Abstract
Trastuzumab remains an important drug in the management of human epidermal growth factor receptor 2 (HER2) overexpressing breast cancer (BC). Several studies reported resistance mechanisms to trastuzumab, including impaired HER2-accessibility caused by mucin 4 (MUC4). Previously, we demonstrated an increase of Zirconium-89-radiolabeled-trastuzumab (89Zr-Trastuzumab) accumulation when MUC4-overexpressing BC-cells were challenged with the mucolytic drug N-Acetylcysteine (NAC). Hereby, using the same approach we investigated whether tumor exposure to NAC would also enhance trastuzumab-efficacy.
Dual SKBr3 (HER2+/MUC4-, sensitive to trastuzumab) and JIMT1 (HER2+/MUC4+, resistant to trastuzumab) HER2-BC-bearing-xenografts were treated with trastuzumab and NAC. Treatment was monitored by molecular imaging evaluating HER2-accessibility/activity (89Zr-Trastuzumab HER2-immunoPET) and glucose metabolism (18F-FDG-PET/CT), as well as tumor volume and the expression of key proteins.
In the MUC4-positive JIMT1-tumors, the NAC-trastuzumab combination resulted in improved tumor-growth control compared to trastuzumab alone; with smaller tumor volume/weight, lower 18F-FDG uptake, lower %Ki67 and pAkt-expression. NAC reduced MUC4-expression, but did not affect HER2-expression or the trastuzumab-sensitivity of the MUC4-negative SKBr3-tumors.
These findings suggest that improving HER2-accessibility by reducing MUC4-masking with the mucolytic drug NAC, results in a higher anti-tumor effect of trastuzumab. This provides a rationale for the potential benefit of this approach to possibly treat a subset of HER2-positive BC overexpressing MUC4.
Keywords: HER2, NAC, trastuzumab, resistance, immunoPET
INTRODUCTION
The Human epidermal growth factor receptor 2 (HER2) transmembrane oncoprotein is overexpressed in 20–30% of breast cancer (BC) patients [1, 2]. HER2 overexpression in BC has been associated with an aggressive biological behavior, translated into shorter disease-free interval and overall survival in patients with early and advanced disease states [3, 4]. Trastuzumab, a recombinant, humanized monoclonal antibody (mAb) was the first clinically approved anti-HER2 therapy. It specifically binds to HER2 on the C-terminal portion of the extracellular domain (ECD) near the juxtamembrane region in domain IV of the HER2 receptor.
The proposed mechanisms of action of trastuzumab are multiple. It's most well-known and crucial mechanism of action is the inhibition of the PI3K/Akt pathways. Trastuzumab interferes with HER2 activation and so results in the suppression of Akt phosphorylation [5, 6].
Trastuzumab used in addition to standard chemotherapy has been shown to improve treatment outcome of early as well as metastatic stage in HER2-positive BC [7, 8]. However, despite this success, responses to trastuzumab have been hampered by several resistances mechanisms [9, 10]. One of these is the overexpression of high molecular weight membrane-anchored mucin MUC4 [9].
MUC4 is overexpressed in 30–95% of all types of BC as well as lymph nodes metastases and tumor vascular emboli. MUC4 has been correlated with progression and higher tumor grade [11–13]. This large glycoprotein can considerably hinder the accessibility and hence the binding of trastuzumab to HER2 ectodomain, thereby impairing efficient trastuzumab-based treatment [14, 15]. MUC4 has also been postulated to interact closely with HER2 to form a ligand-receptor type intramembrane complex. Herein, it has been shown to operate as a ligand/modulator of HER2 activation by inducing the phosphorylation of HER2 on tyr1248 [12, 16–19].
In a previous molecular imaging study, HER2 receptor impaired accessibility by MUC4 in BC was shown to be appropriately assessed by in vivo molecular imaging using PET and Zirconium-89 radiolabeled trastuzumab (89Zr-Trastuzumab). Importantly, mucolytic treatment N-Acetylcysteine (NAC) restored the impaired HER2 accessibility caused by MUC4, and consequently enhanced the binding and uptake of 89Zr-Trastuzumab in vitro and in vivo in a MUC4-expressing HER2-positive BC xenograft mouse model [19].
NAC is known to be a safe, well-tolerated, well-documented and inexpensive drug [18, 19]. Furthermore, it is more effective than any other mucolytic drug and has therefore been the most widely used [20]. NAC presents an acetyl group promoting its binding to the cell membrane and a free thiol group able to reduce intra- or intermolecule disulfide bonds between cysteine residues of mucins [18, 20], leading to reduced masking of HER2 [19]. Of, note NAC has also been reported to indirectly inhibit mTOR, downstream of the PI3K/akt pathway [23].
In the present study, we hypothesized that MUC4-related resistance to trastuzumab can be overcome through the use of a mucolytic drug, by improving the accessibility of the drug to HER2 and hence achieve a better anti-tumor effect. This approach was evaluated in a dual JIMT1 (HER2+/MUC4+; resistant to trastuzumab) and SKBr3 (HER2+/MUC4-; control sensitive to trastuzumab) HER2 human BC-bearing xenograft model by molecular imaging techniques 89Zr-Trastuzumab HER2-immunoPET and 18F-FDG PET-CT; as well as measurement of tumor volume and pathological examination (Figure 1).
Figure 1. Timeline of the animal experimentation.

Athymic nu/nu female mice were inoculated subcutaneously on the right posterior leg with SKBr3 (HER2+/MUC4-) cells and on the left with JIMT1 (HER2+/MUC4+) cells. Imaging and treatments were initiated two weeks after inoculation when tumors reached approximately 0.5 cm3. Mice were randomized to four different groups: (1) the CS group receiving sweetened drinking water and i.p. saline injections (n = 10), (2) the CT group receiving sweetened drinking water and trastuzumab injections (i.p., 5mg/kg (n = 10), (3) the NS group receiving sweetened drinking water supplemented with NAC and saline i.p. injections (n = 9), and (4) the NT group receiving sweetened drinking water supplemented with NAC and trastuzumab injections (i.p., 5mg/kg) (n = 10). Imaging studies were conducted using a μPET-CT and each mouse was scanned three times, with an 18F-FDG and 89Zr-Trastuzumab PET-CT before treatment and an 18F-FDG PET-CT post treatment. Images were recorded for 18F-FDG at 1h and 89Zr-Trastuzumab 6 days after injection respectively. After the last scan, animals were euthanized and an ex-vivo body distribution study was performed. A pathological examination was also conducted on a subset of animals from each cohort with immunostaining of HER2, MUC4, pAkt and Ki67.
RESULTS
Mucolytic drug NAC enhances HER2 accessibility for trastuzumab in MUC4-overexpressing tumors as shown on 89Zr-Trastuzumab immunoPET
Baseline 89Zr-Trastuzumab PET imaging at 6 days post injection revealed tumor uptake in all mice in both NAC and control group. 89Zr-Trastuzumab also showed visible uptake in the liver and bones. Image analysis demonstrated a statistically significant increase in radiotracer uptake, represented by %ΔSUVmax (33.7 ± 7.4%, p < 0.001) of MUC4 overexpressing JIMT1 tumors in mice treated with NAC (2.55 ± 0.05) compared to untreated controls (2.17 ± 0.06). In contrast, no significant differences were observed in 89Zr-Trastuzumab uptake for MUC4-negative SKBr3 tumors implanted in the same animal (NAC: 2.40 ± 0.06 vs. controls: 2.35 ± 0.07) (Figure 2).
Figure 2. Mucolytic drug NAC enhances 89Zr-Trastuzumab uptake in MUC4 overexpressing JIMT1 tumors.
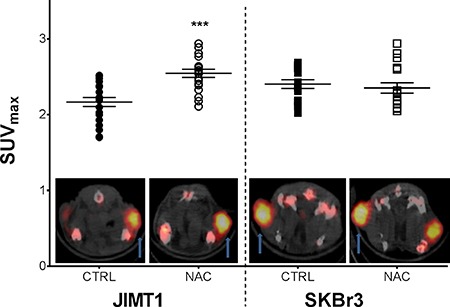
Standardized maximum uptake of 89Zr-Trastuzumab in JIMT1 (HER2+/MUC4+; dot) and SKBr3 (HER2+/MUC4-; squares) tumors under NAC supplementation (n = 19) and control (n = 20) are shown in the graph with a 89Zr-Trastuzumab PET axial image representative of the tumor (arrow) uptake under NAC exposure (NAC) and control (CTRL). All data points and mean ± SEM are shown, both expressed in SUVmax with ***p < 0.001.
NAC supplementation improves the antitumor activity of trastuzumab in MUC4-overexpressing tumors
Tumor volume monitoring
Longitudinal assessment of tumor growth revealed an increase in subcutaneous tumor volume in all tumor types and all treatment arms with different tumor-growth-rates (Figure 3A and 3B). At the end of the treatment period and in the trastuzumab-sensitive SKBr3 tumors, trastuzumab significantly induced slower cell doubling times compared to untreated controls, with or without NAC (CT=32.3 days vs CS=20.3 and NT=30.1 vs NS=20.8, all p < 0.001) and consequently smaller tumor volumes (CT= 0.60 ± 0.10 cm3 vs CS=1.33 ± 0.27, NT=0.60 ± 0.13 vs NS=1.41 ± 0.27, both p < 0.001). In contrast, in the trastuzumab-resistant JIMT1, inhibition of tumor growth was only obtained when trastuzumab was combined with NAC (NT = 33.9 days vs CT = 21.0, NS = 21.7 and CS=21.6, all p < 0.001) resulting in smaller tumor volume in the NT group (NT=0.69 ± 0.10 cm3 vs CT = 1.52 ± 0.22, NS = 1.43 ± 0.18 and CS = 1.37 ± 0.20, all p < 0.001).
Figure 3. The combination of trastuzumab with NAC supplementation results in slower tumor growth.
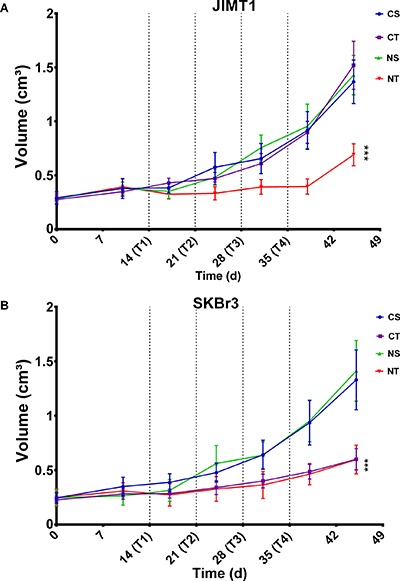
The effect of the different treatment on JIMT1 (HER2+/MUC4+) and SKBr3 (HER2+/MUC4) tumors in dual-tumor-bearing mice randomized to the four treatment arms CS (Control+Saline; blue, n = 10), CT (Control+Trastuzumab; purple, n = 10), NS (NAC+Saline; green, n = 9) and NT (NAC+Trastuzumab; red n = 10) is represented per methodologies as follows: Tumor growth of JIMT1 (A) and SKBr3 (B) tumors monitored through caliper measurements. Data are represented as mean ± SEM, expressed in cm3 for tumor volume, with ***p < 0.001.
These results were corroborated by in vivo measurements on CT as well as confirmed by tumor weight at dissection (Supplementary Figure 1).
18F-FDG PET-CT assessment
After treatment completion, the effects of trastuzumab treatment on tumor metabolism were assessed through changes in 18F-FDG uptake (%ΔSUVmax).
In the MUC4-overexpressing trastuzumab resistant JIMT1 tumors, tumor metabolic changes were observed exclusively in the treatment arm combining trastuzumab and NAC with a significantly lower %ΔSUVmax (NT = 12.6 ± 7.8 % vs CT = 78.6 ± 10.6, NS = 62.1 ± 12.1 and CS = 75.1 ± 15.5; all p < 0.01) (Figure 4B). Whereas in the control SKBr3 tumors, trastuzumab-based treatment led to a significant reduction of tumor activity compared to the respective controls and this independent of NAC supplementation (%ΔSUVmax: CT = 29.2 ± 7.1 % vs CS = 72.5 ± 12.5and NT = 33.8 ± 11.6 vs NS = 75.7 ± 12.6 %; p < 0.001 and p < 0.01) (Figure 4C). Supplementation with NAC alone did not induce any effect on tumor metabolic changes in either tumor types compared to controls.
Figure 4. The combination of trastuzumab with NAC supplementation results in a lower 18F-FDG uptake in trastuzumab resistant tumors.
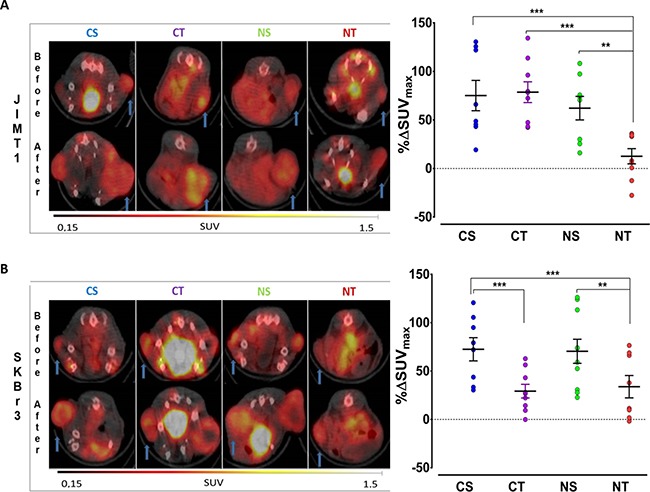
18F-FDG uptake of SKBr3 (HER2+/MUC4-) and JIMT1 (HER2+/MUC4+) tumors in dual-tumor-bearing mice randomized to the four treatment arms CS (Control+Saline; blue, n = 10), CT (Control+Trastuzumab; purple, n = 10), NS (NAC+Saline; green, n = 9) and NT (NAC+Trastuzumab; red n = 10) is represented. (A) The baseline 18F-FDG uptake of JIMT1 and SKBr3 tumors preceding randomization is shown with future treatment group allocation. Representative 18F-FDG PET/CT axial images(left) of the tumor (arrow) before and after treatment are shown together with the changes in 18F-FDG uptake represented as percentage difference in SUVmax (%ΔSUVmax, right) for (B) JIMT1 and SKBr3 tumors in the different treatment arms. Data points as well as mean ± SEM are shown. Data are expressed in SUVmax for the baseline 18F-FDG uptake or as the percentage difference in SUVmax (%ΔSUVmax) when comparing the effect of the different treatment arms; with **p < 0.01 and ***p < 0.001.
Proliferation index Ki67 and activation of the Pi3K/Akt pathway
In the MUC4-overexpressing trastuzumab resistant JIMT1 tumors, the percentage of Ki67 positive cells was decreased only in the NAC-trastuzumab combination arm compared to the other groups (NT=13.8 ± 0.8 % vs CT=25.6 ± 2.7, NS=26.3 ± 1.5 and CS=28.3 ± 1.6; p < 0.001) (Figure 5). The pAkt expression levels of those JIMT1 tumors in the NT combination arm were lower as well (NT=4.5 ± 0.5 vs CT=8.3 ± 0.5, NS=8.3 ± 0.5 and CS=8.2 ± 0.6; p < 0.001) (Figure 5A). In the control SKBr3 tumors, trastuzumab treatment, with or without NAC supplementation, resulted in a decrease in Ki67 positive tumor cells compared to the respective controls (CT = 15.3 ± 1.6 % vs CS = 26.7 ± 1.8 and NT = 14.9 ± 1.6 vs NS=25.8 ± 1.5; p < 0.001 and p = 0.028) (Figure 5B). Also, the pAkt level were about twice lower in trastuzumab-treated SKBr3 tumors compared to the control group (CT = 5.0 ± 0.8 vs CS = 9.2 ± 0.4 and NT = 5.4 ± 0.3 vs NS = 9.5 ± 0.3; both p < 0.001) (Figure 5B). Representative samples of the pathological examination of the Ki67 index and pAkt expression levels are shown in Supplementary Figure 2.
Figure 5. Combining trastuzumab with NAC supplementation reduces proliferation and activation of the molecular Pi3K/Akt signaling pathway in trastuzumab resistant tumors.
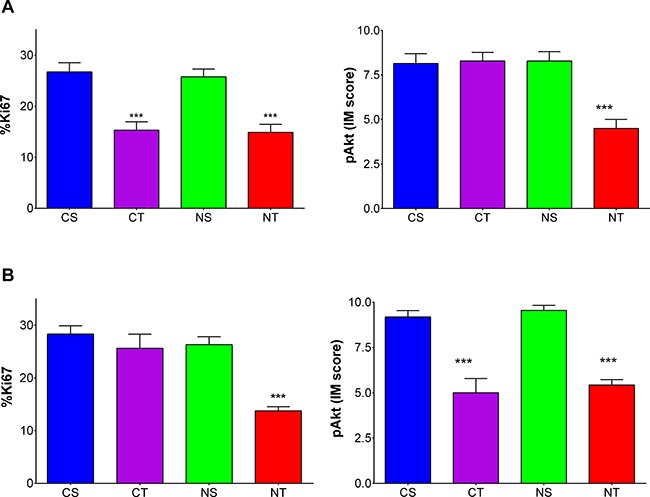
proliferation index Ki67 (left) and pAkt levels (right) for (A) JIMT1 (HER2+/MUC4+) and (B) SKBr3 (HER2+/MUC4-) tumors in dual-tumor-bearing mice randomized to the four treatment arms CS (Control+Saline; blue, n = 5), CT (Control+Trastuzumab; purple, n = 5), NS (NAC+Saline; green, n = 5) and NT (NAC+Trastuzumab; red n = 5). Data are shown as mean ± SEM expressed in % positive cells for Ki67 index values and IM scores for pAkt levels with ***p < 0.001.
Prolonged NAC supplementation does not alter 89Zr-Trastuzumab body distribution in tumor-bearing mice
To assess the possible impact of prolonged supplementation of NAC on body distribution of 89Zr-Trastutumab, organ radioactivity was measured after 60 days on NAC. High uptake occurred in all tumors, liver and spleen and was negligible in the brain as shown in Figure 6. Radioactivity accumulation in non-tumor as well as in the MUC4-negative SKBr3 tumor was not significantly altered by long term NAC exposure.
Figure 6. Prolonged NAC supplementation does not alter 89Zr-Trastuzumab body distribution, with the exception of the maintained enhanced uptake in MUC4-overexpressing tumors.

Ex vivo body distribution of 89Zr-Trastuzumab uptake in JIMT1 (HER2+/MUC4+) and SKBr3 (HER2+/MUC4-) tumors in dual-tumor-bearing mice randomized to the four treatment arms CS (Control+Saline; blue, n = 10), CT (Control+Trastuzumab; purple, n = 10), NS (NAC+Saline; green, n = 9) and NT (NAC+Trastuzumab; red n = 10). Data are expressed in organ-to-blood ratio and represented as mean ± SEM with ***p < 0.001.
In the MUC4-positive JIMT1 tumors treated with NAC, a statistically significant higher radioactivity accumulation was observed compared to respective controls (NS = 2.89 ± 0.25 vs CS = 1.81 ± 0.2 and NT = 2.53 ± 0.28 vs CT = 1.78 ± 0.16; p < 0.001 for both). Furthermore, treatment with trastuzumab (CT and NT) only resulted in a trend for lower 89Zr-Trastuzumab accumulation compared to their corresponding control arm (CS and NS).
Comparison of HER2 expression, HER2 accessibility for trastuzumab metabolic response assessed by 18F-FDG PET
Immunostaining for MUC4 showed overexpression in JIMT1 tumors, with lower MUC4 expression observed in the groups with NAC supplementation compared to the control groups (NS = 5.1 ± 0.2 vs CS = 8.2 ± 0.4 and NT = 5.3 ± 0.4 vs CT = 8.2 ± 0.3; both p < 0.001) (Figure 7A).
Figure 7. NAC supplementation reduces MUC4 expression in MUC4 overexpressing tumors and HER2 expression is not altered in the different treatment arms.
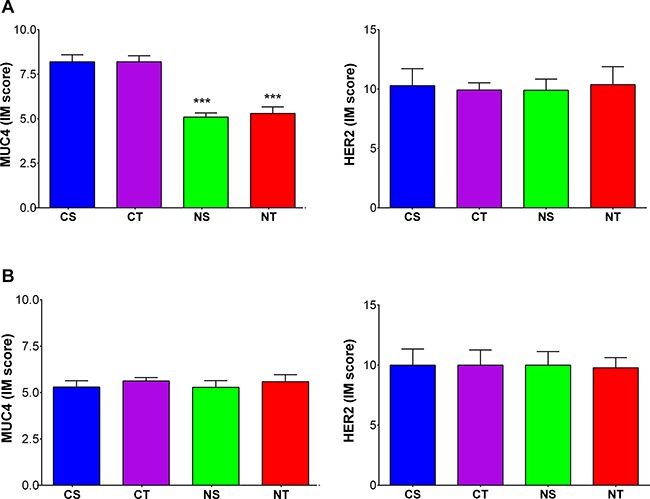
MUC4 (left) and HER2 (right) expression levels in (A) JIMT1 (HER2+/MUC4+) and (B) SKBr3 (HER2+/MUC4-) tumors in dual-tumor-bearing mice randomized to the four treatment arms CS (Control+Saline; blue, n = 5), CT (Control+Trastuzumab; purple, n = 5), NS (NAC+Saline; green, n = 5) and NT (NAC+Trastuzumab; red n = 5). Data are shown as mean ± SEM expressed in IM scores with ***p < 0.001.
On the other hand, heterogeneous immunostaining pattern of intratumor HER2-expression was observed in all tumors. Nonetheless, the mean IM score was similar in all treatment arms (SKBR3: CS = 8.5 ± 1.5, CT = 8.4 ± 1.3, NS=8.3 ± 1.0 and NT = 8.3 ± 0.8; JIMT1: CS=7.3 ± 0.9, CT = 7.3 ± 0.7, NS=7.3 ± 1.1 and NT = 7.3 ± 1.4) (Figure 7B).
Representative samples of the pathological examination of MUC4 and HER2 exspression levels are shown in Supplementary Figure 2. When comparing expression of MUC4 and HER2, a significant correlation was found between the presence of MUC4 and HER2 (R2=0.53; p < 0.001). In contrast, an inverse correlation was observed between MUC4 expression and 89Zr-Trastuzumab uptake (R2 = 0.60; p < 0.001) (Figure 8A).
Figure 8. HER2 expression on immunostaining and HER2 accessibility on 89Zr-Trastuzumab PET are not concordant in regards to their association with MUC4 or metabolic change on 18F-FDG.

The side by side comparison of the association of HER2 expression levels ( left) and 89Zr-Trastuzumab uptake (right) with (A) MUC4 is shown including both JIMT1 (HER2+/MUC4+) and SKBr3 (HER2+/MUC4-) tumors in dual-tumor-bearing mice randomized to the four treatment arms CS (Control+Saline), CT (Control+Trastuzumab), NS (NAC+Saline) and NT (NAC+Trastuzumab). (B) The side by side comparison of the association to 18F-FDG PET metabolic response is limited to mice randomized to the trastuzumab treatment arms CT and NT are also represented. Data shown include both tumor types and are expressed in IM scores for HER2 and MUC4 expression, in SUVmax for 89Zr-Trastuzumab uptake and expressed as the percentage difference in SUVmax (%ΔSUVmax) for 18F-FDG metabolic response.
Furthermore, in the trastuzumab-based treatment arms (CT and NT) a significant correlation was found between the metabolic response assessed by 18F-FDG PET and HER2 accessibility for trastuzumab through 89Zr-Trastuzumab PET (R2 = 0.54; p < 0.001) but not with HER2 expression (R2 < 0.001; p = n.s.) (Figure 8B).
DISCUSSION
This study investigated whether in tumors overexpressing HER2 and MUC4, NAC supplementation could translate into a therapeutic benefit when combined with trastuzumab. Our hypothesis was tested through different methodologies in a dual-BC-xenograft mouse model with human HER2-positive BC tumors, SKBr3 and JIMT1, the latter being resistant to trastuzumab due to MUC4 overexpression. In contrast to the trastuzumab-sensitive SKBr3 tumors, JIMT1 tumors were only responsive when trastuzumab was combined with NAC supplementation. This was demonstrated by (1) an inhibition of tumor growth, (2) smaller tumor volumes as assessed by caliper and CT measurements, (3) a lower 18F-FDG accumulation, (4) a smaller Ki67 proliferation index and (5) a lower Akt phosphorylation for mice receiving the NAC-trastuzumab combination.
All these results converge to the same finding that NAC supplementation can significantly break MUC4-related resistance to trastuzumab by restoring HER2 hampered accessibility.
Our previous study demonstrated that for MUC4 overexpressing tumors HER2 hampered accessibility could be improved as a result of NAC administration [19]. Once more, enhanced accessibility of trastuzumab to HER2 was shown with a 30% higher 89Zr-Trastuzumab uptake in JIMT1 (HER2+/MUC4+; resistant to trastuzumab) tumors exposed to NAC compared to control mice, without any effect on SKBr3 (HER2+/MUC4-; control sensitive to trastuzumab). Additionally, this study confirmed the effect of NAC on MUC4 specifically, with lower MUC4 expression of JIMT1 tumors in the NAC supplementation groups. Besides consistency with our previously published data, these findings confirm the validity of our model and approach in enhancing the accessibility of HER2 for trastuzumab by decreasing MUC4 related hindrance with mucolytic drug NAC.
In accordance with previously published data [23–25], the SKBr3 tumors that served as control were trastuzumab sensitive and presented about 50% inhibition of tumor growth under trastuzumab treatment irrespective of NAC supplementation. In contrast, for the trastuzumab resistant JIMT1 tumors, a significant anti-proliferative effect was seen only when trastuzumab was combined with NAC with a 60% slower tumor doubling time. Consistent tumor volume results were found with CT measurements showing halved tumor volumes in both trastuzumab treatment groups (with or without NAC) for SKBR3 and only in the trastuzumab-NAC arm for JIMT1 tumors when compared to control. Also, the ex vivo measure of tumor weight at dissection validated both results with two times smaller tumors. Trastuzumab did not result in tumor shrinkage/regression but resulted in a change in growth rate. Nevertheless the effect of targeted drugs on the rate of tumor growth have been reported to be more predictive of treatment response rather than tumor shrinkage/regression [27].
Interestingly, the observed overlap at baseline seen in 89Zr-Trastuzumab uptake of JIMT1 tumors of mice in the NAC and control group was not mirrored in terms of trastuzumab's therapeutic response. This is probably due to the fact that the effect of NAC on MUC4 positive tumors increased over time. Accordingly, at dissection, after 60 days of NAC supplementation, the difference in 89Zr-Trastuzumab uptake between the two groups was twice the one observed at baseline.
PET imaging with 18F-FDG, a radioactive analog of glucose, was also implemented. Although all methods of therapy monitoring were concordant, the unequivocal discrimination observed between treatment arms was more pronounced when monitored by 18F-FDG-PET. A 6-fold lesser accumulation of 18F-FDG was found in both trastuzumab groups for SKBR3 and only in the trastuzumab-NAC arm for JIMT1 tumors when compared to control. Also, a significant correlation was found between 18F-FDG uptake and the number of Ki67 positive cells (R2 = 0.79, p < 0.05, data not shown), supporting 18F-FDG-PET as a good surrogate for tumor proliferation activity in ductal breast carcinoma [28], corresponding to the BC cells in our model. Unfortunately an absolute decrease in SUVmax after therapy could not be demonstrated. This was most likely due to tumor growth (up to 40%) in the interval between the baseline 18F-FDG-PET and the start of the trastuzumab to allow the 89Zr-Trastuzumab PET examination. This issue was also previously described in clinical setting by our group [29].
The efficacy of trastuzumab treatment results mostly from the inhibition of the phosphorylation of HER2, hence and foremost the downstream PI3K-Akt pathway [6], which plays a critical role in growth factor-induced cell proliferation and survival [30]. Accordingly in our study, Akt phosphorylation was found significantly decreased in tumors responding to trastuzumab (alone for SKBr3 or only in combination with NAC for JIMT1).
The clinical importance of the Ki67 value in HER2 positive BC has previously been investigated. Studies showed that a Ki67 value ≤20% was predictive of pathological complete response [31] and associated with significantly higher post-recurrence survival [32]. In our study, the Ki67 proliferation index was significantly lowered in both trastuzumab treatment groups for SKBR3 and only in the trastuzumab-NAC arm for JIMT1 tumors, dropping to a value below 15 %. Trastzumab has also been reported to exerts its effect through HER2 downregulation, this effect was not demonstrated here most likely due to heterogeneity in HER2 staining [33].
HER2 immunostaining in our study was done using a clinically available and FDA approved IHC kit with a mAb recognizing the intracellular domain of HER2 (ICD) [26]; whereas immunoPET (and treatment) where based on trastuzumab raised against the extracellular domain (i.e. ECD IV). This epitope difference could explain the non-concordance found between HER2 IHC and 89Zr-Trastuzumab immunoPET in regards to the level of MUC4 as well as the therapeutic effect of trastuzumab.
In the present study we found an inverse correlation between MUC4 expression and 89Zr-Trastuzumab immunoPET in contrast with a positive correlation with HER2 IHC. Although seemingly contradictory, 89Zr-Trastuzumab findings are concordant with MUC4 hindering the accessibility of HER2-ECD henceforth the binding of (radiolabeled) trastuzumab [14, 19], while the HER2 IHC results are agreeing with MUC4 as modulator of the expression of HER2 [17, 18].
This further implies that tumors that would benefit of trastuzumab (or TDM1) are not necessarily the ones with the highest density of HER2, as they also might be the ones with the highest expression of MUC4. On the other hand, these tumors represent the ideal candidates for combining trastuzumab (or T-DM1) with NAC supplementation and as a result be the most favored by our proposed approach.
In addition, a significant correlation was found between 89Zr-Trastuzumab immunoPET (ECD) and the metabolic response after trastuzumab treatment assessed by 18F-FDG PET and not with HER2 expression (ICD).
This relationship between a specific HER2 domain and response to trastuzumab based treatment has also been demonstrated in clinical studies.
Recently, a clinical study on 180 HER2-positive patients under trastuzumab treatment explored the relationship between domain-specific HER2 expression of the tumor on IHC and the benefit of treatment for the patient in terms of disease-free survival (DFS). Carvajal-Hausdorf et al. revealed a differential benefit from trastuzumab therapy based on HER2 ECD expression rather than ICD in BC [35]. Similarly, our group demonstrated that 89Zr-Trastuzumab immunoPET (ECD) before treatment allowed the discrimination of patients benefiting from trastuzumab emtansine (T-DM1) with longer time to treatment failure, rather than their HER2 status (ICD/FISH) [29]. Both findings strongly advocate for a shift to an analysis of HER2 ECD rather than the currently used ICD when determining the eligibility of a patient for a targeted treatment against the ECD.
The present study is in concordance and further shows that increasing the amount of accessible HER2-ECD with NAC (on 89Zr-Trastuzumab), results in an increased therapeutic benefit of trastuzumab regardless of HER2-ICD (on IHC). Furthermore, our results imply that, despite an intact ICD expression, the benefit of trastuzumab-based treatment is greatly diluted when the tumor lesion presents with impaired accessibility to HER2 ECD. Henceforth, NAC supplementation could be of help in those circumstances. It could enhance the amount of available ECD without compromising the therapeutic effect of trastuzumab.
These issues of target accessibility are now even more critical with the advent of new antibody drug conjugates given as single agents, as certain mAbs might be inherently suboptimal for targeted drug delivery due to less accessible target ECD epitopes. Ergo, a possible course of action could be (1) opting for an mAb recognizing a more accessible membrane distal epitope, such as pertuzumab (ECD II [29]) which might represent a superior option for targeted drug delivery to HER2 rather than trastuzumab's ECD IV (eg. T-DM1) or (2) attempt to enhance the accessibility of the epitope for the mAb in question as proposed in this study.
The present study highlighted the potential role of molecular imaging with radiolabeled trastuzumab in gaining information regarding trastuzumab-HER2 interaction, in the investigation of a particular resistance mechanism and as a potential biomarker for response prediction. Although, our study focused on the already longstanding HER2-treatment trastuzumab, the same interactions with HER2 are expected for trastuzumab based treatments such as T-DM1. The latter is now widely offered and has in part substituted trastuzumab in the treatment of HER2 positive BC patients. Nonetheless, up to this day, T-DM1 administration is still preceeded by trastuzumab and also follows when resistance to the former arises. Hence, ways to possibly overcome resistance to trastuzumab remain highly relevant.
Taken together, our results demonstrate that NAC supplementation can significantly break MUC4-related resistance to trastuzumab by restoring HER2 hampered accessibility. The effect was revealed on 89Zr-Trastuzumab HER2-immunoPET, monitored by 18F-FDG-PET/CT and tumor volume measurements, and validated at the molecular level. Tentatively, this safe and promising approach may be offered to a subset of patients with HER2-positive BC tumors overexpressing mucins.
MATERIALS AND METHODS
89Zr-labeling of trastuzumab
Labeling of trastuzumab and quality controls were performed as previously described [19]: 2.5mg of GMP grade N-SucDF-trastuzumab (VU Windesheim-VU Medisch Centrum, Amsterdam, Holland), synthesized by coupling desferrioxamine to trastuzumab, was radiolabeled with 111MBq 89Zr-oxalate (t 1/2=78.4 h; Perkin Elmer). The specific activity was adjusted to 3.7MBq/100μg/100μL by adding unlabeled trastuzumab.
Measurement of 89Zr radioactivity
89Zr-Trastuzumab accumulated activity was measured in a gamma-counter (Wallac Wizard, Perkin-Elmer) with background- and decay-correction as well as reference activity standards of 10%, 20% and 30% of the administered radioactivity.
Cells
The human BC HER2-positive and MUC4-negative SKBr3 (ATCC) and HER2-positive and MUC4-positive JIMT1 (DSMZ) were cultured in DMEM high glucose (Lonza), supplemented with heat inactivated 10% FBS (Fisher) and antibiotics (penicillin-G (Sigma), streptomycin sulphate and kanamycine (both from Gentaur)) at 37°C and 5% CO2 in a fully humidified atmosphere. Cells were subcultured twice a week.
Cells were regularly checked for mycoplasma contamination using MycoAlert® Mycoplasma Detection Kit (Lonza).
Mouse model
Six-week-old athymic nu/nu female mice (Charles River) were inoculated subcutaneously on the right posterior leg with 3 × 106 SKBr3 cells and on the left with 1.5 × 106 JIMT1 cells suspended in 100μL of DMEM and Basement Membrane Extract (1:1; Cultrex, Trevigen). Imaging and treatments (Figure 1) were initiated two weeks after inoculation when tumors reached approximately 0.5 cm3. Mice were randomized to four different groups: (1) the CS group receiving sweetened drinking water and i.p. saline injections (n = 10), (2) the CT group receiving sweetened drinking water and trastuzumab injections (i.p., 5mg/kg [37, 38]) (n = 10), (3) the NS group receiving sweetened drinking water supplemented with NAC and saline i.p. injections (n = 9), and (4) the NT group receiving sweetened drinking water supplemented with NAC and trastuzumab injections (i.p., 5 mg/kg) (n = 10).
The drinking bottles were sweetened and refreshed twice a week until sacrifice for all groups. The drinking water of group NS and NT was supplemented with 4g/L NAC (Sigma Aldrich) and adjusted to pH 6.5–7.5 with sodium hydroxide [39, 40]. NAC supplemented in this manner resulted in a mean dose of 1 g of NAC per kg body weight per day [39].
Mice were observed daily for signs of discomfort or mortality throughout the treatment period and body weight was recorded weekly. All animal experiments were reviewed and approved by the Committee on Animal Ethics and Well Being of the Université Libre de Bruxelles (protocol 495N).
Tumor growth
Tumor growth was monitored weekly by caliper measurements until sacrifice. Tumor volume was calculated using formula and later compared to the volume determined on computed tomography images.
Small-animal PET-CT imaging
Studies were conducted using a μPET-CT (nanoScan®PET/CT, Mediso) on dual-tumor-bearing mice (n = 9–10/group). Each mouse was scanned three times, with an 18F-FDG and 89Zr-Trastuzumab PET-CT before treatment and an 18F-FDG PET-CT post treatment (Figure 1). The PET tracer (18F-FDG = 4.14 ± 0.36 MBq; 89Zr-Trastuzumab = 3.15 ± 0.24 MBq/100 μL) was injected in a lateral tail vein. Images were recorded for 18F-FDG at 1h and 89Zr-Trastuzumab 6 days after injection respectively. Two animals were scanned simultaneously under isoflurane anesthesia (induction: 3% isoflurane, 3L O2; maintenance: 1.5% isoflurane, 1.5L O2) and at 37°C using a thermoregulation unit (Minerve).
Baseline 18F-FDG PET showed homogenous and similar radiotracer uptake in all tumors and in all mice. The subsequent randomization was well balanced, with similar tumor 18F-FDG uptakes for the different arms prior to treatment in both types (SUVmax: SKBR3: CS=0.61 ± 0.06, CT = 0.62 ± 0.06, NS = 0.60 ± 0.05 and NT = 0.64 ± 0.08; JIMT1: CS = 0.69 ± 0.07, CT = 0.56 ± 0.04, NS = 0.66 ± 0.06 and NT = 0.70 ± 0.07).
Ex-vivo body distribution
After the last scan, at 6 days post 89Zr-Trastuzumab administration, animals were euthanized by cervical dislocation under anesthesia and an ex-vivo body distribution study was performed. 89Zr-Trastuzumab activity was measured in 16 organs (including tumors). Tissue uptake is presented as organ to blood ratio.
Immunostaining
After dissection, tumors were immediately fixed in 10% formalin until the ex-vivo study was performed and radioactivity decayed. Samples were then transferred to 70% ethanol and stored at 4°C. Samples were processed and embedded in paraffin, and 4-μm sections were prepared for HE-staining. Tumor sections from a subset of animals from each cohort were also analyzed using immunostaining with different antibodies: anti-HER2 (4B5, Roche), anti-MUC4 (8G-7, Biocare Medical) anti-pAkt (D9E, Santa Cruz) and Ki67 (MIB-1, Dako) and subsequently stained with diaminobensidine (DAB, iview DAB detection kit, Ventana) except for pAkt staining where alkaline phosphatase red (ultraView Universal Alkaline Phosphatase Red, Ventana) was used.
Stained sections were imaged using NDP Slice Scanner (Hamamatsu). Three regions were selected at random on different part of the section and analyzed at ×20 magnification, using ImmunoMembrane and ImmunoRatio web applications. In brief, ImmunoMembrane software segments stained membrane regions from the user-submitted sample image and classifies the image into 0/1+,2+, or 3+ and calculates IM scores based on the membrane staining completeness and intensity; whereas, ImmunoRatio software segments DAB-stained and HE-stained nuclei regions from the user-submitted image and calculates the percentage of DABstained nuclear area over total nuclear area. Both applications generate a pseudo-colored overlay images matching the area segmentation [41].
PET-CT image analysis
PET-CT image analysis and quantification were performed using PMOD software (PMOD Technologies, Switzerland). To define the volumes of interest (VOIs), tumors were manually contoured on the CT image. To quantify radioactivity within the tumor corresponding PET uptake values were normalized to the injected radioactivity per body weight (SUV).
The results are represented as the tumor volume for CT and the maximum uptake value of the VOI (SUVmax) for PET. Changes in 18F-FDG uptake after tumor growth under the different treatment regimens are represented as the percentage difference between SUVmax post-treatment and SUVmax pre-treatment (%ΔSUVmax). Lower %ΔSUVmax denoted lower tumor glucose metabolism in comparisons between treatment regimens.
Statistics
Statistical analysis was done using ANOVA for the 4 animal group differences, student-T test when comparing two data replicates, extra sum-of-squares F test for curve differences, Pearson test for correlation. Statistics and graphs were performed using Graphpad Prism (GraphPad Software, San Diego, CA, USA). Data are presented as mean ± standard error of the mean (SEM) unless stated otherwise.
SUPPLEMENTARY MATERIALS FIGURES
ACKNOWLEDGMENTS AND FUNDING
We express our gratitude to “Les Amis de Bordet” and “Fonds Gaston Ithier” for the grants obtained to conduct this study. We thank the nuMix team of the Center for Microscopy and Molecular Imaging (CMMI) for their assistance in the animal experiment work.
Footnotes
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
REFERENCES
- 1.Sergina NV, Moasser MM. The HER family and cancer: emerging molecular mechanisms and therapeutic targets. Trends Mol Med. 2007;13:527–34. doi: 10.1016/j.molmed.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bailey TA, Luan H, Clubb RJ, Naramura M, Band V, Raja SM, Band H. Mechanisms of Trastuzumab resistance in ErbB2-driven breast cancer and newer opportunities to overcome therapy resistance. J Carcinog. 2011;10:28. doi: 10.4103/1477-3163.90442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schechter AL, Stern DF, Vaidyanathan L, Decker SJ, Drebin JA, Greene MI, Weinberg RA. The neu oncogene: an erb-B-related gene encoding a 185,000-Mr tumour antigen. Nature. 1984;312:513–6. doi: 10.1038/312513a0. [DOI] [PubMed] [Google Scholar]
- 4.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–82. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 5.Junttila TT, Akita RW, Parsons K, Fields C, Lewis Phillips GD, Friedman LS, Sampath D, Sliwkowski MX. Ligand-Independent HER2/HER3/PI3K Complex Is Disrupted by Trastuzumab and Is Effectively Inhibited by the PI3K Inhibitor GDC-0941. Cancer Cell. 2009;15:429–40. doi: 10.1016/j.ccr.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 6.Yakes FM, Chinratanalab W, Ritter CA, King W, Seelig S, Arteaga CL. Herceptin-induced Inhibition of Phosphatidylinositol-3 Kinase and Akt Is Required for Antibody-mediated Effects on p27, Cyclin D1, and Antitumor Action. Cancer Res. 2002;62:4132–41. [PubMed] [Google Scholar]
- 7.Davoli A, Hocevar BA, Brown TL. Progression and treatment of HER2-positive breast cancer. Cancer Chemother Pharmacol. 2010;65:611–23. doi: 10.1007/s00280-009-1208-1. [DOI] [PubMed] [Google Scholar]
- 8.Mitri Z, Constantine T, O’Regan R. The HER2 Receptor in Breast Cancer: Pathophysiology, Clinical Use, and New Advances in Therapy. Chemother Res Pract. 2012;2012:743193. doi: 10.1155/2012/743193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mukohara T. Mechanisms of resistance to anti-human epidermal growth factor receptor 2 agents in breast cancer. Cancer Sci. 2011;102:1–8. doi: 10.1111/j.1349-7006.2010.01711.x. [DOI] [PubMed] [Google Scholar]
- 10.Pohlmann PR, Mayer IA, Mernaugh R. Resistance to Trastuzumab in Breast Cancer. Clin Cancer Res. 2009;15:7479–91. doi: 10.1158/1078-0432.CCR-09-0636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mukhopadhyay P, Chakraborty S, Ponnusamy MP, Lakshmanan I, Jain M, Batra SK. Mucins in the pathogenesis of breast cancer: implications in diagnosis, prognosis and therapy. Biochim Biophys Acta. 2011;1815:224–40. doi: 10.1016/j.bbcan.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carraway KL, Price-Schiavi SA, Komatsu M, Jepson S, Perez A, Carraway CA. Muc4/sialomucin complex in the mammary gland and breast cancer. J Mammary Gland Biol Neoplasia. 2001;6:323–37. doi: 10.1023/a:1011327708973. [DOI] [PubMed] [Google Scholar]
- 13.Workman HC, Miller JK, Ingalla EQ, Kaur RP, Yamamoto DI, Beckett LA, Young LJ, Cardiff RD, Borowsky AD, Carraway KL, Sweeney C, Carraway KL. The membrane mucin MUC4 is elevated in breast tumor lymph node metastases relative to matched primary tumors and confers aggressive properties to breast cancer cells. Breast Cancer Res. 2009;11:R70. doi: 10.1186/bcr2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagy P, Friedländer E, Tanner M, Kapanen AI, Carraway KL, Isola J, Jovin TM. Decreased accessibility and lack of activation of ErbB2 in JIMT-1, a herceptin-resistant, MUC4-expressing breast cancer cell line. Cancer Res. 2005;65:473–82. [PubMed] [Google Scholar]
- 15.Price-Schiavi SA, Jepson S, Li P, Arango M, Rudland PS, Yee L, Carraway KL. Rat Muc4 (sialomucin complex) reduces binding of anti-ErbB2 antibodies to tumor cell surfaces, a potential mechanism for herceptin resistance. Int J Cancer. 2002;99:783–91. doi: 10.1002/ijc.10410. [DOI] [PubMed] [Google Scholar]
- 16.Carraway KL, Theodoropoulos G, Kozloski GA, Carothers Carraway CA. Muc4/MUC4 functions and regulation in cancer. Future Oncol. 2009;5:1631–40. doi: 10.2217/fon.09.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chaturvedi P, Singh AP, Chakraborty S, Chauhan SC, Bafna S, Meza JL, Singh PK, Hollingsworth MA, Mehta PP, Batra SK. MUC4 Mucin Interacts with and Stabilizes the HER2 Oncoprotein in Human Pancreatic Cancer Cells. Cancer Res. 2008;68:2065–70. doi: 10.1158/0008-5472.CAN-07-6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singh AP, Chaturvedi P, Batra SK. Emerging Roles of MUC4 in Cancer: A Novel Target for Diagnosis and Therapy. Cancer Res. 2007;67:433–6. doi: 10.1158/0008-5472.CAN-06-3114. [DOI] [PubMed] [Google Scholar]
- 19.Wimana Z, Gebhart G, Guiot T, Vanderlinden B, Morandini R, Doumont G, Sherer F, Simaeys GV, Goldman S, Ghanem G, Flamen P. Mucolytic Agents Can Enhance HER2 Receptor Accessibility for [89Zr] Trastuzumab, Improving HER2 Imaging in a Mucin-Overexpressing Breast Cancer Xenograft Mouse Model. Mol Imaging Biol. 2015;17:697–703. doi: 10.1007/s11307-015-0840-x. [DOI] [PubMed] [Google Scholar]
- 20.Cotgreave IA. N-acetylcysteine: pharmacological considerations and experimental and clinical applications. Adv Pharmacol. 1997;38:205–27. [PubMed] [Google Scholar]
- 21.Kelly GS. Clinical applications of N-acetylcysteine. Altern Med Rev J Clin Ther. 1998;3:114–27. [PubMed] [Google Scholar]
- 22.Schrier BP, Lichtendonk WJ, Witjes JA. The effect of N-acetyl-L-cysteine on the viscosity of ileal neobladder mucus. World J Urol. 2002;20:64–7. doi: 10.1007/s00345-001-0234-3. [DOI] [PubMed] [Google Scholar]
- 23.Leontieva OV, Blagosklonny MV. Yeast-like chronological senescence in mammalian cells: phenomenon, mechanism and pharmacological suppression. Aging (Albany NY) 2011;3:1078–91. doi: 10.18632/aging.100402. https://doi.org/10.18632/aging.100402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hudziak RM, Lewis GD, Winget M, Fendly BM, Shepard HM, Ullrich A. p185HER2 monoclonal antibody has antiproliferative effects in vitro and sensitizes human breast tumor cells to tumor necrosis factor. Mol Cell Biol. 1989;9:1165–72. doi: 10.1128/mcb.9.3.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tseng PH, Wang YC, Weng SC, Weng JR, Chen CS, Brueggemeier RW, Shapiro CL, Chen CY, Dunn SE, Pollak M, Chen CS. Overcoming Trastuzumab Resistance in HER2-Overexpressing Breast Cancer Cells by Using a Novel Celecoxib-Derived Phosphoinositide-Dependent Kinase-1 Inhibitor. Mol Pharmacol. 2006;70:1534–41. doi: 10.1124/mol.106.023911. [DOI] [PubMed] [Google Scholar]
- 26.Longva KE, Pedersen NM, Haslekås C, Stang E, Madshus IH. Herceptin-induced inhibition of ErbB2 signaling involves reduced phosphorylation of Akt but not endocytic down-regulation of ErbB2. Int J Cancer. 2005;116:359–67. doi: 10.1002/ijc.21015. [DOI] [PubMed] [Google Scholar]
- 27.Kelland LR. “Of mice and men”: values and liabilities of the athymic nude mouse model in anticancer drug development. Eur J Cancer. 2004;40:827–36. doi: 10.1016/j.ejca.2003.11.028. [DOI] [PubMed] [Google Scholar]
- 28.Buck A, Schirrmeister H, Kühn T, Shen C, Kalker T, Kotzerke J, Dankerl A, Glatting G, Reske S, Mattfeldt T. FDG uptake in breast cancer: correlation with biological and clinical prognostic parameters. Eur J Nucl Med Mol Imaging. 2002;29:1317–23. doi: 10.1007/s00259-002-0880-8. [DOI] [PubMed] [Google Scholar]
- 29.Gebhart G, Lamberts LE, Wimana Z, Garcia C, Emonts P, Ameye L, Stroobants S, Huizing M, Aftimos P, Tol J, Oyen WJG, Vugts DJ, Hoekstra OS, et al. Molecular imaging as a tool to investigate heterogeneity of advanced HER2-positive breast cancer and to predict patient outcome under trastuzumab emtansine (T-DM1): the ZEPHIR Trial. Ann Oncol. 2015;27:619–24. doi: 10.1093/annonc/mdv577. [DOI] [PubMed] [Google Scholar]
- 30.Nicholson KM, Anderson NG. The protein kinase B/Akt signalling pathway in human malignancy. Cell Signal. 2002;14:381–95. doi: 10.1016/s0898-6568(01)00271-6. [DOI] [PubMed] [Google Scholar]
- 31.Fasching PA, Heusinger K, Haeberle L, Niklos M, Hein A, Bayer CM, Rauh C, Schulz-Wendtland R, Bani MR, Schrauder M, Kahmann L, Lux MP, Strehl JD, et al. Ki67, chemotherapy response, and prognosis in breast cancer patients receiving neoadjuvant treatment. BMC Cancer. 2011;11:486. doi: 10.1186/1471-2407-11-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ibrahim T, Farolfi A, Scarpi E, Mercatali L, Medri L, Ricci M, Nanni O, Serra L, Amadori D. Hormonal receptor, human epidermal growth factor receptor-2, and Ki67 discordance between primary breast cancer and paired metastases: clinical impact. Oncology. 2013;84:150–7. doi: 10.1159/000345795. [DOI] [PubMed] [Google Scholar]
- 33.Canonici A, Gijsen M, Mullooly M, Bennett R, Bouguern N, Pedersen K, O’Brien NA, Roxanis I, Li JL, Bridge E, Finn R, Siamon D, McGowan P, et al. Neratinib overcomes trastuzumab resistance in HER2 amplified breast cancer. Oncotarget. 2013;4:1592–605. doi: 10.18632/oncotarget.1148. https://doi.org/10.18632/oncotarget.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schrohl AS, Pedersen HC, Jensen SS, Nielsen SL, Brünner N. Human epidermal growth factor receptor 2 (HER2) immunoreactivity: specificity of three pharmacodiagnostic antibodies. Histopathology. 2011;59:975–83. doi: 10.1111/j.1365-2559.2011.04034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carvajal-Hausdorf DE, Schalper KA, Pusztai L, Psyrri A, Kalogeras KT, Kotoula V, Fountzilas G, Rimm DL. Measurement of Domain-Specific HER2 (ERBB2) Expression May Classify Benefit From Trastuzumab in Breast Cancer. J Natl Cancer Inst. 2015;107:djv136. doi: 10.1093/jnci/djv136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Metzger-Filho O, Winer EP, Krop I. Pertuzumab: Optimizing HER2 Blockade. Clin Cancer Res. 2013;19:5552–6. doi: 10.1158/1078-0432.CCR-13-0518. [DOI] [PubMed] [Google Scholar]
- 37.Shi Y, Fan X, Deng H, Brezski RJ, Rycyzyn M, Jordan RE, Strohl WR, Zou Q, Zhang N, An Z. Trastuzumab Triggers Phagocytic Killing of High HER2 Cancer Cells In Vitro and In Vivo by Interaction with Fcγ Receptors on Macrophages. J Immunol. 2015;194:4379–86. doi: 10.4049/jimmunol.1402891. [DOI] [PubMed] [Google Scholar]
- 38.Barok M, Tanner M, Köninki K, Isola J. Trastuzumab-DM1 causes tumour growth inhibition by mitotic catastrophe in trastuzumab-resistant breast cancer cells in vivo. Breast Cancer Res. 2011;13:R46. doi: 10.1186/bcr2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clark J, Clore EL, Zheng K, Adame A, Masliah E, Simon DK. Oral N-acetyl-cysteine attenuates loss of dopaminergic terminals in alpha-synuclein overexpressing mice. PloS One. 2010;5:e12333. doi: 10.1371/journal.pone.0012333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marian AJ, Senthil V, Chen SN, Lombardi R. Antifibrotic Effects of Antioxidant N-Acetylcysteine in a Mouse Model of Human Hypertrophic Cardiomyopathy Mutation. J Am Coll Cardiol. 2006;47:827–34. doi: 10.1016/j.jacc.2005.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tuominen VJ, Tolonen TT, Isola J. ImmunoMembrane: a publicly available web application for digital image analysis of HER2 immunohistochemistry. Histopathology. 2012;60:758–67. doi: 10.1111/j.1365-2559.2011.04142.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


