Abstract
Schistosomes are responsible for the parasitic disease schistosomiasis, an acute and chronic parasitic ailment that affects more than 240 million people in 70 countries worldwide. It is the second most devastating parasitic disease after malaria. At least 200,000 deaths per year are associated with the disease. In the absence of the availability of vaccines, chemotherapy is the main stay for combating schistosomiasis. The antischistosomal arsenal is currently limited to a single drug, Praziquantel, which is quite effective with a single-day treatment and virtually no host-toxicity. Recently, however, the question of reduced activity of Praziquantel has been raised. Therefore, the search for alternative antischistosomal drugs merits the study of new approaches of chemotherapy.
The rational design of a drug is usually based on biochemical and physiological differences between pathogens and host. Pyrimidine metabolism is an excellent target for such studies. Schistosomes, unlike most of the host tissues, require a very active pyrimidine metabolism for the synthesis of DNA and RNA. This is essential for the production of the enormous numbers of eggs deposited daily by the parasite to which the granulomas response precipitate the pathogenesis of schistosomiasis. Furthermore, there are sufficient differences between corresponding enzymes of pyrimidine metabolism from the host and the parasite that can be exploited to design specific inhibitors or “subversive substrates” for the parasitic enzymes. Specificities of pyrimidine transport also diverge significantly between parasites and their mammalian host. This review deals with studies on pyrimidine metabolism in schistosomes and highlights the unique characteristic of this metabolism that could constitute excellent potential targets for the design of safe and effective antischistosomal drugs. In addition, pyrimidine metabolism in schistosomes is compared with that in other parasites where studies on pyrimidine metabolism have been more elaborate, in the hope of providing leads on how to identify likely chemotherapeutic targets which have not been looked at in schistosomes.
Keywords: Schistosomes, parasites, pyrimidine, enzymes, transport, chemotherapy
1. Introduction
Six species of the trematode Schistosoma (Schistosoma mansoni, S. japonicum, S. haematobium, S. mekongi, S. guineensis and S. intercalatum) are the causative agents of the parasitic disease schistosomiasis. Schistosomiasis is an acute and chronic parasitic ailment with a wide range of clinical manifestations that have plagued mankind since ancient times. The clinical disease dates as early as 1500 B.C. Calcified Schistosoma eggs have been identified in Egyptian mummy tissues from the twentieth dynasty (1200 to 1090 B.C.). In China, there are records of schistosomiasis of comparable antiquity. Schistosoma infections in the New World are more recent in origin, probably beginning with African slave trade to the Americas during the sixteenth and seventeenth centuries. At the present time, schistosomiasis affects more than 240 million people in 70 countries worldwide. Most endemic rural populations will have a 40–60% prevalence rate at any one time; but almost everyone (i.e., 95%) has had an infection sometime during his life. These estimates, superimposed on approximately 1.5 billion humans in schistosome endemic areas, easily qualify schistosomiasis as one of the major world public health problems. Indeed, schistosomiasis is the second most devastating parasitic disease after malaria. At least 200,000 deaths per year are associated with the disease.
Since antischistosomal vaccines are not yet available, chemotherapy still is the main stay to control this disease. However, only a small number of effective drugs are currently available. Classical antischistosomal drugs fall into four major groups: the antimonials, the nitrothiazoles, the thioxanthones and the organophosphates. Each of these drugs can be effective against some species of human schistosomes, but none is highly effective against all. In addition, for each of these chemotherapeutic agents, there are known contraindications and/or severe side effects. Thus, none of the classical drugs fulfill the requirements of an ideal antischistosomal compound. The antischistosomal arsenal is currently limited to a single drug, Praziquantel, which has been the drug of choice for the treatment of schistosomiasis for more than 35 years. Praziquantel is orally effective against all six species of schistosomes with a single-day treatment and virtually no toxicity towards the host. Nevertheless, the question of reduced of Praziquantel efficacy was raised recently. Therefore, the search for alternative antischistosomal agents continues to merit the study of new approaches of chemotherapy.
The search for antischistosomal drugs has a long and tortuous history. Most of the currently available drugs were discovered by empirical methods. Rational design of a drug based on biochemical and physiological differences between the pathogen (e.g., cancer, bacteria, parasites, etc.) and the host has been the goal of many investigators for decades. In general, rational design of antischistosomal drugs is lacking because of the paucity of information about the biochemistry, physiology, molecular biology, etc. of this parasite. However, the great phylogenic separation between schistosomes and their host renders the biology and biochemistry of the parasite amenable to better chances of discovering exploitable differences between the host and schistosomes.
The pathogenesis of schistosomiasis (e.g., hepatic fibrosis, portal hypertension, bladder cancer, etc.) results from the granulomas response to the unending accumulation of deposited eggs ranging from approximately 400–4000 eggs/female/day depending on the species. Considering the number of ovipositing worm pairs can reach 2,000 pairs/patient (Gryseels and De Vlas, 1996), the parasites can produce a tremendous number of eggs (800,000–8,000,000 eggs/day/patient). The production of such enormous number of eggs requires a highly active DNA and RNA synthesis which involves a dynamic production of purine and pyrimidine nucleotides, the building blocks of DNA and RNA. Therefore, interference with purine and pyrimidine synthesis in schistosomes would result in the cessation of oviposition and the pathogenesis of schistosomiasis as a result of blocking RNA and DNA synthesis.
The synthesis of purines and pyrimidines can proceed via the de novo and/or the salvage pathways. The de novo pathway utilizes simple compounds for the synthesis of the various purines and pyrimidines. The salvage pathways, on the other hand, are reutilization routes by which the cell can satisfy its purine and pyrimidine requirements from endogenous and/or exogenous preformed purines and pyrimidines. Searching for differences between the parasites and their host in these metabolic pathways could provide highly selective targets for anti-schistosomal chemotherapy. Indeed, one of the most striking differences between schistosomes and its mammalian host is that schistosomes differ significantly from their host with respect to purine metabolism. Senft and co-workers at Brown University (Senft et al., 1972, 1973a and 1973b; Stegman et al., 1973; Crabtree and Senft, 1974; Miech et al., 1975; Senft and Crabtree, 1983) established that schistosomes are incapable of de novo purine biosynthesis and are dependent on the salvage pathway to meet their purine requirements. This was quite surprising since the production of enormous numbers of eggs by the schistosomes requires a highly active purine metabolism for RNA and DNA synthesis. The importance of these differences in nucleotide metabolism between the parasite and its hosts was further highlighted by the discovery that potent nucleoside transport inhibitors of mammalian systems do not significantly inhibit the uptake of nucleosides in S. mansoni (el Kouni et al., 1983a; el Kouni and Cha, 1987). Based on these findings, a successful antischistosomal chemotherapeutic regimen was developed. The highly toxic purine analogues tubercidin and nebularine were made selectively toxic against S. mansoni, S. japonicum and S. haematobium by simultaneous administration of a nucleoside transport inhibitor as an antidote for the host but not the parasite (el Kouni et al., 1983a, 1985, 1987 and 1989; Bear et al., 1988; Baer, 1989; el Kouni, 1991, 1992 and 2003).
Pyrimidines, like purines, are required by all living organisms for the synthesis of DNA, RNA and other metabolites. Studies on the biological, chemical and pharmacological aspects of pyrimidine metabolism in various organisms, from prokaryotes to mammalian systems, are numerous and have been discussed in several reviews by O’Donovan and Neuhard (1970), Henderson and Paterson (1973), Levine et al. (1974), Jaffe (1975), Hurst (1980), Jones (1980), Kensler and Cooney (1981), Munch-Peterson (1983), Hammond and Gutteriddge (1984), Hassan and Coombs (1988), Evans and Guy (2004), Hyde (2007), Garavito et al. (2015), and Krungkrai and Krungkrai (2016). However, in contrast to the relatively extensive work on purine metabolism in schistosomes (Senft et al., 1972, 1973a and 1973b; Stegman et al., 1973; Crabtree and Senft, 1974; Miech et al., 1975; Levy and Read, 1975a and 1975b; Senft and Crabtree, 1983; el Kouni et al., 1983a, 1985, 1987 and 1989; Dovey, et al., 1984 and 1985; el Kouni and Cha, 1987; Baer et al., 1988; el Kouni, 1991; Craig III et al., 1991; Yuan et al., 1993; Kanaaneh et al., 1994: Kanaani et al., 1995 and 1997; Foulk et al., 2002; Pereira et al., 2003, 2005, 2010a and 2010b; da Silveira et al., 2004; Yang et al., 2007; Castilho et al., 2010; D’Muniz-Pereira et al., 2011; Postigo et al., 2010; Marques-Ide et al., 2012; de Moraes et al., 2013; Romanello et al., 2013 and 2017; Saverese and el Kouni, 2014; Torini et al., 2016; Zeraik et al., 2017), little information is available on pyrimidine metabolism in this parasite.
Contrary to their inability to synthesize purines de novo, schistosomes are capable of de novo pyrimidine synthesis in addition to their capacity of pyrimidine salvage. The possession of both the biosynthesis and salvage routes would appear to make pyrimidine metabolism an unattractive drug target. Nevertheless, many of the enzymes involved in these pathways are essential for survival and, unlike purine metabolism, there is little redundancy in the pathways of pyrimidine metabolism. This paper will attempt to review the broad aspects of pyrimidine metabolism in schistosomes and compare them to their mammalian host in an effort to shed light on similarities and differences between this parasite and their host. Elucidation of pyrimidine metabolism in schistosomes not only contributes to the general knowledge of metabolism in this parasite, but may also reveal potential targets for the treatment of schistosomiasis with one or more of the already available chemotherapeutic pyrimidine analogues. Studies on pyrimidine metabolism in other parasites have been more elaborate and sophisticated. Therefore, pyrimidine metabolism in schistosomes is compared with that in other parasites in the hope of providing leads on how to identify likely chemotherapeutic targets.
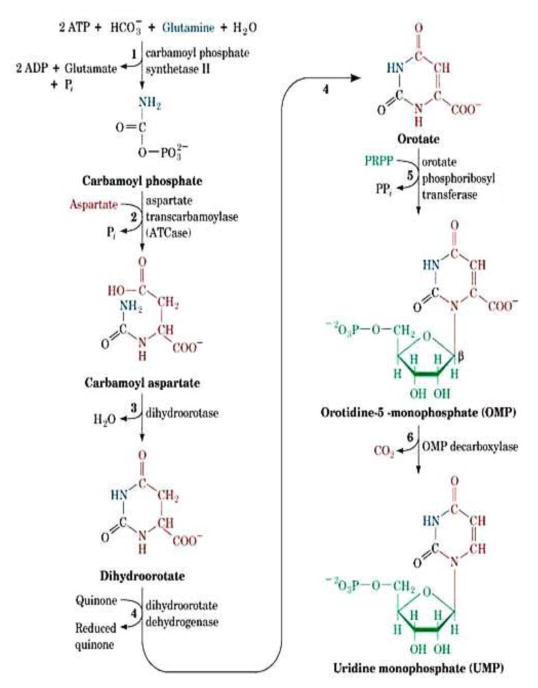
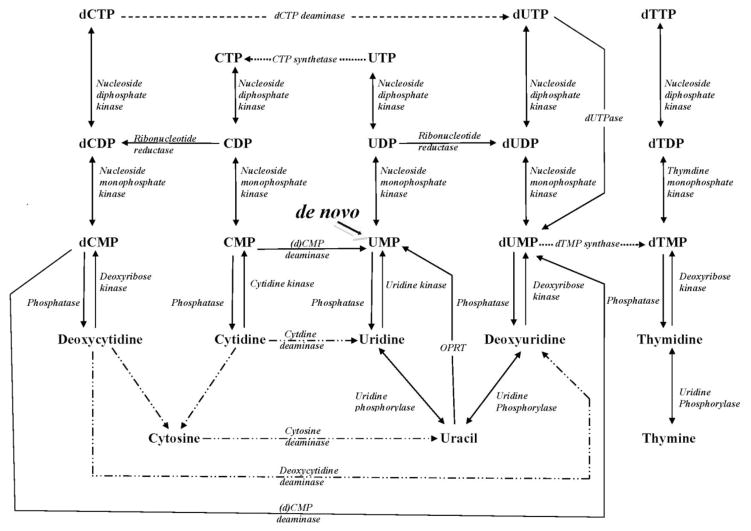
De novo pyrimidine nucleotide biosynthesis is defined as the formation of UMP (uridine 5′-monophosphate) which is considered the “focal point” of pyrimidine metabolism since all other pyrimidine nucleotides can be synthesized from this compound. Fig. 1 summarizes the state of knowledge regarding the de novo biosynthesis of UMP as compiled from studies on various organisms. De novo UMP biosynthesis consists of six sequential enzymatic steps (Fig. 1) which are practically universal in all systems studied from prokaryotes to mammals and have remained intact throughout evolution, although the primary structures of the enzymes responsible for pyrimidine biosynthesis may deviate significantly among prokaryotes, parasitic protozoa, fungi, animals, and mammals including humans.
Fig. 1.
Pyrimidine de novo pathway (After Voet and Voet, 2004).
The first step in the pathway begins with the synthesis of carbamyl phosphate from glutamine, bicarbonate (HCO3−) and 2 moles of ATP and ends by six enzymatic steps later as UMP. The activities of all six enzymes involved in de novo UMP biosynthesis were demonstrated in extracts of S. mansoni by Aoki and Oya (1979), Hill, et al. (1981b), el Kouni et al. (1983b), Iltzsch et al. (1984) and S. japonicum by Huang et al. (1985). These studies established that the parasite is capable of de novo pyrimidine biosynthesis. This is in contrast to the inability of the worms to synthesize purines de novo (Senft et al., 1972, 1973a and 1973b; Stegman et al., 1973; Crabtree and Senft, 1974; Miech et al., 1975; Senft and Crabtree, 1983).
De novo pyrimidine biosynthesis has been detected in most parasites studied except Eimeria tenella (Hill et al., 1981b), Giardia intestinalis (Lindmark and Jarroll, 1982; Aldritt et al., 1985; Jarroll et al., 1989), Trichomonas vaginalis (Hill et al., 1981b; Miller and Lindstead, 1983; Heyworth et al., 1984; Wang and Cheng, 1984a), Tritrichomonas foetus (Wang et al., 1983; Hassan and Coombs, 1988), Cryptosporidium (Striepen et al., 2004), and are thus pyrimidine auxotrophs. It was reported previously that Entamoeba histolytica is able to de novo synthesize pyrimidines (Reeves, 1984). Surprisingly, however, none of the enzymes of the pyrimidine synthesis pathway can be identified within the genome (Anderson and Loftus, 2005).
It is interesting to note that de novo pyrimidine biosynthesis is required for virulence of Toxoplasma gondii (Fox and Bzik, 2002) and Trypanosoma brucei (Ali et al., 2013b; Ong et al., 2013) in vivo. Furthermore, T. brucei mutants that are deficient in de novo UMP synthesis regain virulence when maintained in tissue culture for a long time. Uptake studies showed that these mutants increased their transport of uracil from the media to overcome the inhibition of the de novo pathway (Ong et al., 2013).
2. Enzymes of de novo pyrimidine biosynthesis
2.1. Carbamyl phosphate synthetase II (EC 6.3.5.5), aspartate transcarbamylase (EC 2.1.3.2) and dihydroorotase (EC 3.5.2.3)
Carbamyl phosphate synthetase II, aspartate transcarbamylase and dihydroorotase are the first three enzymes in de novo pyrimidine biosynthesis. Carbamyl phosphate synthetase II catalyzes the following reaction.
Carbamyl phosphate synthetase II is specific for pyrimidine biosynthesis and differs from carbamyl phosphate synthetase I (EC 6.3.4.16) which utilizes N-acetyl-L-glutamate, instead of L- glutamine (or ammonia), as a nitrogen donor for the synthesis of the carbamyl phosphate that becomes the precursor for arginine but not pyrimidine biosynthesis.
In the next step, aspartate transcarbamylase catalyzes the condensation of carbamyl phosphate with L-aspartate to form carbamyl aspartate as follows:
Cyclization of the carbamyl aspartate to produce the pyrimidine ring is catalyzed by dihydroorotase. Dihydroorotase in a reversible condensation reaction eliminating one molecule of water from carbamyl aspartate and close the pyrimidine ring to form 5,6-dihydroorotic acid as follows:
The three enzymes, carbamyl phosphate synthetase II, aspartate transcarbamylase and dihydroorotase have been isolated and partially purified from S. mansoni by Aoki and Oya (1979). These enzymes occur in the cytosol (Aoki and Oya, 1979; el Kouni et al., 1983b). The relative activities of carbamyl phosphate synthetase II, aspartate transcarbamylase and dihydroorotase are 1:45:5 (Aoki and Oya, 1979). The three enzymes are also present in Ascaris suum, Angiostrongylus cantonensis (Aoki et al., 1980), Paragonimus ohirai, Clonorchis sinensis (Kobayashi et al., 1978), Toxoplasma gondii (Schwartzman and Pfefferkorn, 1981; Hill et al., 1981b; Asai et al., 1983), Plasmodium berghei (Hill et al., 1981a), P. falciparum (Hill et al., 1981b; Gero et al., 1984), Crithidia fasciculata, Trypanosoma cruzi, Leishmania major, Fasciola gigantica, Hymenolepis diminuta, Nippostrongylus brasiliensis and Trichuris muris, but not Eimeria tenella (Hill et al., 1981b). In Trichomonas vaginalis, only carbamyl phosphate synthetase II activity was detected (Hill et al., 1981b) explaining why this parasite is devoid of de novo pyrimidine biosynthesis.
The three enzymes, carbamyl phosphate synthetase II, aspartate transcarbamylase and dihydroorotase from Plasmodium falciparum were cloned and expressed and shown to contain unusual protein inserts when compared with sequences of these enzymes from other organisms (Christopherson et al., 2004). Aspartate transcarbamylase (Mejias-Torres and Zimmermann, 2002) and dihydroorotase (Robles Lopez et at., 2006) from Toxoplasma gondii were cloned, purified and characterized.
In Schistosomes, carbamyl phosphate synthetase II is inhibited by uridine 5′-di- and 5′-triphosphates (Aoki and Oya, 1979). Similar results were found in Paragonimus ohirai, Clonorchis sinensis (Kobayashi et al., 1978), Ascaris suum, Angiostrongylus cantonensis (Aoki et al., 1980), and Plasmodium falciparum (Gero et al., 1984). Thus, the enzyme seems to be a rate limiting step of pyrimidine de novo biosynthesis in these parasites.
Schistosomal carbamyl phosphate synthetase II, aspartate transcarbamylase and dihydroorotase appear to exist as a multienzyme complex (Aoki and Oya, 1979), as is the case in Ascaris suum (Aoki et al., 1980) and mammalian systems (c.f. Jones, 1980). The significance of this complex is that the intermediate metabolites are “channeled” from the catalytic site of one enzyme to the other without being diluted in the cell. The result is that these intermediates are protected from degradation by enzymes such as the carbamyl phosphate phosphatases (Black and Jones, 1984), hence, a highly efficient metabolic process. This contrasts with carbamyl phosphate synthetase II, aspartate transcarbamylase and dihydroorotase of Plasmodium berghei (Krungkrai et al., 1990), Crithidia fasciculata (Aoki and Oya, 1987; Krungkrai et al., 1990), Leishmania donovani (Mukherjee et al., 1988), L. mexicana, and Trypanosoma cruzi (Nara et al., 1998; Gao et al., 1999), where these three enzymes are not covalently linked and are present in three discrete monofunctional proteins. Nevertheless, a tri-enzyme complex can be formed in Trypanosoma cruzi from direct interactions between the three enzymes (Nara et al., 2012). It is unclear whether or not this is the case in Plasmodium berghei. Hill et al. (1981a and 1981b) reported that carbamyl phosphate synthetase II and aspartate transcarbamylase is a bifunctional enzyme in P. berghei; whereas, Krungkrai et al. (1990) were able to separate the three enzymatic activities by gel filtration chromatography.
2.2. Dihydroorotate dehydrogenase (EC 1.3.3.1)
Dihydroorotate dehydrogenase is usually mitochondrial and connected to the respiratory chain in mammals. It catalyzes the fourth step in de novo pyrimidine biosynthesis, which involves the ubiquinone-mediated oxidation of dihydroorotate to form orotate in the following manner:
The enzyme is present in extracts of Schistosoma mansoni as a membrane-bound enzyme (el Kouni et al., 1983b). In Toxoplasma gondii (Asai et al., 1983; Hortua Triana et al., 2012), Plasmodium knowlesi, P. berghei, P. gallinaceum (Gutteridge et al., 1979; Krungkari et al., 1991) and P. falciparum (Gutteridge et al., 1979; Gero et al., 1984; Krungkrai, 1995), the enzyme is particulate and probably mitochondrial. The physiological electron acceptor for the schistosome enzyme has not yet been identified but most probably it uses coenzyme Q as an electron acceptor (M. H. el Kouni unpublished). This is the case in Babesia bovis, B. bigemina (Gero et al., 1983), Plasmodium falciparum (Krungkrai, 1995), and Toxoplasma gondii (Hortua Triana et al., 2012), where the enzyme is intimately connected to the electron transport chain to which it passes electrons directly, probably at the ubiquinone level. In contrast, in certain species of Leishmania (Gero and Coombs, 1980; Hammond and Gutteridge, 1982; Feliciano et al., 2006), Crithidia fasciculata (Pascal Jr. et al., 1983) and Trypanosoma (Gutteridge et al., 1979; Hammond and Gutteridge, 1982 and 1984; Pascal Jr. et al., 1983; Takashima et al., 2002; Annoura et al., 2005), dihydroorotate dehydrogenase is located in the cytosol, and utilizes fumarate as an electron acceptor (Takashima et al., 2002; Feliciano et al., 2006).
The enzymes from Toxoplasma gondii (Sierra-Pagan and Zimmermann, 2003), Leishmania major (Feliciano et al., 2006) and Trypanosoma brucei (Arakaki et al., 2008), were cloned, expressed, and characterized. Dihydroorotate dehydrogenase in Toxoplasma gondii is not only essential for de novo pyrimidine biosynthesis but also for mitochondrial function that is not directly dependent on the enzyme activity (Hortua Triana et al., 2016). Similarly, enzyme knockouts of Trypanosoma cruzi could not survive even in the presence of pyrimidine nucleosides, suggesting a vital role of dihydroorotate dehydrogenase activity in the regulation of cellular redox balance (Annoura et al., 2005).
Among the dihydroorotate dehydrogenase inhibitors, atovaquone, an ubiquinone analogue, leflunomide, an antirheumatic drug, brequinar, an immunosuppressive, and triazolopyrimidines, significantly inhibit enzyme activity as well as the growth of Toxoplasma gondii (Araujo et al., 1991; Moshkani and Dalimi, 2000; Hortua Triana et al., 2012; Ferreira et al., 2012), Plasmodium falciparum (Seymour et al., 1994; Basco et al., 1995; Gujjar et al., 2011), Babesia microti (Wittner et al., 1996; Gray and Pudney, 1999; Lawres et al., 2016), and B. bovis (Kamyingkird et al., 2014) in vitro. Knockout of dihydroorotate dehydrogenase in Trypanosoma brucei greatly reduced growth in pyrimidine depleted medium (Arakaki et al., 2008). If a similar case exists in schistosomes, dihydroorotate dehydrogenase could be a target for the development of drug for the treatment of schistosomiasis. Nevertheless, despite the availability of potent dihydroorotate dehydrogenase inhibitors, no study has evaluated the effect of these inhibitors on schistosomal dihydroorotate dehydrogenase. Therefore, the assessment and characterization of schistosomal dihydroorotate dehydrogenase and assessment of its potential as a new drug target for schistosomes remains to be evaluated.
2.3. Orotate phosphoribosyltransferase (EC 2.4.2.10) and orotidine 5′-monophosphate decarboxylase (EC 4.1.1.23)
Orotate phosphoribosyltransferase is the fifth enzyme in the de novo pyrimidine biosynthesis pathway. It catalyzes the transfer of a ribosyl phosphate group from PRPP (α-D-phosphoribosylpyrophosphate) to orotate leading to the formation of OMP (orotidine 5′-monophosphate) as follows:
OMP decarboxylase is the last enzyme in de novo pyrimidine biosynthesis and catalyzes the decarboxylation of OMP to UMP as follows:
Orotate phosphoribosyltransferase and OMP decarboxylase are present in the cytosol of schistosomes (Hill et al, 1981b; el Kouni et al., 1983b; Iltzsch et al., 1984; Huang et al., 1985; el Kouni and Naguib, 1990). However, Schistosoma mansoni has two distinct phosphoribosyltransferases (Iltzsch et al., 1984). These two enzymes differ from one another in their molecular weights as well as substrate and inhibitor specificities. The enzyme with the higher molecular weight, is non-specific for the substrates, as it catalyzes the conversion of orotate, 5-fluorouracil, and uracil to their respective nucleoside 5′-monophosphate. The other enzyme, which has a slightly lower molecular weight, appears to be specific for orotate. Both the specific and non-specific enzymes are inhibited by 5-azaorotic acid (Iltzsch et al., 1984), as is the case with orotate phosphoribosyltransferase from Plasmodium berghei (O’Sullivan and Ketley, 1980) and Toxoplasma gondii (Asai et al., 1983; Javaid et al., 1999), but only the “orotate-specific” enzyme is inhibited by 4,6-dihydroxypyrimidine (Iltzsch et al., 1984). The exact physiological role(s) of these two phosphoribosyltransferases is unclear. It would appear that both enzymes are capable of catalyzing de novo UMP biosynthesis. It is possible, however, that the primary function of the orotate-specific enzyme may be de novo UMP biosynthesis; whereas, the non-specific enzyme may function as a salvage enzyme for uracil by converting it to UMP (See below 6.7.).
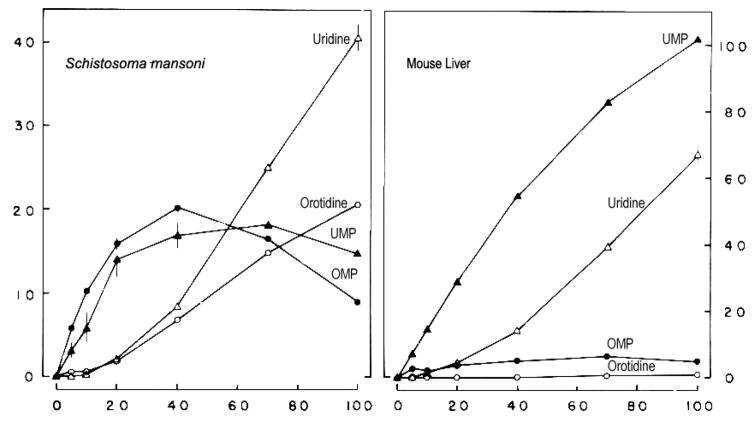
Study of orotate metabolism revealed significant differences between Schistosoma mansoni and mammalian enzymes (Iltzsch et al., 1984). In mouse liver, the major products of orotate metabolism were UMP and uridine with a small amount of OMP and no detectable orotidine (Fig. 2). This is due to the fact that in mammalian cells, orotate phosphoribosyltransferase and OMP decarboxylase exist as a multienzyme complex (Jones, 1980). Therefore, OMP is “channeled” directly to OMP decarboxylase to form UMP (Traut and Jones, 1977; Traut, 1980; Reyes, 1977). As a result, no OMP or orotidine are detected in the cell (Janeway and Cha, 1977). Furthermore, in mammalian cells, OMP decarboxylase activity is greater than orotate phosphoribosyltransferase activity (Kavipurau and Jones, 1977; Brown and O’Sullivan, 1977; Levinson et al., 1979; McClard et al., 1980) presumably to facilitate the “channeling effect”. Iltzsch et al. (1984) showed that this relationship between the two enzyme activities is also present in both S. mansoni and mouse liver. Nevertheless, in contrast to mammalian system, orotidine and uridine were the predominant products of orotate metabolism in S. mansoni (Fig. 2). These results led Iltzsch et al. (1984) to suggest that orotate phosphoribosyltransferase and OMP decarboxylase in S. mansoni may exist as separate enzymes as in most prokaryotes and lower eukaryotes rather than as a multienzyme complex typical of mammalian cells (Jones, 1980), and Trypanosoma cruzi (Gao et al., 1999). However, attempts to separate these two enzymes in Schistosoma mansoni were unsuccessful (Iltzsch et al., 1984). While failure to separate these enzymes does not prove that they are indeed part of a multienzyme complex, it does suggest that orotate phosphoribosyltransferase activity may be associated with OMP decarboxylase activity in S. mansoni.
Fig. 2.
Time course of orotate metabolism by extracts of Schistosoma mansoni (21.8 mg protein/mL) or mouse liver (8.3 mg protein/ml). The amount of extract used was 325 μL for Schistosoma mansoni and 375 μL for mouse liver. (●) OMP; (▲) UMP; (○) orotidine; (△) uridine. (After Iltzsch et al., 1984)
The pattern of product formation of orotate metabolism over time in S. mansoni (Fig. 2) resembles that in Plasmodium falciparum, where OMP accumulates more than UMP at the initial stages of the time course (Rathod and Reyes, 1983). However, orotidine and uridine did not accumulate in P. falciparum as in Schistosoma mansoni. This contradiction may be explained by the fact that Plasmodium falciparum have little OMP phosphatase (EC none) activity (Rathod and Reyes, 1983), as well as the nature of orotate phosphoribosyltransferase and OMP decarboxylase in P. falciparum. The genes encoding the two enzymes in P. falciparum are distinct and located on different chromosomes. The two enzymes are produced separately, but they get cross-linked later and appear as a multienzyme complex with different kinetic properties from the host bifunctional protein complex (Christopherson et al., 2004; Krungkrai et al., 2004 and 2005; Krungkrai and Krungkrai, 2016). Proteomic data and structural modeling showed that an insertion of a low complexity amino acid sequence is responsible for this interaction of orotate phosphoribosyltransferase and OMP decarboxylase in P. falciparum (Imprasittichail et al., 2014). This structural complex of the parasite enzymes provides an efficient functional kinetic advantage. Therefore, it appears that orotate phosphoribosyltransferase and OMP decarboxylase from P. falciparum have unique structural and functional properties, sharing characteristics of the monofunctional pyrimidine-metabolizing enzymes in prokaryotes and bifunctional complexes in eukaryotes (Krungkrai et al., 2005). A similar situation may exist in Schistosoma mansoni and can explain the lack of “channeling” effect along with inability to separate orotate phosphoribosyltransferase and OMP decarboxylase from one another observed by Iltzsch et al. (1984). Regardless of whether or not these enzymes comprise a multienzyme complex in S. mansoni, there does not appear to be a “channeling effect” as seen for mouse liver and other mammalian cells (Traut and Jones, 1977; Reyes, 1977; Traut, 1980).
The subcellular location of phosphoribosyltransferase activity in the cytosol of S. mansoni is similar to many other organisms (see Jones, 1980) as well as most parasites (Hill et al., 1981a and 1981b; Gero and Coombs, 1982; Miller and Linsdtead, 1983; Wang et al., 1983; Asai et al., 1983). In contrast, the enzyme is associated with the glycosomes in Crithidia fasciculata (Gero and Coombs, 1980; Hammond and Gutteridge, 1982), C. luciliae (Pragobpol et al., 1984), certain species of Leishmania (Gutteridge et al., 1979; Hammond et al., 1981; Gero and Coombs, 1980; Hammond and Gutteridge, 1982), and Trypanosoma (Gutteridge et al., 1979; Hammond et al., 1981; Hammond and Gutteridge, 1982 and 1984).
Both orotate phosphoribosyltransferase and OMP decarboxylase activities are present in Toxoplasma gondii (Hill et al., 1981b; Asai et al., 1983), Plasmodium berghei (O’Sullivan and Ketley, 1980; Hill et al., 1981a), P. falciparum (Hill et al., 1981b; Reyes et al., 1982; Gero et al., 1984), Angiostrongylus cantonensis (So et al., 1992). Crithidia fasciculata, Trypanosoma cruzi, Leishmania major, Eimeria tenella, Plasmodium berghei, Fasciola gigantica, Hymenolepis diminuta, Nippostrongylus brasiliensis and Trichuris muris, but are missing from Trichomonas vaginalis (Hill et al., 1981b) explaining why this parasite is devoid of de novo pyrimidine biosynthesis. Orotate phosphoribosyltransferase and OMP decarboxylase are the only enzymes of the de novo pathway that are present in Eimeria tenella (Hill et al., 1981b) which is incapable of de novo pyrimidine biosynthesis. It is interesting to note that in Trypanosoma brucei, orotate phosphoribosyl transferase and OMP decarboxylase are essential for the virulence and survival of the parasite in vivo (Ali et al., 2013b; Ong et al., 2013).
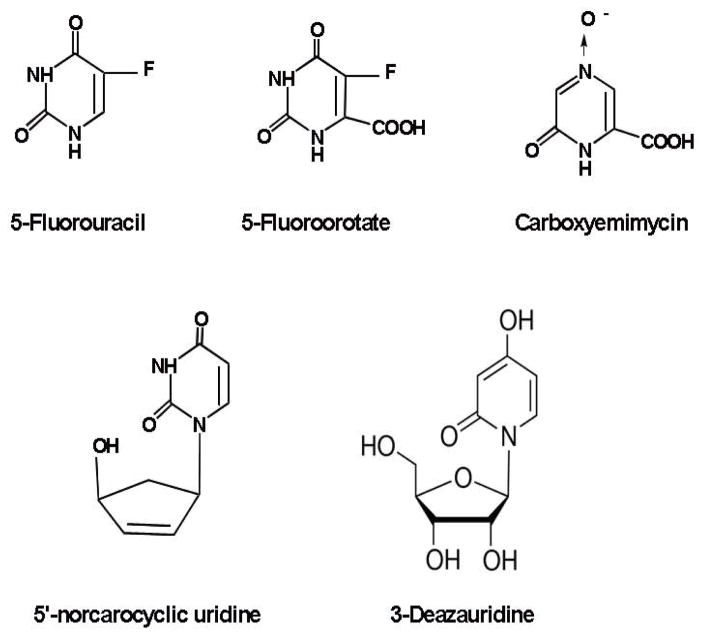
A large number of orotate analogues have been systematically tested against toxoplasma orotate phosphoribosyltransferase and structural features necessary for strong binding were defined (Javaid, et al., 1999). 1-Deazaorotic, 6-iodouracil, 5-bromoorotic acid and 2-methylthioorotic acid were identified by Javaid, et al. (1999) as better ligands of the parasite enzyme than the mammalian counterpart studied earlier by Niedzwicki et al. (1984). The orotic acid analogue, 1,6-dihydro-6-oxo-2-pyrazinecarboxylic acid 4-oxide also known as carboxyemimycin (Fig. 3), exhibited marked anticoccidial activities against Eimeria tenella, E. necatrix, E. acervulina, and E. maxima (Matsuno et al., 1984). In Plasmodium falciparum, which are completely dependent on de novo pyrimidine metabolism, another orotate analogue, 5-fluoroorotate (Fig. 3), was shown to inhibit chloroquine-resistant clones in vitro with an IC50 of 6 nM (Gómez and Rathod, 1990). Mammalian cells are far less sensitive to 5-fluoroorotate, particularly in the presence of uridine. 5-Fluoroorotate, in combination with uridine, cured mice infected with P. yoelii without obvious host-toxicity. The mice were immune to subsequent challenge with a potentially lethal inoculum of P. yoelii (Gómez and Rathod, 1990). The activity of 5-fluoroorotate is mediated through its metabolism to FdUMP (5-fluoro-2′-deoxyuridine 5′-monophosphate), a suicide inhibitor of thymidylate synthase (Santi and McHenry, 1972) and DNA synthesis (see below 2.5.), or by undergoing processing to FUTP (5-fluorouridine 5′-triphosphate) that may inhibit carbamyl phosphate synthetase II (Seymour et al., 1994).
Fig. 3.
Chemical structures of various pyrimidine analogues that exhibited antiparasitic activity.
2.4. Orotidine 5′-monophosphate phosphatase (EC none)
In Schistosoma mansoni, the intermediate metabolite OMP is dephosphorylated to orotidine by a phosphatase (Iltzsch et al., 1984). It is unclear whether OMP phosphatase activity in S. mansoni is due to an OMP-specific enzyme, as in microsomal extracts of mouse liver (el Kouni and Cha, 1982), or to a non-specific phosphatase. Although such a phosphatase is present in mammalian cells, excessive OMP degradation does not occur because of the “channeling mechanism”. Plasmodium falciparum, on the other hand, appear to have little OMP phosphatase activity (Rathod and Reyes, 1983). Cytosolic OMP phosphatase in Schistosoma mansoni may be different from that in mouse liver as it is inhibited by 6-azaUMP; whereas in mouse liver it is not inhibited at all (Iltzsch et al, 1984). Since there are no salvage pathways for orotidine in S. mansoni, the accumulation of orotidine in this parasite would appear to be inefficient metabolism when compared to other organisms.
2.5. Thymidylate synthase (EC 2.1.1.45) and dihydrofolate reductase (EC 1.5.1.3)
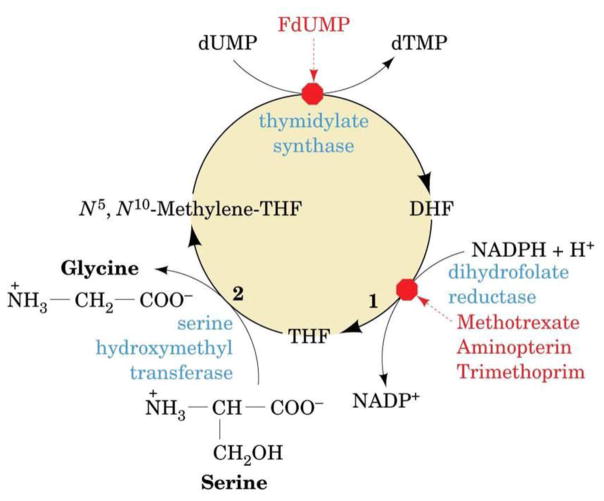
Thymidylate synthase is a key enzyme in DNA synthesis. This enzyme, together with dihydrofolate reductase, are involved in the biosynthesis of dTMP (thymidine 5′-monophosphate). Thymidylate synthase is the sole de novo source of dTMP. It catalyzes the conversion of dUMP (2′-deoxyuridine 5′-monophosphate) and N5,N10-methylenetetrahydrofolate to dTMP and 7,8-dihydrofolate, respectively, as follows:
Subsequently, dihydrofolate reductase reduces 7,8-dihydrofolate by NADPH to generate 5,6,7,8- tetrahydrofolate. The reaction is NADPH dependent. NADH will also work, albeit less efficiently.
Serine hydroxymethyltransferase (EC 2.1.2.1) then catalyzes the reversible transfer of one carbon unit from L-serine to regenerate N5,N10-methylenetetrahydrofolate as follows:
Fig. 4 depicts the dTMP synthesis cycle showing the sequential reactions and metabolic relationship of thymidylate synthase, dihydrofolate reductase, and serine hydroxymethyltransferase.
Fig. 4.
The sequential reactions and metabolic relationships of dihydrofolate reductase and thymidylate synthase for the cycle of dTMP synthesis. DHF, dihydrofolate; THF, tetrahydrofolate. (After Voet and Voet, 2004).
The structure and function of thymidylate synthase and dihydrofolate reductase in parasites have been extensively studied. They are popular chemotherapeutic targets as inhibition of either enzyme will block DNA synthesis. Indeed, as seen in Fig. 4, inhibitors of dihydrofolate reductase (e.g., methotrexate, trimethoprim, aminopterin, pyrimethamine, etc.) and thymidylate synthase (e.g., 5-fluorouracil or 5-fluoroorotate as precursors of FdUMP), are useful in treating several human ailments including parasitic diseases.
Earlier reports indicated that no thymidylate synthase activity was found in schistosomes or filaria (Jaffe, 1971; Jaffe et al., 1972). However, later reports indicated the presence of the enzyme in Dirofilaria and Brugia pahangi (Jaffe and Chrin, 1980). In addition, uracil and uridine were shown to be incorporated into the DNA in filaria and schistosomes (Jaffe et al., 1972; el Kouni and Naguib, 1990), suggesting the presence of the enzyme in these parasites. Thymidylate synthase is also found in the nematodes; Angiostrongylus cantonensis (So et al., 1992), Trichinella spiralis and T. pseudospiralis (Dabrowska et al., 1996; Rode et al., 2000). Comparative studies on inhibition of purified T. spiralis and rat thymidylate synthases by substrate analogues, 4-thio-5-fluoro-dUMP, 2-thio-5-fluoro-dCMP and N4-hydroxy-dCMP, indicated that only dUMP analogues show weak selectivity towards the parasite enzyme (Rode et al., 2000).
The presence of thymidylate synthase activity has been reported also in the protozoa; Crithidia fasciculata, C. oncopelti, the blood forms of Trypanosoma brucei, T. congolense, T. lewisi, and blood, intracellular and culture forms of T. cruzi (Walter et al., 1970). Enzyme activity in the cytosol fractions of these parasites were compared with mammalian enzymes. The parasite enzymes have apparent molecular weights in the range 175,000–200,000, as determined by molecular sieving on Sephadex G-200, which are about three times higher than those of mammalian enzymes. The trypanosomatid enzymes have higher apparent Km values for substrate (dUMP) and cofactor (N5,N10-methylenetetrahydrofolate). No evidence was obtained for the regulation of the parasite enzyme, either by the product, dTMP, or by dTDP (thymidine 5′-diphosphate) or dTTP (thymidine 5′-triphosphate). The trypanosomal enzymes are inhibited by Mg2+, and are more sensitive to mercaptoethanol. Their activities are sensitive to inhibition by fluorinated pyrimidines, a property they share with thymidylate synthases from all sources. The parasite enzymes are also markedly more sensitive to inhibition by suramin (IC50 ~1.8 ×10 −6 M) than foetal rat liver enzyme (IC50 1.1 × 10 −3 M). The trypanosomal enzyme is, therefore, a possible target for chemotherapeutic attack, either on its own or in combination with a dihydrofolate reductase inhibitor (Al Chalabi and Gutteridge, 1977a).
Dihydrofolate reductase is present in Schistosoma mansoni (Jaffe, 1971; Jaffe et al., 1972; Serrão et al., 2017b), and the adult filarial worms; Dirofilaria immitis, Litomosoides carinii, Dipetalonema witei, Brugia pahangi, Onchocerca volvulus (Jaffe, 1971; Jaffe et al., 1972; Jaffe and Chrin, 1980), the nematodes; Aphelenchus Avenae, Nippostrongylus brasiliensis (Platzer, 1974a), Trichinella spiralis, T. pseudospiralis (Rode et al., 2000), and the protozoa Plasmodium lophurae (Platzer, 1974b). The enzyme from of P. lophurae differed from the host enzyme in greater molecular weight, pH optimum, substrate, cofactor specificity, stimulation by salts (Platzer, 1974b), and higher sensitivity to pyrimethamine inhibition (Platzer, 1974b; Bilsland et al., 2011). The schistosomal and filarial dihydrofolate reductases, on the other hand, closely resemble the enzyme from rat liver. The molecular weight of each of the three enzymes is around 20,000. The three enzymes have strong preference for dihydrofolate over folate as substrate, and NADPH over NADH as a cofactor. The apparent Km of dihydrofolate for the parasite enzymes is much higher than that of the rat liver enzyme. There is less of a difference between the apparent KNADPH of the parasite and rat liver enzymes. Both schistosomal and filarial dihydrofolate are less sensitive or equisensitive to inhibition by folate analogues than the mammalian enzyme (Jaffe, 1971; Jaffe et al., 1972; Bilsland et al., 2011). This suggests that it is unlikely that these compounds could be selectively toxic as antischistosomal or antifilarial agents. This suggestion is further supported by recent biochemical, kinetics and structural studies on recombinant schistosomal dihydrofolate reductase (Serrão et al., 2017b). The gathered information showed high structural similarity and the conservation of interactions at the folate and NADP+ binding sites of the parasite and host enzymes which would make it difficult to infer that a specific dihydrofolate reductase inhibitor can be found.
Dihydrofolate reductase and thymidylate synthase usually are distinct monofunctional enzymes in most organisms. In protozoa, however, the two enzymes coexist as a bifunctional protein (Ferone and Roland, 1980; Coderre et al., 1983; Garrett et al., 1984). Though sharing the same protein, the two enzymatic activities are not interdependent. When the gene portion which encodes dihydrofolate reductase is expressed, the protein functions normally (Sirawaraporn et al., 1993). It may be interesting here to note that the bifunctional protein is used as a criterion for identifying protozoa among microorganisms of uncertain taxonomy. For example, by using this distinction, it was decided that Pneumocystis carinii is a fungus rather than a protozoan (Edman et al., 1989).
The bifunctional enzyme dihydrofolate reductase-thymidylate synthase from Cryptosporidium parvum contains novel residues at several positions analogous to those at which point mutations have been shown to produce antifolate resistance in other dihydrofolate reductases (Vásquez et al., 1996). Thus, C. parvum dihydrofolate reductase may be intrinsically resistant to inhibition by some dihydrofolate reductase inhibitors which may explain why cryptosporidiosis is refractory to treatment with the clinically common antibacterial and antiprotozoal antifolates.
Not all parasites have thymidylate synthase and dihydrofolate reductase. Neither enzyme could be detected in Tritrichomonas foetus (Wang et al., 1983), Trichomonas vaginalis (Heyworth et al., 1984; Wang and Cheng, 1984a), Entamoeba histolytica, E. invadens (Garrett et al., 1984), and Giardia intestinalis (Aldritt et al., 1985). These parasites rely on the activities of nucleoside phosphotransferases (see below 6.2.) to salvage exogenous deoxyribosides, including, thymidine, for the synthesis of DNA.
The significance of dihydrofolate reductase-thymidylate synthase in various protozoa has been and still is the focus of intensive research efforts to identify inhibitors of this bifunctional enzyme using different methodologies including: design, synthesis, and evaluation of analogues, the use of crystal structure of wild-type and mutant enzymes as templates for designing novel drugs against resistant-mutant parasites, selective inhibitors interfering with interdomain interactions, molecular dynamics simulation of interactions between rigid and flexible antifolates of wild-type and mutant enzymes, in silico screening for allosteric inhibitors at the interface between the two domains, etc. The discussion of these studies is beyond the scope of the current work and has been reviewed recently by Nyíri and Vértessy (2017).
2.6. Cytidine 5′-triphosphate synthetase (CTP synthetase, 6.3.4.2)
This enzyme carries out the de novo synthesis of CTP (cytidine 5′-triphosphate), the last committed step in pyrimidine nucleotide biosynthesis, and the sole source of CTP from both the de novo and uridine salvage pathways. It is a rate-limiting enzyme. The enzyme catalyzes the ATP (adenosine 5′-triphosphate)-dependent amination of UTP (uridine 5′-triphosphate) to CTP with either L-glutamine or ammonia as a source of nitrogen as follows:
There is no direct evidence that CTP synthetase is present in schistosomes. However, incorporation studies in Schistosoma mansoni (el Kouni and Naguib 1999) showed that uridine and uracil are incorporated into cytidine nucleotides suggesting the existence of the enzyme in these parasites. Similar results were shown in Angiostrongylus cantonensis (So et al., 1992). The enzymes from Giardia intestinalis (Jimenez and O’Sullivan, 1994; Lim et al., 1996), Trypanosoma brucei gambiense, T. b. rhodesiense (Fijolek et al., 2007), and Plasmodium falciparum (Hendriks et al., 1998; Yuan et al., 2005) were cloned, expressed, purified and characterized. The enzyme in Plasmodium falciparum is the largest CTP synthetase found in any organism due to the presence of two novel sequences which are part of the continuous open reading frame and are not introns (Hendriks et al., 1998; Yuan et al., 2005). These features distinguish the parasite enzyme from that of the host making it an attractive target for structure based drug design.
3-Deazauridine (Fig. 3) as well as the glutamine analogues, DON (6-diazo-5-oxo-l-norleucine) and acivicin (α-amino-3-chloro-4,5-dihydro-5-isoxazoleacetic acid) are known inhibitors of CTP synthetase. Acivicin irreversibly inhibits the enzyme from trypanosomes and is trypanocidal in vitro and in vivo (Hofer et al., 2001; Fijolek et al., 2007).
2.7. Ribonucleotide reductase (EC 1.17.4.1)
Ribonucleotide reductase provides the only de novo means of synthesizing both pyrimidine and purine 2′-deoxyribotides, essential precursors for DNA replication and repair. Therefore, the enzyme is highly and allosterically regulated to maintain balanced quantities of the 2′-deoxyriboside 5′-triphosphates required for DNA synthesis. The reaction involves a reduction at the 2′-carbon of the 2′-ribonucleoside 5′-diphosphates as follows:
This reduction is initiated with the generation of a free radical. Following a single reduction, ribonucleotide reductase requires electrons donated from the dithiol groups of the protein thioredoxin. Regeneration of thioredoxin occurs when NADPH provides two hydrogen atoms that are used to reduce the disulfide groups of thioredoxin as follows:
In Trypanosoma brucei, the thioredoxin gene is expressed throughout the life cycle of T. brucei, however, the protein concentration in the parasites is unusually low and the trypanothione/tryparedoxin system seems to be the main donor of reducing equivalents for the parasite synthesis of 2′-deoxyribotides (Dormeyer et al., 2001).
There is no report on the activity of ribonucleotide reductase in schistosomes. Nevertheless, genome analysis indicates the presence of the enzyme in Schistosoma mansoni (Berriman et al., 2009). Furthermore, it was observed that DNA synthesis in male and female S. mansoni was inhibited by hydroxyurea, a potent and selective inhibitor of ribonucleotide reductase, suggesting the presence of active enzyme (Den Hollander and Erasmus, 1984). It should be noted here that, although hydroxyurea has been used to treat a variety of human diseases with few side effects (Parker and Parker, 2004), there are no reports of treating schistosomiasis with this drug.
Hydroxyurea also inhibited the growth and DNA synthesis in Toxoplasma gondii grown in vitro suggesting the presence of ribonucleotide reductase in this parasite (Kasper and Pfefferkorn, 1982). The enzyme was found in the genomes of Entamoeba invadens and E. moshkovskii, but not in the close relatives, E. histolytica and E. dispar, suggesting a recent loss from E. histolytica and E. dispar (Anderson and Loftus, 2005). Giardia lamblia (Baum et al., 1989) and Trichomonas vaginalis (Wang and Cheng, 1984b) also lack ribonucleotide reductase and appear to depend on the transport of deoxyribosides, and activities of nucleoside phosphotransferases (see below 6.2.) to provide deoxyribotides for DNA synthesis.
The genes coding for ribonucleotide reductase in Plasmodium falciparum (Chakrabarti et al., 1993; Rubin et al., 1993), Leishmania mexicana amazonensis (lye et al., 1997), Trypanosoma brucei (Dormeyer et al., 1997), and Cryptosporidium parvum (Akiyoshi et al., 2002) were cloned, sequenced and expressed. The sequences of full-length clones from Plasmodium falciparum showed significant identity with other ribonucleotide reductase sequences in the data base. The gene from Leishmania mexicana amazonensis showed 62% similarity in nucleotide sequence to human and 48.5% to Plasmodium falciparum gene (Lye et al., 1997). Antisense oligodeoxynucleotides against the P. falciparum gene inhibited its growth at concentrations below 0.5 μM (Barker Jr. et al., 1996).
All known ribonucleotide reductases have a quaternary structure where enzymatic activity is dependent on the complex between a large subunit (Rl) dimer and a small subunit (R2) dimer. The interaction between Rl and R2 seems entirely accounted for by C-terminal sequences of R2. The gene coding for the small subunit of ribonucleotide reductase in the nematode Haemonchus contortus was cloned, sequenced, and expressed (Chen et al., 2005). A synthetic oligopeptide corresponding to the C-terminal 7 residues of the small subunits in Plasmodium falciparum inhibited malarial enzyme at concentrations approximately 10-fold lower than that predicted to inhibit the mammalian subunit (Rubin et al., 1993). Furthermore, a recent comparative, annotated, structure-based, multiple-sequence alignment of R2 subunits, identified a clade of R2 subunits unique to Apicomplexa. This novel apicomplexan R2 subunit may be a promising candidate for chemotherapeutic-induced inhibition as it differs greatly from known eukaryotic host ribonucleotide reductases and may be specifically targeted (Munro et al., 2013).
Iron chelators used as ribonucleotide reductases inhibitors were shown to cure Plasmodium berghei malaria in mice (Klayman et al., 1984 and 1986) and are effective against of P. falciparum growth in culture (Pradines et al., 1996; Holland et al., 1998).
2.8. 2′-Deoxyuridine 5′-triphosphate pyrophosphatase (dUTPase, EC 3.6.1.23)
The enzyme dUTPase is a highly active and specific pyrophosphatase that hydrolyze dUTP (2′-deoxyuridine 5′-triphosphate) to dUMP in the following manner:
The nucleotide dUTP is formed by the phosphorylation of dUDP (2′-deoxyuridine 5′-monophosphate), the direct product of ribonucleotide reductase reaction, by nucleoside 5′-diphosphate kinase (Fig. 6). dUTP is a good substrate for DNA polymerase from different species and could end up incorporated into DNA. To prevent such incorporation of dUTP into DNA, dUTPase degrades dUTP to dUMP. The enzyme is quite active resulting in intracellular pools of dUTP at/or less than 0.3 fmol/106 cells and making the incorporation of dUTP into DNA a very rare event (Goulian et al., 1980). Thus, dUTPase has a dual function. First, it produces dUMP, the precursor of dTMP and ultimately dTTP. The second, is preserving the integrity of DNA by removing dUTP from the 2′-deoxyribotide pool, thus reducing the probability of the incorporation of this nucleotide into DNA. Lack or inhibition of dUTP activity leads to harmful perturbations in the nucleotide pool resulting in increased uracil content of DNA that activates a hyperactive futile cycle of DNA repair (Vértessy and Tóth, 2009). Hence, dUTPase is considered an excellent target for chemotherapy.
Fig. 6.
Pyrimidine salvage pathways as gathered from various organisms. In Schistosoma mansoni, solid line, reactions that have been established enzymatically or by genome analysis; dashed lines, reactions that have not been studied yet; dotted lines, reactions that are suggested to exist as inferred from metabolic studies; dotted/dashed lines, reactions that could not be established.
Activity of dUTPase has not been studied in Schistosoma mansoni, but genome analysis indicates the presence of the enzyme in this parasite (Berriman et al., 2009). Enzyme activity of dUTPase has been reported in the nematodes; Trichinella spiralis and T. pseudospiralis (Rode et al., 2000), and the protozoa; Plasmodium falciparum (Whittingham et al., 2005), Leishmania major (Camacho et al., 1997), Trypanosoma cruzi (Hidalgo-Zarco and González-Pazanowska, 2001; Bernier-Villamor et al., 2002) and T. brucei (Castillo-Acosta et al., 2008). The protozoan enzymes were subjected to detailed analysis and characterization.
Currently, there are two recognized major families of dUTPases in protozoa which are unrelated in sequence or structure: dimeric as in Trypanosoma cruzi, T. brucei, Leishmania major, and trimeric as in Plasmodium falciparum. The trimeric dUTPases, including the human enzyme, possess five conserved sequence motifs. These cluster form the substrate recognition site and reaction center, bestowing high selectivity toward dUTP to the exclusion of dCTP (2′-deoxycytidine 5′-triphosphate), dTTP, and UTP. This specificity is achieved through hydrogen bonding patterns that favor the binding of the uracil nucleobase, and intimate interactions with the ribose moiety and the nucleobase that exclude the 2′-hydroxyl group of the pentose sugar and the 5-methyl group of thymine (Persson et al., 2001). The trimeric dUTPase from Plasmodium falciparum has been cloned, overexpressed, and characterized. It has relatively low sequence similarity with its human ortholog (28.4% identity) (Whittingham et al., 2005) making it a suitable drug target for chemotherapy.
The significance of dUTPase in thymidylate biosynthesis in P. falciparum, is enhanced by the lack of salvage pathways in this parasite. Therefore, the enzyme has been and still is the focus of intense research efforts in targeting this enzyme using different methodologies including design, synthesis, and evaluation of analogues (Nguyen et al., 2005 and 2006; Whittingham et al., 2005; McCarthy et al., 2009; Baragaña et al., 2011; Ruda et al., 2011; Hampton et al., 2011), and high throughput searches (Crowther et al., 2011). These studies established that the uracil ring is of utmost importance in binding of ligands to the active site, whereas more variations are allowed at the 3′- and 5′-positions (Recio et al., 2011). Recent ongoing research is focusing on QSAR models (Quantitative Structure–Activity Relationship) to facilitate the design of novel compounds (de Araújo et al., 2015). For a more detailed discussion on the search for inhibitors of P. falciparum dUTPase see review by Nyíri and Vértessy (2017).
Dimeric dUTPases are members of the all-α NTP pyrophosphohydrolase family and represent promising drug targets due to their unique properties regarding substrate specificity and product inhibition as well as significant different structural and biochemical properties which bears no resemblance to typical eukaryotic trimeric dUTPases (Harkiolaki et al., 2004). The catalytic mechanism of dimeric dUTPases was elucidated by Hemsworth et al. (2013).
The enzyme from Leishmania major is encoded by a single gene, and differs significantly from trimeric dUTPases. None of the characteristic five amino acid motifs that are common to all currently known trimeric dUTPases were readily identifiable, and the sequence encoded a larger polypeptide with a molecular weight of 30.4 kDa. The enzyme hydrolyzes both dUTP and dUDP, but not other nucleotides (Camacho et al., 1997 and 2000), is highly dependent on Mg2+ concentrations, and markedly sensitive to the phosphatase inhibitor, NaF (Camacho et al., 2000). Kinetic parameters for dUTP hydrolysis are comparable to that of the human enzyme. However, the binding of dUDP and dUMP suggest differences in the structure of the active sites when compared with the human enzyme (Hidalgo-Zarco et al., 2001). The enzyme was crystalized in complex with substrate analogues, the product dUMP, and a substrate fragment (Hemsworth et al., 2011)
In Trypanosoma brucei, the enzyme is a nuclear enzyme. Down-regulation of its activity by RNAi greatly reduces cell proliferation, causes lethality, and increases the intracellular levels of dUTP. dUTPase-depleted cells presented hypersensitivity to methotrexate, a drug that increases the intracellular pools of dUTP. The knockdown of activity produces numerous DNA strand breaks and defects. It also produced parasites with a single enlarged nucleus as well as an enhanced population of anucleated cells. Defects in growth could be partially reverted by the addition of exogenous thymidine (Castillo-Acosta et al., 2008). Adding uracil, uridine or deoxyuridine could not rescue this phenotype (Castillo-Acosta et al., 2013). Therefore, it appears that dimeric dUTPases are strongly involved in the control of dUTP incorporation into DNA, and that adequate levels of enzyme are indispensable for efficient cell cycle progression and normal DNA replication.
In T. cruzi, dUTPase was cloned and characterized (Bernier-Villamor et al., 2002). The deduced amino acid sequence was similar to that of Leishmania major dUTPase, although it exhibits an amino acid insertion that is sensitive to protease inactivation. The enzyme is a dimer and detailed kinetic characterization showed that it is highly specific for dUTP and dUDP. Crystal structures of T. cruzi dUTPase showed major differences between the substrate binding pocket of dimeric and trimeric dUTPases (Harkiolaki et al., 2004). Asp80Ala substitution in the enzyme induces only a slight conformational change in the active site, yet results in a significant alteration of nucleotide binding and modifies the ability of the enzyme to discriminate between dUTP and dUMP when magnesium is present (Téllez-Sanz et al., 2007). It was also observed for the first time that ligand binding induces large conformational change in dimeric dUTPases (Harkiolaki et al., 2004). Inhibitor designed based on in silico docking were synthesized and tested against T. cruzi dUTPase. However, none of the compounds produced inhibited the enzyme at a concentration of 1 mM. Neither did the compounds inhibit parasite growth at the maximum concentrations studied. It was suggested that the failure of this approach is a result of not considering the flexibility of the protein (Mc Carthy et al., 2006)
3. Uptake of pyrimidine nucleobases and nucleosides
As shown in Table 1, schistosomes are capable of salvaging pyrimidine nucleobases and nucleosides (Levy and Read, 1975a; Nollen et al., 1976; Mattoccia et al., 1981; Mattoccia and Ciolo, 1983; el Kouni and Naguib, 1990). Thymine, cytosine and orotidine are transported into adult S. mansoni, but not incorporated into their nucleic acids (el Kouni and Naguib, 1990). Uridine, cytidine, deoxycytidine, thymidine and uracil were converted to their respective 5′-mono-, di- and triphosphate nucleosides as well as their hexose nucleotides and ultimately incorporated into the nucleic acids of the parasite (Mattoccia et al., 1981; Mattoccia and Cioli, 1983; Huang et al., 1984, el Kouni and Naguib, 1990). Cytidine is the most efficiently utilized pyrimidine. Cytidine, uridine and uracil were incorporated into the worm’s nucleic acids better than orotate by 11-, 4- and 3-fold, respectively, (Table 1) suggesting that the salvage pathways may be more important than the de novo pathway in providing the pyrimidine requirements of the parasite. Orotate, uridine and uracil are incorporated into the various pyrimidines nucleotides; whereas, thymidine, cytidine and deoxycytidine are exclusively incorporated into their respective nucleotides. No incorporation of cytidine or deoxycytidine was detected in uracil nucleotides (el Kouni and Naguib, 1990).
Table 1.
Incorporation of radiolabeled pyrimidine nucleobases and nucleosides into nucleic acids of Schistosoma mansoni
| Compound (45 μM) | Specific activity (Ci/mol) | Incorporation (pmol/10 worm pairs) | Ratio |
|---|---|---|---|
| Orotate | 56.2 | 18.4 | 1 |
| Uracil | 55.2 | 69.3 | 3.8 |
| Cytosine | 4.6 | -a | - |
| Thymine | 55.3 | -a | - |
| Orotidine | 50.0 | -a | - |
| Uridine | 56.0 | 56.3 | 3.1 |
| Cytidine | 51.0 | 210 | 11 |
| Thymidine | 56.0 | 19.4 | 1.1 |
| Deoxycytidine | 56.0 | 18.9 | 1.0 |
Below the sensitivity of the assay (<1 pmol/10 worm pairs). (After el Kouni and Naguib, 1990)
The nematode Angiostrongylus cantonensis can utilize uracil, uridine, and cytidine, but not cytosine, thymine and thymidine for the synthesis of RNA and DNA (So et al., 1992). Trichomonas vaginalis incorporate uridine, cytidine, deoxycytidine, thymidine and uracil, but not thymine or cytosine, into their nucleic acids (Heyworth et al., 1984; Wang and Chen, 1984a). Eimeria tenella incorporate cytidine and uridine, but not thymidine (Ouellette et al., 1973). In contrast, Plasmodium berghei (Van Dyke et al., 1970) and P. falciparum (Krungkrai and Krungkrai, 2016) have little ability to salvage preformed pyrimidine nucleobases and nucleosides from the host cell and plasma, but rely mostly on nucleotide synthesis through the de novo pathway. Pyrimidine salvage was shown not to be an essential function for bloodstream Trypanosoma brucei brucei. However, Trypanosomes lacking de novo pyrimidine biosynthesis are completely dependent on an extracellular pyrimidine source, strongly preferring uracil, and display reduced infectivity (Ali et al., 2013b).
4. Transport of pyrimidines
Transport across the cell membrane is the first step in the uptake of exogenous pyrimidines. An understanding of the mechanisms of transport and membrane function in parasites as well as differences in their properties as compared to those of their mammalian hosts may provide the foundation for rational antiparasitic drug development. Indeed, differences in the properties of nucleoside transport between mammalian and parasitic cells were the basis of a combination therapy approach involving the use of cytotoxic purine nucleoside analogues and host-protecting mammalian nucleoside transport inhibitors (Ogbunude and Ikediobi, 1982; el Kouni et al., 1983a, 1985, 1987, and 1989; el Kouni and Cha, 1987; Gati et al., 1987; Baer et al., 1988; Gero et al., 1989; el Kouni, 1991, 1992 and 2003; Aoki et al., 2009). Pyrimidine transporters, may also be helpful in gene therapy by delivering drugs to particular cells. For example, expression of a trypanosomal adenosine transporter in Saccharomyces cerevisiae rendered yeast hypersensitive to melarsen oxide (Mäser et al., 1999).
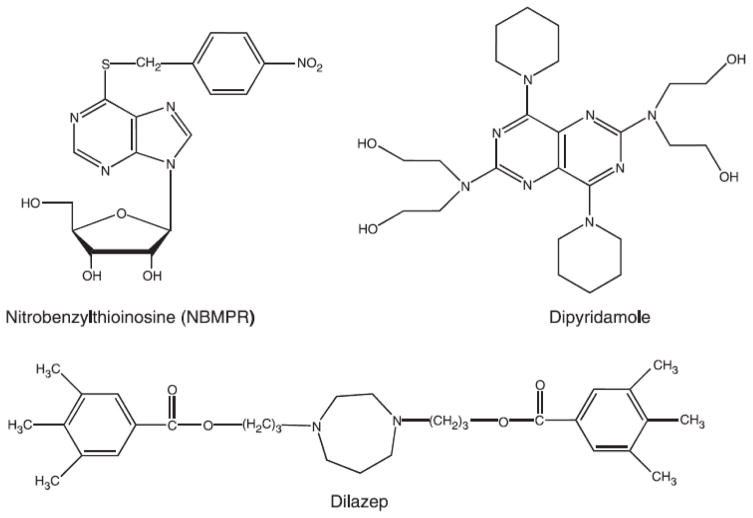
Studies of pyrimidine nucleoside and nucleobase transport in mammalian cells are numerous and have been discussed in several reviews, most recently by Griffith and Jarvis (1996), Buolamwini (1997), Cass et al. (1999), de Koning and Diallinas (2000), Kong et al. (2004), Young et al. (2008 and 2013), and Molina-Arcas el al. (2009). A great deal of the significant progress in understanding nucleoside transport in mammalian cells can be attributed to the discovery and synthesis of a number of highly specific inhibitors of nucleoside transport. The most notable and frequently used among these inhibitors are NBMPR (nitrobenzylthioinosine), dilazep and dipyridamole. The chemical structures of these compounds are shown in Fig. 5. In addition to passive diffusion, there are at least three main classes of nucleoside transporters in mammalian cells. The first (es) is an equilibrative non-concentrative carrier mediated transporter sensitive to NBMPR inhibition and is inhibited by all three nucleoside transport inhibitors mentioned above. The second (ei) is also an equilibrative non-concentrative carrier mediated transporter but insensitive to NBMPR inhibition although inhibited by dipyridamole. The third class is sodium ion driven concentrative nucleoside transporters insensitive to all three inhibitors of the equilibrative nucleoside transport systems. The mammalian nucleoside transporters have broad specificity and vary in their ability to recognize both purine and pyrimidine nucleosides. Therefore, each class can be divided accordingly to several families, the discussion of which is beyond the scope of this review. Nucleobase transport in mammalian cells occurs by passive diffusion or sodium ion dependent transporters (Kraup and Marz, 1995; Griffith and Jarvis, 1996; de Koning and Diallinas, 2000) that are inhibited by papaverine, dilazep or dipyridamole (Griffith and Jarvis, 1993 and 1996; Kraup and Marz, 1995).
Fig. 5.
Chemical structures of 6-nitrobenzylthioinosine (NBMPR), dipyridamole and dilazep.
Few studies have addressed pyrimidine uptake in parasites except where purine transporters were competitively inhibited by some pyrimidines. Pyrimidines are transported into parasites by carriers mediated nucleoside and nucleobase transporters or by passive diffusion. Substrate specificities, inhibition, and affinities for ligands among transporters from parasites are quite different from their host. In addition, none of the parasite transporters have been shown to be a sodium dependent.
4.1. Pyrimidine nucleoside transport
Only one of three nucleoside transporters in Schistosoma mansoni is reported to transport uridine but can also transport adenosine. The second transporter is specific for adenosine. The third transporter has high affinity for adenine but also transports adenosine (Levy and Read, 1975a). Uridine uptake is saturable. At high concentrations, uridine is absorbed, in part, through simple diffusion. Mediated uridine uptake was inhibited completely by adenosine (Pappas and Read, 1975). In another trematode, Fasciola hepatica, uridine uptake occurs only by simple diffusion (Anderson et al., 1993). Studies on three species of cestodes; Hymenolepis diminuta, H. citelli, and H. microstoma, indicated that there is at least one locus involved in the transport of pyrimidine nucleosides. No significant difference was observed in their affinity for ribo- or deoxyribosides (Page 3rd and MacInnis, 1975). In Hymenolepis diminuta, uridine transport is saturable (MacInnis and Ridley, 1969; Pappas and Read, 1974) and diffusion was negligible (Pappas and Read, 1974). The uptake of uridine was stimulated by thymine, unaffected by hypoxanthine or uracil (MacInnis and Ridley, 1969), and inhibited by uridine, adenosine, thymidine, deoxyuridine, cytidine, inosine, AMP (adenosine 5′-monophosphate), and ATP (MacInnis and Ridley 1969; Pappas and Read, 1974). However, thymidine was slightly less effective as an inhibitor of riboside transport than uridine (Page 3rd and MacInnis, 1975). Thymine has no effect on uridine transport in the three cestodes (Page 3rd and MacInnis, 1975).
To the best of our knowledge, there are no other studies on the transport of pyrimidine nucleosides in a multicellular parasites, except these mentioned above in the trematodes; Schistosoma mansoni (Levy and Read, 1975a; Pappas and Read, 1975) and Fasciola hepatica (Uglem and Levy, 1976), and the cestodes; Hymenolepis diminuta, H. citelli, and H. microstoma, (MacInnis and Ridley, 1969; Pappas and Read, 1974; Page 3rd and MacInnis, 1975). Most of the transport studies in parasites were performed on the protozoa because of the ease of growing them in culture.
The protozoa; Toxoplasma gondii (Chiang et al., 1999; Al Safarjalani et al., 2003; de Koning et al., 2003), Plasmodium falciparum (Carter et al., 2000; Downie et al., 2006), Crithidia fasciculata (de Koning et al., 2000), and Plasmodium vivax (Deniskin et al., 2015), all appear to have a single broad-specificity transporter for pyrimidine nucleosides that can also transport purine nucleosides. The nucleoside transporter in P. falciparum was localized to the parasite plasma membrane (Rager et al., 2001). Giardia intestinalis can takeup uridine, cytidine, and thymidine (Jarroll et al., 1987). This parasite has two facilitative transporters for pyrimidine nucleosides; one specific for thymidine (Davey et al., 1991), a second less specific that transport all pyrimidine and purine ribosides and 2′- or 3′-deoxyriboside (Davey et al., 1992; Baum et al., 1993). Trichomonas vaginalis also have two separate nucleoside transporters that can transport pyrimidine nucleosides. The first is non-specific as it takes all pyrimidine and purine nucleosides (Harris et al., 1988). The second transports uridine as well as adenosine, guanosine. In Trypanosoma brucei brucei (Gudin et al., 2006), Leishmania donovani (Ogbunude et al., 1991; Iovannisci et al., 1984; Aronow et al., 1987; Ghosh and Mukherjee, 2000), L. major (Baer et al., 1992; Alzahrani, et al., 2016a), and L. mexicana (Alzahrani, et al., 2016a), uridine is taken up by at least two transporters. One of which also transports thymidine, 5-fluoro-2′-deoxyuridine as well as the nucleobases, uracil and 5-fluorouracil. However, their efficiency for the nucleobases transport are much higher than those for the corresponding nucleosides. The other transporter mediate the uptake of uridine, adenosine, thymidine, 5-fluoro-2′-deoxyuridine and thymidine. Stein et al. (2003) reported that equilibrative pyrimidine nucleoside transporters from Leishmania donovani are nucleoside/proton symporters.
It should be noted here that nucleoside transporters in parasites are not inhibited by NBMPR, dilazep and to a lesser extent dipyridamole at the concentration required to inhibit NBMPR-sensitive mammalian nucleoside transporters (for references see review by el Kouni, 2003). In fact, NBMPR is a permeant in Plasmodium falciparum (Gero et al., 1989), and Toxoplasma gondii (el Kouni et al., 1999; Al Safarjalani et al., 2003; el Kouni 2003 and 2007).
4.2. Pyrimidine nucleobase transport
The uptake of pyrimidine nucleobases (uracil, thymine, and cytosine) by Schistosoma mansoni (Levy and Read, 1975a), and the cestode Taenia crassiceps (Uglem and Levy, 1976) occurs solely by simple diffusion. The uptake rate of each of these pyrimidines was linear with respect to concentration (Levy and Read, 1975; Uglem and Levy, 1976). In the cestode Hymenolepis diminuta, thymine and uracil are transported through a single locus where uracil transport is a mediated system (MacInnis et al., 1965). Both purines and pyrimidines inhibited the uptake of labeled uracil (MacInnis et al., 1965). In addition, pyrimidine nucleobases stimulated or inhibited uracil uptake depending on the inhibitor (I)/substrate (S) ratio. At low I/S ratios, uptake of uracil is inhibited by uracil, thymine, and 5-bromouracil, followed by stimulation of uptake at higher 1/S ratios (MacInnis et al., 1965; Pappas et al., 1973). Thymine stimulated uracil transport in Hymenolepis citelli, but not in H. microstoma, (MacInnis et al., 1965; Pappas et al., 1973; Page 3rd and MacInnis, 1975).
The nature of the chemical group occupying C5 and C6 of the uracil ring was shown to be important in determining the effects of uracil derivatives on uracil uptake by H. diminuta (MacInnis and Ridley, 1969). Uptake of thymine, uracil or 5-bromouracil was unaffected by cytosine, 5-methylcytosine, alloxan, 5-carboxyuracil, thymidine, and uridine. Uracil transport was stimulated by uracil and 5-substituted uracil derivatives, but inhibited by 6-methyluracil (MacInnis and Ridley 1969). Amino acids with ring structures similar to purines and pyrimidines had no effect on the uptake of uracil by H. diminuta (Pappas et al., 1973).
Pyrimidine nucleobase transport mechanisms in protozoa may also be distinct from those in mammalian systems. Tritrichomonas foetus has a pyrimidine nucleobase transporter but can also transport xanthine (Hedstorm and Wang, 1989). Giardia intestinalis has a facilitative transporter specific for uracil/thymine, but not cytosine derivatives (Ey et al., 1992). Leishmania major and L. mexicana have a highly selective, high-affinity uracil transporter which also transports 5-fluorouracil as well as the nucleosides uridine, adenosine, and 5-fluoro-2′-deoxyuridine (Papageorgiou et al., 2004; Alzahrani et al., 2016a). Trypanosoma brucei brucei has a highly selective, high-affinity cytosine transporter (Gudin et al., 2006) as well as a high-affinity uracil transporter (de Koning and Jarvis, 1998; Gudin et al., 2006) that is also capable of transporting orotate, uridine, and deoxyuridine, but with lower affinities (Ali et al., 2013a). In contrast to mammalian cells, the T. brucei brucei nucleobase transporter is a nucleobase/proton symporter, and not a sodium ion dependent transporter (de Koning and Jarvis, 1997a, 1997b and 1980). For a more detailed discussion on the characterization of pyrimidine nucleobase transport in T. brucei brucei see review by Bellofatto (2007)
5. Pathways of pyrimidine salvage
UMP, the focal metabolite in pyrimidine metabolism, can be synthesized by the de novo pathway in almost all organisms but it can also be synthesized by the salvage pathways. These salvage pathways utilize endogenous pre-existing nucleotides and their derivatives or exogenous sources of these compounds. However, unlike the universality of de novo pyrimidine synthesis, differences do exist in the salvage pathways between various organisms (c.f. Munch-Peterson, 1983). Fig. 6 illustrates the reactions of the salvage pathways by which UMP and other pyrimidine nucleobase, nucleoside and nucleotides may be interconverted, as determined from various organisms.
The incorporation of uracil into the nucleic acids of schistosomes (Table 1) takes place via the formation of UMP. As depicted in Fig. 6, the conversion of uracil to UMP can occur either by the sequential reactions of uridine phosphorylase and uridine kinase or by the direct conversion of uracil to UMP by a phosphoribosyltransferase (Iltzsch et al., 1984; el Kouni et al., 1988; el Kouni and Naguib, 1990; Naguib and el Kouni, 2014). As mentioned below (6.7.), schistosomes have a relatively non-specific phosphoribosyltransferase that can utilize uracil under physiological conditions and could be the principal pathway for the conversion of uracil to UMP (Iltzsch et al., 1984).
Thymidine and thymine incorporation into nucleic acids was observed in S. mansoni (Table 1). However, neither the cleavage of thymidine to thymine nor the synthesis of thymidine from thymine was detected in vivo (el Kouni and Naguib, 1990) in spite of the fact that such activities occur in vitro (el Kouni et al., 1988; el Kouni and Naguib, 1990; Naguib and el Kouni, 2014). The utilization of orotate by schistosomes confirms the existence of at least the last part of the de novo UMP biosynthesis (Iltzsch et al., 1984; el Kouni and Naguib, 1990). However, the relatively better efficiency of cytidine, uridine, and uracil utilization suggest that the contribution to pyrimidine metabolism by the salvage pathways may be of greater importance to schistosomes than the de novo pathway. This apparent reliance on the salvage pathway may have evolved from the selective adaptation of these parasites to their environment. Plasma concentration of cytidine and uridine are in the micromolar range in mammals (Rustum, 1978; Moyer et al., 1981; Pellwein et al., 1987) and probably can satisfy the schistosomes requirements for pyrimidines.
6. Enzymes of pyrimidine salvage pathways
Assays of enzyme activities in Schistosoma mansoni cell free extracts can better prove the routes, summarized in Fig. 6, by which pyrimidine nucleobases and nucleosides are metabolized. In the following are the description and characteristics of the enzymes involved in pyrimidine salvage in schistosomes as well as a comparison with these enzymes from other species.
6.1. Alkaline phosphatase (EC 3.1.3.1)
Alkaline phosphatase is ubiquitous and widely distributed enzyme among different organisms. In humans, it is present in almost all tissues throughout the entire body. It is a non-specific hydrolase which is important in the salvage of pyrimidines. The enzyme allows the uptake of impermeable negatively charged nucleotides by removing their organic phosphate group and convert them to the more permeable nucleosides as follows:
Extracts of adult Schistosoma mansoni have high levels of phosphatase activities capable of dephosphorylating pyrimidine nucleoside 5′-monophosphates (Nimmo-Smith and Standen, 1963; Levy and Read, 1975a and 1975b; Cesari et al., 1981; el Kouni and Naguib, 1990). These activities in schistosomes are correlated with activities towards β-glycerophosphate (Table 2) indicating that the dephosphorylation of pyrimidine nucleoside 5′-monophosphates in schistosomes are due to alkaline phosphatases rather than the more specific 5′-nucleotidases (EC 3.1.3.5) (Nimmo-Smith and Standen, 1963; Levy and Read, 1975a and 1975b; Cesari et al., 1981; el Kouni and Naguib, 1990). These activities are associated with the particulate fraction of the parasite which is constituted mainly of the tegument of the parasite (Nimmo-Smith and Standen, 1963; Levy and Read, 1975a and 1975b; Wheater and Wilson, 1976; Ernst, 1976; Simpson et al., 1981; Cesari et al., 1981; el Kouni and Naguib, 1990). The tegument of the adult schistosome is delimited by a double membrane that completely surrounds the parasite (Hockley and McLaren, 1973). The outer of the two membranes plays an important role in nucleotide metabolism as it contains high levels of phosphatase activities that can dephosphorylate pyrimidine nucleoside 5′-monophosphates. Indeed, by the use of various methodologies, alkaline phosphatase was identified on the external surface of the tegument of adult S. mansoni. (Dusanic 1959; Morris and Threadgold, 1968; Levi-Schaffer et al., 1984; Cesari, 1974; Roberts et al., 1983; Payares et al., 1985; van Balkom et al., 2005; Braschi et al., 2006). Therefore, alkaline phosphatase activity is used as marker enzyme in the isolation of surface fragments from S. mansoni (Wheater and Wilson, 1976; Simpson et al., 1981). An immunoglobulin G fraction from sera of mice chronically infected with S. mansoni partially inhibited the parasite alkaline phosphatase activity (Cesari et al., 1981). Alkaline phosphatase from S. mansoni is expressed not only in the tegument but in internal tissues and in life stages outside the mammalian host (Dusanic, 1959; Robinson, 1961; Halton, 1967; Bhardwaj and Skelly, 2011). Alkaline phosphatase activities were also identified in cercariae of S. mansoni (Conde-del Pino et al., 1968; Sodeman Jr. et al., 1968), S. haematobium, and S. japonicum (Sodeman Jr. et al., 1968), but its tissue localization differed among the three species. Nevertheless, the activity appears to be related to the excretory system and the penetration glands. (Sodeman Jr. et al., 1968)
Table 2.
Alkaline phosphatase activities (μmol/min) in various fractions of Schistosoma mansoni towards different substrates
| 30,000 × g | 105,000 × g | ||||
|---|---|---|---|---|---|
|
|
|
||||
| Substrate (1 mM) | Homogenate (39.61 mg)a | Supernatant (17.75 mg) | Pellet (19.35 mg) | Cytosol (12.40 mg) | Microsomes (2.59 mg) |
| CMP | 1.90 ± 0.15(100)b | 0.26 ± 0.09(100) | 1.12 ± 0.19(100) | 0.10 ± 0.03(100) | 0.12 ± 0.02(100) |
| UMP | 1.79 ± 0.15(94) | 0.25 ± 0.09(93) | 1.19 ± 0.00(106) | 0.06 ± 0.01(60) | 0.12 ± 0.03(104) |
| OMP | 1.50 ± 0.14(79) | 0.22 ± 0.07(83) | 0.09 ± 0.03(80) | 0.08 ± 0.03(80) | 0.10 ± 0.02(88) |
| dTMP | 1.71 ± 0.07(90) | 0.22 ± 0.09(84) | 1.04 ± 0.15(93) | 0.09 ± 0.03(90) | 0.10 ± 0.02(84) |
| dCMP | 1.77 ± 0.25(93) | 0.24 ± 0.08(89) | 1.10 ± 0.10(98) | 0.08 ± 0.03(85) | 0.12 ± 0.03(100) |
| dUMP | 1.78 ± 0.32(94) | 0.25 ± 0.08(95) | 1.16 ± 0.10(103) | 0.10 ± 0.03(99) | 0.12 ± 0.03(99) |
| β-Glycerophosphate | |||||
| 1.45 ± 0.20(76) | 0.20 ± 0.06(75) | 1.08 ± 0.08(96) | 0.09 ± 0.02(91) | 0.11 ± 0.01(92) | |
Total amount of protein from 10.8 mL of packed worms.
Percent activity relative to that towards CMP in parenthesis. (After el Kouni and Naguib, 1990)
Alkaline phosphatase was extracted from adult S. mansoni membranes and purified by Payares et al. (1984) who also concluded that the enzyme is not exposed at the schistosome’s surface, and is probably buried in the tegumental membrane network. The enzyme was cloned, expressed and characterized. The cloned enzyme potentially encodes a 536 amino acid protein of ~60 kDa, a pI of 5.92, and possesses six potential N-linked glycosylation sites (Bhardwaj and Skelly, 2011).
Alkaline phosphatases are also found in the trematodes; Dicrocoelium lanceatum, Eurytrema, Paragonimus westermani (Yamao, 1952a and 1952b), Clonorchis sinensis (Yamao, 1952a), Fasciola hepatica (Halton, 1967), the cestodes; Moniezia expansa (Rogers, 1947; Erasmus, 1957b), Taenia pisiformis (Erasmus, 1957a), Cysticercus tenuicollis (Erasmus, 1957b), Hymenolepis microstoma (Bogitsh, 1963), H. diminuta (MacInnis, et al., 1965; Lumsden et al., 1968), the nematode Ascaris lumbricoides (Rogers,1947), and the protozoa Toxoplasma gondii (Iltzsch, 1993). On the other hand, the nematode Trichinella spiralis has a specific 5′-nucleotidase (EC 3.1.3.5) (Gounaris, 2002), and the protozoa Leishmania tropica has a broad substrate specificity nucleotidase (EC 3.1.3.31) (Pereira and Königk, 1981) which convert nucleotides to their respective nucleosides.
6.2. Nucleoside phosphotransferases (EC 2.7.1.77)
These enzymes phosphorylate nucleosides to their respective nucleoside 5′-monophosphates. Using low energy phosphate esters (e.g., various nucleoside 5′-monophosphates, p-nitrophenylphosphate, β-glycerophosphate, etc.) as phosphate donors as follows:
Adult Schistosoma mansoni do not have nucleoside phosphotransferases (el Kouni and Naguib, 1990; Naguib and el Kouni, 2014). No activity was detected when AMP, β-glycerophosphate or p-nitrophenylphosphate was used as a phosphate donor in the extracts of S. mansoni (el Kouni and Naguib, 1990; Naguib and el Kouni, 2014). A similar situation exists in Angiostrongylus cantonensis (So et al., 1992), and Toxoplasma. gondii (Iltzsch, 1993). In contrast, pyrimidine nucleoside phosphotransferases are found in Tritrichomonas foetus (Wang et al., 1983), Trichomonas vaginalis (Wang and Cheng, 1984a and b), Giardia intestinalis (Aldritt et al., 1985), and Tetrahymena pyriformis (Bols and Zimmerman, 1977; Yuyama et al., 1978 and 1979; Fink, 1980).
6.3. Pyrimidine nucleoside kinases [EC 2.7.1.-)
Nucleoside kinases are similar to the nucleoside phosphotransferases as they phosphorylate pyrimidine ribo- and 2′-deoxyribosides to their respective nucleoside 5′-monophosphates. The difference is that the kinase reaction requires a high energy phosphate ester (e.g., ATP) as a phosphate donor as follows:
Among the enzymes involved in pyrimidine salvage, the nucleoside kinases play a pivotal role in the utilization of pyrimidine nucleosides. They are responsible for the first step in the anabolism of pyrimidine nucleosides. Extracts of S. mansoni phosphorylate all pyrimidine nucleosides (except orotidine), in the presence of ATP, to their respective nucleoside 5′-monophosphates, indicating the presence of nucleoside kinases (el Kouni and Naguib, 1990; Naguib and el Kouni, 2014). Competition studies and column chromatography demonstrated that adult S. mansoni contain three cytosolic nucleoside kinases that can phosphorylate pyrimidine nucleosides (Naguib and el Kouni, 2014). The first is a non-specific deoxyriboside kinase (EC 2.7.1.145) which indiscriminately phosphorylates all five naturally occurring pyrimidine (thymidine, 2′-deoxyuridine, and 2′-deoxycytidine) and purine (2′-deoxyguanosine and 2′-deoxyadenosine) deoxyribosides. The second is a kinase specific solely for uridine. The third is a distinct cytidine kinase which differs from uridine kinase. Other than these three nucleoside kinases, the only other nucleoside kinase that is reported in schistosomes is adenosine kinase (EC 2.7.1.20) which is specific for adenosine and its analogues (Senft and Crabtree, 1983; el Kouni et al., 1983a and 1987; Dovey et al., 1984; el Kouni and Cha, 1987).
S. mansoni uridine kinase is a tightly regulated enzyme and is strongly inhibited by its substrate. Unless the appropriate assay conditions are used, this activity cannot be detected (Naguib and el Kouni, 2014). Various nucleoside 5′-triphosphates act as phosphate donors for the three kinases albeit to different degrees. GTP (guanosine 5′-monophosphate) was the best phosphate donor for the kinase reaction when uridine or cytidine, but not when thymidine, was used as a substrate. UTP was the best phosphate donor for the activity towards thymidine. The kinetic parameters of the three nucleoside kinases were characterized as shown in the Table 3. The mechanism of the three kinases is ping pong (Naguib and el Kouni, 2014).
Table 3.
Kinetic Parameters of S. mansoni Various Nucleoside Kinases.
| [14C]Substrate | Km ± S.E. | Vmax ± S.E. (pmol/min/mg) | Competing Nucleoside | Kis ± S.E. (μM) | |
|---|---|---|---|---|---|
|
| |||||
| Nucleoside (μM) | ATP (mM) | ||||
| Uridine | 83a | Cytidine | -b | ||
| Cytidine | 73 ± 13 | 2.1 ± 0.01 | 43 ± 1.3 | Uridine | -b |
| Thymidine | 5.2 ± 0.001 | 2.8 ± 0.001 | 24 ± 0.2 | Cytidine | -b |
| Uridine | -b | ||||
| 2′-Deoxyadenosine | 54 ± 8.2 | ||||
| 2′-Deoxyguanosine | 61 ± 12 | ||||
| Adenosine | -b | ||||
| 2′-Deoxyuridine | 54 ± 0.06 | 13 ± 0.008 | 52 ± 1.0 | Thymidine | 6.3 ± 1.2 |
| 2′-Deoxycytidine | 21 ± 6.4 | ||||
| Cytidine | -b | ||||
| Uridine | -b | ||||
| 2′-Deoxycytidine | 13 ± 0.005 | 2.7 ± 0.001 | 26 ± 0.2 | Thymidine | 4.4 ± 0.9 |
| Cytidine | -b | ||||
| Uridine | -b | ||||
Km value was estimated from V0.5 value because of the allosteric nature of the enzyme.
No competition. (After Naguib and el Kouni, 2014)
Other than S. mansoni, a non-specific deoxyriboside kinase activity was reported so far only in insects; Drosophila melanogaster (Munch-Petersen et al., 1989), silk worm Bombyx mori (Knecht et al., 2002), and the mosquito Anopheles gambiae (Knecht et al., 2003). The presence of separate cytidine and uridine kinases in schistosomes, is a conspicuous difference between pyrimidine metabolism in schistosomes and their mammalian host where uridine and cytidine are phosphorylated by a single uridine-cytidine kinase (EC 2.1.7.48). The presence of a distinctive cytidine kinase along with the absence of cytidine deaminase and phosphorylase can be manipulated for chemotherapy. In this regard, various available cytidine analogues can be used as antischistosomal drugs. These compounds can be phosphorylated to the nucleotide level in the parasites without being degraded by the sequential reaction of cytidine deaminase and uridine phosphorylase (Fig. 6) as is the case in the host.
No pyrimidine nucleoside kinase activity was detected in Plasmodium falciparum (Reyes et al., 1982), Tritrichomonas foetus (Wang et al., 1983), or Toxoplasma gondii (Iltzsch, 1993) indicating that these protozoa lack the ability to directly phosphorylate pyrimidine nucleosides. No uridine kinase was detected in culture promastigote form of Leishmania mexicana amazonensis, the blood trypomastigote and the culture epimastigote of Trypanosoma brucei, blood trypomastigote and intracellular amastigote forms of T. cruzi (Hammond and Gutteridge, 1982), Giardia intestinalis (Aldritt et al., 1985; Vitti et al., 1987), or Leishmania donovani (Wilson et al., 2012). There is a controversy about the presence of uridine kinase in Trichomonas vaginalis. Miller and Lindstead (1983) reported the presence of the activity while Wang and Cheng (1984a) could not detect it.
Activity of uridine-cytidine kinase was detected in Angiostrongylus cantonensis (So et al., 1992) and uridine kinase in Crithidia luciliae (Tampitag and O’Sullivan, 1986). Phosphorylation of uridine and thymidine was observed in Paragonimus ohirai and Clonorchis sinensis (Kobayashi et al., 1978), but it was not clear whether these two nucleosides were phosphorylated by nucleoside kinases or a phosphotransferases. An early report of the existence of uridine kinase in Giardia intestinalis (Lindmark and Jarroll, 1982) was subsequently shown to be incorrect (Vitti et al., 1987). Uridine-cytidine kinase from Entamoeba histolytica was cloned, expressed and purified (Lossani et al., 2009). Striepen et al. (2004) reported the presence of a unique uridine-cytidine kinase activity in Cryptosporidium parvum where the activity is fused with uracil phosphoribosyltransferase in a single bifunctional enzyme.
Thymidine kinase is apparently lacking in the protozoa Trichomonas vaginalis (Wang and Cheng,1984a), the nematodes; Angiostrongylus cantonensis (So et al., 1992), and Ascaris lumbricoides, but found in the cestode Hymenolepis diminuta, the acanthocephalan Moniliformis dubius (Farland and MacInnis, 1974), and the protozoa Cryptosporidium parvum (Striepen et al., 2004). The presence of thymidine kinase in C. parvum is unique within the phylum Apicomplexa. Phylogenetic analysis suggests horizontal gene transfer of thymidine kinase from a proteobacterium (Striepen et al., 2004). Tetrahymena pyriformis has both nucleoside kinase and phosphotransferase activities towards thymidine (Bols and Zimmerman, 1977; Yuyama et al., 1978 and 1979; Fink, 1980). It was suggested that at high thymidine concentrations, nucleoside phosphotransferase is the dominant phosphorylating enzyme while at low thymidine concentrations both nucleoside phosphotransferase and kinase activities can be detected (Fink, 1980).
Thymidine kinases from Trypanosoma brucei rhodesiense (Chello and Jaffe, 1972a and 1972b), Brugia pahangi, Dirofilaria immitis (Jaffe et al., 1982), Giardia intestinalis (Lindmark and Jarroll, 1982; Aldritt et al., 1985; Vitti et al., 1987; Laoworawit et al., 1993), Cryptosporidium parvum (Sun et al., 2010), and Leishmania major (Timm et al., 2015) were partially purified and characterized. The enzyme from Giardia intestinalis can also phosphorylates deoxycytidine (Laoworawit et al., 1993) while that from Leishmania major can phosphorylate uridine, but to a much lower extent (Timm et al., 2015). Thymidine kinases from Entamoeba histolytica (Lossani et al., 2009), and Trypanosoma brucei (Ranjbarian et al., 2012) were cloned, expressed, and characterized. The trypanosomal enzyme is a tandem protein consisting of two homologous parts, domain 1 and domain 2. The two domains are fused into a single open reading frame. Domain 1 is catalytically inactive but improves substrate binding by domain 2. Furthermore, the enzyme is less discriminative against purines than its human counterpart with the following order of efficiency: thymidine > deoxyuridine ≫ deoxyinosine> deoxyguanosine (Ranjbarian et al., 2012). Knockdown of T. brucei thymidine kinase leads to cell death that could not be rescued by exogenous pyrimidines. Metabolite profiling of the thymidine kinase depleted cells revealed a buildup of pyrimidine deoxyribosides (Leija et al., 2016). These findings indicate that thymidine kinase is the sole route for de novo synthesis of thymine nucleotides in T. brucei. Trypanosomatids lack alternative routes found in other eukaryotic cells to synthesize these nucleotides (Leija et al., 2016). For these reasons thymidine kinase was found to be essential to establish infection in mice and, hence, constitutes a strong potential chemotherapeutic target.
6.4. Pyrimidine nucleoside 5′-monophosphate (EC 2.7.4.-) and nucleoside diphosphate (EC 2.7.4.6) kinases
Pyrimidine nucleoside 5′-monophosphate kinases catalyze the reversible phosphorylation of various nucleoside pyrimidine 5′-monophosphates in the presence of a nucleoside 5′-triphosphate, usually ATP, to their respective pyrimidine nucleoside 5′-diphosphate as follows:
There are two pyrimidine nucleoside 5′-monophosphate kinases. The first is a relatively non-specific pyrimidine nucleoside 5′-monophosphate kinase (EC 2.7.4.14) which phosphorylates all pyrimidine nucleoside 5′-monophosphates except dTMP. The other is dTMP kinases (EC 2.7.4.9) which is specific for the phosphorylation of dTMP and probably dUMP. Activity of dTMP kinase is the only known pathway to synthesize dTDP and ultimately dTTP (Fig. 6). Inhibition of dTMP kinase could be very effective in blocking both the de novo and salvage pathways of dTTP production and hence DNA synthesis.
Nucleoside 5′-diphosphate kinases catalyze the exchange of terminal phosphates between various NDP (nucleoside 5′-diphosphates) and NTP (nucleoside 5′-triphosphates) in a reversible manner to produce nucleotide 5′-triphosphates. Many NTP are donors of the phosphate group but the principal donor is ATP.
Schistosoma mansoni contains appreciable levels of nucleoside 5′-monophosphate kinase(s) towards UMP, CMP, dCMP, dUMP and dTMP. Further phosphorylation of nucleoside 5′-diphosphates was also detected, indicating the presence of pyrimidine nucleoside 5′-diphosphate kinases in S. mansoni (el Kouni and Naguib, 1990). A similar situation exists in Plasmodium falciparum (Reyes et al., 1982), and Angiostrongylus cantonensis (So et al., 1992). In Giardia intestinalis, activities were observed towards uridine and cytidine nucleotides (Aldritt et al., 1985). However, in all these cases, there was no information about the nature and specificity of the nucleoside 5′-monophosphate kinase responsible for the phosphorylation of each of the pyrimidine nucleoside 5′-monophosphates. On the other hand, the nucleoside diphosphate kinase from the parasitic nematode Brugia malayi was identified, cloned and characterized. Molecular modeling of the enzyme showed several regions surrounding the conserved catalytic site that may be important in the design of drugs specific for the disruption of NTP synthesis in filarial parasites (Ghosh et al., 1995). A soluble nucleoside diphosphate kinase from epimastigote forms of Trypanosoma cruzi was purified and characterized. The enzyme is a hexamer composed of ~16.5 kDa monomers (Ulloa et al., 1995), and is predominantly localized in the nucleus (Hunger-Glaser et al., 2000). Nucleoside diphosphate kinase was found also in the secretome of Leishmania amazonensis. Activity of the released enzyme prevented ATP-induced cytolysis of infected macrophages. Hence, it appears that, beside its role in nucleotide metabolism in trypanosomatid protozoa, nucleoside diphosphate kinase has other functions related to preserving host-cell integrity for the benefit of the parasite survival (Kolli et al., 2008). Due to their importance for housekeeping functions in the parasite and prolonging the life of host cells in infections with intracellular parasites, nucleoside diphosphate kinase should be considered an attractive target for chemotherapy.
6.5. Uridine phosphorylase (EC 2.4.2.3)
Extracts of schistosomes are capable of cleaving both uridine and thymidine to uracil and thymine, respectively. The activity towards thymidine was approximately 16% of that towards uridine (el Kouni et al., 1988; el Kouni and Naguib, 1990).
There are two different pyrimidine nucleoside phosphorylases that can perform such reversible phosphorolysis: uridine phosphorylase and thymidine phosphorylase (EC 2.4.2.4) as follows:
Substrate specificities of both enzymes vary greatly dependent on the source of the enzymes (el Kouni et al., 1993). In general, however, uridine phosphorylase cleaves primarily pyrimidine ribosides, but is relatively non-specific as it can also cleave pyrimidine 2′- and 5′-deoxyribosides. Thymidine phosphorylase, on the other hand, is more specific for pyrimidine 2′- and 5′-deoxyribosides.
In S. mansoni, the activity towards thymidine is not due to a distinct thymidine phosphorylase but to a non-specific uridine phosphorylase (el Kouni et al., 1988). Uridine phosphorylase is the only pyrimidine nucleoside cleaving activity that can be detected in extracts of S. mansoni. The enzyme is distinct from purine nucleoside phosphorylases (EC 2.4.2.1) and adenine nucleoside phosphorylase (EC none), the two other nucleoside phosphorylases in this parasite (el Kouni et al., 1988; Savarese and el Kouni, 2014; Torini et al., 2016). Although uridine is the preferred substrate, S. mansoni uridine phosphorylase can also catalyze the reversible phosphorolysis of 2′-deoxyuridine and thymidine, but not cytidine, 2′-deoxycytidine, 6-azauridine or orotidine (el Kouni et al., 1988, el Kouni and Naguib, 1990). It should be noted, however, that only the phosphorolysis and reverse phosphorolysis of uridine, but not thymidine, was observed in vivo (el Kouni and Naguib, 1990). The reverse phosphorolysis of uridine in vivo indicates that endogenous ribose-1-phosphate is in adequate amounts for such reaction. The lack of detectable in vivo thymidine cleavage could be due to the competition between the added thymidine and endogenous uridine since schistosomes have only uridine phosphorylase which reacts six-fold better with uridine than thymidine (el Kouni et al., 1988). Such competition by uridine, the better substrate, in addition to the relatively higher ratio of kinase/phosphorylase activity towards thymidine (0.4) than uridine (0.1) (el Kouni and Naguib, 1990) could result in thymidine cleavage activity below the sensitivity of the assay. The competition between the kinases and phosphorylase activities can be further accentuated by the relative intracellular concentrations of co-factors required for their activity, i.e. ATP and phosphate. Similarly, the synthesis of thymidine from thymine may be below the level of detection because of the low intracellular concentration of deoxyribose-1-phosphate.
Schistosomal uridine phosphorylase was purified 170-fold to a specific activity of 2.76 nmol/min/mg protein with a 16% yield. It has a Mr of 56,000 as determined by molecular sieving on Sephadex G-100. The mechanism of uridine phosphorylase is sequential. When uridine was the substrate, the Kuridine = 13 μM and the KPhosphate = 535 μM. When thymidine was used as a substrate the Kthymidine = 55 μM and the KPhosphate = 760 μM. The Vmax with thymidine was 53% that of uridine. Thymidine was a competitive inhibitor when uridine was used as a substrate. The enzyme showed substrate inhibition by uridine, thymidine (>0.125 mM) and phosphate (>10 mM) (el Kouni et al., 1988).
A recent study (da Silva Neto et al., 2016) demonstrated that S. mansoni genome has two isoforms of uridine phosphorylase genes (SmUPa and SmUPb) that share 92% sequence identity. Both SmUP isoforms were successfully cloned, expressed, purified, and crystallized (da Silva Neto et al., 2016). Both enzymes present an overall fold and active site structure similar to other known uridine phosphorylases. They are composed of two monomers related by a two-fold symmetry axis with a large interface. The homodimer has two active sites, each of which include residues from both monomers. The active site is composed of nucleobase, ribose and phosphate binding sites (da Silva Neto et al., 2016). Furthermore, the study showed conclusively that SmUPa is an active uridine phosphorylase while SmUPb has no detectable activity. This is quite strange given the high level of sequence identity between the two isoforms. It is probably the result of the significant loss of available space and hydrogen bonding to the nitrogen pyrimidine ring in the nucleobase binding site of SmUPb rendering this enzyme incapable of binding pyrimidine nucleosides, and consequently incapable of the reversible phosphorolysis of uridine/thymidine (da Silva Neto et al., 2016). It is noteworthy that SmUPa is continuously expressed through the S. mansoni life cycle while SmUPb is mainly expressed in adult parasite phase (da Silva Neto et al., 2016). The reason for the maintenance of the two SmUP genes in the S. mansoni genome remains unknown.
A non-specific uridine-phosphorylase has also been reported in Giardia intestinalis (Vitti et al., 1987; Lee et al., 1988), Toxoplasma gondii (Iltzsch, 1993), the tapeworm Hymenolepis diminuta (Drabikowska, 1996), and Plasmodium falciparum (Krungkrai et al., 2003). The primary structure of the P. falciparum uridine phosphorylase suggests that both uridine phosphorylase and purine nucleoside phosphorylase (EC 2.4.2.1) activities are on the same polypeptide (Kicska et al., 2002). Phosphorolysis of uridine and thymidine was reported in Trichinella pseudospiralis (Rode et al., 2000) but it was not clear whether these activities were due to one or two phosphorylases. A more specific uridine phosphorylase that cannot cleave thymidine was identified in Trichomonas vaginalis (Wang and Cheng, 1984a).
All trypanosomatids investigated; Herpetomonas muscarum muscarum, H. m. ingenoplastis, procyclic and trypomastigotes of Trypanosoma brucei brucei, culture forms of Crithidia fasciculata, amastigotes and cultured promastigotes of Leishmania mexicana, cultured promastigotes of L. m. amazonensis, but not L. donovani and L. tarentolae, are able to convert uracil to uridine in the presence of ribose-l-phosphate (Hammond and Gutteridge, 1982; Hasaan and Coombs, 1986), suggesting that their uridine phosphorylase activities are reversible. It should be emphasized here that since no uridine kinase activity was found in the homogenates of any of these species (Hammond and Gutteridge, 1982). Therefore, it appears that uridine salvage in these parasites is routed via a uridine phosphorylase and uracil phosphoribosyltransferase as shown in Fig. 6. Indeed, uracil phosphoribosyltransferase activity was detected in all organisms tested except the intracellular amastigote form of Trypanosoma cruzi (Hammond and Gutteridge, 1982). It should also be noted that T. brucei brucei can adjust its uridine phosphorylase expression levels to accommodate growth on uridine as its sole pyrimidine source, whether these cells are pyrimidine auxotrophs or prototrophs, (Ong et al., 2013; Ali et al., 2013b). The enzyme from T. brucei was purified, crystalized and characterized. Beside uridine, no other pyrimidine nucleoside can be used as a substrate except 2′-deoxyuridine (Larson et al., 2010).
Thymine was converted to thymidine in vitro by Herpetomonas spp., Trypanosoma brucei brucei, and Crithidia fasciculata, but not by several Leishmania species (Hasaan and Coombs, 1986). The very high catabolic activity of thymidine phosphorylase in C. fasciculata and culture forms of Trypanosoma cruzi prevented incorporation of thymidine into their DNA (Al Chalabi and Gutteridge, 1977b). In the nematode Angiostrongylus cantonensis, uridine, deoxyuridine, thymidine, and surprisingly cytidine were converted to their respective nucleobases by a phosphorylase reaction. However, only uracil and thymine, but not cytosine, could form nucleosides in the reverse reaction (So et al., 1992). It was not clear whether the phosphorolysis of these pyrimidine nucleosides was carried by one or more enzymes. It is also curious that cytidine can be subject to phosphorolysis in A. cantonensis. To the best of our knowledge there is no other report of a cytidine phosphorylase in the literature.
5-(Benzyloxybenzyloxybenzyl)acyclouridine was identified as a potent and specific inhibitor of S. mansoni (Ki = 0.98 μM), but not the host uridine phosphorylase (el Kouni et al., 1988). Other benzylacyclouridines were also identified as inhibitor of S. mansoni uridine phosphorylase but were not specific for the parasite enzyme (el Kouni et al., 1988). The difference in degree of inhibition by the benzylacyclouridines as well as the ranking of the potencies of these inhibitors (Table 4) suggest that there are subtle differences between the binding sites of uridine phosphorylase from mammals and that of the parasite. Structure-activity relationship studies in S. mansoni (el Kouni et al., 1988), Giardia intestinalis (Jiménez et al., 1989), Toxoplasma gondii (Iltzsch and Klenk, 1993; el Kouni et al., 1996), and Hymenolepis diminuta (Drabikowska, 1996) suggest that uridine phosphorylase from these different parasite have common features. All have a hydrophobic pocket adjacent to the 5-position of the pyrimidine ring. This hydrophobic pocket in S. mansoni is larger than that of the mammalian enzyme (el Kouni et al., 1988). The most effective inhibitors were analogues of acyclouridines and 2,2′-anhydrouridines substituted at the C5 position with electron withdrawing (e.g., nitro or halogens) or hydrophobic (e.g., ethyl or benzyl) groups. The inhibitory effect at the 5-position appeared to be further enhanced by substitution at the C6 position with electron releasing groups. The parasite but not the mammalian host uridine phosphorylase can participate in hydrogen bonding with N3 of the pyrimidine ring of nucleoside ligands. Furthermore, the binding of ligands to the enzyme is in the syn/high syn conformation around the N-glycosyl bond. These differences may be useful in designing specific inhibitors for the parasite uridine phosphorylase which will interfere selectively with nucleic acids synthesis in this parasite. Such inhibitors may be used alone or in combination with other pyrimidine analogues as antischistosomal chemotherapeutic agents to interfere selectively with nucleic acids synthesis in this parasite.
Table 4.
Apparent Ki values for various inhibitors of uridine phosphorylase activity in Schistosoma mansoni and mouse liver.
| Compound | Apparent Ki (μM) ± S.E.E.a | Ratiob | |
|---|---|---|---|
|
| |||
| S. mansoni | Mouse | ||
| 5-Benzylacyclouridine | 5.2 ± 0.20 | 3.12 ± 0.21 | 0.60 |
| 5-(Benzyloxybenzyl)acyclouridine | 4.1 ± 0.25 | 1.25 ± 0.10 | 0.30 |
| 5-(Benzyloxybenzyloxybenzyl)acyclouridine | 3.5 ± 0.23 | 519 ± 34 | 148 |
Standard error of estimation,
Ratio of mouse liver apparent Ki to S. mansoni apparent Ki (data from el Kouni et al, 1988).
6.6. Cytosine and its nucleoside and nucleotide deaminases (EC 3.5.4.-)
Three enzymes, cytosine deaminase (EC 3.5.4.1), (2′-deoxy)cytidine deaminase (EC 3.5.4.5), and (d)CMP deaminase (EC 3.5.4.12) catalyze the reversible hydrolytic deamination of cytosine nucleobases, nucleosides and nucleotides to their uracil derivatives in the following manner:
As seen in Fig. 6, there is a fourth cytosine nucleotide deaminase, dCTP deaminase (EC 3.5.4.13). This deaminase has been reported, so far only in bacteria. Hence, it will not be discussed further in this review.
Among these deaminases, (d)CMP deaminase is of particular importance. The enzyme is one of only two enzymes allosterically regulated enzymes in pyrimidine metabolism. The other is ribonucleotide reductase (see above 2.7.). (d)CMP deaminase is activated by dCTP and inhibited by dTTP. As shown in Fig. 6, deamination of dCMP leads to the synthesis of dUMP, the substrate for thymidylate synthase and precursor of dTMP and the only de novo source of dTTP. However, dCMP is also a precursor of dCTP via nucleoside 5′-mono- and diphosphate kinases (Fig. 6). It is assumed that accumulation of dCTP would activate dCMP deaminase to enable the synthesis of thymidine nucleotides. On the other hand, dTTP accumulation can shut off its own synthesis by inhibiting dCMP deaminase. This scenario can explain the allosteric activation of dCMP deaminase activity by dCTP and inhibition by dTTP, and how the necessary balance between the two pyrimidine deoxyribotides required for normal DNA synthesis is achieved.
el Kouni and Naguib (1990) showed that Schistosoma mansoni incorporate cytidine and deoxycytidine exclusively into their respective nucleotides. In addition, enzyme assays indicated that the parasites are incapable of deaminating cytosine, cytidine, deoxycytidine, CMP and dCMP to their respective uracil derivatives. The absence of deoxycytidine deaminase was also observed by Senft et al. (1973a). However, the S. mansoni genome analysis (Berriman et al., 2009) indicates the presence of (d)CMP deaminase. Furthermore, (d)CMP deaminase activity was recently identified in S. mansoni (Serrão et al., 2017a). The enzyme has 175 amino acids and molecular weight of 19.5 kDa with 58% sequence identity to human (d)CMP deaminase, and a KdCMP of 22 μM and kcat, of 54s−1. Its activity, like other known (d)CMP deaminases, requires the presence of dCTP and two metals (Zn2+ and Mg2+), and is feedback inhibited by dTTP (Serrão et al., 2017a). The absence of (d)CMP deaminase activity reported by and el Kouni and Naguib (1990) could due to the use of EDTA in their assay buffer. EDTA is a chelator of metal ions (e.g., Zn2+) required for the activity of cytosine nucleoside/nucleotide deaminases. The same argument could explain the absence of (deoxy)cytidine and cytosine deaminases. However, the S. mansoni genome does not codify for these two enzymes (Berriman et al., 2009). Hence, it will be maintained that S. mansoni lack both (deoxy)cytidine and cytosine deaminases until proven otherwise..
Cytidine and deoxycytidine also are not cleaved by uridine phosphorylase in S. mansoni (el Kouni et al., 1988; Naguib and el Kouni, 2014). Therefore, cytidine and deoxycytidine appear to be exclusively incorporated into their respective nucleotides. The absence of cytidine deaminase and phosphorylase, along with the fact that cytidine is the most efficiently utilized pyrimidine by schistosomes (el Kouni and Naguib, 1990) can be manipulated for chemotherapy. Various available (deoxy)cytidine analogues may be used as antischistosomal drugs. These compounds could be phosphorylated efficiently by the parasites specific cytidine kinase or the non-specific deoxyriboside kinase (Naguib and el Kouni, 2014) without being degraded by sequential reaction of cytidine deaminase and uridine phosphorylase as is the case in the host. Deamination of cytosine nucleoside analogues is the major obstacle in their use in cancer chemotherapy. Cytidine deaminase is found in the post-infective L3 stage of the filarial nematode, Brugia pahangi (Anant et al., 1997). It was also shown that Plasmodium falciparum (Reyes et al., 1982), Tritrichomonas foetus (Wang et al., 1983), Crithidia fasciculata (Kidder, 1984; Hassan and Coombs, 1986)), Trypanosoma cruzi (Kidder, 1984), Trichomonas vaginalis (Wang and Cheng, 1984a), Giardia intestinalis (Aldritt et al., 1985), Angiostrongylus cantonensis (So et al., 1992), and Toxoplasma gondii (Iltzsch, 1993) can deaminate cytidine but not cytosine. Similar results were reported for amastigotes and cultured promastigotes of Leishmania mexicana mexicana, cultured promastigotes of L. m. amazonensis, L. donovani, L. tarentolae, culture forms of Crithidia fasciculata, Herpetomonas muscarum muscarum, H. m. ingenoplastis, and procyclic trypomastigotes of Trypanosoma brucei brucei, (Hassan and Coombs, 1986). However, deamination of deoxycytidine was observed only in Crithidia fasciculata, and cultured promastigotes of Leishmania mexicana mexicana (Hassan and Coombs, 1986). Although, Trypanosoma brucei has cytidine deaminase (Leija et al., 2016), it lacks dCMP deaminase (Castillo-Acosta et al., 2013). Knockdown of cytidine deaminase in T. brucei leads to thymidine/deoxyuridine auxotrophy and cell death despite the fact that the parasite does not require exogenous pyrimidines for growth (Leija et al., 2016).
6.7. Orotate/uracil phosphoribosyltransferase (EC 2.4.2.-)
As mentioned above (2.3.), schistosomes have two phosphoribosyltransferases (Iltzsch et al., 1984). The first is an orotate-specific phosphoribosyltransferase. The second is a non-specific orotate/uracil phosphoribosyltransferase that utilizes orotate, uracil, and 5-fluorouracil as substrates (Iltzsch et al., 1984; el Kouni and Naguib, 1990). Its Km for orotate (2.7 uM) (Iltzsch et al., 1984) is like those of similar enzymes from other parasites (O’Sullivan and Ketley, 1980; Asai et al., 1983; Hammond and Gutteridge, 1982 and 1984), and mammalian cells (Kasbekar et al., 1964; Kessel et al., 1972; Kavipurapu and Jones, 1976; Levinson et al., 1979; Krooth et al., 1979). The Km values for uracil (111 μM) (Iltzsch et al., 1984) is low enough such that uracil may indeed be a substrate for the schistosomal enzyme under physiological conditions. In contrast, the Km values of uracil for the mammalian enzymes (≃6500 μM) are so high that uracil would not serve as a substrate under physiological conditions, particularly in the presence of the strongly competing substrate orotate (Km ≤ 1 μM). (Kessel et al., 1972; Iltzsch et al., 1984).
Therefore, the orotate/uracil phosphoribosyltransferase reaction could be the principal pathway for the conversion of uracil to UMP in schistosomes because of its relatively higher activity with uracil as a substrate (92 ρmol/min/mg protein) (el Kouni and, Naguib 1990) over those of uridine phosphorylase (synthetic direction = 38 ρmol/min/mg protein) (el Kouni et al., 1988) and uridine kinase (16 ρmol/min/mg protein) (el Kouni and, Naguib 1990). The metabolism of uridine to UMP may also proceed via the same pathway for the same reasons. Uridine phosphorylase (catabolic direction = 118 ρmol/min/mg protein) and orotate/uracil phosphoribosyltransferase (92 ρmol/min/mg protein) activities are higher than that of uridine kinase (16 ρmol/min/mg protein) (el Kouni and Naguib, 1990) which is a tightly regulated and strongly inhibited by its substrate (Naguib and el Kouni, 2014). Therefore, it is probable that uridine salvage proceeds by the indirect route via the sequential activities of uridine phosphorylase and orotate/uracil phosphoribosyltransferases as shown in Fig. 6. This contention may also be supported by the similar pattern of incorporation of radioactive uracil and uridine into the nucleotide pool of this parasite (el Kouni and Naguib, 1990). In the final analysis the predominance of one pathway over the other for the salvage of uracil may depend on the efficiency (Vmax/Km) of the various enzymes as well as the availability and intracellular concentrations of the co-factors. Thus, unlike mammalian cells, phosphoribosyltransferase activities in S. mansoni may play a role in both de novo UMP biosynthesis as well as in the salvage of uracil and uridine. A similar situation seems to exist in the nematode Angiostrongylus cantonensis (So et al., 1992).
The results of Iltzsch et al. (1984) clearly indicate that the schistosomal orotate/uracil phosphoribosyltransferase differs significantly from the mammalian enzymes in inhibitor specificities. The ratios of apparent Ki values between mouse liver and S. mansoni differ considerably for various compounds (Table 5). These differences in inhibition constants of various drugs between the S. mansoni and mouse liver enzymes, as well as the difference in orotate metabolism between the two species suggest that this enzyme might be a good target for chemotherapy. In this respect, 5-azaorotic acid may be a potential antischistosomal agent, since its apparent Ki value for the host (mouse) enzyme is 50-fold higher than for the parasite enzyme (Table 5). Furthermore, both orotate/uracil phosphoribosyltransferase and the specific orotate phosphoribosyltransferase are inhibited by 5-azaorotic acid (Iltzsch et al., 1984). Therefore, inhibitors of phosphoribosyltransferases of orotate in schistosomes (e.g., 5-azaorotate) should be selectively toxic against the parasite by blocking the synthesis of UMP by both the salvage and the de novo pathways. Even when an inhibitor blocks orotate phosphoribosyltransferases of both the host and the parasite, selective toxicity can still be achieved by co-administration of uridine. As mentioned above (6.2.), S. mansoni uridine kinase is a tightly regulated enzyme and is strongly inhibited by its substrate (Naguib and el Kouni, 2014). Therefore, the high doses of uridine required to alleviate the inhibition by 5-azaorotate will inhibit the S. mansoni, but not the host, uridine kinase, allowing the host to salvage uridine via the uridine kinase pathway and overcome the block of de novo UMP biosynthesis. Alternatively, co-administration of 5-benzylacyclouridine with 5-azaorotate may rescue the host, but not the parasite, from the possible toxicity of orotate phosphoribosyltransferase inhibitors. 5-Benzylacyclouridine is a potent inhibitor of uridine phosphorylase from various species (Niedzwicki et al., 1982; Park et al., 1986; Naguib et al., 1987; el Kouni et al., 1988; el Kouni et al., 1996), and can alleviate the inhibition of de novo UMP biosynthesis by increasing the plasma levels and salvage of uridine only in the host (Monks et al., 1983; Darnowski and, Handschumacher, 1985; Martin, et al., 1989; Davis et al., 1993; Sommadossi et al., 1995) to overcome pyrimidine starvation. Furthermore, 5-benzylacyclouridine may act synergistically with 5-azaorotate on schistosomes by inhibiting the salvage of uridine by the worms via uridine phosphorylase.
Table 5.
Apparent Ki values for selected pyrimidine nucleobase analogues as inhibitors of phosphoribosyltransferase activity towards 5-fluorouracil (100 μM). in cytosol extracts of Schistosoma mansoni and mouse liver
| Compound | Apparent Ki (μM) ± S.Ea | Ratiob | |
|---|---|---|---|
|
| |||
| S. mansoni | Mouse Liver | ||
| 5-Azaorotic acid (Oxonic acid) | 0.9 ± 0.2 | 45 ± 16 | 50 |
| Barbituric acid | 35 ± 4 | 23 ± 3 | 0.66 |
| 5-Azabarbituric acid (Cyanuric acid) | 392 ± 59 | 212 ± 34 | 0.54 |
| 6-Carboxymethyluracil | 539 ± 86 | 156 ± 28 | 0.29 |
| 4,6-Dihydroxypyrimidine | 8090 ± 1220 | 15 ± 1 | 0.002 |
S.E. = standard error,
Ratio of mouse liver apparent Ki to S. mansoni apparent Ki. (data from Iltzsch et al., 1984).
The non-specific S. mansoni orotate/uracil phosphoribosyltransferase is different from the specific uracil phosphoribosyltransferase (EC 2.4.2.9) reported in several parasites that cannot synthesize pyrimidine de novo such Giardia intestinalis (Lindmark and Jarroll,1982; Aldritt et al., 1985; Jarroll et al., 1989), Tritrichomonas foetus (Wang et al., 1983; Hassan and Coombs, 1988), and Cryptosporidium (Striepen et al., 2004) or the parasites that lack uridine kinase like Toxoplasma gondii (Pfefferkorn, 1978; Iltzsch, 1993), Plasmodium falciparum (Reyes et al., 1982); culture promastigote form of Leishmania mexicana amazonensis, the blood trypomastigote and the culture epimastigote form of Trypanosoma brucei, blood trypomastigote and intracellular amastigote forms of T. cruzi (Hammond and Gutteridge, 1982), Giardia intestinalis (Aldritt et al., 1985; Vitti et al., 1987), Tritrichomonas foetus (Wang et al., 1983), and Leishmania donovani (Wilson et al., 2012). In these parasites UMP synthesis from uridine salvage occurs via the phosphoribosyltransferase reaction (Fig. 6). It should also be mentioned that uracil phosphoribosyltransferase activity in Leishmania mexicana and Trypanosoma brucei, as well as the epimastigote and trypomastigote forms of T. cruzi, is a cytoplasmic enzyme and is quite distinct from the glycosomal orotate phosphoribosyltransferase (Hammond and Gutteridge, 1982). The specific uracil phosphoribosyltransferase was purified from Giardia intestinalis and was found to be a dimer. GTP and dGTP caused a dramatic increase in enzyme activity but not from other eukaryotes (Dai et al., 1995). Genomic analysis indicated that Cryptosporidium parvum has two different uracil phosphoribosyltransferases with one fused with a uridine-cytidine kinase (Striepen et al., 2004). Such a bifunctional uracil phosphoribosyltransferase/uridine-cytidine kinase enzyme has not been previously reported in any parasite or mammalian system.
Pyrimidine auxotrophs of Toxoplasma gondii (Fox and Bzik, 2002), Leishmania donovani (Wilson et al., 2011), Trypanosoma cruzi (Hashimoto el al., 2012), and T. brucei (Ali et al., 2013b; Ong et al., 2013) can grow on medium supplemented with uracil or uridine with a marked preference for uracil. However, such auxotrophs exhibit hypersensitivity to uracil doses which does not affect wild type parasites. Furthermore, that hypersensitivity to uracil is not observed with any of the ribonucleosides (Ali et al., 2013b; Ong et al., 2013). This could be due to substrate inhibition of uracil phosphoribosyltransferase. The enzyme from Leishmania donovani is substrate inhibited (Soysa et al., 2013).
7. Pyrimidine analogues as potential antischistosomal drugs
The literature on the effects of pyrimidine nucleoside analogues in other organisms is voluminous. The majority of these compounds have been studied with regard to their therapeutic and toxic effects. Nevertheless, very few systematic structure activity relationship studies of pyrimidine analogues as ligands of pyrimidine metabolizing enzymes in parasites have been performed, e.g., schistosomes (Iltzsch et al., 1984; el Kouni et al., 1988) as well as toxoplasma (Iltzsch and Klenk, 1993; Iltzsch and Tankersley, 1994; el Kouni et al., 1996; Javid et al., 1999) and some potential chemotherapeutic agents were identified.
Pyrimidine analogues were used sporadically by a number of workers on parasites. With the exception of 5-fluorinated pyrimidines (Jaffe, 1975; Pfefferkorn and Pfefferkorn, 1977; Howells et al., 1981; Gómez, and Rathod, 1990; Papageorgiou et al., 2004; Arakaki et al., 2008; Ali et al., 2013a and 2013b; Alzahrani et al., 2016a), and carboxyemimycin (1,6-dihydro-6-oxo-2-pyrazinecarboxylic acid 4-oxide) (Matsuno et al., 1984) none of the pyrimidine analogues tested were found to be effective against parasites. These results, however, should not discourage the testing of other and newer pyrimidine analogues as antiparasitic agents. Recently, it was shown that analogues of 5′-norcarbocyclic uridine displayed antiparasitic activity in the low to mid-micromolar range against protozoan parasites, specifically the major pathogens Leishmania mexicana and Trypanosoma brucei (Alzahrani et al., 2016b). It is interesting to note that although these 5′-norcarbocyclic nucleoside are pyrimidine analogues they do not act directly on enzymes of pyrimidine metabolism (Alzahrani et al., 2016b), but more likely on S-adenosylhomocysteine hydrolase (EC 3.3.1.1), an enzyme involved in many biological methylation reactions. The chemical structures of these effective antiparasitic pyrimidine analogues are shown in Fig. 3.
In general and in spite of the fact that pyrimidine metabolism in parasites has not been studied with the same degree of thoroughness as has their purine metabolism, the information discussed in this review indicates that parasites are versatile in their ability to synthesize pyrimidine nucleotides. In addition, significant differences exist between different parasites, even among members of the same subgroup with respect to the efficiency of pyrimidine de novo and salvage pathways as well as to the types of enzymes involved in pyrimidine metabolism. These differences may have evolved depending on the availability and accessibility of nutrients in the particular environment in which each parasite developed.
In schistosomes, the presence of a phosphoribosyltransferase that can utilize uracil under physiological conditions (Iltzsch et al., 1984), the presence of a distinctive cytidine kinase (Naguib and el Kouni, 2014), the active metabolism of cytidine, absence of deamination, and lack of phosphorolysis of cytidine derivatives (el Kouni et al., 1988; el Kouni and Naguib, 1990), raises the possibility of using cytidine analogues for the selective treatment of schistosomiasis. Deamination of cytidine analogues is the major obstacle in their use in cancer chemotherapy. Hence the use of pyrimidine analogues as antiparasitic agents in general, and antischistosomal agents in particular remains feasible. Some of the most potent pyrimidine analogues have been synthesized and studied in this laboratory. Acyclouridine and its congeners, the most potent uridine phosphorylase inhibitors (Niedzwicki et al., 1981 and 1982; Naguib et al., 1987 and 1993; el Kouni et al., 1988 and 2000; Al Safarjalani et al., 2005), are potential antischistosomal agents. These compounds have little toxicity to the host, but they may be quite deleterious to parasites which are dependent, or can be made dependent, on the salvage of preformed pyrimidines for their survival. The recent encouraging results with 5′-norcarbocyclic uridines in protozoa (Alzahrani et al., 2016b) tempts the testing of these compound on schistosomes and other parasites. Furthermore, if antischistosomal activity is found in any of the toxic pyrimidine nucleoside analogues, it may still be administered safely. We have demonstrated that even a very toxic nucleoside (e.g., tubercidin) may be used safely as an antischistosomal drug by coadministration of a nucleoside transport inhibitor (el Kouni et al., 1983a, 1985, 1987 and 1989; el Kouni 1991).
8. Conclusions and prospects
It is quite clear that parasites differ from their host in various aspects of pyrimidine metabolism including: substrate specificity of various enzymes, the presence of special enzymes exclusively in the parasites but not the host, nature and type of pyrimidine nucleobase and nucleoside transport, insensitivity of nucleoside transport to inhibitors of mammalian nucleoside transport, etc. It is precisely these marked differences in pyrimidine metabolism between parasites and the host that make pyrimidine metabolism a potential and logical target for antiparasitic chemotherapeutic interventions. However, optimism in this endeavor should be accompanied by caution. In certain situations, multiple independent pathways as well as transport systems for pyrimidine metabolism in parasites may exist. In this case, the design of new antiparasitic chemotherapeutic regimens aimed at blocking pyrimidine metabolism would require inhibiting all these alternative pathways. Therefore, to continue to exploit pyrimidine metabolism in schistosomes and other parasites as a tool of a rational approach to chemotherapy, all the major steps in pyrimidine metabolism by a parasite must first be identified and each subjected to thorough structure-function analysis. An inhibitor against each of these steps must be identified and two or more of these inhibitors should be combined for an effective chemotherapy against the parasite. Indeed, strategies aimed at interfering with pyrimidine metabolism in parasites should be directed towards attacking two or more independent pathways to successfully overcome the ingenuity of the parasites in evading blockage of their pyrimidine metabolism by a single agent.
A final note, this review emphasizes that the ideal drug target is a protein that is essential for the parasite and does not have homologues in the host. The entry of parasitology into the post-genomic age raises hopes for the identification of such novel kinds of drug targets and in turn, new treatments for parasitic diseases. However powerful this functional genomics approach, it will miss some of the attractive targets for the chemotherapy of parasites as many essential proteins tend to be more highly conserved between species than non-essential ones.
Acknowledgments
Research work in the author’s laboratory quoted in this review has been supported by Grant AI-22219, AI-29848, AI-39550 and AI-42975 from the NIAID, DHHS and grants from the UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases.
Abbreviations
- AMP
adenosine 5′-monophosphate
- ADP
adenosine 5′-diphosphate
- ATP
adenosine 5′-triphosphate
- CMP
cytidine 5′-monophosphate
- CDP
cytidine 5′-diphosphate
- CTP
cytidine 5′-triphosphate
- dCMP
2′-deoxycytidine 5′-monophosphate
- dCTP
2′-deoxycytidine 5′-triphosphate
- dTMP
thymidine 5′-monophosphate
- dTDP
thymidine 5′-diphosphate
- dTTP
thymidine 5′-triphosphate
- dUMP
2′-deoxyuridine 5′-monophosphate
- dUDP
2′-deoxyuridine 5′-diphosphate
- dUTP
2′-deoxyuridine 5′-triphosphate
- dUTPase
deoxyuridine 5′-triphosphate pyrophosphatase
- FdUMP
5-fluorodeoxyuridine 5′-monophosphate
- IC50
concentration for 50% inhibition
- NBMPR
nitrobenzylthioinosine
- OMP
orotidine 5′-monophosphate
- UMP
uridine 5′-monophosphate
- UTP
uridine 5′-triphosphate
Footnotes
This paper is dedicated to the memory of Alfred W. Senft, a teacher, a colleague, and a dear friend. His enthusiasm and pioneering work in the field of nucleotide metabolism in schistosomes have inspired us and many others to pursue the study of this fascinating facet of parasitology.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akiyoshi DE, Balakrishnan R, Huettinger C, Widmer G, Tzipor S. Molecular characterization of ribonucleotide reductase from Cryptosporidium parvum. DNA Seq. 2002;13:167–172. doi: 10.1080/10425170290023419. [DOI] [PubMed] [Google Scholar]
- Al Chalabi K, Gutteridge WE. Presence and properties of thymidylate synthase in trypanosomatids. Biochim Biophys Acta. 1977a;481:71–79. doi: 10.1016/0005-2744(77)90138-3. [DOI] [PubMed] [Google Scholar]
- Al Chalabi K, Gutteridge WE. Catabolism of deoxythymidylate in some trypanosomatids. Parasitology. 1977b;74:299–312. doi: 10.1017/s0031182000047922. [DOI] [PubMed] [Google Scholar]
- Aldritt SM, Tien P, Wang CC. Pyrimidine salvage in Giardia lamblia. J Exptl Med. 1985;161:437–445. doi: 10.1084/jem.161.3.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali JAM, Creek DJ, Burgess K, Allison HC, Field MC, Mäser P, de Koning HP. Pyrimidine salvage in Trypanosoma brucei bloodstream forms and the trypanocidal action of halogenated pyrimidines. Mol Pharmacol. 2013a;83:439–453. doi: 10.1124/mol.112.082321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali JAM, Tagoe DN, Munday JC, Donachie A, Morrison LJ, de Koning HP. Pyrimidine biosynthesis is not an essential function for Trypanosoma brucei bloodstream forms. PLoS One. 2013b;8:e58034. doi: 10.1371/journal.pone.0058034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Safarjalani ON, Naguib FNM, el Kouni MH. Uptake of nitrobenzylthioinosine and purine β-L-nucleosides by intracellular Toxoplasma gondii. Antimicrob Agents Chemother. 2003;47:32347–3251. doi: 10.1128/AAC.47.10.3247-3251.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Safarjalani ON, Zhou XJ, Rais RH, Shi J, Schinazi RF, Naguib FNM, el Kouni MH. 5-(Phenylthio)acyclouridine: A powerful enhancer of oral uridine bioavailability: relevance to chemotherapy with 5-fluorouracil and other uridine rescue regimens. Cancer Chemother Pharmacol. 2005;55:541–551. doi: 10.1007/s00280-004-0967-y. [DOI] [PubMed] [Google Scholar]
- Alzahrani KJH, Ali JAM, Eze AA, Looi WL, Tagoe DNA, Creek DJ, Barrett MP, de Koning HP. Functional and genetic evidence that nucleoside transport is highly conserved in Leishmania species: Implications for pyrimidine-based chemotherapy. Int J Parasitol Drugs Drug Resist. 2017a;7:206–226. doi: 10.1016/j.ijpddr.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzahrani KJ, Matyugina ES, Khandazhinskaya AL, Kochetkov SN, Seley-Radtke KL, Koning HP. Evaluation of the antiprotozoan properties of 5′-norcarbocyclic pyrimidine nucleosides. Bioorg Med Chem Lett. 2017b;27:3081–3086. doi: 10.1016/j.bmcl.2017.05.052. [DOI] [PubMed] [Google Scholar]
- Anderson HR, Fairweather I, Bamford DR. Uptake of the nucleoside uridine by the liver fluke Fasciola hepatica. Comp Biochem Physiol A. 1993;106:305–308. [Google Scholar]
- Anderson IJ, Loftus BJ. Entamoeba histolytica: Observations on metabolism based on the genome sequence. Exp Parasitol. 2005;110:173–177. doi: 10.1016/j.exppara.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Anant S, Martin SA, Yu H, MacGinnitie AJ, Devaney E, Davidson NO. A cytidine deaminase expressed in the post-infective L3 stage of the filarial nematode, Brugia pahangi, has a novel RNA-binding activity. Mol Biochem Parasitol. 1997;88:105–14. doi: 10.1016/s0166-6851(97)00083-2. [DOI] [PubMed] [Google Scholar]
- Annoura T, Nara T, Makiuchi T, Hashimoto T, Aoki T. The origin of dihydroorotate dehydrogenase genes of kinetoplastids, with special reference to their biological significance and adaptation to anaerobic, parasitic conditions. J Mol Evol. 2005;60:113–127. doi: 10.1007/s00239-004-0078-8. [DOI] [PubMed] [Google Scholar]
- Aoki JI, Yamashiro-Kanashiro EH, Ramos DC, Cotrim PC. Efficacy of the tubercidin antileishmania action associated with an inhibitor of the nucleoside transport. Parasitol Res. 2009;104:223–228. doi: 10.1007/s00436-008-1177-z. [DOI] [PubMed] [Google Scholar]
- Aoki T, Oya H. Kinetic properties of carbamoyl-phosphate synthetase II (glutamine-hydrolyzing) in the parasitic protozoan Crithidia fasciculata and separation of the enzyme from aspartate carbamoyltransferase. Comp Biochem Physiol B. 1987;87:143–150. doi: 10.1016/0305-0491(87)90481-0. [DOI] [PubMed] [Google Scholar]
- Aoki T, Oya H. Glutamine-dependent carbamoyl-phosphate synthetase and control of pyrimidine biosynthesis in the parasitic Helminth Schistosoma mansoni. Comp Biochem Physiol B. 1979;63:511–515. doi: 10.1016/0305-0491(79)90055-5. [DOI] [PubMed] [Google Scholar]
- Aoki T, Oya H, Mori M, Tatibana M. Control of pyrimidine biosynthesis in the Ascaris ovary: Regulatory properties of glutamine-dependent carbamoyl-phosphate synthetase and copurification of the enzyme with aspartate carbamoyltransferase and dihydroorotase. Mol Biochem Parasitol. 1980;1:55–68. doi: 10.1016/0166-6851(80)90041-9. [DOI] [PubMed] [Google Scholar]
- Arakaki TL, Buckner FS, Gillespie JR, Malmquist NA, Phillips MA, Kalyuzhniy O, Luft JR, Detitta GT, Verlinde CL, Van Voorhis WC, Hol WG, Merritt EA. Characterization of Trypanosoma brucei dihydroorotate dehydrogenase as a possible drug target; Structural, kinetic and RNAi studies. Mol Microbiol. 2008;68:37–50. doi: 10.1111/j.1365-2958.2008.06131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo FG, Huskinson J, Remington JS. Remarkable in vitro and in vivo activities of the hydroxynaphthoquinone 566C80 against tachyzoites and tissue cysts of Toxoplasma gondii. Antimicrob Agents Chemother. 1991;35:293–299. doi: 10.1128/aac.35.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronow B, Kaur K, McCartan K, Ullman B. Two high affinity nucleoside transporters in Leishmania donovani. Mol Biochem Parasitol. 1987;22:29–37. doi: 10.1016/0166-6851(87)90066-1. [DOI] [PubMed] [Google Scholar]
- Asai T, O’Sullivan WJ, Kobayashi M, Gero AM, Yokogawa M, Tatibana M. Enzymes of the de novo pyrimidine biosynthetic pathway in Toxoplasma gondii. Mol Biochem Parasitol. 1983;7:89–100. doi: 10.1016/0166-6851(83)90037-3. [DOI] [PubMed] [Google Scholar]
- Baer HP. Cytotoxic nucleosides and parasitic diseases: A new therapeutic approach. Annals Saudi Med. 1989;9:570–575. [Google Scholar]
- Baer HP, El-Soofi A, Selim A. Treatment of Schistosoma mansoni- and Schistosoma haematobium-infected mice with a combination of tubercidin and nucleoside transport inhibitor. Med Sci Res. 1988;16:919. [Google Scholar]
- Baer HP, Serignese V, Ogbunude PO, Dzimiri M. Nucleoside transporters in Leishmania major: Diversity in adenosine transporter expression or function in different strains. Am J Trop Med Hyg. 1992;47:87–91. doi: 10.4269/ajtmh.1992.47.87. [DOI] [PubMed] [Google Scholar]
- Baragaña B, McCarthy O, Sánchez P, Bosch-Navarrete C, Kaiser M, Brun R, Whittingham JL, Roberts SM, Zhou XX, Wilson KS, Johansson NG, González-Pacanowska D, Gilbert IH. β-Branched acyclic nucleoside analogues as inhibitors of Plasmodium falciparum dUTPase. Bioorg Med Chem. 2011;19:2378–2391. doi: 10.1016/j.bmc.2011.02.012. [DOI] [PubMed] [Google Scholar]
- Barker RH, Jr, Metelev V, Rapaport E, Zamecnik P. Inhibition of Plasmodium falciparum malaria using antisense oligodeoxynucleotides. Proc Natl Acad Sci U S A. 1996;93:514–518. doi: 10.1073/pnas.93.1.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basco LK, Ramiliarisoa O, Le Bras J. In vitro activity of atovaquone against the African isolates and clones of Plasmodium falciparum. Am J Trop Med Hyg. 1995;53:388–391. doi: 10.4269/ajtmh.1995.53.388. [DOI] [PubMed] [Google Scholar]
- Baum KF, Berens RL, Marr JJ. Purine nucleosides and nucleobase cell membrane transport in Giardia lamblia. J Euk Microbiol. 1993;40:643–649. doi: 10.1111/j.1550-7408.1993.tb06122.x. [DOI] [PubMed] [Google Scholar]
- Bellofatto V. Pyrimidine transport activities in trypanosomes. Trends Parasitol. 2007;23:187–189. doi: 10.1016/j.pt.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Berriman M, Haas BJ, LoVerde PT, Wilson RA, Dillon GP, Cerqueira GC, Mashiyama ST, Al-Lazikani B, Andrade LF, Ashton PD, Aslett MA, Bartholomeu DC, Blandin G, Caffrey CR, Coghlan A, Coulson R, Day TA, Delcher A, DeMarco R, Djikeng A, Eyre T, Gamble JA, Ghedin E, Gu Y, Hertz-Fowler C, Hirai H, Hirai Y, Houston R, Ivens A, Johnston DA, Lacerda D, Macedo CD, McVeigh P, Ning Z, Oliveira G, Overington JP, Parkhill J, Pertea M, Pierce RJ, Protasio AV, Quail MA, Rajandream MA, Rogers J, Sajid M, Salzberg SL, Stanke M, Tivey AR, White O, Williams DL, Wortman J, Wu W, Zamanian M, Zerlotini A, Fraser-Liggett CM, Barrell BG, El-Sayed NM. The genome of the blood fluke Schistosoma mansoni. Nature. 2009;460:352–358. doi: 10.1038/nature08160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilsland E, Pir P, Gutteridge A, Johns A, King RD, Oliver SG. Functional expression of parasite drug targets and their human orthologs in yeast. PloS Negl Trop Dis. 2011;5:e1320. doi: 10.1371/journal.pntd.0001320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernier-Villamor V, Sampson DA, Matunis MJ, Lima CD. Characterization of deoxyuridine 5′-triphosphate nucleotidohydrolase from Trypanosoma cruzi. Cell. 2002;108:345–356. doi: 10.1016/s0014-5793(02)03158-7. [DOI] [PubMed] [Google Scholar]
- Bhardwaj R, Skelly J. Characterization of schistosome tegumental alkaline phosphatase (SmAP) PLoS Negl Trop Dis. 2011;5:e1011. doi: 10.1371/journal.pntd.0001011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black MJ, Jones ME. Characterization and significance of carbamyl phosphate phosphatase. Cancer Res. 1984;44:4366–4376. [PubMed] [Google Scholar]
- Bogitsh BJ. Histochemical Studies on Hymenolepis microstoma (Cestoda: Hymenolepididae) J Parasitol. 1963;49:989–997. [PubMed] [Google Scholar]
- Bols NC, Zimmerman AM. Nucleoside phosphotransferase activity through the growth and cell cycle of Tetrahymena pyriformis GL-I. Exptl Cell Res. 1977;108:259–268. doi: 10.1016/s0014-4827(77)80033-5. [DOI] [PubMed] [Google Scholar]
- Braschi S, Borges WC, Wilson RA. Proteomic analysis of the schistosome tegument and its surface membranes. Mem Inst Oswaldo Cruz. 2006;101(Suppl 1):205–212. doi: 10.1590/s0074-02762006000900032. [DOI] [PubMed] [Google Scholar]
- Brown GK, O’Sullivan WJ. Subunit structure of the orotate phosphoribosyltransferase--orotidylate decarboxylase complex from human erythrocytes. Biochemistry. 1977;16:3235–3242. doi: 10.1021/bi00633a030. [DOI] [PubMed] [Google Scholar]
- Buolamwini JK. Nucleoside transport inhibitors: Structure-activity relationships and potential therapeutic applications. Curr Med Chem. 1997;4:35–66. [Google Scholar]
- Camacho A, Arrebola R, Peña-Diaz J, Ruiz-Pérez LM, González-Pacanowska D. Description of a novel eukaryotic deoxyuridine 5′-triphosphate nucleotidohydrolase in Leishmania major. Biochem J. 1997;325(Pt 2):441–447. doi: 10.1042/bj3250441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho A, Hidalgo-Zarco F, Bernier-Villamor V, Ruiz-Pérez LM, González-Pacanowska D. Properties of Leishmania major dUTP nucleotidohydrolase, a distinct nucleotide-hydrolysing enzyme in kinetoplastids. Biochem J. 2000;346(Pt 1):163–168. [PMC free article] [PubMed] [Google Scholar]
- Carter NS, Ben Mamoun C, Liu W, Silva EO, Landfear SM, Goldberg DE, Ullman B. Isolation and functional characterization of the PfNT1 nucleoside transporter gene from Plasmodium falciparum. J Biol Chem. 2000;275:10683–10691. doi: 10.1074/jbc.275.14.10683. [DOI] [PubMed] [Google Scholar]
- Cass CE, Young JD, Baldwin SA, Cabrita MA, Graham KA, Griffiths M, Jennings LL, Mackey JR, Ng AM, Ritzel MW, Vickers MF, Yao SY. Nucleoside transporters of mammalian cells. Pharmaceutical Biotechnology. 1999;12:313–352. doi: 10.1007/0-306-46812-3_12. [DOI] [PubMed] [Google Scholar]
- Castilho MS, Postigo MP, Pereira HM, Oliva G, Andricopulo AD. Structural basis for selective inhibition of purine nucleoside phosphorylase from Schistosoma mansoni: Kinetic and structural studies. Bioorg Med Chem. 2010;18:1421–1427. doi: 10.1016/j.bmc.2010.01.022. [DOI] [PubMed] [Google Scholar]
- Castillo-Acosta VM, Aguilar-Pereyra F, García-Caballero D, Vidal AE, Ruiz-Pérez LM, González-Pacanowska D. Pyrimidine requirements in deoxyuridine triphosphate nucleotidohydrolase deficient Trypanosoma brucei mutants. Mol Biochem Parasitol. 2013;187:9–13. doi: 10.1016/j.molbiopara.2012.11.003. [DOI] [PubMed] [Google Scholar]
- Castillo-Acosta VM, Estévez AM, Vidal AE, Ruiz-Perez LM, González-Pacanowska D. Int. J Biochem Cell Biol. 2008;40:2901–2913. doi: 10.1016/j.biocel.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Cesari IM. Schistosoma mansoni: Distribution and characteristics of alkaline and acid phosphatase. Exptl Parasitol. 1974;36:405–14. doi: 10.1016/0014-4894(74)90080-0. [DOI] [PubMed] [Google Scholar]
- Cesari IM, Simpson AJ, Evans WH. Properties of a series of tegumental membrane-bound phosphatase activities of Schistosoma mansoni. Biochem J. 1981;198:467–473. doi: 10.1042/bj1980467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti D, Schuster SM, Chakrabarti R. Cloning and characterization of subunit genes of ribonucleotide reductase, a cell-cycle-regulated enzyme, from Plasmodium falciparum. Proc Natl Acad Sci U S A. 1993;90:12020–12024. doi: 10.1073/pnas.90.24.12020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chello PL, Jaffe JJ. Isolation, partial purification, and properties of thymidine kinase from Trypanosoma (trypanozoon) brucei rhodesiense. J Parasitol. 1972a;58:298–305. [PubMed] [Google Scholar]
- Chello PL, Jaffe JJ. Comparative properties of trypanosomal and mammalian thymidine kinases. Comp Biochem Physiol B. 1972b;43:543–562. doi: 10.1016/0305-0491(72)90138-1. [DOI] [PubMed] [Google Scholar]
- Chen Y, Donald D, Savin K, Presidente PJ, Hrtman D. Haemonchus contortus: Molecular cloning, sequencing, and expression analysis of the gene coding for the small subunit of ribonucleotide reductase. Exp Parasitol. 2005;111:250–255. doi: 10.1016/j.exppara.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Chiang CW, Carter N, Sullivan WJ, Jr, Donald RGK, Roos DS, Naguib FNM, el Kouni MH, Ullman B, Wilson CM. The adenosine transporter of Toxoplasma gondii. Identification by insertional mutagenesis, cloning and recombinant expression. J Biol Chem. 1999;274:35255–35261. doi: 10.1074/jbc.274.49.35255. [DOI] [PubMed] [Google Scholar]
- Christopherson RI, Cinquin O, Shojaei M, Kuehn D, Menz RI. Cloning and expression of malarial pyrimidine enzymes. Nucleosides Nucleotides Nucleic Acids. 2004;23:1459–1465. doi: 10.1081/NCN-200027678. [DOI] [PubMed] [Google Scholar]
- Coderre JA, Beverley SM, Schimke RT, Santi DV. Overproduction of a bifunctional thymidylate synthetase-dihydrofolate reductase and DNA amplification in methotrexate-resistant Leishmania tropica. Proc. Natl Acad Sci U S A. 1983;80:2132–2136. doi: 10.1073/pnas.80.8.2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde-del Pino E, Annexy-Martínez AM, Pérez-Vilar M, Cintrón-Rivera AA. Studies in Schistosoma mansoni. II. Isoenzyme patterns for alkaline phosphatase, isocitric dehydrogenase, glutamic oxalacetic transaminase, and glucose 6-phosphate dehydrogenase of adult worms and cercariae. Exp Parasitol. 1968;22:288–294. doi: 10.1016/0014-4894(68)90104-5. [DOI] [PubMed] [Google Scholar]
- Crabtree GW, Senft AW. Pathways of nucleotide metabolism in Schistosoma mansoni. V. Adenosine cleavage enzyme and effects of purine analogues on adenosine metabolism in vitro. Biochem Pharmacol. 1974;23:649–660. doi: 10.1016/0006-2952(74)90630-3. [DOI] [PubMed] [Google Scholar]
- Craig SP, III, Yuan L, Kuntz DA, McKerrow JH, Wang CC. High level expression in Escherichia coli of soluble enzymatically active schistosomal hypoxanthine/guanine phosphoribosyltransferase and trypanosomal ornithine decarboxylase. Proc Natl Acad Sci U S A. 1991;88:2500–2504. doi: 10.1073/pnas.88.6.2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowther GJ, Napuli AJ, Gilligan JH, Gagaring K, Borboa R, Francek C, Chen Z, Dagostino EF, Stockmyer JB, Wang Y, Rodenbough PP, Castaneda LJ, Leibly DJ, Bhandari J, Gelb MH, Brinker A, Engels IH, Taylor J, Chatterjee AK, Fantauzzi P, Glynne RJ, Van Voorhis WC, Kuhen KL. Identification of inhibitors for putative malaria drug targets among novel antimalarial compounds. Mol Biochem Parasitol. 2011;175:21–29. doi: 10.1016/j.molbiopara.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabrowska M, Zieliński ZZ, Wranicz M, Michalski R, Pawełczak K, Rode W. Trichinella spiralis thymidylate synthase: Developmental pattern, isolation, molecular properties and inhibition by substrate and cofactor analogues. Biochem Biophys Res Commun. 1996;288:440–445. doi: 10.1006/bbrc.1996.1679. [DOI] [PubMed] [Google Scholar]
- Dai YP, Lee CS, O’Sullivan WJ. Properties of uracil phosphoribosyltransferase from Giardia intestinalis. Int J Parasitol. 1995;25:207–14. doi: 10.1016/0020-7519(94)00090-b. [DOI] [PubMed] [Google Scholar]
- Darnowski JW, Handschumacher RE. Tissue-specific enhancement of uridine utilization and 5-fluorouracil therapy in mice by benzylacyclouridine. Cancer Res. 1985;45:5364–5368. [PubMed] [Google Scholar]
- da Silva Neto AM, Torini de Souza JR, Romanello L, Cassago A, Serrão VH, DeMarco R, Brandão-Neto J, Garratt RC, Pereira HD. Analysis of two Schistosoma mansoni uridine phosphorylases isoforms suggests the emergence of a protein with a non-canonical function. Biochimie. 2016;125:12–22. doi: 10.1016/j.biochi.2016.02.007. [DOI] [PubMed] [Google Scholar]
- da Silveira NJ, Uchôa HB, Canduri F, Pereira JH, Camera JC, Jr, Basso LA, Palma MS, Santos DS, de Azevedo WF., Jr Structural bioinformatics study of PNP from Schistosoma mansoni. Biochem Biophys Res Commun. 2004;322:100–104. doi: 10.1016/j.bbrc.2004.07.088. [DOI] [PubMed] [Google Scholar]
- Davey RA, Ey PL, Mayrhofer G. Characteristics of thymidine transport in Giardia intestinalis trophozoites. Mol Biochem Parasitol. 1991;48:163–171. doi: 10.1016/0166-6851(91)90112-j. [DOI] [PubMed] [Google Scholar]
- Davey RA, Mayrhofer G, Ey PL. Identification of a broad-specificity transporter with affinity for the sugar moiety in Giardia Intestinalis trophozoites. Biochim. Biophys. Acta. 1992;1109:172–178. doi: 10.1016/0005-2736(92)90080-6. [DOI] [PubMed] [Google Scholar]
- Davis ST, Joyner SS, Chandrasurin P, Baccanari DP. Species-dependent differences in the biochemical effects and metabolism of 5-benzylacyclouridine. Biochem Pharmacol. 1993;45:173–181. doi: 10.1016/0006-2952(93)90390-i. [DOI] [PubMed] [Google Scholar]
- de Araújo Santos R, Braz C, Ghasemi J, Safavi-Sohi R, Barbosa E. Mixed 2D–3D-LQTA-QSAR study of a series of Plasmodium falciparum dUTPase inhibitors. Med Chem Res. 2015;24:1098–1111. [Google Scholar]
- de Koning HP, Al-Salabi MI, Cohen AM, Coombs GH, Wastling JM. Identification and characterisation of high affinity nucleoside and nucleobase transporters in Toxoplasma gondii. Int J Parasitol. 2003;33:821–31. doi: 10.1016/s0020-7519(03)00091-2. [DOI] [PubMed] [Google Scholar]
- de Koning HP, Diallinas G. Nucleobase transporters. Mol Membr Biol. 2000;75:75–94. doi: 10.1080/09687680050117101. [DOI] [PubMed] [Google Scholar]
- de Koning HP, Jarvis SM. Hypoxanthine uptake through a purine-selective nucleobase transporter in Trypanosoma brucei brucei procyclic cells is driven by protonmotive force. Eur J Biochem. 1997a;247:1102–1110. doi: 10.1111/j.1432-1033.1997.01102.x. [DOI] [PubMed] [Google Scholar]
- de Koning HP, Jarvis SM. Purine nucleobase transport in bloodstream forms of Trypanosoma brucei brucei is mediated by two novel transporters. Molec Biochem Parasitol. 1997b;89:245–258. doi: 10.1016/s0166-6851(97)00129-1. [DOI] [PubMed] [Google Scholar]
- de Koning HP, Jarvis SM. A highly selective, high-affinity transporter for uracil in Trypanosoma brucei brucei: Evidence for proton-dependent transport. Biochem Cell Biol. 1998;76:853–858. doi: 10.1139/bcb-76-5-853. [DOI] [PubMed] [Google Scholar]
- de Koning HP, Watson CJ, Sutcliffe L, Jarvis SM. Differential regulation of nucleoside and nucleobase transport in Crithidia fasciculata and Trypanosoma brucei in response to purine stress. Molec Biochem Parasitol. 2000;106:93–107. doi: 10.1016/s0166-6851(99)00203-0. [DOI] [PubMed] [Google Scholar]
- de Moraes MC, Cardoso CL, Cass QB. Immobilized purine nucleoside phosphorylase from Schistosoma mansoni for specific inhibition studies. Anal Bioanal Chem. 2013;405:4871–4878. doi: 10.1007/s00216-013-6872-7. [DOI] [PubMed] [Google Scholar]
- Deniskin R, Frame IJ, Sosa Y, Akabas MH. Targeting the Plasmodium vivax equilibrative nucleoside transporter 1 (PvENT1) for antimalarial drug development. Int J Parasitol Drugs Drug Resist. 2015;28:1–11. doi: 10.1016/j.ijpddr.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Den Hollander JE, Erasmus DA. Schistosoma mansoni: DNA synthesis in males and females from mixed and single-sex infections. Parasitology. 1984;88:463–76. doi: 10.1017/s0031182000054731. [DOI] [PubMed] [Google Scholar]
- D’Muniz-Pereira H, Oliva G, Garratt RC. Purine nucleoside phosphorylase from Schistosoma mansoni in complex with ribose-1-phosphate. J Synchrotron Radiat. 2011;8:62–65. doi: 10.1107/S0909049510027718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dormeyer M, Reckenfelderbäumer N, Ludemann H, Krauth-Siegel RL. Trypanothione-dependent synthesis of deoxyribonucleotides by Trypanosoma brucei ribonucleotide reductase. J Biol Chem. 2001;276:10602–10106. doi: 10.1074/jbc.M010352200. [DOI] [PubMed] [Google Scholar]
- Dormeyer M, Schöneck R, Dittmar GA, Krauth-Siegel RL. Cloning, sequencing and expression of ribonucleotide reductase R2 from Trypanosoma brucei. FEBS Lett. 1997;414:449–453. doi: 10.1016/s0014-5793(97)01036-3. [DOI] [PubMed] [Google Scholar]
- Downie MJ, Saliba KJ, Howitt SM, Bröer S, Kirk K. Transport of nucleosides across the Plasmodium falciparum parasite plasma membrane has characteristics of PfENT1. Mol Microbiol. 2006;60:738–48. doi: 10.1111/j.1365-2958.2006.05125.x. [DOI] [PubMed] [Google Scholar]
- Dovey HF, McCarey JH, Wang CC. Purine salvage in Schistosoma mansoni schistosomules. Mol Biochem Parasitol. 1984;11:157–167. doi: 10.1016/0166-6851(84)90062-8. [DOI] [PubMed] [Google Scholar]
- Dovey HF, McKerrow JH, Wang CC. Action of tubercidin and other adenosine analogs on Schistosoma mansoni schistosomules. Mol Biochem Parasitol. 1985;16:185–198. doi: 10.1016/0166-6851(85)90086-6. [DOI] [PubMed] [Google Scholar]
- Drabikowska AK. Uridine phosphorylase from Hymenolepis diminuta (Cestoda): Kinetics and inhibition by pyrimidine nucleoside analogs. Acta Biochim Pol. 1996;43:733–741. [PubMed] [Google Scholar]
- Dusanic DG. Histochemical observations of alkaline phosphatase in Schistosoma mansoni. J Infect Dis. 1959;105:1–8. doi: 10.1093/infdis/105.1.1. [DOI] [PubMed] [Google Scholar]
- Edman JC, Edman U, Lundgren B, Santi DV. Isolation and expression of the Pneumocystis carinii thymidylate synthase gene. Proc Natl Acad Sci U S A. 1989;86:6503–6507. doi: 10.1073/pnas.86.17.6503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el Kouni MH. Efficacy of combination therapy with tubercidin and nitrobenzylthioinosine 5′-monophosphate against chronic and advanced stages of schistosomiasis. Biochem Pharmacol. 1991;41:815–820. doi: 10.1016/0006-2952(91)90085-j. [DOI] [PubMed] [Google Scholar]
- el Kouni MH. Chemotherapy of Schistosomiasis. R I Medicine. 1992;75:212–216. [PubMed] [Google Scholar]
- el Kouni MH. Potential Chemotherapeutic Targets in the Purine Metabolism of Parasites. Pharmacol Ther. 2003;99:283–309. doi: 10.1016/s0163-7258(03)00071-8. [DOI] [PubMed] [Google Scholar]
- el Kouni MH. Adenosine metabolism in Toxoplasma gondii: Potential targets for chemotherapy. Curr Pharm Des. 2007;13:581–597. doi: 10.2174/138161207780162836. [DOI] [PubMed] [Google Scholar]
- el Kouni MH, Cha S. Isolation and partial characterization of a 5′-nucleotidase specific for orotidine-5′-monophosphate. Proc Natl Acad Sci U S A. 1982;79:1037–1041. doi: 10.1073/pnas.79.4.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el Kouni MH, Cha S. Metabolism of adenosine analogues by Schistosoma mansoni and the effect of nucleoside transport inhibitors. Biochem Pharmacol. 1987;36:1099–1106. doi: 10.1016/0006-2952(87)90420-5. [DOI] [PubMed] [Google Scholar]
- el Kouni MH, Diop D, Cha S. Combination therapy of schistosomiasis by tubercidin and nitrobenzylthioinosine 5′-monophosphate. Proc Natl Acad Sci U S A. 1983a;80:6667–6670. doi: 10.1073/pnas.80.21.6667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el Kouni MH, Diop D, O’Shea P, Carlisle R, Sommadossi J-P. Prevention of tubercidin host toxicity by nitrobenzylthioinosine 5′-monophosphate for the treatment of schistosomiasis. Antimicrob Agents Chemother. 1989;33:824–827. doi: 10.1128/aac.33.6.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el Kouni MH, el Kouni MM, Naguib FNM. Differences in activities and substrate specificity of human and murine pyrimidine nucleoside phosphorylases: Implications for chemotherapy with 5-fluoropyrimidines. Cancer Res. 1993;53:3687–3693. [PubMed] [Google Scholar]
- el Kouni MH, Knopf PM, Cha S. Combination therapy of Schistosoma japonicum by tubercidin and nitrobenzylthioinosine 5′-monophosphate. Biochem Pharmacol. 1985;34:3921–3923. doi: 10.1016/0006-2952(85)90445-9. [DOI] [PubMed] [Google Scholar]
- el Kouni MH, Messier NJ, Cha S. Treatment of schistosomiasis by purine nucleoside analogues in combination with nucleoside transport inhibitors. Biochem Pharmacol. 1987;36:3815–3821. doi: 10.1016/0006-2952(87)90443-6. [DOI] [PubMed] [Google Scholar]
- el Kouni MH, Goudgaon NM, Rafeeq M, Al Safarjalani ON, Schinazi RF, Naguib FNM. 5-Phenylthioacyclouridine: a potent and specific inhibitor of uridine phosphorylase. Biochem Pharmacol. 2000;60:851–856. doi: 10.1016/s0006-2952(00)00410-x. [DOI] [PubMed] [Google Scholar]
- el Kouni MH, Guarcello V, Al Safarjalani ON, Naguib FNM. Metabolism and selective toxicity of 6-nitrobenzylthioinosine in Toxoplasma gondii. Antimicrob Agents Chemother. 1999;43:2437–2443. doi: 10.1128/aac.43.10.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el Kouni MH, Naguib FNM. Pyrimidine salvage pathways in adult Schistosoma mansoni Int. J Parasitol. 1990;20:37–44. doi: 10.1016/0020-7519(90)90170-r. [DOI] [PubMed] [Google Scholar]
- el Kouni MH, Naguib FNM, Niedzwicki JG, Iltzsch MH, Cha S. Uridine phosphorylase from Schistosoma mansoni. J Biol Chem. 1988;263:6081–6086. [PubMed] [Google Scholar]
- el Kouni MH, Naguib FNM, Panzica RP, Otter BA, Chu SH, Gosselin G, Chu CK, Schinazi RF, Shealy YF, Goudgaon N, Ozerov AA, Ueda T, Iltzsch MH. Effects of modifications in the pentose moiety and conformational changes on the binding of nucleoside ligands to uridine phosphorylase from Toxoplasma gondii. Biochem Pharmacol. 1996;51:1687–1700. doi: 10.1016/0006-2952(96)00213-4. [DOI] [PubMed] [Google Scholar]
- el Kouni MH, Niedzwicki JG, Lee K-Y, Iltzsch MH, Senft AW, Cha S. Enzymes of pyrimidine metabolism in Schistosoma mansoni. Fed Proc. 1983b;42:2207. [Google Scholar]
- Erasmus DA. Studies on phosphatase systems of cestodes. I. Studies on Taenia pisiformis (cysticercus and adult) Parasitology. 1957a;47:70–80. doi: 10.1017/s0031182000021776. [DOI] [PubMed] [Google Scholar]
- Erasmus DA. Studies on phosphatase systems of cestodes. II. Studies on Cysticercus tenuicollis and Moniezia expansa (adult) Parasitology. 1957b;47:781–91. doi: 10.1017/s0031182000021788. [DOI] [PubMed] [Google Scholar]
- Ernst SC. Biochemical and cytochemical studies of alkaline phosphatase activity in Schistosoma mansoni. Rice Univ. Studies. 1976;62:81–95. [Google Scholar]
- Evans DR, Guy HI. Mammalian pyrimidine biosynthesis: Fresh insights into an ancient pathway. J Biol Chem. 2004;279:33035–33038. doi: 10.1074/jbc.R400007200. [DOI] [PubMed] [Google Scholar]
- Ey PL, Davey RA, Duffield GA. A low affinity nucleobase transport in the protozoan parasite Giardia intestinalis. Biochim. Biophys. Acta. 1992;1109:179–186. doi: 10.1016/0005-2736(92)90081-v. [DOI] [PubMed] [Google Scholar]
- Farland WH, MacInnis AJ. In vitro thymidine kinase activity: Present in Hymenolepis diminuta (Cestoda) and Moniliformis dubius (Acanthocephala), but apparently lacking in Ascaris lumbricoides (Nematoda) J Parasitol. 1978;64:564–565. [PubMed] [Google Scholar]
- Feliciano PR, Cordeiro A, Costa-Filho AJ, Nonato MC. Cloning, expression, purification, and characterization of Leishmania major dihydroorotate dehydrogenase. Protein Expr Purif. 2006;48:98–103. doi: 10.1016/j.pep.2006.02.010. [DOI] [PubMed] [Google Scholar]
- Ferone R, Roland S. Dihydrofolate reductase:thymidylate synthase, a bifunctional polypeptide from Crithidia fasciculata. Proc Natl Acad Sci U S A. 1980;77:5802–5806. doi: 10.1073/pnas.77.10.5802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira RA, de Oliveira AB, Gualberto SA, Miguel Del Corral JM, Fujiwara RT, Guimarães PHG, de Almeida Vitor RW. New naphthoquinones and an alkaloid with in vitro activity against Toxoplasma gondii RH and EGS strains. Exptl Parasitol. 2012;132:450–457. doi: 10.1016/j.exppara.2012.09.003. [DOI] [PubMed] [Google Scholar]
- Fijolek A, Hofer A, Thelander L. Expression, purification, characterization, and in vivo targeting of trypanosome CTP synthetase for treatment of African sleeping sickness. J Biol Chem. 2007;282:11858–11865. doi: 10.1074/jbc.M611580200. [DOI] [PubMed] [Google Scholar]
- Fink K. Thymidine phosphorylation in synchronous culture of Tetrahymena pyriformis GL. Exptl Cell Res. 1980;127:438–441. doi: 10.1016/0014-4827(80)90449-8. [DOI] [PubMed] [Google Scholar]
- Foulk BW, Pappas G, Hirai Y, Hirai H, Williams DL. Adenylosuccinate lyase of Schistosoma mansoni: Gene structure, mRNA expression, and analysis of the predicted peptide structure of a potential chemotherapeutic target. Int J Parasitol. 2002;32:1487–1495. doi: 10.1016/s0020-7519(02)00161-3. [DOI] [PubMed] [Google Scholar]
- Fox BA, Bzik DJ. De novo pyrimidine biosynthesis is required for virulence of Toxoplasma gondii. Nature. 2002;415:926–929. doi: 10.1038/415926a. [DOI] [PubMed] [Google Scholar]
- Gao G, Nara T, Shimada JN, Aoki T. Novel organization and sequence of five genes encoding all six enzymes for de novo pyrimidine biosynthesis in Trypanosoma cruzi. J Mol Biol. 1999;285:149–161. doi: 10.1006/jmbi.1998.2293. [DOI] [PubMed] [Google Scholar]
- Garavito MF, Narváez-Ortiz HY, Zimmermann BH. Pyrimidine metabolism: Dynamic and versatile pathways in pathogens and cellular development. J Genet Genomics. 2015;42:195–205. doi: 10.1016/j.jgg.2015.04.004. [DOI] [PubMed] [Google Scholar]
- Garrett CE, Coderre JA, Meek TD, Garvey EP, Claman DM, Beverley SM, Santi DV. A bifunctional thymidylate synthetase-dihydrofolate reductase in protozoa. Mol Biochem Parasitol. 1984;11:257–265. doi: 10.1016/0166-6851(84)90070-7. [DOI] [PubMed] [Google Scholar]
- Gati WP, Stoyke AF, Gero AM, Paterson AR. Nucleoside permeation in mouse erythrocytes infected with Plasmodium yoelii. Biochem Biophys Res Commun. 1987;145:1134–1141. doi: 10.1016/0006-291x(87)91555-5. [DOI] [PubMed] [Google Scholar]
- Gero AM, Coombs GH. Orotate phosphoribosyltransferase and orotidine-5′-phosphate decarboxylase in two parasitic kinetoplastid flagellates. FEBS Lett. 1980;118:130–132. doi: 10.1016/0014-5793(80)81234-8. [DOI] [PubMed] [Google Scholar]
- Gero AM, Brown GV, O’Sullivan WJ. Pyrimidine de novo synthesis during the life cycle of the intraerythrocytic stage of Plasmodium falciparum. J Parasitol. 1984;70:536–541. [PubMed] [Google Scholar]
- Gero AM, O’Sullivan WJ, Wright IG, Mahoney DF. The enzymes of pyrimidine biosynthesis in Babesia bovis and Babesia bigemina. Aust J Exp Biol Med Sci. 1983;61:239–243. doi: 10.1038/icb.1983.22. [DOI] [PubMed] [Google Scholar]
- Gero AM, Scott HV, O’Sullivan WJ, Christopherson RI. Antimalarial action of nitrobenzylthioinosine in combination with purine nucleoside antimetabolites. Molec Biochem Parasitol. 1989;34:87–98. doi: 10.1016/0166-6851(89)90023-6. [DOI] [PubMed] [Google Scholar]
- Ghosh I, Raghavan N, FitzGerald PC, Scott AL. Nucleoside diphosphate kinase from the parasitic nematode Brugia malayi. Gene. 1995;164:261–266. doi: 10.1016/0378-1119(95)00500-6. [DOI] [PubMed] [Google Scholar]
- Ghosh M, Mukherjee T. Stage-specific development of a novel adenosine transporter in Leishmania donovani amastigotes. Mol Biochem Parasitol. 2000;108:93–99. doi: 10.1016/s0166-6851(00)00208-5. [DOI] [PubMed] [Google Scholar]
- Gómez ZM, Rathod PK. Antimalarial activity of a combination of 5-fluoroorotate and uridine in mice. Antimicrob Agents Chemother. 1990;34:1371–1375. doi: 10.1128/aac.34.7.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulian M, Bleile B, Tseng BY. Methotrexate-induced misincorporation of uracil into DNA. Proc Natl Acad Sci U S A. 1980;77:1956–1960. doi: 10.1073/pnas.77.4.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gounaris K. Nucleotidase cascades are catalyzed by secreted proteins of the parasitic nematode Trichinella spiralis. Infect. Immun. 2002;70:4917–4924. doi: 10.1128/IAI.70.9.4917-4924.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JS, Pudney M. Activity of atovaquone against Babesia microti in the Mongolian gerbil, Meriones unguiculatus. J Parasitol. 1999;85:723–728. [PubMed] [Google Scholar]
- Griffith DA, Jarvis SM. High affinity sodium-dependent nucleobase transport in cultured renal epithelial cells (LLC-PK) J Biol Chem. 1993;268:20085–20090. [PubMed] [Google Scholar]
- Gryseels B, De Vlas SJ. Worm burdens in schistosome infections. Parasitol Today. 1996;12:115–119. doi: 10.1016/0169-4758(96)80671-5. [DOI] [PubMed] [Google Scholar]
- Gudin S, Quashie NB, Candlish D, Al-Salabi MI, Jarvis SM, Ranford-Cartwright LC, de Koning HP. Trypanosoma brucei: A survey of pyrimidine transport activities. Exp Parasitol. 2006;114:118–125. doi: 10.1016/j.exppara.2006.02.018. [DOI] [PubMed] [Google Scholar]
- Gujjar R, Mazouni FE, White KL, White J, Creason S, Shackleford DM, Deng X, Charman WN, Bathurst I, Burrows J, Floyd DM, Matthews D, Buckner FS, Charman SA, Phillips MA, Rathod PK. Lead-optimization of aryl and aralkyl amine based triazolopyrimidine inhibitors of Plasmodium falciparum dihydroorotate dehydrogenase with antimalarial activity in mice. J Med Chem. 2011;54:3935–3949. doi: 10.1021/jm200265b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutteridge WE, Dave D, Richards WH. Conversion of dihydroorotate to orotate in parasitic protozoa. Biochim Biophys Acta. 1979;582:390–401. doi: 10.1016/0304-4165(79)90131-4. [DOI] [PubMed] [Google Scholar]
- Halton DW. Studies on phosphatase activity in Trematoda. J Parasitol. 1967;53:46–55. [PubMed] [Google Scholar]
- Hammond DJ, Gutteridge WE. UMP synthesis in the kinetoplastida. Biochim Biophys Acta. 1982;718:1–10. doi: 10.1016/0304-4165(82)90002-2. [DOI] [PubMed] [Google Scholar]
- Hammond DJ, Gutteridge WE. Purine and pyrimidine metabolism in the Trypanosomatidae. Molec Biochem Parasitol. 1984;13:243–261. doi: 10.1016/0166-6851(84)90117-8. [DOI] [PubMed] [Google Scholar]
- Hammond DJ, Gutteridge WE, Opperdoes FR. A novel location for two enzymes of de novo pyrimidine biosynthesis in trypanosomes and Leishmania. FEBS Lett. 1981;128:27–29. doi: 10.1016/0014-5793(81)81070-8. [DOI] [PubMed] [Google Scholar]
- Hampton SE, Baragaña B, Schipani A, Bosch-Navarrete C, Musso-Buendía JA, Recio E, Kaiser M, Whittingham JL, Roberts SM, Shevtsov M, Brannigan JA, Kahnberg P, Brun R, Wilson KS, González-Pacanowska D, Johansson NG, Gilbert IH. Design, synthesis, and evaluation of 5′-diphenyl nucleoside analogues as inhibitors of the Plasmodium falciparum dUTPase. Chem Med Chem. 2011;6:1816–1831. doi: 10.1002/cmdc.201100255. [DOI] [PubMed] [Google Scholar]
- Harkiolaki M, Dodson EP, Bernier-Villamor V, Turkenburg JP, González-Pacanowska D, Wilson KS. The crystal structure of Trypanosoma cruzi dUTPase reveals a novel dUTP/dUDP binding fold. Structure. 2004;12:41–53. doi: 10.1016/j.str.2003.11.016. [DOI] [PubMed] [Google Scholar]
- Harris DI, Beechey RB, Linstead D, Barrett J. Nucleoside uptake by Trichomonas vaginalis. Molec Biochem Parasitol. 1988;29:105–116. doi: 10.1016/0166-6851(88)90065-5. [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Morales J, Fukai Y, Suzuki S, Takamiya S, Tsubouchi A, Inoue S, Inoue M, Kita K, Harada S, Tanaka A, Aoki T, Nara T. Critical importance of the de novo pyrimidine biosynthesis pathway for Trypanosoma cruzi growth in the mammalian host cell cytoplasm. Biochem Biophys Res Commun. 2012;417:1002–1006. doi: 10.1016/j.bbrc.2011.12.073. [DOI] [PubMed] [Google Scholar]
- Hassan HF, Coombs GH. Comparative study of purine- and pyrimidine metabolizing enzymes of range of trypanosomatids. Comp Biochem Physiol. 1986;84B:217–223. [PubMed] [Google Scholar]
- Hassan HF, Coombs GH. Purine and pyrimidine metabolism in parasitic protozoa. FEMS Microbiol Rev. 1988;4:47–83. doi: 10.1111/j.1574-6968.1988.tb02708.x-i1. [DOI] [PubMed] [Google Scholar]
- Hedstorm L, Wang CC. Purine base transport in wild-type and mycophenolic acid-resistant Tritrichomonas foetus. Molec Biochem Parasitol. 1989;35:219–228. doi: 10.1016/0166-6851(89)90208-9. [DOI] [PubMed] [Google Scholar]
- Hemsworth GR, González-Pacanowska D, Wilson KS. On the catalytic mechanism of dimeric dUTPases. Biochem J. 2013;456:81–88. doi: 10.1042/BJ20130796. [DOI] [PubMed] [Google Scholar]
- Hemsworth GR, Moroz OV, Fogg MJ, Scott B, Bosch-Navarrete C, González-Pacanowska D, Wilson KS. The crystal structure of the Leishmania major deoxyuridine triphosphate nucleotidohydrolase in complex with nucleotide analogues, dUMP, and deoxyuridine. J Biol Chem. 2011;286:16470–81. doi: 10.1074/jbc.M111.224873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson JF, Paterson ARP. Nucleotide Metabolism: An Introduction. Acad. Press, Inc; N.Y., N.Y: 1973. [Google Scholar]
- Hendriks EF, O’Sullivan WJ, Stewart TS. Molecular cloning and characterization of the Plasmodium falciparum cytidine triphosphate synthetase gene. Biochim. Biophys. Acta. 1998;1399:213–218. doi: 10.1016/s0167-4781(98)00108-0. [DOI] [PubMed] [Google Scholar]
- Heyworth PG, Gutteridge WE, Ginger CD. Pyrimidine metabolism in Trichomonas vaginalis. FEBS Lett. 1984;176:55–60. doi: 10.1016/0014-5793(84)80910-2. [DOI] [PubMed] [Google Scholar]
- Hidalgo-Zarco F, Camacho AG, Bernier-Villamor V, Nord J, Ruiz-Pérez LM, González-Pacanowska D. Kinetic properties and inhibition of the dimeric dUTPase-dUDPase from Leishmania major. Protein Sci. 2001;10:1426–33. doi: 10.1110/ps.48801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidalgo-Zarco F, González-Pazanowska D. Trypanosomal dUTPases as potential targets for drug design. Curr Protein Pept Sci. 2001;2:389–397. doi: 10.2174/1389203013381026. [DOI] [PubMed] [Google Scholar]
- Hill B, Kilsby J, McIntosh RT, Wrigglesworth R, Ginger CD. Pyrimidine biosynthesis in Plasmodium berghei. Int J Biochem. 1981a;13:303–310. doi: 10.1016/0020-711x(81)90082-3. [DOI] [PubMed] [Google Scholar]
- Hill B, Kilsby J, Rogerson GW, McIntosh RT, Ginger CD. The enzymes of pyrimidine biosynthesis in a range of parasitic protozoa and helminths. Mol Biochem Parasitol. 1981b;2:123–134. doi: 10.1016/0166-6851(81)90094-3. [DOI] [PubMed] [Google Scholar]
- Hockley DJ, McLaren DJ. Schistosoma mansoni: Changes in the outer membrane of the tegument during development from cercaria to adult worm. Int J Parasitol. 1973;3:13–25. doi: 10.1016/0020-7519(73)90004-0. [DOI] [PubMed] [Google Scholar]
- Hofer A, Steverding D, Chabes A, Brun R, Thelander L. Trypanosoma brucei CTP synthetase: A target for the treatment of African sleeping sickness. Proc Natl Acad Sci U S A. 2001;98:6412–6416. doi: 10.1073/pnas.111139498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland KP, Elford HL, Bracchi V, Annis CG, Schuster SM, Chakrabarti D. Antimalarial activities of polyhydroxyphenyl and hydroxamic acid derivatives. Antimicrob Agents Chemother. 1998;42:2456–4568. doi: 10.1128/aac.42.9.2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hortua Triana MA, Cajiao Herrera D, Zimmermann BH, Fox BA, Bzik DJ. Pyrimidine pathway-dependent and -independent functions of the Toxoplasma gondii mitochondrial dihydroorotate dehydrogenase. Infect Immun. 2016;84:2974–2981. doi: 10.1128/IAI.00187-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hortua Triana MA, Huynh MH, Garavito MF, Fox BA, Bzik DJ, Carruthers VB, Löffler M, Zimmermann BH. Biochemical and molecular characterization of the pyrimidine biosynthetic enzyme dihydroorotate dehydrogenase from Toxoplasma gondii. Mol Biochem Parasitol. 2012;84:71–81. doi: 10.1016/j.molbiopara.2012.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howells RE, Tinsley J, Devaney E, Smith G. The effect of 5-fluorouracil and 5-fluorocytosine on the development of the filarial nematodes Brugia pahangi and Dirofilaria immitis. Acta Trop. 1981;38:289–304. [PubMed] [Google Scholar]
- Huang Z-Y, Chang H-L, Chen G-Z. The metabolism of pyrimidine and purine in Schistosoma japonicum. Chinese Med J. 1984;97:698–706. [PubMed] [Google Scholar]
- Hunger-Glaser I, Hemphill A, Shalaby T, Hanni M, Seebeck T. Nucleoside diphosphate kinase of Trypanosoma brucei. Gene. 2000;257:251–257. doi: 10.1016/s0378-1119(00)00401-7. [DOI] [PubMed] [Google Scholar]
- Hurst DT. Introduction to the Chemistry and Biochemistry of Pyrimidine, Purines, and Pteridines. John Wiley & Sons Ltd; Chichester, N.Y: 1980. [Google Scholar]
- Hyde JE. Targeting purine and pyrimidine metabolism in human apicomplexan parasites. Curr Drug Targets. 2007;8:31–47. doi: 10.2174/138945007779315524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iltzsch MH. Pyrimidine salvage pathways in Toxoplasma gondii. J Eukaryot Microbiol. 1993;40:24–28. doi: 10.1111/j.1550-7408.1993.tb04877.x. [DOI] [PubMed] [Google Scholar]
- Iltzsch MH, Klenk EE. Structure-activity relationship of nucleobase ligands of uridine phosphorylase from Toxoplasma gondii. Biochem Pharmacol. 1993;46:1849–1858. doi: 10.1016/0006-2952(93)90592-k. [DOI] [PubMed] [Google Scholar]
- Iltzsch MH, Niedzwicki JG, Senft AW, Cha S, el Kouni MH. Enzymes of uridine 5′-monophosphate biosynthesis in Schistosoma mansoni. Mol Biochem Parasitol. 1984;12:153–171. doi: 10.1016/0166-6851(84)90132-4. [DOI] [PubMed] [Google Scholar]
- Iltzsch MH, Tankersley KO. Structure-activity relationship of ligands of uracil phosphoribosyltransferase from Toxoplasma gondii. Biochem Pharmacol. 1994;48:781–792. doi: 10.1016/0006-2952(94)90057-4. [DOI] [PubMed] [Google Scholar]
- Imprasittichail W, Roytrakul S, Krungkrai SR, Krungkrail J. A unique insertion of low complexity amino acid sequence underlies protein-protein interaction in human malaria parasite orotate phosphoribosyltransferase and orotidine 5′-monophosphate decarboxylase. Asian Pac J Trop Med. 2014;7:184–192. doi: 10.1016/S1995-7645(14)60018-3. [DOI] [PubMed] [Google Scholar]
- Iovannisci DM, Goebel D, Allen K, Kaur K, Ullman B. Genetic analysis of adenine metabolism in Leishmania donovani promastigotes. J Biol Chem. 1984;259:14617–14623. [PubMed] [Google Scholar]
- Jaffe JJ. Dihydrofolate reductase from filarial worms and schistosomes. Ann N Y Acad Sci. 1971;186:113–114. doi: 10.1111/j.1749-6632.1971.tb31132.x. [DOI] [PubMed] [Google Scholar]
- Jaffe JJ. Nucleoside analogs as antiparasitic agents. Ann N Y Acad Sci. 1975;255:306–316. doi: 10.1111/j.1749-6632.1975.tb29238.x. [DOI] [PubMed] [Google Scholar]
- Jaffe JJ, Chrin LR. Folate metabolism in filariae: enzymes associated with 5,10-methylenetetrahydrofolate. J Parasitol. 1980;66:53–58. [PubMed] [Google Scholar]
- Jaffe JJ, Comley JCW, Chrin LR. Thymidine kinase activity and thymidine salvage in adult Brugia pahangi and Dirofilaria immitis. Mol. Biochem Parasitol. 1982;5:361–370. doi: 10.1016/0166-6851(82)90009-3. [DOI] [PubMed] [Google Scholar]
- Jaffe JJ, McCormack JJ, Meymarian E. Comparative properties of schistosomal and filarial dihydrofolate reductases. Biochem Pharmacol. 1972;21:719–31. doi: 10.1016/0006-2952(72)90064-0. [DOI] [PubMed] [Google Scholar]
- Janeway CM, Cha S. Effects of 6-azauridine on nucleotides, orotic acid, and orotidine in L1578Y mouse lymphoma cells in vitro. Cancer Res. 1977;37:4382–4388. [PubMed] [Google Scholar]
- Jarroll EL, Hammond MM, Lindmark DG. Giardia lamblia: uptake of pyrimidine nucleosides. Exptl Parasitol. 1987;63:152–156. doi: 10.1016/0014-4894(87)90156-1. [DOI] [PubMed] [Google Scholar]
- Javaid ZZ, el Kouni MH, Iltzsch MH. Pyrimidine nucleobase ligands of orotate phosphoribosyltransferase from Toxoplasma gondii. Biochem Pharmacol. 1999;58:1457–1465. doi: 10.1016/s0006-2952(99)00231-2. [DOI] [PubMed] [Google Scholar]
- Jiménez BM, Kranz P, Lee CS, Gero AM, O’Sullivan WJ. Inhibition of uridine phosphorylase from Giardia lamblia by pyrimidine analogs. Biochem Pharmacol. 1989;38:3785–3789. doi: 10.1016/0006-2952(89)90586-8. [DOI] [PubMed] [Google Scholar]
- Jimenez BM, O’Sullivan WJ. CTP synthetase and enzymes of pyrimidine ribonucleotide metabolism in Giardia intestinalis. Int. J Parasitol. 1994;24:713–718. doi: 10.1016/0020-7519(94)90125-2. [DOI] [PubMed] [Google Scholar]
- Jones ME. Pyrimidine nucleotide biosynthesis in animals: Genes, enzymes, and regulation of UMP biosynthesis. Ann Rev Biochem. 1980;49:253–279. doi: 10.1146/annurev.bi.49.070180.001345. [DOI] [PubMed] [Google Scholar]
- Kamyingkird K, Cao S, Masatani T, Moumouni PF, Vudriko P, Mousa AA, Terkawi MA, Nishikawa Y, Igarashi I, Xuan X. Babesia bovis dihydroorotate dehydrogenase (BboDHODH) is a novel molecular target of drug for bovine babesiosis. J Vet Med Sci. 2014;76:323–330. doi: 10.1292/jvms.13-0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaaneh J, Craig SP, III, Wang CC. Differential inhibitory effects of GMP-2′,3′-dialdehyde on human and schistosomal hypoxanthine-guanine phosphoribosyltransferases. Eur J Biochem. 1994;223:595–601. doi: 10.1111/j.1432-1033.1994.tb19030.x. [DOI] [PubMed] [Google Scholar]
- Kanaani J, Maltby D, Focia P, Wang CC. Identification of the active sites of human schistosomal hypoxanthine-guanine phosphoribosyltransferases by GMP-2′,3′-dialdehyde affinity labeling. Biochemistry. 1995;34:14987–14996. doi: 10.1021/bi00046a005. [DOI] [PubMed] [Google Scholar]
- Kanaani J, Maltby D, Somoza JR, Wang CC. Inactivation of Trichomonas foetus and Schistosoma mansoni purine phosphoribosyltransferases by arginine-specific reagents. Eur J Biochem. 1997;244:810–817. doi: 10.1111/j.1432-1033.1997.00810.x. [DOI] [PubMed] [Google Scholar]
- Kasbekar DK, Nagabhushanam A, Greenberg D. Purification and properties of orotic acid-decarboxylating enzymes from calf thymus. J Biol Chem. 1964;239:4245–4249. [PubMed] [Google Scholar]
- Kasper LH, Pfefferkorn ER. Hydroxyurea inhibition of growth and DNA synthesis in Toxoplasma gondii: Characterization of a resistant mutant. Mol Biochem Parasitol. 1982;6:141–150. doi: 10.1016/0166-6851(82)90073-1. [DOI] [PubMed] [Google Scholar]
- Kavipurapu PR, Jones ME. Purification, size, and properties of the complex of orotate phosphoribosyltransferase:orotidylate decarboxylase from mouse Ehrlich ascites carcinoma. J Biol Chem. 1976;251:5589–5599. [PubMed] [Google Scholar]
- Kensler TW, Cooney DA. Chemotherapeutic inhibitors of the enzymes of the de novo pyrimidine pathway. Adv Pharmacol Chemother. 1981;18:273–352. doi: 10.1016/s1054-3589(08)60257-4. [DOI] [PubMed] [Google Scholar]
- Kessel D, Deacon J, Coffey B, Bakamjian A. Some properties of a pyrimidine phosphoribosyltransferase from murine leukemia cells. Mol Pharmacol. 1972;8:731–739. [PubMed] [Google Scholar]
- Kicska GA, Tyler PC, Evans GB, Furneaux RH, Kim K, Schramm VL. Transition state analogue inhibitors of purine nucleoside phosphorylase from Plasmodium falciparum. J Biol Chem. 2002;277:3219–3225. doi: 10.1074/jbc.M105905200. [DOI] [PubMed] [Google Scholar]
- Kidder GW. Characteristics of cytidine aminohydrolase activity in Trypanosoma cruzi and Crithidia fasciculata. J Protozool. 1984;31:298–300. doi: 10.1111/j.1550-7408.1984.tb02965.x. [DOI] [PubMed] [Google Scholar]
- Klayman DL, Acton N, Scovill JP. 2-Acetylpyridine thiosemicarbazones 12. Derivatives of 3-acetylisoquinoline as potential antimalarial agents. Arzneim-Forsch. 1986;136:10–13. doi: 10.1002/chin.198619236. [DOI] [PubMed] [Google Scholar]
- Klayman DL, Mason CJ, Scovill JP. 2-Acetylpyridine thiosemicarbazones 10. 2-Propionyl-2-butryl-, and 2-(2-methylpropionyl)pyridine thiosemicarbazones as potential antimalarial agents. Arzneim-Forsch. 1984;34:1701–1703. [PubMed] [Google Scholar]
- Knecht W, Petersen GE, Munch-Petersen B, Piškur J. Deoxyribonucleoside kinases belonging to the thymidine kinase 2 (TK2)-like group vary significantly in substrate specificity, kinetics and feed-back regulation. J Mol Biol. 2002;315:529–540. doi: 10.1006/jmbi.2001.5257. [DOI] [PubMed] [Google Scholar]
- Knecht W, Petersen GE, Sandrini MPB, Søndergaard L, Munch-Petersen B, Piškur J. Mosquito has a single multisubstrate deoxyribonucleoside kinase characterized by unique substrate specificity. Nucleic Acids Res. 2003;31:1665–1672. doi: 10.1093/nar/gkg257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M, Yokogawa M, Mori M, Tatibana M. Pyrimidine nucleotide biosynthesis in Clonorchis sinensis and Paragonimus ohirai. Int J Parasitol. 1978;8:471–477. doi: 10.1016/0020-7519(78)90067-x. [DOI] [PubMed] [Google Scholar]
- Kolli BK, Kostal J, Zaborina O, Chakrabarty AM, Chang KP. Leishmania-released nucleoside diphosphate kinase prevents ATP-mediated cytolysis of macrophages. Mol Biochem Parasitol. 2008;158:163–75. doi: 10.1016/j.molbiopara.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong W, Engel K, Wang J. Mammalian nucleoside transporters. Curr Drug Metab. 2004;5:63–84. doi: 10.2174/1389200043489162. [DOI] [PubMed] [Google Scholar]
- Kraup M, Marz R. Membrane transport of nucleobases: Interaction with inhibitors. Gen Pharmacol. 1995;26:1185–1190. doi: 10.1016/0306-3623(95)00005-l. [DOI] [PubMed] [Google Scholar]
- Krooth RS, Hsiao W-L, Potvin BW. Resistance to 5-fluoroorotic acid and pyrimidine auxotrophy: a new bidirectional selective system for mammalian cells. Somatic Cell Gen. 1979;5:551–569. doi: 10.1007/BF01542694. [DOI] [PubMed] [Google Scholar]
- Krungkrai J. Purification, characterization and localization of mitochondrial dihydroorotate dehydrogenase in Plasmodium falciparum, human malaria parasite. Biochim. Biophys. Acta. 1995;1243:351–360. doi: 10.1016/0304-4165(94)00158-t. [DOI] [PubMed] [Google Scholar]
- Krungkrai J, Cerami A, Henderson GB. Pyrimidine biosynthesis in parasitic protozoa: Purification of a monofunctional dihydroorotase from Plasmodium berghei and Crithidia fasciculata. Biochemistry. 1990;29:6270–6275. doi: 10.1021/bi00478a023. [DOI] [PubMed] [Google Scholar]
- Krungkrai J, Cerami A, Henderson GB. Purification and characterization of dihydroorotate dehydrogenase from the rodent malaria parasite Plasmodium berghei. Biochemistry. 1991;30:1934–1939. doi: 10.1021/bi00221a029. [DOI] [PubMed] [Google Scholar]
- Krungkrai J, Prapunwatana P, Wichitkul C, Reungprapavut S, Krungkrai SR, Horii T. Molecular biology and biochemistry of malarial parasite pyrimidine biosynthetic pathway. Southeast Asian J Trop Med Public Health. 2003;34(Suppl 2):32–43. [PubMed] [Google Scholar]
- Krungkrai SR, Aoki S, Palacpac NM, Sato D, Mitamura T, Krungkrai J, Horii T. Human malaria parasite orotate phosphoribosyltransferase: Functional expression, characterization of kinetic reaction mechanism and inhibition profile. Mol Biochem Parasitol. 2004;134:245–55. doi: 10.1016/j.molbiopara.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Krungkrai SR, DelFraino BJ, Smiley JA, Prapunwattana P, Mitamura T, Horii T, Krungkrai J. A novel enzyme complex of orotate phosphoribosyltransferase and orotidine 5′-monophosphate decarboxylase in human malaria parasite Plasmodium falciparum: Physical association, kinetics, and inhibition characterization. Biochemistry. 2005;44:1643–1652. doi: 10.1021/bi048439h. [DOI] [PubMed] [Google Scholar]
- Krungkrai SR, Krungkrai J. Insights into the pyrimidine biosynthetic pathway of human malaria parasite Plasmodium falciparum as chemotherapeutic target. Asian Pac J Trop Med. 2016;9:525–34. doi: 10.1016/j.apjtm.2016.04.012. [DOI] [PubMed] [Google Scholar]
- Krungkrai SR, Prapunwattana P, Horii T, Krungkrai J. Orotate phosphoribosyltransferase and orotidine 5′-monophosphate decarboxylase exist as multienzyme complex in human malaria parasite Plasmodium falciparum Biochem. Biophys Res Commun. 2004;318:1012–1018. doi: 10.1016/j.bbrc.2004.04.124. [DOI] [PubMed] [Google Scholar]
- Laoworawit P, Lee CS, O’Sullivan WJ. Deoxynucleoside kinases of Giardia intestinalis. Mol. Biochem Parasitol. 1993;60:37–44. doi: 10.1016/0166-6851(93)90026-t. [DOI] [PubMed] [Google Scholar]
- Larson ET, Mudeppa DG, Gillespie JR, Mueller N, Napuli AJ, Arif JA, Ross J, Arakaki TL, Lauricella A, Detitta G, Luft J, Zucker F, Verlinde CL, Fan E, Van Voorhis WC, Buckner FS, Rathod PK, Hol WG, Merritt EA. The crystal structure and activity of a putative trypanosomal nucleoside phosphorylase reveal it to be a homodimeric uridine phosphorylase. J Mol Biol. 2010;396:1244–1259. doi: 10.1016/j.jmb.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawres LA, Garg A, Kumar V, Bruzual I, Forquer IP, Renard I, Virji AZ, Boulard P, Rodriguez EX, Allen AJ, Pou S, Wegmann KW, Winter RW, Nilsen A, Mao J, Preston DA, Belperron AA, Bockenstedt LK, Hinrichs DJ, Riscoe MK, Doggett JS, Ben Mamoun C. Radical cure of experimental babesiosis in immunodeficient mice using a combination of an endochin-like quinolone and atovaquone. J Exp Med. 2016;213:1307–1318. doi: 10.1084/jem.20151519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CS, Jiménez BM, O’Sullivan WJ. Purification and characterization of uridine (thymidine) phosphorylase from Giardia lamblia. Mol Biochem Parasitol. 1988;30:271–277. doi: 10.1016/0166-6851(88)90096-5. [DOI] [PubMed] [Google Scholar]
- Leija C, Rijo-Ferreira F, Kinch LN, Grishin NV, Nischan N, Kohler JJ, Hu Z, Phillips MA. Pyrimidine salvage enzymes are essential for de novo biosynthesis of deoxypyrimidine nucleotides in Trypanosoma brucei. PLoS Pathog. 2016;12:e1006010. doi: 10.1371/journal.ppat.1006010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi-Schaffer F, Tarrab-Hazdai R, Schryer MD, Arnon R, Smolarsky M. Isolation and partial characterization of the tegumental outer membrane of schistosomula of Schistosoma mansoni. Mol Biochem Parasitol. 1984;13:283–300. doi: 10.1016/0166-6851(84)90120-8. [DOI] [PubMed] [Google Scholar]
- Levine RL, Hoogenraad NJ, Kretchmer N. A review: Biological and clinical aspects of pyrimidine metabolism. Pediatr Res. 1974;8:724–734. doi: 10.1203/00006450-197407000-00008. [DOI] [PubMed] [Google Scholar]
- Levinson BB, Ullman B, Martin DW. Pyrimidine pathway variants of cultured mouse lymphoma cells with altered levels of both orotate phosphoribosyltransferase and orotidylate decarboxylase. J Biol Chem. 1979;254:4396–4401. [PubMed] [Google Scholar]
- Levy MG, Read CP. Purine and pyrimidine transport in Schistosoma mansoni. J Parasitol. 1975a;61:627–632. [PubMed] [Google Scholar]
- Levy MG, Read CP. Relation of tegumentary phosphatase to purine and pyrimidine transport in Schistosoma mansoni. J Parasitol. 1975b;61:648–656. [PubMed] [Google Scholar]
- Lim RL, O’Sullivan WJ, Stewart TS. Isolation, characterization and expression of the gene encoding cytidine triphosphate synthetase from Giardia intestinalis. Mol. Biochem Parasitol. 1996;78:249–257. doi: 10.1016/s0166-6851(96)02635-7. [DOI] [PubMed] [Google Scholar]
- Lindmark DG, Jarroll EL. Pyrimidine metabolism in Giardia lamblia trophozoites. Mol Biochem Parasitol. 1982;5:291–296. doi: 10.1016/0166-6851(82)90036-6. [DOI] [PubMed] [Google Scholar]
- Lossani A, Torti A, Gatti S, Bruno A, Maserati R, Wright GE, Focher F. Thymidine kinase and uridine-cytidine kinase from Entamoeba histolytica: Cloning, characterization, and search for specific inhibitors. Parasitology. 2009;136:595–602. doi: 10.1017/S0031182009005964. [DOI] [PubMed] [Google Scholar]
- Lumsden RD, Gonzalez G, Mills RR, Viles JM. Cytological studies on the absorptive surfaces of cestodes. III. Hydrolysis of phosphate esters. J Parasitol. 1968;54:524–35. [PubMed] [Google Scholar]
- Lye LF, Hsieh YH, Su KE, Lee ST. Cloning and functional characterization of the ribonucleotide reductase gene small subunit from hydroxyurea-resistant Leishmania mexicana amazonensis. Mol Biochem Parasitol. 1997;90:353–358. doi: 10.1016/s0166-6851(97)00159-x. [DOI] [PubMed] [Google Scholar]
- Mc Carthy OK, Schipani A, Buendía AM, Ruiz-Perez LM, Kaiser M, Brun R, Pacanowska DG, Gilbert IH. Design, synthesis and evaluation of novel uracil amino acid conjugates for the inhibition of Trypanosoma cruzi dUTPase. Bioorg Med Chem Lett. 2006;16:3809–3812. doi: 10.1016/j.bmcl.2006.04.027. [DOI] [PubMed] [Google Scholar]
- McCarthy O, Musso-Buendia A, Kaiser M, Brun R, Ruiz-Perez LM, Johansson NG, Pacanowska DG, Gilbert IH. Design, synthesis and evaluation of novel uracil acetamide derivatives as potential inhibitors of Plasmodium falciparum dUTP nucleotidohydrolase. Eur J Med Chem. 2009;44:678–688. doi: 10.1016/j.ejmech.2008.05.018. [DOI] [PubMed] [Google Scholar]
- MacInnis AJ, Fisher FM, Jr, Read CP. Membrane transport of purines and pyrimidines in a Cestode. J Parasitol. 1965;5:260–267. [PubMed] [Google Scholar]
- MacInnis AJ, Ridley RK. The molecular configuration of pyrimidines that causes allosteric activation of uracil transport in Hymenolepis diminuta. J Parasitol. 1969;55:1134–1140. [PubMed] [Google Scholar]
- de Marques IA, Romanello L, DeMarco R, Pereira HD. Structural and kinetic studies of Schistosoma mansoni adenylate kinases. Mol Biochem Parasitol. 2012;185:157–160. doi: 10.1016/j.molbiopara.2012.07.003. [DOI] [PubMed] [Google Scholar]
- Martin DS, Stolfi RL, Sawyer RC. Use of oral uridine as a substitute for parenteral uridine rescue of 5-fluorouracil therapy, with and without the uridine phosphorylase inhibitor 5-benzylacyclouridine. Cancer Chemother Pharmacol. 1989;24:9–14. doi: 10.1007/BF00254098. [DOI] [PubMed] [Google Scholar]
- Mäser P, Sütterlin C, Kralli A, Kaminsky R. A nucleoside transporter from Trypanosoma brucei involved in drug resistance. Science. 1999;285:242–244. doi: 10.1126/science.285.5425.242. [DOI] [PubMed] [Google Scholar]
- Matsuno T, Kobayashi N, Hariguchi F, Yamazaki T, Imai K, Onaga H. Eimeria tenella, E. necatrix, E. acervulina, and E. maxima: Anticoccidial activity of 1,6-dihydro-6-oxo-2-pyrazinecarboxylic acid 4-oxide. Exp Parasitol. 1984;57:55–61. doi: 10.1016/0014-4894(84)90062-6. [DOI] [PubMed] [Google Scholar]
- Mattoccia LP, Cioli D. Effect of hycanthone administered in vivo upon the incorporation of radioactive precursors into macromolecules of Schistosoma mansoni. Mol Biochem Parasitol. 1983;8:99–107. doi: 10.1016/0166-6851(83)90002-6. [DOI] [PubMed] [Google Scholar]
- Mattoccia LP, Lelli A, Cioli D. Effect of hycanthone on Schistosoma mansoni macromolecular synthesis in vitro. Mol Biochem Parasitol. 1981;2:295–307. doi: 10.1016/0166-6851(81)90082-7. [DOI] [PubMed] [Google Scholar]
- McClard RW, Black MJ, Livingstone LR, Jones ME. Isolation and initial characterization of the single polypeptide that synthesizes uridine 5′-monophosphate from orotate in Ehrlich ascites carcinoma. Purification by tandem affinity chromatography of uridine-5′-monophosphate synthase. Biochemistry. 1980;19:4699–4706. doi: 10.1021/bi00561a024. [DOI] [PubMed] [Google Scholar]
- Mejias-Torres IA, Zimmermann BH. Molecular cloning, recombinant expression and partial characterization of the aspartate transcarbamoylase from Toxoplasma gondii. Mol Biochem Parasitol. 2002;119:191–201. doi: 10.1016/s0166-6851(01)00415-7. [DOI] [PubMed] [Google Scholar]
- Miech RP, Senft AW, Senft DG. Pathways of nucleotide metabolism in Schistosoma mansoni - VI. Adenosine phosphorylase. Biochem Pharmacol. 1975;24:407–411. doi: 10.1016/0006-2952(75)90226-9. [DOI] [PubMed] [Google Scholar]
- Miller RL, Lindstead D. Purine and pyrimidine metabolizing activities in Trichomonas vaginalis extracts. Molec Biochem Parasitol. 1983;7:41–51. doi: 10.1016/0166-6851(83)90115-9. [DOI] [PubMed] [Google Scholar]
- Molina-Arcas M, Casado FJ, Pastor-Anglada M. Nucleoside transporter proteins. Curr Vasc Pharmacol. 2009;7:426–34. doi: 10.2174/157016109789043892. [DOI] [PubMed] [Google Scholar]
- Monks A, Ayers O, Cysyk RL. Effect of 5-benzylacyclouridine, a potent inhibitor of uridine phosphorylase, on the metabolism of circulating uridine by the isolated rat liver. Biochem Pharmacol. 1983;32:2003–2009. doi: 10.1016/0006-2952(83)90419-7. [DOI] [PubMed] [Google Scholar]
- Morris GP, Threadgold LT. Ultrastructure of the tegument of adult Schistosoma mansoni. J Parasitol. 1968;54:15–27. [PubMed] [Google Scholar]
- Moshkani SK, Dalimi A. Evaluation of the efficacy of atovaquone alone or in combination with azithromycin against acute murine toxoplasmosis. Vet Res Commun. 2000;24:169–177. doi: 10.1023/a:1006404314523. [DOI] [PubMed] [Google Scholar]
- Moyer JD, Oliver JT, Handschumacher RE. Salvage of circulating pyrimidine nucleosides in the rat. Cancer Res. 1981;41:3010–3017. [PubMed] [Google Scholar]
- Mukherjee T, Ray M, Bhaduri A. Aspartate transcarbamylase from Leishmania donovani. A discrete, nonregulatory enzyme as a potential chemotherapeutic site. J Biol Chem. 1988;263:708–713. [PubMed] [Google Scholar]
- Munch-Petersen A. Metabolism of Nucleotides, Nucleosides and Nucleobases in Microorganisms. Acad Press, Inc; N.Y., N.Y: 1983. [Google Scholar]
- Munch-Petersen B, Piškur J, Søndergaard L. Four deoxynucleoside kinase activities from Drosophila melanogaster are contained within a single monomeric enzyme, a new multifunctional deoxynucleoside kinase. J Biol Chem. 1998;273:3926–3931. doi: 10.1074/jbc.273.7.3926. [DOI] [PubMed] [Google Scholar]
- Munro JB, Jacob CG, Silva JC. A novel clade of unique eukaryotic ribonucleotide reductase R2 subunits is exclusive to apicomplexan parasites. J Mol Evol. 2013;77:92–106. doi: 10.1007/s00239-013-9583-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naguib FNM, el Kouni MH. Nucleoside kinases in adult Schistosoma mansoni: phosphorylation of pyrimidine nucleosides. Mol Biochem Parasitol. 2014;194:53–55. doi: 10.1016/j.molbiopara.2014.04.007. [DOI] [PubMed] [Google Scholar]
- Naguib FNM, el Kouni MH, Chu SH, Cha S. New analogues of benzylacyclouridines, specific and potent inhibitors of uridine phosphorylase from human and mouse livers. Biochem Pharmacol. 1987;36:2195–2201. doi: 10.1016/0006-2952(87)90150-x. [DOI] [PubMed] [Google Scholar]
- Naguib FNM, Levesque DL, Wang EC, Panzica RP, el Kouni MH. 5-Benzylbarbituric acid derivatives, potent and specific inhibitors of uridine phosphorylase. Biochem Pharmacol. 1993;46:1273–1283. doi: 10.1016/0006-2952(93)90477-e. [DOI] [PubMed] [Google Scholar]
- Nara T, Gao G, Yamasaki H, Nakajima-Shimada J, Aoki T. Carbamoyl-phosphate synthetase II in kinetoplastids. Biochim Biophys Acta Protein Structure and Molecular Enzymology. 1998;1387:462–468. doi: 10.1016/s0167-4838(98)00127-7. [DOI] [PubMed] [Google Scholar]
- Nara T, Hashimoto M, Hirawake H, Liao CW, Fukai Y, Suzuki S, Tsubouchi A, Morales J, Takamiya S, Fujimura T, Taka H, Mineki R, Fan CK, Inaoka DK, Inoue M, Tanaka A, Harada S, Kita K, Aoki T. Molecular interaction of the first 3 enzymes of the de novo pyrimidine biosynthetic pathway of Trypanosoma cruzi. Biochem Biophys Res Commun. 2012;418:140–143. doi: 10.1016/j.bbrc.2011.12.148. [DOI] [PubMed] [Google Scholar]
- Nguyen C, Kasinathan G, Leal-Cortijo I, Musso-Buendia A, Kaiser M, Brun R, Ruiz-Pérez LM, Johansson NG, González-Pacanowska D, Gilbert IH. Deoxyuridine triphosphate nucleotidohydrolase as a potential antiparasitic drug target. J Med Chem. 2005;48:5942–5954. doi: 10.1021/jm050111e. [DOI] [PubMed] [Google Scholar]
- Nguyen C, Ruda GF, Schipani A, Kasinathan G, Leal I, Musso-Buendia A, Kaiser M, Brun R, Ruiz-Pérez LM, Sahlberg BL, Johansson NG, Gonzalez-Pacanowska D, Gilbert IH. Acyclic nucleoside analogues as inhibitors of Plasmodium falciparum dUTPase. J Med Chem. 2006;49:4183–4195. doi: 10.1021/jm060126s. [DOI] [PubMed] [Google Scholar]
- Niedzwicki JG, Chu SH, el Kouni MH, Rowe EC, Cha S. 5-Benzylacyclouridine and 5-benzyloxybenzylacyclouridine, potent inhibitors of uridine phosphorylase. Biochem Pharmacol. 1982;31:1857–1861. doi: 10.1016/0006-2952(82)90488-9. [DOI] [PubMed] [Google Scholar]
- Niedzwicki JG, el Kouni MH, Chu SH, Cha S. Pyrimidine acyclonucleosides, inhibitors of uridine phosphorylase. Biochem Pharmacol. 1981;30:2097–2101. doi: 10.1016/0006-2952(81)90228-8. [DOI] [PubMed] [Google Scholar]
- Niedzwicki JG, Iltzsch MH, el Kouni MH, Cha S. Structure-activity relationship of pyrimidine base analogs as ligands of orotate phosphoribosyltransferase. Biochem Pharmacol. 1984;33:2383–2395. doi: 10.1016/0006-2952(84)90710-x. [DOI] [PubMed] [Google Scholar]
- Nimmo-Smith RH, Standen OD. Phosphomonoesterases of Schistosoma mansoni. Exptl Parasitol. 1963;13:305–322. doi: 10.1016/0014-4894(63)90082-1. [DOI] [PubMed] [Google Scholar]
- Nollen PM, Floyd RD, Kolzow RG, Deter DL. The timing of reproductive cell development and movement in Schistosoma mansoni, S. japonicum, and S. haematobium, using techniques of autoradiography and transplantation. J Parasitol. 1976;62:227–231. [PubMed] [Google Scholar]
- Nyíri K, Vértessy BG. Perturbation of genome integrity to fight pathogenic microorganisms. Biochim Biophys Acta. 2017;1861(1 Pt B):3593–3612. doi: 10.1016/j.bbagen.2016.05.024. [DOI] [PubMed] [Google Scholar]
- Ogbunude PO, al-Jaser MH, Baer HP. Leishmania donovani: Characteristics of adenosine and inosine transporters in promastigotes of two different strains. Exp Parasitol. 1991;73:369–375. doi: 10.1016/0014-4894(91)90109-a. [DOI] [PubMed] [Google Scholar]
- Ogbunude P0J, Ikediobi C0. Effect of nitrobenzylthioinosinate on the toxicity of tubercidin and ethidium against Trypanosoma gambiense. Acta Tropica. 1982;39:219–224. [PubMed] [Google Scholar]
- O’Donovan GA, Neuhard J. Pyrimidine metabolism in microorganisms. Bacteriol Rev. 1970;34:278–343. doi: 10.1128/br.34.3.278-343.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong HB, Sienkiewicz N, Wyllie S, Patterson S, Fairlamb AH. Trypanosoma brucei (UMP synthase null mutants) are avirulent in mice, but recover virulence upon prolonged culture in vitro while retaining pyrimidine auxotrophy. Mol Microbiol. 2013;90:443–455. doi: 10.1111/mmi.12376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Sullivan WJ, Ketley K. Biosynthesis of uridine monophosphate in Plasmodium berghei. Ann Trop Med Parasitol. 1980;74:109–114. doi: 10.1080/00034983.1980.11687320. [DOI] [PubMed] [Google Scholar]
- Ouellette CA, Strout RG, McDougald LR. Incorporation of radioactive pyrimidine nucleosides into DNA and RNA of Eimria tenella (Coccidia) cultured in vitro. J Protozool. 1973;20:150–153. doi: 10.1111/j.1550-7408.1973.tb06022.x. [DOI] [PubMed] [Google Scholar]
- Page CR, 3rd, MacInnis AJ. Characterization of nucleoside transport in hymenolepidid cestodes. J Parasitol. 1975;61:281–290. [PubMed] [Google Scholar]
- Papageorgiou IG, Yakob L, Diallinas G, Soteriadou KP, de Koning HP. Identification of the first pyrimidine nucleobase transporter in Leishmania: Similarities with the Trypanosoma brucei U1 transporter and antileishmanial activity of uracil analogues. Parasitology. 2004;130:275–283. doi: 10.1017/s0031182004006626. [DOI] [PubMed] [Google Scholar]
- Pappas PW, Read CP. Relation of nucleoside transport and surface phosphohydrolase activity in Hymenolepis diminuta. J Parasitol. 1974;60:447–452. [PubMed] [Google Scholar]
- Pappas PW, Read CP. Membrane transport in helminth parasites: A review. Exp Parasitol. 1975;73:469–530. doi: 10.1016/0014-4894(75)90016-8. [DOI] [PubMed] [Google Scholar]
- Pappas PW, Uglem GL, Read CP. The influx of purines and pyrimidines across the brush border of Hymenolepis diminuta. Parasitology. 1973;66:525–538. doi: 10.1017/s0031182000046072. [DOI] [PubMed] [Google Scholar]
- Park KS, el Kouni MH, Krenitsky TA, Chu SH, Cha S. Inhibition of uridine phosphorylase from Escherichia coli by benzylacyclouridines. Biochem Pharmacol. 1986;35:3853–3855. doi: 10.1016/0006-2952(86)90675-1. [DOI] [PubMed] [Google Scholar]
- Parker JN, Parker PM. A medical dictionary, bibliography, and annotated research guide to internet references. ICON Health Publications; San Diego: 2004. Hydroxyurea. [Google Scholar]
- Pascal RA, Jr, Le Trang N, Cerami A, Walsh C. Purification and properties of dihydroorotate oxidase from Crithidia fasciculata and Trypanosoma brucei. Biochemistry. 1983;22:171–178. doi: 10.1021/bi00270a025. [DOI] [PubMed] [Google Scholar]
- Payares G, McLaren DJ, Evans WH, Smithers SR. Antigenicity and immunogenicity of the tegumental outer membrane of adult Schistosoma mansoni. Parasite Immunol. 1985;7:45–61. doi: 10.1111/j.1365-3024.1985.tb00478.x. [DOI] [PubMed] [Google Scholar]
- Payares G, Smithers SR, Evans WH. Purification and topographical location of tegumental alkaline phosphatase from adult Schistosoma mansoni. Mol Biochem Parasitol. 1984;13:343–360. doi: 10.1016/0166-6851(84)90125-7. [DOI] [PubMed] [Google Scholar]
- Pellwein K, Jayaram HN, Weber G. Effect of ischemia on nucleosides and bases in rat liver and hepatoma 3924A. Cancer Res. 1987;47:3092–3096. [PubMed] [Google Scholar]
- Pereira HD, Franco GR, Cleasby A, Garratt RCJ. Structures for the potential drug target purine nucleoside phosphorylase from Schistosoma mansoni causal agent of schistosomiasis. Mol Biol. 2005;353:584–599. doi: 10.1016/j.jmb.2005.08.045. [DOI] [PubMed] [Google Scholar]
- Pereira HM, Berdini V, Ferri MR, Cleasby A, Garratt RC. Crystal structure of Schistosoma purine nucleoside phosphorylase complexed with a novel monocyclic inhibitor. Acta Trop. 2010a;114:97–102. doi: 10.1016/j.actatropica.2010.01.010. [DOI] [PubMed] [Google Scholar]
- Pereira HM, Cleasby A, Pena SSD, Franco GR, Garratt RC. Cloning, expression and preliminary crystallographic studies of the potential drug target purine nucleoside phosphorylase from Schistosoma mansoni. Acta Crystallogr D Biol Crystallogr. 2003;59(Pt 6):1096–1099. doi: 10.1107/s090744490300773x. [DOI] [PubMed] [Google Scholar]
- Pereira HM, Rezende MM, Castilho MS, Oliva G, Garratt RC. Adenosine binding to low-molecular-weight purine nucleoside phosphorylase: The structural basis for recognition based on its complex with the enzyme from Schistosoma mansoni. Acta Crystallogr D Biol Crystallogr. 2010b;66(Pt 1):73–79. doi: 10.1107/S0907444909045715. [DOI] [PubMed] [Google Scholar]
- Pereira NM, Königk E. A nucleotidase from Leishmania tropica Promastigotes: Partial purification and properties. Tropenmed Parasitol. 1981;32:209–214. [PubMed] [Google Scholar]
- Persson R, Cedergren-Zeppezauer E, Wilson KS. Homotrimeric dUTPases; Structural solutions for specific recognition and hydrolysis of dUTP. Curr Protein Pept Sci. 2001;2:287–300. doi: 10.2174/1389203013381035. [DOI] [PubMed] [Google Scholar]
- Pfefferkorn ER. Toxoplasma gondii: The enzymic defect of a mutant resistant to 5-fluorodeoxyuridine. Exptl Parasitol. 1978;44:26–35. doi: 10.1016/0014-4894(78)90077-2. [DOI] [PubMed] [Google Scholar]
- Pfefferkorn ER, Pfefferkorn LC. Toxoplasma gondii: Characterization of a mutant resistant to 5-fluorodeoxyuridine. Exp Parasitol. 1977;41:95–104. doi: 10.1016/0014-4894(77)90060-1. [DOI] [PubMed] [Google Scholar]
- Platzer EG. Comparative biochemistry of folate metabolism in Aphelenchus avenae and Nippostrongylus brasiliensis (Nematoda) Comp Biochem Physiol B. 1974a;49:3–13. doi: 10.1016/0305-0491(74)90216-8. [DOI] [PubMed] [Google Scholar]
- Platzer EG. Dihydrofolate reductase in Plasmodium lophurae and duckling erythrocytes. J Protozool. 1974b;21:400–405. doi: 10.1111/j.1550-7408.1974.tb03678.x. [DOI] [PubMed] [Google Scholar]
- Postigo MP, Guido RV, Oliva G, Castilho MS, da R, Pitta I, de Albuquerque JF, Andricopulo AD. Discovery of new inhibitors of Schistosoma mansoni PNP by pharmacophore-based virtual screening. Chem Inf Model. 2010;50:1693–1705. doi: 10.1021/ci100128k. [DOI] [PubMed] [Google Scholar]
- Pradines B, Ramiandrasoa F, Basco LK, Bricard L, Kunesch G, Le Bras J. In vitro activities of novel catecholate siderophores against Plasmodium falciparum. Antimicrob Agents Chemother. 1996;40:2094–2098. doi: 10.1128/aac.40.9.2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pragobpol S, Gero AM, Lee CS, O’Sullivan WJ. Orotate phosphoribosyltransferase and orotidylate decarboxylase from Crithidia luciliae: subcellular location of the enzymes and a study of substrate channeling. Arch Biochem Biophys. 1984;230:285–293. doi: 10.1016/0003-9861(84)90109-7. [DOI] [PubMed] [Google Scholar]
- Rager N, Mamoun CB, Carter NS, Goldberg DE, Ullman B. Localization of the Plasmodium falciparum PfNT1 nucleoside transporter to the parasite plasma membrane. J Biol Chem. 2001;276:41095–41099. doi: 10.1074/jbc.M107037200. [DOI] [PubMed] [Google Scholar]
- Ranjbarian F, Vodnala M, Vodnala SM, Rofougaran R, Thelander L, Hofer A. Trypanosoma brucei thymidine kinase is tandem protein consisting of two homologous parts, which together enable efficient substrate binding. J Biol Chem. 2012;287:17628–17636. doi: 10.1074/jbc.M112.340059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathod PK, Reyes P. Orotidylate-metabolizing enzymes of the human malarial parasite, Plasmodium falciparum, differ from host cell enzymes. J Biol Chem. 1983;258:2852–2855. [PubMed] [Google Scholar]
- Recio E, Musso-Buendía A, Vidal AE, Ruda GF, Kasinathan G, Nguyen C, Ruiz-Pérez LM, Gilbert IH, González-Pacanowska D. Site-directed mutagenesis provides insights into the selective binding of trityl derivatives to Plasmodium falciparum dUTPase. Eur J Med Chem. 2011;46:3309–3314. doi: 10.1016/j.ejmech.2011.04.052. [DOI] [PubMed] [Google Scholar]
- Reeves RE. Metabolism of Entamoeba histolytica Schaudinn, 1903. Advances in Parasitology. 1984;23:105–142. doi: 10.1016/s0065-308x(08)60286-9. [DOI] [PubMed] [Google Scholar]
- Reyes P. The rapid separation of orotate, orotidylate, and uridylate by thin-layer chromatography. Anal Biochem. 1977;77:362–369. doi: 10.1016/0003-2697(77)90249-4. [DOI] [PubMed] [Google Scholar]
- Reyes P, Rathod PK, Sanchez DJ, Mrema JEK, Rieckmann KH, Heidrich HG. Enzymes of purine and pyrimidine metabolism from the human malaria parasite, Plasmodium falciparum. Molec Biochem Parasitol. 1982;5:275–290. doi: 10.1016/0166-6851(82)90035-4. [DOI] [PubMed] [Google Scholar]
- Roberts SM, Aitken R, Vojvodic M, Wells E, Wilson RA. Identification of exposed components on the surface of adult Schistosoma mansoni by lactoperoxidase-catalysed iodination. Mol Biochem Parasitol. 1983;9:129–143. doi: 10.1016/0166-6851(83)90105-6. [DOI] [PubMed] [Google Scholar]
- Robinson DLH. Phosphatases in Schistosoma mansoni. Nature. 1961;191:473–474. doi: 10.1038/191473a0. [DOI] [PubMed] [Google Scholar]
- Robles Lopez SM, Hortua Triana MA, Zimmermann BH. Cloning and preliminary characterization of the dihydroorotase from Toxoplasma gondii. Mol Biochem Parasitol. 2006;148:93–98. doi: 10.1016/j.molbiopara.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Rode W, Dąbrowska M, Zieliński Z, Gołos B, Wranicz M, Felczak K, Kulikowski T. Trichinella spiralis and Trichinella pseudospiralis: Developmental patterns of enzymes involved in thymidylate biosynthesis and pyrimidine salvage. Parasitology. 2000;120:593–600. doi: 10.1017/s0031182099005880. [DOI] [PubMed] [Google Scholar]
- Rogers WP. Histological distribution of alkaline phosphatase in helminth parasites. Nature. 1947;159:374–375. doi: 10.1038/159374b0. [DOI] [PubMed] [Google Scholar]
- Romanello L, Bachega JF, Cassago A, Brandão-Neto J, DeMarco R, Garratt RC, Pereira HD. Adenosine kinase from Schistosoma mansoni: structural basis for the differential incorporation of nucleoside analogues. Acta Crystallogr D Biol Crystallogr. 2013;69(Pt 1):126–136. doi: 10.1107/S0907444912044800. [DOI] [PubMed] [Google Scholar]
- Romanello L, Serrão VHB, Torini JR, Bird LE, Nettleship JE, Rada H, Reddivari Y, Owens RJ, DeMarco R, Brandão-Neto J, Pereira HD. Structural and kinetic analysis of Schistosoma mansoni adenylosuccinate lyase (SmADSL) Mol Biochem Parasitol. 2017;214:27–35. doi: 10.1016/j.molbiopara.2017.03.006. [DOI] [PubMed] [Google Scholar]
- Rubin H, Salem JS, Li LS, Yang FD, Mama S, Wang ZM, Fisher A, Hamann CS, Cooperman BS. Cloning, sequence determination, and regulation of the ribonucleotide reductase subunits from Plasmodium falciparum: A target for antimalarial therapy. Proc Natl Acad Sci U S A. 1993;90:9280–9284. doi: 10.1073/pnas.90.20.9280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruda GF, Nguyen C, Ziemkowski P, Felczak K, Kasinathan G, Musso-Buendia A, Sund C, Zhou XX, Kaiser M, Ruiz-Pérez LM, Brun R, Kulikowski T, Johansson NG, González-Pacanowska D, Gilbert IH. Modified 5′-trityl nucleosides as inhibitors of Plasmodium falciparum dUTPase. Chem Med Chem. 2011;6:309–320. doi: 10.1002/cmdc.201000445. [DOI] [PubMed] [Google Scholar]
- Rustum YM. High-pressure liquid chromatography. I. Quantitative separation of purine and pyrimidine nucleosides and bases. Anal Biochem. 1978;90:289–299. doi: 10.1016/0003-2697(78)90033-7. [DOI] [PubMed] [Google Scholar]
- Santi DV, McHenry CS. 5-Fluoro-2′-deoxyuridylate: covalent complex with thymidylate synthetase. Proc Natl Acad Sci U S A. 1972;69:1855–1857. doi: 10.1073/pnas.69.7.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savarese TM, el Kouni MH. Isolation and substrate specificity of an adenine nucleoside phosphorylase from adult Schistosoma mansoni. Mol Biochem Parasitol. 2014;194:44–47. doi: 10.1016/j.molbiopara.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzman JD, Pfefferkorn ER. Pyrimidine synthesis by intracellular Toxoplasma gondii. J Parasitol. 1981;67:150–158. [PubMed] [Google Scholar]
- Senft AW, Crabtree GW. Purine metabolism in the schistosomes: Potential targets for chemotherapy. Pharmacol Ther. 1983;20:341–356. doi: 10.1016/0163-7258(83)90031-1. [DOI] [PubMed] [Google Scholar]
- Senft AW, Crabtree GW, Agarwal KC, Scholar EM, Agarwal RP, Parks RE., Jr Pathways of nucleotide metabolism in Schistosoma mansoni. III. Identification of enzymes in cell-free extracts. Biochem Pharmacol. 1973a;22:449–458. doi: 10.1016/0006-2952(73)90286-4. [DOI] [PubMed] [Google Scholar]
- Senft AW, Miech RP, Brown PR, Senft DG. Purine metabolism in Schistosoma mansoni. Int J Parasitol. 1972;2:249–260. doi: 10.1016/0020-7519(72)90013-6. [DOI] [PubMed] [Google Scholar]
- Senft AW, Senft DG, Miech RP. Pathways of nucleotide metabolism in Schistosoma mansoni. II. Disposition of adenosine by whole worms. Biochem Pharmacol. 1973b;22:437–447. doi: 10.1016/0006-2952(73)90285-2. [DOI] [PubMed] [Google Scholar]
- Serrão VHB, Pereira HD, de Souza JRT, Romanello L. Schistosoma mansoni energetic pathway: Structures and kinetics experiments in the search for the best therapeutic target. Curr Pharm Des. 2017a doi: 10.2174/1381612823666171011100532. In Press. [DOI] [PubMed] [Google Scholar]
- Serrão VHB, Romanello L, Cassago A, Cheleski J, DeMarco R, Brandão-Neto J, Pereira HD. Structure and kinetics assays of recombinant Schistosoma mansoni dihydrofolate reductase. Acta Trop. 2017b;70:190–196. doi: 10.1016/j.actatropica.2017.03.007. [DOI] [PubMed] [Google Scholar]
- Seymour KK, Lyons SD, Phillips L, Rieckmann KH, Christopherson RI. Cytotoxic effects of inhibitors of de novo pyrimidine biosynthesis upon Plasmodium falciparum. Biochemistry. 1994;33:5268–5274. doi: 10.1021/bi00183a033. [DOI] [PubMed] [Google Scholar]
- Sierra Pagan ML, Zimmermann BH. Cloning and expression of the dihydroorotate dehydrogenase from Toxoplasma gondii. Biochim Biophys Acta. 2003;1637(2):178–181. doi: 10.1016/s0925-4439(02)00226-0. [DOI] [PubMed] [Google Scholar]
- Simpson AJ, Schryer MD, Cesari IM, Evans WH, Smithers SR. Isolation and partial characterization of the tegumental outer membrane of adult Schistosoma mansoni. Parasitology. 1981;83:163–177. doi: 10.1017/s0031182000050137. [DOI] [PubMed] [Google Scholar]
- Sirawaraporn W, Prapunwattana P, Sirawaraporn R, Yuthavong Y, Santi DV. The dihydrofolate reductase domain of Plasmodium falciparum thymidylate synthase-dihydrofolate reductase. Gene synthesis, expression, and anti-folate-resistant mutants. J Biol Chem. 1993;268:21637–21644. [PubMed] [Google Scholar]
- So NN, Wong PC, Ko RC. Precursors of pyrimidine nucleotide biosynthesis for gravid Angiostrongylus cantonensis (Nematoda: Metastrongyloidea) Int J Parasitol. 1992;22:427–433. doi: 10.1016/0020-7519(92)90143-9. [DOI] [PubMed] [Google Scholar]
- Sodeman WA, Jr, Berry EG, Fors MB. Schistosomal phosphatases. Histochemical localization of alkaline and acid phosphatase in cercariae of Schistosoma mansoni, Schistosoma haematobium, and Schistosoma japonicum. Am J Trop Med Hyg. 1968;17:851–857. [PubMed] [Google Scholar]
- Sommadossi JP, Cretton EM, Kidd LB, McClure HM, Anderson DC, el Kouni MH. Effects of 5-benzylacyclouridine, an inhibitor of uridine phosphorylase, on the pharmacokinetics of uridine in rhesus monkeys: Implications for chemotherapy. Cancer Chemother Pharmacol. 1995;37:14–22. doi: 10.1007/BF00685624. [DOI] [PubMed] [Google Scholar]
- Soysa R, Wilson ZN, Elferich J, Forquer I, Shinde U, Riscoe MK, Yates PA, Ullman B. Substrate inhibition of uracil phosphoribosyltransferase by uracil can account for the uracil growth sensitivity of Leishmania donovani pyrimidine auxotrophs. J Biol Chem. 2013;288:29954–29964. doi: 10.1074/jbc.M113.478826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegman RJ, Senft AW, Brown PR, Parks RE., Jr Pathways of nucleotide metabolism in Schistosoma mansoni. IV. Incorporation of adenosine analogs in vitro. Biochem Pharmacol. 1973;22:459–468. doi: 10.1016/0006-2952(73)90287-6. [DOI] [PubMed] [Google Scholar]
- Stein A, Vaseduvan G, Carter NS, Ullman B, Landfear SM, Kavanaugh MP. Equilibrative nucleoside transporter family members from Leishmania donovani are electrogenic proton symporters. J Biol Chem. 2003;278:35127–35134. doi: 10.1074/jbc.M306188200. [DOI] [PubMed] [Google Scholar]
- Striepen B, Pruijssers AJ, Huang J, Li C, Gubbels MJ, Umejiego NN, Hedstrom L, Kissinger JC. Gene transfer in the evolution of parasite nucleotide biosynthesis. Proc Natl Acad Sci U S A. 2004;101:3154–3159. doi: 10.1073/pnas.0304686101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun XE, Sharling L, Muthalagi M, Mudeppa DG, Pankiewicz KW, Felczak K, Rathod PK, Mead J, Striepen B, Hedstrom L. Prodrug activation by Cryptosporidium thymidine kinase. J Biol Chem. 2010;285:15916–15922. doi: 10.1074/jbc.M110.101543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashima E, Inaoka DK, Osanai A, Nara T, Odaka M, Aoki T, Inaka K, Harada S, Kita K. Characterization of the dihydroorotate dehydrogenase as a soluble fumarate reductase in Trypanosoma cruzi. Mol Biochem Parasitol. 2002;122:189–200. doi: 10.1016/s0166-6851(02)00100-7. [DOI] [PubMed] [Google Scholar]
- Tampitag S, O’Sullivan WJ. Enzymes of pyrimidine biosynthesis in Crithidia luciliae. Mol. Biochem Parasitol. 1986;19:125–134. doi: 10.1016/0166-6851(86)90117-9. [DOI] [PubMed] [Google Scholar]
- Téllez-Sanz R, Yassin Z, Bernier-Villamor V, Ortiz-Salmerón E, Musso-Buendia JA, Barón C, Ruíz-Pérez LM, González-Pacanowska D, García-Fuentes L. Effect of an Asp80Ala substitution on the binding of dUTP and dUMP to Trypanosoma cruzi dUTPase. Biochimie. 2007;89:972–980. doi: 10.1016/j.biochi.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Timm J, Bosch-Navarrete C, Recio E, Nettleship JE, Rada H, González-Pacanowska D, Wilson KS. Structural and Kinetic characterization of thymidine kinase from Leishmania major. PLoS Negl Trop Dis. 2015;9:e0003781. doi: 10.1371/journal.pntd.0003781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torini JR, Brandão-Neto J, DeMarco R, Pereira HD. Crystal structure of Schistosoma mansoni adenosine phosphorylase/5′-methylthioadenosine phosphorylase and its importance on adenosine salvage pathway. PLoS Negl Trop Dis. 2016;10:e0005178. doi: 10.1371/journal.pntd.0005178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traut TW. Significance of the enzyme complex that synthesizes UMP in Ehrlich ascites cells. Arch Biochem Biophys. 1980;200:590–594. doi: 10.1016/0003-9861(80)90391-4. [DOI] [PubMed] [Google Scholar]
- Traut TW, Jones ME. Kinetic and conformational studies of the orotate phosphoribosyltransferase:orotidine-5′-phosphate decarboxylase enzyme complex from mouse Ehrlich ascites cells. J Biol Chem. 1977;252:8374–8381. [PubMed] [Google Scholar]
- Uglem GL, Levy MG. Absorption kinetics of some purines, pyrimidines, and nucleosides in Taenia Crassiceps larvae. Rice Univ. Studies. 1976;62:225–236. [Google Scholar]
- Ulloa RM, Muschietti JP, Veron M, Torres HN, Tellez-Iñón MT. Purification and characterization of a soluble nucleoside diphosphate kinase in Trypanosoma cruzi. Mol Biochem Parasitol. 1995;70:119–129. doi: 10.1016/0166-6851(95)00016-t. [DOI] [PubMed] [Google Scholar]
- van Balkom BW, van Gestel RA, Brouwers JF, Krijgsveld J, Tielens AG, Heck AJ, van Hellemond JJ. Mass spectrometric analysis of the Schistosoma mansoni tegumental sub-proteome. J Proteome Res. 2005;4:958–966. doi: 10.1021/pr050036w. [DOI] [PubMed] [Google Scholar]
- Van Dyke K, Tremblay GC, Lantz CH, Szustkiewicz C. The source of purines and pyrimidines in Plasmodium berghei. Am J Trop Med Hyg. 1970;19:202–208. doi: 10.4269/ajtmh.1970.19.202. [DOI] [PubMed] [Google Scholar]
- Vásquez JR, Goozé L, Kim K, Gut J, Petersen C, Nelson RG. Potential antifolate resistance determinants and genotypic variation in the bifunctional dihydrofolate reductase-thymidylate synthase gene from human and bovine isolates of Cryptosporidium parvum. Mol Biochem Parasitol. 1996;79:153–165. doi: 10.1016/0166-6851(96)02647-3. [DOI] [PubMed] [Google Scholar]
- Vértessy BG, Tóth J. Keeping uracil out of DNA: Physiological role, structure and catalytic mechanism of dUTPases. Acc Chem Res. 2009;42:97–106. doi: 10.1021/ar800114w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitti GF, O’Sullivan WJ, Gero AM. The biosynthesis of uridine 5′-monophosphate in Giardia lamblia. Int J Parasitol. 1987;17:805–812. doi: 10.1016/0020-7519(87)90062-2. [DOI] [PubMed] [Google Scholar]
- Voet D, Voet JG. Biochemistry. 3. John Wiley and Sons; N.Y., N.Y: 2004. [DOI] [PubMed] [Google Scholar]
- Walter RD, Mühlpfordt H, Königk E. Comparative studies of the desoxythymidylate synthesis in Plasmodium chabaudi, Trypanosoma gambiense and Trypanosoma lewisi. Z Tropenmed Parasitol. 1970;21:347–357. [PubMed] [Google Scholar]
- Wang CC, Cheng HW. Salvage of pyrimidine nucleosides by Trichomonas vaginalis. Mol Biochem Parasitol. 1984a;10:171–184. doi: 10.1016/0166-6851(84)90005-7. [DOI] [PubMed] [Google Scholar]
- Wang CC, Cheng HW. The deoxyribonucleoside phosphotransferase of Trichomonas vaginalis. A potential target for anti-trichomonial chemotherapy. J Exp Med. 1984b;160:987–1000. doi: 10.1084/jem.160.4.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CC, Verham R, Tzeng SF, Aldritt S, Cheng HW. Pyrimidine metabolism in Tritrichomonas foetus. Proc Natl Acad Sci U S A. 1983;80:2564–2568. doi: 10.1073/pnas.80.9.2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheater PR, Wilson RA. The tegument of Schistosoma mansoni: A histochemical investigation. Parasitology. 1976;72:99–109. doi: 10.1017/s0031182000043237. [DOI] [PubMed] [Google Scholar]
- Whittingham JL, Leal I, Nguyen C, Kasinathan G, Bell E, Jones AF, Berry C, Benito A, Turkenburg JP, Dodson EJ, Ruiz Perez LM, Wilkinson AJ, Johansson NG, Brun R, Gilbert IH, Gonzalez Pacanowska D, Wilson KS. dUTPase as a platform for antimalarial drug design: structural basis for the selectivity of a class of nucleoside inhibitors. Structure. 2005;13:329–338. doi: 10.1016/j.str.2004.11.015. [DOI] [PubMed] [Google Scholar]
- Wilson ZN, Gilroy CA, Boitz JM, Ullman B, Yates PA. Genetic dissection of pyrimidine biosynthesis and salvage in Leishmania donovani. J Biol Chem. 2012;287:12759–12770. doi: 10.1074/jbc.M112.346502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittner M, Lederman J, Tanowitz HB, Rosenbaum GS, Weiss LM. Atovaquone in the treatment of Babesia microti infections in hamsters. Am J Trop Med Hyg. 1996;55:219–222. doi: 10.4269/ajtmh.1996.55.219. [DOI] [PubMed] [Google Scholar]
- Yamao Y. Histochemical studies on endoparasites. V. Distribution of the glyceromonophosphatases in the tissues of flukes Eurytrema, sp., Dicrocoelium lanceatum and Clonorchis sinensis. J Coll Arts and Sci Chiba Univ Nat Sci Series. 1952a;1:9–13. [Google Scholar]
- Yamao Y. Histochemical studies on endoparasites. VI. Distribution of the glyceromonophosphatases in the tissues of the lung flukes Paragonimus westermani. Jikken Seibutsugaku Hó (Bull, Exp Biol) 1952b;2:159–162. [Google Scholar]
- Yang Z, Xu B, Wang W, Feng Z, Wei DZ, Hu W. Cloning and fusion expression of adenosine deaminase of Schistosoma japonicum. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi. 2007;28:6–11. [PubMed] [Google Scholar]
- Young JD, Yao SY, Baldwin JM, Cass CE, Baldwin SA. The human concentrative and equilibrative nucleoside transporter families, SLC28 and SLC29. Mol Aspects Med. 2013;34:529–47. doi: 10.1016/j.mam.2012.05.007. [DOI] [PubMed] [Google Scholar]
- Young JD, Yao SY, Sun L, Cass CE, Baldwin SA. Human equilibrative nucleoside transporter (ENT) family of nucleoside and nucleobase transporter proteins. Xenobiotica. 2008;38:995–1021. doi: 10.1080/00498250801927427. [DOI] [PubMed] [Google Scholar]
- Yuan P, Hendriks EF, Fernandez HR, O’Sullivan WJ, Stewart TS. Functional expression of the gene encoding cytidine triphosphate synthetase from Plasmodium falciparum which contains two novel sequences that are potential antimalarial targets. Mol Biochem Parasitol. 2005;143:200–208. doi: 10.1016/j.molbiopara.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Yuan L, Wu CSC, Craig SP, III, Liu AF, Wang CC. Comparing the human and schistosomal hypoxanthine-guanine phosphoribosyltransferases by circular dichroism. Biochim Biophys Acta. 1993;1162:10–16. doi: 10.1016/0167-4838(93)90121-7. [DOI] [PubMed] [Google Scholar]
- Yuyama S, Corff S, Young PG. Identification of true thymidine kinase in Tetrahymena pyriformis ST. Arch Biochem Biophys. 1978;188:64–69. doi: 10.1016/0003-9861(78)90356-9. [DOI] [PubMed] [Google Scholar]
- Yuyama S, Corff S, Young PG. Levels of true thymidine kinase and nucleoside phosphotransferase in two strains of Tetrahymena pyriformis under different growth conditions. Comp Biochem Physiol B. 1979;62:325–328. doi: 10.1016/0305-0491(79)90097-x. [DOI] [PubMed] [Google Scholar]
- Zeraik AE, Balasco Serrão VH, Romanello L, Torini JR, Cassago A, DeMarco R, Pereira HD. Schistosoma mansoni displays an adenine phosphoribosyltransferase preferentially expressed in mature female gonads and vitelaria. Mol Biochem Parasitol. 2017;214:82–86. doi: 10.1016/j.molbiopara.2017.04.004. [DOI] [PubMed] [Google Scholar]