This meta-analysis asssesses whether the risk of cardiovasular disease is increased among patients with type 2 diabetes and diabetic macular edema or proliferative diabetic retinopathy.
Key Points
Question
Do patients with type 2 diabetes and diabetic macular edema or proliferative diabetic retinopathy have an increased risk of cardiovascular disease?
Findings
In this systematic review and meta-analysis of 7604 participants with type 2 diabetes, patients with diabetic macular edema or proliferative diabetic retinopathy had a higher risk of incident cardiovascular disease and fatal cardiovascular disease compared with patients without diabetic macular edema or proliferative diabetic retinopathy.
Meaning
Persons with diabetic macular edema or proliferative diabetic retinopathy have an increased risk of incident cardiovascular disease, and thus these individuals should be followed up more actively to prevent cardiovascular disease.
Abstract
Importance
Previous studies on the relationship between diabetic retinopathy (DR) and cardiovascular disease (CVD) focused on the early stages of DR. Understanding whether patients with type 2 diabetes and severe stages of DR (diabetic macular edema [DME] and proliferative diabetic retinopathy [PDR]) have a higher risk of CVD will allow physicians to more effectively counsel patients.
Objective
To examine the association of severe stages of DR (DME and PDR) with incident CVD in patients with type 2 diabetes.
Data Sources
English-language publications were reviewed for articles evaluating the relationship of DR and CVD in MEDLINE, EMBASE, Current Contents, and the Cochrane Library from inception (January 1, 1950) to December 31, 2014, using the search terms diabetic retinopathy OR macular edema AND stroke OR cerebrovascular disease OR coronary artery disease OR heart failure OR myocardial infarction OR angina pectoris OR acute coronary syndrome OR coronary artery disease OR cardiomyopathy.
Study Selection
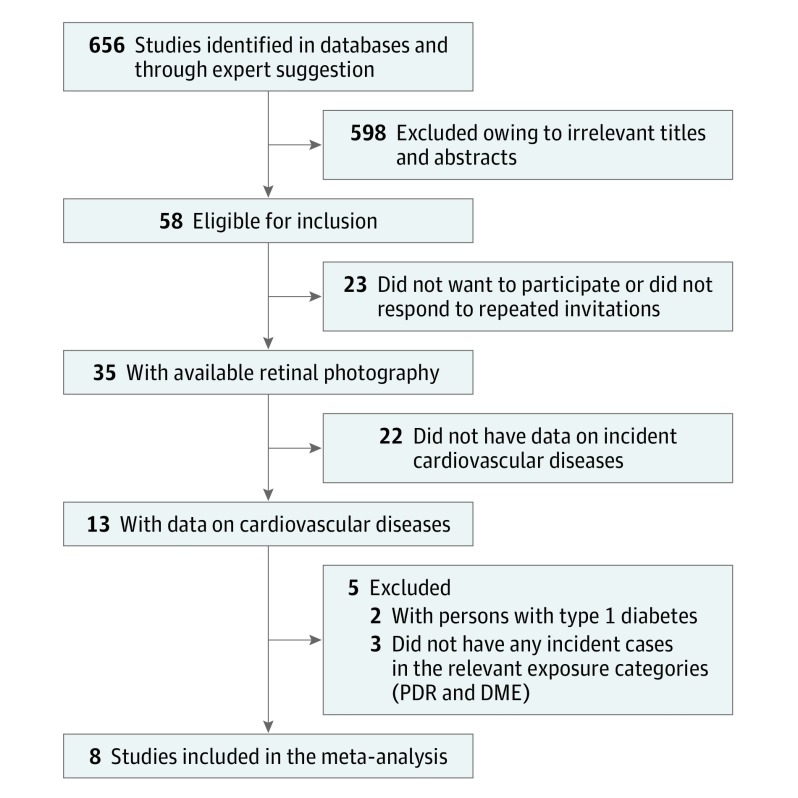
Among 656 studies screened for eligibility, 7604 individuals were included from 8 prospective population-based studies with data on photographic-based DR grading, follow-up visits, and well-defined incident CVD end point.
Data Extraction and Synthesis
Two independent reviewers conducted a systematic search of the 4 databases, and a single pooled database was developed. Incidence rate ratios (IRRs) were estimated for patients with DME, PDR, and vision-threatening DR, compared with persons without these conditions, by using individual participant data followed by a standard inverse-variance meta-analysis (2-step analysis). The review and analyses were performed from January 1, 2009, to January 1, 2017.
Main Outcome and Measures
Incident CVD, including coronary heart disease, stroke, or death from cardiovascular causes.
Results
Among 7604 patients with type 2 diabetes, the prevalence of DME was 4.6% and PDR, 7.4%. After a mean follow-up of 5.9 years (range, 3.2-10.1 years), 1203 incident CVD events, including 916 coronary heart disease cases, were reported. Persons with DME or PDR were more likely to have incident CVD (IRR, 1.39; 95% CI, 1.16-1.67) and fatal CVD (IRR, 2.33; 95% CI, 1.49-3.67) compared with those without DME or PDR.
Conclusions and Relevance
Patients with type 2 diabetes and DME or PDR have an increased risk of incident CVD, which suggests that these persons should be followed up more closely to prevent CVD.
Introduction
Diabetic retinopathy (DR) is a frequent microvascular complication of diabetes. In the past few decades, studies have shown that presence of any DR is associated with a range of cardiovascular diseases (CVDs), such as stroke, coronary heart disease (CHD), and myocardial infarction, and CVD-associated mortality. The Atherosclerosis Risk in Communities study reported a 3-fold higher risk (hazard ratio [HR], 3.35; 95% CI, 1.40-8.01) of fatal CHD events among persons with any DR, whereas the Wisconsin Epidemiologic Study on Diabetic Retinopathy revealed a nonsignificant increase in CHD-associated mortality among older persons with mild nonproliferative DR (HR, 1.21; 95% CI, 0.95-1.53) and proliferative DR (PDR) (HR, 1.43; 95% CI, 0.94-2.17). Evidence from other studies has been inconclusive, possibly because of differences in the exposures compared (eg, nonproliferative DR vs PDR), methods used (eg, fundus photography vs clinical ophthalmoscopy), and outcome definitions (eg, fatal CHD vs nonfatal stroke). However, these studies were focused mainly on the early stages of DR, because the prevalence of severe DR stages was relatively low in individual studies.
A meta-analysis showed that persons with any DR were twice as likely to have the composite outcome of all-cause mortality and/or cardiovascular events. That meta-analysis also reported that severe DR, defined as PDR or severe nonproliferative DR, was associated with a 4-fold higher risk of the composite mortality and CVD outcomes. Of importance, the meta-analysis did not include diabetic macular edema (DME), nor did it have the ability to adjust for confounding effects of cardiovascular risk factors in the analysis. Compared with any DR, understanding the specific relationship of the most severe stages (ie, DME and PDR) with incident CVD is clinically relevant because of the widespread use of anti–vascular endothelial growth factor (anti-VEGF) agents and other novel therapies for DME and PDR. In particular, some studies have found that anti-VEGF agents could increase the risk of CVD. Understanding whether patients with type 2 diabetes and DME or PDR have a higher risk of CVD will allow physicians to effectively counsel patients.
To address this critical gap, we conducted a systematic review and an individual participant meta-analysis to specifically determine the association of DME and PDR with CVD outcomes by using data from population-based cohort studies.
Methods
Data Sources and Searches and Study Selection
We conducted a systematic review of the literature. All prospective population-based cohort studies that reported associations between the presence of DR and incident CVD were considered for inclusion. Our overall search strategy targeted studies that satisfied the following 5 criteria: (1) evaluated any cardiovascular outcome by DR status, (2) followed up participants with diabetes recruited from the general population for at least 1 year, (3) included analysis of original data, (4) used retinal photography findings for the diagnosis, and (5) reported a risk estimate (eg, HR or relative risk [RR] adjusting for age, sex, and key potential confounders) relating preexisting DR to subsequent CVD by using survival analysis regression models.
Two independent reviewers (J.X. and M.K.I.) conducted a systematic search of 4 databases, including MEDLINE, EMBASE, Current Contents, and the Cochrane Library, from January 1, 1950, to December 31, 2014, for articles evaluating the relationship of DR and CVD. We used the following MeSH (Medical Subject Headings) search terms: diabetic retinopathy OR macular edema AND stroke OR cerebrovascular disease OR coronary artery disease OR heart failure OR myocardial infarction OR angina pectoris OR acute coronary syndrome OR coronary artery disease OR cardiomyopathy. No limits were placed on the year of publication, but we limited our search to English-language publications. The reference lists of included articles were also reviewed to identify other potentially relevant studies. Studies were systematically excluded if the titles and abstracts did not meet the 5 aforementioned inclusion criteria.
Data Extraction
We contacted principal authors of eligible studies and requested individual participant data regarding CVD, CHD, stroke, presence and severity of DR, DME status, age, sex, race/ethnicity, diabetes type and duration, hemoglobin A1c (HbA1c) level, systolic and diastolic blood pressure, lipid profile, smoking status, body mass index, and current use of diabetes, antihypertensive, and lipid-lowering medications. Data were checked for completeness and explanations for coding changes, and uncertain or missing data items were clarified with principal authors; a single pooled database was developed. The review and analyses were performed from January 1, 2009, to January 1, 2017. All studies had institutional review board approval and provided appropriately deidentified data for individual-level meta-analysis.
Definition of Exposures
In all included studies, diabetes was defined as a self-reported physician diagnosis of diabetes in combination with use of oral hypoglycemic medication or insulin. In addition, in each of the 8 studies, undiagnosed diabetes was determined by one of the following blood glucose measurements: a fasting glucose level of 126 mg/dL or higher (to convert to millimoles per liter, multiply by 0.0555), a non-fasting glucose level of 199.8 mg/dL or higher, or an HbA1c level of 7.0% or higher. Not all studies reported information on diabetes type. In those studies, if data on age at diagnosis of diabetes were available, participants were classified as having type 1 diabetes if they were diagnosed before 30 years of age and as having type 2 diabetes if they were diagnosed with diabetes after 30 years of age. In the present study, the analyses were limited to persons with type 2 diabetes.
Included studies graded DR on the basis of fundus photography findings using the Early Treatment Diabetic Retinopathy Scale (ETDRS), as described previously. Diabetic retinopathy severity was categorized as nonproliferative DR (ETDRS score, 20-53) or PDR (ETDRS score, ≥60). DME was also defined using fundus photography findings as hard exudates in the presence of microaneurysms and blot hemorrhage within 1 disc diameter from the foveal center or the presence of focal photocoagulation scars in the macular area. The primary exposures for the present study (DME and PDR) were based on the severity in the worse eye or in the single eye that was photographed. In addition, a combined end point was analyzed: vision-threatening DR (VTDR), defined as the presence of DME and/or PDR.
Cardiovascular Outcomes
In each study, information on incident cardiovascular outcomes was obtained through follow-up interviews, identifying hospitalizations and deaths during follow-up, and reviewing local hospital discharge lists and death certificates from state statistics offices.
Coronary heart disease was defined as evidence of a first physician-diagnosed myocardial infarction, coronary artery bypass graft surgery, or percutaneous transluminal coronary angioplasty abstracted from medical records (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] codes 402, 410, 411, 412, 413, 414, 427, 428, 429, 429.2, 440, 518.4, 798, and 799).
Stroke was defined as a first cerebral ischemic (embolic or thrombotic) or hemorrhagic (subarachnoid or intracerebral) event with associated clinical features of sudden focal neurological deficits diagnosed by a physician and abstracted from medical records (ICD-9-CM codes 430-438). A case was also eligible if review of the medical record revealed diagnostic computed tomography or magnetic resonance imaging with cerebrovascular findings.
Coronary vascular disease was defined as a diagnosis of CHD or stroke. Furthermore, we distinguished studies that reported nonfatal, fatal, or any (either fatal or nonfatal) CHD, stroke, or CVD outcomes. The following 4 outcomes were used for the present meta-analysis: (1) first CVD event, (2) fatal CVD, (3) first CHD event, and (4) fatal CHD.
Data Synthesis and Analysis
Funnel plots were constructed to assess potential publication bias in our meta-analysis. Multiple regression models with Poisson distribution and robust error variance were used to estimate incidence rate ratios (IRRs) among patients with DME, PDR, and VTDR compared with persons without these conditions by using individual participant data followed by a standard inverse-variance meta-analysis (2-step analysis). This method has advantages when analyzing rare events in individual participant data meta-analyses, particularly when studies have different follow-up times. Because of the small numbers of patients with stroke, this outcome was not analyzed as an individual outcome but instead was included only in the analysis of first-ever and fatal CVD. Because the Blue Mountains Eye Study and the Cardiovascular Health Study had shorter diabetes duration and shorter follow-up time, we performed a sensitivity analysis by excluding these 2 studies. We used the Cochran Q test to assess between-study differences and the I2 statistic to quantify the proportion of observed inconsistency across study results not explained by chance. Furthermore, a Galbraith plot was constructed to assess heterogeneity. A fixed-effect meta-analysis was used if there was no statistical heterogeneity in the true effects underlying the studies; otherwise, a random-effect meta-analysis was used. Furthermore, forest plots were constructed. Studies that did not have data on all required confounders were excluded from subsequent multivariable analyses. All statistical analyses were performed using Stata, version 14.1 (StataCorp). A 2-tailed P < .05 was considered as statistically significant.
Results
Study Characteristics
A total of 656 titles and abstracts were screened for eligibility. After exclusion of studies on the basis of irrelevant titles and abstracts or failure to meet inclusion criteria (Figure), 8 population-based studies including a total of 7604 patients with type 2 diabetes were included in our meta-analysis. Table 1 gives the baseline characteristics of participants with type 2 diabetes from the 8 studies. Table 2 presents the number of prevalent DME, PDR, and VTDR cases and incident CVD outcomes in each study. After a mean follow-up time of 5.9 years (range 3.2-10.1 years) across studies, there was a total of 1203 incident cases of first CVD events (with 286 fatal cases), including 916 CHD cases (with 242 fatal cases).
Figure. Flow Diagram of Selection of Studies for Inclusion in the Meta-analysis.
DME indicates diabetic macular edema; PDR, proliferative diabetic retinopathy.
Table 1. Characteristics of Patients With Type 2 Diabetes From the 8 Studies Included in the Meta-analysisa.
| Characteristic | Study | |||||||
|---|---|---|---|---|---|---|---|---|
| ARIC14 | AusDiab15 | BDES16 | BMES17 | CHS18 | LALES19 | MESA20 | WESDR21 | |
| Country | United States | Australia | United States | Australia | United States | United States | United States | United States |
| Race/ethnicity | 65 EU, 35 AA | 95 EU, 5 AS | 100 EU | 97 EU | 75 EU, 25 AA | 100 HI | 22 EU, 14 AS, 35 AA, 29 HI | 99 EU, 1 AA |
| Year(s) of DR grading | 1993-1995 | 1999-2000 | 1988-1990 | 1992-1994 | 1997-1998 | 2000-2003 | 2002-2004 | 1980-1982 |
| Sample size, No. | 1843 | 821 | 446 | 281 | 405 | 1234 | 794 | 1780 |
| Age, mean (SD), y | 60.8 (5.7) | 63.4 (12.2) | 65.9 (10.0) | 67.9 (8.7) | 78.0 (4.3) | 58.7 (10.4) | 65.8 (9.2) | 66.6 (11.3) |
| Males, No. (%) | 890 (48.3) | 420 (51.2) | 197 (44.2) | 149 (53.0) | 186 (45.9) | 545 (44.2) | 414 (52.1) | 801 (45.0) |
| Follow-up time, median (25th to 75th percentile), y | 6.0 (3.0-8.0) | 5.0 (2.0-11.0) | 6.0 (2.0-15.0) | 3.0 (0.1-10.0) | 8.0 (3.0-15.0) | 7.0 (3.0-14.0) | 6.0 (4.0-12.0) | 9.9 (4.8-18.1) |
| Current smoking, No. (%) | 290 (15.7) | 99 (12.1) | 61 (13.7) | 37 (13.2) | 25 (6.2) | 152 (12.3) | 83 (10.5) | 195 (11.0) |
| SBP, mean (SD), mm Hg | 130.1 (19.5) | 144.5 (19.7) | 138.5 (21.1) | 151.6 (23.8) | 134.7 (20.0) | 130.1 (20.0) | 130.6 (21.7) | 147.4 (24.0) |
| Received BP treatment, No. (%) | 949 (51.5) | 349 (42.5) | 242 (54.3) | 123 (43.8) | 308 (76.0) | NA | 542 (68.3) | 624 (35.1) |
| BMI, mean (SD) | 31.7 (6.1) | 30.1 (6.1) | 31.4 (6.2) | 28.0 (5.2) | 29.1 (4.9) | 32.1 (6.2) | 30.6 (6.1) | 28.7 (5.6) |
| Total cholesterol level, mean (SD), mg/dL | 208.8 (42.2) | 218.9 (39.8) | 235.1 (46.0) | 222.7 (44.9) | 193.3 (39.4) | NA | 182.1 (36.3) | NA |
| Received lipid treatment, No. (%) | 274 (14.9) | 209 (25.5) | 22 (4.9) | 15 (5.3) | 82 (20.3) | NA | 283 (35.6) | NA |
| HbA1c level, median (25th to 75th percentile), % | 6.8 (6.0-8.4) | 6.1 (5.6-7.1) | 9.1 (7.0-11.5) | NA | 7.5 (6.3-9.4) | 8.1 (7.0-9.9) | 6.8 (6.2-7.7) | 9.5 (8.1-10.9)b |
| Diabetes duration, median (25th to 75th percentile), y | 6.0 (3.0-8.0) | 5.0 (2.0-11.0) | 6.0 (2.0-15.0) | 3.0 (0.1-10.0) | 8.0 (3.0-15.0) | 7.0 (3.0-14.0) | 6.0 (4.0-12.0) | 9.9 (4.8-18.1) |
| Received diabetes treatment, No. (%) | 945 (51.3) | 276 (33.6) | 275 (61.7) | 144 (51.3) | 288 (71.1) | 897 (72.7) | 534 (67.3) | 1141 (64.1) |
Abbreviations: AA, African-American; ARIC, Atherosclerosis Risk in Communities; AS, Asian; AusDiab, Australian Diabetes, Obesity, and Lifestyle Study; BDES, Beaver Dam Eye Study; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); BMES, Blue Mountains Eye Study; BP, blood pressure; CHS, Cardiovascular Health Study; DME, diabetic macular edema; DR, diabetic retinopathy; EU, white, European ancestry; HbA1c, hemoglobin A1c; HI, Hispanic; LALES, Los Angeles Latino Eye Study; MESA, Multi-Ethnic Study of Atherosclerosis; NA, not available; SBP, systolic blood pressure; WESDR, Wisconsin Epidemiologic Study of Diabetic Retinopathy.
SI conversion factor: to convert to millimoles per liter, multiply by 0.0259.
In all studies, the Early Treatment Diabetic Retinopathy Scale was used to grade DR and DME.
In WESDR, HbA1 level was measured instead of HbA1c level.
Table 2. Cases of Prevalent Severe DR and Incident Cardiovascular Outcomes Included in This Meta-analysis.
| DR Type or Outcome | Study, Participants, No. (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| ARIC | AusDiab | BDES | BMES | CHS | LALES | MESA | WESDR | |
| Type | ||||||||
| DME | 9 (0.5) | 11 (1.3) | 13 (2.9) | 12 (4.3) | 6 (1.5) | 119 (9.6) | 48 (6.1) | 136 (7.6) |
| PDR | 313 (17.0) | 7 (0.9) | 14 (3.1) | 4 (3.1) | 8 (2.0) | NA | 8 (1.0) | 115 (6.5) |
| VTDR | 321 (17.4) | 14 (1.7) | 24 (5.4) | 15 (5.3) | 10 (2.5) | 119 (9.6) | 50 (6.3) | 198 (11.2) |
| Outcome | ||||||||
| First CVD | 379 (20.6) | 63 (7.7) | 147 (33.0) | 62 (22.1) | 133 (33.8) | 87 (4.7) | 37 (4.6) | 295 (16.6) |
| Fatal CVD | 29 (1.6) | 31 (3.8) | 82 (18.4) | 49 (17.4) | 90 (22.2) | NA | 5 (0.6) | NA |
| First CHD | 287 (15.6) | 55 (6.7) | 125 (28.0) | 48 (17.1) | 99 (24.4) | 59 (4.8) | 33 (4.2) | 210 (11.8) |
| Fatal CHD | 29 (1.6) | 25 (3.1) | 70 (15.7) | 39 (13.9) | 75 (18.5) | NA | 4 (0.5) | NA |
Abbreviations: ARIC, Atherosclerosis Risk in Communities; AusDiab, Australian Diabetes, Obesity, and Lifestyle Study; BDES, Beaver Dam Eye Study; BMES, Blue Mountains Eye Study; CHD, coronary heart disease; CHS, Cardiovascular Health Study; CVD, cardiovascular disease; DME, diabetic macular edema; DR, diabetic retinopathy; LALES, Los Angeles Latino Eye Study; MESA, Multi-Ethnic Study of Atherosclerosis Study; NA, not applicable; PDR, proliferative diabetic retinopathy; VTDR, vision-threatening diabetic retinopathy; WESDR, Wisconsin Epidemiologic Study of Diabetic Retinopathy.
Meta-analysis
Funnel plot results for potential publication bias are shown in eFigure 1 in the Supplement. The Gilbraith plot showed no significant heterogeneity in this meta-analysis (eFigure 2 in the Supplement). Both DME and PDR were related to an increased risk of first-ever CVD (IRR for DME, 1.65 [95% CI, 1.24-2.19]; IRR for PDR, 1.28 [95% CI, 1.03-1.58]) and fatal CVD (IRR for DME, 2.85 [95% CI, 1.43-5.68]; IRR for PDR, 1.85 [95% CI, 1.04-3.28]) (Table 3 eFigure 3 in the Supplement). These associations were consistent after multivariable adjustment for vascular risk factors, including smoking, systolic blood pressure, use of hypertension medication, total cholesterol level, and body mass index. When duration of diabetes, use of any treatment for diabetes, and glycated HbA1c level were included in the multivariable model, the association between the presence of VTDR (HR, 2.20; 95% CI, 1.10-4.39) and fatal CVD remained significant.
Table 3. Summary of the Meta-analysis for Cardiovascular Outcomes in Persons With Type 2 Diabetes.
| Outcome, DR Type | Model 1a | Model 2b | Model 3c | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Studies, No. | Participants, No. | Events, No. | IRR (95% CI) | Studies, No. | Participants, No. | Events, No. | IRR (95% CI) | Studies, No. | Participants, No. | Events, No. | IRR (95% CI) | |
| First CVD | ||||||||||||
| DME | 7 | 5553 | 910 | 1.65 (1.24-2.19) | 6 | 4662 | 810 | 1.46 (1.04-2.05) | 4 | 2285 | 427 | 1.48 (0.76-2.87) |
| PDR | 7 | 5514 | 1016 | 1.28 (1.03-1.58) | 4 | 3106 | 604 | 1.29 (1.00-1.66) | 3 | 2306 | 430 | 1.21 (0.85-1.72) |
| VTDR | 8 | 6343 | 1100 | 1.39 (1.16-1.67) | 5 | 3872 | 635 | 1.34 (1.07-1.69) | 4 | 2396 | 451 | 1.26 (0.92-1.73) |
| Fatal CVD | ||||||||||||
| DME | 5 | 3719 | 176 | 2.85 (1.43-5.68) | 5 | 3603 | 162 | 3.03 (1.43-6.43) | 4 | 2278 | 101 | 3.53 (1.35-9.25) |
| PDR | 5 | 3552 | 14 | 1.85 (1.04-3.28) | 4 | 3106 | 164 | 2.05 (1.03-4.07) | 3 | 2036 | 99 | 1.50 (0.56-4.03) |
| VTDR | 6 | 4309 | 258 | 2.33 (1.49-3.67) | 5 | 4012 | 185 | 2.40 (1.44-4.02) | 4 | 2389 | 101 | 2.20 (1.10-4.39) |
| First CHD | ||||||||||||
| DME | 7 | 4950 | 740 | 1.57 (1.16-2.14) | 5 | 3426 | 468 | 1.80 (1.00-3.26) | 4 | 2156 | 359 | 1.45 (0.73-2.88) |
| PDR | 6 | 4580 | 743 | 1.39 (1.09-1.76) | 4 | 2884 | 477 | 1.36 (1.02-1.81) | 3 | 1898 | 357 | 1.20 (0.82-1.76) |
| VTDR | 7 | 5288 | 802 | 1.47 (1.20-1.79) | 5 | 3648 | 504 | 1.44 (1.11-1.87) | 4 | 2256 | 375 | 1.32 (0.94-1.85) |
| Fatal CHD | ||||||||||||
| DME | 5 | 3519 | 150 | 3.55 (1.76-7.15) | 5 | 3411 | 136 | 3.80 (1.77-8.18) | 3 | 1810 | 89 | 3.77 (1.39-10.21) |
| PDR | 5 | 3287 | 208 | 2.11 (1.19-3.76) | 4 | 2884 | 139 | 2.33 (1.17-4.66) | 3 | 1898 | 89 | 1.70 (0.63-4.60) |
| VTDR | 6 | 4076 | 218 | 2.74 (1.74-4.32) | 5 | 3633 | 142 | 2.81 (1.67-4.74) | 3 | 1898 | 89 | 2.47 (1.21-5.06) |
Abbreviations: CVD, cardiovascular disease; CHD, coronary heart disease; DME, diabetic macular edema; DR, diabetic retinopathy; IRR, incidence rate ratio; PDR, proliferative diabetic retinopathy; VTDR, vision-threatening diabetic retinopathy.
Model 1: adjusted for age, sex, and race/ethnicity.
Model 2: model 1 additionally adjusted for current smoking, systolic blood pressure, use of hypertension medication, total cholesterol level, and body mass index.
Model 3: model 2 additionally adjusted for duration of diabetes, use of treatment for diabetes, and hemoglobin A1c level.
With respect to CHD, DME and PDR were related to an increased risk of first-ever CHD (IRR for DME, 1.57 [95% CI, 1.16-2.14]; IRR for PDR, 1.39 [95% CI, 1.09-1.76]) and fatal CHD (IRR for DME, 3.55 [95% CI, 1.76-7.15]; IRR for PDR, 2.11 [95% CI, 1.19-3.76]) (Table 3). These associations were consistent after multivariable adjustment for vascular risk factors, including smoking, systolic blood pressure, use of hypertension medication, total cholesterol level, and body mass index. When duration of diabetes, use of treatment for diabetes, and glycosylated hemoglobin level were included in the multivariable model, the association between the presence of VTDR (HR, 2.47; 95% CI, 1.21-5.06) and fatal CHD remained significant.
Finally, in an analysis excluding the Blue Mountains Eye Study and the Cardiovascular Health Study (Table 4), results were similar to our main analysis.
Table 4. Summary of the Meta-analysis for Cardiovascular Outcomes in Persons With Type 2 Diabetes After Excluding the CHS and BMESa.
| Outcome, DR Type | Model 1b | Model 2c | Model 3d | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Studies, No. | Participants, No. | Events, No. | IRR (95% CI) | Studies, No. | Participants, No. | Events, No. | IRR (95% CI) | Studies, No. | Participants, No. | Events, No. | IRR (95% CI) | |
| First CVD | ||||||||||||
| DME | 6 | 5316 | 852 | 1.63 (1.21-2.19) | 5 | 4404 | 752 | 1.47 (1.04-2.10) | 4 | 2285 | 427 | 1.48 (0.76-2.87) |
| PDR | 5 | 4983 | 861 | 1.27 (1.02-1.59) | 3 | 2863 | 553 | 1.27 (1.00-1.64) | 3 | 2306 | 430 | 1.21 (0.85-1.72) |
| VTDR | 6 | 5779 | 939 | 1.38 (1.15-1.66) | 4 | 3628 | 584 | 1.33 (1.05-1.69) | 4 | 2396 | 451 | 1.26 (0.92-1.73) |
| Fatal CVD | ||||||||||||
| DME | 4 | 3541 | 129 | 3.42 (1.47-7.98) | 4 | 3359 | 123 | 3.86 (1.60-9.30) | 3 | 1937 | 99 | 3.45 (1.29-9.28) |
| PDR | 3 | 2982 | 133 | 1.87 (1.00-3.50) | 3 | 2863 | 125 | 1.80 (0.87-3.70) | 3 | 2036 | 99 | 1.50 (0.56-4.03) |
| VTDR | 4 | 3739 | 137 | 2.47 (1.47-4.13) | 4 | 3739 | 137 | 2.40 (1.36-4.24) | 4 | 2389 | 101 | 2.20 (1.10-4.39) |
| First CHD | ||||||||||||
| DME | 6 | 4682 | 693 | 1.50 (1.09-2.07) | 4 | 3182 | 431 | 1.87 (0.98-3.60) | 4 | 2156 | 359 | 1.45 (0.73-2.88) |
| PDR | 5 | 4313 | 696 | 1.37 (1.08-1.74) | 3 | 2641 | 440 | 1.33 (1.00-1.77) | 3 | 1898 | 132 | 1.20 (0.82-1.76) |
| VTDR | 6 | 5020 | 544 | 1.41 (1.15-1.73) | 4 | 3404 | 467 | 1.41 (1.08-1.84) | 4 | 2256 | 375 | 1.32 (0.94-1.85) |
| Fatal CHD | ||||||||||||
| DME | 4 | 3277 | 113 | 4.22 (1.78-9.98) | 4 | 3167 | 107 | 4.72 (1.93-11.55) | 3 | 1810 | 89 | 3.77 (1.39-10.21) |
| PDR | 3 | 2724 | 117 | 2.07 (1.10-3.88) | 3 | 2531 | 110 | 1.98 (0.96-4.10) | 3 | 1898 | 89 | 1.70 (0.63-4.60) |
| VTDR | 4 | 3480 | 120 | 2.81 (1.67-4.74) | 4 | 3389 | 113 | 2.72 (1.53-4.83) | 3 | 1898 | 89 | 2.47 (1.21-5.06) |
Abbreviations: BMES, Blue Mountains Eye Study; CHS, Cardiovascular Health Study; CHD, coronary heart disease; CVD, cardiovascular disease; DME, diabetic macular edema; DR, diabetic retinopathy; IRR. incidence rate ratio; PDR, proliferative diabetic retinopathy; VTDR, vision-threatening diabetic retinopathy.
Because BMES and CHS had shorter durations of diabetes and shorter follow-up, we performed a sensitivity analysis by excluding the 2 studies.
Model 1: adjusted for age, sex, and race/ethnicity.
Model 2: model 1 additionally adjusted for current smoking, systolic blood pressure, use of hypertension medication, total cholesterol level, and body mass index.
Model 3: model 2 additionally adjusted for duration of diabetes, use of treatment for diabetes, and hemoglobin A1c level.
Discussion
In this individual participant meta-analysis, we revealed that among patients with type 2 diabetes, the presence of DME or PDR was associated with an increased risk of incident CVD, including CHD, compared with those without these conditions. These associations persisted after adjusting for other cardiovascular risk factors.
Diabetic macular edema and PDR are distinct constellations of retinal microvascular signs that reflect small-vessel disease caused by hyperglycemia. The presence of microvascular disease in the eye raises the possibility of more generalized microangiopathic processes affecting not only the eye but also other sites including the myocardium and brain. Furthermore, it has been suggested that in addition to macrovascular disease, as reflected by the effects of accelerated atherosclerosis and large-vessel occlusive disease, microvascular pathology plays an important role in these end organs. Our findings suggest that the presence of DME or PDR may be a marker of generalized microvascular disease, which may contribute to the development of CVD in persons with diabetes. In support of this hypothesis, histopathologic findings have shown that small-vessel disease present in the retina is also evident in the heart and brain of individuals with diabetes. In addition, evidence from histologic studies have shown that DR is associated with subclinical coronary microvascular pathologic findings, such as endothelial dysfunction, impaired coronary hypoxic-vasodilation, myocardial perfusion defects, and poorer coronary flow reserve. Cardiac failure has also been reported in diabetes, even in the absence of angiographically detectable coronary artery disease. These findings suggest a role for microvascular disease in the development of CHD in persons with type 2 diabetes. The pathophysiologic pathways underlying these observations are still uncertain, but several putative factors, such as oxidative stress, inflammation, endothelial dysfunction, and advanced glycation end products, have been reported.
Unlike previous studies, our findings address the specific relationship of the most severe clinically relevant stages of DR (ie, DME and PDR) with incident CVD. These data are important because anti-VEGF treatments for DME and possibly PDR are the current standard of care. A major concern with anti-VEGF agents is the potential increased risk of CVD secondary to long-term systemic suppression of VEGF. Interim analysis of the Safety Assessment of Intravitreal Lucentis for Age-Related Macular Degeneration trial revealed an increased risk of stroke in patients treated with 0.5 mg of intravitreal ranibizumab injection compared with patients treated with 0.3 mg (1.2% vs 0.3%; P = .02). Although subsequent analysis at completion of that trial showed no significant increase in stroke risk, it raised a concern about the safety of ranibizumab. Furthermore, the Comparison of Age-related Macular Degeneration Treatments Trials suggested that bevacizumab treatment may also result in a higher risk of serious adverse events among persons with age-related macular degeneration. This raises a concern that treatment with anti-VEGF for persons with VTDR, who already have a greater risk of fatal CHD and CVD events, could lead to an additional increase in risk of severe CVD events. However, because the studies included were conducted before the introduction of anti-VEGF therapy for DME and DR, data on whether anti-VEGF use modified the relationship are not available. Nevertheless, our study provides an assessment of the baseline risk of the relationship that will provide a foundation for future studies.
Strengths and Limitations
Strengths of our study include its comprehensive, systematic review of the literature using a broad search strategy to identify all relevant studies and the inclusion of primary data from high-quality studies. The availability of individual participant data provided by authors allowed for more robust calculation of the multivariable adjusted risk estimates. Our data collection enabled us to stratify risk estimates by specific fatal and nonfatal CVD end points and to provide adjustment for key potential confounders. Our study had several limitations. First, DME grading was based on solely fundus photography findings because no data were available from optical coherence tomography at baseline. This factor could have led to an underestimation of the number of patients with DME. Despite this underestimation, we found a significant association between DME and incident CVD. Second, we could not include all 8 studies in subsequent multivariable analyses (models 2 and 3 in Table 3 and eFigure 3 in the Supplement) because of the lack of data on key confounders in some studies. Therefore, a smaller sample size was available for the fully adjusted models, leading to wider CIs and loss of statistical significance. However, the point estimates remained stable. Third, because of the small number of patients with type 1 diabetes and incident stroke, we were not able to examine separately the association of type 1 diabetes with incident stroke. Finally, most of these studies were conducted at a time when the clinical management and standard of care of diabetes and DR might have been different. For example, tighter glucose and blood pressure control could be expected in the current management of type 2 diabetes. As already indicated, studies were conducted before the era of anti-VEGF therapy, and the impact of this on our results are unknown. However, because of the nature of our study, in which we required sufficient incident CVD and CHD cases over time, it was not possible to include newer studies because there would be too few incident CVD and CHD cases for analysis.
Conclusions
In conclusion, persons with type 2 diabetes and DME or PDR had an increased risk of incident CVD and CHD events compared with those with mild or no DR. These data suggest that persons with type 2 diabetes and more severe stages of DR should be followed up more actively by their physicians, with interventions as appropriate, to prevent CVD complications.
eFigure 1. Funnel plots showing the studies including the different meta-analyses
eFigure 2. Galbraith plot
eFigure 3. Forest plots for the association of diabetic macular edema, proliferative diabetic retinopathy, and of vision-threatening diabetic retinopathy with first-ever cardiovascular disease
References
- 1.Yau JW, Rogers SL, Kawasaki R, et al. ; Meta-analysis for Eye Disease (META-EYE) Study Group . Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35(3):556-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klein R, Klein BE, Moss SE. Epidemiology of proliferative diabetic retinopathy. Diabetes Care. 1992;15(12):1875-1891. [DOI] [PubMed] [Google Scholar]
- 3.Cheung N, Wong TY. Diabetic retinopathy and systemic vascular complications. Prog Retin Eye Res. 2008;27(2):161-176. [DOI] [PubMed] [Google Scholar]
- 4.Cheung N, Wang JJ, Klein R, Couper DJ, Sharrett AR, Wong TY. Diabetic retinopathy and the risk of coronary heart disease: the Atherosclerosis Risk in Communities Study. Diabetes Care. 2007;30(7):1742-1746. [DOI] [PubMed] [Google Scholar]
- 5.Klein R, Klein BE, Moss SE, Cruickshanks KJ. Association of ocular disease and mortality in a diabetic population. Arch Ophthalmol. 1999;117(11):1487-1495. [DOI] [PubMed] [Google Scholar]
- 6.Lip GY, Clementy N, Pierre B, Boyer M, Fauchier L. The impact of associated diabetic retinopathy on stroke and severe bleeding risk in diabetic patients with atrial fibrillation: the loire valley atrial fibrillation project. Chest. 2015;147(4):1103-1110. [DOI] [PubMed] [Google Scholar]
- 7.Hägg S, Thorn LM, Putaala J, et al. ; FinnDiane Study Group . Incidence of stroke according to presence of diabetic nephropathy and severe diabetic retinopathy in patients with type 1 diabetes. Diabetes Care. 2013;36(12):4140-4146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kramer CK, Rodrigues TC, Canani LH, Gross JL, Azevedo MJ. Diabetic retinopathy predicts all-cause mortality and cardiovascular events in both type 1 and 2 diabetes: meta-analysis of observational studies. Diabetes Care. 2011;34(5):1238-1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liew G, Mitchell P. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2007;356(7):747-748. [DOI] [PubMed] [Google Scholar]
- 10.Ciulla TA, Rosenfeld PJ. Antivascular endothelial growth factor therapy for neovascular age-related macular degeneration. Curr Opin Ophthalmol. 2009;20(3):158-165. [DOI] [PubMed] [Google Scholar]
- 11.Crowther MJ, Riley RD, Staessen JA, Wang J, Gueyffier F, Lambert PC. Individual patient data meta-analysis of survival data using Poisson regression models. BMC Med Res Methodol. 2012;12:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mitchell P, Smith W, Wang JJ, Attebo K. Prevalence of diabetic retinopathy in an older community: the Blue Mountains Eye Study. Ophthalmology. 1998;105(3):406-411. [DOI] [PubMed] [Google Scholar]
- 13.Carnethon MR, Biggs ML, Barzilay J, et al. . Diabetes and coronary heart disease as risk factors for mortality in older adults. Am J Med. 2010;123(6):556.e1-556.e9. doi: 10.1016/j.amjmed.2009.11.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The ARIC Investigators The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129(4):687-702. [PubMed] [Google Scholar]
- 15.Dunstan DW, Zimmet PZ, Welborn TA, et al. ; Australian Diabetes, Obesity and Lifestyle Study (AusDiab) . The Australian Diabetes, Obesity and Lifestyle Study (AusDiab)–methods and response rates. Diabetes Res Clin Pract. 2002;57(2):119-129. [DOI] [PubMed] [Google Scholar]
- 16.Klein R, Klein BEK, Linton KLP, De Mets DL. The Beaver Dam Eye Study: visual acuity. Ophthalmology. 1991;98(8):1310-1315. [DOI] [PubMed] [Google Scholar]
- 17.Attebo K, Mitchell P, Smith W. Visual acuity and the causes of visual loss in Australia: the Blue Mountains Eye Study. Ophthalmology. 1996;103(3):357-364. [DOI] [PubMed] [Google Scholar]
- 18.Fried LP, Borhani NO, Enright P, et al. . The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1(3):263-276. [DOI] [PubMed] [Google Scholar]
- 19.Varma R, Paz SH, Azen SP, et al. ; Los Angeles Latino Eye Study Group . The Los Angeles Latino Eye Study: design, methods, and baseline data. Ophthalmology. 2004;111(6):1121-1131. [DOI] [PubMed] [Google Scholar]
- 20.Bild DE, Detrano R, Peterson D, et al. . Ethnic differences in coronary calcification: the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation. 2005;111(10):1313-1320. [DOI] [PubMed] [Google Scholar]
- 21.Klein R, Klein BE, Moss SE, Cruickshanks KJ. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. XIV. Ten-year incidence and progression of diabetic retinopathy. Arch Ophthalmol. 1994;112(9):1217-1228. [DOI] [PubMed] [Google Scholar]
- 22.Ong YT, De Silva DA, Cheung CY, et al. . Microvascular structure and network in the retina of patients with ischemic stroke. Stroke. 2013;44(8):2121-2127. [DOI] [PubMed] [Google Scholar]
- 23.Karnik AA, Fields AV, Shannon RP. Diabetic cardiomyopathy. Curr Hypertens Rep. 2007;9(6):467-473. [DOI] [PubMed] [Google Scholar]
- 24.Brooks BA, Franjic B, Ban CR, et al. . Diastolic dysfunction and abnormalities of the microcirculation in type 2 diabetes. Diabetes Obes Metab. 2008;10(9):739-746. [DOI] [PubMed] [Google Scholar]
- 25.Akasaka T, Yoshida K, Hozumi T, et al. . Retinopathy identifies marked restriction of coronary flow reserve in patients with diabetes mellitus. J Am Coll Cardiol. 1997;30(4):935-941. [DOI] [PubMed] [Google Scholar]
- 26.Miura H, Wachtel RE, Loberiza FR Jr, et al. . Diabetes mellitus impairs vasodilation to hypoxia in human coronary arterioles: reduced activity of ATP-sensitive potassium channels. Circ Res. 2003;92(2):151-158. [DOI] [PubMed] [Google Scholar]
- 27.Faglia E, Favales F, Calia P, et al. ; Milan Study on Atherosclerosis and Diabetes (Mi SAD) . Cardiac events in 735 type 2 diabetic patients who underwent screening for unknown asymptomatic coronary heart disease: 5-year follow-up report from the Milan Study on Atherosclerosis and Diabetes (MiSAD). Diabetes Care. 2002;25(11):2032-2036. [DOI] [PubMed] [Google Scholar]
- 28.Yoon JK, Lee KH, Park JM, et al. . Usefulness of diabetic retinopathy as a marker of risk for thallium myocardial perfusion defects in non-insulin-dependent diabetes mellitus. Am J Cardiol. 2001;87(4):456-459, A6. [DOI] [PubMed] [Google Scholar]
- 29.Sari I, Soydinc S, Davutoglu V, Sezen Y, Aksoy M. Uncomplicated diabetes mellitus is equivalent for coronary artery disease: new support from novel angiographic myocardial perfusion-myocardial blush. Int J Cardiol. 2008;127(2):262-265. [DOI] [PubMed] [Google Scholar]
- 30.Yamagishi S, Matsui T, Ueda S, Nakamura K, Imaizumi T. Advanced glycation end products (AGEs) and cardiovascular disease (CVD) in diabetes. Cardiovasc Hematol Agents Med Chem. 2007;5(3):236-240. [DOI] [PubMed] [Google Scholar]
- 31.Takenaka K, Yamagishi S, Matsui T, Nakamura K, Imaizumi T. Role of advanced glycation end products (AGEs) in thrombogenic abnormalities in diabetes. Curr Neurovasc Res. 2006;3(1):73-77. [DOI] [PubMed] [Google Scholar]
- 32.Farhangkhoee H, Khan ZA, Kaur H, Xin X, Chen S, Chakrabarti S. Vascular endothelial dysfunction in diabetic cardiomyopathy: pathogenesis and potential treatment targets. Pharmacol Ther. 2006;111(2):384-399. [DOI] [PubMed] [Google Scholar]
- 33.Cooper ME. Importance of advanced glycation end products in diabetes-associated cardiovascular and renal disease. Am J Hypertens. 2004;17(12, pt 2):31S-38S. [DOI] [PubMed] [Google Scholar]
- 34.Cai L, Kang YJ. Oxidative stress and diabetic cardiomyopathy: a brief review. Cardiovasc Toxicol. 2001;1(3):181-193. [DOI] [PubMed] [Google Scholar]
- 35.Williams JG, Rincon-Skinner T, Sun D, et al. . Role of nitric oxide in the coupling of myocardial oxygen consumption and coronary vascular dynamics during pregnancy in the dog. Am J Physiol Heart Circ Physiol. 2007;293(4):H2479-H2486. [DOI] [PubMed] [Google Scholar]
- 36.Giugliano D, Ceriello A, Paolisso G. Oxidative stress and diabetic vascular complications. Diabetes Care. 1996;19(3):257-267. [DOI] [PubMed] [Google Scholar]
- 37.Dafer RM, Schneck M, Friberg TR, Jay WM. Intravitreal ranibizumab and bevacizumab: a review of risk. Semin Ophthalmol. 2007;22(3):201-204. [DOI] [PubMed] [Google Scholar]
- 38.Martin DF, Maguire MG, Fine SL, et al. ; Comparison of Age-related Macular Degeneration Treatments Trials (CATT) Research Group . Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology. 2012;119(7):1388-1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Funnel plots showing the studies including the different meta-analyses
eFigure 2. Galbraith plot
eFigure 3. Forest plots for the association of diabetic macular edema, proliferative diabetic retinopathy, and of vision-threatening diabetic retinopathy with first-ever cardiovascular disease