Abstract
Anticoagulant drugs, like vitamin K antagonists and heparin, have been the mainstay for the treatment and prevention of venous thromboembolic disease for many years. Although effective if appropriately used, traditional anticoagulants have several limitations such as unpredictable pharmacologic and pharmacokinetic responses and various adverse effects including serious bleeding complications. New oral anticoagulants have recently emerged as an alternative because of their rapid onset/offset of action, predictable linear dose-response relationships and fewer drug interactions. However, they are still associated with problems such as bleeding, lack of reversal agents and standard laboratory monitoring. In an attempt to overcome these drawbacks, key steps of the hemostatic pathway are investigated as targets for anticoagulation. Here we reviewed the traditional and new anticoagulants with respect to their targets in the coagulation cascade, along with their therapeutic advantages and disadvantages. In addition, investigational anticoagulant drugs currently in the development stages were introduced.
Keywords: Anticoagulant, Vitamin K antagonist, Heparin, Venous thromboembolism
INTRODUCTION
Venous thromboembolism (VTE) refers to thrombosis within the vein, commonly in the legs or pelvis (deep vein thrombosis, DVT) and its complication, pulmonary embolism (PE), the condition of thrombi departing from their original generation site into a pulmonary artery (Hyers, 1999). It is the third leading cause of cardiovascular-related deaths, following acute coronary syndrome and stroke (Piazza and Goldhaber, 2010), with an annual incidence of 1 to 3 times per 1,000 people (Heit et al., 2016; Puurunen et al., 2016). Moreover, it often leads to long-term complications such as post-thrombotic syndrome and chronic thromboembolic pulmonary hypertension, which impose a significant burden on both patients and the healthcare systems (Ruppert et al., 2010; Bruni-Fitzgerald, 2015).
Pathologic thrombosis or bleeding may occur whenever the hemostatic balance is disturbed due to various health conditions including surgery, trauma, malignancy, and congenital disorders (Previtali et al., 2011) and even following chronic cigarrete smoking (Park et al., 2016). In normal circumstances, hemostasis is maintained through the complex interactions between the vascular system (Kwon et al., 2016), coagulation system, fibrinolytic system (Lee et al., 2015) and platelets (Kim et al., 2016). Natural anticoagulants such as tissue factor pathway inhibitors (TFPI), protein C, protein S, and anti-thrombin (AT) also regulate the coagulation process. The fibrinolytic system plays a role by dissolving the fibrin clot during the healing process of an injured blood vessel (Weitz, 1997; Chapin and Hajjar, 2015).
Anticoagulants can inhibit thrombosis by altering various pathways within the coagulation system or through targeting thrombin directly by attenuating its generation (Mega and Simon, 2015). For many years, unfractionated heparins (UFHs) and vitamin K antagonists (VKAs) have been the main options for the prevention and treatment of VTE (Franchini et al., 2016). The treatment changed little until low molecular weight heparins (LMWHs), fragments of UFHs, were introduced in the 1980s, simplifying the management of thromboembolism by saving the trouble of frequent coagulation monitoring (Weitz, 1997). In the 2000s, ultra-low molecular heparins (ULMWHs) were developed in an effort to improve the pharmacokinetic profile of conventional heparin formulations and to lower the risk of heparin-induced thrombocytopenia (HIT) (Masuko and Linhardt, 2012). However, all forms of heparin require parenteral administration, which is cumbersome for long-term use (Fareed et al., 2008). Similarly, oral VKAs have several drawbacks including a wide range of food and drug interactions, as well as the need for frequent monitoring and dose adjustment (Hirsh et al., 2007).
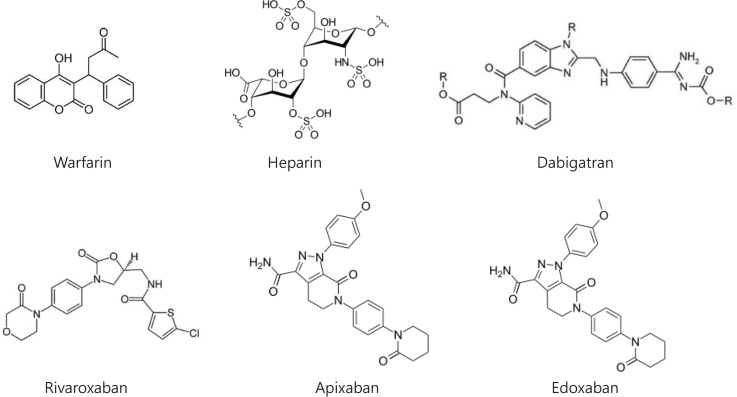
Over the past decades new oral anticoagulants (NOACs), which more directly and selectively target specific proteins in the coagulation cascade, have been developed, as shown in Fig. 1. They are conveniently administered in oral, fixed doses without routine monitoring and have fewer interactions than VKAs with foods or drugs (Mekaj et al., 2015). But NOACs have their own limitations such as lack of reliable coagulation monitoring methods and selective antidotes (except dabigatran), as shown in Table 1. This review summarizes the pharmacologic characteristics of traditional and new anticoagulants, as well as anticoagulants under development, focusing on their advantages and disadvantages.
Fig. 1.
Chemical structures of current anticoagulants.
Table 1.
Traditional and novel anticoagulants in the market and development
| Generic Name | Mechanism of action | Reversal agents | Anticoagulation monitoring |
|---|---|---|---|
| Traditional drugs | |||
| Warfarin | Deplete coagulation factors II VII, IX, and X through inhibition of cyclic interconversion of vitamin K and its epoxide | Vitamin K | INR |
| UFH | Indirectly inhibit thrombin (factor II), factor X, IX, XI, and XII via enhancing the activity of antithrombin | Protamine sulfate | PT, aPTT |
| LMWH | Inhibit thrombin and factor X via enhancing the activity of antithrombin | Protamine sulfate | Anti-Xa assay |
| ULMWH | Inhibit factor X via enhancing the activity of antithrombin | - | Anti-Xa assay |
| New drugs | |||
| Dabigatran | Inhibit free and fibrin-bound thrombin via direct binding | Idarucizumab | aPTT, ECT |
| Rivaroxaban | Inhibit free and fibrin-bound factor Xa via direct binding | Andexanet alfa, PER977 | Anti-Xa assay |
| Apixaban | Inhibit free and fibrin-bound factor Xa via direct binding | Andexanet alfa, PER977 | Anti-Xa assay |
| Edoxaban | Inhibit free and fibrin-bound factor Xa via direct binding | Andexanet alfa, PER977 | Anti-Xa assay |
| Drugs under development | |||
| Tifacogin | Inhibit tissue factor-factor VIIa complex | - | - |
| TB-402 | Inhibit factor VIII via direct binding | - | - |
| Pegnivacogin | Inhibit factor IX via direct binding | - | - |
| Factor XI-ASO | Inhibit factor XI via direct binding | - | - |
| rHA-infestin-4 | Inhibit factor XII | - | - |
| Recomodulin | Inhibit factor V and VIII via activating protein C through thrombin-thrombomodulin complex | - | - |
aPTT: activated partial thromboplastin time, ASO: antisense oligonucleotide, INR: international Normalized Ratio, ECT: ecarin clotting time, LMWH: low molecular weight heparin, PT: Prothrombin time, UFH: unfractionated heparin, ULMWH: ultra-low molecular weight heparin.
TRADITIONAL ANTICOAGULANTS
Vitamin K antagonists
VKAs such as coumarin derivatives (e.g., warfarin, acenocoumarol, and phenprocoumon) exert their anticoagulant effects by interfering with the cyclic interconversion of vitamin K and its 2,3 epoxide (KO), therefore depleting the vitamin K hydroquinone (KH2; Wessler and Gitel, 1986). The coagulation factors II (thrombin), VII, IX, and X, as well as proteins C and S, require carboxylation, by converting glutamic acid to gamma-carboxyglutamic acid, for their normal functions. The carboxylation procedure requires KH2 (Ageno et al., 2012). VKAs inhibit vitamin K epoxide reductase complex 1 (VKORC1), an enzyme that catalyzes the reduction of KO to vitamin K, which is then converted to KH2 and then oxidized back to KO, concomitantly with gamma-glutamyl carboxylation. The full anticoagulation effect of warfarin is not achieved until the clearance of factor X and prothrombin that have half-lives of 36 and 50 h, respectively (Loke et al., 2012). Because proteins C and S, with relatively short half-lives, initially exert their procoagulant effects, the combined use of VKAs with parenteral agents is required.
The anticoagulant response to warfarin is largely affected by diet, concurrent drugs, and genetic polymorphisms (Hirsh et al., 2003). The anticoagulant effect of warfarin can be counteracted by vitamin K intake either through food or supplements. A large amount of vitamin K causes warfarin resistance for up to a week because vitamin K accumulated in the liver can bypass VKORC (Lurie et al., 2010). The cytochrome P450 enzyme (CYP2C9) is responsible for oxidative metabolism of the warfarin S-isomer, which is five times more potent than the R-isomer. Therefore, the dose-response of warfarin can be influenced by CYP2C9 inhibiting drugs that affect the metabolic clearance of warfarin, especially the S-isomers, such as phenylbutazone, sulfinpyrazone, metronidazole, trimethoprim-sulfamethoxazole, or amiodarone. It is also influenced by genetic polymorphisms in CYP2C9 and VKORC unit 1 genes (Fung et al., 2012). The individuals who carry CYP2C9*2 or CYP2C9*3 tend to have higher levels of S-warfarin due to the impaired ability to metabolize it. Since CYP2C9 is also responsible for the metabolism of acenocoumarol, and less importantly for phenprocoumon, polymorphisms of CYP2C9 also affect the efficacy of acenocoumarol and phenprocoumon, although with a lesser extent than with warfarin (Verhoef et al., 2014). Genetic mutations or an altered expression of the VKORC1 gene can also lead to variable responses, either hyper-sensitivity or resistance to warfarin therapy. Acenocoumarol and phenprocoumon are also influenced by the VKORC1 genotype, especially in the first few months.
Hemorrhage is the most significant and frequent complication related to warfarin, with an annual incidence of major bleeding at a rate of 13 per 100 patients (Linkins et al., 2003). The risk of bleeding associated with warfarin is related not only to the degree of anticoagulation but also to patient-related factors and the concurrent use of antiplatelet agents or other drugs (Fitzmaurice et al., 2002). Bleeding complications can be managed by administering vitamin K, fresh frozen plasma (FFP), prothrombin complex concentrates (PCCs), or recombinant factor VIIa (Tran et al., 2013), which can antagonize the effects of warfarin therapy.
Heparins
Heparins indirectly inhibit thrombin by enhancing the activity of antithrombin (AT), a proteinase inhibitor of coagulation enzymes such as thrombin and factors Xa of the common pathway, as well as IXa, XIa and XIIa of the intrinsic coagulation pathway (Hirsh and Raschke, 2004). Following a conformational change induced by heparin, AT irreversibly inhibits thrombin via binding its active site. For inactivation of thrombin, heparin must bind simultaneously to thrombin at exosite 2 and AT, forming a ternary complex which requires at least 18 saccharide units (Liaw et al., 2001). In contrast, heparin only binds to AT via high-affinity pentasaccharides, for the inhibition of factor Xa, without requiring a bridge between factor Xa and AT. Since most heparin molecules are at least 18 units long, inhibitory activities of heparin against the thrombin and factor Xa are equivalent (Hirsh, 1991). However, the AT-bound heparin weakly inhibits the thrombin, once formed as a ternary heparin-fibrin-thrombin complex, because it can no longer gain access to the exosite 2 already occupied. Furthermore, AT-bound heparin is unable to inhibit factor Xa bound to activated platelets (Teitel and Rosenberg, 1983).
Unfractionated heparins (UFH): Unfractionated heparin (UFH) is heterogeneous in terms of molecular size, anticoagulant activity and pharmacokinetics (Garcia et al., 2012). The molecular weight of UFH ranges from 3,000 to 30,000 Da, with an average of 15,000 Da (approximately 45 saccharide units). Only 20–50% of UFH chains contain the high-affinity pentasaccharide unit necessary for activating AT (Marmur, 2002). Heparin molecules without a pentasaccharide unit have minimal activity at therapeutic concentrations. Low-affinity heparin can inhibit thrombin via heparin cofactor (Tollefsen et al., 1982) as well as factor Xa generation, through AT-independent mechanisms (Garcia et al., 2012).
Besides the multiple anticoagulant mechanisms, heparin involves multiple clearance mechanisms including both rapid, saturable and slow, non-saturable processes (Hirsh and Fuster, 1994). The rapid phase of heparin clearance occurs through binding to macrophages and endothelial cells at saturable sites on the cell membrane and subsequent depolymerization, whereas the slow clearance mechanism is through the kidneys. Because low doses of heparins initially undergo the saturable and dose-dependent clearance, the effect of heparin is not linear, although both intensity and duration of heparin activity may increase with escalating doses. As a result, the pharmacokinetics and pharmacodynamics of UFH are unpredictable.
Additionally, UFH has a number of limitations such as a short duration of action with a half-life of 60 min, poor bioavailability after subcutaneous injection, and an immune-mediated reaction, a life-threatening adverse event (Krishnaswamy et al., 2010). Heparin complexes with an endogenous platelet factor 4 (PF4), which undergoes conformational changes and becomes immunogenic, leading to the generation of heparin-PF4 antibodies (Kreimann et al., 2014). The heparin-PF4-IgG immune complex then activates platelets and causes the release of microparticles, platelet consumption and peripheral thrombocytopenia, and also endothelial injury and activation (Rauova et al., 2006). Heparin’s affinity for PF4 depends on molecular weights and chain lengths (Amiral et al., 1995), thus accounting for the increased incidence of thrombocytopenia by UFH when compared with LMWH.
The effect of UFH can be reversed by intravenously administering protamine sulfate that binds to heparin and forms a stable salt (Greinacher et al., 2015). This can be advantageous in situations of cardiac surgery or treating critically ill patients who may require rapid reversal of the anticoagulation effect.
Low molecular weight heparins (LMWH): LMWH is a mixture of polymers with molecular weights that vary from 1,000 to 10,000 Da, with a mean molecular weight between 4,000 and 5,000 Da, approximately one third the size of UFH (Hirsh, 1998). Since LMWHs are prepared by various chemical or physical depolymerizations of heparin, each LMWH has unique characteristics in terms of molecular weight, polysaccharide chain length distributions, and pharmacological properties that may influence pharmacokinetic properties and anticoagulant activity profiles (Merli and Groce, 2010). Since 50 to 75% of LMWH species have a length of less than 18 saccharides, which will inhibit only factor Xa, the selectivity ratio of activity against factor Xa to thrombin varies between 4:1 and 2:1, depending on their preparations (Holmer et al., 1986). Because LMWH mostly undergoes renal elimination, its biologic half-life may be prolonged in cases of renal insufficiency, especially for those with lower molecular weights such as enoxaparin or nadroparin (Schmid et al., 2009).
In addition to the convenience of subcutaneous administration and almost 100% subcutaneous bioavailability, LMWH has several advantages over UFH in terms of pharmacological characteristics (Hirsh and Raschke, 2004). The low protein binding of LMWH makes anticoagulant effects more predictable, which allows for a fixed or body weight-based dose regimen without the need for frequent monitoring (Hirsh and Raschke, 2004). Low nonspecific binding to macrophages and endothelial cells increases the plasma half-life of LMWH. In addition, the lower binding to platelets, PF4 and osteoclasts may reduce the risk of HIT and osteoporosis.
Unlike UFH’s complete neutralization activity of anti-factor Xa, protamine sulfate reverses only about 60% of the anti-factor Xa activity of LMWH (Wolzt et al., 1995). This may be due to the fact that protamine favors the regions of larger heparin chains, and an effective antidote for the residual smaller chains in LMWHs is not available (Schroeder et al., 2011). Furthermore, the subcutaneous administration of heparins is more difficult to completely reverse.
Ultra-low molecular weight heparins (ULMWHs): Ultra-low molecular weight heparins (ULMWHs), also known as an indirect factor Xa inhibitors, are synthetic analogues of the pentasaccharide contained within heparins, with an average molecular weight of less than 3,000 Da (Walenga and Lyman, 2013). These small, homogeneous drugs have been developed on the basis that higher selectivity in the activity against factor Xa or thrombin would produce similar or better efficacy than LMWHs, but have a lower risk of bleeding and HIT. ULM-WHs also exhibit anticoagulant efficacy through the selective inhibition of factor Xa via the unique pentasaccharide unit (Hirsh, 1998).
ULMWHs only exhibit the anti-factor Xa effect when binding to AT and are devoid of other functional components of heparins such as the release of TFPI from the vascular endothelium, the formation of complexes with PF4, and profibrinolytic actions. It is likely that the heparin chains must be of a sufficient length to form a complex with PF4 for binding to antibodies, which is a pathological mechanism of HIT (Rauova et al., 2005). Besides anticoagulant activity which is weaker than that of UFH or LMWHs, ULMWHs show anti-angiogenic, anti-metastatic and anti-inflammatory activities (Gandhi and Mancera, 2010).
The hepatic clearance of heparin is believed to involve the stabilin-2-receptor that requires heparin chains longer than decasaccharides for binding (Pempe et al., 2012). Therefore, unlike UFH, ULMWHs never reach the size needed for hepatic clearance and therefore depend heavily on renal clearance (Rupprecht and Blank, 2010).
ULMWHs offer several advantages over conventional heparins such as a higher bioavailability, rapid onset of action with longer biological half-lives, and a lower risk of bleeding, as well as osteoporosis. Because of the absence of binding to other plasma proteins, ULMWHs have predictable pharmacokinetics with almost 100% bioavailability. However, no antidote is available for bleeding associated with ULMWHs, whereas protamine sulfate can neutralize UFH completely and LMWHs partially. Unlike the impermeability of LMWHs or UFHs through the placental or blood-brain barrier, ULMWHs are able to partially pass the blood-brain barrier (Hoppensteadt et al., 2003).
NEW ANTICOAGULANTS
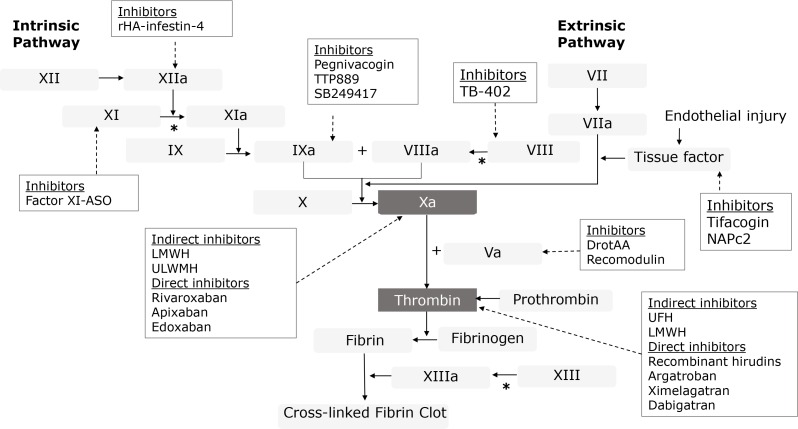
The newer anticoagulants offer superior therapeutic control over coagulations with minimal bleeding complications. They directly target either thrombin or factor Xa in the coagulation cascade, which is pharmacologically distinct from traditional anticoagulant agents (Fig. 2). Since the amount of activated coagulation factors is amplified at each level of the coagulation cascade, direct inhibition of the final products from both the intrinsic and extrinsic coagulation pathways (factor Xa and thrombin) can provide more effective anticoagulation.
Fig. 2.
Targets of various anticoagulants in the coagulation pathways. VKA: vitamin K antagonists, UFH: unfractionated heparin, LMWH: low molecular weight heparin, ULMWH: ultra-low molecular weight heparin, NAP: nematode anticoagulant protein, ASO: antisense oligonucleotide, DrotAA: drotecogin alpha (activated), *catalyzed by thrombin.
Direct thrombin inhibitors
Thrombin is an end product in the coagulation cascade, which converts soluble fibrinogen to insoluble fibrin. It amplifies coagulation by activating factors V and VIII on the surface of platelets and platelet-bound factor XI, stimulating platelets and generating more thrombin. By activating XIII, it also accelerates the formation of cross-linked fibrins and clot stabilization. In addition to its procoagulant role, thrombin plays a role in growth factor synthesis, cell proliferation, prostaglandin I2 synthesis, and chemotaxis of polymorphonuclear cells (Coughlin, 1994). Therefore, inhibition of thrombin may provide benefits in addition to anticoagulation (Bea et al., 2006).
The antithrombotic action of heparin occurs through binding to both AT and thrombin’s exosite 2, a heparin-binding domain, simultaneously. Heparin also can act as a bridge between fibrin and thrombin, enhancing thrombin’s affinity for fibrin and increasing the amount of fibrin-bound thrombin. Because the fibrin-heparin-thrombin complex, therefore, occupies not only exosite 2 but also exosite 1 (fibrin-binding site), fibrin-bound thrombin is protected from inhibition by the heparin-AT complex and remains active, resulting in further thrombus generation (Weitz et al., 1990). As such, heparin is relatively ineffective at inhibiting thrombin propagation (Di Nisio et al., 2005).
Unlike heparins, direct thrombin inhibitors (DTIs) act without a preceding interaction with AT and directly suppress thrombin, as well as its interaction with its substrates (Di Nisio et al., 2005). DTIs block the action of thrombin by binding to the catalytic site (univalent) or to both the catalytic site and exosite 1 (bivalent) (Bates and Weitz, 2000). Therefore, DTIs can inhibit both free and fibrin-bound thrombin. In addition, there are other advantages such as more predictable anticoagulant effects due to the absence of interaction with plasma proteins, not being neutralized by PF4, the inhibition of thrombin-induced platelet aggregation and absence of immune-mediated thrombocytopenia (Lee and Ansell, 2011).
Bivalent DTIs include recombinant hirudins (e.g., lepirudin and desirudin) and a synthetic hirudin, bivalirudin (Di Nisio et al., 2005). Bivalent DTIs form an irreversible complex with thrombin, but bivalirudin, which is slowly cleaved by thrombin once bound, restores the catalytic function of thrombin (Lee and Ansell, 2011). As a result, thrombin inhibition using bivalirudin is temporary, which may contribute to its low bleeding risk compared with recombinant hirudins (Nawarskas and Anderson, 2001). Bivalirudin is mainly cleared by proteolysis and hepatic metabolism, whereas recombinant hirudins predominantly undergo renal excretion.
Univalent DTIs, such as argatroban, ximelagatran, and dabigatran exteilate, non-covalently and reversibly bind to thrombin, leaving a small fraction of free thrombin (Di Nisio et al., 2005). Reversible and selective binding to thrombin accompanies a minimal risk of bleeding and rapid restoration of hemostasis to baseline upon discontinuation. Like recombinant hirudins, argatroban is a parenteral DTI but is metabolized by the liver (Koster et al., 2007).
Ximelagatran, a prodrug of melagatran, is the first oral DTI, which represents a new era of anticoagulation for the prevention and treatment of VTE. Although it was withdrawn from the market due to a risk of significant hepatotoxicity, ximelagatran demonstrated improved antithrombotic efficacy when compared with traditional anticoagulation therapies (Evans et al., 2004). A few years later, dabigatran etexilate, the second oral DTI, was developed with some improvements such as no risk of hepatotoxicity and low potential for food or drug interactions. Following oral absorption, dabigatran etexilate is rapidly converted into its active form, dabigatran, by nonspecific serum esterase without the involvement of cytochrome P450 enzymes or other oxidoreductases. Therefore, dabigatran etexilate has a low potential for interacting with drugs (Stangier and Clemens, 2009). Approximately 80% of circulating dabigatran is excreted unchanged via the kidneys and the remainder is conjugated with glucuronic acid. The conjugated dabigatran, which exhibits similar properties to the unconjugated form, is predominantly excreted via the bile.
Currently, none of the DTIs, except dabigatran, have direct reversal agents available for use. Recombinant factor VIIa, activated prothrombin complex concentrate (aPCC), activated charcoal, desmopressin and von Wilebrand factor concentrate have been tried in various studies (Majeed and Schulman, 2013; Baumann Kreuziger et al., 2014). Dabigatran effects can be reversed within minutes of intravenous administration of idarucizumab, a humanized monoclonal antibody, which binds tightly and prevents dabigatran from binding to thrombin (Sie, 2016).
Direct factor Xa inhibitors
Factor Xa is a primary site of amplification for coagulation factors, generating about 1,000 thrombin molecules from a single Xa molecule (Mann et al., 2003). Factor Xa binds to negatively charged phospholipid surfaces, which are exposed on activated platelets, together with factor Va to form the prothrombinase complex, the activator that converts prothrombin into thrombin. The conversion of fibrinogen to fibrin, the basic building block of all blood clots, is then catalyzed by thrombin. The rate of prothrombin activation by factor Xa in a prothrombinase complex is dramatically increased, thereby rapidly facilitating thrombin generation and plug formation at sites of injury. Whereas heparin inhibits factor Xa and thrombin to a similar degree, LMWHs have a relatively greater inhibitory effect against factor Xa, which has drawn attention as a potential anticoagulant target (Garcia et al., 2012). The interest of factor Xa as a drug target was further solidified by positive results from the use of fondapariunux, a parenteral indirect factor Xa inhibitor (Yeh et al., 2012).
Unlike indirect factor Xa inhibitors which are dependent on AT, direct factor Xa inhibitors interact directly and selectively with factor Xa and inhibit both free and bound forms of factor Xa without affecting platelet aggregation (Rupprecht and Blank, 2010). They are also associated with reduced incidence of rebound thrombosis compared to direct and indirect thrombin inhibitors (Perzborn et al., 2011). Because direct factor Xa inhibitors have good bioavailability with rapid onset of action, there is no need for bridging therapy with a parenteral agent (Cabral and Ansell, 2015). In general, they exhibit linear pharmacokinetics and display predictable anticoagulation effects following oral administration. All three agents, rivaroxaban, apixaban, and edoxaban, are excreted through the kidneys to varying degrees and have elimination half-lives much shorter than the VKAs. Rivaroxaban has a dual mechanism of excretion, with two-thirds of the administered dose excreted through the urine as either unchanged or inactive metabolites and one-third of the dose excreted through feces (Perzborn et al., 2011). Only 25% of an apixaban dose is eliminated by the kidneys with the remainder excreted via the fecal route (Eriksson et al., 2009). Edoxaban undergoes multiple elimination pathways with 35% excreted in the urine. Over 70% of the dose is excreted unchanged (Bounameaux and Camm, 2014).
The substantial benefits of oral factor Xa inhibitors are unfortunately accompanied by a high incidence of major and clinically relevant bleeding including gastrointestinal bleeding (Connolly and Spyropoulos, 2013). Moreover, all three factor Xa inhibitors are CYP3A4 and P-glycoprotein (P-gp) substrates that carry potential drug interaction issues. The CYP3A4 and/or P-gp inhibitors, as well as inducers, might impact the concentration of oral factor Xa inhibitors, leading to increased risk of bleeding or thrombosis (Short and Connors, 2014).
Potential antidotes for reversing anticoagulation caused by factor Xa inhibitors are currently under development (Ahmed et al., 2016). Andexanet alfa is a recombinant, modified factor Xa protein with a mutation on the catalytic site that abolishes the procoagulant property, which binds to direct and indirect factor Xa inhibitors in the blood (Connors, 2015). PER977 (e.g., arapazine and ciraparantag) binds to factor Xa inhibitors, as well as direct and indirect thrombin inhibitors, through noncovalent bonds and electrical charge interactions (Das and Liu, 2015).
ANTICOAGULANTS UNDER DEVELOPMENT
Both thrombin and factor Xa inhibitors have been extensively evaluated in several large clinical trials for the prevention and treatment of thromboembolic disorders. Despite their excellent efficacy compared to traditional agents, these drugs have their own drawbacks. Bleeding is still a major issue, with no reliable diagnostic test available to safely monitor the therapeutic dosage, as well as a lack of effective reversal agents (Hyers, 1999; Miller et al., 2012; Hu et al., 2016).
A variety of anticoagulant strategies, targeting other steps in coagulation, are in development to attempt to overcome the limitations of currently used agents. Drugs that target the tissue factor (TF)-factor VIIa complex inhibit the initiation of coagulation. Propagation of coagulation can be inhibited by drugs that target factors IXa or Xa or by agents that inactivate their respective cofactors, factors VIIIa and Va.
Tissue factor pathway inhibitors
Following vascular injury, TF, also known as thromboplastin, is exposed to the blood and binds to factor VIIa, which sets off the extrinsic coagulation pathway (Wood et al., 2014). The TF-factor VIIa complex activates factors X and IX. Additionally, activated factor IX forms a complex with factor VIIIa, which also activates factor X. Factor Xa then binds to factor Va to form prothrombinase, an enzymatic complex which rapidly converts prothrombin to thrombin. The TF activity and the extrinsic pathway are regulated by the tissue factor pathway inhibitor (TFPI). It inhibits factor Xa directly and the TF-factor VIIa complex in an Xa-dependent fashion. The factor Xa-dependent inhibition of the TF-factor VIIa complex generates an inactive quaternary complex in the plasma membrane.
Inhibition of TF-factor VIIa complex by recombinant TFPI was examined in various models of disseminated intravascular coagulation such as sepsis. Tifacogin, a recombinant TFPI expressed in Saccharomyces cerevisae, inhibits factor VIIa in a factor Xa-dependent fashion (Matyal et al., 2005). The drug requires intravenous infusion since the drug has a short plasma half-life and easily eliminated by the liver. The benefits of tifacogin administration in sepsis, pneumonia, and bacteremia have been investigated without promising results (Abraham et al., 2003; Hardy et al., 2006; Laterre et al., 2009). The synthetic nematode anticoagulant protein (NAPc2), which was originally isolated from the canine hookworm Ancylostoma canimum, binds to a non-catalytic site on factor Xa to form a NAPc2-factor-Xa complex and inhibits factor VIIa from binding to TF (Vlasuk and Rote, 2002). Because of its high affinity binding, NAPc2 has a half-life of about 50 h after subcutaneous administration. Factor VIIa with its active site blocked competes with factor VIIa for TF binding sites, thereby attenuating the initiation of coagulation by the TF-factor VIIa complex (Dickinson and Ruf, 1997)
Factor VIII inhibitors
Factor VIII (i.e., anti-hemophilic factor) acts as a cofactor for factor IXa, which activates factor X, thereby, forming an amplification loop (Lenting et al., 1998). Partial inhibition of factor VIII appears to be essential to reduce the risk of bleeding because complete inhibition will induce pathological hemophilia. TB-402 is a recombinant human monoclonal antibody that binds with a high affinity to factor VIII, partially inhibiting the action of factor VIII (Verhamme et al., 2010). It is under phase 2 clinical trials and the exact target of factor VIII inhibition and the degree of inhibition need to be established in further research.
Factor IXa inhibitors
The TF-factor VIIa complex activates factor IX, which is relatively stable and diffuses toward activated platelets (Howard et al., 2007). The activated platelets then bind the factor VIIa-IXa complex and recruit factor X for its activation. The activation of factor X by the factor VIIa-IXa complex is nearly 50 times more efficient than the TF-factor VIIa complex (Butenas et al., 2002). Therefore, factor IXa represents a prime target for anticoagulation. Defects in factor IXa lead to hemophilia B, while increased concentrations of factor IXa in the blood result in a significantly increased risk of thrombosis formation.
Factor IXa inhibitors including factor IX-directed monoclonal antibodies, factor IXa-directed RNA aptamers (e.g., pegnivacogin), and oral factor IXa inhibitors (e.g., TTP889) have been investigated in humans. SB249417, a chimeric monoclonal antibody directed against the factor IXa, completed a phase I clinical trial, showing a dose-dependent effect on clotting times after continuous infusion (Chow et al., 2002). The REG1 system consisted of pegnivacogin (RB006) and anivamersen (RB007), its complementary control agent being an aptamer-base factor IXa inhibitor that is being investigated for acute coronary syndrome (Vavalle and Cohen, 2012). Aptamers are small oligonucleotides with high affinity that are used as active drugs. Partial inhibition using TTP889 was not an effective strategy for VTE prophylaxis and TTP889 is currently being investigated for advanced heart failure to determine the potential benefit of attenuated thrombin generation (Roser-Jones et al., 2011). Natural factor IX binding proteins and factor IXai are under pre-clinical trials.
Factor XI inhibitors
A study showed that factor XI deficiency was associated with a less severe bleeding tendency and a lower incidence of venous thrombosis and stroke, compared to deficiencies of factors VIII or IX, which suggests factor XI is a safe target for anticoagulation. Factor XI inhibition has been extensively studied in both arterial and venous thrombosis in diverse animal models. Antibodies and antisense oligonucleotides (FXI-ASO) against factor XI both showed protective effects in thrombosis without an increased risk of bleeding (Büller et al., 2015). The anti-human factor XI monoclonal antibody was used to prevent vascular graft occlusion in a primate thrombosis model. Similar studies are currently being conducted to ensure the safety of factor XI inhibitors in humans.
Factor XII inhibitors
Available data on factor XII was limited but factor XII knockout mice were observed to have protection against pathologic thrombosis while having no hemostasis changes. The selective factor XIIa inhibitor, recombinant human albumin fused to the factor XIIa inhibitor infestin-4 (rHA-infestin-4), was developed (Hagedorn et al., 2010). Inhibition of factor XII is apparently a safe and efficient way of thrombosis prevention, at least in animals. Factor XII antisense, Pro-Phe-Arg-chloromethylketone, Ir-CPI, and several non-specific protein inhibitors are under pre-clinical trials.
Factor Va inhibitors
Factor V acts as a cofactor of factor Xa and forms a prothrombinase complex, together with platelet membrane phospholipids. Factor Va inhibitors include drotecogin alpha (activated; DrotAA) and Recomodulin (ART-123), which were initially developed for sepsis-induced thrombosis treatment. DrotAA is a recombinant form of activated protein C with antithrombotic, anti-inflammatory and pro-fibrinolytic properties (Dellinger, 2003). It showed some beneficial effects for coagulation abnormalities associated with severe sepsis but failed to show improvement of patients with severe sepsis. As of 2011, DrotAA was withdrawn from the market.
Recomodulin, a recombinant human thrombomodulin alpha, has shown to be efficacious for VTE prophylaxis following total hip replacement surgery and sepsis-associated disseminated intravascular coagulation (DIC) (Kearon et al., 2005; Vincent et al., 2013). Thrombomodulin is a thrombin receptor and the thrombin-thrombomodulin complex activates protein C to form activated protein C, which inactivates factors Va and VIIIa (Esmon, 2005). It has a long plasma half-life after a subcutaneous injection of two to three days, such that it can be given once every five to six days to maintain anticoagulant activity.
Polyphosphate inhibitors
Polyphosphate is a polymer of inorganic phosphate residues and is secreted by activated platelets and mast cells. It may initiate and/or accelerate coagulation, acting at several points in the coagulation cascade (Ruiz et al., 2004). It accelerates the activation of factor V, as well as factor XI by thrombin, and enhances fibrin clot structure increasing its resistance to fibrinolysis (Smith and Morrissey, 2008; Smith et al., 2012). A variety of compounds that inhibit polyphosphate and reduce thrombosis are under investigation in animal disease models. Universal heparin reversal agent (UHRA) compounds were studied in mouse models of thrombosis and hemostasis to ensure reduced toxicity and bleeding risk, compared to the toxic substances such as polyethylenimine, polyamidoamine dendrimers, and polymyxin B (Travers et al., 2014).
CONCLUSIONS
The prevention and treatment of VTE is evolving. The new target-specific oral anticoagulants such as oral DTIs and direct factor Xa inhibitors have shifted a paradigm from hospitals to outpatient settings exempting drug monitoring. Current oral anticoagulants available offer predictable, reversible anticoagulant effects with no need for invasive monitoring. However, the major complication of these drugs, bleeding, especially gastrointestinal bleeding, continues to persist, and an optimal management strategy needs to be provided. To date, different categories of anticoagulants are currently under development with unique profiles, along with benefits and potential drawbacks. In the future, the search for safer and more effective oral anticoagulants that have an antidote for rapid reversal will continue.
Acknowledgments
This work is supported by the National Research Foundation of Korea (Grant No. NRF-2015R1D1A1A01057931).
REFERENCES
- Abraham E, Reinhart K, Opal S, Demeyer I, Doig C, Rodriguez AL, Beale R, Svoboda P, Laterre PF, Simon S, Light B, Spapen H, Stone J, Seibert A, Peckelsen C, De Deyne C, Postier R, Pettilä V, Sprung CL, Artigas A, Percell SR, Shu V, Zwingelstein C, Tobias J, Poole L, Stolzenbach JC, Creasey AA. Efficacy and safety of tifacogin (recombinant tissue factor pathway inhibitor) in severe sepsis: A randomized controlled trial. JAMA. 2003;290:238–247. doi: 10.1001/jama.290.2.238. [DOI] [PubMed] [Google Scholar]
- Ageno W, Gallus AS, Wittkowsky A, Crowther M, Hylek EM, Palareti G. Oral anticoagulant therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American college of chest physicians evidence-based clinical practice guidelines. Chest. 2012;141:e44S–e88S. doi: 10.1378/chest.11-2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed Z, Hassan S, Salzman GA. Novel oral anticoagulants for venous thromboembolism with special emphasis on risk of hemorrhagic complications and reversal agents. Curr Drug Ther. 2016;11:3–20. doi: 10.2174/1574885511666160421145036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiral J, Bridey F, Wolf M, Boyer-Neumann C, Fressinaud E, Vissac AM, Peynaud-Debayle E, Dreyfus M, Meyer D. Antibodies to macromolecular platelet factor 4-heparin complexes in heparin-induced thrombocytopenia: a study of 44 cases. Thromb Haemost. 1995;73:21–28. [PubMed] [Google Scholar]
- Bates SM, Weitz JI. The mechanism of action of thrombin inhibitors. J. Invasive Cardiol. 2000;12(Suppl F):27F–32. [PubMed] [Google Scholar]
- Baumann Kreuziger LM, Keenan JC, Morton CT, Dries DJ. Management of the bleeding patient receiving new oral anticoagulants: A role for prothrombin complex concentrates. BioMed Res Int. 2014;2014:583794. doi: 10.1155/2014/583794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bea F, Kreuzer J, Preusch M, Schaab S, Isermann B, Rosenfeld ME, Katus H, Blessing E. Melagatran reduces advanced atherosclerotic lesion size and may promote plaque stability in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2006;26:2787–2792. doi: 10.1161/01.ATV.0000246797.05781.ad. [DOI] [PubMed] [Google Scholar]
- Bounameaux H, Camm AJ. Edoxaban: an update on the new oral direct factor Xa inhibitor. Drugs. 2014;74:1209–1231. doi: 10.1007/s40265-014-0261-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruni-Fitzgerald KR. Venous thromboembolism: an overview. J Vasc Nurs. 2015;33:95–99. doi: 10.1016/j.jvn.2015.02.001. [DOI] [PubMed] [Google Scholar]
- Butenas S, Brummel KE, Branda RF, Paradis SG, Mann KG. Mechanism of factor VIIa-dependent coagulation in hemophilia blood. Blood. 2002;99:923–930. doi: 10.1182/blood.V99.3.923. [DOI] [PubMed] [Google Scholar]
- Büller HR, Bethune C, Bhanot S, Gailani D, Monia BP, Raskob GE, Segers A, Verhamme P, Weitz JI. Factor XI antisense oligonucleotide for prevention of venous thrombosis. N Engl J Med. 2015;372:232–240. doi: 10.1056/NEJMoa1405760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabral KP, Ansell JE. The role of factor Xa inhibitors in venous thromboembolism treatment. Vasc Health Risk Manage. 2015;11:117–123. doi: 10.2147/VHRM.S39726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapin JC, Hajjar KA. Fibrinolysis and the control of blood coagulation. Blood Rev. 2015;29:17–24. doi: 10.1016/j.blre.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow FS, Benincosa LJ, Sheth SB, Wilson D, Davis CB, Minthorn EA, Jusko WJ. Pharmacokinetic and pharmacodynamic modeling of humanized anti-factor IX antibody (SB 249417) in humans. Clin Pharmacol Ther. 2002;71:235–245. doi: 10.1067/mcp.2002.122276. [DOI] [PubMed] [Google Scholar]
- Connolly G, Spyropoulos AC. Practical issues, limitations, and periprocedural management of the NOAC’s. J. Thromb. Thrombolysis. 2013;36:212–222. doi: 10.1007/s11239-013-0911-2. [DOI] [PubMed] [Google Scholar]
- Connors JM. Antidote for factor Xa anticoagulants. N Engl J Med. 2015;373:2471–2472. doi: 10.1056/NEJMe1513258. [DOI] [PubMed] [Google Scholar]
- Coughlin SR. Thrombin receptor function and cardiovascular disease. Trends Cardiovasc Med. 1994;4:77–83. doi: 10.1016/1050-1738(94)90013-2. [DOI] [PubMed] [Google Scholar]
- Das A, Liu D. Novel antidotes for target specific oral anti-coagulants. Exp Hematol Oncol. 2015;4:25. doi: 10.1186/s40164-015-0020-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellinger RP. Inflammation and coagulation: Implications for the septic patient. Clin Infect Dis. 2003;36:1259–1265. doi: 10.1086/374835. [DOI] [PubMed] [Google Scholar]
- Dickinson CD, Ruf W. Active site modification of factor VIIa affects interactions of the protease domain with tissue factor. J Biol Chem. 1997;272:19875–19879. doi: 10.1074/jbc.272.32.19875. [DOI] [PubMed] [Google Scholar]
- Di Nisio M, Middeldorp S, Büller HR. Direct thrombin inhibitors. N Engl J Med. 2005;353:1028–1040. doi: 10.1056/NEJMra044440. [DOI] [PubMed] [Google Scholar]
- Eriksson BI, Quinlan DJ, Weitz JI. Comparative pharmacodynamics and pharmacokinetics of oral direct thrombin and factor Xa inhibitors in development. Clin Pharmacokinet. 2009;48:1–22. doi: 10.2165/0003088-200948010-00001. [DOI] [PubMed] [Google Scholar]
- Esmon CT. The interactions between inflammation and coagulation. Br J Haematol. 2005;131:417–430. doi: 10.1111/j.1365-2141.2005.05753.x. [DOI] [PubMed] [Google Scholar]
- Evans HC, Perry CM, Faulas D. Ximelagatran/melagatran. Drugs. 2004;64:649–678. doi: 10.2165/00003495-200464060-00010. [DOI] [PubMed] [Google Scholar]
- Fareed J, Hoppensteadt DA, Fareed D, Demir M, Wahi R, Clarke M, Adiguzel C, Bick R. Survival of heparins, oral anticoagulants, and aspirin after the year 2010. Semin Thromb Hemost. 2008;34:58–73. doi: 10.1055/s-2008-1066025. [DOI] [PubMed] [Google Scholar]
- Fitzmaurice DA, Blann AD, Lip GYH. Bleeding risks of antithrombotic therapy. BMJ. 2002;325:828–831. doi: 10.1136/bmj.325.7368.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchini M, Liumbruno GM, Bonfanti C, Lippi G. The evolution of anticoagulant therapy. Blood Transfus. 2016;14:175–184. doi: 10.2450/2015.0096-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung E, Patsopoulos NA, Belknap SM, O’Rourke DJ, Robb JF, Anderson JL, Shworak NW, Moore JH. Effect of genetic variants, especially CYP2C9 and VKORC1, on the pharmacology of warfarin. Semin Thromb Hemost. 2012;38:893–904. doi: 10.1055/s-0032-1328891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi NS, Mancera RL. Heparin/heparan sulphate-based drugs. Drug Discov. Today. 2010;15:1058–1069. doi: 10.1016/j.drudis.2010.10.009. [DOI] [PubMed] [Google Scholar]
- Garcia DA, Baglin TP, Weitz JI, Samama MM. Parenteral anticoagulants: antithrombotic therapy and prevention of thrombosis, 9th ed: American college of chest physicians evidence-based clinical practice guidelines. Chest. 2012;141:e24S–e43S. doi: 10.1378/chest.11-2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greinacher A, Thiele T, Selleng K. Reversal of anticoagulants: an overview of current developments. Thromb Haemost. 2015;113:931–942. doi: 10.1160/TH14-11-0982. [DOI] [PubMed] [Google Scholar]
- Hagedorn I, Schmidbauer S, Pleines I, Kleinschnitz C, Kronthaler U, Stoll G, Dickneite G, Nieswandt B. Factor XIIa inhibitor recombinant human albumin Infestin-4 abolishes occlusive arterial thrombus formation without affecting bleeding. Circulation. 2010;121:1510–1517. doi: 10.1161/CIRCULATIONAHA.109.924761. [DOI] [PubMed] [Google Scholar]
- Hardy S, Schirm S, Liu X, Dai Y. Tifacogin increases bacterial clearance from blood. Crit. Care. 2006;10:P155. doi: 10.1186/cc4502. [DOI] [Google Scholar]
- Heit JA, Spencer FA, White RH. The epidemiology of venous thromboembolism. J. Thromb. Thrombolysis. 2016;41:3–14. doi: 10.1007/s11239-015-1311-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsh J. Heparin. N Engl J Med. 1991;324:1565–1574. doi: 10.1056/NEJM199105303242206. [DOI] [PubMed] [Google Scholar]
- Hirsh J. Low-molecular-weight heparin. Circulation. 1998;98:1575–1582. doi: 10.1161/01.CIR.98.15.1575. [DOI] [PubMed] [Google Scholar]
- Hirsh J, Fuster V, Ansell J, Halperin JL. American heart association/American college of cardiology foundation guide to warfarin therapy. J Am Coll Cardiol. 2003;41:1633–1652. doi: 10.1016/S0735-1097(03)00416-9. [DOI] [PubMed] [Google Scholar]
- Hirsh J, O’Donnell M, Eikelboom JW. Beyond unfractionated heparin and warfarin: current and future advances. Circulation. 2007;116:552–560. doi: 10.1161/CIRCULATIONAHA.106.685974. [DOI] [PubMed] [Google Scholar]
- Hirsh J, Fuster V. Guide to anticoagulant therapy. Part I: heparin. Circulation. 1994;89:1449–1468. doi: 10.1161/01.CIR.89.3.1449. [DOI] [PubMed] [Google Scholar]
- Hirsh J, Raschke R. Heparin and low-molecular-weight heparin: The seventh ACCP conference on antithrombotic and thrombolytic therapy. Chest. 2004;126:188S–203S. doi: 10.1378/chest.126.3_suppl.188S. [DOI] [PubMed] [Google Scholar]
- Holmer E, Söderberg K, Bergqvist D, Lindahl U. Heparin and its low molecular weight derivatives: Anticoagulant and antithrombotic properties. Haemostasis. 1986;16(Suppl 2):1–7. doi: 10.1159/000215348. [DOI] [PubMed] [Google Scholar]
- Hoppensteadt D, Walenga JM, Fareed J, Bick RL. Heparin, low-molecular-weight heparins, and heparin pentasaccharide: Basic and clinical differentiation. Hematol Oncol Clin North Am. 2003;17:313–341. doi: 10.1016/S0889-8588(02)00091-6. [DOI] [PubMed] [Google Scholar]
- Howard EL, Becker KCD, Rusconi CP, Becker RC. Factor IXa inhibitors as novel anticoagulants. Arterioscler Thromb Vasc Biol. 2007;27:722–727. doi: 10.1161/01.ATV.0000259363.91070.f1. [DOI] [PubMed] [Google Scholar]
- Hu TY, Vaidya VR, Asirvatham SJ. Reversing anticoagulant effects of novel oral anticoagulants: Role of ciraparantag, andexanet al fa, and idarucizumab. Vasc Health Risk Manag. 2016;12:35–44. doi: 10.2147/VHRM.S89130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyers TM. Venous thromboembolism. Am J Respir Crit Care Med. 1999;159:1–14. doi: 10.1164/ajrccm.159.1.9803109. [DOI] [PubMed] [Google Scholar]
- Kearon C, Comp P, Douketis J, Royds R, Yamada K, Gent M. Dose-response study of recombinant human soluble thrombomodulin (ART-123) in the prevention of venous thromboembolism after total hip replacement. J Thrombo Haemost. 2005;3:962–968. doi: 10.1111/j.1538-7836.2005.01251.x. [DOI] [PubMed] [Google Scholar]
- Kim JH, Lee J, Kang S, Moon H, Chung KH, Kim KR. Antiplatelet and antithrombotic effects of the extract of lindera obtusiloba leaves. Biomol. Ther. (Seoul) 2016;24:659–664. doi: 10.4062/biomolther.2016.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster A, Fischer KG, Harder S, Mertzlufft F. The direct thrombin inhibitor argatroban: a review of its use in patients with and without HIT. Biologics. 2007;1:105–112. [PMC free article] [PubMed] [Google Scholar]
- Kreimann M, Brandt S, Krauel K, Block S, Helm CA, Weitschies W, Greinacher A, Delcea M. Binding of anti-platelet factor 4/heparin antibodies depends on the thermodynamics of conformational changes in platelet factor 4. Blood. 2014;124:2442–2449. doi: 10.1182/blood-2014-03-559518. [DOI] [PubMed] [Google Scholar]
- Krishnaswamy A, Lincoff AM, Cannon CP. The use and limitations of unfractionated heparin. Crit Pathw Cardiol. 2010;9:35–40. doi: 10.1097/HPC.0b013e3181d29713. [DOI] [PubMed] [Google Scholar]
- Kwon IS, Yim JH, Lee HK, Pyo S. Lobaric acid inhibits VCAM-1 expression in TNF-alpha-stimulated vascular smooth muscle cells via modulation of NF-kappaB and MAPK signaling pathways. Biomol. Ther. (Seoul) 2016;24:25–32. doi: 10.4062/biomolther.2015.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laterre PF, Opal SM, Abraham E, LaRosa SP, Creasey AA, Xie F, Poole L, Wunderink RG. A clinical evaluation committee assessment of recombinant human tissue factor pathway inhibitor (tifacogin) in patients with severe community-acquired pneumonia. Critic. Care. 2009;13:R36. doi: 10.1186/cc7747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CJ, Ansell JE. Direct thrombin inhibitors. Br J Clin Pharmacol. 2011;72:581–592. doi: 10.1111/j.1365-2125.2011.03916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DH, Kim HH, Lim DH, Kim JL, Park HJ. Effect of cordycepin-enriched WIB801C from cordyceps militaris suppressing fibrinogen binding to glycoprotein IIb/IIIa. Biomol. Ther. (Seoul) 2015;23:60–70. doi: 10.4062/biomolther.2014.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenting PJ, van Mourik JA, Mertens K. The life cycle of coagulation factor VIII in view of its structure and function. Blood. 1998;92:3983–3996. [PubMed] [Google Scholar]
- Liaw PC, Becker DL, Stafford AR, Fredenburgh JC, Weitz JI. Molecular basis for the susceptibility of fibrin-bound thrombin to inactivation by heparin cofactor II in the presence of dermatan sulfate but not heparin. J Biol Chem. 2001;276:20959–20965. doi: 10.1074/jbc.M010584200. [DOI] [PubMed] [Google Scholar]
- Linkins LA, Choi PT, Douketis JD. Clinical impact of bleeding in patients taking oral anticoagulant therapy for venous thromboembolism: A meta-analysis. Ann Intern Med. 2003;139:893–900. doi: 10.7326/0003-4819-139-11-200312020-00007. [DOI] [PubMed] [Google Scholar]
- Loke C, Ali SS, Johari V. Pharmacology of anticoagulants. Dis Mon. 2012;58:424–430. doi: 10.1016/j.disamonth.2012.04.005. [DOI] [PubMed] [Google Scholar]
- Lurie Y, Loebstein R, Kurnik D, Almog S, Halkin H. Warfarin and vitamin K intake in the era of pharmacogenetics. Br J Clin Pharmacol. 2010;70:164–170. doi: 10.1111/j.1365-2125.2010.03672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majeed A, Schulman S. Bleeding and antidotes in new oral anticoagulants. Best Pract Res Clin Haematol. 2013;26:191–202. doi: 10.1016/j.beha.2013.07.001. [DOI] [PubMed] [Google Scholar]
- Mann KG, Brummel K, Butenas S. What is all that thrombin for? J Thromb Haemost. 2003;1:1504–1514. doi: 10.1046/j.1538-7836.2003.00298.x. [DOI] [PubMed] [Google Scholar]
- Marmur JD. Direct versus indirect thrombin inhibition in percutaneous coronary intervention. J. Invasive Cardiol. 2002;14(Suppl B):8B–18B. [PubMed] [Google Scholar]
- Masuko S, Linhardt RJ. Chemoenzymatic synthesis of the next generation of ultralow MW heparin therapeutics. Future Med Chem. 2012;4:289–296. doi: 10.4155/fmc.11.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matyal R, Mahmood F, Park KW. Tifacogin, recombinant tissue factor pathway inhibitor. Int Anesthesiol Clin. 2005;43:135–144. doi: 10.1097/01.aia.0000157499.41843.a3. [DOI] [PubMed] [Google Scholar]
- Mega JL, Simon T. Pharmacology of antithrombotic drugs: an assessment of oral antiplatelet and anticoagulant treatments. Lancet. 2015;386:281–291. doi: 10.1016/S0140-6736(15)60243-4. [DOI] [PubMed] [Google Scholar]
- Mekaj YH, Mekaj AY, Duci SB, Miftari EI. New oral anticoagulants: their advantages and disadvantages compared with vitamin K antagonists in the prevention and treatment of patients with thromboembolic events. Ther Clin Risk Manag. 2015;11:967–977. doi: 10.2147/TCRM.S84210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merli GJ, Groce JB. Pharmacological and clinical differences between low-molecular-weight heparins: Implications for prescribing practice and therapeutic interchange. P T. 2010;35:95–105. [PMC free article] [PubMed] [Google Scholar]
- Miller CS, Grandi SM, Shimony A, Filion KB, Eisenberg MJ. Meta-analysis of efficacy and safety of new oral anti-coagulants (dabigatran, rivaroxaban, apixaban) versus warfarin in patients with atrial fibrillation. Am J Cardio. 2012;110:453–460. doi: 10.1016/j.amjcard.2012.03.049. [DOI] [PubMed] [Google Scholar]
- Nawarskas JJ, Anderson JR. Bivalirudin: A new approach to anticoagulation. Heart Dis. 2001;3:131–137. doi: 10.1097/00132580-200103000-00010. [DOI] [PubMed] [Google Scholar]
- Park JM, Chang KH, Park KH, Choi SJ, Lee K, Lee JY, Satoh M, Song SY, Lee MY. Differential effects between cigarette total particulate matter and cigarette smoke extract on blood and blood vessel. Toxicol Res. 2016;32:353–358. doi: 10.5487/TR.2016.32.4.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pempe EH, Xu Y, Gopalakrishnan S, Liu J, Harris EN. Probing structural selectivity of synthetic heparin binding to stabilin protein receptors. J Biol Chem. 2012;287:20774–20783. doi: 10.1074/jbc.M111.320069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perzborn E, Roehrig S, Straub A, Kubitza D, Misselwitz F. The discovery and development of rivaroxaban, an oral, direct factor Xa inhibitor. Nat Rev Drug Discov. 2011;10:61–75. doi: 10.1038/nrd3185. [DOI] [PubMed] [Google Scholar]
- Piazza G, Goldhaber SZ. Venous thromboembolism and atherothrombosis: an integrated approach. Circulation. 2010;121:2146–2150. doi: 10.1161/CIRCULATIONAHA.110.951236. [DOI] [PubMed] [Google Scholar]
- Previtali E, Bucciarelli P, Passamonti SM, Martinelli I. Risk factors for venous and arterial thrombosis. Blood Transfus. 2011;9:120–138. doi: 10.2450/2010.0066-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puurunen MK, Gona PN, Larson MG, Murabito JM, Magnani JW, O’Donnell CJ. Epidemiology of venous thromboembolism in the framingham heart study. Thromb Res. 2016;145:27–33. doi: 10.1016/j.thromres.2016.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauova L, Poncz M, McKenzie SE, Reilly MP, Arepally G, Weisel JW, Nagaswami C, Cines DB, Sachais BS. Ultralarge complexes of PF4 and heparin are central to the pathogenesis of heparin-induced thrombocytopenia. Blood. 2005;105:131–138. doi: 10.1182/blood-2004-04-1544. [DOI] [PubMed] [Google Scholar]
- Rauova L, Zhai L, Kowalska MA, Arepally GM, Cines DB, Poncz M. Role of platelet surface PF4 antigenic complexes in heparin-induced thrombocytopenia pathogenesis: diagnostic and therapeutic implications. Blood. 2006;107:2346–2353. doi: 10.1182/blood-2005-08-3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roser-Jones C, Chan M, Howard EL, Becker KC, Rusconi CP, Becker RC. Factor IXa as a target for pharmacologic inhibition in acute coronary syndrome. Cardiovasc Ther. 2011;29:e22–e35. doi: 10.1111/j.1755-5922.2010.00134.x. [DOI] [PubMed] [Google Scholar]
- Ruiz FA, Lea CR, Oldfield E, Docampo R. Human platelet dense granules contain polyphosphate and are similar to acidocalcisomes of bacteria and unicellular eukaryotes. J Biol Chem. 2004;279:44250–44257. doi: 10.1074/jbc.M406261200. [DOI] [PubMed] [Google Scholar]
- Ruppert A, Lees M, Steinle T. Clinical burden of venous thromboembolism. Curr Med Res Opin. 2010;26:2465–2473. doi: 10.1185/03007995.2010.516090. [DOI] [PubMed] [Google Scholar]
- Rupprecht HJ, Blank R. Clinical pharmacology of direct and indirect factor Xa inhibitors. Drugs. 2010;70:2153–2170. doi: 10.2165/11538030-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Schmid P, Fischer AG, Wuillemin WA. Low-molecular-weight heparin in patients with renal insufficiency. Swiss Med. Wkly. 2009;139:438–452. doi: 10.4414/smw.2009.11284. [DOI] [PubMed] [Google Scholar]
- Schroeder M, Hogwood J, Gray E, Mulloy B, Hackett AM, Johansen KB. Protamine neutralisation of low molecular weight heparins and their oligosaccharide components. Anal. Bio-anal Chem. 2011;399:763–771. doi: 10.1007/s00216-010-4220-8. [DOI] [PubMed] [Google Scholar]
- Short NJ, Connors JM. New oral anticoagulants and the cancer patient. Oncologist. 2014;19:82–93. doi: 10.1634/theoncologist.2013-0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sie P. Spotlight on idarucizumab and its potential for the reversal of anticoagulant effects of dabigatran. Drug Des Devel Ther. 2016;10:1683–1689. doi: 10.2147/DDDT.S94167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SA, Choi SH, Collins JN, Travers RJ, Cooley BC, Morrissey JH. Inhibition of polyphosphate as a novel strategy for preventing thrombosis and inflammation. Blood. 2012;120:5103–5110. doi: 10.1182/blood-2012-07-444935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SA, Morrissey JH. Polyphosphate enhances fibrin clot structure. Blood. 2008;112:2810–2816. doi: 10.1182/blood-2008-03-145755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stangier J, Clemens A. Pharmacology, pharmacokinetics, and pharmacodynamics of dabigatran etexilate, an oral direct thrombin inhibitor. Clin. Appl. Thromb. Hemost. 2009;15(Suppl 1):9S–16S. doi: 10.1177/1076029609343004. [DOI] [PubMed] [Google Scholar]
- Teitel JM, Rosenberg RD. Protection of factor Xa from neutralization by the heparin-antithrombin complex. J Clin Invest. 1983;71:1383–1391. doi: 10.1172/JCI110891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollefsen DM, Majerus DW, Blank MK. Heparin cofactor II. Purification and properties of a heparin-dependent inhibitor of thrombin in human plasma. J Biol Chem. 1982;257:2162–2169. [PubMed] [Google Scholar]
- Tran HA, Chunilal SD, Harper PL, Tran H, Wood EM, Gallus AS. An update of consensus guidelines for warfarin reversal. Med J Aust. 2013;198:198–199. doi: 10.5694/mja12.10614. [DOI] [PubMed] [Google Scholar]
- Travers RJ, Shenoi RA, Kalathottukaren MT, Kizhakkedathu JN, Morrissey JH. Nontoxic polyphosphate inhibitors reduce thrombosis while sparing hemostasis. Blood. 2014;124:3183–3190. doi: 10.1182/blood-2014-05-577932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vavalle JP, Cohen MG. The REG1 anticoagulation system: A novel actively controlled factor IX inhibitor using RNA aptamer technology for treatment of acute coronary syndrome. Future Cardiol. 2012;8:371–382. doi: 10.2217/fca.12.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhamme P, Pakola S, Jensen TJ, Berggren K, Sonesson E, Saint-Remy JM, Balchen T, Belmans A, Cahillane G, Stassen JM, Peerlinck K, Glazer S, Jacquemin M. Tolerability and pharmacokinetics of TB-402 in healthy male volunteers. Clin Ther. 2010;32:1205–1220. doi: 10.1016/j.clinthera.2010.06.012. [DOI] [PubMed] [Google Scholar]
- Verhoef TI, Redekop WK, Daly AK, van Schie RM, de Boer A, Maitland-van der Zee AH. Pharmacogenetic-guided dosing of coumarin anticoagulants: algorithms for warfarin, acenocoumarol and phenprocoumon. Br J Clin Pharmacol. 2014;77:626–641. doi: 10.1111/bcp.12220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Ramesh MK, Ernest D, LaRosa SP, Pachl J, Aikawa N, Hoste E, Levy H, Hirman J, Levi M, Daga M, Kutsogiannis DJ, Crowther M, Bernard GR, Devriendt J, Puigserver JV, Blanzaco DU, Esmon CT, Parrillo JE, Guzzi L, Henderson SJ, Pothirat C, Mehta P, Fareed J, Talwar D, Tsuruta K, Gorelick KJ, Osawa Y, Kaul I. A randomized, double-blind, placebo-controlled, Phase 2b study to evaluate the safety and efficacy of recombinant human soluble thrombomodulin, ART-123, in patients with sepsis and suspected disseminated intravascular coagulation. Crit Care Med. 2013;41:2069–2079. doi: 10.1097/CCM.0b013e31828e9b03. [DOI] [PubMed] [Google Scholar]
- Vlasuk GP, Rote WE. Inhibition of factor VIIa/tissue factor with nematode anticoagulant protein c2: from unique mechanism to a promising new clinical anticoagulant. Trends Cardiovasc Med. 2002;12:325–331. doi: 10.1016/S1050-1738(02)00185-8. [DOI] [PubMed] [Google Scholar]
- Walenga JM, Lyman GH. Evolution of heparin anticoagulants to ultra-low-molecular-weight heparins: a review of pharmacologic and clinical differences and applications in patients with cancer. Crit Rev Oncol Hematol. 2013;88:1–18. doi: 10.1016/j.critrevonc.2013.06.007. [DOI] [PubMed] [Google Scholar]
- Weitz JI. Low-molecular-weight heparins. N Engl J Med. 1997;337:688–698. doi: 10.1056/NEJM199709043371007. [DOI] [PubMed] [Google Scholar]
- Weitz JI, Hudoba M, Massel D, Maraganore J, Hirsh J. Clot-bound thrombin is protected from inhibition by heparin-antithrombin III but is susceptible to inactivation by antithrombin III-independent inhibitors. J Clin Invest. 1990;86:385–391. doi: 10.1172/JCI114723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessler S, Gitel SN. Pharmacology of heparin and warfarin. J Am Coll Cardiol. 1986;8:10B–20B. doi: 10.1016/S0735-1097(86)80003-1. [DOI] [PubMed] [Google Scholar]
- Wolzt M, Weltermann A, Nieszpaur-Los M, Schneider B, Fassolt A, Lechner K, Eichler HG, Kyrle PA. Studies on the neutralizing effects of protamine on unfractionated and low molecular weight heparin (Fragmin) at the site of activation of the coagulation system in man. Thromb Haemost. 1995;73:439–443. [PubMed] [Google Scholar]
- Wood JP, Ellery PE, Maroney SA, Mast AE. Biology of tissue factor pathway inhibitor. Blood. 2014;123:2934–2943. doi: 10.1182/blood-2013-11-512764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh CH, Fredenburgh JC, Weitz JI. Oral direct factor Xa inhibitors. Circ Res. 2012;111:1069–1078. doi: 10.1161/CIRCRESAHA.112.276741. [DOI] [PubMed] [Google Scholar]