Abstract
Chronic hepatitis C virus (HCV) infection affects millions of people worldwide and is associated with cancer. Direct-acting antivirals (DAAs) have changed HCV treatment paradigms, but little is known about management of HCV infection in patients with cancer. The substantial burden of HCV infection in patients with cancer and the inconclusive evidence regarding detection and management of HCV infection in patients with cancer prompted us to review the literature and formulate recommendations. Patients for whom HCV screening is recommended included all patients with hematological malignancies, hematopoietic cell transplant candidates or patients with hepatocellular carcinoma (HCC). There is lack of consensus-based recommendations for the identification of other cancer patients but physicians may consider screening patients belonging to groups at heightened risk of HCV infection including persons born during 1945–1965 and those at high risk for infection. Patients with evidence of HCV infection should be assessed by an expert to evaluate liver disease severity, comorbidities associated with HCV infection, and treatment opportunities. DAA therapy should be tailored on the basis of patient prognosis, type of cancer, cancer treatment plan, and hepatic and virologic parameters. HCV-infected cancer patients with cirrhosis (or even advanced fibrosis) and those at risk for liver disease progression, especially patients with HCV-associated comorbidities, should have ongoing follow-up, regardless of whether there is a sustained virologic response, to ensure timely detection and treatment of HCC. HCV infection and its treatment should not be considered contraindications to cancer treatment and should not delay the initiation of an urgent cancer therapy.
Keywords: HCV, cancer, primary liver cancer, hepatocellular carcinoma, non-Hodgkin lymphoma, direct-acting antiviral
Introduction
Chronic hepatitis C virus (HCV) infection affects millions of people worldwide.1 The oncologic burden of HCV infection is substantial. First, HCV infection is associated with the development of several different types of cancer.2,3 In 2012, a total of 170,000 new cancer cases, or approximately 7.8% of all new cancers, were attributable to HCV in GLOBOCAN 2012.4 Second, chronic HCV infection in cancer patients causes significant additional morbidity and mortality and can interfere with cancer treatment.5–10
Management of chronic HCV infection has historically been neglected in cancer centers, likely due to reservation of treating concomitantly with chemotherapy and older HCV therapy, such as interferon.6 Today, direct-acting antivirals (DAAs) have changed the treatment paradigm for chronic HCV infection and improved virologic outcomes, even in HCV-infected cancer patients.11 Rates of sustained virologic response (SVR), regarded as indicating HCV cure, are now similar in HCV-infected patients with and without cancer.11 Differences in the presentation, natural history, and management of HCV infection in patients with and without cancer are presented in Table 1.5–7,12–15
TABLE 1.
Characteristics of HCV infection in patients with and without cancer
| Characteristic | HCV Infection in Patients with Cancer | HCV Infection in Patients without Cancer |
|---|---|---|
| Findings on serologic assays for HCV antibodies | False-negative results possible (negative findings on screening for HCV antibodies with detectable HCV RNA in serum) | Reliable |
| Risk of development of fibrosis progression and early cirrhosis | High, particularly in HCT recipients | Low |
| Viral reactivation | Reported after certain types of cancer treatment | Uncommon in patients without immunosuppression |
| Standard-of-care anti-HCV therapy | Not defined | Well defined |
| Rate of sustained virologic response after treatment with DAAs | High | High |
| Risk of drug-drug interactions with DAAs | High | Low to moderate |
| Use of DAAs in patients with decompensated cirrhosis | Potentially contraindicated | Recommended but to be managed by providers with expertise in that condition, ideally in a liver transplant center |
| Provision of care | Frequently requires a transdisciplinary team | Frequently managed by single provider |
DAA, direct-acting antiviral; HCT, hematopoietic cell transplant; HCV, hepatitis C virus
The burden of HCV in patients with cancer and the inconclusive evidence regarding detection and management of HCV infection in patients with cancer prompted us to review the literature and to summarize the available data on HCV epidemiology and risk factors; associations between HCV and cancer; the carcinogenic potential of HCV; HCV screening; complications of HCV infection, and treatment of HCV infection, including with the use of new DAAs.
We searched PubMed and Web of Science for articles published from January 1, 1966, through March 24, 2017, using the terms “hepatitis C virus”, “HCV”, “prevalence”, “screening”, “cancer”, “chemotherapy”, and “reactivation”. We also searched the reference lists of articles identified by this search strategy and selected those references we judged relevant for each specific issue discussed. Abstracts were included only when they related directly to subsequently published work. We used the Grades of Recommendation, Assessment, Development and Evaluation (GRADE) system to grade the quality of evidence.16
Global epidemiology of HCV infection
The main mode of transmission of HCV was the use of contaminated medical materials. In high-income countries, widespread use of shared needles for medical treatments led to an epidemic of HCV infection in the 1940s. In the United States, contaminated blood products were also a mode of transmission of HCV infection before July 1992. Intravenous drug use has been and is still a leading contributor to the epidemic of HCV infection worldwide. 17,18 In low-to-middle-income countries, iatrogenic factors are still key contributors to the epidemic of HCV infection.
Estimates of the prevalence of HCV antibodies worldwide range from 1.6%19 to 2.8%.20 The highest prevalences are reported in low-income countries, including Egypt (15%), Pakistan (4.7%), and Taiwan (4.4%)21; prevalences are lower in North America (1.1%–1.3%), Australia (1.7%), and Eastern and Western Europe (0.5%–4.5%).19 The latest epidemiologic reports suggest that there are currently 80 million HCV-RNA-positive individuals around the globe.19,22
In the United States, it is estimated that at least 3.5 million people (range, 2.5 million to 4.7 million) live with HCV infection23 and that about half of them are unaware they are infected.24 Individuals born during 1945–1965 have a 3% prevalence of HCV antibodies, which is five times the prevalence in adults born in other years.25
Natural history of HCV infection
HCV infection evolves to chronic infection in more than two-thirds of cases, and 4% to 25% of patients with chronic HCV infection develop cirrhosis within 20 to 30 years after HCV infection is first diagnosed.26 Once cirrhosis develops in a patient with chronic HCV infection, the risk of hepatocellular carcinoma (HCC) is estimated to be 1% to 4% annually, similar to the risk of end-stage liver disease.27,28 A small proportion of HCC cases, fewer than 10%, develop in patients with HCV infection without cirrhosis.29 In the Global Burden of Disease Study 2013, in which liver-related deaths were classified as secondary to hepatitis B virus (HBV), HCV, or alcohol use, the annual number of HCV-attributable deaths worldwide increased from approximately 450,000 in 1990 to 520,000 in 2013.22
Many factors affect the natural history of HCV infection. Liver disease progression in patients with chronic HCV infection is closely related to age at infection, sex, and HCV-associated comorbidities, including HIV and HBV co-infections, alcohol use disorders, and diabetes.30 Given the many factors affecting the natural progression of HCV infection, it is very difficult to determine exactly which factors have the biggest impact.31,32 For example, in historical, community-based studies, liver disease progression in patients with HCV infection remained rare over decades after diagnosis with HCV infection, and liver disease progression was associated with obesity and alcohol use. In cohorts in which intravenous drug use was the main mode of transmission of HCV, alcohol use disorders were reported in about 30% to 50% of individuals; among male military service veterans with HCV infection, the proportion with alcohol use disorders was even higher. In Sub-Saharan Africa, where alcohol abuse is rare, HCV transmission is associated with HBV infection. In Egypt and the Middle East, obesity and diabetes mellitus are endemic.33
Comorbidities associated with HCV infection probably underlie the unexpected observations of early HCC occurrence and recurrence among patients at risk for liver disease progression following HCV eradication with DAAs.34–37 These observations from uncontrolled studies contrast with previous models of liver disease progression that support widespread use of DAAs to reduce the burden of HCV infection.38 These discrepancies regarding the benefits of DAA therapy can most likely be explained by underestimation of the prevalence of HCV-associated comorbidities and their impact on liver disease progression; the historical practice of selecting healthier patients without comorbidities for interferon-based HCV treatments, a selection bias that does not apply to treatment with DAAs; and maximization of the benefits of HCV treatment with systematic implementation of care pathways and accompanying lifestyle modifications, which include cure of HCV-associated comorbidities, in observational cohorts.39–42
Some cancer patients with HCV infection will probably remain at risk for liver disease progression after a SVR, and the risk of progression is probably greatest in patients with HCV-associated comorbidities, including alcohol use disorders. Alcohol use has not been entered in most liver disease progression models, including those that support current cost-effectiveness strategies in industrialized countries, and therefore the benefits of treatment of HCV infection with DAAs could have been overestimated.38,43 In an individual patient, treating HCV-associated comorbidities could be as important as eradicating HCV in terms of improving outcomes, and there is enough evidence to recommend abstinence from alcohol together with treatment of HCV-associated comorbidities in patients with advanced liver disease, regardless of whether or not they have a SVR. 41
Associations between HCV infection and cancer
Among adults with cancer who were newly registered at The University of Texas MD Anderson Cancer Center from January 2004 through April 2011, the prevalence of HCV antibodies was 1.5% overall,44 and much higher in certain subtypes – e.g. 10.6% in selected solid tumors other than HCC.45 In some regions of Europe and Asia, HCV antibodies have been reported in up to 2.8% of patients with solid tumors and 30% of those with hematological malignancies.46,47
Such data are limited, however, in that they come from single cancer centers and were derived using various study methodologies, cancer subpopulations, and diagnostic tests (antibody or nucleic acid testing). Original studies reporting associations between HCV infection and hepatic and extrahepatic malignancies are summarized in Supplementary Table 1. The strength of the evidence supporting associations between HCV infection and cancer is presented in Table 2 and in previous meta-analyses.48–59
TABLE 2.
Summary of evidence on the association between HCV and cancer
| Type of cancer | Type(s) of studiesa | Number of studies analyzed | No. of patients with HCV infection/total no. of patientsa | Quality of evidenceb |
|---|---|---|---|---|
| Primary liver cancer | ||||
| Hepatocellular carcinoma | Retrospective and prospective cohort, case-control, and case series | 10 | 19,185/128,049 | High |
| Intrahepatic cholangiocarcinoma | Retrospective and prospective cohort, case-control, and case series | 11 | 5415/426,561 | High |
| Hematologic malignancy | ||||
| NHL | Retrospective and prospective cohort, case-control, and case series | 30 | 115,999/1,379,245 | High |
| Myelodysplastic syndrome | Case Control | 1 | 17,320/217,320 | Very Low |
| Waldenström macroglobulinemia | Retrospective cohort | 1 | 165/719,687 | Very low |
| Digestive cancers | ||||
| Pancreas | Retrospective and prospective cohort and case-control | 6 | 52,045/1,065,206 | Low |
| Esophagus | Retrospective cohort | 3 | 36/155,023 | Very low |
| Rectum | Retrospective cohort | 1 | 12/12,126 | Very low |
| Anus | Case-control | 1 | 4015/204,015 | Very low |
| Head and neck | Retrospective and prospective cohort and case-control | 5 | 637/185,522 | Low |
| Thyroid | Retrospective and prospective cohort and case-control | 7 | 11,613/1,091,516 | Very low |
| Kidney | Retrospective and prospective cohort and case-control | 7 | 36,633/398,051 | Very low |
| Prostate | Retrospective cohort and case-control | 3 | 283,428/562,556 | Very low |
| Lung | Retrospective cohort | 1 | 67/12,126 | Very low |
| Nonepithelial skin | Retrospective cohort and case-control | 1 | 6650/206,650 | Very low |
All studies are detailed in Supplementary Table 1.
The quality of evidence was assessed using the GRADE approach.16 Using this approach, the quality of evidence is rated as high, moderate, low, or very low depending on the presence of 5 factors: type of evidence; quality of evidence; consistency of evidence; directness of evidence; and effect size based on the reported odds ratio, relative risk, or hazard ratio for comparison and the amplitude of the ratio. Meta-analyses were not taken into account when the quality of evidence was rated.
HCV and primary liver cancer
The strongest reported association between HCV infection and cancer is the association between HCV and primary liver cancer, including HCC and intrahepatic cholangiocarcinoma.
The association between HCV and HCC was first reported in 1989, in reports of 2 European case-control studies.60,61 Both studies demonstrated the synergistic carcinogenic effects of HCV infection and HCV-associated comorbidities, including alcohol use and HBV infection.60,61 Between 2005 and 2015, deaths from HCV-attributable HCC increased by 21.1% and deaths from alcohol-attributable HCC increased by 26.1%, during which time deaths from HCC attributable to all other causes, including HBV, remained stable.62 From 2001 to 2012, 38.4% of patients in New York City with newly diagnosed HCC had HCV infection, 17.9% had HBV infection, and 2.2% had both.63
The association between HCV and intrahepatic cholangiocarcinoma was first suggested in a case-control study from Korea published in 1996 and was confirmed in a case-control study from Italy published in 2001.64,65 Two meta-analyses54,66 and a cohort study67 showed that the odds ratio (OR) for intrahepatic cholangiocarcinoma in patients with HCV infection compared to patients without HCV infection ranged from 3.42 (95% CI, 1.96–5.99) to 4.84 (95% CI, 2.41–9.71). In contrast, no significant association has been reported between HCV infection and extrahepatic cholangiocarcinoma.
HCV and extrahepatic solid tumors
The literature on the relationship between HCV infection and extrahepatic solid tumors is inconclusive. In a large non-veteran cohort study from the United States, HCV infection was associated with carcinomas of the pancreas (standardized rate ratio [SRR], 2.5; 95% CI, 1.7–3.2), rectum (SRR, 2.1; 95% CI, 1.3–2.8), kidney (SRR, 1.7; 95% CI, 1.1–2.2), and lung (SRR, 1.6; 1.3–1.9)2; however, this study was unadjusted for major confounders, including alcohol and tobacco uses. In fact, alcohol-related and tobacco-related cancers were overrepresented in this cohort, suggesting that HCV could be a lifestyle marker. A prospective community-based cohort study from Taiwan68 showed that chronic HCV infection was associated with increased risks of esophagus, prostate, and thyroid cancer. In another case-control study,45 HCV infection was associated with non-oropharyngeal (except nasopharyngeal) and human-papillomavirus-positive oropharyngeal head and neck cancers (OR, 2.97; 95% CI, 1.31–6.76).
HCV and hematological malignancies
The second most robust association between HCV infection and cancer is the association between HCV and B-cell non-Hodgkin lymphoma (NHL). The association was first reported in 1994 in a large multicenter case-control study from Italy. 69 In a large European multicenter case-control-study,70 HCV infection was associated with increased risks of diffuse large B-cell NHL (OR, 2.24; 95% CI, 1.68–2.99), marginal zone lymphoma (OR, 2.47; 95% CI, 1.44–4.23), and lymphoplasmacytic lymphoma (OR, 2.57; 95% CI, 1.14–5.79). In a large population-based study in the United States,71 HCV infection was associated with increased risks of Burkitt lymphoma (OR, 5.21; 95% CI 1.62–16.8) and follicular lymphoma (OR, 1.88; 95% CI, 1.17–3.02). Only weak associations between HCV and other hematological malignancies have been reported (Table 2, Supplementary Table 1).
Mechanisms by which HCV contributes to development of cancer
HCV infection and primary liver cancer
Currently, evidence of a direct oncogenic effect of HCV on liver cells is scant, although HCV core protein has transforming effects and impairs oxidative stress metabolism in animal models.72,73 In a cellular model of HCV, NS3/4A (HCV viral protease) and NS5B (RNA-dependent RNA polymerase) impeded DNA repair via interaction with the ATM-driven response pathway.74 Selected genotypes of HCV also might be associated with risk of development of HCC: greater carcinogenic potential has been reported for some variants, mostly genotypes 1 [1b] and 3,75,76 although definitive studies are lacking.77
The main mechanism by which HCV contributes to development of HCC seems related to chronic inflammation, which mediates cycles of death and regeneration and eventually can lead to cirrhosis and to HCC.78,79 Most cases of HCC arise from hepatocytes or liver stem cells in cirrhotic nodules that have accumulated enough mutations to re-enter the cell cycle, reactivate telomerase, and progress through cancer checkpoints.70,80,81 This pattern accounts for the clinical and molecular heterogeneity of HCC and probably explains the limited benefit of targeted therapies. Reactive oxygen species are also thought to play a central role in the development of HCV-associated HCC. 82,83 A small proportion of HCC cases develop in patients with HCV infection without cirrhosis; in almost all such cases, the patients also had or have HBV infection.29
Genetic studies suggest that mutations accumulate in the premalignant stage of HCC and that mutation of the TERT promoter is an early genetic event.84 The other genes most commonly mutated in HCC are TP53 and CTNNB1.81,85 Recurrent mutations in epigenetic modifier genes ARID1A and ARID2 are also often present.80 These and other genetic defects impact telomere maintenance and oxidative stress. Other pathways dysregulated in HCC include the PI3K-AKT-mTOR, RAS/RAF/MAPK, and JAK/STAT pathways. 81,85
Confounders hinder determination of the precise impact of HCV infection on the development of HCC. Common risk factors for development of HCC include environmental exposures to hepatotoxins (e.g., alcohol and aflatoxins), misdirected host immune and DNA damage responses, co-infections (e.g., with HBV and HIV), advanced age, male sex, and metabolic factors (nonalcoholic fatty liver disease, obesity, metabolic syndrome, and type 2 diabetes). Diabetes77,86 and the metabolic syndrome with steatosis are important risk factors for HCC, associated with alcohol use disorders but also with the worldwide obesity epidemic.87 Furthermore, evidence is accumulating that even past heavy alcoholism in people who have since stopped drinking can contribute to liver disease progression to cirrhosis and liver cancer in HCV-infected patients, including those with a SVR.41,88
Just as evidence of a direct oncogenic effect of HCV on liver cells is scant, there is no clear evidence of a direct oncogenic effect of HCV on biliary cells, and intrahepatic cholangiocarcinoma seems to be associated more with chronic liver disease and cirrhosis, regardless of its origin, than specifically with HCV infection. Overall, the main risk factor for intrahepatic cholangiocarcinoma seems to be liver regeneration and hepatic-progenitor-cell turnover with selection of transformed cells.89
HCV infection and other extrahepatic solid tumors
For other extrahepatic cancers, it is important to keep in mind that correlation does not imply causation. As mentioned, most epidemiologic studies showing associations between HCV and extrahepatic cancers are unadjusted for potential confounders, including alcohol use disorders and tobacco smoking.
There could be a mechanistic link with HCV for oral and head and neck cancers, especially oropharyngeal cancers, as HCV is found in precancerous lesions.90 Potential synergism between HCV and human papillomavirus has also been suggested in the development of oral and head and neck cancers.45 To date, potentiation of human papillomavirus by HCV has not been reported in other cancers.
HCV infection and hematological malignancies
Evidence for a mechanistic contribution of HCV to cancer is strongest for B-cell HNL. A subset of HCV-associated lymphomas produce immunoglobulins against E2 (HCV envelope glycoprotein), suggesting that these malignancies originate from B cells activated by HCV recognition.91 The mechanism or mechanisms of HCV-related lymphomagenesis remain to be elucidated but emerging data are now reported regarding the genetic basis for lymphotropism of HCV.92 Mechanisms that have been postulated to explain the HCV-induced transformation process include cryoglobulinemia, t(14;18), chronic lymphocyte proliferation stimulated by viral antigens, HCV replication in B cells mediated by viral proteins, and virus-induced B-cell damage.93,94 Treatment of HCV is thought to reduce the risk of development of NHL and may aid in the treatment of some HCV-associated NHLs.94 The best evidence of a direct relationship between HCV and B-cell NHL is the demonstration of regression of B-cell NHL with anti-HCV therapy with interferon, which has antiproliferative properties.95 Early reports of the use of interferon-free regimens also suggest benefit.96–98
Natural history of HCV infection in patients with cancer
The natural course of HCV infection can be altered by cancer treatment. Patients with HCV infection are at risk for liver disease progression, including decompensated cirrhosis and HCC, after HCC treatment.8,99 Hepatitis flare occurs in 26% to 45% of HCV-infected cancer patients and can lead to liver dysfunction that necessitates discontinuation or dose reduction of potentially life-saving chemotherapy.5,100
Hepatitis flare is defined as an increase in alanine aminotransferase (ALT) level to at least 3 times the upper limit of normal in the absence of liver infiltration by tumor, use of hepatotoxic drugs other than chemotherapeutics,101 or other active systemic infection (bacterial or fungal infection or infection with hepatitis A virus, HBV, hepatitis E virus, cytomegalovirus, Epstein-Barr virus, herpes simplex virus, varicella-zoster virus, adenovirus, or HIV).5
Both HCV reactivation and hepatitis flare have been described in association with various chemotherapy regimens5,100. However, except for rituximab and high-dose steroids, the level of evidence is low. At the MD Anderson Cancer Center HCV clinic, HCV RNA is ordered as confirmatory test for patients with positive anti–HCV antibody testing during screening. Among patients with proven infection (detectable HCV RNA), HCV specialists monitor for hepatitis flare checking ALT level before the initiation of chemotherapy (baseline) and every 2 to 8 weeks during chemotherapy. HCV RNA level is also checked before the initiation of chemotherapy (baseline), every 12 weeks during chemotherapy, and at the time of suspected hepatitis flare looking for simultaneous viral reactivation.
The risk of liver failure or liver-related death associated with HCV reactivation or hepatitis flare is low, including for chemotherapy regimens incorporating rituximab.5,100 Therefore, because chemotherapy and hematopoietic cell transplant (HCT) are potentially life-saving, the presence of HCV infection should not be considered a contraindication to cancer treatment and should not delay the initiation of an urgent cancer treatment.
Patients with hematological malignancies
The highest risks of HCV reactivation, hepatitis flare, and increased rate of liver disease progression have been reported among patients with hematological malignancies. Among HCV-infected patients with hematological malignancies, HCT recipients have faster progression to cirrhosis than patients who do not undergo HCT.12 Among HCT recipients, patients with HCV are at higher risk for fatal sinusoidal obstruction syndrome (previously known as veno-occlusive disease),102 hepatitis flare,102,103 liver decompensation,104 and fatal fibrosing cholestatic hepatitis C (rare) than patients without HCV.105 Use of mycophenolate mofetil has been linked to development of fibrosing cholestatic hepatitis C in patients undergoing HCT.105,106
HCV reactivation is more common in patients with lymphoma and in patients treated with rituximab than in patients with other types of cancer. HCV reactivation is defined as an increase in HCV RNA level of at least 1 log10 IU/mL over baseline following administration of chemotherapy5 as chronically infected patients generally have stable HCV RNA levels that vary within 0.5 log10 IU/mL.107 HCV reactivation due to chemotherapy is less common than HBV reactivation.108 HCV reactivation is rarely fatal109; most patients have an indolent course.100 HCV reactivation mainly occurs during the weeks of chemotherapy treatment and frequently regresses at cessation of it.5,100
Patients with solid tumors
Evidence concerning the potential for HCV reactivation and hepatitis flare in HCV-infected patients receiving chemotherapy is weaker for patients with solid tumors than for patients with hematological malignancies, and is based on only limited published data.100,110
Natural history of cancer in patients with HCV infection
A recent epidemiological study in the New York City population found that viral hepatitis was the primary risk factor for HCC (>50% of patients) and that the median survival time for HCV-infected patients who developed HCC was 13.1 months from the time of HCC diagnosis. Of note, this median survival time was significantly shorter than the median survival time for HBV-infected patients who developed HCC, 22.3 months.63 Similar results were found in a nationwide study from France.111
To date, there have been no randomized clinical studies directly comparing the impact of cancer therapy in HCV-related and HBV-related HCC. However, subanalyses of the pivotal trials of systemic therapy with sorafenib and local therapy with transarterial chemoembolization have been reported that address the question. The key results, summarized in Supplementary Table 2, suggest that both treatments may be more effective in HCV-related than in HBV-related HCC. Given the heterogeneity of these studies with respect to patient populations and performance status, the small numbers of patients studied in some groups, and the fact that HBV-related HCC is more lethal than HCV-related HCC, the impact of cancer therapy in HCV-related versus HBV-related HCC merits further study. Because HCV-driven cirrhosis progression can make a patient with HCC ineligible for treatment, viral eradication before sorafenib treatment could improve oncologic outcomes such as post-progression survival and overall survival among patients with HCV-associated advanced HCC.112
HCV-infected patients are at risk for development of a second primary HCV-associated cancer after cancer treatment, including HCC and B-cell NHL, if the infection is left untreated.8,10
HCV Screening in patients with cancer
The diagnosis of HCV infection in cancer patients is a neglected topic.6 The objective of HCV screening in cancer patients is to ensure that patients with HCV infection are identified to help prevent the spread of this infection, while also increasing opportunities for preventive care and potentially reducing HCV associated morbidity and mortality in infected patients. They can receive proper care for this infection which could slow liver disease progression,12,99,113 and give patients access to a maximum of cancer drugs.11
In the United States, the Centers for Disease Control and Prevention (CDC) recommends one-time HCV testing for persons born between 1945 and 1965 (so-called baby boomers), without prior ascertainment of risk.25 Worldwide, one-time HCV testing is recommended by professional societies for persons with past behaviors, exposures, and conditions associated with an increased risk of HCV infection (e.g., injection-drug use, long-term hemodialysis, prior recipients of transfusions or organ transplants before July 1992, HIV infection, etc.).15,114,115 Periodic testing is also recommended for persons with ongoing risk factors for exposure to HCV.
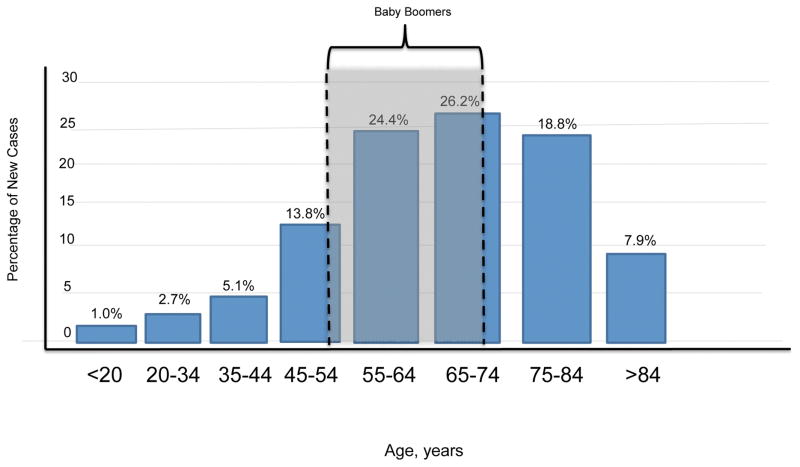
The importance of screening the 1945–1965 birth cohort is illustrated in the Annual Report to the Nation on the Status of Cancer (1975–2012), which showed that HCV-associated and liver cancer–associated death rates were highest among persons born during 1945–1965.116 NCI Surveillance, Epidemiology, and End Results 1975–2013 data show that 50.6% of all new cancers in the United States are diagnosed among people aged 55 to 74 years.117 In 2017, the baby boomers, who have a high rate of HCV infection, will be in approximately that age range (aged 52 to 72 years) (gray box, Figure 1), meaning that the number of HCV-infected cancer patients in the United States may peak around this time. Certainly, birth cohort–based HCV screening of new cancer patients would allow the identification of a substantial number of patients but still leave many patients unknowingly infected (Figure 1).
FIGURE 1. Relationship between ages of baby boomers in 2017 and ages at which cancer is most commonly diagnosed.
Bar graph shows the percentage of new cancer cases by age at diagnosis (SEER 18 2009–2013, any site, all races, both sexes). In 2017, baby boomers (individuals born in 1945–1965), the targets of birth-cohort-based HCV screening, will be 52 to 72 years old. Birth-cohort-based HCV screening of new cancer patients would identify a substantial proportion of HCV-infected patients but leave many patients with undiagnosed HCV infection.
Unfortunately, the large majority of cancer centers in the United States do not even adhere to the birth cohort–based HCV screening recommendation for the general population. Only 14% of patients receiving chemotherapy were screened for HCV at MD Anderson Cancer Center from January 2004 to April 2011.118 Predictors of HCV screening included not only birth after 1965 and traditional HCV risk factors but also Asian race and rituximab therapy,118 indicating that decisions about screening for HCV were extrapolated from HBV screening recommendations.119
Of interest, a more recent community-based study demonstrated that screening only patients in the 1945–1965 birth cohort would mean missing HCV infection in approximately one-quarter of HCV-infected patients.120 The risk of such selective screening in a cancer center was illustrated in a 2008–2011 retrospective cohort of adult patients with hematological malignancies. Of the HCV-infected patients, only three-fourths had at least 1 HCV risk factor identified by the oncologists or were born during 1945–1965, meaning that one-quarter of the seropositive cancer patients would have been missed without universal screening.121 A prospective study from France also found that selective screening using a questionnaire on HCV risk factors had low sensitivity in the identification of HCV-infected patients receiving chemotherapy for solid tumors.122 As a result of the findings from this study, authors recommended that HCV screening be conducted routinely and systematically in every cancer patient.
For over a decade, universal HCV screening has been standard practice among the hematological services at MD Anderson Cancer Center. Professional societies such as the American Society for Blood and Marrow Transplantation,7 the European Conference on Infection in Leukemia (ECIL-5), a joint initiative of the Infectious Diseases Working Party of the European Group for Blood and Marrow Transplantation (EBMT), European Organization for Research and Treatment of Cancer (EORTC), Immunocompromised Host Society (ICHS), and European Leukemia Net (ELN)9 have generated guidance documents for diagnosis and treatment of HCV infection in patients with hematological malignancies and HCT recipients; universal screening is recommended in this population. In terms of those with solid tumors, all patients with HCC should undergo screening for HCV but the optimal screening strategy for patients with other solid tumors is less clear. One-time HCV screening in cancer patients belonging to the 1945 to 1965 birth cohort and other patients based on exposures, behaviors, and conditions that increase risk for HCV infection will be in alignment with recommendations of the CDC and the US Preventive Services Task Force (USPSTF) in non-cancer patients.25,123 A possible approach also to identify such infected cancer patients is combining risk-based and birth cohort–based strategies with education for oncologists.44 Since 2016, the standard practice at MD Anderson of universal HCV screening in the hematologic patient populations has been expanded to all other cancer patients. The rationale to that approach is that identification and elimination of HCV from infected patients have the potential for virologic, hepatic, and oncologic benefits not only in those with hematologic malignancies,106 but also with solid tumors. For instance, viral eradication may normalize transaminases, allowing access to chemotherapeutic agents that would otherwise be contraindicated,11,106,124 eliminate the risk of developing HCV reactivation that can lead to discontinuation of chemotherapy,5 prevent liver disease progression,6 or the development of HCC or NHL as a secondary cancer,8,10 and allow access to multiple clinical trials of cancer chemotherapies11,106 (see more details in section Potential benefits of antiviral therapy). Exceptions to screening could be patients with contraindications for DAAs (e.g., uncontrolled cancer with limited life expectancy or decompensated cirrhosis), patient decline, or other medical reasons. HCV infection in patients with cancer warrants better awareness and well-designed research to identify the optimal screening strategy.
The U.S. Food and Drug Administration has approved 2 methods of diagnosing HCV: serologic assays that detect antibodies are used for screening, and molecular assays that detect HCV RNA (e.g., nucleic acid testing) are used to confirm HCV infection. The specificity of current serologic assays is greater than 99%. Guidelines from the U.S. Public Health Service, Infectious Diseases Society of America, Veterans Affairs Hepatitis C Resource Center Program, and National Hepatitis C Program Office recommend HCV antibody screening in immunocompromised patients, such as those with HIV infection.125 However, little is known about the optimal approach to diagnosis of HCV infection in cancer patients, particularly those who are immunocompromised, such as patients with hematological malignancies and HCT recipients. Previous studies have established that the prevalence of seronegative HCV infection in HIV-infected patients is as high as 6.9%, but no similar data exist for cancer patients. Furthermore, serologic assays could be unreliable for diagnosing HCV infection in HCT recipients. One study demonstrated that 13% of HCV-infected HCT recipients had false-negative antibody to HCV test results after transplant.13 Therefore, molecular diagnostic methods (e.g., nucleic acid testing) could be more reliable than serologic assays in HCT recipients. Professional societies recommend that all HCT candidates should be screened for HCV.7,9
Evaluation and treatment of HCV-infected cancer patients
HCV infection is a supplementary burden in patients with a new diagnosis cancer. HCV-infected cancer patients are at risk for serious adverse effects of cancer treatment, including sinusoidal obstruction syndrome and chemotherapy-associated steatohepatitis resulting from oxaliplatin or irinotecan.126,127 Ischemic hepatitis, cholangiopathy, impaired liver regeneration, sinusoidal obstruction syndrome, and death during HCT and liver surgery have also been reported in HCV-infected cancer patients.128,129 Moreover, patients with chronic viral hepatitis are often excluded from cancer trials: approximately 48% of early-phase cancer clinical trials at MD Anderson Cancer Center exclude HCV-infected patients.
New entry into the healthcare system with close follow-up and subsequent lifestyle modifications can improve outcomes of patients with HCV infection.40 Treatment and eradication of HCV infection with subsequent normalization of liver function tests might also open access to cancer clinical trials to patients originally excluded because of HCV infection.11
Evaluation of the HCV-infected patient
As mentioned above, patients with HCV infection are at risk for liver fibrosis and cirrhosis, which are both associated with serious adverse events during cancer treatment, including HCT. Cirrhosis can also be associated with abnormal clearance and metabolism of anticancer agents.130 Therefore, evaluation of liver fibrosis in HCV-infected cancer patients is mandatory, regardless of whether or not there is a SVR.
Liver stiffness as measured by transient elastography is a surrogate marker for cirrhosis in HCV-RNA-positive patients. A liver stiffness value below 7.1 kPa rules out significant liver fibrosis, whereas a liver stiffness value above 12.5 kPa is very predictive of cirrhosis.131 Between these thresholds, liver stiffness value is inconclusive and liver biopsy should remain a standard. Transient elastography also performs poorly, as do all other noninvasive tests, in HCV-infected patients with a SVR to treatment, and decisions regarding care should not be based on such measurements.132 Therefore, liver biopsy should remain the standard for diagnosis of cirrhosis in HCV-RNA-negative patients.133
The simplest method for classification of patients with HCV infection and cirrhosis is to classify patients according to whether they have compensated or decompensated cirrhosis. Decompensated cirrhosis is defined by the presence of ascites, variceal bleeding, encephalopathy, and/or jaundice.134 Median survival is significantly longer in patients with compensated cirrhosis than in those with decompensated cirrhosis (>12 years vs. 2 years).135 As the outcome of HCV-infected cancer patients with decompensated cirrhosis is related more to liver dysfunction than to cancer, these patients are generally not candidates for cytotoxic treatments.
A more precise method for classification of patients with HCV infection and cirrhosis is based on the Child-Pugh score. The score is based on bilirubin and albumin levels, prothrombin time, and the presence or absence of ascites and encephalopathy. Patients with Child-Pugh C disease are usually not eligible for cancer treatment. For patients with Child-Pugh B disease, it is essential to carefully evaluate both the effective degree of hepatic dysfunction and tumor and patient characteristics (e.g., chemosensitivity, site of disease, kind and degree of symptoms).
Despite the fundamental role of the liver in drug metabolism, the impact of late-stage cirrhosis on metabolism and clearance of cancer drugs is not understood. The pharmacokinetics of hepatically cleared chemotherapy may be affected by multiple factors, including altered clearance due to decreased hepatocyte uptake or impaired liver blood flow, altered biliary excretion, altered metabolic capacity, decreased albumin level leading to increased free drug, or altered oral drug absorption from portal hypertension.136 No single method allows estimation of the pharmacokinetics and pharmacodynamics of a drug in an individual patient with cirrhosis. However, the evidence supporting systematic drug dose reduction in patients with compensated cirrhosis is very weak, and most patients with normal liver function can receive cancer drugs without liver damage. Care for such patients should be managed by physicians and doctors of pharmacy with expertise in treating patients with compensated cirrhosis to avoid systematic dose reduction that could be associated with poor cancer outcome.
Patients with cirrhosis and portal hypertension are at risk for postoperative hepatic decompensation after liver resection or bleeding due to treatment with an antiangiogenic agent.137,138 Measurement of hepatic venous pressure gradient or gastrointestinal endoscopy may be used to rule out esophagogastric varices and clinically significant portal hypertension. A low platelet count (<100,000 per microliter) or the presence of large varices on preoperative imaging rules out patients with cirrhosis as candidates for major liver resection. Patients with a liver stiffness value of less than 20 kPa with a platelet count of greater than 150,000 per microliter have a very low risk of clinically significant portal hypertension, but whether this holds true among HCV-infected patients with a SVR to treatment has not been validated. 139 The future liver remnant volume after liver resection should always be assessed before surgery by experts in liver volume manipulations.129
Patient selection for treatment
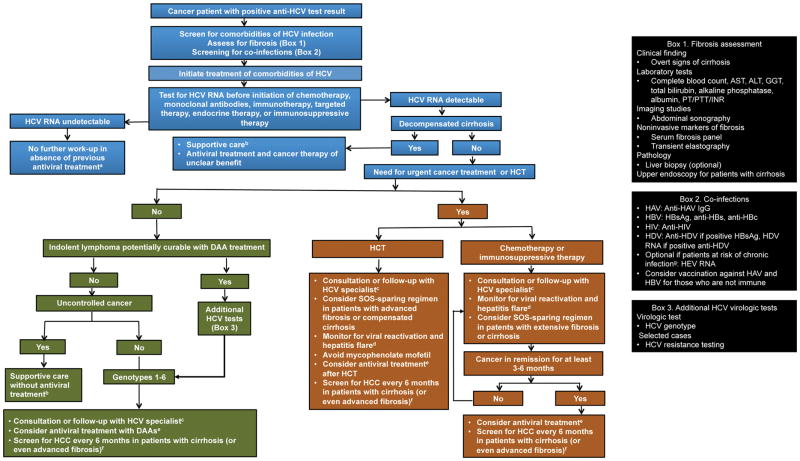
Data on treatment of HCV infection in cancer patients are limited, largely because studies of anti-HCV therapy typically exclude patients with cancer. There is currently no standard of care antiviral therapy; however, data are becoming available, and SVR rates seem similar to those observed in HCV-infected patients without cancer.11 Unlike interferon-based regimens, DAA therapy can be used in cancer patients with bone marrow compromise or cytopenias without dose adjustments or discontinuation. 11,13,103 Our algorithm for treatment of HCV-infected cancer patients is depicted in Figure 2.
FIGURE 2.
Management algorithm for patients with cancer and HCV infection
Antiviral therapy should be offered to HCV-infected cancer patients except those with contraindications to antiviral therapy, including uncontrolled cancer, comorbidity associated with a life expectancy of less than 12 months, pregnancy or pregnant partner (if ribavirin is considered), anticipated major drug-drug interaction with a chemotherapy or immunosuppressive agent that cannot be temporarily discontinued, and/or known hypersensitivity or intolerance to DAAs.15,106
Antiviral therapy could also be contraindicated in HCV-infected cancer patients with Child-Pugh class B or C cirrhosis because incurable extrahepatic malignancy is considered a contraindication to listing for liver transplant,140 the only available treatment for decompensated cirrhosis.15,141 In addition, decompensated cirrhosis is a contraindication to DAA therapy in oncologic patients because of an increased risk of lactic acidosis that could be even higher with cancer treatment.142,143
Selection and delivery of DAA therapy
In general, HCV-infected patients with cancer can be treated in the same way as HCV-infected patients without cancer. However, the DAA regimen should be individualized on the basis of the patient’s prior antiviral therapy, HCV genotype, and degree of liver disease; the potential hematologic toxic effects of the DAA regimen; and the potential for drug-drug interactions (details on interactions are provided later in this article). If a HCV-infected cancer patient is deemed to be a candidate for treatment with DAAs, he or she should receive antiviral therapy according to the guidelines for patients without cancer. 15,115
To avoid drug-drug interactions and overlapping toxic effects, we recommend starting anti-HCV therapy when the patient is not receiving chemotherapy. However, DAAs can be used concomitantly with selected antineoplastic agents if patients are closely monitored. In selected patients, the concomitant approach could be appropriate to achieve viral clearance and normalization of ALT levels just before initiation of chemotherapy. Simultaneous antiviral and oncologic therapy may also prevent delays in the administration of chemotherapy in HCV-infected cancer patients.144 In some major cancer centers, HCV-treating physicians (infectious disease specialists and hepatologists), oncologists, and pharmacists work together in the diagnostic work-up, monitoring, and treatment of HCV-infected patients.14
In HCV-infected patients without cancer, SVR at 12 weeks has a positive predictive value for SVR at 24 weeks of greater than 97%. However, confirmation of SVR by repeat HCV RNA testing at 24 weeks is still appropriate as late relapses are reported following treatment with DAAs.145 For some chronic and incurable viral infections, such as HBV infection, long-term antiviral therapy is used to prevent viral reactivation. This approach is not applicable to HCV infection as DAAs eradicate HCV in most infected patients within 2 to 3 months without viral relapse after cancer therapy.146
About 40% of patients with HCV infection (more in areas with high HBV endemicity) harbor HBV markers and are at risk for HBV reactivation during cancer treatment.9,147 Reactivation of HBV infection in HCV-coinfected patients receiving DAAs has also been reported, and there are a few reported cases of fulminant hepatic failure requiring liver transplantation.148,149 Most of these reports were from patients with detectable HBV surface antigen (HBsAg), none of them on anti-HBV therapy.149 Of interest, a few cases of reactivation of HBV infection in HCV-coinfected patients occurred in patients with isolated hepatitis B core antibody,148,150 even in the absence of immunomodulatory medications.151 However, on the basis of currently available data, the impact of HBV reactivation in HCV-coinfected patients receiving DAAs appears to be minor.150,151 Cancer patients with HBV and HCV co-infection are at risk for HBV reactivation, but it is expected that patients with detectable HBsAg will receive anti-HBV therapy during chemotherapy, which lowers the HBV reactivation risk.152 We recommend periodic monitoring of ALT, HBsAg, and HBV DNA according to the guidelines for HBV-monoinfected cancer patients153 for early detection of hepatitis flare and HBV reactivation in cancer patients with HBV and HCV co-infection receiving DAAs. Recommendations are less clear for cancer patients with isolated hepatitis B core antibody, but close observation is still advised as most of those patients are not routinely given anti-HBV therapy.152,153
Potential benefits of antiviral therapy
The benefit of holistic treatment of HCV-infected patients, including close follow-up with subsequent lifestyle modifications and interferon-based treatment, is demonstrated.40 Eradication of HCV with interferon-based treatment was associated with a 75% reduction in the risk of liver cancer.116,154,155
DAA therapy with close follow-up has been reported to improve liver function in patients with decompensated cirrhosis,113,156 although there have also been cases of DAA-associated lactic acidosis and sudden hepatic decompensation.142,143,157 Decompensated cirrhosis is the main cause of death among HCV-infected patients in complete remission of cancer.99 The benefit of DAA therapy in infected cancer patients with advanced liver disease has not been demonstrated yet.
HCV treatment may halt, delay, or prevent progression to cirrhosis and end-stage liver disease in patients with cancer and in HCT recipients. In the DAA era, studies of fibrosis regression versus progression after SVR are limited, but similar outcomes are anticipated in HCV-infected cancer patients, as long as HCV-associated comorbidities are controlled.
Viral eradication with anti-HCV therapy may improve liver function and normalize the ALT level, allowing patients access to multiple cancer chemotherapies, including agents with hepatic metabolism, and prevent HCV reactivation.5,100,124,158 HCV eradication might also open access to cancer clinical trials to patients originally excluded because of HCV-associated chronic liver disease.11
HCV-infected patients with selected subtypes B-cell NHL should be treated with DAAs first and then with chemotherapy. Antiviral treatment of HCV infection in patients with certain cancers, such as indolent B-cell NHL, has induced tumor regression and improved hematological outcomes with the use of interferon-containing94 and interferon-free regimens.97,98 Our group and others have demonstrated that antiviral therapy including DAA-based therapy can improve oncologic outcomes, including survival, of patients who have developed HCV-associated NHL,13,97,98,159 including patients with HCV-associated B-cell NHL undergoing HCT.6,13 In fact, the National Comprehensive Cancer Network guidelines on splenic marginal zone lymphoma recommend treatment of HCV without chemotherapy as first-line therapy for HCV-infected patients.160
Furthermore, anti-HCV therapy may reduce the risk of HCC or NHL as second primary cancers8,10 and improve the oncologic outcomes of patients with selected HCV-related cancers, such as NHL.13
Nonrandomized studies of the effect of HCV eradication with DAAs on HCC progression or recurrence have yielded disparate results.161 One early report summarizing the outcomes of 58 Spanish patients with radiologically negative HCC who had completed DAA therapy revealed a surprisingly high rate of tumor recurrence (28%).35 However, the retrospective nature of the study, the lack of consistency in obtaining imaging at baseline and follow-up, and the lack of consistency in the timing of DAA therapy, as detailed by our group,162 preclude drawing firm conclusions from this study. In another large study from Spain, rates of recurrence of HCC were high and similar to those in previous reports,163 but the authors acknowledged that their results must be interpreted with caution given the lack of a routine surveillance monitoring protocol. Larger retrospective analyses from a French population and a meta-analysis did not reveal an increased risk of HCC recurrence or negative impact on overall survival and recurrence-free survival.42,164
Whether the risk of HCC recurrence is increased with DAA therapy is controversial.35,162 HCC recurrence is closely related to other risk factors, including age at infection, sex, and HCV-associated comorbidities, including HIV and HBV co-infections, alcohol abuse disorders, and diabetes.30 Loss of cancer immune monitoring and progression of HCC favored by interferon-free treatments could be another mechanism underlying HCC recurrence.165–168 Regardless of whether antiviral therapy is ultimately shown to increase the risk of cancer recurrence among HCV-infected cancer patients, the main message is that surveillance for cancer occurrence or recurrence is important among patients with a SVR at risk for liver disease progression. The field awaits prospective trials to determine the merits of DAA therapy in HCV-infected cancer patients as well as the characteristics of patients who will likely to experience cancer progression after DAA therapy, including those who have undergone resection or locoregional therapy for HCC.164
Potential interactions between DAAs and chemotherapy, targeted therapy, and supportive care medications
DAAs are highly effective and well tolerated in HCV-infected cancer patients. However, the potential for interactions between DAAs and other drugs used in the treatment of cancer poses a challenge.
To our knowledge, there is only 1 published prospective study of interactions between DAAs and chemotherapy.144 This study found no significant interactions when DAAs and various chemotherapy agents were administered concomitantly to treat various malignancies. No toxic effects were observed that required discontinuation of either agent. Additionally, there was a trend toward normalization of ALT levels during and after combination therapy. Until further research establishes a better understanding of clinically significant interactions between DAAs and chemotherapy, clinicians should utilize pharmacodynamic and pharmacokinetic data to guide treatment decisions.
Pharmacodynamic drug interactions result from physiological activities of 2 interacting drugs, whereas pharmacokinetic drug interactions may lead to altered concentrations of drugs or metabolites due to changes in absorption, distribution, metabolism, or elimination. The most common sites for drug-drug interactions are hepatocytes and intestinal enterocytes via the cytochrome P450 enzyme system, as well as the P-glycoprotein (P-gp) membrane transporter. Furthermore, additional drug transporters, such as breast cancer resistance protein and organic anion transporting polypeptides,169 have known metabolic pathways that may contribute to interactions with DAAs.
DAAs used to treat HCV infection and their metabolism, transporter effects, and potential for interactions with chemotherapy, targeted agents, and supportive care medications commonly used to treat cancer are outlined in Tables 3 to 6.170–187 For more detailed information, please refer to www.hep-druginteractions.org, Lexicomp Online (Hudson, Ohio: Lexicomp, Inc.; November 2016) and medication package labeling.
TABLE 3.
Metabolism and transporter effects of direct-acting antivirals
| Direct-acting antiviral | Metabolism | Transporter effects |
|---|---|---|
| Sofosbuvir | Hepatic; forms active nucleoside analog triphosphate GS461203 | P-gp and BCRP substrate |
| Sofosbuvir-ledipasvira | Slow oxidative metabolism via unknown mechanism | P-gp and BCRP substrate Inhibits P-gp, BCRP, OATP1B1, OATP1B3, and bile salt export pump |
| Sofosbuvir-velpatasvira | Hepatic via CYP2B6, CYP2C8, and CYP3A4 | P-gp and BCRP substrate Inhibits P-gp, BCRP, OATP1B1, OATP1B3, and OATP2B1 |
| Ritonavir-boosted paritaprevir-omibitasvir-dasabuvirb |
Ritonavir-boosted
paritaprevir Hepatic via CYP3A4 (primary) and CYP3A5 Ombitasvir Hydrolysis Dasabuvir Hepatic- via CY2C8 (primary) and CYP2C8 |
Ritonavir-boosted
paritaprevir P-gp, BCRP, OATP1B1, and OATP1B3 substrate Inhibits P-gp, BCRP, UGT1A1, OATP1B1, and OATP1B3 Ombitasvir P-gp and BCRP substrate Inhibits P-gp, BCRP, and UGT1A1 Dasabuvir P-gp and BCRP substrate Inhibits P-gp, BCRP, and UGT1A1 |
| Grazoprevir-elbasvir | Hepatic via CYP3A4 (partial) |
Grazoprevir OATP1B1 and OATP1B3 substrate; transported by P-gp Inhibits BCRP (intestinal level), and CYP3A(weak) Elbasvir Inhibits BCRP (intestinal level) |
| Daclatasvir | Hepatic via CYP3A | CYP3A4 and P-gp substrate Inhibits BCRP, P-gp, OATP1B1, and OATP1B3 (moderate) |
| Simeprevir | Hepatic via CYP3A (primary), CYP2C8, and CYP2C19 | CYP3A4, P-gp, BCRP, OATP1B1, OATP1B3, and
OATP2B1 substrate; transported by OATP1B1 and OATP1B3 Inhibits OATP1B1, OATP1B3, P-gp, BCRP, BSEP, CYP1A2 (mild), and CYP3A4 (mild) |
| Ribavirinc | Hepatic and intracellular | Inhibits CYP1A2 and IMDH |
BCRP, breast cancer resistance protein; CYP, cytochrome P450 enzymatic system; IMDH, inosine monophosphate dehydrogenase; OATP, organic anion transporting polypeptide; P-gp, p-glycoprotein.
Metabolism and transporter effects do not include sofosbuvir; refer to sofosbuvir for information.
Ritonavir has no activity against HCV but is given as a low-dose CYP3A inhibitor to boost paritaprevir concentration.
Ribavirin is often used in combination with DAAs; therefore, it is included to provide a comprehensive overview.
TABLE 6.
Major interactions between HCV direct-acting antivirals and common supportive care medications in oncologya
| Medication | Sofosbuvir | Sofosbuvir-ledipasvir | Sofosbuvir-velpatasvir | Ritonavir-boosted paritaprevir-ombitasvir-dasabuvir | Grazoprevir-elbasvir | Daclatasvir | Simeprevir | Ribavirinb |
|---|---|---|---|---|---|---|---|---|
| Acid-reducing agents | ||||||||
| H2 antagonist | ◆ | ■ | ■ | ◆ | ◆ c | ◆ | ◆ | ◆ |
| Proton pump inhibitor | ◆ | ■ | ● | ■ c | ◆ c | ◆ | ◆ c | ❖ |
| Anticoagulant | ||||||||
| Apixaban | ◆ | ■ | ■ | ● | ■ | ■ | ■ | ◆ |
| Dabigatran | ◆ | ■ | ■ | ■ | ■ | ■ | ■ | ◆ |
| Edoxaban | ◆ | ■ | ◆ | ■ | ■ | ■ | ■ | ◆ |
| Rivaroxaban | ◆ | ■ | ■ | ● | ■ | ■ | ■ | ◆ |
| Warfarin | ■ c | ■ c | ■ c | ■ c | ◆ | ◆ | ■ c | ■c |
| Antiemetic | ||||||||
| Aprepitant | ◆ | ◆ | ◆ | ■ | ◆ | ■ | ■ | ◆ |
| Antifungals | ||||||||
| Fluconazole | ◆ | ◆ | ◆ | ◆ | ◆ | ◆ | ● | ❖ |
| Isavuconazole | ▲ | ▲ | ▲ | ▼ | ▲ | ▲ | ▼ | ▲ |
| Posaconazole | ◆ | ◆ | ◆ | ● | ◆ | ■ | ● | ❖ |
| Voriconazole | ◆ | ◆ | ◆ | ● | ◆ | ■ | ● | ❖ |
| Bisphosphonate | ||||||||
| Pamidronate | ◆ | ◆ | ◆ | ◆ | ◆ | ◆ | ◆ | ◆ |
| Immunosuppressants | ||||||||
| Cyclosporine | ◆ c | ◆ | ◆ | ■ c | ● c | ◆ c | ● c | ❖ |
| Mycophenolate | ◆ | ◆ | ◆ | ■ | ◆ c | ◆ | ◆ | ◆ |
| Sirolimus | ◆ | ◆ | ◆ | ■ | ■ | ◆ | ■ | ❖ |
| Tacrolimus | ◆ c | ◆ | ◆ | ■ c | ■ c | ◆ c | ■ c | ❖ |
| Growth factor | ||||||||
| Eltrombopag | ◆ | ◆ | ◆ | ◆ | ● | ■ | ■ | ❖ |
| Filgrastim | ◆ | ◆ | ◆ | ◆ | ◆ | ◆ | ◆ | ❖ |
| Steroids | ||||||||
| Dexamethasone | ◆ | ■ | ◆ | ■ | ■ | ● | ● | ❖ |
| Methylprednisolone | ◆ | ◆ | ◆ | ■ | ◆ | ◆ | ■ | ❖ |
| Prednisone | ◆ | ◆ | ◆ | ■ | ◆ c | ◆ | ◆ | ❖ |
H2, histamine 2 receptor.
Table does not include all supportive care medications in oncology. Drug interactions are based on metabolism and clearance data rather than direct study of co-administration except where otherwise indicated. Symbols used to rank clinical significance of drug interactions are based on HEP Drug Interactions (www.hep-druginteractions.org; University of Liverpool), Lexicomp Online (Hudson, Ohio: Lexi-Comp, Inc.; November 2016), and medication package inserts. For additional drug-drug interactions and more extensive range of drugs, detailed pharmacokinetic interaction data, and dosage adjustments, refer to the above-mentioned websites, medication package inserts, and specific references.176–178,187
Ribavirin is often used in combination with DAAs; therefore, it is included to provide a comprehensive drug-drug interaction overview.
Co-administration studied directly.
Legend
| ❖ | No clear data |
| ◆ | No clinically significant interaction expected |
| ■ | Potential interaction that may require a dosage adjustment, altered timing of administration, or additional monitoring |
| ● | These drugs should not be co-administered |
| ▲ | Not included in HEP Drug Interactions; however, metabolism and clearance data suggest that an interaction is unlikely |
| ▼ | Not included in HEP Drug Interactions; however, metabolism and clearance data suggest that an interaction may occur |
Conclusions
The oncologic burden of HCV infection is twofold. First, HCV infection is associated with the development of several cancers, an effect often intertwined with the consequences of HCV-associated comorbidities. Second, chronic HCV infection causes significant morbidity and mortality in cancer patients. Anti-HCV therapy is associated with viral clearance, improved liver function, and in many cases favorable oncologic outcomes of patients who have already developed HCV-associated malignancies (e.g., B-cell NHL). Chronic HCV infection can affect the cancer treatment plan (e.g., necessitating chemotherapy dose reduction), but this infection is manageable and in most cases virologically cured. HCV-infected patients should not be systematically deprived of effective treatment of their cancer, including HCT if indicated, as only a very small proportion of HCV-infected cancer patients have a contraindication to cancer treatment or HCT. The optimal timing of DAA therapy for HCV-infected cancer patients and HCT recipients remains to be determined. At the same time that HCV infection is treated with antiviral therapy, HCV-associated comorbidities should be addressed. Patients with cirrhosis (or even advanced fibrosis)188 and those with HCV-associated comorbidities, including alcohol use disorder, should be maintained in the healthcare system even if viral eradication is achieved. HCV-infected cancer patients will require specialized care delivered by a multidisciplinary team of HCV-treating physicians, oncologists, and pharmacists who work together towards optimizing patient care and outcomes. However, management of HCV infection in cancer patients should not be limited to tertiary cancer centers. Unmet needs and knowledge gaps with respect to HCV infection in patients with cancer are shown in Table 7.
TABLE 7.
Unmet clinical and research needs and knowledge gaps on HCV infection in patients with cancer
| Optimal screening strategy in cancer patients and HCT recipients |
| Value of routine HCV screening in cancer centers |
| Need for systematic cancer screening of HCV-infected cancer patients to identify HCV-related second primary cancers |
| Optimal strategy for monitoring HCV infection during cancer treatment |
| Impact of DAA-based therapy in cancer prevention |
| Most favorable strategy for early detection and treatment of coinfections caused by carcinogenic viruses (e.g., hepatitis B virus, HIV, and human papillomavirus) in HCV-infected cancer patients |
| Studies on HBV reactivation in cancer patients with HBV and HCV co-infection receiving DAAs |
| Optimal timing of antiviral therapy in relation to chemotherapy and HCT |
| Efficacy and safety of various DAA regimens in cancer patients and HCT recipients |
| Predictors of liver disease progression, even if a SVR is achieved |
| Reliability of serologic markers of fibrosis and FibroScan VCTE in predicting the presence of advanced fibrosis and cirrhosis in cancer patients and HCT candidates |
| Inclusion of HCV-infected cancer patients in clinical trials of both cancer treatment and DAAs |
| Virologic, hepatic and oncologic impact of treating HCV in people with various cancers |
| Large well-designed prospective studies focused on cancer patients from distinct geographic areas analyzing the impact of geographic variations of HCV genotypes |
| Characterization of interactions between DAAs and chemotherapeutic agents |
DAA, direct-acting antiviral; HBV, hepatitis B virus ; HCT, hematopoietic cell transplant; HCV, hepatitis C virus ; SVR, sustained virologic response; VCTE, vibration-controlled transient elastography
Supplementary Material
TABLE 4.
Major interactions between HCV direct-acting antivirals and standard chemotherapy drugsa
| Chemotherapy drug | Sofosbuvir | Sofosbuvir-ledipasvir | Sofosbuvir-velpatasvir | Ritonavir-boosted paritaprevir-ombitasvir-dasabuvir | Grazoprevir-elbasvir | Daclatasvir | Simeprevir | Ribavirinb |
|---|---|---|---|---|---|---|---|---|
| Alkylating agents | ||||||||
| Chlorambucil | ◆ | ◆ | ◆ | ◆ | ◆ | ◆ | ◆ | ◆ |
| Cyclophosphamide | ▲ | ▲ | ▲ | ▼ | ▲ | ▲ | ▲ | ▲ |
| Antifolate | ||||||||
| Methotrexate | ◆ | ◆ | ■ | ◆ | ■ | ■ | ◆ | ■ |
| Antitumor antibiotics | ||||||||
| Doxorubicin | ◆ | ■ | ■ | ■ | ■ | ■ | ■ | ◆ |
| Idarubicin | ◆ | ◆ | ◆ | ◆ | ◆ | ◆ | ◆ | ◆ c |
| Cytidine analog | ||||||||
| Gemcitabine | ◆ | ◆ | ◆ | ◆ | ◆ | ◆ | ◆ | ■ |
| 5-Fluoropyrimidine | ||||||||
| Capecitabine | ◆ | ◆ | ◆ | ◆ | ◆ | ◆ | ◆ | ■ |
| Microtubule agents | ||||||||
| Docetaxel | ▲ | ▼ | ▼ | ▼ | ▼ | ▼ | ▼ | ▲ |
| Paclitaxel | ▲ | ▼ | ▼ | ▼ | ▼ | ▼ | ▼ | ▲ |
| Platinum analogs | ||||||||
| Carboplatin | ◆ | ◆ | ◆ | ◆ | ◆ | ◆ | ◆ | ◆ |
| Cisplatin | ◆ | ◆ | ◆ | ■ | ◆ | ◆ | ◆ | ◆ |
| Oxaliplatin | ◆ | ◆ | ◆ | ■ | ◆ | ◆ | ◆ | ◆ |
| Purine antimetabolites | ||||||||
| Fludarabine | ◆ | ◆ | ◆ | ◆ | ◆ | ◆ | ◆ | ◆ |
| Mercaptopurine | ◆ | ◆ | ◆ | ◆ | ◆ | ◆ | ◆ | ● c |
| Topoisomerase I/II inhibitors | ||||||||
| Etoposide | ◆ | ◆ | ◆ | ■ | ◆ | ◆ | ■ | ◆ |
| Irinotecan | ◆ | ■ | ■ | ■ | ■ | ■ | ■ | ◆ |
| Topotecan | ▲ | ▲ | ▼c | ▲ | ▼ | ▼ | ▲ | ▲ |
| Vinca alkaloids | ||||||||
| Vinblastine | ◆ | ◆ | ◆ | ■ | ◆ | ◆ | ■ | ◆ |
| Vincristine | ◆ | ◆ | ◆ | ■ | ◆ | ◆ | ■ | ◆ |
| Vinorelbine | ◆ | ■ | ■ | ■ | ■ | ■ | ■ | ◆ |
Table does not include all chemotherapeutic agents. Drug interactions are based on metabolism and clearance data rather than direct study of co-administration except where otherwise indicated. Symbols used to indicate clinical significance of drug interactions are based on HEP Drug Interactions (www.hep-druginteractions.org; University of Liverpool), Lexicomp Online (Hudson, Ohio: Lexi-Comp, Inc.; November 2016), and medication package inserts. For additional drug-drug interactions and more extensive range of drugs, detailed pharmacokinetic interaction data, and dosage adjustments, refer to the above-mentioned websites, medication package inserts, and specific references.172,173,179
Ribavirin is often used in combination with DAAs; therefore, it is included to provide a comprehensive drug-drug interaction overview.
Co-administration studied directly.
Legend
| ◆ | No clinically significant interaction expected |
| ■ | Potential interaction that may require a dosage adjustment, altered timing of administration, or additional monitoring |
| ● | These drugs should not be co-administered |
| ▲ | Not included in HEP Drug Interactions; however, metabolism and clearance data suggest that an interaction is unlikely |
| ▼ | Not included in HEP Drug Interactions; however, metabolism and clearance data suggest that an interaction may occur |
TABLE 5.
Major interactions between HCV direct-acting antivirals and monoclonal antibodies, targeted chemotherapy, and endocrine therapya
| Drug | Sofosbuvir | Sofosbuvir-ledipasvir | Sofosbuvir-velpatasvir | Ritonavir-boosted paritaprevir-ombitasvir-dasabuvir | Grazoprevir-elbasvir | Daclatasvir | Simeprevir | Ribavirinb |
|---|---|---|---|---|---|---|---|---|
| Endocrine agents | ||||||||
| Anastrozole | ◆ | ◆ | ◆ | ◆ | ◆ | ◆ | ◆ | ◆ |
| Exemestane | ◆ | ◆ | ◆ | ◆ | ◆ | ◆ | ◆ | ◆ |
| Letrozole | ◆ | ◆ | ◆ | ■ | ◆ | ◆ | ■ | ◆ |
| Tamoxifen | ◆ | ◆ | ◆ | ■ | ◆ | ◆ | ■ | ◆ |
| HDAC inhibitors | ||||||||
| Vorinostat | ▲ | ▲ | ▲ | ▲ | ▲ | ▲ | ▲ | ▲ |
| Romidepsin | ▲ | ▼ | ▼ | ▼ | ▼ | ▼ | ▼ | ▲ |
| Immunotherapy | ||||||||
| Ipilimumab | ◆ | ◆ | ◆ | ◆ | ◆ | ◆ | ◆ | ◆ |
| Nivolumab | ▲ | ▲ | ▲ | ▲ | ▲ | ▲ | ▲ | ▲ |
| Tocilizumab | ▲ | ▲ | ▲ | ▲ | ▲ | ▼ | ▼ | ▲ |
| Immunomodulators | ||||||||
| Lenalidomide | ▲ | ▼ | ▼ | ▼ | ▼ | ▼ | ▼ | ▲ |
| Thalidomide | ◆ | ◆ | ◆ | ◆ | ◆ | ◆ | ◆ | ❖ c |
| Monoclonal antibodies | ||||||||
| Rituximab | ◆ | ◆ | ◆ | ◆ | ◆ | ◆ | ◆ | ◆ |
| Brentuximab | ▲ | ▲ | ▲ | ▲ | ▲ | ▲ | ▲ | ▲ |
| mTOR kinase inhibitor | ||||||||
| Everolimus | ◆ | ■ | ■ | ■ | ■ | ■ | ■ | ◆ |
| Proteasome inhibitor | ||||||||
| Bortezomib | ◆ | ◆ | ◆ | ■ | ◆ | ◆ | ■ | ◆ |
| TKIs | ||||||||
| Dasatinib | ◆ | ◆ | ◆ | ■ | ◆ | ■ | ■ | ◆ |
| Erlotinib | ◆ | ■ | ■ | ● | ■ | ■ | ■ | ◆ |
| Gefitinib | ◆ | ◆ | ◆ | ■ | ◆ | ◆ | ■ | ◆ |
| Imatinib | ◆ | ◆ | ■ | ■ | ■ | ◆ | ■ | ❖ c |
| Lapatinib | ◆ | ■ | ■ | ■ | ■ | ■ | ■ | ◆ |
| Nilotinib | ◆ | ◆ | ■ | ■ | ■ | ■ | ■ | ◆ |
| Ponatinib | ▲ | ▲ | ▼ | ▼ | ▼ | ▲ | ▼ | ▲ |
| Sorafenib | ◆ | ◆ | ◆ | ■ | ◆ | ◆ | ■ | ❖ c |
| Sunitinib | ◆ | ◆ | ◆ | ■ | ◆ | ◆ | ■ | ◆ |
HDAC, histone deacetylase; mTOR, mammalian target of rapamycin; TKI, tyrosine kinase inhibitor.
Drug interactions are based on metabolism and clearance data rather than direct study of co-administration except where otherwise indicated. Symbols used to indicate clinical significance of drug interactions are based on HEP Drug Interactions (www.hep-druginteractions.org; University of Liverpool), Lexicomp Online (Hudson, Ohio: Lexi-Comp, Inc.; November 2016), and medication package inserts. For additional drug-drug interactions and more extensive range of drugs, detailed pharmacokinetic interaction data, and dosage adjustments, refer to the above-mentioned websites, medication package inserts, and specific references170,171,173–175.
Ribavirin is often used in combination with DAAs; therefore, it is included to provide a comprehensive drug-drug interaction overview.
Co-administration studied directly.
Legend
| ❖ | No clear data |
| ◆ | No clinically significant interaction expected |
| ■ | Potential interaction that may require a dosage adjustment, altered timing of administration, or additional monitoring |
| ● | These drugs should not be co-administered |
| ▲ | Not included in HEP Drug Interactions; however, metabolism and clearance data suggest that an interaction is unlikely |
| ▼ | Not included in HEP Drug Interactions; however, metabolism and clearance data suggest that an interaction may occur |
Acknowledgments
The authors thank Doctor Jean-Charles Nault, and Professor Olivier Scatton for comments on the manuscript and Stephanie Deming from the Department of Scientific Publications at MD Anderson Cancer Center for editorial assistance.
Funding source: This study was supported by the NIH/NCI under award number P30CA016672. Prof. Mallet is funded by la Fondation ARC pour la Recherche sur le Cancer.
Footnotes
Author Contributions: Harrys A. Torres: Conceptualization, writing original draft, review and editing, project administration, and supervision. Terri Lynn Shigle: Writing original draft and review and editing. Nassim Hammoudi: Writing original draft and review and editing. J. T. Link: Writing original draft and review and editing. Felipe Samaniego: Writing original draft and review and editing. Ahmed Kaseb: Writing original draft and review and editing. Vincent Mallet: Conceptualization, writing original draft, and review and editing.
Disclosures: Dr. Torres is or has been the principal investigator for research grants from Gilead Sciences, Merck & Co., Inc., and Vertex Pharmaceuticals, with all funds paid to MD Anderson. He also is or has been a paid scientific advisor for Gilead Sciences, Janssen Pharmaceuticals, Inc., Merck & Co., Inc., Dynavax Technologies, Vertex Pharmaceuticals, Genentech, Novartis, Astellas Pharma, Pfizer Inc., and Theravance Biopharma, Inc.; the terms of these arrangements are being managed by MD Anderson in accordance with its conflict of interest policies. Dr. Mallet is a speaker for BMS, Gilead, Janssen, Merck & Co., and Roche; a board member of Gilead, Janssen, and Merck & Co.; and a consultant for Translational Health Economics Network. He is funded by la Fondation ARC pour la Recherche sur le Cancer. The other authors have no conflicts to disclose.
References
- 1.Webster DP, Klenerman P, Dusheiko GM. Hepatitis C. Lancet. 2015;385(9973):1124–1135. doi: 10.1016/S0140-6736(14)62401-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allison RD, Tong X, Moorman AC, et al. Increased incidence of cancer and cancer-related mortality among persons with chronic hepatitis C infection, 2006–2010. J Hepatol. 2015;63(4):822–828. doi: 10.1016/j.jhep.2015.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mahale P, Torres HA, Kramer JR, et al. Hepatitis C virus infection and the risk of cancer among elderly US adults: A registry-based case-control study. Cancer. 2017;123(7):1202–1211. doi: 10.1002/cncr.30559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Plummer M, de Martel C, Vignat J, Ferlay J, Bray F, Franceschi S. Global burden of cancers attributable to infections in 2012: a synthetic analysis. Lancet Glob Health. 2016;4(9):e609–616. doi: 10.1016/S2214-109X(16)30143-7. [DOI] [PubMed] [Google Scholar]
- 5.Mahale P, Kontoyiannis DP, Chemaly RF, et al. Acute exacerbation and reactivation of chronic hepatitis C virus infection in cancer patients. J Hepatol. 2012;57(6):1177–1185. doi: 10.1016/j.jhep.2012.07.031. [DOI] [PubMed] [Google Scholar]
- 6.Torres HA, Mahale P, Blechacz B, et al. Effect of hepatitis C virus infection in patients with cancer: addressing a neglected population. J Natl Compr Canc Netw. 2015;13(1):41–50. doi: 10.6004/jnccn.2015.0007. [DOI] [PubMed] [Google Scholar]
- 7.Torres HA, Chong PP, De Lima M, et al. Hepatitis C Virus Infection among Hematopoietic Cell Transplant Donors and Recipients: American Society for Blood and Marrow Transplantation Task Force Recommendations. Biol Blood Marrow Transplant. 2015;21(11):1870–1882. doi: 10.1016/j.bbmt.2015.07.033. [DOI] [PubMed] [Google Scholar]
- 8.Mahale P, Kaseb AO, Hassan MM, Torres HA. Hepatocellular carcinoma as a second primary cancer in patients with chronic hepatitis C virus infection. Dig Liver Dis. 2015;47(4):348–349. doi: 10.1016/j.dld.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 9.Mallet V, van Bommel F, Doerig C, et al. Management of viral hepatitis in patients with haematological malignancy and in patients undergoing haemopoietic stem cell transplantation: recommendations of the 5th European Conference on Infections in Leukaemia (ECIL-5) Lancet Infect Dis. 2016;16(5):606–617. doi: 10.1016/S1473-3099(16)00118-3. [DOI] [PubMed] [Google Scholar]
- 10.Economides MP, Mahale P, Turturro F, et al. Development of non-Hodgkin lymphoma as a second primary cancer in hepatitis C virus-infected patients with a different primary malignancy. Leuk Lymphoma. 2016:1–4. doi: 10.1080/10428194.2016.1196817. [DOI] [PubMed] [Google Scholar]
- 11.Torres HA, Economides MP, Kyvernitakis A, et al. Sofosbuvir-Based Therapy in Patients with Chronic Hepatitis C Virus Infection and Malignancies - A Prospective Observational Study of 143 Patients. Paper presented at: 53rd Annual Meeting of American Society of Clinical Oncology (ASCO); June 2–6, 2017; Chicago, IL. [Google Scholar]
- 12.Peffault de Latour R, Levy V, Asselah T, et al. Long-term outcome of hepatitis C infection after bone marrow transplantation. Blood. 2004;103(5):1618–1624. doi: 10.1182/blood-2003-06-2145. [DOI] [PubMed] [Google Scholar]
- 13.Kyvernitakis A, Mahale P, Popat UR, et al. Hepatitis C Virus Infection in Patients Undergoing Hematopoietic Cell Transplantation in the Era of Direct-Acting Antiviral Agents. Biol Blood Marrow Transplant. 2016;22(4):717–722. doi: 10.1016/j.bbmt.2015.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Torres HA, Roach LR, Mahale P, et al. Transdisciplinary Approach to Managing Hepatitis C Virus Infection in Patients at a Tertiary Care Cancer Center. J Natl Compr Canc Netw. 2016;14(9):1185–1188. doi: 10.6004/jnccn.2016.0125. [DOI] [PubMed] [Google Scholar]
- 15.AASLD/IDSA HCV Guidance Panel. [Accessed March 29, 2017];Recommendations for testing, managing, and treating hepatitis C. 2017 http://www.hcvguidelines.org.
- 16.Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. Bmj. 2008;336(7650):924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Degenhardt L, Charlson F, Stanaway J, et al. Estimating the burden of disease attributable to injecting drug use as a risk factor for HIV, hepatitis C, and hepatitis B: findings from the Global Burden of Disease Study 2013. Lancet Infect Dis. 2016;16(12):1385–1398. doi: 10.1016/S1473-3099(16)30325-5. [DOI] [PubMed] [Google Scholar]
- 18.Zibbell JE, Iqbal K, Patel RC, et al. Increases in hepatitis C virus infection related to injection drug use among persons aged </=30 years - Kentucky, Tennessee, Virginia, and West Virginia, 2006–2012. MMWR Morb Mortal Wkly Rep. 2015;64(17):453–458. [PMC free article] [PubMed] [Google Scholar]
- 19.Gower E, Estes C, Blach S, Razavi-Shearer K, Razavi H. Global epidemiology and genotype distribution of the hepatitis C virus infection. J Hepatol. 2014;61(1 Suppl):S45–57. doi: 10.1016/j.jhep.2014.07.027. [DOI] [PubMed] [Google Scholar]
- 20.Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology. 2013;57(4):1333–1342. doi: 10.1002/hep.26141. [DOI] [PubMed] [Google Scholar]
- 21.Sievert W, Altraif I, Razavi HA, et al. A systematic review of hepatitis C virus epidemiology in Asia, Australia and Egypt. Liver Int. 2011;31(Suppl 2):61–80. doi: 10.1111/j.1478-3231.2011.02540.x. [DOI] [PubMed] [Google Scholar]
- 22.Stanaway JD, Flaxman AD, Naghavi M, et al. The global burden of viral hepatitis from 1990 to 2013: findings from the Global Burden of Disease Study 2013. Lancet. 2016;388(10049):1081–1088. doi: 10.1016/S0140-6736(16)30579-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edlin BR, Eckhardt BJ, Shu MA, Holmberg SD, Swan T. Toward a more accurate estimate of the prevalence of hepatitis C in the United States. Hepatology. 2015;62(5):1353–1363. doi: 10.1002/hep.27978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Denniston MM, Klevens RM, McQuillan GM, Jiles RB. Awareness of infection, knowledge of hepatitis C, and medical follow-up among individuals testing positive for hepatitis C: National Health and Nutrition Examination Survey 2001–2008. Hepatology. 2012;55(6):1652–1661. doi: 10.1002/hep.25556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith BD, Morgan RL, Beckett GA, et al. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945–1965. MMWR Recomm Rep. 2012;61(RR-4):1–32. [PubMed] [Google Scholar]
- 26.Freeman AJ, Dore GJ, Law MG, et al. Estimating progression to cirrhosis in chronic hepatitis C virus infection. Hepatology. 2001;34(4 Pt 1):809–816. doi: 10.1053/jhep.2001.27831. [DOI] [PubMed] [Google Scholar]
- 27.Tong MJ, el-Farra NS, Reikes AR, Co RL. Clinical outcomes after transfusion-associated hepatitis C. N Engl J Med. 1995;332(22):1463–1466. doi: 10.1056/NEJM199506013322202. [DOI] [PubMed] [Google Scholar]
- 28.Niederau C, Lange S, Heintges T, et al. Prognosis of chronic hepatitis C: results of a large, prospective cohort study. Hepatology. 1998;28(6):1687–1695. doi: 10.1002/hep.510280632. [DOI] [PubMed] [Google Scholar]
- 29.Demitri MS, Poussin K, Baccarini P, et al. HCV-associated liver cancer without cirrhosis. Lancet. 1995;345(8947):413–415. doi: 10.1016/s0140-6736(95)90400-x. [DOI] [PubMed] [Google Scholar]
- 30.Schwarzinger M, Baillot S, Yazdanpanah Y, Rehm J, Mallet V. Contribution of Alcohol Use Disorders on the Burden of Chronic Hepatitis C in France, 2008–2013: A Nationwide Retrospective Cohort Study. J Hepatol. 2017 doi: 10.1016/j.jhep.2017.03.031. In press. [DOI] [PubMed] [Google Scholar]
- 31.von Wulffen M, Clark PJ, Macdonald GA, et al. Liver-related mortality in countries of the developed world: an ecological study approach to explain the variability. Aliment Pharmacol Ther. 2016;44(1):68–77. doi: 10.1111/apt.13657. [DOI] [PubMed] [Google Scholar]
- 32.Madden R, Mallet V. Editorial: the burden of chronic liver disease - an ecological method sees the wood for the trees. Aliment Pharmacol Ther. 2016;44(5):531–532. doi: 10.1111/apt.13698. [DOI] [PubMed] [Google Scholar]
- 33.http://www.who.int/gho/ncd/risk_factors/overweight/en/.
- 34.Conti F, Buonfiglioli F, Scuteri A, et al. Early occurrence and recurrence of hepatocellular carcinoma in HCV-related cirrhosis treated with direct-acting antivirals. J Hepatol. 2016;65(4):727–733. doi: 10.1016/j.jhep.2016.06.015. [DOI] [PubMed] [Google Scholar]
- 35.Reig M, Marino Z, Perello C, et al. Unexpected high rate of early tumor recurrence in patients with HCV-related HCC undergoing interferon-free therapy. J Hepatol. 2016;65(4):719–726. doi: 10.1016/j.jhep.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 36.Yang JD, Aqel BA, Pungpapong S, Gores GJ, Roberts LR, Leise MD. Direct acting antiviral therapy and tumor recurrence after liver transplantation for hepatitis C-associated hepatocellular carcinoma. J Hepatol. 2016;65(4):859–860. doi: 10.1016/j.jhep.2016.06.023. [DOI] [PubMed] [Google Scholar]
- 37.Kozbial K, Moser S, Schwarzer R, et al. Unexpected high incidence of hepatocellular carcinoma in cirrhotic patients with sustained virologic response following interferon-free direct-acting antiviral treatment. J Hepatol. 2016;65(4):856–858. doi: 10.1016/j.jhep.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 38.Najafzadeh M, Andersson K, Shrank WH, et al. Cost-effectiveness of novel regimens for the treatment of hepatitis C virus. Ann Intern Med. 2015;162(6):407–419. doi: 10.7326/M14-1152. [DOI] [PubMed] [Google Scholar]
- 39.van der Meer AJ, Wedemeyer H, Feld JJ, et al. Life expectancy in patients with chronic HCV infection and cirrhosis compared with a general population. JAMA. 2014;312(18):1927–1928. doi: 10.1001/jama.2014.12627. [DOI] [PubMed] [Google Scholar]
- 40.Innes HA, McDonald SA, Dillon JF, et al. Toward a more complete understanding of the association between a hepatitis C sustained viral response and cause-specific outcomes. Hepatology. 2015;62(2):355–364. doi: 10.1002/hep.27766. [DOI] [PubMed] [Google Scholar]
- 41.Vandenbulcke H, Moreno C, Colle I, et al. Alcohol intake increases the risk of HCC in hepatitis C virus-related compensated cirrhosis: A prospective study. J Hepatol. 2016;65(3):543–551. doi: 10.1016/j.jhep.2016.04.031. [DOI] [PubMed] [Google Scholar]
- 42.ANRS collaborative study group on hepatocellular carcinoma (ANRS CO22 HEPATHER; CO12 CirVir and CO23 CUPILT cohorts) Lack of evidence of an effect of direct-acting antivirals on the recurrence of hepatocellular carcinoma: Data from three ANRS cohorts. J Hepatol. 2016;65(4):734–740. doi: 10.1016/j.jhep.2016.05.045. [DOI] [PubMed] [Google Scholar]
- 43.Innes HA, Hutchinson SJ, Barclay S, et al. Quantifying the fraction of cirrhosis attributable to alcohol among chronic hepatitis C virus patients: implications for treatment cost-effectiveness. Hepatology. 2013;57(2):451–460. doi: 10.1002/hep.26051. [DOI] [PubMed] [Google Scholar]
- 44.Hwang JP, Suarez-Almazor ME, Torres HA, et al. Hepatitis C virus screening in patients with cancer receiving chemotherapy. J Oncol Pract. 2014;10(3):e167–174. doi: 10.1200/JOP.2013.001215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mahale P, Sturgis EM, Tweardy DJ, Ariza-Heredia EJ, Torres HA. Association Between Hepatitis C Virus and Head and Neck Cancers. J Natl Cancer Inst. 2016;108(8) doi: 10.1093/jnci/djw035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Silvestri F, Pipan C, Barillari G, et al. Prevalence of hepatitis C virus infection in patients with lymphoproliferative disorders. Blood. 1996;87(10):4296–4301. [PubMed] [Google Scholar]
- 47.Oguz A, Aykas F, Unal D, et al. Hepatitis B and C Seroprevalence in Solid Tumors - Necessity for Screening During Chemotherapy. Asian Pacific Journal of Cancer Prevention. 2014;15(3):1411–1414. doi: 10.7314/apjcp.2014.15.3.1411. [DOI] [PubMed] [Google Scholar]
- 48.Dal Maso L, Franceschi S. Hepatitis C virus and risk of lymphoma and other lymphoid neoplasms: a meta-analysis of epidemiologic studies. Cancer Epidemiol Biomarkers Prev. 2006;15(11):2078–2085. doi: 10.1158/1055-9965.EPI-06-0308. [DOI] [PubMed] [Google Scholar]
- 49.Fiorino S, Bacchi-Reggiani L, de Biase D, et al. Possible association between hepatitis C virus and malignancies different from hepatocellular carcinoma: A systematic review. World J Gastroenterol. 2015;21(45):12896–12953. doi: 10.3748/wjg.v21.i45.12896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fiorino S, Chili E, Bacchi-Reggiani L, et al. Association between hepatitis B or hepatitis C virus infection and risk of pancreatic adenocarcinoma development: a systematic review and meta-analysis. Pancreatology. 2013;13(2):147–160. doi: 10.1016/j.pan.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 51.Gisbert JP, Garcia-Buey L, Pajares JM, Moreno-Otero R. Prevalence of hepatitis C virus infection in B-cell non-Hodgkin’s lymphoma: systematic review and meta-analysis. Gastroenterology. 2003;125(6):1723–1732. doi: 10.1053/j.gastro.2003.09.025. [DOI] [PubMed] [Google Scholar]
- 52.Matsuo K, Kusano A, Sugumar A, Nakamura S, Tajima K, Mueller NE. Effect of hepatitis C virus infection on the risk of non-Hodgkin’s lymphoma: a meta-analysis of epidemiological studies. Cancer Sci. 2004;95(9):745–752. doi: 10.1111/j.1349-7006.2004.tb03256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Negri E, Little D, Boiocchi M, La Vecchia C, Franceschi S. B-cell non-Hodgkin’s lymphoma and hepatitis C virus infection: a systematic review. Int J Cancer. 2004;111(1):1–8. doi: 10.1002/ijc.20205. [DOI] [PubMed] [Google Scholar]
- 54.Palmer WC, Patel T. Are common factors involved in the pathogenesis of primary liver cancers? A meta-analysis of risk factors for intrahepatic cholangiocarcinoma. J Hepatol. 2012;57(1):69–76. doi: 10.1016/j.jhep.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pozzato G, Mazzaro C, Dal Maso L, et al. Hepatitis C virus and non-Hodgkin’s lymphomas: Meta-analysis of epidemiology data and therapy options. World J Hepatol. 2016;8(2):107–116. doi: 10.4254/wjh.v8.i2.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shin HR, Oh JK, Masuyer E, et al. Epidemiology of cholangiocarcinoma: an update focusing on risk factors. Cancer Sci. 2010;101(3):579–585. doi: 10.1111/j.1349-7006.2009.01458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang Z, Sheng YY, Dong QZ, Qin LX. Hepatitis B virus and hepatitis C virus play different prognostic roles in intrahepatic cholangiocarcinoma: A meta-analysis. World J Gastroenterol. 2016;22(10):3038–3051. doi: 10.3748/wjg.v22.i10.3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xu JH, Fu JJ, Wang XL, Zhu JY, Ye XH, Chen SD. Hepatitis B or C viral infection and risk of pancreatic cancer: a meta-analysis of observational studies. World J Gastroenterol. 2013;19(26):4234–4241. doi: 10.3748/wjg.v19.i26.4234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhou YM, Zhao YF, Li B, et al. Hepatitis viruses infection and risk of intrahepatic cholangiocarcinoma: evidence from a meta-analysis. BMC Cancer. 2012;12:7. doi: 10.1186/1471-2407-12-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bruix J, Barrera JM, Calvet X, et al. Prevalence of antibodies to hepatitis C virus in Spanish patients with hepatocellular carcinoma and hepatic cirrhosis. Lancet. 1989;2(8670):1004–1006. doi: 10.1016/s0140-6736(89)91015-5. [DOI] [PubMed] [Google Scholar]
- 61.Colombo M, Kuo G, Choo QL, et al. Prevalence of antibodies to hepatitis C virus in Italian patients with hepatocellular carcinoma. Lancet. 1989;2(8670):1006–1008. doi: 10.1016/s0140-6736(89)91016-7. [DOI] [PubMed] [Google Scholar]
- 62.GBD 2015 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1459–1544. doi: 10.1016/S0140-6736(16)31012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Moore MS, Ivanina E, Bornschlegel K, Qiao B, Schymura MJ, Laraque F. Hepatocellular Carcinoma and Viral Hepatitis in New York City. Clin Infect Dis. 2016;63(12):1577–1583. doi: 10.1093/cid/ciw605. [DOI] [PubMed] [Google Scholar]
- 64.Shin H-R, Lee C-U, Park H-J, et al. Hepatitis B and C Virus, Clonorchis sinensis for the Risk of Liver Cancer: A Case-Control Study in Pusan, Korea. International Journal of Epidemiology. 1996;25(5):933–940. doi: 10.1093/ije/25.5.933. [DOI] [PubMed] [Google Scholar]
- 65.Donato F, Gelatti U, Tagger A, et al. Intrahepatic cholangiocarcinoma and hepatitis C and B virus infection, alcohol intake, and hepatolithiasis: a case-control study in Italy. Cancer Causes Control. 2001;12(10):959–964. doi: 10.1023/a:1013747228572. [DOI] [PubMed] [Google Scholar]
- 66.Zhou Y, Zhao Y, Li B, et al. Hepatitis viruses infection and risk of intrahepatic cholangiocarcinoma: evidence from a meta-analysis. BMC Cancer. 2012;12:289. doi: 10.1186/1471-2407-12-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.El-Serag HB, Engels EA, Landgren O, et al. Risk of hepatobiliary and pancreatic cancers after hepatitis C virus infection: A population-based study of U.S. veterans. Hepatology. 2009;49(1):116–123. doi: 10.1002/hep.22606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee MH, Yang HI, Lu SN, et al. Chronic hepatitis C virus infection increases mortality from hepatic and extrahepatic diseases: a community-based long-term prospective study. J Infect Dis. 2012;206(4):469–477. doi: 10.1093/infdis/jis385. [DOI] [PubMed] [Google Scholar]
- 69.Ferri C, Caracciolo F, Zignego AL, et al. Hepatitis C virus infection in patients with non-Hodgkin’s lymphoma. Br J Haematol. 1994;88(2):392–394. doi: 10.1111/j.1365-2141.1994.tb05036.x. [DOI] [PubMed] [Google Scholar]
- 70.de Sanjose S, Benavente Y, Vajdic CM, et al. Hepatitis C and non-Hodgkin lymphoma among 4784 cases and 6269 controls from the International Lymphoma Epidemiology Consortium. Clin Gastroenterol Hepatol. 2008;6(4):451–458. doi: 10.1016/j.cgh.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Anderson LA, Pfeiffer R, Warren JL, et al. Hematopoietic malignancies associated with viral and alcoholic hepatitis. Cancer Epidemiol Biomarkers Prev. 2008;17(11):3069–3075. doi: 10.1158/1055-9965.EPI-08-0408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Moriya K, Fujie H, Shintani Y, et al. The core protein of hepatitis C virus induces hepatocellular carcinoma in transgenic mice. Nat Med. 1998;4(9):1065–1067. doi: 10.1038/2053. [DOI] [PubMed] [Google Scholar]
- 73.Okuda M, Li K, Beard MR, et al. Mitochondrial injury, oxidative stress, and antioxidant gene expression are induced by hepatitis C virus core protein. Gastroenterology. 2002;122(2):366–375. doi: 10.1053/gast.2002.30983. [DOI] [PubMed] [Google Scholar]
- 74.Ariumi Y, Kuroki M, Dansako H, et al. The DNA damage sensors ataxia-telangiectasia mutated kinase and checkpoint kinase 2 are required for hepatitis C virus RNA replication. J Virol. 2008;82(19):9639–9646. doi: 10.1128/JVI.00351-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Silini E, Bottelli R, Asti M, et al. Hepatitis C virus genotypes and risk of hepatocellular carcinoma in cirrhosis: a case-control study. Gastroenterology. 1996;111(1):199–205. doi: 10.1053/gast.1996.v111.pm8698200. [DOI] [PubMed] [Google Scholar]
- 76.Torres HA, Nevah MI, Barnett BJ, et al. Hepatitis C virus genotype distribution varies by underlying disease status among patients in the same geographic region: a retrospective multicenter study. J Clin Virol. 2012;54(3):218–222. doi: 10.1016/j.jcv.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 77.El-Serag HB, Kanwal F, Richardson P, Kramer J. Risk of hepatocellular carcinoma after sustained virological response in Veterans with hepatitis C virus infection. Hepatology. 2016;64(1):130–137. doi: 10.1002/hep.28535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yu GY, He G, Li CY, et al. Hepatic expression of HCV RNA-dependent RNA polymerase triggers innate immune signaling and cytokine production. Mol Cell. 2012;48(2):313–321. doi: 10.1016/j.molcel.2012.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tomasetti C, Li L, Vogelstein B. Stem cell divisions, somatic mutations, cancer etiology, and cancer prevention. Science. 2017;355(6331):1330–1334. doi: 10.1126/science.aaf9011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Guichard C, Amaddeo G, Imbeaud S, et al. Integrated analysis of somatic mutations and focal copy-number changes identifies key genes and pathways in hepatocellular carcinoma. Nat Genet. 2012;44(6):694–698. doi: 10.1038/ng.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fujimoto A, Furuta M, Totoki Y, et al. Whole-genome mutational landscape and characterization of noncoding and structural mutations in liver cancer. Nat Genet. 2016;48(5):500–509. doi: 10.1038/ng.3547. [DOI] [PubMed] [Google Scholar]
- 82.Barbaro G, Di Lorenzo G, Asti A, et al. Hepatocellular mitochondrial alterations in patients with chronic hepatitis C: ultrastructural and biochemical findings. Am J Gastroenterol. 1999;94(8):2198–2205. doi: 10.1111/j.1572-0241.1999.01294.x. [DOI] [PubMed] [Google Scholar]
- 83.Korenaga M, Wang T, Li Y, et al. Hepatitis C virus core protein inhibits mitochondrial electron transport and increases reactive oxygen species (ROS) production. J Biol Chem. 2005;280(45):37481–37488. doi: 10.1074/jbc.M506412200. [DOI] [PubMed] [Google Scholar]
- 84.Nault JC, Calderaro J, Di Tommaso L, et al. Telomerase reverse transcriptase promoter mutation is an early somatic genetic alteration in the transformation of premalignant nodules in hepatocellular carcinoma on cirrhosis. Hepatology. 2014;60(6):1983–1992. doi: 10.1002/hep.27372. [DOI] [PubMed] [Google Scholar]
- 85.Takeda H, Takai A, Inuzuka T, Marusawa H. Genetic basis of hepatitis virus-associated hepatocellular carcinoma: linkage between infection, inflammation, and tumorigenesis. J Gastroenterol. 2017;52(1):26–38. doi: 10.1007/s00535-016-1273-2. [DOI] [PubMed] [Google Scholar]
- 86.Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127(5 Suppl 1):S35–50. doi: 10.1053/j.gastro.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 87.Risk NCD, Collaboration F. Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19·2 million participants. The Lancet. 2016;387(10026):1377–1396. doi: 10.1016/S0140-6736(16)30054-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Delarocque-Astagneau E, Roudot-Thoraval F, Campese C, Desenclos JC, Hepatitis CSS. Past excessive alcohol consumption: A major determinant of severe liver disease among newly referred hepatitis C virus infected patients in hepatology reference centers, France, 2001. Annals of Epidemiology. 2005;15(8):551–557. doi: 10.1016/j.annepidem.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 89.Komuta M, Spee B, Vander Borght S, et al. Clinicopathological study on cholangiolocellular carcinoma suggesting hepatic progenitor cell origin. Hepatology. 2008;47(5):1544–1556. doi: 10.1002/hep.22238. [DOI] [PubMed] [Google Scholar]
- 90.Nagao Y, Sata M, Noguchi S, et al. Detection of hepatitis C virus RNA in oral lichen planus and oral cancer tissues. J Oral Pathol Med. 2000;29(6):259–266. doi: 10.1034/j.1600-0714.2000.290604.x. [DOI] [PubMed] [Google Scholar]
- 91.Quinn ER, Chan CH, Hadlock KG, Foung SK, Flint M, Levy S. The B-cell receptor of a hepatitis C virus (HCV)-associated non-Hodgkin lymphoma binds the viral E2 envelope protein, implicating HCV in lymphomagenesis. Blood. 2001;98(13):3745–3749. doi: 10.1182/blood.v98.13.3745. [DOI] [PubMed] [Google Scholar]
- 92.Chen CL, Huang JY, Wang CH, et al. Hepatitis C virus has a genetically determined lymphotropism through co-receptor B7.2. Nat Commun. 2017;8:13882. doi: 10.1038/ncomms13882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hartridge-Lambert SK, Stein EM, Markowitz AJ, Portlock CS. Hepatitis C and non-Hodgkin lymphoma: the clinical perspective. Hepatology. 2012;55(2):634–641. doi: 10.1002/hep.25499. [DOI] [PubMed] [Google Scholar]
- 94.Peveling-Oberhag J, Arcaini L, Hansmann ML, Zeuzem S. Hepatitis C-associated B-cell non-Hodgkin lymphomas. Epidemiology, molecular signature and clinical management. J Hepatol. 2013;59(1):169–177. doi: 10.1016/j.jhep.2013.03.018. [DOI] [PubMed] [Google Scholar]
- 95.Hermine O, Lefrere F, Bronowicki JP, et al. Regression of splenic lymphoma with villous lymphocytes after treatment of hepatitis C virus infection. N Engl J Med. 2002;347(2):89–94. doi: 10.1056/NEJMoa013376. [DOI] [PubMed] [Google Scholar]
- 96.Sultanik P, Klotz C, Brault P, Pol S, Mallet V. Regression of an HCV-associated disseminated marginal zone lymphoma under IFN-free antiviral treatment. Blood. 2015;125(15):2446–2447. doi: 10.1182/blood-2014-12-618652. [DOI] [PubMed] [Google Scholar]
- 97.Arcaini L, Besson C, Frigeni M, et al. Interferon-free antiviral treatment in B-cell lymphoproliferative disorders associated with hepatitis C virus infection. Blood. 2016 doi: 10.1182/blood-2016-05-714667. [DOI] [PubMed] [Google Scholar]
- 98.Maciocia N, O’Brien A, Ardeshna K. Remission of Follicular Lymphoma after Treatment for Hepatitis C Virus Infection. N Engl J Med. 2016;375(17):1699–1701. doi: 10.1056/NEJMc1513288. [DOI] [PubMed] [Google Scholar]
- 99.Cabibbo G, Petta S, Barbara M, et al. Hepatic Decompensation is the Major Driver of Death In HCV-infected cirrhotic patients with successfully treated early hepatocellular carcinoma. J Hepatol. 2017 doi: 10.1016/j.jhep.2017.01.033. [DOI] [PubMed] [Google Scholar]
- 100.Torres HA, Hosry J, Mahale P, Economides MP, Lok AS. Hepatitis C virus reactivation in patients with cancer: A prospective observational study of 91 patients. Paper presented at: The Liver Meeting®; November 11–15, 2016; Boston, MA. [Google Scholar]
- 101.Navarro VJ, Senior JR. Drug-related hepatotoxicity. N Engl J Med. 2006;354(7):731–739. doi: 10.1056/NEJMra052270. [DOI] [PubMed] [Google Scholar]
- 102.Strasser SI, Myerson D, Spurgeon CL, et al. Hepatitis C virus infection and bone marrow transplantation: a cohort study with 10-year follow-up. Hepatology. 1999;29(6):1893–1899. doi: 10.1002/hep.510290609. [DOI] [PubMed] [Google Scholar]
- 103.Oliver NT, Nieto YL, Blechacz B, Anderlini P, Ariza-Heredia E, Torres HA. Severe hepatitis C reactivation as an early complication of hematopoietic cell transplantation. Bone Marrow Transplant. 2017;52(1):138–140. doi: 10.1038/bmt.2016.196. [DOI] [PubMed] [Google Scholar]
- 104.Nakasone H, Kurosawa S, Yakushijin K, et al. Impact of hepatitis C virus infection on clinical outcome in recipients after allogeneic hematopoietic cell transplantation. Am J Hematol. 2013;88(6):477–484. doi: 10.1002/ajh.23436. [DOI] [PubMed] [Google Scholar]
- 105.Evans AT, Loeb KR, Shulman HM, et al. Fibrosing cholestatic hepatitis C after hematopoietic cell transplantation: report of 3 fatal cases. Am J Surg Pathol. 2015;39(2):212–220. doi: 10.1097/PAS.0000000000000345. [DOI] [PubMed] [Google Scholar]
- 106.Torres HA, McDonald GB. How I treat hepatitis C virus infection in patients with hematologic malignancies. Blood. 2016;128(11):1449–1457. doi: 10.1182/blood-2016-05-718643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.McGovern BH, Birch CE, Bowen MJ, et al. Improving the diagnosis of acute hepatitis C virus infection with expanded viral load criteria. Clin Infect Dis. 2009;49(7):1051–1060. doi: 10.1086/605561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Torres HA, Davila M. Reactivation of hepatitis B virus and hepatitis C virus in patients with cancer. Nat Rev Clin Oncol. 2012;9(3):156–166. doi: 10.1038/nrclinonc.2012.1. [DOI] [PubMed] [Google Scholar]
- 109.Vento S, Cainelli F, Mirandola F, et al. Fulminant hepatitis on withdrawal of chemotherapy in carriers of hepatitis C virus. Lancet. 1996;347(8994):92–93. doi: 10.1016/s0140-6736(96)90212-3. [DOI] [PubMed] [Google Scholar]
- 110.Melisko ME, Fox R, Venook A. Reactivation of hepatitis C virus after chemotherapy for colon cancer. Clin Oncol (R Coll Radiol) 2004;16(3):204–205. doi: 10.1016/j.clon.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 111.Goutte N, Sogni P, Bendersky N, Barbare JC, Falissard B, Farges O. Geographical variations in incidence, management and survival of hepatocellular carcinoma in a Western country. J Hepatol. 2017;66(3):537–544. doi: 10.1016/j.jhep.2016.10.015. [DOI] [PubMed] [Google Scholar]
- 112.Kawaoka T, Aikata H, Teraoka Y, et al. Impact of Hepatitis C Virus Eradication on the Clinical Outcome of Patients with Hepatitis C Virus-Related Advanced Hepatocellular Carcinoma Treated with Sorafenib. Oncology. 2017 doi: 10.1159/000458532. [DOI] [PubMed] [Google Scholar]
- 113.Cheung MC, Walker AJ, Hudson BE, et al. Outcomes after successful direct-acting antiviral therapy for patients with chronic hepatitis C and decompensated cirrhosis. J Hepatol. 2016;65(4):741–747. doi: 10.1016/j.jhep.2016.06.019. [DOI] [PubMed] [Google Scholar]
- 114.Omata M, Kanda T, Wei L, et al. APASL consensus statements and recommendations for hepatitis C prevention, epidemiology, and laboratory testing. Hepatol Int. 2016;10(5):681–701. doi: 10.1007/s12072-016-9736-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.European Association for the Study of the Liver; Electronic address: easloffice@easloffice.eu. EASL Recommendations on Treatment of Hepatitis C 2016. J Hepatol. 2017;66(1):153–194. doi: 10.1016/j.jhep.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 116.Ryerson AB, Eheman CR, Altekruse SF, et al. Annual Report to the Nation on the Status of Cancer, 1975–2012, featuring the increasing incidence of liver cancer. Cancer. 2016;122(9):1312–1337. doi: 10.1002/cncr.29936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2013. National Cancer Institute; Bethesda, MD: Apr, 2016. http://seer.cancer.gov/csr/1975_2013/, based on November 2015 SEER data submission, posted to the SEER web site. [Google Scholar]
- 118.Hwang JP, Suarez-Almazor ME, Palla SL, et al. Hepatitis C virus screening prior to chemotherapy. Paper presented at: The MASCC/ISOO 2013 International Cancer Care Symposium; June 27–29 2013; Berlin, Germany. [Google Scholar]
- 119.Terrault NA, Hassanein TI. Management of the patient with SVR. J Hepatol. 2016;65(1 Suppl):S120–129. doi: 10.1016/j.jhep.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 120.Hsieh YH, Rothman RE, Laeyendecker OB, et al. Evaluation of the Centers for Disease Control and Prevention Recommendations for Hepatitis C Virus Testing in an Urban Emergency Department. Clin Infect Dis. 2016;62(9):1059–1065. doi: 10.1093/cid/ciw074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Saxena A, Hassan M, Mahale P, Kontoyiannis DP, Torres HA. Screening for hepatitis C viral Infection in a tertiary care cancer center. Hepatology. 2012;56:662A–663A. [Google Scholar]
- 122.Brasseur M, Heurgue-Berlot A, Barbe C, et al. Prevalence of hepatitis B and C and sensibility of a selective screening questionnaire in patients receiving chemotherapy for solid tumors. BMC Cancer. 2015;15:999. doi: 10.1186/s12885-015-2033-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.USPSTF. US Preventive Services Task Force. [Accessed on May 3, 2017];Screening for hepatitis C virus infection in adults: US Preventive Services Task Force recommendation statement. http://www.uspreventiveservicestaskforce.org/uspstf/uspshepc.htm.
- 124.Torres HA, Mahale P, Miller ED, Oo TH, Frenette C, Kaseb AO. Coadministration of telaprevir and transcatheter arterial chemoembolization in hepatitis C virus-associated hepatocellular carcinoma. World J Hepatol. 2013;5(6):332–335. doi: 10.4254/wjh.v5.i6.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tien PC Veterans Affairs Hepatitis CRCP, National Hepatitis CPO. Management and treatment of hepatitis C virus infection in HIV-infected adults: recommendations from the Veterans Affairs Hepatitis C Resource Center Program and National Hepatitis C Program Office. Am J Gastroenterol. 2005;100(10):2338–2354. doi: 10.1111/j.1572-0241.2005.00222.x. [DOI] [PubMed] [Google Scholar]
- 126.Vauthey JN, Pawlik TM, Ribero D, et al. Chemotherapy regimen predicts steatohepatitis and an increase in 90-day mortality after surgery for hepatic colorectal metastases. J Clin Oncol. 2006;24(13):2065–2072. doi: 10.1200/JCO.2005.05.3074. [DOI] [PubMed] [Google Scholar]
- 127.Yakushijin K, Atsuta Y, Doki N, et al. Sinusoidal obstruction syndrome after allogeneic hematopoietic stem cell transplantation: Incidence, risk factors and outcomes. Bone Marrow Transplant. 2016;51(3):403–409. doi: 10.1038/bmt.2015.283. [DOI] [PubMed] [Google Scholar]
- 128.McDonald GB. Hepatobiliary Complications of Hematopoietic Cell Transplantation, 40 Years On. Hepatology. 2010;51(4):1450–1460. doi: 10.1002/hep.23533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Clavien P, Petrowsky H, DeOliveira ML, Graf R. Medical progress: Strategies for safer liver surgery and partial liver transplantation. New England Journal of Medicine. 2007;356(15):1545–1559. doi: 10.1056/NEJMra065156. [DOI] [PubMed] [Google Scholar]
- 130.Lewis JH, Stine JG. Review article: prescribing medications in patients with cirrhosis - a practical guide. Aliment Pharmacol Ther. 2013;37(12):1132–1156. doi: 10.1111/apt.12324. [DOI] [PubMed] [Google Scholar]
- 131.Castera L, Vergniol J, Foucher J, et al. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128(2):343–350. doi: 10.1053/j.gastro.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 132.Sultanik P, Kramer L, Soudan D, et al. The relationship between liver stiffness measurement and outcome in patients with chronic hepatitis C and cirrhosis: a retrospective longitudinal hospital study. Aliment Pharmacol Ther. 2016;44(5):505–513. doi: 10.1111/apt.13722. [DOI] [PubMed] [Google Scholar]
- 133.Bravo AA, Sheth SG, Chopra S. Current concepts: Liver biopsy. New England Journal of Medicine. 2001;344(7):495–500. doi: 10.1056/NEJM200102153440706. [DOI] [PubMed] [Google Scholar]
- 134.Saunders JB, Walters JR, Davies AP, Paton A. A 20-year prospective study of cirrhosis. Br Med J (Clin Res Ed) 1981;282(6260):263–266. doi: 10.1136/bmj.282.6260.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.D’Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44(1):217–231. doi: 10.1016/j.jhep.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 136.Superfin D, Iannucci AA, Davies AM. Commentary: Oncologic drugs in patients with organ dysfunction: a summary. Oncologist. 2007;12(9):1070–1083. doi: 10.1634/theoncologist.12-9-1070. [DOI] [PubMed] [Google Scholar]
- 137.Bruix J, Castells A, Bosch J, et al. Surgical resection of hepatocellular carcinoma in cirrhotic patients: Prognostic value of preoperative portal pressure. Gastroenterology. 1996;111(4):1018–1022. doi: 10.1016/s0016-5085(96)70070-7. [DOI] [PubMed] [Google Scholar]
- 138.Iavarone M, Primignani M, Vavassori S, et al. Determinants of esophageal varices bleeding in patients with advanced hepatocellular carcinoma treated with sorafenib. United European Gastroenterol J. 2016;4(3):363–370. doi: 10.1177/2050640615615041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.de Franchis R. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63(3):743–752. doi: 10.1016/j.jhep.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 140.Martin P, DiMartini A, Feng S, Brown R, Jr, Fallon M. Evaluation for liver transplantation in adults: 2013 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Hepatology. 2014;59(3):1144–1165. doi: 10.1002/hep.26972. [DOI] [PubMed] [Google Scholar]
- 141.Curry MP. Direct acting antivirals for decompensated cirrhosis. Efficacy and safety are now established. J Hepatol. 2016;64(6):1206–1207. doi: 10.1016/j.jhep.2016.02.041. [DOI] [PubMed] [Google Scholar]
- 142.Welker MW, Luhne S, Lange CM, et al. Lactic acidosis in patients with hepatitis C virus cirrhosis and combined ribavirin/sofosbuvir treatment. J Hepatol. 2016;64(4):790–799. doi: 10.1016/j.jhep.2015.11.034. [DOI] [PubMed] [Google Scholar]
- 143.Oberg CL, Hiensch RJ, Poor HD. Ombitasvir-Paritaprevir-Ritonavir-Dasabuvir (Viekira Pak)-Induced Lactic Acidosis. Crit Care Med. 2017;45(3):e321–e325. doi: 10.1097/CCM.0000000000002086. [DOI] [PubMed] [Google Scholar]
- 144.Economides MP, Mahale P, Kyvernitakis A, et al. Concomitant use of direct-acting antivirals and chemotherapy in hepatitis C virus-infected patients with cancer. Aliment Pharmacol Ther. 2016 doi: 10.1111/apt.13825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sarrazin C, Isakov V, Svarovskaia ES, et al. Late Relapse Versus Hepatitis C Virus Reinfection in Patients With Sustained Virologic Response After Sofosbuvir-Based Therapies. Clin Infect Dis. 2017;64(1):44–52. doi: 10.1093/cid/ciw676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mahale P, Okhuysen PC, Torres HA. Does chemotherapy cause viral relapse in cancer patients with hepatitis C infection successfully treated with antivirals? Clin Gastroenterol Hepatol. 2014;12(6):1051–1054. e1051. doi: 10.1016/j.cgh.2013.10.034. [DOI] [PubMed] [Google Scholar]
- 147.Lok AS, Everhart JE, Di Bisceglie AM, et al. Occult and Previous Hepatitis B Virus Infection are Not Associated with Hepatocellular Carcinoma in United States Patients with Chronic Hepatitis C. Hepatology. 2011;54(2):434–442. doi: 10.1002/hep.24257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ende AR, Kim NH, Yeh MM, Harper J, Landis CS. Fulminant hepatitis B reactivation leading to liver transplantation in a patient with chronic hepatitis C treated with simeprevir and sofosbuvir: a case report. J Med Case Rep. 2015;9:164. doi: 10.1186/s13256-015-0630-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wang C, Ji D, Chen J, et al. Hepatitis due to Reactivation of Hepatitis B Virus in Endemic Areas Among Patients With Hepatitis C Treated With Direct-acting Antiviral Agents. Clin Gastroenterol Hepatol. 2017;15(1):132–136. doi: 10.1016/j.cgh.2016.06.023. [DOI] [PubMed] [Google Scholar]
- 150.Londono MC, Lens S, Marino Z, et al. Hepatitis B reactivation in patients with chronic hepatitis C undergoing anti-viral therapy with an interferon-free regimen. Aliment Pharmacol Ther. 2017 doi: 10.1111/apt.13985. [DOI] [PubMed] [Google Scholar]
- 151.Belperio PS, Shahoumian TA, Mole LA, Backus LI. Evaluation of Hepatitis B Reactivation among 62,920 Veterans treated with Oral Hepatitis C Antivirals. Hepatology. 2017 doi: 10.1002/hep.29135. [DOI] [PubMed] [Google Scholar]
- 152.Di Bisceglie AM, Lok AS, Martin P, Terrault N, Perrillo RP, Hoofnagle JH. Recent US Food and Drug Administration warnings on hepatitis B reactivation with immune-suppressing and anticancer drugs: just the tip of the iceberg? Hepatology. 2015;61(2):703–711. doi: 10.1002/hep.27609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hwang JP, Somerfield MR, Alston-Johnson DE, et al. Hepatitis B Virus Screening for Patients With Cancer Before Therapy: American Society of Clinical Oncology Provisional Clinical Opinion Update. J Clin Oncol. 2015;33(19):2212–2220. doi: 10.1200/JCO.2015.61.3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.van der Meer AJ, Veldt BJ, Feld JJ, et al. Association Between Sustained Virological Response and All-Cause Mortality Among Patients With Chronic Hepatitis C and Advanced Hepatic Fibrosis. JAMA. 2012;308(24):2584–2593. doi: 10.1001/jama.2012.144878. [DOI] [PubMed] [Google Scholar]
- 155.Morgan RL, Baack B, Smith BD, Yartel A, Pitasi M, Falck-Ytter Y. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta-analysis of observational studies. Ann Intern Med. 2013;158(5 Pt 1):329–337. doi: 10.7326/0003-4819-158-5-201303050-00005. [DOI] [PubMed] [Google Scholar]
- 156.Foster GR, Irving WL, Cheung MC, et al. Impact of direct acting antiviral therapy in patients with chronic hepatitis C and decompensated cirrhosis. J Hepatol. 2016;64(6):1224–1231. doi: 10.1016/j.jhep.2016.01.029. [DOI] [PubMed] [Google Scholar]
- 157.Dyson JK, Hutchinson J, Harrison L, et al. Liver toxicity associated with sofosbuvir, an NS5A inhibitor and ribavirin use. J Hepatol. 2016;64(1):234–238. doi: 10.1016/j.jhep.2015.07.041. [DOI] [PubMed] [Google Scholar]
- 158.van Schaik RH. CYP450 pharmacogenetics for personalizing cancer therapy. Drug Resist Updat. 2008;11(3):77–98. doi: 10.1016/j.drup.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 159.Hosry J, Mahale P, Turturro F, et al. Antiviral therapy improves overall survival in hepatitis C virus-infected patients who develop diffuse large B-cell lymphoma. Int J Cancer. 2016;139(11):2519–2528. doi: 10.1002/ijc.30372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.National Comprehensive Cancer Network N. Splenic Marginal Zone Lymphoma. 2016 http://www.nccn.org/professionals/physician_gls/f_guidelines.asp, Version 1.
- 161.Reig M, Boix L, Bruix J. The impact of direct antiviral agents on the development and recurrence of hepatocellular carcinoma. Liver Int. 2017;37(Suppl 1):136–139. doi: 10.1111/liv.13321. [DOI] [PubMed] [Google Scholar]
- 162.Torres HA, Vauthey JN, Economides MP, Mahale P, Kaseb A. Hepatocellular carcinoma recurrence after treatment with direct-acting antivirals: First, do no harm by withdrawing treatment. J Hepatol. 2016;65(4):862–864. doi: 10.1016/j.jhep.2016.05.034. [DOI] [PubMed] [Google Scholar]
- 163.Calleja JL, Crespo J, Rincon D, et al. Effectiveness, safety and clinical outcomes of direct-acting antiviral therapy in HCV genotype 1 infection: results from a Spanish real world cohort. J Hepatol. 2017 doi: 10.1016/j.jhep.2017.01.028. [DOI] [PubMed] [Google Scholar]
- 164.Manthravadi S, Paleti S, Pandya P. Impact of sustained viral response postcurative therapy of hepatitis C-related hepatocellular carcinoma: a systematic review and meta-analysis. Int J Cancer. 2017;140(5):1042–1049. doi: 10.1002/ijc.30521. [DOI] [PubMed] [Google Scholar]
- 165.Alanio C, Nicoli F, Sultanik P, et al. Bystander hyperactivation of preimmune CD8+ T cells in chronic HCV patients. Elife. 2015:4. doi: 10.7554/eLife.07916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Langhans B, Nischalke HD, Kramer B, et al. Increased peripheral CD4+ regulatory T cells persist after successful direct-acting antiviral treatment of chronic hepatitis C. J Hepatol. 2016 doi: 10.1016/j.jhep.2016.12.019. [DOI] [PubMed] [Google Scholar]
- 167.Tatsumi T, Takehara T. Impact of natural killer cells on chronic hepatitis C and hepatocellular carcinoma. Hepatol Res. 2016;46(5):416–422. doi: 10.1111/hepr.12619. [DOI] [PubMed] [Google Scholar]
- 168.Villani R, Facciorusso A, Bellanti F, et al. DAAs Rapidly Reduce Inflammation but Increase Serum VEGF Level: A Rationale for Tumor Risk during Anti-HCV Treatment. PLoS One. 2016;11(12):e0167934. doi: 10.1371/journal.pone.0167934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Giacomini KM, Huang SM. Transporters in drug development and clinical pharmacology. Clin Pharmacol Ther. 2013;94(1):3–9. doi: 10.1038/clpt.2013.86. [DOI] [PubMed] [Google Scholar]
- 170.Milazzo L, Biasin M, Gatti N, et al. Thalidomide in the treatment of chronic hepatitis C unresponsive to alfa-interferon and ribavirin. Am J Gastroenterol. 2006;101(2):399–402. doi: 10.1111/j.1572-0241.2006.00350.x. [DOI] [PubMed] [Google Scholar]
- 171.Caseiro MM. Treatment of chronic hepatitis C in non-responsive patients with pegylated interferon associated with ribavirin and thalidomide: report of six cases of total remission. Rev Inst Med Trop Sao Paulo. 2006;48(2):109–112. doi: 10.1590/s0036-46652006000200011. [DOI] [PubMed] [Google Scholar]
- 172.Peyrin-Biroulet L, Cadranel JF, Nousbaum JB, et al. Interaction of ribavirin with azathioprine metabolism potentially induces myelosuppression. Aliment Pharmacol Ther. 2008;28(8):984–993. doi: 10.1111/j.1365-2036.2008.03812.x. [DOI] [PubMed] [Google Scholar]
- 173.Kraljacic BC, Arguello M, Amri A, Cormack G, Borden K. Inhibition of eIF4E with ribavirin cooperates with common chemotherapies in primary acute myeloid leukemia specimens. Leukemia. 2011;25(7):1197–1200. doi: 10.1038/leu.2011.57. [DOI] [PubMed] [Google Scholar]
- 174.Pardo-Yules B, Gallego-Duran R, Eslam M, et al. Thalidomide with peginterferon alfa-2b and ribavirin in the treatment of non-responders genotype 1 chronic hepatitis C patients: proof of concept. Rev Esp Enferm Dig. 2011;103(12):619–625. doi: 10.4321/s1130-01082011001200003. [DOI] [PubMed] [Google Scholar]
- 175.Shi F, Len Y, Gong Y, et al. Ribavirin Inhibits the Activity of mTOR/eIF4E, ERK/Mnk1/eIF4E Signaling Pathway and Synergizes with Tyrosine Kinase Inhibitor Imatinib to Impair Bcr-Abl Mediated Proliferation and Apoptosis in Ph+ Leukemia. PLoS One. 2015;10(8):e0136746. doi: 10.1371/journal.pone.0136746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Britnell SR, Willets AE, Vanderman AJ, Woodard CL, Britt RB. Influence of Successful Chronic Hepatitis C Virus Treatment with Ledipasvir/Sofosbuvir on Warfarin Dosing Requirements in Four Veterans. Pharmacotherapy. 2016;36(11):1173–1179. doi: 10.1002/phar.1845. [DOI] [PubMed] [Google Scholar]
- 177.DeCarolis DD, Westanmo AD, Chen YC, Boese AL, Walquist MA, Rector TS. Evaluation of a Potential Interaction Between New Regimens to Treat Hepatitis C and Warfarin. Ann Pharmacother. 2016 doi: 10.1177/1060028016660325. [DOI] [PubMed] [Google Scholar]
- 178.Schulman S. Inhibition of warfarin activity by ribavirin. Ann Pharmacother. 2002;36(1):72–74. doi: 10.1345/aph.1A181. [DOI] [PubMed] [Google Scholar]
- 179.EPCLUSA® (sofosbuvir and velpatasvir) Gilead Sciences IFC; CA, USA: Prescribing Information Revised: 6/2016. [Google Scholar]
- 180.DAKLINZA® (daclatasvir) Bristol-Myers Squibb Company; Princeton N, USA: Prescribing Information Revised: 4/2016. [Google Scholar]
- 181.COPEGUS® (ribavirin) Genentech ISF; CA, USA: Prescribing Information Revised: 8/2011. [Google Scholar]
- 182.HARVONI® (ledipasvir and sofosbuvir) Gilead Sciences IFC; CA, USA: Prescribing Information Revised: 6/2016. [Google Scholar]
- 183.OLYSIO® (simeprevir) Janssen Therapeutics DoJP; LP Titusville NJ, USA: Prescribing Information Revised: 5/2016. [Google Scholar]
- 184.SOVALDI® (sofosbuvir) Gilead Sciences IFC; CA, USA: Prescribing Information Revised: 8/2015. [Google Scholar]
- 185.ZEPATIER® (elbasvir and grazoprevir) Merck Sharp & Dohme Corp; NJ U: Prescribing Information Revised: 1/2016. [Google Scholar]
- 186.VIEKIRA PAK® (ombitasvir paritaprevir and ritonavir tablets; dasabuvir tablets) AbbVie Inc; NC, IL, USA: Prescribing Information Revised: 6/2016. [Google Scholar]
- 187.Puglisi GM, Smith SM, Jankovich RD, Ashby CR, Jr, Jodlowski TZ. Paritaprevir/ritonavir/ombitasvir+dasabuvir plus ribavirin therapy and inhibition of the anticoagulant effect of warfarin: a case report. J Clin Pharm Ther. 2017;42(1):115–118. doi: 10.1111/jcpt.12475. [DOI] [PubMed] [Google Scholar]
- 188.Hoofnagle JH, Sherker AH. Hepatitis C: Down but Not Out. Ann Intern Med. 2017 doi: 10.7326/M17-0567. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.