Abstract
We report on biochemical pathways perturbed upon chronic fluoxetine administration to juvenile macaques using global metabolomics analyses of fibroblasts derived from skin biopsies. After exposure to tissue culture conditions confounding environmental factors are eliminated and identification of metabolites whose levels are affected by the drug become apparent with a better signal-to-noise ratio compared to data obtained from plasma and cerebrospinal fluid (CSF). Levels of more than 200 metabolites were analyzed to interrogate affected molecular pathways and identify biomarkers of drug response. In addition, we have correlated the metabolomics results with monoamine oxidase (MAOA) genotype and impulsivity behavioral data. Affected pathways include Purine and Pyrimidine metabolisms that have been previously implicated to contribute to neuropsychiatric disorders.
Keywords: Fluoxetine, Neurodevelopmental, disorders, Impulsivity, Metabolomics, Fibroblasts, Biomarkers
1. Introduction
Research on the molecular mechanisms that govern higher brain function, disease and drug action in the CNS requires non-human primates (NHP) which exhibit similar brain function and structure as humans. The long postnatal period during which the primate brain matures provides an appropriate animal model for investigating juvenile brain development and its response to drugs. Juvenile macaque monkeys, the most common laboratory NHP, are becoming a preferred model for studying short- and long-term effects of psychoactive agents used in children (Popke et al. 2001; Patterson et al., 2010; Rodriguez et al., 2010; Soto et al., 2012; Shrestha et al., 2014; Golub et al., 2015).
Several clinical studies have found a wide individual variability in children’s response to fluoxetine and other psychopharmacologic agents. These drugs are commonly prescribed for a variety of childhood diseases with neurobehavioral symptoms including major depressive disorder (MDD) and autism spectrum disorders (ASD) (Henry et al., 2012; Geller et al., 2001; Birmaher et al., 2003; Nilsson et al., 2004; Hollander et al., 2005; Hetrick et al., 2007, 2010; Quintana et al., 2007; Strawn et al., 2015). As in other areas of medicine the prediction of response to treatment in childhood pharmacotherapy is critical for an optimal therapeutic response with minimal adverse side effects. In the current study we have attempted to address this issue by examining peripheral metabolome biosignatures in response to fluoxetine administration. In other studies focused on the molecular pathology of mental disorders cultured fibroblast cells obtained from patient skin biopsies have already been successfully used (Gassó et al., 2014; Kálmán et al., 2014). Following up on a previous study where we analyzed plasma and cerebrospinal fluid (CSF) specimens, the focus of the current investigation was fibroblasts obtained from rhesus monkey’s skin biopsies as a novel source for the identification of metabolomic markers and perturbed molecular pathways associated with juvenile fluoxetine administration.
Blood and CSF are the most common sample types for biomarker development for psychoactive agents since they are readily available and used in the clinical laboratory. However, both fluids contain metabolites that have been produced and processed in multiple tissues and do not reflect the response of primary cellular targets of the drug. Hence they are prone to reflecting many life style and environmental influences on the metabolome.
We hypothesized that brain metabolic pathways affected by fluoxetine treatment will also reveal themselves through metabolomics analyses in other cells of the body. Skin fibroblasts can be retrieved by punch biopsy, which is a less invasive procedure and carries a much lower risk of infection compared to lumbar puncture. After replication, fibroblast cells established in tissue culture are removed from other environmental influences that are confounding metabolomics analyses in blood and CSF. We therefore reasoned that drug primary induced pathway activities that persists in cellular metabolism are reflected more accurately in cultured fibroblasts than in blood and CSF body fluids. In addition, metabolite signature variations in the fibroblasts may also serve as candidates for predicting individual differences in therapeutic response by associating them with behavioral phenotypes of the rhesus monkeys. We report on our metabolomics analyses of fibroblasts from skin biopsies and compare the results with those obtained from CSF and plasma. Our data implicate Purine metabolism to be affected in response to long-term fluoxetine administration and to correlate with impulsive behavior of the rhesus monkeys. Purinergic signaling and mitochondrial energy homeostasis have been implicated in psychiatric disorder pathology. Our results support the notion that these molecular pathways are also relevant in current psychopharmacology (Lindberg et al., 2015).
2. Experimental procedures
2.1. Assurance of compliance with animal codes
All procedures followed the Guide for the Care and Use of Laboratory Animals of the National Research Council. The California National Primate Research Center (CNPRC) is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care. Protocols for this project were approved prior to implementation by the UC Davis Institutional Animal Care and Use Committee.
2.2. Animals and animal care
The cohort of male rhesus monkeys (Macaca mulatto) that were sampled for this study has been described previously (He et al., 2014; Golub et al., 2015). At approximately 1 year of age animals were selected from the outdoor colony at the CNPRC, relocated to indoor caging, and assigned to treatment (fluoxetine) or control (vehicle) groups. There were two transfer groups relocated two weeks apart based on birth dates and sampling at necropsy for metabolomics studies was conducted separately on the two groups after one-year of dosing. The impulsivity test reported here was also conducted after one-year of dosing. Dosing continued for another year, followed by a one-year post dosing evaluation, sacrifice and necropsy.
In addition to fluoxetine dosing, genetic polymorphisms of MAOA, which affect serotonin mediated brain functions, were identified by genotyping and included as a variable in the study. The entire cohort was housed in the same cage room in double cages that allowed socialization in pairs. All subjects received identical, standardized husbandry and enrichment according to CNPRC protocols. Animal health was evaluated daily. No conditions resulting in veterinary diagnosis were reported, with the exception of episodes of diarrhea treated with Tylosin®. Linear and ponderal growth were measured at intervals throughout the study without indication of fluoxetine effects during the dosing period (Supplementary Table 1). Based on experience, cage location variables and transfer group were screened as potential covariates in data analyses.
2.3. Fluoxetine dosing
Fluoxetine dose selection was based on information in the human and non-human primate literature and on a preliminary pharmacokinetic/pharmacodynamic study to provide steady-state circulating levels of fluoxetine/norfluoxetine in the range reported for therapeutic use of fluoxetine in children (Golub and Hogrefe, 2014). Dosing was initiated at one-year of age at 1.6mg/kg/day and adjusted to 2.4 mg/kg/day after 11 months, one month before the one-year sampling reported here. The initial dose for the present study was set lower (1.6 mg/kg) while the monkeys were being adapted to indoor housing, daily dosing and behavioral testing environments. At the end of 11 months, plasma fluoxetine was analyzed and the dose was reset to 2.4 mg/kg to obtain the desired plasma levels. Liquid fluoxetine (20 mg/5 mL, Webster Veterinary) was prepared for oral dosing by dilution in flavored commercial flavoring syrup (Torani®). Monkeys were trained to come to the front of the cage and receive the dose from the end of a 6 mL syringe which was inserted into the cage. The vehicle administration consisted of fluid with the same taste and volume.
2.4. Impulsivity test
To compare fluoxetine’s metabolic profile and behavioral effects we used a reward delay test adapted for monkeys as described previously (He et al., 2014) from similar tests in children to measure impulsivity (Golub et al., 2005, 2007). The test was hand administered in the Wisconsin General Test Apparatus (WGTA) in one session of 40 trials. To obtain the reward on each trial, the monkey had to withhold responding while a screen was moved back 2.54 cm every 2 s for 7 screen intervals until the food reward was fully disclosed. At interval 7 the box covering the reward was fully revealed. It was at this point that the animal was able to displace the box and retrieve the reward. The trial length was up to 15 intervals. The number of intervals was recorded when the animal attempted to displace the box. We defined an impulsive response if the recorded interval was smaller than 7. The apical endpoint for analysis was the impulsive rate, defined as percentage of trials with responses smaller than 7 intervals. Testing was blinded and randomized for group.
2.5. Blood and CSF sampling
Venous blood samples for plasma extraction were collected under ketamine anesthesia (10 mg/kg i.m.) after an overnight fast. The anesthesia was then supplemented with dexmedetomidine (0.0075–0.015 mg/kg, i.m.) for CSF collection (0.5–1 mL), which was performed via the drip method from the cisterna magna under sterile conditions.
2.6. Fibroblast sampling and culture
Skin explants were collected at the same time as blood and CSF samples. A 6 mm punch biopsy was taken from the lower back and submerged immediately in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% Fetal Bovine Serum (FBS), L-glutamine (2 mM/L), 0.001X Gentamicin and Amino-Max™ in a petri dish. The biopsy was then washed with Hanks solution and transferred to a new petri dish and covered with media. The skin was cut into 2 × 3 mm pieces with a scalpel and placed in wells with a coverslip on top. Silicon grease was put on the four corners and the coverslip pressed down firmly to minimize space between coverslip and plate. Two ml DMEM was put into each well and the 6-well plate incubated in 5% CO2 at 37°C. After 2–3 weeks when fibroblasts were confluent media was aspirated and cells briefly washed with trypsin EDTA. The coverslip was removed, 1 ml trypsin EDTA added and incubated for 5–20 min until the monolayer was disrupted. One ml media was added to stop trypsinization and remaining tissue removed and disposed of. Cells were split 1:5 to 1:20 into T75 and T175 filter cap flasks or plates and fed with DMEM in 5% CO2 at 37°C. Media was exchanged every 4–7 days and cells split when confluent. When sufficient cells were cultured they were washed twice with Hanks solution and counted. After the last wash, 1–5 million cells were resuspended in Hanks solution. The cell suspension was then spun for 1 min in a microfuge at 10,000 RPM. After centrifugation the supernatant was poured off, excess liquid removed and cells stored at −80°C.
2.7. Metabolite extraction
Fibroblasts. The cell pellet (107 cells) was resuspended in 0.5 ml of −20°C p.a. methanol/milliQ grade water (80:20) followed by flash freezing in liquid nitrogen. After thawing on ice, samples were vortexed for 30 s, centrifuged at 800×g at 4°C and the supernatant removed. The pellet was then resuspended in 0.5 ml of 4°C p.a. methanol/milliQ grade water (80:20) and the extraction repeated. The extracts were pooled, centrifuged at 15,000g for 1 min at 4°C, the supernatant removed, Speedvac dried and stored at −20°C.
Plasma and CSF. 100 μl frozen plasma or CSF were thawed on ice, 400 μl −20°C p.a. methanol/milliQ grade water (80:20) added and mixed by vortexing for 2 min. After keeping the samples on ice for 2 h the protein precipitate was centrifuged at 2053g for 10 min at 4°C. Centrifugation was repeated to remove remaining particles at 15,000g for 1 min at 4°C. Supernatants were Speedvac dried and the metabolite extracts stored at −20°C.
2.8. Targeted metabolomics analysis
Samples were dissolved in 20 μl liquid chromatography-mass spectrometry grade water. Ten microliters were injected and analyzed using a 5500 QTRAP triple quadrupole mass spectrometer (AB/ SCIEX, Framingham, MA, USA) coupled to a Prominence UFLC high-performance liquid chromatography system (Shimadzu, Columbia, MD, USA) via selected reaction monitoring of a total of 280 endogenous water-soluble metabolites for steady-state analyses of samples. Samples were delivered to the mass spectrometer via normal phase chromatography using a 4.6 mm i.d. × 10 cm Amide Xbridge HILIC column (Waters, Milford, MA, USA) at 350 μl min−1. Gradients were run starting from 85% buffer B (high-performance liquid chromatography grade acetonitrile) to 42% B from 0 to 5 min; 42%–0% B from 5 to 16 min; 0% B was held from 16 to 24 min; 0%–85% B from 24 to 25 min; 85% B was held for 7 min to re-equilibrate the column. Buffer A comprised 20 mM ammonium hydroxide/20 mM ammonium acetate (pH=9.0) in 95:5 water:acetonitrile. Some metabolites were targeted in both positive and negative ion modes for a total of 291 selected reaction monitoring transitions using positive/negative polarity switching. Electrospray ionization voltage was +4900 V in positive ion mode and −4500 V in negative ion mode. The dwell time was 4 ms per selected reaction monitoring transition and the total cycle time was 1.89 s. Approximately 9–12 data points were acquired per detected metabolite. Peak areas from the total ion current for each metabolite-selected reaction monitoring transition were integrated using the MultiQuant v2.0 software (AB/SCIEX). Animals from the same cohort were used for all metabolomics analyses.
2.9. Data processing and normalization
To remove the bias from sample, all metabolite intensities from each sample were normalized by the median such that all samples have the same median intensity. We removed the metabolites with at least 60% missing values. The remaining missing values were then estimated using k nearest neighbors (KNN). We further filtered out non-informative metabolites. The filtering was performed by variance, with the 5% metabolites with lowest variance being filtered out. Finally, we applied log transformation to scale metabolite intensities. After removing missing and non-informative metabolites, 196, 205, and 235 metabolites remained for analysis in CSF, plasma and fibroblast datasets, respectively.
2.10. Statistical analysis
Statistical analyses were performed using R3.0.2 (http://www.r-project.org/). Metabolite intensities were analyzed by two-way ANCOVA testing the effect of treatment, MAOA genotype and the treatment*genotype interaction. Transfer group was identified as a covariate for fibroblast culture results, and was added to the ANCOVA model covariate. When interactions were not significant, the model was reduced to a one-way ANCOVA, where treatment and transfer group variables were kept in the model. For behavior data, we calculated percentage of impulsive responses for each monkey over 40 trials, denoted as impulsive response rate and used Beta regression, with metabolite intensity as independent variable and the impulsive response rate as dependent variable. Pathway enrich ment analyses were conducted using MetaboAnalyst (http://www.metaboanalyst.ca). All metabolites of pathways in the database were used as background reference when performing hypergeo metric tests. Statistical significance was defined as p<0.05.
2. Results
2.1. Metabolite profiling in response to chronic fluoxetine treatment
In the current study we have focused on metabolite profiling of fibroblast cultures established from skin biopsies of fluoxetine-and vehicle-treated monkeys. Fibroblast metabolite profiles were then compared to plasma and CSF results to find out to what extent they are reflected in peripheral body fluids. Fibroblast culture extracts were analyzed for 235 metabolites with known identities. Fifteen fibroblast metabolites showed significant intensity level differences (p<0.05) between control and fluoxetine groups (Table 1). Of those, 11 metabolites displayed significant interactions between fluoxetine and MAOA genotype, indicating that treatment changes for these metabolites are affected by the genotype. Post hoc comparisons showed that Glycerol 3-phosphate, Allantoin, Orotic acid, Allantoic acid, Oxoglutaric acid and D-Phenyllactic acid were up-regulated as a result of fluoxetine treatment in the hi-MAOA group with fold changes 1.7, 1.9, 2.0, 2.2, 2.2 and 2.5, respectively (Figure 1), and exhibit no treatment changes in low-MAOA group, whereas 2-Isopropylmalic acid and Glucosamine were down-regulated in hi-MAOA group with fold changes 0.3 and 0.5, respectively and up-regulated in low-MAOA group with fold changes 2.3 and 4.1, respectively (Figure 2).
Table 1.
Metabolites with significant intensity level differences (<0.05) between control (n = 8) and fluoxetine (n = 8) groups in fibroblasts.
| Metabolite | P-value | FC | FC-hiMAOA | FC-low-MAOA |
|---|---|---|---|---|
| AICAR | 0.0449# | 0.69 | – | – |
| Allantoic acid | 0.026 | – | 2.21* | 0.59 |
| Allantoin | 0.0062 | – | 1.87* | 0.85 |
| Cytidine | 0.0256# | 0.71 | – | – |
| Cytosine | 0.0148# | 0.88 | – | – |
| Glucosamine | 0.0341 | – | 0.48* | 4.08* |
| Glycerol 3-phosphate | 0.0057 | – | 1.70* | 0.76 |
| Guanosine triphosphate | 0.0406 | – | 1.52 | 0.36 |
| Hypoxanthine | 0.027 | – | 1.76 | 0.95 |
| 2-Isopropylmalic acid | 0.0101 | – | 0.35* | 2.26* |
| 3-Methylphenylacetic acid | 0.0497 | – | 0.49* | 1.6 |
| Nicotinic acid | 0.0479# | 1.24 | – | – |
| Orotic acid | 0.0131 | – | 1.99* | 0.7 |
| Oxoglutaric acid | 0.0292 | – | 2.15* | 0.83 |
| D-Phenyllactic acid | 0.0375 | – | 2.50* | 0.88 |
FC: Fold change metabolite level of fluoxetine-treated relative to untreated control.
No interaction between treatment and genotype (one-way ANCOVA).
No overlap in standard error (SE) bar of fluoxetine and control in Post hoc comparisons.
Fig. 1.
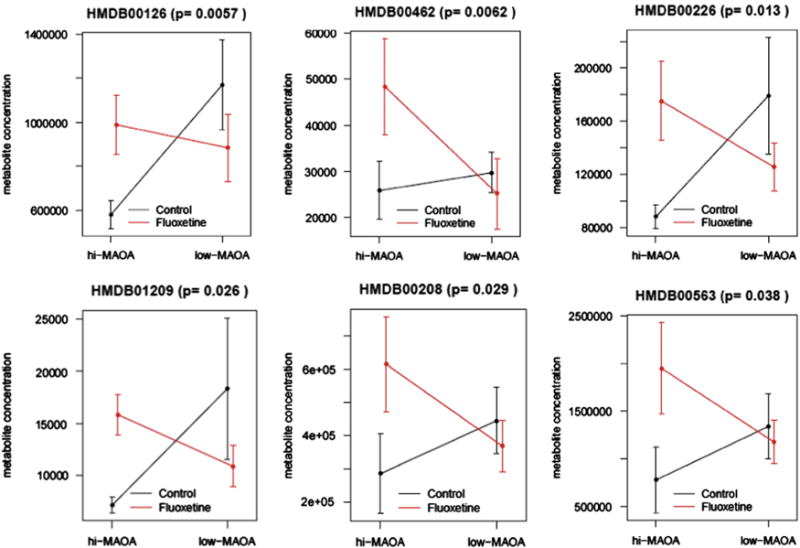
Post hoc comparisons of Glycerol 3-phosphate (HMDB00126), Allantoin (HMDB00426), Orotic acid (HMDB00226), Allantoic acid (HMDB01209), Oxoglutaric acid (HMDB00208) and D-Phenyllactic acid (HMDB00563). Data are mean ± SEM (n = 8 controls, n=8 fluoxetine).
Fig. 2.
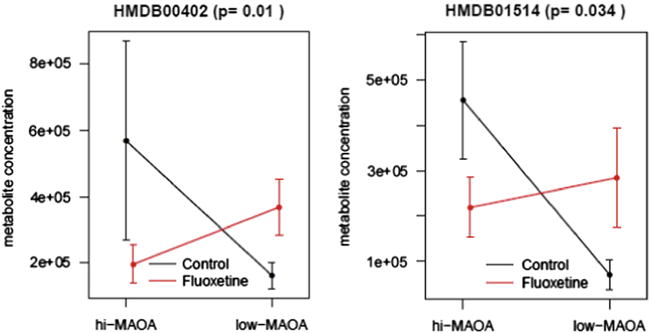
Post hoc comparisons of 2-Isopropylmalic acid (HMDB00402) and Glucosamine (HMDB01514). Data are mean ± SEM (n=8 controls, n=8 fluoxetine).
When compared with peripheral fluids we found that levels of 16 and 26 metabolites changed in CSF and plasma, respectively from fluoxetine treated animals (Tables 2 and 3). Only one metabolite, Cytidine, was shared between plasma and fibroblast data sets, and no common metabolite differences were found between CSF and fibroblasts.
Table 2.
Metabolites with significant intensity level differences (<0.05) between control (n = 8) and fluoxetine (n = 8) groups in CSF.
| Metabolite | P-value | FC | FC-hiMAOA | FC-low-MAOA |
|---|---|---|---|---|
| N-Acetyl-D-glucosamine | 0.0088# | 1.19 | – | – |
| N1-Acetylspermidine | 0.0471 | – | 2.73 | 0.53 |
| Adenosine | 0.0088 | – | 0.36 | 2.21 |
| Adenosine monophosphate | 0.006 | – | 0.51 | 4.79 |
| Ascorbic acid | 0.024 | – | 0.48 | 1.64 |
| L-Asparagine | 0.0075 | – | 0.77 | 1.38 |
| L-Cystine | 0.0373 | – | 0.29 | 1.53 |
| Glyoxylic acid | 0.0427# | 1.35 | – | – |
| Guanosine | 0.012 | – | 0.83 | 2.94 |
| L-Homocysteic acid | 0.0469 | – | 0.52 | 1.03 |
| Homocysteine | 0.024 | – | 0.46 | 1.35 |
| L-Leucine | 0.0115 | – | 0.79 | 1.33 |
| L-Lysine | 0.0348 | – | 0.74 | 1.33 |
| L-Threonine | 0.0036 | – | 0.59 | 1.29 |
| Pantothenic acid | 0.039 | – | 1.94 | 0.75 |
| Pyruvic acid | 0.0135 | – | 0.95 | 1.44 |
FC: Fold change metabolite level of fluoxetine-treated relative to untreated control.
No interaction between treatment and genotype (one-way ANCOVA).
Table 3.
Metabolites with significant intensity level differences (<0.05) between control (n = 8) and fluoxetine (n = 8) groups in plasma.
| Metabolites | P-value | FC | FC-hiMAOA | FC-low-MAOA |
|---|---|---|---|---|
| Acetoacetic acid | 0.0352# | 0.75 | – | – |
| N-Acetylornithine | 0.0301 | – | 0.52 | 1.65 |
| N-Acetylputrescine | 0.0074 | – | 0.7 | 2.72 |
| O-Acetylserine | 0.0348# | 0.74 | – | – |
| Adenosine triphosphate | 0.0052 | – | 2.94 | 0.43 |
| Aminoadipic acid | 0.0260# | 1.3 | – | – |
| p-Aminobenzoic acid | 0.0016# | 2.35 | – | – |
| Citrulline | 0.0069 | – | 1.62 | 0.69 |
| Cytidine | 0.0386 | – | 0.71 | 1.32 |
| Dimethylglycine | 0.0414 | – | 1.36 | 0.68 |
| Glyceric acid 1,3-biphosphate | 0.0408 | – | 2.07 | 1.03 |
| dGTP | 0.0113 | – | 1.94 | 0.55 |
| 3-Hydroxybutyric acid | 0.0121 | – | 0.7 | 1.84 |
| 4-Hydroxyproline | 0.0125 | – | 2.08 | 0.84 |
| Kynurenic acid | 0.0006# | 1.45 | – | – |
| 3-Methylhistidine | 0.0223 | – | 0.8 | 1.18 |
| Methylmalonic acid | 0.012 | – | 0.67 | 1.21 |
| 3-Phosphoglyceric acid | 0.0035 | – | 3.04 | 0.7 |
| L-Proline | 0.0248 | – | 1.8 | 0.81 |
| Pyrophosphate | 0.0328 | – | 1.81 | 0.66 |
| D-Ribulose 5-phosphate | 0.0009 | – | 2.14 | 0.6 |
| Shikimic acid | 0.037 | – | 3.1 | 0.48 |
| Succinic acid | 0.0293 | – | 0.69 | 1.24 |
| Urea | 0.0003 | – | 1.07 | 1.51 |
| Xanthine | 0.0284# | 1.23 | – | – |
| Xanthosine | 0.0141 | – | 0.76 | 1.27 |
FC: Fold change metabolite level of fluoxetine-treated relative to untreated control.
No interaction between treatment and genotype (one-way ANCOVA was used).
2.2. Affected pathways in response to fluoxetine treatment
To explore the biological function and pathways that were affected by fluoxetine in fibroblasts, we applied pathway enrichment. Three main pathways were affected upon fluoxetine treatment in fibroblasts. They include Purine metabolism, Pyrimidine metabolism and Histidine metabolism (Table 4).
Table 4.
Affected pathways in response to fluoxetine treatment in fibroblasts.
| Pathway | P-value | Significant metabolites |
|---|---|---|
| Purine metabolism | 0.0015 | AICAR, Allantoic acid, Guanosine triphosphate, Hypoxanthine |
| Pyrimidine metabolism | 0.0044 | Cytidine, Orotic acid, Cytosine |
| Histidine metabolism | 0.0259 | Oxoglutaric acid, AICAR |
Purine and Pyrimidine metabolites and their receptors have previously been associated with neuropsychiatric disorders. Specifically, dysfunctional purinergic signaling mechanisms have been implicated in MDD, schizophrenia, bipolar disorder and ASD (Lindberg et al., 2015; Lucae et al., 2006). Children with Purine and Pyrimidine metabolism dysfunction have shown to exhibit neurodevelopmental and behavioral abnormalities related to ASD (Micheli et al., 2011). Furthermore, metabolites that are part of the Purine pathway were shown to be effective therapeutic targets for treating ASD-like symptoms in the maternal immune activation (MIA) mouse model. Weekly treatment with the anti-purinergic drug Suramin was able to ameliorate behavioral, molecular and neuropathological abnormalities in this mouse model (Naviaux et al., 2014).
We identified 9 and 7 significantly enriched pathways for the CSF and plasma datasets, respectively (Table 5). Apart from Purine metabolism and Pyrimidine metabolism, additional pathways were also identified, suggesting that environmental influences impact pathways in the periphery, reflected in blood and CSF metabolite levels. The information of enriched pathways provides a more systematic assessment of the molecular function than the individual metabolites. We found 15 metabolites in fibroblasts whose levels were altered between fluoxetine- and vehicle-treated animals that enriched 3 pathways. On the other hand 16 metabolites in CSF and 27 metabolites in plasma enriched 9 and 7 pathways, respectively. This finding further supports our hypothesis that the interrogation of the fibroblast metabolome more accurately reflects the fluoxetine treatment effect due to removal of environmental confounders in cultured cells.
Table 5.
Affected pathways in response to fluoxetine treatment in CSF and plasma.
| CSF
|
Plasma
|
||
|---|---|---|---|
| Pathway | P-value | Pathway | P-value |
| Valine, leucine and isoleucine biosynthesis | 0.0006 | Arginine and proline metabolism | 0.0001 |
| Aminoacyl-tRNA biosynthesis | 0.0012 | Purine metabolism | 0.0003 |
| Purine metabolism | 0.0026 | Synthesis and degradation of ketone bodies | 0.0016 |
| Glycine, serine and threonine metabolism | 0.0035 | Propanoate metabolism | 0.0058 |
| Alanine, aspartate and glutamate metabolism | 0.0105 | Butanoate metabolism | 0.0085 |
| Pantothenate and CoA biosynthesis | 0.0132 | Caffeine metabolism | 0.0208 |
| Nitrogen metabolism | 0.0266 | Pyrimidine metabolism | 0.0255 |
| Ascorbate and aldarate metabolism | 0.0347 | ||
| Glyoxylate and dicarboxylate metabolism | 0.0422 | ||
2.3. Metabolites associated with impulsivity
Impulsivity is a commonly reported side effect of fluoxetine treatment in children (Lee et al, 2015; Safer, 2011). Impulsive behavior in macaques was previously shown to be a behavioral effect of fluoxetine treatment (He et al., 2014). For correlation with metabolomics data we carried out Beta regression to identify metabolites associated with impulsive behavior in the fluoxetine treated group. In fibroblasts we found that 17 metabolites are associated with the impulsive behavior (Table 6). Specifically, Uridine, a metabolite that is part of Pyrimidine metabolism showed a strong negative correlation with impulsive response rate (p=9.5E–08). Other studies have shown that Uridine has antidepressant-like effects in rodents (Carlezon et al., 2005). Most of the metabolites correlating with impulsivity in fibroblasts did not show an overlap with those found in CSF and plasma (results not shown). Comparing these behavioral effect related metabolites with treatmenteffect related metabolites, we also found no overlaps (Figure 3). This indicates that different mechanisms may be involved in fluoxetine treatment and behavioral effect.
Table 6.
Metabolites showing significant association with animal impulsive behavior (fluoxetine n=8, vehicle n=8).
| Metabolite | Correlation | P-value |
|---|---|---|
| Cis-Aconitic acid | + | 0.0319 |
| S-Adenosylmethiornnamine | − | 0.0272 |
| dCDP | − | 0.0444 |
| Citric acid | + | 0.0017 |
| Deoxyinosine | + | 0.0377 |
| Deoxyribose 5-phosphate | + | 0.0147 |
| Fructose 6-phosphate | − | 0.0095 |
| Glucose 6-phosphate | − | 0.0059 |
| Glutathione | − | 0.0148 |
| Glyoxylic acid | − | 0.0151 |
| Guanidoacetic acid | − | 0.0334 |
| Guanine | − | 0.0011 |
| Guanosine | − | 0.0046 |
| 5′-Methylthioadenosine | − | 0.0038 |
| Thymidine 5′-triphosphate | − | 0.0121 |
| Ureidosuccinic acid | − | 0.0227 |
| Uridine | − | 9.50E – 08 |
+ / −: Positive/negative correlation between metabolite intensity and impulsive response rate.
Fig. 3.
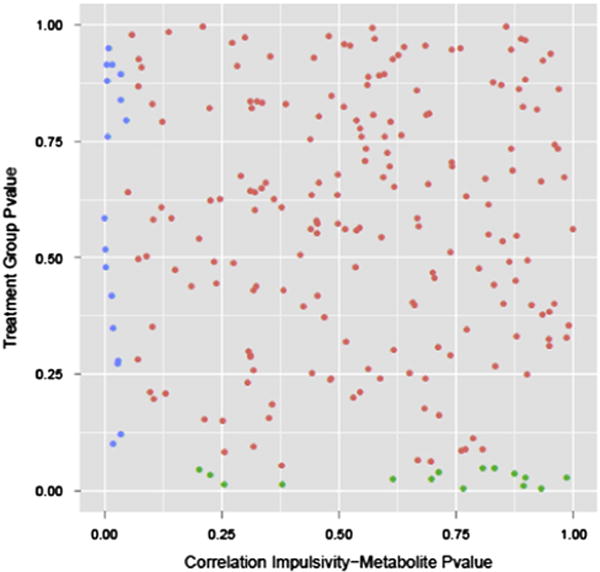
Scatter plot of p value of all metabolites testing for the fluoxetine treatment effect (y axis) and correlation with impulsive response (x axis). Each dot represents one metabolite. Green and blue are metabolites significantly associated with fluoxetine treatment and impulsive behavioral effect reaction, respectively (n=8 controls, n=8 fluoxetine). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3. Discussion
Antidepressants of the Selective Serotonin Reuptake Inhibitor (SSRI) type suffer from a number of side effects and a significant number of patients do not respond to drug treatment. The SSRI fluoxetine is frequently prescribed for children suffering from neurobehavioral disorders including MDD and ASD and severe side effects have been observed. As in other areas of medicine the prediction of response to treatment in childhood pharmacotherapy is critical for an optimal therapeutic response with minimal adverse side effects.
Although the primary pharmacological function of fluoxetine is the prevention of serotonin re-uptake in the synaptic cleft, there is good reason to assume that the drug has also other biological actions relevant to brain function causing a number of undesired side effects (Ni and Miledi, 1997; Norrholm and Ouimet, 2000; Bymaster et al., 2002; Koch et al., 2002; De Foubert et al., 2004; Pinna et al., 2006; Musazzi et al., 2009; Makkonen et al., 2011; Imoto et al., 2015). Since SSRIs are typically used for extended time periods they may affect a number of different pathways resulting in altered protein and metabolite levels. The downstream effects responsible for neural adaptation events affecting synaptic plasticity are presumably responsible for therapeutic effects of these agents. Proteins or metabolites that reflect pathway activity, also referred to as ‘Authentic Biomarkers’, provide a metric for predicting treatment response and undesired side effects. Ideally this is done in an animal model with brain functions similar to humans. In the current study we used rhesus monkeys that differed in two common gene polymorphisms for MAOA, an enzyme that selectively metabolizes serotonin. The two MAOA isoforms are of relevance for a fluoxetine treatment study based on SSRI action on serotonin reuptake.
All -omics investigations in humans are plagued by confounding life style and environmental factors which make data interpretation challenging. Lifestyle, diet, drugs, and other environmental factors are difficult to control in a patient cohort and complicate the identification of biomarkers related to disease or drug treatment. Although our metabolomics studies involved juvenile rhesus monkeys that were housed under controlled conditions and fed an identical diet there are other confounders that can affect peripheral fluid constituents and the interpretation of metabolite profiling data. These include variations in growth, health and maturation typically seen in genetically heterogeneous populations of non-human primates as in humans. Since blood is circulating through the entire organism its contents is a reflection of many life style and environmental influences including nutrition and exercise. Although CSF is circulating in the CNS there is an exchange with blood and hence an infiltration of other molecular components that are not derived from CNS. As a consequence many metabolites present in blood and CSF can confound the interpretation of metabolomics data related to the drug response in the CNS. Other studies have successfully used skin fibroblast cultures to identify molecular mechanisms associated with MDD, schizophrenia and developmental disorders (Lindberg et al., 2015; Gassó et al., 2014; Kálmán et al., 2014).
Our goal was to identify biomarkers that are part of biochemical pathways perturbed upon chronic fluoxetine administration using global metabolomics analyses of fibroblasts derived from skin biopsies. Our data demonstrate that fluoxetine administration induced metabolome alterations are more accurately reflected in fibroblasts compared with plasma and CSF. Cells in tissue culture are not plagued by confounding noise encountered in plasma and CSF. Focusing on fibroblast data should therefore aid in the discovery of pathways affected by drug treatment.
In our study only a few metabolites identified in the cells were also found significantly altered in peripheral body fluids obtained from the same animals. The majority of peripheral fluid metabolites was not altered in fibroblasts and may be unrelated to drug treatment. This result further underscores the potential utility of fibroblast culture for elucidating molecular mechanisms in response to in vivo drug administration.
Overall, we found levels of metabolites that are part of Purine and Pyrimidine metabolisms to be affected and correlating with impulsivity behavior. Both pathways have been linked to psychiatric disorders (Micheli et al., 2011; Lindberg et al., 2015). Polymorphisms of the gene encoding the purinergic ion channel P2RX7 have been associated with the development of MDD (Lucae et al., 2006). Cytidine and Uridine, pyrimidine metabolites, have antidepressant-like activities in rodents, reducing immobility in the Forced Swim Test (FST) in a dose-dependent manner (Carlezon et al., 2005). How precisely fluoxetine affects Purine and Pyrimidine metabolisms is unclear at the present time. A study with platelets that were treated with SSRIs suggests that purinergic P2Y1 and P2Y12 receptors signaling pathways are affected by fluoxetine (Tseng et al., 2013).
In addition to analyzing metabolome changes in response to chronic fluoxetine administration we also correlated metabolite levels with a behavioral effect, in our case impulsivity, caused by drug therapy during juvenile development. We again found levels of metabolites that are part of Purine and Pyrimidine metabolisms to be affected and correlating with impulsivity behavior with Uridine representing a significant biomarker for the impulsivity behavioral effect.
Ultimately it is hoped that metabolite biomarkers will aid in stratifying treatment responders and non-responders by correlating them with genetic and behavioral data. This may accelerate translation of biomarkers for response to psychopharmacological agents in children. Affected pathway information will also improve our understanding of adverse effects caused by fluoxetine in children.
Supplementary Material
Acknowledgments
The authors appreciate the technical assistance of CNPRC Primate Medicine in obtaining the fibroblast samples, JoAnn Yee and the CNPRC Pathogen Detection Laboratory Core in conducting the fibroblast cultures, and Mine Palazoglu of the West Coast Metabolomics Center for processing the samples prior to analysis. This work was supported by NIH Grants HD065826 (PI MSG), and OD011107 (PI Harris Lewin).
Role of funding source
Supported by NIH Grants HD065826, HD065826 (supplement) (Mari Golub PI), OD011107 (Harris Lewin, PI), and the Max Planck Society (Christoph Turck PI). The NIH and the Max Planck Society had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Abbreviations
- 5HTTLPR
Serotonin transporter length polymorphic region
- AICAR
5-Aminoimidazole-4-carboxamide ribonucleotide
- ASD
Autism spectrum disorders
- CSF
Cerebrospinal fluid
- DMEM
Dulbecco’s Modified Eagle’s Medium
- FBS
Fetal bovine serum
- FST
Forced swim test
- KNN
k nearest neighbors
- MAOA
Monoamine oxidase A
- MDD
Major depressive disorder
- NHP
Non-human primates
- SSRI
Selective serotonin reuptake inhibitor
- WGTA
Wisconsin general test apparatus
Appendix A. Supplementary material
Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.euroneuro.2016.03.017.
Footnotes
Contributors
S-YS performed all statistical analyses. CEH-P and JMA carried out behavioral and metabolomics studies, respectively. SY-S, CWT and MSG designed the study and wrote the manuscript. All authors contributed to and have approved the final manuscript.
Conflict of interest
The authors declare no conflicts of interest.
References
- Birmaher B, Axelson DA, Monk K, Kalas C, Clark DB, Ehmann M, Bridge J, Heo J, Brent DA. Fluoxetine for the treatment of childhood anxiety disorders. J Am Acad Child Adolesc Psychiatry. 2003;42:415–423. doi: 10.1097/01.CHI.0000037049.04952.9F. [DOI] [PubMed] [Google Scholar]
- Bymaster FP, Zhang W, Carter PA, Shaw J, Chernet E, Phebus L, Wong DT, Perry KW. Fluoxetine, but not other selective serotonin uptake inhibitors, increases norepinephrine and dopamine extracellular levels in prefrontal cortex. Psychopharmacology. 2002;160:353–361. doi: 10.1007/s00213-001-0986-x. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Mague SD, Parow AM, Stoll AL, Cohen BM, Renshaw PF. Antidepressant-like effects of uridine and omega-3 fatty acids are potentiated by combined treatment in rats. Biol Psychiatry. 2005;57:343–350. doi: 10.1016/j.biopsych.2004.11.038. [DOI] [PubMed] [Google Scholar]
- De Foubert G, Carney SL, Robinson CS, Destexhe EJ, Tomlinson R, Hicks CA, Murray TK, Gaillard JP, Deville C, Xhenseval V, Thomas CE, O’Neill MJ, Zetterström TS. Fluoxetine-induced change in rat brain expression of brain-derived neurotrophic factor varies depending on length of treatment. Neuroscience. 2004;128:597–604. doi: 10.1016/j.neuroscience.2004.06.054. [DOI] [PubMed] [Google Scholar]
- Gassó P, Mas S, Molina O, Lafuente A, Bernardo M, Parellada E. Increased susceptibility to apoptosis in cultured fibroblasts from antipsychotic-naïve first-episode schizophrenia patients. J Psychiatr Res. 2014;48:94–101. doi: 10.1016/j.jpsychires.2013.09.017. [DOI] [PubMed] [Google Scholar]
- Geller DA, Hoog SL, Heiligenstein JH, Ricardi RK, Tamura R, Kluszynski S, Jacobson JG, Team FPOS. Fluoxetine treatment for obsessive-compulsive disorder in children and adolescents: a placebo-controlled clinical trial. J Am Acad Child Adolesc Psychiatry. 2001;40:773–779. doi: 10.1097/00004583-200107000-00011. [DOI] [PubMed] [Google Scholar]
- Golub MS, Hogrefe CE. Fluoxetine: juvenile pharmacokinetics in a non-human primate model. Psychopharmacology. 2014;231:4041–4047. doi: 10.1007/s00213-014-3537-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golub MS, Hogrefe CE, Germann SL. Iron deprivation during fetal development changes the behavior of juvenile rhesus monkeys. J Nutr. 2007;137:979–984. doi: 10.1093/jn/137.4.979. [DOI] [PubMed] [Google Scholar]
- Golub MS, Bulleri AM, Hogrefe CE, Sherwood RJ. Bone growth in juvenile rhesus monkeys is influenced by 5HTTLPR polymorphisms and interactions between 5HTTLPR polymorphisms and fluoxetine. Bone. 2015;79:162–169. doi: 10.1016/j.bone.2015.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golub MS, Hogrefe CE, Germann SL, Tran TT, Beard JL, Crinella FM, Lonnerdal B. Neurobehavioral evaluation of rhesus monkey infants fed cow’s milk formula, soy formula, or soy formula with added manganese. Neurotoxicol Teratol. 2005;27:615–627. doi: 10.1016/j.ntt.2005.04.003. [DOI] [PubMed] [Google Scholar]
- He Y, Hogrefe CE, Grapov D, Palazoglu M, Fiehn O, Turck CW, Golub MS. Identifying individual differences of fluoxetine response in juvenile rhesus monkeys by metabolite profiling. Transl Psychiatry. 2014;4:e478. doi: 10.1038/tp.2014.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry A, Kisicki MD, Varley C. Efficacy and safety of antidepressant drug treatment in children and adolescents. Mol Psychiatry. 2012;17:1186–1193. doi: 10.1038/mp.2011.150. [DOI] [PubMed] [Google Scholar]
- Hetrick S, Merry S, McKenzie J, Sindahl P, Proctor M. Selective serotonin reuptake inhibitors (SSRIs) for depressive disorders in children and adolescents. Cochrane Database Syst Rev. 2007:CD004851. doi: 10.1002/14651858.CD004851.pub2. [DOI] [PubMed] [Google Scholar]
- Hetrick SE, McKenzie JE, Merry SN. The use of SSRIs in children and adolescents. Curr Opin Psychiatry. 2010;23:53–57. doi: 10.1097/YCO.0b013e328334bc92. [DOI] [PubMed] [Google Scholar]
- Hollander E, Phillips A, Chaplin W, Zagursky K, Novotny S, Wasserman S, Iyengar R. A placebo controlled crossover trial of liquid fluoxetine on repetitive behaviors in childhood and adolescent autism. Neuropsychopharmacology. 2005;30:582–589. doi: 10.1038/sj.npp.1300627. [DOI] [PubMed] [Google Scholar]
- Imoto Y, Kira T, Sukeno M, Nishitani N, Nagayasu K, Nakagawa T, Kaneko S, Kobayashi K, Segi-Nishida E. Role of the 5-HT4 receptor in chronic fluoxetine treatment-induced neurogenic activity and granule cell dematuration in the dentate gyrus. Mol Brain. 2015;8:29. doi: 10.1186/s13041-015-0120-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kálmán S, Garbett KA, Vereczkei A, Shelton RC, Korade Z, Mirnics K. Metabolic stress-induced micro-RNA and mRNA expression profiles of human fibroblasts. Exp Cell Res. 2014;320:343–353. doi: 10.1016/j.yexcr.2013.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch S, Perry KW, Nelson DL, Conway RG, Threlkeld PG, Bymaster FP. R-fluoxetine increases extracellular DA, NE, as well as 5-HT in rat prefrontal cortex and hypothalamus: an in vivo microdialysis and receptor binding study. Neuropsychopharmacology. 2002;27:949–959. doi: 10.1016/S0893-133X(02)00377-9. [DOI] [PubMed] [Google Scholar]
- Lee CS, Williamson LR, Martin SE, DeMarco M, Majczak M, Martini J, Hunter HL, Fritz G, Boekamp J. Adverse events in very young children prescribed psychotropic medications: preliminary findings from an acute clinical sample. J Child Adolesc Psychopharmacol. 2015;25:509–513. doi: 10.1089/cap.2015.0034. [DOI] [PubMed] [Google Scholar]
- Lindberg D, Shan D, Ayers-Ringler J, Oliveros A, Benitez J, Prieto M, McCullumsmith R, Choi DS. Purinergic signaling and energy homeostasis in psychiatric disorders. Curr Mol Med. 2015;15:275–295. doi: 10.2174/1566524015666150330163724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucae S, Salyakina D, Barden N, Harvey M, Gagné B, Labbé M, Binder EB, Uhr M, Paez-Pereda M, Sillaber I, Ising M, Brückl T, Lieb R, Holsboer F, Müller-Myhsok B. P2RX7, a gene coding for a purinergic ligand-gated ion channel, is associated with major depressive disorder. Hum Mol Genet. 2006;15:2438–2445. doi: 10.1093/hmg/ddl166. [DOI] [PubMed] [Google Scholar]
- Makkonen I, Kokki H, Kuikka J, Turpeinen U, Riikonen R. Effects of fluoxetine treatment on striatal dopamine transporter binding and cerebrospinal fluid insulin-like growth factor-1 in children with autism. Neuropediatrics. 2011;42:207–209. doi: 10.1055/s-0031-1291242. [DOI] [PubMed] [Google Scholar]
- Micheli V, Camici M, Tozzi MG, Ipata PL, Sestini S, Bertelli M, Pompucci G. Neurological disorders of purine and pyrimidine metabolism. Curr Top Med Chem. 2011;11:923–947. doi: 10.2174/156802611795347645. [DOI] [PubMed] [Google Scholar]
- Musazzi L, Cattaneo A, Tardito D, Barbon A, Gennarelli M, Barlati S, Racagni G, Popoli M. Early raise of BDNF in hippocampus suggests induction of posttranscriptional mechanisms by antidepressants. BMC Neurosci. 2009;10:48. doi: 10.1186/1471-2202-10-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naviaux JC, Schuchbauer MA, Li K, Wang L, Risbrough VB, Powell SB, Naviaux RK. Reversal of autism-like behaviors and metabolism in adult mice with single-dose anti-purinergic therapy. Transl Psychiatry. 2014;4:e400. doi: 10.1038/tp.2014.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni YG, Miledi R. Blockage of 5HT2C serotonin receptors by fluoxetine (Prozac) Proc Natl Acad Sci USA. 1997;94:2036–2040. doi: 10.1073/pnas.94.5.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson M, Joliat MJ, Miner CM, Brown EB, Heiligenstein JH. Safety of subchronic treatment with fluoxetine for major depressive disorder in children and adolescents. J Child Adolesc Psychopharmacol. 2004;14:412–417. doi: 10.1089/cap.2004.14.412. [DOI] [PubMed] [Google Scholar]
- Norrholm SD, Ouimet CC. Chronic fluoxetine administration to juvenile rats prevents age-associated dendritic spine proliferation in hippocampus. Brain Res. 2000;883:205–215. doi: 10.1016/s0006-8993(00)02909-7. [DOI] [PubMed] [Google Scholar]
- Patterson TA, Li M, Hotchkiss CE, Mauz A, Eddie M, Greischel A, Stierstorfer B, Deschl U, Paule MG. Toxicity assessment of pramipexole in juvenile rhesus monkeys. Toxicology. 2010;276:164–171. doi: 10.1016/j.tox.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Pinna G, Costa E, Guidotti A. Fluoxetine and norfluoxetine stereospecifically and selectively increase brain neurosteroid content at doses that are inactive on 5-HT reuptake. Psychopharmacology. 2006;186:362–372. doi: 10.1007/s00213-005-0213-2. [DOI] [PubMed] [Google Scholar]
- Popke EJ, Allen RR, Pearson EC, Hammond TG, Paule MG. Differential effects of two NMDA receptor antagonists on cognitive-behavioral performance in young non-human primates II. Neurotoxicol Teratol. 2001;23:333–347. doi: 10.1016/s0892-0362(01)00138-6. [DOI] [PubMed] [Google Scholar]
- Quintana H, Butterbaugh GJ, Purnell W, Layman AK. Fluoxetine monotherapy in attention-deficit/hyperactivity disorder and comorbid non-bipolar mood disorders in children and adolescents. Child Psychiatry Hum Dev. 2007;37:241–253. doi: 10.1007/s10578-006-0032-7. [DOI] [PubMed] [Google Scholar]
- Rodriguez JS, Morris SM, Hotchkiss CE, Doerge DR, Allen RR, Mattison DR, Paule MG. The effects of chronic methylphenidate administration on operant test battery performance in juvenile rhesus monkeys. Neurotoxicol Teratol. 2010;32:142–151. doi: 10.1016/j.ntt.2009.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safer DJ. Age-grouped differences in adverse drug events from psychotropic medication. J Child Adolesc Psychopharma-col. 2011;21:299–309. doi: 10.1089/cap.2010.0152. [DOI] [PubMed] [Google Scholar]
- Shrestha SS, Nelson EE, Liow JS, Gladding R, Lyoo CH, Noble PL, Morse C, Henter ID, Kruger J, Zhang B, Suomi SJ, Svenningsson P, Pike VW, Winslow JT, Leiben-luft E, Pine DS, Innis RB. Fluoxetine administered to juvenile monkeys: effects on the serotonin transporter and behavior. Am J Psychiatry. 2014;171:323–331. doi: 10.1176/appi.ajp.2013.13020183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto PL, Wilcox KM, Zhou Y, Kumar A, Ator NA, Riddle MA, Wong DF, Weed MR. Long-term exposure to oral methylphenidate or dl-amphetamine mixture in peri-adolescent rhesus monkeys: effects on physiology, behavior, and dopamine system development. Neuropsychopharmacology. 2012;37:2566–2579. doi: 10.1038/npp.2012.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strawn JR, Welge JA, Wehry AM, Keeshin B, Rynn MA. Efficacy and tolerability of antidepressants in pediatric anxiety disorders: a systematic review and meta-analysis. Depress Anxiety. 2015;32:149–157. doi: 10.1002/da.22329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng YL, Chiang ML, Lane HY, Su KP, Lai YC. Selective serotonin reuptake inhibitors reduce P2Y12 receptor-mediated amplification of platelet aggregation. Thromb Res. 2013;131:325–332. doi: 10.1016/j.thromres.2013.02.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


