Significance
Venous thromboembolism (VTE) is a common disease characterized by the formation of inappropriate blood clots. Inheritance of specific genetic variants, such as the Factor V Leiden polymorphism, increases VTE susceptibility. However, only ∼10% of people inheriting Factor V Leiden develop VTE, suggesting the involvement of other genes that are currently unknown. By inducing random genetic mutations into mice with a genetic predisposition to VTE, we identified two genomic regions that reduce VTE susceptibility. The first includes the gene for blood coagulation, Factor 3, and its role was confirmed by analyzing mice with an independent mutation in this gene. The second contains a mutation in the Actr2 gene. These findings identify critical genes for the regulation of blood-clotting risk.
Keywords: venous thromboembolism, Factor V Leiden, ENU mutagenesis, tissue factor pathway inhibitor, genetic screen
Abstract
Factor V Leiden (F5L) is a common genetic risk factor for venous thromboembolism in humans. We conducted a sensitized N-ethyl-N-nitrosourea (ENU) mutagenesis screen for dominant thrombosuppressor genes based on perinatal lethal thrombosis in mice homozygous for F5L (F5L/L) and haploinsufficient for tissue factor pathway inhibitor (Tfpi+/−). F8 deficiency enhanced the survival of F5L/L Tfpi+/− mice, demonstrating that F5L/L Tfpi+/− lethality is genetically suppressible. ENU-mutagenized F5L/L males and F5L/+ Tfpi+/− females were crossed to generate 6,729 progeny, with 98 F5L/L Tfpi+/− offspring surviving until weaning. Sixteen lines, referred to as “modifier of Factor 5 Leiden (MF5L1–16),” exhibited transmission of a putative thrombosuppressor to subsequent generations. Linkage analysis in MF5L6 identified a chromosome 3 locus containing the tissue factor gene (F3). Although no ENU-induced F3 mutation was identified, haploinsufficiency for F3 (F3+/−) suppressed F5L/L Tfpi+/− lethality. Whole-exome sequencing in MF5L12 identified an Actr2 gene point mutation (p.R258G) as the sole candidate. Inheritance of this variant is associated with suppression of F5L/L Tfpi+/− lethality (P = 1.7 × 10−6), suggesting that Actr2p.R258G is thrombosuppressive. CRISPR/Cas9 experiments to generate an independent Actr2 knockin/knockout demonstrated that Actr2 haploinsufficiency is lethal, supporting a hypomorphic or gain-of-function mechanism of action for Actr2p.R258G. Our findings identify F8 and the Tfpi/F3 axis as key regulators in determining thrombosis balance in the setting of F5L and also suggest a role for Actr2 in this process.
Venous thromboembolism (VTE) is a common disease that affects 1–3 per 1,000 individuals each year (1). VTE susceptibility exhibits a complex etiology involving contributions of both genes and environment. Genetic risk factors explain ≈60% of the overall risk for VTE (2). Recent large-scale genome-wide association studies (GWAS) confirmed ABO, F2 F5, F11, FGG, and PROCR as thrombosis susceptibility genes, with only two additional novel loci, TSPAN15 and SLC44A2, identified (3–6), leaving the major component of VTE genetic risk still unexplained.
The Factor V Leiden variant (F5L) is a common inherited risk factor for VTE with an average allele frequency of 3.5% in the European population (7–9). F5L is estimated to account for up to 25% of the genetically attributable thrombosis risk in this population (7). However, penetrance is incomplete, with only ∼10% of F5L heterozygotes developing thrombosis in their lifetimes. The severity of thrombosis also varies widely among affected individuals (10), limiting the clinical utility of F5L genotyping in the management of VTE (11).
The incomplete penetrance and variable expressivity of thrombosis among F5L patients can at least partially be explained by genetic interactions between F5L and other known thrombotic risk factors such as hemizygosity for antithrombin III or proteins C or S, as well as the common prothrombin 20210 polymorphism (10, 12, 13). However, <2% of F5L heterozygotes would be expected to coinherit a mutation at one or more of these loci, suggesting that a large number of additional genetic risk factors for VTE and/or modifiers of F5L remain to be identified (3, 10).
Mice carrying the orthologous F5L mutation exhibit a mild to moderate prothrombotic phenotype closely mimicking the human disorder (14). We previously reported a synthetic lethal interaction between F5L homozygosity (F5L/L) and hemizygosity for tissue factor pathway inhibitor (Tfpi+/−) (15). Nearly all mice with this lethal genotype combination (F5L/L Tfpi+/−) succumb to widespread, systemic thrombosis in the immediate perinatal period (15).
N-ethyl-N-nitrosourea (ENU) mutagenesis in mice has been used effectively to identify novel genes involved in a number of biological processes (16, 17). ENU-induced germline mutations transmitted from a mutagenized male mouse (G0) occur at ∼1.5 mutations per megabase, at least 50-fold higher than the endogenous background mutation rate (18). Several previous reports have successfully applied an existing phenotype as a sensitizer to identify modifier genes. A dominant suppressor screen in Mecp2-deficient mice (Rett syndrome) identified a mutation in squalene epoxidase (Sqle) as a heritable suppressor, resulting in prolonged survival and amelioration of neurologic manifestations (19). Other successful sensitized screens include analysis of mouse mutants predisposed to diabetic nephropathy (20), a screen in Sox10 haploinsufficient mice identifying the Gli3 gene as a modifier of neurocristopathy (21), and identification of a mutation in the c-Myb gene as a dominant modifier for platelet count in Mpl-deficient mice (congenital thrombocytopenia) (22). We now report the results of a dominant, sensitized ENU mutagenesis screen for suppressors of F5L/L Tfpi+/−-dependent lethal thrombosis.
Results and Discussion
F8 Deficiency Suppresses F5L/L Tfpi+/− Lethality.
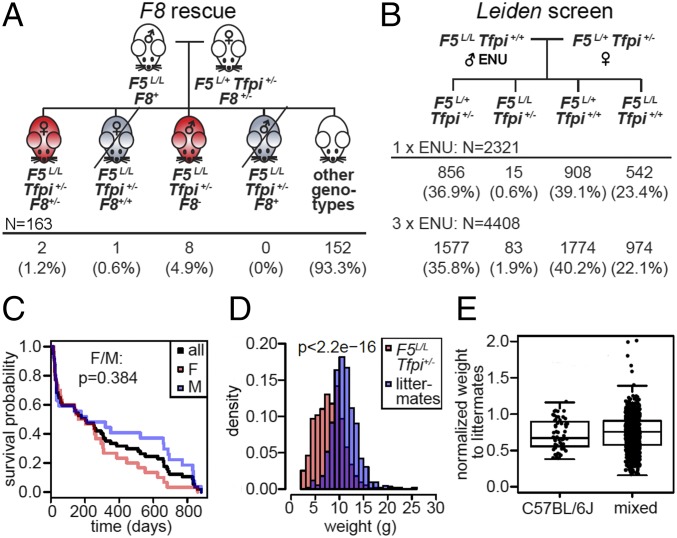
X-linked hemophilia A results in a moderate-to-severe bleeding disorder in humans and is caused by mutations in the F8 gene. To test whether the F5L/L Tfpi+/− lethal thrombotic phenotype is suppressible by hemophilia A in mice, triple-heterozygous F5L/+ Tfpi+/− F8+/− female mice were generated and crossed to F5L/L male mice (Fig. 1A). One quarter of conceptuses are expected to carry the F5L/L Tfpi+/− genotype, with half of the total expected male conceptuses completely F8 deficient (F8−). Thus, 1/16th of the overall offspring from this mating are expected to be F5L/L Tfpi+/− F8− males. Similarly, 1/16th of the progeny should be F5L/L Tfpi+/− F8+/− females. A total of 163 progeny from this cross were genotyped at weaning, resulting in eight F5L/L Tfpi+/− F8− male mice observed (and 0 F5L/L Tfpi+/− F8+ male mice, P = 0.02) and two F5L/L Tfpi+/− F8+/− female mice (and one F5L/L Tfpi+/− F8+/+ female mouse, P = 0.9). These results demonstrate that F5L/L Tfpi+/− thrombosis is genetically suppressible by F8 deficiency with nearly complete penetrance in F8− male mice and are consistent with human studies demonstrating F8 level as an important VTE risk factor (23).
Fig. 1.
F8-deficient thrombosuppression and design of the Leiden ENU mutagenesis screen. (A) The mating scheme and observed distributions of the F5L/+ Tfpi+/− F8 deficiency rescue experiments. F8− results in suppression of the F5L/L Tfpi+/− phenotype. (B) The mating scheme and observed distribution of the Leiden screen. F5L/L Tfpi+/+ male mice were mutagenized with either 1 × 150 mg/kg or 3 × 90 mg/kg ENU and were bred with nonmutagenized F5L/+ Tfpi+/− females. Fifteen and eighty-three F5L/L Tfpi+/− progeny, respectively were observed in each of the dosing regimens, with more than twice the rate of F5L/L Tfpi+/− survivors in the progeny of the 3 × 90 mg/kg-treated mice. (C) There was no significant difference in survival between male and female F5L/L Tfpi+/− putative suppressor mice (P = 0.384). Normal weaning and breeding ages are 20 d and 42 d, respectively. (D) F5L/L Tfpi+/− putative suppressor mice were significantly smaller than their nonF5L/L Tfpi+/− littermates. (E) F5L/L Tfpi+/− putative suppressors were smaller than their littermates of other genotypes (P < 8.8 × 10−12 for B6 and P = 2.2 × 10−16 for mixed B6-129S1) regardless of whether they were on the pure B6 or mixed B6-129S1 genetic backgrounds (P = 0.327 between B6 and mixed backgrounds).
The F5L/L Tfpi+/− Phenotype Is Suppressed by Dominant ENU-Induced Mutations.
A sensitized, genome-wide ENU mutagenesis screen for dominant thrombosis suppressor genes was implemented as depicted in Fig. 1B. ENU-mutagenized G0 F5L/L males were crossed to F5L/+ Tfpi+/− females to generate G1 mice, which were screened by genotyping at weaning for F5L and Tfpi+/−. Previously described visible dominant mutant phenotypes (24), including belly spotting and skeletal abnormalities, were observed in ≈5.9% of G1 offspring, similar to the ∼4.2% rate of observable mutants in previous studies (24). This is consistent with the ∼20–30 functionally significant mutations per G1 mouse expected with this ENU mutagenesis protocol (25). Although 25% of G1 embryos from this cross are expected to carry the synthetic lethal F5L/L Tfpi+/− genotype, most are lost at birth. Given a total of 6,631 G1s for the other three genotypes observed at weaning (∼1/3 for each genotype), a similar number of F5L/L Tfpi+/− G1 conceptuses, ∼2,210 (6,631 ÷ 3), would have been expected. The 98 live F5L/L Tfpi+/− mice (45 females, 53 males) thus represented 4.4% of the number expected with this genotype. Survival data were collected for 57 of the F5L/L Tfpi+/− G1 mice, 34 of which lived past 70 d of age; precise dates of death were not available for the remaining 41 mice. No significant sex-specific differences in survival were observed (Fig. 1C).
Heritability for each of the 44 G1 putative suppressor mutants who lived to breeding age was evaluated by a progeny test backcross to C57BL/6J (B6) F5L/L mice. The observation of one or more F5L/L Tfpi+/− offspring surviving to weaning increased the likelihood that a particular modifier of Factor 5 Leiden (MF5L) line carries a transmissible suppressor mutation. Of the original 98 surviving F5L/L Tfpi+/− G1 mice, 75 produced no offspring surviving to weaning, either due to infertility or the above-mentioned early lethality, with >50% of these mice (37 of 75) exhibiting a grossly runted appearance. Approximately half of the F5L/L Tfpi+/− G1 mice that attained breeding age (23/44) produced one or more G2 progeny surviving to weaning; seven (two males and five females) produced no F5L/L Tfpi+/− G2s, including four G1s with eight or more offspring of other genotypes. Sixteen F5L/L Tfpi+/− G1 mice produced one or more F5L/L Tfpi+/− progeny when bred to B6 F5L/L mice (Materials and Methods). These 16 potential thrombosuppressor mouse lines are designated MF5L1–16. The number of total progeny, genotypic distribution, and penetrance of the F5L/L Tfpi+/− mice in each line are listed in Table 1. Within these suppressor lines, mice with the F5L/L Tfpi+/− genotype were ∼30% smaller than their F5L/L littermates at the time of weaning (P < 2.2 × 10−16) (Fig. 1D), and this difference was maintained after outcrossing to the 129S1 strain (Fig. 1E).
Table 1.
Progeny genotypes and penetrance of putative MF5L thrombosuppressor genes
| ENU line | Sex of G1 | Total no. of progeny | Total no. of F5L/L Tfpi+/− mice | No. of F5L/L Tfpi+/+ littermates | Penetrance, % | No. of genotyped genetically informative F5L/L Tfpi+/− Mice |
| MF5L1 | M | 654 | 184 | 470 | 78.3 | 27 |
| MF5L2 | F | 14 | 1 | 13 | 15.4 | 0 |
| MF5L3 | F | 50 | 3 | 47 | 12.8 | 0 |
| MF5L4 | M | 3 | 1 | 2 | 100.0 | 0 |
| MF5L5 | M | 255 | 50 | 205 | 48.8 | 0 |
| MF5L6 | M | 1,393 | 336 | 1,057 | 63.6 | 98 |
| MF5L7 | F | 42 | 1 | 41 | 4.9 | 0 |
| MF5L8 | M | 543 | 132 | 411 | 64.2 | 0 |
| MF5L9 | M | 1,127 | 264 | 863 | 61.2 | 84 |
| MF5L10 | M | 111 | 15 | 96 | 31.3 | 0 |
| MF5L11 | M | 459 | 121 | 338 | 71.6 | 0 |
| MF5L12 | M | 200 | 46 | 154 | 59.7 | 0 |
| MF5L13 | M | 115 | 13 | 102 | 25.5 | 0 |
| MF5L14 | M | 47 | 3 | 44 | 13.6 | 0 |
| MF5L15 | F | 40 | 3 | 37 | 16.2 | 0 |
| MF5L16 | M | 442 | 119 | 323 | 73.7 | 14 |
Penetrance was calculated as total F5L/L Tfpi+/− divided by half of the number of F5L/L Tfpi+/+ littermates.
Previous reports based on gene function in the specific-locus test estimate an ENU-induced mutation rate of 1/700 loss-of-function mutations per locus for the ENU dosing regimen used here (26). This mutation rate predicts that our screen of 6,729 G1 progeny (2,210 F5L/L Tfpi+/− expected) should have produced approximately three mutations per gene averaged over the entire genome, with 54% of these mutations expected to be null (16), resulting in 1.5× genome coverage for loss-of-function mutations.
The MF5L6 Suppressor Mutation Maps to a Chromosome 3 Interval Containing F3.
To map putative ENU-induced suppressor mutations, surviving F5L/L Tfpi+/− mice were intercrossed with F5L/L mice that had been backcrossed onto the 129S1/SvIMJ strain (129S1). Crosses between F5L/L and F5L/+ Tfpi+/− mice (both F5L and Tfpi− backcrossed >12 generations onto 129S1) confirmed the lethality of the F5L/L Tfpi+/− genotype on the 129S1 background (Table S1). The four lines containing the largest number of genetically informative B6-129S1 mixed background F5L/L Tfpi+/− offspring (MF5L1, 6, 9, and 16) were used for gene mapping. Although the MF5L1, MF5L9, and MF5L16 lines were successfully expanded to pedigrees containing 27, 84, and 14 F5L/L Tfpi+/− informative offspring, respectively, genotyping for a total of ∼800 markers in each cross failed to identify any loci with a logarithm of the odds (LOD) score greater than or equal to 3.3 (maximum LODs for MF5L1 = 1.15, MF5L9 = 2.5, and MF5L16 = 1.61). Although we cannot exclude cosegregation of more than one suppressor mutation, the absence of a clear linkage signal for each of these lines likely reflects complex mouse strain modifier gene interactions, which are known to significantly impact mouse phenotypes (10, 27) and confound linkage analysis (28). Consistent with this hypothesis, we have previously documented poorer survival to weaning in mixed B6-129S1 F5L/L mice compared with littermates (14). We extended these observations by the analysis of additional F5L/+ and F5L/L littermates, with mice of the F5L/L genotype demonstrating a 50% reduction in survival in the 129S1 versus B6 strain backgrounds (Table S1).
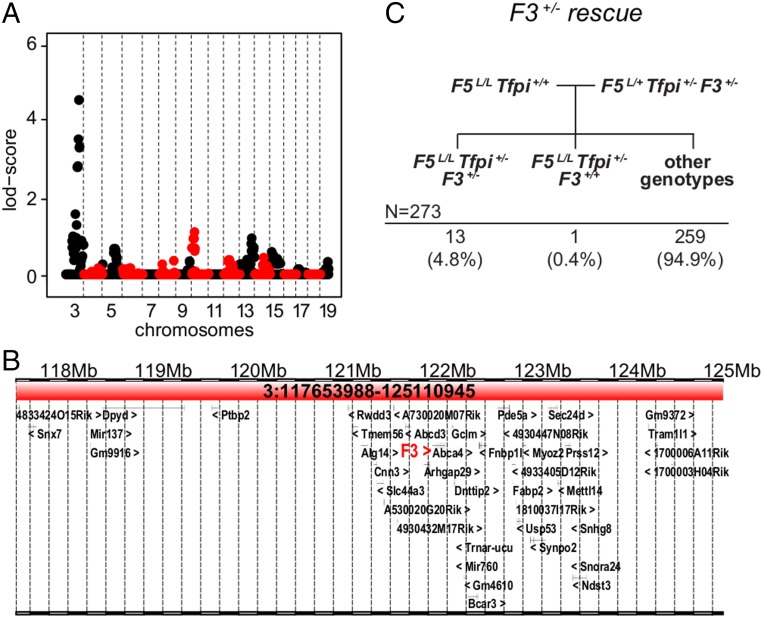
MF5L6 was maintained for 12 generations on both the mixed and B6 backgrounds and produced a total of 336 F5L/L Tfpi+/− mice (98 on the mixed B6-129S1 background and therefore useful for linkage analysis; see Table 1). Genome-wide SNP genotyping was performed on DNA from these 98 genetically informative F5L/L Tfpi+/− mice; multipoint linkage analysis is shown in Fig. 2A. Since the genetic intervals around the F5 and Tfpi loci cannot be accurately assessed for linkage, these regions of chromosomes (Chr) 1 and 2 were excluded from linkage analysis (Materials and Methods and Fig. 2A). A single locus with a significant LOD score of 4.49 was identified on Chr 3, with the 1 LOD interval (117.3–124.8 Mb) containing 43 National Center for Biotechnology Information reference sequence (RefSeq)-annotated genes (Fig. 2B).
Fig. 2.
The MF5L6 suppressor locus maps to Chr3. (A) Linkage analysis for the MF5L6 line. Alternating red and black are used to highlight the chromosomes. Chrs 1 and 2 were excluded from further analysis since they contain the F5 and Tfpi genes, whose segregation was restricted by required genotypes at these loci. The Chr3 peak had the highest LOD score in the Chr3 subregion: 117.3–124.8 Mb [maximum LOD = 4.49, one LOD interval, significance threshold of LOD >3.3 (44)]. (B) The Chr3 candidate interval (Chr3:117.3–124.8 Mb) contains 43 RefSeq-annotated genes, including F3. (C) The mating scheme and observed distribution of offspring to test F3 deficiency as a suppressor of F5L/L Tfpi+/−. F3+/− results in incompletely penetrant suppression of the F5L/L Tfpi+/− phenotype.
The F3 gene located within this interval (Chr3:121.7 Mb) (Fig. 2B) encodes tissue factor (TF), a procoagulant component of the hemostatic pathway that has Tfpi as its major regulator. Quantitative or qualitative deficiencies in F3 are thus highly plausible candidates to suppress the F5L/L Tfpi+/− phenotype. To test F3 as a candidate suppressor of the F5L/L Tfpi+/− phenotype, an independent F3-null allele was introduced, and triple-heterozygous F5L/+ Tfpi+/− F3+/− mice were crossed to F5L/L B6 mice (Fig. 2C). Of 273 progeny genotyped at weaning, 13 F5L/L Tfpi+/− F3+/− mice and one F5L/L Tfpi+/− F3+/+ mouse (P = 9.7 × 10−5) were observed. We also observed significantly fewer male than female F5L/L Tfpi+/− F3+/− mice (2 vs. 11, P = 0.03). Thus, haploinsufficiency for F3+/− suppresses the synthetic lethal F5L/L Tfpi+/− phenotype with incomplete penetrance (33%) that also differs by sex (10% for males and 67% for females). In contrast, the MF5L6 line exhibited an overall penetrance of 72.4%, with similar male/female penetrance. Gender-specific differences in venous thrombosis rates have previously been reported, including contributions from oral contraceptives and hormone replacement therapy (29–31). This difference in penetrance could be due to 129S1 strain effects in the MF5L6 line or differences between a F3 regulatory mutation in MF5L6 compared with the F3 loss-of-function allele used here.
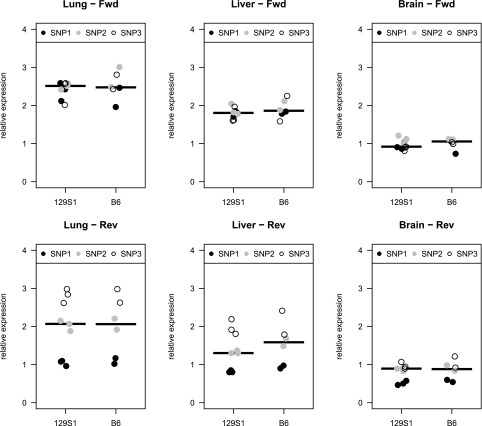
Whole-exome sequencing data analysis of a F5L/L Tfpi+/− mouse from MF5L6 failed to identify an ENU variant in F3 or in any other genes in the nonrecombinant interval or more broadly on the entire Chr3. This is a particularly gene-rich region (Fig. 2B), and errors in annotation could obscure the responsible variant. Of note, this interval also includes Slc44a3, a paralog of Slc44a2, the latter previously identified as a potential modifier of VTE risk in humans (6). Although additional ENU variants were identified on other chromosomes, none cosegregated with the survival phenotype in line MF5L6 (Table S2). Sanger sequencing analysis of the full set of F3 exons and introns, as well as 5 kb upstream of exon 1, also failed to identify an ENU-induced mutation. In addition, analysis of F3 mRNA levels in liver, lung, and brain tissues of adult mice failed to identify any differences in the level of expression from the ENU-mutant compared with the WT allele (Fig. S1).
Fig. S1.
Relative expression analysis of F3 alleles. Expression differences between 129S1 and B6 alleles were estimated at three different SNPs: (i) rs30268372, (ii) rs30269285, and (iii) 30269288 using both forward (Fwd) and reverse (Rev) sequences of cDNA extracted from lung, liver, and whole-brain tissues of adult mice. No significant differences were observed between the alleles in any of the tested tissues. Relative expression (y axis) represents the expression levels of B6 and 129S1 alleles normalized to the level of the DBA allele (Methods).
Taken together, these data suggest that an ENU-induced F3 regulatory mutation outside the sequenced segment may be responsible for thrombosuppression in MF5L6, although we cannot exclude a regulatory mutation in another gene. Nonetheless, our findings demonstrate that F3/Tfpi balance plays a key role in thrombosis in the mouse, particularly in the setting of F5L, and suggest that modest variations in either F3 or Tfpi could be important modifiers of VTE susceptibility in humans.
Whole-Exome Sequencing Identifies Candidate ENU Suppressor Variants for Eight MF5L Lines.
Whole-exome next-generation sequencing (NGS) was performed on genomic DNA from an index F5L/L Tfpi+/− mouse (from the G2–G5 generation) from each of eight MF5L lines, including the four lines described above and four additional lines with large pedigrees (MF5L5, MF5L8, MF5L11, MF5L12). The mean coverage of sequenced exomes was more than 90×, with >97% of the captured region covered with at least six independent reads (Table S3). A total of 125 heterozygous variants were identified as candidate suppressor mutations, with 79 variants affecting protein sequence (Table S2). Of the total mutations, 54.4% were nonsynonymous single-nucleotide variants (SNVs), followed by UTRs (17.6%) and synonymous (14.4%) and stop-gain SNVs (7.2%), with the remainder being comprised of indels, splicing, and stop-loss mutations. The most common mutation events were A/T→G/C transitions (35.3%), while C/G→G/C transversions were the least represented (2.5%). This spectrum of mutations is consistent with previously published ENU experiments (32). Variants exhibiting no recombination with the Tfpi locus on Chr2 (17 variants) were excluded from further analysis (Materials and Methods). Sanger sequencing confirmation was performed for 62 variants, including all nonsynonymous and stop-gain mutations. These variants were then checked for parent of origin (either the G1 mutagenized progeny or its nonmutagenized mate) as well as the original mutagenized G0 male. Forty-seven of these variants were identified in the G1 mouse but not in the G0 or nonmutagenized parent, consistent with ENU-induced mutations. The remaining 15 mutations were NGS sequencing errors (11/15), de novo mutations (2/15) or mutations transmitted from the nonmutagenized parent (2/15) (Table S2).
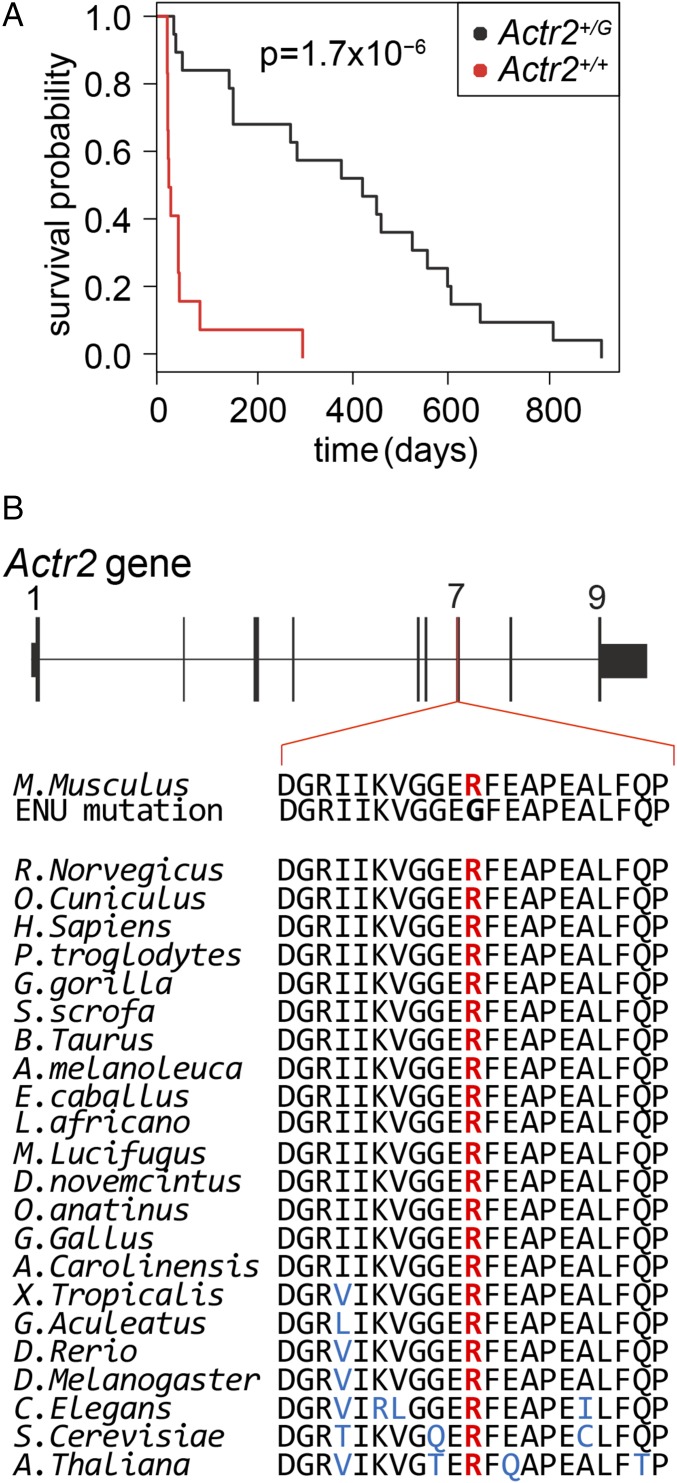
Each SNV was analyzed in additional MF5L mice from the line in which it was identified. None of the thrombosuppressive exonic ENU-induced variants identified in lines MF5L1, 5, 6, 8, 9, 11, and 16 segregated with the lethal phenotype as tested by Kaplan–Meier analysis using a significance threshold of P < 0.05 (33). Of the seven candidate ENU-induced SNVs identified from whole-exome sequence analysis for the MF5L12 line, one was an NGS sequencing error, and six were validated by Sanger sequencing as consistent with ENU-induced mutations in the G0 mice (Table S2). For each of these six SNVs, cosegregation with the survival phenotype was tested by Kaplan–Meier analysis of the first 31 F5L/L Tfpi+/− mice generated from the MF5L12 line. Only one variant, a nonsynonymous SNV in the actin-related protein 2 (Actr2) gene (c.772C > G, p.R258G, Actr2G), demonstrated a significant survival advantage when coinherited with the F5L/L Tfpi+/− genotype (P = 1.7 × 10−6) (Fig. 3A).
Fig. 3.
The Actr2 R258G ENU-induced mutation is a potential thrombosis suppressor gene. (A) Kaplan–Meier survival plot for F5L/L Tfpi+/− mice with and without the Actr2G mutation. F5L/L Tfpi+/− Actr2+/G mice exhibit significantly better survival than F5L/L Tfpi+/− Actr2+/+ littermates (n = 19 for Actr2+/G, n = 12 for Actr2+/+, n = 31 total). (B) ARP2 amino acid R258 is highly conserved in animals, plants, and fungi.
Actr2 as a Thrombosuppressor Gene.
The gene Actr2 encodes the ARP2 protein, which is an essential component of the Arp2/3 complex (34). ARP2 along with ARP3 and five other independent protein subunits (ARPC1–5) form the evolutionarily conserved seven-subunit Arp2/3 complex (35). Arp2/3 is a major component of the actin cytoskeleton and is found in most eukaryotic cells, including platelets (36). Arp2/3 binds to the sides of actin filaments and initiates the growth of a new filament, leading to the creation of branched actin networks that are important for many cellular processes (37). Loss of Arp2/3 function can have severe consequences, as illustrated by the embryonic lethality of mice homozygous for an ARP3 hypomorph (38). In hemostasis, the Arp2/3 complex is necessary for actin-dependent platelet cytoskeletal remodeling events, which are essential for platelet activation and degranulation (37, 39, 40). The Actr2+/G mutation results in a p.R258G substitution in exon 7 of Actr2 at a highly conserved amino acid position, with arginine present at this position for all 60 available vertebrate sequences (https://genome.ucsc.edu), as well as in plants and fungi (Fig. 3B). In addition, no variants at this position have been identified to date in over 120,000 human alleles (41).
Actr2 Hemizygosity Is Incompatible with Survival.
We attempted to generate an independent Actr2 knockin (Actr2G) allele by CRISPR/Cas9 genome editing (Materials and Methods and Figs. S2 and S3 and Table S4). Although highly efficient gene targeting was observed in blastocysts (Fig. S3), transfer of 275 injected embryos into foster mothers resulted in no surviving pups with a successfully targeted Actr2 allele. These data suggest that heterozygous loss of function for Actr2 may be incompatible with survival to term. Consistent with this hypothesis, human sequencing data from the Exome Aggregation Consortium (ExAC), which includes 60,706 individual exomes, reports a probability of loss-of-function intolerance for ACTR2 of 0.997 (41). ACTR2 mutations have not been previously associated with human disease (https://omim.org/entry/604221) (42), again as is consistent with early embryonic lethality. In addition, of 373,692 mouse ENU-induced mutations listed in the Mutagenetix website (https://mutagenetix.utsouthwestern.edu/), only 16 are located in the Actr2 gene, with no predicted loss-of-function mutations (43). Taken together, these data strongly suggest that haploinsufficiency for Actr2 is not tolerated in humans or mice. The viability of Actr2+/G mice suggests that the Actr2G allele is either hypomorphic or a unique gain-of-function mutation distinct from simple haploinsufficiency. Similarly, analysis of ExAC data suggests that four of the six other members of the Arp2/3 complex are intolerant of heterozygous loss of function in humans (41). Our findings suggest that subtle alterations in Actr2 function, and potentially in other components of the actin cytoskeleton, could alter hemostatic balance and play a previously unappreciated role in thrombosis susceptibility.
Fig. S2.
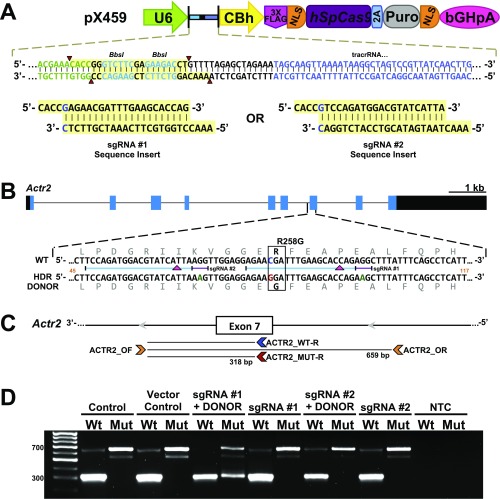
Strategy to generate the independent Actr2 knockin (Actr2G) allele by CRISPR/Cas9 technology. (A) Schematic representation of the pX459 vector and the designed guide sequence inserts. The image is an adaptation from Ran et al. (55). The guide oligos contain overhangs for ligation into the pair of BbsI sites (highlighted in yellow) in pX459. Digestion of pX459 with BbsI allows the replacement of the type II restriction sites (red arrowheads) with direct insertion of annealed oligos. Likewise, a G-C base pair (blue type in sgRNA insert sequences) is added at the 5′ end of the guide sequence for U6 transcription. Color coding of the sequence correlates with positions within the guide RNA expression cassette located in the pX459 vector (green type is the 3′ end of the U6 promoter; bright blue type on yellow highlighting identifies BbsI sites; black type is the chimeric guide RNA backbone; and blue type is the tracrRNA sequence. (B) sgRNA and HDR donor design for targeting the mouse Actr2 locus for R258G incorporation. The Actr2 gene is shown in the forward direction. Black and blue filled boxes indicate exons and the ORF, respectively. Gray type indicates the in-frame amino acid sequence; blue and red type indicate the C→G nucleotide change resulting in the R258G substitution; green type indicates synonymous mutations to block subsequent CRISPR binding following sequence replacement. Numbers in orange indicate the position within the 161-bp HDR template that is depicted in the figure. The blue and purple bars indicate the sgRNA and PAM, respectively, for sgRNA #1 and #2, and red arrowheads indicate the DSB position within Actr2 for each guide. (Scale bar for the Actr2 locus, 1 kb; intronic regions are reduced for fit.) (C) Schematic representation of Actr2 primer locations. Note reverse orientation. HDR-mediated DNA integration within the Actr2 gene was tested in the N2a cell line using the two sgRNAs and their respective ssDNA donor sequences for HDR-mediated knockin (Table S4). (D) Genotyping of heterogeneous N2a cell populations after puromycin selection. PCR was performed as described in Materials and Methods and depicted in C. Mut and Wt indicate competitive PCR with primers ACTR_OF/R and MUT-R or WT-R, respectively; NTC, no template control. In postselected heterogeneous N2a cells, the 318-bp band indicating the presence of the Actr2G allele within the heterogeneous cell population was observed when PCR was performed with the mutant-specific primer (Mut) on gDNA isolated from cells cotransfected with sgRNA #1 + DONOR.
Fig. S3.
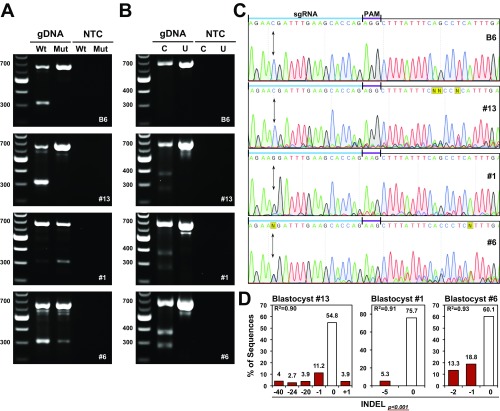
Actr2 hemizygosity is incompatible with survival. Thirty blastocysts were injected, and the 13 surviving to the 60-cell stage were analyzed. Genotyping of the 13 blastocysts produced from the sgRNA #1-injected oocytes exhibited a high degree of mosaicism, with all 13 positive for DNA cleavage events and five (38%) positive for Actr2G substitution. (A) Actr2 R258G genotyping of three mouse blastocysts (#13, #1, and #6) injected at the 60-cell stage and tail DNA from a B6 control mouse. This is the same PCR scheme shown in Fig. S2D. As expected, no mutant amplification is seen in the B6 tail DNA sample. Amplification of the mutant allele is present in blastocysts #1 and #6 but not in #13. (B) Surveyor assay for indel detection from products amplified with ACTR2_OF/R. C, cut; U, uncut. (C) Sanger sequencing chromatograms from the mouse blastocysts and B6 control. (D) Indel frequency for the three blastocysts determined by decomposition using TIDE software (60). Results indicate that all three blastocysts contain indels. Integration of the HDR donor template carrying the Actr2 mutation and synonymous PAM change within the blastocysts were detected by both PCR (A and B) and Sanger sequencing (D). While integration of the HDR donor template is evident in blastocysts #1 and #6 (B–D), they also contained multiple indels (2). Genetic mosaicism can occur with high frequency in founder mice derived using CRISPR/Cas9 genome editing (61). In our targeting strategy, the degree of observed mosaicism was likely due to persistent Cas9 expression. Subsequent injection of 305 fertilized eggs and subsequent transfer of 275 embryos into nine foster mothers resulted in the generation of only 18 surviving pups at weaning, all of which were WT for the Actr2 allele. Thus, the high efficiency of CRISPR/Cas9 in generating compound heterozygous loss-of-function variants together with the less efficient Actr2G substitution likely explains the outcome of our Actr2 genome-editing experiments. Taken together with the high Actr2-targeting efficiency, these data suggest that heterozygous loss of function for Actr2 may be incompatible with survival to term.
The identification of novel factors involved in the regulation of hemostasis is challenging; genes leading to marked shifts in hemostatic balance resulting in either severe bleeding or thrombosis are straightforward to identify clinically in humans, whereas subtle shifts are likely to escape detection given the multiple layers of buffering built into the complex hemostatic system (10). Homozygous deficiency (which would not be tested by our dominant suppressor strategy) for a number of hemostatic factors results in clinical bleeding, whereas heterozygous carriers remain asymptomatic. Although a single mutation in the X-chromosomal Factor VIII (or IX) gene produces severe bleeding in humans and rescues the lethal F5L/L Tfpi+/− mouse phenotype (Fig. 1A), an F8 gene mutation would not be transmitted from the ENU-mutagenized male to male offspring and thus would be undetected. Indeed, the dominant sensitized suppressor screen reported here was undertaken to identify genes for which a modest (≤50%) reduction in function would significantly shift the overall hemostatic balance. Such loci represent likely candidates for common human variation contributing to thrombosis and bleeding disorders. Gene variants with subtle yet significant antithrombotic effects represent attractive therapeutic targets because of a potentially wide therapeutic window with few unintended side effects. The finding of 98 F5L/L Tfpi+/− mice carrying putative thrombosis suppressor mutations (at an estimated 1.5× genome coverage) suggests that subtle alterations at a number of loci are capable of suppressing the F5L/L Tfpi+/− lethal thrombotic phenotype. The complex strain-specific genetic modifiers that confounded the genetic linkage analysis are consistent with this model. Nonetheless, our findings illustrate the particular importance of the F3/Tfpi axis in thrombosis regulation (especially in the setting of F5L) as well as the identification of Actr2 and the Arp2/3 complex as another potentially sensitive regulatory pathway for maintaining hemostatic balance.
Materials and Methods
The University of Michigan Institutional Committee on the Use and Care of Animals approved all experiments using mice (protocol numbers PRO00007371, PRO00005191, and PRO00005913). Detailed descriptions of mouse strains and procedures for ENU mutagenesis, breeding, genetic mapping and genotyping, Sanger and whole-exome sequencing, estimation of F3 allelic expression, generation of Actr2 CRISPR/Cas9-targeted mice and cells, the SURVEYOR nuclease assay, and statistical data analyses are provided in Supporting Information. Primers used in these studies are listed in Table S5.
SI Materials and Methods
Mice.
C57BL/6J (B6, stock number 000664), DBA2/J (DBA, stock number 000671), 129S1/SvImJ (129S1, stock number 002448), and B6D2F1 (stock number 100006) mice were purchased from the Jackson Laboratory. F5L/L (F5tm2Dgi/J, stock number 004080) mice were previously generated (14). F3- and Tfpi-deficient mice were a generous gift of George Broze, Division of Hematology & Oncology, Washington University School of Medicine, St. Louis (45, 46). F8-deficient mice were a generous gift of Haig Kazazian, Department of Molecular Biology & Genetics, Johns Hopkins School of Medicine, Baltimore (47). All mice designated to be on the B6 background were backcrossed more than eight generations to B6. F5L/L breeding stock for the 129S1 modifier gene crosses and genetic mapping experiments were generated from F5L mice serially backcrossed more than 12 generations to the 129S1 strain to create B6-129S1 F5L congenic mice. Tfpi− breeding stock for the 129S1 modifier gene crosses were generated by serially backcrossing more than 12 generations to the 129S1 strain to create B6-129S1 Tfpi+/− congenic mice. G1 suppressor mutant heritability was evaluated by a progeny test backcross to B6 F5L/L mice. Among the 16 mice able to produce surviving F5L/L Tfpi+/− offspring, males were overrepresented (four females, 12 males), likely because of larger numbers of offspring resulting from breeding to multiple female partners. These 16 potential thrombosuppressor mouse lines were crossed onto 129S1 to generate suppressor lines of genetically informative progeny for genetic mapping. The University of Michigan Institutional Committee on the Use and Care of Animals approved all experiments using mice (protocol numbers PRO00007371, PRO00005191, and PRO00005913).
Genotyping.
DNA was isolated from tail biopsies, and mice were genotyped for Tfpi+/− and F5L as previously described (15). Mice were genotyped for F3 deficiency using custom primers listed in Table S5. All primers were purchased from Integrated DNA Technologies (IDT).
ENU Mutagenesis and Breeding.
ENU was purchased (Sigma Aldrich) in ISOPAC vials and prepared according to the protocol given at www.sigmaaldrich.com/catalog/product/sigma/n3385?lang=en®ion=US. A single ENU dose of 150 mg/kg was administered i.p. into an initial cohort of 159 F5L/L B6 male mice (referred to as “generation 0” or “G0” mice). For a second cohort of 900 male F5L/L G0 mice, the protocol was changed to three weekly i.p. injections of ENU (90 mg/kg). After a 10-wk recovery period, each G0 mouse was bred to F5L/+ Tfpi+/− mice (Fig. 1B) on the B6 genetic background to produce G1 generation offspring, which were genotyped at 2 wk of age. G1 mice of the F5L/L Tfpi+/− genotype surviving to weaning age (3 wk of age) were considered to carry a suppressor mutation.
Modifier Gene Transmission.
F5L/L Tfpi+/− G1 founders were crossed to F5L/L mice on the B6 genetic background to produce G2 generation offspring. G2 mice were outcrossed to F5L/L mice on the 129S1 genetic background for two or more generations.
Genetic Mapping.
Genetic markers distinguishing the B6 and 129S1 strains distributed across the genome were genotyped using the Illumina GoldenGate Genotyping Universal-32 platform (Illumina) at the University of Michigan DNA Sequencing Core. Linkage analysis was performed on the Mendel platform version 14.0 (48) using 806 informative markers from the total of 1,449 genotyped markers. LOD scores ≥3.3 were considered significant (44). Chr1 and 2 contain the F5 and Tfpi genes, respectively, and therefore these chromosomes were excluded from further analysis. The number of mice for each of the mapped pedigrees are listed in Table 1.
Sanger Sequencing of the F3 Gene and Analysis of Candidate Mutations.
Genomic DNA was extracted from mouse tail biopsies using the Gentra Puregene Tissue Kit (Qiagen). A total of 48 overlapping pairs of amplicons (primers: F3gene_1-F3gene_35; upstreamF3_1-upstreamF3_13) (Table S5) were used to Sanger sequence the entire F3 gene (∼11 kb) and an additional ∼5 kb of upstream sequences on both strands. Sanger sequencing was performed at the University of Michigan Sequencing Core. For the analysis of candidate mutations, amplicons were generated harboring the nucleotide of interest using a custom outer primer pair. Inner forward and reverse primers were used to bidirectionally sequence these amplicons. Sequencing chromatograms were visualized and manually scored using FinchTV (PerkinElmer).
Estimation of F3 Allelic Expression.
The F3 exonic region harbors three known B6-129S1/DBA SNPs (rs30268372, rs30269285, and rs30269288; https://www.ncbi.nlm.nih.gov/SNP/) that were used for relative expression analysis. F5L/L Tfpi+/− mice with one B6 allele (in cis with ENU-induced variants) and one 129S1 allele at the Chr3 candidate region were outcrossed to DBA WT females introducing exonic B6-129S1/DBA SNPs. Five progeny from this cross (two B6/DBA and three 129S1/DBA allele carriers, identified by DNA genotyping) were tested for differential allelic expression. Three tissue samples (lung, liver, whole brain) were obtained from each mouse as previously described (49). RNA was extracted from the tissue samples using the RNeasy Plus Mini Kit (Qiagen) according to the manufacturer's recommendations and was reverse transcribed using SuperScript II (Invitrogen). cDNA corresponding to exon 3–exon 5 was amplified with primers F3-exon-F and F3-exon-R using GoTaq Green Master Mix (Promega). Primers F3-exon-F and F3-exon-R were also used to Sanger sequence the F3 exonic region.
Relative expression was estimated at SNP sites by dividing the area under the Sanger sequencing peak of the B6-129S1 allele by the DBA allele (49, 50). Next, the relative expression of each SNP was compared between the B6 and 129S1 allele-carrying progeny.
Mouse Whole-Exome Sequencing.
Libraries were prepared using Agilent (Agilent Technologies) or NimbleGen (Roche NimbleGen) mouse whole-exome capture kits. One hundred-base pair paired-end sequencing was performed on the Illumina HiSeq. 2000 platform at the University of Michigan DNA Sequencing Core. A detailed overview of the whole-exome sequencing pipeline is available at GitHub (https://github.com/tombergk/FVL_SUP). Briefly, sequence reads were aligned using Burrows–Wheeler Alignment software (51) to the mouse reference genome (genome assembly GRCm38, Ensembl release 73). Reads were sorted and duplications removed using Picard tools (picard.sourceforge.net). Coverage statistics were estimated using QualiMap software (52). Variants were called across eight samples using GATK HaplotypeCaller software (53). Standard hard filters recommended by the Broad Institute were applied using GATK VariantFiltration (53) followed by a pipeline developed in house to remove variants between the B6 and 129S1 strains, shared variants within our mouse cohort, and variants in closer proximity than 200 bp to each other. Variants were annotated using ANNOVAR software (54) with RefSeq annotation (release 61). Heterozygous variants within exonic regions with >6× coverage unique for only one mouse in the cohort were regarded as potential ENU-induced variants. A total of 125 heterozygous variants were identified as candidate suppressor mutations, using an in-house filtering pipeline (50), with 79 variants occurring within the coding sequence. The number of ENU variants identified in each exome-sequenced mouse varied by genealogical distance from the G1 MF5L founder. The candidate ENU-induced variants were confirmed by Sanger sequencing.
Generation of Actr2 CRISPR/Cas9-Targeted Mice and Cells.
Actr2 targeting sequence design and cloning.
The pX459 [pSpCas9(BB)-2A-Puro; plasmid ID: 48139] (55) biscistronic expression vector for human-codon optimized Streptococcus pyogenes Cas9, chimeric single-guide RNA (sgRNA), and the puromycin-resistance gene was obtained from Addgene. The vector was digested with BbsI, and a pair of annealed oligos (the sgRNA) was independently cloned into the backbone vector as described (55) and as depicted in Fig. S2A. Colonies were screened for successful guide insertion by BbsI/AgeI double digestion to discriminate between positive and negative clones. Clones that displayed only an ∼8.5-kb linearized AgeI-digested fragment were used in downstream applications.
The Actr2-specific sgRNA sequences used in these experiments are listed in Table S4. sgRNAs were selected based on (i) their proximity to the mutation site (<100 bp away; 10 bp optimal); (ii) the presence of a protospacer-adjacent motif (PAM) NGG sequence adjacent to the sgRNA; (iii) the ability to incorporate a synonymous variant within the PAM to protect the homology-directed repair (HDR) donor template from Cas9-targeted degradation; and (iv) an inverse likelihood of off-target binding score of >70 and no fewer than three mismatches within exonic regions of the genome as determined by the CRISPR design tool (crispr.mit.edu).
ssDNA donor for homology-directed repair.
The ssDNA oligonucleotides that served as HDR donor templates were ordered as Ultramer DNA oligos from IDT and are listed in Table S4. The HDR donor template consists of a 161-bp genomic sequence homologous to a region spanning −44 to +117 bp from the splice junction of intron 6 and exon 7 of the mouse Actr2 gene (Fig. S2B). The HDR donor encodes an arginine (R)-to-glycine (G) mutation at the 258th amino acid position (c.772C > G) and a synonymous mutation within the PAM to prevent donor DNA cleavage by Cas9. In this design, 80-bp homology arms flank the C-to-G transversion mutation with the position of the double-strand break (DSB) occurring 11–12 bp downstream or 17–18 bp upstream of the homology arm junction for sgRNA #1 and #2, respectively.
Transfection of Neuro-2a cells for validation of sgRNA and HDR efficiency.
Mouse Neuro-2a (N2a) cells (ATCC CCL-131) were routinely cultured in Eagle’s Minimum Essential Medium (EMEM) (HyClone) supplemented with 10% (vol/vol) FBS (HyClone) and were incubated at 37 °C in the presence of 5% CO2. N2a cells (passage 4) were seeded in triplicate into 24-well plates at a density of 1 × 105 cells per well in 0.5 mL EMEM containing 10% FBS 24 h before transfection. Cells in each well were cotransfected for 24 h with 0.5 μg pX459 plasmid (expressing either sgRNA #1 or #2) and 0.5 μg HDR donor template using 0.75 μL Lipofectamine 3000 (Invitrogen/Thermo Fisher Scientific) diluted in Opti-Mem I medium (Gibco/Thermo Fisher Scientific) following the manufacturer’s instructions. Transfected cells were isolated by antibiotic selection using 2 μg/mL Puromycin dihydrochloride (Gibco/Thermo Fisher Scientific) for 72 h. Transfected cells were passaged once before harvesting for genomic DNA extraction using the PureLink Genomic DNA mini kit (Invitrogen/Thermo Fisher Scientific) according to the manufacturer’s protocol.
Mouse pronuclear injection.
Pronuclear microinjection was carried out essentially as described in Brinster et al. (56). Following pronuclear microinjection, mouse zygotes were cultured in vitro to the blastocyst stage before DNA extraction and analysis for the presence of indels and mutations. Establishing stable CRISPR mouse lines by pronuclear coinjection of Cas9 mRNA, sgRNA, and oligo donors is highly efficient, as it was reported that for every 100 embryos that underwent this process, ∼13 genetically modified embryos were produced (57). CRISPR/Cas9 Actr2-edited embryos and mice were generated in collaboration with the University of Michigan Transgenic Animal Model Core (TAMC). A premixed solution of 5 ng/μL of pX459 plasmid containing sgRNA #1 targeting Actr2 exon 7 and 10 ng/µL HDR donor template was prepared in RNase-free microinjection buffer [10 mM Tris⋅HCl (pH 7.4), 0.25 mM EDTA] and was microinjected into the male pronucleus of fertilized mouse eggs obtained from the mating of B6 male mice with superovulated B6 female mice. Microinjected eggs were transferred to pseudopregnant B6DF1 female mice.
Genome extraction from blastocyst embryos.
Mouse blastocyst DNA extraction was performed based on the method described by Sakurai et al. (58) Briefly, 60-cell expanded blastocysts cultured for 3 d in vitro at the TAMC were individually collected into 0.2 mL tubes in 10 μL of ultrapure water. Ten microliters of 2× blastocyst extraction buffer [100 mM Tris⋅HCl (pH 8.3), 100 mM KCl, 0.02% gelatin, 0.45% Tween-20 supplemented with 60 μg/mL yeast tRNA and 125 μg/mL Proteinase K] were added, and the samples were incubated at 56 °C for 10 min followed by 95 °C for 10 min and were immediately placed on ice to prevent heteroduplex formation. Crude lysates were stored at −20 °C.
Multiplex PCR genotyping.
We designed a two-reaction multiplex PCR strategy sensitive enough to detect WT and R258G alleles that differ by a single nucleotide. Common ACTR2_OF forward and ACTR2_OR reverse primers were used in separate reactions with the WT-specific reverse primer (ACTR2_WT-R) or the R258G-specific reverse primer (ACTR2_MUT-R) that differ only in the −1 position on the 3′ end (Table S5). Inclusion of the common primers (659-bp product) provided amplification competition and acted as a positive PCR control. Amplification of the 318-bp product using ACTR2_WT-R or ACTR2_MUT-R allele-specific primers indicates the presence of the WT or mutant allele, respectively (Figs. S2D and S3A).
SURVEYOR nuclease assay.
The genomic region flanking the CRISPR target site for Actr2 was PCR amplified using genomic primers ACTR2_OF and ACTR2_OR (Table S5). Unpurified PCR products (30 μL) were subjected to a denaturing and reannealing process to enable heteroduplex formation: 95 °C for 10 min; 95 °C to 85 °C ramping at −2 °C/s; 85 °C to 25 °C at −0.3 °C/s; 25 °C for 1 min, and 4 °C hold. After reannealing, ∼250-ng DNA products were treated with SURVEYOR nuclease and SURVEYOR enhancer S (IDT) following the manufacturer’s recommended protocol for GoTaq DNA polymerase-amplified products. Digested and undigested (cut/uncut) products were analyzed by standard gel electrophoresis using 2.0% Tris-acetate EDTA (TAE) agarose gels containing ethidium bromide and were imaged with a Chemi-Doc Touch gel imaging system (Bio-Rad).
Statistical Data Analysis.
Statistical differences among the potential progeny of mouse crosses were determined using the Fisher’s exact test. The Student’s t test was used for estimating statistical differences between the weights of F5L/L Tfpi+/− mice and their littermates. Relative expression differences for F3 alleles were estimated using the Wilcoxon rank-sum test. All the above statistical analyses were performed using the stats package in R software. Kaplan–Meier survival curves with log-rank test to estimate significant differences in mouse survival and the significance for putative suppressors identified by exome sequencing were performed using the survival package in R (59).
Supplementary Material
Acknowledgments
We thank the expertise of the Transgenic Animal Model Core staff of the University of Michigan’s Biomedical Research Core Facilities for their assistance with this study. Research reported in this publication was supported by the National Cancer Institute of the NIH under Award P30CA046592 by the use of the following Cancer Center Shared Resources: Transgenic Animal Models. This research was supported by NIH Grants P01-HL057346 (to D.G.) and R15-HL133907 and R01-HL135035 (to R.J.W.). R.J.W. was supported by the Oakland University Research Excellence Fund, an Aniara Diagnostica Coagulation Research Grant, an American Heart Association (AHA) Predoctoral Fellowship, and AHA Innovative Research and Scientist Development Grants. K.T. was an International Fulbright Science and Technology Fellow and the recipient of an AHA Predoctoral Fellowship. M.A.B. and A.J.J. were recipients of AHA Undergraduate Fellowships. D.G. is a member of the University of Michigan Cancer Center and is an Investigator of the Howard Hughes Medical Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1705762114/-/DCSupplemental.
References
- 1.Silverstein MD, et al. Trends in the incidence of deep vein thrombosis and pulmonary embolism: A 25-year population-based study. Arch Intern Med. 1998;158:585–593. doi: 10.1001/archinte.158.6.585. [DOI] [PubMed] [Google Scholar]
- 2.Souto JC, et al. Genetic determinants of hemostasis phenotypes in Spanish families. Circulation. 2000;101:1546–1551. doi: 10.1161/01.cir.101.13.1546. [DOI] [PubMed] [Google Scholar]
- 3.Trégouët DA, et al. Is there still room for additional common susceptibility alleles for venous thromboembolism? J Thromb Haemost. 2016;14:1798–1802. doi: 10.1111/jth.13392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dentali F, et al. Non-O blood type is the commonest genetic risk factor for VTE: Results from a meta-analysis of the literature. Semin Thromb Hemost. 2012;38:535–548. doi: 10.1055/s-0032-1315758. [DOI] [PubMed] [Google Scholar]
- 5.Rosendaal FR, Reitsma PH. Genetics of venous thrombosis. J Thromb Haemost. 2009;7:301–304. doi: 10.1111/j.1538-7836.2009.03394.x. [DOI] [PubMed] [Google Scholar]
- 6.Germain M, et al. Cardiogenics Consortium Meta-analysis of 65,734 individuals identifies TSPAN15 and SLC44A2 as two susceptibility loci for venous thromboembolism. Am J Hum Genet. 2015;96:532–542. doi: 10.1016/j.ajhg.2015.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dahlbäck B. Advances in understanding pathogenic mechanisms of thrombophilic disorders. Blood. 2008;112:19–27. doi: 10.1182/blood-2008-01-077909. [DOI] [PubMed] [Google Scholar]
- 8.Lijfering WM, Rosendaal FR, Cannegieter SC. Risk factors for venous thrombosis - current understanding from an epidemiological point of view. Br J Haematol. 2010;149:824–833. doi: 10.1111/j.1365-2141.2010.08206.x. [DOI] [PubMed] [Google Scholar]
- 9.Clark JS, Adler G, Salkic NN, Ciechanowicz A. Allele frequency distribution of 1691G >A F5 (which confers Factor V Leiden) across Europe, including Slavic populations. J Appl Genet. 2013;54:441–446. doi: 10.1007/s13353-013-0166-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Westrick RJ, Ginsburg D. Modifier genes for disorders of thrombosis and hemostasis. J Thromb Haemost. 2009;7:132–135. doi: 10.1111/j.1538-7836.2009.03362.x. [DOI] [PubMed] [Google Scholar]
- 11.Evaluation of Genomic Applications in Practice and Prevention (EGAPP) Working Group Recommendations from the EGAPP Working Group: Routine testing for Factor V Leiden (R506Q) and prothrombin (20210G>A) mutations in adults with a history of idiopathic venous thromboembolism and their adult family members. Genet Med. 2011;13:67–76. doi: 10.1097/GIM.0b013e3181fbe46f. [DOI] [PubMed] [Google Scholar]
- 12.De Stefano V, et al. The risk of recurrent deep venous thrombosis among heterozygous carriers of both factor V Leiden and the G20210A prothrombin mutation. N Engl J Med. 1999;341:801–806. doi: 10.1056/NEJM199909093411104. [DOI] [PubMed] [Google Scholar]
- 13.van Boven HH, et al. Factor V Leiden (FV R506Q) in families with inherited antithrombin deficiency. Thromb Haemost. 1996;75:417–421. [PubMed] [Google Scholar]
- 14.Cui J, et al. Spontaneous thrombosis in mice carrying the factor V Leiden mutation. Blood. 2000;96:4222–4226. [PubMed] [Google Scholar]
- 15.Eitzman DT, et al. Lethal perinatal thrombosis in mice resulting from the interaction of tissue factor pathway inhibitor deficiency and factor V Leiden. Circulation. 2002;105:2139–2142. doi: 10.1161/01.cir.0000017361.39256.82. [DOI] [PubMed] [Google Scholar]
- 16.Cordes SP. N-ethyl-N-nitrosourea mutagenesis: Boarding the mouse mutant express. Microbiol Mol Biol Rev. 2005;69:426–439. doi: 10.1128/MMBR.69.3.426-439.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moresco EM, Li X, Beutler B. Going forward with genetics: Recent technological advances and forward genetics in mice. Am J Pathol. 2013;182:1462–1473. doi: 10.1016/j.ajpath.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bull KR, et al. Unlocking the bottleneck in forward genetics using whole-genome sequencing and identity by descent to isolate causative mutations. PLoS Genet. 2013;9:e1003219. doi: 10.1371/journal.pgen.1003219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buchovecky CM, et al. A suppressor screen in Mecp2 mutant mice implicates cholesterol metabolism in Rett syndrome. Nat Genet. 2013;45:1013–1020. doi: 10.1038/ng.2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tchekneva EE, et al. A sensitized screen of N-ethyl-N-nitrosourea-mutagenized mice identifies dominant mutants predisposed to diabetic nephropathy. J Am Soc Nephrol. 2007;18:103–112. doi: 10.1681/ASN.2006020164. [DOI] [PubMed] [Google Scholar]
- 21.Matera I, et al. A sensitized mutagenesis screen identifies Gli3 as a modifier of Sox10 neurocristopathy. Hum Mol Genet. 2008;17:2118–2131. doi: 10.1093/hmg/ddn110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carpinelli MR, et al. Suppressor screen in Mpl-/- mice: c-Myb mutation causes supraphysiological production of platelets in the absence of thrombopoietin signaling. Proc Natl Acad Sci USA. 2004;101:6553–6558. doi: 10.1073/pnas.0401496101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bank I, et al. Elevated levels of FVIII:C within families are associated with an increased risk for venous and arterial thrombosis. J Thromb Haemost. 2005;3:79–84. doi: 10.1111/j.1538-7836.2004.01033.x. [DOI] [PubMed] [Google Scholar]
- 24.Nolan PM, et al. A systematic, genome-wide, phenotype-driven mutagenesis programme for gene function studies in the mouse. Nat Genet. 2000;25:440–443. doi: 10.1038/78140. [DOI] [PubMed] [Google Scholar]
- 25.Justice MJ, et al. Effects of ENU dosage on mouse strains. Mamm Genome. 2000;11:484–488. doi: 10.1007/s003350010094. [DOI] [PubMed] [Google Scholar]
- 26.Davis AP, Justice MJ. An Oak Ridge legacy: The specific locus test and its role in mouse mutagenesis. Genetics. 1998;148:7–12. doi: 10.1093/genetics/148.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lusis AJ. Genetics of atherosclerosis. Trends Genet. 2012;28:267–275. doi: 10.1016/j.tig.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoo YJ, Mendell NR. The power and robustness of maximum LOD score statistics. Ann Hum Genet. 2008;72:566–574. doi: 10.1111/j.1469-1809.2008.00442.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kyrle PA, et al. The risk of recurrent venous thromboembolism in men and women. N Engl J Med. 2004;350:2558–2563. doi: 10.1056/NEJMoa032959. [DOI] [PubMed] [Google Scholar]
- 30.Roach RE, et al. Sex difference in the risk of recurrent venous thrombosis: A detailed analysis in four European cohorts. J Thromb Haemost. 2015;13:1815–1822. doi: 10.1111/jth.13116. [DOI] [PubMed] [Google Scholar]
- 31.Vandenbroucke JP, et al. Oral contraceptives and the risk of venous thrombosis. N Engl J Med. 2001;344:1527–1535. doi: 10.1056/NEJM200105173442007. [DOI] [PubMed] [Google Scholar]
- 32.Justice MJ, Noveroske JK, Weber JS, Zheng B, Bradley A. Mouse ENU mutagenesis. Hum Mol Genet. 1999;8:1955–1963. doi: 10.1093/hmg/8.10.1955. [DOI] [PubMed] [Google Scholar]
- 33.Rich JT, et al. A practical guide to understanding Kaplan-Meier curves. Otolaryngol Head Neck Surg. 2010;143:331–336. doi: 10.1016/j.otohns.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rotty JD, Wu C, Bear JE. New insights into the regulation and cellular functions of the ARP2/3 complex. Nat Rev Mol Cell Biol. 2013;14:7–12. doi: 10.1038/nrm3492. [DOI] [PubMed] [Google Scholar]
- 35.Rottner K, Hänisch J, Campellone KG. WASH, WHAMM and JMY: Regulation of Arp2/3 complex and beyond. Trends Cell Biol. 2010;20:650–661. doi: 10.1016/j.tcb.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 36.Veltman DM, Insall RH. WASP family proteins: Their evolution and its physiological implications. Mol Biol Cell. 2010;21:2880–2893. doi: 10.1091/mbc.E10-04-0372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Falet H, et al. Importance of free actin filament barbed ends for Arp2/3 complex function in platelets and fibroblasts. Proc Natl Acad Sci USA. 2002;99:16782–16787. doi: 10.1073/pnas.222652499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vauti F, et al. Arp3 is required during preimplantation development of the mouse embryo. FEBS Lett. 2007;581:5691–5697. doi: 10.1016/j.febslet.2007.11.031. [DOI] [PubMed] [Google Scholar]
- 39.Li Z, Kim ES, Bearer EL. Arp2/3 complex is required for actin polymerization during platelet shape change. Blood. 2002;99:4466–4474. doi: 10.1182/blood.v99.12.4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koseoglu S, et al. VAMP-7 links granule exocytosis to actin reorganization during platelet activation. Blood. 2015;126:651–660. doi: 10.1182/blood-2014-12-618744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lek M, et al. Exome Aggregation Consortium Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;536:285–291. doi: 10.1038/nature19057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McKusick VA. Mendelian inheritance in man and its online version, OMIM. Am J Hum Genet. 2007;80:588–604. doi: 10.1086/514346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beutler B. 2017 MUTAGENETIX. Available at www.mutagenetix.net/home.cfm. Accessed April 6, 2017.
- 44.Lander E, Kruglyak L. Genetic dissection of complex traits: Guidelines for interpreting and reporting linkage results. Nat Genet. 1995;11:241–247. doi: 10.1038/ng1195-241. [DOI] [PubMed] [Google Scholar]
- 45.Toomey JR, Kratzer KE, Lasky NM, Stanton JJ, Broze GJ., Jr Targeted disruption of the murine tissue factor gene results in embryonic lethality. Blood. 1996;88:1583–1587. [PubMed] [Google Scholar]
- 46.Huang ZF, Higuchi D, Lasky N, Broze GJ., Jr Tissue factor pathway inhibitor gene disruption produces intrauterine lethality in mice. Blood. 1997;90:944–951. [PubMed] [Google Scholar]
- 47.Bi L, et al. Targeted disruption of the mouse factor VIII gene produces a model of haemophilia A. Nat Genet. 1995;10:119–121. doi: 10.1038/ng0595-119. [DOI] [PubMed] [Google Scholar]
- 48.Lange K, et al. Mendel: The Swiss army knife of genetic analysis programs. Bioinformatics. 2013;29:1568–1570. doi: 10.1093/bioinformatics/btt187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mohlke KL, et al. Mvwf, a dominant modifier of murine von Willebrand factor, results from altered lineage-specific expression of a glycosyltransferase. Cell. 1999;96:111–120. doi: 10.1016/s0092-8674(00)80964-2. [DOI] [PubMed] [Google Scholar]
- 50.Tomberg K, et al. Spontaneous 8bp deletion in Nbeal2 recapitulates the gray platelet syndrome in mice. PLoS One. 2016;11:e0150852. doi: 10.1371/journal.pone.0150852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.García-Alcalde F, et al. Qualimap: Evaluating next-generation sequencing alignment data. Bioinformatics. 2012;28:2678–2679. doi: 10.1093/bioinformatics/bts503. [DOI] [PubMed] [Google Scholar]
- 53.DePristo MA, et al. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat Genet. 2011;43:491–498. doi: 10.1038/ng.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang K, Li M, Hakonarson H. ANNOVAR: Functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010;38:e164. doi: 10.1093/nar/gkq603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ran FA, et al. Genome engineering using the CRISPR-Cas9 system. Nat Protoc. 2013;8:2281–2308. doi: 10.1038/nprot.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brinster RL, Chen HY, Trumbauer ME, Yagle MK, Palmiter RD. Factors affecting the efficiency of introducing foreign DNA into mice by microinjecting eggs. Proc Natl Acad Sci USA. 1985;82:4438–4442. doi: 10.1073/pnas.82.13.4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang H, et al. One-step generation of mice carrying reporter and conditional alleles by CRISPR/Cas-mediated genome engineering. Cell. 2013;154:1370–1379. doi: 10.1016/j.cell.2013.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sakurai T, Watanabe S, Kamiyoshi A, Sato M, Shindo T. A single blastocyst assay optimized for detecting CRISPR/Cas9 system-induced indel mutations in mice. BMC Biotechnol. 2014;14:69. doi: 10.1186/1472-6750-14-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Therneau TM, Grambsch PM. 2000. Modeling Survival Data: Extending the Cox Model (Springer, New York), 1st Ed, pp xiv, 350.
- 60.Brinkman EK, Chen T, Amendola M, van Steensel B. Easy quantitative assessment of genome editing by sequence trace decomposition. Nucleic Acids Res. 2014;42:e168. doi: 10.1093/nar/gku936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Singh P, Schimenti JC, Bolcun-Filas E. A mouse geneticist’s practical guide to CRISPR applications. Genetics. 2015;199:1–15. doi: 10.1534/genetics.114.169771. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.