Abstract
Acute respiratory distress syndrome (ARDS) is a serious complication of acute lung injury. Severe systemic inflammation is the main cause of multiple organ dysfunction and high mortality. Removal of reactive oxygen species by anti-oxidants has been applied in clinical practice. N-acetylcysteine (NAC) is the most commonly used anti-oxidant. However, the benefit of anti-oxidant therapy was not consistently demonstrated by previous studies. In the present study, a meta-analysis was performed to evaluate the effects of NAC for adult patients with ARDS. The PubMed, Cochrane and EMBASE databases were searched to retrieve all of the available randomized controlled trials (RCTs) published until October 2015. Quality evaluation of included studies was performed according to the modified Jadad scale score. The Cochrane Collaboration Review Manager 5.3 software was used to perform the meta-analysis. Five RCTs comprising 183 patients were found to be eligible for inclusion in the meta-analysis. Pooled analysis showed that NAC did not contribute to reduce short-term mortality [risk ratio (RR)=0.73; 95% confidence interval (CI): 0.50–1.07; P=0.10] or 30-day mortality (RR=0.72; 95% CI: 0.44–1.19; P=0.20) when compared with those in the control group. However, duration of intensive care unit (ICU) stay in the NAC group was shortened [weighted mean difference (WMD), −4.56; 95% CI: (−7.32 to −1.80); P=0.001]. There was no significant difference in the ratio of partial arterial oxygen pressure to the fraction of inspired oxygen between the two groups [WMD, 54.34; 95% CI: (−30.50 to 139.17); P=0.21]. No severe adverse reactions were observed in the patients included. Although the duration of ICU stay was shortened, the clinical benefits of NAC were limited for ARDS based on the present meta-analysis. As the number of included trials and patients was small, additional trials are required to provide sufficient evidence for the efficacy of NAC in ARDS.
Keywords: N-acetylcysteine, acute respiratory distress syndrome, randomized controlled trials, mortality, systematic review
Introduction
Acute respiratory distress syndrome (ARDS) is characterized by rapid progression and devastating hypoxemic respiratory failure (1). In clinical and animal models, the term acute lung injury (ALI) has been widely applied to describe a mild form of ARDS (2). ARDS has been a hotspot of clinical research since it was first described in 1967 (3). The estimated overall mortality of ARDS has remained at a high level of 44.3% (4). Survivors of ARDS usually suffer from significant physical and psychological disability, which leads to an increased medical cost and requires the use of social health care at the same time (5).
In the last decade, numerous approaches have been proposed to treat ARDS, such as improvements in fluid administration, non-invasive mechanical ventilation strategies and ventilator management (6–9). Although in-hospital and 1-year mortality rates have decreased by a certain degree in recent years, ARDS still leads to a heavy social burden and high health-care cost (10,11).
It has been evidenced that oxidative stress is associated with poorer outcome in critical illnesses, including ARDS (12). Effective removal of reactive oxygen species (ROS) by anti-oxidants has been an attractive strategy to treat ARDS (13). N-acetylcysteine (NAC) is a common anti-oxidant and has been tested in multiple trials on lung injury and sepsis (14,15). However, the benefit of anti-oxidant therapy is not consistent among studies (16). According to a Cochrane review from 2004, NAC treatment did not significantly reduce early mortality due to ARDS (17). Other benefits, including 30-day mortality, duration of intensive care unit (ICU) stay and oxygenation, were not analyzed in that review. In the last decade, several further clinical studies have attempted to optimize the dose and timing of NAC application. Certain studies had positive results, as the study by Moradi et al (18) from 2009. Therefore, the present study performed a meta-analysis including these new data to evaluate whether the use of NAC may provide a benefit to patients with ARDS.
Materials and methods
Data sources and search strategies
All available randomized controlled trials (RCTs) using NAC in ARDS patients were identified from the following data sources: PubMed/MEDLINE, EMBASE and Cochrane Library (inception until October 2015). The following keywords were used: (‘N-acetylcysteine’ OR ‘NAC’ OR ‘acetylcysteine’) AND (‘acute respiratory distress syndrome’ OR ‘adult respiratory distress syndrome’ OR ‘ARDS’ OR ‘acute lung injury’ OR ‘ALI’). In addition, the reference lists of the eligible studies were manually searched. No language restrictions were applied.
The retrieved studies were assessed by two reviewers. They identified the titles, abstracts and citations independently. Based on the criteria presented below, the reviewers assessed all the retrieved studies for inclusion. Disagreements in all phases were resolved by discussing with a third reviewer. The reviewers selected the eligible studies which satisfied the inclusion and exclusion criteria. Neither ethical approval nor patient consent were required in this meta-analysis, as all the studies included had already been published.
Inclusion criteria were as follows: i) RCTs, which were reviewed either published in full or in abstract form; ii) trials with additional use of NAC in patients with ALI or ARDS; iii) the definition or the diagnostic criteria of ARDS were clear and specific, and diagnosis was required to be based on at least the following criteria: a) Bilateral infiltration evidence on chest radiography, and b) hypoxemia evidence via arterial partial pressure of oxygen/fraction of inspired oxygen (PaO2/FiO2); iv) short-term mortality (30-day or hospital or ICU mortality), length of ICU stay, ratio of PaO2/FiO2 or adverse events were presented.
Exclusion criteria were as follows: i) The study was not an original research article; ii) the study was not an RCT; iii) the trial was limited to animals or cells; iv) no extractable outcome was included in the study.
Quality assessment and data extraction
The modified Jadad quality score was used to evaluate the methodological quality of included trials (19). It evaluates the method of randomization, allocation concealment, double-blinding and information on withdrawals and drop-outs to follow-up. According to these criteria, the studies were divided into two groups: High-quality group (score, ≥4) and low-quality group (score, ≤3).
The primary outcomes of the present meta-analysis were short-term mortality and 30-day mortality. The secondary outcomes were duration of ICU stay and PaO2/FiO2 ratio. The following data were extracted and recorded according to a pre-designed form: Name of first author, year of publication, number of patients, dosage of NAC, PaO2/FiO2 ratio, application of positive end expiratory pressure (PEEP), diagnostic criteria and adverse events. Additional information, including sex and age of patients, the number of cases of organ dysfunction and days of mechanical ventilation was also recorded. The data were extracted by two independent reviewers. Disagreement was resolved by discussion with the third reviewer when necessary.
Statistical analysis
According to the design, the weighted mean difference (WMD) was determined to analyze continuous variables and the 95% confidence interval (CI) was used to indicate the effect size. Similarly, the relative risk (RR) was used to analyze dichotomous data and the effect size was also indicated by the 95% CI. I2 statistics were used to measure statistical heterogeneity (20). The fixed-effects model was applied if there was no obvious heterogeneity among studies (I2<50%) (21) and otherwise, a random-effects model was applied (I2>50%) (22). Publication bias was investigated via Begg's funnel plot method (23). P-values were 2-tailed P<0.05 was considered to indicate a statistically significant difference. The Review Manager version 5.3 (the Cochrane Collaboration, Copenhagen, Denmark) was used to perform statistical analyses. There was no registered protocol in the present meta-analysis.
Results
Study identification
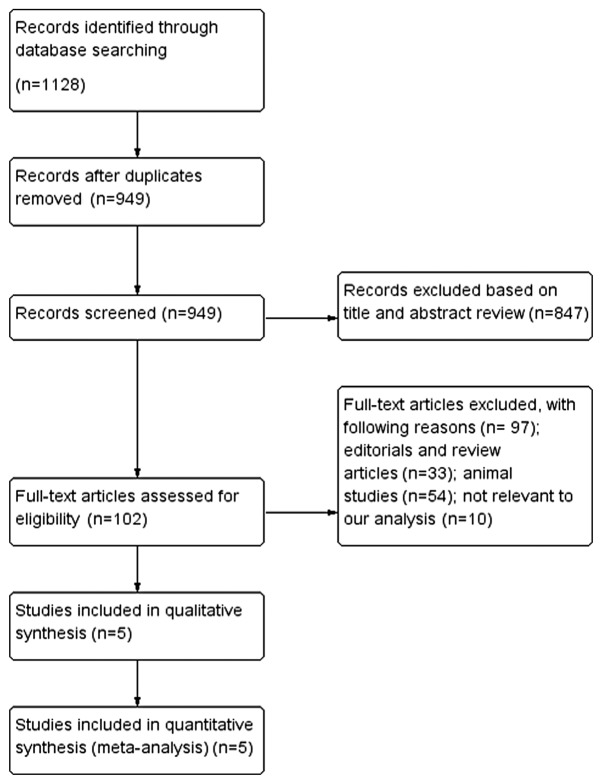
Initially, 1,131 articles were found to be eligible according to the inclusion criteria applied to the search strategy. After removal of duplicates, 182 trials were excluded. A total of 102 trials remained after a preliminary screen, and 97 trials were excluded for the reasons listed in Fig. 1, which included editorials and review articles (n=33), animal studies (n=54) and studies not relevant to the present analysis (n=10). Five RCTs met the inclusion criteria and were subjected to further analysis (15,18,24–26).
Figure 1.
Flow chart of the meta-analysis.
Characteristics of studies
There was no obvious difference in age, gender or any other basic information among all of the included trials. There were 183 patients in total, including 94 in the experimental group and 89 in the control group. Table I illustrates the characteristics of patients in the five studies (15,18,24–26), including basic information, intervention strategies, PEEP, oxygenation index and diagnostic criteria Table II presents the results of the quality evaluation. The studies by Bernard et al (24) and Domenighetti et al (25) were considered to be of high quality and the other three were of low quality. As <10 articles were included, publication bias was estimated using Begg's funnel-plot method. As presented in Fig. 2, no significant publication bias was found among those articles according to Begg's test (P=0.086).
Table I.
Characteristics of included studies.
| Author, year | Group, n | Intervention | Males, n (%) | Age (years) | PEEP (cmH2O) | OI (mmHg) | Main inclusion criteria | (Refs.) |
|---|---|---|---|---|---|---|---|---|
| Bernard, 1997 | E, 14 | NAC 210 mg/kg/day i.v. × 10 days | – | 43±6 | 10±1 | 176±23 | Mechanical Ventilation, bilateral CXR infiltrates, OI≤200 mmHg or 225 mmHg if PEEP>10 cmH2O, duration of ARDS <24 h | (24) |
| C, 15 | Placebo | – | 47±4 | 12±2 | 159±19 | |||
| Domenighetti, 1997 | E, 22 | NAC 190 mg/kg/day i.v. × 3 days | 19 (86.4) | 52.1±17.8 | 5.6±3 | 140±49 | AECC definition for ARDS | (25) |
| C, 20 | Placebo | 14 (70.0) | 52.4±17 | 5.4±3 | 133±37 | |||
| Moradi, 2009 | E, 14 | NAC 150 mg/kg bolus, 50 mg/kg/day i.v. × 3 days | 9 (63.4) | 48.4±5.5 | – | 194.5±40.5 | AECC definition for ARDS | (18) |
| C, 13 | 5% dextrose | 8 (61.5) | 49.2±4.5 | – | 139.1±15.3 | |||
| Ortolani, 2000 | E, 12 | NAC 150 mg/kg/day i. v. × 9 days | 6 (50.0) | 57±14 | 10±2 | 168±18 | Mechanical ventilation, bilateral CXR infiltrates, OI≤200 mmHg or 225 mmHg if PEEP>10 cmH2O, duration of ARDS <24 h | (15) |
| C, 12 | 5% dextrose | 7 (58.3) | 55±13 | 12±3 | 160±21 | |||
| Suter, 1994 | E, 32 | NAC 40 mg/kg/day i.v. × 9 days | 24 (75.0) | 46.6±19.7 | – | 255±113 | Risk factor and mild moderate ALI (LIS 0.1–2.5) | (26) |
| C, 29 | Placebo | 23 (79.3) | 48.1±21.9 | – | 248±99 |
E, experimental group; C, control group; NAC, N-acetylcysteine; i.v., intravenously; PEEP, positive end-expiratory pressure; OI, oxygenation index; AECC, American-European Consensus conference; CXR, chest radiograph; ARDS, acute respiratory distress syndrome; LIS, lung injury score.
Table II.
Quality assessment of included studies.
| Author, year | Generation of allocation sequence | Allocation concealment | Blindness | Withdrawal and drop-out | Jadad scorea | (Refs.) |
|---|---|---|---|---|---|---|
| Bernard, 1997 | 2 | 2 | 2 | 1 | 7 | (24) |
| Domenighetti, 1997 | 1 | 1 | 2 | 1 | 5 | (25) |
| Moradi, 2009 | 1 | 1 | 0 | 1 | 3 | (18) |
| Ortolani, 2000 | 1 | 1 | 0 | 1 | 3 | (15) |
| Suter, 1994 | 1 | 1 | 2 | 1 | 5 | (26) |
The Jadad scale score ranges from 1 to 7; a higher score indicates better a quality of a randomized controlled trial.
Figure 2.
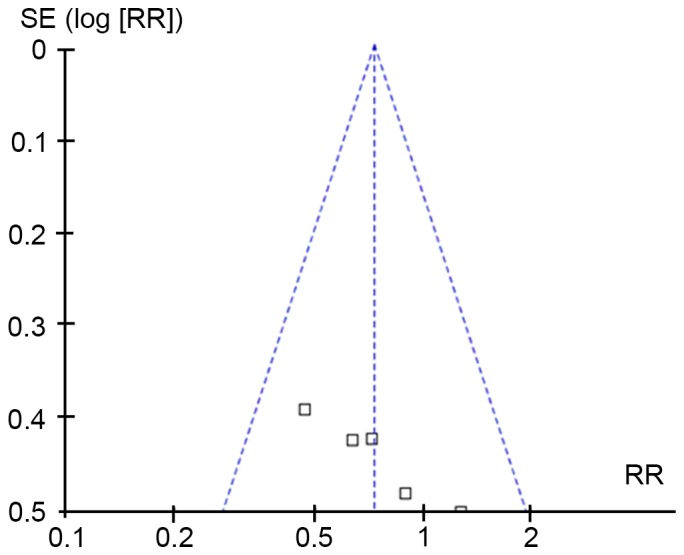
Publication bias evaluated by Begg's funnel plot. Each empty spot represents one publication. RR, relative risk; SE, standard error.
Mortality
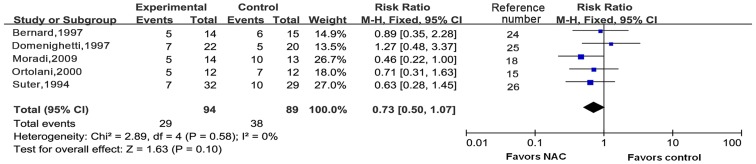
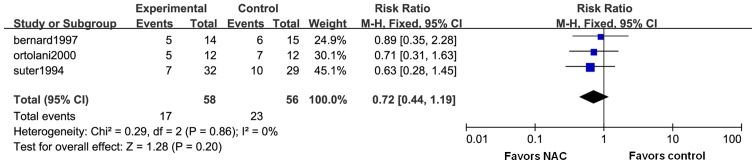
All of the five trials reported on short-term mortality (Fig. 3). As there was no significant heterogeneity (I2=0%; P=0.58), the fixed-effects model was used to merge the data. After pooling of the results from all RCTs, the meta-analysis indicated that NAC did not reduce short-term mortality (RR=0.73; 95% CI: 0.50–1.07; P=0.10). Three of the trials contained data on 30-day mortality (Fig. 4). The fixed-effects model was used according to the test of heterogeneity (I2=0%; P=0.86). The meta-analysis revealed that additional use of NAC did not contribute to reduce 30-day mortality as compared with conventional therapy (RR=0.72; 95% CI: 0.44–1.19; P=0.20).
Figure 3.
Forest plots for short-term mortality. M-H, Mantel-Haetszel; df, degrees of freedom; CI, confidence interval; NAC, N-acetylcysteine.
Figure 4.
Forest plots for 30-day mortality. M-H, Mantel-Haetszel; df, degrees of freedom; CI, confidence interval; NAC, N-acetylcysteine.
Duration of ICU stay
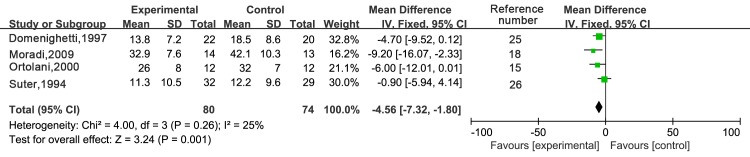
Four trials reported on the duration of ICU stay (Fig. 5). The fixed-effects model was used according to the test of heterogeneity (I2=25%; P=0.26). The meta-analysis revealed that NAC treatment significantly shortened the duration of ICU stay when compared with that in the control treatment group [WMD, −4.56; 95% CI: (−7.32 to −1.80); P=0.001].
Figure 5.
Forest plots for duration of Intensive Care Unit stay. IV, inverse variance; df, degrees of freedom; CI, confidence interval; NAC, N-acetylcysteine; SD, standard deviation.
Oxygenation
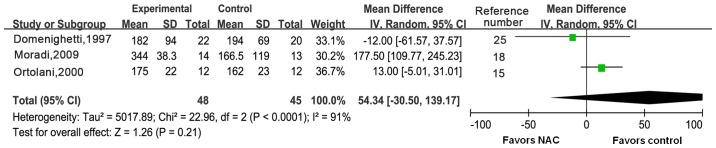
Three trials reported on changes in oxygenation in ARDS patients. There was obvious heterogeneity between the three trials (I2=91%, P<0.0001), so a random-effects model was used (Fig. 6). There was no significant difference in the ratio of PaO2/FiO2 between the two groups [WMD, 54.34; 95% CI: (−30.50 to 139.17); P=0.21]. The heterogeneity may have been caused by inconsistent intervention measures and different stages of disease. As the number of included trials was small, no subgroup analysis was performed.
Figure 6.
Forest plots for ratio of arterial partial pressure of oxygen/fraction of inspired oxygen using the random-effects model. IV, inverse variance; df, degrees of freedom; CI, confidence interval; NAC, N-acetylcysteine; SD, standard deviation.
Adverse events
All of the five trials reported that no adverse events were caused by the study medication.
Discussion
The principal finding of the present meta-analysis was that the clinical benefits of NAC for ARDS are limited. The application of NAC did not significantly reduce short-term mortality, 30-day mortality or the PaO2/FiO2 ratio. However, analysis of the pooled data indicated that NAC reduced the duration of ICU stay. It is worth mentioning that the study by Moradi et al (18) was of low quality according to the Jadad score. Therefore, this single study may have caused bias for the pooled effect. Generally, the present data did not support the effectiveness of NAC treatment. A large clinical trial on 1,223 critically ill adult patients also reported a negative effect of anti-oxidant supplementation (27). Caution is therefore warranted in using anti-oxidants for ARDS treatment. One optimistic result is that no adverse events were reported in all of the trials, which means that NAC is at least safe for use.
Compared with other reviews, the present study added more detail on the effects of NAC. The Cochrane meta-analysis from 2004 only summarized the impact of NAC on early mortality of ARDS (17), while the Cochrane meta-analysis from 2012 analyzed ARDS patients together with other sepsis patients (16). The present study focused on ARDS only and performed a literature search. Compared with the Cochrane meta-analysis from 2004, one additional study by Moradi et al (18) was added and the pooled results to provide more detail regarding the effects of NAC. Compared with the Cochrane meta-analysis from 2012, ARDS patients were separately analyzed.
The definitions of ARDS has been revised several times. The newest Berlin definition was made in 2012 (2). In the studies included in the present meta-analysis, the study by Suter et al (26) used a four-point lung-injury scoring (LIS) system proposed in 1988 and the others basically used the definition of the American-European Consensus Conference (AECC) in 1994. The criteria and advantages of these definitions were previously reviewed (28). Although different definitions were used, these diagnostic criteria have continuity and comparability. The LIS system and the AECC definition have similar sensitivity, while the specificity of LIS is higher than that of the AECC definition (29). The Berlin definition suggests that different measures are taken based on the severity of the disease, as patients with severe ARDS may have a greater benefit from rescue therapies (30). In addition, the inflammatory response profiles changed at different disease stages and the anti-oxidants were likely to interfere with patient's immune status (13,31). Thus, it may be more complicated than originally thought to fully evaluate the efficacy of anti-oxidants such as NAC. Perhaps the efficacy of NAC is only significant in certain patient subgroups. At present, there are very few well-designed trials associated with this topic. In the present study, only five RCT studies comprising 183 patients were included and the statistical power may therefore have been impacted. Furthermore, none of the RCTs discussed the association between NAC efficacy and disease severity. In the future, studies with large samples and rigorous design, including short-term as well as long-term outcomes as end-points, may help to improve the quality of evidence. However, it is worth mentioning that no more novel RCTs are available since the study by Moradi et al (18) from 2009. This may be due to the continuous concerns regarding the efficacy and safety of the drug. Therefore, future RCTs with a large sample size are less likely to be performed.
Acknowledgements
The authors would like to thank Dr Wenli Lu and Dr Yuan Wang (Department of Health Statistics, School of Public Health, Tianjin Medical University) for their help and advice regarding the preparation of the manuscript.
References
- 1.Vincent JL, Sakr Y, Ranieri VM. Epidemiology and outcome of acute respiratory failure in intensive care unit patients. Crit Care Med. 2003;31:S296–S299. doi: 10.1097/01.CCM.0000057906.89552.8F. (4 Suppl) [DOI] [PubMed] [Google Scholar]
- 2.ARDS Definition Task Force, corp-author. Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS. Acute respiratory distress syndrome: The Berlin definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 3.Ashbaugh DG, Bigelow DB, Petty TL, Levine BE. Acute respiratory distress in adults. Lancet. 1967;2:319–323. doi: 10.1016/S0140-6736(67)90168-7. [DOI] [PubMed] [Google Scholar]
- 4.Phua J, Badia JR, Adhikari NK, Friedrich JO, Fowler RA, Singh JM, Scales DC, Stather DR, Li A, Jones A, et al. Has mortality from acute respiratory distress syndrome decreased over time?: A systematic review. Am J Respir Crit Care Med. 2009;179:220–227. doi: 10.1164/rccm.200805-722OC. [DOI] [PubMed] [Google Scholar]
- 5.Herridge MS, Tansey CM, Matté A, Tomlinson G, Diaz-Granados N, Cooper A, Guest CB, Mazer CD, Mehta S, Stewart TE, et al. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364:1293–1304. doi: 10.1056/NEJMoa1011802. [DOI] [PubMed] [Google Scholar]
- 6.Neamu RF, Martin GS. Fluid management in acute respiratory distress syndrome. Curr Opin Crit Care. 2013;19:24–30. doi: 10.1097/MCC.0b013e32835c285b. [DOI] [PubMed] [Google Scholar]
- 7.Piquilloud L, Tassaux D, Bialais E, Lambermont B, Sottiaux T, Roeseler J, Laterre PF, Jolliet P, Revelly JP. Neurally adjusted ventilatory assist (NAVA) improves patient-ventilator interaction during non-invasive ventilation delivered by face mask. Intensive Care Med. 2012;38:1624–1631. doi: 10.1007/s00134-012-2626-9. [DOI] [PubMed] [Google Scholar]
- 8.Antonelli M, Conti G, Esquinas A, Montini L, Maggiore SM, Bello G, Rocco M, Maviglia R, Pennisi MA, Gonzalez-Diaz G, Meduri GU. A multiple-center survey on the use in clinical practice of noninvasive ventilation as a first-line intervention for acute respiratory distress syndrome. Crit Care Med. 2007;35:18–25. doi: 10.1097/01.CCM.0000251821.44259.F3. [DOI] [PubMed] [Google Scholar]
- 9.Sinha P, Flower O, Soni N. Deadspace ventilation: A waste of breath! Intensive Care Med. 2011;37:735–746. doi: 10.1007/s00134-011-2194-4. [DOI] [PubMed] [Google Scholar]
- 10.Chen W, Chen YY, Tsai CF, Chen SC, Lin MS, Ware LB, Chen CM. Incidence and outcomes of acute respiratory distress syndrome: A nationwide registry-based study in Taiwan, 1997 to 2011. Medicine (Baltimore) 2015;94:e1849. doi: 10.1097/MD.0000000000001849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rubenfeld GD, Herridge MS. Epidemiology and outcomes of acute lung injury. Chest. 2007;131:554–562. doi: 10.1378/chest.06-1976. [DOI] [PubMed] [Google Scholar]
- 12.Park HS, Kim SR, Lee YC. Impact of oxidative stress on lung diseases. Respirology. 2009;14:27–38. doi: 10.1111/j.1440-1843.2008.01447.x. [DOI] [PubMed] [Google Scholar]
- 13.Jain M, Chandel NS. Rethinking antioxidants in the intensive care unit. Am J Respir Crit Care Med. 2013;188:1283–1285. doi: 10.1164/rccm.201307-1380CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rank N, Michel C, Haertel C, Lenhart A, Welte M, Meier-Hellmann A, Spies C. N-acetylcysteine increases liver blood flow and improves liver function in septic shock patients: Results of a prospective, randomized, double-blind study. Crit Care Med. 2000;28:3799–3807. doi: 10.1097/00003246-200012000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Ortolani O, Conti A, De Gaudio AR, Masoni M, Novelli G. Protective effects of N-acetylcysteine and rutin on the lipid peroxidation of the lung epithelium during the adult respiratory distress syndrome. Shock. 2000;13:14–18. doi: 10.1097/00024382-200013010-00003. [DOI] [PubMed] [Google Scholar]
- 16.Szakmany T, Hauser B, Radermacher P. N-acetylcysteine for sepsis and systemic inflammatory response in adults. Cochrane Database Syst Rev: CD006616. 2012 doi: 10.1002/14651858.CD006616.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adhikari N, Burns KE, Meade MO. Pharmacologic therapies for adults with acute lung injury and acute respiratory distress syndrome. Cochrane Database Syst Rev: CD004477. 2004 doi: 10.1002/14651858.CD004477.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moradi M, Mojtahedzadeh M, Mandegari A, Soltan-Sharigi MS, Najafi A, Khajavi MR, Hajibabayee M, Ghahremani MH. The role of glutathione-S-transferase polymorphisms on clinical outcome of ALI/ARDS patient treated with N-acetylcysteine. Respir Med. 2009;103:434–441. doi: 10.1016/j.rmed.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 19.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 20.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 21.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 22.Montori V, Guyatt G, Oxman A, Cook D. Summarizing the evidence: Fixed-effects and random-effects models. In: Guyatt G, Rennie D, editors. Users' guides to the medical literature: a manual for evidence-based clinical practice. AMA Press; Chicago, IL: 2002. pp. 539–546. [Google Scholar]
- 23.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bernard GR, Wheeler AP, Arons MM, Morris PE, Paz HL, Russell JA, Wright PE. A trial of antioxidants N-acetylcysteine and procysteine in ARDS. The Antioxidant in ARDS Study Group. Chest. 1997;112:164–172. doi: 10.1378/chest.112.1.164. [DOI] [PubMed] [Google Scholar]
- 25.Domenighetti G, Suter PM, Schaller MD, Rits R, Perret C. Treatment with N-acetylcysteine during acute respiratory distress syndrome: A randomized, double-blind, placebo-controlled clinical study. J Crit Care. 1997;12:177–182. doi: 10.1016/S0883-9441(97)90029-0. [DOI] [PubMed] [Google Scholar]
- 26.Suter PM, Domenighetti G, Schaller MD, Laverrière MC, Rits R, Perret C. N-acetylcysteine enhances recovery from acute lung injury in man. A randomized, double-blind, placebo-controlled clinical study. Chest. 1994;105:190–194. doi: 10.1378/chest.105.1.190. [DOI] [PubMed] [Google Scholar]
- 27.Heyland D, Muscedere J, Wischmeyer PE, Cook D, Jones G, Albert M, Elke G, Berger MM, Day AG. Canadian Critical Care Trials Group: A randomized trial of glutamine and antioxidants in critically ill patients. N Engl J Med. 2013;368:1489–1497. doi: 10.1056/NEJMoa1212722. [DOI] [PubMed] [Google Scholar]
- 28.Ware LB, Matthay MA. The acute respiratory distress syndrome. New Engl J Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 29.de Hemptinne Q, Remmelink M, Brimioulle S, Salmon I, Vincent JL. ARDS: A clinicopathological confrontation. Chest. 2009;135:944–949. doi: 10.1378/chest.08-1741. [DOI] [PubMed] [Google Scholar]
- 30.Ferguson ND, Fan E, Camporota L, Antonelli M, Anzueto A, Beale R, Brochard L, Brower R, Esteban A, Gattinoni L, et al. The Berlin definition of ARDS: An expanded rationale, justification, and supplementary material. Intensive Care Med. 2012;38:1573–1582. doi: 10.1007/s00134-012-2698-6. [DOI] [PubMed] [Google Scholar]
- 31.Hotchkiss RS, Monneret G, Payen D. Immunosuppression in sepsis: A novel understanding of the disorder and a new therapeutic approach. Lancet Infect Dis. 2013;13:260–268. doi: 10.1016/S1473-3099(13)70001-X. [DOI] [PMC free article] [PubMed] [Google Scholar]