Abstract
Genetic risk factors for autism spectrum disorder (ASD) have yet to be fully elucidated. Postzygotic mosaic mutations (PMMs) have been implicated in several neurodevelopmental disorders and overgrowth syndromes. By leveraging whole-exome sequencing data on a large family-based ASD cohort, the Simons Simplex Collection, we systematically evaluated the potential role of PMMs in autism risk. Initial re-evaluation of published single-nucleotide variant (SNV) de novo mutations showed evidence consistent with putative PMMs for 11% of mutations. We developed a robust and sensitive SNV PMM calling approach integrating complementary callers, logistic regression modeling, and additional heuristics. In our high-confidence call set, we identified 470 PMMs in children, increasing the proportion of mosaic SNVs to 22%. Probands have a significant burden of synonymous PMMs and these mutations are enriched for computationally predicted impacts on splicing. Evidence of increased missense PMM burden was not seen in the full cohort. However, missense burden signal increased in subcohorts of families where probands lacked nonsynonymous germline mutations, especially in genes intolerant to mutations. Parental mosaic mutations that were transmitted account for 6.8% of the presumed de novo mutations in the children. PMMs were identified in previously implicated high-confidence neurodevelopmental disorder risk genes, such as CHD2, CTNNB1, SCN2A, and SYNGAP1, as well as candidate risk genes with predicted functions in chromatin remodeling or neurodevelopment, including ACTL6B, BAZ2B, COL5A3, SSRP1, and UNC79. We estimate that PMMs potentially contribute risk to 3%-4% of simplex ASD case subjects and future studies of PMMs in ASD and related disorders are warranted.
Keywords: autism spectrum disorder, postzygotic, mosaicism, somatic, mutation, neurodevelopment, exome, splicing
Introduction
Autism spectrum disorder (ASD [MIM: 209850]) has a strong genetic component and a complex genetic architecture. Technological advances have allowed the discovery of rare inherited and de novo mutations in ASD cohorts, including copy-number variants (CNVs), structural variants, single-nucleotide variants (SNVs), and small insertions and deletions (indels).1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13 These studies, especially those focused on simplex cohorts (single affected individual within a family), have revealed a significant contribution of de novo mutations implicating hundreds of independent loci in ASD risk. However, the full complement of ASD risk factors and mechanisms have yet to be fully elucidated.
Postzygotic mutations occur after fertilization of the embryo. Depending on their timing and cell lineage, these mutations may be found in the soma, resulting in somatic mosaicism, or the germ cells, resulting in gonadal mosaicism. Mutations occurring during early embryonic development can result in both types of mosaicism.14 For simplicity, we will refer to these mutations generally as postzygotic mosaic mutations (PMMs), because in most cases their contribution to the germline is unknown. In addition to the well-known role of somatic mutations in cancer, PMMs have been firmly implicated in several neurodevelopmental/brain disorders including epilepsy, cortical malformations, RASopathies, and overgrowth syndromes.15, 16, 17, 18, 19, 20, 21 Pathways underlying some of these syndromes, e.g., PI3K/ATK/mTOR and RAS-MAPK, are also implicated in syndromic and nonsyndromic ASD.
The mosaic nature of these mutations can make them difficult to identify with current clinical testing, even when targeting specific genes, leading to no diagnosis, misdiagnosis, or misinterpretation of recurrence risk.16, 22 It has also been hypothesized that sporadic conditions may be caused by PMMs at loci where germline mutations are embryonic lethal.23 Importantly, when and where mutations occur in development can have a dramatic effect on the phenotypic presentation as exemplified by PIK3CA-related overgrowth spectrum (PROS).15, 24 Moreover, recent data have suggested that even low-level mosaicism (∼1% in affected tissue) can be clinically significant, as shown in the affected skin/brain of individuals with Sturge-Weber syndrome (MIM: 185300).25
In previous work focusing on discovering germline de novo mutations (GDMs) in simplex ASD families, we were surprised to validate 4.2% of de novo mutations as likely mosaic in origin, including nine PMMs and two gonadal mosaic mutations (from a total 260 mutations), suggesting that mosaic mutations might be a common and under-recognized contributor to ASD risk.2 A similar observation has been made from de novo mutations identified in whole-genome sequencing from simplex intellectual disability (ID) trios.26 However, the mutation calling approaches used previously were tuned to detect GDMs.
Here, we systematically evaluate the role of PMMs in ASD by leveraging a harmonized dataset12 of existing whole-exome sequences (WES) from a well-characterized cohort of ∼2,300 families—the Simons Simplex Collection (SSC), including parents, probands, and unaffected siblings. Our goal was to answer several fundamental questions. (1) What are the rates of PMMs (detectable in whole blood DNA) in children and do they play a role in ASD risk? (2) What are the rates of PMMs in parents and how often are these events transmitted to offspring? (3) Do the target genes of GDMs and PMMs in individuals with ASD overlap? To answer these questions, we first re-evaluated all previously published de novo mutations using a binomial approach and found evidence that 11% of SNVs and 26% of indels called with methods intended for germline mutation detection show allele skewing consistent with mosaicism. We then developed a systematic method for identifying, specifically, SNVs that are likely PMMs from WES (or other next-generation sequencing [NGS] data), which integrates calls from complementary approaches and extensive validation data.
We recalled genotypes on the SSC cohort and estimate that 22% of de novo SNVs are, in fact, PMMs arising in children. Unexpectedly, the strongest signal for mutation burden in probands was observed for synonymous PMMs. Furthermore, synonymous PMMs occurring in probands are enriched for mutations predicted to impact splicing. Evidence of missense PMM burden in the full cohort was not observed; however, burden signal did increase in subsets of the cohort without germline mutations, which is strongest in genes that are intolerant to mutations. Parental mosaic mutations occurred at a higher rate and were frequently transmitted to children. Nonsynonymous (NS) PMMs were identified in high-confidence ASD/ID risk genes and candidate risk genes involved with chromatin remodeling or neurodevelopment. Overall, these findings suggest that future studies of PMMs in ASD and related disorders are warranted.
Material and Methods
Family Selection and Sequence Data
We obtained the initially published1, 2, 4, 5, 11 and harmonized reprocessed12 WES data from 2,506 families of the Simons Simplex Collection (SSC).27 Harmonized data are available from NIMH Data Archive (NDAR: 10.15154/1169193) or SFARI base. Informed consents were obtained by each SSC recruitment site, in accordance with their local institutional review board (IRB). Oregon Health & Science University IRB approved our study as human subjects exempt because only de-identified data was accessed. Exome libraries were previously generated from whole-blood (WB)-derived DNA and captured with NimbleGen EZ Exome v.2.0 or similar custom reagents (Roche Nimblegen) and sequenced using Illumina chemistry at one of three centers: Cold Spring Harbor Laboratory (CSHL), University of Washington (UW), or Yale University School of Medicine. Where individuals had been sequenced by multiple centers, the library with the highest mean coverage was included in the harmonized reprocessed dataset (N. Krumm, personal communication).12
We selected 24 family quads (“pilot 24”) for initial methods development that had WES independently performed by all three centers.11 WES data were merged and then reprocessed to match the harmonized dataset.12 We then expanded to a cohort of 400 additional independent quad families (“pilot 400”) with high median WES coverage, also requiring proportionate distribution across the three centers (Yale, 193; CSHL, 118; UW, 89). The full SSC harmonized reprocessed dataset12 contained 2,366 families, of which 1,781 are quads and 585 are trios (Table S1), after removing samples with known Mendelian inconsistencies or contamination issues (N. Krumm, personal communication). One hundred and two families with individuals showing elevated GDM or PMM calls were excluded post variant calling (Supplemental Material and Methods, Figure S1). The cohort used in the downstream analyses included 2,264 families, of which 1,698 are quads and 566 are trios. Additional families with low joint coverage values were removed depending on the minimum coverage requirement for analyzing variants of different minimum allele fractions (AF) (see Supplemental Material and Methods).
Evaluating Potential Mosaic Mutations in Previously Published De Novo Calls
Reported de novo mutations for the SSC were evaluated (Table S2).1, 2, 4, 5, 11, 12 Allele counts from prior analysis were used where available (N. Krumm, personal communication) and otherwise extracted on a quality-aware basis from mpileups of the corresponding WES using a custom script (samtools mpileup -B -d 1500 | mPUP -m -q 20 -a count). Reported mutation calls that had no variant reads from the quality-aware mpileup data were excluded. We focused our analysis on exonic and canonical intronic splice site regions (±2 base pairs [bp]). Mutations were considered putative PMMs if significantly skewed from the heterozygosity expectation of 0.5 AF for autosomal and X chromosome sites of females (binomial p ≤ 0.001). Sex chromosome sites of males were evaluated under a hemizygous expectation. Robustness of the data was evaluated using additional filters for observed AF (5%–35%, 10%–35%, 10%–25%, or corresponding hemizygous values) or at more strict deviations from the binomial expectation (p ≤ 0.0001). The observed rates of AF skewed de novo mutations were compared with expected null distributions of randomly sampled rare inherited variants by simulation (Supplemental Material and Methods).
Raw Variant Calling and Annotation
SNVs were recalled on individual samples using VarScan 2.3.2, LoFreq 2.1.1, and our in-house script mPUP (Supplemental Material and Methods). All caller outputs were combined at the individual level and used to generate family-level variant tables. Variants were annotated with ANNOVAR (03/22/15 release, see Web Resources)28 against the following databases: RefSeq genes (obtained 2015-12-11), segmental duplications (UCSC track genomicSuperDups, obtained 2015-03-25), repetitive regions (UCSC track simpleRepeat, obtained 2015-03-25), Exome Aggregation Consortium (ExAC) release 0.3 (prepared 2015-11-29), Exome Sequencing Project (ESP) 6500 (prepared 2014-12-22), and 1000 Genomes Phase 3 version 5 (prepared 2014-12-16). Annotation tracks did not include added flanking sequences. Population frequency databases were obtained from the ANNOVAR website. Initially, variants with AFs significantly below 50% (binomial p ≤ 0.001) were considered putative PMMs. For putative transmitted parental PMMs, which also had skewed AFs in child(ren), we required a significant difference between parent and child AF (Fisher’s exact p ≤ 0.01), with child AF > parental AF. Only PMM (child or parental) or GDM calls were considered for validation.
smMIP Design, Capture, and Sequencing
Three to four independent smMIPs were designed against candidate variant sites using the 11-25-14 release of MIPGEN29 and a custom in-house selection script (Supplemental Material and Methods). The selected smMIPs were divided into pools with roughly equal numbers (Table S3). Single strand capture probes were prepared similarly to previous approaches with modifications (Supplemental Material and Methods).29 DNA samples prepared from WB (entire pilot 24; 78 families pilot 400) and lymphoblastoid cell lines (LCLs) (entire pilot 24) were obtained from the SSC through Rutgers University Cell and DNA Repository (Piscataway, NJ). Probe captures and PCRs to append sequencing adaptors and barcodes were performed as previously described with minor modifications.30
Purified capture pools were then combined together for sequencing with NextSeq500 v2 chemistry (Illumina). Overlapping reads were merged and aligned using BWA 0.7.1. For each unique smMIP tag, the read with the highest sum of quality scores was selected to serve as the single read for the tag group. Validation outcomes were compared across WB and LCL data (where available) (Table S4).
Establishing a Systematic PMM Calling Pipeline
We iteratively developed best practices and heuristics through multiple rounds of validation and model development (Supplemental Note: Model Development and Material and Methods). Initial evaluation and smMIP validation was performed on the higher-depth pilot 24 dataset (Figures S2–S8, Supplemental Note: Model Development and Material and Methods). An initial logistic regression model was trained on the pilot 24 resolutions, using only calls validated as true PMMs or false positives in the smMIP data. Candidate model predictors were derived from WES data (Supplemental Material and Methods).
We next evaluated pilot 400 quad families (Figures S9–S12). Based on results from the initial validations, for all putative parental transmitted PMMs, we required more significant skew in parental AF (binomial p ≤ 0.0001), significant difference between parent and child AF (Fisher’s exact p ≤ 0.01), and child AF > parental AF (Figure S8). All putative PMMs scoring < 0.2 in the initial logistic regression model were excluded. Validations using smMIPs were conducted on calls from 78 of the pilot 400 families. All initial validation-positive calls, from both pilot sets, were then subjected to an additional manual review of the WES and smMIP alignments to flag potentially problematic sites prior to modeling.
A refined logistic regression model was trained based on the pilot 400 validation data (Supplemental Material and Methods, Figure S9). We further evaluated this refined model, applying the same filtering parameters as the training set, using the pilot 24 validation calls, which had been selected prior to any modeling or validations.
A third set of calls was evaluated from both pilot sets that had not previously been validated due to data missingness in population frequency datasets (Supplemental Note: Model Development). To better separate germline from mosaic calls based on our empirical validations, 90% binomial confidence intervals (CI) (Agresti-Coull method) for the variant AFs derived from the WES data were calculated using the R binom package. Based upon the distribution of germline resolutions in these data, putative PMMs were re-classified as germline if the upper bound of their observed AF was ≥0.4 (95% CI, one-tailed) (Figure S10). Additionally, calls were excluded that annotated as segmental duplication regions/tandem repeat finder (SD/TRF) sites or mPUP-only calls as they had a significantly higher false positive and smMIP probe failure rate (Figure S11). Putative PMMs passing filters from this third set of calls were scored with the refined logistic regression model and excluded from validations if they scored < 0.26. We retroactively applied our refined filtering scheme to all validation calls in order to develop a harmonized set of high-confidence resolutions and evaluated sensitivity and PPV of the refined model (Figure S12). Variants with a refined logistic model score ≥ 0.518 were included for additional analyses.
Cohort Variant Calling and Burden Analysis
Variants were called from all WES data in the harmonized reprocessed dataset and filtered with our best practice filtering scheme (Supplemental Material and Methods). To improve PPV for true PMMs, we required all variants be supported by at least five variant reads and present in no more than two families throughout the cohort (Figure S11). Eight variants were removed that had skewed AFs in both the child(ren) and parent. We defined our high-confidence dataset as those variants with AF ≥ 5% (based on the AF upper 90% CI) and 45× minimum joint coverage in all family members (Table S5).
For burden analysis, five minimum variant AFs thresholds were evaluated (5%, 7.5%, 10%, 12.5%, 15%). For each AF threshold, we determined the minimum total depth (130×, 85×, 65×, 50×, 45×) at which we had approximately 80% binomial probability to observe five or more variant reads (Figure S13). A variant was included for each subanalysis if its AF upper 90% CI met the minimum AF and if it met minimum coverage requirements in all family members. For each AF burden analysis, the total number of jointly sequenced bases at or above each depth threshold in each family was determined. Based on these joint coverage values, families in the 5th percentile or lower were excluded; in the 130× analysis the bottom decile was excluded (Figure S14).
Mutation burden and in the unique autosomal sequence was determined by first calculating the rate of mutation in each individual by summing all SNVs within a given functional class or gene set, e.g., for missense variants, and dividing by the total number of jointly sequenced bases (diploid, 2n) meeting the minimum coverage thresholds. Rates of mutation were then compared between groups (probands versus siblings or fathers versus mothers) using, as appropriate, paired or unpaired nonparametric rank tests. To control for multiple comparisons, we used the Benjamini-Yekutieli approach,31 which allows for dependent data structures, setting a false discovery rate (FDR) of 0.05. Families of tests were defined based on the dataset and mutation functional class (Supplemental Material and Methods).
To calculate mean population rates for each group of individuals (e.g., probands) for plotting and extrapolating variant counts to a full-coverage exome, all SNVs within a given functional class or gene set were summed and divided by the total number of jointly sequenced bases (diploid, 2n) for all families meeting the minimum coverage thresholds. Poisson 95% confidence intervals for mean rates were estimated using the Poisson exact method based on the observed number of SNVs.
Subcohort burden analyses were performed by separating families based on whether or not probands had previously identified GDMs in published call sets.1, 2, 4, 5, 11, 12, 32 Mutations with no read support or flagged as potentially mosaic from our initial analysis of published de novo calls were removed (binomial p ≤ 0.001). Two levels of disruption were considered: whether probands had germline de novo likely gene disrupting (LGD) mutations, which we define as SNVs, indels, or de novo CNVs that affect at least one gene (germline LGD list); or alternatively, whether probands had any germline de novo NS SNVs or indels (any germline NS list). The probands with any germline NS list is inclusive of probands with germline LGDs.
Burden in genes that show evidence of selection against new mutations was evaluated using the recently updated essential gene set,33 which contains human orthologs of mouse genes associated with lethality in the Mouse Genome Database;33, 34 and the ExAC intolerant dataset, which denotes the probability of a gene being loss-of-function intolerant.35
Analysis of PMM Properties
The AF distributions between children and parents PMMs were compared by Wilcoxon-rank sum test using the high-confidence dataset. To determine the fraction of parental PMMs that may be attributed to lack of grandparental data, variant calls were regenerated from the non-merged reprocessed WES data12 for the pilot 24/400 families applying the same refined logistic model and final filters, but ignoring family data. The observed bimodal AF distributions were fit to normal mixed models using R package mixtools, function normalmixEM(), which defined two Gaussian distributions. Calls were separated into two discrete sets. G1 was defined by the mean plus or minus two standard deviations of the leftmost Gaussian model (lower AFs, μ1 = 0.09, σ1 = 0.046). G2 included the remaining higher AF calls. The fraction of calling remaining in each set after applying transmission filters was calculated and used to estimate the number of variants expected to remain in the parents if the grandparental generation was available.
Splice site distances for variants were annotated using Variant Effect Predictor (see Web Resources). The absolute value of the shorter of the two distances between donor or acceptor site was chosen as the distance to nearest splice site. Potential impacts of synonymous mutations on splicing were evaluated using Human Splice Finder (HSF) v.3.0 and SPANR alpha version (see Web Resources).36, 37 For HSF, the multiple transcript analysis was used with default settings and results were extracted from HTML format outputs with an in-house script (Table S6). Variants contained within multiple overlapping transcripts with disparate calls were manually filtered based on whether transcripts were coding or had complete stop/start information in the UCSC genome browser (Feb. 2009; GRCh37/hg19). SPANR analysis was performed with default settings and splice altering variants defined as described previously (5% > dPSI percentile or dPSI percentile > 95%).
Gene Set Enrichment
Five different gene set lists that have previously been evaluated using de novo mutations,11 including an updated version of the essential gene list,34 were downloaded from GenPhenF (see Web Resources) and then mapped to gene symbols based on our RefSeq ANNOVAR annotations. To determine enrichment, we took a similar approach as previously described, using the null length model.11 However, we calculated joint coverage for all genes within a set as well as all the genes outside of that set (across the cohort) and used this value to estimate the expected proportion of mutations (p). Since more than one gene can overlap any genomic position, all genes impacted were counted in this analysis. For example, if a mutation or genomic position overlapped a gene within the set and outside of the set, it was counted twice. Gene set enrichment was evaluated using a binomial test in R binom.test(x, n, p), where x = number of genes impact within set, n = total number of genes impacted, and p = expected mean based on joint coverage.
Genome-wide gene rankings generated from two previous studies33, 38 were used to determine whether genes targeted by missense or synonymous mutations in probands showed enrichment for ASD candidate gene rankings. The LGD intolerance ranking is based on the load of LGD mutations observed per gene.33 The LGD-RVIS is the average rank between LGD and RVIS (another measure of constraint) scores.33, 39 ASD association rankings are the results of a machine learning approach that uses the connections of ASD candidate genes within a brain-specific interaction network to predict the degree of ASD association for every gene.38
Intersection of PMMs with Previously Published GDMs
Degree of overlap of GDMs and PMMs for different functional classes between probands and siblings was determined using Fisher’s exact test. Both the high-confidence and burden (15%-45×) datasets were evaluated. Our high-confidence risk gene set was curated using the 27 ASD genes reported by Iossifov et al. and 65 ASD genes reported by Sanders et al. (FDR ≤ 0.1)11, 32 as well as 94 genes enriched for GDMs in developmental disorders from the Deciphering Developmental Disorders study.40 Combined, the high-confidence risk gene sets includes 139 unique genes.
Results
Reanalysis of Previously Reported De Novo Mutations
We began by analyzing the existing set of previously reported exonic or canonical intronic splice site de novo mutations in the SSC.1, 2, 4, 5, 11, 12 We evaluated 5,076 SNVs (probands, 2,996; siblings, 2,080) and 416 small indels (probands, 273; siblings, 143) (Table S2). Variants had a mean depth of 77.5×. We found an excess of mutations with observed AFs lower than expected for germline events using a binomial threshold of 0.001 (Figures 1A–1D; Table S7). We evaluated the likelihood of this excess specifically within the autosomal sequence by simulating a null distribution from rare inherited SNVs (Supplemental Materials and Methods; Figure 1B; Table S7). For autosomal de novo SNVs, we observed that 305/2,893 (11%) of affected proband calls and 191/1,993 (10%) of unaffected sibling calls show evidence of being PMMs. In contrast, we never observed the same degree of skewing of calls with lower AFs for rare inherited SNVs (simulation means: probands, 2.8%; siblings, 2.9%; p < 0.0001, by simulation). A higher potential PMM rate is observed in sites that annotated as SD/TRF loci, 55/231 (24%) in probands and 28/144 (20%) in siblings (p = 0.0166 and 0.41, respectively, by simulation). These SD/TRF sites are known to be more prone to false PMM calls due to uncertain mapping of WES reads. However, these SD/TRF loci represent only 9% of the called mutations and thus have a modest effect on the overall rate. We observed a similar rate of potential SNV PMMs (8%–9%) when applying a range of additional AF cutoffs (5%–35%, 10%–35%, 10%–25%), more strict binomial deviations (p ≤ 0.0001), or both, suggesting that these are robust estimates. In sharp contrast, we did not observe an excess of calls with higher than expected AFs (Table S7).
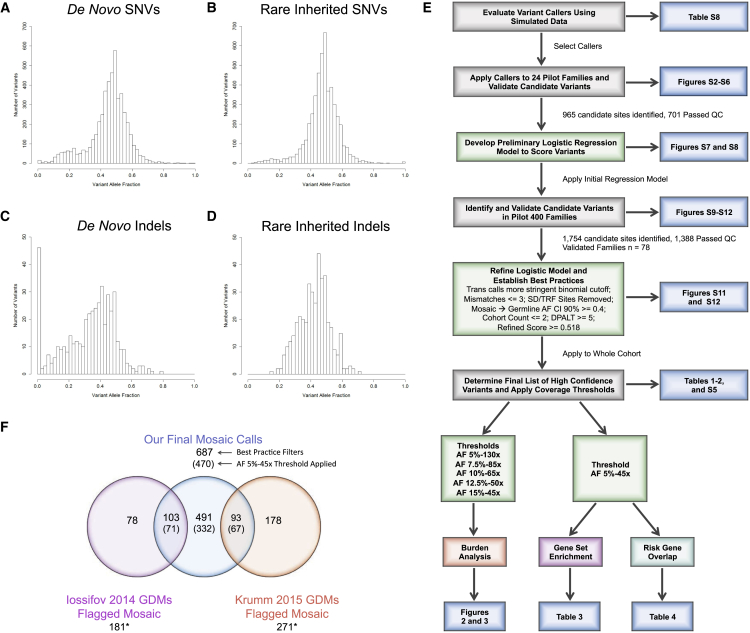
Figure 1.
Re-Evaluation De Novo Mutations in the Simons Simplex Collection (SSC)
(A–D) Histograms showing the allele fraction distributions of previously published autosomal de novo or rare inherited variants in the SSC.
(A) Published de novo SNVs (n = 2,893) show an elevated number of low allele fraction calls that are potentially PMMs (left tail).
(B) Representative histogram from a random sampling of 2,893 published autosomal rare inherited SNVs. The number of low allele fraction calls is substantially fewer compared to de novo SNVs (left tail).
(C) Published de novo indels (n = 268) show an elevated number of low allele fraction calls (left tail) that are potentially PMMs as well as an overall shifted distribution.
(D) Representative histogram from a random sampling of 268 published rare inherited indels. Similar to SNVs, the number of low allele fraction calls is substantially fewer compared to de novo indels (left tail).
(E) Schematic showing an overview of our systematic approach to developing a robust PMM calling pipeline and applying it to the SSC. Key analyses and display items are indicated. Abbreviations: Trans calls, calls showing evidence of transmission from parent to child; SD/TRF, segmental duplications/tandem repeats; AF, allele fraction; CI, confidence interval; and DPALT, Q20 alternative allele depth.
(F) Venn diagram showing the intersection of previously published de novo mutations initially flagged as potentially PMMs (binomial p ≤ 0.001) and our PMM calls after applying final filters. Numbers in parentheses are calls remaining after applying an AF 5%-45× joint coverage threshold. ∗Our pipeline identified an additional 37 calls (29 from Iossifov et al.11 and 8 from Krumm et al.12), which overlapped the published calls flagged as potentially mosaic but were re-classified as likely germline based on their AF CIs. Note: Krumm et al.12 dataset only reported newly identified calls and therefore does not intersect the Iossifov et al.11 dataset.
For indels, we also observed a large number of potential PMMs exceeding the binomial expectation (Figures 1C and 1D; Table S7), with more variability overall between probands and siblings (57/268 [22%] versus 48/140 [35%], respectively, p = 0.005, two-sided Fisher’s exact). For rare inherited indels, we never observed the same degree of skewing of calls with lower AFs (simulation means: probands, 6%; siblings, 17%; p < 0.0001, by simulation) (Figure 1D; Table S7). Similar to SNVs, we found an elevation in the rate for SD/TRF loci (probands, 7/18 [39%]; siblings, 9/16 [56%]; p = 0.0003 and < 0.0001, respectively, by simulation). However, the percent PMM estimates were less robust, compared with SNVs, when applying additional AF cutoffs, more strict binomial deviations, or both. For example, the overall PMM rates using the stricter binomial threshold reduced to 40/268 (15%) for probands and 33/140 (24%) for siblings (p = 0.045, two-sided Fisher’s exact), which nevertheless still exceeded the null expectation (p < 0.0001, by simulation) (Table S7). We observed no de novo indels with significantly deviated higher AFs.
From validation data previously reported or available for a subset (63/545) of the predicted mosaic calls, which included Sanger and NGS data, we found that 39/63 (62%) calls showed strong evidence of allele skewing (Table S2). These data argue that the majority of these calls are bona fide PMMs but that systematic approaches tuned to detecting PMMs are still needed.
Developing a Systematic Mutation Calling Framework
We sought to perform a systematic analysis of PMMs with methods specifically geared toward SNV mosaic mutations, which do not require a matched “normal” tissue data comparison (Figure 1E). Moreover, we expected a large number of suspected PMM calls to be false because of random sampling biases, mapping artifacts, or systematic sequencing errors. Therefore, we worked to build a robust calling framework that would integrate different approaches and could be empirically tuned based on validation data. We first evaluated several standalone (single sample) SNV mosaic mutation callers, including Altas2,41 LoFreq,42 Varscan2,43 and a custom read parser (mPUP) using simulated data containing artificial variants at 202 loci. Based on their complementary performances at different depths and AFs, we selected Varscan2, LoFreq, and mPUP for further evaluation (Tables S8 and S9).
We took advantage of the fact that 24 quad families (96 individuals) had WES independently generated by three centers, providing an opportunity to empirically evaluate these methods on a combined remapped and merged high-depth WES dataset (merged pilot 24: average mean coverage 208×) (Figures S2B and S14A). We obtained high-confidence validation data from at least one DNA source using smMIPs and Illumina sequencing for 645/902 (72%) of the predicted PMM and 56/63 (84%) of the GDM sites (Figure S3; Table S4). Not surprisingly, we found that the majority of the PMMs predicted by a single variant caller were false positives (345/347, 99%), whereas those called by at least two other approaches had a better PPV (162/298, 54%) (Figure S7). In addition, a small number of PMMs (13%) were in cis with existing heterozygous polymorphisms. PMM alleles tracked with specific haplotypes but were absent from a number of overlapping reads, strongly suggesting that these are bona fide postzygotic events (Figure S4). We further found that for transmitted variants, we could eliminate most of the mischaracterized calls that validated as parental germline by requiring a more significant binomial deviation and performing a Fisher’s exact test of the read counts from the parent-child pair (Figure S8). Some of these transmitted variants showed consistently skewed AFs that transmitted in a Mendelian fashion, suggesting that they are systematically biased (Figure S5).
Using these pilot 24 validation data, we constructed an initial logistic regression model (Supplemental Material and Methods). We then applied this initial logistic regression model and additional filters for ambiguous transmitted sites to an independent set of 400 quad families (Material and Methods, Figure S9). We performed smMIPs validation on WB DNA samples from 78 of these quads and obtained high-confidence validation data on 1,388/1,754 sites.
Based on manual inspection of the WES and smMIP alignment data, we identified additional features associated with poor prediction outcomes or problematic genomic regions, including multiple mismatches within the variant reads and presence in multiple families (Figures S6, S11A, and S11B). We added filters based on these features to the pilot 400 validation set and built a refined logistic regression model (Figure S9). The model performed well in 3-way cross validations with sensitivity estimated at 92% and PPV at 80% (threshold 0.26) (Figure S12A). To further evaluate this model, we rescored the pilot 24 validation sites with and without additional filters (Material and Methods). Importantly, these calls were selected and validated prior to model development, giving an independent set of data to evaluate performance. These data performed better than the training data (after removing mPUP only calls), likely due to the increased WES coverage of the pilot 24 samples with sensitivity of 94% and PPV of 83% (threshold 0.26) (Figures S12C and S12D).
We identified additional heuristics that enabled further distinction between true mosaic calls and calls that validated as germline. We observed that calls validating germline tended to have higher observed WES AFs. We calculated the 90% binomial CI (95% one-sided) for the observed AF as a potential complement to the observed significant binomial deviations. We found that the vast majority—112/113 (99%)—of validated PMM calls had upper CI bounds that remained below 0.4, while bounds for the majority of true germline calls—25/33 (76%)—fell above this threshold (Figure S10). In addition, we observed that a significant fraction of the false positive calls exceeding our logistic score threshold (5/26 [19%]) were annotated as SD or TRF sites (Figures S11C and S11D). Moving forward, we chose to remove these SD/TRF sites and re-classify mosaic versus germline status based on the AF binomial CI.
We conducted a third set of validations on PMM and GDM calls not previously evaluated (Supplemental Note: Model Development) in the pilot cohorts using these new filtering parameters and model scores (Figures S12E and S12F). We observed that across all test sets (excluding training data), both sensitivity and PPV converged at a logistic score of 0.518 (sensitivity 0.83, PPV 0.85). At this score threshold, 21/22 (95%) of mosaic predictions that validated as true variants were confirmed as mosaic in children (all test sets). We chose to use this more stringent score threshold for our subsequent burden analysis. In addition, we removed calls with less than five variant allele reads as these disproportionately contributed to false calls (Figure S11E).
Evaluation of Mutation Rates and Burden in Children with ASD
Using this approach, we recalled SNVs in the SSC, in both children and parents, from the existing harmonized reprocessed WES data (average mean coverage 89×).12 We identified 687 total PMMs originating in the children from 1,699 quads and 567 trios passing SNV QC metrics (Tables 1 and S5). We re-identified 3,445/4,198 previously published SNV GDMs, which were not flagged as potentially mosaic, and 1,064 novel calls, i.e., not included in the published call set. Applying our high-confidence call set criteria (5% minimum AF and 45× joint coverage) resulted in 470 PMMs, of which 332 were not part of the published de novo mutation calls (Figure 1F and Table 1). Of the 452 previously published SNV GDMs that we initially flagged as potentially mosaic, 233 were called by our approach (196 as mosaic), of which 157 remained in our high-confidence call set (138 as mosaic, 19 as re-classified germline) (Figure 1F). Likewise, applying the high-confidence call set criteria reduced the GDM count to 1,677, of which only 10 were novel. Compared to our analysis of previously published de novo SNVs, we observed a higher fraction of mosaic mutations among the de novo calls in children, 470/2,147 (22%), consistent with increased sensitivity of our mosaic targeted approach (Table 1).
Table 1.
PMM Counts in Children across Different Allele Fraction and Coverage Thresholds
| syn | mis | non+splice | Total | ||
|---|---|---|---|---|---|
| Best Practice Filters | |||||
| Quads | Pro | 94 | 195 | 20 | 309 |
| Sib | 62 | 203 | 15 | 280 | |
| Trios | Pro | 26 | 63 | 6 | 95 |
| Total Pro | 120 | 258 | 26 | 404 | |
| AF 5%-45× High Confidence | |||||
| Quads | Pro | 58 | 131 | 12 | 201 |
| Sib | 42 | 133 | 10 | 185 | |
| Trios | Pro | 22 | 53 | 6 | 81 |
| Total Pro | 80 | 184 | 18 | 282 | |
| Total germlinea | Pro | 246 | 704 | 73 | 1,023 |
| Sib | 186 | 431 | 26 | 643 | |
| AF 15%-45× Burdenb | |||||
| Quads | Pro | 24 | 65 | 5 | 94 |
| Sib | 20 | 66 | 5 | 91 | |
| Jointly covered bases: 24.5 | |||||
| Trios | Pro | 8 | 30 | 0 | 38 |
| Total Pro | 32 | 95 | 5 | 132 | |
| Jointly covered bases: 9.7 | |||||
| AF 12.5%-50× Burdenb | |||||
| Quads | Pro | 32 | 67 | 5 | 104 |
| Sib | 16 | 80 | 6 | 102 | |
| Jointly covered bases: 22.3 | |||||
| Trios | Pro | 12 | 31 | 2 | 45 |
| Total Pro | 44 | 98 | 7 | 149 | |
| Jointly covered bases: 8.9 | |||||
| AF 10%-65× Burdenb | |||||
| Quads | Pro | 38 | 63 | 6 | 107 |
| Sib | 20 | 76 | 4 | 100 | |
| Jointly covered bases: 16.7 | |||||
| Trios | Pro | 12 | 31 | 1 | 44 |
| Total Pro | 50 | 94 | 7 | 151 | |
| Jointly covered bases: 6.8 | |||||
| AF 7.5%-85× Burdenb | |||||
| Quads | Pro | 31 | 56 | 6 | 93 |
| Sib | 18 | 66 | 5 | 89 | |
| Jointly covered bases: 11.4 | |||||
| Trios | Pro | 11 | 28 | 4 | 43 |
| Total Pro | 42 | 84 | 10 | 136 | |
| Jointly covered bases: 4.7 | |||||
| AF 5%-130× Burdenb | |||||
| Quads | Pro | 20 | 35 | 4 | 59 |
| Sib | 12 | 35 | 2 | 49 | |
| Jointly covered bases: 5.1 | |||||
| Trios | Pro | 10 | 18 | 5 | 33 |
| Total Pro | 30 | 53 | 9 | 92 | |
| Jointly covered bases: 2.0 | |||||
Abbreviations are as follows: AF, allele fraction; Pro, proband; Sib, sibling; syn, synonymous; mis, missense; non + splice, nonsense and canonical splicing. Bases in billions. Mutations with other annotations not shown.
Germline de novo mutations identified using our pipeline.
PMMs in sex chromosomes were excluded in this set.
The burden of PMMs in individuals affected with ASD compared to their unaffected siblings may differ based on embryonic timing, as an early embryonic mutation would contribute more substantially to postembryonic tissues. Therefore, we evaluated burden across the entire SSC cohort at several defined minimum AFs, as a surrogate for embryonic time, and corresponding joint family coverage thresholds (AF-COV): 5%-130×, 7.5%-85×, 10%-65×, 12.5%-50×, and 15%-45× (Figure S13 and Table 1).
We first examined the mutation burden of the unique autosomal coding regions in quad families exclusively as they provided a matched set of child samples (Material and Methods). Within our 15%-45× GDM calls, we recapitulated the previously observed mutation burdens for missense (p = 0.003, one-sided Wilcoxon signed-rank test [WSRT]) and nonsense/splice (p = 0.00025, one-sided WSRT) mutations and lack of burden for synonymous mutations, demonstrating that previous findings are robust to removing potential PMM calls. Given the low number of nonsense/splice mutations (Figure 2A), we restricted our mosaic burden analyses to synonymous and missense PMMs. We did not observe burden signal for missense PMMs within the cohort of quad families (Figure 2B). Unexpectedly, we observed an increased burden of synonymous PMMs in probands (Figure 2C). The signal was strongest in the 12.5%-50× subanalysis with probands having twice as many mutations (32 in probands or 7.2 × 10−10/base pair versus 16 in siblings or 3.6 × 10−10/base pair, p = 0.0024, two-sided WSRT, FDR < 0.05). This trend continued for the three lower AF windows, but these did not exceed an FDR of 0.05. We extrapolated the observed mean per base rates to the full unique autosomal RefSeq exome (31,854,496 bases/haplotype, including canonical splice sites) in order to calculate the average differential between probands and siblings, similar to the analysis performed previously for GDMs.11 Based on the 12.5%-50× data, we found that probands had a rate of 0.046 synonymous PMMs per exome and siblings 0.023, suggesting that 50% of proband synonymous PMMs contribute to ASD risk. The differential between probands and siblings was 0.023, which translates to 2.3% of simplex case subjects in the overall cohort harboring a synonymous PMM related to ASD risk.
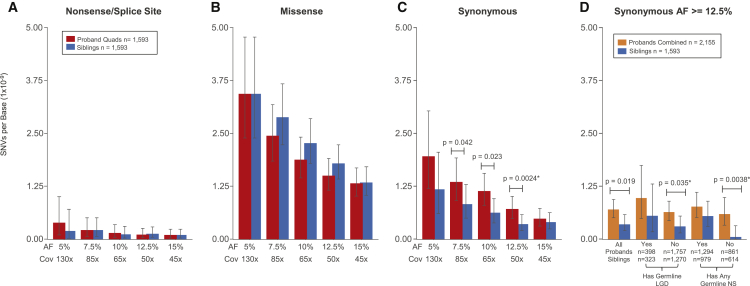
Figure 2.
Rates and Burden of SNV PMMs in the Simons Simplex Collection (SSC)
(A–C) Rates and burden analyses of PMMs in quad families of the SSC. Mean rates with 95% Poisson CIs (exact method) are shown.
(A) Nonsense/splice PMM rates are similar and not evaluated further given their low frequency.
(B) Missense PMMs show no evidence of burden in probands from quad families.
(C) Synonymous PMMs show an unexpected burden in probands from quad families. Significance determined using a two-sided Wilcoxon signed-rank test. ∗FDR < 0.05 using the Benjamini-Yekutieli approach.
(D) Analysis of synonymous PMMs at AF 12.5%-50× in the full SSC and subcohorts. Mean rates with 95% Poisson CIs (exact method) are shown for combined probands (quad + trio families) and unaffected siblings. Abbreviations are as follows: SSC subcohorts all, all families within the cohort passing quality criteria; Has Germline LGD, denotes whether or not proband in family has a LGD GDM or gene disrupting de novo CNV; Has Any Germline NS, denotes whether or not proband in family has any NS GDM (includes the LGD set). Significance determined using a two-sided Wilcoxon rank sum test. ∗FDR < 0.05 using the Benjamini-Yekutieli approach.
We next combined the data from quad and trio-only (father, mother, proband) families to increase the number of mutations and conducted an exploratory analysis of mutation rates in subsets of the full cohort. Since a large fraction of the SSC has germline mutation events that are likely contributory,8, 11, 44 we reasoned that grouping families by presence or absence of proband GDMs of different severity (LGD/disruptive CNV versus any NS) might improve our ability to detect any PMM signal that might be present. Based on the 12.5%-50× data in families without a germline LGD, we observed synonymous burden signal similar to the full cohort. However, the full cohort data did not meet the FDR threshold using the less powerful unpaired test data. In contrast, for the families without any reported NS GDMs, we observed a dramatic depletion of synonymous PMM events in the unaffected siblings, with a proband to sibling rate ratio of 10 (p = 0.0038, two-sided Wilcoxon rank-sum test [WRST], FDR < 0.05) (Figure 2D). In this group without NS GDMs, this equates to 0.038 synonymous PMM events per proband exome and 0.004 per sibling exome (differential of 0.034), suggesting that 89% of this mutation class contributes to ASD risk.
Next, we examined missense PMMs using the two cohort subgroupings at the 15%-45× threshold. We observed a non-significant trend toward burden of missense PMMs in probands for families either without any LGD GDMs (rate ratio 1.28) or without any NS GDMs (rate ratio 1.49) (p = 0.085 and p = 0.076, respectively, one-sided WRST) (Figure 3A). It has now been well documented using several approaches that LGD GDMs in probands show enrichments for genes that are highly conserved/intolerant to LGD mutations.11, 44, 45 We reasoned that missense PMMs relating to ASD risk could also show similar enrichments. We selected two intolerant gene sets, an updated set of essential genes (n = 2,455)34 and the recently published ExAC intolerant set (n = 3,232).35 These subanalyses showed increased effect sizes, but none of these results exceeded a FDR of 0.05. For both essential and ExAC intolerant sets, we observed similar trends for enrichments of missense PMMs in probands (rate ratios 1.4, p = 0.093 and p = 0.13, respectively, one-sided WRST).
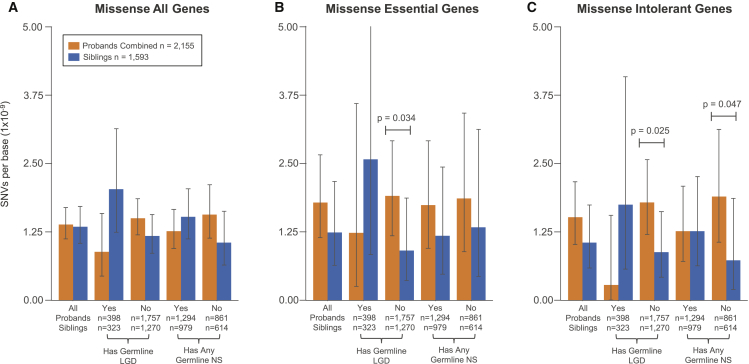
Figure 3.
Rates and Burden of Missense PMMs in Subcohorts and Gene Sets
For all plots, the 15%-45× burden call set was used and mean rates with 95% Poisson CIs (exact method) are shown. Abbreviations are as follows: SSC subcohorts: All, all families within the cohort passing quality criteria; Has Germline LGD, denotes whether or not proband in family has a LGD GDM or gene disrupting de novo CNV; Has Any Germline NS, denotes whether or not proband in family has any NS GDM (includes the LGD set). Significance determined using a one-sided Wilcoxon rank sum test. No comparisons met a FDR < 0.05 using the Benjamini-Yekutieli approach.
(A) Splitting by subcohort shows trends for increased missense PMM burden in families where probands do not have reported germline mutations.
(B) Evaluating mutations specific for the essential gene set shows stronger proband burden in the without any germline LGD subcohort.
(C) Similarly, evaluating mutations specific for the intolerant gene set shows stronger proband burden without any germline LGD or without any germline NS subcohorts.
When combining these two approaches, which subdivide the cohort and gene targets, we saw the strongest effects. In the subset of families without LGD GDMs, we saw a stronger effect for both essential and ExAC intolerant genes (rate ratios 2.1 and 2, p = 0.034 and p = 0.025, respectively, one-sided WRST). We observed similar results when restricting to quad only families. Missense PMMs in essential genes occur at a rate of 0.022 events per exome in probands who do not have a LGD GDM and at a rate of 0.031 for intolerant genes (0.011 and 0.015 for siblings, respectively, differentials 0.011 and 0.016). The families without any NS GDMs showed the largest effect in the ExAC intolerant set (ratio 2.6, p = 0.047, one-sided WRST) but similar rates to the full cohort in the essential gene set (ExAC: 0.033 events per proband, 0.013 per sibling, 0.02 differential). Based on these differentials, we estimate that 1%–2% of probands without LGD or NS GDMs have a missense PMM in an essential/intolerant gene potentially contributing to risk. Adjusted to the full cohort, this gives a range of 0.8%-1.3% of probands harboring a missense PMM in an essential/intolerant gene potentially related to ASD risk.
Parental PMM Rates and Transmission
We also identified PMMs arising in the SSC parents (Table 2; Figure S4). We identified 1,293 nontransmitted (654 in fathers and 639 in mothers) and 92 transmitted (52 in fathers and 40 in mothers) total PMMs in our high-confidence call set. For transmitted mutations, which by definition require the postzygotic mutation contribution to both soma and germline, we required a stricter deviation from the binomial expectation based on empirical validation data (p ≤ 0.0001). The overall PMM rates were similar between fathers and mothers (Figure S15). Comparing children and parents in the high-confidence call set, we found the PMM rate to be 2.6-fold greater in the SSC parents relative to their children. However, we suspect that some fraction of this elevated rate may be due to biases in filtering out transmitted sites that show false mosaic signal, as we do not have the previous generation, i.e., grandparents, to compare to as we do for the children. Therefore, we looked at variants in a subset of the cohort and determined the fraction of variants remaining in children before and after applying transmission filters. Using this rate, we estimated the number of PMMs expected to be filtered from the parental calls based on transmission. We estimate that 40% of our parental PMM calls are in excess of what is expected and likely attributed to incomplete filtering (Figure S16). Applying this correction reduces the parental excess PMM rate to only 1.6-fold greater. Based on the children, two-thirds of filtered calls appear to be systematically biased as they are skewed in both generations. The remaining one-third of calls are skewed in only a single generation with AFs > 20%, suggesting that they are likely stochastic events.
Table 2.
PMM Counts in Parents across Different Allele Fraction Coverage Thresholds
| syn | mis | non+splice | Total | ||
|---|---|---|---|---|---|
| Best Practice Filters | |||||
| Nontrans | Fa | 259 | 543 | 54 | 856 |
| Mo | 266 | 570 | 41 | 877 | |
| Trans | Fa | 21 | 41 | 1 | 63 |
| Mo | 12 | 37 | 0 | 49 | |
| AF 5%-45× High Confidence | |||||
| Nontrans | Fa | 196 | 418 | 40 | 654 |
| Mo | 199 | 405 | 35 | 639 | |
| Trans | Fa | 19 | 32 | 1 | 52 |
| Mo | 7 | 33 | 0 | 40 | |
| AF 15%-45× Burdena | |||||
| Nontrans | Fa | 114 | 261 | 19 | 394 |
| Mo | 130 | 267 | 15 | 412 | |
| Trans | Fa | 19 | 32 | 1 | 52 |
| Mo | 6 | 31 | 0 | 37 | |
| Jointly Covered Bases: 34.2 | |||||
| AF 12.5%-50× Burdena | |||||
| Nontrans | Fa | 126 | 276 | 22 | 424 |
| Mo | 130 | 281 | 18 | 429 | |
| Trans | Fa | 16 | 30 | 1 | 47 |
| Mo | 6 | 30 | 0 | 36 | |
| Jointly Covered Bases: 31.2 | |||||
| AF 10%-65× Burdena | |||||
| Nontrans | Fa | 121 | 229 | 18 | 368 |
| Mo | 110 | 229 | 19 | 358 | |
| Trans | Fa | 11 | 23 | 1 | 35 |
| Mo | 4 | 20 | 0 | 24 | |
| Jointly Covered Bases: 16.7 | |||||
| AF 7.5%-85× Burdena | |||||
| Nontrans | Fa | 90 | 177 | 19 | 286 |
| Mo | 92 | 180 | 19 | 291 | |
| Trans | Fa | 5 | 15 | 1 | 21 |
| Mo | 2 | 13 | 0 | 15 | |
| Jointly Covered Bases: 16.1 | |||||
| AF 5%-130× Burdena | |||||
| Nontrans | Fa | 53 | 110 | 15 | 178 |
| Mo | 49 | 101 | 9 | 159 | |
| Trans | Fa | 3 | 4 | 0 | 7 |
| Mo | 1 | 5 | 0 | 6 | |
Abbreviations are as follows: AF, allele fraction; Fa, father; Mo, mother; syn, synonymous; mis, missense; non + splice, nonsense and canonical splicing. Bases in billions. Mutations with other annotations not shown.
PMMs in sex chromosomes were excluded in this set.
The increased rate of PMMs in parents compared to children is in line with previous observations that PMMs accumulate with age.46, 47 We also observed an overall trend toward an increase in the rate of PMMs with parental age for both sexes (Figure S17A). The rate of PMMs markedly increases after age 45 and there is a significant difference in rate between parents younger than 45 as compared to those 45 and older (mothers-rate ratio 1.2, p = 0.04; fathers-rate ratio 1.3, p = 0.01, one-sided WRST) (Figure S17B). We also saw that the number of individuals with multiple PMMs (adjusted for coverage differences) within a given age range increased as well (Figure S17C). Recent studies have also demonstrated a rise in PMMs in particular genes that result in aberrant clonal expansions (ACEs) that are specific to blood cells.47, 48, 49, 50 We did not find strong evidence for enrichment of PMMs in 42 genes with recurrent ACE-associated mutations from three studies of hematopoietic clonal expansion (parents-obs: 9, exp: 6.6, p = 0.17; children-obs: 5, exp: 2.3, p = 0.07; two-sided binomial).48, 49, 50 However, among the parents we did find recurrent nontransmitted PMMs in two of the most frequently mutated ACE-related genes, DNMT3A (four nonsense and one missense) and TET2 (two missense). These PMMs did occur in relatively older individuals for our cohort, 45–50 years old. Two missense PMMs in TET2 were also observed in the children.
Within the 45× joint coverage data, we found that 7%–10% of parental PMMs were transmitted to one or more children depending on the minimum AF threshold (high confidence 5% versus burden 15%) (Table 2). Moreover, in our high-depth validation data with final filters applied, we found that 1/164 GDM predictions showed evidence of low AF in parental DNA, which was not detected by WES (Table S4). We also identified six obligate mosaics given their de novo presence in two offspring, i.e., gonadal mosaic mutations (Table S5). Within the quad families of our high-confidence call set, we did observe skewing of transmission to siblings (18 to both, 39 siblings, 22 probands), suggesting that as a class, transmitted mosaic mutations are not associated with ASD within this cohort. However, individual mutations at ASD risk loci may still be relevant to the disorder.
Properties of PMMs
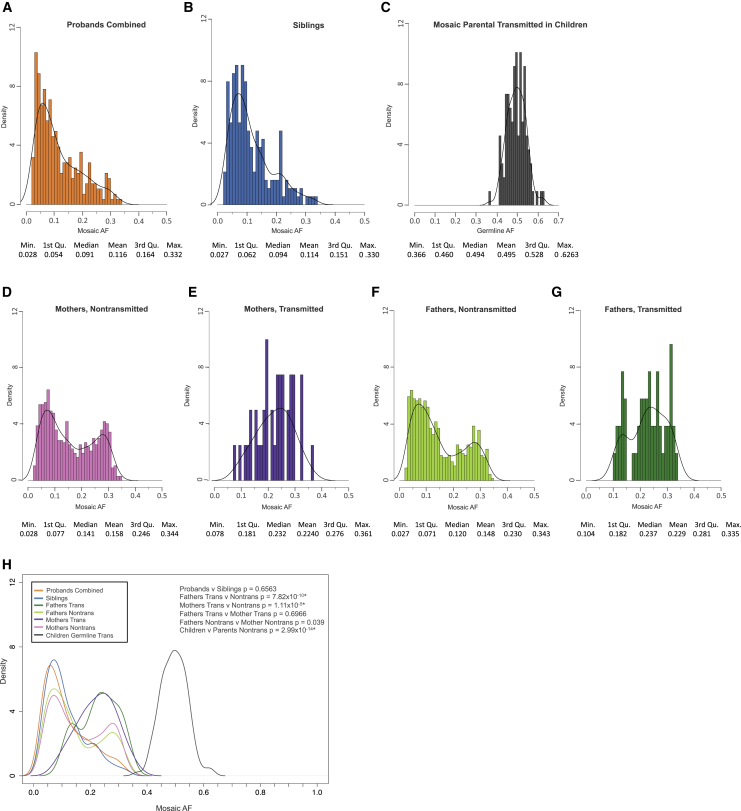
Using the high-confidence call set (Table S5), we examined whether general properties of PMMs differed between parents and children and how mutational mechanisms compare with GDMs. We found that AF distributions of PMMs between parents (fathers and mothers), and likewise between children (probands and siblings), were similar; therefore, we combined parental calls and child calls, respectively (Figure 4). Nontransmitted parental PMMs have a distinct AF distribution, which is bimodal, and significantly different from both transmitted parental PMM and child PMM distributions (nontransmitted parental versus transmitted, p = 7.07 × 10−14, nontransmitted parental versus children, p = 2.99 × 10−14, two-sided WRST, FDR < 0.05). Similar to how we empirically separated germline and mosaic calls in children, we calculated the confidence intervals of the parental PMM AFs (Figure S18). We found that the vast majority of transmitted PMMs had AF CIs in excess of 10% (92/94 [98%]), suggesting early embryonic origin for PMMs within this AF range and consequently the largest risk for transmission.
Figure 4.
Mosaic Variant Allele Fraction Distributions
For all plots, all PMMs from the 5%-45× high-confidence call set were used.
(A) Distribution of allele fractions for variants in probands combined (quad + trio families).
(B) Distribution of allele fractions for variants in siblings.
(C) Distribution of allele fractions for germline variants in children that were transmitted from mosaic parents.
(D and E) Distribution of allele fractions for variants in mothers that were not (D) and were (E) transmitted to children.
(F and G) Distribution of allele fractions for variants in fathers that were not (F) and were (G) transmitted to children.
(H) Combined data plotted as kernel density curves. Parental transmitted are significantly shifted toward a higher allele fraction than nontransmitted or child mosaic variants. Children have a significantly different distribution than parental nontransmitted. Significance determined using a two-sided Wilcoxon rank sum test. ∗FDR < 0.05 using the Benjamini-Yekutieli approach.
The mutational spectra and signatures of GDMs and PMMs were similar (Figure S19). For both GDMs and PMMs, the relative frequency of mutations within trinucleotides showed strongest correlation with previously described51 cancer signature 1, followed by 6 (Figures S19B and S19C). Signature 1, which is characterized by spontaneous deamination of 5-methylcytosine, is indicative of endogenous mutational processes and associated with all cancer types.51 Signature 6 is associated with defective DNA mismatch repair.51
Potential Impact of Synonymous PMMs on Splicing
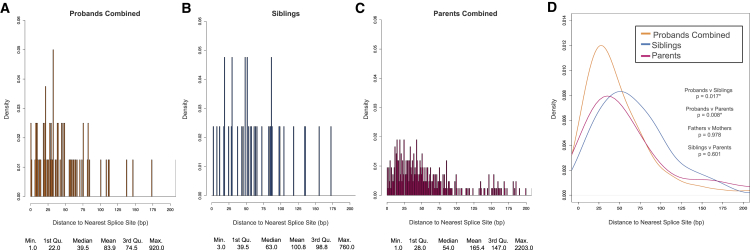
A possible mechanism for synonymous variants contributing to ASD risk would be by disrupting splicing. Exonic splice-affecting variants are preferentially localized near existing canonical splicing sites, i.e., the starts or ends of exons.52, 53 Therefore, we calculated the absolute minimum distances of all synonymous PMMs and GDMs to their closest splicing site (Figure 5). We found the proband synonymous PMM distribution to be shifted toward splicing sites compared to both sibling and parental synonymous PMM distributions (p = 0.017 and p = 0.008, respectively, two-sided WRST, FDR < 0.05), while the sibling distribution was similar to the parental (p = 0.601, two-sided WRST). We observed a similar shift toward splice sites for GDMs in probands as compared to siblings (p = 0.005, two-sided WRST, FDR < 0.05).
Figure 5.
Distance to Nearest Splice Site for Synonymous PMMs
For all plots, all synonymous PMMs from the 5%-45× high-confidence call set were used. Splice site distances were calculated as absolute minimum distance to nearest canonical splice site.
(A) Distribution of distance to nearest splice site in probands combined (quad + trio families).
(B) Distribution of distance to nearest splice site in siblings.
(C) Distribution of distance to nearest splice site in combined parents (quad + trio families).
(D) Combined data plotted as kernel density curves. Proband distribution is significantly shifted toward the canonical splice sites compared to both parents and siblings. Significance was determined using a two-sided Wilcoxon rank sum test. ∗FDR < 0.05 using the Benjamini-Yekutieli approach.
We further evaluated potential effects of synonymous mutations on splicing computationally using HSF, which utilizes a collection of different splicing prediction approaches.36 HSF reported significantly more instances of putative splice altering mutations for proband synonymous PMMs (70/78) when compared to siblings (25/41) (p = 0.0005, odds ratio, 5.506, 95% CI 1.946–16.836, two-sided Fisher’s exact) (Table S6). Synonymous GDMs showed no enrichment (proband 188/235 versus sibling 137/177, p = 0.544, odds ratio, 1.168, 95% CI 0.726–1.879, two-sided Fisher’s exact). When restricting to synonymous PMMs that occur within 50 bp of the start or end of an exon, where splicing regulatory elements are enriched,54 we observed a stronger enrichment (proband 45/53 versus sibling 5/12, p = 0.00378, odds ratio, 7.53, 95% CI 1.618–38.861, two-sided Fisher’s exact). We did not observe a similar enrichment for proband synonymous GDMs near splice junctions. To assess the robustness of HSF findings, given the high call rate of splice-altering variants, we removed the two most frequently called matrices and reclassified variants. We still observed an enrichment of proband synonymous PMMs predicted to alter splicing (all variants: proband 53/79, sibling 18/41, p = 0.019, odds ratio, 2.60, 95% CI 1.20–5.66; within 50 bp: probands 34/50, sibling 5/15, p = 0.033, odds ratio, 4.25, 95% CI 1.24–14.5, two-sided Fisher’s exact).
To independently assess splice altering variant enrichment, we applied a recently reported machine-learning-based approach, SPANR.37 SPANR requires a variant to be within 100 bp from an exon start or end site and be located within an exon flanked by an exon on either side, which limited our analysis to 68 proband and 29 sibling PMMs. SPANR reported a significant enrichment of splice-altering synonymous PMMs in probands (proband 15/68 versus sibling 1/29, p = 0.03, odds ratio, 7.81, 95% CI 1.09–344.8, two-sided Fisher’s exact). Similarly, proband PMMs remained enriched for splice-altering variants (though not significantly) when restricting to mutations within 50 bp of a canonical splice site (proband 14/46, sibling 1/13, p = 0.15, odds ratio 5.13, CI 95% 0.64–239.9, two-sided Fisher’s exact).
Gene Set Enrichment
We applied a similar approach as Iossifov and colleagues to look for enrichments of PMMs within different gene sets using our high-confidence dataset.11 Using expected values generated from joint coverage for the cohort, we examined whether our PMMs/GDMs showed more or fewer mutations than expected independently for probands and siblings. Our GDM dataset showed similar enrichments or lack thereof to previous reports (Table 3). In probands, we found enrichment (1.8-fold) for missense PMMs intersecting chromatin modifiers (p = 0.043, two-sided binomial) and depletion of missense PMMs in embryonically expressed genes (p = 0.024, two-sided binomial). Interestingly, missense GDMs showed no evidence of enrichment or depletion for these gene sets, while LGD GDMs have previously been shown to be enriched.11
Table 3.
Enrichment of Missense Germline and Postzygotic Mutations in Gene Sets
| Total No. of Genes |
Mis GDM (Pro) |
Mis GDM (Sib) |
Mis PMM (Pro) |
Mis PMM (Sib) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 701 | 426 | 177 | 129 | |||||||||||
| Set | p | Genes in Seta | Obs | Exp | p | Obs | Exp | p | Obs | Exp | p | Obs | Exp | p |
| Chromatin | 0.0372 | 388 | 32 | 26.1 | 0.230 | 20 | 15.8 | 0.303 | 12 | 6.6 | 0.043 | 2 | 4.8 | 0.247 |
| Embryonic | 0.1433 | 1,797 | 114 | 100.5 | 0.178 | 60 | 61.1 | 0.835 | 16 | 25.4 | 0.024 | 25 | 18.5 | 0.103 |
| Essential | 0.1967 | 2,402 | 160 | 137.8 | 0.036 | 83 | 83.7 | 0.903 | 41 | 34.8 | 0.256 | 24 | 25.4 | 0.825 |
| PSD | 0.0701 | 879 | 58 | 49.1 | 0.183 | 35 | 29.9 | 0.346 | 17 | 12.4 | 0.183 | 14 | 9.0 | 0.167 |
| FMRP | 0.1005 | 775 | 100 | 70.3 | 4 × 10−4 | 57 | 42.7 | 0.036 | 20 | 17.8 | 0.53 | 13 | 12.9 | 1.000 |
45× joint coverage, 5% AF call set. Variants in sex chromosomes excluded. Expected (Exp) and p values obtained from two-sided binomial test, based on gene length model (p). Abbreviations are as follows: Obs, observed; Mis GDM, missense germline de novo mutation; Mis PMM, missense postzygotic mutation; Pro, proband; Sib, sibling; PSD, post synaptic density associated genes; FMRP, fragile X mental retardation protein-associated genes.
Total number of genes differs from full lists as we used only genes that we were able to map to our gene symbol annotations and genes on sex chromosomes were excluded.
Recently, several groups have taken different approaches to generate genome-wide ASD candidate risk gene rankings and predict novel gene targets.33, 38 These approaches have largely been validated on LGD GDMs. We explored whether our high-confidence PMM calls showed any shift in ASD candidate gene rankings for probands compared with their unaffected siblings (Table S10). We evaluated rankings based on gene mutation intolerance (LGD rank, LGD-RVIS average rank)33 or based on a human brain-specific gene functional interaction network (ASD association).38 At the population level, we found only non-significant increases in LGD-RVIS rankings for proband synonymous and essential missense PMMs in the subcohort of families without any proband NS GDMs (p = 0.029 and p = 0.073, one-sided WRST). We also observed no significant shifts in rankings for missense GDMs.
Intersecting Proband Mosaic and Germline Mutation Gene Targets
To determine whether germline and mosaic mutations in probands share common target genes, we intersected missense PMMs from the high-confidence call set and the burden subset (15%-45×) with the re-classified published GDM calls. We observed no enrichment of proband missense PMMs with genes that are targets of sibling GDMs of any type. However, we did find an apparent enrichment of genes that are targets of proband missense GDMs within proband missense PMMs from the burden call set (proband: 25/100; sibling: 9/69, p = 0.042, OR, 2.222, 95% CI 0.904–5.582, one-sided Fisher’s exact), suggesting that some common ASD risk targets for mosaic and germline mutations.
In addition, we intersected all predicted NS PMMs (our high-confidence call set plus re-classified published [unique CDS]) with 139 genes that have reached high-confidence levels for their risk contribution for ASD and/or developmental disorders.11, 32, 40 In probands, 12/496 PMMs intersect (8 missense, 4 LGD) while only 4/354 PMMs intersect in siblings (3 missense and 1 LGD). The novel, i.e., not published in the GDM call set,11, 12 proband events included missense PMMs in CHD2 (MIM: 602119, GenBank: NM_001042572.2; c.272A>G [p.Glu91Gly]), CTNNB1 (MIM: 116806, GenBank: NM_001098209.1; c.1127G>A [p.Arg376His]), KIF1A (MIM: 601255, GenBank: NM_001244008.1; c.655G>A [p.Ala219Thr]), and KMT2C (MIM: 606833, GenBank: NM_170606.2; c.14416C>G [p.Arg4806Gly]) (Table 4). We also identified a novel missense mutation in SCN2A (MIM: 182390, GenBank: NM_001040142.1; c.3370A>T [p.Ser1124Cys]) that was transmitted to the proband from the mother. Our SNV PMM pipeline re-identified published de novo calls that we re-classified as likely mosaic events, including KANSL1 (MIM: 612452, GenBank: NM_001193465.1; c.729A>C [p.Gln243His]), KAT2B (MIM: 602303, GenBank: NM_003884.4; c.1151−1G>A [splicing]), INTS6 (MIM: 604331, GenBank: NM_001039937.1; c.1789C>T [p.Arg596Ter]), SYNGAP1 (MIM: 612621, GenBank: NM_006772.2; c.3055C>T [p.Arg1019Cys]), and TBL1XR1 (MIM: 608628, GenBank: NM_024665.4; c.845T>C [p.Leu282Pro]) (Table 4). Only the KANSL1 and INTS6 PMMs met the high confidence 45× joint coverage criteria. Mosaic re-classified indels included DIP2A (MIM: 607711, GenBank: NM_001146114.1; c.1646_1652dup7 [p.Leu552ValfsTer34]) and GIGYF1 (MIM: 612064, GenBank: NM_022574.4; c.1140_1156del17 [p.Thr381ArgfsTer13]) (Table 4). With the exception of probands with the CHD2 and DIP2A PMMs, none of these other probands have NS GDMs in other strong risk genes.
Table 4.
Highlighted Mosaic Mutations in Candidate ASD Risk Genes
| Person:Sex | NVIQ/VIQ | Gene | Func | Gene Lista |
SSC Pro GDM Counta |
SSC Sib GDM Counta |
AF | HGVSc | HGVSp | Pub | Other Pub NS GDM | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LGD | Mis | LGD | Mis | ||||||||||
| 13073.p1:M | 60/25 | CHD2 | mis | HC11, 32, 40 | 3 | 0 | 0 | 0 | 14/125 (11%) | NM_001042572.2; c.272A>G | p.Glu91Gly | N | SYNGAP1:fs del |
| 12139.p1:M | 106/86 | CTNNB1 | mis | HC40 | 1 | 1 | 0 | 0 | 8/103 (8%) | NM_001098209.1; c.1127G>A | p.Arg376His | N | GPBP1:mis |
| 14687.p1:M | 38/62 | INTS6 | ns | HC40 | 0 | 0 | 0 | 0 | 13/54 (24%) | NM_001039937.1; c.1789C>T | p.Arg597Ter | Y | ATP2A1:mis |
| 12028.p1:M | 93/80 | KIF1A | mis | HC40 | 0 | 1 | 0 | 1 | 29/250 (12%) | NM_001244008.1; c.655G>A | p.Ala219Thr | N | NA |
| 11305.p1:M | 35/60 | KANSL1 | mis | HC40 | 0 | 0 | 0 | 0 | 40/126 (32%) | NM_001193465.1; c.729A>C | p.Gln243His | Yb | OR1S1:misc |
| 11592.p1:M | 109/122 | KAT2B | sp | HC32 | 0 | 0 | 0 | 0 | 20/80 (25%) | NM_003884.4; c.1151−1G>A | – | Yb | NA |
| 13897.p1:M | 91/78 | KMT2C | mis | HC32, 40 | 1 | 1 | 0 | 0 | 8/115 (7%) | NM_170606.2; c.14416C>G | p.Arg4806Gly | N | CGGBP1:mis |
| 13522.mo:Md | 87/70 | SCN2A | mis | HC11, 32, 40 | 2 | 4 | 0 | 0 | 11/50 (22%) | NM_001040142.1; c.3370A>T | p.Ser1124Cys | N | NA |
| 14001.p1:M | 63/38 | SYNGAP1 | mis | HC11, 32, 40 | 1 | 1 | 0 | 0 | 18/74 (24%) | NM_006772.2; c.3055C>T | p.Arg1019Cys | Yb | NA |
| 12335.p1:F | 47/66 | TBL1XR1 | mis | HC40 | 1 | 0 | 0 | 0 | 9/40 (22%) | NM_024665.4; c.845T>C | p.Leu282Pro | Yb | STK36:mis; SPATA32:mis |
| 13012.p1:M | 60/21 | DIP2A | fs ins | HC11, 32, 40 | 1 | 0 | 0 | 0 | 34/164 (21%) | NM_001146114.1; c.1646_1652dup7 | p.Leu552ValfsTer34 | Ye | RELN:mis |
| 11232.p1:M | 68/91 | GIGYF1 | fs del | HC32 | 2 | 0 | 0 | 0 | 15/65 (23%) | NM_022574.4; c.1140_1156del17 | p.Thr381ArgfsTer13 | Ye | NA |
| 13694.p1:M | 26/17 | BAZ2B | ns | GLGD | 1 | 0 | 0 | 1 | 9/163 (6%) | NM_013450.2; c.3868C>T | p.Arg1290Ter | N | NA |
| 11411.fa:Md | 67/51 | COL5A3 | mis | GLGD | 1 | 0 | 0 | 0 | 16/68 (24%) | NM_015719.3; c.3338C>T | p.Pro1113Leu | N | SNRK:mis; TSNARE1:mis |
| 14051.p1:M | 115/107 | CTNNA3 | mis | GLGD | 1 | 0 | 0 | 0 | 9/295 (3%) | NM_001127384.1; c.152G>C | p.Arg51Pro | N | SEC16B:mis; RFC5:mis |
| 12120.p1:M | 115/85 | SPEN | mis | GLGD | 1 | 1 | 0 | 0 | 15/58 (26%) | NM_015001.2; c.4651G>A | p.Glu1551Lys | Y | OR5J2:mis |
| 14420.p1:M | 101/80 | SSPO | mis | GLGD | 1 | 1 | 0 | 0 | 29/98 (30%) | NM_198455.2; c.14150C>G | p.Ala4717Gly | Y | SH3BP5L:mis; ZMIZ2:mis |
| 14547.p1:M | 95/60 | UNC79 | ns | GLGD | 1 | 0 | 0 | 0 | 9/106 (8%) | NM_020818.3; c.6208C>T | p.Arg2070Ter | N | UQCRC2:mis |
| 12025.p1:M | 96/69 | USP15 | ns | GLGD | 1 | 0 | 0 | 0 | 8/164 (5%) | NM_001252078.1; c.813T>G | p.Tyr271Ter | N | NA |
| 12837.p1:M | 92/89 | BIRC6 | mis | GMIS | 0 | 1 | 0 | 2 | 23/123 (19%) | NM_016252.3; c.9578G>C | p.Arg3193Pro | Y | SH3RF3:mis |
| 13215.p1:M | 69/87 | CFAP74 | mis | GMIS | 0 | 1 | 0 | 0 | 8/157 (5%) | NM_001080484.1; c.1127G>A | p.Arg376Lys | N | JUP:mis |
| 11942.p1:M | 44/62 | DMXL2 | mis | GMIS | 0 | 2 | 0 | 0 | 19/256 (7%) | NM_001174116.1; c.3455A>G | p.Asp1152Gly | N | NA |
| 14248.p1:F | 83/94 | DNAH10 | mis | GMIS | 0 | 2 | 0 | 0 | 13/125 (10%) | NM_207437.3; c.3599G>A | p.Arg1200His | Y | MYO1E:mis; ELAVL2:fs del; ITGA2B:mis |
| 11627.p1:M | 100/83 | DNAH17 | mis | GMIS | 0 | 2 | 0 | 1 | 11/77 (14%) | NM_173628.3; c.7979C>T | p.Ser2660Phe | Y | RGMA:mis |
| 11521.p1:M | 101/128 | MTUS1 | ns | GMIS | 0 | 1 | 0 | 0 | 17/111 (15%) | NM_001001924.2; c.707C>G | p.Ser236Ter | Y | HERC2:misc |
| 14168.p1:M | 140/123 | OBSCN | mis | GMIS | 0 | 2 | 0 | 0 | 14/61 (23%) | NM_001098623.2; c.18344G>A | p.Arg6115Gln | Y | FCGBP:misc |
| 11947.p1:M | 33/28 | SSRP1 | ns | GMIS | 0 | 1 | 0 | 0 | 13/143 (9%) | NM_003146.2; c.159G>A | p.Trp53Ter | N | MDM2:mis; CCR7:mis |
| 13793.p1:M | 56/48 | SYNE1 | mis | GMIS | 0 | 2 | 0 | 1 | 13/225 (6%) | NM_033071.3; c.2330C>T | p.Ala777Val | N | PCDHB4:misc; SBF1:mis |
| 12108.p1:M | 63/74 | VPS13D | ns | GMIS | 0 | 1 | 0 | 0 | 11/133 (8%) | NM_015378.2; c.10552C>T | p.Arg3518Ter | N | KAT6A:fs del; SMG6:mis |
| 14059.p1:M | 105/89 | ACTL6B | syn | novel | 0 | 0 | 0 | 0 | 8/212 (4%) | NM_016188.4; c.360C>T | p.Ser120 = | N | NA |
| 11506.p1:F | 92/82 | COL5A3 | syn | GLGD | 1 | 0 | 0 | 0 | 25/356 (7%) | NM_015719.3; c.2460G>A | p.Ser820 = | N | PSMB4:mis; KIAA17:mis; INPP5D:mis |
| 11115.p1:F | 46/19 | CCT6B | syn | GMIS | 0 | 1 | 0 | 0 | 13/179 (7%) | NM_001193529.1; c.885C>T | p.Ala295 = | N | NA |
| 11336.p1:M | 124/114 | FYN | syn | novel | 0 | 0 | 0 | 0 | 8/129 (6%) | NM_002037.5; c.1051C>T | p.Leu351 = | N | DXO:mis; SLC26A5:mis |
| 14471.p1:M | 96/96 | STMN1 | syn | novel | 0 | 0 | 0 | 0 | 7/90 (8%) | NM_001145454.1; c.219T>C | p.Ala73 = | N | NA |
Abbreviations are as follows: NVIQ, nonverbal IQ; VIQ, verbal IQ; mis, missense; ns, nonsense; syn, synonymous; sp, canonical splicing site; fs, frameshifting mutation; ins, insertion; del, deletion; SSC, Simons Simplex Collection; Pro, proband; Sib, sibling; LGD, likely gene disrupting; GDM, germline de novo mutation; GLGD, overlaps gene with germline LGD mutation; GMIS, overlaps gene with germline missense mutation; HC, overlaps high-confidence risk gene list; AF, allele fraction; HGVSc, Human Genome Variation Society format cDNA; HGVSp, Human Genome Variation Society format protein; Pub, published in de novo mutation calls; NS, nonsynonymous.
Lists and counts compiled after re-classification of published calls (binomial p ≤ 0.001, see Table S2).
Call did not meet 45× joint coverage threshold.
Published GDM call in segmental duplication or tandem repeat loci.
Phenotypic data is for proband.
Indels were identified from re-classification of published calls.
Among the remaining NS PMMs, we found seven mutations in genes overlapping proband LGD GDMs (sibling NS GDM count ≤ 1) (Table 4). Of particular interest are novel nonsense PMMs in BAZ2B (MIM: 605633, GenBank: NM_013450.2; c.3868C>T [p.Arg1290Ter]), UNC79 (MIM: 616884, GenBank: NM_020818.3; c.6208C>T [p.Arg2070Ter]), and USP15 (MIM: 604731, GenBank: NM_001252078.1; c.813T>G [p.Tyr217Ter]). BAZ2B is part of the bromodomain gene family involved in chromatin remodeling.55 UNC79 works in concert with UNC80 to regulate the excitability of hippocampal neurons through activation of sodium channel NALCN.56 USP15 is a deubiquitinase that plays many roles across the cell including modulating immune response through TGF-β and NF-κB pathways.57
Ten of the remaining NS PMMs intersect gene targets of missense GDMs (sibling NS GDM count ≤ 2) (Table 4). Of note are novel nonsense PMMs in the chromatin remodeling factor SSRP1 (MIM: 604328, GenBank: NM_003146.2; c.159G>A, [p.Trp53Ter]) and the membrane trafficking protein VSP13D (MIM: 608877, GenBank: NM_015378.2; c.10552C>T [p.Arg3518Ter]). Novel missense PMMs included were DMXL2 (MIM: 612186, GenBank: NM_001174116.1; c.3455A>G [p.Asp1152Gly]), SYNE1 (MIM: 608441, GenBank: NM_033071.3; c.2330C>T [p.Ala777Val]), and CFAP74 (GenBank: NM_001080484.1; c.1127G>A [p.Arg376Lys]).
Among the synonymous PMMs, we identified four candidate genes based on known roles in neurodevelopment, predicted creation of a new exonic silencing site, and no other NS GDM events in ASD risk genes in the proband: ACTL6B (MIM: 612458, GenBank: NM_016188.4; c.360C>T [p.Ser120 = ]), CCT6B (MIM: 610730, GenBank: NM_001193529.1; c.885C>T [p.Ala295 = ]), FYN (MIM: 137025, GenBank: NM_002037.5; c.1051C>T [p.Leu351 = ]), and STMN1 (MIM: 151442, GenBank: NM_001145454.1; c.219T>C [p.Ala73 = ]). Notably, ACTL6B is a neuron-specific component of the SWI/SNF chromatin remodeling complex.58 We also highlight a synonymous PMM in COL5A3 (GenBank: NM_015719.3; c.2460G>A [p.Ser820 = ]) because it has a high likelihood of impacting splicing by altering the wild-type 3′ exonic donor site, a missense PMM (GenBank: NM_015719.3; c.3338C>T [p.Pro1113Leu]), and a LGD GDM are present at this locus, and we found no other NS GDMs associated with ASD risk in the proband. Taken together, these new mosaic calls provide additional support for high-confidence ASD risk genes and highlight candidates as potential contributors to ASD risk.
Discussion
The aim of our study was to systematically evaluate exonic PMMs in a large family-based SSC cohort and their potential role in ASD. Historically, PMMs, much like GDMs, have been intractable to systematical genome-wide study. However, NGS technologies have now made this class of genomic variation accessible, genome-wide, at single-base resolution. A number of recent reports have demonstrated that PMMs are relatively common in both healthy and neurodevelopmental disorder cohorts, including intellectual disability, ASD, or general developmental delays.2, 26, 46, 59, 60 However, how frequent and widespread these events might be in early and/or late development and how much risk they contribute to complex disorders has yet to be fully elucidated.
We found evidence for 11% of SNVs and 26% of indels previously reported as de novo mutations from the SSC WES data having AFs consistent with a PMM arising in the child. This is in excess of our original observation of 3.5% (9/260) of mutation events consistent with child PMMs, using only 209 families.2 A similar analysis of de novo mutations identified from whole-genome sequencing of simplex ID trios validated 6.5% (7/107) as PMMs.26 We reasoned that re-analyzing the WES data systematically with approaches tuned to detect PMMs would reveal novel mutations, especially those with lower AFs (<20%). We developed a SNV calling approach to detect PMMs without matched normal data but in the context of nuclear families (Figure 1E). Using this approach, the rate of de novo SNVs that are PMMs arising in children increased to 22%. Given that the depth of sequence directly affects the observable minimum mutation AF, we used varying AF-COV thresholds (e.g., 15%-45×, 5%-130×) to evaluate mosaic mutation burden. Surprisingly, in the full cohort, we found the strongest signal for PMM burden with synonymous SNVs (Figure 2C). The distribution of proband PMMs showed a significant shift in distance to nearest splice site (Figure 5D). Moreover, proband synonymous PMMs showed enrichments for splice altering predictions using two independent approaches.
It has recently been shown that in some cancers, synonymous mutations may have a modest enrichment in oncogenes.52 Within 16 oncogenes, the signal was specific to the mutations within 30 base pairs (“near-splice”) of the exon boundary and showed gains of exonic splicing enhancer (ESE) motifs and loss of exonic splicing silencer (ESS) motif sequences. Conducting an analysis of the intersection of ASD and schizophrenia WES GDMs and regulatory elements, Takata and colleagues recently reported an enrichment of near-splice synonymous GDMs in ASD probands (odds ratio ∼2) and to a lesser extent schizophrenia probands, relative to control subjects.53 Stronger signal in their initial ASD cohort was seen for sites predicted to cause ESE/ESS changes, but reduced in a replication dataset (odds ratios 2.52 and 1.55, respectively). In their analysis they compared the fraction of near-splice or those also disrupting ESE/ESS sites mutations in case versus control subjects (Fisher’s exact test), which does not take into account coverage differences across individuals/cohorts. We repeated our analysis of the distance to splice site distributions for the high-confidence 45×-joint coverage SSC synonymous GDMs, finding them to be significantly closer to splice sites in probands as compared to siblings (p = 0.005), similar to the PMM calls. However, we observed no corresponding enrichment of splice-altering variant predictions. Taken together, these data are consistent with a possible role of synonymous postzygotic mutations that functionally disrupt splicing regulation in ASD.
While computational splice regulation predictions can provide useful information at the population level, we advise interpreting the effect of individual variants with caution given the uncertainty of splice regulatory mechanisms, cell-type-specific splicing patterns, limited training sets, and high reported false positive rates. For example, HSF has a reported false positive rate of 43%.36 This is due in part to the wide breadth of splicing signals it attempts to capture. Additional functional validation of these mutations using in vitro approaches, e.g., minigene assays, or in vivo approaches, e.g., genome editing of cell lines, is warranted.
From the synonymous PMMs predicted to impact splicing, we identified a number of genes that have roles in neurodevelopment and are associated with other ASD risk genes. In particular, we highlight genes ACTL6B, a member of the chromatin remodeler complex SWI/SNF;58 CCT6B, a postsynaptic density gene recently implicated in recessive intellectual disability;61 FYN, which encodes a non-receptor tyrosine kinase that is involved in axon outgrowth;62 and STMN1, which encodes a microtubule destabilizing protein that is involved in the regulation of axon outgrowth.63 Also notable is COL5A3, which encodes a scaffolding protein that is directly regulated by ASD and Pitt-Hopkins (MIM: 610954)-associated gene TCF4 (MIM: 602272).64 Individuals with duplications that span COL5A3 have phenotypic characteristics similar to those of TCF4-related syndromes including seizures, facial dysmorphia, and developmental delay.64
We did not observe evidence of missense PMM burden in the full cohort of ASD probands. This is perhaps not surprising given the strong contribution of GDMs to ASD in the SSC and that most de novo events will be missense changes by chance, i.e., form most of the background non-disorder-related mutations. Our sample size is too small given their rate of mutations to fully evaluate nonsense/splice PMMs as a separate class. Based on the differential between probands and siblings, it has been reported that LGD GDMs have a 40% likelihood of contributing to ASD (90% of loci with recurrent LGD), while the likelihood for missense variants is ∼35%.11 We reasoned that restricting our analysis to families without proband germline mutations would increase our power to detect any effect of missense PMMs, even though we would be removing a significant fraction of families with germline events unrelated to ASD. Indeed, if we subdivide the SSC cohort into families that have or do not have a proband LGD GDM/de novo CNVs, or, alternatively, any NS germline mutation, we observed a difference emerging. This difference is strongest in the subset of genes predicted to be essential/intolerant to mutation (Figures 3B and 3C). Similarly, we also saw a further increase in synonymous PMM burden in the subcohort without any reported NS GDMs (Figure 2).
Freed and Pevsner recently reported on PMM burden in probands and siblings in the SSC.59 While our two studies used the same SSC datasets, we each used different computational and validation approaches. Restricting our comparison to SNVs at exonic/canonical splice sites, our 45× high-confidence call set contains 470 PMMs in children, 384 that are unique to our study. Their 20× final call set contained 167 PMMs, 81 of which are absent from our high-confidence calls. The majority of these absent calls failed to meet our 45× threshold (67%) or was present in families we excluded as outliers (30%). Our two criteria for including variants for mutation burden analyses were similar, but with several key differences. Most importantly, they restricted their burden analysis to their PMM calls that overlapped the previously published de novo datasets, met 40× joint-coverage, and also included indel calls. Unlike our study, they did not restrict their analysis to different minimum AF-COV thresholds. They report the burden of all classes of variants combined (e.g., synonymous, missense, LGD, and other) as significant. After correcting for germline misclassification, they estimate that 5.1% of probands have PMMs related to ASD risk. Moreover, they found nominal contributions across all classes of mutations.
Comparing our 45× PMM burden analysis to their data, we similarly observed differences in synonymous mutation rates. However, we did not observe higher missense mutation rates among probands in the full cohort. These differences are likely driven by our different computational approaches and our use of a larger number of PMM calls unique to our pipeline (164/231). Freed and Pevsner included 122 exonic/splice SNV calls in their burden analysis, 55 of which were absent from our call set. Again, the majority of these absent calls failed to meet our 45× threshold (62%) or was present in families we excluded as outliers (33%). With our approach, we estimate that PMMs as a group contribute to 3%-4% of simplex ASD, with an ∼2% contribution from synonymous mutations. Combined, our two analyses suggest that exonic PMMs as a whole are likely contributing to ASD risk in the SSC at rates similar to other classes of de novo mutations.11, 32
We found that proband missense PMMs were more likely than sibling missense PMMs to intersect with genes that are targets of proband missense GDMs (odds ratio ∼2). A number of our novel nonsense PMMs in probands overlapped genes with GDMs including BAZ2B, SSRP1, UNC79, USP15, and VPS13D (Table 4). Consistent with our observation of enrichment of chromatin modifiers in proband missense PMMs, we found that many of our PMMs overlapping genes with NS GDMs are also involved in chromatin regulation: e.g., BAZ2B, CHD2, COL5A3, KAT2B, KMT2C, and SSRP1. Recent studies have found that ASD risk genes are highly co-expressed during the mid-fetal period of cortical development.65, 66 Several PMMs intersect genes that occupy the same co-expression modules, which are significantly enriched for ASD risk genes. For example, BIRC6 (MIM: 605638), DMXL2, OBSCN (MIM: 60861), SPEN (MIM: 613484), SSRP1, and UNC79 all occupy modules 2 and 3, which peak between post conception weeks 10 and 22 and are enriched for chromatin modifiers/transcriptional regulators.65 COL5A3, KIF1A, SCN2A, and SYNE1 are found in modules 13/16/17, which are turned on later in development, after post conception weeks 10, and are enriched for synaptic genes.65
Moreover, we found missense PMMs in some of the highest-confidence ASD risk genes identified in the SSC or other combined studies, for example: CHD2, CTNNB1, KMT2C, SCN2A, and SYNGAP1 (Table 4).30, 32, 33, 67 Interestingly, small de novo deletions targeting CHD2, SYNGAP1, CTNNB1, and KMT2C have been reported in the SSC as well,32 demonstrating that new mutations of multiple types and origins at these sites contribute to ASD risk. Taken together, our data argue that proband PMMs and GDMs target many common risk genes. Finally, mutations in some of these genes are not restricted to ASD as these genes have also been found to be disrupted in cohorts primarily defined on diagnoses of epileptic encephalopathy, ID, and congenital heart defects with additional features.68, 69, 70, 71 Understanding how mutations impact these important genes that blur our diagnostic constructs will be an important area of future research. These and other data suggest that the creation of more broadly defined cohorts and better integration of genetic studies of developmental disorders are warranted.
We also performed our PMM analyses in the parental data, identifying both nontransmitted and transmitted PMMs. Transmitted PMMs are obligated to be present in both the soma and the germline. Given the low number of offspring of each parent, we cannot rule out the possibility that a fraction of the nontransmitted parental events are also present in the parental germ cells. Our observed postzygotic mutation rate is much higher in the SSC parents compared to the SSC children. Moreover, the nontransmitted PMM AFs have a bimodal distribution that is distinct from both the child PMMs and parental transmitted PMMs. There are several potential explanations for the increased rate of mutation and AF differences. As parents in this cohort were several decades older at time of DNA collection, this increase could be explained by the accumulation of PMMs in the blood, some of which might drift to or be selected for higher AF. We found very little evidence for enrichment of PMMs in genes related to blood ACEs, except DNMT3A. The number of parents with PMMs in ACE-related genes is < 1%, which is consistent with estimates that ACE-associated mutations occur in fewer than 1% of individuals under 50 and do not begin to rise until after 65.48, 49, 50 Our analysis on a subset of the cohort suggests that ∼40% of the excess in nontransmitted parental PMM calls could be explained by incomplete filtering of recurrently biased and randomly skewed sites, while the remainder are likely true events (Figure S16). The parental transmitted PMM distribution closely resembles the rightmost Gaussian of the nontransmitted distribution, suggesting that this subset is still representative of likely early embryonic events, a fraction of which are also found in the germ cells. Recurrently biased sites are likely to have higher AFs (>20%). Parental (or non-family based) PMMs with AF that fall in this upper range that are not clearly transmitted should be interpreted with caution. However, importantly, Xie and colleagues report this same bimodal distribution in a case-control study of ACE, which did not benefit from transmission-based filtering.49
Rahbari and colleagues recently performed whole-genome sequencing on moderately sized pedigrees followed by the identification and characterization of de novo mutations in multiple children, spanning approximately a decade.46 In validating their de novo calls using target capture and deep sequencing, they identified a number of mutations that were at low levels in the parental blood-derived DNA. Importantly in contrast to our study, PMMs were not directly identified in the parents and calls with greater than 5% of reads showing the alternative allele in a parent were excluded from the de novo call set. Nevertheless, they found that 4.2% of apparent germline mutations are present in the blood of parents at >1% AF. However, the rate we observed in our high-confidence smMIP validation data, of similar calls (without parental WES signal), is 0.6% (1 out of 164). In our 45× WES dataset, we found 0.66% of GDMs in children are also obligate gonadal mosaic. Overall, our data support that at least 7%–11% (depending on the AF) of parental PMM events are also present in the parental germ cells and can be transmitted to the next generation. Together these two sets of parental postzygotic mutations account for 6.8% of the presumed de novo mutations in the children from our high-confidence call set (Table S5). Importantly, many of these events would be missed by de novo calling pipelines that eliminate any sites with variant reads present in a parent. This rate is higher than what has been recently reported for de novo CNVs (4%).22 These findings have important implications for recurrence risk and clinical testing, which are still not widely appreciated.14, 22, 46, 72, 73 While the recurrence risk for de novo mutations is generally thought to be low (∼1%), finding the presence of a mutation, even at low levels, in a parent dramatically increases this risk to a previously estimated >5%.46, 72, 73 The risk may be dramatically higher for specific mutations, depending on their embryonic timing and distribution within the germ cells.
We were limited by the availability of DNA from a single peripheral blood source and WES data that is non-uniform. Future studies in this area would greatly benefit from deep uniform whole-genome sequencing, access to multiple peripheral and other tissue types of different embryonic origin, and improved indel variant calling approaches. This could include brain tissue in cases of surgical resection to control intractable epilepsy. Moreover, we strongly suggest that new efforts to establish autism brain banks obtain peripheral DNA samples from the donor and their parents. These DNA would greatly aid in the classification of variant types, i.e., PMMs, GDMs, or inherited variants, identified in bulk brain and single-cell sequencing studies as well as help determine their likely embryonic timing.
In summary, our data support the conclusion that exonic postzygotic mosaicism contributes to the overall genetic architecture of ASD, in potentially 3%-4% of all ASD simplex cases, and that future studies of mosaicism in ASD and related disorders are warranted. We present a general approach for identifying PMMs that overcomes many of the inherent detection and validation challenges for these events in family-based and unmatched samples. The methods developed will allow continued discovery of PMMs in future datasets, including unsolved genetic disorders, and our findings have potential translational implications for clinical detection, case management, interventions, and genetic counseling.
Acknowledgments
This work was supported by a grant from the Simons Foundation (SFARI 305927, B.J.O.) and the Agence Nationale de la Recherche (ANR-13-PDOC-0029, Y.D. and J.-B.R.). B.J.O. is currently a Klingenstein-Simons Fellow in Neurosciences and Alfred P. Sloan Foundation Fellow in Neuroscience (FG-2015-65608) and was supported by the NARSAD Young Investigator Award (22935) from the Brain and Behavior Research Foundation. We are grateful for the use of the Exacloud high performance computing environment developed jointly by OHSU and Intel and the technical support of the OHSU Advanced Computing Center. We would like to thank S.J. Webb, A.C. Adey, K.M. Wright, I. Iossifov, S. Bedrick, J. Burchard, and A. Presmanes Hill for helpful discussions regarding the manuscript. We also thank I. Fisk, N. Volfovsky, N. Krumm, and T.N. Turner for their assistance accessing the WES datasets. We are grateful to all of the families at the participating Simons Simplex Collections (SSC) sites, as well as the principal investigators (A. Beaudet, R. Bernier, J. Constantino, E. Cook, E. Fombonne, D. Geschwind, R., Goin-Kochel, E. Hanson, D. Grice, A. Klin, D. Ledbetter, C. Lord, C. Martin, D. Martin, R. Maxim, J. Miles, O. Ousley, K. Pelphrey, B. Peterson, J. Piggot, C. Saulnier, M. State, W. Stone, J. Sutcliffe, C. Walsh, Z. Warren, E. Wijsman). Approved researchers can obtain the SSC population dataset described in this study by applying at https://base.sfari.org.
Published: August 31, 2017
Footnotes
Supplemental Data include Supplemental Note (Material and Methods, Model Development, and Case Reports), 19 figures, and 11 tables and can be found with this article online at http://dx.doi.org/10.1016/j.ajhg.2017.07.016.
Web Resources
GenBank, http://www.ncbi.nlm.nih.gov/genbank/
GenPhenF, https://iossifovlab.com/gpf
Human Splicing Finder, http://www.umd.be/HSF/
National Database for Autism Research, https://ndar.nih.gov
OMIM, http://www.omim.org/
SFARI, https://sfari.org/
Simulated NGS Data, http://www.ebi.ac.uk/goldman-srv/simNGS
UCSC Genome Browser, http://genome.ucsc.edu
Variant Effect Predictor, http://useast.ensembl.org/Homo_sapiens/Tools/VEP
Supplemental Data
References
- 1.O’Roak B.J., Deriziotis P., Lee C., Vives L., Schwartz J.J., Girirajan S., Karakoc E., Mackenzie A.P., Ng S.B., Baker C. Exome sequencing in sporadic autism spectrum disorders identifies severe de novo mutations. Nat. Genet. 2011;43:585–589. doi: 10.1038/ng.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O’Roak B.J., Vives L., Girirajan S., Karakoc E., Krumm N., Coe B.P., Levy R., Ko A., Lee C., Smith J.D. Sporadic autism exomes reveal a highly interconnected protein network of de novo mutations. Nature. 2012;485:246–250. doi: 10.1038/nature10989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neale B.M., Kou Y., Liu L., Ma’ayan A., Samocha K.E., Sabo A., Lin C.F., Stevens C., Wang L.S., Makarov V. Patterns and rates of exonic de novo mutations in autism spectrum disorders. Nature. 2012;485:242–245. doi: 10.1038/nature11011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sanders S.J., Murtha M.T., Gupta A.R., Murdoch J.D., Raubeson M.J., Willsey A.J., Ercan-Sencicek A.G., DiLullo N.M., Parikshak N.N., Stein J.L. De novo mutations revealed by whole-exome sequencing are strongly associated with autism. Nature. 2012;485:237–241. doi: 10.1038/nature10945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iossifov I., Ronemus M., Levy D., Wang Z., Hakker I., Rosenbaum J., Yamrom B., Lee Y.H., Narzisi G., Leotta A. De novo gene disruptions in children on the autistic spectrum. Neuron. 2012;74:285–299. doi: 10.1016/j.neuron.2012.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Itsara A., Cooper G.M., Baker C., Girirajan S., Li J., Absher D., Krauss R.M., Myers R.M., Ridker P.M., Chasman D.I. Population analysis of large copy number variants and hotspots of human genetic disease. Am. J. Hum. Genet. 2009;84:148–161. doi: 10.1016/j.ajhg.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marshall C.R., Noor A., Vincent J.B., Lionel A.C., Feuk L., Skaug J., Shago M., Moessner R., Pinto D., Ren Y. Structural variation of chromosomes in autism spectrum disorder. Am. J. Hum. Genet. 2008;82:477–488. doi: 10.1016/j.ajhg.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sebat J., Lakshmi B., Malhotra D., Troge J., Lese-Martin C., Walsh T., Yamrom B., Yoon S., Krasnitz A., Kendall J. Strong association of de novo copy number mutations with autism. Science. 2007;316:445–449. doi: 10.1126/science.1138659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levy D., Ronemus M., Yamrom B., Lee Y.H., Leotta A., Kendall J., Marks S., Lakshmi B., Pai D., Ye K. Rare de novo and transmitted copy-number variation in autistic spectrum disorders. Neuron. 2011;70:886–897. doi: 10.1016/j.neuron.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 10.Sanders S.J., Ercan-Sencicek A.G., Hus V., Luo R., Murtha M.T., Moreno-De-Luca D., Chu S.H., Moreau M.P., Gupta A.R., Thomson S.A. Multiple recurrent de novo CNVs, including duplications of the 7q11.23 Williams syndrome region, are strongly associated with autism. Neuron. 2011;70:863–885. doi: 10.1016/j.neuron.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iossifov I., O’Roak B.J., Sanders S.J., Ronemus M., Krumm N., Levy D., Stessman H.A., Witherspoon K.T., Vives L., Patterson K.E. The contribution of de novo coding mutations to autism spectrum disorder. Nature. 2014;515:216–221. doi: 10.1038/nature13908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krumm N., Turner T.N., Baker C., Vives L., Mohajeri K., Witherspoon K., Raja A., Coe B.P., Stessman H.A., He Z.-X. Excess of rare, inherited truncating mutations in autism. Nat. Genet. 2015;47:582–588. doi: 10.1038/ng.3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Rubeis S., He X., Goldberg A.P., Poultney C.S., Samocha K., Cicek A.E., Kou Y., Liu L., Fromer M., Walker S., DDD Study. Homozygosity Mapping Collaborative for Autism. UK10K Consortium Synaptic, transcriptional and chromatin genes disrupted in autism. Nature. 2014;515:209–215. doi: 10.1038/nature13772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Campbell I.M., Shaw C.A., Stankiewicz P., Lupski J.R. Somatic mosaicism: implications for disease and transmission genetics. Trends Genet. 2015;31:382–392. doi: 10.1016/j.tig.2015.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poduri A., Evrony G.D., Cai X., Walsh C.A. Somatic mutation, genomic variation, and neurological disease. Science. 2013;341:1237758. doi: 10.1126/science.1237758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jamuar S.S., Lam A.T., Kircher M., D’Gama A.M., Wang J., Barry B.J., Zhang X., Hill R.S., Partlow J.N., Rozzo A. Somatic mutations in cerebral cortical malformations. N. Engl. J. Med. 2014;371:733–743. doi: 10.1056/NEJMoa1314432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee J.H., Huynh M., Silhavy J.L., Kim S., Dixon-Salazar T., Heiberg A., Scott E., Bafna V., Hill K.J., Collazo A. De novo somatic mutations in components of the PI3K-AKT3-mTOR pathway cause hemimegalencephaly. Nat. Genet. 2012;44:941–945. doi: 10.1038/ng.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kurek K.C., Luks V.L., Ayturk U.M., Alomari A.I., Fishman S.J., Spencer S.A., Mulliken J.B., Bowen M.E., Yamamoto G.L., Kozakewich H.P., Warman M.L. Somatic mosaic activating mutations in PIK3CA cause CLOVES syndrome. Am. J. Hum. Genet. 2012;90:1108–1115. doi: 10.1016/j.ajhg.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lindhurst M.J., Parker V.E., Payne F., Sapp J.C., Rudge S., Harris J., Witkowski A.M., Zhang Q., Groeneveld M.P., Scott C.E. Mosaic overgrowth with fibroadipose hyperplasia is caused by somatic activating mutations in PIK3CA. Nat. Genet. 2012;44:928–933. doi: 10.1038/ng.2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rivière J.B., Mirzaa G.M., O’Roak B.J., Beddaoui M., Alcantara D., Conway R.L., St-Onge J., Schwartzentruber J.A., Gripp K.W., Nikkel S.M., Finding of Rare Disease Genes (FORGE) Canada Consortium De novo germline and postzygotic mutations in AKT3, PIK3R2 and PIK3CA cause a spectrum of related megalencephaly syndromes. Nat. Genet. 2012;44:934–940. doi: 10.1038/ng.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adviento B., Corbin I.L., Widjaja F., Desachy G., Enrique N., Rosser T., Risi S., Marco E.J., Hendren R.L., Bearden C.E. Autism traits in the RASopathies. J. Med. Genet. 2014;51:10–20. doi: 10.1136/jmedgenet-2013-101951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Campbell I.M., Yuan B., Robberecht C., Pfundt R., Szafranski P., McEntagart M.E., Nagamani S.C., Erez A., Bartnik M., Wiśniowiecka-Kowalnik B. Parental somatic mosaicism is underrecognized and influences recurrence risk of genomic disorders. Am. J. Hum. Genet. 2014;95:173–182. doi: 10.1016/j.ajhg.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Happle R. Lethal genes surviving by mosaicism: a possible explanation for sporadic birth defects involving the skin. J. Am. Acad. Dermatol. 1987;16:899–906. doi: 10.1016/s0190-9622(87)80249-9. [DOI] [PubMed] [Google Scholar]
- 24.Keppler-Noreuil K.M., Rios J.J., Parker V.E., Semple R.K., Lindhurst M.J., Sapp J.C., Alomari A., Ezaki M., Dobyns W., Biesecker L.G. PIK3CA-related overgrowth spectrum (PROS): diagnostic and testing eligibility criteria, differential diagnosis, and evaluation. Am. J. Med. Genet. A. 2015;167A:287–295. doi: 10.1002/ajmg.a.36836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shirley M.D., Tang H., Gallione C.J., Baugher J.D., Frelin L.P., Cohen B., North P.E., Marchuk D.A., Comi A.M., Pevsner J. Sturge-Weber syndrome and port-wine stains caused by somatic mutation in GNAQ. N. Engl. J. Med. 2013;368:1971–1979. doi: 10.1056/NEJMoa1213507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Acuna-Hidalgo R., Bo T., Kwint M.P., van de Vorst M., Pinelli M., Veltman J.A., Hoischen A., Vissers L.E., Gilissen C. Post-zygotic point mutations are an underrecognized source of de novo genomic variation. Am. J. Hum. Genet. 2015;97:67–74. doi: 10.1016/j.ajhg.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fischbach G.D., Lord C. The Simons Simplex Collection: a resource for identification of autism genetic risk factors. Neuron. 2010;68:192–195. doi: 10.1016/j.neuron.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 28.Wang K., Li M., Hakonarson H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010;38:e164. doi: 10.1093/nar/gkq603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boyle E.A., O’Roak B.J., Martin B.K., Kumar A., Shendure J. MIPgen: optimized modeling and design of molecular inversion probes for targeted resequencing. Bioinformatics. 2014;30:2670–2672. doi: 10.1093/bioinformatics/btu353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O’Roak B.J., Stessman H.A., Boyle E.A., Witherspoon K.T., Martin B., Lee C., Vives L., Baker C., Hiatt J.B., Nickerson D.A. Recurrent de novo mutations implicate novel genes underlying simplex autism risk. Nat. Commun. 2014;5:5595. doi: 10.1038/ncomms6595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Benjamini Y., Yekutieli D. The control of the false discovery rate in multiple testing under dependency. Ann. Stat. 2001;29:1165–1188. [Google Scholar]
- 32.Sanders S.J., He X., Willsey A.J., Ercan-Sencicek A.G., Samocha K.E., Cicek A.E., Murtha M.T., Bal V.H., Bishop S.L., Dong S., Autism Sequencing Consortium Insights into autism spectrum disorder genomic architecture and biology from 71 risk loci. Neuron. 2015;87:1215–1233. doi: 10.1016/j.neuron.2015.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iossifov I., Levy D., Allen J., Ye K., Ronemus M., Lee Y.H., Yamrom B., Wigler M. Low load for disruptive mutations in autism genes and their biased transmission. Proc. Natl. Acad. Sci. USA. 2015;112:E5600–E5607. doi: 10.1073/pnas.1516376112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Georgi B., Voight B.F., Bućan M. From mouse to human: evolutionary genomics analysis of human orthologs of essential genes. PLoS Genet. 2013;9:e1003484. doi: 10.1371/journal.pgen.1003484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lek M., Karczewski K.J., Minikel E.V., Samocha K.E., Banks E., Fennell T., O’Donnell-Luria A.H., Ware J.S., Hill A.J., Cummings B.B., Exome Aggregation Consortium Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;536:285–291. doi: 10.1038/nature19057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Desmet F.O., Hamroun D., Lalande M., Collod-Béroud G., Claustres M., Béroud C. Human Splicing Finder: an online bioinformatics tool to predict splicing signals. Nucleic Acids Res. 2009;37:e67. doi: 10.1093/nar/gkp215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xiong H.Y., Alipanahi B., Lee L.J., Bretschneider H., Merico D., Yuen R.K., Hua Y., Gueroussov S., Najafabadi H.S., Hughes T.R. RNA splicing. The human splicing code reveals new insights into the genetic determinants of disease. Science. 2015;347:1254806. doi: 10.1126/science.1254806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krishnan A., Zhang R., Yao V., Theesfeld C.L., Wong A.K., Tadych A., Volfovsky N., Packer A., Lash A., Troyanskaya O.G. Genome-wide prediction and functional characterization of the genetic basis of autism spectrum disorder. Nat. Neurosci. 2016;19:1454–1462. doi: 10.1038/nn.4353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Petrovski S., Wang Q., Heinzen E.L., Allen A.S., Goldstein D.B. Genic intolerance to functional variation and the interpretation of personal genomes. PLoS Genet. 2013;9:e1003709. doi: 10.1371/journal.pgen.1003709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Deciphering Developmental Disorders Study Prevalence and architecture of de novo mutations in developmental disorders. Nature. 2017;542:433–438. doi: 10.1038/nature21062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Challis D., Yu J., Evani U.S., Jackson A.R., Paithankar S., Coarfa C., Milosavljevic A., Gibbs R.A., Yu F. An integrative variant analysis suite for whole exome next-generation sequencing data. BMC Bioinformatics. 2012;13:8. doi: 10.1186/1471-2105-13-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wilm A., Aw P.P., Bertrand D., Yeo G.H., Ong S.H., Wong C.H., Khor C.C., Petric R., Hibberd M.L., Nagarajan N. LoFreq: a sequence-quality aware, ultra-sensitive variant caller for uncovering cell-population heterogeneity from high-throughput sequencing datasets. Nucleic Acids Res. 2012;40:11189–11201. doi: 10.1093/nar/gks918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koboldt D.C., Zhang Q., Larson D.E., Shen D., McLellan M.D., Lin L., Miller C.A., Mardis E.R., Ding L., Wilson R.K. VarScan 2: somatic mutation and copy number alteration discovery in cancer by exome sequencing. Genome Res. 2012;22:568–576. doi: 10.1101/gr.129684.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Samocha K.E., Robinson E.B., Sanders S.J., Stevens C., Sabo A., McGrath L.M., Kosmicki J.A., Rehnström K., Mallick S., Kirby A. A framework for the interpretation of de novo mutation in human disease. Nat. Genet. 2014;46:944–950. doi: 10.1038/ng.3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ji X., Kember R.L., Brown C.D., Bućan M. Increased burden of deleterious variants in essential genes in autism spectrum disorder. Proc. Natl. Acad. Sci. USA. 2016;113:15054–15059. doi: 10.1073/pnas.1613195113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rahbari R., Wuster A., Lindsay S.J., Hardwick R.J., Alexandrov L.B., Turki S.A., Dominiczak A., Morris A., Porteous D., Smith B., UK10K Consortium Timing, rates and spectra of human germline mutation. Nat. Genet. 2016;48:126–133. doi: 10.1038/ng.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Forsberg L.A., Gisselsson D., Dumanski J.P. Mosaicism in health and disease - clones picking up speed. Nat. Rev. Genet. 2017;18:128–142. doi: 10.1038/nrg.2016.145. [DOI] [PubMed] [Google Scholar]
- 48.Jaiswal S., Fontanillas P., Flannick J., Manning A., Grauman P.V., Mar B.G., Lindsley R.C., Mermel C.H., Burtt N., Chavez A. Age-related clonal hematopoiesis associated with adverse outcomes. N. Engl. J. Med. 2014;371:2488–2498. doi: 10.1056/NEJMoa1408617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xie M., Lu C., Wang J., McLellan M.D., Johnson K.J., Wendl M.C., McMichael J.F., Schmidt H.K., Yellapantula V., Miller C.A. Age-related mutations associated with clonal hematopoietic expansion and malignancies. Nat. Med. 2014;20:1472–1478. doi: 10.1038/nm.3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Genovese G., Kähler A.K., Handsaker R.E., Lindberg J., Rose S.A., Bakhoum S.F., Chambert K., Mick E., Neale B.M., Fromer M. Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. N. Engl. J. Med. 2014;371:2477–2487. doi: 10.1056/NEJMoa1409405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alexandrov L.B., Nik-Zainal S., Wedge D.C., Aparicio S.A., Behjati S., Biankin A.V., Bignell G.R., Bolli N., Borg A., Børresen-Dale A.L., Australian Pancreatic Cancer Genome Initiative. ICGC Breast Cancer Consortium. ICGC MMML-Seq Consortium. ICGC PedBrain Signatures of mutational processes in human cancer. Nature. 2013;500:415–421. doi: 10.1038/nature12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Supek F., Miñana B., Valcárcel J., Gabaldón T., Lehner B. Synonymous mutations frequently act as driver mutations in human cancers. Cell. 2014;156:1324–1335. doi: 10.1016/j.cell.2014.01.051. [DOI] [PubMed] [Google Scholar]
- 53.Takata A., Ionita-Laza I., Gogos J.A., Xu B., Karayiorgou M. De novo synonymous mutations in regulatory elements contribute to the genetic etiology of autism and schizophrenia. Neuron. 2016;89:940–947. doi: 10.1016/j.neuron.2016.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cáceres E.F., Hurst L.D. The evolution, impact and properties of exonic splice enhancers. Genome Biol. 2013;14:R143. doi: 10.1186/gb-2013-14-12-r143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jones M.H., Hamana N., Nezu Ji., Shimane M. A novel family of bromodomain genes. Genomics. 2000;63:40–45. doi: 10.1006/geno.1999.6071. [DOI] [PubMed] [Google Scholar]
- 56.Lu B., Zhang Q., Wang H., Wang Y., Nakayama M., Ren D. Extracellular calcium controls background current and neuronal excitability via an UNC79-UNC80-NALCN cation channel complex. Neuron. 2010;68:488–499. doi: 10.1016/j.neuron.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Reyes-Turcu F.E., Ventii K.H., Wilkinson K.D. Regulation and cellular roles of ubiquitin-specific deubiquitinating enzymes. Annu. Rev. Biochem. 2009;78:363–397. doi: 10.1146/annurev.biochem.78.082307.091526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vogel-Ciernia A., Matheos D.P., Barrett R.M., Kramár E.A., Azzawi S., Chen Y., Magnan C.N., Zeller M., Sylvain A., Haettig J. The neuron-specific chromatin regulatory subunit BAF53b is necessary for synaptic plasticity and memory. Nat. Neurosci. 2013;16:552–561. doi: 10.1038/nn.3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Freed D., Pevsner J. The contribution of mosaic variants to autism spectrum disorder. PLoS Genet. 2016;12:e1006245. doi: 10.1371/journal.pgen.1006245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.D’Gama A.M., Pochareddy S., Li M., Jamuar S.S., Reiff R.E., Lam A.T., Sestan N., Walsh C.A. Targeted DNA sequencing from autism spectrum disorder brains implicates multiple genetic mechanisms. Neuron. 2015;88:910–917. doi: 10.1016/j.neuron.2015.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Riazuddin S., Hussain M., Razzaq A., Iqbal Z., Shahzad M., Polla D.L., Song Y., van Beusekom E., Khan A.A., Tomas-Roca L., UK10K Exome sequencing of Pakistani consanguineous families identifies 30 novel candidate genes for recessive intellectual disability. Mol. Psychiatry. 2016 doi: 10.1038/mp.2016.109. Published online July 26, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu G., Beggs H., Jürgensen C., Park H.T., Tang H., Gorski J., Jones K.R., Reichardt L.F., Wu J., Rao Y. Netrin requires focal adhesion kinase and Src family kinases for axon outgrowth and attraction. Nat. Neurosci. 2004;7:1222–1232. doi: 10.1038/nn1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wen H.L., Lin Y.T., Ting C.H., Lin-Chao S., Li H., Hsieh-Li H.M. Stathmin, a microtubule-destabilizing protein, is dysregulated in spinal muscular atrophy. Hum. Mol. Genet. 2010;19:1766–1778. doi: 10.1093/hmg/ddq058. [DOI] [PubMed] [Google Scholar]
- 64.Chen E.S., Gigek C.O., Rosenfeld J.A., Diallo A.B., Maussion G., Chen G.G., Vaillancourt K., Lopez J.P., Crapper L., Poujol R. Molecular convergence of neurodevelopmental disorders. Am. J. Hum. Genet. 2014;95:490–508. doi: 10.1016/j.ajhg.2014.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Parikshak N.N., Luo R., Zhang A., Won H., Lowe J.K., Chandran V., Horvath S., Geschwind D.H. Integrative functional genomic analyses implicate specific molecular pathways and circuits in autism. Cell. 2013;155:1008–1021. doi: 10.1016/j.cell.2013.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Willsey A.J., Sanders S.J., Li M., Dong S., Tebbenkamp A.T., Muhle R.A., Reilly S.K., Lin L., Fertuzinhos S., Miller J.A. Coexpression networks implicate human midfetal deep cortical projection neurons in the pathogenesis of autism. Cell. 2013;155:997–1007. doi: 10.1016/j.cell.2013.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.de Ligt J., Willemsen M.H., van Bon B.W., Kleefstra T., Yntema H.G., Kroes T., Vulto-van Silfhout A.T., Koolen D.A., de Vries P., Gilissen C. Diagnostic exome sequencing in persons with severe intellectual disability. N. Engl. J. Med. 2012;367:1921–1929. doi: 10.1056/NEJMoa1206524. [DOI] [PubMed] [Google Scholar]
- 68.Carvill G.L., Heavin S.B., Yendle S.C., McMahon J.M., O’Roak B.J., Cook J., Khan A., Dorschner M.O., Weaver M., Calvert S. Targeted resequencing in epileptic encephalopathies identifies de novo mutations in CHD2 and SYNGAP1. Nat. Genet. 2013;45:825–830. doi: 10.1038/ng.2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Homsy J., Zaidi S., Shen Y., Ware J.S., Samocha K.E., Karczewski K.J., DePalma S.R., McKean D., Wakimoto H., Gorham J. De novo mutations in congenital heart disease with neurodevelopmental and other congenital anomalies. Science. 2015;350:1262–1266. doi: 10.1126/science.aac9396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rauch A., Wieczorek D., Graf E., Wieland T., Endele S., Schwarzmayr T., Albrecht B., Bartholdi D., Beygo J., Di Donato N. Range of genetic mutations associated with severe non-syndromic sporadic intellectual disability: an exome sequencing study. Lancet. 2012;380:1674–1682. doi: 10.1016/S0140-6736(12)61480-9. [DOI] [PubMed] [Google Scholar]
- 71.Allen A.S., Berkovic S.F., Cossette P., Delanty N., Dlugos D., Eichler E.E., Epstein M.P., Glauser T., Goldstein D.B., Han Y., Epi4K Consortium. Epilepsy Phenome/Genome Project De novo mutations in epileptic encephalopathies. Nature. 2013;501:217–221. doi: 10.1038/nature12439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Campbell I.M., Stewart J.R., James R.A., Lupski J.R., Stankiewicz P., Olofsson P., Shaw C.A. Parent of origin, mosaicism, and recurrence risk: probabilistic modeling explains the broken symmetry of transmission genetics. Am. J. Hum. Genet. 2014;95:345–359. doi: 10.1016/j.ajhg.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Acuna-Hidalgo R., Veltman J.A., Hoischen A. New insights into the generation and role of de novo mutations in health and disease. Genome Biol. 2016;17:241. doi: 10.1186/s13059-016-1110-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.