Summary
Background
Local cancer relapse risk after breast conservation surgery followed by radiotherapy has fallen sharply in many countries, and is influenced by patient age and clinicopathological factors. We hypothesise that partial-breast radiotherapy restricted to the vicinity of the original tumour in women at lower than average risk of local relapse will improve the balance of beneficial versus adverse effects compared with whole-breast radiotherapy.
Methods
IMPORT LOW is a multicentre, randomised, controlled, phase 3, non-inferiority trial done in 30 radiotherapy centres in the UK. Women aged 50 years or older who had undergone breast-conserving surgery for unifocal invasive ductal adenocarcinoma of grade 1–3, with a tumour size of 3 cm or less (pT1–2), none to three positive axillary nodes (pN0–1), and minimum microscopic margins of non-cancerous tissue of 2 mm or more, were recruited. Patients were randomly assigned (1:1:1) to receive 40 Gy whole-breast radiotherapy (control), 36 Gy whole-breast radiotherapy and 40 Gy to the partial breast (reduced-dose group), or 40 Gy to the partial breast only (partial-breast group) in 15 daily treatment fractions. Computer-generated random permuted blocks (mixed sizes of six and nine) were used to assign patients to groups, stratifying patients by radiotherapy treatment centre. Patients and clinicians were not masked to treatment allocation. Field-in-field intensity-modulated radiotherapy was delivered using standard tangential beams that were simply reduced in length for the partial-breast group. The primary endpoint was ipsilateral local relapse (80% power to exclude a 2·5% increase [non-inferiority margin] at 5 years for each experimental group; non-inferiority was shown if the upper limit of the two-sided 95% CI for the local relapse hazard ratio [HR] was less than 2·03), analysed by intention to treat. Safety analyses were done in all patients for whom data was available (ie, a modified intention-to-treat population). This study is registered in the ISRCTN registry, number ISRCTN12852634.
Findings
Between May 3, 2007, and Oct 5, 2010, 2018 women were recruited. Two women withdrew consent for use of their data in the analysis. 674 patients were analysed in the whole-breast radiotherapy (control) group, 673 in the reduced-dose group, and 669 in the partial-breast group. Median follow-up was 72·2 months (IQR 61·7–83·2), and 5-year estimates of local relapse cumulative incidence were 1·1% (95% CI 0·5–2·3) of patients in the control group, 0·2% (0·02–1·2) in the reduced-dose group, and 0·5% (0·2–1·4) in the partial-breast group. Estimated 5-year absolute differences in local relapse compared with the control group were −0·73% (−0·99 to 0·22) for the reduced-dose and −0·38% (−0·84 to 0·90) for the partial-breast groups. Non-inferiority can be claimed for both reduced-dose and partial-breast radiotherapy, and was confirmed by the test against the critical HR being more than 2·03 (p=0·003 for the reduced-dose group and p=0·016 for the partial-breast group, compared with the whole-breast radiotherapy group). Photographic, patient, and clinical assessments recorded similar adverse effects after reduced-dose or partial-breast radiotherapy, including two patient domains achieving statistically significantly lower adverse effects (change in breast appearance [p=0·007 for partial-breast] and breast harder or firmer [p=0·002 for reduced-dose and p<0·0001 for partial-breast]) compared with whole-breast radiotherapy.
Interpretation
We showed non-inferiority of partial-breast and reduced-dose radiotherapy compared with the standard whole-breast radiotherapy in terms of local relapse in a cohort of patients with early breast cancer, and equivalent or fewer late normal-tissue adverse effects were seen. This simple radiotherapy technique is implementable in radiotherapy centres worldwide.
Funding
Cancer Research UK.
Research in context.
Evidence before this study
A comprehensive literature search using PubMed and MEDLINE was done before the trial opened to identify all previous pathological and clinical breast radiotherapy studies investigating patterns of recurrence within the ipsilateral breast, and also to identify results of previous partial-breast radiotherapy studies. Search terms included “early breast cancer”, “partial irradiation”, and “partial breast radiotherapy”. Existing research suggests that most local relapses occur in the vicinity of the original tumour bed and that older trials testing partial-breast radiotherapy were uninformative because of suboptimal patient selection, poor localisation of the tumour, and hence, inaccurate radiotherapy. We hypothesised that partial-breast radiotherapy using modern methods of radiotherapy planning and treatment would be non-inferior in terms of local relapse incidence and might have reduced normal-tissue toxicity in a low-risk of relapse population. This formed part of our peer-reviewed funding application for the trial.
Added value of this study
IMPORT LOW is the first phase 3 trial reporting 5-year outcome data for local relapses and adverse effects after partial-breast radiotherapy delivered using standard external beam radiotherapy techniques, and is the only trial, to the best of our knowledge, testing the importance of treatment volume unconfounded by radiotherapy dose-time factors. Additionally, the study is unique because it includes very comprehensive patient-reported outcome measures.
At 5 years, partial-breast radiotherapy delivered using a simple and standard technique, showed no increase in local relapse rates compared with whole-breast radiotherapy, and produced equivalent or reduced late adverse effects. Follow-up is continuing and 10-year local relapse incidence and toxicity will be reported in future.
Implications of all the available evidence
IMPORT LOW has similar local relapse incidence to the recently reported GEC-ESTRO brachytherapy partial-breast radiotherapy trial that also confirmed non-inferiority of partial-breast versus whole-breast radiotherapy. Our method of partial-breast radiotherapy seems to be safe and effective and has a key advantage of being relatively simple compared with conformal or inverse-planned intensity-modulated radiotherapy or brachytherapy. The use of standard medial and lateral tangential beams also minimises the mean heart dose without the need for breath hold in most patients with left-sided breast cancer, given that most patients have tumours in the upper half of the breast and above the level of the heart. Implementation of this technique will not require additional resources or training in most countries worldwide.
Introduction
Breast radiotherapy after breast-conserving surgery has been shown to reduce the risk of any recurrence of breast cancer by a half and breast cancer-related mortality by a sixth in patients with early breast cancer.1 Whole-breast radiotherapy is the standard of care in the UK and internationally.2, 3, 4, 5 Current treatment guidelines discuss partial-breast radiotherapy for selected patients at low risk of recurrence because of age, small tumour size, and early stage, the evidence for which comes mainly from retrospective and prospective cohort studies in patients who received treatment using the MammoSite system and long-term results of a single, small, well conducted randomised trial of interstitial brachytherapy.6, 7, 8, 9, 10
One challenge in treating patients with early breast cancer is to reduce the morbidity of radiotherapy without compromising its ability to cure the cancer. The rationale for investigating partial-breast radiotherapy is based on international reports of reductions in local relapse incidence, and the recognition that the majority of ipsilateral local relapses occur close to the region of the index tumour (the so-called tumour bed).11, 12 Rapid technical advances in radiotherapy combined with accurate localisation of the tumour bed using titanium surgical clips enable more precise matching of radiotherapy dose intensity to the spatial variation in local relapse risk. Precise matching can now be achieved using a linear accelerator.13, 14, 15 This approach is predicted to have fewer chronic adverse effects than whole-breast radiotherapy, given the lower exposure of organs at risk, including breast tissue, ribcage, lung, and heart, without loss of local tumour control. Thousands of patients are currently being followed up in randomised studies, but long-term data (5 years or older) are available for few patients.7, 16, 17, 18 We report 5-year results of the first phase 3 trial testing partial-breast radiotherapy using a standard external beam technique and delivered after complete local tumour excision of low-risk early breast cancer.
Methods
Study design
IMPORT LOW is a multicentre, randomised, controlled, phase 3, non-inferiority trial comparing the safety and efficacy of standard whole-breast radiotherapy (control, whole-breast group) with experimental schedules of radiotherapy to the whole breast and partial breast (reduced-dose group), and to the partial breast only (partial-breast group). For the study protocol, see appendix pp 11–85. All treatment groups received simple forward-planned intensity-modulated radiation techniques (IMRT) to optimise dose homogeneity. In addition to the main study, two substudies addressing late adverse effects were done in a subset of centres, including photographic assessments of the breast and comprehensive patient-reported outcomes; centres declared upfront whether they wished to participate in the substudies. Patients were recruited to the substudies from the participating centres until the planned sample size had been obtained, and separate consent was given for the main trial and substudies. The study was approved by the Oxfordshire Research Ethics Committee B (06/Q1605/128) and done in accordance with the principles of Good Clinical Practice.
Participants
Women who were aged 50 years or older who had breast-conserving surgery for unifocal invasive ductal adenocarcinoma (excluding invasive carcinoma of classical lobular type) of any grade (1–3) were recruited. Other inclusion criteria were pathological tumour size 3 cm or less (pT1–2), axillary node negative or one to three positive nodes (pN0–1), and minimum microscopic margins of non-cancerous tissue of 2 mm or more. Patients were not eligible if they had distant metastases, a previous malignancy of any kind (unless non-melanomatous skin cancer), undergone a mastectomy, or received neoadjuvant chemotherapy or concurrent adjuvant chemoradiotherapy. Primary endocrine therapy was allowed as long as the tumour was less than 3·0 cm, all other inclusion criteria were met, and breast-conserving surgery had been done. Eligibility criteria were amended twice during the trial. Women with grade 3 tumours or tumours with a diameter greater than 2 cm, or both, were excluded before a protocol amendment (approved March 4, 2008). A subsequent amendment (approved May 7, 2009) allowed inclusion of lymphovascular invasion and patients with one to three positive nodes (pN1; original criteria were that patients were node negative). The reduced local relapse incidence in the START trial19 and other recent studies compared with older trials indicated that broadening of the eligibility criteria was safe.11 All patients provided written informed consent. The study was sponsored by The Institute of Cancer Research. The Institute of Cancer Research Clinical Trials and Statistics Unit (ICR-CTSU; London, UK) was responsible for study management and central statistical data monitoring and all analyses. The Trial Management Group was responsible for day-to-day running of the trial and was overseen by an independent trial steering committee (TSC) and interim data reviewed confidentially by an independent data monitoring committee (IDMC). Patient advocates were involved at every stage of the trial, from initial study design through to preparation of the final manuscript.
Randomisation and masking
Women were randomly assigned (in a 1:1:1 ratio) to receive conventional whole-breast radiotherapy or one of the two experimental schedules (reduced-dose or partial-breast radiotherapy). To randomly assign a patient, research staff at the centres telephoned ICR-CTSU to obtain the treatment allocation and trial ID number. Computer-generated random permuted blocks (mixed sizes of six and nine) were used to assign patients to groups, stratifying patients by radiotherapy treatment centre. Treatment allocation was not masked from patients, clinicians, or those analysing the data.
Procedures
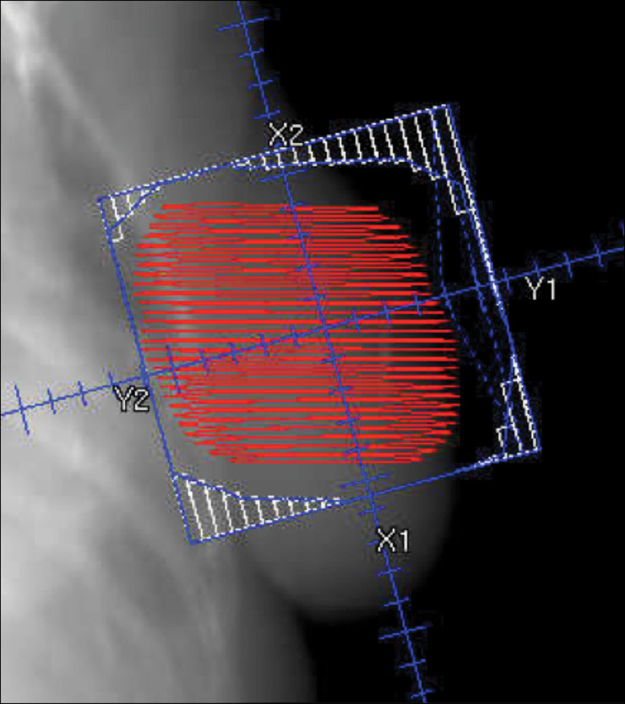
Patients assigned to whole-breast radiotherapy (control) received 40 Gy in 15 fractions to the whole breast, those assigned to the reduced-dose group received 36 Gy in 15 fractions to the whole breast and 40 Gy in 15 fractions to the partial breast containing the tumour bed, and those assigned to the partial-breast group received 40 Gy in 15 fractions to the partial breast only. For localisation of the tumour bed, it was strongly recommended by the Trial Management Group to insert surgical clips, but if this was not possible, ultrasound, MRI, or CT was used.13, 20 If one of the recommended localisation procedures could not be done, entry into the study was permissible if the clinician was confident that clinical localisation was accurate—eg, if an obvious palpable tissue deficit was detected (appendix p 2).13, 20 The protocol specified forward-planned field-in-field IMRT delivered by standard medial and lateral tangential beams reduced in length but not in width. Non-target breast tissue medial or lateral to the planning target volume was thereby included in the high-dose zone (figure 1). Details of contouring and planning are described in the IMPORT LOW radiotherapy planning pack, which was used in addition to the clinical protocol (appendix p 1) and developed in partnership with the UK Radiotherapy Trials Quality Assurance (RTTQA) team. Each centre completed an initial questionnaire to establish details of their intended technique. Additionally, the RTTQA team visited each radiotherapy centre before opening of recruitment to independently validate the technique in use against the information given in the questionnaire. Measurements were made of the treatment volume with a purpose-made breast phantom, with particular reference to dose homogeneity. All plans together with corresponding CT datasets were collected electronically and stored at the RTTQA repository. Additionally, a subset of approximately one in ten patients (every tenth patient enrolled) were selected at randomisation to have thermoluminescence dosimetry measurements, which were also sent to the RTTQA team.
Figure 1.
Radiotherapy technique for partial-breast group
Red shows the partial-breast planning target volume and blue shows the radiotherapy field arrangements shaped with multileaf collimators. See appendix p 9 for further details.
After radiotherapy, patients were scheduled for annual follow-up for 10 years. The mammography schedule was followed according to local practice, and was typically done annually for the first 5 years and then every 3 years as part of the national screening programme. Normal-tissue effects were assessed by clinicians, patients, and using photographs. Clinicians assessed breast shrinkage, distortion, induration, breast oedema, and telangiectasia at 1, 2, 5, and 10 years using a four-point scale (not at all, a little, quite a bit, or very much), comparing the ipsilateral breast with the contralateral breast when relevant.21 The assessment after 1 year was only required after protocol amendment (approved March 4, 2008). For the photographic substudy, photographs were taken at baseline (after surgery and before radiotherapy), at 2 years, and at 5 years.22 Patients in the patient-reported outcomes substudy completed the European Organisation for Research and Treatment of Cancer (EORTC) QLQ-C30 core questionnaire, EORTC QLQ-BR23 breast cancer module, body-image scale, protocol-specific questions (has skin appearance changed, overall breast appearance changed, breast become smaller, breast become harder or firmer to touch, or is shoulder stiffness present?), Hospital Anxiety and Depression Scale, and the EuroQol EQ-5D-3L health status questionnaire. These were scheduled at baseline (before randomisation), 6 months, and 1, 2, and 5 years. Symptomatic rib fracture, symptomatic lung fibrosis, and ischaemic heart disease incidence were recorded at 1, 2, 5, and 10-year follow-up.
Outcomes
The primary outcome measure was local relapse, defined as the presence of any invasive or non-invasive carcinoma in any location in the ipsilateral breast parenchyma or overlying skin, assessed at each centre. Secondary efficacy outcomes were location of local tumour relapse, time to regional relapse (axilla, supraclavicular fossa, and internal mammary chain), time to distant relapse, disease-free survival (an event was defined as any local, regional, or distant relapse, contralateral breast cancer, or death due to breast cancer), overall survival, contralateral breast cancers, and other second primary cancers. Secondary outcomes relating to late-onset normal-tissue effects were assessed by clinicians for all patients, and also by patients and from photographs in the substudies.
Patient-reported outcomes focused on key items (arm or shoulder and breast) from the EORTC QLQ-BR23 module and protocol-specific questions that were recorded on the same 4-point scale as for the clinician assessments (not at all, a little, quite a bit, or very much). This manuscript reports on selected items from the BR23 breast cancer module and protocol-specific questions that correspond to clinician-reported assessments. Further analysis of patient-reported outcomes will be reported separately.
Digital photographs were scored as showing no change (none), mild, or marked change in breast appearance at 2 and 5 years compared with baseline by three observers (CC, AK, and JRY) using a previously described and validated consensus method.22 These observers were masked to treatment allocation but not to year of follow-up.
Statistical analysis
The trial was powered to assess non-inferiority of the cumulative incidence of local relapse for each of the experimental groups compared with the control group. A 2·5% incidence of local relapse at 5 years was assumed with whole-breast radiotherapy, and the trial aimed to show that an increase of more than 2·5% in the cumulative incidence of local relapse would not occur in either experimental group. 645 patients were needed in each group to give 80% power with an α of 2·5% (one sided), allowing for 5% of patients to be lost to follow-up by 5 years. A target number of events was not stated in the protocol but data maturity was reviewed and discussed by the IDMC and TSC. The IDMC considered data to be sufficiently mature once at least 80% of forms were returned at 5 years.
The photographic substudy required 400 patients per group to have more than 90% power to detect at least a 10% difference in change of overall breast appearance for each experimental group compared with control (two-sided α of 0·025). With 400 patients per group, the patient-reported outcome substudy had more than 80% power to detect differences of at least 15% in the prevalence of normal-tissue effects (two-sided α of 0·005 to allow for multiple testing) and allowing for 10% attrition (due to death or illness). The same 0·005 threshold for significance was used for the clinician-reported normal-tissue effects.
Survival analysis methods were used to compare efficacy outcomes between the control group and experimental schedules with time measured from randomisation. For time to local relapse, patients were censored at death or at final follow-up for those who had no events. For distant relapse, disease-free survival, and overall survival, patients who had no events were censored at final follow-up. Nelson-Aalen cumulative hazard functions were plotted by treatment group.
Kaplan-Meier analyses were used to estimate event rates at 5 years with 95% CIs. Estimates of treatment effect were made using unadjusted Cox regression models, with hazard ratios (HRs) less than 1 indicating a decreased risk of the event in the experimental group compared with the control group. Absolute treatment differences in local relapses were calculated on the basis of the Kaplan-Meier estimate of patients who did not have local relapse in the control group and the HR. Each experimental group could be considered non-inferior to the control group if the upper limit of the two-sided 95% CI for local relapse HR was less than 2·03 (critical HR; excluding an increase in local relapse from 2·5% to 5·0%). Superiority of each experimental group compared with the control could be tested if non-inferiority could be claimed (using a 0·025 significance level). Analyses were done in the intention-to-treat population. The primary outcome was also analysed in the per-protocol population (all patients who completed their protocol-defined radiotherapy regimen) because this was a non-inferiority trial.
Patient and clinician-reported late normal tissue effects were dichotomised for the analysis as none or a little versus quite a bit or very much (defined as none or mild versus moderate or marked). The proportion of late moderate or marked events at 5 years is reported for each clinician-reported and patient-reported late normal-tissue event. Fisher's exact tests were used to compare each experimental schedule with the control group. All analyses of late normal tissue effects were done on a modified intention-to-treat basis—ie, all patients with available data, according to randomised treatment allocation. Time to first moderate or marked event was analysed using Kaplan-Meier analysis. Patients with no events were censored at last assessment of normal tissues (by clinician or patient as appropriate) or death. For the patient-reported outcomes, the Cox model was adjusted for baseline scores. Photographic data is presented as the proportion of patients who had photographs taken, with no change (none) or mild or marked change in breast appearance at 2 and 5 years compared with baseline. The Fisher's exact test was used to compare each experimental schedule with the control group at both time points. There was no imputation of missing normal-tissue data.
For all time-to-event analyses, the proportional hazards assumption of the Cox model was tested using Schoenfeld residuals and found to hold. Analyses were based on a database snapshot taken on June 15, 2016, and done using STATA version 13. This study is registered in the ISRCTN registry, number ISRCTN12852634, and ClinicalTrials.gov, number NCT00814567.
Role of the funding source
Cancer Research UK provided peer-reviewed approval for the trial but had no other role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the study data and had final responsibility for the decision to submit for publication. CLG and JMB also had full access to study data.
Results
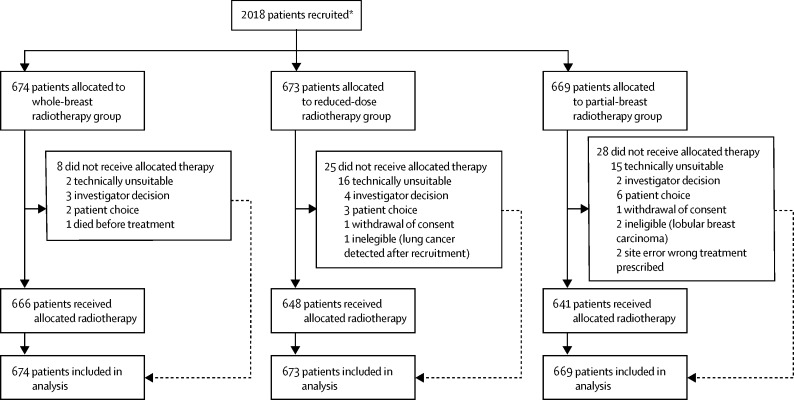
Between May 3, 2007, and Oct 5, 2010, 2018 patients were recruited to the study. Two individuals withdrew consent for use of their data in the analysis; these two patients were removed from the intention-to-treat population. Patients were randomly assigned to either the whole-breast group (n=675), reduced-dose group (n=674), or the partial-breast group (n=669). Five patients were found to be ineligible after randomisation (three patients had lobular breast carcinoma, one had renal cell carcinoma, and one had lung cancer). Three of these patients did not receive their allocation treatment, but the other two were in the control group and so received standard treatment regardless. Seven patients did not receive any radiotherapy and 54 did not receive their allocated treatment (figure 2). 1482 (74%) of 2016 patients had surgical clips, 494 (25%) had imaging (either CT or ultrasound), and for 40 (2%), clinical methods alone were used to localise the tumour bed. Demographic and clinical characteristics were similar across the three treatment groups (table 1). 104 (5%) of 2016 women had chemotherapy, 1826 (91%) had endocrine therapy, and 36 (2%) had trastuzumab.
Figure 2.
Trial profile
*Two patients withdrew consent for any of their data to be used in the analysis.
Table 1.
Demographic and clinical characteristics at randomisation by treatment group (n=2016*)
| Whole-breast radiotherapy (n=674) | Reduced-dose radiotherapy (n=673) | Partial-breast radiotherapy (n=669) | ||
|---|---|---|---|---|
| Age, years | 62 (57–67) | 63 (57–67) | 62 (57–67) | |
| Side of primary tumour | ||||
| Left breast | 336/674 (50%) | 344/673 (51%) | 348/669 (52%) | |
| Right breast | 338/674 (50%) | 329/673 (49%) | 321/669 (48%) | |
| Pathological tumour size, cm† | 1·2 (0·8–1·5) | 1·1 (0·8–1·6) | 1·2 (0·8–1·6) | |
| Tumour grade‡ | ||||
| 1 | 298/672 (44%) | 272/673 (40%) | 284/668 (43%) | |
| 2 | 310/672 (46%) | 328/673 (49%) | 320/668 (48%) | |
| 3 | 64/672 (10%) | 73/673 (11%) | 63/668 (9%) | |
| Re-excision | ||||
| Yes | 93/673 (14%) | 78/673 (12%) | 87/667 (13%) | |
| No | 580/673 (86%) | 595/673 (88%) | 580/667 (87%) | |
| Axillary surgery | ||||
| Yes | 672/673 (>99%) | 673/673 (100%) | 666/667 (>99%) | |
| No | 1/673 (<1%) | 0 | 1/667 (<1%) | |
| Pathological node status | ||||
| Positive | 24/674 (4%) | 19/673 (3%) | 16/669 (2%) | |
| Negative | 650/674 (96%) | 654/673 (97%) | 653/669 (98%) | |
| Histological type | ||||
| Infiltrating ductal | 578/671 (86%) | 581/672 (86%) | 563/665 (85%) | |
| Mixed | 14/671 (2%) | 18/672 (3%) | 22/665 (3%) | |
| Other | 79/671 (12%) | 73/672 (11%) | 80/665 (12%) | |
| Lymphovascular invasion | ||||
| Present | 34/493 (7%) | 47/492 (10%) | 35/494 (7%) | |
| Absent | 459/493 (93%) | 445/492 (90%) | 459/494 (93%) | |
| ER status | ||||
| Positive | 640/672 (95%) | 638/672 (95%) | 633/667 (95%) | |
| Poor§ | 32/672 (5%) | 34/672 (5%) | 34/667 (5%) | |
| PR status | ||||
| Positive | 400/493 (81%) | 393/477 (82%) | 380/475 (80%) | |
| Poor§ | 93/493 (19%) | 84/477 (18%) | 95/475 (20%) | |
| HER2 status | ||||
| Negative | 599/622 (96%) | 603/628 (96%) | 580/614 (94%) | |
| Positive | 23/622 (4%) | 25/628 (4%) | 34/614 (6%) | |
| Adjuvant therapy received¶ | ||||
| Chemotherapy | 29/673 (4%) | 42/670 (6%) | 33/665 (5%) | |
| Endocrine therapy | 610/673 (91%) | 614/670 (92%) | 602/665 (91%) | |
| Trastuzumab | 7/673 (1%) | 15/670 (2%) | 14/665 (2%) | |
Data are n/N (%) or median (IQR). N is total number of patients for whom the test result or measurement was available. ER=oestrogen receptor. PR=progesterone receptor.
Two patients withdrew consent for any of their data to be used in analysis.
Result unknown in one patient from partial-breast radiotherapy group.
Tumours of two patients in the whole-breast group and one patient in the partial-breast group were ungradeable.
Poor refers to less than 10% receptor staining.
Not mutually exclusive (ie, patients could have had more than one type of therapy).
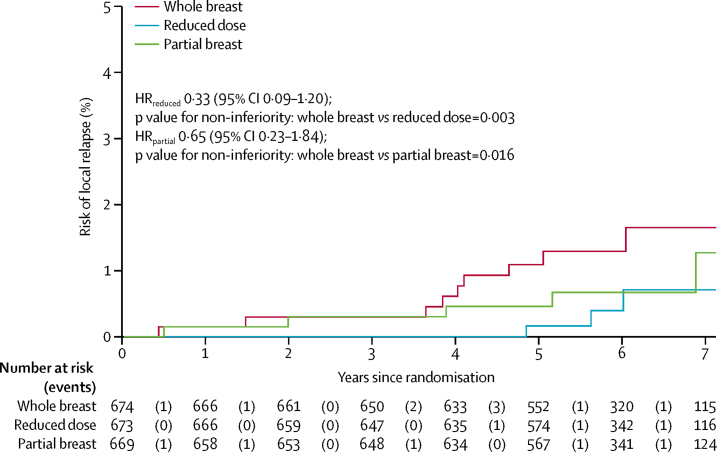
After a median follow-up of 72·2 months (IQR 61·7–83·2), local relapse had been reported for 18 patients, nine (1%) of whom were in the whole-breast group, three (<1%) in the reduced-dose group, and six (1%) in the partial-breast group. 5-year estimated cumulative incidence of local relapse was 1·1% (95% CI 0·5–2·3) in the whole-breast group, 0·2% (0·02–1·2) in the reduced-dose group, and 0·5% (0·2–1·4) in the partial-breast group. The estimated absolute differences in local relapse by 5 years in the experimental groups compared with whole-breast radiotherapy at 5 years was −0·73% (95% CI −0·99 to 0·22) for the reduced-dose group and −0·38% (−0·84 to 0·90) for the partial-breast group. Since the upper limit of the two-sided 95% CI ruled out a greater than 2·5% increase in local relapse risk for each of the test schedules, non-inferiority can be claimed for both reduced-dose and partial-breast radiotherapy. Confirmation of this assertion is illustrated by a test against the critical HR greater than 2·03, with p=0·003 for the reduced-dose group and p=0·016 for the partial-breast group compared with the whole-breast radiotherapy group (table 2; figure 3). Analyses in the per-protocol population were consistent (p=0·003 for the reduced-dose group and p=0·017 for the partial-breast group; full data for per-protocol analyses not shown because treatment compliance was high). Local relapses occurred most frequently in patients with at least one high-risk feature (appendix p 86).
Table 2.
Relapse and mortality by treatment group
| Cumulative number of events, n/N (%) | 5-year cumulative incidence, % (95% CI) | Hazard ratio*(95% CI) | p value† | |
|---|---|---|---|---|
| Local relapse | ||||
| Whole breast | 9/674 (1%) | 1·1% (0·5–2·3) | 1 | ·· |
| Reduced dose | 3/673 (<1%) | 0·2% (0·02–1·2) | 0·33 (0·09–1·20) | 0·077 |
| Partial breast | 6/669 (1%) | 0·5% (0·2–1·4) | 0·65 (0·23–1·84) | 0·420 |
| Local-regional relapse | ||||
| Whole breast | 9/674 (1%) | 1·1% (0·5–2·3) | 1 | ·· |
| Reduced dose | 3/673 (<1%) | 0·2% (0·02–1·2) | 0·33 (0·09–1·21) | 0·077 |
| Partial breast | 8/669 (1%) | 0·8% (0·3–1·8) | 0·88 (0·34–2·27) | 0·761 |
| Distant relapse | ||||
| Whole breast | 13/674 (2%) | 1·4% (0·7–2·6) | 1 | ·· |
| Reduced dose | 10/673 (1%) | 1·5% (0·8–2·8) | 0·77 (0·34–1·75) | 0·525 |
| Partial breast | 12/669 (2%) | 1·6% (0·8–2·9) | 0·92 (0·42–2·03) | 0·838 |
| Any breast-cancer-related event | ||||
| Whole breast | 33/674 (5%) | 3·7% (2·5–5·4) | 1 | ·· |
| Reduced dose | 24/673 (4%) | 3·4% (2·2–5·1) | 0·72 (0·43–1·22) | 0·223 |
| Partial breast | 33/669 (5%) | 4·0% (2·8–5·9) | 1·00 (0·62–1·62) | 0·982 |
| All-cause mortality | ||||
| Whole breast | 40/674 (6%) | 5·0% (3·6–7·0) | 1 | ·· |
| Reduced dose | 39/673 (6%) | 4·1% (2·8–5·9) | 0·97 (0·62–1·50) | 0·883 |
| Partial breast | 37/669 (6%) | 3·7% (2·5–5·4) | 0·91 (0·58–1·42) | 0·693 |
A hazard ratio of less than 1 favours the experimental group.
Log-rank test, for each experimental group compared with whole-breast radiotherapy.
Figure 3.
Cumulative hazard of local relapse by treatment group
HR=hazard ratio.
Four regional relapses were reported: one in the whole-breast group, one in the reduced-dose group, and two in the partial-breast group. Two of these relapses coincided with local relapse and two were isolated axillary relapses. Incidence of distant relapse, disease-free survival, and overall survival were similar across treatment groups, with low numbers of overall events and no statistically significant differences observed between experimental and control groups (table 2). 32 (2%) of 2016 patients developed invasive contralateral breast primary cancers: ten (1%) of 674 in the whole-breast group, 11 (2%) of 673 in the reduced-dose group, and 11 (2%) of 669 in the partial-breast group (table 3). Non-breast second primary cancers were reported for 96 (5%) of 2016 patients: 35 (5%) of 674 in the whole-breast group, 37 (5%) of 673 in the reduced-dose group, and 24 (4%) of 669 in the partial-breast group. Colorectal, lung, and gynaecological cancers were the most common. 18 of the 19 cases of lung cancer developed within 5 years of randomisation and similar numbers were ipsilateral and contralateral to the treated breast (appendix p 87).
Table 3.
Local relapse, second cancers, and deaths by treatment group
| Whole breast (n=674) | Reduced dose (n=673) | Partial breast (n=669) | Total (n=2016) | ||
|---|---|---|---|---|---|
| Local relapse | 9* (1%) | 3† (<1%) | 6 (1%) | 18 (1%) | |
| Within radiotherapy field‡ | 9 (1%) | 1 (<1%) | 4 (1%) | 14 (1%) | |
| Borderline with radiotherapy field | 0 | 0 | 1 (<1%) | 1 (<1%) | |
| Not documented | 0 | 2 (<1%) | 1 (<1%) | 3 (<1%) | |
| Contralateral breast second primary | 12 (2%) | 13 (2%) | 13 (2%) | 38 (2%) | |
| Invasive | 10 (1%) | 11 (2%) | 11 (2%) | 32 (2%) | |
| DCIS | 2 (<1%) | 2 (<1%) | 2 (<1%) | 6 (<1%) | |
| Non-breast second primary | 35 (5%) | 37 (5%) | 24 (4%) | 96 (5%) | |
| Colorectal | 10§ (1%) | 7 (1%) | 3 (<1%) | 20 (1%) | |
| Lung | 11§ (2%) | 4 (1%) | 4 (1%) | 19 (1%) | |
| Gynaecological | 5 (1%) | 8 (1%) | 4 (1%) | 17 (1%) | |
| Other¶ | 4 (1%) | 3 (<1%) | 1 (<1%) | 8 (<1%) | |
| Oesophagus | 0 | 3 (<1%) | 3 (<1%) | 6 (<1%) | |
| Pancreas | 1 (<1%) | 2 (<1%) | 3 (<1%) | 6 (<1%) | |
| Lymphoma | 0 | 2 (<1%) | 3 (<1%) | 5 (<1%) | |
| Genitourinary | 3 (<1%) | 1 (<1%) | 0 | 4 (<1%) | |
| Head and neck | 1 (<1%) | 2 (<1%) | 0 | 3 (<1%) | |
| Liver | 0 | 2 (<1%) | 1 (<1%) | 3 (<1%) | |
| Cancer of unknown primary | 0 | 0 | 2 (<1%) | 2 (<1%) | |
| Peritoneal | 0 | 2 (<1%) | 0 | 2 (<1%) | |
| Sarcoma | 1 (<1%) | 1|| (<1%) | 0 | 2 (<1%) | |
| Deaths | 40 (6%) | 39 (6%) | 37 (6%) | 116 (6%) | |
| Breast cancer | 9** (1%) | 7†† (1%) | 10‡‡ (1%) | 26 (1%) | |
| Second cancer | 14 (2%) | 16 (2%) | 12 (2%) | 42 (2%) | |
| Cardiac | 5 (1%) | 2 (<1%) | 2 (<1%) | 9 (<1%) | |
| Cerebrovascular accident | 1 (<1%) | 2 (<1%) | 1 (<1%) | 4 (<1%) | |
| Pulmonary embolism | 0 | 2 (<1%) | 0 | 2 (<1%) | |
| Other | 11 (2%) | 10 (1%) | 10 (1%) | 31 (2%) | |
| Unknown | 0 | 0 | 2 (<1%) | 2 (<1%) | |
Data are n (%). DCIS=ductal carcinoma in situ.
Two patients with DCIS.
One patient with DCIS.
No relapses were documented outside of the radiotherapy field.
One patient reported a colorectal second cancer followed by a lung second cancer and is included in both categories.
Other includes adrenal, squamous cell carcinoma of the skin, melanoma, leukaemia, and mesothelioma.
Angiosarcoma developed in the treated breast.
One patient with distant relapse before death died from mesothelioma.
One patient with distant relapse before death died from renal failure.
Two patients with distant relapse before death also died from other causes, one sepsis and one cardiac related.
116 (6%) of 2016 patients died: 26 (1%) from breast cancer, 88 (4%) from other causes (including 42 [2%] from second cancers and nine [<1%] cardiac-related), and two (<1%) with unknown cause of death with no evidence of disease relapse before death (table 3). Numbers of cardiac deaths were similar between patients with left-side and right-sided breast cancers (appendix p 88).
In relation to normal-tissue effects, at the 5-year assessment, patients generally reported fewer moderate or marked events for the protocol-specific questions (skin change, overall breast appearance change, breast smaller, and breast harder or firmer to touch) in the partial-breast group than in the whole-breast group (table 4), although this reduction was statistically significant for change in breast appearance only (p<0·0001). At 5 years, change in breast appearance had the highest cumulative incidence of items reported as moderate or marked by patients in all groups. Reports of breast becoming harder or firmer were significantly reduced in both the reduced-dose group (p=0·002) and partial-breast group (p<0·0001) compared with the whole-breast group. Cumulative incidence of reports of the breast becoming harder or firmer were higher than the point prevalence at 5 years because this value included events reported earlier in follow-up, many of which were likely to be temporary post-surgical effects. The proportion of patients reporting any arm and shoulder symptoms as moderate or marked at 5 years was low in all groups with no significant differences for either experimental schedule compared with the control group. Similarly, cumulative incidence estimates indicated similar rates of arm and shoulder symptoms between groups.
Table 4.
Patient assessments of moderate or marked late adverse events
|
Cumulative number of adverse events |
Adverse events at 5 years |
|||||
|---|---|---|---|---|---|---|
| n/N (%) | 5-year cumulative incidence*, % (95% CI) | HR (95% CI), p value† | n/N (%) | p value‡ | ||
| Protocol-specific items | ||||||
| Breast appearance changed | ||||||
| Whole breast | 158/411 (38%) | 47·7% (41·1–54·8) | 1 | 80/295 (27%) | ·· | |
| Reduced dose | 123/433 (28%) | 36·7% (30·6–43·6) | 0·74 (0·54–1·00), p=0·051 | 66/325 (20%) | 0·047 | |
| Partial breast | 113/421 (27%) | 35·1% (28·7–42·5) | 0·64 (0·46–0·89), p=0·007 | 49/331 (15%) | <0·0001 | |
| Breast smaller | ||||||
| Whole breast | 119/411 (29%) | 37·3% (30·9–44·4) | 1 | 66/294 (22%) | ·· | |
| Reduced dose | 110/433 (25%) | 31·9% (26·3–38·4) | 0·83 (0·59–1·16), p=0·280 | 63/326 (19%) | 0·373 | |
| Partial breast | 104/421 (25%) | 34·7% (27·5–43·0) | 0·78 (0·54–1·11), p=0·162 | 56/331 (17%) | 0·086 | |
| Breast harder or firmer | ||||||
| Whole breast | 115/411 (28%) | 35·3% (28·4–43·3) | 1 | 27/292 (9%) | ·· | |
| Reduced dose | 74/433 (17%) | 21·0% (16·2–26·9) | 0·53 (0·36–0·79), p=0·002 | 23/325 (7%) | 0·376 | |
| Partial breast | 58/421 (14%) | 15·3% (12·0–19·5) | 0·47 (0·32–0·71), p<0·0001 | 15/330 (5%) | 0·024 | |
| Shoulder stiffness | ||||||
| Whole breast | 56/411 (14%) | 19·3% (14·0–26·5) | 1 | 12/296 (4%) | ·· | |
| Reduced dose | 56/433 (13%) | 19·3% (13·9–26·4) | 0·93 (0·64–1·35), p=0·701 | 22/328 (7%) | 0·161 | |
| Partial breast | 58/421 (14%) | 15·3% (12·0–19·5) | 1·06 (0·73–1·54), p=0·756 | 13/331 (4%) | 0·999 | |
| Skin appearance changed | ||||||
| Whole breast | 63/411 (15%) | 21·0% (15·5–27·9) | 1 | 22/294 (7%) | ·· | |
| Reduced dose | 59/433 (14%) | 17·9% (13·2–24·0) | 1·07 (0·68–1·68), p=0·775 | 23/325 (7%) | 0·878 | |
| Partial breast | 49/421 (12%) | 14·6% (10·4–20·5) | 0·87 (0·54–1·40), p=0·569 | 12/330 (4%) | 0·051 | |
| EORTC QLQ-BR23 | ||||||
| Arm or shoulder pain | ||||||
| Whole breast | 98/411 (24%) | 32·6% (26·3–39·9) | 1 | 33/297 (11%) | ·· | |
| Reduced dose | 104/433(24%) | 30·1% (24·7–36·4) | 0·94 (0·71–1·25), p=0·678 | 43/329 (13%) | 0·465 | |
| Partial breast | 97/421 (23%) | 27·2% (21·9–33·6) | 0·97 (0·73–1·28), p=0·809 | 24/331 (7%) | 0·097 | |
| Swollen arm or hand | ||||||
| Whole breast | 21/411 (5%) | 6·2% (4·1–9·5) | 1 | 5/295 (2%) | ·· | |
| Reduced dose | 26/433 (6%) | 9·8% (6·2–15·3) | 1·19 (0·67–2·11), p=0·558 | 15/330 (5%) | 0·066 | |
| Partial breast | 16/421 (4%) | 4·4% (2·7–7·3) | 0·59 (0·30–1·15), p=0·123 | 2/330 (1%) | 0·264 | |
| Difficulty raising arm | ||||||
| Whole breast | 42/411 (10%) | 13·6% (9·2–19·8) | 1 | 10/297 (3%) | ·· | |
| Reduced dose | 45/433 (10%) | 14·0% (9·8–19·8) | 0·98 (0·64–1·50), p=0·913 | 17/328 (5%) | 0·326 | |
| Partial breast | 47/421 (11%) | 13·5% (10·1–18·0) | 1·08 (0·71–1·64), p=0·726 | 15/331 (5%) | 0·542 | |
| Breast pain | ||||||
| Whole breast | 67/411 (16%) | 19·1% (14·9–24·3) | 1 | 13/295 (4%) | ·· | |
| Reduced dose | 65/433 (15%) | 16·9% (12·9–22·1) | 0·96 (0·68–1·35), p=0·812 | 18/330 (5%) | 0·584 | |
| Partial breast | 64/421 (15%) | 18·2% (14·1–23·4) | 0·96 (0·68–1·36), p=0·830 | 13/328 (4%) | 0·842 | |
| Breast swollen | ||||||
| Whole breast | 31/411 (8%) | 8·1% (5·7–11·3) | 1 | 1/295 (<1%) | ·· | |
| Reduced dose | 26/433 (6%) | 6·8% (4·7–9·9) | 0·84 (0·49–1·41), p=0·503 | 4/329 (1%) | 0·377 | |
| Partial breast | 17/421 (4%) | 4·7% (2·9–7·6) | 0·49 (0·27–0·89), p=0·019 | 1/328 (<1%) | 0·999 | |
| Breast oversensitive | ||||||
| Whole breast | 64/411 (16%) | 17·2% (13·7–21·5) | 1 | 9/296 (3%) | ·· | |
| Reduced dose | 59/433 (14%) | 16·5% (12·0–22·4) | 0·89 (0·62–1·27), p=0·526 | 16/330 (5%) | 0·308 | |
| Partial breast | 54/421 (13%) | 18·3% (13·0–25·5) | 0·80 (0·55–1·14), p=0·220 | 13/330 (4%) | 0·665 | |
| Skin problems in breast | ||||||
| Whole breast | 50/411 (12%) | 15·7% (11·1–21·9) | 1 | 7/296 (2%) | ·· | |
| Reduced dose | 42/433 (10%) | 13·4% (9·2–19·2) | 0·78 (0·52–1·18), p=0·237 | 10/328 (3%) | 0·632 | |
| Partial breast | 35/421 (8%) | 9·2% (6·7–12·7) | 0·64 (0·42–0·99), p=0·045 | 9/330 (3%) | 0·806 | |
EORTC=European Organisation for Research and Treatment of Cancer.
Estimated at 5 years and 3 months.
Wald test.
Fisher's exact test.
1319 women consented to the photographic substudy, and baseline photographs were received and assessed for 1222 patients. Photographs taken at 2 years were assessed in 1000 women. The most common reasons for photographs not being available were centre administrative oversight so that photographic appointments were not made, patients not attending hospital visits, and patients withdrawing consent from the substudy. At 2 years, mild or marked changes in breast appearance were observed in 37 (11%) of 332 in the whole-breast group, 32 (10%) of 335 in the reduced-dose group, and 31 (10%) of 333 people in the partial-breast group. At 5 years, photographs were available for 805 women and, compared with the 2-year results, the proportion of patients with mild or marked changes had increased across all groups (whole-breast 60 (23%) of 262, reduced-dose 59 (22%) of 264, and partial-breast 50 (18%) of 279). No evidence of a statistically significant difference was seen in the proportion of patients with a change in breast appearance for either experimental schedule compared with whole-breast radiotherapy at 2 years (reduced-dose p=0·527; partial-breast p=0·446) or 5 years (reduced-dose p=0·917; partial-breast p=0·165).
Clinical assessment of late normal-tissue effects at 5 years showed a low occurrence of moderate or marked events across all treatment groups (table 5). At 5 years, breast shrinkage had the highest prevalence of moderate or marked events (whole-breast 41 (9%) of 452, reduced-dose 37 (8%) of 478, and partial-breast 33 (7%) of 472), whereas breast oedema was rare (whole-breast four (1%) of 446; reduced-dose two (<1%) of 468; partial-breast none of 468). The cumulative incidences also indicated breast shrinkage to be the most common late normal-tissue effect. The HRs for all late effects were consistently less than 1, but no evidence of statistically significant differences for individual events was seen. Severe late adverse effects were rare, and included four confirmed reports of rib fractures, eight of lung fibrosis, and five of ischaemic heart disease (appendix p 89).
Table 5.
Clinician assessment of moderate or marked late adverse events
|
Cumulative number of adverse events |
Adverse events at 5 years |
||||
|---|---|---|---|---|---|
| n/N (%) | 5-year cumulative incidence*, % (95% CI) | HR (95% CI), p value† | n/N (%) | p value‡ | |
| Worst normal-tissue effects | |||||
| Whole breast | 134/674 (20%) | 27·6% (22·5–33·6) | 1 | 60/457 (13%) | ·· |
| Reduced dose | 108/673 (16%) | 21·1% (17·2–25·7) | 0·77 (0·60–0·99), p=0·043 | 48/480 (10%) | 0·152 |
| Partial breast | 94/669 (14%) | 20·0% (15·6–25·4) | 0·69 (0·53–0·90), p=0·006 | 49/474 (10%) | 0·221 |
| Breast shrinkage | |||||
| Whole breast | 79/674 (12%) | 18·4% (13·7–24·5) | 1 | 41/452 (9%) | ·· |
| Reduced dose | 70/673 (10%) | 13·6% (10·6–17·5) | 0·86 (0·62–1·18), p=0·345 | 37/478 (8%) | 0·480 |
| Partial breast | 61/669 (9%) | 13·9% (10·1–19·0) | 0·78 (0·56–1·08), p=0·134 | 33/472 (7%) | 0·276 |
| Breast induration (index) | |||||
| Whole breast | 63/674 (9%) | 12·7% (9·5–16·8) | 1 | 21/453 (5%) | ·· |
| Reduced dose | 43/673 (6%) | 8·4% (6·0–11·6) | 0·66 (0·45–0·98), p=0·040 | 13/474 (3%) | 0·161 |
| Partial breast | 48/669 (7%) | 10·8% (7·7–15·1) | 0·77 (0·53–1·12), p=0·165 | 24/471 (5%) | 0·762 |
| Breast induration (outside index)§ | |||||
| Whole breast | 15/674 (2%) | 2·3% (1·4–3·8) | 1 | 2/450 (<1%) | ·· |
| Reduced dose | 10/673 (1%) | 2·1% (1·0–4·1) | 0·66 (0·30–1·48), p=0·310 | 2/464 (<1%) | >0·999 |
| Telangiectasia | |||||
| Whole breast | 8/674 (1%) | 1·6% (0·8–3·3) | 1 | 3/445 (1%) | ·· |
| Reduced dose | 8/673 (1%) | 3·0% (1·3–6·8) | 0·96 (0·36–2·57), p=0·976 | 6/468 (1%) | 0·507 |
| Partial breast | 5/669 (1%) | 0·6% (0·2–1·7) | 0·62 (0·21–1·92), p=0·401 | 4/465 (1%) | >0·999 |
| Breast oedema | |||||
| Whole breast | 24/674 (4%) | 4·0% (2·6–6·2) | 1 | 4/446 (1%) | ·· |
| Reduced dose | 18/673 (3%) | 3·2% (2·0–5·3) | 0·74 (0·40–1·37), p=0·338 | 2/468 (<1%) | 0·441 |
| Partial breast | 11/669 (2%) | 1·7% (0·9–3·0) | 0·46 (0·23–0·94), p=0·029 | 0/468 | 0·056 |
| Other radiotherapy related | |||||
| Whole breast | 11/674 (2%) | 1·7% (1·0–3·1) | 1 | 3/457 (1%) | ·· |
| Reduced dose | 9/673 (1%) | 1·4% (0·7–2·6) | 0·81 (0·34–1·97), p=0·646 | 0/480 | 0·263 |
| Partial breast | 6/669 (1%) | 0·9% (0·4–2·0) | 0·55 (0·20–1·49), p=0·234 | 0/474 | 0·221 |
HR=hazard ratio.
Estimated at 5 years and 3 months.
Log-rank test.
Fisher's exact test.
No cases of moderate or marked breast induration (outside index) were reported in the partial-breast group.
Discussion
Our 5-year results confirm that local relapse was scarce across all trial groups and that non-inferiority was shown for both partial-breast and reduced-dose radiotherapy. Late normal-tissue effects were also uncommon across all groups, and significantly fewer patients reported breast hardness in the partial-breast radiotherapy group compared with control. These findings support our hypothesis that partial-breast radiotherapy using a standard radiation technique can reduce late toxicity without jeopardising local tumour control.
IMPORT LOW is the only phase 3 trial of partial-breast radiotherapy to use the same dose-fractionation regimen and radiation technique in the whole-breast and partial-breast radiotherapy groups. Because the same regimen is used, differences in treatment outcome can be attributed more reliably to differences in radiotherapy volume. The Danish Breast Cancer Group phase 2 partial-breast radiotherapy trial is similarly designed to have breast volume as the only variable, but has a primary endpoint of grade 2 or higher breast induration at 3 years (Offersen B, Aarhus University, personal communication; NCT00892814). Other phase 3 partial-breast radiotherapy trials report a variety of different dose-fractionation regimens from a single intraoperative dose to 1–2 weeks of treatment.18, 23, 24 These differences make it challenging to distinguish whether variations in outcome are caused by differences in treated volume or radiation dose-time effects. This difficulty is illustrated by the interim results at 3 years from the RAPID trial25 (NCT00282035) that compared three-dimensional (3D) conformal partial-breast radiotherapy using 38·5 Gy in ten fractions over 5 days, with whole-breast radiotherapy using 42·5 Gy in 16 fractions or 50 Gy in 25 fractions with an optional boost. Cosmetic outcome and late normal-tissue toxicity were worse in the partial-breast radiotherapy group in the RAPID trial, which suggests that dose-time effects were the dominant factor over reduced irradiated volume within this study. Other randomised trials (NSABP NCT00103181, SHARE NCT01247233, IRMA NCT01803958) using similar dose-fractionation regimens to RAPID have yet to publish mature outcome data, although early reports suggest minor toxicity.
Another strength of IMPORT LOW is that patients were specifically engaged with the aim of producing the most comprehensive patient-reported outcomes in any published partial-breast radiotherapy trial to date. The patient's viewpoint is clearly important, but previous breast radiotherapy trials have shown that patient-reported outcomes are very sensitive when distinguishing between different dose-fractionation regimens.26 The results of IMPORT LOW suggest that patient-reported outcomes are also able to detect a radiotherapy volume effect, which is highly relevant for the design of future breast cancer radiotherapy trials, because patient-reported outcomes could be the most cost-effective yet sensitive and patient-centred method of outcome assessment. We analysed and presented the late normal-tissue toxicity for both patient-reported and clinician-reported outcomes both using discrete 5-year timepoints and cumulative incidence. The purpose of dual analysis is to convey different information, in that the longitudinal results capture the maximum grades of toxicity, whereas the cross-sectional 5-year results take into account that some side-effects were resolved, such as oedema, which might reduce over time. We acknowledge that multiple statistical tests were done for the normal-tissue toxicity analysis, but we accounted for this by using a stringent significance level of 0·005 for clinician-reported and patient-reported outcomes.
The simplicity of IMPORT LOW is also a strength. The partial-breast radiotherapy technique uses standard tangential fields that are simply shortened to encompass the tumour bed and margin of healthy tissue. This technique means that a larger volume of breast is treated than with other 3D conformal or IMRT and brachytherapy techniques, but tangential beams minimise dose to surrounding organs at risk such as the heart and lungs by keeping the exit beams within the breast. This method might be important in minimising second radiation-induced cancers. It might also minimise the mean heart dose without the need for breath-hold in most patients with left-sided breast cancer, given that most patients have tumours in the upper half of the breast and above the level of the heart.27, 28 The tangential field arrangement is more likely to deliver at least some dose to the lower axilla in comparison with more conformal partial-breast radiotherapy techniques that are likely to deliver none, which might be important in minimising axillary recurrences.29 A simple form of forward-planned IMRT was used to optimise dose homogeneity, but this is now standard in most centres,30, 31 so implementation of this technique does not require additional resources or training in most countries.
The original estimates of local relapse on which the sample size was based were high, given recent general improvements in local tumour control.11 Retrospective power calculations, based on year 5 data being available for 1832 (91%) of 2016 patients and an observed local relapse 5-year cumulative incidence in the control group of 1·1% confirm that a clinically relevant absolute 2·0% increase in 5-year local relapse could be excluded for each test group, assuming 80% power and 2·5% α (one-sided). The demonstration of non-inferiority is expected to be stable with longer follow-up, although proportion of patients with local relapse in IMPORT LOW is likely to be in the range of 1–3% by 10 years. This expectation is based on the ELIOT trial23 in which the cumulative incidence of local relapse in the intraoperative group rose in an apparently linear fashion between 5 and 9 years. Compliance with photographic assessments was not as high as anticipated in IMPORT LOW. However, given the few reported changes in breast appearance at 5 years in the control group (23%), retrospective power calculations indicate that this photographic substudy has 75% power to detect a difference of 10% (with a 2·5% significance level).
Furthermore, our study might have been limited by biased reporting of late normal-tissue toxicity because treatment allocation could not be masked. However, the panel of assessors doing the photographic assessments were masked to treatment groups, although photographic assessments seem to be less sensitive to subtle changes in normal-tissue toxicity than patient-reported assessments.
A major question raised by this trial is which patients should be selected for partial-breast radiotherapy? IMPORT LOW was originally designed to recruit patients with very low-risk disease; however, eligibility criteria were widened during recruitment to include some slightly higher-risk features after the publication of evidence of low recurrence rates from other breast radiotherapy trials, such as START.19 However, looking at the baseline characteristics in IMPORT LOW, most of the women who were recruited had small, low-grade, ER-positive, node-negative tumours. The appendix (p 86) shows that despite the low proportion of patients with high-risk disease, these patients had eight of the 18 local relapses. However, this observation should be taken with caution because the overall number of events was low. The UK has taken a pragmatic approach to patient selection for partial-breast radiotherapy by producing a consensus statement,32 which states that partial-breast radiotherapy can be considered for patients who are 50 years or older, with grade 1–2 cancer, a tumour of 30 mm or less, ER positive, HER2 negative, and N0 with minimum 1 mm radial excision margins for invasive disease. Given the small proportion of participants in IMPORT LOW who were node positive, we support the UK Breast Radiotherapy Consensus in not recommending partial-breast radiotherapy for this group. Consistent with the findings of ACOSOG Z0011,33 IBSCG 23–01,34 NCIC MA20,35 and EORTC 22922,36 we recommend that patients who are node positive receive whole-breast radiotherapy as standard of care.
A further controversy raised by this and other reported studies,37 is the definition of ipsilateral local relapse. For example, the IMPORT LOW definition is recurrence of any preinvasive or invasive carcinoma in the ipsilateral breast regardless of histology or location of the index breast cancer. The GEC-ESTRO trial8 definition does not take into account location within the breast, but does exclude tumours with differing histology, and the Cochrane review37 only includes relapses within the index quadrant with the same histology. Clearly, inclusion or exclusion of local relapses could make a substantial difference in reported results given the low number of events in this patient group.
Finally, the results of IMPORT LOW are not consistent with the 2016 overview by the Cochrane Collaboration37 that was based on the published data of phase 3 trials, six of which contributed to analyses of local relapses and four to analyses of toxicity endpoints. This overview reported inferior results for both local relapse and late normal-tissue toxicity with partial-breast radiotherapy. The small number of contemporary partial-breast radiotherapy trials described in the Cochrane report37 might explain the difference between the findings.31 Four other phase 2 trials testing partial-breast radiotherapy are yet to report 5-year results (NSABP/RTOG NCT00103181, RAPID NCT00282035, SHARE NCT01247233, and IRMA NCT01803958). The mature results from over 10 000 patients recruited within these important trials will add to the literature in future.
The results from as yet unpublished partial-breast radiotherapy trials are clearly needed, but because of the huge heterogeneity in dose-fractionation regimen, radiotherapy technique, irradiated volume, and inconsistencies in the definition of ipsilateral breast tumour recurrence, these data might prove challenging to interpret. A large individual patient data meta-analysis might resolve this potential dilemma and we strongly support this initiative.
We also recognise the importance of investigating possible effects of partial-breast radiotherapy on the development of radiation-induced second cancer and major cardiac events. However, this research will require thousands of patients followed up for many years before robust conclusions can be made and might be best achieved by future interrogation of routine health data.
Another approach is to investigate the biology of local relapse and its relationship to partial-breast radiotherapy. For example, what constitutes a true ipsilateral recurrence from an ipsilateral new primary at the molecular level is still unclear and requires further investigation.
At 5 years, partial-breast radiotherapy delivered using a simple intensity-modulated technique achieved non-inferiority in incidence of local relapse compared with whole-breast radiotherapy and similar or reduced late adverse effects. This method of partial-breast radiotherapy seems to be safe and effective and could be implemented easily within most radiotherapy centres worldwide.
Acknowledgments
Acknowledgments
We thank the patients who participated in this study and all investigators and research support staff, past and present, at participating centres (appendix p 90). Recognition goes to all trials unit staff at The Institute of Cancer Research Clinical Trials and Statistics Unit who contributed to the central coordination of the study. We thank the members of the Trial Management Group past and present (appendix p 92), the independent members of the Trial Steering Committee (Malcolm Mason (chair), Vivian Cosgrove, and Philip Poortmans) and the Independent Data Monitoring Committee (Helen Lucraft [chair], Matthew Sydes, and John Staffurth) for overseeing the study. We acknowledge support from Cancer Research UK (grant number C1491/A6035), the National Institute for Health Research (NIHR) Cancer Research Network (CRN), National Health Service Research Scotland, Health and Care Research Wales, and the National Institute of Health Research Royal Marsden/Institute of Cancer Research Biomedical Research Centre. CEC is supported by the Cambridge National Institute of Health Research Biomedical Research Centre. We are grateful to the NCRI Radiotherapy Trials Quality Assurance group at Mount Vernon Hospital for overseeing the radiotherapy planning and delivery throughout the study. The IMPORT Trialists' Group consists of the Trial Management Group, Trial Steering Committee, Independent Data Monitoring Committee, and the principal and main co-investigators at the participating centres. IMPORT LOW is sponsored by The Institute of Cancer Research, London.
Contributors
CEC and JRY are the current and previous chief investigators. AMK is the chief clinical coordinator. JMB is the scientific lead for the study within the Institute of Cancer Research Clinical Trials and Statistics Unit and provided oversight and guidance for trial management and statistical analysis throughout. JRY, JMB, CEC, and JSH were responsible for the study design. JMB, CEC, JRY, AMK, and CLG were responsible for data interpretation and writing the report. CLG and JSH did the statistical analysis. JT, ISB, MAE, and RK managed the study and data collection. MLJ and MW are patient advocate members of the trial management group and provided guidance for study documentation and reports. YMT and LC were the quality assurance radiographer and physicist and contributed to radiotherapy planning and quality assurance. EMD was involved in radiotherapy planning and set-up. RKA, AA, AMB, CC, ANH, EJS, IS, and DAW are principal investigators (past or present) at participating centres, were members of the trial management group, were responsible for patient recruitment, and contributed to the report through review and discussion. PH is the patient-reported outcomes lead. All authors reviewed the manuscript.
Declaration of interests
We declare no competing interests.
Contributor Information
Charlotte E Coles, Email: colesc@doctors.org.uk.
IMPORT Trialists:
Wail Al Sarakbi, Sarah Barber, Gillian Barnett, Peter Bliss, John Dewar, David Eaton, Stephen Ebbs, Ian Ellis, Philip Evans, Emma Harris, Hayley James, Cliona Kirwan, Julie Kirk, Helen Mayles, Anne McIntyre, Judith Mills, Andrew Poynter, Elena Provenzano, Christine Rawlings, Mark Sculpher, Georges Sumo, Mark Sydenham, Andrew Tutt, Nicola Twyman, Karen Venables, Anna Winship, John Winstanley, Gordon Wishart, and Alastair Thompson
Supplementary Material
References
- 1.Early Breast Cancer Trialists' Collaborative Group (EBCTCG) Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10 801 women in 17 randomised trials. Lancet. 2011;378:1707–1716. doi: 10.1016/S0140-6736(11)61629-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Breast radiotherapy after breast-conserving surgery The Steering Committee on Clinical Practice Guidelines for the Care and Treatment of Breast Cancer. Canadian Association of Radiation Oncologists. CMAJ. 1998;158(suppl 3):S35–S42. [PubMed] [Google Scholar]
- 3.Sedlmayer F, Sautter-Bihl ML, Budach W. DEGRO practical guidelines: radiotherapy of breast cancer I: radiotherapy following breast conserving therapy for invasive breast cancer. Strahlenther Onkol. 2013;189:825–833. doi: 10.1007/s00066-013-0437-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belkacemi Y, Kaidar-Person O, Poortmans P. Patterns of practice of regional nodal irradiation in breast cancer: results of the European Organization for Research and Treatment of Cancer (EORTC) NOdal Radiotherapy (NORA) survey. Ann Oncol. 2015;26:529–535. doi: 10.1093/annonc/mdu561. [DOI] [PubMed] [Google Scholar]
- 5.Dundas KL, Pogson EM, Batumalai V. Australian survey on current practices for breast radiotherapy. J Med Imaging Radiat Oncol. 2015;59:736–742. doi: 10.1111/1754-9485.12348. [DOI] [PubMed] [Google Scholar]
- 6.Smith BD, Arthur DW, Buchholz TA. Accelerated partial breast irradiation consensus statement from the American Society for Radiation Oncology (ASTRO) Int J Radiat Oncol Biol Phys. 2009;74:987–1001. doi: 10.1016/j.ijrobp.2009.02.031. [DOI] [PubMed] [Google Scholar]
- 7.Polgár C, Major T, Fodor J. Accelerated partial-breast irradiation using high-dose-rate interstitial brachytherapy: 12-year update of a prospective clinical study. Radiother Oncol. 2010;94:274–279. doi: 10.1016/j.radonc.2010.01.019. [DOI] [PubMed] [Google Scholar]
- 8.Polgár C, Van Limbergen E, Pötter R. Patient selection for accelerated partial-breast irradiation (APBI) after breast-conserving surgery: recommendations of the Groupe Européen de Curiethérapie-European Society for Therapeutic Radiology and Oncology (GEC-ESTRO) breast cancer working group based on clinical evidence (2009) Radiother Oncol. 2010;94:264–273. doi: 10.1016/j.radonc.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 9.Wilkinson JB, Beitsch PD, Shah C. Evaluation of current consensus statement recommendations for accelerated partial breast irradiation: a pooled analysis of William Beaumont Hospital and American Society of Breast Surgeon MammoSite Registry Trial Data. Int J Radiat Oncol Biol Phys. 2013;85:1179–1185. doi: 10.1016/j.ijrobp.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 10.Shah C, Vicini F, Wazer DE, Arthur D, Patel RR. The American Brachytherapy Society consensus statement for accelerated partial breast irradiation. Brachytherapy. 2013;12:267–277. doi: 10.1016/j.brachy.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 11.Mannino M, Yarnold JR. Local relapse rates are falling after breast conserving surgery and systemic therapy for early breast cancer: can radiotherapy ever be safely withheld? Radiother Oncol. 2009;90:14–22. doi: 10.1016/j.radonc.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 12.Salvadori B, Marubini E, Miceli R. Reoperation for locally recurrent breast cancer in patients previously treated with conservative surgery. Br J Surg. 1999;86:84–87. doi: 10.1046/j.1365-2168.1999.00961.x. [DOI] [PubMed] [Google Scholar]
- 13.Coles CE, Wilson CB, Cumming J. Titanium clip placement to allow accurate tumour bed localisation following breast conserving surgery: audit on behalf of the IMPORT Trial Management Group. Eur J Surg Oncol. 2009;35:578–582. doi: 10.1016/j.ejso.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 14.Harris EJ, Donovan EM, Yarnold JR, Coles CE, Evans PM, IMPORT Trial Management Group Characterization of target volume changes during breast radiotherapy using implanted fiducial markers and portal imaging. Int J Radiat Oncol Biol Phys. 2009;73:958–966. doi: 10.1016/j.ijrobp.2008.10.030. [DOI] [PubMed] [Google Scholar]
- 15.Coles CE, Donovan E, Haviland J, Yarnold J. Intensity-modulated radiotherapy for the treatment of breast cancer. Clin Oncol. 2013;25:215. doi: 10.1016/j.clon.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 16.Théberge V, Whelan T, Shaitelman SF, Vicini FA. Altered fractionation: rationale and justification for whole and partial breast hypofractionated radiotherapy. Semin Radiat Oncol. 2011;21:55–65. doi: 10.1016/j.semradonc.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 17.Marta GN, Macedo CR, Carvalho Hde A, Hanna SA, da Silva JL, Riera R. Accelerated partial irradiation for breast cancer: systematic review and meta-analysis of 8653 women in eight randomized trials. Radiother Oncol. 2015;114:42–49. doi: 10.1016/j.radonc.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 18.Strnad V, Ott OJ, Hildebrandt G, on behalf of the Groupe Européen de Curiethérapie of European Society for Radiotherapy and Oncology 5-year results of accelerated partial breast irradiation using sole interstitial multicatheter brachytherapy versus whole-breast irradiation with boost after breast-conserving surgery for low-risk invasive and in-situ carcinoma of the female breast: a randomised, phase 3, non-inferiority trial. Lancet. 2016;387:229–238. doi: 10.1016/S0140-6736(15)00471-7. [DOI] [PubMed] [Google Scholar]
- 19.Haviland JS, Owen JR, Dewar JA, on behalf of the START Trialists' Group The UK Standardisation of Breast Radiotherapy (START) randomised trials of radiotherapy hypofractionation for treatment of early breast cancer; 10-year follow-up results (CRUK/96/001) Lancet Oncol. 2013;14:1086–1094. doi: 10.1016/S1470-2045(13)70386-3. [DOI] [PubMed] [Google Scholar]
- 20.Schaverien MV, Stallard S, Dodwell D, Doughty JC. Use of boost radiotherapy in oncoplastic breast-conserving surgery—a systematic review. Eur J Surg Oncol. 2013;39:1179–1185. doi: 10.1016/j.ejso.2013.07.240. [DOI] [PubMed] [Google Scholar]
- 21.Yarnold J, Ashton A, Bliss J. Fractionation sensitivity and dose response of late adverse effects in the breast after radiotherapy for early breast cancer: long-term results of a randomised trial. Radiother Oncol. 2005;75:9–17. doi: 10.1016/j.radonc.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 22.Haviland JS, Ashton A, Broad B. Evaluation of a method for grading late photographic change in breast appearance after radiotherapy for early breast cancer. Clin Oncol. 2008;20:497–501. doi: 10.1016/j.clon.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 23.Veronesi U, Orecchia R, Maisonneuve P. Intraoperative radiotherapy versus external radiotherapy for early breast cancer (ELIOT): a randomised controlled equivalence trial. Lancet Oncol. 2013;14:1269–1277. doi: 10.1016/S1470-2045(13)70497-2. [DOI] [PubMed] [Google Scholar]
- 24.Livi L, Meattini I, Marrazzo L. Accelerated partial breast irradiation using intensity-modulated radiotherapy versus whole breast irradiation: 5-year survival analysis of a phase 3 randomised controlled trial. Eur J Cancer. 2015;51:451–463. doi: 10.1016/j.ejca.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 25.Olivotto IA, Whelan TJ, Parpia S. Interim cosmetic and toxicity results from RAPID: a randomized trial of accelerated partial breast irradiation using three-dimensional conformal external beam radiation therapy. J Clin Oncol. 2013;31:4038–4045. doi: 10.1200/JCO.2013.50.5511. [DOI] [PubMed] [Google Scholar]
- 26.Haviland JS, Hopwood P, Mills J, Sydenham M, Bliss JM, Yarnold JR, on behalf of the START Trialists' Group Do patient-reported outcome measures agree with clinical and photographic assessments of normal tissue effects after breast radiotherapy? The Experience of the Standardisation of Breast Radiotherapy (START) trials in early breast cancer. Clin Oncol. 2016;28:345–353. doi: 10.1016/j.clon.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 27.Donovan EM, James H, Bonora M, Yarnold JR, Evans PM. Second cancer incidence risk estimates using BEIR VII models for standard and complex external beam radiotherapy for early breast cancer. Med Phys. 2012;39:5814–5824. doi: 10.1118/1.4748332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kirby AM, Evans PM, Donovan EM, Convery HM, Haviland JS, Yarnold JR. Prone versus supine positioning for whole and partial-breast radiotherapy: a comparison of non-target tissue dosimetry. Radiother Oncol. 2010;96:178–184. doi: 10.1016/j.radonc.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 29.Gentilini O, Botteri E, Leonardi MC. Ipsilateral axillary recurrence after breast conservative surgery: the protective effect of whole breast radiotherapy. Radiother Oncol. 2017;122:37–44. doi: 10.1016/j.radonc.2016.12.021. [DOI] [PubMed] [Google Scholar]
- 30.Mukesh MB, Barnett GC, Wilkinson JS. Randomized controlled trial of intensity-modulated radiotherapy for early breast cancer: 5-year results confirm superior overall cosmesis. J Clin Oncol. 2013;31:4488–4495. doi: 10.1200/JCO.2013.49.7842. [DOI] [PubMed] [Google Scholar]
- 31.Vaidya JS, Wenz F, Bulsara M, on behalf of the TARGIT trialists' group Risk-adapted targeted intraoperative radiotherapy versus whole-breast radiotherapy for breast cancer: 5-year results for local control and overall survival from the TARGIT-A randomised trial. Lancet. 2014;383:603–613. doi: 10.1016/S0140-6736(13)61950-9. [DOI] [PubMed] [Google Scholar]
- 32.Bloomfield DJ, on behalf of the Core Group facilitated by The Royal College of Radiologists Development of postoperative radiotherapy for breast cancer: UK consensus statements—a model of patient, clinical and commissioner engagement? Clin Oncol. 2017 doi: 10.1016/j.clon.2017.06.011. DOI:10.1016/j.clon.2017.06.011 published online July 4. [DOI] [PubMed] [Google Scholar]
- 33.Giuliano AE, Ballman K, McCall L. Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: long-term follow-up from the American College of Surgeons Oncology Group (alliance) ACOSOG Z0011 Randomized Trial. Ann Surg. 2016;264:413–420. doi: 10.1097/SLA.0000000000001863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Galimberti V, Cole BF, Zurrida S, for the International Breast Cancer Study Group Trial 23–01 investigators Axillary dissection versus no axillary dissection in patients with sentinel-node micrometastases (IBCSG 23-01): a phase 3 randomised controlled trial. Lancet Oncol. 2013;14:297–305. doi: 10.1016/S1470-2045(13)70035-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Whelan TJ, Olivotto IA, Parulekar WR, for the MA.20 Study Investigators Regional nodal irradiation in early-stage breast cancer. N Engl J Med. 2015;373:307–316. doi: 10.1056/NEJMoa1415340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Poortmans PM, Collette S, Kirkove C, for the EORTC Radiation Oncology and Breast Cancer Groups Internal mammary and medial supraclavicular irradiation in breast cancer. N Engl J Med. 2015;373:317–327. doi: 10.1056/NEJMoa1415369. [DOI] [PubMed] [Google Scholar]
- 37.Hickey BE, Lehman M, Francis DP, See AM. Partial breast irradiation for early breast cancer. Cochrane Database Syst Rev. 2016;7 doi: 10.1002/14651858.CD007077.pub3. CD007077. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.