it with new untreated DMEM immediately after the direct CAP treatment (Fig. 4c and d). Thus, the anti-cancer effect of the direct CAP treatment on cancer cells is still based on a rise of intracellular ROS due to the extracellular reactive species dissolved in DMEM. Moreover, the volume of DMEM does not show a noticeable impact on the anti-cancer effect of the direct or the indirect CAP treatment, though corresponding H2O2 concentration in DMEM varies drastically with volume (Fig. 1). This difference indicates that other factors such as the whole reactive species amount rather than the concentration of reactive species may dominate the anti-cancer effect. In addition to the general trend introduced above, a slight difference on the anti-cancer effect due to the volume of DMEM still can be observed on MDA-MB-231 cells. The strongest anti-cancer effect of the direct CAP treatment on MDA-MB-231 cells occurs when the volume of DMEM is 50 μL.
Figure 4.
Under the same experimental conditions, the anti-cancer capacity of the direct CAP treatment is significantly stronger than the indirect CAP treatment. For the indirect CAP treatment, the CAP-stimulated DMEM was used to affect the growth of cancer cells. Comparison between the direct CAP treatment and the indirect treatment is performed on (a) MDA-MB-231 cells and (b) PA-TU-8988T cells. Pre-treating cancer cells with 6 mM NAC an intracellular scavenger for 3 hr or immediately (about 1 min) renewing the DMEM after the direct CAP treatment can effectively weakens the anti-cancer capacity of the direct CAP treatment on (c) MDA-MB-231 cells and (d) PA-TU-8988T cells. Results are presented as the mean ± s.d. of three independently repeated experiments. Student’s t-test was performed between the results from the direct CAP treatment and the results from the indirect CAP treatment. The significance is indicated as *p < 0.05.
Discussion
Since the first observation that CAP treatment could cause apoptosis in lung cancer cells line in 2004 and the first complete study on the anti-cancer effect of dielectric barrier discharge (DBD) on melanoma cells in 2007, the research on the application of CAP in cancer treatment experienced an exponential development7, 8, 11. Despite the understanding on the anti-cancer mechanism at the molecular and cellular level is far from clear, it is widely acknowledged that the CAP-originated reactive species are the main anti-cancer factors inhibiting the growth of cancer cells in vitro 6–8, 10, 11. In all previous studies, however, cancer cells have just been regarded as having a passive role in plasma medicine to sustain the attack from the reactive species such as H2O2 6–8, 10, 11. This study first demonstrates that specific cancer cells such as pancreatic adenocarcinoma cells and breast adenocarcinoma cells will generate significant amount of H2O2 as a response to the direct CAP treatment. For example, MDA-MB-231 cells generate about 85% more H2O2 than the CAP treatment does when the volume of DMEM is 50 μL. Despite only two cell lines has shown noticeable cell-based H2O2 generation capacity in this study, such cellular response may commonly exist in other cancer cell lines which has not been studied. And, it is completely possible that such feature may also be shared by other cells including normal mammalian cells, bacteria, as well as yeasts. These cells have been investigated in plasma medicine for decades37, 39, 40.
We hypothesize that the short-lived species in CAP may stimulate the H2O2 production by cancer cells. First, the H2O2 generation by cancer cells occurs only during CAP treatment. The cessation of CAP treatment will immediately cause the consumption of H2O2 to be the dominate response of cancer cells (Fig. 2). Consuming H2O2 is a basic response of cancer cells in an H2O2-containing environment (Fig. S1). It is possible that H2O2 consumption capacity and the H2O2 generation capacity coexist during CAP treatment. U87MG cells show a much stronger H2O2 consumption capacity compared with breast cancer cell lines11, 24. Thus, the weak cell-based H2O2 generation by U87MG cells may be due to its too strong H2O2 consumption capacity which dominates the interaction between U87MG cells and CAP.
The short-lived species in CAP has been regarded as a main source to form H2O2 in the CAP-stimulated solutions, though the corresponding mechanism is still disputable41, 42. Such H2O2 formation is an aqueous solution-based reaction. Hydroxyl radicals may form H2O2 by a simple reaction: OH. + OH. → H2O2 27, 28. H2O2 may also be formed by the recombination reaction based on hydroperoxyl radicals (HO2): HO2 + HO2 → H2O2 + O2 27. HO2 may be formed by a reaction involving O2 −: O2 − + H+ → HO2 27. For the cell-based H2O2 generation, H2O2 may be formed by a dismutation reaction catalyzed by the extracellular superoxide dismutase (Ex-SOD, SOD3) on the cytoplasmic membrane of cancer cells22, 23. Superoxide is provided by CAP39. The expression of SOD3 have been found in human pancreatic adenocarcinoma tissues, estrogen-induced breast cancer tissues, and breast carcinoma cells (MCF-7, MDA-MB-231), but not in glioblastoma cells, which may explain why cell-based H2O2 production has not been noticeably observed in the CAP-treated U87MG cells43–45.
The discovery of cell-based H2O2 generation provides a new feature which distinguishes CAP treatment and simple chemical treatments such as a H2O2 treatment. The direct CAP treatment causes a much stronger anti-cancer effect on pancreatic adenocarcinoma cells and breast adenocarcinoma cells than the indirect CAP treatment does (Fig. 4). Such difference may be at least partially due to the cell-based H2O2 generation during CAP treatment (Fig. 1). Nonetheless, it is necessary to emphasize that the weak anti-cancer capacity of the indirect CAP treatment revealed in this study is mainly due to the small diameter of the wells in 96-wells plate. As we demonstrated in previous study, the diameter of multi-wells plate was proportional to the reactive species concentration and the anti-cancer effect of PSS24. Consequently, the PSS made on 6-wells plate will be much more toxic to cancer cells than the PSS made on 96-wells plate24.
More importantly, this study provides a new perspective to understand the potential cellular interaction during CAP treatment, which also provide clues to understand the anti-cancer capacity of CAP in vivo. In addition to the proven selective anti-cancer capacity of CAP in vitro to cancer cells, another attractive feature of CAP is its promising anti-cancer effect seen in vivo. A series of investigations have achieved a similar conclusion that the growth of subcutaneous xenograft tumors or melanoma in mice can be significantly halted by a CAP treatment just above the skins of mice or on the exposed cancerous tissues in mice13–15, 46. The mechanism governing these descriptions is nearly completely unknown. ROS and RNS may also be the main factors contributing to the decreased tumor cell viability in vivo by CAP treatment through directly attacking tumor or indirectly activating immune response in vivo to further kill tumor cells18, 47, 48. The trans-skin motion (diffusion, transportation or other physical ways) of reactive species may be a key to understand the anti-cancer capacity in vivo. Several attempts have been performed to understand such processes. The diffusion of reactive species across the skin analogue has been observed49, 50. Our study provides a novel explanation for the trans-skin motion of reactive species. The short-lived reactive species, may activate cells to generate long-lived reactive species such as H2O2 through the similar way revealed in this study. The tumorous tissue may be immersed in a H2O2-rich environment even the CAP treatment does not directly generate a significant amount of H2O2 on the relative dry skin. The cells in skin and the cells in tumor may have similar mutual interaction through generating toxic chemicals such as H2O2 to neighbor cells. H2O2 is a second messenger in lymphocyte activation51, 52. μM level of H2O2 rapidly induced the activation of the transcription factor NF-κB and early gene expression of interleukin-2 (IL-2) and the IL-2 receptor α chain51. The H2O2-generating tumor tissues may be a potential target for immune system. The anti-cancer capacity of CAP treatment in vivo may involve the H2O2-activated immune attack on tumorous tissues.
Conclusions
A new previously unknown basic cellular response to CAP treatment is demonstrated in this study. Only direct CAP treatment on breast adenocarcinoma cells and pancreatic adenocarcinoma cells immersed in a thin layer of medium results in a μM level of cell-based H2O2 generation. The measured maximum H2O2 generation based on the CAP-stimulated MDA-MB-231 cells immersed in a thin layer of DMEM is about 85% more than that formed in the CAP-stimulated same medium but lacking cells. Controlling the volume of medium, the cell confluence, and the plasma discharge voltage can regulate the cell-based H2O2 generation. The abundant short-lived reactive species in CAP may trigger this unique cellular response, which gives a new perspective to understand the interaction between CAP and cells in vitro and in vivo.
Materials and Methods
CAP device
The CAP device used in this study was a typical CAP jet generator using helium as the carrying gas. The noticeable anti-cancer effect of this device has been demonstrated through a series of previous investigations from our lab24, 53. The detailed introduction for this device was illustrated in previous reports24, 53. Here, a short introduction is given. A violet plasma jet was generated between a central anode and a ring grounded cathode. The discharge was driven by an alternating current high voltage (3.16 kV) with a frequency of 30 kHz. The generated CAP was ejected out from a quartz tube with a diameter of 4.5 mm. The flow rate of helium was about 4.7 L/min. The input voltage of DC power was 11.5 V. According to the emission spectrum, the CAP in the gas phase was mainly composed of ROS (OH., O), RNS (NO, N2 +), and helium (He)38.
Cell cultures
Human pancreas adenocarcinoma cells (PA-TU-8988T) and human glioblastoma cells (U87MG) were provided by Dr. Murad’s lab at the George Washington University. Human breast adenocarcinoma cells (MDA-MB-231) were provided by Dr. Zhang’s lab at the George Washington University, and cultured in the same protocol as previous studies. The Dulbecco’s modified Eagle’s medium (DMEM) was purchased from Life Technologies (11965-118). DMEM was mixed with 1% (v/v) penicillin and streptomycin solution (Life Technologies, 15140122). The medium used in the cell culture and seeding was composed of DMEM supplemented with 10% (v/v) fetal bovine serum (ThermoFisher Scientific, 26140079) and 1% (v/v) penicillin and streptomycin solution (Life Technologies, 15140122). In each experiment, 100 μL of cancer cells harvesting solutions were seeded in a well on a 96-wells plate (Corning, 62406-081) a day prior to the CAP treatment. These cancer cells were cultured 24 hr under the standard cell culture conditions (a humidified, 37 °C, 5% CO2 environment). All wells on the margins of 96-wells plate were not used.
Making (N-Acetyl-L-cysteine) NAC-DMEM and pre-treating cancer cells
6 mM NAC-DMEM was made by dissolving NAC powder (A7250, Sigma-Aldrich) in DMEM. The whole medium which has been used to culture cancer cells overnight were removed first. Then, 100 μL of NAC-rich DMEM was used to culture cancer cells in the well of a 96-wells plate. After 3 hr, 6 mM NAC-DMEM was removed before the further treatment.
The direct and the indirect CAP treatment on cancer cells
The gap between the bottom of 12-wells plate and the CAP source was set to be 3 cm. All DMEM used in the CAP treatment was made of mixing 1% (v/v) penicillin-streptomycin solution (Life Technologies, 15140122) with standard DMEM (Life Technologies, 11965-118). The schematically illustration of the protocols was presented in Fig. S3. Prior to the direct CAP treatment, the medium which has been used to culture cells overnight was removed. After this step, for the direct CAP treatment, the DMEM with a specific volume such as 20 μL was transferred to cover the cancer cells in a well on a 96-wells plate. After that, the CAP jet was used to vertically treat the cancer cells for 1 min. For the indirect CAP treatment, the CAP-stimulated DMEM (PSM) was used to affect the growth of cancer cells cultured in a 96-wells plate. To make PSM, the DMEM with a specific volume such as 30 μL in a well on a 96-wells plate was treated by CAP jet vertically for 1 min. Such PSM was then transferred to affect the cancer cells which have been cultured overnight. For both direct and indirect CAP treatment, the control group corresponded to the cancer cells grown in the new DMEM with a specific volume without the CAP treatment. Then, cancer cells were cultured in the incubator under the standard culture conditions for 3 hr. Subsequently, the thin layer of DMEM around the cancer cells in each well was removed. 100 μL of new untreated DMEM was added in the well and cultured cancer cells for 3 days before the final cell viability assay. In this study, the treatment just using helium rather than CAP was also performed. The protocols were the same as the description above except that no discharge was used. In another case, the DMEM covering the cells needed to be replaced immediately after the CAP treatment. After removing the CAP-treated DMEM, 100 μL of new untreated DMEM was added to culture cells for 3 days before the final cell viability assay.
Cell viability assay
MTT (3-(4,5-Dimethyl-2-thiazol)-2,5-Diphenyl-2H-tetrazolium Bromide) assay was performed following the protocol provided by manufacturer (M2128, Sigma-Aldrich). The original experimental data about the cell viability was the absorbance at 570 nm measured by a H1 microplate reader (Hybrid Technology). The original absorbance at 570 nm was processed to be a relative cell viability through the division between the absorbance of the CAP-treated cancer cells and the absorbance of the cancer cells without the CAP treatment.
Extracellular H2O2 assay
The H2O2 concentration in DMEM or PBS was measured by using Fluorimetric Hydrogen Peroxide Assay Kit (Sigma-Aldrich, MAK165-1KT) following the protocols provided by manufacturer (Sigma-Aldrich). The fluorescence was measured by a H1 microplate reader (Hybrid Technology) at 540/590 nm. The final fluorescence of the experimental group was obtained by deducting the measured fluorescence of the control group from the measured fluorescence of the experimental group. Based on the standard curve, the H2O2 concentration in DMEM or PBS was obtained. Because the recommended volume of the sample solution was 50 μL, when the volume of sample solution was less than 50 μL but larger than 10 μL, we just collected 10 μL of the sample solution and mixed it with 40 μL of the untreated DMEM to make a 50 μL of the diluted sample solution. The H2O2 concentration of such case was 5 times larger than the measured concentration.
Measuring the H2O2 consumption speed by cancer cells
The protocols for different cancer cell lines are the same. Here, we just use PA-TU-8988T cells as an example. First, 100 μL of the PA-TU-8988T cells harvesting solution was seeded in a well on a 96-wells plate with a confluence of 6 × 104 cells/mL. 3 wells were seeded as 3 samples for one experiment. Cells were cultured in incubator for 6 hr under the standard conditions. Then, 1 mL of DMEM or PBS in a well on a 12-wells plate was treated by CAP for 1 min. After that, the medium which has been used to culture cells for 6 hr was removed. 120 μL of the CAP-treated DMEM or H2O2-containing DMEM (H2O2-DMEM) was transferred to culture PA-TU-8988T cells in one well on a 96-wells plate. 36.3 μM H2O2-DMEM was made by adding 9.8 M H2O2 standard solution (216763, Sigma-Aldrich) in DMEM. Since then until the third hour, 50 μL of the DMEM which has been used to culture cells was transferred to a well on a black clear bottom 96-wells plate in triplicate every hour. Ultimately, the residual H2O2 concentration in DMEM was measured using fluorimetric hydrogen peroxide assay kit illustrated above.
Electronic supplementary material
Acknowledgements
This work was supported by National Science Foundation, grant 1465061.
Author Contributions
D.Y. and M.K. designed all experiments. D.Y., H.C., W.Z. and A.T., performed all experiments. L.Z. and J.S. contributed to the materials preparation. All authors contributed to the data analysis. D.Y. and M.K. wrote the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-11480-x
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Dayun Yan, Email: ydy2012@gwmail.gwu.edu.
Michael Keidar, Email: keidar@gwu.edu.
References
- 1.López-Lázaro M. Dual role of hydrogen peroxide in cancer: Possible relevance to cancer chemoprevention and therapy. Cancer. Lett. 2007;252:1–8. doi: 10.1016/j.canlet.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 2.Szatrowski TP, Nathan CF. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer. Res. 1991;51:794–799. [PubMed] [Google Scholar]
- 3.Zieba M, et al. Comparison of hydrogen peroxide generation and the content of lipid peroxidation products in lung cancer tissue and pulmonary parenchyma. Respir. Med. 2000;94:800–805. doi: 10.1053/rmed.2000.0825. [DOI] [PubMed] [Google Scholar]
- 4.Lim SD, et al. Increased Nox1 and hydrogen peroxide in prostate cancer. Prostate. 2005;62:200–207. doi: 10.1002/pros.20137. [DOI] [PubMed] [Google Scholar]
- 5.Chen Q, et al. Pharmacologic ascorbic acid concentrations selectively kill cancer cells: action as a pro-drug to deliver hydrogen peroxide to tissues. Proc. Natl. Acad. Sci. USA. 2005;102:13604–13609. doi: 10.1073/pnas.0506390102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu X, et al. Reactive species in non-equilibrium atmospheric-pressure plasmas: Generation, transport, and biological effects. Phys. Rep. 2016;630:1–84. doi: 10.1016/j.physrep.2016.03.003. [DOI] [Google Scholar]
- 7.Keidar M. Plasma for cancer treatment. Plasma. Sources. Sci. Technol. 2015;24:33001. doi: 10.1088/0963-0252/24/3/033001. [DOI] [Google Scholar]
- 8.Yan D, Sherman JH, Keidar M. Cold atmospheric plasma, a novel promising anti-cancer treatment modality. Oncotarget. 2017;8:15977–15995. doi: 10.18632/oncotarget.13304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keidar M, et al. Cold atmospheric plasma in cancer therapy. Phys. Plasmas. 2013;20:057101. doi: 10.1063/1.4801516. [DOI] [Google Scholar]
- 10.Hirst AM, Frame FM, Arya M, Maitland NJ, O’Connell D. Low temperature plasmas as emerging cancer therapeutics: the state of play and thoughts for the future. Tumor. Biol. 2016;37:7021–7031. doi: 10.1007/s13277-016-4911-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yan D, et al. Toward understanding the selective anticancer capacity of cold atmospheric plasma–a model based on aquaporins (Review) Biointerphases. 2015;10:40801. doi: 10.1116/1.4938020. [DOI] [PubMed] [Google Scholar]
- 12.Ishaq M, Bazaka K, Ostrikov K. Intracellular effects of atmospheric-pressure plasmas on melanoma cancer cells. Phys. Plasmas. 2015;22:122003. doi: 10.1063/1.4933366. [DOI] [Google Scholar]
- 13.Keidar M, et al. Cold plasma selectivity and the possibility of a paradigm shift in cancer therapy. Br. J. Cancer. 2011;105:1295–301. doi: 10.1038/bjc.2011.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yajima I, et al. Non-equilibrium atmospheric pressure plasmas modulate cell cycle-related gene expressions in melanocytic tumors of RET-transgenic mice. Exp. Dermatol. 2014;23:424–425. doi: 10.1111/exd.12415. [DOI] [PubMed] [Google Scholar]
- 15.Chernets N, Kurpad DS, Alexeev V, Rodrigues DB, Freeman TA. Reaction Chemistry generated by nanosecond pulsed dielectric barrier discharge treatment is responsible for the tumor eradication in the B16 melanoma mouse model. Plasma. Process. Polym. 2015;12:1400–1409. doi: 10.1002/ppap.201500140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Graves DB. The emerging role of reactive oxygen and nitrogen species in redox biology and some implications for plasma applications to medicine and biology. J. Phys. D. Appl. Phys. 2012;45:263001. doi: 10.1088/0022-3727/45/26/263001. [DOI] [Google Scholar]
- 17.Shashurin A, Shneider MN, Dogariu A, Miles RB, Keidar M. Temporary-resolved measurement of electron density in small atmospheric plasmas. Appl. Phys. Lett. 2010;96:171502. doi: 10.1063/1.3389496. [DOI] [Google Scholar]
- 18.Ratovitski EA, et al. Anti-cancer therapies of 21st century: Novel approach to treat human cancers using cold atmospheric plasma. Plasma. Process. Polym. 2014;11:1128–1137. doi: 10.1002/ppap.201400071. [DOI] [Google Scholar]
- 19.Ja Kim S, Min Joh H, Chung TH. Production of intracellular reactive oxygen species and change of cell viability induced by atmospheric pressure plasma in normal and cancer cells. Appl. Phys. Lett. 2013;103:153705. doi: 10.1063/1.4824986. [DOI] [Google Scholar]
- 20.Thiyagarajan M, Anderson H, Gonzales XF. Induction of apoptosis in human myeloid leukemia cells by remote exposure of resistive barrier cold plasma. Biotechnol. Bioeng. 2014;111:565–574. doi: 10.1002/bit.25114. [DOI] [PubMed] [Google Scholar]
- 21.Kim GJ, Kim W, Kim KT, Lee JK. DNA damage and mitochondria dysfunction in cell apoptosis induced by nonthermal air plasma. Appl. Phys. Lett. 2010;96:021502. doi: 10.1063/1.3292206. [DOI] [Google Scholar]
- 22.Nozik-Grayck E, Suliman HB, Piantadosi CA. Extracellular superoxide dismutase. Int. J. Biochem. Cell Biol. 2005;37:2466–2471. doi: 10.1016/j.biocel.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 23.Ameziane-El-Hassani R, Schlumberger M, Dupuy C. NADPH oxidases: new actors in thyroid cancer? Nat. Rev. Endocrinol. 2016;12:485–494. doi: 10.1038/nrendo.2016.64. [DOI] [PubMed] [Google Scholar]
- 24.Yan D, et al. Principles of using cold atmospheric plasma stimulated media for cancer treatment. Sci. Rep. 2015;5:18339. doi: 10.1038/srep18339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adachi T, et al. Plasma-activated medium induces A549 cell injury via a spiral apoptotic cascade involving the mitochondrial-nuclear network. Free Radic. Biol. Med. 2015;79:28–44. doi: 10.1016/j.freeradbiomed.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 26.Yan D, et al. Stabilizing the cold plasma-stimulated medium by regulating medium’s composition. Sci. Rep. 2016;6:26016. doi: 10.1038/srep26016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Locke BR, Shih K-Y. Review of the methods to form hydrogen peroxide in electrical discharge plasma with liquid water. Plasma. Sources. Sci. Technol. 2011;20:34006. doi: 10.1088/0963-0252/20/3/034006. [DOI] [Google Scholar]
- 28.Yue YF, Mohades S, Laroussi M, Lu X. Measurements of plasma-generated hydroxyl and hydrogen peroxide concentrations for plasma medicine applications. IEEE. Trans. Plasma. Sci. 2016;44:2754–2758. doi: 10.1109/TPS.2016.2550805. [DOI] [Google Scholar]
- 29.Ishaq M, et al. Atmospheric gas plasma-induced ROS production activates TNF-ASK1 pathway for the induction of melanoma cancer cell apoptosis. Mol. Biol. Cell. 2014;25:1523–31. doi: 10.1091/mbc.E13-10-0590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ahn HJ, et al. Atmospheric-pressure plasma jet induces apoptosis involving mitochondria via generation of free radicals. PLoS One. 2011;6:6–12. doi: 10.1371/journal.pone.0028154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang X, Li M, Zhou R, Feng K, Yang S. Ablation of liver cancer cells in vitro by a plasma needle. Appl. Phys. Lett. 2008;93:021502. doi: 10.1063/1.2959735. [DOI] [Google Scholar]
- 32.Fridman G, et al. Floating electrode dielectric barrier discharge plasma in air promoting apoptotic behavior in Melanoma skin cancer cell lines. Plasma. Chem. Plasma. Process. 2007;27:163–176. doi: 10.1007/s11090-007-9048-4. [DOI] [Google Scholar]
- 33.Utsumi F, et al. Effect of indirect nonequilibrium atmospheric pressure plasma on anti-proliferative activity against chronic chemo-resistant ovarian cancer cells in vitro and in vivo. PLoS One. 2013;8:1–10. doi: 10.1371/journal.pone.0081576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tanaka H, Nakamura K, Mizuno M, Ishikawa K, Takeda K. Non-thermal atmospheric pressure plasma activates lactate in Ringer’s solution for anti-tumor effects. Sci. Rep. 2016;6:36282. doi: 10.1038/srep36282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yan D, et al. Controlling plasma stimulated media in cancer treatment application. Appl. Phys. Lett. 2014;105:224101. doi: 10.1063/1.4902875. [DOI] [Google Scholar]
- 36.Kaushik NK, Kaushik N, Park D, Choi EH. Altered antioxidant system stimulates dielectric barrier discharge plasma-induced cell death for solid tumor cell treatment. PLoS. One. 2014;9:1–11. doi: 10.1371/journal.pone.0103349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arjunan KP, Friedman G, Fridman A, Clyne AM. Non-thermal dielectric barrier discharge plasma induces angiogenesis through reactive oxygen species. J. R. Soc. Interface. 2012;9:147–157. doi: 10.1098/rsif.2011.0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheng X, et al. The effect of tuning cold plasma composition on glioblastoma cell viability. PLoS One. 2014;9:e98652. doi: 10.1371/journal.pone.0098652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kalghatgi S, et al. Effects of non-thermal plasma on mammalian cells. PLoS One. 2011;6:e16270. doi: 10.1371/journal.pone.0016270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ma RN, et al. An atmospheric-pressure cold plasma leads to apoptosis in Saccharomyces cerevisiae by accumulating intracellular reactive oxygen species and calcium. J. Phys. D: Appl. Phys. 2013;46:285401. doi: 10.1088/0022-3727/46/28/285401. [DOI] [Google Scholar]
- 41.Nakajima N, et al. The expresssion of Mdm2 on Helicobacter pylori infected intestinal metaplasia and gastric cancer. J. Clin. Biochem. Nutr. 2009;44:196–202. doi: 10.3164/jcbn.08-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tanaka H, et al. Plasma medical science for cancer therapy: toward cancer therapy using nonthermal atmospheric pressure plasma. IEEE. Trans. Plasma. Sci. 2014;42:3760–3764. doi: 10.1109/TPS.2014.2353659. [DOI] [Google Scholar]
- 43.O’Leary BR, et al. Loss of SOD3 (EcSOD) expression promotes an aggressive phenotype in human pancreatic ductal adenocarcinoma. Clin. Cancer. Res. 2015;21:1741–1751. doi: 10.1158/1078-0432.CCR-14-1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Singh B, Bhat HK. Superoxide dismutase 3 is induced by antioxidants, inhibits oxidative DNA damage and is associated with inhibition of estrogen-induced breast cancer. Carcinogenesis. 2012;33:2601–2610. doi: 10.1093/carcin/bgs300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Teoh MLT, Fitzgerald MP, Oberley LW, Domann FE. Overexpression of extracellular superoxide dismutase attenuates heparanase expression and inhibits breast carcinoma cell growth and invasion. Cancer. Res. 2009;69:6355–6363. doi: 10.1158/0008-5472.CAN-09-1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mirpour S, et al. Utilizing the micron sized non-thermal atmospheric pressure plasma inside the animal body for the tumor treatment application. Sci. Rep. 2016;6:29048. doi: 10.1038/srep29048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miller V, Lin A, Fridman A. Why target Immune cells for plasma treatment of cancer. Plasma. Chem. Plasma. Process. 2016;36:259–268. doi: 10.1007/s11090-015-9676-z. [DOI] [Google Scholar]
- 48.Lin A, et al. Uniform nanosecond pulsed dielectric barrier discharge plasma enhances anti-tumor effects by induction of immunogenic cell death in tumors and stimulation of macrophages. Plasma. Process. Polym. 2015;12:1392–1399. doi: 10.1002/ppap.201500139. [DOI] [Google Scholar]
- 49.Szili EJ, Oh J-S, Hong S-H, Hatta A, Short RD. Probing the transport of plasma-generated RONS in an agarose target as surrogate for real tissue: dependency on time, distance and material composition. J. Phys. D. Appl. Phys. 2015;48:202001. doi: 10.1088/0022-3727/48/20/202001. [DOI] [Google Scholar]
- 50.Gaur N, et al. Combined effect of protein and oxygen on reactive oxygen and nitrogen species in the plasma treatment of tissue. Appl. Phys. Lett. 2015;107:103703. doi: 10.1063/1.4930874. [DOI] [Google Scholar]
- 51.Los M, et al. Hydrogen peroxide as a potent activator of T-lymphocyte functions. Eur. J. Immunol. 1995;25:159–165. doi: 10.1002/eji.1830250127. [DOI] [PubMed] [Google Scholar]
- 52.Reth M. Hydrogen peroxide as second messenger in lymphocyte activation. Nat. Immunol. 2002;3:1129–1134. doi: 10.1038/ni1202-1129. [DOI] [PubMed] [Google Scholar]
- 53.Shashurin A, et al. Influence of cold plasma atmospheric jet on surface integrin expression of living cells. Plasma. Process. Polym. 2010;7:294–300. doi: 10.1002/ppap.200900086. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.