Significance
Alterations in nonduplex structures could play roles during DNA replication in the progression of cancer and other intractable diseases. These noncanonical structures differ topologically from each other. However, the role of these differences in diseases remains unknown. In this study, we found that the presence of i-motif structures in the template caused the DNA polymerase to stall immediately before these structures. The i-motif structures are more efficient than other structures, such as G-quadruplexes and hairpins, although their thermodynamic stabilities are similar. This inhibition effect of the DNA polymerase was regulated by molecular crowding, which mimics conditions in the cell. Therefore, it is possible that the i-motif could impede DNA replication or repair and cause genomic instability.
Keywords: replication, i-motif, G-quadruplex, thermodynamics, molecular crowding
Abstract
Noncanonical DNA structures that stall DNA replication can cause errors in genomic DNA. Here, we investigated how the noncanonical structures formed by sequences in genes associated with a number of diseases impacted DNA polymerization by the Klenow fragment of DNA polymerase. Replication of a DNA sequence forming an i-motif from a telomere, hypoxia-induced transcription factor, and an insulin-linked polymorphic region was effectively inhibited. On the other hand, replication of a mixed-type G-quadruplex (G4) from a telomere was less inhibited than that of the antiparallel type or parallel type. Interestingly, the i-motif was a better inhibitor of replication than were mixed-type G4s or hairpin structures, even though all had similar thermodynamic stabilities. These results indicate that both the stability and topology of structures formed in DNA templates impact the processivity of a DNA polymerase. This suggests that i-motif formation may trigger genomic instability by stalling the replication of DNA, causing intractable diseases.
Noncanonical intramolecular structures of nucleic acids, such as a triplex and a quadruplex, are stabilized under conditions that mimic the crowded cellular conditions (1), and have been detected in cells (2, 3). In vitro and in vivo, guanine-quadruplex (G-quadruplex or G4) formation inhibits transcription and translation of template nucleic acids (4–8). It is possible that the noncanonical structures act as “functional codes” triggered by different molecular environments, which regulate gene expression epigenetically (6, 7). As sequences capable of forming the noncanonical structures are found in telomeres and promoter regions of known oncogenes, alterations of the noncanonical structures could play important roles in the progression of cancer and in other diseases (9).
One of the remarkable features of noncanonical structures of nucleic acids is the diversity of topologies. In the case of G4s, antiparallel, mixed, and parallel type structures have been characterized (Fig. 1 A–C). The sequences complementary to regions capable of G4 formation are composed of tandem repeats of cytosine, and these C-rich regions can form a different type of tetraplex topology, which is the i-motif (10, 11). An intramolecular i-motif is formed upon the interaction of four C-rich regions. The structure has three loops and two parallel-stranded hairpin-like units stabilized by cytosine and protonated cytosine (C:C+) base pairs that are vertically intercalated antiparallel to one another (Fig. 1 D and E). The i-motif structures are categorized into class I and class II based on the lengths of the loops. C-rich sequences are found in telomeres and in the promoter regions of about 40% of human genes (12, 13). As the i-motif is stabilized by hydrogen bonding in C:C+ base pairs (14, 15), acidic conditions stabilize the structure. As the i-motif can mediate transcriptional regulation of B-cell lymphoma 2 (Bcl2) oncogene in cells (16), the environment inside these cells might be favorable to i-motif formation. Interestingly, the promoter region of the gene that encodes hypoxia-induced transcription factor (Hif1a), which is highly expressed in cancer cells, forms a very stable i-motif structure in vitro (12, 17). The thermodynamic stability of the i-motif structure with the Hif1a sequence under slightly acidic conditions is comparable to that of G4 formed by the opposite strand (18). Because G4 formation in the template DNA causes breakage of the genomic DNA due to stalling of DNA polymerase during replication (19, 20), the presence of an i-motif on the template DNA may be responsible for the mechanism of intractable diseases, including cancer. However, the effects of these topological differences, particularly the i-motif, remain unknown.
Fig. 1.
Schematic illustrations of topologies of antiparallel G4 (A), mixed G4 (B), parallel G4 (C), class I i-motif (D), and class II i-motif (E) having longer loops than class I are shown.
In this study, we investigated the effect of structures formed in the template strand on the replication reaction. Under slightly acidic conditions, i-motif formation decreased the rate of replication by Klenow fragment DNA polymerase (KF). Gel electrophoretic analyses revealed that the DNA polymerase stalled immediately before the i-motif–forming region, indicating that the i-motif is an obstruction to the polymerase. We compared the inhibition resulting from i-motif formation with that resulting from G4 and hairpin formation, and found that the level of inhibition was determined not only by the stability but also by the topology of noncanonical DNA structures. The i-motif, antiparallel G4s, and parallel G4s repressed replication more effectively than mixed G4 or hairpins with similar thermodynamic stabilities. These findings suggest that not only G4 but also the i-motif can induce genomic instability more than other noncanonical structures, which causes intractable diseases.
Results
DNA Replication Reaction.
In the template DNA, the structure-forming sequence was adjacent to the region complementary to the primer (SI Appendix, Fig. S1). The 5′ terminus of primer DNA was labeled with fluorescein (FAM) to enable quantification of product formation. The gap between the region of a noncanonical structure and the primer-binding region was four bases, which is a length selected to ensure that the structure adopted does not interfere with the formation of the elongation complex of KF and initial polymerization (21). This design enabled us to discriminate stalled product from unreacted primer and full-length product. The i-motif–forming sequences used are derived from the human telomeric sequence (C3TA2)4, the promoter region of the Hif1a gene, a region complementary to a portion of the insulin-linked polymorphic region (cILPR), which is a regulatory sequence upstream of the gene encoding insulin, and the complementary sequence of an oncogene of Bcl2 (cBcl2). Also tested were G4-forming sequences (T2AG3)4, Q6, and ILPR (complementary G-rich sequence of the cILPR) and hairpin-forming sequences (H1, H2, and H3). Q6 possesses four repeats of the G4 unit linked with the loop of the thrombin-binding aptamer (6). The control was a linear sequence not expected to form any stable structures. All of the sequences are shown in SI Appendix, Table S1. Formation of the i-motif and G4 structures, which have different topologies, was confirmed by circular dichroism (CD) analyses at 37 °C in the buffer used for replication assays (SI Appendix, Figs. S2 and S3).
Replication of DNA Containing an I-Motif Structure.
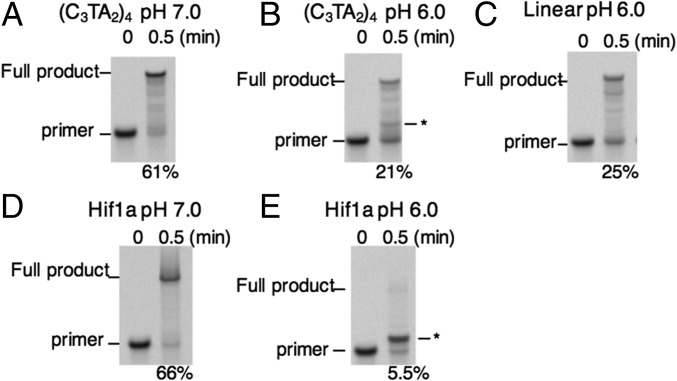
Although inhibition of DNA replication by a G4 formed within a template DNA strand has been studied before (22), there have been no reports on the effects of i-motif structures on DNA replication. Therefore, we first examined replication of a template that contained the human telomeric sequence (C3TA2)4 forming the i-motif structure. The replication reaction was carried out with 1 μM KF and 250 μM dNTPs for 0.5 min at 37 °C, and the progress of the reaction was analyzed by quantification of fluorescently labeled products on denaturing polyacrylamide gel electrophoresis (PAGE). At pH 7.0, a condition in which (C3TA2)4 does not form the i-motif structure, the fluorescently labeled primer was completely converted to a longer product within 0.5 min (Fig. 2A). By staining of DNA using SYBR Gold dye, we confirmed that the fluorescently labeled replication product had the same mobility as the template DNA (SI Appendix, Fig. S4). Thus, the replication of the (C3TA2)4-containing template yielded a full-length product. Under a slightly acidic condition at pH 6.0, replication of the i-motif–forming template was repressed (Fig. 2B). A band that migrated just above the primer band was observed in the reaction with the (C3TA2)4-containing template at pH 6.0 (Fig. 2B). The presence of this band indicates that the replication was stalled after primer extension of less than 10 nucleotides (SI Appendix, Fig. S4). Such a short band was not observed in the case of the replication of the linear template (Fig. 2C). These data suggest that the i-motif structure formed at low pH transiently stalled replication immediately before the structure. When the experiment was performed on a template containing the i-motif–forming sequence found in the promoter region of the Hif1a gene, which forms a particularly stable i-motif structure in vitro (12, 17), the results were more striking. At pH 7.0, the replication of the Hif1a template was efficient (Fig. 2D), but virtually no full-length product was detected at pH 6.0 (Fig. 2E). We did observe a small amount of a shorter product, indicating that the replication reaction was inhibited after elongation of less than 10 nucleotides (SI Appendix, Fig. S4). It was shown previously that KF stalls immediately before a G4 structure on a template (22). To confirm that the shorter products resulted from stalling of the polymerase during progressive replication, we analyzed the replication from FAM-labeled primer S, which was 10 bases shorter than the original primer. Reactions using this primer generated the same length of stalled product as those using the longer primer, indicating that the noncanonical structure did not disrupt template loading (SI Appendix, Fig. S5). Thus, during replication of the Hif1a template DNA, we confirmed that KF was stalled by the i-motif structure formed within the template. We also confirmed replication stall by T7 DNA polymerase, which replicates the genome of T7 phage (SI Appendix, Fig. S6). Therefore, stalling of replication at an i-motif can be considered to generally occur.
Fig. 2.
Representative PAGE analyses of replication reactions with (C3TA2)4 template DNA at pH 7.0 (A), (C3TA2)4 template DNA at pH 6.0 (B), linear template DNA at pH 6.0 (C), Hif1a template DNA at pH 7.0 (D), and Hif1a template DNA at pH 6.0 (E) are shown. The images were captured using a fluorescent imager; thus, only DNA containing the fluorescent label is visualized. Images of the same gels stained to visualize all DNA are shown in SI Appendix, Fig. S2. Reactions were carried out with 1 μM KF, 1 μM DNAs, and 250 μM dNTPs in 40 mM MES buffer (pH 7.0 or pH 6.0) for 2 min at 37 °C. The asterisk indicates the position of major stalled product. The values at the bottom of the gel image are the percentages of full-length product.
Dependence of Replication Efficiency on i-Motif Stability.
To understand the relationship between the replication efficiency and i-motif stability, a UV melting analysis was performed on strands containing only the i-motif–forming region of the template sequences (Table 1 and SI Appendix, Figs. S7 and S8 and Table S2). At pH 6.0, (C3TA2)4 had a melting temperature (Tm) of 31.4 °C and a free energy change at 37 °C (−∆G°37) of −0.70 kcal⋅mol−1, whereas the Hif1a sequence had a Tm of 57.3 °C and a −∆G°37 of 3.1 kcal⋅mol−1 (Table 1). These data suggest that the stalling effect of KF is related to the magnitude of −∆G°37. We previously showed that transcription by RNA polymerase was halted at a G4 structure in the template DNA (6) and that the magnitude of the inhibition depended on the stability of G4. There has been no quantitative analysis of the effect of stabilities of noncanonical structures present in the template DNA on replication, but a previous study showed that replication was inhibited at a G4 structure and that the inhibition was more efficient in the presence of a G4-binding ligand expected to increase the stability of the G4 structure (5). The data reported here suggest that the i-motif can also inhibit replication in a stability-dependent manner.
Table 1.
Thermodynamic and kinetic parameters of the structured sequence region on each template DNA at pH 6.0
| DNA topology | Sequence | Tm, °C | −∆G°37, kcal⋅mol−1 | ks, min−1 |
| i-motif | (C3TA2)4 | 31.4 ± 0.1 | −0.70 ± 0.01 | 4.5 ± 0.1 |
| Hif1a | 57.3 ± 0.1 | 3.1 ± 0.2 | 0.39 ± 0.11 | |
| Hairpin | H2 | 75.8 ± 2.2 | 4.0 ± 0.2 | 3.7 ± 0.1 |
| Mixed G4* | (T2AG3)4 | 62.8 ± 0.5 | 3.0 ± 0.2 | 2.6 ± 0.3 |
| Parallel G4 | ILPR | 58.1 ± 0.2 | 2.1 ± 0.3 | 0.54 ± 0.4 |
| Antiparallel G4 | Q6 | 71.1 ± 0.3 | 4.5 ± 0.1 | 0.08 ± 0.03 |
All experiments were performed in 40 mM MES (pH 6.0) and 8 mM MgCl2 without both KCl and PEG, except for the case of (T2AG3)4. Oligonucleotides corresponding to the structured region of indicated templates were evaluated at 10 μM strand concentration.
Experiments for (T2AG3)4 were performed in 40 mM MES (pH 6.0), 8 mM MgCl2, and 30 mM KCl.
Effect of Molecular Crowding on the Replication of i-Motif–Forming DNA.
To further investigate the replication of i-motif–forming DNA in cell-mimicking conditions, we examined replication in more physiologically relevant conditions in the presence of salt and crowding reagents using polyethylene glycol (PEG). In the presence of 100 mM KCl and 20 wt% PEG200 (average molecular weight is 200) at pH 6.0, the amount of full-length product replicated from the template containing the (C3TA2)4 sequence was quite similar to that in the absence of PEG200 (SI Appendix, Fig. S9A). Because the higher molecular weight of PEG shows more stabilization of i-motif due to the excluded volume effect (23, 24), the replication might not be effectively stalled by the addition of 20 wt% PEG200. In fact, the addition of 20 wt% PEG200 did not increase thermal stability of the (C3TA2)4 i-motif, whose Tm was 28.7 °C and −∆G°37 was −1.4 kcal⋅mol−1 (SI Appendix, Table S2). In the presence of 20 wt% PEG1000 (average molecular weight is 1,000) at pH 6.0, a short product was detected on PAGE (SI Appendix, Fig. S9B), indicating that KF was stalled immediately before the i-motif structure. The stabilizing effect of PEG1000 was observed in the melting temperature of (C3TA2)4 (SI Appendix, Fig. S8 and Table S2). The Tm in the presence of 20 wt% PEG1000 was 37.3 °C and the −∆G°37 was 0.1 kcal⋅mol−1. In the case of the Hif1a sequence, the thermodynamic stability was also not affected in the presence of PEG200 (Tm = 51.2 °C and −∆G°37 = 3.1 kcal⋅mol−1) but increased in the presence of PEG1000 (Tm = 59.7 °C and −∆G°37 = 4.2 kcal⋅mol−1) (SI Appendix, Fig. S8 and Table S2). The Hif1a sequence stalled KF immediately before the i-motif structure in the presence of PEG1000, although PEG200 did not have an effect (SI Appendix, Fig. S9 C and D). Interestingly, stalled replication could be observed even at pH 7.0 in the presence of 100 mM KCl and 20 wt% PEG1000 (SI Appendix, Fig. S9E). Although the detailed mechanism of stabilization of the i-motif structure by PEG1000 is unknown, the higher molecular-weight PEG shifts the pKa of N3 in the cytosine base (23, 24). As the i-motif structure is very compact compared with a random coil, a more pronounced excluded volume effect of higher molecular-weight PEG1000 compared with that of PEG200 may contribute to the observed effect of PEG1000 on the replication of an i-motif–forming DNA. In summary, our results suggest that the cellular condition crowded with biomacromolecules stabilizes the i-motif structure, which facilitates the inhibition of replication.
Replication Rate Is Inversely Correlated with Stability of i-Motif.
To further analyze the correlation between the thermodynamic stability and replication rate, we evaluated the amount of product as a function of time (SI Appendix, Fig. S10). In the reaction with the (C3TA2)4 template in the absence of PEG at pH 6.0, stalled product was observed at 0.16 min (SI Appendix, Fig. S10A). The amount of stalled product was decreased at 0.33 min and had almost disappeared by 1 min. On the other hand, full-length product was detected at 0.33 min, and the conversion to full-length product was complete at 2 min. In the case of the replication of the Hif1a sequence, stalled product was detected at 0.5 min and the amount gradually decreased with time. A small amount of full-length product was detected at 1 min, and the amount increased at longer time points (SI Appendix, Fig. S10B). For both the (C3TA2)4 and Hif1a templates, the increase in full-length product corresponded to a decrease in amount of the stalled product (Fig. 3A). The generation curves of full-length product were analyzed by global fitting (SI Appendix, Fig. S11) and rate constants, ks (min−1) for the reaction step of the stall of replication, including the prior replication to the stalling position and kf (min−1) for the reaction step of generating full-length product after resolving the replication stall were calculated. The ks values were 4.5 min−1 and 0.38 min−1 at 37 °C and pH 6.0 for the templates containing the (C3TA2)4 sequence and the Hif1a sequence, respectively (Table 1). These results indicate that KF took about a 12-fold longer time to overcome the more stable i-motif formed by the Hif1a sequence than to overcome that formed by the (C3TA2)4 sequence. The kf values were 4.5 min−1 and 6.3 min−1 at 37 °C and pH 6.0 for the templates containing the (C3TA2)4 sequence and the Hif1a sequence, respectively (SI Appendix, Table S2), suggesting that the replication stall did not occur for the (C3TA2)4 sequence but that the stalling step was the rate-determining step in the replication of the Hif1a sequence.
Fig. 3.
Kinetics of replication of DNA templates containing noncanonical structures. (A) Ratios of stalled (dotted lines) and full-length (solid lines) to total product of (C3TA2)4 (blue), Hif1a (red), and linear (green) templates as a function of time. (B) Plot of −∆G°37 values vs. lnks for reactions to dissolve the stall from reaction start along i-motif–forming templates (blue), G4-forming templates (red), and linear template (green). Data on the G4 ILPR template were excluded from fitting of the G4 data. (C) Ratios of stalled product and full-length product to total products of (T2AG3)4 replication in the presence of 1 mM KCl (blue) and 30 mM KCl (red) and of linear (green) replication in the absence of KCl as a function of time. (D) Ratios of full-length product to total product of Hif1a in the presence of 100 mM KCl and 20 wt% PEG200 (blue) and in the presence of 100 mM KCl and 20 wt% PEG1000 (red). All of the reactions were carried in 40 mM MES (pH 6.0), 8 mM MgCl2, 1 μM KF, 1 μM DNAs, and 250 μM dNTPs at 37 °C.
Fig. 3B shows a plot of the logarithm of replication rate constant (lnks) vs. −∆G°37 for the template sequences investigated. The −∆G°37 value for the linear control template was assumed to be zero. This plot includes data on an additional i-motif formed by the cILPR. The cILPR is a portion of the complementary sequence of the insulin-linked polymorphic region, which forms a previously characterized i-motif (18). The values of lnks decreased with increasing −∆G°37, and there is a good linear correlation. As ∆G° is equal to −RT lnK (R is a gas constant and K is the equilibrium constant), ∆G° also can be described as −RT ln(k1/k−1), where k1 is the rate constant of structure formation and k−1 is the deformation rate constant. Because the stabilities of DNA structures depend on hydrogen bonding and stacking interactions between bases, k1 values for DNAs of similar chain lengths are similar; thus, k−1 is a dominant factor in stability (25). Therefore, the linear relationship between lnks and −∆G°37 indicates that the replication rate through an i-motif is mainly proportional to the rate of the unfolding of the i-motif structure (k−1). As the temperature was constant in our analysis, we infer that lnks is proportional to the activation free energy, ∆G‡. The ∆G‡ of the unfolding of a DNA duplex (∆G‡off) has a linear correlation with the stability of the DNA duplex (−∆G°) (26), and the activation free energy of dissociation of a DNA duplex depends on the sequence (GC content) and length (27, 28). Therefore, to unwind structured DNAs, the polymerase must decrease the ∆G‡off or rely on spontaneous dissociation of the structured DNA. It is possible that reduced enzyme processivity due to low pH and PEG may be the factor in the replication stall. Low pH and PEG do affect enzyme processivity because the ks value of linear template at pH 7.0 in the absence of salt and PEG was 18 min−1, which was about fivefold larger than that at pH 6.0 (SI Appendix, Figs. S10 and S11 and Table S2). However, the slope of the lnks vs. −∆G°37 plot of i-motifs, which indicates the magnitude of ∆G‡ for the unwinding process of the i-motif by KF, showed similar values at pH 6.0 in the absence of salt and PEG (−0.83) and in the presence of 100 mM KCl without PEG (−0.73), and at pH 7.0 in the presence of 100 mM KCl and 20 wt% PEG1000 (−0.55) (SI Appendix, Figs. S12–S14) (PEG200 is discussed in the next section). The similar value obtained for the slope indicated that the unwinding of i-motif was dominated by the same mechanism, not by enzyme properties, in various conditions. Thus, the increased stalling at the i-motif was due to folding of the i-motif and not to reduced enzyme processivity at low pH or in PEG.
Effect of Topology of Structured DNAs on the Replication Reaction.
We next analyzed the replication of template DNAs having sequences that can form different structural topologies. First, we investigated the replication of a template DNA containing a hairpin structure. The sequences contained stems of 4 (H1), 9 (H2), and 12 (H3) base pairs, and all had a four-nucleotide loop characterized previously (6). The −∆G°37 values obtained from UV melting experiments in 40 mM MES (pH 6.0) and 8 mM MgCl2 were 2.2, 4.0, and 8.3 kcal⋅mol−1 for H1, H2, and H3, respectively (Table 1 and SI Appendix, Fig. S7 and Table S2). Thus, each of these hairpins is more stable than the i-motif structure adopted by (C3TA2)4, which has a −∆G°37 of −0.70 kcal⋅mol−1. Interestingly, KF did not stall on any of the hairpin-containing templates (SI Appendix, Fig. S10).
We also analyzed replication of templates that contained regions able to form G4 in different KCl concentrations. For the template containing (T2AG3)4 in the presence of 30 mM KCl at 37 °C and pH 6.0, stalled product was observed at 0.16 min (Fig. 3C and SI Appendix, Fig. S10) and the full-length product increased correspondingly. UV melting showed that the −∆G°37 value of (T2AG3)4 was 3.0 kcal⋅mol−1 in the presence of 30 mM KCl (Table 1 and SI Appendix, Fig. S7). We also confirmed that the stalled product was due to a replication block at the G4 structure (SI Appendix, Fig. S5D). Interestingly, although Hif1a, H2, and (T2AG3)4 in 30 mM KCl showed similar stabilities (Table 1), the ks values differed, indicating that the replication stall depended on not only the stability but also the topology.
The plots of lnks vs. −∆G°37 for hairpin DNAs and (T2AG3)4 at 1, 10, 30, and 50 mM KCl were linear, as shown in the case of i-motif templates (Fig. 3B). The slope of the lnks vs. −∆G°37 was −0.048 for hairpin structures and −0.28 for (T2AG3)4, which is different from that of −0.83 for the i-motif, indicating that the activation free energy (∆G°37‡) required to unwind the i-motif structures was about 17-fold or threefold higher than that required to unwind hairpin or (T2AG3)4 G4, which forms a mixed structure (SI Appendix, Fig. S2). The data for the template containing the G4 ILPR, which forms a parallel structure (29), and Q6 sequences at 1 mM KCl, which form an antiparallel structure, also showed a different stability tendency and ks value (Table 1). Those plots did not fall on the line with data for (T2AG3)4-containing templates. This suggests that the topology of G4 affects the efficiency of unwinding. Interestingly, the data for the ILPR and Q6 template showed a correlation with data on i-motifs, suggesting that the i-motif is a strong block for replication as well as parallel/antiparallel G4s.
To investigate the effect of i-motif topology on replication, we tested the class II i-motif cBcl2 sequence (Fig. 1E) that is found in the promoter region of the Bcl2 oncogene. In this case, we observed two major bands shorter than the full-length product (SI Appendix, Fig. S15A). The Bcl2 sequence can adopt i-motif structures of different conformations, and the sequence can also form a hairpin structure, due to the presence of six cytosine tracts. If multiple conformers are present, more than one stalled product might be observed. Moreover, there were unexpected smeared bands above the full-length product band. Because a nascent DNA product from the replication of a triplet repeat, such as (CGG)n or (CAG)n, which forms a hairpin-like structure, potentially causes longer product than the template (30), the transient hairpin structure of the nascent DNA product from the cBcl2 template might increase the size of product. Therefore, we calculated the rate of full-length product formation, including the smeared bands. As a result, the lnks value was −0.18, and −∆G°37 value was 3.0 kcal⋅mol−1 (SI Appendix, Fig. S15 B and C). On the lnks vs. −∆G°37 plot, the cBcl2 i-motif conformed to the linear correlation of class I i-motifs (Fig. 3B), which indicates that the topology of the i-motif does not influence the replication stall. Based on these experiments, we conclude that the class II i-motifs with longer loops and class I motifs are unwound by a similar mechanism.
The topology and stability of noncanonical structures are also affected by the crowding condition. Experiments were also performed in the presence of 20 wt% PEG200 (Fig. 3D and SI Appendix, Fig. S12). In the case of the i-motif, the slope of lnks vs. −∆G°37 was −0.12 (SI Appendix, Fig. S16), whose magnitude was about sixfold smaller than that in the absence of PEG200 (−0.74) (SI Appendix, Fig. S14B). Thus, the stalling effect of the i-motif decreased in 20 wt% PEG200 compared with conditions without PEG200. Our recent study suggested that ethylene glycol binds to the duplex and disrupts the hydrogen network around Watson–Crick base pairing (31). All stabilities of the i-motif in the presence of 20 wt% PEG200 decreased compared with stabilities in corresponding conditions without PEG200. Therefore, PEG200 might uniquely interact with the base pairs of the i-motif and decrease stability, thereby increasing replication efficiency. On the other hand, (T2AG3)4 was transformed from a mixed to a parallel topology in the presence of 20 wt% PEG200 and 30 mM KCl (32) (SI Appendix, Fig. S3). Replication was effectively repressed and showed a similar slope of the lnks vs. −∆G°37 plot (−0.87) to that of the i-motif observed in the absence of PEG200 (−0.83) (SI Appendix, Figs. S14A and S16). Thus, this transformation may inhibit replication more efficiently, as observed in the case of ILPR. In the presence of PEG1000, the replication of i-motifs was effectively repressed (Fig. 3D). The slope of lnks vs. −∆G°37 was −0.56, which was close to that observed in the absence of PEG200 (−0.83) (SI Appendix, Figs. S14C and S16). Therefore, a specific molecular environment with various conditions of molecular crowding regulates the processivity of KF along a template DNA based on the activation free energy for unwinding by changing the stability and the topology of the DNA structure formed.
Discussion
In this study, we found a linear correlation between the lnks and the stability (−∆G°37) of particular structural topologies of noncanonical structures in the template DNA, indicating that the rate-limiting step of structure unwinding by KF is determined by both the stability and topology of the structure formed within the DNA template. We reported previously that the processivity of T7 RNA polymerase in the transcription reaction was influenced by both hairpin and G4 structures formed within the template DNAs (6). The rates of transcription reaction were correlated with the stabilities of the structures, and the lack of relationship with topology may indicate that the unwinding mechanisms of RNA polymerase and KF are different (33). Some G4-specific helicases show topological specificity. For example, the telomere protein TPP1 more efficiently unwinds antiparallel G4 structures like Q6 than it does parallel G4 (34). In contrast, RNA helicase RHAU preferentially binds to parallel G4 structures like that of ILPR (35). These results imply that the processivity of DNA polymerase requires the assistance of different types of helicases able to unwind various topologies.
KF has an ability to unwind hairpin structures, presumably through an unzipping mechanism, which is essentially the reverse of the folding process. Based on the nearest neighbor model, stability of a duplex region of a hairpin is determined by the sequence and number of stacked base pairs (36, 37). Terminal base pairs “breathe” and are easily dissociated (38). Therefore, KF may induce unzipping of a hairpin in a mechanism that does not depend on the overall free energy of the hairpin structure (Fig. 4A). In the case of the i-motif, the structural topology encountered by the polymerase is quite different from those in hairpin and mixed G4 structures, and the loop structure of the i-motif likely presents a steric obstruction to unwinding of C:C+ base pairs (Fig. 4B). Furthermore, in the i-motif structure, two parallel-strand C:C+ base pairs mutually intercalate with each other and form the tetraplex. In this conformation, the base pair next to the terminal base pair belongs to the other parallel strand. Therefore, the consecutive unzipping from the terminal base pairs by breathing should be repressed due to the stacking by the intercalated base pairs from the other parallel strand. Replication through an i-motif–forming region may require the complete unfolding of the structure, leading to our observations. This might be a reason why G4 showed different enzyme processivities. In the case of mixed G4, although the stability of a terminal quartet is presumably higher than that of a terminal base pair of a duplex, once the terminal quartet is unzipped by breathing, the stability of the overall structure is decreased and KF can proceed (Fig. 4C). As for other G4 topologies, the breathing of the terminal quartet might be repressed, because metastable intermediates formed due to strand slippage via triplex formation occur during unfolding (39) (Fig. 4D). Data on the ILPR and Q6 G4s fall on the line of lnks vs. −∆G°37 data on i-motif–forming sequences. This suggests that it is also possible that metastable intermediates in the unfolding reaction might prolong dissociation of the i-motif.
Fig. 4.
Proposed mechanism of unwinding of structured DNAs in templates containing various structures. (A) Terminal base pair of a hairpin is relatively unstable. Thus, polymerase can induce unzipping one base pair at a time to proceed through a hairpin. (B) In the i-motif structure, the loop region can be a block for polymerase, and the base pair has a different topology from the first, which likely stalls the polymerase. (C) Replication of mixed G4 structure presents an obstacle as the terminal quartet is more stable than the terminal base pair of a hairpin. Once the terminal G-quartet is unzipped, the stability of G4 is significantly reduced and replication proceeds rapidly. (D) Parallel/antiparallel G4s have a different unfolding pathway, which repressed the terminal breathing and enzyme processivity as well as the i-motif.
In cells, helicases assist DNA polymerase in replication of structured DNAs. A variety of helicases that unwind G4 have been identified, and defects in helicases that unwind these structures are associated with genetic diseases (9). This phenomenon implies that the topological properties of structures formed in DNA templates are important. A recent study showed that the antiparallel G4 on the leading strand with Tm values even less than physiological temperature could cause genomic instability (40). This phenomenon implies that the replication stall due to the topology of noncanonical structures is also influenced by helicase polarity. Helicases that unwind i-motif structures have not yet been identified. However, i-motif–binding proteins, such as hnRNPA1 and hnRNPLL, induce unwinding of i-motif structures (41–43). It has also been suggested that negative supercoiling induced by RNA polymerases promote i-motif formation in cells (44). Our data suggest that i-motif formation can block replication, and this, in turn, may result in genomic instability. Genomic instability is associated with cancer development and also with chronic diseases, such as diabetes (45, 46). It is possible that mutation in or dysregulation of expression of i-motif–binding proteins or the acidic pH of the cancer cell environment may promote or lead to stabilization of i-motif formation. Moreover, there exists a lower number of the free water in tumor cells than normal cells (47), which might also stabilize i-motif formation.
In conclusion, template DNA that forms an i-motif especially decreased the processivity of DNA polymerase through a mechanism likely due to the unique topology of base pairs that stabilize the i-motif. Unwinding of i-motif structure by processive activity of KF had an activation free energy barrier (∆G°37‡) about threefold higher than that for unwinding of mixed G4s. The stalling was correlated with stability of the i-motif structure and was regulated by crowding molecule size. Therefore, it is possible that the i-motif may cause genomic instability in cancer cells. Our physicochemical approach can be applied to the screening of the ligands capable of inducing formation of specific topologies that stall replication. Development of topology-specific binders may enable site-specific control of genomic instability and expression of genes triggered by the noncanonical structures targeted.
Materials and Methods
The characterization of structure and melting analysis of DNAs were investigated by CD and UV spectrometry. In the replication assay, FAM-labeled DNA was used as a primer strand for the quantification of replicated product by PAGE analyses. Detailed information about the materials and methods used in this study is provided in SI Appendix, Materials and Methods.
Supplementary Material
Acknowledgments
We thank M. Izumi, J. Inoue, and A. Matsuyama for their help. This work was supported by grants-in-aid for scientific research from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) and Japan Society for the Promotion of Science (JSPS), especially a Grant-in-Aid for Scientific Research on Innovative Areas “Chemistry for Multimolecular Crowding Biosystems” (JSPS KAKENHI Grant JP17H06351); MEXT-Supported Program for the Strategic Research Foundation at Private Universities (2014–2019), Japan; The Hirao Taro Foundation of Konan Gakuen for Academic Research; The Okazaki Kazuo Foundation of Konan Gakuen for Advanced Scientific Research; The Chubei Itoh Foundation; and the Hyogo Science and Technology Association.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. S.A.W. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1704258114/-/DCSupplemental.
References
- 1.Nakano S, Miyoshi D, Sugimoto N. Effects of molecular crowding on the structures, interactions, and functions of nucleic acids. Chem Rev. 2014;114:2733–2758. doi: 10.1021/cr400113m. [DOI] [PubMed] [Google Scholar]
- 2.Biffi G, Tannahill D, McCafferty J, Balasubramanian S. Quantitative visualization of DNA G-quadruplex structures in human cells. Nat Chem. 2013;5:182–186. doi: 10.1038/nchem.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fadloun A, et al. Chromatin signatures and retrotransposon profiling in mouse embryos reveal regulation of LINE-1 by RNA. Nat Struct Mol Biol. 2013;20:332–338. doi: 10.1038/nsmb.2495. [DOI] [PubMed] [Google Scholar]
- 4.Bochman ML, Paeschke K, Zakian VA. DNA secondary structures: Stability and function of G-quadruplex structures. Nat Rev Genet. 2012;13:770–780. doi: 10.1038/nrg3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siddiqui-Jain A, Grand CL, Bearss DJ, Hurley LH. Direct evidence for a G-quadruplex in a promoter region and its targeting with a small molecule to repress c-MYC transcription. Proc Natl Acad Sci USA. 2002;99:11593–11598. doi: 10.1073/pnas.182256799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tateishi-Karimata H, Isono N, Sugimoto N. New insights into transcription fidelity: Thermal stability of non-canonical structures in template DNA regulates transcriptional arrest, pause, and slippage. PLoS One. 2014;9:e90580. doi: 10.1371/journal.pone.0090580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Endoh T, Kawasaki Y, Sugimoto N. Suppression of gene expression by G-quadruplexes in open reading frames depends on G-quadruplex stability. Angew Chem Int Ed Engl. 2013;52:5522–5526. doi: 10.1002/anie.201300058. [DOI] [PubMed] [Google Scholar]
- 8.Bugaut A, Balasubramanian S. 5′-UTR RNA G-quadruplexes: Translation regulation and targeting. Nucleic Acids Res. 2012;40:4727–4741. doi: 10.1093/nar/gks068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu Y, Brosh RM., Jr G-quadruplex nucleic acids and human disease. FEBS J. 2010;277:3470–3488. doi: 10.1111/j.1742-4658.2010.07760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benabou S, Aviñó A, Eritja R, González C, Gargallo R. Fundamental aspects of the nucleic acid i-motif structures. RSC Advances. 2014;4:26956–26980. [Google Scholar]
- 11.Phan AT, Mergny JL. Human telomeric DNA: G-quadruplex, i-motif and Watson-Crick double helix. Nucleic Acids Res. 2002;30:4618–4625. doi: 10.1093/nar/gkf597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brazier JA, Shah A, Brown GD. I-motif formation in gene promoters: Unusually stable formation in sequences complementary to known G-quadruplexes. Chem Commun (Camb) 2012;48:10739–10741. doi: 10.1039/c2cc30863k. [DOI] [PubMed] [Google Scholar]
- 13.Kendrick S, Hurley LH. The role of G-quadruplex/i-motif secondary structures as cis-acting regulatory elements. Pure Appl Chem. 2010;82:1609–1621. doi: 10.1351/PAC-CON-09-09-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gehring K, Leroy J-L, Guéron M. A tetrameric DNA structure with protonated cytosine.cytosine base pairs. Nature. 1993;363:561–565. doi: 10.1038/363561a0. [DOI] [PubMed] [Google Scholar]
- 15.Phan AT, Guéron M, Leroy J-L. The solution structure and internal motions of a fragment of the cytidine-rich strand of the human telomere. J Mol Biol. 2000;299:123–144. doi: 10.1006/jmbi.2000.3613. [DOI] [PubMed] [Google Scholar]
- 16.Kendrick S, et al. The dynamic character of the BCL2 promoter i-motif provides a mechanism for modulation of gene expression by compounds that bind selectively to the alternative DNA hairpin structure. J Am Chem Soc. 2014;136:4161–4171. doi: 10.1021/ja410934b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gurung SP, Schwarz C, Hall JP, Cardin CJ, Brazier JA. The importance of loop length on the stability of i-motif structures. Chem Commun (Camb) 2015;51:5630–5632. doi: 10.1039/c4cc07279k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dhakal S, et al. G-quadruplex and i-motif are mutually exclusive in ILPR double-stranded DNA. Biophys J. 2012;102:2575–2584. doi: 10.1016/j.bpj.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brosh RM., Jr DNA helicases involved in DNA repair and their roles in cancer. Nat Rev Cancer. 2013;13:542–558. doi: 10.1038/nrc3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paeschke K, et al. Pif1 family helicases suppress genome instability at G-quadruplex motifs. Nature. 2013;497:458–462. doi: 10.1038/nature12149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beese LS, Derbyshire V, Steitz TA. Structure of DNA polymerase I Klenow fragment bound to duplex DNA. Science. 1993;260:352–355. doi: 10.1126/science.8469987. [DOI] [PubMed] [Google Scholar]
- 22.Chen H, et al. Exploring the formation and recognition of an important G-quadruplex in a HIF1α promoter and its transcriptional inhibition by a benzo[c]phenanthridine derivative. J Am Chem Soc. 2014;136:2583–2591. doi: 10.1021/ja412128w. [DOI] [PubMed] [Google Scholar]
- 23.Rajendran A, Nakano S, Sugimoto N. Molecular crowding of the cosolutes induces an intramolecular i-motif structure of triplet repeat DNA oligomers at neutral pH. Chem Commun (Camb) 2010;46:1299–1301. doi: 10.1039/b922050j. [DOI] [PubMed] [Google Scholar]
- 24.Cui J, Waltman P, Le VH, Lewis EA. The effect of molecular crowding on the stability of human c-MYC promoter sequence I-motif at neutral pH. Molecules. 2013;18:12751–12767. doi: 10.3390/molecules181012751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Howorka S, Movileanu L, Braha O, Bayley H. Kinetics of duplex formation for individual DNA strands within a single protein nanopore. Proc Natl Acad Sci USA. 2001;98:12996–13001. doi: 10.1073/pnas.231434698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rauzan B, et al. Kinetics and thermodynamics of DNA, RNA, and hybrid duplex formation. Biochemistry. 2013;52:765–772. doi: 10.1021/bi3013005. [DOI] [PubMed] [Google Scholar]
- 27.Crothers DM, Bloomfield VA, Tinoco I. Nucleic Acids: Structures, Properties, and Functions. University Science Books; Sausalito, CA: 2000. [Google Scholar]
- 28.Gu XB, Nakano S, Sugimoto N. Consecutive GC base pairs determine the energy barrier of DNA duplex formation under molecularly crowded conditions. Chem Commun (Camb) 2007;(26):2750–2752. doi: 10.1039/b702865b. [DOI] [PubMed] [Google Scholar]
- 29.Yu Z, et al. ILPR G-quadruplexes formed in seconds demonstrate high mechanical stabilities. J Am Chem Soc. 2009;131:1876–1882. doi: 10.1021/ja806782s. [DOI] [PubMed] [Google Scholar]
- 30.Mirkin EV, Mirkin SM. To switch or not to switch: At the origin of repeat expansion disease. Mol Cell. 2014;53:1–3. doi: 10.1016/j.molcel.2013.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakano M, et al. Thermodynamic properties of water molecules in the presence of cosolute depend on DNA structure: A study using grid inhomogeneous solvation theory. Nucleic Acids Res. 2015;43:10114–10125. doi: 10.1093/nar/gkv1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu H, Gu X, Nakano S, Miyoshi D, Sugimoto N. Beads-on-a-string structure of long telomeric DNAs under molecular crowding conditions. J Am Chem Soc. 2012;134:20060–20069. doi: 10.1021/ja305384c. [DOI] [PubMed] [Google Scholar]
- 33.Yin YW, Steitz TA. Structural basis for the transition from initiation to elongation transcription in T7 RNA polymerase. Science. 2002;298:1387–1395. doi: 10.1126/science.1077464. [DOI] [PubMed] [Google Scholar]
- 34.Ray S, Bandaria JN, Qureshi MH, Yildiz A, Balci H. G-quadruplex formation in telomeres enhances POT1/TPP1 protection against RPA binding. Proc Natl Acad Sci USA. 2014;111:2990–2995. doi: 10.1073/pnas.1321436111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heddi B, Cheong VV, Martadinata H, Phan AT. Insights into G-quadruplex specific recognition by the DEAH-box helicase RHAU: Solution structure of a peptide-quadruplex complex. Proc Natl Acad Sci USA. 2015;112:9608–9613. doi: 10.1073/pnas.1422605112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Turner DH, Sugimoto N, Kierzek R, Dreiker SD. Free energy increments for hydrogen bonds in nucleic acid base pairs. J Am Chem Soc. 1987;109:3783–3785. [Google Scholar]
- 37.Turner DH, Sugimoto N, Freier SM. RNA structure prediction. Annu Rev Biophys Biophys Chem. 1988;17:167–192. doi: 10.1146/annurev.bb.17.060188.001123. [DOI] [PubMed] [Google Scholar]
- 38.Phelps C, Lee W, Jose D, von Hippel PH, Marcus AH. Single-molecule FRET and linear dichroism studies of DNA breathing and helicase binding at replication fork junctions. Proc Natl Acad Sci USA. 2013;110:17320–17325. doi: 10.1073/pnas.1314862110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stadlbauer P, Krepl M, Cheatham TE, 3rd, Koca J, Sponer J. Structural dynamics of possible late-stage intermediates in folding of quadruplex DNA studied by molecular simulations. Nucleic Acids Res. 2013;41:7128–7143. doi: 10.1093/nar/gkt412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schiavone D, et al. Determinants of G quadruplex-induced epigenetic instability in REV1-deficient cells. EMBO J. 2014;33:2507–2520. doi: 10.15252/embj.201488398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kang HJ, Kendrick S, Hecht SM, Hurley LH. The transcriptional complex between the BCL2 i-motif and hnRNP LL is a molecular switch for control of gene expression that can be modulated by small molecules. J Am Chem Soc. 2014;136:4172–4185. doi: 10.1021/ja4109352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roy B, et al. Interaction of individual structural domains of hnRNP LL with the BCL2 promoter i-motif DNA. J Am Chem Soc. 2016;138:10950–10962. doi: 10.1021/jacs.6b05036. [DOI] [PubMed] [Google Scholar]
- 43.Miglietta G, Cogoi S, Pedersen EB, Xodo LE. GC-elements controlling HRAS transcription form i-motif structures unfolded by heterogeneous ribonucleoprotein particle A1. Sci Rep. 2015;5:18097. doi: 10.1038/srep18097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brooks TA, Hurley LH. The role of supercoiling in transcriptional control of MYC and its importance in molecular therapeutics. Nat Rev Cancer. 2009;9:849–861. doi: 10.1038/nrc2733. [DOI] [PubMed] [Google Scholar]
- 45.Palazzo RP, Bagatini PB, Schefer PB, de Andrade FM, Maluf SW. Genomic instability in patients with type 2 diabetes mellitus on hemodialysis. Rev Bras Hematol Hemoter. 2012;34:31–35. doi: 10.5581/1516-8484.20120011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gaillard H, García-Muse T, Aguilera A. Replication stress and cancer. Nat Rev Cancer. 2015;15:276–289. doi: 10.1038/nrc3916. [DOI] [PubMed] [Google Scholar]
- 47.Damadian R, Zaner K, Hor D, DiMaio T. Human tumors detected by nuclear magnetic resonance. Proc Natl Acad Sci USA. 1974;71:1471–1473. doi: 10.1073/pnas.71.4.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.