Abstract
Numerous clinical trials have examined the role of anthocyanins on cardiometabolic health, but their effects have not been quantitatively synthesized and systematically evaluated. The aim of our study was to conduct a systematic review and meta-analysis of randomized controlled trials (RCTs) assessing the effects of anthocyanins on glycemic regulation and lipid profiles in both healthy populations and those with cardiometabolic diseases. The MEDLINE, EMBASE, Cochrane database, OVID EBM Reviews, and clinicaltrials.gov databases were searched until February 2017. RCTs with a duration of ≥2 wk that evaluated the effects of anthocyanins on glycemic control, insulin sensitivity, and lipids as either primary or secondary outcomes were included. The Cochrane Risk of Bias tool was used to assess the study quality. Standardized mean differences (SMDs) were determined by random-effects models. Meta-regression, sensitivity, and subgroup analyses were performed to explore the influence of covariates on the overall effects. Thirty-two RCTs (1491 participants) were eligible for meta-analysis. Anthocyanins significantly reduced fasting glucose (SMD: −0.31; 95% CI: −0.59, −0.04; I2 = 80.7%), 2-h postprandial glucose (SMD: −0.82; 95% CI: −1.49, −0.15; I2 = 77.7), glycated hemoglobin (SMD: −0.65; 95% CI: −1.00, −0.29; I2 = 72.7%), total cholesterol (SMD: −0.33; 95% CI: −0.62, −0.03; I2 = 86.9%), and LDL (SMD: −0.35; 95% CI: −0.66, −0.05; I2 = 85.2%). Sensitivity analyses showed that the overall effects remained similar by excluding the trials with a high or unclear risk of bias. The significant improvements in glycemic control and lipids support the benefits of anthocyanins in the prevention and management of cardiometabolic disease. Further well-designed RCTs are needed to evaluate the long-term effects of anthocyanins on metabolic profiles and to explore the optimal formula and dosage. The protocol for this review was registered at https://www.crd.york.ac.uk/PROSPERO/#index.php as CRD42016033210.
Keywords: anthocyanins, cardiovascular disease, meta-analysis, randomized controlled trial, type 2 diabetes
Introduction
Dietary modification or supplementation is a safe and cost-effective strategy for preventing and managing metabolic disease (1) and is being pursued as an alternative or supplement to pharmaceutical treatments. Anthocyanins are a subgroup of natural pigments in the major group of polyphenols that are responsible for dark color ranging from red-orange to blue-violet in plants, such as flowers, vegetables, grains, and fruits. The potential benefits of anthocyanins in prevention and management of cardiometabolic diseases have sparked substantial interest in recent decades (2). Epidemiologic evidence indicates that incorporating anthocyanin-rich foods into the diet may lower the risk of type 2 diabetes (3), blood pressure (4), and cardiovascular diseases (CVDs) (5). These findings are supported by animal experiments and clinical studies that have shown the improvement in cardiometabolic features after the consumption of anthocyanin supplements or berry fruits (6). Experimental studies suggest that the beneficial effect mechanisms of anthocyanins mainly involve insulin-dependent (7) and insulin-independent pathways (8).
Several reviews have summarized the effects of purified anthocyanins or anthocyanin-rich foods on metabolic markers or disease risk. A recent review reported the beneficial impacts of blueberries on insulin resistance and glucose intolerance (9). Wallace et al. (10) systematically reviewed 10 randomized controlled trials (RCTs) on the effects of anthocyanin supplementation on lipids and blood pressure, and the results indicated that anthocyanins significantly improved LDL. Another systematic review (11) explored the potential action mechanisms and cardiovascular effects of anthocyanins in animal models and human trials and revealed significant improvements on lipids and antioxidant capacity. However, most of the systematic reviews on anthocyanins were based on observational studies. A number of RCTs have been performed to test the effects of anthocyanin-rich foods or supplements on cardiometabolic markers, and the findings have been ambiguous. To our knowledge, the meta-analysis assessing the effects of anthocyanins on cardiometabolic markers based on RCTs is limited. Current evidence from human trials has not yet been comprehensively evaluated and quantitatively synthesized. We therefore conducted a systematic review and meta-analysis to provide a more precise estimate of the overall effects of anthocyanins on glycemic regulation and lipid profiles and sought to improve clinical practice of using anthocyanins for the prevention and therapy of cardiometabolic disorders.
Methods
Search strategy and selection criteria
The current systematic review was performed according to the Preferred Reporting Items for Systematic Review and Meta-Analysis guidelines (12). The protocol was registered in the PROSPERO international prospective register of systematic review (CRD42016033210). Literature searches were performed until February 2017 from the database of MEDLINE, EMBASE, Cochrane Library, and OVID EBM Reviews, and clinical trial registries were searched in clinicaltrials.gov.
Published studies were screened by 2 reviewers based on their titles, abstracts, and full texts according to the following inclusion criteria: 1) clinical RCTs using purified anthocyanins or anthocyanins-rich foods as treatment compared with a placebo or nonexposed concurrent controls and 2) participants with cardiometabolic diseases, such as type 2 diabetes, metabolic syndromes, dyslipidemia, hypertension, or healthy individuals. Moreover, the references of the included studies or reviews were further screened to identify other eligible studies. Studies that were excluded fit the following criteria: participants younger than 18 y of age, or with end-stage diseases (such as carcinoma or organ failure) or hormone-related disorders (such as polycystic ovary syndrome or being post-menopause); trials that used multifactorial or crossover design without parallel controls or applied multistage interventions; a duration of <2 wk or just a single supplementation; and studies that used non-English language or provided incomplete data for data extraction.
Data extraction and quality assessment
Data extraction was carried out by 2 independent reviewers and then double checked. The differences were resolved by discussion with a third reviewer. The following information was extracted from each study: 1) study characteristics, including study design, sample size, control varieties, duration of treatment, dosage, and sources of anthocyanins; 2) participant characteristics, such as age, sex, and baseline BMI (in kg/m2) ; and 3) metabolic variables measured at baseline and posttreatment or the differences. The biomarkers included glycemic regulation markers, such as fasting blood glucose, fasting insulin, glycated hemoglobin (HbA1c), HOMA-IR; lipid profiles, such as LDL, HDL, total cholesterol, and TGs; inflammation markers, such as C-reactive protein, TNF-α, and IL-6; and blood pressure. The authors were contacted to request further data if they were not available in the paper.
The quality of the selected trials was assessed according to the criteria of Cochrane Handbook for Systematic Reviews of Interventions (13), which consisted of 4 main aspects: random-sequence generation, allocation concealment, blinding, and selective reporting. The risk of bias was classified as low, high, or unclear. The completeness of the data and potential selective reporting bias of each study were assessed by comparing the outcome of the published report with the original protocol registered in the database.
Data synthesis and analysis
This meta-analysis was performed by Stata (version 12.0; StataCorp) and Review Manager (Version 5.3; the Cochrane Collaboration). The means and SDs at the baseline and posttreatment had been directly extracted from articles. If not available, SDs were calculated from SEs or 95% CIs. Because some outcomes were measured by different methods or with different units, standardized mean differences (SMDs) were applied to estimate the effect size. Pooled SMDs and their 95% CIs are shown in forest plots to assess the overall effects of anthocyanins compared with controls. Heterogeneity between studies was tested by using Cochran’s Q test (study by treatment interaction, P < 0.1), and an I2 value >50% was considered significant heterogeneity across studies (14). In light of the significant heterogeneity, a random-effects model was used to estimate the pooled effects. For selected outcomes, SDs of mean differences between the initial and final values were calculated by using a conservative correlation coefficient of 0.5 (15).
Meta-regression, sensitivity, and subgroup analyses were conducted to evaluate the influence of potential confounding factors and sources of heterogeneity. These factors included participant characteristics (disease or risk), baseline BMI, dosage of anthocyanins, treatment duration, formula of supplements (purified or extracted anthocyanins or anthocyanin-rich foods), sources of anthocyanins (berry or nonberry sources), and the methodological quality (according to allocation concealment, adequate concealment of allocation is deemed a low risk of bias, and inadequate concealment a high or unclear risk of bias). Sensitivity analyses were performed by using the leave-one-out approach to examine whether the results are robust by removing individual trials. Potential publication bias was examined by visual inspection of the funnel plots as well as the Egger’s test (16, 17). P values <0.05 were considered significant.
Results
Literature search and study selection
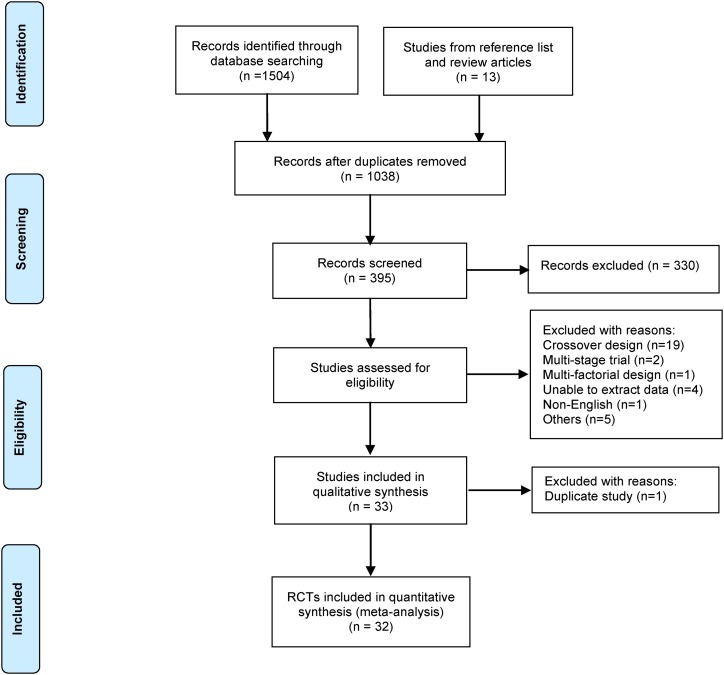
A total of 1504 potential studies were screened in the initial electronic search. After removing duplicate studies, the remaining 1038 articles were assessed for relevance according to the inclusion criteria for this review. The identification process for eligible studies is shown in Figure 1 and is based on the Preferred Reporting Items for Systematic Review and Meta-Analysis flow diagram (18). Sixty-five trials with anthocyanins as treatment were identified and further examined as full texts, and 33 were included for the meta-analysis. Among the 33 eligible studies, 6 enrolled patients with hyperglycemia, either type 2 diabetes mellitus (19–24) or prediabetes (25); 6 enrolled overweight or obese subjects (26–31); 6 included patients with metabolic syndromes (32–37); 4 studies included patients with dyslipidemia (38–41); and other studies included subjects with nonalcoholic fatty liver disease (42), hypertension (43), and CVD risk (44, 45) and healthy volunteers (46–50). Two publications came from the same trial with different treatment durations (41, 51). Only the one with the longer duration (41) was included. For studies with >2 groups within a study, the 2 comparisons (30) or only the higher dosage (25) compared with the control was extracted to avoid using duplicate control subjects. Most studies were placebo-controlled trials, except 3 that were diet (34) or water (33, 43) controlled. Finally, 32 eligible RCTs were included in the meta-analysis. A summary of the characteristics of all the included studies are presented in Table 1, and all excluded RCTs are listed in the Supplemental Table 1.
FIGURE 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram of the study selection. RCT, randomized controlled trial.
TABLE 1.
Characteristics of included trials in the meta-analysis of the effect of anthocyanins on cardiometabolic health1
| Study (ref), year | Cardiometabolic disease | Subjects, n, T/C | Duration | Intervention material | Total dose2 | Anthocyanin, mg/d |
| Soltani et al. (19), 2015 | T2DM | 30/30 | 6 wk | Cornus mas L. fruit extracts | 500 mg × 4 | 600 |
| Li et al. (20), 2015 | T2DM | 29/29 | 24 wk | Purified anthocyanins | 320 mg | 307.2 |
| Lee et al. (21), 2008 | T2DM | 15/15 | 12 wk | Cranberry extracts | 1500 mg | NA |
| Kianbakht et al. (22), 2013 | T2DM | 37/37 | 4 wk | Whortleberry extracts | 1050 mg | 7.35 |
| Fukuda et al. (23), 2015 | T2DM | 41/39 | 8 wk | Brazilian green propolis | 226.8 mg | NA |
| Shidfar et al. (24), 2012 | T2DM | 29/29 | 12 wk | Cranberry juice | 240 mL | NA |
| An et al. (25), 2016 | Prediabetes | 17/13 | 12 wk | Black raspberry extracts | 1800 mg | NA |
| Wright et al. (26), 2013 | Overweight and obesity | 8/8 | 4 wk | Dried purple carrot | 8.3 g × 3 | 118.5 |
| Stull et al. (27), 2010 | Obesity | 15/17 | 6 wk | Blueberry powder | 45 g | 668 |
| Rebello et al. (28), 2015 | Overweight and obesity | 14/14 | 4 wk | Blueberry anthocyanins | 8.8 g × 2 | 325 |
| Davinelli et al. (29), 2015 | Overweight | 26/16 | 4 wk | Maqui berry extracts | 450 mg | 162 |
| Basu et al. (30), 2014 (LD) | Abdominal adiposity | 15/15 | 12 wk | Freeze-dried strawberries | 494 mL | 78 |
| Basu et al. (30), 2014 (HD) | Abdominal adiposity | 15/15 | 12 wk | Freeze-dried strawberries | 494 mL | 155 |
| Lee et al. (31), 2016 | Overweight and obesity | 32/31 | 8 wk | Black soybean extracts | 2500 mg | 162.5 |
| Basu et al. (32), 2011 | Metabolic syndrome | 15/16 | 8 wk | Cranberry juice | 480 mL | 24.8 |
| Basu et al. (33), 2010 | Metabolic syndrome | 25/23 | 8 wk | Freeze-dried blueberries | 480 mL | 742 |
| Gurrola-Diaz et al. (34), 2010 | Metabolic Syndrome | 18/11 | 31 d | Hibiscus sabdariffa extracts | 100 mg | 19.24 |
| Jeong et al. (35), 2014 | Metabolic syndrome | 39/38 | 12 wk | Black raspberry extracts | 750 mg | NA |
| Puupponen-Pimiä et al. (36), 2013 | Metabolic syndrome | 20/12 | 12 wk | Berry constituents | 300 g | 70.7 |
| Stull et al. (37), 2015 | Metabolic syndrome | 23/21 | 6 wk | Blueberry | 45 g | 580.6 |
| Kianbakht et al. (38), 2014 | Dyslipidemia | 40/40 | 2 mo | Whortleberry extracts | 1050 mg | 7.35 |
| Qin et al. (39), 2009 | Dyslipidemia | 60/60 | 12 wk | Purified anthocyanins | 320 mg | 307.2 |
| Soltani et al. (40), 2014 | Hyperlipidemia | 25/25 | 4 wk | Vaccinium arctostaphylos extracts | 1000 mg | 90 |
| Zhu et al. (41), 2013 | Hypercholesterolemia | 73/73 | 24 wk | Purified anthocyanins | 320 mg | 307.2 |
| Zhang et al. (42), 2015 | NAFLD | 34/29 | 12 wk | Purified anthocyanins | 320 mg | 307.2 |
| Asgary et al. (43), 2014 | Hypertension | 11/10 | 2 wk | Pomegranate juice | 150 mL | 8.7 |
| Erlund et al. (44), 2008 | Cardiovascular risk | 35/36 | 8 wk | Berry constituents | 150 g | 515 |
| Sumner et al. (45), 2005 | Coronary heart disease | 26/19 | 3 mo | Pomegranate juice | 240 mL | NA |
| Karlsen et al. (46), 2007 | None | 59/59 | 3 wk | Purified anthocyanins | 300 mg | 300 |
| Duthie et al. (47), 2006 | None | 11/9 | 2 wk | Cranberry juice | 750 mL | 2.2 |
| Murkovic et al. (48), 2004 | None | 17/17 | 2 wk | Elderberry juice | 1200 mg | 120 |
| Novotny et al. (49), 2015 | None | 29/27 | 8 wk | Cranberry juice | 480 mL | 21 |
| Lynn et al. (50), 2014 | None | 25/21 | 6 wk | Tart cherry juice | 250 mL | 273.5 |
Duration refers to the duration of treatment. HD, high dose; LD, low dose; NA, not available; NAFLD, nonalcoholic fatty liver disease; ref, reference; T/C, treatment/control; T2DM, type 2 diabetes.
Amounts shown are for 1 dose/d unless otherwise indicated.
Study characteristics
A total of 1491 adults (759 in treatment groups and 732 in control groups) were included in the present meta-analysis, including 360 subjects with hyperglycemia and 396 with dyslipidemia. The variety of supplements included purified anthocyanins, composite anthocyanin extracts, and anthocyanins-rich foods, all of which were provided as powders, capsules, tablets, or beverages. The principal sources of anthocyanins were various berries, such as blueberries, strawberries, whortleberries, and cranberries. The dosage of pure anthocyanins ranged from 2.2 to 742 mg/d, and the treatment duration ranged from 2 to 24 wk. Of the included 32 RCTs in the meta-analysis, only 1 reported adverse events (25).
Risk of bias assessment
The graphs for the risk of bias are shown in Supplemental Figures 1 and 2. Most included studies (27 of 32) were double-blind. Allocation concealment was adequate in 22 trials, unclear in 5 studies, and not implemented in 5 studies. Twenty-five studies masked the participants, and 29 studies masked the outcome investigators. No obvious selective reporting bias was observed through comparison of the published outcomes and the limited available protocols. The funnel plots were symmetrical (figure not shown), and the Egger’s tests suggested that no significant publication bias existed for the overall effects of most glycolipid metabolism markers (P values for Egger’s testing are presented in Table 2).
TABLE 2.
Meta-analysis for the effects of anthocyanins on cardiometabolic markers1
| Outcome | Comparisons, n | Effect size | I2, % | P2 |
| Fasting glucose | 22 | −0.31 (−0.59, −0.04) | 80.70 | 0.37 |
| 2-h glucose | 4 | −0.82 (−1.49, −0.15) | 77.30 | 0.92 |
| HbA1c | 11 | −0.65 (−1.00, −0.29) | 72.70 | 0.12 |
| Fasting insulin | 12 | −0.002 (−0.38, 0.38) | 78.20 | 0.52 |
| HOMA-IR | 9 | −0.2 (−0.80, 0.40) | 87.10 | 0.73 |
| TGs | 24 | −0.2 (−0.45, 0.06) | 76.50 | 0.08 |
| Total cholesterol | 30 | −0.33 (−0.62, −0.03) | 86.90 | 0.19 |
| LDL cholesterol | 27 | −0.35 (−0.66, −0.05) | 85.20 | 0.86 |
| HDL cholesterol | 29 | 0.24 (−0.00, 0.49) | 81.10 | 0.51 |
| apoA-I | 6 | 0.21 (−0.12, 0.54) | 65.90 | 0.81 |
| apoB | 6 | −0.1 (−0.36, 0.17) | 47.60 | 0.63 |
| Systolic blood pressure | 19 | −0.17 (−0.55, 0.21) | 87.90 | 0.63 |
| Diastolic blood pressure | 20 | −0.17 (−0.56, 0.21) | 88.20 | 0.29 |
| TNF-α | 7 | −0.09 (−0.40, 0.21) | 67.70 | 0.33 |
| C-reactive protein | 7 | −0.51 (−1.14, 0.13) | 88.40 | 0.3 |
| IL-6 | 5 | −0.1 (−0.43, 0.22) | 52.50 | 0.1 |
Data are pooled standardized mean differences (95% CIs) by a random-effects model. HbA1c, glycated hemoglobin; 2-h glucose, 2-h postprandial glucose by oral glucose tolerance test.
Publication bias by Egger's regression.
Effects of anthocyanins on glycemic control and insulin sensitivity
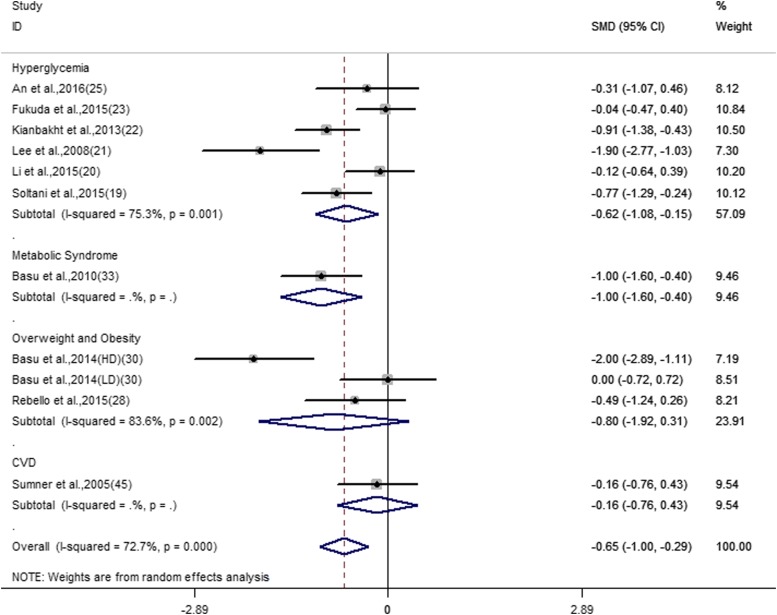
The overall effect for all outcomes is displayed in Table 2. For heterogeneity testing, the I2 ranged from 47.6% to 88.4%. Based on random-effects models, our meta-analyses suggested relevant favorable effects of anthocyanins on fasting and 2-h postprandial glucose and HbA1c. The pooled analyses suggested that anthocyanins significantly reduced fasting glucose concentrations (SMD: −0.31; 95% CI: −0.59, −0.04; I2 = 80.7%). Four trials (189 subjects) reported the effect of anthocyanins on 2-h postprandial blood glucose (SMD: −0.82; 95% CI: −1.49, −0.15; I2 = 77.7). Eleven trials, which included 510 subjects, reported results on HbA1c (SMD: −0.65; 95% CI: −1.00, −0.29; I2 = 72.7%) (Figure 2). There were nonsignificant pooled effects of anthocyanins on fasting insulin (SMD: −0.002; 95% CI: −0.38, 0.38; I2 = 78.2%) and HOMA-IR (SMD: −0.2; 95% CI: −0.8, 0.4; I2 = 87.1%).
FIGURE 2.
Forest plot of the meta-analysis for the effect of anthocyanins on glycated hemoglobin. Data are pooled SMDs with 95% CIs and are calculated by a random-effects model. Subgroup analysis was performed according to different cardiometabolic risk status. The 2014 trial by Basu et al. (33) has 4 study arms: HD, LD, and 2 placebo controls. CVD, cardiovascular disease; HD, high dose; ID, identifier; LD, low dose; SMD, standardized mean difference.
Effects of anthocyanins on lipid profiles and inflammation
Twenty-seven trials reported outcomes on lipid profiles and indicated that anthocyanin treatment was associated with decreased LDL (SMD: −0.35; 95% CI: −0.66, −0.05; I2 = 85.2%) and total cholesterol (SMD: −0.33; 95% CI: −0.62, −0.03; I2 = 86.9%) and marginally increased HDL (SMD: 0.24; 95% CI: −0.00, 0.49; I2 = 81.1%). There was no significant pooled effect of anthocyanins on systolic blood pressure, diastolic blood pressure, or inflammatory markers, including C-reactive protein, TNF-α, and IL-6.
Subgroup and sensitivity analyses and meta-regression
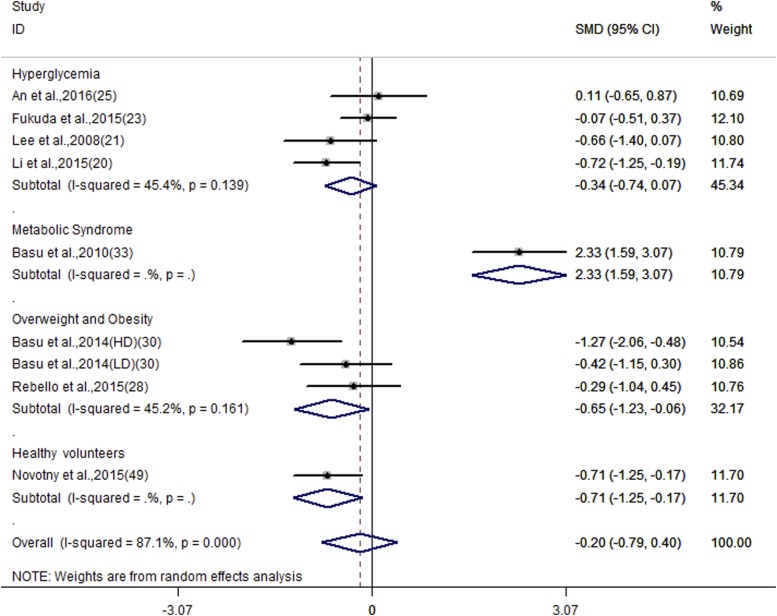
Subgroup analyses (Table 3) revealed that anthocyanin treatment substantially reduced fasting glucose in subjects with hyperglycemia, LDL in subjects with dyslipidemia, and HOMA-IR among overweight and obese subjects (SMD: −0.65; 95% CI: −1.23, −0.06; I2 = 45.2%) (Figure 3). Subgroup analyses among anthocyanin sources and formulas indicated decreased heterogeneity within subgroups. Meanwhile, a larger reduction of fasting glucose and LDL was observed in treatments that used purified anthocyanins or anthocyanin-rich extracts than in that of anthocyanin-rich foods, and in treatments that used berry sources than in other sources for anthocyanins.
TABLE 3.
Subgroup analyses for the effects of anthocyanins on fasting glucose and LDL cholesterol1
| Fasting glucose |
LDL cholesterol |
|||||||
| Subgroups | Comparisons, n | Mean difference | I2,% | P2 | Comparisons, n | Mean difference | I2,% | P2 |
| Cardiometabolic disease | ||||||||
| Dyslipidemia | 3 | −0.05 (−0.26, 0.16) | 0 | 0.91 | 5 | −0.59 (−0.87, −0.31) | 52.9 | 0.47 |
| None | 1 | −2.61 (−3.33, −1.89) | — | 0.004 | 3 | −0.04 (−0.91, 0.83) | 78.5 | 0.8 |
| Hyperglycemia | 7 | −0.5 (−0.85, −0.15) | 63.4 | 0.47 | 4 | −1.27 (−2.59, 0.06) | 93.5 | 0.23 |
| Metabolic syndrome | 4 | 0.01 (−0.94, 0.97) | 87.6 | 0.76 | 6 | −0.69 (−1.53, 0.16) | 89.8 | 0.41 |
| Overweight or obesity | 5 | −0.1 (−0.62, 0.43) | 66.9 | 0.93 | 7 | 0.16 (−0.54, 0.86) | 84.4 | 0.97 |
| CVD | 2 | −0.11 (−0.6, 0.38) | 0 | — | 2 | 0.28 (−0.21, 0.77) | 0 | — |
| Duration, wk | ||||||||
| 2–11 | 12 | −0.41 (−0.89, 0.07) | 86.5 | 0.55 | 16 | −0.37 (−0.75, 0.001) | 81.3 | 0.99 |
| 12–24 | 10 | −0.21 (−0.49, 0.08) | 64.1 | — | 11 | −0.33 (−0.86, 0.19) | 89.5 | — |
| BMI, kg/m2 | ||||||||
| <28 | 8 | −0.1 (−0.27, 0.07) | 0 | — | 11 | −0.49 (−0.86, −0.13) | 80.8 | — |
| ≥28 | 14 | −0.41 (−0.87, 0.05) | 87 | 0.46 | 15 | −0.17 (−0.67, 0.33) | 88.2 | 0.37 |
| Source | ||||||||
| Berries | 16 | −0.36 (−0.74, 0.01) | 85.7 | — | 21 | −0.41 (−0.78, −0.04) | 87.8 | — |
| Other | 6 | −0.16 (−0.39, 0.07) | 0 | 0.65 | 6 | −0.17 (−0.54, 0.20) | 47.8 | 0.67 |
| Formula | ||||||||
| Purified or extracts | 11 | −0.28 (−0.49, −0.07) | 44.6 | 0.86 | 13 | −0.61 (−0.94, −0.29) | 78.7 | 0.17 |
| Nonpurified | 11 | −0.31 (−0.90, 0.27) | 89 | — | 14 | −0.04 (−0.55, 0.47) | 86.7 | — |
| Study quality, risk of bias | ||||||||
| Low | 16 | −0.47 (−0.76, −0.18) | 79.8 | — | 19 | −0.5 (−0.82, −0.16) | 84.8 | — |
| High or unclear | 6 | 0.16 (−0.47, 0.79) | 77.2 | 0.08 | 8 | 0.06 (−0.67, 0.8) | 86.1 | 0.26 |
Data are pooled standardized mean differences (95% CIs) by a random-effects model. BMI could not be extracted in some studies. Source means anthocyanins from berries or other sources, and the other sources include pomegranate juice, Hibiscus sabdariffa extracts, purple carrot, Cornus mas L. fruit extracts, Vaccinium arctostaphylos extracts, etc. Formula refers to purified anthocyanins or anthocyanins-rich extracts and nonpurified anthocyanins-rich food. The study quality was stratified by the risk of bias of allocation concealment. CVD, cardiovascular disease.
Univariable meta-regression model.
FIGURE 3.
Forest plot of the meta-analysis for the effect of anthocyanins on HOMA-IR. Data are pooled SMDs with 95% CIs and are calculated by a random-effects model. Subgroup analysis was performed according to different cardiometabolic risk status. HD, high dose; ID, identifier; LD, low dose; SMD, standardized mean difference.
Subgroup analysis by dosage of anthocyanins indicated reduced overall heterogeneity within strata (Table 4). A more notable effect was observed on HbA1c and TGs with a higher dosage of >400 mg/d than with lower dosages of anthocyanins. The optimal dosage for LDL reduction was at 200–400 mg anthocyanins/d. The overall effects on TGs and LDL had a dose-response trend, although it was nonsignificant. We did not assess the effect of an anthocyanin dose on 2-h postprandial glucose and inflammatory markers because there were inadequate studies for subgrouping or meta-regression. There were no clear effects of anthocyanin dose on blood pressure or other metabolic variables.
TABLE 4.
Subgroup analyses for anthocyanin dose on the overall effects of cardiometabolic markers1
| Outcome and anthocyanin dose, mg/d | Comparisons, n | Mean difference | I2,% | P2 |
| Fasting glucose | ||||
| <200 | 8 | −0.47 (1.19, 0.24) | 89.1 | 0.59 |
| 200–400 | 5 | −0.11 (0.30, 0.08) | 0 | — |
| >400 | 4 | −0.1 (−0.92, 0.72) | 86.4 | 0.85 |
| HbA1c | ||||
| <200 | 3 | −0.94 (−1.90, 0.03) | 83.2 | 0.37 |
| 200–400 | 2 | −0.24 (−0.67, 0.19) | 0 | — |
| >400 | 2 | −0.87 (−1.26, −0.47) | 0 | 0.42 |
| HOMA-IR | ||||
| <200 | 3 | −0.77 (−1.20, −0.34) | 19.9 | 0.6 |
| 200–400 | 2 | −0.58 (−1.00, −0.14) | 0 | — |
| >400 | 1 | 2.33 (1.59, 3.07) | — | — |
| Insulin | ||||
| <200 | 3 | −0.42 (−1.12, 0.29) | 70 | 0.67 |
| 200–400 | 3 | −0.21 (−0.52, 0.10) | 0 | — |
| >400 | 3 | 0.26 (−0.52, 1.05) | 80.3 | 0.3 |
| TGs | ||||
| <200 | 12 | 0.04 (−0.47, 0.55) | 86 | 0.51 |
| 200–400 | 3 | −0.32 (−0.80, 0.17) | 60.9 | — |
| >400 | 5 | −0.55 (−0.84, −0.26) | 22 | 0.68 |
| Total cholesterol | ||||
| <200 | 14 | −0.18 (−0.73, 0.37) | 88.5 | 0.93 |
| 200–400 | 7 | −0.07 (−0.23, 0.09) | 0 | — |
| >400 | 4 | −0.76 (−1.89, 0.37) | 92.4 | 0.37 |
| LDL cholesterol | ||||
| <200 | 14 | −0.15 (−0.57, 0.28) | 81.7 | 0.58 |
| 200–400 | 5 | −0.49 (−0.69, −0.29) | 3.4 | — |
| >400 | 3 | −0.8 (−2.78, 1.18) | 95.7 | 0.67 |
| HDL cholesterol | ||||
| <200 | 14 | 0.23 (−0.24, 0.70) | 84.9 | 0.38 |
| 200–400 | 7 | 0.55 (0.09, 1.02) | 86 | — |
| >400 | 4 | 0.10 (−0.18, 0.38) | 0 | 0.39 |
| Systolic blood pressure | ||||
| <200 | 9 | −0.19 (−1.04, 0.66) | 91.8 | 0.95 |
| 200–400 | 5 | −0.14 (−0.44, 0.16) | 57.4 | — |
| >400 | 4 | −0.26 (−1.40, 0.87) | 92.7 | 0.89 |
| Diastolic blood pressure | ||||
| <200 | 9 | −0.33 (−1.24, 0.58) | 92.7 | 0.78 |
| 200–400 | 5 | −0.04 (−0.23, 0.16) | 6.2 | — |
| >400 | 4 | −0.42 (1.54, 0.70) | 92.5 | 0.76 |
Data are pooled standardized mean differences (95% CIs) by a random-effects model. Anthocyanin dose could not be extracted in some studies. HbA1c, glycated hemoglobin.
P value for univariable meta-regression model.
Sensitivity analyses, which exclude each study and subgroup analysis that was stratified by study quality (pooled separately from the trials with high or unclear risk of bias), were performed to test the robustness of the results. The studies with a low risk of bias had more marked effects on fasting glucose and LDL than did those with a high or unclear risk of bias. The exclusion of each study from the meta-analysis did not influence the overall effects on the main glucose and lipid metabolism measurements, such as fasting glucose, HbA1c, and LDL.
Discussion
This meta-analysis of the 32 RCTs revealed that anthocyanin treatment significantly improved fasting and 2-h postprandial glucose, HbA1c, total cholesterol, and LDL. No relevant effects were observed on blood pressure or inflammation markers. Subgroup analyses suggested that anthocyanins substantially decreased fasting glucose in subjects with hyperglycemia, LDL in subjects with dyslipidemia, and HOMA-IR among overweight and obese subjects. Sensitivity analyses showed that the overall effects of anthocyanins remained robust by excluding each trial or the trials with a high or unclear risk of bias.
Our study included a wide range of populations with different cardiometabolic statuses. The trials included in this meta-analysis also involved a large variety of dosages and sources of anthocyanins. To identify the sources of heterogeneity across the trials, we performed a range of analyses, including meta-regression, sensitivity, and subgroup analyses. The results suggested an approximate dose-response trend for TGs and LDL and decreased heterogeneity within subgroups. However, meta-regression showed no significant impact of the anthocyanin dose on the overall effects. This may be because the applied anthocyanins have different molecular structures and contain diverse bioactive constituents. Additionally, there are few dose-response studies. Further well-designed clinical trials are warranted to explore the dose-response relation of anthocyanin treatment within one study, as explored by Basu et al. (30).
Our results are consistent with the reports on whortleberry (22) and cranberry (21) that anthocyanins from berry sources had more marked effects than other sources on main glycolipid metabolism markers of fasting glucose and LDL. This could be because anthocyanins from berry sources contain more of certain active ingredients. Anthocyanins and proanthocyanins were mainly localized in the skin of fruits (52). Food processing may alter the components or molecule structure of anthocyanins (53). Moreover, we found that purified or extracted anthocyanins have more notable effects than nonpurified anthocyanin-rich foods, which could be because the presence of other components in anthocyanin-rich foods or the interaction between food ingredients may interfere with the effect of purified anthocyanins. The processing procedures in anthocyanin extraction or purification might be crucial for the biological functions of anthocyanins (54). Studies have shown that background dietary structure and physiological relevance could have an impact on the biological activity of anthocyanins (55). Further clinical trials are necessary to identify the specific molecular structure or certain processing methods that could affect the biological function of anthocyanins.
Our analysis indicated that anthocyanin treatment could significantly reduce markers of glycemic control. Subgroup analysis further suggested that anthocyanins improved fasting glucose and HbA1c with greater effects observed among patients with hyperglycemia. These findings would have important public health implications in primary and secondary prevention of type 2 diabetes (56) and CVDs (57). Studies suggested that postprandial hyperglycemia could be a better predictor of CVD than fasting glucose (58), and lowering postprandial blood glucose concentrations is an important clinical practice for diabetes control. Our analysis indicated that the 2-h postprandial glucose concentration was reduced by anthocyanin treatment. These findings support that anthocyanins could be a candidate for pharmacological management of hyperglycemia.
Insulin resistance occurs early in the progression of metabolic disorders and plays a pivotal pathogenic role in the development of type 2 diabetes. Animal and human studies have demonstrated that anthocyanins could improve insulin sensitivity (20, 59). Despite the nonsignificant effect that was observed in the overall effect of HOMA-IR, studies have shown that HOMA-IR could be significantly reduced by anthocyanins in patients with type 2 diabetes mellitus (19) and prediabetes (25), and the subgroup analysis showed a marked pooled effect of HOMA-IR in overweight and obese subjects. Obesity is considered a known risk factor of insulin resistance and type 2 diabetes (60, 61) as well as CVD (62–64). Adipose tissues could secrete certain cytokines or molecules, such as FFAs and adipokines that contribute to the development of metabolic disorders (65, 66). In an obese mouse model, blueberry anthocyanins had the potency to inhibit weight gain and body fat accumulation (67). Further clinical studies are required to confirm whether anthocyanins have additional benefits on insulin resistance and weight control among overweight or obese populations.
The present meta-analysis indicates that anthocyanins have favorable overall effects on LDL, which concurs with the previous systematic review (10). It was reported that anthocyanins could significantly improve LDL, but not other markers of CVD, among diseased individuals. Another systematic review discussed the cardiovascular protection of anthocyanins and revealed that the possible mechanism may be related to its effect on lipid peroxidation (11). These existing systematic reviews mainly focused on CVD molecular biomarkers, but a few reviews focused on glycemic regulation. Blumberg et al. (6) discussed the glucoregulation effect of cranberries from limited clinical trials. Stull (9) reported the improvements on insulin resistance but could not draw conclusions on the antidiabetic effect of blueberries because of the small number of clinical studies. Edirisinghe et al. (68) reviewed how the antidiabetic actions of berries might involve insulin-dependent or -independent mechanisms, such as the modification of inflammation, as demonstrated in experimental studies (69, 70). Several studies reported that anthocyanins could significantly reduce inflammatory biomarkers (25, 41, 46), whereas our meta-analysis showed a nonsignificant combined effect on inflammatory biomarkers. The inconsistencies could be due to the incomplete literature search on these outcomes, because the effect of anthocyanins on inflammatory markers was not our primary outcome, and only limited trials that reported the data on inflammation were included.
Our meta-analysis has several strengths. First, to our knowledge, this is the first meta-analysis that comprehensively assessed the effects of anthocyanins from available RCT data on cardiometabolic markers. We included a large number of studies with a randomized controlled design, extracted comprehensive cardiometabolic markers as outcomes, and included participants with a range of cardiometabolic risks. The relatively large number of participants allowed us a greater statistical power to detect a small treatment effect. Second, most of the included trials had a relatively good quality, and no significant publication bias was tested by Egger's regression. However, several limitations should be noted. First, the applied material of anthocyanins was heterogeneous in nature. To reduce the effect of heterogeneity, we conducted a number of subgroup and regression analyses. Standardized-pooled-effect (SMD) and random-effect models were also used to address the heterogeneity. Although there were wide CIs, the pooled effects were close to the average effect of each study but were still statistically significant. Second, there were limited studies with long-term durations and dose-response effects. In addition, a number of trials using anthocyanin-rich foods as treatment had an unclear or wide range of anthocyanin content, and there could be other nonquantified matrix compounds. It is possible that other components contained in these foods or their interactions with anthocyanins might have influenced the effects of anthocyanins. However, the inclusion of these studies in the current meta-analyses provided an additional perspective to the overall effect of anthocyanins. Finally, because of the missing medication-usage data of the selected RCTs, we could not analyze the influence of medication on the overall effects of anthocyanin treatment.
In conclusion, our meta-analysis of 32 RCTs indicated that anthocyanin supplementation or consumption of anthocyanin-rich foods has beneficial effects on glycolipid metabolism by reducing fasting and 2-h postprandial glucose, HbA1c, and LDL. The significant improvements in multiple cardiometabolic biomarkers support anthocyanins as an alternative for the prevention and treatment of cardiometabolic disorders. Further well-designed long-term trials are required to explore the optimal dosage, duration, and anthocyanins formula.
Acknowledgments
We thank Candida J Rebello for providing us with additional details on his data to complete the analysis and Giovanni Scapagnini for kindly responding to our e-mail and providing the correct data for our query. The authors’ responsibilities were as follows—WL: designed the study; LiLi Yang: drafted the protocol and modified the final manuscript; SD and DL: screened and selected the trials; LiPing Yang and ZL: extracted the data; ZD and YC: analyzed the data; LiPing Yang: drafted the manuscript; and all authors: read and approved the final manuscript.
References
- 1.Franz MJ, Powers MA, Leontos C, Holzmeister LA, Kulkarni K, Monk A, Wedel N, Gradwell E. The evidence for medical nutrition therapy for type 1 and type 2 diabetes in adults. J Am Diet Assoc 2010;110:1852–89. [DOI] [PubMed] [Google Scholar]
- 2.Wallace TC. Anthocyanins in cardiovascular disease. Adv Nutr 2011;2:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guo X, Yang B, Tan J, Jiang J, Li D. Associations of dietary intakes of anthocyanins and berry fruits with risk of type 2 diabetes mellitus: a systematic review and meta-analysis of prospective cohort studies. Eur J Clin Nutr 2016;70:1360–7. [DOI] [PubMed] [Google Scholar]
- 4.Zhu Y, Sun J, Lu W, Wang X, Wang X, Han Z, Qiu C. Effects of blueberry supplementation on blood pressure: a systematic review and meta-analysis of randomized clinical trials. J Hum Hypertens 2017;31:165–71. [DOI] [PubMed] [Google Scholar]
- 5.Cassidy A, Bertoia M, Chiuve S, Flint A, Forman J, Rimm EB. Habitual intake of anthocyanins and flavanones and risk of cardiovascular disease in men. Am J Clin Nutr 2016;104:587–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blumberg JB, Basu A, Krueger CG, Lila MA, Neto CC, Novotny JA, Reed JD, Rodriguez-Mateos A, Toner CD. Impact of cranberries on gut microbiota and cardiometabolic health: proceedings of the Cranberry Health Research Conference 2015. Adv Nutr 2016;7:759S–70S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seymour EM, Tanone II, Urcuyo-Llanes DE, Lewis SK, Kirakosyan A, Kondoleon MG, Kaufman PB, Bolling SF. Blueberry intake alters skeletal muscle and adipose tissue peroxisome proliferator-activated receptor activity and reduces insulin resistance in obese rats. J Med Food 2011;14:1511–8. [DOI] [PubMed] [Google Scholar]
- 8.Takikawa M, Inoue S, Horio F, Tsuda T. Dietary anthocyanin-rich bilberry extract ameliorates hyperglycemia and insulin sensitivity via activation of AMP-activated protein kinase in diabetic mice. J Nutr 2010;140:527–33. [DOI] [PubMed] [Google Scholar]
- 9.Stull AJ. Blueberries’ impact on insulin resistance and glucose intolerance. Antioxidants (Basel) 2016;5:E44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wallace TC, Slavin M, Frankenfeld CL. Systematic review of anthocyanins and markers of cardiovascular disease. Nutrients 2016;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reis JF, Monteiro VV, de Souza Gomes R, do Carmo MM, da Costa GV, Ribera PC, Monteiro MC. Action mechanism and cardiovascular effect of anthocyanins: a systematic review of animal and human studies. J Transl Med 2016;14:315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 2009;62:e1–34. [DOI] [PubMed] [Google Scholar]
- 13.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA; Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Julian PT, Higgins SG. Cochrane handbook for systematic reviews of interventions version 5.1.0 [Internet]. c2011 [updated 2011 Mar; cited 2016 Feb 18]. Available from: http://www.handbook.cochrane.org/.
- 16.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, Carpenter J, Rcker G, Harbord RM, Schmid CH, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011;343:d4002. [DOI] [PubMed] [Google Scholar]
- 18.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soltani R, Gorji A, Asgary S, Sarrafzadegan N, Siavash M.. Evaluation of the effects of Cornus mas L. fruit extract on glycemic control and insulin level in type 2 diabetic adult patients: a randomized double-blind placebo-controlled clinical trial. Evid Based Complement Alternat Med 2015;2015:740954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li D, Zhang Y, Liu Y, Sun R, Xia M. Purified anthocyanin supplementation reduces dyslipidemia, enhances antioxidant capacity, and prevents insulin resistance in diabetic patients. J Nutr 2015;145:742–8. [DOI] [PubMed] [Google Scholar]
- 21.Lee IT, Chan YC, Lin CW, Lee WJ, Sheu WHH. Effect of cranberry extracts on lipid profiles in subjects with Type 2 diabetes. Diabet Med 2008;25:1473–7. [DOI] [PubMed] [Google Scholar]
- 22.Kianbakht S, Abasi B, Dabaghian FH. Anti-hyperglycemic effect of vaccinium arctostaphylos in type 2 diabetic patients: a randomized controlled trial. Forsch Komplementmed 2013;20:17–22. [DOI] [PubMed] [Google Scholar]
- 23.Fukuda T, Fukui M, Tanaka M, Senmaru T, Iwase H, Yamazaki M, Aoi W, Inui T, Nakamura N, Marunaka Y. Effect of Brazilian green propolis in patients with type 2 diabetes: a double-blind randomized placebo-controlled study. Biomed Rep 2015;3:355–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shidfar F, Heydari I, Hajimiresmaiel SJ, Hosseini S, Shidfar S, Amiri F. The effects of cranberry juice on serum glucose, apoB, apoA-I, Lp(a), and Paraoxonase-1 activity in type 2 diabetic male patients. J Res Med Sci 2012;17:355–60. [PMC free article] [PubMed] [Google Scholar]
- 25.An JH, Kim DL, Lee TB, Kim KJ, Kim SH, Kim NH, Kim HY, Choi DS, Kim SG. Effect of Rubus occidentalis extract on metabolic parameters in subjects with prediabetes: a proof-of-concept, randomized, double-blind, placebo-controlled clinical trial. Phytother Res 2016;30:1634–40. [DOI] [PubMed] [Google Scholar]
- 26.Wright OR, Netzel GA, Sakzewski AR. A randomized, double-blind, placebo-controlled trial of the effect of dried purple carrot on body mass, lipids, blood pressure, body composition, and inflammatory markers in overweight and obese adults: the QUENCH trial. Can J Physiol Pharmacol 2013;91:480–8. [DOI] [PubMed] [Google Scholar]
- 27.Stull AJ, Cash KC, Johnson WD, Champagne CM, Cefalu WT. Bioactives in blueberries improve insulin sensitivity in obese, insulin-resistant men and women. J Nutr 2010;140:1764–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rebello CJ, Burton J, Heiman M, Greenway FL. Gastrointestinal microbiome modulator improves glucose tolerance in overweight and obese subjects: a randomized controlled pilot trial. J Diabetes Complications 2015;29:1272–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davinelli S, Bertoglio JC, Zarrelli A, Pina R, Scapagnini G. A randomized clinical trial evaluating the efficacy of an anthocyanin-maqui berry extract (Delphinol®) on oxidative stress biomarkers. J Am Coll Nutr 2015;34 Suppl 1:28–33. [DOI] [PubMed] [Google Scholar]
- 30.Basu A, Betts NM, Nguyen A, Newman ED, Fu D, Lyons TJ. Freeze-dried strawberries lower serum cholesterol and lipid peroxidation in adults with abdominal adiposity and elevated serum lipids. J Nutr 2014;144:830–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee M, Sorn SR, Park Y, Park HK. Anthocyanin rich-black soybean testa improved visceral fat and plasma lipid profiles in overweight/obese Korean adults: a randomized controlled trial. J Med Food 2016;19:995–1003. [DOI] [PubMed] [Google Scholar]
- 32.Basu A, Betts NM, Ortiz J, Simmons B, Wu M, Lyons TJ. Low-energy cranberry juice decreases lipid oxidation and increases plasma antioxidant capacity in women with metabolic syndrome. Nutr Res 2011;31:190–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Basu A, Du M, Leyva MJ, Sanchez K, Betts NM, Wu M, Aston CE, Lyons TJ. Blueberries decrease cardiovascular risk factors in obese men and women with metabolic syndrome. J Nutr 2010;140:1582–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gurrola-Diaz CM, Garcia-Lopez PM, Sanchez-Enriquez S, Troyo-Sanroman R, Andrade-Gonzalez I, Gomez-Leyva JF. Effects of Hibiscus sabdariffa extract powder and preventive treatment (diet) on the lipid profiles of patients with metabolic syndrome (MeSy). Phytomedicine 2010;17:500–5. [DOI] [PubMed] [Google Scholar]
- 35.Jeong HS, Hong SJ, Lee TB, Kwon JW, Jeong JT, Joo HJ, Park JH, Ahn CM, Yu CW, Lim DS. Effects of black raspberry on lipid profiles and vascular endothelial function in patients with metabolic syndrome. Phytother Res 2014;28:1492–8. [DOI] [PubMed] [Google Scholar]
- 36.Puupponen-Pimiä R, Seppänen-Laakso T, Kankainen M, Maukonen J, Törrönen R, Kolehmainen M, Leppänen T, Moilanen E, Nohynek L, Aura AM, et al. Effects of ellagitannin-rich berries on blood lipids, gut microbiota, and urolithin production in human subjects with symptoms of metabolic syndrome. Mol Nutr Food Res 2013;57:2258–63. [DOI] [PubMed] [Google Scholar]
- 37.Stull AJ, Cash KC, Champagne CM, Gupta AK, Boston R, Beyl RA, Johnson WD, Cefalu WT. Blueberries improve endothelial function, but not blood pressure, in adults with metabolic syndrome: a randomized, double-blind, placebo-controlled clinical trial. Nutrients 2015;7:4107–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kianbakht S, Abasi B, Hashem Dabaghian F. Improved lipid profile in hyperlipidemic patients taking Vaccinium arctostaphylos fruit hydroalcoholic extract: a randomized double-blind placebo-controlled clinical trial. Phytother Res 2014;28:432–6. [DOI] [PubMed] [Google Scholar]
- 39.Qin Y, Xia M, Ma J, Hao Y, Liu J, Mou H, Cao L, Ling W. Anthocyanin supplementation improves serum LDL- and HDL-cholesterol concentrations associated with the inhibition of cholesteryl ester transfer protein in dyslipidemic subjects. Am J Clin Nutr 2009;90:485–92. [DOI] [PubMed] [Google Scholar]
- 40.Soltani R, Hakimi M, Asgary S, Ghanadian SM, Keshvari M, Sarrafzadegan N. Evaluation of the effects of Vaccinium arctostaphylos L. fruit extract on serum lipids and hs-CRP levels and oxidative stress in adult patients with hyperlipidemia: randomized, double-blind, placebo-controlled clinical trial. Evid Based Complement Alternat Med 2014;2014:217451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu Y, Ling W, Guo H, Song F, Ye Q, Zou T, Li D, Zhang Y, Li G, Xiao Y, et al. Anti-inflammatory effect of purified dietary anthocyanin in adults with hypercholesterolemia: a randomized controlled trial. Nutr Metab Cardiovasc Dis 2013;23:843–9. [DOI] [PubMed] [Google Scholar]
- 42.Zhang PW, Chen FX, Li D, Ling WH, Guo HH. A CONSORT-compliant, randomized, double-blind, placebo-controlled pilot trial of purified anthocyanin in patients with nonalcoholic fatty liver disease. Medicine 2015;94:e758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Asgary S, Sahebkar A, Afshani MR, Keshvari M, Haghjooyjavanmard S, Rafieian-Kopaei M. Clinical evaluation of blood pressure lowering, endothelial function improving, hypolipidemic and anti-inflammatory effects of pomegranate juice in hypertensive subjects. Phytother Res 2014;28:193–9. [DOI] [PubMed] [Google Scholar]
- 44.Erlund I, Koli R, Alfthan G, Marniemi J, Puukka P, Mustonen P, Mattila P, Jula A. Favorable effects of berry consumption on platelet function, blood pressure, and HDL cholesterol. Am J Clin Nutr 2008;87:323–31. [DOI] [PubMed] [Google Scholar]
- 45.Sumner MD, Elliott-Eller M, Weidner G, Daubenmier JJ, Chew MH, Marlin R, Raisin CJ, Ornish D. Effects of pomegranate juice consumption on myocardial perfusion in patients with coronary heart disease. Am J Cardiol 2005;96:810–4. [DOI] [PubMed] [Google Scholar]
- 46.Karlsen A, Retterstøl L, Laake P, Paur I, Bøhn SK, Sandvik L, Blomhoff R. Anthocyanins inhibit nuclear factor-kappaB activation in monocytes and reduce plasma concentrations of pro-inflammatory mediators in healthy adults. J Nutr 2007;137:1951–4. [DOI] [PubMed] [Google Scholar]
- 47.Duthie SJ, Jenkinson AM, Crozier A, Mullen W, Pirie L, Kyle J, Yap LS, Christen P, Duthie GG. The effects of cranberry juice consumption on antioxidant status and biomarkers relating to heart disease and cancer in healthy human volunteers. Eur J Nutr 2006;45:113–22. [DOI] [PubMed] [Google Scholar]
- 48.Murkovic M, Abuja PM, Bergmann AR, Zirngast A, Adam U, Winklhofer-Roob BM, Toplak H. Effects of elderberry juice on fasting and postprandial serum lipids and low-density lipoprotein oxidation in healthy volunteers: a randomized, double-blind, placebo-controlled study. Eur J Clin Nutr 2004;58:244–9. [DOI] [PubMed] [Google Scholar]
- 49.Novotny JA, Baer DJ, Khoo C, Gebauer SK, Charron CS. Cranberry juice consumption lowers markers of cardiometabolic risk, including blood pressure and circulating C-reactive protein, triglyceride, and glucose concentrations in adults. J Nutr 2015;145:1185–93. [DOI] [PubMed] [Google Scholar]
- 50.Lynn A, Mathew S, Moore CT, Russell J, Robinson E, Soumpasi V, Barker ME. Effect of a tart cherry juice supplement on arterial stiffness and inflammation in healthy adults: a randomised controlled trial. Plant Foods Hum Nutr 2014;69:122–7. [DOI] [PubMed] [Google Scholar]
- 51.Zhu Y, Xia M, Yang Y, Liu F, Li Z, Hao Y, Mi M, Jin T, Ling W. Purified anthocyanin supplementation improves endothelial function via NO-cGMP activation in hypercholesterolemic individuals. Clin Chem 2011;57:1524–33. [DOI] [PubMed] [Google Scholar]
- 52.Grace MH, Massey AR, Mbeunkui F, Yousef GG, Lila MA. Comparison of health-relevant flavonoids in commonly consumed cranberry products. J Food Sci 2012;77:H176–83. [DOI] [PubMed] [Google Scholar]
- 53.He B, Zhang LL, Yue XY, Liang J, Jiang J, Gao XL, Yue PX. Optimization of ultrasound-assisted extraction of phenolic compounds and anthocyanins from blueberry (Vaccinium ashei) wine pomace. Food Chem 2016;204:70–6. [DOI] [PubMed] [Google Scholar]
- 54.Vagiri M, Jensen M. Influence of juice processing factors on quality of black chokeberry pomace as a future resource for colour extraction. Food Chem 2017;217:409–17. [DOI] [PubMed] [Google Scholar]
- 55.Ley SH, Hamdy O, Mohan V, Hu FB. Prevention and management of type 2 diabetes: dietary components and nutritional strategies. Lancet 2014;383:1999–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Diabetes Prevention Program Research Group. HbA1c as a predictor of diabetes and as an outcome in the diabetes prevention program: a randomized clinical trial. Diabetes Care 2015;38:51–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Selvin E, Steffes MW, Zhu H, Matsushita K, Wagenknecht L, Pankow J, Coresh J, Brancati FL. Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N Engl J Med 2010;362:800–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brutsaert EF, Shitole S, Biggs ML, Mukamal KJ, deBoer IH, Thacker EL, Barzilay JI, Djousse L, Ix JH, Smith NL, et al. Relations of postload and fasting glucose with incident cardiovascular disease and mortality late in life: the cardiovascular health study. J Gerontol A Biol Sci Med Sci 2016;71:370–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sasaki R, Nishimura N, Hoshino H, Isa Y, Kadowaki M, Ichi T, Tanaka A, Nishiumi S, Fukuda I, Ashida H, et al. Cyanidin 3-glucoside ameliorates hyperglycemia and insulin sensitivity due to downregulation of retinol binding protein 4 expression in diabetic mice. Biochem Pharmacol 2007;74:1619–27. [DOI] [PubMed] [Google Scholar]
- 60.Khodabandehloo H, Gorgani-Firuzjaee S, Panahi G, Meshkani R. Molecular and cellular mechanisms linking inflammation to insulin resistance and beta-cell dysfunction. Transl Res 2016;167:228–56. [DOI] [PubMed] [Google Scholar]
- 61.Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006;444:840–6. [DOI] [PubMed] [Google Scholar]
- 62.Skinner AC, Perrin EM, Moss LA, Skelton JA. Cardiometabolic risks and severity of obesity in children and young adults. N Engl J Med 2015;373:1307–17. [DOI] [PubMed] [Google Scholar]
- 63.Li W, Katzmarzyk PT, Horswell R, Zhang Y, Zhao W, Wang Y, Johnson J, Hu G. Body mass index and stroke risk among patients with type 2 diabetes mellitus. Stroke 2015;46:164–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tirosh A, Shai I, Afek A, Dubnov-Raz G, Ayalon N, Gordon B, Derazne E, Tzur D, Shamis A, Vinker S, et al. Adolescent BMI trajectory and risk of diabetes versus coronary disease. N Engl J Med 2011;364:1315–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Daniele G, Eldor R, Merovci A, Clarke GD, Xiong J, Tripathy D, Taranova A, Abdul-Ghani M, DeFronzo RA. Chronic reduction of plasma free fatty acid improves mitochondrial function and whole-body insulin sensitivity in obese and type 2 diabetic individuals. Diabetes 2014;63:2812–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Defronzo RA. Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 2009;58:773–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Prior RL, Wu X, Gu L, Hager TJ, Hager A, Howard LR. Whole berries versus berry anthocyanins: interactions with dietary fat levels in the C57BL/6J mouse model of obesity. J Agric Food Chem 2008;56:647–53. [DOI] [PubMed] [Google Scholar]
- 68.Edirisinghe I, Burton-Freeman B, Seeram NP, Shukkitt-Hale B. Anti-diabetic actions of berry polyphenols – review on proposed mechanisms of action. J Berry Res 2016;6:237–50. [Google Scholar]
- 69.DeFuria J, Bennett G, Strissel KJ, Perfield JW, Milbury PE, Greenberg AS, Obin MS. Dietary blueberry attenuates whole-body insulin resistance in high fat-fed mice by reducing adipocyte death and its inflammatory sequelae. J Nutr 2009;139:1510–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Speciale A, Canali R, Chirafisi J, Saija A, Virgili F, Cimino F. Cyanidin-3-O-glucoside protection against TNF-alpha-induced endothelial dysfunction: involvement of nuclear factor-kappaB signaling. J Agric Food Chem 2010;58:12048–54. [DOI] [PubMed] [Google Scholar]