INTRODUCTION
The Trial Innovation Network, an initiative of the Clinical and Translational Science Award (CTSA) Program, is a national multidisciplinary platform that will enable multicenter clinical trials and studies. A key goal of the Trial Innovation Network is to increase the efficiency and effectiveness of clinical trials and studies by focusing on innovation, excellence, and collaboration; leveraging the strength and expertise of the CTSA Program; and fostering partnerships among the Institutes and Centers of the National Institutes of Health (NIH), researchers, participants, providers, industry, and other stakeholders.
Challenges in clinical trials
The road from understanding pathophysiology to treating a disease is long, slow, and expensive. It may take more than a decade to develop a basic discovery into a new treatment, with more than half of the time and resources spent testing new therapies in a clinical trials ecosystem that is often fragmented, redundant, and slowed by inefficiencies.1, 2
Typical avenues that currently are used to conduct clinical trials are marked by considerable delays and roadblocks. Trial start‐up may lag due to prolonged institutional review board (IRB) review and contract procedures.3, 4 Trial implementation may be slowed because investigators and funders often spend significant time and resources building new research infrastructure for individual studies with less focus on creating durable, interoperable, and sustainable research systems. Other challenges are caused by initiating studies before gathering input from key stakeholders on trial design or budgets and developing complex protocols that require a large number of procedures and extensive data collection. These lead to problems with recruitment and retention, the need for study amendments, protocol deviations, and a mismatch between study budgets and the actual costs of a trial, which are primarily driven by data collection, data management, and study administration.5 These inefficiencies culminate in fewer high‐quality trials, a decreased return on research investments, less data to inform clinical care, and most importantly, fewer evidence‐based treatments for patients. Other challenges to conducting clinical trials at academic medical centers are institutional bureaucracy and high costs. The scientific community, federal agencies, participants, providers, and other key stakeholders need to work together to modernize the roadways of clinical trials with disruptive new ideas.
Early vision and goals of the Trial Innovation Network
One such idea is to create a national platform for clinical trials—much like our national highway system, which connects people, ideas, and resources across our country. Similarly, a clinical trials “highway” could link stakeholders and infrastructure, systematically address roadblocks in clinical trials from start‐up to closeout, and serve as a catalyst to encourage researchers, funders, participants, and providers to join together in a true community of learning. A federally funded clinical trials platform would be a national resource that could have an extraordinary impact on public health and the future of clinical research. This is the vision for the Trial Innovation Network, a collaborative initiative of the CTSA Program launched in July 2016 that is supported by the National Center for Advancing Translational Sciences.
The long‐term goal is for the Trial Innovation Network to be a national multidisciplinary platform that will enable multicenter clinical trials and studies that answer clinical questions and inform clinical care. The mission of the Trial Innovation Network is to increase the efficiency and effectiveness of clinical trials and studies by focusing on operational innovation and operational excellence—that is, by inventing and deploying new ways of doing the work of clinical trials and by executing this work reliably, cost‐effectively, and within agreed upon timeframes. The Trial Innovation Network aims to achieve this mission by embedding innovation and critical evaluation into the clinical trials process, building quality into the design of protocols, aligning Network infrastructure with existing local institutional infrastructure, streamlining IRB and contracting processes, employing novel approaches to project management, and using data‐driven approaches to optimize clinical trials at every point—from protocol design to the publication of results. The Trial Innovation Network will function as a national laboratory to study and improve the process of multicenter clinical trials and studies, as a platform to test cutting‐edge innovations to enhance clinical trial operations, as a forerunner for new NIH policies and approaches to optimize clinical trials stewardship, and as a vanguard for innovative approaches to manage NIH clinical trial networks.
Key partners of the Trial Innovation Network
The Trial Innovation Network is composed of key collaborative components—the three Trial Innovation Centers, one Recruitment Innovation Center, and more than 60 CTSA Program Hubs (Figure 1; Table 1). The Trial Innovation Centers are charged with coordinating and providing innovative high‐quality operational support for clinical trials and studies and critically evaluating key elements of clinical trial operations, such as trial design, data collection, and Data Safety and Monitoring Board processes. The Trial Innovation Centers will also function as central IRBs for Trial Innovation Network trials and studies and will develop innovative approaches to implement the NIH single IRB policy. They will also collect metrics and data about the single IRB process and will work with key partners in the CTSA Program and other stakeholders to harmonize single IRB definitions, processes, and procedures.
Figure 1.
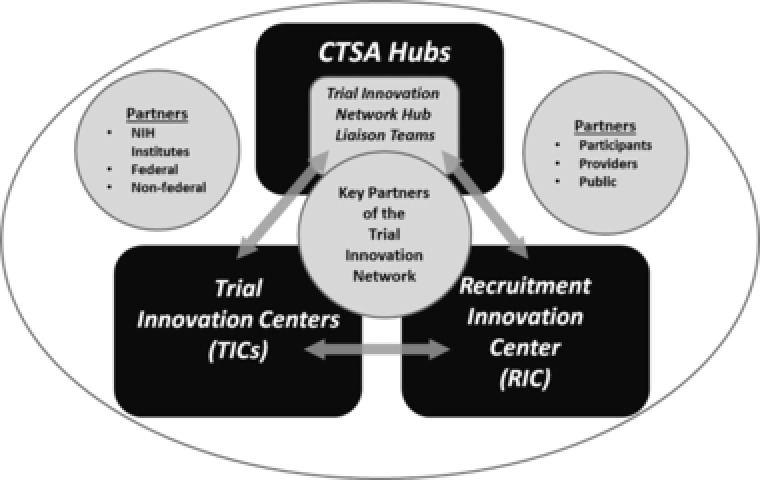
Key partners of the Trial Innovation Network. CTSA, Clinical and Translational Science Award; NIH, National Institutes of Health.
Table 1.
Trial Innovation Network—Roles and responsibilities of key partners
| Key partners of the Trial Innovation Network |
|---|
Trial innovation centers
|
Recruitment innovation center
|
CTSA Program hubs
|
CTSA, Clinical and Translational Science Award; IRB, institutional review board; RIC, Recruitment Innovation Center; TIC, Trial Innovation Center.
The Recruitment Innovation Center will provide tools and services to enhance participant engagement, recruitment, and retention and will focus on minority and underrepresented participant engagement and recruitment, optimizing the informed consent process, training Hub Liaison Teams on best practices to optimize recruitment, and leveraging the electronic health record, other existing databases, and novel technology to boost recruitment and retention. The CTSA Program hubs are the frontline of the Trial Innovation Network, and their Trial Innovation Network Hub Liaison Teams will operationalize the Network at the hub level, leveraging their knowledge of the local environment to efficiently and effectively conduct trials and studies.
Leveraging innovations from the CTSA Program
Key elements of the Trial Innovation Network will build on the established local and regional strengths at the CTSA Program hubs and groundbreaking work by CTSA Program investigators on the standard agreement template, standard IRB reliance tools, standards for good clinical practice training and scientific review, and tools to facilitate data‐driven recruitment and retention. The Trial Innovation Network will help disseminate these operational and translational innovations and will be a vanguard to implement and test current and future innovations from the CTSA Program and the scientific community. Examples of collaborative initiatives led by CTSA Program investigators that are being leveraged in the Trial Innovation Network include Accelerated Research Agreements (https://www.ara4us.org/); Streamlined Multi‐Site Accelerated Resource for Trials (SMART) IRB (https://smartirb.org/); CTSA Program consensus guidelines for training in good clinical practice (http://www.ctsa-gcp.org/about.html) and scientific review of clinical protocols; Accrual to Clinical Trials (https://www.act-network.org/); and ResearchMatch (https://www.researchmatch.org/).6, 7, 8, 9, 10
Key elements of the Trial Innovation Network
Key components of the Trial Innovation Network currently being organized include three harmonized central IRBs that will use the SMART IRB Authorization Agreement and a standard contract agreement and template to expedite contract negotiations and the development of study budgets. In addition, the Trial Innovation Network will use innovative trial designs, streamlined data collection, harmonized data standards, synchronized approaches to rational study budgeting, and coordinated processes for Data Safety and Monitoring Boards and safety reporting. The Trial Innovation Network will engage key stakeholders—participants, providers, and site investigators—early to provide input during the protocol development process. The Trial Innovation Network website will provide information as well as an environment for collaboration where key stakeholders can share ideas, thoughts, and comments. The website will include a library of tools and webinars to share innovations, knowledge, best practices, and lessons learned about clinical trials and research processes.
Leveraging the strengths of the CTSA Program
The Trial Innovation Network is part of the larger CTSA Program and can leverage the consortium's expertise, size, diversity, and geographic distribution. The CTSA Program hubs include leading medical research centers across the nation, with large catchment areas and diverse patient populations. The Trial Innovation Network will aim to address the challenge of conducting clinical trials at academic centers by leveraging the infrastructure of the CTSA Program. The Trial Innovation Network will also leverage the size and diversity of the CTSA Program to streamline identification of sites and investigators, a time‐consuming process that often starts de novo with each new trial. The idea is for investigators from any discipline to leverage an existing and established consortium of sites and to use the CTSA hubs and affiliated sites as a sustainable platform for future trials. The Trial Innovation Network will also use data‐driven approaches to identify hubs and sites with appropriate patient populations and match investigators to studies. Collaboration is a guiding principle of the Trial Innovation Network, and the goal is to ensure potential sites have the opportunity to comment on protocol feasibility and study budgets before funding decisions are made.
Recognizing that NIH Institutes, investigators, and industry may have varying reasons to partner with a public clinical trials platform, the Trial Innovation Network is aiming to provide tailored options for different users. For example, NIH investigators may have an interest in leveraging the operational infrastructure of the Network, while industry may have an interest in partnering to recruit specific patient populations and to gain access to the clinical expertise at the CTSA Program hubs.
Using an adaptive management approach to build the Trial Innovation Network
To achieve the ambitious goals of the Trial Innovation Network, the program will be built in stages. The first stage will focus on building teams, engaging stakeholders, and organizing the key elements of the Trial Innovation Network—the Network Hub Liaison Teams, the central IRBs, processes for standard contract agreements, and the proposal submission and review process. The Network will begin with providing services and consultations and launching pilot studies. Some of the initial consultations may be selected for further development into protocols that could be implemented in the Trial Innovation Network. The next stage will focus on implementing full‐scale clinical trials and studies. Building the Trial Innovation Network iteratively will allow the investigators to ensure that critical factors to optimize success are in place—organized teams, stakeholder engagement, clear communication plans, robust Network infrastructure, and performance metrics. Developing the Trial Innovation Network in “sprints,” an approach adopted from software development, is a management strategy that allows investigators to flexibly adapt and respond to rapidly evolving needs. Important elements of this approach include piloting initial models and iteratively developing key deliverables based on user requirements.
The Trial Innovation Network has opened the mechanism for Network project proposals and will launch early projects designed to build the major elements of the Network, provide key performance metrics, and develop a pipeline of future clinical trials and studies. During the early stages, the Trial Innovation Network will start with consultations and services and will focus on building partnerships with NIH Institutes and Centers, the scientific community, and other stakeholders. The goal is to then move to launch full‐scale Trial Innovation Network clinical trials and studies.
CONCLUSION
The Trial Innovation Network represents a unique opportunity for CTSA Program institutions, researchers, providers, participants, and NIH and other funders to join together as a learning community to transform the clinical trials enterprise. While the roadblocks to changing clinical trials and the culture of clinical research are formidable, these challenges may also open doors to accomplish what may seem impossible—significantly accelerate the translation process, bring together stakeholders from many disciplines, and create an unprecedented federally funded national platform that will have impacts on public health and the future of clinical research.
Acknowledgments
The authors thank Daniel Benjamin, Gordon Bernard, Michael Dean, Daniel Ford, Daniel Hanley, Paul Harris, Harry Selker, and Consuelo Wilkins for their helpful review and comments.
Conflict of Interest
The authors declared no conflict of interest.
References
- 1. Berndt, E.R. & Cockburn, I.M. Price indexes for clinical trial research: a feasibility study. Mon. Labor Rev. 1–30 (2014). [Google Scholar]
- 2. Reith, C. et al Randomized clinical trials – removing unnecessary obstacles. N. Eng. J. Med. 369, 1061–1065 (2013). [DOI] [PubMed] [Google Scholar]
- 3. Mascette, A.M. et al Are central institutional review boards the solution? The National Heart, Lung, and Blood Institute Working Group's report on optimizing the IRB process. Acad. Med. 87, 1710–1714 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kiriakis, J. et al Observational study of contracts processing at 29 CTSA sites. Clin. Transl. Sci. 6, 279–285 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Eisenstein, E.L. , Lemons, P.W. , Tardiff, B.E. , Schulman, K.A. , Jolly, M.K. & Califf, R.M. Reducing the costs of phase III cardiovascular clinical trials. Am. Heart J. 149, 482–488 (2005). [DOI] [PubMed] [Google Scholar]
- 6. Accelerated Research Agreements . March 22, 2017. https://www.ara4us.org/.
- 7. SMART IRB . March 22, 2017. https://smartirb.org/.
- 8. Enhancing Clinical Research Professionals’ Training and Qualifications . March 22, 2017. http://www.ctsa-gcp.org/about.html. [DOI] [PMC free article] [PubMed]
- 9. Accrual to Clinical Trials Network . March 22, 2017. https://www.act-network.org/.
- 10. ResearchMatch . March 22, 2017. https://www.researchmatch.org/.


